Abstract
Monoclonal antibodies (mAbs) that efficiently neutralize SARS-CoV-2 have been developed at an unprecedented speed. Notwithstanding, there is a vague understanding of the various Ab functions induced beyond antigen binding by the heavy-chain constant domain. To explore the diverse roles of Abs in SARS-CoV-2 immunity, we expressed a SARS-CoV-2 spike protein (SP) binding mAb (H4) in the four IgG subclasses present in human serum (IgG1-4) using glyco-engineered Nicotiana benthamiana plants. All four subclasses, carrying the identical antigen-binding site, were fully assembled in planta and exhibited a largely homogeneous xylose- and fucose-free glycosylation profile. The Ab variants ligated to the SP with an up to fivefold increased binding activity of IgG3. Furthermore, all H4 subtypes were able to neutralize SARS-CoV-2. However, H4-IgG3 exhibited an up to 50-fold superior neutralization potency compared with the other subclasses. Our data point to a strong protective effect of IgG3 Abs in SARS-CoV-2 infection and suggest that superior neutralization might be a consequence of cross-linking the SP on the viral surface. This should be considered in therapy and vaccine development. In addition, we underscore the versatile use of plants for the rapid expression of complex proteins in emergency cases.
Keywords: engineered IgG3, plant-based expression, antibodies, SARS-CoV-2, virus neutralization
Pre- and postexposure immunotherapies with neutralizing antibodies (nAbs) are presently being explored for the prevention and treatment of COVID-19. This has, at an unprecedented speed, led to the isolation of mAbs that efficiently neutralize SARS-CoV-2 (e.g., H4) (1). These mAbs, mainly directed against spike protein (SP) on the virion`s surface, are predominantly of the IgG1 subtype. However, a strong prevalence of other subclasses (like IgG3 and IgG4) was observed in the sera of COVID-19 patients (2), indicating a protective effect during the viral infection. While the role of IgG1 is intensively investigated, data on the functional impact of the other IgG subclasses, discriminated mainly by diverse Fc domains, are rare. Notably, the first SARS-CoV-2 studies on distinct IgG-Fc functional profiling point to enhancement of disease (3), in line with data from SARS-CoV experiments (4). These data underscore the need for a better understanding of the molecular properties of SARS-CoV-2 Ab variants prior to application in therapy or prophylaxis.
To further explore the role of Ab domains beyond antigen-binding in SARS-CoV-2 immunity, we focused on the expression and functional analyses of a neutralizing mAb with identical variable domains but different constant heavy chains (HCs), representing all four IgG subclasses present in human serum (IgG1-4).
Results
Production of Recombinant Monoclonal H4-IgG Subclasses.
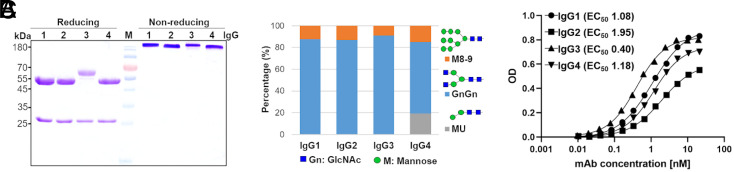
H4 SARS-CoV-2 neutralizing monoclonal IgG1 antibody that binds to an epitope at the receptor-binding domain of the SP (1) served as a template in this study. Four plant expression constructs with identical variable regions but different HC domains, representing human subclasses IgG1-4, were generated (H4-IgGHC1-4) (SI Appendix). The IgG3 isotype refers to allotype G3m5, containing a 62-amino-acid hinge domain. For light-chain expression, a single construct carrying the variable region of H4 and κ-light chain constant (κ-LC) domain was used for all subclasses (H4-IgGLC). Respective vectors of H4-IgGHC1-4 and H4-IgGLC were coexpressed in Nicotiana benthamiana glycosylation mutant ΔXTFT by agroinfiltration (5, 6) and purified at 4 d postinfiltration by protein A–based affinity and subsequent size-exclusion chromatography. SDS/PAGE analysis of purified mAbs revealed the correct expression, assembly, and high purity of all four subclasses (Fig. 1A).
