Abstract
Diabetes mellitus (DM) and also anemia are common in the elderly and have a negative impact on the clinical outcomes of patients. The coexistence of anemia and DM seems to be insufficiently recognized; therefore, the aim of our study is to analyze the incidence and clinical consequences of this coexistence, including mortality, in the population of people aged ≥60. A retrospective study was conducted on 981 primary care clinic patients aged ≥60 during 2013-2014. The prevalence of coexistence of DM and anemia (defined in accordance with WHO) and data on the incidence of comorbidities, hospitalization, medical procedures, and all-cause mortality were analyzed. In the study population, 25% had DM, while 5.4% had both DM and anemia. Peripheral artery disease (PAD) was found in 48 patients (4.89%) of the entire study population, more often in men (p < 0.001). Diabetic patients with anemia compared to nonanemic diabetics had more comorbidities (median 4 (4, 5) vs. 3 (2–4); p < 0.001)—PAD more often (p = 0.004), more hospitalization (median 2 (0–11) vs. 0 (0–11); p < 0.001), and more frequent medical procedures (e.g., percutaneous coronary intervention (p < 0.001), coronary artery bypass surgery (p = 0.027), arteriography (p < 0.001), and bypass surgery or endovascular treatments of lower limb ischemia (p < 0.001)). The cumulative survival of patients with both DM and anemia vs. nonanemic diabetics at 36 months was 86.4% vs. 99.3% (p < 0.001). A multivariate logistic regression model showed anemia to be a significant risk factor for death in diabetic patients (p = 0.013). Patients with both DM and anemia have more comorbidities than nonanemic diabetic patients; they are more often hospitalized, require medical procedures more frequently, and are at a higher risk of death. Effective treatment of anemia in patients with DM is advisable and may well improve the prognosis of patients.
1. Introduction
Both diabetes mellitus (DM) and anemia are common in the elderly population, and both are associated with the severity of the course of many comorbidities (especially cardiovascular diseases) and an increase in the risk of death [1–5]. In 2019, 463 million people worldwide were diagnosed with diabetes and approximately 20% of them were in their 60s [6]. The prevalence of anemia in the elderly population ranges from about 3% to over 60% [7]. The long-term course of diabetes leads to micro- and macrovascular damage, which negatively affects the circulatory system, nervous system, kidneys, and eyes [8]. DM is a known factor influencing the development of atherosclerosis and as a result the course of diseases such as peripheral arterial disease (PAD) or coronary heart disease. Anemia also, especially in elderly patients, has a negative impact on the circulatory system and leads to left ventricular hypertrophy [9], exacerbation of symptoms of ischemic heart disease, heart failure [10, 11], or the formation of atherosclerotic changes in the vessels [12]. The most common etiologies of anemia in the elderly are anemia of chronic diseases (ACD), unexplained anemia, and iron deficiency anemia [13]. A significant group of elderly anemic patients remains untreated due to the lack of a known cause of the decrease in hemoglobin (Hb) [14]; hence, anemia can negatively affect health for many years.
The coexistence of anemia and diabetes is not often discussed in literature, and this problem seems to be insufficiently recognized [15]. Therefore, the aim of our study is to analyze the frequency of the simultaneous occurrence of anemia and DM and to study the clinical consequences of this coexistence, including mortality in the population of people aged ≥60.
2. Materials and Methods
This is a retrospective analysis of the data of patients aged ≥60 years who were under the care of the primary care clinic in Poland for the years 2013-2014. The collected data (laboratory tests, diseases, treatments, hospitalizations, and medical procedures) come from the medical records of primary care clinics, specialist consultation cards, and patient discharge records. The patients' data after the age of 60 were analyzed.
Anemia was defined according to WHO criteria (Hb < 13 g/dL in men, Hb < 12 g/dL in women) [16]. The definitions of the types of anemia, severity, and definitions of diseases were presented earlier [1]. Diseases were defined in accordance with the recommendations of international societies which were in force at the time of diagnosis [17–27]. DM was diagnosed on the basis of random blood glucose levels ≥ 11.1 mmol/L (200 mg/dL), in the presence of typical symptoms of diabetes (such as increased thirst, polyuria, and weakness) or twice confirmed blood glucose after fasting ≥ 7.0 mmol/L (126 mg/dL), blood glucose values ≥ 11.1 mmol/L (200 mg/dL) at 120 minutes after an oral glucose load of 75 g. Using antidiabetic drugs and/or insulin confirmed DM [28]. DM with complications was defined as diabetes with the presence of at least one of the macroangiopathic complications (presence of ischemic heart disease, lower extremity ischemia, and stroke history) or microangiopathic complications (presence of diabetic nephropathy, diabetic neuropathy, diabetic retinopathy, and diabetic foot). Complicated arterial hypertension was diagnosed in the presence of subclinical or clinical signs of organ damage in the course of arterial hypertension.
The frequency of the following medical procedures was recorded: coronary angiography, percutaneous coronary intervention, coronary artery bypass grafting, and device therapy (implantable cardioverter defibrillator (ICD), cardiac resynchronization therapy (CRT)). The frequency of lower extremity amputations due to ischemia and other invasive treatments of lower extremity arterial disease were also analyzed: bypass surgery and endovascular treatment (percutaneous angioplasty with stenting, balloon angioplasty).
Data on all-cause mortality in patients between January 2013 and December 2014 were obtained from reports by the National Health Fund. In death, analysis of the risk factors in diabetic patients, various details were taken into account: age, presence of anemia, selected comorbidities, some laboratory tests, history of acute coronary syndrome before age 60, and stroke before age 60.
The study was compiled in accordance with the Declaration of Helsinki and was approved by the Bioethical Committee of Poznan University of Medical Sciences.
