Abstract
Impaired bone healing occurs in 5–10% of cases following injury, leading to a significant economic and clinical impact. While an inflammatory response upon injury is necessary to facilitate healing, its resolution is critical for bone tissue repair as elevated acute or chronic inflammation is associated with impaired healing in patients and animal models. This process is governed by important crosstalk between immune cells through mediators that contribute to resolution of inflammation in the local healing environment. Approaches modulating the initial inflammatory phase followed by its resolution leads to a pro-regenerative environment for bone regeneration. In this review, we discuss the role of inflammation in bone repair, the negative impact of dysregulated inflammation on bone tissue regeneration, and how timely resolution of inflammation is necessary to achieve normal healing. We will discuss applications of biomaterials to treat large bone defects with a specific focus on resolution of inflammation to modulate the immune environment following bone injury, and their observed functional benefits. We conclude the review by discussing future strategies that could lead to the realization of anti-inflammatory therapeutics for bone tissue repair.
Keywords: Resolution of inflammation, biomaterials, bone healing, drug delivery, immunomodulation
1. Introduction
Bone has a strong intrinsic healing capability, however, 5–10% of injuries result in impaired healing such as delayed union, malunion, or nonunion [1]. Bone healing is a topic of great clinical and economic importance. Depending on the location and cues from the microenvironment, bone tissue healing occurs by two biological processes– primary (direct) healing or secondary (indirect) healing [2]. Flat bones, such as cranial bones, form by direct healing that undergoes intramembranous ossification which involves direct conversion of progenitor cells to osteoblasts that lay down new bone matrix [3]. On the other hand, fracture healing of long bone tissues mostly involves indirect healing that undergoes both intramembranous and endochondral ossification, especially in non-stable conditions (i.e., lacking rigidly stable fixation). In this type of healing, progenitor cells along the periosteal and endosteal surfaces undergo intramembranous ossification, while in the fracture gap, where there is motion, the progenitor cells contribute to bone formation through endochondral ossification. The endochondral ossification involves a cartilaginous intermediate phase that serves as a template for new bone formation. The chondrocytes in the cartilaginous template undergo a series of sequential phenotypic and functional changes, which are tightly regulated by both systemic and locally secreted factors that act upon receptors to affect intracellular signaling and cellular commitments in a spatiotemporal manner. For a comprehensive review on the biology of fracture repair, see Bahney et al. [4].
Regardless of the location and type of injury, bone tissue regeneration undergoes three continuing and overlapping phases: inflammation, regeneration, and remodeling [2]. While the inflammatory process is crucial for tissue repair, dysregulated inflammation, including either decreased or elevated levels, is detrimental to bone healing [5–9]. Similarly, the duration of inflammation is also crucial for normal tissue repair. During normal bone repair, inflammation is initiated after injury and resolved almost immediately to stimulate a pro-regenerative environment enriched with pro-osteogenic and pro-angiogenic factors, and relevant cell populations to ensure normal tissue repair and optimal bone regeneration [4]. Similar to the onset of inflammation, the resolution of inflammation is also coordinated by a plethora of molecular mediators which includes proteins, lipids, and purine molecules, as well as synchronized cellular events involving different cell populations [10–13]. The pro-osteogenic and pro-angiogenic molecules have also been shown to regulate immune cell phenotype and function, thus potentially impacting bone healing by contributing to the immune environment as well [14–19]. Note that the resolution of inflammation is different to anti-inflammation. Resolution of inflammation indicates an inherent activation of events leading to apoptosis and efferocytosis at the end of the acute inflammatory phase whereas anti-inflammation mainly refers to inhibition of inflammatory signaling. Injuries and diseases that produce a heightened inflammatory state hinder the pro-regenerative environment and thereby the bone tissue repair [8, 20–25]. Specifically, impaired healing is observed when massive tissue damage occurs with excessive acute inflammation such as in the case of severe fractures, polytrauma, and concomitant muscle trauma [8, 22, 24, 25], or in pre-existing health conditions with chronic inflammation such as diabetes and rheumatoid arthritis [21–23]. Delayed healing is also a hallmark of aging [20] and compromised bone tissue regeneration with aging is very well established both in animal models and clinically in humans [26]. While several factors have been identified to be responsible for the altered tissue regeneration with aging, emerging studies show the potential role played by this activated inflammatory stage (termed as “inflammaging”) on impaired bone tissue regeneration.
The fundamental understanding of inflammation and tissue healing shows how a spatiotemporal and contextual regulation of inflammatory phases is necessary for normal tissue repair and regeneration of bone tissues with functions similar to native tissue. Recent advancements suggest that resolution of inflammation following the pro-inflammatory phase could be an effective therapeutic strategy to improve bone tissue regeneration. An active strategy to resolve inflammation not only prevents progression of acute inflammation into a persistent chronic inflammation stage but also generate a pro-regenerative environment. Biomaterial-assisted delivery of biomolecules is an integral strategy to these efforts, especially in regard to local intervention following bone injury. The intrinsic properties of the biomaterial itself can have an effect on inflammation and its regulation. In bone tissue regeneration, biomaterials have been used as scaffolds/bone grafts for neo-tissue formation, and/or as carriers of therapeutic molecules to modulate various pathways relevant to bone tissue formation, such as resolution of inflammation, angiogenesis and osteogenesis, to promote neo-tissue formation resulting in bone tissue repair.
The therapeutic molecules that target inflammation include proteins, small molecules or nucleic acids [27]; not to mention, delivery of cells such as bone-marrow derived mesenchymal progenitor (MSCs) that have also been shown to reduce the inflammatory response [28]. In this review, we will discuss the role of inflammation and how its timely resolution is necessary for normal tissue healing with a focus on bone tissue repair. We further discuss the application of biomaterials to treat bone defects that modulate the immune environment to resolve inflammation and their observed functional benefits. We conclude the review by discussing future strategies that may improve anti-inflammatory therapeutics for bone tissue repair.
2. Inflammation and its resolution in bone healing
2.1. Acute inflammatory response in bone healing
Inflammation is a universal response despite the type and location of injury and is an indispensable process during wound healing. The inflammatory phase includes the initial inflammatory response and its resolution characterized by immune cell infiltration, clearance of cellular debris, and secretion of cytokines and growth factors that enable tissue repair [29]. Immediately after injury, the rupture of blood vessels causes formation of a hematoma enriched with fibrin and serves as an initial frame for healing [30]. This process initiates the inflammatory phase where chemokines and inflammatory cytokines are released from the hematoma during platelet degranulation and recruitment of inflammatory cells [31]. For example, the C-C motif chemokine ligand 2 (CCL2) cytokine, also known as monocyte chemoattractant protein-1 (MCP-1), released from the platelets recruits monocytes and neutrophils [32]. The recruited neutrophils and monocytes release additional chemokines to recruit other cell types such as lymphocytes and eosinophils to the fracture site [31]. A myriad of cytokines is present at the fracture environment, however, whether they are indispensable or play a particular role in various phases of inflammation is less understood [33]. The monocytes within this environment differentiate into classically activated pro-inflammatory M1-like macrophages in response to the pro-inflammatory chemokines like interferon gamma (IFN-gamma) [34] and their numbers are found to increase over the first 7 days following fracture in a mouse model [35]. These macrophages contribute to a pro-inflammatory milieu enriched with cytokines such as IL-1, IL-6, and Tumor Necrosis Factor (TNF) alpha [36]. IL-1 regulates expression of Cox-1 and Cox-2 (cyclooxygenases) that synthesize inflammatory prostaglandins (PGs) such as PGE2 from arachidonic acid (AA), an omega-6 fatty acid [12]. On the other hand, depletion of M1 macrophages showed significantly reduced cytokines including IL-6, TNF-α, and IP-10, and impaired fracture healing [37]. These studies demonstrate that M1 macrophages and the pro-inflammatory phase are important for fracture healing.
2.2. Resolution of inflammation for normal bone repair
The process of resolution is the latter part of the inflammatory phase that is orchestrated by a variety of immune cells and different anti-inflammatory mediators such as cytokines/growth factors, lipids, and purine molecules [13]. In the context of bone healing, studies have shown that resolution of inflammation is driven mainly by the alternatively activated anti-inflammatory (M2) macrophages, regulatory T cells (Treg), and T helper 2 (Th2) cells [12, 38–40], of which the M2 macrophages have attracted the most attention (Figure 1). However, this process is initiated by cells and molecules that promote inflammation. Although neutrophils are first responders of the immune system that infiltrate the injury site and contribute to the active phase of inflammation, they also contribute to the onset of resolution of inflammation by secreting pro-resolving cytokines such as lipoxins and resolvins [41], and proteases that degrade the ECM and downregulate inflammatory cytokines such as IL-1β and TNF-α [42]. Similarly, the cytokines that are produced during the inflammatory phase and participate in the inflammatory process also contribute to the resolution of inflammation. For example, interleukin-6 (IL-6) produced at the site of inflammation, with a key role in the acute inflammatory phase, has also been recognized to contribute to resolution of inflammation. Treatment of macrophages undergoing M2 polarization with IL-6 significantly enhanced expression of M2 markers [43]. IL-6 derived from antigen-presenting cells (APCs) has been shown to induce anti-inflammatory IL-4 production in naive CD4+ T cells and thereby polarizes them to Th2 cells [44] and also promotes secretion of IL-10 [45]. Thus, many of the well-recognized pro-inflammatory agents themselves kick-start the resolution of inflammation and create a pro-regenerative environment.
Figure 1.
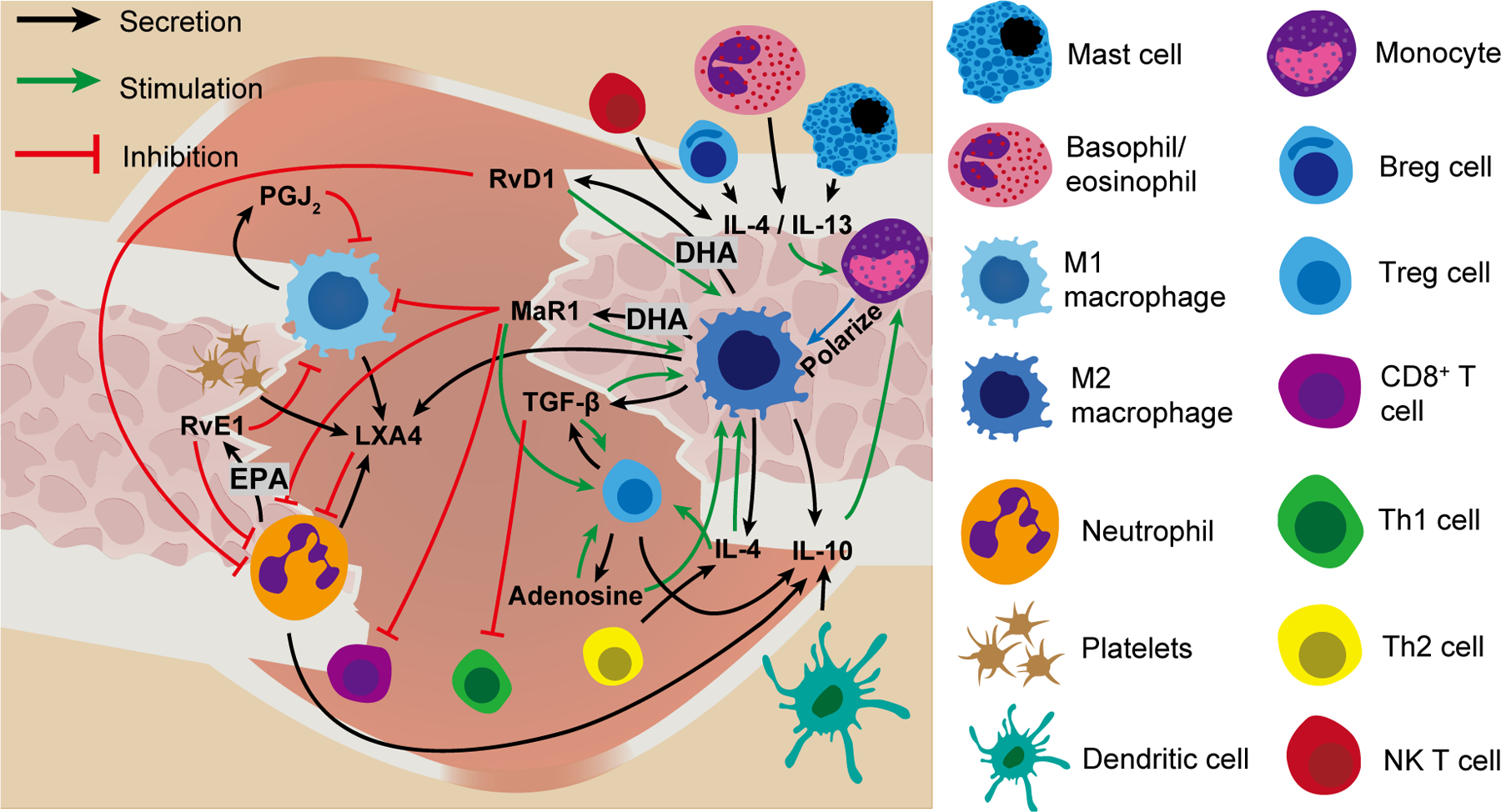
Illustration of proposed immune cell and molecule interactions that contribute to resolution of inflammation during bone healing. DHA: docosahexaenoic acid, EPA: eicosapentaenoic acid, RvD1: resolving D1, PGJ2: prostaglandin J2, MaR1: maresin 1, RvE1: resolvin E1, LXA4: lipoxin A4, TGF-β: transforming growth factor-beta, IL-4: interleukin-4, IL-10: interleukin-10, IL-13: interleukin-13, Breg: Regulatory B cell, Treg: regulatoru T cell, Th1: T helper 1. Th2: T helper 2.
