Abstract
A low intake of fruits and vegetables is a risk factor for gastric cancer, though there is uncertainty regarding the magnitude of the associations. In this study, the relation between fruits and vegetables intake and gastric cancer was assessed, complementing a previous work on the association between consumption of citrus fruits and gastric cancer.
Data from 25 studies (8,456 cases and 21,133 controls) with information on fruits and/or vegetables intake were used. A two-stage approach based on random effects models was used to pool study-specific adjusted (sex, age, and the main known risk factors for gastric cancer) odds ratios (ORs) and the corresponding 95% confidence intervals (CIs). Exposure-response relations, including linear and non-linear associations, were modelled using one and two-order fractional polynomials.
Gastric cancer risk was lower for a higher intake of fruits (OR: 0.76, 95%CI: 0.64–0.90), non-citrus fruits (OR: 0.86, 95%CI: 0.73–1.02), vegetables (OR: 0.68, 95%CI: 0.56–0.84), and fruits and vegetables (OR: 0.61, 95%CI: 0.49–0.75); results were consistent across sociodemographic and lifestyles categories, as well as study characteristics. Exposure-response analyses showed an increasingly protective effect of portions/day of fruits (OR: 0.64, 95%CI: 0.57–0.73 for six portions), non-citrus fruits (OR: 0.71, 95%CI: 0.61–0.83 for six portions), vegetables (OR: 0.51, 95%CI: 0.43–0.60 for ten portions).
A protective effect of all fruits, non-citrus fruits and vegetables was confirmed, supporting further dietary recommendations to decrease the burden of gastric cancer.
Keywords: fruits, gastric cancer, nutrition, pooled analyses, vegetables
Introduction
A low intake of fruits and vegetables has long been acknowledged as a risk factor for gastric cancer.1, 2 However, the findings supporting the classification of this relationship as “probable” by the World Cancer Research Fund (WCRF)3 have not been corroborated by the most recent studies.4–7 This observation has led the WCRF to reclassify the evidence as “limited though suggestive” of a protective role of citrus fruits for cardia cancers and an increase in the risk of gastric cancer associated with a low intake of fruits. For vegetables, the classification of the evidence regarding a potential protective effect on gastric cancer has varied over time, and was classified as “limited and inconclusive” in the most recent WCRF report.8 The inconsistency and heterogeneity of risk estimates, as well as the small number of studies addressing the different gastric cancer anatomical locations and histological types were pointed as limitations of the evidence currently available.8
The Stomach Cancer Pooling (StoP) Project, a consortium of case-control studies, which uses an individual participant data approach for the evaluation of the associations between risk factors and gastric cancer,9 allows for some of these limitations to be overcome. A recent report, based on StoP data, showed a significant reduction in the risk of gastric cancer with a high intake of citrus fruits, with similar magnitudes of association between cardia and non-cardia cancers as well as between histological types; the protective effect increased until three servings/week and leveled off thereafter.10
The present study aimed to expand this analysis and further evaluate the association between the intake of fruits, non-citrus and vegetables and gastric cancer, through pooled analyses of individual participant data from studies participating in the StoP Project.
Methods
Study population
For this analysis, version 2.0 of the StoP Project dataset was used, which included a total of 14,016 cases of incident histologically confirmed gastric cancer (4,769 women and 9,247 men) and 33,704 controls (13,352 women and 20,352 men) from 30 case-control or nested case-control studies, as previously described.9 Briefly, studies became involved by personal contacts of participating investigators, which were identified through searches in electronic databases, including MEDLINE and Embase, backward citation tracking and contact with experts. Principal investigators of studies were contacted and invited to participate in the consortium with those agreeing to participate providing a signed data transfer agreement and, thereafter, the complete original data set of the study. All data were collected and harmonized according to a pre-specified format at the data coordinating center. Ethical approval was obtained by each individual study and the StoP Project was granted approval by the University of Milan Review Board (reference 19/15 on 01/04/2015).
The present analyses used data from 25 studies (23 case-control and two nested case-control),11 including 8,456 cases and 21,133 controls with information on fruits and/or vegetables intake, they were conducted in Brazil (two studies),12, 13 Canada,14 China (four studies),15–18 Greece,19 Iran (two studies),20, 21 Italy (four studies),22–25 Japan,26 Mexico (three studies),27–29 Portugal,30 Russia,31 Spain (two studies),32, 33 Sweden (two studies)11 and the United States of America.34
The quality of studies included was assessed using the Newcastle-Ottawa (NOS) quality assessment scale for case-control studies.35 The scale evaluates the quality of studies based on three different categories: selection, exposure and comparability. A study can be awarded a maximum of nine stars, which indicates the highest quality.
Variables defining the exposure
Food frequency questionnaires (FFQs) were used to gather information on the dietary habits of participants’ for the period of one, two, three or five years before diagnosis (for cases), onset of disease or hospital admission (for hospital-based controls) or recruitment (for population-based controls). Most studies (n=20) included face-to-face interviews by trained researchers for the application of FFQs, while five used self-administered FFQs. Fourteen of the included studies reported that the questionnaire used was previously validated by comparison with multiple 24-hour recall interviews and/or diet records (Supplementary Table 1). The FFQs used in the different studies included between 19 and 147 individual food and beverage items; most FFQs included fruits, such as apples, pears, oranges, bananas, grapes, peaches, berries (e.g., strawberries, cranberries) and watermelon, and vegetables, such as cauliflower, broccoli, carrots, lettuce, cabbage, tomato, green pepper, cucumber, onions and garlic were the most common (Supplementary Table 1). When the consumption of each item was expressed in grams, the weight of the item reported was converted into portions/day considering the standard size of fruits and vegetables retrieved from the tables of reference amounts for foods from various countries36–38.
Statistical analysis
The frequency of consumption of each food group (portions/day) for each study was obtained by adding up the frequencies of consumption of the individual items described above, and then categorizing them into tertiles, based on the distribution of fruits, non-citrus fruits, vegetables, and fruits and vegetables intake among controls in each study.
A two-stage modeling approach was used to quantify the association between fruits and vegetables intake and gastric cancer.39 First, through multivariable unconditional logistic regression models, the study-specific odds ratios (ORs) and corresponding 95% confidence intervals (95% CI) were estimated for the association between fruits and vegetables consumption and gastric cancer, compared to the lowest intake tertile as the reference group. Considering that the proportion of missing data was low, a complete case approach was adopted. Models included terms for sex, age (five-year age groups: <40;40–45; …; 70–75; >75), socioeconomic status (low, intermediate, or high, as defined in each original study based on education, income or occupation), smoking status (never, former and current smokers of ≤10 cigarettes/day; 11 to 20 cigarettes/day; >20 cigarettes/day), alcohol drinking (never, low: ≤12g of ethanol/day, intermediate: >12 to 47 g of ethanol/day, high: >47g of ethanol/day), salt intake (study-specific tertiles), red and processed meat intake (study-specific tertiles), other fruits or total vegetables intake (study-specific tertiles), total energy intake (study-specific quintiles), study center (for multicenter studies) and race/ethnicity (White, Black/African American, Asian, Hispanic/Latino, other), when appropriate and available (Supplementary Table 3).
Then, for the second stage, summary (pooled) effects estimates were computed using random-effects models;40 heterogeneity between studies was quantified using the I2 statistics.41
Stratified analyses were also performed to further explore the effect of high consumption of fruits and vegetables across categories of sex, age, geographical region of the studies, socioeconomic status, smoking status, alcohol drinking, type of controls (hospital-based, population-based), cancer anatomical subsite (cardia, non-cardia) and histological type (intestinal, diffuse and undifferentiated, as defined by the Lauren classification). For the strata of cancer subsite and histological type, multinomial logistic regression models were used to estimate the ORs for each type of cancer separately (i.e., cardia and non-cardia or intestinal, diffuse and undifferentiated).The difference between groups was assessed through the Q test for heterogeneity.42, 43
Several sensitivity analyses were performed: first, by defining the same categories of exposure for all studies according to the distribution of all fruits, non-citrus and vegetables consumption in all controls. Second, the categories of exposure were defined using as reference the minimum amounts of consumption recommended by the World Health Organization (WHO) to prevent non-communicable diseases and their risk factors, i.e. at least two portions/day for fruits, three portions/day for vegetables, and five portions/day for fruits and vegetables.44 The cut-offs that describe consumption of less than half of the recommended amount, between half and the recommended amount or more than the recommended amount were used, resulting in three categories. Third, excluding the consumption of fruit juice from fruit and non-citrus fruit intake, and excluding the consumption of legumes, such as beans, lentils, chickpeas and peas, from vegetable intake. Fourth, removing studies that used a self-administered FFQ (n=5) and non-validated FFQs (n=11), as well as studies which scored five or less stars in the NOS (n=5). Fifth, analyses were restricted to studies evaluating participants more than one year before the gastric cancer diagnosis, and to case-control studies. Further sensitivity analyses were carried out in order to compare the estimates adjusted and unadjusted for total energy intake, as well as adjusted for the presence of H. pylori infection, among studies with information on energy intake and infection status, respectively. Finally, the influence of specific studies to the overall estimates was also analyzed by excluding one study at a time.
