Abstract
Background:
The public health significance of the reported higher incidence of chronic kidney disease (CKD) with intensive systolic blood pressure (SBP) lowering is unclear.
Objective:
To examine the effects of intensive SBP lowering on kidney and cardiovascular outcomes and contrast its apparent beneficial and adverse effects.
Design:
Subgroup analyses of SPRINT (Systolic Blood Pressure Intervention Trial). (ClinicalTrials.gov: NCT01206062)
Setting:
Adults with high blood pressure and elevated cardiovascular risk.
Participants:
6662 participants with a baseline estimated glomerular filtration rate (eGFR) of at least 60 mL/min/1.73 m2.
Intervention:
Random assignment to an intensive or standard SBP goal (120 or 140 mm Hg, respectively).
Measurements:
Differences in mean eGFR during follow-up (estimated with a linear mixed-effects model), prespecified incident CKD (defined as a >30% decrease in eGFR to a value <60 mL/min/1.73 m2), and a composite of all-cause death or cardiovascular event, with surveillance every 3 months.
Results:
The difference in adjusted mean eGFR between the intensive and standard groups was −3.32 mL/min/1.73 m2 (95% CI, −3.90 to −2.74 mL/min/1.73 m2) at 6 months, was −4.50 mL/min/1.73 m2 (CI, −5.16 to −3.85 mL/min/1.73 m2) at 18 months, and remained relatively stable thereafter. An incident CKD event occurred in 3.7% of participants in the intensive group and 1.0% in the standard group at 3-year follow-up, with a hazard ratio of 3.54 (CI, 2.50 to 5.02). The corresponding percentages for the composite of death or cardiovascular event were 4.9% and 7.1% at 3-year follow-up, with a hazard ratio of 0.71 (CI, 0.59 to 0.86).
Limitation:
Long-term data were lacking.
Conclusion:
Intensive SBP lowering increased risk for incident CKD events, but this was outweighed by cardiovascular and all-cause mortality benefits.
Primary Funding Source:
National Institutes of Health.
About 1.3 billion adults worldwide are believed to have hypertension, defined as systolic blood pressure (SBP) of at least 140 mm Hg, diastolic blood pressure (DBP) of at least 90 mm Hg, or the need for blood pressure medication (1). High blood pressure is one of the most important risk factors for cardiovascular disease (CVD) events (2–5), end-stage renal disease (ESRD) (3), and all-cause death (3). Nearly two thirds of U.S. adults aged 60 years or older have hypertension (6), and the population of persons aged 65 years or older is projected to reach 83.7 million by 2050, almost double the estimate of 43.1 million in 2012 (7). Therefore, the prevalence and public health burden of hypertension will probably continue to increase unless effective interventions are implemented.
Although SBP is generally considered a more important CVD risk factor than DBP in patients older than 50 years, the optimal goal for SBP during antihypertensive therapy has been controversial (2, 8). SPRINT (Systolic Blood Pressure Intervention Trial) examined the effects of intensive (target <120 mm Hg) versus standard (target <140 mm Hg) SBP control (9). The intervention was stopped early after a median follow-up of 3.26 years because intensive therapy resulted in a substantial reduction in the primary outcome—a composite of CVD events (hazard ratio [HR], 0.75 [95% CI, 0.64 to 0.89])—and in the secondary outcome of all-cause mortality (HR, 0.73 [CI, 0.60 to 0.90]). However, in persons without kidney disease at baseline, the intensive SBP group had a 3.5-fold higher hazard of incident chronic kidney disease (CKD), based on an a priori definition of a reduction in estimated glomerular filtration rate (eGFR) of at least 30% to a confirmed level less than 60 mL/min/1.73 m2.
We performed detailed analyses of the SPRINT prespecified kidney outcomes of incident CKD (as defined earlier) and incident albuminuria (defined as a doubling of urinary albumin–creatinine ratio from a value <10 mg/g to a value >10 mg/g) in participants with a baseline eGFR of at least 60 mL/min/1.73 m2. In addition, we evaluated the following post hoc hypotheses. First, the effect of intensive SBP control on incident CKD is due to an acute effect occurring in the first several months of follow-up, without subsequent effects on the long-term rate of kidney disease progression. If this is true, the difference in mean eGFR between treatment groups would be expected to increase during the early phase of follow-up but remain constant during the later phase of treatment. Second, elderly participants (aged ≥65 years at baseline) and those with baseline albuminuria are at higher risk for kidney function decline with intensive treatment. Finally, the higher risk for incident CKD in the intensive treatment group is outweighed by protective cardiovascular and all-cause mortality effects.
Methods
SPRINT was a randomized, controlled, open-label trial that compared the effects of intensive (target <120 mm Hg) versus standard (target <140 mm Hg) SBP control in 9361 participants at 102 clinical centers across the United States and Puerto Rico (9). Details of the SPRINT protocol have been published (10, 11). The study was approved by the institutional review boards at each participating study site.
This article describes a secondary analysis of prespecified secondary outcomes (incident CKD and incident albuminuria) performed in the prespecified subgroup of participants without CKD at baseline. Secondary post hoc analyses examined the acute effects of the intervention on eGFR, outcomes by subgroup, and the numbers needed to treat for benefit or harm.
Study Population
Participants were recruited between November 2010 and March 2013 and were required to meet all of the following inclusion criteria: age 50 years or older, SBP of 130 to 180 mm Hg, and increased risk for CVD (defined as ≥1 of the following: clinical or subclinical CVD other than stroke; 10-year risk for CVD ≥15%, based on the Framingham global risk indicator [12]; aged ≥75 years; or eGFR of 20 to <60 mL/min/1.73 m2). Major exclusion criteria included presence of diabetes, prior stroke, advanced CKD (eGFR <20 mL/min/1.73 m2), proteinuria greater than 1 g/d, polycystic kidney disease, congestive heart failure (symptoms or ejection fraction <35%), dementia, or residence in a nursing home. Further details of the SPRINT inclusion and exclusion criteria have been published elsewhere (9, 10). This article is restricted to the prespecified subgroup of 6662 SPRINT participants with baseline eGFR of at least 60 mL/min/1.73 m2.
Intervention, Follow-up, and Measurements
Volunteers who met the eligibility criteria and agreed to participate were randomly assigned to either intensive or standard SBP control. Randomization was stratified by clinical site but not by baseline presence of CKD. Neither the clinical investigators nor the participants were blinded to the intervention. Details of the SPRINT intervention algorithm are provided elsewhere (9, 10). In brief, participants were seen monthly for the first 3 months and every 3 months thereafter at standardized visits by trained study staff following requirements specified in the protocol. An automated system (Model 907 [Omron Healthcare]) was used to measure blood pressure at an office visit while the participant was seated and after 5 minutes of quiet rest. The mean of 3 blood pressure readings, each taken 1 minute apart, was used to estimate blood pressure.
Medication doses were initially adjusted every month in the intensive treatment group to target an SBP less than 120 mm Hg, and doses were adjusted to target an SBP of 135 to 139 mm Hg in the standard treatment group. The dose was reduced in the standard group if SBP was less than 130 mm Hg at a single visit or less than 135 mm Hg at 2 consecutive visits. Although achievement of SBP goals was emphasized in both groups, the investigators were allowed to adjust medication doses on the basis of clinical judgment. Lifestyle modification was encouraged.
Serum specimens were obtained at each visit for the first 3 months and quarterly thereafter for estimation of serum creatinine concentrations at the central laboratory at the University of Minnesota using an enzymatic creatinine assay (Roche). Fasting visits occurred at baseline and at 12, 24, and 48 months. The 4-variable MDRD (Modification of Diet in Renal Disease) study equation was used to estimate GFR (13).
Urine albumin and creatinine concentrations were measured at baseline and at 6, 12, 24, and 48 months. Albumin was measured using an immunoturbidimetric assay (Roche) at the central laboratory, and creatinine was measured using an enzymatic method (Roche). Event ascertainment and safety assessments were performed per protocol.
A decision to discontinue the SPRINT blood pressure intervention was made on 20 August 2015 after interim analyses of the primary outcome exceeded the monitoring boundary on 2 consecutive occasions, accompanied by a statistically significant difference in all-cause mortality (9). Only events that occurred on or before 20 August 2015 are included in this analysis; however, some eGFR or albuminuria outcomes were confirmed by samples collected after that date. Data for this analysis were frozen on 31 January 2016. As a result, 13 “new” incident CKD events in the intensive group and 3 in the standard group are included in addition to those in the previous report. Further details on additional event outcomes are provided in Appendix 2 (available at Annals.org) (9).
SPRINT Outcomes
The primary outcome in SPRINT was a composite of nonfatal myocardial infarction, acute coronary syndrome not resulting in myocardial infarction, stroke, acute decompensated heart failure, or death from CVD. Secondary outcomes included the components of the primary outcome, all-cause death, and a composite of the primary outcome or all-cause death. All outcome events were adjudicated by an outcomes committee that was blinded to the intervention.
Incident CKD, defined as a greater than 30% decrease in eGFR with a confirmed value less than 60 mL/min/1.73 m2 (confirmed by the next available official SPRINT laboratory value), was a prespecified secondary renal outcome in participants without CKD at baseline (eGFR ≥60 mL/min/1.73 m2). Incident albuminuria was prespecified as a doubling of urinary albumin–creatinine ratio from a value less than 10 mg/g to a value greater than 10 mg/g (confirmed by the next available official SPRINT laboratory value). Incident ESRD was defined as a need for long-term dialysis or kidney transplantation.
Statistical Analysis
All randomly assigned participants without CKD at baseline were included in the analyses, which were based on the intention-to-treat principle. The number of antihypertensive medications was determined as the number of distinct classes prescribed for each participant at each visit. Follow-up SBP was compared between treatment groups by using a mixed linear model with an unstructured variance–covariance matrix to control for within-subject correlation. A mixed-effect linear model with an unstructured variance–covariance matrix was used to estimate mean eGFR at follow-up by treatment group, with baseline eGFR included as a covariate. Seventy-two participants (31 in the intensive group and 41 in the standard group) without postrandomization serum creatinine measurements did not contribute to estimation of mean eGFR at follow-up or the analysis of incident CKD.
Separate Cox proportional hazards regression analyses with stratification by clinic were used to examine the effect of treatment group assignment on the primary renal outcome (defined as time from randomization to incident CKD, with a confirmed 30% decrease in eGFR from baseline) and on time to incident albuminuria and CVD and death outcomes. Follow-up was censored at the participant’s date of last creatinine measurement for incident CKD (or last urine sample for urinary albumin–creatinine ratio) and the date of the last assessment of study events for CVD outcomes. Information on vital status was supplemented with data from the National Death Index. Follow-up for all-cause mortality was censored at the end of calendar year 2014 for participants who were lost to follow-up.
Analyses of the cumulative incidence and absolute risks for renal and nonfatal CVD outcomes were performed using competing-risks models, in which non-CVD death was treated as a competing risk for the CVD outcomes and all-cause death was treated as a competing risk for incident CKD and incident albuminuria (14). The proportionality assumption for treatment effects was assessed by examining martingale residuals; no significant departures were found. Interactions between treatment effect and prespecified subgroups based on age, sex, race, and baseline albuminuria were assessed by likelihood ratio tests, with P value adjustment to account for the 4 comparisons (15). Tests of significance were performed using a 2-sided 5% level of significance. The numbers needed to treat for benefit or harm and the corresponding 95% CIs were calculated for CVD outcomes and incident CKD as the inverse of the absolute risk reduction (number needed to treat for benefit) or increase (number needed to treat for harm) (16), using estimates of event-free survival from cumulative incidence curves adjusted for competing risks (nonfatal outcomes) or stratified Kaplan–Meier survival estimates (fatal outcomes) at 3 years.
All analyses were performed using SAS, version 9.4 (SAS Institute). Additional details on the statistical methods are presented in Appendix 2.
Role of the Funding Source
The steering committee designed SPRINT, gathered the data (in collaboration with investigators at the clinics and other study units), and approved the decision to submit the manuscript for publication. The writing committee wrote the manuscript and vouches for the completeness and accuracy of the data and analysis. The coordinating center was responsible for analyzing the data. Scientists at the National Institutes of Health participated in the design of the study and, as a group, had 1 vote on the steering committee.
Results
Of the 9361 SPRINT participants, 6662 (71.6%) had an eGFR of at least 60 mL/min/1.73 m2 at baseline and were included in the current analysis (Appendix Figure 1, available at Annals.org). Median follow-up was 39.6 months (range, 0 to 57.4 months) in the intensive group and 39.4 months (range, 0 to 57.4 months) in the standard group. Summary values of baseline demographic, clinical, and laboratory characteristics were similar in both groups (Table 1). A high Framingham risk score was the most common indicator (60.6%) of increased cardiovascular risk in both groups. The mean age of the study population was 66.3 years (SD, 9.0), 33.7% were women, and 32.2% were black. Mean eGFR was 81.2 mL/min/1.73 m2 (SD, 15.5), mean SBP was 139.9 mm Hg (SD, 15.4), and mean DBP was 79.4 mm Hg (SD, 11.6).
Table 1.