The anti-inflammatory cytokine IL-10 has been shown to play a key role in resolution of inflammation and bone healing. IL-10 is secreted by a repertoire of immune cells including neutrophils, macrophages, dendritic cells, Th2, and Tregs [46] and induces polarization of macrophages to an anti-inflammatory state [47]. In recombination activating gene 1 knockout (RAG1−/−) mice lacking an adaptive immune system, the fracture repair was found to be accelerated and is correlated with an upregulation of IL-10 [48]. In addition, IL-10 knockout mice show accelerated alveolar bone resorption and diminished bone formation [49]. The prototypical cytokines of the type II (Th2) inflammatory response IL-4 and IL-13—produced by CD4+ T cells, regulatory B cells, basophils, eosinophils, mast cells, and NK T cells [50, 51] also polarize macrophages to the alternatively activated M2 phenotype [47]. Unlike the IL-10 knockout mice which displayed diminished bone formation, the IL-4/IL-13 double KO mice exhibited normal fracture healing [52]. However, the delivery of IL-4 and IL-13 was reported to promote bone regeneration in multiple studies [39, 53–55].
In addition to the above discussed interleukins, growth factors such as TGF-β and IGF-1 also exhibit pleiotropic functions that directly modulate immune cells and elicit a pro-regenerative environment by acting on endothelial cells (contributing to angiogenesis) or osteogenic cells (bone formation). TGF-β proteins— TGF-β1, TGF-β2, and TGF-β3— are stored as latent proteins in the extracellular matrix (ECM) and are activated by various pathways in a context-dependent manner [56]. Storage of inactive TGF-β in the ECM enables its rapid temporal and spatial activation during tissue homeostasis, a property that is distinct to other growth factors or cytokines [56]. Many studies have shown that TGF-β1 and TGF-β3 promote resolution of inflammation [57–59] though they are well known for their direct role on chondrocyte differentiation and osteoblast commitment [60]. Inflammatory cytokines such as IL-6 have been shown to interact with TGF-β and enhance TGF-β1 signaling [61]. The infusion of IL-6 in circulation contributes to increased levels of IL-10 in plasma [62], and its blockade reverses TGF-β1 activation [63]. Using animal models with deletion of TGF-βRI/TGF-βRII or supplementation of TGF-β1, studies have shown that TGF-β induces T cell differentiation to Treg cells, polarization of macrophages to M2 phenotype, suppression of mast cell proliferation, and attenuation of TNF-α expression [14, 58, 64–67]. In addition, TGF-β1 has also been shown to be released by both Treg cells and M2 macrophages [68]. TGF-β1 knockout mice die within 3 weeks following birth due to an increased inflammatory response and tissue necrosis of vital organs [59]. Similarly, the insulin growth factor 1 (IGF-1) which is known to strongly influence bone formation [69], has also been shown to regulate inflammation in various organs including bone [70]. IGF-1 has been shown to increase IL-10 expression in peripheral blood mononuclear cells, which could contribute to an anti-inflammatory environment [71], and promote microglial M2 and inhibit M1 phenotypes in the brain [18]. Furthermore, studies have shown that knockout of IGF-1 impairs M2 macrophage polarization in a mouse model of muscle regeneration [72], and IGF-1-deficient mice exhibit persistent inflammation and associated pathologies in different organs and cells [73].
Lipids are another key molecule involved in resolution of inflammation during bone healing. Specialized pro-resolving mediators (SPMs) are lipid mediators derived from omega-3 fatty acids (docosahexaenoic acid [DHA] and eicosapentaenoic acid [EPA] [51]), or omega-6 fatty acids (arachidonic acid [AA]) [12, 74, 75]. The role of omega-3 fatty acids on bone healing was established by studies involving fat-1 transgenic mice that encode an omega-3 fatty acid desaturase which converts omega-6 to omega-3 fatty acids [76, 77]. Results showed accelerated healing in femoral fracture of transgenic fat-1 mice [77], demonstrating the importance of omega-3 fatty acid, and possibly its metabolites, on bone healing. The EPA–derived Resolvin E1 (RvE1) is one such lipid mediator and is secreted by neutrophils [78]. Local treatment of RvE1 in a cranial defect model accelerated regeneration in both wildtype and ChemR23 overexpressing mice [79]. Similarly, maresin 1 (MaR1), a DHA metabolite secreted by macrophages, acting as an autocrine and paracrine molecule, is shown to decrease macrophage-associated inflammation [80]. Systemic administration of MaR1 in aged mice with tibial fracture significantly decreased levels of IL-6, IL-10, TNF-α, KC, IL-1β, and MCP-1 in the circulation, decreased pro-inflammatory macrophages at the fracture callus, and improved bone healing [81]. The study showed improved bone healing when administration of MaR1 was initiated 3 days following injury, whereas treatment immediately after the injury did not reduce inflammation nor improve the outcome of fracture repair. These findings highlight the importance of timing of intervention on therapeutic benefit when pro-resolving mediators are administered. They demonstrate that a tightly regulated window of inflammatory response is required for normal bone healing. The conflicting and inconclusive results regarding nonsteroidal anti-inflammatory drugs (NSAIDs) that suppress bone healing further highlight the importance of timing and therapeutic window on treatment outcome. NSAIDs are commonly prescribed to manage pain arising from bone injury by inhibiting cyclooxygenase-2 (COX-2) activity, a key enzyme involved in the onset of inflammation. However, they have been shown to increase the rate of delayed union and nonunion in both patients and animals [5, 82]. The negative effect of NSAIDs on healing is most apparent with their long-term use, while short-term use has shown no significant effect [82, 83]. This could be explained by the apparent dual role of COX-2 in the inflammatory process, initially contributing to the onset of inflammation and later helping to resolve the process. NSAIDs are anti-inflammatory drugs by inhibiting the actions of Cox enzymes and production of PGE2 [84], yet they also inhibit the production of anti-inflammatory PGs and SPMs, thus severely disrupting the resolution of inflammation [85, 86].
While most attention is focused on cytokines and SPMs, the purine adenosine is less described yet functions as a key molecule for the resolution of inflammation. Adenosine is a small molecule that exerts multiple functions in bone tissue regeneration such as resolution of inflammation, angiogenesis, and osteogenesis. It is a purine nucleoside that regulates cell function by activating G-protein-coupled adenosine A1, A2A, A2B and A3 receptors on cell membranes [87]. Under normal conditions, adenosine is ubiquitously present and is detected in the extracellular environment at nanomolar concentrations. Following cellular damage or stress, increased levels of adenosine are secreted by different cell types through its intracellular formation and export via nucleoside transporters, or extracellular degradation of adenine nucleotides (ATP, ADP, or AMP). It is released immediately after trauma during the inflammatory phase and serves as a pro-resolving mediator [13]. Adenosine inhibits recruitment and activation of neutrophils and suppresses T cell function [16]. Furthermore, A2A receptor signaling has been shown to be a potent inhibitor of pro-inflammatory M1 macrophage activation by suppressing cytokine and chemokine production while increasing production of the anti-inflammatory cytokine IL-10 [16, 88]. On the other hand, it strongly promotes IL-4- and IL-13-induced M2 macrophage activation through A2A and A2B receptors, as indicated by upregulation of arginase-1 and TIMP-1 [16]. Besides its anti-inflammatory function, extracellular adenosine also plays an important role in angiogenesis, which is crucial for bone regeneration [4]. It has a direct mitogenic effect on endothelial cells and stimulates the production of pro-angiogenic molecules including interleukin-8 (IL-8), basic fibroblast growth factor (bFGF), and vascular endothelial growth factor (VEGF), while inhibiting production of the anti-angiogenic factor thrombospondin in hypoxic tissues [89]. Adenosine also directly promotes osteogenic differentiation of progenitor cells and osteoblasts [90–99]. Extracellular adenosine has been shown to promote proliferation of osteoprogenitors through the A2A receptor [100] and induce osteogenic differentiation of progenitor/stem cells via the A2B receptor [94–96]. In animals, aberrant adenosine signaling has been shown to impair bone healing. For example, mice lacking ecto-5'-nucleotidase CD73, a cell membrane enzyme that generates extracellular adenosine, exhibit delayed bone repair [101]. Similarly, knockout of the adenosine A2A receptor decreased bone formation in a cranial defect model while A2B receptor null animals displayed delayed tibial fracture healing [90, 102].
3. Elevated acute and chronic inflammation impacts bone healing
Although an initial transient inflammatory phase is essential for proper bone healing, injuries with excess acute inflammation and pathological conditions with chronic systemic inflammation negatively impact fracture healing and result in increased rates of delayed healing and nonunion. Severe traumatic injuries such as isolated severe fractures, polytrauma, and concomitant muscle trauma display an elevated acute inflammatory response and compromised fracture healing [103]. Similarly, conditions associated with chronic inflammation such as physiological aging, diabetes, and rheumatoid arthritis also show compromised fracture healing [20, 21, 23]. Despite the distinction between excess acute inflammation and chronic inflammation, their pathologies exhibit similarities and are characterized by high levels of pro-inflammatory cytokines such as TNF-α and NF-kB signaling [8, 104, 105]. Antibody-based therapeutic interventions such as those against TNF-α, have been successfully used to resolve inflammation and associated pathologies [23]. For example, mice overexpressing TNF-α displayed symptoms of rheumatoid arthritis and impaired fracture healing but exhibited normal healing upon treatment with anti-TNF-α antibody [23]. Since, TNF-α levels are increased in various conditions associated with chronic inflammation, blocking TNF-α activity could be a potential strategy to mitigate inflammation and thereby promote tissue regeneration and healing. It is plausible that other inflammatory factors might also be contributing to the resolution of inflammation, warranting further investigation.
3.1. Elevated acute inflammation in severe injuries is associated with impaired healing
Severe injuries with multi-trauma are associated with impaired bone healing and have been postulated to be due to an increase in both local and systemic inflammation. Systemic inflammation, manifested by elevated inflammatory markers, was found in animal models of multi-trauma [25, 106]. The detrimental role of acute systemic inflammation on healing was also demonstrated in mice where experimentally induced acute systemic inflammation using lipopolysaccharide reduced the amount and quality of regenerated femoral bone [107]. Weckbach et al. studied the inflammatory alterations in mice using a combination of traumatic injuries [25], which led to an elevated level of systemic inflammatory response compared to individual injuries. For example, a combination of head injury with femoral fracture showed higher levels of IL-6 and MCP-1 in circulation and decreased neutrophil apoptosis [25]. Similarly, combined thoracic and femoral fracture injury required prolonged healing time, which was associated with increased recruitment of polymorphonuclear cells, decreased numbers of CD68+ macrophages, and elevated IL-6 levels at the fracture callus [7]. Fractures involving muscle trauma exhibit increased inflammatory response. Substantial muscle trauma and tissue loss results in a higher and prolonged local inflammation, including elevated levels of CD3+ lymphocytes at 3 days post-injury and presence of a higher number of CD68+ macrophages 14 days post-injury in the callus [8]. Based on the findings that a fracture with unstable fixation results in delayed bone healing compared to those with stable fixation, studies have used non-stable fixation as a model of severe fracture [108]. The non-stabilized group exhibiting delayed healing had significantly more cytotoxic T cells, T helper cells, and leukocytes in the hematoma and bone marrow neighboring the injury when compared to the stabilized group [24]. These studies suggest that elevated systemic and local inflammation are detrimental to bone repair by prolonging the inflammatory phase as shown by decreased apoptosis of neutrophils and protracted presence of inflammatory cells, most likely by undermining the resolution of inflammation.
3.2. Chronic inflammatory conditions are associated with impaired bone regeneration
Multivariate analysis of a cohort database has found the odds ratio of nonunion to be increased in patients with osteoarthritis, type 1 diabetes, obesity, and rheumatoid arthritis [22]. Chronic inflammation is an underlying factor in all these conditions [109–114]. Similar to patients, the association of these chronic inflammatory states with poor bone repair has also been demonstrated in animals [9, 20, 23, 115]. Patients with diabetes exhibit poor healing with a healing time 87% longer than their counterparts and a 3.4-fold higher risk of complications including nonunion [9]. Excess systemic inflammation associated with diabetes has also been shown to correlate with poor healing in animals, which is thought to be mediated by upregulation of the pro-inflammatory cytokine TNF- α that inhibits angiogenesis, stimulates chondrocyte apoptosis, and enhances osteoclast formation [104, 116–118]. In Zucker diabetic fatty rats, increased levels of the inflammatory cytokine macrophage inflammatory protein 1 (MIP-1) have been shown to be associated with delayed healing [119]. Type 1 diabetic mice with aberrant NF-κB activation in skeletal stem cells (SSCs) have enhanced inflammation, while inhibition of NF-κB in SSCs rescued the negative impact of diabetes on inflammation, SSC expansion, and tissue formation [120]. Diabetes caused defective pro-resolving M2 macrophage polarization by reducing TGF-β1 expression in SSCs, which was recovered by NF-κB inhibition or exogenous TGF-β1 treatment [120]. Similarly, obesity accompanied by perturbances in inflammatory cytokines has been shown to affect healing in animals. Fracture healing was delayed in obese mice (ob/ob) and was correlated to the presence of low TGF-β1 and high TNF-α levels in blood plasma [115]. These studies further underscore the key role played by growth factors such as TGF-β1 in inflammatory phases. Since TNF-α is a common inflammatory cytokine elevated in all chronic inflammatory conditions with poor healing, an experimental approach overexpressing TNF-α in mice with symptoms of rheumatoid arthritis was used to examine its impact on fracture healing [23]. Results showed that high levels of TNF-α in circulation negatively affected fracture healing, exhibited reduced cartilage, increased soft tissue, and decreased bone biomechanical stability.