A one-stage strategy of analysis was used to assess the shape of the dose-response relationship for all exposures considered, first by considering the variable as continuous in the logistic model and assessing the significance of a linear trend,39 and second through fractional polynomial regression models45 that take into account the non-linear trend between the exposure and the outcome. First and second order transformations were computed for the continuous term of fruits, non-citrus and vegetables intake, and the model minimizing the deviance difference with respect to the linear model was selected.45
The statistical analysis was performed with STATA, version 15.1 (Stata Corporation, College Station, TX, USA).
Results
The consumption of fruits and vegetables among the participants in each study is described in Table 1. In most studies, controls had a higher median consumption of both fruits and vegetables, when compared with cases. For fruits, the median consumption ranged between 0.0 (China 4) and 4.2 (Greece) portions/day for cases, and 0.3 (China 2 and China 4) and 4.7 (Greece) portions/day for controls. For non-citrus, the median consumption ranged from 0.1 (China 2 and Iran 1) and 3.0 (Greece) portions/day for cases, and 0.1 (Iran 1) and 3.1 (Greece) portions/day for controls. Regarding vegetables, the median consumption ranged between 0.4 (China 1 and Iran 2) and 3.9 (Russia, Mexico 1 and Mexico 3) portions/day for cases, and 0.4 (China 1) and 4.4 (Japan 3) portions/day for controls. For fruits and vegetables together, the median consumption ranged from 1.2 (Iran 1) to 7.8 (Greece) portions/day among cases, and 1.5 (Iran 1) to 9.0 (Greece) portions/day among controls. The main sociodemographic characteristics of the cases and controls are described in Supplementary Table 2.
Table 1.
Median and percentiles 25 and 75 (portions/day) of fruits, non-citrus fruits, vegetables, and fruits and vegetables consumption by area and study.
| Cases | Controls | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | Median (P25-P75) portions/day |
N | % | Median (P25-P75) portions/day |
|||||||
| Fruits | Non-citrus fruits | Vegetables | Fruits and vegetables | Fruits | Non-citrus fruits | Vegetables | Fruits and vegetables | |||||
| Total | 8,456 | 1.7 (0.9–2.9) | 1.4 (0.7–2.4) | 1.7 (0.8–3.1) | 3.6 (2.2–5.8) | 21,133 | 1.8 (1.0–3.0) | 1.4 (0.8–2.4) | 2.1 (1.1–3.4) | 4.1 (2.6–6.3) | ||
| Study | ||||||||||||
| EUROPE | 4,345 | 51.4 | 1.9 (1.1–3.0) | 1.5 (0.9–2.4) | 1.6 (0.6–2.9) | 3.6 (2.2–5.7) | 11,003 | 52.1 | 2.1 (1.3–3.3) | 1.7 (1.0–2.6) | 2.1 (1.0–3.4) | 4.5 (2.8–6.5) |
| Greece (Lagiou et al., 2004) | 110 | 1.3 | 4.2 (2.6–6.4) | 3.0 (1.9–4.9) | 3.1 (2.2–4.4) | 7.8 (5.5.−10.6) | 100 | 0.5 | 4.7 (3.7–5.9) | 3.1 (2.1–4.1) | 3.9 (2.9–5.2) | 9.0 (6.7–10.6) |
| Italy 1 (La Vecchia et al., 1995) | 769 | 9.1 | 2.4 (1.6–3.6) | 2.0 (1.3–3.0) | 2.2 (1.6–3.1) | 4.8 (3.5–6.4) | 2,081 | 9.8 | 3.0 (2.0–4.0) | 2.1 (1.4–3.1) | 2.7 (2.1–3.7) | 5.6 (4.2–7.3) |
| Italy 2 (Lucenteforte et al, 2008) | 230 | 2.7 | 3.9 (1.9–5.4) | 2.8 (1.4–4.0) | 0.9 (0.6–1.4) | 4.8 (2.8–6.6) | 547 | 2.6 | 3.6 (2.1–5.4) | 2.7 (1.6–4.1) | 0.9 (0.6–1.4) | 4.7 (2.9–6.8) |
| Italy 3 (De Feo et al., 2012) | 157 | 1.9 | 1.6 (1.0–1.6) | NA | 1.0 (1.0–1.6) | 2.6 (2.0–3.3) | 429 | 2.0 | 1.0 (1.0–1.6) | NA | 1.0 (1.0–1.6) | 2.0 (1.6–3.0) |
| Italy 4 (Buiatti et al., 1989) | 1,016 | 12.0 | 1.6 (1.0–2.1) | 1.2 (0.8–1.7) | 0.5 (0.3–0.7) | 2.1 (1.5–2.7) | 1,159 | 5.5 | 1.7 (1.2–2.2) | 1.3 (0.9–1.7) | 0.5 (0.4–0.7) | 2.2 (1.7–2.9) |
| Portugal (Lunet et al., 2007) | 633 | 7.5 | 1.5 (0.9–2.2) | 1.3 (0.8–1.9) | 1.8 (1.1–2.7) | 3.4 (2.2–4.8) | 1,600 | 7.6 | 2.0 (1.4–2.8) | 1.6 (1.1–2.4) | 2.1 (1.3–3.1) | 4.3 (3.0–5.8) |
| Russia (Zaridze et al., 2000) | 444 | 5.2 | 2.7 (1.5–4.5) | 2.2 (1.1–4.0) | 3.9 (2.0–6.4) | 7.3 (3.9–10.7) | 606 | 2.9 | 2.6 (1.4–4.4) | 2.1 (1.0–3.7) | 4.1 (2.4–6.1) | 7.0 (4.1–10.4) |
| Spain 1 (Castaño-Vinyals, 2012) | 339 | 4.0 | 2.5 (1.6–3.5) | 1.7 (1.0–2.5) | 2.6 (1.5–3.8) | 5.2 (3.6–7.2) | 3,040 | 14.4 | 2.5 (1.5–3.5) | 1.6 (0.9–2.4) | 2.6 (1.7–3.8) | 5.3 (3.6–7.1) |
| Spain 2 (Santibanez et al., 2012) | 398 | 4.7 | 1.8 (1.2–2.5) | 1.1 (0.8–1.6) | 2.0 (1.2–3.2) | 4.0 (2.9–5.4) | 455 | 2.1 | 2.0 (1.4–2.7) | 1.1 (0.8–1.6) | 2.2 (1.5–3.5) | 4.5 (3.2–6.0) |
| Sweden 1 (Harris et al, 2013) | 88 | 1.0 | 1.5 (0.5–2.0) | NA | 2.0 (1.5–3.8) | 4.0 (2.5–5.5) | 352 | 1.7 | 1.5 (1.0–2.5) | NA | 2.5 (1.5–4.0) | 4.0 (3.0–6.0) |
| Sweden 2 (Harris et al, 2013) | 161 | 1.9 | 1.0 (0.5–1.5) | NA | 2.0 (1.0–3.0) | 3.0 (2.0–4.5) | 644 | 3.0 | 1.0 (0.5–2.0) | NA | 2.0 (1.0–3.0) | 3.0 (2.0–4.5) |
| ASIA | 1,863 | 22.0 | 1.2 (0.3–2.8) | 1.1 (0.4–2.5) | 1.6 (0.6–3.2) | 3.6 (2.2–5.7) | 3,005 | 14.2 | 1.0 (0.3–2.6) | 1.0 (0.3–2.6) | 1.5 (0.5–3.0) | 3.7 (1.9–6.8) |
| China 1 (Deandrea et al, 2010) | 266 | 3.1 | NA | NA | 0.4 (0.3–0.5) | NA | 533 | 2.5 | NA | NA | 0.4 (0.2–0.5) | NA |
| China 2 (Mu et al., 2005) | 201 | 2.4 | 0.2 (0.0–0.6) | 0.2 (0.0–0.6) | 2.1 (1.1–3.6) | 2.5 (1.4–4.0) | 410 | 1.9 | 0.3 (0.0–0.6) | 0.3 (0.0–0.6) | 2.1 (1.3–3.4) | 2.6 (1.9–4.4) |
