Abstract
Gastric cancer (GC) is one of the most aggressive malignancies, currently ranking third among cancers leading to death worldwide. Despite the recent advancements in GC research, it is most often diagnosed during the terminal stages and with limited treatment modalities contributing to its poor prognosis and a lower survival rate.
Much research has provided conflicting results between a vitamin D deficient status and the development of GC. Vitamin D is a well-known and essential hormone classically known to regulate calcium and phosphate absorption, enabling adequate mineralization of the skeletal system. However, the function of vitamin D is multidimensional. It possesses unique roles, including acting as antioxidants or immunomodulators while crossing the cell membrane, performing several intracellular functions, participating in gene regulation, and controlling the proliferation and invasion of cancer cells, including those of GC.
In light of this, it is imperative to analyze the causes of GC, review the factors that can be used to enhance the effectiveness of treatments, and discover the tools to determine prognosis, reduce mortality, and prevent GC development. In this review, we have summarized recent investigations on multiple associations between vitamin D and GC, emphasizing genetic associations, vitamin D receptors, and the prevalence of hormone deficiency in those developing this aggressive malignancy.
Keywords: vitamin d, cholecalciferol, gastric cancer, ergocalciferol, vitamin d receptor, genetic polymorphism, vitamin d deficiency
Introduction and background
Gastric cancer (GC) is one of the five most common cancers to occur globally despite its abatement in incidence during recent years [1,2]. This aggressive malignancy manifests a poor prognosis due to its advanced stage of diagnosis and restricted treatment alternatives, placing GC third among cancers leading to mortality worldwide [3,4]. Furthermore, numerous risk factors such as genetics, Helicobacter pylori (most common), smoked foods, red meat, smoking, and alcohol also contribute to the development of GC [5-8]. Additionally, many investigations also support the significance of vitamin D in the overall pathogenesis of GC at both genetic and molecular levels.
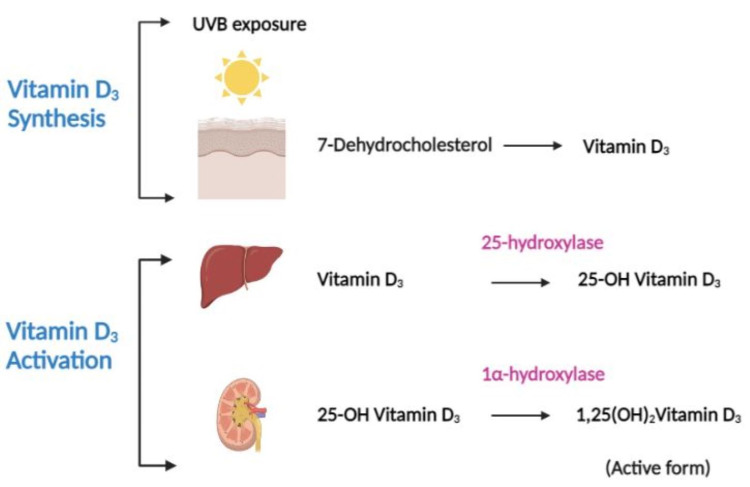
Despite its essential properties as a fat-soluble vitamin, vitamin D is also an active steroid hormone. Its primary function is bone mineralization by regulating calcium and phosphorus homeostasis. We can obtain vitamin D from various foods, supplements and via dermal production under the influence of sun exposure. It is available in two forms, vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol). However, since both forms are inactive, they must be hydroxylated twice before activating the final product. The initial activation happens in the liver, followed by the second in the kidneys by enzymes 25-hydroxylase and 1alpha-hydroxylase (1α-hydroxylase) respectively to produce 1,25-dihydroxyvitamin D3 (1,25(OH)2Vitamin D3) (active form), which exerts the physiological effect [6,9,10]. Figure 1 shows the formation and activation of vitamin D3.
Figure 1. Schematic representation of the dermal synthesis of vitamin D3 and its activation.
Under the effect of ultraviolet B light from the sun, vitamin D3 is produced in the dermis from 7-dehydrocholesterol. Following production, it enters the liver, where the enzyme 25-hydroxylase converts it to 25-hydroxyvitamin D3 (25-OH Vitamin D3). It subsequently travels to the kidneys, where it is transformed into 1,25-dihydroxyvitamin D3 (1,25(OH)2Vitamin D3), the physiologically active form of vitamin D.
[Original illustration]
Moreover, many studies have highlighted the active participation of vitamin D in other processes like the immune system, inflammation, gene regulation, signal transduction, and finally, the development of cancer. Epidemiological and animal studies mentioned earlier showed that vitamin D exerts an antineoplastic effect via its vitamin D receptor (VDR). Its interaction stimulates apoptosis and differentiation while inhibiting invasion, angiogenesis, proliferation, inflammation, and metastasis [11,12]. In addition, the VDR functions as a transcription factor, regulating several gene expressions.