In addition to chronic conditions, advanced age is another risk factor that results in a higher rate of nonunion in patients [121, 122]. Physiological aging is thought to be a chronic inflammatory state and recent studies suggest that “inflammaging” is a key factor contributing to delayed healing, as the ability to cope with the stressors decreases with increasing age [123]. Cellular senescence is a hallmark of aging. Senescent cells create a proinflammatory environment by senescence-associated secretory phenotype (SASP), which consists of proinflammatory mediators [124]. Senescence is a protective arrested state that cells enter into either to be repaired or discarded. The SASP inflammatory factors are released to attract immune cells for clearance of the senescent cells [125]. However, in aging, the immune system is not as efficient in clearing these senescent cells in a timely manner, thereby creating sustained levels of proinflammatory signals in the microenvironment. These increased levels of SASP can also influence nearby cells to enter a senescent state and release SASP, further increasing pro-inflammatory signals [126]. Macrophages play a central role in the aberrant healing of aged animals, and the secretion of inflammatory cytokines is a similar phenomenon shared by altered macrophage and senescent cells [127]. Macrophages are sustained and promoted by an accumulation of senescent cells during aging, and blocking macrophage recruitment and activity at the fracture site in old mice exhibits better fracture healing [128]. The delayed healing observed in the old mice was thought to be due to the upregulation of M1 pro-inflammatory gene signatures in macrophages residing in fracture calluses of aged mice [129, 130]. This sustained callus inflammation in old compared to young mice is attributed to the increase of M1 macrophage numbers and CD8+ cell gene expression [131]. Similarly studies in rat have shown that increased M1 macrophage function is accompanied by impaired M2 macrophage function and leads to decreased bone healing with aging [132]. Not only are there differences in the balance of M1:M2 macrophages with increased age, but also their function and secretome are different. For example, macrophages found in young mice secrete lipoprotein receptor-related protein 1 (Lrp1) which provides the ability for normal fracture repair [133]. Macrophages from aged mice displaying delayed healing have been shown to secrete less Lrp1. The altered Lrp1 with aging is alleviated when the aged mice were anastomosed with young mice, which allowed the aged mice to regain fracture healing potential comparable to their young counterparts. Consistent with these observations, Lrp1 deletion in myeloid cells has been shown to exacerbate inflammation in mice as characterized by increased levels of proinflammatory cytokines and chemokines, [134] while its expression modulates inflammation for tissue repair [135, 136]. These studies illustrate the importance of immune regulation to achieve normal bone regeneration.
4. Application of biomaterials in modulating inflammation
Though our understanding of how elevated or protracted inflammatory response impairs healing is limited, studies have demonstrated the importance of regulating inflammatory response during repair, and how therapeutic strategies that restore the normal process of inflammation or its resolution, result in normal healing of bone tissues. Biomaterials that are immunomodulatory could be an important treatment strategy to modulate inflammation and promote healing. This result could be achieved by using materials that inherently modulate the inflammatory response or release active molecules (i.e., therapeutics) to promote resolution. Therapeutics can be incorporated into the biomaterial via conjugation or physical adsorption [137]. The release of drug molecules can be engineered to occur through one or a combination of mechanisms such as hydrolysis, presence of tissue-specific enzymes, chemical moieties, or mechanical loading [17, 138–141]. Depending upon the complexity of injury and location, biomaterials are used in different forms (nano/microparticles, hydrogels, or solid scaffolds) to support bone tissue regeneration. The form of biomaterial is mostly determined by the type of bone injury. For instance, biomaterial scaffolds for large bone defects require additional structural features such as porosity and microarchitectures to promote infiltration of endogenous cells for vascularized bone tissue formation [142, 143]. However, almost all biomaterials elicit a foreign body response (FBR) immediately upon implantation, with the level of response dependent on the physical and chemical properties of the implant. The immune reaction to the scaffold plays a key role in determining the quality of tissue repair. Additionally, the degradation products can also contribute to inflammation. Therefore, it is importance to design biomaterials that elicit minimal acute response in vivo. Given the key role played by the proteins from the host tissue milieu on FBR, early attempts to design biomaterials were focused on using inert materials to minimize the host response. Some of the naturally occurring biomaterials such as high molecular weight hyaluronic acid and chitosan have been touted to exhibit some level of anti-inflammatory functions [144, 145]. Emerging studies demonstrate how biomaterials can be designed to interact with the host through tailored, mechanical, chemical, interfacial and topographical characteristics [146–148]. In the case of treating large defects, an ideal scaffold should contribute to immunomodulation, osteogenic properties, and angiogenesis to activate the necessary crosstalk between the cells in different phases of bone healing. While autologous bone grafts are still the gold standard to treat large defects, synthetic biomaterials containing calcium phosphate and bioceramics have been extensively used towards bone tissue repair. Besides degradation, it is very well established that many of the calcium phosphate biomaterials can serve as reservoirs of Ca2+ and PO43- ions. We have shown that biomineralized scaffolds containing calcium phosphate minerals with dynamic dissolution/precipitation are osteoinductive and can be used to promote endogenous bone tissue repair [149]. Calcium and phosphate ions are the major dissolution products, and their presence in the extracellular milieu is known to be involved in inflammatory signaling pathways in a context-dependent and tissue-specific manner [150–152]. For example, the high extracellular Ca2+ -driven activation of the calcium receptor signaling cascade leading to the production of Wnt5A can downregulate TNF-α via NF-κB inhibition and thereby reduce inflammation [153–155]. We have shown that PO43- ions functioning through SLC20a1 on progenitor cells including bone marrow derived cells can increase the extracellular adenosine levels [96]. As described earlier, extracellular adenosine is well documented to contribute to anti-inflammatory signaling pathways [16, 88] and is also considered as a pro-resolving mediator.
In addition to the chemistry, the interfacial (wettability and surface microstructures) and physical properties (porosity and pore architecture) can also contribute to immunomodulation [156, 157]. For example, rough hydrophilic titanium implant surfaces polarize the adaptive immune system towards a pro-regenerative phenotype by enabling a faster resolution of inflammation that drives macrophages to recruit MSC and Th2 cell populations (Figure 3) [158]. Beyond the physicochemical properties, direct incorporation of bioactive molecules has also been widely used to stimulate a specific immune response and cell-cell crosstalk. For instance, the positive effects of extracellular matrix (ECM) proteins on promoting M2/M1 ratio have been used to promote the immunomodulatory function of the biomaterial implants or scaffolds [159, 160]. Mansour et al. have incorporated bone ECM extracts into synthetic dicalcium phosphate bioceramics as a means to regulate immune response [161]. The scaffold coated with bone extract enriched with Ca+-binding ECM molecules showed decreased levels of pro-inflammatory cytokines (e.g., IL-1, TNF-α) and enhanced bone formation in a rat tibia fracture model. Similarly, heparinization of β-TCP has been proposed as a strategy to achieve immunomodulatory effects [162]. While the above discussion focused on tailoring material properties to regulate the inflammatory environment, biomaterials have also been extensively used towards the application of various mediators that resolve inflammation to improve bone healing. Biomaterials-assisted local delivery of the biomolecules is central to most of these approaches and is used in other applications including those in periodontal disease (Table 1).
Figure 3.
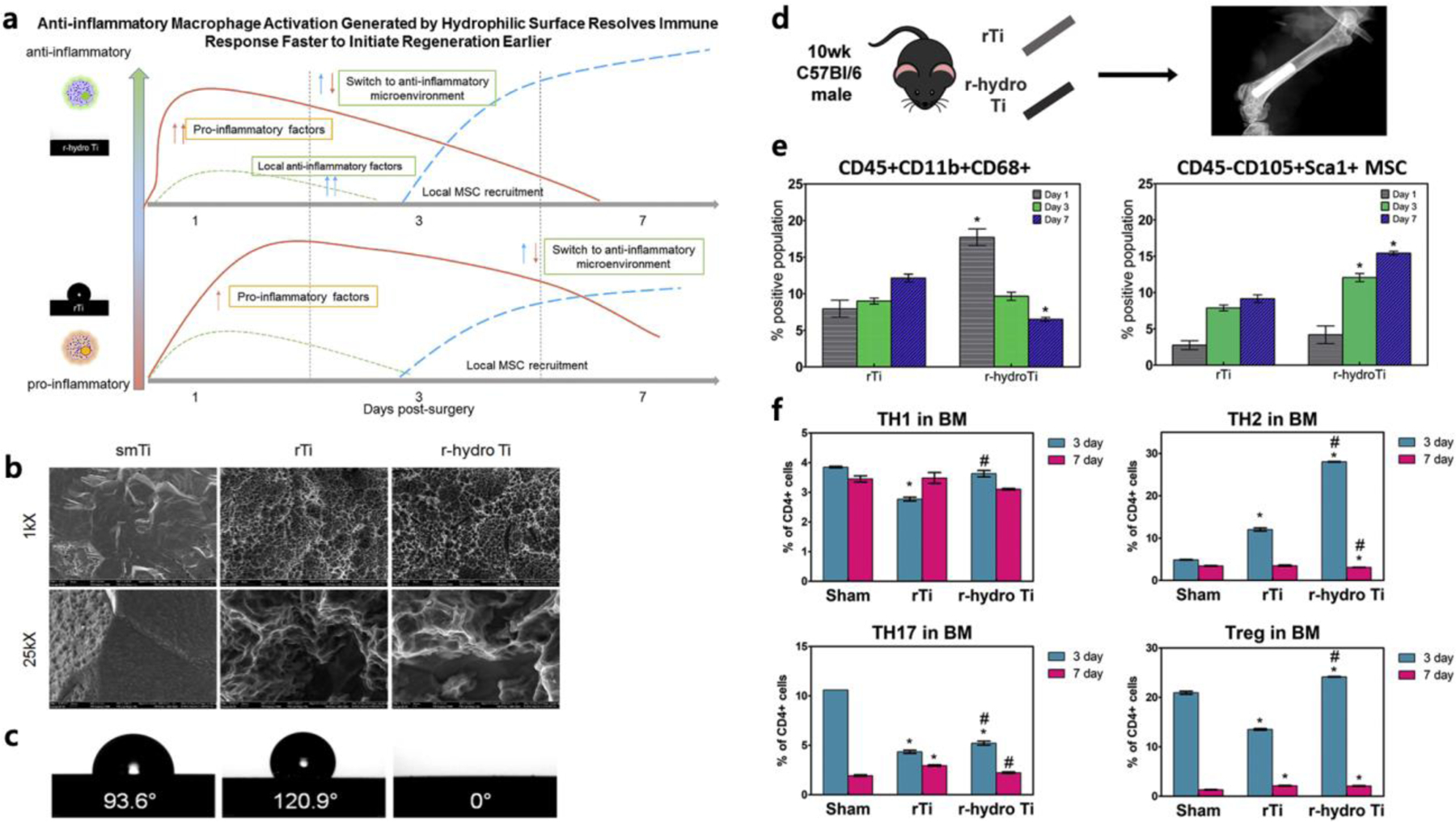
Anti-inflammatory macrophage activation generated by biomaterial surface properties. a) Summary of immune response generated by a hydrophilic surface. b) Qualitative assessment of surface topography through scanning electron microscopy at 1kX and 25kX of Ti implants. c) Indirect measurement of surface wettability through contact angle of smTi, rTi, r-hydro Ti. d) Schematic of surgery. e) CD45 + CD11b + CD68 + Macrophages adhered to implant. Changes in CD45−CD90+Sca-1+ MSCs at implant surface *p < 0.05 vs. rTi. f) Changes in T-cell populations at implant, (n = 6) The greatest changes in T-helper cell populations with surface properties was found in anti-inflammatory Th2 and Treg populations. The greatest percent of these populations were found on r-hydro Ti implants. *p < 0.05 vs. sham, # vs. rTi. (smTi = smooth titanium, rTi = rough titanium, r-hydro Ti= rough-hydrophilic titanium). Reprinted with permission from [158].
Table 1.
