Abstract
A 94-year-old woman with rheumatoid arthritis who had been treated with low-dose methotrexate was referred to our hospital because of a 3-day history of a fever and pancytopenia. With a diagnosis of febrile neutropenia of unknown origin, empirical antibiotic treatment and folinic acid therapy were initiated. Despite a recovery from pancytopenia, the high fever remained, and dyspnea developed. She was clinically diagnosed with Pneumocystis jirovecii pneumonia (PCP) and successfully treated with trimethoprim/sulfamethoxazole and adjunctive corticosteroid therapy. Folinic acid treatment effectively brought about rapid immune recovery but might have led to a clinical manifestation of PCP resembling immune reconstruction inflammatory syndrome.
Keywords: methotrexate, folinic acid, Pneumocystis jirovecii, Pneumocystis pneumonia, immune reconstitution inflammatory syndrome
Introduction
Pneumocystis jirovecii pneumonia (PCP) is a severe opportunistic fungal infection in patients with a decline in cell-mediated immunity, known classically as an acquired immune deficiency syndrome (AIDS)-defining disease (1). The prevalence of PCP in patients infected by human immunodeficiency virus (HIV) has dropped due to the spread of highly active antiretroviral therapy and a PCP prophylaxis strategy (2). However, there has been a growing number of PCP patients without HIV infection, due to the increasing use of corticosteroids and immunosuppressive drugs (2). The clinical significance and awareness of PCP are thus increasing in the clinical practice of internal medicine.
PCP can manifest after the administration of highly active antiretroviral therapy to HIV patients, a phenomenon thought to be caused by an exaggerated inflammatory response to microorganisms associated with immune recovery (3,4). This phenomenon, known as “immune reconstitution inflammatory syndrome (IRIS),” has been investigated mainly in the realm of HIV/AIDS, but it has also been reported to occur in non-HIV patients due to steroid tapering or discontinuation of immunosuppressive treatment (5).
We herein report the case of a patient with rheumatoid arthritis (RA) who presented with an IRIS-like condition associated with PCP during folinic acid treatment for low-dose methotrexate (MTX)-related pancytopenia.
Case Report
A 94-year-old woman who was living in a nursing home was referred to our department with a 3-day history of a fever, cough, and pancytopenia based on laboratory data. She had been diagnosed with RA when she was 60 years old and treated with low-dose MTX (oral 6 mg weekly) for >1 year (exact duration of treatment unknown), without folic acid or prophylactic antimicrobial drugs. One day before coming to our hospital, she was administered levofloxacin by her family physician. She had a fever of 37.7°C, and the laboratory data revealed pancytopenia (white blood cell count 530 /μL, hemoglobin 7.6 g/dL, platelet count 3.7×104/μL) at that time.
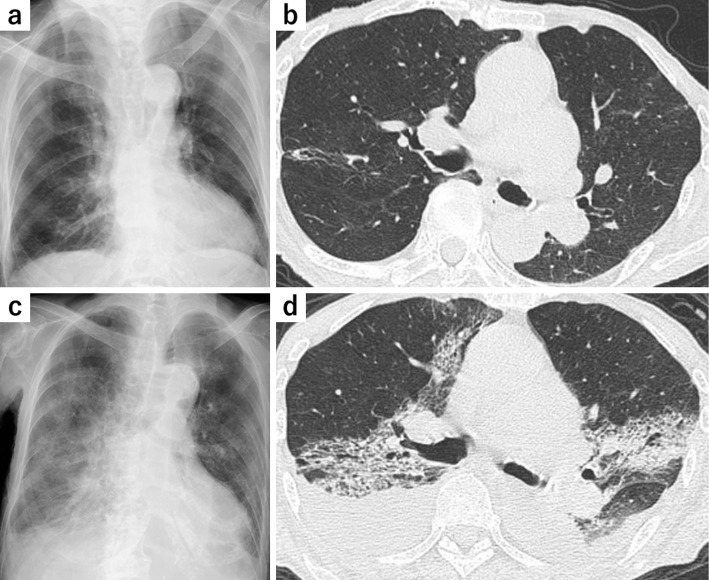
At presentation, she was emaciated but had few respiratory symptoms. Her vital signs were as follows: body temperature 37.4°C, blood pressure 157/78 mmHg, heart rate 85 bpm, and percutaneous oxygen saturation (SpO2) 96% on room air. On a physical examination, her wrist joints were highly deformed but neither tender nor swollen. Slight coarse crackles were audible on the lower left lung. Laboratory findings on admission were pancytopenia (white blood cell count 820 /μL, hemoglobin 9.2 g/dL, platelet count 3.1×104/μL) and an elevated C-reactive protein (CRP) level (17.5 mg/dL) (Table). Chest radiography and chest computed tomography (CT) showed no finding of active inflammation except some old inflammatory changes (Fig. 1a, b). Abdominal CT also showed no findings regarding a source of infection.