Since vitamin D is essential in the control and maintainance of many of the crucial tasks within the human body, its deficiency can contribute to immune dysregulation. In infections like Helicobacter pylori, vitamin D deficiency can lead to the failure of its removal [13,14]. Similarly, GC patients may demonstrate a greater survival rate with adequate vitamin D levels as compared to individuals with VDD [10], suggesting that vitamin D could be a powerful constituent in the GC mechanism. This review focuses on various associations between vitamin D and the pathogenesis of GC, which may be beneficial for early diagnosis and treatment. Overall, it provides an understanding of delaying the progression of GC and lowering its associated mortality rate.
Review
Methods
The literature was searched in the PubMed database. The regular keywords used in the search for vitamin D are as follows: Vitamin D, Cholecalciferol, Calcitriol, Drisdol, 1,25dihydroxycholecalciferol, Ergocalciferol, VitaminD2, VitaminD3; For GC, we used keywords such as Stomach cancer, Stomach neoplasm, Stomach tumor, Stomach carcinoma, Gastric carcinoma, Gastric cancer, Gastric tumor, Gastric neoplasm. The Boolean search strategy was applied using "OR" in the regular keywords, giving 92,292 and 161,726 results for vitamin D and GC, respectively. Regular keywords were then combined using the Boolean term "AND" that generated 1,48,274 papers. We also used Medical Subject Headings (MeSH) keywords such as “Vitamin D” and “Stomach Neoplasms” that produced 61,722 and 99,898 articles, respectively. The Boolean term “AND” was implemented on MeSH keywords, which gave us 48 papers. Finally, both regular and MeSH keywords together yielded 119 articles.
Results
Only studies in the English language were included, which reduced the number of articles from 119 to 113. These 113 papers were screened based on the relevant topic, and any articles with animal studies were excluded. Fifty articles were retrieved that were either free full text or abstracts. Table 1 and Table 2 present a summary of the most relevant study characteristics.
Table 1. A table outlining the studies exploring the genetic involvement of vitamin D in gastric cancer.
BMP3: Bone morphogenetic protein 3; GC: Gastric cancer; Hh: hedgehog; miR: miRNA; PTEN: Phosphatase and tensin homolog deleted on chromosome 10; VD: vitamin D; VDR: vitamin D receptor; VDD: vitamin D deficiency; VDBP: vitamin D binding protein
| Author | Country | Year | Study design | Key findings |
| Pan et al. [15] | China | 2010 | Experimental | VD, in combination with trichostatin A/sodium butyrate and 5-aza-2’deoxycytidine, promotes apoptosis in gastric cancer cells via raising PTEN expression. |
| Baek et al. [16] | Korea | 2011 | Experimental | VD reduces gastric cancer cell survival by suppressing Hh signaling and is synergistic with anti-cancer drugs (Adriamycin, Vinblastine, and Paclitaxel). |
| Cong et al. [17] | China | 2015 | Case-Control | VDR FokI polymorphism and GC risk have a positive relationship. |
| Chang et al. [18] | China | 2015 | Experimental | VD restricts gastric cancer cell proliferation by stimulating miR-145 expression. |
| Zhao et al. [19] | China | 2019 | Experimental | VD increases BMP3 expression and delays GC progression. |
| Parsamanesh et al. [20] | Iran | 2019 | Case-Control | A negative association has been observed between VDR FokI polymorphism and GC risk, whereas VDR TaqI polymorphism has shown a positive relationship with GC. |
| Durak et al. [21] | Turkey | 2019 | Case-Control | GC risk has not been correlated with VDBP, TaqI, and FokI VDR polymorphisms; VDD deficiency increases GC risk. |
| Chang et al. [22] | China | 2019 | Experimental | VD inhibits GC cell proliferation by upregulating miR-99b-3p expression. |
| Calcagno et al. [2] | Brazil | 2019 | Review | VD produces an anti-cancer effect in gastric cancer by regulating histone acetylation. |
| Qadir et al. [23] | India | 2021 | Case-Control | The BsmI VDR polymorphism has been linked to GC risk; ApaI and TaqI VDR polymorphisms have not been attributed to GC risk. |
| Hoseinkhani et al. [24] | Iran | 2021 | Case-Control | FokI VDR polymorphism is associated with GC risk; However, TaqI, ApaI, and BsmI VDR polymorphisms are not related to GC risk. |
Table 2. A table summarizing the association of vitamin D deficiency with gastric cancer.
GC: gastric cancer; VD: vitamin D; VDD: vitamin D deficiency; VDR: vitamin D receptor.
