Abstract
A 72-year-old man presented with chorea while undergoing treatment for recurrence of nodal peripheral T-cell lymphoma with T follicular helper (TFH) phenotype. An examination by brain N-isopropyl-p-iodoamphetamine (123I-IMP)-single photon emission computed tomography (SPECT) revealed no abnormalities other than a decreased cerebral blood flow (CBF) in the left striatum. After four courses of salvage chemotherapy, his clinical symptoms and asymmetric cerebral perfusion improved, suggesting that the decreased CBF had caused chorea. The significance of brain SPECT has not been fully clarified in patients with chorea-associated malignant lymphoma, warranting further investigations. Brain SPECT is an alternative approach to identify abnormalities in such patients.
Keywords: peripheral T-cell lymphoma, chorea, single photon-emission computed tomography
Introduction
Chorea is characterized by repetition of short and fast involuntary movements at irregular intervals, typically causing abnormalities on the face, mouth, trunk, and extremities. Huntington's disease (HD) is the most common cause of chorea in adults, but cerebrovascular disorders, autoimmune diseases, metabolic diseases, and neoplasms are also reported as causative factors (1). Patients with malignant lymphoma rarely but occasionally experience chorea due to either paraneoplastic neurological syndrome (PNS) (2,3) or direct invasion of the tumor into the basal ganglia (4-7).
We herein report a case of nodal peripheral T-cell lymphoma (PTCL) with T follicular helper (TFH) phenotype in a patient who presented with chorea during treatment. We also present a short literature review to elucidate the clinical characteristics of lymphoma patients with chorea.
Case Report
A 72-year-old man presented to a hospital with a fever, night sweats, and weight loss. Whole-body computed tomography (CT) revealed supraclavicular, mediastinal, para-aortic, and inguinal lymphadenopathy, and subsequently, a biopsy of the left inguinal lymph node was performed. Histological studies of the biopsy specimens showed scattered atypical cells with large irregular nuclei, and immunohistochemistry studies revealed that the cells were cluster of differentiation (CD)3+, CD4+, CD5+, CD7+, CD10-, CD30+, PD1-, BCL6+, and CXCL13+. He was diagnosed with nodal PTCL with TFH phenotype and achieved his first complete remission (CR) after six courses of dose-adjusted EPOCH (etoposide, prednisolone, vincristine, cyclophosphamide and doxorubicin).
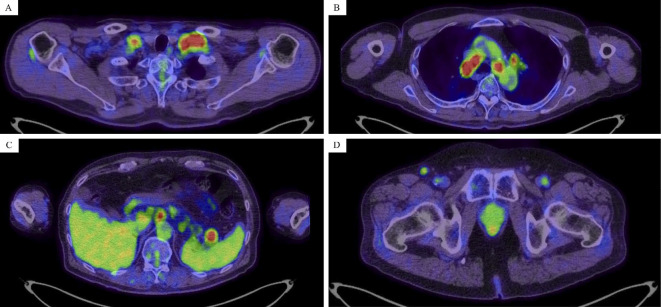
Approximately 1.5 years after the first CR, he had recurrence of the lymphoma and therefore received salvage chemotherapy. He then achieved partial response with the reduction of supraclavicular, mediastinal, para-aortic, and inguinal lymphadenopathy. However, seven months after the start of salvage chemotherapy, positron emission tomography (PET)-CT showed re-growth of these lymph nodes and the uptake of fluorodeoxyglucose at the same site (Fig. 1), suggesting progression of the lymphoma. Furthermore, personality changes and the presence of chorea in the right upper and lower extremities were observed.
Figure 1.
Positron emission tomography and computed tomography findings show the fluorodeoxyglucose uptake into the bilateral supraclavicular, mediastinum, para-aortic, spleen hilum, and right inguinal lymphoma (A-D).