Table.
Laboratory Data on Admission.
| <Hematology> | Total bilirubin | 1.2 | mg/dL | |||||
| White blood cells | 820 | /μL | Blood urea nitrogen | 18.8 | mg/dL | |||
| Neutrophils | 66.0 | % | Serum creatinine | 0.87 | mg/dL | |||
| Lymphocytes | 20.0 | % | AST | 25 | IU/L | |||
| Monocytes | 10.0 | % | ALT | 12 | IU/L | |||
| Eosinophils | 4.0 | % | ALP | 216 | IU/L | |||
| Red blood cells | 262×104 | /μL | LDH | 373 | IU/L | |||
| Hemoglobin | 9.2 | g/dL | γ-GTP | 20 | IU/L | |||
| Platelets | 3.1×104 | /μL | Amylase | 139 | IU/L | |||
| <Biochemistry> | Total protein | 5.7 | g/dL | |||||
| Serum sodium | 136 | mEq/L | Albumin | 2.6 | g/dL | |||
| Serum potassium | 3.0 | mEq/L | Creatine kinase | 40 | IU/L | |||
| Serum chloride | 86 | mEq/L | <Serology> | |||||
| Serum calcium | 8.9 | mg/dL | C-reactive protein | 17.52 | mg/dL | |||
ALT: alanine aminotransferase, ALP: alkaline phosphatase, AST: aspartate aminotransferase, γ-GTP: γ-glutamyltransferase, LDH: lactate dehydrogenase
Figure 1.
Radiological findings. Chest X-ray (a) and chest CT (b) on admission showing some old inflammatory changes but no findings of active inflammation. Chest radiograph (c) and chest CT (d) on day 6 post-admission showing bilateral symmetric ground-glass opacities associated with consolidations and bilateral pleural effusions. CT: computed tomography
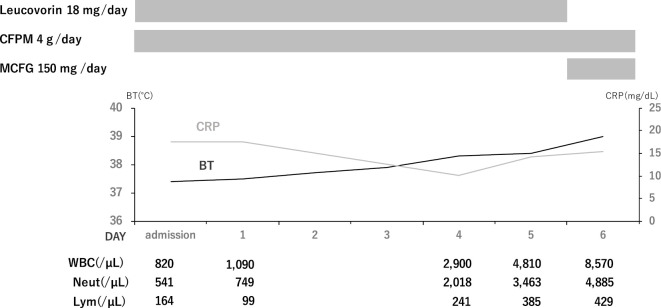
The patient was admitted to our hospital and treated with 2 g of cefepime every 12 hours. Since her pancytopenia was suspected of having been caused by the long-term low-dose MTX therapy, we discontinued MTX, administered sufficient intravenous fluids, and initiated a 6-day course of intravenous folinic acid (leucovorin, 18 mg/day). As expected, the patient's blood cell counts began to recover, but her body temperature and CRP level remained high (Fig. 2). A urine culture, a sputum culture, and two sets of blood cultures on admission were all negative.
Figure 2.
Clinical course during the initial treatment (from day 0 to day 6). The patient’s body temperature and CRP remained high post-admission despite the blood cell recovery. BT: body temperature, CFPM: cefepime, CRP: C-reactive protein, Lym: lymphocytes, MCFG: micafungin, Neut: neutrophils, WBC: white blood cells
On day 6 after her admission, the patient's levels of lactate dehydrogenase (LDH) (522 U/L) and serum β-D-glucan (151.9 pg/mL) were elevated. An empirical antifungal therapy (150 mg/day of micafungin) was started. On the same day, the patient developed hypoxia requiring supplemental oxygen. Chest X-ray and chest CT revealed bilateral symmetric ground-glass opacities (GGOs) and consolidations with bilateral pleural effusions (Fig. 1c, d). Serum KL-6 (615 U/L) levels were also elevated. Based on these findings, we initiated 2.25 g of piperacillin/tazobactam every 6 hours as an escalation of antibiotic therapy, and we consulted the department of respiratory medicine for a diagnostic and therapeutic strategy for suspected PCP.