| Author | Country | Year | Study design | Key findings |
| Ren et al. [10] | China | 2012 | Retrospective-Observational | VDD has been linked to a poor prognosis of gastric cancer. |
| Bao et al. [7] | China | 2014 | Experimental | VD and Cisplatin promote apoptosis and cell cycle arrest in gastric cancer cells in a synergistic fashion. |
| Wen et al. [25] | China | 2015 | Experimental | VDR expression is lowest in gastric cancer compared to precancerous and normal gastric tissue. |
| Khayatzadeh et al. [26] | Iran | 2015 | Meta-analysis | GC has no relationship with VD level or consumption. |
| Vyas et al. [8] | USA | 2016 | Case-Control | VDD is correlated with a higher risk of gastric adenocarcinoma. |
| Du et al. [27] | China | 2017 | Review | The risk and mortality from gastric cancer are higher in VDD. |
| Yildirim et al. [14] | Turkey | 2017 | Prospective-Observational | VDD is correlated with the failure of Helicobacter pylori elimination. |
| Parizadeh et al. [28] | Iran | 2019 | Review | Ultraviolet B radiation reduces the risk of gastric cancer; VDD increases the risk of mortality in GC. |
| Kwak et al. [29] | Korea | 2020 | Cross-Sectional | VDD is considered a risk factor for GC. |
| Hedayatizadeh-Omran et al. [30] | Iran | 2020 | Case-Control | An increased prevalence of VDD has been found in GC; VDD is more prominent in high-grade GC. |
Genetic role of vitamin D in gastric cancer
Genetic Polymorphism
Vitamin D is a vital hormone synthesized for the adequate functioning of normal and healthy tissues. It performs its function by binding to VDR plus one of the retinoid X receptors (RXR) to produce a complex. The complex translocates inside the cell nucleus and binds to vitamin D response elements (VDRE) to further regulate the transcription of its target genes [31]. The gene encoding VDR is located on chromosome 12q13.1 [32,33]. Several VDR gene polymorphisms are linked to cancers, including colon, breast, ovarian, prostate cancer, and melanoma [34]. The most common VDR gene polymorphisms along with their location are: FokI (rs2228570) in exon2 in the 5’ end of the VDR gene [35], and TaqI (rs731236) in exon9, BsmI (rs544410), and ApaI (rs7975232) in intron 8 of the 3’ end region of the VDR gene [36,37]. Numerous studies have mentioned the association of these polymorphisms with the development and progression of GC.
Cong et al. demonstrated the relationship between the FokI polymorphism of the VDR gene to the risk of developing GC. In the FokI polymorphism, the nucleotide ATG becomes substituted with ACG in the first start codon, where translation begins. The latter results in the allele change from “f” to “F.” The f allele, when compared to F, bears an association with an increased risk of GC, a higher level of c-reactive protein (CRP), and more inadequate GC differentiation, contributing to the poor prognosis produced by GC [17]. Similarly, another study describes a positive correlation between the FokI polymorphism with GC susceptibility (p= 0.021). In contrast, other VDR polymorphisms (BsmI, ApaI, and TaqI) have no significant associations with the risk of GC compared to the healthy groups [24].
However, Parsamanesh et al. found that rather than FokI, the TC genotype of TaqI VDR polymorphism is related to the risk of GC (p = 0.002, OR: 2.39) [20]. Likewise, Durak et al. observed that FokI and TaqI polymorphisms of the VDR did not correlate with the susceptibility of GC (p > 0.05), yet found a higher number of the t allele of the TaqI variant in the advanced stage GC, suggesting a nexus between the t allele and a poorer prognosis for GC. In their research, authors assert that VDD strongly correlates with increased GC susceptibility, but the gene polymorphism of vitamin D binding protein (VDBP; rs7041) does not [21]. In addition, another investigation discovered that the BsmI polymorphism is strongly related to GC development, particularly in patients with a high BMI, yet ApaI and TaqI are not. Additionally, ApaI, TaqI, and BsmI variants of the VDR gene markedly limit GC survival [23].
Epigenetics
Calcagno et al. describe the effect of vitamin D on epigenetics. Epigenetics refers to a heritable trait that induces alteration in gene expression without changing the DNA sequence [38]. One of the unique epigenetic mechanisms is histone post-translational alterations. Histone acetylation and DNA methylation have a significant impact on cancer prognosis and treatment results. Histone acetylation results from a dynamic equilibrium between histone acetyltransferases (HATs) and deacetylases (HDACs). Histone acetylation causes gene activation, whereas its deacetylation leads to the silencing of genes. HDAC expression is high in GC, causing a lower expression of tumor suppressor genes (TSGs). Interestingly, vitamin D activates HATs and boosts the expression of TSGs, serving as an anti-cancer agent by modifying epigenetic pathways, which VDRs usually regulate in GC [2].
Pan et al. established the relation between vitamin D and the TSG phosphatase and tensin homolog deleted on chromosome 10 (PTEN). Vitamin D enhances GC cell death by triggering PTEN upregulation through VDR-mediated suppression of the PTEN promoter methylation. VDR synergistically stimulated PTEN overexpression with transcription factors like early growth response gene-1 (Egr-1) and HAT (P300). Additionally, vitamin D amplifies PTEN expression and accelerates apoptosis in GC cells, particularly when accompanied by epigenetic modifiers like HDAC inhibitors (trichostatin A/TSA and sodium butyrate) and DNA methylation inhibitors (5-aza-2’deoxycytidine/5aza). These data suggest that vitamin D coupled with epigenetic modifiers could benefit patients with GC as a promising treatment modality [15].
According to Zhao et al., cancer pathogenesis involves the blockage of several TSGs due to their promoter's methylation. Bone morphogenetic protein 3 (BMP3) is a known TSG, downregulated and expressed in low quantities in GC, owing to promoter hypermethylation. Moreover, VDRE is present in the methylation region of the BMP3 gene promoter. Vitamin D, along with its receptor, is found to bind to these VDREs (p= <0.05), inhibiting BMP3 promoter methylation and increasing the expression of the TSGs in gastric cancer cells. The apparent strength in association suggests the relation between vitamin D and the BMP3 gene, plus the anti-cancer effect of vitamin D in GC via suppression of BMP3 methylation [19].