2.1. Statistical Analysis
Interval data is presented as means and standard deviations. In this case, the data does not follow normal distribution; the descriptive statistics are presented as medians and interquartile ranges. The numerical data was compared using the Mann–Whitney test. Categorical data were analyzed by the chi-square test, and its results are presented as numbers and percentages or odds ratios (OR) and 95% CI. To find significant factors that may increase the risk of occurrence of anemia in diabetic patients, a logistic regression was performed. The results are presented as OR and 95% CI. The logistic regression was performed both as a univariate and multivariate model. The survival was performed with the Kaplan-Meier estimate. The differences between survival curves were analyzed by the log-rank test. The influence of the studied parameters on the risk of death was done by the Cox proportional hazard model. The results are presented as hazard ratio (HR) and its 95% CI.
The analysis was performed using the statistical package TIBCO Software Inc. (2017); Statistica (data analysis software system), version 13 (http://statistica.io). All tests were considered significant at p < 0.05.
3. Results
In the group of 981 patients in the study (aged ≥60 years), there were 245 diabetic patients (140 women; 105 men)—24.97% of the entire study population (Figure 1). The majority of the diabetic group had type 2 DM (81%), of whom 47% were receiving insulin.
Figure 1.

Study population.
DM with complications was found in 143 patients (74 women: 51.75%; 69 men: 48.25%)—58.37% of the diabetic group. 53 patients had both anemia and DM, including 40 (75.47%) patients with DM with complications, whereas the diabetics without anemia amounted to 192 patients (of whom 103 (53.65%) have DM with complications). The demographic characteristics of the patients with DM are presented in Table 1.
Table 1.
Characteristics of the diabetic group.
| Diabetes n = 245 |
Diabetes with anemia n = 53 |
Diabetes without anemia n = 192 |
p value | |
|---|---|---|---|---|
| Female, n (%) | 140 (57.1) | 28 (52.8) | 112 (58.3) | 0.475 |
| Age mean ± SD | 71.4 ± 8.28 | 73.5 ± 8.51 | 70.8 ± 8.14 | |
| Age median (LQ-UQ) | 69.9 (64.26-77.93) | 74.6 (65.53-81.02) | 68.7 (63.97-77.55) | 0.294 |
LQ: lower quartile; UQ: upper quartile.
The patients with DM with complications were more than 2.5 times more likely to have anemia (OR = 2.659; 95% CI 1.337–5.286; p = 0.004). Women with DM with complications suffered from anemia 4 times more often than men (OR = 4.231; 95% CI 1.594–11.23; p = 0.002).
3.1. Comorbidities
The group with both DM and anemia had more comorbidities and was more often hospitalized compared to the nonanemic diabetic patients (respectively; p < 0.001) (Table 2).
Table 2.
Differences in the number of comorbidities and the frequency of hospitalization.
| Variable | Diabetes with anemia | Diabetes without anemia | p value | ||
|---|---|---|---|---|---|
| Median (min–max) | Lower-upper quartile | Median (min–max) | Lower-upper quartile | ||
| Number of comorbidities | 4 (1–9) | 4–5 | 3 (1–8) | 2–4 | <0.001 |
| Number of hospitalizations | 2 (0–11) | 1–4 | 0 (0–11) | 0–1 | <0.001 |
The clinical characteristics of diabetic patients are presented in Table 3. Significant differences were found only in the case of PAD (p = 0.004) and hypertension with complications (90.6% of patients with both DM and anemia; 63.5% of patients with DM without anemia) (p < 0.001). In the entire study population of 981 patients, PAD was found in 48 (4.89%) patients, including 17 (2.86%) women and 31 (8.01%) men (p < 0.001). In the group of patients with DM, PAD was found in 18 (17.14%) men and 7 (5%) women (p = 0.002).
Table 3.
Clinical characteristics of diabetic patients with and without anemia.
| Characteristics | Diabetes n = 245 |
Diabetes with anemia n = 53 |
Diabetes without anemia n = 192 |
p value |
|---|---|---|---|---|
| Level of hemoglobin in the blood | ||||
| Female (g/dL), mean ± SD | 12.97 ± 1.76 | 10.09 ± 1.33 | 13.69 ± 0.91 | <0.001 |
| Male (g/dL), mean ± SD | 13.99 ± 1.85 | 11.34 ± 1.43 | 14.81 ± 0.98 | <0.001 |
|
| ||||
| Other laboratory tests | ||||
| WBC (109/L), mean ± SD | 7.33 ± 1.97 | 7.3 ± 2.27 | 7.34 ± 1.89 | 0.312 |
| RBC (1012/L), mean ± SD | 4.43 ± 0.60 | 3.7 ± 0.54 | 4.63 ± 0.44 | 0.301 |
| PLT (109/L), mean ± SD | 236.37 ± 83.69 | 276.77 ± 130.58 | 225.57 ± 61.95 | 0.288 |
| ESR (mm/h), mean ± SD | 24.33 ± 22.80 | 37.56 ± 32.75 | 19.96 ± 16.38 | 0.034 |