| China 3 (Setiawan et al, 2005) | 702 | 8.3 | 2.0 (0.8–4.7) | 1.9 (0.8–4.5) | 2.8 (1.9–3.9) | 5.1 (3.2–8.9) | 696 | 3.3 | 2.4 (1.1–5.5) | 2.2 (1.0–5.1) | 2.8 (1.9–3.9) | 5.4 (3.5–9.8) |
| China 4 (Setiawan et al., 2001) | 115 | 1.4 | 0.0 (0.0–0.3) | NA | NA | 390 | 1.8 | 0.3 (0.0–0.3) | NA | NA | NA | |
| Iran 1 (Pourfarzi et al., 2009) | 216 | 2.5 | 0.3 (0.1–0.9) | 0.1 (0.1–0.5) | 0.7 (0.5–1.1) | 1.2 (0.7–2.0) | 392 | 1.9 | 0.4 (0.3–1.0) | 0.1 (0.1–0.5) | 1.0 (0.4–1.4) | 1.5 (0.8–2.4) |
| Iran 2 (Pakseresht et al, 2011) | 210 | 2.5 | 1.8 (1.0–2.8) | 1.1 (0.6–1.7) | 0.5 (0.3–1.1) | 2.6 (1.6–3.6) | 281 | 1.3 | 1.6 (1.0–2.9) | 1.0 (0.6–1.8) | 0.7 (0.4–1.4) | 2.5 (1.5–4.3) |
| Japan 3 (Machida-Montani et al, 2004) | 153 | 1.8 | 2.5 (1.6–3.9) | 2.3 (1.5–3.6) | 3.7 (2.3–5.8) | 6.5 (4.2–9.6) | 303 | 1.4 | 3.0 (1.9–4.1) | 2.7 (1.7–3.7) | 4.4 (2.6–6.3) | 7.7 (5.0–10.2) |
| AMERICAS | 2,248 | 26.6 | 1.6 (0.8–2.6) | 1.3 (0.6–2.2) | 2.1 (1.2–3.4) | 3.8 (2.4–5.8) | 7,115 | 33.7 | 1.5 (0.8–2.5) | 1.1 (0.6–2.0) | 2.1 (1.3–3.4) | 3.8 (2.4–5.6) |
| Brazil 1 (Nishimoto et al, 2002) | 226 | 2.7 | 1.2 (0.5–2.0) | NA | 1.2 (0.4–1.7) | 2.5 (1.4–3.6) | 226 | 1.1 | 1.5 (1.0–2.2) | NA | 1.4 (0.7–2.0) | 3.1 (2.1–4.0) |
| Brazil 2 (Hamada et al, 2002) | 93 | 1.1 | 1.5 (1.0–2.2) | NA | 1.5 (1.2–2.2) | 3.5 (2.2–4.5) | 186 | 0.9 | 1.4 (1.0–2.2) | NA | 2.0 (1.2–2.2) | 3.5 (2.6–4.4) |
| Canada (Mao et al., 2002) | 1,170 | 13.8 | 1.3 (0.6–2.1) | 1.0 (0.4–1.7) | 1.8 (1.1–2.6) | 3.3 (2.1–4.7) | 5,023 | 23.7 | 1.4 (0.6–2.1) | 1.1 (0.5–1.7) | 1.8 (1.1–2.6) | 3.3 (2.1–4.6) |
| Mexico 1 (Hernandez-Ramirez et al, 2009) | 248 | 2.9 | 1.9 (1.2–3.0) | 1.6 (1.0–2.5) | 3.9 (3.1–4.5) | 5.8 (4.6–7.5) | 478 | 2.3 | 1.4 (0.7–2.5) | 1.1 (0.6–2.1) | 3.9 (3.1–4.5) | 5.4 (4.2–7.0) |
| Mexico 2 (Lopez-Carrillo et al., 1994) | 220 | 2.6 | 2.7 (1.6–4.1) | 2.3 (1.3–3.4) | 3.7 (2.5–4.8) | 6.4 (4.5–9.0) | 752 | 3.6 | 2.9 (1.6–4.5) | 2.3 (1.3–3.6) | 4.2 (3.3–5.6) | 7.3 (5.1–9.7) |
| Mexico 3 (Lopez-Carrillo et al., 2003) | 159 | 1.9 | 3.5 (1.9–5.9) | 2.2 (1.3–3.8) | 3.9 (3.0–5.0) | 7.5 (5.3–10.5) | 318 | 1.5 | 3.4 (1.9–6.2) | 2.3 (1.3–4.1) | 3.9 (3.2–4.9) | 7.7 (5.5–10.8) |
| USA 1 (Zhang et al., 1999) | 132 | 1.6 | 1.5 (0.7–2.7) | 1.4 (0.6–2.4) | 1.7 (1.1–2.7) | 3.6 (2.0–5.4) | 132 | 0.6 | 1.6 (0.6–2.9) | 1.4 (0.6–2.5) | 1.9 (1.1–3.1) | 3.7 (2.0–5.5) |
NA – not available; P25-P75 – percentile 25 - percentile 75; USA – United States of America.
A significantly lower risk of gastric cancer was observed for a higher consumption of fruits, vegetables, and fruits and vegetables (Table 2), with the strongest associations being observed for the comparisons of the highest vs. the lowest tertiles (fruits, OR: 0.76, 95%CI: 0.64–0.90, I2: 59.7%; vegetables, OR: 0.68, 95%CI: 0.56–0.84, I2: 74.5%; fruits and vegetables, OR: 0.61, 95%CI: 0.49–0.75, I2: 75.5%). Although not statistically significant, a higher consumption of non-citrus fruits also had a lower risk of gastric (OR: 0.86, 95%CI: 0.73–1.02, I2: 55.0%) (Table 2 and Figure 1).
Table 2.
Pooled odds ratios of gastric cancer according to study-specific tertiles of fruits, non-citrus fruits, vegetables, and fruits and vegetables consumption (portions/day).
| Cases | Controls | OR (CI 95%)a | I2 (%) | |||||
|---|---|---|---|---|---|---|---|---|
| N | % | portions/day Median (P25-P75) |
N | % | portions/day Median (P25-P75) |
|||
| Fruits b | ||||||||
| 1st tertile | 3,164 | 37.6 | 0.8 (0.4–1.2) | 7,041 | 33.3 | 0.8 (0.4–1.2) | 1 | |
| 2nd tertile | 2,604 | 31.0 | 1.9 (1.5–2.5) | 6,841 | 32.3 | 1.9 (1.4–2.6) | 0.81 (0.71–0.93) | 49.2 |
| 3rd tertile | 2,2350 | 27.9 | 3.6 (2.5–5.3) | 6,617 | 31.2 | 3.6 (2.5–4.7) | 0.76 (0.64–0.90) | 59.7 |
| Missing | 292 | 3.5 | 673 | 3.2 | ||||
| P value for trend | <0.001 | |||||||
| Non-citrus fruits c | ||||||||
| 1st tertile | 2,686 | 35.6 | 0.6 (0.3–1.0) | 6,121 | 32.4 | 0.6 (0.3–1.0) | 1 | |
| 2nd tertile | 2,353 | 31.2 | 1.4 (1.1–2.1) | 6,181 | 32.7 | 1.4 (1.1–2.0) | 0.83 (0.70–0.98) | 61.8 |
| 3rd tertile | 2,234 | 29.6 | 3.0 (2.0–4.4) | 5,956 | 31.5 | 2.8 (2.1–3.9) | 0.86 (0.73–1.02) | 55.0 |
| Missing | 275 | 3.6 | 625 | 3.3 | ||||
| P value for trend | <0.001 | |||||||
| Vegetables d | ||||||||
| 1st tertile | 3,311 | 38.8 | 1.0 (0.4–1.6) | 7,028 | 33.0 | 1.0 (0.5–1.5) | 1 | |
| 2nd tertile | 2,552 | 29.9 | 2.1 (1.1–2.8) | 6,826 | 32.1 | 2.2 (1.6–2.8) | 0.81 (0.69–0.95) | 65.8 |
| 3rd tertile | 2,471 | 28.9 | 3.6 (2.3–5.1) | 6,867 | 32.3 | 3.8 (2.8–5.0) | 0.68 (0.56–0.84) | 74.5 |
| Missing | 208 | 2.4 | 547 | 2.6 | ||||
| P value for trend | <0.001 | |||||||
| Fruits and vegetables e | ||||||||
| 1st tertile | 3,200 | 38.7 | 2.0 (1.4–3.1) | 6,888 | 33.2 | 2.2 (1.5–3.2) | 1 | |
| 2nd tertile | 2,493 | 30.1 | 4.1 (2.8–5.6) | 6,532 | 31.5 | 4.3 (3.1–5.6) | 0.76 (0.65–0.88) | 59.2 |
| 3rd tertile | 2,303 | 27.8 | 7.0 (4.7–9.9) | 6,667 | 27.8 | 7.1 (5.3–9.1) | 0.61 (0.49–0.75) | 75.5 |
| Missing | 280 | 3.4 | 648 | 3.1 | ||||
| P value for trend | <0.001 | |||||||
95% CI – 95% Confidence Interval; OR – Odds Ratio; P25-P75 – percentile 25 - percentile 75.