Vitamin D regulation and cancer progression
Vitamin D has been related to cancer through many signaling mechanisms [39]. The hedgehog (Hh) signaling system has been associated with the advancement of many cancers, including GC [40]. An experimental investigation uncovered a relationship between vitamin D and the Hh signaling system, promoting the survival of GC cells. GC cells treated with vitamin D lowered Hh signaling target genes, signifying that the vitamin can weaken the GC cell survival. Furthermore, vitamin D acts synergistically with anti-cancer drugs such as Adriamycin®, paclitaxel, and vinblastine, thereby increasing the survival of GC patients [16].
Vitamin D regulates several genes, including micro RNAs (miRNA), which play a significant role in cancer development [41-43] and anti-tumor activities [44,45]. miRNAs are chief regulators of messenger RNA (mRNA) and can potentially modify the cell cycle, cell proliferation, cell invasion, and apoptosis. Alteration of specific miRNA can lead to cancer development, partially due to their behavior as oncogenes or TSGs in cancer cells. GC shows lower miRNA-145 (miR-145) than normal gastric tissue [46]. Chang et al. describe vitamin D's effect on miR-145 expression in GC cells. The authors observed that vitamin D promoted miR-145 expression, resulting in expanding cells in the S-phase and decreasing cells in the G2/M-phase of the cell cycle, explaining that miR-145 prevented the S to G2 transition in GC cells in vitro. Vitamin D also inhibits E2F transcription factor 3 (E2F3) found to be upregulated in GC, cyclin-dependent kinase 6 (CDK6), prime targets of miR-145, and the subsequent E2F3 regulated cell cycle genes such as cyclin-dependent kinase2 (CDK2) and cyclinA2 (CCNA2). As a result, vitamin D can exert its anti-cancer activity via overexpression of miR-145 [18].
Chang et al. have additionally revealed another vitamin D-regulated miRNA (miR-99b-3p). They discovered that like miR-145, miR-99b-3p was reduced in GC cells and that VDR increased miR-99b-3p expression in GC cells. Unlike miR-145, vitamin D and miR-99b-3p inhibit homeobox D3 (HOXD3) protein, primarily expressed in GC cells. In contrast to miR-145, miR-99b-3p increases cells in the G1 phase and reduces them in the S phase, implying that miRNA-99b-3p prevents the G1-S transition in the GC cell cycle [22]. VDR-mediated miRNA regulation appears to be one of the critical mechanisms in vitamin D's antiproliferative activity based on the studies mentioned above. Figure 2 illustrates the anti-tumor action of vitamin D.
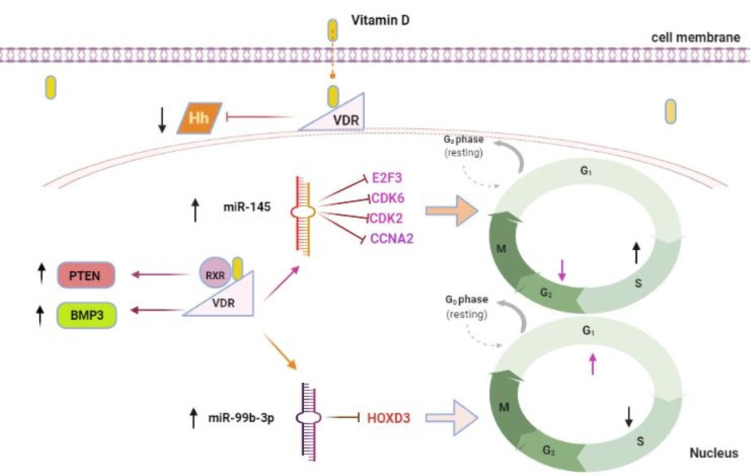
Figure 2. The anti-cancer effect of vitamin D is mediated by the vitamin D receptor.
Vitamin D upregulates the tumor suppressor genes (PTEN and BMP3), causing GC cell death. It blocks the Hh signaling system, reducing GC cell viability. Vitamin D increases the expression of miR-145 and inhibits E2F3, CDK6, and the subsequent cell cycle regulatory genes CDK2 and CCNA2, causing the arrest of the S to G2 transition of the GC cell cycle. Additionally, vitamin D inhibits HOXD3 and upregulates the miR-99b-3p expression resulting in the cessation of G1 to S transition of the GC cell cycle.
VDR: vitamin D receptor; GC: gastric cancer; RXR: retinoid X receptor; PTEN: phosphatase and tensin homolog deleted on chromosome 10; BMP3: bone morphogenetic protein 3; Hh: hedgehog; E2F3: E2F transcription factor3; CDK: cyclin-dependent kinase; CCNA2: cyclinA2; HOXD3: homeobox D3; miR: miRNA.