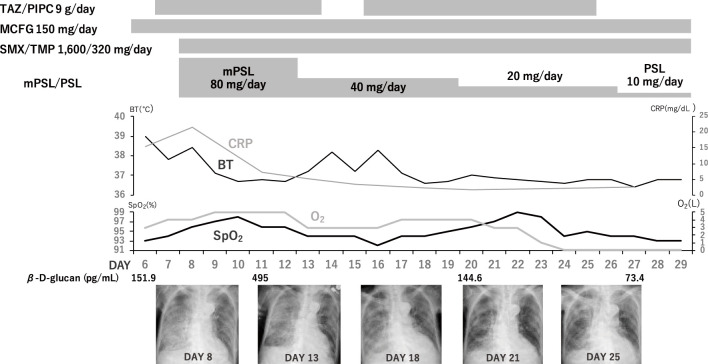
Given the patient's age and clinical condition, bronchoscopy with bronchoalveolar lavage (BAL) was not conducted. On the 8th day of hospitalization, a sputum test was performed for a P. jirovecii-specific polymerase chain reaction (PCR), and we initiated sulfamethoxazole/trimethoprim (TMP/SMX) treatment (sulfamethoxazole 1,600 mg/trimethoprim 320 mg/day, intravenous) and corticosteroid therapy (methylprednisolone 80 mg/day, intravenous). The patient's temperature and CRP level began to decrease (Fig. 3). We diagnosed her with PCP based on the positive PCR result, serum β-D-glucan elevation, and clinical course.
Figure 3.
Clinical course after the development of dyspnea (from day 6 to day 29). After the initiation of SMX/TMP treatment and adjunctive corticosteroid therapy, the patient’s clinical condition and radiographic findings gradually improved. MCFG: micafungin, mPSL: methylprednisolone, PSL: prednisolone, SMX/TMP: trimethoprim/sulfamethoxazole, TAZ/PIPC: tazobactam/piperacillin
The dose of corticosteroid therapy was tapered, and the TMP/SMX treatment was continued for 22 days until the patient's respiratory symptoms and radiography findings improved. The serum β-D-glucan level transiently increased just after the initiation of the treatment (Fig. 3) but decreased over the course of treatment. The patient was released from the hospital on day 68 of her hospitalization.
Discussion
We described a case of PCP that became clinically apparent after the introduction of folinic acid treatment for low-dose MTX-induced pancytopenia in a patient with RA. The patient's initial presentation was febrile neutropenia of unknown origin. In addition to empirical antibiotic therapy, we discontinued the patient's MTX regimen and started folinic acid treatment. Despite the recovery of her blood cell counts, the patient developed PCP, but she was successfully treated with SMX/TMP combined with corticosteroid therapy.
Our patient was clinically diagnosed with PCP based on the positive PCR findings, an elevated β-D-glucan level, and radiologic findings. The diagnosis of PCP is difficult to make because P. jirovecii cannot be cultured. Microscopic staining with BAL fluid, the best diagnostic method for PCP (6), could not be performed in this patient because of her advanced age and clinical condition. Since P. jirovecii-specific PCR has a false-positive issue with colonization, a serum β-D-glucan assay is regarded as a useful additional tool for diagnosing PCP. The cut-off value for differentiating PCP from colonization was reported to be 33.5 pg/mL, with a positive predictive value was 0.925 (7). In the present case, β-D glucan was elevated to 495 pg/mL, which exceeded the diagnostic threshold. Serum KL-6 elevation was consistent with the diagnosis of PCP.
Chest CT findings on the 6th hospital day showed bilateral diffuse GGOs associated with consolidations and bilateral pleural effusions, both of which are atypical findings in PCP. The combination of GGOs and consolidations can be presented in PCP mainly among non-HIV patients (8). These findings can also be suggestive of heart failure. However, the finding of pleural effusion was considered to reflect hypoalbuminemia and excessive intravenous fluid. In addition, the BNP level (69.1 pg/mL) on the 7th day of hospitalization did not support clinically significant heart failure. Cardiogenic pulmonary edema was therefore considered to account for only a small part of the extensive GGOs and consolidations. Cytomegalovirus (CMV) pneumonia, a frequent opportunistic viral infection in immunocompromised patients, is sometimes difficult to differentiate from PCP on CT (8). However, the result of the CMV pp65 antigenemia assay was negative.