Pooled ORs were computed using random-effects models. Study-specific ORs were adjusted, when available and applicable, for sex, age (five-year age groups: <40;40–45; …; 70–75; >75), socioeconomic status (low, intermediate, or high, as defined in each original study based on education, income or occupation), smoking status (never, former and current smokers of ≤10 cigarettes/day; 10 to 20 cigarettes per day; >20 cigarettes/day), alcohol drinking (never, low: ≤12g of ethanol/day, intermediate: >12 to ≤47 g/day, high: >47g/day), salt intake (study-specific tertiles), red and processed meat intake (study-specific tertiles), other fruits/vegetables intake (study-specific tertiles), total energy intake (study-specific quintiles), study center (for multicenter studies) and race/ethnicity (White, Black/African American, Asian, Hispanic/Latino, other).
No information for study China 1.17
No information for studies Brazil 1,13 Brazil 2,12 China 1,17 China 4,64 Italy 3,24 Sweden 111 and Sweden 2.11
No information for study China 4.64
Fig. 1.

Forest plots describing the association between the intake of fruits, non-citrus fruits, vegetables, and fruits and vegetables (highest vs. lowest tertile, portions/day) and gastric cancer using the estimates from the Stomach Cancer Pooling (Stop) Project database.
95% CI – 95% Confidence Interval; NA – Not available; OR – Odds Ratio.
The protective effect of a high consumption of all these food groups was consistent across most strata of sociodemographic and lifestyle variables (Table 3). Though the difference was not statistically significant, individuals belonging to the low socioeconomic status strata presented the highest protection for a higher consumption of fruits (OR: 0.66, 95%CI: 0.52–0.84, I2: 56.9%) and non-citrus fruits (OR: 0.72, 95%CI: 0.56–0.93, I2: 54.6%), compared with subjects in intermediate (fruits: OR: 0.96, 95%CI: 0.75–1.23, I2: 26.9%; non-citrus fruits: OR: 1.06, 95%CI: 0.81–1.38, I2: 36.0%) and high socioeconomic status (fruits: OR: 0.95, 95%CI: 0.60–1.51, I2: 32.1%; non-citrus fruits: OR: 1.14, 95%CI: 0.78–1.66, I2: 19.9%). There were also slight differences according to the site of gastric cancer, for vegetables, with a stronger association being observed among noncardia gastric cancer (OR: 0.61, 95%CI: 0.50–0.73, I2: 60.3%) when compared to those with cardia gastric cancer (OR: 0.86, 95%CI: 0.64–1.14, I2: 18.9%).
Table 3.
Pooled odds ratio of gastric cancer for the highest vs. the lowest study-specific tertile of fruits, non-citrus fruits, vegetables, and fruits and vegetables consumption (portions/day) according to strata of selected variables.
| Fruits | Non-citrus fruits | Vegetables | Fruits and vegetables | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI)a | I2 (%) | OR (95% CI)a | I2 (%) | OR (95% CI)a | I2 (%) | OR (95% CI)a | I2 (%) | |
| Overall | 0.76 (0.64–0.90) | 59.7 | 0.86 (0.73–1.02) | 55.0 | 0.68 (0.56–0.84) | 74.5 | 0.61 (0.49–0.75) | 75.5 |
| Sex | ||||||||
| Men | 0.74 (0.57–0.95) | 67.9 | 0.76 (0.59–0.97) | 63.3 | 0.72 (0.57–0.91) | 67.7 | 0.62 (0.48–0.80) | 72.7 |
| Women | 0.75 (0.60–0.94) | 31.4 | 0.94 (0.68–1.30) | 60.9 | 0.66 (0.51–0.86) | 46.6 | 0.59 (0.45–0.78) | 55.3 |
| p for interaction | 0.938 | 0.308 | 0.627 | 0.796 | ||||
| Age (years) | ||||||||
| ≤55 | 0.83 (0.66–1.05) | 18.4 | 0.92 (0.76–1.12) | 0.0 | 0.72 (0.55–0.95) | 46.0 | 0.67 (0.52–0.86) | 32.1 |
| >55 to ≤65 | 0.65 (0.48–0.88) | 44.6 | 0.83 (0.59–1.15) | 51.0 | 0.82 (0.57–1.19) | 67.6 | 0.60 (0.41–0.88) | 70.5 |
| >65 | 0.79 (0.63–0.99) | 39.9 | 0.89 (0.71–1.11) | 34.9 | 0.67 (0.54–0.84) | 40.2 | 0.65 (0.53–0.80) | 37.5 |
| p for interaction | 0.438 | 0.871 | 0.650 | 0.894 | ||||
| Area | ||||||||
| Europe | 0.79 (0.64–0.98) | 60.5 | 0.94 (0.76–1.16) | 57.6 | 0.61 (0.46–0.80) | 76.3 | 0.59 (0.46–0.76) | 72.5 |
| Asia | 0.73 (0.49–1.09) | 18.45.6 | 0.69 (0.40–1.19) | 63.7 | 0.99 (0.65–1.51) | 66.5 | 0.75 (0.59–0.95) | 0.0 |
| Americas | 0.69 (0.44–1.08) | 65.2 | 0.80 (0.55–1.18) | 38.4 | 0.62 (0.44–0.89) | 52.0 | 0.53 (0.28–0.98) | 84.3 |
| p for interaction | 0.843 | 0.502 | 0.142 | 0.310 | ||||
| Socioeconomic status b | ||||||||
| Low | 0.66 (0.52–0.84) | 56.9 | 0.72 (0.56–0.93) | 54.6 | 0.66 (0.50–0.88) | 70.0 | 0.59 (0.46–0.75) | 64.1 |
| Intermediate | 0.96 (0.75–1.23) | 26.9 | 1.06 (0.81–1.38) | 36.0 | 0.79 (0.62–1.00) | 31.4 | 0.75 (0.56–0.99) | 54.7 |
| High | 0.95 (0.60–1.51) | 32.1 | 1.14 (0.78–1.66) | 19.9 | 0.63 (0.36–1.11) | 50.2 | 0.75 (0.49–1.17) | 41.7 |
| p for interaction | 0.079 | 0.052 | 0.561 | 0.387 | ||||
| Cigarette smoking c | ||||||||
| Never | 0.75 (0.61–0.91) | 34.7 | 0.90 (0.70–1.15) | 48.4 | 0.69 (0.54–0.88) | 56.7 | 0.60 (0.47–0.78) | 62.5 |
| Former | 0.86 (0.62–1.19) | 48.6 | 0.96 (0.69–1.36) | 49.6 | 0.72 (0.54–0.98) | 37.9 | 0.60 (0.44–0.81) | 48.6 |
| Current | 0.63 (0.46–0.86) | 36.1 | 0.60 (0.42–0.86) | 45.5 | 0.78 (0.56–1.08) | 49.5 | 0.55 (0.38–0.78) | 53.1 |
| p for interaction | 0.397 | 0.119 | 0.842 | 0.917 | ||||
| Alcohol intake d | ||||||||
| Non drinker | 0.61 (0.43–0.86) | 54.0 | 0.64 (0.43–0.95) | 56.0 | 0.60 (0.44–0.84) | 54.4 | 0.48 (0.34–0.69) | 60.9 |
| Drinker | ||||||||
| ≤12g of ethanol/day | 0.84 (0.60–1.17) | 48.3 | 0.99 (0.72–1.35) | 34.7 | 0.71 (0.48–1.01) | 55.5 | 0.62 (0.43–0.88) | 59.0 |
| >12–47g of ethanol/day | 0.82 (0.57–1.18) | 59.7 | 0.86 (0.67–1.09) | 21.8 | 0.75 (0.51–1.08) | 63.8 | 0.66 (0.47–0.93) | 59.1 |
| >47 g of ethanol/day | 0.78 (0.50–1.22) | 23.7 | 1.02 (0.56–1.84) | 49.9 | 0.57 (0.42–0.79) | 0.0 | 0.55 (0.37–0.82) | 18.9 |
| p for interaction | 0.559 | 0.359 | 0.647 | 0.605 | ||||
| Controls | ||||||||
| Hospital-basede | 0.67 (0.56–0.79) | 0.0 | 0.88 (0.73–1.06) | 0.0 | 0.60 (0.39–0.95) | 82.9 | 0.50 (0.37–0.68) | 51.6 |
| Population-basedf | 0.74 (0.58–0.94) | 69.3 | 0.78 (0.61–1.00) | 68.1 | 0.74 (0.59–0.91) | 63.9 | 0.63 (0.50–0.81) | 74.1 |
| p for interaction | 0.511 | 0.445 | 0.406 | 0.243 | ||||
| Site g | ||||||||
| Cardia | 0.81 (0.62–1.07) | 11.1 | 0.82 (0.55–1.21) | 39.8 | 0.86 (0.64–1.14) | 18.9 | 0.75 (0.57–1.00) | 22.9 |
| Noncardia | 0.74 (0.60–0.91) | 64.9 | 0.88 (0.72–1.08) | 59.3 | 0.61 (0.50–0.73) | 60.3 | 0.58 (0.45–0.74) | 78.4 |
| p for interaction | 0.606 | 0.755 | 0.051 | 0.179 | ||||
| Histotype h | ||||||||
| Intestinal | 0.83 (0.61–1.13) | 54.3 | 0.87 (0.61–1.24) | 60.0 | 0.72 (0.52–1.00) | 62.5 | 0.73 (0.55–0.98) | 52.3 |
| Diffuse | 0.74 (0.55–1.00) | 34.3 | 0.89 (0.66–1.20) | 30.4 | 0.62 (0.48–0.80) | 19.8 | 0.58 (0.42–0.81) | 50.2 |
| Undifferentiated | 1.04 (0.71–1.41) | 47.5 | 1.08 (0.84–1.40) | 28.9 | 0.77 (0.57–1.02) | 40.6 | 0.93 (0.64–1.36) | 65.3 |
| p for interaction | 0.337 | 0.508 | 0.526 | 0.179 | ||||
| Studies with information on energy intake i | ||||||||
| Adjusting for energy intake | 0.66 (0.54–0.82) | 60.6 | 0.82 (0.68–1.00) | 56.2 | 0.64 (0.49–0.84) | 78.2 | 0.54 (0.42–0.69) | 74.7 |
| Not adjusting for energy intake | 0.81 (0.66–1.00) | 65.1 | 0.99 (0.79–1.25) | 72.1 | 0.75 (0.62–0.90) | 60.1 | 0.68 (0.60–0.78) | 29.6 |
| Studies with information on H. pylori (HP) infection status j | ||||||||
| Adjusting for HP infection | 0.70 (0.49–1.00) | 58.0 | 0.76 (0.49–1.19) | 64.7 | 0.69 (0.51–0.93) | 45.4 | 0.59 (0.41–0.84) | 62.6 |
| Not adjusting for HP infection | 0.70 (0.50–1.00) | 58.4 | 0.76 (0.48–1.19) | 66.5 | 0.68 (0.51–0.91) | 43.9 | 0.59 (0.42–0.83) | 61.2 |
95% CI – 95% Confidence Interval; HP – Helicobacter pylori; OR – Odds Ratio.