Fig. 1.
Biochemical characterization and antigen-binding activities of recombinant H4-IgG1-4 produced in ΔXTFT. (A) Purified H4-IgG1-4 separated by reducing and nonreducing SDS/PAGE (Coomassie brilliant blue stained), 4 µg protein was loaded at each lane. (B) LC-ESI-MS/MS–derived glycosylation profiles of purified H4-IgG1-4. Bars represent the relative abundance (%) of glycoforms present at the conserved Fc glycosite. (C) ELISA-binding activity EC50 values in nanomoles of purified H4-IgG1-4 to SP using antibodies against κ-LC for detection.
Glycosylation Status of Plant-Derived H4-IgG Subclasses.
It is well known that the conserved Fc glycosylation at position N297 plays a vital role in Fcγ receptor (FcγR) interactions and effector function. In line, recent results demonstrate glycosylation-dependent functional effects during COVID-19 (7). Thus, we aimed at the production of mAbs with a targeted glycosylation profile. Liquid chromatography–electrospray ionization–tandem mass spectrometry (LC-ESI-MS/MS) (SI Appendix) of H4-IgG1-4 revealed a largely homogeneous Fc glycosylation profile, with ∼90% GnGn structures (i.e., core xylose and fucose-free N-glycans) (Fig. 1B).
Antigen-Binding and Neutralization Potency of H4-IgG Subclasses.
Antigen-binding properties of H4-IgG1-4 were determined by ELISA using Chinese hamster ovary–produced SARS-CoV-2 SP. The following binding activities, expressed by EC50 values, were obtained: IgG2 < IgG1 = IgG4 < IgG3 (EC50 1.95, 1.08, 1.18, and 0.4 nM, respectively) with an up to fivefold increased binding of IgG3 compared to the lowest binder, IgG2 (Fig. 1C).
To further determine functional activities of Ab subclasses, Vero cell–based SARS-CoV-2 neutralization (NT) assays were performed, as described previously (8) (SI Appendix). IgG1, IgG2, and IgG4 exhibited similar NT potencies (NT100 295.88, 296.49, and 222.36 nM, respectively) (Table 1). However, IgG3 displayed an up to 50-fold increased activity (NT100 5.91 nM) (Table 1) in comparison with the other subclasses. Our results point to an important role of HC domains that are not directly involved in antigen binding.
Table 1.
SARS-CoV-2 NT activities (NT100 values) of H4-IgG subclasses
| Ab subtype | NT100 (nM) |
| H4-IgG1 | 295.88 |
| H4-IgG2 | 296.49 |
| H4-IgG3 | 5.91 |
| H4-IgG4 | 222.36 |
Discussion
While major differences in IgG subtype prevalence in human sera during and after viral infections compared to healthy individuals are frequently observed, the consequences thereof are not well understood. Here we performed a side-by-side comparison of a SARS-CoV-2 neutralizing mAb with identical antigen-binding domains but four different HCs. Our results demonstrate substantially increased NT activities of IgG3 compared to the other three subclasses, despite relative modest variations in antigen binding.
Major differences of IgG3 compared to the other IgG subclasses are the long O-glycosylated hinge region and single point mutations in the Fc domain that enable, for example, high-affinity interaction with activating FcγRs. Such characteristics make IgG3 a uniquely potent immunoglobulin, with the potential for triggering effector functions and enhanced viral NT (9). Since we used Vero cells that do not carry FcγR in our studies, superior SARS-CoV-2 NT induced by Fc-mediated effector activities can be excluded. More likely, our data suggest that the unusual hinge region of IgG3 is a key factor that mediates altered activities. Indeed, previous studies, particularly in HIV settings, report a significant impact of the hinge region on the Ab’s functional activities (10). It has been suggested that augmented Ab-based NT potency toward HIV is driven by “intratrimeric cross-linking” of the two Fab arms, thus increasing the overall avidity of the spike–Ab interaction (11, 12). Our data are in line with such studies and indicate that enhanced SARS-CoV-2 NT is a consequence of cross-linking SP on the viral surface, induced by the unique structural features of IgG3. Also in accordance with HIV data (11), we hypothesize that cross-linking lowers the concentration of Abs required for NT and low spike densities facilitate Ab evasion. Interestingly, the SP copy number per SARS-CoV-2 virion is comparable with HIV, but 5 to 10 times less than that of other enveloped viruses, such as the influenza virus (13, 14). Notably, epitope-specific induction of neutralizing IgG3 Abs has been reported (e.g., HIV, Chikungunya virus) (9). This is an additional factor that cannot be excluded in our studies.