Selected list of mediators involved in resolution of inflammation used as therapeutics for bone regeneration
| Class | Molecule | Biomaterial | Injury model | Treatment outcome | References |
|---|---|---|---|---|---|
| Cytokines | IL-4+IL-13 | Collagen sponge | Mouse (C57BL/6N); femoral osteotomy (0.7 mm) | Enhanced bone formation (microCT); increased M2 macrophages | Claudia Schlundt, 2018 |
| IL-4+SDF-1 | Gelatin | Rat (Sprague-Dawley); periodontal defect (2×2 mm) | Improved periodontal regeneration (histology, microCT); decreased M1 macrophages; increased M2 macrophages; increased MSCs | He, 2019 | |
| IL-4 | Nanofibrous gelatin microspheres | Rat (Sprague-Dawley), diabetic; mandibular periodontal defect (3×2×1 mm) | Restored bone regeneration (histology, microCT); recovered M2/M1 macrophage ratio; reduced TNF-alpha | Hu, 2018 | |
| IL-4 | Decellularized bone matrix reservoir | Rat (Sprague Dawley); cranial defect (5 mm) | decreased TNF-α levels | Zheng, 2018 | |
| Anti TNF-alpha antibody (Infliximab) | Systemic (free molecule) delivery | Mouse (Tg197); femoral fracture (closed) | Benefited fracture healing (histology, biomechanical test) | Timmen, 2014 | |
| TGF-beta1 | Cellulose | Rabbit; cranial defect (15 mm) | Enhanced bone formation (histology and X-ray) | McKinney, 1996 | |
| TGF-beta1 | Chitosan/collagen microgranules | Rabbit (New Zealand); cranial defect (8 mm) | Enhanced bone regeneration (histology) | Lee, 2006 | |
| TGF-beta1 | Porous oly(propylene fumarate) scaffold | Rabbit (New Zealand); cranial defect (6.3 mm) | Adequately induced bone formation (histology) | Vehof, 2002 | |
| TGF-beta1 | CaP/Gelain microparticles | Rabbit (New Zealand); femoral | Enhanced bone remodeling (histology, biomechanical | Link, 2008 | |
| IGF-1 | Poly(lactide-co-glycolide) microspheres (PLGA MS) | Ovine; methaphyseal drill hole (8-mm) and segmental tibia defect (10-mm) | New bone formation and bridging, reduced inflammation | Meinel, 2003 | |
| IGF-1/IGF-2 | ColI gel | Rat (Sprague-Dawley); facial critical-sized defect (5×15 mm) |
Osseous healing, IGF-2<IGF-1/IGF/2<IGF-1 | Toung, 1999 | |
| Lipids | Resolvin E1 | Local injection, no scaffold | Mouse (FVB and chemR23tg); cranial defect (1 mm) | Accelerated bone healing (histology) | Gao, 2013 |
| Maresin1 | Systemic (free molecule) delivery | Mouse (C57BL/6J), aged; transverse tibial fracture | Improved fracture healing (histology, microCT, biomechanical testing); decreased local pro-inflammatory macrophages; decreased circulating IL-6, IL-10, TNF-alpha, KC, IL-1beta, MCP-1 | Huang, 2020 | |
| Resolvin D1 | Chitosan scaffold | Rat (Wistar); femoral defect (3 mm) | Improved bone repair (histology, micrCT) | Vasconcelos, 2018 | |
| Resolvin D1 | Macrophage cell membrane-coated gold nanocage in boron-containing mesoporous bioactive glass scaffold | Mouse (C57BL/6); femoral defect (1 mm) | Enhance bone regeneration (histology); increased M2 macrophages | Yin, 2020 | |
| 15-Deoxy-prostaglandin J2 | Collagen sponge/PLGA nanoparticle | Rat (Wistar); femoral defect (5 mm) | Increased bone formation (histology); decreased IL-6, IL-1beta, TNF-alpha; increased BMP-6 and PDGF-B | Tang, 2017 | |
| 15-Deoxy-prostaglandin J2 | Collagen sponge | Rat (Sprague Dawley); fibula segmental defect (7 mm) | Small improvement in bone formation (X-ray, histology, microCT) | Nam, 2019 | |
| PGI2 analog (Iloprost) | Biphasic fibrin | Mouse (C56BL/6N); femoral osteotomy (0.7 mm) | Improved fracture healing (Histology, microCT); decreased CD8+IFNγ+ T cells; decreased M1 macrophages; increased M2 macrophages | Wendler, 2019 | |
| Lipoxin A4 analog (Benzo-lipoxin A4) | Neutrophil microparticles | Miniature swine; chronic periodontal defect | Improved bone formation (histology, microCT); decreased inflammatory cell infiltration; increased lipoxins and resolvins; decreased PGE2 and PGD2 | Van Dyke, 2015 | |
| Oleic acid | Porous PCL scaffold | Rat (Wistar); tibial defect (3 mm) | Improved bone formation (histology) | Cardoso, 2017 | |
| Purine (Adenosine) | Adenosine | Layered MgFe hydroxide nanocarriers/gelatin gel | Rat (Sprague-Dawley); tibial defect (4 mm) | Increased bone healing (histology, microCT) | Kang, 2017 |
| Adenosine | PEG-PBA macroporous scaffold | Mouse (C57BL/6J); transverse tibial fracture | Improved bone healing (histology, microCT); increased angiogenesis | Zeng, 2020 | |
| A2A receptor agonist (CGS 21680) | Collagen sponge | Mouse (C57BL/6 and A2A KO); cranial defect (3 mm) | Enhanced bone regeneration (histology, microCT) | Mediero, 2015 | |
| ENT inhibitor (Dipyridamole) | Collagen sponge | Mouse (C57BL/6 and A2A KO); cranial defect (3 mm) | Enhanced bone formation (histology, microCT) | Mediero, 2015 | |
| ENT inhibitor (Dipyridamole) | HAp/beta-TCP scaffold | Mouse (C57BL/6); cranial defect (3 | Enhanced bone regeneration (histology, microCT) | Ishack, 2017 | |
| A2A receptor agonist (CGS 21680) | Fibrin gel | Rat (Sprague-Dawley); transverse tibial fracture, burnt periosteum | Accelerated bone healing (histology, X-ray, microCT) | Zheng, 2020 | |
| ENT inhibitor (Dipyridamole) | Beta-TCP scaffold | Sheep (Dorset/Finn); cranial defect (11 mm) | Improved bone regeneration (histology, microCT) | Bekisz, 2018 |
4.1. Biomaterial-assisted delivery of cytokines
As the cytokines IL-4, IL-13, and IL-10 have been shown to resolve inflammation, delivery of these molecushowed decreased levels of pro-inflammatory cytokinesles have been extensively explored to promote fracture healing. Combined delivery of IL-4 and IL-13 from a collagen sponge increased the M2 macrophage population and enhanced bone healing of a mouse femoral fracture [39]. Another study showed that co-delivery of IL-4 and the chemoattractant SDF-1 from gelatin hydrogels improved periodontal bone regeneration in diabetic rats, decreased the number of M1 macrophages, and increased the number of M2 macrophages and MSCs in the callus [53]. Local delivery of IL-4 alone reduced bone loss in a polyethylene particle-induced calvarial osteolysis model [54] and increased bone mineral density with transplantation of MSCs overexpressing IL-4 in a murine femoral defect [163]. Delivery of IL-4 using gelatin microspheres restored bone regeneration in a mandibular periodontal defect in diabetic rats which was associated with improved M2/M1 macrophage ratio and reduced TNF-α expression [55]. Similarly, injection of different doses of IL-4 to pre-implanted decellularized bone matrix in a rat cranial defect was found to increase the local M2/M1 macrophage ratio and IL-10 levels in the milieu while decreasing TNF-α and improving bone formation [164]. In this study, a low dose (10 ng) of IL-4 showed better bone healing compared to the higher doses (50 and 100 ng). The authors speculate that the low dose creates an optimal M2/M1 macrophage ratio, while the higher dose results in abundant M2 macrophages in the local milieu contributing to the formation of foreign body giant cells and fibrosis [165].
Growth factors are well known for their direct role on chondrocyte differentiation and osteoblast commitment aside from their effect on resolving inflammation [60] and have thus been extensively used to promote bone healing. Given the key role played by TGF-β in osteoblast commitment, its delivery has been extensively used to promote bone tissue repair [15, 166, 167]. While most of these regenerative studies have primarily focused on the ability of TGF-β to promote bone formation, given its role in resolving inflammation, it is plausible that the positive effects of TGF-β could also arise from its function as a pro-resolving mediator. While there is a lack of studies that have directly examined whether delivery of TGF-β1 elicits immunomodulation during bone tissue repair, studies involving other tissues clearly demonstrate the role of immunomodulation during tissue repair. For example, delivery of TGF-β1 by poly-lactide-co-glycolide (PLG) scaffolds implanted into epididymal fat pads has been shown to decrease leukocyte numbers and inflammatory molecules such as TNF-α, IL-12, and MCP-1 [168]. The inflammatory response was also found to be reduced when polyurethane (PU) conjugated with TGF-β1 was implanted into skeletal muscle as evident by decreased monocyte/macrophage infiltration [169]. These studies suggest that the improved bone healing observed following intervention using TGF-β1 may also involve regulation of the local osteoimmune environment. Similar to TGF-β1, the growth factor IGF-1 has been delivered from biomaterials for bone repair. A study encapsulated IGF-1 in PLGA microspheres and implanted them into sheep long bone defects (humeral or femoral drill holes, and tibial segmental osteotomy) [19]. In both models, the biomaterial-mediated delivery of IGF-1 augmented bone healing and reduced the expression of inflammatory markers IL-6, IL-1β, and iNOS. Toung et al. investigated the effect of IGF-1 and IGF-2 for critical-sized facial defects in rats using collagen type I gels to deliver IGF-1, IGF-2, or a combination of both [170]. All IGF-infused scaffolds resulted in significantly enhanced bone healing when compared to non-loaded gels. Given its role in mitigating inflammation and promoting bone regeneration, it would be interesting to examine how IGF-1 regulates the resolution of inflammation in the context of bone regeneration and how it promotes bone healing through immunomodulation.
4.2. Lipid mediators
Lipid mediators are the prototypical molecules characterized for the resolution of inflammation. Resolvin D1 (RvD1), a DHA metabolite that is secreted by M2 macrophages [171], improved bone formation in a rat femoral defect when delivered using a chitosan scaffold [172]. It also increased the presence of M2 macrophages and enhanced bone formation in mouse femoral defects when loaded in gold nanocages coated with LPS-stimulated macrophage cell membranes (BANC) and implanted with a mesoporous bioactive scaffold [173]. Prostaglandins are another type of lipid mediator derived from omega-6 fatty acids that are particularly important in bone repair. The prostaglandin 15-Deoxy-∆12,14-prostaglandin J2 (15d-PGJ2) can be released by M1 macrophages and exhibits anti-inflammatory properties acting as a pro-resolving molecule [174–176]. Treatment of a rat femoral defect with a collagen sponge loaded with free 15d-PGJ2 or a collagen sponge containing 15d-PGJ2-loaded PLGA nanoparticles decreased expression of inflammatory cytokines IL-6, IL-1β, and TNF-α, upregulated the levels of bone regenerating molecules BMP-6 and PDGF-B, and promoted bone formation [177]. Furthermore, 15d-PGJ2-loaded PLGA nanoparticles in a collagen sponge provided better outcomes when compared to free 15d-PGJ2 in a collagen sponge, possibly due to its prolonged release and bioavailability. In another study, delivery of free 15-dPGJ2 by a collagen sponge in a fibula segmental defect demonstrated some evidence of regeneration, although not significant [178], further signifying how the use of PGs can be used to promote fracture healing. Using synthetic analogs of PG is another strategy used to activate PG signaling. Iloprost, a synthetic analog of prostacyclin (PGI2), has been used to modulate the inflammatory response during bone repair [179]. PGI2 receptor (IP) is present on different immune cell populations and exhibits anti-inflammatory and immunosuppressive properties [180]. Results showed that treatment with Iloprost loaded in a fibrin scaffold decreased CD8+IFNγ+ T cells, decreased M1 macrophages, increased M2 macrophages, and improved fracture repair of the femur. Lipoxins are a group of lipid mediators that are derived from omega-6 fatty acids and are synthesized and released by various cells including platelets and neutrophils [181]. Delivery of lipoxin A4 analog, benzo-lipoxin A4, with the help of carriers derived from neutrophils improved bone formation in chronic periodontal defects of miniature swine [182]. Furthermore, direct delivery of fatty acids such as oleic acid has also been utilized for bone repair [183]. Oleic acid is an omega-9 monounsaturated fatty acid with anti-inflammatory properties [184], and its release from polycaprolactone (PCL) scaffolds implanted in a rat tibial defect has been shown to promote bone regeneration [183].
4.3. Adenosine
Fundamental understandings in animal models demonstrate that adenosine and its receptor signaling are required for normal healing, and several studies have explored the feasibility of delivering pharmaceutics that target adenosine receptors for bone healing. Current understandings of adenosine suggest that its contribution to bone healing is multi-faceted. While substantial efforts have focused on the direct effect of adenosine receptor signaling on osteogenesis of the fracture environment, minimal studies have examined the role of adenosine and its receptors on the fracture immune environment. A recent study found that local delivery of the A2A receptor agonist CGS 21680 using fibrin in rat transverse tibial fractures improved healing that corresponds to diminished inflammatory response as demonstrated by a decrease of IL-6, increased Treg, and decreased Th17 [185]. Bone formation was improved by delivering adenosine from layered magnesium iron hydroxide nanocarriers (MgFe-Ado-LDH) into rat tibial defects [186]. Given that injuries like bone trauma induce adenosine release, our group has designed a scaffold that reversibly sequesters adenosine in the fracture environment to leverage the surge of extracellular adenosine following injury to prolong local adenosine signaling (Figure 2) [17]. Our results showed implantation of these scaffolds increased angiogenesis and accelerated bone healing in situ. Another method to increase extracellular adenosine levels is to inhibit their cellular uptake by equilibrative nucleoside transporter 1 (ENT1). One such compound is dipyridamole that inhibits cellular uptake of extracellular adenosine by targeting ENT1 and subsequently increasing the extracellular adenosine level. A number of studies have shown that local release of dipyridamole from a collagen sponge or HAp/βTCP scaffold increased bone formation in cranial defects of mice and sheep [102, 187]. The potent effect of dipyridamole on bone healing was also demonstrated in a rabbit model of segmental radial defect when released from bioactive ceramics [188]. Since the A2A receptor promotes osteoblast activity, its effect on bone tissue repair was also tested using an agonist. Local delivery of A2A receptor agonist CGS 21680 using a collagen sponge to treat mouse cranial defects and using fibrin to treat rat transverse tibial fractures showed improved healing [102, 185]. Despite extensive understandings with respect to the potent anti-inflammatory and bone-forming effect of adenosine signaling, these studies were carried out independently. A detailed and systematic study is needed to unravel how adenosine directly regulates the inflammatory immune response and subsequently contributes to bone regeneration. Given the multi-functionality of adenosine signaling, it is plausible that extracellular adenosine influences different phases of bone healing in a context-dependent manner.
Figure 2.