Pooled ORs were computed using random-effects models. Study-specific ORs were adjusted, when available and applicable, for sex, age (five-year age groups: <40;40–45; …; 70–75; >75), socioeconomic status (low, intermediate, or high, as defined in each original study based on education, income or occupation), smoking status (never, former and current smokers of ≤10 cigarettes/day; 10 to 20 cigarettes per day; >20 cigarettes/day), alcohol drinking (never, low: ≤12g of ethanol/day, intermediate: >12 to ≤47 g/day, high: >47g/day), salt intake (study-specific tertiles), red and processed meat intake (study-specific tertiles), other fruits/vegetables intake (study-specific tertiles), total energy intake (study-specific quintiles), study center (for multicenter studies) and race/ethnicity (White, Black/African American, Asian, Hispanic/Latino, other).
As defined in each original study based on education, income or occupation.
Excluding study China 4.18
Includes studies Brazil 1,13 China 1,17 Greece,19 Italy 1,22 Italy 2,23 Italy 3,24 Japan 3,26 Mexico 3,29 Spain 233 and USA 1.34 Excluding studies Brazil 212 and Russia31 as they include both hospital- and population-based controls.
Includes studies Canada,14 China 2,15 China 3,16 China 4,18 Iran 1,20 Iran 2,21 Italy 4,25 Mexico 1,27 Mexico 2,28 Portugal,30 Spain 1,32 Sweden 111 and Sweden 2.11 Excluding studies Brazil 212 and Russia31 as they include both hospital- and population-based controls.
Excluding studies China 1,17 China 2,15 China 3,16 China 4,18 Greece,19 Italy 1,22 Japan 3,26 Mexico 2,28 Sweden 111 and Sweden 2.11
No information for studies Brazil 1,13 Brazil 2,12 Canada,14 China 1,17 China 2,15 China 4,18 Iran 1,20 Italy 3, Russia,31 Sweden 1,11 Sweden 211 and USA1.34
No information for studies Canada,14 China 1,17 China 3,16 China 4,18 Greece,19 Italy 1,22 Italy 2,23 Italy 3,24 Italy 4,25 Mexico 2,28 Spain 2,33 Sweden 1,11 Sweden 211 and USA1.34 H. pylori infection was defined using the same criteria of the original studies, according to the following serological tests: enzyme-linked immunosorbent assay (ELISA) tests (nine studies)12, 13, 15, 20, 26, 27, 29–31 or Western Blot (one study)21 to determine immunoglobulin G (IgG) antibody titers in serum, and in one study through multiplex serology.32 When anti-H. pylori serum IgG titers were assessed using an ELISA-based method, participants with borderline results were classified as testing positive for H. pylori infection.
Sensitivity analyses did not result in changes in the direction or magnitude of the associations; a significantly lower risk of gastric cancer was still observed when considering OR estimates adjusted for total energy intake or accounting for H. pylori infection (Table 3). Other strategies to reduce heterogeneity among studies, namely using the same cut-off for all studies, defined either by the overall distribution on controls or taking the amounts recommended by the WHO into account, led to estimates of the same magnitude, with slightly lower heterogeneity, particularly for non-citrus and vegetables intake (Supplementary Table 4).
Additional stratified analyses according to study characteristics also yielded similar and consistent results throughout (Supplementary Table 5). The results excluding fruit juices and legumes from the fruit and vegetable intakes, respectively, also did not materially differ from those of the main analyses. Similarly, the magnitude of estimates remained essentially unchanged when considering the validity of the FFQ, method of administration, as well as the period of assessment. Finally, applying the NOS to the included studies and removing those with five stars or less, also did not substantively change the associations observed in the overall analyses.
Figure 2 shows the dose-response relationships between the intake of fruits, non-citrus fruits and vegetables and gastric cancer risk. There was an increasingly protective effect of portions/day of fruits (OR: 0.64, 95%CI: 0.57–0.73 for six portions), non-citrus fruits (OR: 0.71, 95%CI: 0.61–0.83 for six portions), vegetables (OR: 0.51, 95%CI: 0.43–0.60 for ten portions).
Fig. 2.
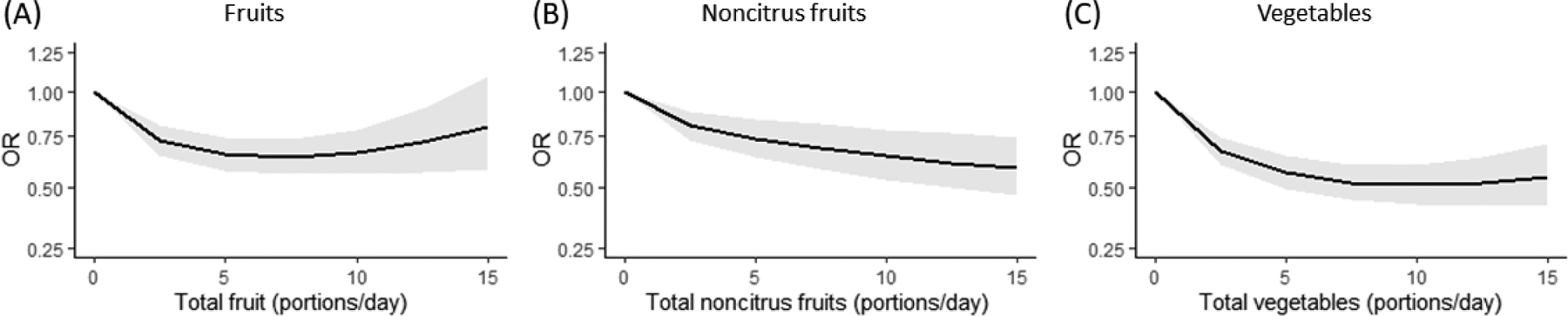
Dose-response relationship between fruits (a), non-citrus fruits (b), vegetables (c), and fruits and vegetables (d) and gastric cancer, fitted by a fractional polynomial.
95% CI – 95% Confidence Interval; OR – Odds Ratio.
Discussion
With this uniquely large individual participant pooled analysis, we observed and quantified, better than previously available, a protective effect of fruits and vegetables on the occurrence of gastric cancer, consistent across sociodemographic categories and study characteristics, and further confirmed through analyses of the dose-response association.