| CRP (mg/L), mean ± SD | 24.18 ± 56.94 | 46.93 ± 78.07 | 4.57 ± 7.27 | 0.288 |
|
| ||||
| eGFR leveln(%) | ||||
| eGFR < 60 mL/min/1.73 m2 | 46 (20.5) | 16 (34.04) | 30 (16.95) | 0.010 |
| eGFR ≥ 60 mL/min/1.73 m2 | 178 (75.5) | 31 (65.96) | 147 (83.05) | 0.010 |
|
| ||||
| Comorbiditiesn(%) | ||||
| Hypertension | 232 (94.7) | 51 (96.2) | 181 (94.3) | 0.582 |
| Hypertension with complications | 170 (69.4) | 48 (90.6) | 122 (63.5) | 0.001 |
| Coronary heart disease | 121 (49.4) | 31 (58.5) | 90 (46.9) | 0.265 |
| Heart failure | 33 (13.5) | 17 (32.1) | 16 (8.3) | 0.092 |
| Atrial fibrillation | 29 (11.8) | 10 (18.9) | 19 (9.9) | 0.495 |
| PAD | 25 (10.2) | 13 (24.53) | 12 (6.25) | 0.004 |
| Venous thromboembolism | 6 (2.4) | 2 (3.8) | 4 (2.1) | 0.903 |
| Thyroid diseases | 47 (19.2) | 14 (26.4) | 33 (17.2) | 0.469 |
| Pulmonary disease | 25 (10.2) | 8 (15.1) | 17 (8.9) | 0.640 |
| Asthma | 13 (5.3) | 3 (5.7) | 10 (5.2) | 0.976 |
| COPD | 8 (3.3) | 3 (5.7) | 5 (2.6) | 0.826 |
| Chronic kidney disease | 41 (16.7) | 18 (34) | 23 (12) | 0.090 |
| Chronic liver diseases | 15 (6.1) | 10 (18.9) | 5 (2.6) | 0.384 |
| Rheumatic diseases | 0 (0) | 0 (0) | 0 (0) | — |
| Dementia | 6 (2.4) | 2 (3.8) | 4 (2.1) | 0.903 |
| Cancer | 29 (11.8) | 13 (24.5) | 16 (8.3) | 0.232 |
|
| ||||
| Drugsn(%) | ||||
| Insulin | 60 (24.5) | 20 (37.7) | 40 (20.8) | 0.011 |
| Metformin | 91 (37.1) | 20 (37.7) | 71 (37) | 0.951 |
| ACE inhibitors | 111 (45.3) | 38 (71.7) | 73 (38) | <0.001 |
| Aspirin | 106 (43.3) | 36 (67.9) | 70 (36.5) | <0.001 |
| DOACs+VKA | 27 (11) | 13 (24.5) | 14 (7.3) | 0.001 |
ALT: aminotransferase alanine; AST: aminotransferase aspartate; COPD: chronic obstructive pulmonary disease; CRP: C-reactive protein; DOACs: direct oral anticoagulants; ESR: erythrocyte sedimentation rate; PAD: peripheral artery disease; PLT: platelets; VKA: vitamin K antagonists; WBC: white blood cells; RBC: red blood cells.
3.2. Medical Procedures
In the anemic diabetic group, the following procedures were conducted significantly more often: coronary angiography (p < 0.001), percutaneous coronary intervention (p < 0.001), coronary artery bypass surgery (p = 0.027), arteriography (p < 0.001), and invasive treatment of lower extremity ischemia (bypass surgery or endovascular treatments; p < 0.001) (Table 4). Arteriography and coronary angiography were performed significantly more often in diabetic patients in comparison with nondiabetic patients (p = 0.027).
Table 4.
Comparison of the frequency of selected medical procedures in different patient groups.
| Procedures | Diabetes n (%) |
Diabetes with anemia n (%) |
Diabetes without anemia n (%) |
p value |
|---|---|---|---|---|
| Coronary angiography | 56 (22.9) | 25 (47.2) | 31 (16.1) | <0.001 |
| Percutaneous coronary intervention | 34 (13.9) | 15 (28.3) | 19 (9.9) | 0.001 |
| Coronary artery bypass surgery | 10 (4.1) | 5 (9.4) | 5 (2.6) | 0.027 |
| Arteriography | 13 (5.3) | 8 (15.1) | 5 (2.6) | <0.001 |
| Invasive treatment of chronic lower extremity ischemia∗ | 9 (3.7) | 7 (13.2) | 2 (1.0) | <0.001 |
| Lower extremity amputations | 2 (0.8) | 1 (1.9) | 1 (0.5) | 0.328 |
| Device therapies∗∗ | 9 (3.7) | 3 (5.7) | 6 (3.1) | 0.385 |
∗Bypass surgery and endovascular treatment (percutaneous angioplasty with stenting, balloon angioplasty). ∗∗Implantable cardioverter defibrillator (ICD) and cardiac resynchronization therapy (CRT).
3.3. Characteristics of Anemia in the DM Group
The three most common types of anemia in the diabetic group were unexplained anemia (32.1%), anemia of chronic diseases (28.3%), and iron deficiency anemia (18.9%). Other types of anemia were presented as follows: chemo- and/or radiotherapy-induced anemia (9.4%), renal insufficiency anemia (3.8%), vitamin B12 deficiency anemia (1.9%), iron and vit. B12 deficiency anemia (1.9%), vit. B12 and folate deficiency anemia (1.9%), and hemorrhagic anemia (1.9%).
Mild anemia was the most prevalent (71.7%); the others were moderate anemia in 22.6%, severe in 3.8%, and very severe in 1.9%.
3.4. All-Cause Mortality
Of the 245 diabetic patients, 7 (2.86%) died during the 36-month follow-up, including 6 (11.32%) in the anemic diabetic group and 1 (0.52%) in the nonanemic diabetic group.
Cumulative survival probability rate after 36 months in both groups, anemic diabetics vs. nonanemic diabetics, was 86.4% vs. 99.3%, respectively (p < 0.001) (Figure 2).
Figure 2.
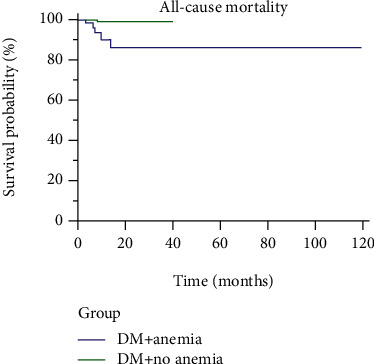
Cumulative survival probability of the anemic and nonanemic diabetic patients.