[Original illustration]
Vitamin D deficiency and GC association
Several solid tumors (particularly the stomach, colon, liver and gallbladder, pancreatic, lung, female breast, prostate, bladder, and kidney cancers) appear to be reduced by adequate vitamin D levels [47]. Ren et al. measured vitamin D in GC and discovered that 8.1% of patients had sufficient levels (>75nmol/L), 34% had inadequate levels (50-75 nmol/L), and 57.9% had deficient levels (50nmol/L). Vitamin D level was also inversely related to tumor staging and lymph node metastasis, while tumor size, position, differentiation, and distant metastasis had no significant relationship. Furthermore, the five-year survival rate was 57.8% in the group with high vitamin D levels and 43% in the group with low vitamin D levels; vitamin D levels were indicated to be an independent prognostic factor and were comparable to bad prognosis in GC [10].
As VDR mediates vitamin D activity, Wen et al. evaluated the expression of VDR in normal, precancerous, and cancerous gastric tissues. They compared it with the clinicopathological characteristics of GC patients; They found VDR expression was markedly reduced in GC tissues versus healthy and precancerous tissues; secondly, well and moderately differentiated tissues demonstrated profound VDR expression in contrast with the poorly differentiated ones. Finally, VDR was expressed in large quantities in smaller gastric tumors than larger ones [25].
Vyas et al. concluded that the prevalence of VDD in patients with gastric adenocarcinoma was significantly higher when compared to patients with normal vitamin D levels (83.7 % vs. 63.27 %), suggesting a significant relationship between VDD and gastric adenocarcinoma. However, they declared no association between the degree of deficiency and the staging of GC like the above study [8].
To further understand the impact of vitamin D in GC, various studies with distinctive designs have been performed. The majority of ecological investigations using ultraviolet B exposure (UVB) reduced the incidence and mortality of GC [27,28]. A recent study by Kwak et al. discovered that patients with low vitamin D levels have a higher predilection for developing GC. They observed that the odds ratio (OR) for GC was 0.52 (95% CI: 0.30, 0.92) in the arm with increased total vitamin D levels (≤ 20ng/mL), compared to the decreased total vitamin D levels (<12ng/mL) with a p-value of 0.030; This signifies that higher vitamin D levels have a lower prevalence of GC and vice versa [29].
Hedayatizadeh et al. noticed a higher prevalence of VDD in GC cases than controls, with an evident decline in vitamin D concentration in high-grade GC cases. However, no correlations were found between lymph node metastasis, distant metastasis, tumor site, and vitamin D concentration [30]. On the contrary, in a meta-analysis conducted by Khayatzadeh et al., no significant relationship between vitamin D intake/serum vitamin D status and GC development was elucidated [26]. Consistent with this, a few more further studies revealed no correlation between vitamin D level and risk of GC [9,48].
The function of vitamin D in the immune system is widely understood. Numerous investigations have established the relation between VDD and infectious diseases [49, 50]. Yildirim et al. disclosed the relationship between the failure of eliminating HP in vitamin D deficient patients. In their study of 220 patients, elimination was achieved in 170 patients (77.2%), and 50 patients (22.7%) had a failure, with mean vitamin D levels substantially lower in the elimination failure group than in the successful treatment group (9.13 ± 4.7 vs. 19.03 ± 8.13; p= 0.001) [14]. A recent study that supports this notion was published by Yang et al., including results that patients with VDD had a slower elimination rate of HP (OR=0.09; 95%CI=0.2,0.4). Moreover, they reported that the average vitamin D level was higher in HP-negative individuals than the HP-positive individuals [13]. Based on the above findings, VDD could be a potential cause for the failure to eliminate HP, and adequate levels of vitamin D might be beneficial for the effective elimination of the infection.
On the other hand, Bao et al. reported the synergistic action of vitamin D and anti-cancer drug, cisplatin, against GC. Vitamin D amplified the effect of cisplatin by upregulating the pro-apoptotic protein like BCL2-associated X protein (Bax), enhancing cell cycle regulators such as p21 and p27 [51], and reducing the phosphorylation of phosphatidylinositol 3-kinase/AKT and extracellular-signal-related kinase 1/ERK, kinases implicated in GC cell proliferation and apoptosis [52,53]. Vitamin D potentiates the anti-cancer action of cisplatin by controlling cell proliferation, promoting apoptosis, and arresting GC cells in the G0/G1 phase of the cell cycle [7].
Limitation
Certain limitations exist in our study as the data was gathered only from one database (PubMed); only studies published in English were selected; studies with free full text and pertinent abstracts were solely included, and most of the collected studies were only in vitro studies.
Conclusions
Several studies have been conducted to explore the relationship between vitamin D and GC. We observed a variety of correlations between vitamin D and GC. In most studies, GC patients have shown an increased prevalence of VDD, although few studies on VDD prevalence in GC patients are paradoxical. UVB radiation has been shown in most ecological studies to lessen the incidence and mortality of GC. An adequate vitamin D level has been associated with an increase in the survival rate of GC patients, and a low vitamin D level can be considered as a poor prognostic factor in GC.
Furthermore, variations in the VDR gene have been attributed to an increased risk of various malignancies, including GC. Many VDR gene polymorphisms are associated with the risk of GC. However, various research on VDR gene polymorphisms and GC risk have yielded inconsistent results.