Baseline Characteristics of SPRINT Participants Without CKD, by Treatment Group*
| Characteristic | Intensive Group |
Standard Group |
Total |
|||
|---|---|---|---|---|---|---|
| Participants, n | Value | Participants, n | Value | Participants, n | Value | |
| Criteria for increased cardiovascular risk, n (%) | ||||||
| Age ≥75 y | 3326 | 726 (21.8) | 3336 | 732 (21.9) | 6662 | 1458 (21.9) |
| Clinical cardiovascular disease | 3326 | 473 (14.2) | 3336 | 460 (13.8) | 6662 | 933 (14.0) |
| Subclinical cardiovascular disease | 3326 | 258 (7.8) | 3336 | 270 (8.1) | 6662 | 528 (7.9) |
| Framingham riskscore ≥15% | 3321 | 2004 (60.4) | 3331 | 2020 (60.8) | 6652 | 4024 (60.6) |
| Clinical characteristics | ||||||
| Mean age (SD), y | 3326 | 66.3 (9.0) | 3336 | 66.3 (9.0) | 6662 | 66.3 (9.0) |
| Female, n (%) | 3326 | 1131 (34.0) | 3336 | 1113 (33.4) | 6662 | 2244 (33.7) |
| Race/ethnicity, n (%) | 3326 | – | 3336 | – | 6662 | – |
| Non-Hispanic black | – | 1045 (31.4) | – | 1101 (33.0) | – | 2146 (32.2) |
| Hispanic | – | 405 (12.2) | – | 380 (11.4) | – | 785 (11.8) |
| Non-Hispanic white | – | 1804 (54.2) | – | 1794 (53.8) | – | 3598 (54.0) |
| Other race | – | 72 (2.2) | – | 61 (1.8) | – | 133 (2.0) |
| Mean blood pressure (SD), mm Hg | 3324 | – | 3335 | – | 6659 | – |
| Systolic | – | 139.9 (15.6) | – | 139.8 (15.1) | – | 139.9 (15.4) |
| Diastolic | – | 79.5 (11.5) | – | 79.3 (11.7) | – | 79.4 (11.6) |
| Mean serum creatinine level (SD) | 3326 | – | 3336 | – | 6662 | – |
| μmol/L | – | 82 (15) | – | 83 (16) | – | 82 (15) |
| mg/dL | – | 0.93 (0.17) | – | 0.94 (0.18) | – | 0.93 (0.17) |
| Mean eGFR (SD), mL/min/1.73 m2 | 3326 | 81.3 (15.5) | 3336 | 81.2 (15.5) | 6662 | 81.2 (15.5) |
| Median urinary albumin-creatinine ratio (IQR), mg/g | 3167 | 8.8 (5.5–17.2) | 3165 | 8.5 (5.4–16.8) | 6332 | 8.6 (5.5–17.1) |
| Mean fasting total cholesterol level (SD) | 3326 | – | 3336 | – | 6662 | – |
| mmol/L | – | 4.97 (1.08) | – | 4.97 (1.06) | – | 4.97 (1.07) |
| mg/dL | – | 191.7 (41.6) | – | 192.0 (40.8) | – | 191.8 (41.2) |
| Mean fasting HDL cholesterol level (SD) | 3326 | – | 3336 | – | 6662 | – |
| mmol/L | – | 1.37 (0.37) | – | 1.37 (0.38) | – | 1.37 (0.37) |
| mg/dL | – | 53.0 (14.2) | – | 52.9 (14.5) | – | 53.0 (14.4) |
| Mean fasting triglyceride level (SD) | 3326 | – | 3336 | – | 6662 | – |
| mmol/L | – | 1.41 (1.04) | – | 1.41 (1.10) | – | 1.41 (1.07) |
| mg/dL | – | 124.8 (91.6) | – | 124.6 (97.2) | – | 124.7 (94.4) |
| Mean fasting plasma glucose level (SD) | 3326 | – | 3336 | – | 6662 | – |
| mmol/L | – | 5.50 (0.76) | – | 5.49 (0.76) | – | 5.50 (0.76) |
| mg/dL | – | 99.1 (13.7) | – | 99.0 (13.7) | – | 99.1 (13.7) |
| Statin use, n (%) | 3308 | 1318 (39.8) | 3312 | 1364 (41.2) | 6620 | 2682 (40.5) |
| Aspirin use, n (%) | 3316 | 1646 (49.6) | 3330 | 1609 (48.3) | 6646 | 3255 (49.0) |
| Angiotensin-converting enzyme inhibitor/aldosterone-receptor blocker use, n (%) | 3326 | 1299 (39.1) | 3336 | 1248 (37.4) | 6662 | 2547 (38.2) |
| Smoking status, n (%) | 3321 | – | 3331 | – | 6652 | – |
| Never | – | 1436 (43.2) | – | 1461 (43.9) | – | 2897 (43.5) |
| Former | – | 1354 (40.8) | – | 1385 (41.6) | – | 2739 (41.1) |
| Current | – | 531 (16.0) | – | 485 (14.6) | – | 1016 (15.3) |
| Mean Framingham risk score (SD) | 3321 | 23.9 (11.7) | 3331 | 23.9 (11.6) | 6652 | 23.9 (11.7) |
| Mean body mass index (SD), kg/m2 | 3305 | 30.1 (5.8) | 3313 | 30.0 (5.7) | 6618 | 30.0 (5.8) |
| Mean antihypertensive agents (SD), n | 3326 | 1.74 (1.03) | 3336 | 1.72 (1.04) | 6662 | 1.73 (1.03) |
| Not using antihypertensive agents, n (%) | 3326 | 370 (11.1) | 3336 | 387 (11.6) | 6662 | 757 (11.4) |
CKD = chronic kidney disease; eGFR = estimated glomerular filtration rate; HDL = high-density lipoprotein; IQR = interquartile range; SPRINT = Systolic Blood Pressure Intervention Trial.
Percentages may not sum to 100 due to rounding.
The intervention achieved SBP separation between groups by 6 months, and this separation was stable throughout the remainder of follow-up (Appendix Figure 2, available at Annals.org). Systolic BP decreased in both treatment groups, with the reduction being most pronounced during the first 6 months and steeper in the intensive group than the standard group. The average between-group difference in SBP after 6 months was 15.0 mm Hg (CI, 14.7 to 15.4 mm Hg).
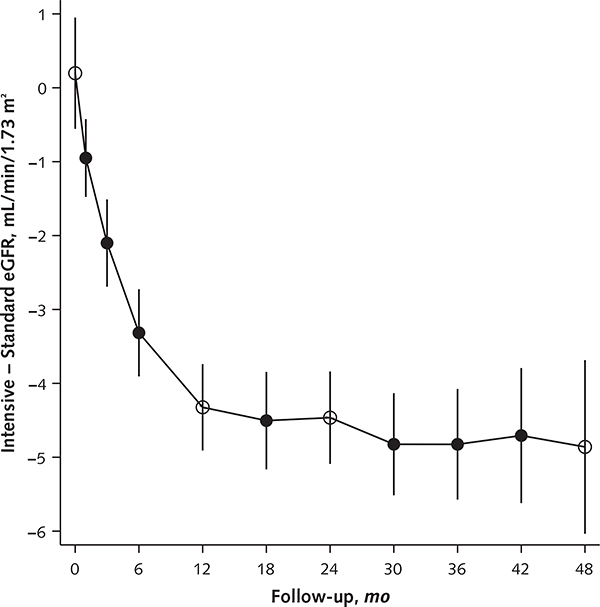
The number of participants who were lost to follow-up or withdrew consent was similar in the intensive and standard groups (5.7% vs. 5.5%; P = 0.69). Fasting resulted in a lower estimated eGFR. Fasting and nonfasting eGFRs are presented in Figure 1. The difference in adjusted mean eGFR between groups was −3.32 mL/min/1.73 m2 (CI, −3.90 to −2.74 mL/min/1.73 m2) at 6 months; increased to −4.50 mL/min/1.73 m2 (CI, −5.16 to −3.85 mL/min/1.73 m2) at 18 months; and was relatively stable for the remainder of follow-up, with differences of −4.83 mL/min/1.73 m2 (CI, −5.51 to −4.14 mL/min/1.73 m2) at month 30 and −4.71 mL/min/1.73 m2 (CI, −5.61 to −3.80 mL/min/1.73 m2) at month 42 (Figure 1; Appendix Figure 3, available at Annals.org).
Figure 1.
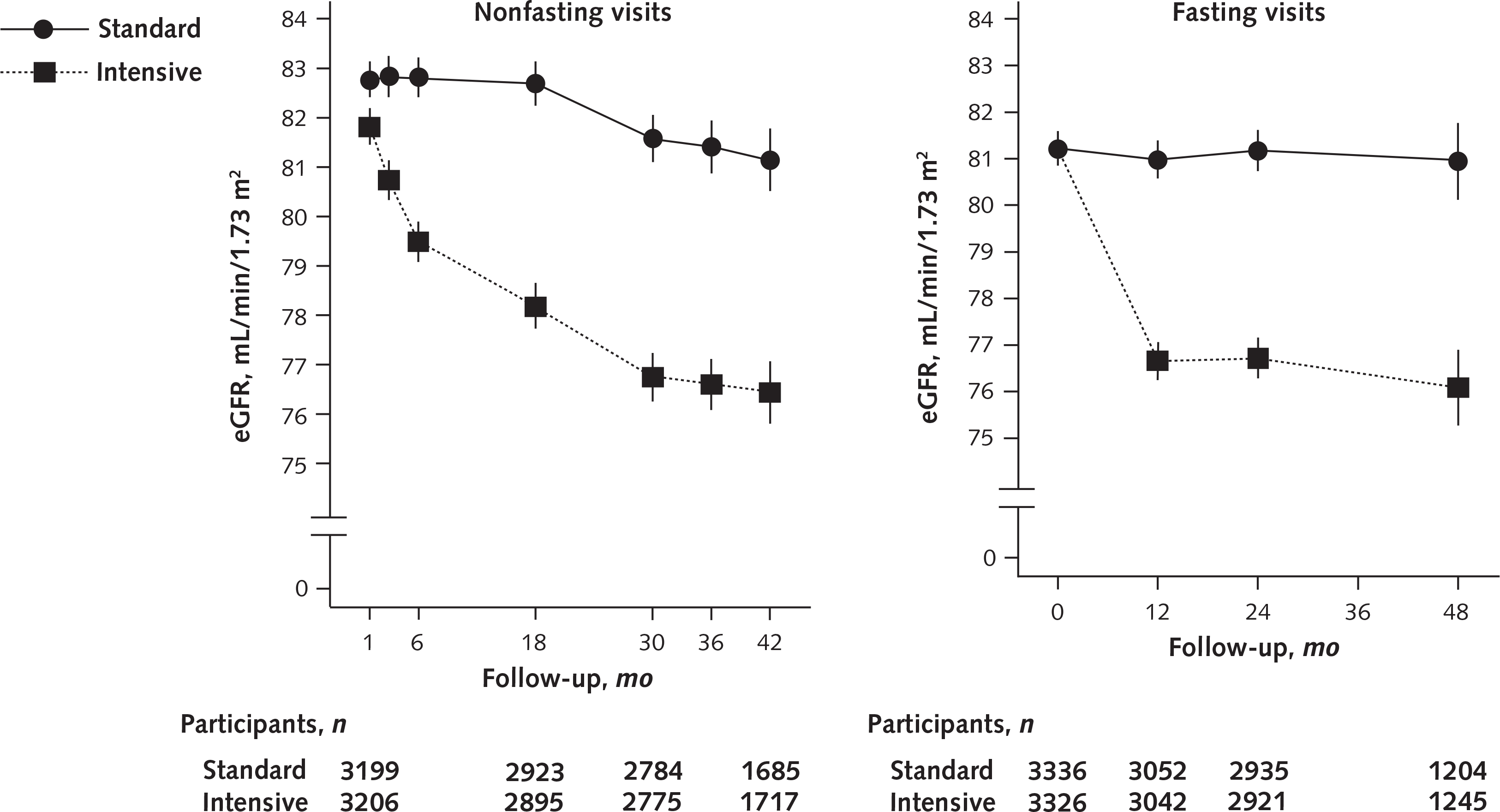
Predicted mean eGFRs and 95% CIs (error bars) from a linear model accounting for baseline eGFR in the non-CKD population.
Results from nonfasting (left) and fasting (right) visits, including the raw (unadjusted) baseline mean. CKD = chronic kidney disease; eGFR = estimated glomerular filtration rate.
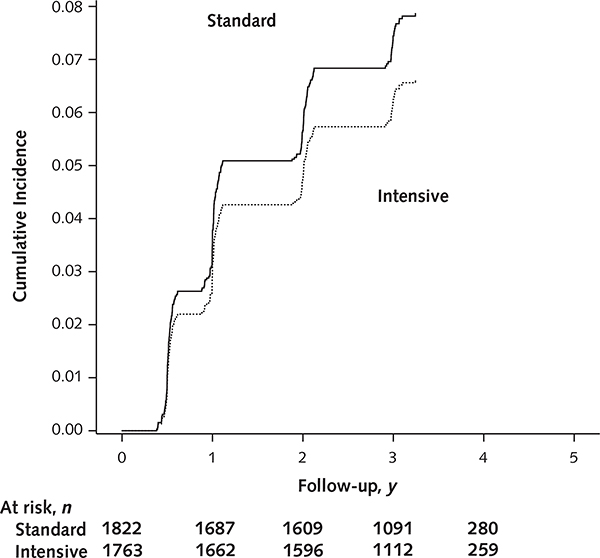
Overall, 140 of 3326 participants (4.2%) in the intensive group and 40 of 3336 (1.1%) in the standard group had an incident CKD event, based on the a priori protocol definition of a decrease in eGFR of at least 30% and a confirmed value less than 60 mL/min/1.73 m2 (Table 2). Compared with the standard group, the intensive group had a significantly higher rate of incident CKD (1.33 vs. 0.37 events per 100 person-years; HR, 3.54 [CI, 2.50 to 5.02]; P < 0.001). At 3 years, the cumulative incidence of CKD was an absolute 2.6% higher in the intensive group (Figure 2 [panel A] and Table 2). The number needed to treat for 3 years to produce 1 incident CKD event (that is, the number needed to treat for harm) was 38 (CI, 29 to 53).