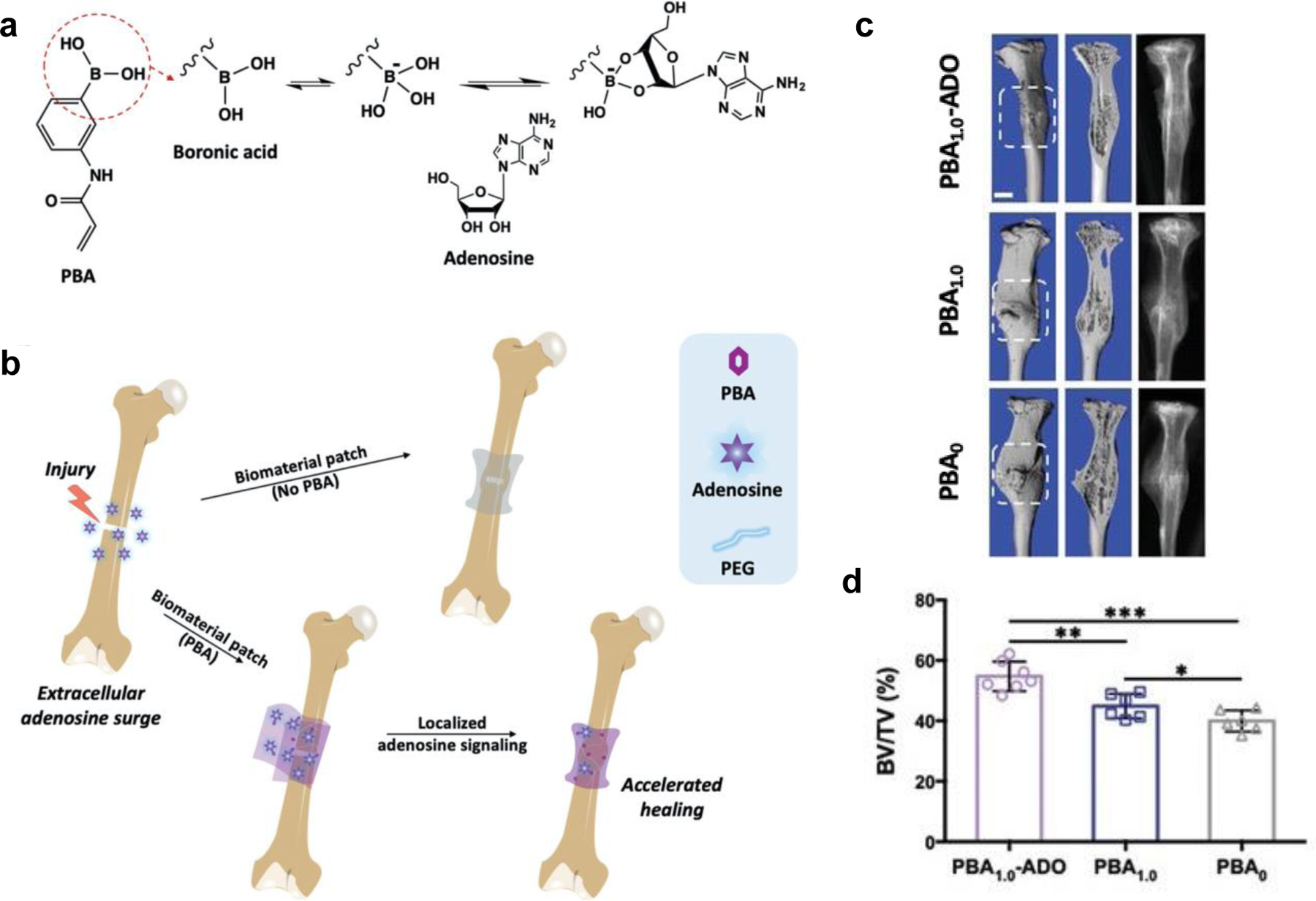
Schematic of PBA- mediated adenosine sequestration. a) 3-(Acrylamido)phenylboronic acid (PBA) contains a boronic acid moiety (circled) that forms a dynamic covalent complex of cyclic boronate ester with cis- diol- bearing adenosine. b) A PBA-conjugated biomaterial sequesters surge of extracellular adenosine at the fracture site after injury and sustains elevated and localized adenosine levels to accelerate fracture repair. c) 3D reconstructions and corresponding radiographs of the fractured tibiae at 21 d post-injury treated with various biomaterial patches. White boxes enclose the callus regions. Scale bars: 1 mm. d) Bone volume ratio (BV/TV) of calluses quantified from figure (c) at 21 d post-fracture. Reprinted with permission from [17].
5. Conclusion and Future Perspective
The emerging understanding of the role of inflammation in tissue regeneration has led to a paradigm shift in approaches that harness the inflammatory cells to promote healing. While inflammation is an essential mechanism to initiate healing, a timely and efficient resolution of inflammation is necessary for the formation of a pro-regenerative environment. Spatiotemporal modulation of immune cell functions and their interactions with tissue-specific cells, including progenitor cells, is vital to promote bone tissue regeneration. Further understanding of the anti-inflammatory and/or pre-resolving molecules and their temporal and cell-specific functions will significantly contribute to their successful therapeutic applications.
Application of specialized pro-resolving mediators that orchestrate key cellular process driving resolution of acute inflammation and generating a pro-regenerative environment to promote bone healing is highly attractive. Local delivery of these molecules at the injury site circumvents the challenges of systemic pharmacokinetics and off-target effects. Biomaterials play a central role in these strategies as they can aid localized delivery of pro-resolving mediators into the injury site. Bone tissue regeneration is multi-factorial and involves multiple cytokines and cell populations. Hence, therapeutic interventions that integrate multiple signaling pathways and inflammatory mediators should be developed to promote better therapeutic outcomes. However, a greater understanding of the action of these molecules and specific cell populations involved, selectivity, and downstream signaling in bone tissue in health and diseases is needed to provide a foundation for therapeutic targeting and pharmacokinetic studies. Emerging studies also imply that the time and mode of administration of the pro-resolving mediators needs to be custom tailored to achieve the therapeutic outcome. For example, studies utilizing SPMs like MaR1 required administration of the molecule following 3 days post-fracture to observe any therapeutic outcome, while application of adenosine immediately following injury showed therapeutic benefits. These observations suggest the need for a candidate-dependent delivery strategy to meet the spatiotemporal function. The advent of stimuli-responsive materials can be harnessed to precisely control the time, dose, and duration of these therapeutic molecules [140, 189]. A biomaterial-based approach that senses the environment and accordingly releases the appropriate biomolecule to temporally regulate the immune and progenitor cells to stimulate a regenerative environment and restore tissue homeostasis will advance the translational potential. Further studies in bone injury, including local interventions and temporal analyses of cells at the single cell resolution and microenvironmental factors, will provide much needed insight into the mediators and processes that govern resolution of inflammation at the injury site.
It is interesting to note that some molecules have pleiotropic functions in immunomodulation, osteogenesis and/or angiogenesis. These multi-functional molecules could be ideal therapeutic candidates to promote bone repair. Leveraging such multi-functional molecules that can regulate various relevant signaling pathways and cell populations will further advance the current efforts focusing on endogenous healing mechanisms to promote bone regeneration and fracture repair. In the case of bone tissue repair, biomaterials have also been used as bone grafts for treating large bone defects. Most of these approaches target a single biological event and lack approaches that can target different phases of bone healing and drive dynamic crosstalk between various cell populations. Incorporation of molecules with pleiotropic functions could also have a significant impact in improving the functions of bone grafts.
To date, although many biomaterials have been developed that promote fracture healing and also contribute to immunomodulation, most of the research has yet to be translated into the clinic. Currently, autografts or allografts are the most frequently used bone grafts, and given their biological origin, they could provide some beneficial effect towards resolution of inflammation although this is not very well established. Other widely used biomaterials containing calcium phosphate minerals that are shown to promote fracture healing can potentially contribute to immune modulation via sequestration of growth factors [190, 191], other pro-resolving mediators, or by contributing to the extracellular adenosine [96]. Recently the FDA approved a bioresorbable thermoset screw that releases citrate molecules, which has been shown to exhibit anti-inflammatory functions [192, 193]. Although still in its infancy, the emerging evidence overall strongly indicates that therapeutic approaches integrating molecules that can actively resolve acute inflammation will be an efficient therapeutic strategy to promote bone tissue repair and mitigate delayed healing and non-unions.
Acknowledgement
The authors thank Unghyeon Ko for assistance in figure preparation. This work is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number NIH R01 AR071552 and R01 AR079189.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Miranda M, Moon M, Treatment Strategy for Nonunions and Malunions
- [2].Marsell R, Einhorn TA, The biology of fracture healing, Injury 42(6) (2011) 551–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Frost HM, The biology of fracture healing. An overview for clinicians. Part II, Clin Orthop Relat Res (248) (1989) 294–309. [PubMed] [Google Scholar]
- [4].Bahney CS, Zondervan RL, Allison P, Theologis A, Ashley JW, Ahn J, Miclau T, Marcucio RS, Hankenson KD, Cellular biology of fracture healing, J Orthop Res 37(1) (2019) 35–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wheatley BM, Nappo KE, Christensen DL, Holman AM, Brooks DI, Potter BK, Effect of NSAIDs on Bone Healing Rates: A Meta-analysis, J Am Acad Orthop Surg 27(7) (2019) e330–e336. [DOI] [PubMed] [Google Scholar]
- [6].Gerstenfeld LC, Cho TJ, Kon T, Aizawa T, Tsay A, Fitch J, Barnes GL, Graves DT, Einhorn TA, Impaired fracture healing in the absence of TNF-alpha signaling: the role of TNF-alpha in endochondral cartilage resorption, J Bone Miner Res 18(9) (2003) 1584–92. [DOI] [PubMed] [Google Scholar]
- [7].Recknagel S, Bindl R, Brochhausen C, Gockelmann M, Wehner T, Schoengraf P, Huber-Lang M, Claes L, Ignatius A, Systemic inflammation induced by a thoracic trauma alters the cellular composition of the early fracture callus, J Trauma Acute Care Surg 74(2) (2013) 531–7. [DOI] [PubMed] [Google Scholar]
- [8].Hurtgen BJ, Ward CL, Garg K, Pollot BE, Goldman SM, McKinley TO, Wenke JC, Corona BT, Severe muscle trauma triggers heightened and prolonged local musculoskeletal inflammation and impairs adjacent tibia fracture healing, J Musculoskelet Neuronal Interact 16(2) (2016) 122–34. [PMC free article] [PubMed] [Google Scholar]
- [9].Jiao H, Xiao E, Graves DT, Diabetes and Its Effect on Bone and Fracture Healing, Curr Osteoporos Rep 13(5) (2015) 327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Maruyama M, Rhee C, Utsunomiya T, Zhang N, Ueno M, Yao Z, Goodman SB, Modulation of the Inflammatory Response and Bone Healing, Front Endocrinol (Lausanne) 11 (2020) 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Muire PJ, Mangum LH, Wenke JC, Time Course of Immune Response and Immunomodulation During Normal and Delayed Healing of Musculoskeletal Wounds, Front Immunol 11 (2020) 1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].O’Connor JP, Manigrasso MB, Kim BD, Subramanian S, Fracture healing and lipid mediators, Bonekey Rep 3 (2014) 517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gonçalves WA, Melão ACR, Teixeira MM, Rezende BM, Pinho V, Pro-resolving Mediators, in: Riccardi C, Levi-Schaffer F, Tiligada E (Eds.), Immunopharmacology and Inflammation, Springer International Publishing, Cham, 2018, pp. 133–175. [Google Scholar]
- [14].Gong D, Shi W, Yi SJ, Chen H, Groffen J, Heisterkamp N, TGFbeta signaling plays a critical role in promoting alternative macrophage activation, BMC Immunol 13 (2012) 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].McKinney L, Hollinger JO, A bone regeneration study: transforming growth factor-beta 1 and its delivery, J Craniofac Surg 7(1) (1996) 36–45. [PubMed] [Google Scholar]
- [16].Hasko G, Cronstein B, Regulation of inflammation by adenosine, Front Immunol 4 (2013) 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zeng Y, Shih YV, Baht GS, Varghese S, In Vivo Sequestration of Innate Small Molecules to Promote Bone Healing, Adv Mater 32(8) (2020) e1906022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Labandeira-Garcia JL, Costa-Besada MA, Labandeira CM, Villar-Cheda B, Rodriguez-Perez AI, Insulin-Like Growth Factor-1 and Neuroinflammation, Front Aging Neurosci 9 (2017) 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Meinel L, Zoidis E, Zapf J, Hassa P, Hottiger MO, Auer JA, Schneider R, Gander B, Luginbuehl V, Bettschart-Wolfisberger R, Illi OE, Merkle HP, von Rechenberg B, Localized insulin-like growth factor I delivery to enhance new bone formation, Bone 33(4) (2003) 660–72. [DOI] [PubMed] [Google Scholar]
- [20].Clark D, Nakamura M, Miclau T, Marcucio R, Effects of Aging on Fracture Healing, Curr Osteoporos Rep 15(6) (2017) 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Brem H, Tomic-Canic M, Cellular and molecular basis of wound healing in diabetes, J Clin Invest 117(5) (2007) 1219–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zura R, Xiong Z, Einhorn T, Watson JT, Ostrum RF, Prayson MJ, Della Rocca GJ, Mehta S, McKinley T, Wang Z, Steen RG, Epidemiology of Fracture Nonunion in 18 Human Bones, JAMA Surg 151(11) (2016) e162775. [DOI] [PubMed] [Google Scholar]
- [23].Timmen M, Hidding H, Wieskotter B, Baum W, Pap T, Raschke MJ, Schett G, Zwerina J, Stange R, Influence of antiTNF-alpha antibody treatment on fracture healing under chronic inflammation, BMC Musculoskelet Disord 15 (2014) 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Schmidt-Bleek K, Schell H, Schulz N, Hoff P, Perka C, Buttgereit F, Volk HD, Lienau J, Duda GN, Inflammatory phase of bone healing initiates the regenerative healing cascade, Cell Tissue Res 347(3) (2012) 567–73. [DOI] [PubMed] [Google Scholar]
- [25].Weckbach S, Hohmann C, Braumueller S, Denk S, Klohs B, Stahel PF, Gebhard F, Huber-Lang MS, Perl M, Inflammatory and apoptotic alterations in serum and injured tissue after experimental polytrauma in mice: distinct early response compared with single trauma or “double-hit” injury, J Trauma Acute Care Surg 74(2) (2013) 489–98. [DOI] [PubMed] [Google Scholar]
- [26].Liu M, Nakasaki M, Shih YV, Varghese S, Effect of age on biomaterial-mediated in situ bone tissue regeneration, Acta Biomater 78 (2018) 329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chung R, Foster BK, Zannettino AC, Xian CJ, Potential roles of growth factor PDGF-BB in the bony repair of injured growth plate, Bone 44(5) (2009) 878–85. [DOI] [PubMed] [Google Scholar]
- [28].Hwang NS, Zhang C, Hwang YS, Varghese S, Mesenchymal stem cell differentiation and roles in regenerative medicine, Wiley Interdiscip Rev Syst Biol Med 1(1) (2009) 97–106. [DOI] [PubMed] [Google Scholar]
- [29].Julier Z, Park AJ, Briquez PS, Martino MM, Promoting tissue regeneration by modulating the immune system, Acta Biomater 53 (2017) 13–28. [DOI] [PubMed] [Google Scholar]