Vitamin D exerts an anti-cancer effect by different mechanisms, such as regulating epigenetic pathways, upregulating the expression of miRNAs, boosting the action of cisplatin, stimulating TSGs, and regulating intracellular signal transduction. We also found that serum vitamin D is lower in the HP-positive patients than negative ones, and vitamin D deficient patients fail to eliminate HP. Based on the facts presented above, we may conclude that vitamin D is a protective factor in GC. It can be utilized as a promising technique to treat GC and increase survival rates by correcting the deficiency of vitamin D. However, additional studies are required to fully assess the genetic association of VDR in GC for a more profound knowledge of how to diagnose and treat the aggressive malignancy early and effectively to maximize the survival outcomes.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. Fitzmaurice C, Allen C, Barber RM, et al. JAMA Oncol. 2017;3:524–548. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Role of histone acetylation in gastric cancer: implications of dietetic compounds and clinical perspectives. Calcagno DQ, Wisnieski F, Mota ER, et al. Epigenomics. 2019;11:349–362. doi: 10.2217/epi-2018-0081. [DOI] [PubMed] [Google Scholar]
- 3.Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Ferlay J, Soerjomataram I, Dikshit R, et al. Int J Cancer. 2015;136:0–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 4.Gastric cancer epidemiology and risk factors. Guggenheim DE, Shah MA. J Surg Oncol. 2013;107:230–236. doi: 10.1002/jso.23262. [DOI] [PubMed] [Google Scholar]
- 5.Manganese superoxide dismutase (MnSOD Val-9Ala) gene polymorphism and susceptibility to gastric cancer. Moradi MT, Yari K, Rahimi Z, Kazemi E, Shahbazi M. Asian Pac J Cancer Prev. 2015;16:485–488. doi: 10.7314/apjcp.2015.16.2.485. [DOI] [PubMed] [Google Scholar]
- 6.Vitamin D and GI cancers: shedding some light on dark diseases. Hargrove L, Francis T, Francis H. Ann Transl Med. 2014;2:9. doi: 10.3978/j.issn.2305-5839.2013.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.1,25-Dihydroxyvitamin D₃ and cisplatin synergistically induce apoptosis and cell cycle arrest in gastric cancer cells. Bao A, Li Y, Tong Y, Zheng H, Wu W, Wei C. Int J Mol Med. 2014;33:1177–1184. doi: 10.3892/ijmm.2014.1664. [DOI] [PubMed] [Google Scholar]
- 8.Association between serum vitamin D levels and gastric cancer: a retrospective chart analysis. Vyas N, Companioni RC, Tiba M, et al. World J Gastrointest Oncol. 2016;8:688–694. doi: 10.4251/wjgo.v8.i9.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prospective study of serum 25(OH)-vitamin D concentration and risk of oesophageal and gastric cancers. Chen W, Dawsey SM, Qiao YL, et al. Br J Cancer. 2007;97:123–128. doi: 10.1038/sj.bjc.6603834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prognostic effects of 25-hydroxyvitamin D levels in gastric cancer. Ren C, Qiu MZ, Wang DS, et al. J Transl Med. 2012;10:16. doi: 10.1186/1479-5876-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.1alpha,25-dihydroxyvitamin D3 (Calcitriol) inhibits hypoxia-inducible factor-1/vascular endothelial growth factor pathway in human cancer cells. Ben-Shoshan M, Amir S, Dang DT, Dang LH, Weisman Y, Mabjeesh NJ. Mol Cancer Ther. 2007;6:1433–1439. doi: 10.1158/1535-7163.MCT-06-0677. [DOI] [PubMed] [Google Scholar]
- 12.The role of vitamin D in reducing cancer risk and progression. Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. Nat Rev Cancer. 2014;14:342–357. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- 13.Effect of vitamin D on Helicobacter pylori infection and eradication: a meta-analysis. Yang L, He X, Li L, Lu C. Helicobacter. 2019;24:0. doi: 10.1111/hel.12655. [DOI] [PubMed] [Google Scholar]
- 14.The influence of vitamin D deficiency on eradication rates of Helicobacter pylori. Yildirim O, Yildirim T, Seckin Y, Osanmaz P, Bilgic Y, Mete R. Adv Clin Exp Med. 2017;26:1377–1381. doi: 10.17219/acem/65430. [DOI] [PubMed] [Google Scholar]
- 15.Vitamin D stimulates apoptosis in gastric cancer cells in synergy with trichostatin A /sodium butyrate-induced and 5-aza-2'-deoxycytidine-induced PTEN upregulation. Pan L, Matloob AF, Du J, et al. FEBS J. 2010;277:989–999. doi: 10.1111/j.1742-4658.2009.07542.x. [DOI] [PubMed] [Google Scholar]
- 16.Vitamin D3 regulates cell viability in gastric cancer and cholangiocarcinoma. Baek S, Lee YS, Shim HE, Yoon S, Baek SY, Kim BS, Oh SO. Anat Cell Biol. 2011;44:204–209. doi: 10.5115/acb.2011.44.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.FokI polymorphism of the vitamin D receptor gene is associated with susceptibility to gastric cancer: a case-control study. Cong L, Wang WB, Liu Q, Du JJ. Tohoku J Exp Med. 2015;236:219–224. doi: 10.1620/tjem.236.219. [DOI] [PubMed] [Google Scholar]