3.5. Risk Factors for Death in a Diabetic Patient
A number of risk factors for death in diabetic patients were examined (Table 5), and the multivariate regression analysis showed anemia to be the only significant risk factor (p = 0.013).
Table 5.
Death risk factors in diabetic patients: the univariate and multivariate analysis.
| Parameter/variables | HR | 95% CI | p value |
|---|---|---|---|
| Univariate analysis | |||
| Sex (female) | 3.957 | 0.462–33.870 | 0.209 |
| Age (years) | |||
| 70–79 | 1.985 | 0.124–31.848 | 0.628 |
| ≥80 | 10.771 | 1.202–96.531 | 0.034 |
| Anemia | 18.442 | 2.151–158.080 | 0.008 |
| Heart failure | 0.239 | 0.003–18.869 | 0.521 |
| Coronary heart disease | 1.803 | 0.330–9.853 | 0.496 |
| Cancer | 4.228 | 0.774–23.099 | 0.096 |
| Acute coronary syndrome before age 60 | 2.619 | 0.306–22.449 | 0.380 |
| Stroke before age 60 | 0 | 0 | 0.996 |
| Atrial fibrillation | 1.644 | 0.192–14.085 | 0.650 |
| Chronic kidney disease | 0 | 0 | 0.996 |
| Pulmonary disease (asthma, COPD) | 2.077 | 0.241–17.858 | 0.505 |
| PAD | 0.770 | 0.180–3.240 | 0.774 |
| ESR | 1.020 | 0.980–1.050 | 0.211 |
|
| |||
| Multivariate analysis | |||
| Anemia | 15.232 | 1.767–131.295 | 0.013 |
ESR: erythrocyte sedimentation rate; CI: confidence interval; COPD: chronic obstructive pulmonary disease; HR: hazard ratio; PAD: peripheral artery disease.
4. Discussion
The prevalence of diabetes is increasing worldwide, and the problem is repeatedly referred to as the diabetes pandemic [29]. The estimated global average prevalence of DM in elderly patients was 19.3% in 2019 [30]. In our study population of 981 outpatients aged ≥60, one quarter of patients suffered from DM and the coexistence of anemia and DM was found in 21.6% of patients. This is in accordance with the studies conducted in China (22%) [31], Australia (23.3%) [32], and Ethiopia (19%) [33]. Some studies reported more frequent simultaneous occurrence of anemia and DM, e.g., hospitalized patients in Ethiopia (34.8%) [34] and endocrinology department outpatients in Iran (30.4%) [35]. Even higher incidence was recorded in Tobago (in the Caribbean) (46.5%) [36], significantly higher in the group of outpatients aged ≥75 in the United Kingdom (59%) [37], and the highest in hospitalized patients in the internal medicine department in Pakistan (63%) [38].
Differences in the frequency coexistence of anemia and DM may be due to a number of factors. Based on the current literature, it can be concluded that this coexistence is more often found in patients who are hospitalized or patients under the care of specialized clinics. The age of the studied patients is of high importance (the prevalence of anemia and DM increases with age) as well as their race (the Negroid race is more prone to the coexistence of both diseases). Other possible factors are the duration of anemia, the type of DM, and coexistence and severity of CKD. The level of health care, the economic situation of the country in which the study was conducted, and the geographical location (altitude) are also of importance.
The pathogenesis of anemia in diabetes is multifactorial and is associated mainly with the presence of chronic inflammation, diabetic nephropathy, hyporeninemia, and nutritional deficiencies [15, 39]. Vitamin B12 and folate deficiency is frequently observed among diabetic patients [15, 40]. Erythropoietin (EPO) deficiency and/or resistance, iron deficiencies (resulting from reduced dietary intake, impaired enteral absorption, blood loss), and proteinuria (with loss of transferrin or EPO) are mechanisms leading to anemia development in patients with diabetic kidney disease [41, 42]. In men with type 2 DM, obesity, and insulin resistance, low testosterone levels are often found, which may also increase the risk of anemia [43]. In the course of DM, especially type 2, the expression of proinflammatory cytokines (mainly IL-6) and CRP increases [44]. Both anemia and DM are associated with inflammation, and our study showed that patients with anemia and DM more frequently presented increased ESR. Hyperglycemia and obesity are important contributors to inflammation, which result in EPO deficiency or lack of iron availability for erythropoiesis. Apart from exacerbating inflammation, hyperglycemia can also affect cardiovascular complications, e.g., increase the size of some types of infarct [45]. The common coexisting conditions in type 1 diabetes are autoimmune diseases (especially celiac disease, Addison's disease, Hashimoto's thyroiditis, and autoimmune gastritis) which may lead to anemia [15, 46]. Diabetic acidosis promotes the occurrence of hemolytic anemia in patients with congenital deficiency of glucose-6-phosphate dehydrogenase or in sickle cell anemia [47]. The treatment of diabetes can also contribute to the onset of anemia, e.g., the use of biguanides [48], thiazolidinediones [49], and renin-angiotensin aldosterone (RAA) system blockers [50]. Data from a randomized controlled trial determined the association between the use of metformin and the risk of anemia in type 2 DM. The mechanism of this phenomenon remains unknown so far, and vitamin B12 deficiency does not seem to be the only reason [51]. However, not all diabetic patients are anemic. Certain correlation between insulin resistance and erythropoiesis has been observed. Patients with a higher insulin resistance had a higher erythrocyte count [52]. A proper diet and treatment of DM can reduce inflammation and thus have a positive impact on erythropoiesis and atherosclerotic progression. Metformin combined with a low-calorie diet has been shown to reduce the inflammation and oxidative stress associated with obesity and insulin resistance in prediabetic patients [53]. GLP-1 therapy reduces the inflammation and oxidative stress of atherosclerotic plaque, which probably affects its stability [54]. Sodium glucose cotransporter-2 (SGLT2) inhibitors can improve erythropoiesis, reducing the risk of anemia or the need to use erythropoiesis-stimulating agents (ESA) [55]. Canagliflozin in diabetic patients with nephropathy leads to an increase in reticulocyte count, erythrocyte count, and hematocrit [56], which is mediated by EPO [56]. SGLT2 inhibitors may also counterbalance the negative effects of RAA system blockers on erythropoiesis [50].