Table 2.
Effects of the SPRINT Intervention on Incident CKD, Cardiovascular Outcomes, and All-CAUSE Mortality in Participants Without CKD
| Variable | Intensive Group |
Standard Group |
Hazard Ratio per 100 Person-Years (95% CI)* | Absolute Risk Reduction at 3 Years (95% CI), %† | ||||
|---|---|---|---|---|---|---|---|---|
| Participants, n | Events per 100 Person-Years, n | Events/Total Years of Follow-up, n/N | Participants, n | Events per 100 Person-Years, n | Events/Total Years of Follow-up, n/N | |||
| Incident CKD | 3326 | 1.33 | 140/10 564 | 3335 | 0.37 | 40/10 715 | 3.54 (2.50 to 5.02) | −2.6 (−3.4 to −1.9) |
| SPRINT primary outcome‡ or all-cause death | 3326 | 1.78 | 189/10 631 | 3336 | 2.51 | 264/10 530 | 0.71 (0.59 to 0.86) | 2.2 (1.1 to 3.3) |
| SPRINT primary outcome‡ | 3326 | 1.28 | 136/10 615 | 3326 | 1.92 | 202/10 509 | 0.67 (0.54 to 0.84) | 1.8 (0.8 to 2.8) |
| All-cause death | 3326 | 0.77 | 83/10 831 | 3336 | 1.05 | 114/10 814 | 0.74 (0.55 to 0.98) | 0.9 (0.2 to 1.7) |
| Incident albuminuria | 1763 | 1.98 | 109/5497 | 1822 | 2.39 | 133/5575 | 0.82 (0.64 to 1.05) | 1.2 (−0.5 to 2.8) |
CKD = chronic kidney disease; SPRINT = Systolic Blood Pressure Intervention Trial.
Calculated from Cox proportional hazards regression models.
The difference in the survival estimate (i.e., 1 minus the cumulative incidence) at 3-y follow-up in the intensive group minus that in the standard group, calculated from a proportional hazards regression model. Models for incident CKD, incident albuminuria, and the SPRINT primary outcome were adjusted for competing risk for death not caused by cardiovascular disease. Negative values indicate increase in absolute risk.
First occurrence of myocardial infarction, acute coronary syndrome, stroke, heart failure, or cardiovascular death. Surveillance for cardiovascular outcomes and death was performed every 3 mo.
Figure 2.
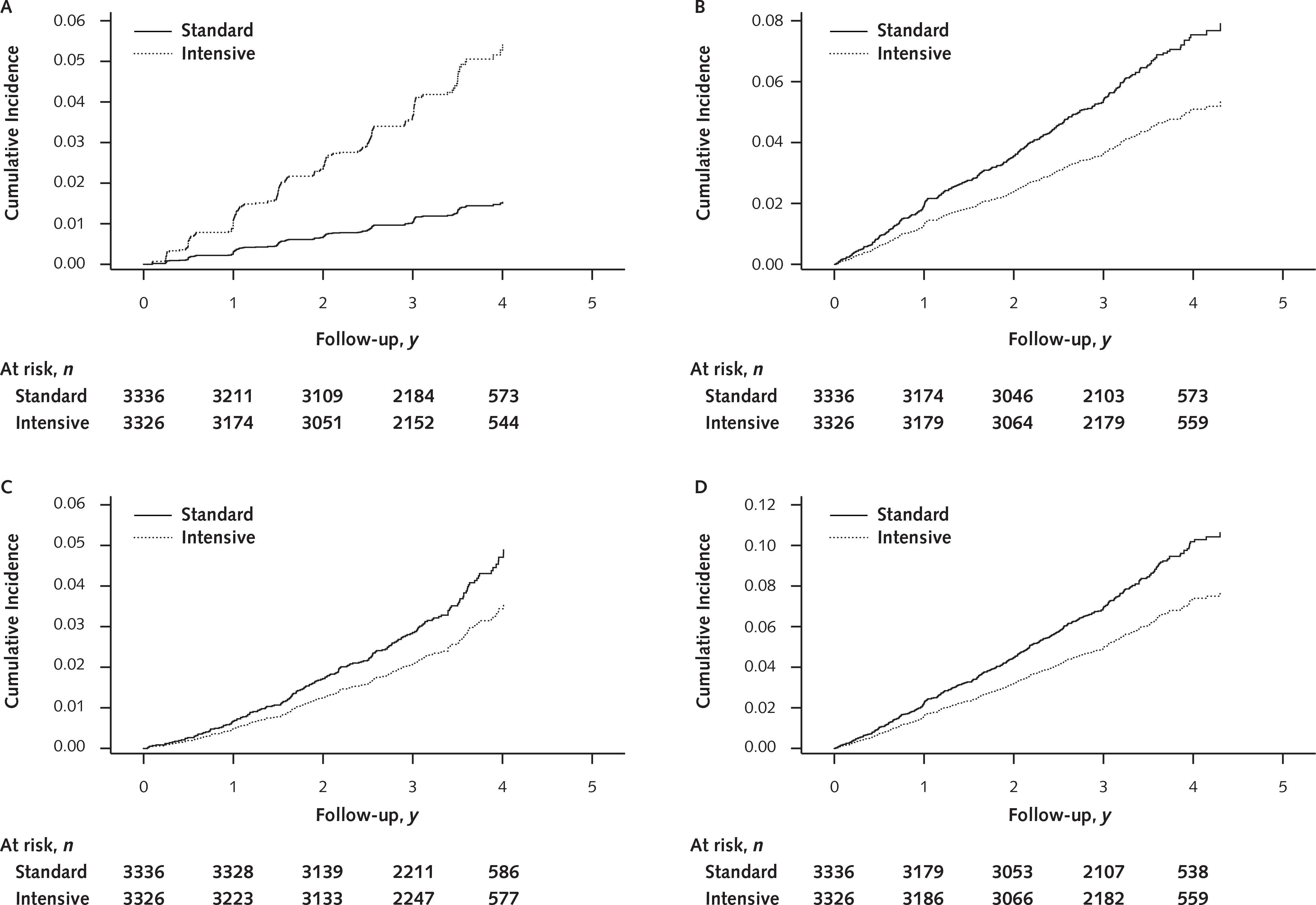
Cumulative incidence plots for incident CKD (A), primary CVD outcome (B), all-cause death (C), and the composite of primary CVD outcome or all-cause death (D) in the non-CKD population, by treatment group.
CKD = chronic kidney disease; CVD = cardiovascular disease.
The effect of the intensive treatment intervention on incident CKD was similar across baseline age, sex, race, and albuminuria subgroups (Appendix Figure 4, available at Annals.org). The intervention significantly reduced risk for the composite of a primary CVD event or all-cause death (absolute risk reduction, 2.2% [CI, 1.1% to 3.3%]; HR, 0.71 [CI, 0.59 to 0.86]), primary CVD event (absolute risk reduction, 1.8% [CI, 0.8% to 2.8%]; HR, 0.67 [CI, 0.54 to 0.84]), and all-cause death (absolute risk reduction, 0.9% [CI, 0.2% to 1.7%]; HR, 0.74 [CI, 0.55 to 0.98]) (Table 2 and Figure 2 [panels B to D]). The numbers needed to treat for 3 years to prevent 1 occurrence of the composite outcome, the primary cardiovascular outcome, and all-cause death were 46 (CI, 29 to 94), 57 (CI, 36 to 129), and 108 (CI, 59 to 541), respectively.
Appendix Figure 5 (available at Annals.org) shows the cumulative incidence of albuminuria. Assessment of albuminuria during follow-up was limited to 3585 participants (1763 in the intensive group and 1822 in the standard group) with a baseline urinary albumin–creatinine ratio less than 10 mg/g. In this subgroup, the incidence of albuminuria did not differ significantly between the intensive and standard groups (absolute risk reduction for the intensive group, 1.2% [CI, −0.5% to 2.8%]; HR, 0.82 [CI, 0.64 to 1.05]) (Table 2).
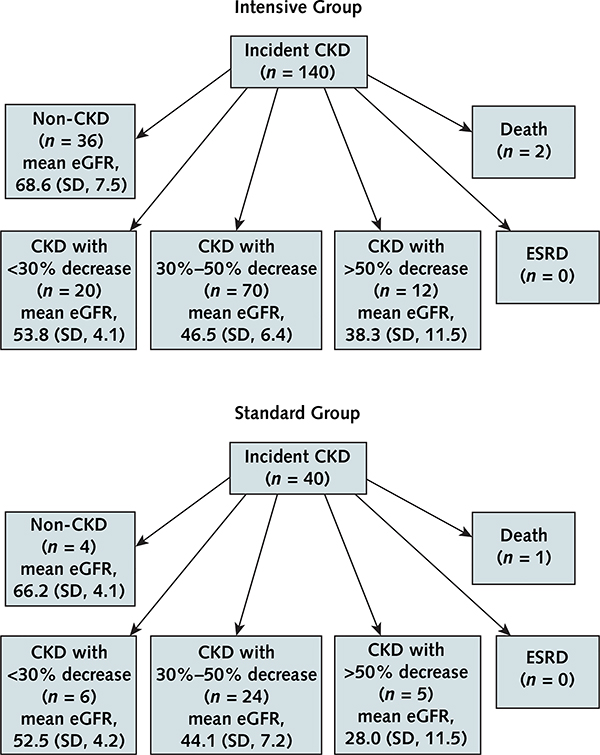
The final status of participants who had incident CKD is shown in Figure 3. At the final study visit, 36 (25.7%) of those with incident CKD in the intensive group and 4 (10%) in the standard group recovered renal function and were deemed to no longer have incident CKD. None of the participants with incident CKD in either group developed ESRD. One of the 40 participants (2.5%) with incident CKD in the standard group and 2 of the 140 participants (1.4%) in the intensive group died.
Figure 3.
Final status of participants with incident CKD.
Final disposition (categorized as non-CKD, CKD with varying decreases in eGFR, ESRD, or death) of persons who met the criteria for incident CKD during follow-up in the intensive (top) and standard (bottom) groups. The mean (SD) end-of-study eGFR is provided for each category. The 180 incident CKD events occurred a median of 21.0 mo (range, 1.2 to 47.9 mo) after randomization. The final eGFR assessments for patients with incident CKD occurred 42.4 mo (range, 11.4 to 58.9 mo) after randomization. CKD = chronic kidney disease; eGFR = estimated glomerular filtration rate; ESRD = end-stage renal disease.
Sensitivity analyses of incident CKD based on a 40% or 50% reduction in eGFR from baseline, a fixed decrease in eGFR of 27 mL/min/1.73 m2 from baseline (regardless of whether eGFR decreased to <60 mL/min/1.73 m2), and use of the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation instead of the MDRD study equation are presented in Appendix Tables 1, 2, and 3 (available at Annals.org), respectively. In general, these results were consistent with those of the main analyses.
Discussion
In SPRINT participants without CKD at baseline, intensive SBP treatment resulted in an absolute 2.6% higher risk for incident CKD at 3 years of follow-up than standard SBP treatment, based on our a priori definition of a reduction in eGFR of at least 30% with a confirmed level less than 60 mL/min/1.73 m2. On the other hand, the intensive treatment group had an absolute 2.2% lower risk for the composite of a primary CVD event or all-cause death at 3 years.
Because glomerular filtration is highly dependent on the hydrostatic pressure gradient across the glomerular basement membrane, intensive SBP lowering would be expected to result in a reduction in glomerular capillary pressure and a corresponding decrease in eGFR (17). Hence, the early reduction in eGFR associated with intensive SBP lowering (Figure 1) was expected and is consistent with decreases noted during intensive blood pressure lowering in the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial (18), the SPS3 (Secondary Prevention of Small Subcortical Strokes) (19) trial, AASK (African American Study of Kidney Disease and Hypertension) (20), and the MDRD study (21). In addition, similar decreases in eGFR have been observed with the use of angiotensin-converting enzyme inhibitors (22) or aldosterone-receptor blockers (23) and with dietary protein restriction (24). Despite the acute decrease in eGFR during treatment with angiotensin-converting enzyme inhibitors and aldosterone-receptor blockers, such therapy is known to slow the progression of kidney disease compared with placebo in adults with diabetes mellitus and a high risk for CVD or nephropathy (25, 26). The long-term consequences of the acute reduction in eGFR noted during intensive treatment in SPRINT are unclear. However, the mean difference in eGFR between treatment groups remained relatively stable after 18 months (Figure 1 [panel B] and Appendix Figure 3), and no one in either group developed ESRD during follow-up (Figure 3).
In contrast to analyses of the difference in mean eGFR between groups, which involved averages across the full cohort, the time-to-event analyses of incident CKD focused on patients with a substantial decrease in eGFR during follow-up. These analyses indicated that the intensive group had an absolute risk increase of 2.6% over 3 years and a 3.5-fold higher hazard of incident CKD compared with the standard group. These differences persisted if the 30% threshold for decrease in eGFR was increased to 40% (Appendix Table 1) and were not fully attenuated when we attempted to account for the initial presumed hemodynamic effect of the treatments by restricting the analysis to after the first 6 months of treatment (Appendix Tables 1 and 2). These differences were observed across subgroups based on age, sex, race, and albuminuria (Appendix Figure 4).
The clinical and public health significance of CKD is a reflection of increases in risk for cardiovascular events, all-cause death, and ESRD (27). In the current study, none of the participants with incident CKD progressed to ESRD during the relatively short follow-up (Figure 3). Furthermore, 25.7% of those with incident CKD in the intensive group recovered renal function and were classified as no longer having CKD at the final study assessment (Figure 3).