- 18.miR-145 mediates the antiproliferative and gene regulatory effects of vitamin D3 by directly targeting E2F3 in gastric cancer cells. Chang S, Gao L, Yang Y, et al. Oncotarget. 2015;6:7675–7685. doi: 10.18632/oncotarget.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.1,25-Dihydroxyvitamin D3 affects gastric cancer progression by repressing BMP3 promoter methylation. Zhao Y, Cai LL, Wang HL, et al. Onco Targets Ther. 2019;12:2343–2353. doi: 10.2147/OTT.S195642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Positive correlation between vitamin D receptor gene TaqI variant and gastric cancer predisposition in a sample of Iranian population. Parsamanesh N, Moossavi M, Tavakkoli T, Javdani H, Fakharian T, Moossavi SZ, Naseri M. J Cell Physiol. 2019;234:15044–15047. doi: 10.1002/jcp.28145. [DOI] [PubMed] [Google Scholar]
- 21.The effects of serum levels, and alterations in the genes of binding protein and receptor of vitamin D on gastric cancer. Durak Ş, Gheybi A, Demirkol Ş, et al. Mol Biol Rep. 2019;46:6413–6420. doi: 10.1007/s11033-019-05088-9. [DOI] [PubMed] [Google Scholar]
- 22.miR-99b-3p is induced by vitamin D3 and contributes to its antiproliferative effects in gastric cancer cells by targeting HoxD3. Chang S, Gao Z, Yang Y, et al. Biol Chem. 2019;400:1079–1086. doi: 10.1515/hsz-2019-0102. [DOI] [PubMed] [Google Scholar]
- 23.Association of Vitamin D receptor gene variations with gastric cancer risk in Kashmiri population. Qadir J, Majid S, Khan MS, Wani MD. Mol Biol Rep. 2021;48:3313–3325. doi: 10.1007/s11033-021-06376-z. [DOI] [PubMed] [Google Scholar]
- 24.Association of vitamin D receptor polymorphisms (FokI (Rs2228570), ApaI (Rs7975232), BsmI (Rs1544410), and TaqI (Rs731236)) with gastric cancer in a Kurdish population from west of Iran. Hoseinkhani Z, Rastegari-Pouyani M, Tajemiri F, Yari K, Mansouri K. Rep Biochem Mol Biol. 2021;9:435–441. doi: 10.52547/rbmb.9.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alterations in vitamin D signaling pathway in gastric cancer progression: a study of vitamin D receptor expression in human normal, premalignant, and malignant gastric tissue. Wen Y, Da M, Zhang Y, Peng L, Yao J, Duan Y. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4680461/ Int J Clin Exp Pathol. 2015;8:13176–13184. [PMC free article] [PubMed] [Google Scholar]
- 26.Vitamin D intake, serum Vitamin D levels, and risk of gastric cancer: a systematic review and meta-analysis. Khayatzadeh S, Feizi A, Saneei P, Esmaillzadeh A. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4652314/ J Res Med Sci. 2015;20:790–796. doi: 10.4103/1735-1995.168404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pathogenic roles of alterations in vitamin D and vitamin D receptor in gastric tumorigenesis. Du C, Yang S, Zhao X, Dong H. Oncotarget. 2017;8:29474–29486. doi: 10.18632/oncotarget.15298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The relationship between vitamin D status and risk of gastric cancer. Parizadeh SM, Ghandehari M, Jafarzadeh-Esfehani R, et al. https://pubmed.ncbi.nlm.nih.gov/31272234/ Nutr Cancer. 2020;72:15–23. doi: 10.1080/01635581.2019.1616779. [DOI] [PubMed] [Google Scholar]
- 29.Vitamin D status and gastric cancer: a cross-sectional study in Koreans. Kwak JH, Paik JK. Nutrients. 2020;12:2004. doi: 10.3390/nu12072004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Association between pre-chemotherapy serum levels of vitamin D and clinicopathologic findings in gastric cancer. Hedayatizadeh-Omran A, Janbabaei G, Alizadeh-Navaei R, Amjadi O, Mahdavi Izadi J, Omrani-Nava V. Caspian J Intern Med. 2020;11:290–294. doi: 10.22088/cjim.11.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)₂vitamin D₃: genomic and non-genomic mechanisms. Haussler MR, Jurutka PW, Mizwicki M, Norman AW. Best Pract Res Clin Endocrinol Metab. 2011;25:543–559. doi: 10.1016/j.beem.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Association of vitamin-D receptor (Fok-I) gene polymorphism with bladder cancer in an Indian population. Mittal RD, Manchanda PK, Bhat S, Bid HK. BJU Int. 2007;99:933–937. doi: 10.1111/j.1464-410X.2007.06657.x. [DOI] [PubMed] [Google Scholar]
- 33.Molecular epidemiology of vitamin D receptor gene variants. Zmuda JM, Cauley JA, Ferrell RE. Epidemiol Rev. 2000;22:203–217. doi: 10.1093/oxfordjournals.epirev.a018033. [DOI] [PubMed] [Google Scholar]