The lack of significant CT findings on admission might have delayed the diagnosis of PCP. Given that the patient presented to our hospital with a high fever and elevated CRP and LDH levels, she might have already had PCP on admission to our hospital with as-yet-unnoticed radiographical findings. One case report described the absence of GGOs in HIV-related PCP, which caused the authors to mistakenly rule out PCP (9). Another case of PCP with normal chest CT findings was reported in a patient with HIV infection (10). To our knowledge, however, there has been no report of PCP showing no relevant chest CT findings in a non-HIV patient. One possible reason for this is that because PCP in patients without HIV is generally characterized by an acute onset and fast development of disease (6), it is already progressive when CT is performed. The present patient was as severely immunosuppressed as one with AIDS, so the presentation of PCP might have been slowly progressive, like HIV-related PCP, before admission. Since latent PCP without abnormal findings on chest CT can occur under immunosuppressive conditions, such as in cases of AIDS and severe leucopenia, the early recognition of PCP should be based not merely on CT findings but also on the patient's background and serum β-D glucan values.
The present case can be regarded as a rare case of PCP presenting with an IRIS-like phenomenon related to leucovorin rescue therapy for MTX-induced pancytopenia. An IRIS-like condition has been reported to occur in non-HIV patients, due typically to steroid withdrawal or the cessation of immunosuppressants (5). In our patient, PCP became clinically apparent after her recovery from leucopenia, resembling unmasking IRIS, which is defined as a clinical manifestation of latent infection (11). This phenomenon can be triggered by reactivation of T-cell mediated immunity due to the discontinuation of MTX therapy and folinic acid treatment. Although not fully understood, T-cell-mediated immunity is believed to play an essential role in the pathogenesis of IRIS (12). Indeed, there is a case report of a patient with chronic lymphocytic leukemia who developed an IRIS-like syndrome associated with PCP after the completion of alemtuzumab therapy (13). The restoration of the CD4-positive T-lymphocyte count, the value of which was suppressed by alemtuzumab, was speculated to lead to the development of PCP (13).
Our patient was likely to have had long-term myelosuppression due to the low-dose MTX regimen administered prior to her admission. Risk factors for HIV-associated IRIS include a low CD4-positive T lymphocyte count and high pathogen burden at the initiation of anti-HIV therapy (14). The patient exhibited severe leucopenia with lymphocyte counts <200 cells/μL. Furthermore, the high level of serum β-D glucan (495 pg/mL) indicated a high burden of P. jirovecii, despite the fact that the fungal load is generally lower in non-HIV patients than in HIV-infected patients (6). In light of the report that a CD4-positive T lymphocyte count <200 cells/μL is a major risk factor for the development of PCP (15), we suspect that for our patient, the significant deterioration in her immunity, especially T cell-mediated immunity, allowed for the growth of P. jirovecii in her lungs, as seen in HIV-related PCP. We therefore infer that the patient was at a high risk for an IRIS-like syndrome. In this condition, the rapid recovery of white blood cells, including T lymphocytes, due to the cessation of MTX and the folinic acid therapy promoted immune reconstitution, which could have caused an exaggerated inflammatory reaction to P. jirovecii. Therefore, the lung consolidations on chest CT that emerged on the 6th hospital day may have reflected host immune-mediated pulmonary damage in PCP (16).
Folinic acid therapy might have contributed substantially to the rapid recovery of blood cells in the present case. Folinic acid treatment, also known as leucovorin rescue therapy, is mainly used in cases of high-dose MTX-induced hematotoxicity. The Japanese clinical guideline of MTX for RA patients also recommends using it for severe adverse effects of low-dose MTX (17). The recommended daily dose of folinic acid is 3 times as much as the MTX dose, so we administered 18 mg of leucovorin per day (17). Folinic acid treatment was reported to significantly shorten the recovery time from pancytopenia due to low-dose MTX therapy in RA patients (18). This finding suggests that folinic acid therapy might have accelerated the host immune reaction to the pathogen through the mechanism described above.
Invasive pulmonary aspergillosis is a frequent opportunistic fungal pneumonia associated with serum β-D glucan elevation in neutropenic patients. We considered it unlikely based on the radiological findings but should have considered performing further examinations, including antigen testing.