This study complements a previous work with the same set of studies on the association between citrus fruits and gastric cancer10 by showing that the protective effect is not only restricted to this small subgroup of food items. Citrus fruits contain, besides vitamin C and other carotenoid antioxidants, particular flavanones, such as hesperitin and naringenin, that have anti-oxidant activity and, in animal models, inhibit human gastric cancer cell proliferation and migration.46, 47 However, other classes of flavonoids with similar activity can be found in other fruits, such as apples48 or berries.49 Additionally, fruits and vegetables are also rich in fiber, which can act as a scavenger of nitrates, preventing the formation of carcinogenic N-nitroso compounds50, and possibly other cancer preventive agents. Regarding vegetables, our estimates are in line with previous evidence, showing a similar degree of protection against gastric cancer as the one observed for a high consumption of allium vegetables (OR: 0.68, 95%CI: 0.57–0.81), garlic (OR:0.60, 95%CI: 0.47–0.76), onion (OR: 0.55, 95%CI: 0.41–0.73)51 or cruciferous vegetables (OR: 0.78, 95%CI: 0.71–0.86).52 These vegetables have high contents of organosulfur compounds, which may have protective effects, as well as vitamins, carotenoids and other phytochemicals with potential anti-inflammatory and antioxidant activity, conveying anti-carcinogenic effects.53–55
Most previous meta-analyses of cohort studies have shown a protective effect of a high consumption of fruit,4, 6, 7 leading the WCRF to conclude that “there is some evidence that suggests consuming little or no fruit increases the risk of stomach cancer”.8 However, evidence regarding vegetable intake has been less consistent and the most recent WCRF report was unable to come to any conclusion.8 In particular, a pooled analysis of prospective studies in China, Japan and Korea showed a weak, non-dose response of an inverse association of vegetable intake with non-cardia gastric cancer risk;7 while, a reanalysis of the European Prospective Investigation into Cancer and Nutrition study did not find an association between total or specific vegetables intake and gastric cancer risk.4 Nevertheless, the results of the current study add to previous evidence pooled estimates, including the characterization of the exposure-relationships for all fruits and vegetables, which show that a higher consumption of fruits and vegetables was associated with a lower risk of gastric cancer.
Generally, cohort studies have not confirmed the strong associations often seen in case-control studies; likewise, our stratified analysis including only case-control studies had a stronger estimate than that using nested case-control studies. This was also observed in our dose-response analyses, for which strong estimates were obtained for the consumption of six portions/day of vegetables. These results may be partially explained by the bias due to dietary recall or dietary changes accompanying disease associated with case-control studies. However, a previous systematic review and meta-analysis of cohort studies on the effect of fruit and vegetable consumption on gastric cancer showed that the association is stronger among studies with longer follow-up times,56 which may suggest different effects of exposures depending on when they occur.
We observed a higher risk reduction among individuals in the low socioeconomic group for the consumption of fruits and non-citrus fruits, though, differences were not statistically significant, while in the StoP Projectś citrus fruits study, the interaction was statistically significant.10 This suggests that, not only citrus fruits but all fruits and vegetables might counterbalance the negative effects of the lifestyle risk factors associated with low socioeconomic status.57 Regional differences were also observed, reflecting not only the different diets but also the detail of the FFQs applied regarding the number and types of food items included. For non-citrus fruits, the association was strongest among Asian studies, as also observed in the citrus fruits study.10 While the items that constitute the ‘non-citrus’ group are comparable among Asian studies, there is a wider variation of items across studies from the other regions. Moreover, the Canadian study had a particular weight to the Americas risk estimate, since it used a FFQ sent by mail rather than one applied face-to-face, possibly resulting in a less accurate assessment of fruits intake.
Heterogeneity was high for all the food groups considered, which is common in studies evaluating dietary associations,58 mainly due to the different methods used by each study to collect dietary data, particularly the period of dietary assessment, the number and the items present in each food questionnaire. Within the StoP consortium, most studies used FFQ designed not only to be representative of the countries’ diet but also to take into account the seasonality of the items included. However, the diversity of items present in each questionnaire and the disagreement regarding what constitutes a portion or a serving of fruit and vegetable likely contributed to the heterogeneity observed.6 Nevertheless, 14 studies in the StoP project used previously validated FFQs, while 20 studies collected data using face-to-face interviewers, which have been shown to have lower random within-person variation than other dietary assessment and have an acceptable validity when compared to reference measures.59, 60 In fact, our sensitivity analyses showed no significant differences, providing further support to the robustness of our findings.
Studies were considered for analysis regardless of having addressed the association between fruits and vegetables intake and gastric cancer in a previous report, which prevented publication bias. The harmonization of adjustment strategies and control of confounding throughout the studies of the StoP consortium, further contributes to the validity of our estimates. Additionally, the protective effect of fruits and vegetables detected in the main analysis was consistently observed among strata of different sociodemographic and lifestyles variables, as well as study characteristics. Sensitivity analyses, either removing one study at a time or considering the same cut-off for all studies, yielded estimates similar to those observed in the main analysis, albeit with less heterogeneity, particularly for non-citrus and vegetables intake.
Both cases and controls reported low levels of fruits and vegetables intake, with the median of consumption not reaching the amount recommended of five portions a day (at least two of fruits and three of vegetables)44 in most studies. The worldwide consumption of fruits and vegetables is low, particularly in low and middle income countries61 and, when assuming a causal relation between fruits and vegetables intake and the occurrence of gastric cancer, an increase in the overall consumption to at least 300g of fruits/day and 400 g vegetables/day, was estimated to prevent 6.0 to 11.5% of gastric cancer cases in these settings, by 2025.62
The main limitation of the current study is the case-control design of the included studies, which may have potentially yielded inaccurate measures of fruit and vegetable consumption. As past dietary habits were reported by participants, recall bias may have occurred, particularly among patients, as changes in lifestyle may occur as cancer develops and becomes symptomatic.63 Nevertheless, all studies recruited incident, histologically confirmed gastric cancer cases, and most obtained dietary information regarding at least the year before diagnosis or the period before changes in dietary habits. We conducted a sensitivity analysis excluding studies in which FFQs were within one year of gastric cancer diagnosis, and the estimates obtained were essentially the same. Additionally, case-control studies may be prone to selection bias. It is possible that hospital-based controls include individuals with conditions that could potentially be related to fruit and vegetable intake, while population-based controls are considered to be more representative of the population under study, however, the latter may be healthier and have higher fruit and vegetable intake. Nevertheless, the results of our stratified analysis by type of controls showed negligible differences.
This study adds a pooled analysis to previous evidence, allowing to perform stratified analyses namely by cancer anatomical location and histological type, and exposure-response analyses. Despite the differences between the food items that constitute these heterogeneous food groups, a protective effect was observed for all those that were analyzed. This contributes to reinforce the recommendations for healthier lifestyles, including an increased intake of fruits and vegetables.
Supplementary Material
Novelty:
This pooled analysis within a global consortium of case-control studies shows that the possible protective effect of a high intake of fruits and vegetables is not restricted to citrus fruits, and is observed regardless of gastric cancer location and histological type.
Funding:
The authors thank the European Cancer Prevention (ECP) Organization for providing support for the project meetings. This study is supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC), Project no. 21378 (Investigator Grant), the Italian Ministry of Health (Young Researchers, GR-2011-02347943 to SB) and the Italian League for the Fight against Cancer (LILT). This project was also supported by FEDER through the Operational Programme Competitiveness and Internationalization and national funding from the Foundation for Science and Technology – FCT (Portuguese Ministry of Science, Technology and Higher Education) under the Unidade de Investigação em Epidemiologia – Instituto de Saúde Pública da Universidade do Porto (EPIUnit) (POCI-01-0145-FEDER-006862; Ref. UID/DTP/04750/2019). AF (PD/BD/105823/2014) was awarded with an individual scholarship through national funding from FCT/MCTES. Individual grants attributed to ARC (SFRH/BD/102181/2014) and BP (SFRH/BPD/108751/2015) were funded by the FCT and the “Programa Operacional Capital Humano” (POCH/FSE). SM was funded under the project “NEON-PC - Neuro-oncological complications of prostate cancer: longitudinal study of cognitive decline “ (POCI-01-0145-FEDER-032358; Ref. PTDC/SAU-EPI/32358/2017). We also thank all MCC-Spain study collaborators (CIBERESP, ISCIII, ISGlobal, ICO, University of Huelva, University of Oviedo, University of Cantabria, University of León, Granada, Instituto Salud Pública de Navarra, FISABIO, Murcia Regional Health Authority and collaborators).
Role of funding source:
The funding source had no role in the study design; collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Abbreviations:
- CI
Confidence Interval
- FFQ
Food Frequency Questionnaire
- NOS
Newcastle-Ottawa Scale
- OR
Odds Ratios
- StoP Project
Stomach Cancer Pooling Project
- WCRF
World Cancer Research Fund
- WHO
World Health Organization
Footnotes
Conflict of interest statement: The authors declare that they have no conflict of interest.
Data accessibility:
The data that support the findings of this study are available from the StoP Project but restrictions apply to the availability of these data, which were used under license for the current study and so are not publicly available. Data are, however, available from the authors upon reasonable request and permission of the Steering Committee of the StoP Project.