- 34.Vitamin D receptor polymorphism and cancer: an update. Rai V, Abdo J, Agrawal S, Agrawal DK. Anticancer Res. 2017;37:3991–4003. doi: 10.21873/anticanres.11784. [DOI] [PubMed] [Google Scholar]
- 35.The role of vitamin D in the carcinogenesis of breast and ovarian cancer [Article in Polish] Walentowicz-Sadłecka M, Sadłecki P, Walentowicz P, Grabiec M. Ginekol Pol. 2013;84:305–308. doi: 10.17772/gp/1581. [DOI] [PubMed] [Google Scholar]
- 36.Vitamin D receptor gene polymorphisms and breast cancer risk among postmenopausal Egyptian women. Abd-Elsalam EA, Ismaeil NA, Abd-Alsalam HS. Tumour Biol. 2015;36:6425–6431. doi: 10.1007/s13277-015-3332-3. [DOI] [PubMed] [Google Scholar]
- 37.Polymorphisms in the vitamin D receptor gene and bone mass, bone turnover and osteoporotic fractures. Langdahl BL, Gravholt CH, Brixen K, Eriksen EF. Eur J Clin Invest. 2000;30:608–617. doi: 10.1046/j.1365-2362.2000.00686.x. [DOI] [PubMed] [Google Scholar]
- 38.What do you mean, "epigenetic"? Deans C, Maggert KA. Genetics. 2015;199:887–896. doi: 10.1534/genetics.114.173492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Deeb KK, Trump DL, Johnson CS. Nat Rev Cancer. 2007;7:684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 40.Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Berman DM, Karhadkar SS, Maitra A, et al. Nature. 2003;425:846–851. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- 41.Vitamin D manipulates miR-181c, miR-20b and miR-15a in human umbilical vein endothelial cells exposed to a diabetic-like environment. Zitman-Gal T, Green J, Pasmanik-Chor M, Golan E, Bernheim J, Benchetrit S. Cardiovasc Diabetol. 2014;13:8. doi: 10.1186/1475-2840-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Downregulation of miR-302c and miR-520c by 1,25(OH)2D3 treatment enhances the susceptibility of tumour cells to natural killer cell-mediated cytotoxicity. Min D, Lv XB, Wang X, Zhang B, Meng W, Yu F, Hu H. Br J Cancer. 2013;109:723–730. doi: 10.1038/bjc.2013.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MicroRNA-627 mediates the epigenetic mechanisms of vitamin D to suppress proliferation of human colorectal cancer cells and growth of xenograft tumors in mice. Padi SK, Zhang Q, Rustum YM, Morrison C, Guo B. Gastroenterology. 2013;145:437–446. doi: 10.1053/j.gastro.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Identification of microRNA-98 as a therapeutic target inhibiting prostate cancer growth and a biomarker induced by vitamin D. Ting HJ, Messing J, Yasmin-Karim S, Lee YF. J Biol Chem. 2013;288:1–9. doi: 10.1074/jbc.M112.395947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MicroRNA-22 is induced by vitamin D and contributes to its antiproliferative, antimigratory and gene regulatory effects in colon cancer cells. Alvarez-Díaz S, Valle N, Ferrer-Mayorga G, et al. Hum Mol Genet. 2012;21:2157–2165. doi: 10.1093/hmg/dds031. [DOI] [PubMed] [Google Scholar]
- 46.MicroRNA profiling of human gastric cancer. Yao Y, Suo AL, Li ZF, et al. Mol Med Rep. 2009;2:963–970. doi: 10.3892/mmr_00000199. [DOI] [PubMed] [Google Scholar]
- 47.Lower vitamin-D production from solar ultraviolet-B irradiance may explain some differences in cancer survival rates. Grant WB. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2576130/ J Natl Med Assoc. 2006;98:357–364. [PMC free article] [PubMed] [Google Scholar]
- 48.Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, Willett WC. J Natl Cancer Inst. 2006;98:451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 49.Vitamin D deficiency is associated with community-acquired clostridium difficile infection: a case-control study. Sahay T, Ananthakrishnan AN. BMC Infect Dis. 2014;14:661. doi: 10.1186/s12879-014-0661-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Variations in vitamin D production could possibly explain the seasonality of childhood respiratory infections in Hawaii. Grant WB. Pediatr Infect Dis J. 2008;27:853. doi: 10.1097/INF.0b013e3181817bc1. [DOI] [PubMed] [Google Scholar]
- 51.Disorder-function relationships for the cell cycle regulatory proteins p21 and p27. Mitrea DM, Yoon MK, Ou L, Kriwacki RW. Biol Chem. 2012;393:259–274. doi: 10.1515/hsz-2011-0254. [DOI] [PubMed] [Google Scholar]
- 52.Targeting AKT protein kinase in gastric cancer. Almhanna K, Strosberg J, Malafa M. https://ar.iiarjournals.org/content/31/12/4387.long. Anticancer Res. 2011;31:4387–4392. [PubMed] [Google Scholar]
- 53.ERK inhibition enhances TSA-induced gastric cancer cell apoptosis via NF-κB-dependent and Notch-independent mechanism. Yao J, Qian CJ, Ye B, Zhang X, Liang Y. Life Sci. 2012;91:186–193. doi: 10.1016/j.lfs.2012.06.034. [DOI] [PubMed] [Google Scholar]