Although more intensive treatment increased the risk for incident CKD—which has been found in observational studies to be a risk factor for future cardiovascular events and all-cause death—it resulted in a substantial decrease in CVD and all-cause mortality during the median of 3.26 years of treatment in SPRINT. The number needed to treat for harm for an asymptomatic incident CKD event within 3 years was 38 (CI, 29 to 53), whereas the number needed to treat to prevent a composite of cardiovascular event or all-cause death was 46 (CI, 29 to 94). In other words, for each cardiovascular event or all-cause death prevented over 3 years, we noted 1.2 incident CKD events. We believe that an asymptomatic CKD event is benign compared with a cardiovascular event or death; therefore, the benefits of the intervention outweigh the risks, at least for the duration of the current study. However, we acknowledge that some patients and providers might consider incident CKD to be more important than a cardiovascular event or death.
When the SPRINT intervention is adopted in routine clinical practice, the incidence and, therefore, the prevalence of CKD might increase at the population level. Chronic kidney disease due to blood pressure lowering might not have the same clinical and public health significance as CKD due to progression of underlying intrinsic renal disease, but long-term follow-up of the SPRINT participants will be important to study this issue.
Although randomization of participants in the non-CKD subgroup was not stratified by eGFR, all participants were randomly allocated to intervention assignments. The subset of participants without CKD is a proper subgroup because it is based on information known at baseline. Furthermore, the probability of an important imbalance in a given covariate, measured or unmeasured, is very small in a sample this large (>70% of the entire SPRINT cohort of 9361 participants). Indeed, the baseline characteristics of the intensive and standard groups within the non-CKD stratum were similar. Therefore, we believe that the outcomes reported in this article are based on a randomized comparison. Limitations of this study include short follow-up, which limits inferences on long-term effects, such as progression to ESRD.
In summary, although an acute decrease in eGFR was observed in the intensive treatment group, the differences in mean eGFR remained relatively stable between groups. Intensive SBP lowering increased the risk for incident CKD events, but this was outweighed by the potential for cardiovascular and all-cause mortality benefits over the relatively short follow-up. None of the participants with incident CKD progressed to ESRD. The long-term consequences of incident CKD due to intensive SBP lowering need to be established.
Acknowledgment:
The SPRINT investigators thank Takeda Pharmaceuticals International for contributing study medications (azilsartan and azilsartan–chlorthalidone).
Financial Support: SPRINT received federal funds from the National Institutes of Health, including the National Heart, Lung, and Blood Institute; the National Institute of Diabetes and Digestive and Kidney Diseases; the National Institute on Aging; and the National Institute of Neurological Disorders and Stroke, under contract numbers HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200 900048C, and HHSN268200900049C and interagency agreement number A-HL-13–002-001. It was also supported in part with resources and use of facilities through the U.S. Department of Veterans Affairs. Additional support was provided by the following Clinical and Translational Science Awards funded by the National Center for Advancing Translational Sciences: Case Western Reserve University: UL1TR000439; Ohio State University: UL1RR025755; University of Pennsylvania: UL1RR024134 and UL1TR000003; Boston University: UL1RR025771; Stanford University: UL1TR000093; Tufts University: UL1RR025752, UL1TR000073, and UL1TR001064; University of Illinois: UL1TR000050; University of Pittsburgh: UL1TR000005; University of Texas Southwestern Medical Center: 9U54TR000017–06; University of Utah: UL1TR000105–05; Vanderbilt University: UL1 TR000445; George Washington University: UL1TR000075; University of California, Davis: UL1 TR000002; University of Florida: UL1 TR000064; University of Michigan: UL1TR000433; and Tulane University: P30GM10 3337 (National Institute of General Medical Sciences Centers of Biomedical Research Excellence Award).
Disclosures: Dr. Beddhu reports grants from Bayer and AbbVie outside the submitted work. Dr. Toto reports other support from Amgen, Boehringer Ingelheim, Reata Pharmaceuticals, Novo Nordisk, Bayer Pharmaceuticals, and AstraZeneca outside the submitted work. Dr. Greene reports personal fees from Janssen Pharmaceuticals and Pfizer outside the submitted work. Dr. Freedman reports a grant from Novartis Pharmaceuticals and personal fees from Ionis Pharmaceuticals and AstraZeneca outside the submitted work. Dr. Killeen reports personal fees from Roche Diagnostics outside the submitted work. Dr. Rahman reports a grant from Bayer outside the submitted work. Dr. Rastogi reports grants from Cubist, Relypsa, Sanofi, Kadmon, AMAG, Amgen, AstraZeneca, Bayer, Genzyme, GlaxoSmithKline, Omeros, Otsuka, Overture, Questcor, Sandoz, VPI, and SPRINT; personal fees from Cubist, Fresenius Medical Care, Medscape, Relypsa, and Sanofi; nonfinancial support from Relypsa, AstraZeneca, and Bayer; and other support from Fresenius Medical Care, Kadmon, and Janssen outside the submitted work. Authors not named here have disclosed no conflicts of interest. Disclosures can also be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M16-2966.
Appendix 1: Sprint Research Group
Study Leadership: Paul Whelton (Chair), Karen C. Johnson (Vice-Chair), Lawrence Fine (Project Officer), Joni Snyder (Deputy Project Officer).
Program Office: National Institutes of Health, Bethesda, Maryland: Diane Bild (Project Scientist), Denise Bonds (Project Scientist), Nakela Cook (Project Scientist), Jeffrey Cutler (Project Scientist), Lawrence Fine (Project Officer), Peter Kaufmann (Project Scientist), Paul Kimmel (Project Scientist), Lenore Launer (Project Scientist), Claudia Moy (Project Scientist), William Riley (Project Scientist), Laurie Ryan (Project Scientist), Joni Snyder (Deputy Project Officer), Eser Tolunay (Project Scientist), Song Yang (Biostatistician).
SPRINT Clinical Center Networks: Case Western Reserve University, Cleveland, OH: Jackson T Wright Jr (CCN PI), Mahboob Rahman (CCN Co-PI), Alan J Lerner (CCN MIND PI), Mahboob Rahman (CCN Co-PI), Carolyn Still (CCN Project Manager, Co-I), Alan Wiggers (Co-I), Sara Zamanian, (CCN Asst. Project Manager), Alberta Bee (former CCN Asst. Project Manager), Renee Dancie (former CCN Project Manager); Memphis Veteran Affairs Medical Center, Memphis, TN: William Cushman (PI), Barry Wall (Co-I), Linda Nichols (MIND PI), Robert Burns (MIND Consultant), Jennifer Martindale-Adams (MIND Consultant), Dan Berlowitz (Economic & HRQL Consultant), Elizabeth Clark (CCN Coordinator), Sandy Walsh (CCN Coordinator) Terry Geraci (CCN Coordinator) Carol Huff (Budget Analyst), Linda Shaw (CCN Research Assistant). University of Alabama, Birmingham, AL: Suzanne Oparil (PI), Cora E. Lewis (Co-PI), Virginia Bradley (MIND Co-I), David Calhoun (Co-I), Stephen Glasser (Co-I), Kim Jenkins (CCN Coordinator), Tom Ramsey (CCN Coordinator); University of Utah, Salt Lake City, UT: Alfred K. Cheung (PI), Srinivasan Beddhu (Co-I), Gordon Chelune (MIND Co-I), Jeffrey Childs (Associate Director of Operations), Lisa Gren (Director of Operations), Anne Randall (CCN Coordinator); Wake Forest University Health Sciences, Winston-Salem, NC: Michael Rocco (PI), David Goff (Co-PI), Carlos Rodriguez (Co-I), Laura Coker (Co-I), Amret Hawfield (Co-I), Joseph Yeboah (Co-I), Lenore Crago (CCN Coordinator) John Summerson (CCN Coordinator), Anita Hege (MIND Coordinator).
SPRINT Central Coordinating Center: Wake Forest University Health Sciences, Winston-Salem, NC: David Reboussin (PI), Jeff Williamson (Co-PI), Walter Ambrosius (Co-I), William Applegate (Co-I), Greg Evans (Co-I), Capri Foy (Co-I), Barry I. Freedman (Co-I), Dalane Kitzman (Co-I), Mary Lyles (Co-I), Nick Pajewski (Co-I), Steve Rapp (Co-I), Scott Rushing (Co-I), Neel Shah (Co-I), Kaycee M. Sink (Co-I, Safety Officer), Mara Vitolins (Co-I), Lynne Wagenknecht (Co-I), Valerie Wilson (Co-I), Letitia Perdue (Project Coordinator), Nancy Woolard (MIND Project Coordinator), Tim Craven (Biostatistician), Katelyn Garcia (Biostatistician), Sarah Gaussoin (Biostatistician), Laura Lovato (Biostatistician), Jill Newman (Biostatistician), Bobby Amoroso (Programmer), Patty Davis (Programmer), Jason Griffin (Programmer), Darrin Harris (Programmer), Mark King (Programmer), Kathy Lane (Programmer), Wes Roberson (Programmer), Debbie Steinberg (Programmer), Donna Ashford (Project Manager), Phyllis Babcock (Project Manager), Dana Chamberlain (Project Manager), Vickie Christensen (Project Manager), Loretta Cloud (Project Manager), Christy Collins (Project Manager), Delilah Cook (Project Manager), Katherine Currie (Project Manager), Debbie Felton (Project Manager), Stacy Harpe (Project Manager), Marjorie Howard (Project Manager), Michelle Lewis (Project Manager), Pamela Nance (Project Manager), Nicole Puccinelli-Ortega (Project Manager), Laurie Russell (Project Manager), Jennifer Walker (Project Manager), Brenda Craven (former Project Coordinator), Candace Goode (Data Coordinator), Margie Troxler (Fiscal Coordinator), Janet Davis (Administrative Support), Sarah Hutchens (Administrative Support). WFU CC Publication Acknowledgement: Wake Forest University Claude D. Pepper Older Americans Independence Center (P30-AG21332).
SPRINT Central Laboratory: University of Minnesota Advanced Research and Diagnostic Laboratory: Anthony A. Killeen (PI), Anna M. Lukkari (coordinator). Acknowledgement statement: This project was supported by Award Number N01-HC-95240. The content is solely the responsibility of the author and does not reflect the official views of the NHLBI.
SPRINT Drug Distribution Center: VA Cooperative Studies Program Clinical Research Pharmacy Coordinating Center: Robert Ringer (PI), Brandi Dillard (coordinator), Norbert Archibeque, (coordinator) Stuart Warren (Co-I), Mike Sather (PI), James Pontzer (programmer), Zach Taylor (programmer).
SPRINT ECG Reading Center: Epidemiological Cardiology Research Center (EPICARE), Winston Salem, NC: Elsayed Z Soliman (PI), Zhu-Ming Zhang (Co-I), Yabing Li (coordinator), Chuck Campbell (coordinator), Susan Hensley (coordinator), Julie Hu (coordinator), Lisa Keasler (coordinator), Mary Barr (coordinator), Tonya Taylor (coordinator).
SPRINT MRI Reading Center: University of Pennsylvania-Philadelphia, PA: R. Nick Bryan (PI), Christos Davatzikos (Co-I), Ilya Nasarallah (Co-I), Lisa Desiderio (Project Manager), Mark Elliott (MRI Physicist), Ari Borthakur (MRI Physicist), Harsha Battapady (Data Analyst), Guray Erus (Postdoctoral Fellow), Alex Smith (Postdoctoral Fellow), Ze Wang (Research Associate), Jimit Doshi (Data Analyst). SPRINT MRI by site: University of Pennsylvania-Philadelphia, PA: Raymond Townsend (Clinic PI), Debbie Cohen (Co-I), Yonghong Huan (Co-I), Mark Duckworth (Research Coordinator), Virginia Ford (Research Coordinator), Kelly Sexton (MRI Coordinator). University Hospital Case Medical Center- Cleveland, OH: Jackson T. Wright, Jr. (PI), Alan Lerner (Co-I), Mahboob Rahman (Co-I), Carolyn Still (Project Manager), Alberta Bee (Research Coordinator), Debra Lee Stokes, (MRI coordinator), Shonte Smith (MRI coordinator), Jeffrey Sunshine (Site Radiologist), Mark Clampitt (MRI Technologist). Vanderbilt University: Seth Smith (MRI Director), Brian Welch (MRI Research Manager), Manus Donahue (MRI Physicist), Alex Dagley (Researcher Coordinator), Dave Pennell (MRI Technologist), Chris Cannistraci (Imaging Research Specialist), Kristin Merkle (MRI Research Coordinator), Julie Lewis (Clinic PI) Mohammed Sika (Research Coordinator). University of Miami: Clinton Wright (Co-I), Mohammad Sabati (MRI Director), Edward Campuzano (Chief MRI Technologist), Hector Martin (MRI Technologist), Andrea Roman (MRI Technologist), Jesus Cruz (MRI Technologist), Natalya Nagornaya (Site Radiologist). Wake Forest University: Laura Coker (Co-I), Anita Hege (Project Coordinator), Joseph Maldjian (Site Radiologist), Sandra Kaminsky (MRI Technologist), Debra Fuller (MRI Technologist), Youngkoo Jung (MRI Physicist). University of Alabama at Birmingham: Suzanne Oparil (Network PI), Beth Lewis (Co-PI), Virginia Wadley (MIND Co-I), Kim Jenkins (Project Coordinator), Tom Ramsey (Project Coordinator), William Evanochko (MRI Physicist), Glenn Roberson (Site Radiologist), Trina Corbitt (MRI Technologist), William Fisher (MRI Technologist), Cathy Clements (MRI Technologist). Boston University: Daniel Weiner (Clinic PI), Andrew Wells (Research Coordinator), Amanda Civiletto (Research Coordinator), Gerard P. Aurigemma (Clinic PI), Noelle Bodkin (Research Coordinator), Alex Norbash (Co-I,) Margaret Lavoye (Research Administrator), Andrew Ellison (MRI Technologist), Ronald Killiany (Imaging Center Director), Osama Sakai (Site Radiologist).