In conclusion, we reported a rare case of an IRIS-like condition associated with PCP in a patient with RA who received folinic acid treatment for low-dose MTX-induced pancytopenia. Clinicians should be aware of the possibility of unmasking opportunistic infections after a patient's rapid immune recovery from an immunosuppressive status.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Thomas CF Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med 350: 2487-2498, 2004. [DOI] [PubMed] [Google Scholar]
- 2. Roux A, Gonzalez F, Roux M, et al. Update on pulmonary Pneumocystis jirovecii infection in non-HIV patients. Med Mal Infect 44: 185-198, 2014. [DOI] [PubMed] [Google Scholar]
- 3. Achenbach CJ, Harrington RD, Dhanireddy S, Crane HM, Casper C, Kitahata MM. Paradoxical immune reconstitution inflammatory syndrome in HIV-infected patients treated with combination antiretroviral therapy after AIDS-defining opportunistic infection. Clin Infect Dis 54: 424-433, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. French MA. HIV/AIDS: immune reconstitution inflammatory syndrome: a reappraisal. Clin Infect Dis 48: 101-107, 2009. [DOI] [PubMed] [Google Scholar]
- 5. Fujita J. Immune reconstitution inflammatory syndrome in the lung in non-human immunodeficiency virus patients. Respir Investig 58: 36-44, 2020. [DOI] [PubMed] [Google Scholar]
- 6. Salzer HJF, Schäfer G, Hoenigl M, et al. Clinical, diagnostic, and treatment disparities between HIV-infected and non-HIV-infected immunocompromised patients with Pneumocystis jirovecii pneumonia. Respiration 96: 52-65, 2018. [DOI] [PubMed] [Google Scholar]
- 7. Tasaka S, Kobayashi S, Yagi K, et al. Serum (1→3) β-D-glucan assay for discrimination between Pneumocystis jirovecii pneumonia and colonization. J Infect Chemother 20: 678-681, 2014. [DOI] [PubMed] [Google Scholar]
- 8. Cereser L, Dallorto A, Candoni A, et al. Pneumocystis jirovecii pneumonia at chest high-resolution computed tomography (HRCT) in non-HIV immunocompromised patients: spectrum of findings and mimickers. Eur J Radiol 116: 116-127, 2019. [DOI] [PubMed] [Google Scholar]
- 9. Block BL, Mehta T, Ortiz GM, et al. Unusual radiographic presentation of pneumocystis pneumonia in a patient with AIDS. Case Rep Infect Dis 2017: 3183525, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maki Y, Fujikura Y, Sasaki H, et al. A case of Pneumocystis pneumonia diagnosed by elevation of serum 1,3-beta-D-glucan and demonstration of a cyst in bronchoalveolar lavage fluid despite no abnormal CT findings. Kikannshi Gaku (J Jpn Soc Respir Endosc) 41: 284-288, 2019(in Japanese, Abstract in English). [Google Scholar]
- 11. Haddow LJ, Colebunders R, Meintjes G, et al. Cryptococcal immune reconstitution inflammatory syndrome in HIV-1-infected individuals: proposed clinical case definitions. Lancet Infect Dis 10: 791-802, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gopal R, Rapaka RR, Kolls JK. Immune reconstitution inflammatory syndrome associated with pulmonary pathogens. Eur Respir Rev 26: 160042, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Otahbachi M, Nugent K, Buscemi D. Granulomatous Pneumocystis jiroveci pneumonia in a patient with chronic lymphocytic leukemia: a literature review and hypothesis on pathogenesis. Am J Med Sci 333: 131-135, 2007. [DOI] [PubMed] [Google Scholar]
- 14. Walker NF, Scriven J, Meintjes G, Wilkinson RJ. Immune reconstitution inflammatory syndrome in HIV-infected patients. HIV AIDS (Auckl) 7: 49-64, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Glück T, Geerdes-Fenge HF, Straub RH, et al. Pneumocystis carinii pneumonia as a complication of immunosuppressive therapy. Infection 28: 227-230, 2000. [DOI] [PubMed] [Google Scholar]
- 16. Tasaka S, Tokuda H, Sakai F, et al. Comparison of clinical and radiological features of Pneumocystis pneumonia between malignancy cases and acquired immunodeficiency syndrome cases: a multicenter study. Intern Med 49: 273-281, 2010. [DOI] [PubMed] [Google Scholar]
- 17. Japan College of Rheumatology. Clinical practice guideline of methotrexate for patients with rheumatoid arthritis: 2016 update version. Yodosha, Tokyo, 2016: 50. [Google Scholar]
- 18. Cansu DÜ, Teke HÜ, Bodakçi E, Korkmaz C. How should we manage low-dose methotrexate-induced pancytopenia in patients with rheumatoid arthritis? Clin Rheumatol 37: 3419-3425, 2018. [DOI] [PubMed] [Google Scholar]