Anemia leads to hypoxia and the impairment of many tissues and organs, especially the circulatory and renal. Poorer cardiovascular and renal functions, in turn, are factors contributing to anemia [41, 42, 57]. This results in a “vicious cycle” and seems to be an important factor in the coexistence of anemia and diabetes. Additionally, anemia causes falsely low levels of glycosylated hemoglobin in diabetic patients and as a consequence of a lack of proper treatment, which may lead to complications of DM [58].
Strong associations of the coexistence of DM and anemia with diabetic nephropathy, neuropathy, and retinopathy [35, 59] as well as a significant number of comorbidities with this coexistence [60–62] have been reported previously. The links between anemia and diabetes and the complications can be partially explained by the influence of EPO, which apart from affecting the hematopoiesis has a number of other metabolic effects. EPO receptors have been detected in various tissues, especially in adipose tissue, pancreas, brain tissue, and in the retina. It is believed that the presence of EPO receptors in the hypothalamus regulates metabolism of glucose, while in adipose tissue it has an anti-inflammatory effect and increases insulin sensitivity [63]. However, additional studies to better understand the key pathomechanisms and consequences of the coexistence of anemia and diabetes are required.
Our results demonstrated that patients with DM and anemia (compared to the nonanemic diabetics) had more comorbidities (most commonly DM with complications, hypertension with complications, and PAD), were more often hospitalized, and had a higher risk of death. A decrease in Hb in diabetic patients increases the risk of hospitalization and death, as has been shown in previous studies [11, 64, 65]. Patients with DM have an increased risk of death in general from any cause [66, 67], but mainly from cardiovascular diseases [3, 59]. The main factors are the duration of DM and the age of patients [66]. In a retrospective 10-year analysis of patients over 65 years of age, the increased risk of death in diabetic patients was approximately 70% versus without DM [68]. Anemia seems to be a risk multiplier for all-cause mortality [41]. In our analysis, patients with anemia and DM were not only hospitalized more often but also more often underwent various medical procedures, such as coronary angiography, percutaneous coronary intervention, coronary artery bypass surgery, arteriography, and invasive treatment of chronic lower limb ischemia (bypass surgery, endovascular treatments). Our results cast a new light on the consequences of anemia in diabetic patients. To our knowledge, the previous studies only covered medical procedures performed in patients with either anemia or diabetes and not in the presence of both disorders. A Polish study which analyzed patients who underwent vascular angioplasty procedures showed the anemic patients had multivessel disease in angiography, more often experienced myocardial infarction, more frequently needed coronary artery bypass graft, and had a higher risk of death during the one-year follow-up [69]. Anselmino and colleagues showed that patients with DM required percutaneous coronary intervention or coronary artery bypass grafting more often than nondiabetic patients [70].
The findings of our study revealed that 4.89% of all included elderly patients had PAD and it was diagnosed more often in men than in women. Based on the literature, it is estimated that the incidence of PAD is 3-10%, is higher in men, and increases with age [27]. Our study confirms previously established data that in diabetic patients the incidence of PAD is about 2 times more frequent coexisting than in nondiabetic patients. Our research showed that patients with DM and anemia vs. nonanemic diabetics suffered from PAD more often; they more frequently had invasive procedures related to lower limb ischemia, such as bypass surgery and endovascular treatment. Invasive procedures for PAD are performed when the patient presents clinical symptoms. Anemia may worsen these symptoms. There is a mutual correlation between anemia, DM, and medical procedures. Medical procedures may affect the development and severity of anemia. While aggravating symptoms of circulatory system, anemia may be a deciding factor when evaluating the need to perform medical procedures.
Our study has its limitations, mostly due to the retrospective character of the analysis. There was no possibility to obtain some missing information, such as the duration of DM and some laboratory data. The data comes from a healthcare clinic, where there was only a small group of patients with CKD. However, the study examined almost 1000 patients and detailed data was collected on many parameters such as patients' diseases, hospitalization, medical procedures, and death.
5. Conclusions
The comparison of the two groups with both DM and anemia vs. nonanemic diabetics showed the following characteristics of the former group: more comorbidities, including PAD, more frequent hospitalizations, and more various medical procedures performed. Our study confirmed that anemia has a negative impact on the survival of patients with DM. We believe anemia to be one of the underrecognized risk factors for DM. Therefore, proper treatment of anemia in patients with DM is highly recommended, as it may positively affect the course of DM and the patients' prognosis. Further studies on the coexistence of anemia and diabetes are needed.
Data Availability
The reader can access the data by correspondence with the authors.
Conflicts of Interest
All authors declare that they have no conflict of interest.