References
- 1.Correa P Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res 1992;52: 6735–40. [PubMed] [Google Scholar]
- 2.World Cancer Research Fund & American Institute for Cancer Research. Food, Nutrition and the Prevention of Cancer: a Global Perspective. Washington, DC: AIRC, 1997. [DOI] [PubMed] [Google Scholar]
- 3.World Cancer Research Fund & American Institute for Cancer Research, Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington, DC: AIRC, 2007. [Google Scholar]
- 4.Gonzalez CA, Lujan-Barroso L, Bueno-de-Mesquita HB, Jenab M, Duell EJ, Agudo A, Tjonneland A, Boutron-Ruault MC, Clavel-Chapelon F, Touillaud M, Teucher B, Kaaks R, et al. Fruit and vegetable intake and the risk of gastric adenocarcinoma: a reanalysis of the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) study after a longer follow-up. Int J Cancer 2012;131: 2910–9. [DOI] [PubMed] [Google Scholar]
- 5.Shimazu T, Wakai K, Tamakoshi A, Tsuji I, Tanaka K, Matsuo K, Nagata C, Mizoue T, Inoue M, Tsugane S, Sasazuki S, Research Group for the D, et al. Association of vegetable and fruit intake with gastric cancer risk among Japanese: a pooled analysis of four cohort studies. Ann Oncol 2014;25: 1228–33. [DOI] [PubMed] [Google Scholar]
- 6.Wang Q, Chen Y, Wang X, Gong G, Li G, Li C. Consumption of fruit, but not vegetables, may reduce risk of gastric cancer: results from a meta-analysis of cohort studies. Eur J Cancer 2014;50: 1498–509. [DOI] [PubMed] [Google Scholar]
- 7.Wang T, Cai H, Sasazuki S, Tsugane S, Zheng W, Cho ER, Jee SH, Michel A, Pawlita M, Xiang YB, Gao YT, Shu XO, et al. Fruit and vegetable consumption, Helicobacter pylori antibodies, and gastric cancer risk: a pooled analysis of prospective studies in China, Japan, and Korea. Int J Cancer 2017;140: 591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Cancer Research Fund International & American Institute for Cancer Research, Continuous Update Project Report: Diet, Nutrition, Physical Activity and Stomach Cancer. Washington, DC: AIRC, 2016. [Google Scholar]
- 9.Pelucchi C, Lunet N, Boccia S, Zhang ZF, Praud D, Boffetta P, Levi F, Matsuo K, Ito H, Hu J, Johnson KC, Ferraroni M, et al. The Stomach cancer Pooling (StoP) project: study design and presentation. Eur J Cancer Prev 2015;24: 16–23. [DOI] [PubMed] [Google Scholar]
- 10.Bertuccio P, Alicandro G, Rota M, Pelucchi C, Bonzi R, Galeone C, Bravi F, Johnson KC, Hu J, Palli D, Ferraroni M, Lopez-Carrillo L, et al. Citrus fruit intake and gastric cancer: the Stomach cancer Pooling (StoP) project consortium. Int J Cancer 2019;144: 2936–44. [DOI] [PubMed] [Google Scholar]
- 11.Harris H, Håkansson N, Olofsson C, Julin B, Åkesson A, Wolk A. The Swedish Mammography Cohort and the Cohort of Swedish Men: study design and characteristics of 2 population-based longitudinal cohorts. OA Epidemiology 2013;1: 16. [Google Scholar]
- 12.Hamada GS, Kowalski LP, Nishimoto IN, Rodrigues JJ, Iriya K, Sasazuki S, Hanaoka T, Tsugane S, Sao Paulo--Japan Cancer Project Gastric Cancer Study G. Risk factors for stomach cancer in Brazil (II): a case-control study among Japanese Brazilians in Sao Paulo. Jpn J Clin Oncol 2002;32: 284–90. [DOI] [PubMed] [Google Scholar]
- 13.Nishimoto IN, Hamada GS, Kowalski LP, Rodrigues JG, Iriya K, Sasazuki S, Hanaoka T, Tsugane S, Sao Paulo--Japan Cancer Project Gastric Cancer Study G. Risk factors for stomach cancer in Brazil (I): a case-control study among non-Japanese Brazilians in Sao Paulo. Jpn J Clin Oncol 2002;32: 277–83. [DOI] [PubMed] [Google Scholar]
- 14.Mao Y, Hu J, Semenciw R, White K, Canadian Cancer Registries Epidemiology Research G. Active and passive smoking and the risk of stomach cancer, by subsite, in Canada. Eur J Cancer Prev 2002;11: 27–38. [DOI] [PubMed] [Google Scholar]
- 15.Mu LN, Lu QY, Yu SZ, Jiang QW, Cao W, You NC, Setiawan VW, Zhou XF, Ding BG, Wang RH, Zhao J, Cai L, et al. Green tea drinking and multigenetic index on the risk of stomach cancer in a Chinese population. Int J Cancer 2005;116: 972–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Setiawan VW, Yu GP, Lu QY, Lu ML, Yu SZ, Mu L, Zhang JG, Kurtz RC, Cai L, Hsieh CC, Zhang ZF. Allium vegetables and stomach cancer risk in China. Asian Pac J Cancer Prev 2005;6: 387–95. [PMC free article] [PubMed] [Google Scholar]
- 17.Deandrea S, Foschi R, Galeone C, La Vecchia C, Negri E, Hu J. Is temperature an effect modifier of the association between green tea intake and gastric cancer risk? Eur J Cancer Prev 2010;19: 18–22. [DOI] [PubMed] [Google Scholar]
- 18.Setiawan VW, Zhang ZF, Yu GP, Li YL, Lu ML, Tsai CJ, Cordova D, Wang MR, Guo CH, Yu SZ, Kurtz RC. GSTT1 and GSTM1 null genotypes and the risk of gastric cancer: a case-control study in a Chinese population. Cancer Epidemiol Biomarkers Prev 2000;9: 73–80. [PubMed] [Google Scholar]
- 19.Lagiou P, Samoli E, Lagiou A, Peterson J, Tzonou A, Dwyer J, Trichopoulos D. Flavonoids, vitamin C and adenocarcinoma of the stomach. Cancer Causes Control 2004;15: 67–72. [DOI] [PubMed] [Google Scholar]
- 20.Pourfarzi F, Whelan A, Kaldor J, Malekzadeh R. The role of diet and other environmental factors in the causation of gastric cancer in Iran--a population based study. Int J Cancer 2009;125: 1953–60. [DOI] [PubMed] [Google Scholar]
- 21.Pakseresht M, Forman D, Malekzadeh R, Yazdanbod A, West RM, Greenwood DC, Crabtree JE, Cade JE. Dietary habits and gastric cancer risk in north-west Iran. Cancer Causes Control 2011;22: 725–36. [DOI] [PubMed] [Google Scholar]
- 22.La Vecchia C, D’Avanzo B, Negri E, Decarli A, Benichou J. Attributable risks for stomach cancer in northern Italy. Int J Cancer 1995;60: 748–52. [DOI] [PubMed] [Google Scholar]
- 23.Lucenteforte E, Scita V, Bosetti C, Bertuccio P, Negri E, La Vecchia C. Food groups and alcoholic beverages and the risk of stomach cancer: a case-control study in Italy. Nutr Cancer 2008;60: 577–84. [DOI] [PubMed] [Google Scholar]
- 24.De Feo E, Simone B, Persiani R, Cananzi F, Biondi A, Arzani D, Amore R, D’Ugo D, Ricciardi G, Boccia S. A case-control study on the effect of Apolipoprotein E genotypes on gastric cancer risk and progression. BMC Cancer 2012;12: 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buiatti E, Palli D, Decarli A, Amadori D, Avellini C, Bianchi S, Biserni R, Cipriani F, Cocco P, Giacosa A, et al. A case-control study of gastric cancer and diet in Italy. Int J Cancer 1989;44: 611–6. [DOI] [PubMed] [Google Scholar]
- 26.Machida-Montani A, Sasazuki S, Inoue M, Natsukawa S, Shaura K, Koizumi Y, Kasuga Y, Hanaoka T, Tsugane S. Association of Helicobacter pylori infection and environmental factors in non-cardia gastric cancer in Japan. Gastric Cancer 2004;7: 46–53. [DOI] [PubMed] [Google Scholar]
- 27.Hernandez-Ramirez RU, Galvan-Portillo MV, Ward MH, Agudo A, Gonzalez CA, Onate-Ocana LF, Herrera-Goepfert R, Palma-Coca O, Lopez-Carrillo L. Dietary intake of polyphenols, nitrate and nitrite and gastric cancer risk in Mexico City. Int J Cancer 2009;125: 1424–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez-Carrillo L, Hernandez Avila M, Dubrow R. Chili pepper consumption and gastric cancer in Mexico: a case-control study. Am J Epidemiol 1994;139: 263–71. [DOI] [PubMed] [Google Scholar]
- 29.Lopez-Carrillo L, Lopez-Cervantes M, Robles-Diaz G, Ramirez-Espitia A, Mohar-Betancourt A, Meneses-Garcia A, Lopez-Vidal Y, Blair A. Capsaicin consumption, Helicobacter pylori positivity and gastric cancer in Mexico. Int J Cancer 2003;106: 277–82. [DOI] [PubMed] [Google Scholar]
- 30.Lunet N, Valbuena C, Vieira AL, Lopes C, Lopes C, David L, Carneiro F, Barros H. Fruit and vegetable consumption and gastric cancer by location and histological type: case-control and meta-analysis. Eur J Cancer Prev 2007;16: 312–27. [DOI] [PubMed] [Google Scholar]
- 31.Zaridze D, Borisova E, Maximovitch D, Chkhikvadze V. Alcohol consumption, smoking and risk of gastric cancer: case-control study from Moscow, Russia. Cancer Causes Control 2000;11: 363–71. [DOI] [PubMed] [Google Scholar]
- 32.Castano-Vinyals G, Aragones N, Perez-Gomez B, Martin V, Llorca J, Moreno V, Altzibar JM, Ardanaz E, de Sanjose S, Jimenez-Moleon JJ, Tardon A, Alguacil J, et al. Population-based multicase-control study in common tumors in Spain (MCC-Spain): rationale and study design. Gac Sanit 2015;29: 308–15. [DOI] [PubMed] [Google Scholar]
- 33.Santibanez M, Alguacil J, de la Hera MG, Navarrete-Munoz EM, Llorca J, Aragones N, Kauppinen T, Vioque J, Group PS. Occupational exposures and risk of stomach cancer by histological type. Occup Environ Med 2012;69: 268–75. [DOI] [PubMed] [Google Scholar]
- 34.Zhang ZF, Kurtz RC, Klimstra DS, Yu GP, Sun M, Harlap S, Marshall JR. Helicobacter pylori infection on the risk of stomach cancer and chronic atrophic gastritis. Cancer Detect Prev 1999;23: 357–67. [DOI] [PubMed] [Google Scholar]
- 35.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, 2013.