SPRINT Sub-Committee Chairs: Ancillary Science: Alfred Cheung, Design and Analysis: Walter Ambrosius, Economic Evaluation/Health Related Quality of Life: Dan Berlowitz, Intervention: William Cushman, Measurements, Procedures and Quality Control: Beth Lewis, Mortality and Morbidity: Suzanne Oparil, Presentations and Publications: Jackson T. Wright, Jr., Recruitment, Retention and Adherence: David Goff, Safety: Kaycee Sink, SPRINT MIND: Jeff Williamson.
SPRINT Clinical Centers by Network: OHIO Network: Cleveland Clinic Foundation- Cleveland, OH: George Thomas (PI), (Co-PI), Martin Schreiber, Jr (Co-I), Sankar Dass Navaneethan (Co-I), John Hickner (Co-I), Michael Lioudis (Co-I), Michelle Lard (Co-I), Susan Marczewski (former coordinator), Jennifer Maraschky (coordinator), Martha Colman (former coordinator) Andrea Aaby (coordinator), Stacey Payne (coordinator), Melanie Ramos, (coordinator), Carol Horner (former coordinator). Louis Stokes Cleveland VA Medical Center-Cleveland, OH: Mahboob Rahman (PI), Paul Drawz (Co-I), Pratibha P. Raghavendra (Co-I), Scott Ober (Co-I), Ronda Mourad (Co-I), Muralidhar Pallaki (Co-I), Peter Russo (Co-I), Pratibha Raghavendra, Co-I), Pual Fantauzzo (Co-I), Lisa Tucker (coordinator), Bill Schwing (coordinator). MetroHealth Medical Center-Cleveland, OH: John R. Sedor (PI), Edward J. Horwitz (Co-PI), Jeffrey R. Schellling (Co-I), John F. O’Toole (Co-I), Lisa Humbert (coordinator), Wendy Tutolo (coordinator). North East Ohio Neighborhood Health Center- Cleveland, OH: Suzanne White (PI), Alishea Gay (Former Co-I), Walter Clark, Jr (former PI), Robin Hughes (coordinator). University Hospital Case Medical Center-Cleveland, OH: Mirela Dobre (PI), Jackson T. Wright, Jr. (Co-I), Carolyn H. Still (Co-I), Alberta Bee (coordinator), Monique Williams (coordinator). The Ohio State University Medical Center, Division of Nephrology and Hypertension-Columbus, OH: Udayan Bhatt (PI), Lee Hebert (former PI) Anil Agarwal (Co-PI), Melissa Brown Murphy (coordinator), Nicole Ford (former coordinator), Cynthia Stratton (coordinator), Jody Baxter (former coordinator), Alicia A. Lykins (former coordinator), Alison McKinley Neal (former coordinator) Leena Hirmath (former coordinator). The Ohio State University Medical Center, Division of Endocrine, Diabetes, and Metabolism-Columbus, OH: Osei Kwame (PI), Kyaw Soe (Co-I), William F. Miser (former Co-PI), Colleen Sagrilla (coordinator), Jan Johnston (coordinator), Amber Anaya (coordinator), Ashley Mintos (coordinator), Angel A. Howell (coordinator), Kelly Rogers (former coordinator), Sara Taylor (former Co-I). University Hospitals Landerbrook Health Center-Mayfield Height, OH: Donald Ebersbacher (PI), Lucy Long (coordinator), Beth Bednarchik (coordinator). University Hospitals Glenridge Office Park-North Royalton, OH: Alan Wiggers (PI), Lucy Long (coordinator). University Hospitals Suburban Health- Cleveland, OH: Adrian Schnall (PI), Jonathan Smith (coordinator), Lori Peysha (coordinator), Lori Peysha (coordinator), Beth Bednarchik (coordinator), Lisa Leach (coordinator), Megan Tribout (coordinator). University Hospitals Otis Moss Jr. Health Center-Cleveland, OH: Carla Harwell (PI), Pinkie Ellington (coordinator). SUNY Downstate Medical Center, New York: Mary Ann Banerji (PI), Pranav Ghody (Co-I), Melissa Vahídeh Rambaud (coordinator). University of Pennsylvania-Philadelphia, PA: Raymond Townsend (PI), Debbie Cohen (Co- I), Yonghong Huan (Co-I), Mark Duckworth (former coordinator), Virginia Ford (coordinator), Juliet Leshner (coordinator), Ann Davison (coordinator), Sarah Vander Veen (coordinator). Temple University-Philadelphia, PA: Crystal A Gadegbeku (PI), Avi Gillespie (Co-I), Anuradha Paranjape (Co-I), Sandra Amoroso (coordinator), Zoe Pfeffer (coordinator), Sally B. Quinn (coordinator). Tulane University-New Orleans, LA: Jiang He (PI), Jing Chen (Co-I), Eva Lustigova (coordinator), Erin Malone (coordinator). Ochsner Clinic Foundation-New Orleans, LA: Marie Krousel-Wood (PI), Richard Deichmann (Co-I), Patricia Ronney (Co-I), Susan Muery (coordinator), Donnalee Trapani (coordinator). CWRU CCN Publication Acknowledgements: CWRU: This publication was made possible by the Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. OSU: The project described was supported by Award Number UL1RR025755 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. U Penn: The project described was supported by the National Center for Research Resources, Grant UL1RR024134, and is now at the National Center for Advancing Translational Sciences, Grant UL1TR000003. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. SOUTHEAST Network: Georgia Regents University, Augusta, GA: Matt Diamond (PI), Laura Mulloy (PI), Marcella Hodges (coordinator), Michelle Collins (coordinator), Charlene Weathers (coordinator), Heather Anderson (former coordinator), Emily Stone (former coordinator), Walida Walker (former coordinator). Carolinas Medical Center, Charlotte, NC: Andrew McWilliams (PI), Michael Dulin (Co-I), Lindsay Kuhn (Co-PI), Susan Standridge (coordinator), Lindsay Lowe (coordinator), Kelly Everett (coordinator), Kelry Preston (former coordinator), Susan Norton (former coordinator), Silena Gaines (former coordinator). University of South Carolina, Columbia, SC: Ali A. Rizvi (PI), Andrew W. Sides (Co-PI), Diamond Herbert (coordinator), Matthew M. Hix (coordinator), Melanie Whitmire (former coordinator (former coordinator), Brittany Arnold (former coordinator), Philip Hutchinson (former coordinator), Joseph Espiritu (former coordinator). Duke University, Durham, NC: Mark Feinglos (PI), Eugene Kovalik (Co-PI), Georgianne Gedon-Lipscomb (coordinator), Kathryn Evans (coordinator), Connie Thacker (coordinator), Ronna Zimmer (coordinator), Mary Furst (coordinator), MaryAnn Mason (former coordinator). East Carolina University, Greenville, NC: James Powell (PI), Paul Bolin (Co-PI), Junhong Zhang (Co-PI), Mary Pinion (coordinator), Gail Davis (coordinator), Winifred Bryant (former coordinator), Presley Phelps (former coordinator), Connie Garris-Sutton (former coordinator), Beatrice Atkinson (former coordinator). University of Miami, Miami, FL: Gabriele Contreras (PI), Maritza Suarez (Co-PI), Ivonne Schulman (Co-PI), Don Koggan (coordinator), Jackie Vassallo (coordinator), Gloria Peruyera (former coordinator). Wake Forest Nephrology, Winston Salem, NC: Michael Rocco (PI), Amret Hawfield (Co-PI), Cassandra Bethea (coordinator), Sheri Mayer (coordinator), Laura Gilliam (former coordinator). Wake Forest Downtown Health Plaza, Winston Salem, NC: Carolyn Pedley (PI), Geraldine Zurek (coordinator), Miriam Baird (coordinator), Charles Herring (Pharm D), Mary Martha Smoak (former coordinator). Wake Forest Geriatrics, Winston Salem, NC: Julie Williams (PI), Samantha Rogers (Co-PI), Lindsay Gordon (coordinator), Erin Kennedy (coordinator), Beverly Belle (coordinator), Jessica McCorkle-Doomy (former coordinator), Jonathan Adams (former coordinator), Dana Chamberlain (former coordinator). University of South Florida, Tampa, FL: Ramon Lopez (PI), Juris Janavs (coordinator). Emory University, Atlanta, GA: Frederic Rahbari-Oskoui (PI), Arlene Chapman (former PI), Allen Dollar (former Co-PI), Olubunmi Williams (coordinator), Yoosun Han (former coordinator). The Mayo Clinic Jacksonville, Jacksonville, FL: William Haley (PI), Peter Fitzpatrick (Co-PI), Joseph Blackshear (Co-PI), Brian Shapiro (Co-PI), Anna Harrell (coordinator), Arta Palaj (coordinator), Katelyn Henderson (coordinator), Ashley Johnson (former coordinator), Heath Gonzalez (former coordinator), Jermaine Robinson (former coordinator). Miami VA, Miami, FL: Leonardo Tamariz (PI), Ivonne Schulman (Co-PI), Jennifer Denizard (coordinator), Rody Barakat (former coordinator), Dhurga Krishnamoorthy (former coordinator). Pennington Biomedical Research, Baton Rouge, LA: Frank Greenway (PI), Ron Monce (Co-I), Timothy Church (former PI), Chelsea Hendrick (coordinator), Aimee Yoches (coordinator), Leighanne Sones (coordinator), Markee Baltazar (former coordinator). Morehouse School of Medicine, Atlanta, GA: Priscilla Pemu (PI), Connie Jones (coordinator), Derrick Akpalu (coordinator). UTAH Network: Boston University Medical Center, Boston MA: Laura Dember (PI), Denise Soares (coordinator). Henry Ford Hospital, Detroit MI: Jerry Yee (PI), Kausik Umanath (Co-PI), Naima Ogletree (Sub-I), Schawana Thaxton (Sub-I), Karen Campana (coordinator), Dayna Sheldon (coordinator), Krista MacArthur (coordinator). Intermountain Health Care, Salt Lake City UT: J. Brent Muhlestein (PI), Nathan Allred (Co-I), Brian Clements (Co-I), Ritesh Dhar (Co-I), Kent Meredith (Co-I), Viet Le (Co-I), Edward Miner (Co-I), James Orford (Co-I), Erik R. Riessen (Co-I), Becca Ballantyne (coordinator), Ben Chisum (coordinator), Kevin Johnson (coordinator), Dixie Peeler (coordinator). Stanford University, Palo Alto CA: Glenn Chertow (PI), Manju Tamura (Co-PI), Tara Chang (Co-I), Kevin Erickson (Co-I), Jenny Shen (Co-I), Randall S. Stafford (Co-I), Gregory Zaharchuk (Co-I), Margareth Del Cid (coordinator), Michelle Dentinger (coordinator), Jennifer Sabino (coordinator), Rukmani Sahay (coordinator), Ekaterina (Katie) Telminova (coordinator). Tufts Medical Center, Boston MA: Daniel E. Weiner (PI), Mark Sarnak (Co-I), Lily Chan (coordinator), Amanda Civiletto (coordinator), Alyson Heath (coordinator), Amy Kantor (coordinator), Priyanka Jain (coordinator), Bethany Kirkpatrick (coordinator), Andrew Well (coordinator), Barry Yuen (coordinator). University of Colorado, Denver, Denver CO: Michel Chonchol (PI), Beverly Farmer (coordinator), Heather Farmer (coordinator), Carol Greenwald (coordinator), Mikaela Malaczewski (coordinator). University of Illinois, Chicago, Chicago IL: James Lash (PI), Anna Porter (Co-I), Ana Ricardo (Co-I), Robert T. Rosman (Co-I), Janet Cohan (coordinator), Nieves Lopez Barrera (coordinator), Daniel Meslar (coordinator), Patricia Meslar (coordinator). University of Pittsburgh, Pittsburgh PA: Margaret (Molly) Conroy (PI), Mark Unruh (PI), Rachel Hess (Co-PI), Manisha Jhamb (Co-I), Holly Thomas (Co-I), Pam Fazio (coordinator), Elle Klixbull (coordinator), Melissa Komlos-Weimer (coordinator), LeeAnne Mandich (coordinator), Tina Vita (coordinator). University of Texas Southwestern, Dallas TX: Robert Toto (PI), Peter Van Buren (Co-I), Julia Inrig (Co-I), Martha Cruz (coordinator), Tammy Lightfoot (coordinator), Nancy Wang (coordinator), Lori Webster (coordinator). University of Utah, Salt Lake City UT: Srinivasan Beddhu (PI), Kalani Raphael (Co-I), Barry Stults (Co-I), Tahir Zaman (Co-I), Debra Simmons (Co-I), Tooran Lavasani (nurse practitioner), Rebecca Filipowicz (Sr. research analyst), Guo Wei (Sr research analyst), Gracie Mary Miller (coordinator), Jenice Harerra (coordinator), Jeff Christensen (Clinical research assistant), Ajay Giri (Clinical research assistant), Xiaorui Chen (graduate research assistant), Natalie Anderton (graduate research assistant), Arianna Jensen (undergraduate research assistant). Vanderbilt University, Nashville TN: Julia Lewis (PI), Anna Burgner (Co-I), Jamie P. Dwyer (Co-I), Gerald Schulman (Co-I), Terri Herrud (coordinator), Ewanda Leavell (coordinator), Tiffany McCray (coordinator), Edwina McNeil-Simaan (coordinator), Munmun Poudel (coordinator), Malia Reed (coordinator), Mohammed Sika (coordinator), Delia Woods (coordinator), Janice L. Zirkenbach (coordinator). George Washington University, Washington DC: Dominic S. Raj (PI), Scott Cohen (Co-I), Samir Patel (Co-I), Manuel Velasquez (Co-I), Roshni S. Bastian (coordinator), Maria Wing (coordinator) Akshay Roy-Chaudhury (Coordinator). University of California, Davis, Sacramento CA: Thomas Depner (PI), Lorien Dalyrymple (Co-I), George Kaysen (Co-I), Susan Anderson (coordinator). Salt Lake City VA, Salt Lake City UT: Srinivasan Beddhu (PI), John Nord (Co-I), Debra Simmons (Co-I), Gracie Mary Miller (coordinator), Jenice Harerra (coordinator), Ajay Giri (Clinical research assistant). Veterans Medical Research Foundation, San Diego CA: Joachim H. Ix (PI), Leonard Goldenstein (Co-PI), Cynthia M. Miracle (Co-I), Nketi Forbang (coordinator), Maja Mircic (coordinator), Brenda Thomas (coordinator), Tiffany Tran (coordinator). UCLA, Los Angeles CA: Anjay Rastogi (PI), Mihae Kim (Sub-PI), Mohamad Rashid (Co-PI), Bianca Lizarraga (coordinator), Amy Hocza (coordinator), Kristine Sarmosyan (coordinator), Jason Norris (coordinator), Tushar Sharma (coordinator), Amanda Chioy (coordinator), Eric Bernard (coordinator), Eleanore Cabrera (coordinator), Christina Lopez (coordinator), Susana Nunez (coordinator), Joseph Riad (coordinator), Suzanne Schweitzer (coordinator), Siran Sirop (coordinator), Sarah Thomas (coordinator), Lauren Wada (coordinator). Loyola University Medical Center, Chicago IL: Holly Kramer (PI), Vinod Bansal (Co-PI), Corliss E. Taylor (coordinator). University of Florida, Gainesville FL: Mark Segal (PI), Karen L. Hall (Co-I), Amir Kazory (Co-I), Lesa Gilbert (coordinator), Linda Owens (coordinator), Danielle Poulton (coordinator), Elaine Whidden (coordinator). University of Michigan, Ann Arbor MI: Jocelyn (Jo) Wiggins (PI), Caroline Blaum (PI), Linda Nyquist (Co-I), Lillian Min (Co-I), Tanya Gure (Co-I), Ruth Lewis (coordinator), Jennifer Mawby (coordinator), Eileen Robinson (coordinator). UTAH CCN Publication Acknowledgments: Boston: The trial was supported by a CTSA grant UL1 RR025771. Stanford: Spectrum is part of the Clinical and Translational Science Award (CTSA) program, funded by the National Center for Advancing Translational Sciences (Grant: UL1 TR000093) at the National Institutes of Health (NIH). All publications resulting from the use of Spectrum resources must cite this grant number. Tufts: The project described was supported by the National Center for Research Resources Grant Number UL1RR025752 and the National Center for Advancing Translational Sciences, national Institutes of Health, Grant Numbers UL1 TR000073 and UL1 TR001064. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. University of Illinois: The project described was supported by Grant number UL1TR000050, Clinical Interface Core. UT Southwestern: The current CTSA grant number is 9U54TR000017–06. University of Texas Southwestern Clinical and Translational Alliance for Research. University of Utah: CTSA Grant number is UL1TR000105–05. Center for Clinical and Translational Science. Vanderbilt University: Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institute of Health under Award Number UL1 TR000445. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Vanderbilt Institute for Clinical and Translational Research (VICTR). University of CA, Davis: CTSA Grant number UL1 TR000002. National Center for Advancing Translational Sciences. University of Florida: This work is supported in part by the NIH/NCATS Clinical and Translational Science Award to the University of Florida, Award Number UL1 TR000064; UF Clinical Research Center (UF CRC). University of Michigan: This work is supported in part by the Michigan Institute for Clinical and Health Research, Award Number UL1TR000433. UAB Network: Athens Internal Medicine, Athens, AL: Nauman Qureshi (PI), Karen Ferguson (coordinator), Sumrah Haider (coordinator), Mandy James (coordinator), Christy Jones (coordinator), Kim Renfroe (coordinator), April Seay (coordinator), Carrie Weigart (coordinator). UAB - The Chronic Kidney Disease Clinic, Birmingham, AL: Denyse Thornley-Brown (PI), Dana Rizik (Co-I), Bari Cotton (coordinator), Meredith Fitz- Gerald (coordinator), Tiffany Grimes (coordinator), Carolyn Johnson (coordinator), Sara Kennedy (coordinator), Chanel Mason (coordinator), Lesa Rosato-Burson (coordinator), Robin Willingham (coordinator). UAB - Vascular Biology and Hypertension Clinic, Birmingham, AL: David Calhoun (PI), Eric Judd (Co-I), Tonya Breaux-Shropshire (coordinator), Felice Cook (coordinator), Julia Medina (coordinator). Nephrology Associates, P.C., Birmingham, AL: James Lewis (PI), Roman Brantley (Co-I), John Brouilette (Co-I), Jeffrey Glaze (Co-I), Stephanie Hall (Co-I), Nancy Hiott (Co-I), David Tharpe (Co-I), Spencer Boddy (coordinator), Catherine Mack (coordinator). University of Tennessee Health Science Center, Memphis, TN: Karen C. Johnson (PI) Catherine Womack (Co –I), Beate Griffin (coordinator), Carol Hendrix (coordinator), Karen Johnson (coordinator), Lisa Jones (coordinator), Chelsea Towers (coordinator). Punzi Medical Center and Trinity Hypertension Research, Carrollton, TX: Henry Punzi (PI), Kathy Cassidy (coordinator), Kristin Schumacher (coordinator). Family Care Practice, Fajardo, Puerto Rico: Carmen Irizarry (PI), Ilma Colon (coordinator). Centro Cardiovascular de Caguas, El Verde, Caguas, Puerto Rico: Pedro Colon-Ortiz (PI), Pedro ColonHernandez (Co-I), Merari Carrasquillo (coordinator), Nivea Vazquez (coordinator). Miguel Sosa-Padilla, Private Practice San Juan, Puerto Rico: Miguel Sosa-Padilla (PI), Alex Cintron-Pinero (Co-I), Mayra Ayala (coordinator), Olga Pacheco (coordinator), Catalina Rivera (coordinator) Irma Sotomayor-Gonzalez (coordinator). Altamira Family Practice and Research Institute Center, San Juan, Puerto Rico: Jamie Claudio (PI), Jose Lazaro (coordinator), Migdalia Arce (coordinator), Lourdes Heres (coordinator), Alba Perez (coordinator). Centro Clinico San Patricio, San Juan, Puerto Rico: Jose Tavarez-Valle (PI), Ferlinda Arocho (coordinator), Mercedes Torres (coordinator), Melvaliz Vazquez (coordinator). University of Massachusetts – Worchester, MA: Gerard P. Aurigemma (PI), Rebecca Takis- Smith (Co-I), Julia Andrieni (Co-I), Noelle Bodkin (coordinator), Kiran Chaudhary (coordinator), Paula Hu (coordinator). Rutgers-Robert Wood Johnson Medical School, New Brunswick, New Jersey: John Kostis (PI), William J. Kostis (Co-PI) Nora Cosgrove (coordinator), Denise Bankowski (coordinator), Monica Boleyn (coordinator), Laurie Casazza (coordinator), Victoria Giresi (coordinator), Tosha Patel (coordinator), Erin Squindo (coordinator), Yan Wu (coordinator). University of Mississippi Medical Center CRP – Jackson, MS: Marion Wofford (PI), Michael Flessner (Co-I), Cathy Adair (coordinator). Nashville Medical Group, Nashville, TN: Jordan Asher (PI), Debbie Loope (coordinator), Rita Cobb (coordinator), Reiner Venegas (coordinator). New York Irving Pavilion Research, Columbia University, New York, NY: Thomas Bigger (Director), Daniel Donovan (PI), Carlos Lopez-Jimenez (Co-I), Amilcar Tirado (coordinator). New York, Irving Pavilion Research Unit – CTSA Satellite, Columbia University, New York, NY: Thomas Bigger (Director), Asqual Getaneh (PI), Rocky Tang (coordinator), Sabrina Durant (coordinator). Clinical Cardiovascular Research Lab for the Elderly, Columbia University, New York, NY: Thomas Bigger (Director), Mathew Maurer (PI), Sergio Teruya (Co-I) Stephen Helmke (coordinator), Julissa Alvarez (coordinator). Medical University of South Carolina Nephrology, Charleston, SC: Ruth Campbell (PI), Roberto Pisoni (Co-I), Rachel Sturdivant (Co-I), Caroline Counts (coordinator), Vickie Hunt (coordinator), Lori Spillers (coordinator). Great Lakes Medical Research, Westfield, NY: Donald Brautigam (PI), Timothy Kitchen (Co- I), Timothy Gorman (Co-I) Jessica Sayers (coordinator), Sarah Button (coordinator), June Chiarot (coordinator), Rosemary Fischer (coordinator), Melissa Lyon (coordinator), Maria Resnick (coordinator). VA Network: New Mexico VA Healthcare System – Albuquerque, NM: Karen Servilla (PI), Darlene Vigil (Co-I), Terry Barrett (coordinator). Atlanta VAMC – Atlanta GA: Mary Ellen Sweeney (PI), Rebecca Johnson (Co-I), Susan McConnell (Co-I), Khadijeh Shahid Salles (Co-I), Francoise Watson (Co-I), Cheryl Schenk (coordinator), Laura Whittington (coordinator), Maxine Maher (coordinator). VA Boston Healthcare System – Jamaica Plain, MA: Jonathan Williams (PI), Stephen Swartz (PI), Paul Conlin (Co-I), George Alexis (coordinator), Rebecca Lamkin (coordinator), Patti Underwood (coordinator), Helen Gomes (coordinator). James J. Peters VAMC – Bronx, NY: Clive Rosendorff (PI), Stephen Atlas (Co-I), Lawrence Kwon (Co-I), Matar Matar (coordinator). Ralph H. Johnson VAMC – Charleston, SC: Roberto Pisoni (PI), Jan Basile (PI), Joseph John (PI), Deborah Ham (coordinator), Hadi Baig (coordinator). Dayton VAMC – Dayton, OH: Mohammed Saklayen (PI), Jason Yap (Co-I), Helen Neff (coordinator), Carol Miller (coordinator), Ling Zheng-Phelan (coordinator). John D. Dingell VAMC – Detroit, MI: Saib Gappy (PI), Shiva Rau (Co-I), Arathi Raman (Co-I), Vicki Berchou (coordinator), Elizabeth Jones (coordinator), Erin Olgren (coordinator). VA New Jersey Healthcare System – East Orange, NJ: Michael Yudd (PI), Sithiporn Sastrasinh (PI), Jennine Michaud (Co-I), Jessica Fiore (coordinator), Marianne Kutza (coordinator). Malcom Randall VAMC – Gainesville, FL: Ronald Shorr (PI), Rattana Mount (Co-I), Jeremy Thoms (Co-I), Helen Dunn (coordinator), Susan Stinson (coordinator), Jessica Hunter (coordinator). Michael E. DeBakey VAMC – Houston, TX: Addison Taylor (PI), Jeffery Bates (Co-I), Catherine Anderson (coordinator). G.V. (Sonny) Montgomery VAMC – Jackson, MS: Kent Kirchner (PI), Jodi Stubbs (Co-I), Ardell Hinton (coordinator), Anita (Kaye) Spencer (coordinator). Kansas City VAMC – Kansas City, MO: Santosh Sharma (PI), Thomas Wiegmann (PI), Smita Mehta (coordinator). John L. McClellan Memorial Veterans Hospital – Little Rock, AR: Michelle Krause (PI), Kate Dishongh (coordinator). Memphis VAMC – Memphis, TN: Barry Wall (PI), Richard Childress (Co-I), William Cushman (Co-I), Geeta Gyamlani (Co-I), Atossa Niakan (Co-I), Cathy Thompson (Co-I), Janelle Moody (coordinator). Clement J. Zablocki VAMC – Milwaukee, WI: Jeffrey Whittle (PI), Gary Barnas (Co-I), Dawn Wolfgram, (Co-I), Heidi Cortese (coordinator), Jonette Johnson (coordinator). Nashville VAMC/TVHS-GRECC – Nashville, TN: Christianne Roumie (PI), Adriana Hung (Co-I), Jennifer Wharton (coordinator), Kurt Niesner (coordinator). VA New York Harbor Healthcare System – New York, NY: Lois Katz (PI), Elizabeth Richardson (coordinator), George Brock (coordinator). Northport VAMC – Northport, NY: Joanne Holland (PI), Troy Dixon (PI), Athena Zias (Co-I), Christine Spiller (coordinator). Phoenix VA Healthcare System – Phoenix, AZ: Penelope Baker (PI), James Felicetta (PI), Shakaib Rehman (Co-I), Kelli Bingham (coordinator). Portland VAMC – Portland, OR: Suzanne Watnick (PI), Jessica Weiss (Co-I), Tera Johnston (coordinator). St. Louis VA Healthcare System – St. Louis, MO: Stephen Giddings (PI), Andrew Klein (PI), Caroline Rowe (Co-I), Kristin Vargo (coordinator), Kristi Waidmann (coordinator). Washington, D.C. VAMC – Washington, D.C.: Vasilios Papademetriou (PI), Jean Pierre Elkhoury (Co-I), Barbara Gregory (coordinator), Susan Amodeo (coordinator), Mary Bloom (coordinator). West Los Angeles VA Healthcare Center/Greater Los Angeles Healthcare System – Los Angeles, CA: Dalia Goldfarb-Waysman (PI), Richard Treger (Co-I), Karen Knibloe (coordinator). Minneapolis VAMC – Minneapolis, MN: Areef Ishani (PI), Yelena Slinin (Co-I), Christine Olney (coordinator), Jacqueline Rust (coordinator). Audie L. Murphy Memorial Veterans Hospital – South Texas Veterans Healthcare System – San Antonio, TX: Paolo Fanti (PI), Shweta Bansal (Co-I), Monica Dunnam (Co-I), Christopher Dyer (Co-I), Lih-Lan Hu (coordinator), Perla Zarate-Abbott (coordinator).