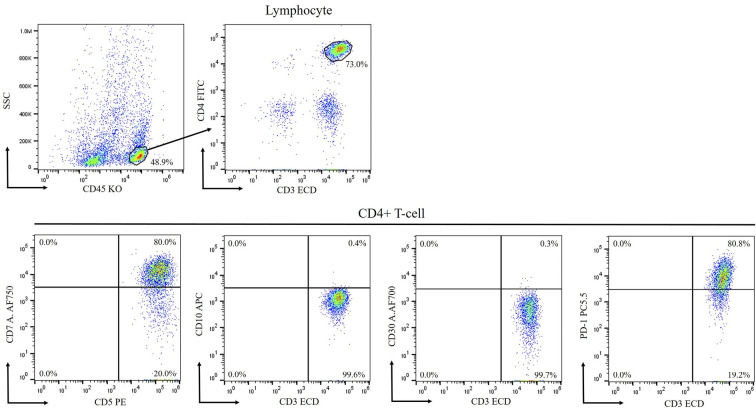
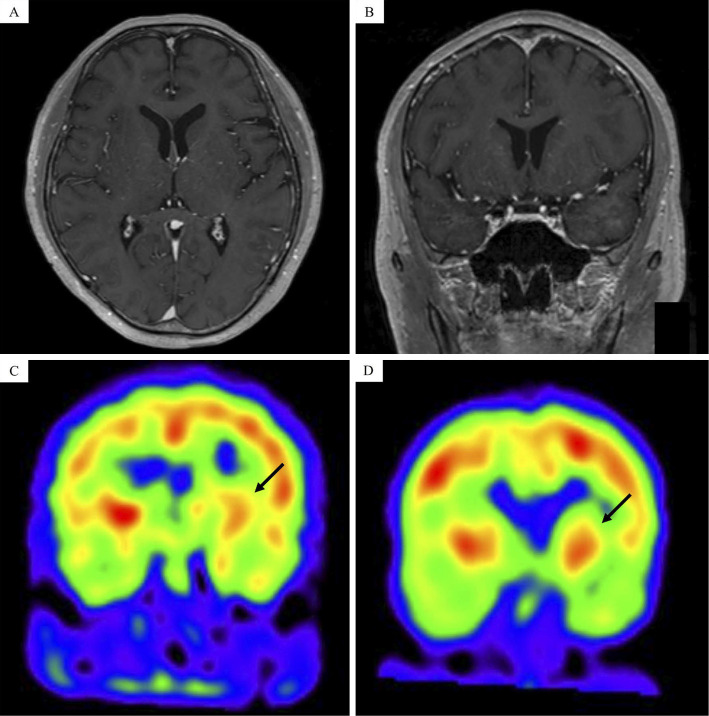
The patient had never received antipsychotic drugs and had no family history of diseases associated with involuntary movements, such as HD. Furthermore, he had no history of thrombosis. Complete blood counts and biochemistry tests showed mild elevation in lactate dehydrogenase and immunoglobulin G (IgG)-κ-type M-protein. Tumor markers almost showed normal findings and no PNS-associated auto-antibodies were detected (Table 1). Although a cerebrospinal fluid (CSF) examination revealed mild pleocytosis (18/μL; normal range, 0-5/μL) and slightly increased levels of protein (78 mg/dL; normal range, 10-40 mg/dL), flow cytometry (FCM) indicated no evidence of leptomeningeal invasion of lymphoma cells (Fig. 2). Imaging examinations, including head CT and gadolinium-enhanced brain magnetic resonance imaging (MRI), showed unremarkable findings. However, N-isopropyl-p-iodoamphetamine (123I-IMP)-single-photon emission computed tomography (SPECT) of the brain showed decreased perfusion in the left striatum compared to that in the right striatum, which suggested that the decreased perfusion might have caused the clinical symptoms (Fig. 3). He received high-dose methotrexate and cytarabine, because the cause of his neurological symptom was suspected to be PNS or direct tumor invasion.
Table 1.