- [30].Yuasa M, Mignemi NA, Nyman JS, Duvall CL, Schwartz HS, Okawa A, Yoshii T, Bhattacharjee G, Zhao C, Bible JE, Obremskey WT, Flick MJ, Degen JL, Barnett JV, Cates JM, Schoenecker JG, Fibrinolysis is essential for fracture repair and prevention of heterotopic ossification, J Clin Invest 125(8) (2015) 3117–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Edderkaoui B, Potential Role of Chemokines in Fracture Repair, Front Endocrinol (Lausanne) 8 (2017) 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gschwandtner M, Derler R, Midwood KS, More Than Just Attractive: How CCL2 Influences Myeloid Cell Behavior Beyond Chemotaxis, Front Immunol 10 (2019) 2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pountos I, Walters G, Panteli M, Einhorn TA, Giannoudis PV, Inflammatory Profile and Osteogenic Potential of Fracture Haematoma in Humans, J Clin Med 9(1) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ellis TN, Beaman BL, Interferon-gamma activation of polymorphonuclear neutrophil function, Immunology 112(1) (2004) 2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].McCauley J, Bitsaktsis C, Cottrell J, Macrophage subtype and cytokine expression characterization during the acute inflammatory phase of mouse bone fracture repair, J Orthop Res 38(8) (2020) 1693–1702. [DOI] [PubMed] [Google Scholar]
- [36].Huang R, Wang X, Zhou Y, Xiao Y, RANKL-induced M1 macrophages are involved in bone formation, Bone Res 5 (2017) 17019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hozain S, Cottrell J, CDllb+ targeted depletion of macrophages negatively affects bone fracture healing, Bone 138 (2020) 115479. [DOI] [PubMed] [Google Scholar]
- [38].Sun G, Wang Y, Ti Y, Wang J, Zhao J, Qian H, Regulatory B cell is critical in bone union process through suppressing proinflammatory cytokines and stimulating Foxp3 in Treg cells, Clin Exp Pharmacol Physiol 44(4) (2017) 455–462. [DOI] [PubMed] [Google Scholar]
- [39].Schlundt C, El Khassawna T, Serra A, Dienelt A, Wendler S, Schell H, van Rooijen N, Radbruch A, Lucius R, Hartmann S, Duda GN, Schmidt-Bleek K, Macrophages in bone fracture healing: Their essential role in endochondral ossification, Bone 106 (2018) 78–89. [DOI] [PubMed] [Google Scholar]
- [40].Schlundt C, Reinke S, Geissler S, Bucher CH, Giannini C, Mardian S, Dahne M, Kleber C, Samans B, Baron U, Duda GN, Volk HD, Schmidt-Bleek K, Individual Effector/Regulator T Cell Ratios Impact Bone Regeneration, Front Immunol 10 (2019) 1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Serhan CN, Chiang N, Van Dyke TE, Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators, Nat Rev Immunol 8(5) (2008) 349–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gresnigt MS, Joosten LA, Verschueren I, van der Meer JW, Netea MG, Dinarello CA, van de Veerdonk FL, Neutrophil-mediated inhibition of proinflammatory cytokine responses, J Immunol 189(10) (2012) 4806–15. [DOI] [PubMed] [Google Scholar]
- [43].Fernando MR, Reyes JL, Iannuzzi J, Leung G, McKay DM, The pro-inflammatory cytokine, interleukin-6, enhances the polarization of alternatively activated macrophages, PLoS One 9(4) (2014) e94188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Rincon M, Anguita J, Nakamura T, Fikrig E, Flavell RA, Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells, J Exp Med 185(3) (1997) 461–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Jin JO, Han X, Yu Q, Interleukin-6 induces the generation of IL-10-producing Tr1 cells and suppresses autoimmune tissue inflammation, J Autoimmun 40 (2013) 28–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ng TH, Britton GJ, Hill EV, Verhagen J, Burton BR, Wraith DC, Regulation of adaptive immunity; the role of interleukin-10, Front Immunol 4 (2013) 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Roszer T, Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms, Mediators Inflamm 2015 (2015) 816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Toben D, Schroeder I, El Khassawna T, Mehta M, Hoffmann JE, Frisch JT, Schell H, Lienau J, Serra A, Radbruch A, Duda GN, Fracture healing is accelerated in the absence of the adaptive immune system, J Bone Miner Res 26(1) (2011) 113–24. [DOI] [PubMed] [Google Scholar]
- [49].Zhang Q, Chen B, Yan F, Guo J, Zhu X, Ma S, Yang W, Interleukin-10 inhibits bone resorption: a potential therapeutic strategy in periodontitis and other bone loss diseases, Biomed Res Int 2014 (2014) 284836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Junttila IS, Tuning the Cytokine Responses: An Update on Interleukin (IL)-4 and IL-13 Receptor Complexes, Front Immunol 9 (2018) 888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, Johnson LL, Swain SL, Lund FE, Reciprocal regulation of polarized cytokine production by effector B and T cells, Nat Immunol 1(6) (2000) 475–82. [DOI] [PubMed] [Google Scholar]
- [52].Silfversward CJ, Sisask G, Larsson S, Ohlsson C, Frost A, Ljunggren O, Nilsson O, Bone formation in interleukin-4 and interleukin-13 depleted mice, Acta Orthop 79(3) (2008) 410–20. [DOI] [PubMed] [Google Scholar]
- [53].He XT, Li X, Xia Y, Yin Y, Wu RX, Sun HH, Chen FM, Building capacity for macrophage modulation and stem cell recruitment in high-stiffness hydrogels for complex periodontal regeneration: Experimental studies in vitro and in rats, Acta Biomater 88 (2019) 162–180. [DOI] [PubMed] [Google Scholar]
- [54].Rao AJ, Nich C, Dhulipala LS, Gibon E, Valladares R, Zwingenberger S, Smith RL, Goodman SB, Local effect of IL-4 delivery on polyethylene particle induced osteolysis in the murine calvarium, J Biomed Mater Res A 101(7) (2013) 1926–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hu Z, Ma C, Rong X, Zou S, Liu X, Immunomodulatory ECM-like Microspheres for Accelerated Bone Regeneration in Diabetes Mellitus, ACS Appl Mater Interfaces 10(3) (2018) 2377–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Xu X, Zheng L, Yuan Q, Zhen G, Crane JL, Zhou X, Cao X, Transforming growth factor-beta in stem cells and tissue homeostasis, Bone Res 6 (2018) 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Komai T, Inoue M, Okamura T, Morita K, Iwasaki Y, Sumitomo S, Shoda H, Yamamoto K, Fujio K, Transforming Growth Factor-beta and Interleukin-10 Synergistically Regulate Humoral Immunity via Modulating Metabolic Signals, Front Immunol 9 (2018) 1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Oh SA, Li MO, TGF-beta: guardian of T cell function, J Immunol 191(8) (2013) 3973–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, et al. , Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease, Nature 359(6397) (1992) 693–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Wu M, Chen G, Li YP, TGF-beta and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease, Bone Res 4 (2016) 16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Zhang XL, Topley N, Ito T, Phillips A, Interleukin-6 regulation of transforming growth factor (TGF)-beta receptor compartmentalization and turnover enhances TGF-beta1 signaling, J Biol Chem 280(13) (2005) 12239–45. [DOI] [PubMed] [Google Scholar]
- [62].Steensberg A, Fischer CP, Keller C, Moller K, Pedersen BK, IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans, Am J Physiol Endocrinol Metab 285(2) (2003) E433–7. [DOI] [PubMed] [Google Scholar]
- [63].Denton CP, Ong VH, Xu S, Chen-Harris H, Modrusan Z, Lafyatis R, Khanna D, Jahreis A, Siegel J, Sornasse T, Therapeutic interleukin-6 blockade reverses transforming growth factor-beta pathway activation in dermal fibroblasts: insights from the faSScinate clinical trial in systemic sclerosis, Ann Rheum Dis 77(9) (2018) 1362–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Mia S, Warnecke A, Zhang XM, Malmstrom V, Harris RA, An optimized protocol for human M2 macrophages using M-CSF and IL-4/IL-10/TGF-beta yields a dominant immunosuppressive phenotype, Scand J Immunol 79(5) (2014) 305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Bogdan C, Paik J, Vodovotz Y, Nathan C, Contrasting mechanisms for suppression of macrophage cytokine release by transforming growth factor-beta and interleukin-10, J Biol Chem 267(32) (1992) 23301–8. [PubMed] [Google Scholar]
- [66].Broide DH, Wasserman SI, Alvaro-Gracia J, Zvaifler NJ, Firestein GS, Transforming growth factor-beta 1 selectively inhibits IL-3-dependent mast cell proliferation without affecting mast cell function or differentiation, J Immunol 143(5) (1989) 1591–7. [PubMed] [Google Scholar]
- [67].Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM, Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3, J Exp Med 198(12) (2003) 1875–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Liu Z, Kuang W, Zhou Q, Zhang Y, TGF-beta1 secreted by M2 phenotype macrophages enhances the stemness and migration of glioma cells via the SMAD2/3 signalling pathway, Int J Mol Med 42(6) (2018) 3395–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kawai M, Rosen CJ, The insulin-like growth factor system in bone: basic and clinical implications, Endocrinol Metab Clin North Am 41(2) (2012) 323–33, vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Reible B, Schmidmaier G, Moghaddam A, Westhauser F, Insulin-Like Growth Factor-1 as a Possible Alternative to Bone Morphogenetic Protein-7 to Induce Osteogenic Differentiation of Human Mesenchymal Stem Cells in Vitro, Int J Mol Sci 19(6) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Wolters TLC, Netea MG, Hermus A, Smit JWA, Netea-Maier RT, IGF1 potentiates the pro-inflammatory response in human peripheral blood mononuclear cells via MAPK, J Mol Endocrinol 59(2) (2017) 129–139. [DOI] [PubMed] [Google Scholar]
- [72].Tonkin J, Temmerman L, Sampson RD, Gallego-Colon E, Barberi L, Bilbao D, Schneider MD, Musaro A, Rosenthal N, Monocyte/Macrophage-derived IGF-1 Orchestrates Murine Skeletal Muscle Regeneration and Modulates Autocrine Polarization, Mol Ther 23(7) (2015) 1189–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Arroba AI, Rodriguez-de la Rosa L, Murillo-Cuesta S, Vaquero-Villanueva L, Hurle JM, Varela-Nieto I, Valverde AM, Autophagy resolves early retinal inflammation in Igf1-deficient mice, Dis Model Mech 9(9) (2016) 965–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Serhan CN, Pro-resolving lipid mediators are leads for resolution physiology, Nature 510(7503) (2014) 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Basil MC, Levy BD, Specialized pro-resolving mediators: endogenous regulators of infection and inflammation, Nat Rev Immunol 16(1) (2016) 51–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kang JX, Wang J, Wu L, Kang ZB, Transgenic mice: fat-1 mice convert n-6 to n-3 fatty acids, Nature 427(6974) (2004) 504. [DOI] [PubMed] [Google Scholar]
- [77].Chen Y, Cao H, Sun D, Lin C, Wang L, Huang M, Jiang H, Zhang Z, Jin D, Zhang B, Bai X, Endogenous Production of n-3 Polyunsaturated Fatty Acids Promotes Fracture Healing in Mice, J Healthc Eng 2017 (2017) 3571267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Flower RJ, Perretti M, Controlling inflammation: a fat chance?, J Exp Med 201(5) (2005) 671–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Gao L, Faibish D, Fredman G, Herrera BS, Chiang N, Serhan CN, Van Dyke TE, Gyurko R, Resolvin E1 and chemokine-like receptor 1 mediate bone preservation, J Immunol 190(2) (2013) 689–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Tang S, Wan M, Huang W, Stanton RC, Xu Y, Maresins: Specialized Proresolving Lipid Mediators and Their Potential Role in Inflammatory-Related Diseases, Mediators Inflamm 2018 (2018) 2380319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Huang R, Vi L, Zong X, Baht GS, Maresin 1 resolves aged-associated macrophage inflammation to improve bone regeneration, FASEB J 34(10) (2020) 13521–13532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Geusens P, Emans PJ, de Jong JJ, van den Bergh J, NSAIDs and fracture healing, Curr Opin Rheumatol 25(4) (2013) 524–31. [DOI] [PubMed] [Google Scholar]
- [83].Goodman SB, Ma T, Mitsunaga L, Miyanishi K, Genovese MC, Smith RL, Temporal effects of a COX-2-selective NSAID on bone ingrowth, J Biomed Mater Res A 72(3) (2005) 279–87. [DOI] [PubMed] [Google Scholar]
- [84].Scher JU, Pillinger MH, The anti-inflammatory effects of prostaglandins, J Investig Med 57(6) (2009) 703–8. [DOI] [PubMed] [Google Scholar]
- [85].Serhan CN, Levy BD, Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators, J Clin Invest 128(7) (2018) 2657–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Sugimoto MA, Sousa LP, Pinho V, Perretti M, Teixeira MM, Resolution of Inflammation: What Controls Its Onset?, Front Immunol 7 (2016) 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Borea PA, Gessi S, Merighi S, Vincenzi F, Varani K, Pharmacology of Adenosine Receptors: The State of the Art, Physiol Rev 98(3) (2018) 1591–1625. [DOI] [PubMed] [Google Scholar]