Appendix 2: Additional Statistical Details
Sensitivity Analyses
Because time-to-event analyses of incident CKD based on a decrease in eGFR of 30% are believed to be sensitive to hemodynamic effects, we performed sensitivity analyses in which similar Cox regressions were applied for incident CKD with 40% and 50% decreases in eGFR from baseline, as well as for incident CKD with 30%, 40%, or 50% decreases in eGFR from the 6-month visit (Appendix Table 1). Furthermore, because relative changes from 6 months could be influenced by an acute effect leading to different eGFRs at 6 months, we also examined the time to a fixed decrease in eGFR of 27 mL/min/1.73 m2 (representing a 30% decrease from a baseline eGFR of 90 mL/min/1.73 m2) from 6 months, with and without requiring the threshold of 60 mL/min/1.73 m2 (Appendix Table 2). Finally, analyses of incident CKD based on 30%, 40%, and 50% change in eGFR calculated using the CKD-EPI equation rather than the MDRD study equation were performed (Appendix Table 3).
Additional Event Outcomes
This article is limited to the 6662 randomly assigned participants with baseline eGFR of at least 60 mL/min/1.73 m2 and excludes 53 without baseline serum creatinine measurements who were included in the “non-CKD” group in the primary results manuscript that was published previously (9). The primary CKD outcome in the non-CKD subgroup was a greater than 30% decrease in eGFR to a value less than 60 mL/min/1.73 m2, with a consecutive confirmatory value at least 90 days later. The addition of potential confirmatory laboratory values subsequent to the cutoff date for follow-up events (20 August 2015) resulted in identification of 16 CKD events (13 in the intensive group and 3 in the standard group) in addition to those reported in the primary results manuscript. Likewise, continued review of serious adverse events and surveillance after the cutoff date resulted in identification of 10 primary CVD outcomes (1 in the intensive group and 9 in the standard group) and 1 more fatal event in the standard group that occurred before the cutoff date. All of these events were included in the current analysis.
Appendix Figure 1.
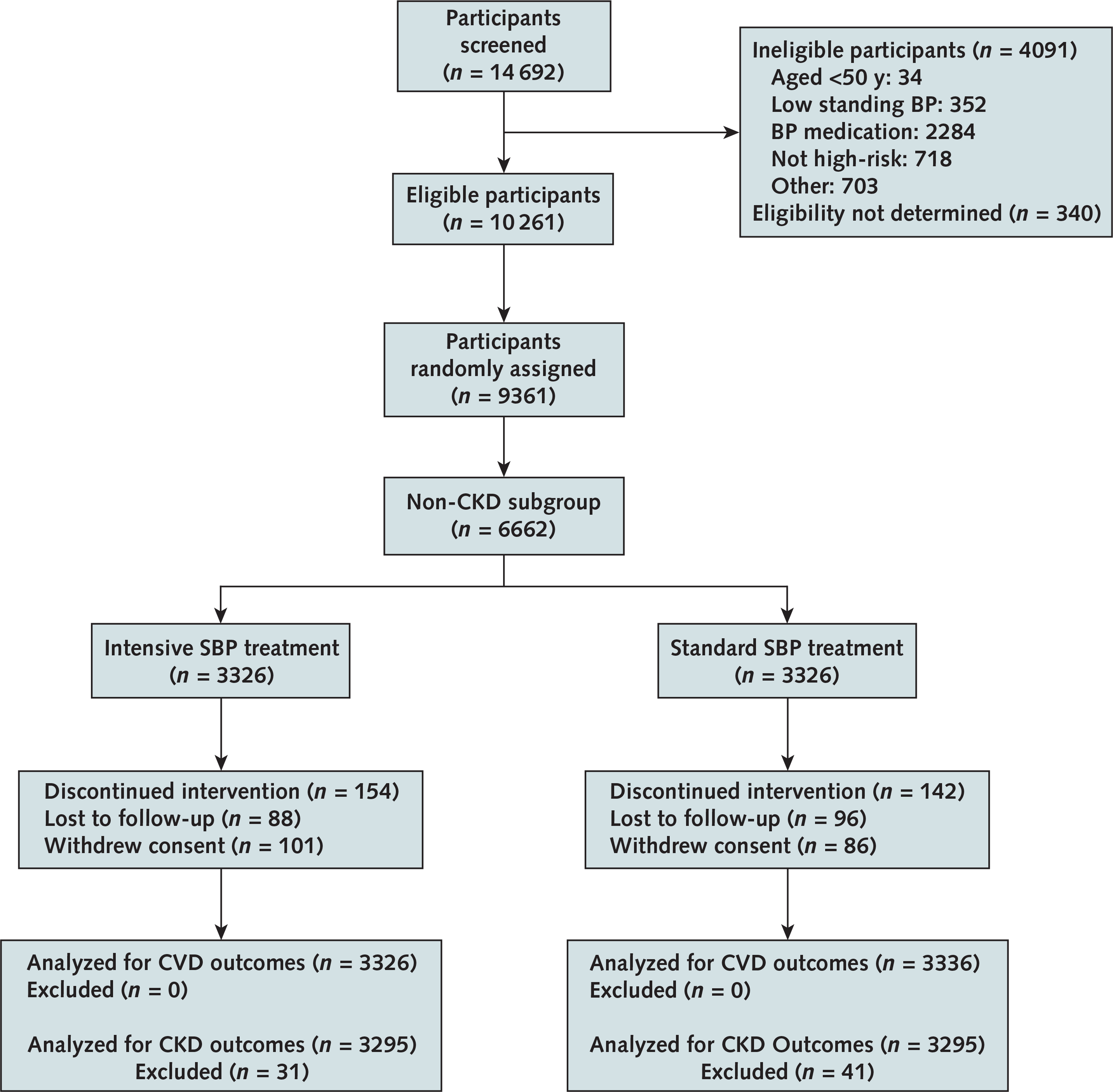
CONSORT flow diagram of study participants.
BP = blood pressure; CKD = chronic kidney disease; CONSORT = Consolidated Standards of Reporting Trials; CVD = cardiovascular disease; SBP = systolic blood pressure.
Appendix Figure 2.
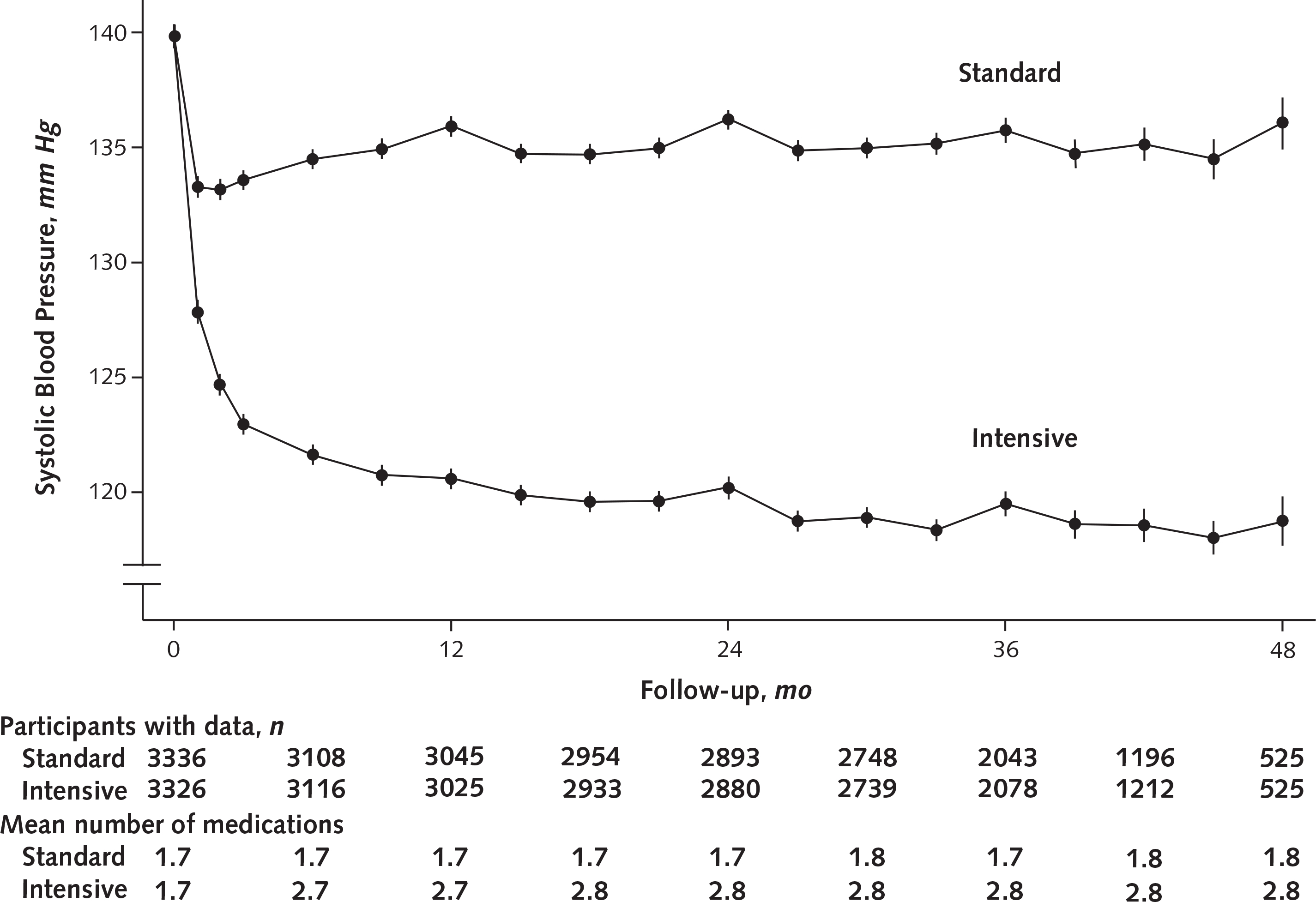
Systolic blood pressure separation in the non-CKD population, by treatment group.
The mean number of medications was the average number of antihypertensive medication classes prescribed per participant. Closed circles depict raw means. Error bars indicate 95% CIs. CKD = chronic kidney disease.
Appendix Figure 3.
Difference in eGFR during follow-up in the non-CKD population.
Adjusted means (intensive minus standard group) with 95% CIs (error bars) are shown. Open circles depict fasting visits; closed circles depict nonfasting visits. CKD = chronic kidney disease; eGFR = estimated glomerular filtration rate.
Appendix Figure 4.
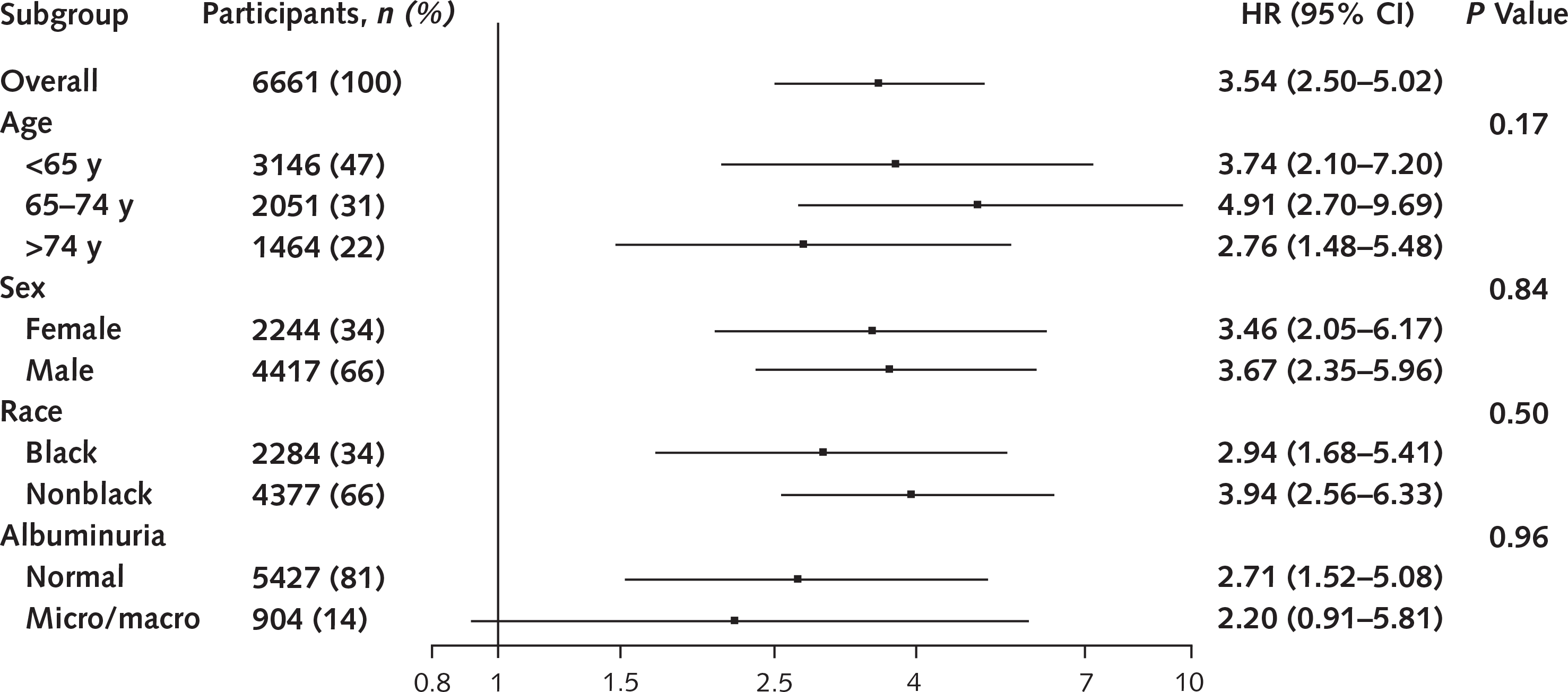
Forest plot of HRs for incident CKD (>30% decrease from baseline) in the entire non-CKD population and in subgroups defined by age, sex, race, and albuminuria (urinary albumin–creatinine ratio <30 vs. ≥30 mg/g).