- 36.Health Canada, Nutrition labelling: table of reference amounts for food. Health Canada, 2016. [Google Scholar]
- 37.U.S. Food & Drug Administration, Title 21 -- Food and drugs. U.S. Food & Drug Administration, 2019. [Google Scholar]
- 38.Joint Research Center, Food-based dietary guidelines in Europe. European Commission, 2019. [Google Scholar]
- 39.Smith-Warner SA, Spiegelman D, Ritz J, Albanes D, Beeson WL, Bernstein L, Berrino F, van den Brandt PA, Buring JE, Cho E, Colditz GA, Folsom AR, et al. Methods for pooling results of epidemiologic studies: the Pooling Project of Prospective Studies of Diet and Cancer. Am J Epidemiol 2006;163: 1053–64. [DOI] [PubMed] [Google Scholar]
- 40.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7: 177–88. [DOI] [PubMed] [Google Scholar]
- 41.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21: 1539–58. [DOI] [PubMed] [Google Scholar]
- 42.Borenstein M, Hedges LV, Higgins J, Rothstein HR. Introduction to meta-analysis. John Wiley & Sons, 2011. [Google Scholar]
- 43.Sedgwick P Meta-analyses: heterogeneity and subgroup analysis. BMJ 2013;346: f4040. [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization. Diet, nutrition, and the prevention of chronic diseases: report of a joint WHO/FAO expert consultationed., vol. 916: World Health Organization, 2003. [PubMed] [Google Scholar]
- 45.Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol 1999;28: 964–74. [DOI] [PubMed] [Google Scholar]
- 46.Zhang J, Wu D, Vikash, Song J, Wang J, Yi J, Dong W. Hesperetin induces the apoptosis of gastric cancer cells via activating mitochondrial pathway by increasing reactive oxygen species. Dig Dis Sci 2015;60: 2985–95. [DOI] [PubMed] [Google Scholar]
- 47.Bao L, Liu F, Guo HB, Li Y, Tan BB, Zhang WX, Peng YH. Naringenin inhibits proliferation, migration, and invasion as well as induces apoptosis of gastric cancer SGC7901 cell line by downregulation of AKT pathway. Tumour Biol 2016;37: 11365–74. [DOI] [PubMed] [Google Scholar]
- 48.Hyson DA. A comprehensive review of apples and apple components and their relationship to human health. Adv Nutr 2011;2: 408–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Govers C, Berkel Kasikci M, van der Sluis AA, Mes JJ. Review of the health effects of berries and their phytochemicals on the digestive and immune systems. Nutr Rev 2018;76: 29–46. [DOI] [PubMed] [Google Scholar]
- 50.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological agents. Volume 100 B. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum 2012;100: 1–441. [PMC free article] [PubMed] [Google Scholar]
- 51.Turati F, Pelucchi C, Guercio V, La Vecchia C, Galeone C. Allium vegetable intake and gastric cancer: a case-control study and meta-analysis. Mol Nutr Food Res 2015;59: 171–9. [DOI] [PubMed] [Google Scholar]
- 52.Wu QJ, Yang Y, Wang J, Han LH, Xiang YB. Cruciferous vegetable consumption and gastric cancer risk: a meta-analysis of epidemiological studies. Cancer Sci 2013;104: 1067–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Metere A, Giacomelli L. Absorption, metabolism and protective role of fruits and vegetables polyphenols against gastric cancer. Eur Rev Med Pharmacol Sci 2017;21: 5850–8. [DOI] [PubMed] [Google Scholar]
- 54.Slavin JL, Lloyd B. Health benefits of fruits and vegetables. Adv Nutr 2012;3: 506–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.World Cancer Research Fund International, American Institute for Cancer Research, Continuous Update Project Expert Report 2018. Wholegrains, vegetables and fruit and the risk of cancer, 2018.
- 56.Lunet N, Lacerda-Vieira A, Barros H. Fruit and vegetables consumption and gastric cancer: a systematic review and meta-analysis of cohort studies. Nutr Cancer 2005;53: 1–10. [DOI] [PubMed] [Google Scholar]
- 57.Rota M, Alicandro G, Pelucchi C, Bonzi R, Bertuccio P, Jinfu H, Zhang ZF, Johnson KC, Palli D, Ferraroni M, Yu GP, Galeone C, et al. Education and gastric cancer risk – an individual participant data meta-analysis in the StoP project consortium. Int J Cancer. Epub ahead of print, 2019. [DOI] [PubMed] [Google Scholar]
- 58.Boeing H Nutritional epidemiology: New perspectives for understanding the diet-disease relationship? Eur J Clin Nutr 2013;67: 424–9. [DOI] [PubMed] [Google Scholar]
- 59.Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, McIntosh A, Rosenfeld S. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America’s Table Study. Am J Epidemiol 2001;154: 1089–99. [DOI] [PubMed] [Google Scholar]
- 60.Cade J, Thompson R, Burley V, Warm D. Development, validation and utilisation of food-frequency questionnaires – a review. Public Health Nutr 2002;5: 567–87. [DOI] [PubMed] [Google Scholar]
- 61.Miller V, Mente A, Dehghan M, Rangarajan S, Zhang X, Swaminathan S, Dagenais G, Gupta R, Mohan V, Lear S, Bangdiwala SI, Schutte AE, et al. Fruit, vegetable, and legume intake, and cardiovascular disease and deaths in 18 countries (PURE): a prospective cohort study. Lancet 2017;390: 2037–49. [DOI] [PubMed] [Google Scholar]
- 62.Peleteiro B, Padrao P, Castro C, Ferro A, Morais S, Lunet N. Worldwide burden of gastric cancer in 2012 that could have been prevented by increasing fruit and vegetable intake and predictions for 2025. Br J Nutr 2016;115: 851–9. [DOI] [PubMed] [Google Scholar]
- 63.Botterweck AA, van den Brandt PA, Goldbohm RA. A prospective cohort study on vegetable and fruit consumption and stomach cancer risk in The Netherlands. Am J Epidemiol 1998;148: 842–53. [DOI] [PubMed] [Google Scholar]
- 64.Setiawan VW, Zhang ZF, Yu GP, Lu QY, Li YL, Lu ML, Wang MR, Guo CH, Yu SZ, Kurtz RC, Hsieh CC. GSTP1 polymorphisms and gastric cancer in a high-risk Chinese population. Cancer Causes Control 2001;12: 673–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the StoP Project but restrictions apply to the availability of these data, which were used under license for the current study and so are not publicly available. Data are, however, available from the authors upon reasonable request and permission of the Steering Committee of the StoP Project.