In addition, we show the expression of Ab variants with a single dominant N-glycan species (i.e., GnGn or G0). This glycan form, known to confer proinflammatory activities on Abs, is detected only in minor amounts in human-derived Abs. Here, we do not expect functional alterations due to this modification; however, fucose-free SARS-CoV-2 Abs may exhibit increased in vivo activities. This needs to be proven. In addition, the conserved core GnGn structures (5) simplify further engineering to elucidate so far potentially unknown glycosylation-dependent activities of SARS-CoV-2 antibodies, as suggested recently (7). Collectively, our results deliver insights into SARS-CoV-2 immunology and should perchance be considered for therapy or passive SARS-CoV-2 protection.
Materials and Methods
In planta expression of IgG subtypes was accomplished by agroinfiltration of respective DNA constructs. Subsequently, affinity- and size exclusion chromatography–purified mAbs were subjected to ELISA antigen-binding assays and NT activity was measured by a Vero cell–based SARS-CoV-2 infection assay, as described recently (8). Materials and methods are detailed in SI Appendix, Supplementary Text Materials and Methods.
Acknowledgments
We thank Jutta Hutecek (Center for Virology, Medical University of Vienna) for excellent technical assistance and Victor Klimyuk (Icon Genetics GmbH) for providing MagnICON vectors TMVα and PVXα. This work was supported by Austrian Science Fund Grants I 4328-B and I 3721-B30 (to H.S.) and the BOKU COVID‐19 Initiative.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2107249118/-/DCSupplemental.
Data Availability
All study data are included in the article and SI Appendix.
References
- 1.Wu Y., et al., A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science 368, 1274–1278 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amanat F., et al., A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 26, 1033–1036 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atyeo C., et al., Dissecting strategies to tune the therapeutic potential of SARS-CoV-2-specific monoclonal antibody CR3022. JCI Insight 6, 143129 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu L., et al., Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight 4, 123158 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kallolimath S., et al., Expression profiling and glycan engineering of IgG subclass 1-4 in Nicotiana benthamiana. Front. Bioeng. Biotechnol. 8, 825 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strasser R., et al., Generation of glyco-engineered Nicotiana benthamiana for the production of monoclonal antibodies with a homogeneous human-like N-glycan structure. Plant Biotechnol. J. 6, 392–402 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Larsen M. D., et al.; Amsterdam UMC COVID-19; Biobank Study Group, Afucosylated IgG characterizes enveloped viral responses and correlates with COVID-19 severity. Science 371, eabc8378 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koblischke M., et al., Dynamics of CD4 T cell and antibody responses in COVID-19 patients with different disease severity. Front. Med. (Lausanne) 7, 592629 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damelang T., Rogerson S. J., Kent S. J., Chung A. W., Role of IgG3 in infectious diseases. Trends Immunol. 40, 197–211 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Chu T. H., et al., Hinge length contributes to the phagocytic activity of HIV-specific IgG1 and IgG3 antibodies. PLoS Pathog. 16, e1008083 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bournazos S., Gazumyan A., Seaman M. S., Nussenzweig M. C., Ravetch J. V., Bispecific anti-HIV-1 antibodies with enhanced breadth and potency. Cell 165, 1609–1620 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galimidi R. P., et al., Intra-spike crosslinking overcomes antibody evasion by HIV-1. Cell 160, 433–446 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao H., et al., Molecular architecture of the SARS-CoV-2 virus. Cell 183, 730–738.e13 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ke Z., et al., Structures and distributions of SARS-CoV-2 spike proteins on intact virions. Nature 588, 498–502 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All study data are included in the article and SI Appendix.