CKD = chronic kidney disease; HR = hazard ratio.
Appendix Figure 5.
Cumulative incidence of albuminuria in non-CKD subgroup.
CKD = chronic kidney disease.
Appendix Table 1.
Rates of Incident CKD, Defined as eGFR <60 mL/min/1.73 m2 and Calculated by the MDRD Study Equation, With a Confirmed 30%, 40%, or 50% Decrease in eGFR From Baseline or Month 6
| Decrease in eGFR | Intensive Group |
Standard Group |
Hazard Ratio (95% CI) | P Value | ||
|---|---|---|---|---|---|---|
| Events per 100 Person-Years, n | Events/Total Years of Follow-up, n/N | Events per 100 Person-Years, n | Events/Total Years of Follow-up, n/N | |||
| From baseline* | ||||||
| >30% | 1.32 | 140/10 584 | 0.37 | 40/10 751 | 3.55 (2.52–5.11) | <0.001 |
| >40% | 0.43 | 46/10 748 | 0.12 | 13/10 794 | 3.51 (1.95–6.77) | <0.001 |
| >50% | 0.10 | 11/10 812 | 0.05 | 5/10 803 | 2.11 (0.76–6.71) | 0.15 |
| From 6 mo† | ||||||
| >30% | 0.57 | 58/10 195 | 0.23 | 24/10 284 | 2.50(1.57–4.11) | <0.001 |
| >40% | 0.19 | 19/10 265 | 0.11 | 11/10 304 | 1.70(0.82–3.69) | 0.16 |
| >50% | 0.04 | 4/10 282 | 0.02 | 2/10 315 | 1.97 (0.38–14.23) | 0.40 |
CKD = chronic kidney disease; eGFR = estimated glomerular filtration rate; MDRD = Modification of Diet in Renal Disease.
n = 3332 for the intensive group and 3345 for the standard group.
n = 3097 for the intensive group and 3106 for the standard group.
Appendix Table 2.
Rates of Incident CKD, Defined as eGFR <60 mL/min/1.73 m2 and Calculated by the MDRD Study Equation, With a Fixed Decrease in eGFR of 27 mL/min/1.73 m2 From Month 6
| Variable | Intensive Group |
Standard Group |
Hazard Ratio (95% CI) | P Value | ||
|---|---|---|---|---|---|---|
| Events per 100 Person-Years, n | Events/Total Years of Follow-up, n/N | Events per 100 Person-Years, n | Events/Total Years of Follow-up, n/N | |||
| Fixed 27-unit decrease in eGFR to <60 mL/min/1.73 m2 | 0.28 | 29/10 248 | 0.14 | 14/10 298 | 2.05 (1.10–4.00) | 0.023 |
| Fixed 27-unit decrease in eGFR to any eGFR | 0.73 | 74/10 174 | 0.53 | 54/10 240 | 1.43 (1.01–2.05) | 0.044 |
CKD = chronic kidney disease; eGFR = estimated glomerular filtration rate; MDRD = Modification of Diet in Renal Disease.
Appendix Table 3.
Rates of Incident CKD, Defined as eGFR <60 mL/min/1.73 m2 and Calculated by the CKD-EPI Equation, With a Confirmed 30%, 40%, or 50% Decrease in eGFR From Baseline or Month 6
| Decrease in eGFR | Intensive Group |
Standard Group |
Hazard Ratio (95% CI) | P Value | ||
|---|---|---|---|---|---|---|
| Events per 100 Person-Years, n | Events/Total Years of Follow-up, n/N | Events per 100 Person-Years, n | Events/Total Years of Follow-up, n/N | |||
| From baseline* | ||||||
| >30% | 1.56 | 164/10 584 | 0.47 | 50/10 751 | 3.34 (2.45–4.63) | <0.001 |
| >40% | 0.53 | 57/10 748 | 0.15 | 16/10 794 | 3.67 (2.16–6.62) | <0.001 |
| >50% | 0.13 | 14/10 812 | 0.05 | 5/10 803 | 2.75(1.05–8.53) | 0.039 |
| From 6 mo† | ||||||
| >30% | 0.65 | 66/10 195 | 0.30 | 31/10 284 | 2.22 (1.46–3.46) | <0.001 |
| >40% | 0.21 | 22/10 265 | 0.12 | 12/10 304 | 1.86 (0.93–3.88) | 0.079 |
| >50% | 0.04 | 4/10 282 | 0.02 | 2/10 315 | 1.94(0.38–14.00) | 0.40 |
CKD = chronic kidney disease; CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration; eGFR = estimated glomerular filtration rate.
n = 3332 for the intensive group and 3345 for the standard group.
n = 3097 for the intensive group and 3106 for the standard group.
Footnotes
All components of the SPRINT study protocol were designed and implemented by the investigators. The investigative team collected, analyzed, and interpreted the data. All manuscript writing and revising were done by the authors.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the U.S. Department of Veterans Affairs, or the U.S. government.
Reproducible Research Statement: Study protocol: See the Supplement (available at Annals.org). Statistical code: Not available. Data set: Partially available at https://biolincc.nhlbi.nih.gov/studies/sprint_pop.
Contributor Information
Srinivasan Beddhu, Division of Nephrology & Hypertension, University of Utah School of Medicine, 295 Chipeta Way, Suite 4000, Salt Lake City, UT 84108.
Michael V. Rocco, Section on Nephrology, Wake Forest School of Medicine, 1 Medical Center Boulevard, Winston-Salem, NC 27157-1053.
Robert Toto, University of Texas Southwestern Medical Center, 5623 Harry Hines Boulevard, Dallas, TX 75390-8592..
Timothy E. Craven, Department of Biostatistical Sciences, Wake Forest School of Medicine, 1 Medical Center Boulevard, Winston-Salem, NC 27157-1063..
Tom Greene, Division of Nephrology & Hypertension, University of Utah School of Medicine, 295 Chipeta Way, Suite 4000, Salt Lake City, UT 84108..
Udayan Bhatt, Ohio State University, Ground Floor, 395 West 12th Avenue, Columbus, OH 43210..
Alfred K. Cheung, Division of Nephrology & Hypertension, University of Utah School of Medicine, 295 Chipeta Way, Suite 4000, Salt Lake City, UT 84108..
Debbie Cohen, Renal, Electrolyte and Hypertension Division, University of Pennsylvania, 1 Founders Building, 3400 Spruce Street, Philadelphia, PA 19104..
Barry I. Freedman, Section on Nephrology, Wake Forest School of Medicine, 1 Medical Center Boulevard, Winston-Salem, NC 27157-1053.
Amret T. Hawfield, Section on Nephrology, Wake Forest School of Medicine, 1 Medical Center Boulevard, Winston-Salem, NC 27157-1053.
Anthony A. Killeen, Department of Laboratory Medicine & Pathology, University of Minnesota, 420 Delaware Street SE, MMC 609, Minneapolis, MN 55455..
Paul L. Kimmel, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, 6707 Democracy Boulevard, Bethesda, MD 20892..
James Lash, Division of Nephrology, University of Illinois at Chicago, 820 South Wood Street (M/C 793), Chicago, IL 60612-7315..
Vasilios Papademetriou, VA Medical Center, 50 Irving Street NW, Room 1D137, Washington, DC 20422..
Mahboob Rahman, Case Western Reserve University, 11100 Euclid Avenue, Cleveland, OH 44196..
Anjay Rastogi, Division of Nephrology, University of California, Los Angeles, 10630 Santa Monica Boulevard, Los Angeles, CA 90025-4837..
Karen Servilla, New Mexico Veterans Administration Health Care System, Medicine Service, Renal Section, 120 San Pedro SE, Albuquerque, NM 87108..
Raymond R. Townsend, Nephrology & Hypertension, University of Pennsylvania, 122 Founders Building, 3400 Spruce Street, Philadelphia, PA 19104..
Barry Wall, Section 111b, Division of Nephrology, VA Medical Center, 1030 Jefferson Avenue, Memphis, TN 38104..
Paul K. Whelton, Department of Epidemiology #8318, Tulane University School of Public Health and Tropical Medicine, 1440 Canal Street, Room 2018, New Orleans, LA 70112..
References
- 1.Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. 2016;134:441–50. doi: 10.1161/CIRCULATIONAHA.115.018912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–20. doi: 10.1001/jama.2013.284427 [DOI] [PubMed] [Google Scholar]
- 3.Hsu CY, McCulloch CE, Darbinian J, Go AS, Iribarren C. Elevated blood pressure and risk of end-stage renal disease in subjects without baseline kidney disease. Arch Intern Med. 2005;165:923–8. [DOI] [PubMed] [Google Scholar]
- 4.Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet. 2014;383:1899–911. doi: 10.1016/S0140-6736(14)60685-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13. [DOI] [PubMed] [Google Scholar]
- 6.Yoon SS, Burt V, Louis T, Carroll MS. Hypertension Among Adults in the United States, 2009–2010. NCHS Data Brief no. 107. Hyattsville, MD: National Center for Health Statistics; October 2012. Accessed at www.cdc.gov/nchs/data/databriefs/db107.pdf on 4 May 2017. [Google Scholar]
- 7.Ortman JM, Velkoff VA, Hogan H. An Aging Nation: The Older Population in the United States. Current Population Reports. Washington, DC: U.S. Census Bureau; May 2014. Accessed at www.census.gov/prod/2014pubs/p25-1140.pdf on 4 May 2016. [Google Scholar]
- 8.Wright JT Jr, Fine LJ, Lackland DT, Ogedegbe G, Dennison Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med. 2014;160:499–503. doi: 10.7326/M13-2981 [DOI] [PubMed] [Google Scholar]
- 9.Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, et al. ; SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015; 373:2103–16. doi: 10.1056/NEJMoa1511939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, et al. ; SPRINT Study Research Group. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. 2014;11:532–46. doi: 10.1177/1740774514537404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Systolic Blood Pressure Intervention Trial Protocol Version 4.0. National Heart, Lung, and Blood Institute. 1 November 2012. Accessed at www.sprinttrial.org/public/Protocol_Current.pdf on 20 March 2016. [Google Scholar]
- 12.D’Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–53. doi: 10.1161/CIRCULATIONAHA.107.699579 [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002 [DOI] [PubMed] [Google Scholar]
- 14.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 15.Hommel G A stagewise rejective multiple test procedure based on a modified Bonferroni test. Biometrika. 1988;75:383–6. [Google Scholar]
- 16.Altman DG. Confidence intervals for the number needed to treat. BMJ. 1998;317:1309–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palmer BF. Renal dysfunction complicating the treatment of hypertension. N Engl J Med. 2002;347:1256–61. [DOI] [PubMed] [Google Scholar]
- 18.Cushman WC, Evans GW, Byington RP, Goff DC Jr, Grimm RH Jr, Cutler JA, et al. ; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010; 362:1575–85. doi: 10.1056/NEJMoa1001286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peralta CA, McClure LA, Scherzer R, Odden MC, White CL, Shlipak M, et al. Effect of intensive versus usual blood pressure control on kidney function among individuals with prior lacunar stroke: a post hoc analysis of the Secondary Prevention of Small Subcortical Strokes (SPS3) randomized trial. Circulation. 2016;133:584–91. doi: 10.1161/CIRCULATIONAHA.115.019657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Appel LJ, Wright JT Jr, Greene T, Agodoa LY, Astor BC, Bakris GL, et al. ; AASK Collaborative Research Group. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med. 2010;363:918–29. doi: 10.1056/NEJMoa0910975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarnak MJ, Greene T, Wang X, Beck G, Kusek JW, Collins AJ, et al. The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the Modification of Diet in Renal Disease Study. Ann Intern Med. 2005;142:342–51. doi: 10.7326/0003-4819-142-5-200503010-00009 [DOI] [PubMed] [Google Scholar]
- 22.Toto RD, Mitchell HC, Lee HC, Milam C, Pettinger WA. Reversible renal insufficiency due to angiotensin converting enzyme inhibitors in hypertensive nephrosclerosis. Ann Intern Med. 1991;115:513–9. doi: 10.7326/0003-4819-115-7-513 [DOI] [PubMed] [Google Scholar]
- 23.Holtkamp FA, de Zeeuw D, Thomas MC, Cooper ME, de Graeff PA, Hillege HJ, et al. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int. 2011;80:282–7. doi: 10.1038/ki.2011.79 [DOI] [PubMed] [Google Scholar]
- 24.Short-term effects of protein intake, blood pressure, and antihypertensive therapy on glomerular filtration rate in the Modification of Diet in Renal Disease Study. J Am Soc Nephrol. 1996;7:2097–109. [DOI] [PubMed] [Google Scholar]
- 25.Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet. 2000;355:253–9. [PubMed] [Google Scholar]
- 26.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. ; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–9. [DOI] [PubMed] [Google Scholar]
- 27.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305. [DOI] [PubMed] [Google Scholar]