- [88].Csoka B, Selmeczy Z, Koscso B, Nemeth ZH, Pacher P, Murray PJ, Kepka-Lenhart D, Morris SM Jr., Gause WC, Leibovich SJ, Hasko G, Adenosine promotes alternative macrophage activation via A2A and A2B receptors, FASEB J 26(1) (2012) 376–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Hankenson KD, Dishowitz M, Gray C, Schenker M, Angiogenesis in bone regeneration, Injury 42(6) (2011) 556–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Carroll SH, Wigner NA, Kulkarni N, Johnston-Cox H, Gerstenfeld LC, Ravid K, A2B adenosine receptor promotes mesenchymal stem cell differentiation to osteoblasts and bone formation in vivo, J Biol Chem 287(19) (2012) 15718–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Kara FM, Doty SB, Boskey A, Goldring S, Zaidi M, Fredholm BB, Cronstein BN, Adenosine A(1) receptors regulate bone resorption in mice: adenosine A(1) receptor blockade or deletion increases bone density and prevents ovariectomy-induced bone loss in adenosine A(1) receptor-knockout mice, Arthritis Rheum 62(2) (2010) 534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Katebi M, Soleimani M, Cronstein BN, Adenosine A2A receptors play an active role in mouse bone marrow-derived mesenchymal stem cell development, J Leukoc Biol 85(3) (2009) 438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Kang H, Shih YR, Varghese S, Biomineralized matrices dominate soluble cues to direct osteogenic differentiation of human mesenchymal stem cells through adenosine signaling, Biomacromolecules 16(3) (2015) 1050–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Kang H, Shih YR, Nakasaki M, Kabra H, Varghese S, Small molecule-driven direct conversion of human pluripotent stem cells into functional osteoblasts, Sci Adv 2(8) (2016) e1600691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Rao V, Shih YR, Kang H, Kabra H, Varghese S, Adenosine Signaling Mediates Osteogenic Differentiation of Human Embryonic Stem Cells on Mineralized Matrices, Front Bioeng Biotechnol 3 (2015) 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Shih YR, Hwang Y, Phadke A, Kang H, Hwang NS, Caro EJ, Nguyen S, Siu M, Theodorakis EA, Gianneschi NC, Vecchio KS, Chien S, Lee OK, Varghese S, Calcium phosphate-bearing matrices induce osteogenic differentiation of stem cells through adenosine signaling, Proc Natl Acad Sci U S A 111(3) (2014) 990–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Gharibi B, Abraham AA, Ham J, Evans BA, Adenosine receptor subtype expression and activation influence the differentiation of mesenchymal stem cells to osteoblasts and adipocytes, J Bone Miner Res 26(9) (2011) 2112–24. [DOI] [PubMed] [Google Scholar]
- [98].Takedachi M, Oohara H, Smith BJ, Iyama M, Kobashi M, Maeda K, Long CL, Humphrey MB, Stoecker BJ, Toyosawa S, Thompson LF, Murakami S, CD73-generated adenosine promotes osteoblast differentiation, J Cell Physiol 227(6) (2012) 2622–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Shih YV, Liu M, Kwon SK, Iida M, Gong Y, Sangaj N, Varghese S, Dysregulation of ectonucleotidase-mediated extracellular adenosine during postmenopausal bone loss, Sci Adv 5(8) (2019) eaax1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Costa MA, Barbosa A, Neto E, Sa-e-Sousa A, Freitas R, Neves JM, Magalhaes-Cardoso T, Ferreirinha F, Correia-de-Sa P, On the role of subtype selective adenosine receptor agonists during proliferation and osteogenic differentiation of human primary bone marrow stromal cells, J Cell Physiol 226(5) (2011) 1353–66. [DOI] [PubMed] [Google Scholar]
- [101].Bradaschia-Correa V, Josephson AM, Egol AJ, Mizrahi MM, Leclerc K, Huo J, Cronstein BN, Leucht P, Ecto-5 '-nucleotidase (CD73) regulates bone formation and remodeling during intramembranous bone repair in aging mice, Tissue Cell 49(5) (2017) 545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Mediero A, Wilder T, Perez-Aso M, Cronstein BN, Direct or indirect stimulation of adenosine A2A receptors enhances bone regeneration as well as bone morphogenetic protein-2, FASEB J 29(4) (2015) 1577–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Karladani AH, Granhed H, Karrholm J, Styf J, The influence of fracture etiology and type on fracture healing: a review of 104 consecutive tibial shaft fractures, Arch Orthop Trauma Surg 121(6) (2001) 325–8. [DOI] [PubMed] [Google Scholar]
- [104].Lim JC, Ko KI, Mattos M, Fang M, Zhang C, Feinberg D, Sindi H, Li S, Alblowi J, Kayal RA, Einhorn TA, Gerstenfeld LC, Graves DT, TNFalpha contributes to diabetes impaired angiogenesis in fracture healing, Bone 99 (2017) 26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Baker RG, Hayden MS, Ghosh S, NF-kappaB, inflammation, and metabolic disease, Cell Metab 13(1) (2011) 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Relja B, Yang B, Bundkirchen K, Xu B, Kohler K, Neunaber C, Different experimental multiple trauma models induce comparable inflammation and organ injury, Sci Rep 10(1) (2020) 20185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Behrends DA, Hui D, Gao C, Awlia A, Al-Saran Y, Li A, Henderson JE, Martineau PA, Defective Bone Repair in C57Bl6 Mice With Acute Systemic Inflammation, Clin Orthop Relat Res 475(3) (2017) 906–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Schell H, Thompson MS, Bail HJ, Hoffmann JE, Schill A, Duda GN, Lienau J, Mechanical induction of critically delayed bone healing in sheep: radiological and biomechanical results, J Biomech 41(14) (2008) 3066–72. [DOI] [PubMed] [Google Scholar]
- [109].Sokolove J, Lepus CM, Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations, Ther Adv Musculoskelet Dis 5(2) (2013) 77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Clark M, Kroger CJ, Tisch RM, Type 1 Diabetes: A Chronic Anti-Self-Inflammatory Response, Front Immunol 8 (2017) 1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Bruunsgaard H, Pedersen M, Pedersen BK, Aging and proinflammatory cytokines, Curr Opin Hematol 8(3) (2001) 131–6. [DOI] [PubMed] [Google Scholar]
- [112].Sohn DH, Sokolove J, Sharpe O, Erhart JC, Chandra PE, Lahey LJ, Lindstrom TM, Hwang I, Boyer KA, Andriacchi TP, Robinson WH, Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4, Arthritis Res Ther 14(1) (2012) R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y, Obesity and inflammation: the linking mechanism and the complications, Arch Med Sci 13(4) (2017) 851–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS, Kavanaugh A, McInnes IB, Solomon DH, Strand V, Yamamoto K, Rheumatoid arthritis, Nat Rev Dis Primers 4 (2018) 18001. [DOI] [PubMed] [Google Scholar]
- [115].Gao F, Lv TR, Zhou JC, Qin XD, Effects of obesity on the healing of bone fracture in mice, J Orthop Surg Res 13(1) (2018) 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Kayal RA, Siqueira M, Alblowi J, McLean J, Krothapalli N, Faibish D, Einhorn TA, Gerstenfeld LC, Graves DT, TNF-alpha mediates diabetes-enhanced chondrocyte apoptosis during fracture healing and stimulates chondrocyte apoptosis through FOXO1, J Bone Miner Res 25(7) (2010) 1604–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Alblowi J, Kayal RA, Siqueira M, McKenzie E, Krothapalli N, McLean J, Conn J, Nikolajczyk B, Einhorn TA, Gerstenfeld L, Graves DT, High levels of tumor necrosis factor-alpha contribute to accelerated loss of cartilage in diabetic fracture healing, Am J Pathol 175(4) (2009) 1574–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Kayal RA, Tsatsas D, Bauer MA, Allen B, Al-Sebaei MO, Kakar S, Leone CW, Morgan EF, Gerstenfeld LC, Einhorn TA, Graves DT, Diminished bone formation during diabetic fracture healing is related to the premature resorption of cartilage associated with increased osteoclast activity, J Bone Miner Res 22(4) (2007) 560–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Fontaine JL, Hunt NA, Curry S, Kearney T, Jupiter D, Shibuya N, Lavery LA, Fracture healing and biomarker expression in a diabetic Zucker rat model, J Am Podiatr Med Assoc 104(5) (2014) 428–33. [DOI] [PubMed] [Google Scholar]
- [120].Ko KI, Syverson AL, Kralik RM, Choi J, DerGarabedian BP, Chen C, Graves DT, Diabetes-Induced NF-kappaB Dysregulation in Skeletal Stem Cells Prevents Resolution of Inflammation, Diabetes 68(11) (2019) 2095–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Robinson CM, Court-Brown CM, McQueen MM, Wakefield AE, Estimating the risk of nonunion following nonoperative treatment of a clavicular fracture, J Bone Joint Surg Am 86(7) (2004) 1359–65. [DOI] [PubMed] [Google Scholar]
- [122].Court-Brown CM, McQueen MM, Nonunions of the proximal humerus: their prevalence and functional outcome, J Trauma 64(6) (2008) 1517–21. [DOI] [PubMed] [Google Scholar]
- [123].Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G, Inflamm-aging. An evolutionary perspective on immunosenescence, Ann N Y Acad Sci 908 (2000) 244–54. [DOI] [PubMed] [Google Scholar]
- [124].Balistreri CR, Candore G, Accardi G, Colonna-Romano G, Lio D, NF-kappaB pathway activators as potential ageing biomarkers: targets for new therapeutic strategies, Immun Ageing 10(1) (2013) 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Olivieri F, Prattichizzo F, Grillari J, Balistreri CR, Cellular Senescence and Inflammaging in Age-Related Diseases, Mediators Inflamm 2018 (2018) 9076485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Campisi J, d’Adda di Fagagna F, Cellular senescence: when bad things happen to good cells, Nat Rev Mol Cell Biol 8(9) (2007) 729–40. [DOI] [PubMed] [Google Scholar]
- [127].Behmoaras J, Gil J, Similarities and interplay between senescent cells and macrophages, J Cell Biol 220(2) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Slade Shantz JA, Yu YY, Andres W, Miclau T 3rd, Marcucio R, Modulation of macrophage activity during fracture repair has differential effects in young adult and elderly mice, J Orthop Trauma 28 Suppl 1 (2014) S10–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Clark D, Brazina S, Yang F, Hu D, Hsieh CL, Niemi EC, Miclau T, Nakamura MC, Marcucio R, Age-related changes to macrophages are detrimental to fracture healing in mice, Aging Cell 19(3) (2020) e13112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Zhao SJ, Kong FQ, Fan J, Chen Y, Zhou S, Xue MX, Yin GY, Bioinformatics Analysis of the Molecular Mechanism of Aging on Fracture Healing, Biomed Res Int 2018 (2018) 7530653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Hebb JH, Ashley JW, McDaniel L, Lopas LA, Tobias J, Hankenson KD, Ahn J, Bone healing in an aged murine fracture model is characterized by sustained callus inflammation and decreased cell proliferation, J Orthop Res 36(1) (2018) 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Loffler J, Sass FA, Filter S, Rose A, Ellinghaus A, Duda GN, Dienelt A, Compromised Bone Healing in Aged Rats Is Associated With Impaired M2 Macrophage Function, Front Immunol 10 (2019) 2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Vi L, Baht GS, Soderblom EJ, Whetstone H, Wei Q, Furman B, Puviindran V, Nadesan P, Foster M, Poon R, White JP, Yahara Y, Ng A, Barrientos T, Grynpas M, Mosely MA, Alman BA, Macrophage cells secrete factors including LRP1 that orchestrate the rejuvenation of bone repair in mice, Nat Commun 9(1) (2018) 5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Mantuano E, Brifault C, Lam MS, Azmoon P, Gilder AS, Gonias SL, LDL receptor-related protein-1 regulates NFkappaB and microRNA-155 in macrophages to control the inflammatory response, Proc Natl Acad Sci U S A 113(5) (2016) 1369–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Potere N, Del Buono MG, Mauro AG, Abbate A, Toldo S, Low Density Lipoprotein Receptor-Related Protein-1 in Cardiac Inflammation and Infarct Healing, Front Cardiovasc Med 6 (2019) 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Wujak L, Schnieder J, Schaefer L, Wygrecka M, LRP1: A chameleon receptor of lung inflammation and repair, Matrix Biol 68–69 (2018) 366–381. [DOI] [PubMed] [Google Scholar]
- [137].Zeng Y, Hoque J, Varghese S, Biomaterial-assisted local and systemic delivery of bioactive agents for bone repair, Acta Biomater 93 (2019) 152–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Yang F, Zhang X, Song L, Cui H, Myers JN, Bai T, Zhou Y, Chen Z, Gu N, Controlled drug release and hydrolysis mechanism of polymer-magnetic nanoparticle composite, ACS Appl Mater Interfaces 7(18) (2015) 9410–9. [DOI] [PubMed] [Google Scholar]
- [139].Gu G, Xia H, Hu Q, Liu Z, Jiang M, Kang T, Miao D, Tu Y, Pang Z, Song Q, Yao L, Chen H, Gao X, Chen J, PEG-co-PCL nanoparticles modified with MMP-2/9 activatable low molecular weight protamine for enhanced targeted glioblastoma therapy, Biomaterials 34(1) (2013) 196–208. [DOI] [PubMed] [Google Scholar]
- [140].Kar M, Vernon Shih YR, Velez DO, Cabrales P, Varghese S, Poly(ethylene glycol) hydrogels with cell cleavable groups for autonomous cell delivery, Biomaterials 77 (2016) 186–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Di J, Yao S, Ye Y, Cui Z, Yu J, Ghosh TK, Zhu Y, Gu Z, Stretch-Triggered Drug Delivery from Wearable Elastomer Films Containing Therapeutic Depots, ACS Nano 9(9) (2015) 9407–15. [DOI] [PubMed] [Google Scholar]