References
- 1.Michalak S. S., Rupa-Matysek J., Gil L. Comorbidities, repeated hospitalizations, and age ≥80 years as indicators of anemia development in the older population. Annals of Hematology . 2018;97(8):1337–1347. doi: 10.1007/s00277-018-3321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barr E. L. M., Zimmet P. Z., Welborn T. A., et al. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab) Circulation . 2007;116(2):151–157. doi: 10.1161/CIRCULATIONAHA.106.685628. [DOI] [PubMed] [Google Scholar]
- 3.Yu O. H. Y., Suissa S. Identifying causes for excess mortality in patients with diabetes: closer but not there yet. Diabetes Care . 2016;39(11):1851–1853. doi: 10.2337/dci16-0026. [DOI] [PubMed] [Google Scholar]
- 4.Olum R., Bongomin F., Kaggwa M. M., Andia-Biraro I., Baluku J. B. Anemia in diabetes mellitus in Africa: a systematic review and meta-analysis. Diabetes and Metabolic Syndrome: Clinical Research & Reviews . 2021;15(5):p. 102260. doi: 10.1016/j.dsx.2021.102260. [DOI] [PubMed] [Google Scholar]
- 5.Katwal P. C., Jirjees S., Htun Z. M., Aldawudi I., Khan S. The effect of anemia and the goal of optimal HbA1c control in diabetes and non-diabetes. Cureus . 2020;12(6, article e8431) doi: 10.7759/cureus.8431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saeedi P., Petersohn I., Salpea P., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas. Diabetes research and clinical practice . 2019;157, article 107843 doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 7.Beghé C., Wilson A., Ershler W. B. Prevalence and outcomes of anemia in geriatrics: a systematic review of the literature. The American Journal of Medicine . 2004;116(7):3–10. doi: 10.1016/j.amjmed.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Harding J. L., Pavkov M. E., Magliano D. J., Shaw J. E., Gregg E. W. Global trends in diabetes complications: a review of current evidence. Diabetologia . 2019;62(1):3–16. doi: 10.1007/s00125-018-4711-2. [DOI] [PubMed] [Google Scholar]
- 9.Levin A., Singer J., Thompson C. R., Ross H., Lewis M. Prevalent left ventricular hypertrophy in the predialysis population: identifying opportunities for intervention. American Journal of Kidney Diseases . 1996;27(3):347–354. doi: 10.1016/S0272-6386(96)90357-1. [DOI] [PubMed] [Google Scholar]
- 10.Szachniewicz J., Petruk-Kowalczyk J., Majda J., et al. Anaemia is an independent predictor of poor outcome in patients with chronic heart failure. International Journal of Cardiology . 2003;90(2–3):303–308. doi: 10.1016/S0167-5273(02)00574-0. [DOI] [PubMed] [Google Scholar]
- 11.McClellan W. M., Flanders W. D., Langston R. D., Jurkovitz C., Presley R. Anemia and renal insufficiency are independent risk factors for death among patients with congestive heart failure admitted to community hospitals: a population-based study. Journal of the American Society of Nephrology . 2002;13(7):1928–1936. doi: 10.1097/01.ASN.0000018409.45834.FA. [DOI] [PubMed] [Google Scholar]
- 12.Grammer T. B., Kleber M. E., Silbernagel G., et al. Hemoglobin, iron metabolism and angiographic coronary artery disease (the Ludwigshafen risk and cardiovascular health study) Atherosclerosis . 2014;236(2):292–300. doi: 10.1016/j.atherosclerosis.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Bianchi V. E. Anemia in the elderly population. Journal of Hematology . 2014;3(4):95–106. doi: 10.14740/jh182w. [DOI] [Google Scholar]
- 14.Michalak S. S., Rupa-Matysek J., Hus I., Gil L. Unexplained anemia in the elderly – a real life analysis of 981 patients. Archives of medical science: AMS . 2019;15(1) doi: 10.5114/aoms.2019.82723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angelousi A., Larger E. Anaemia, a common but often unrecognized risk in diabetic patients: a review. Diabetes & Metabolism . 2015;41(1):18–27. doi: 10.1016/j.diabet.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Blanc B., Finch C., Hallberg L., Herbert V. Nutritional anaemias. Report of a WHO scientific group. World Health Organization Technical Report Series . 1968;405:5–37. [PubMed] [Google Scholar]
- 17.McMurray J. J. V., Adamopoulos S., Anker S. D., et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. European heart journal . 2012;14(8):803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 18.Mancia G., Fagard R., Narkiewicz K., et al. 2013 ESH/ESC guidelines for the management of arterial hypertension. Journal of Hypertension . 2013;31(7):1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 19.Stevens P. E., Levin A., Disease K. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Annals of internal medicine . 2013;158(11):825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 20.Cosentino F., Grant P. J., Aboyans V., et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. European Heart Journal . 2020;41(2):255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 21.Vestbo J., Hurd S. S., Agustí A. G., et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. American Journal of Respiratory and Critical Care Medicine . 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 22.Boulet L.-P., FitzGerald J. M., Levy M. L., et al. A guide to the translation of the Global Initiative for Asthma (GINA) strategy into improved care. The European Respiratory Journal . 2012;39(5):1220–1229. doi: 10.1183/09031936.00184511. [DOI] [PubMed] [Google Scholar]
- 23.Edge S. B., Compton C. C. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Annals of Surgical Oncology . 2010;17(6):1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 24.Aletaha D., Neogi T., Silman A. J., et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis and Rheumatism . 2010;62(9):2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 25.Runyon B. A. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology . 2013;57(4):1651–1653. doi: 10.1002/hep.26359. [DOI] [PubMed] [Google Scholar]
- 26.News| ESMO clinical practice guidelines |ESMO. 2018. http://www.esmo.org/Guidelines/Guidelines-News .
- 27.Norgren L., Hiatt W. R., Dormandy J. A., Nehler M. R., Harris K. A., Fowkes F. G. R. Inter-society consensus for the management of peripheral arterial disease (TASC II) Journal of vascular surgery . 2007;33(1):S1–75. doi: 10.1016/j.ejvs.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 28.Task Force Members, Rydén L., Grant P. J., et al. ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. European heart journal . 2013;34(39):3035–3087. doi: 10.1093/eurheartj/eht108. [DOI] [PubMed] [Google Scholar]
- 29.Dragan L., Alexia P., Ioanna Z., Haralambos G., Andreas P., Athanasios M. The growing epidemic of diabetes mellitus. Current Vascular Pharmacology . 2020;18(2):104–109. doi: 10.2174/1570161117666190405165911. [DOI] [PubMed] [Google Scholar]
- 30.Sinclair A., Saeedi P., Kaundal A., Karuranga S., Malanda B., Williams R. Diabetes and global ageing among 65-99-year-old adults: findings from the International Diabetes Federation Diabetes Atlas. Diabetes research and clinical practice . 2020;162, article 108078 doi: 10.1016/j.diabres.2020.108078. [DOI] [PubMed] [Google Scholar]
- 31.He B. B., Xu M., Wei L., et al. Relationship between anemia and chronic complications in Chinese patients with type 2 diabetes mellitus. Archives of Iranian Medicine . 2015;18(5):277–283. [PubMed] [Google Scholar]
- 32.Thomas M. C., Cooper M. E., Tsalamandris C., MacIsaac R., Jerums G. Anemia with impaired erythropoietin response in diabetic patients. Archives of Internal Medicine . 2005;165(4):466–469. doi: 10.1001/archinte.165.4.466. [DOI] [PubMed] [Google Scholar]
- 33.Abate A., Birhan W., Alemu A. Association of anemia and renal function test among diabetes mellitus patients attending Fenote Selam Hospital, West Gojam, Northwest Ethiopia: a cross sectional study. BMC Blood Disorders . 2013;13(1):1–7. doi: 10.1186/2052-1839-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bekele A., Teji Roba K., Egata G., Gebremichael B. Anemia and associated factors among type-2 diabetes mellitus patients attending public hospitals in Harari region, eastern Ethiopia. PloS One . 2019;14(12, article e0225725) doi: 10.1371/journal.pone.0225725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hosseini M. S., Rostami Z., Saadat A., Saadatmand S. M., Naeimi E. Anemia and microvascular complications in patients with type 2 diabetes mellitus. Nephro-urology monthly . 2014;6(4, article e19976) doi: 10.5812/numonthly.19976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ezenwaka C. E., Jones-LeCointe A., Nwagbara E., Seales D., Okali F. Anaemia and kidney dysfunction in Caribbean type 2 diabetic patients. Cardiovascular Diabetology . 2008;7(1):p. 25. doi: 10.1186/1475-2840-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trevest K., Treadway H. Prevalence and determinants of anemia in older people with diabetes attending an outpatient clinic: a cross-sectional audit. Clinical diabetes: a publication of the American Diabetes Association . 2014;32(4):158–162. doi: 10.2337/diaclin.32.4.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shams N., Osmani M. H. Newly diagnosed anemia in admitted diabetics, frequency, etiology and associated factors. Journal of the College of Physicians and Surgeons--Pakistan : JCPSP . 2015;25(4):242–246. [PubMed] [Google Scholar]
- 39.Thomas M. C. Anemia in diabetes: marker or mediator of microvascular disease? Nature Clinical Practice Nephrology . 2007;3(1):20–30. doi: 10.1038/ncpneph0378. [DOI] [PubMed] [Google Scholar]
- 40.Kibirige D., Mwebaze R. Vitamin B12 deficiency among patients with diabetes mellitus: is routine screening and supplementation justified? Journal of Diabetes and Metabolic Disorders . 2013;12(1):p. 17. doi: 10.1186/2251-6581-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mehdi U., Toto R. D. Anemia, diabetes, and chronic kidney disease. Diabetes Care . 2009;32(7):1320–1326. doi: 10.2337/dc08-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanna R. M., Streja E., Kalantar-Zadeh K. Burden of anemia in chronic kidney disease: beyond erythropoietin. Advances in Therapy . 2021;38(1):52–75. doi: 10.1007/s12325-020-01524-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grossmann M., Thomas M. C., Panagiotopoulos S., et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. The Journal of Clinical Endocrinology and Metabolism . 2008;93(5):1834–1840. doi: 10.1210/jc.2007-2177. [DOI] [PubMed] [Google Scholar]
- 44.Pradhan A. D., Manson J. E., Rifai N., Buring J. E., Ridker P. M. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. Journal of the American Medical Association . 2001;286(3):327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 45.Paolisso P., Foà A., Bergamaschi L., et al. Hyperglycemia, inflammatory response and infarct size in obstructive acute myocardial infarction and MINOCA. Cardiovascular Diabetology . 2021;20(1):p. 33. doi: 10.1186/s12933-021-01222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Devendra D., Eisenbarth G. S. 17\. Immunologic endocrine disorders. The Journal of Allergy and Clinical Immunology . 2003;111(2):S624–S636. doi: 10.1067/mai.2003.81. [DOI] [PubMed] [Google Scholar]
- 47.Meloni G., Meloni T. Glyburide-induced acute haemolysis in a G6PD-deficient patient with NIDDM. British Journal of Haematology . 1996;92(1):159–160. doi: 10.1046/j.1365-2141.1996.275810.x. [DOI] [PubMed] [Google Scholar]
- 48.Ting R. Z.-W., Szeto C. C., Chan M. H.-M., Ma K. K., Chow K. M. Risk factors of vitamin B (12) deficiency in patients receiving metformin. Archives of Internal Medicine . 2006;166(18):1975–1979. doi: 10.1001/archinte.166.18.1975. [DOI] [PubMed] [Google Scholar]
- 49.Berria R., Glass L., Mahankali A., et al. Reduction in hematocrit and hemoglobin following pioglitazone treatment is not hemodilutional in type II diabetes mellitus. Clinical Pharmacology and Therapeutics . 2007;82(3):275–281. doi: 10.1038/sj.clpt.6100146. [DOI] [PubMed] [Google Scholar]