- [142].Abdul Ameer LA, Raheem ZJ, Abdulrazaq SS, Ali BG, Nasser MM, Khairi AWA, The anti-inflammatory effect of the platelet-rich plasma in the periodontal pocket, Eur J Dent 12(4) (2018) 528–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Zhu L, Luo D, Liu Y, Effect of the nano/microscale structure of biomaterial scaffolds on bone regeneration, Int J Oral Sci 12(1) (2020) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Litwiniuk M, Krejner A, Speyrer MS, Gauto AR, Grzela T, Hyaluronic Acid in Inflammation and Tissue Regeneration, Wounds 28(3) (2016) 78–88. [PubMed] [Google Scholar]
- [145].Fong D, Hoemann CD, Chitosan immunomodulatory properties: perspectives on the impact of structural properties and dosage, Future Sci OA 4(1) (2018) FSO225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Pacelli S, Manoharan V, Desalvo A, Lomis N, Jodha KS, Prakash S, Paul A, Tailoring biomaterial surface properties to modulate host-implant interactions: implication in cardiovascular and bone therapy, J Mater Chem B 4 (2016) 1586–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Amani H, Arzaghi H, Bayandori M, Dezfuli AS, Pazoki-Toroudi H, Shafiee A, Moradi L, Controlling Cell Behavior through the Design of Biomaterial Surfaces: A Focus on Surface Modification Techniques, Advanced Materials Interfaces 6(13) (2019) 1900572. [Google Scholar]
- [148].Ayala R, Zhang C, Yang D, Hwang Y, Aung A, Shroff SS, Arce FT, Lal R, Arya G, Varghese S, Engineering the cell-material interface for controlling stem cell adhesion, migration, and differentiation, Biomaterials 32(15) (2011) 3700–11. [DOI] [PubMed] [Google Scholar]
- [149].Gonzalez Diaz EC, Shih YV, Nakasaki M, Liu M, Varghese S, Mineralized Biomaterials Mediated Repair of Bone Defects Through Endogenous Cells, Tissue Eng Part A 24(13–14) (2018) 1148–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Barrere F, van Blitterswijk CA, de Groot K, Bone regeneration: molecular and cellular interactions with calcium phosphate ceramics, Int J Nanomedicine 1(3) (2006) 317–32. [PMC free article] [PubMed] [Google Scholar]
- [151].Velard F, Braux J, Amedee J, Laquerriere P, Inflammatory cell response to calcium phosphate biomaterial particles: an overview, Acta Biomater 9(2) (2013) 4956–63. [DOI] [PubMed] [Google Scholar]
- [152].Humbert P, Brennan MA, Davison N, Rosset P, Trichet V, Blanchard F, Layrolle P, Immune Modulation by Transplanted Calcium Phosphate Biomaterials and Human Mesenchymal Stromal Cells in Bone Regeneration, Front Immunol 10 (2019) 663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].MacLeod RJ, Hayes M, Pacheco I, Wnt5a secretion stimulated by the extracellular calcium-sensing receptor inhibits defective Wnt signaling in colon cancer cells, Am J Physiol Gastrointest Liver Physiol 293(1) (2007) G403–11. [DOI] [PubMed] [Google Scholar]
- [154].Li Z, Zhang K, Li X, Pan H, Li S, Chen F, Zhang J, Zheng Z, Wang J, Liu H, Wnt5a suppresses inflammation-driven intervertebral disc degeneration via a TNF-alpha/NF-kappaB-Wnt5a negative-feedback loop, Osteoarthritis Cartilage 26(7) (2018) 966–977. [DOI] [PubMed] [Google Scholar]
- [155].Yang X, Zhao S, Yuan H, Shi R, Gu W, Gu Z, Lu X, Zhao H, Knockdown of Ror2 suppresses TNFalphainduced inflammation and apoptosis in vascular endothelial cells, Mol Med Rep 22(4) (2020) 2981–2989. [DOI] [PubMed] [Google Scholar]
- [156].Vishwakarma A, Bhise NS, Evangelista MB, Rouwkema J, Dokmeci MR, Ghaemmaghami AM, Vrana NE, Khademhosseini A, Engineering Immunomodulatory Biomaterials To Tune the Inflammatory Response, Trends Biotechnol 34(6) (2016) 470–482. [DOI] [PubMed] [Google Scholar]
- [157].Andorko JI, Jewell CM, Designing biomaterials with immunomodulatory properties for tissue engineering and regenerative medicine, Bioeng Transl Med 2(2) (2017) 139–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Hotchkiss KM, Clark NM, Olivares-Navarrete R, Macrophage response to hydrophilic biomaterials regulates MSC recruitment and T-helper cell populations, Biomaterials 182 (2018) 202–215. [DOI] [PubMed] [Google Scholar]
- [159].Kajahn J, Franz S, Rueckert E, Forstreuter I, Hintze V, Moeller S, Simon JC, Artificial extracellular matrices composed of collagen I and high sulfated hyaluronan modulate monocyte to macrophage differentiation under conditions of sterile inflammation, Biomatter 2(4) (2012) 226–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Luo M, Zhao F, Liu L, Yang Z, Tian T, Chen X, Cao X, Chen D, Chen X, IFN-gamma/SrBG composite scaffolds promote osteogenesis by sequential regulation of macrophages from M1 to M2, J Mater Chem B 9(7) (2021) 1867–1876. [DOI] [PubMed] [Google Scholar]
- [161].Mansour A, Abu-Nada L, Al-Waeli H, Mezour MA, Abdallah MN, Kinsella JM, Kort-Mascort J, Henderson JE, Ramirez-Garcialuna JL, Tran SD, Elkashty OA, Mousa A, El-Hadad AA, Taqi D, Al-Hamad F, Alageel O, Kaartinen MT, Tamimi F, Bone extracts immunomodulate and enhance the regenerative performance of dicalcium phosphates bioceramics, Acta Biomater 89 (2019) 343–358. [DOI] [PubMed] [Google Scholar]
- [162].Diez-Escudero A, Espanol M, Bonany M, Lu X, Persson C, Ginebra MP, Heparinization of Beta Tricalcium Phosphate: Osteo-immunomodulatory Effects, Adv Healthc Mater 7(5) (2018). [DOI] [PubMed] [Google Scholar]
- [163].Lin T, Pajarinen J, Kohno Y, Maruyama M, Romero-Lopez M, Huang JF, Nathan K, Khan TN, Yao Z, Goodman SB, Transplanted interleukin-4--secreting mesenchymal stromal cells show extended survival and increased bone mineral density in the murine femur, Cytotherapy 20(8) (2018) 1028–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Zheng ZW, Chen YH, Wu DY, Wang JB, Lv MM, Wang XS, Sun J, Zhang ZY, Development of an Accurate and Proactive Immunomodulatory Strategy to Improve Bone Substitute Material-Mediated Osteogenesis and Angiogenesis, Theranostics 8(19) (2018) 5482–5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Wynn TA, Vannella KM, Macrophages in Tissue Repair, Regeneration, and Fibrosis, Immunity 44(3) (2016) 450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Lee JY, Kim KH, Shin SY, Rhyu IC, Lee YM, Park YJ, Chung CP, Lee SJ, Enhanced bone formation by transforming growth factor-beta1-releasing collagen/chitosan microgranules, J Biomed Mater Res A 76(3) (2006) 530–9. [DOI] [PubMed] [Google Scholar]
- [167].Vehof JW, Fisher JP, Dean D, van der Waerden JP, Spauwen PH, Mikos AG, Jansen JA, Bone formation in transforming growth factor beta-1-coated porous poly(propylene fumarate) scaffolds, J Biomed Mater Res 60(2) (2002) 241–51. [DOI] [PubMed] [Google Scholar]
- [168].Liu JMH, Zhang J, Zhang X, Hlavaty KA, Ricci CF, Leonard JN, Shea LD, Gower RM, Transforming growth factor-beta 1 delivery from microporous scaffolds decreases inflammation post-implant and enhances function of transplanted islets, Biomaterials 80 (2016) 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Shi D, Xiao J, Gu R, Wu G, Liao H, Polyurethane conjugating TGF-beta on surface impacts local inflammation and endoplasmic reticulum stress in skeletal muscle, J Biomed Mater Res A 105(4) (2017) 1156–1165. [DOI] [PubMed] [Google Scholar]
- [170].Toung JS, Ogle RC, Morgan RF, Lindsey WH, Insulinlike growth factor 1- and 2-augmented collagen gel repair of facial osseous defects, Arch Otolaryngol Head Neck Surg 125(4) (1999) 451–5. [DOI] [PubMed] [Google Scholar]
- [171].Werz O, Gerstmeier J, Libreros S, De la Rosa X, Werner M, Norris PC, Chiang N, Serhan CN, Human macrophages differentially produce specific resolvin or leukotriene signals that depend on bacterial pathogenicity, Nat Commun 9(1) (2018) 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Vasconcelos DP, Costa M, Neves N, Teixeira JH, Vasconcelos DM, Santos SG, Aguas AP, Barbosa MA, Barbosa JN, Chitosan porous 3D scaffolds embedded with resolvin D1 to improve in vivo bone healing, J Biomed Mater Res A 106(6) (2018) 1626–1633. [DOI] [PubMed] [Google Scholar]
- [173].Yin C, Zhao Q, Li W, Zhao Z, Wang J, Deng T, Zhang P, Shen K, Li Z, Zhang Y, Biomimetic anti-inflammatory nano-capsule serves as a cytokine blocker and M2 polarization inducer for bone tissue repair, Acta Biomater 102 (2020) 416–426. [DOI] [PubMed] [Google Scholar]
- [174].Surh YJ, Na HK, Park JM, Lee HN, Kim W, Yoon IS, Kim DD, 15-Deoxy-Delta(1)(2),(1)(4)-prostaglandin J(2), an electrophilic lipid mediator of anti-inflammatory and pro-resolving signaling, Biochem Pharmacol 82(10) (2011) 1335–51. [DOI] [PubMed] [Google Scholar]
- [175].Shibata T, Kondo M, Osawa T, Shibata N, Kobayashi M, Uchida K, 15-deoxy-delta 12,14-prostaglandin J2. A prostaglandin D2 metabolite generated during inflammatory processes, J Biol Chem 277(12) (2002) 10459–66. [DOI] [PubMed] [Google Scholar]
- [176].Buckner MM, Antunes LC, Gill N, Russell SL, Shames SR, Finlay BB, 15-Deoxy-Delta12,14-prostaglandin J2 inhibits macrophage colonization by Salmonella enterica serovar Typhimurium, PLoS One 8(7) (2013) e69759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Tang Q, Chen LL, Wei F, Sun WL, Lei LH, Ding PH, Tan JY, Chen XT, Wu YM, Effect of 15-Deoxy-Delta(12,14)-prostaglandin J2Nanocapsules on Inflammation and Bone Regeneration in a Rat Bone Defect Model, Chin Med J (Engl) 130(3) (2017) 347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Nam JW, Kwon JI, Hong JY, Kim HJ, The effect of two locally administered anti-resorptive agents on bone regeneration in a rat fibula model: Alendronate and 15-deoxy-Delta(12,14)-prostaglandin J2, J Craniomaxillofac Surg 47(11) (2019) 1758–1766. [DOI] [PubMed] [Google Scholar]
- [179].Wendler S, Schlundt C, Bucher CH, Birkigt J, Schipp CJ, Volk HD, Duda GN, Schmidt-Bleek K, Immune Modulation to Enhance Bone Healing-A New Concept to Induce Bone Using Prostacyclin to Locally Modulate Immunity, Front Immunol 10 (2019) 713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Dorris SL, Peebles RS Jr., PGI2 as a regulator of inflammatory diseases, Mediators Inflamm 2012 (2012) 926968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Romano M, Cianci E, Simiele F, Recchiuti A, Lipoxins and aspirin-triggered lipoxins in resolution of inflammation, Eur J Pharmacol 760 (2015) 49–63. [DOI] [PubMed] [Google Scholar]
- [182].Van Dyke TE, Hasturk H, Kantarci A, Freire MO, Nguyen D, Dalli J, Serhan CN, Proresolving nanomedicines activate bone regeneration in periodontitis, J Dent Res 94(1) (2015) 148–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Cardoso GB, Chacon E, Chacon PG, Bordeaux-Rego P, Duarte AS, Saad STO, Zavaglia CA, Cunha MR, Fatty acid is a potential agent for bone tissue induction: In vitro and in vivo approach, Exp Biol Med (Maywood) 242(18) (2017) 1765–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Palomer X, Pizarro-Delgado J, Barroso E, Vazquez-Carrera M, Palmitic and Oleic Acid: The Yin and Yang of Fatty Acids in Type 2 Diabetes Mellitus, Trends Endocrinol Metab 29(3) (2018) 178–190. [DOI] [PubMed] [Google Scholar]
- [185].Zheng X, Wang D, The Adenosine A2A Receptor Agonist Accelerates Bone Healing and Adjusts Treg/Th17 Cell Balance through Interleukin 6, Biomed Res Int 2020 (2020) 2603873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Kang H, Kim M, Feng Q, Lin S, Wei K, Li R, Choi CJ, Kim TH, Li G, Oh JM, Bian L, Nanolayered hybrid mediates synergistic co-delivery of ligand and ligation activator for inducing stem cell differentiation and tissue healing, Biomaterials 149 (2017) 12–28. [DOI] [PubMed] [Google Scholar]
- [187].Ishack S, Mediero A, Wilder T, Ricci JL, Cronstein BN, Bone regeneration in critical bone defects using three-dimensionally printed beta-tricalcium phosphate/hydroxyapatite scaffolds is enhanced by coating scaffolds with either dipyridamole or BMP-2, J Biomed Mater Res B Appl Biomater 105(2) (2017) 366–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Witek L, Alifarag AM, Tovar N, Lopez CD, Cronstein BN, Rodriguez ED, Coelho PG, Repair of Critical-Sized Long Bone Defects Using Dipyridamole-Augmented 3D-Printed Bioactive Ceramic Scaffolds, J Orthop Res 37(12) (2019) 2499–2507. [DOI] [PubMed] [Google Scholar]
- [189].Hoque J, Sangaj N, Varghese S, Stimuli-Responsive Supramolecular Hydrogels and Their Applications in Regenerative Medicine, Macromol Biosci 19(1) (2019) e1800259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Lee JS, Suarez-Gonzalez D, Murphy WL, Mineral coatings for temporally controlled delivery of multiple proteins, Adv Mater 23(37) (2011) 4279–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Belair DG, Le NN, Murphy WL, Design of growth factor sequestering biomaterials, Chem Commun (Camb) 50(99) (2014) 15651–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Morochnik S, Zhu Y, Duan C, Cai M, Reid RR, He TC, Koh J, Szleifer I, Ameer GA, A thermoresponsive, citrate-based macromolecule for bone regenerative engineering, J Biomed Mater Res A 106(6) (2018) 1743–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Acuitive Technologies Granted FDA 510(k) Clearance for CITREGEN™ CITRELOCK™ Tendon Interference Screw System https://www.prnewswire.com/news-releases/acuitive-technologies-granted-fda-510k-clearance-for-citregen-citrelock-tendon-interference-screw-system-301156417.html. 2021).