- 50.Marathias K. P., Lambadiari V. A., Markakis K. P., et al. Competing effects of renin angiotensin system blockade and sodium-glucose cotransporter-2 inhibitors on erythropoietin secretion in diabetes. American Journal of Nephrology . 2020;51(5):349–356. doi: 10.1159/000507272. [DOI] [PubMed] [Google Scholar]
- 51.Donnelly L. A., Dennis J. M., Coleman R. L., et al. Risk of anemia with metformin use in type 2 diabetes: a MASTERMIND study. Diabetes Care . 2020;43(10):2493–2499. doi: 10.2337/dc20-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barbieri M., Ragno E., Benvenuti E., et al. New aspects of the insulin resistance syndrome: impact on haematological parameters. Diabetologia . 2001;44(10):1232–1237. doi: 10.1007/s001250100634. [DOI] [PubMed] [Google Scholar]
- 53.Sardu C., Pieretti G., D’Onofrio N., et al. Inflammatory cytokines and SIRT1 levels in subcutaneous abdominal fat: relationship with cardiac performance in overweight pre-diabetics patients. Frontiers in physiology . 2018;9, article 1030 doi: 10.3389/fphys.2018.01030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balestrieri M. L., Rizzo M. R., Barbieri M., et al. Sirtuin 6 expression and inflammatory activity in diabetic atherosclerotic plaques: effects of incretin treatment. Diabetes . 2015;64(4):1395–1406. doi: 10.2337/db14-1149. [DOI] [PubMed] [Google Scholar]
- 55.Oshima M., Neuen B. L., Jardine M. J., et al. Effects of canagliflozin on anaemia in patients with type 2 diabetes and chronic kidney disease: a post-hoc analysis from the CREDENCE trial. The Lancet Diabetes and Endocrinology . 2020;8(11):903–914. doi: 10.1016/S2213-8587(20)30300-4. [DOI] [PubMed] [Google Scholar]
- 56.Maruyama T., Takashima H., Oguma H., et al. Canagliflozin improves erythropoiesis in diabetes patients with anemia of chronic kidney disease. Diabetes Technology & Therapeutics . 2019;21(12):713–720. doi: 10.1089/dia.2019.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deray G., Heurtier A., Grimaldi A., Launay Vacher V., Isnard B. C. Anemia and diabetes. American Journal of Nephrology . 2004;24(5):522–526. doi: 10.1159/000081058. [DOI] [PubMed] [Google Scholar]
- 58.Camargo J. L., Gross J. L. Conditions associated with very low values of glycohaemoglobin measured by an HPLC method. Journal of Clinical Pathology . 2004;57(4):346–349. doi: 10.1136/jcp.2002.007088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomas M. C., Tsalamandris C., MacIsaac R. J., Jerums G. The epidemiology of hemoglobin levels in patients with type 2 diabetes. American journal of kidney diseases . 2006;48(4):537–545. doi: 10.1053/j.ajkd.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 60.Zoppini G., Targher G., Chonchol M., et al. Anaemia, independent of chronic kidney disease, predicts all-cause and cardiovascular mortality in type 2 diabetic patients. Atherosclerosis . 2010;210(2):575–580. doi: 10.1016/j.atherosclerosis.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 61.Gambert S. R., Pinkstaff S. Emerging epidemic: diabetes in older adults: demography, economic impact, and pathophysiology. Diabetes Spectrum: A Publication of the American Diabetes Association . 2006;19(4):221–228. doi: 10.2337/diaspect.19.4.221. [DOI] [Google Scholar]
- 62.Kalyani R. R., Saudek C. D., Brancati F. L., Selvin E. Association of diabetes, comorbidities, and A1C with functional disability in older adults: results from the National Health and Nutrition Examination Survey (NHANES), 1999-2006. Diabetes Care . 2010;33(5):1055–1060. doi: 10.2337/dc09-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woo M., Hawkins M. Beyond erythropoiesis: emerging metabolic roles of erythropoietin. Diabetes . 2014;63(7):2229–2231. doi: 10.2337/db14-0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Holland D. C., Lam M. Predictors of hospitalization and death among pre-dialysis patients: a retrospective cohort study. Nephrology Dialysis Transplantation . 2000;15(5):650–658. doi: 10.1093/ndt/15.5.650. [DOI] [PubMed] [Google Scholar]
- 65.Collins A. J., Ma J. Z., Ebben J. Impact of hematocrit on morbidity and mortality. Seminars in Nephrology . 2000;20(4):345–349. [PubMed] [Google Scholar]
- 66.Huang E. S., Laiteerapong N., Liu J. Y., John P. M., Moffet H. H., Karter A. J. Rates of complications and mortality in older patients with diabetes mellitus: the diabetes and aging study. JAMA Internal Medicine . 2014;174(2):251–258. doi: 10.1001/jamainternmed.2013.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rao Kondapally Seshasai S., Kaptoge S., Thompson A., et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. The New England Journal of Medicine . 2011;364(9):829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chi M.-J., Liang C.-K., Lee W.-J., Peng L.-N., Chou M.-Y., Chen L.-K. Association of new-onset diabetes mellitus in older people and mortality in Taiwan: a 10-year nationwide population-based study. The Journal of Nutrition, Health & Aging . 2017;21(2):227–232. doi: 10.1007/s12603-016-0751-9. [DOI] [PubMed] [Google Scholar]
- 69.Wańha W., Kawecki D., Roleder T., et al. Impact of anaemia on long-term outcomes in patients treated with first- and second-generation drug-eluting stents; Katowice-Zabrze Registry. Kardiologia Polska (Polish Heart Journal) . 2016;74(6):561–569. doi: 10.5603/KP.a2015.0217. [DOI] [PubMed] [Google Scholar]
- 70.Anselmino M., Malmberg K., Ohrvik J., Rydén L. Euro Heart Survey Investigators. Evidence-based medication and revascularization: powerful tools in the management of patients with diabetes and coronary artery disease: a report from the Euro Heart Survey on diabetes and the heart. European Journal of Preventive Cardiology . 2008;15(2):216–223. doi: 10.1097/HJR.0b013e3282f335d0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The reader can access the data by correspondence with the authors.