Patient’s Laboratory Data on Admission.
| <Complete blood count> | Cl | 104 | mmol/L | <Tumor marker> | |||||||||
| WBC | 5,850 | /μL | Ca | 9.3 | mg/dL | PSA | 1.76 | ng/mL | |||||
| RBC | 355 | ×104/μL | CRP | 0.05 | mg/dL | CYFRA | 2.9 | ng/dL | |||||
| Hb | 12.0 | g/dL | Ferritin | 444.0 | ng/mL | SCC | 0.9 | mg/dL | |||||
| Ht | 36.2 | % | IgG | 873.7 | mg/dL | CEA | 1.760 | ng/mL | |||||
| MCV | 101.9 | fL | IgA | 70.0 | mg/dL | SLX | 21.2 | ng/mL | |||||
| MCH | 33.8 | pg | IgM | 17.9 | mg/dL | NSE | 23.30 | ng/mL | |||||
| MCHC | 33.2 | g/dL | IFE | IgG-κ | ProGRP | 48.5 | mmol/L | ||||||
| Plt | 21.2 | ×104/μL | NH3 | 24 | μg/dL | SIL-2R | 444.0 | IU/mL | |||||
| PBG | 139 | mg/dL | |||||||||||
| <Biochemistry> | HbA1c | 5.2 | % | <Serology> | |||||||||
| TP | 6.9 | g/dL | FT3 | 2.10 | pg/mL | Amphiphysin | N/D | ||||||
| Alb | 4.6 | g/dL | FT4 | 1.30 | pg/mL | CV2/CRMP5 | N/D | ||||||
| T-Bil | 1.3 | mg/dL | TSH | 1.11 | μIU/mL | PNMA2(Ma2/Ta) | N/D | ||||||
| AST | 35 | IU/L | Vit B1 | 30 | ng/mL | Ri | N/D | ||||||
| ALT | 29 | IU/L | ANA | N/D | Yo | N/D | |||||||
| ALP | 223 | IU/L | Hu | N/D | |||||||||
| γ-GTP | 30 | IU/L | <Coagulation> | recoverin | N/D | ||||||||
| LDH | 279 | IU/L | PT | 10.7 | s | SOX1 | N/D | ||||||
| BUN | 22.2 | mg/dL | APTT | 26.5 | s | titin | N/D | ||||||
| Cr | 0.91 | mg/dL | Fib | 258 | mg/dL | zic4 | N/D | ||||||
| UA | 5.1 | mg/dL | FDP | <2.5 | μg/mL | GAD65 | N/D | ||||||
| Na | 135 | mmol/L | D-dimer | <0.5 | μg/mL | Tr(DNER) | N/D | ||||||
| K | 4.0 | mmol/L | AT3 | 104 | % | ||||||||
WBC: white blood cells, RBC: red blood cells, Hb: hemoglobin, Ht: hematocrit, MCV: mean corpuscular volume, MCH: mean corpuscular hemoglobin, MCHC: mean corpuscular hemoglobin concentration, Plt: platelets, TP: total protein, Alb: albumin, T-Bil: total bilirubin, AST: aspartate aminotransferase, ALT: alanine aminotransferase, ALP: alkaline phosphatase, γ-GTP: gamma glutamyltranspeptidase, LDH: lactate dehydrogenase, BUN: blood urea nitrogen, Cr: creatinine, UA: uric acid, Na: natrium, K: kalium, Cl: chlorine, Ca: calcium, CRP: C-reactive protein, IgG: immunoglobulin G, IgA: immunoglobulin A, IgM: immunoglobulin M, IFE: immunofixation electrophoresis, PBG: postprandial blood glucose, HbA1c: hemoglobin A1c, FT3: free T3, FT4: free T4, TSH: thyroid-stimulating hormone, VitB1: vitamin B1, ANA: anti-nuclear antibody, N/D: not detectable, PT: prothrombin time, APTT: activated partial thromboplastin time, Fib: fibrinogen, FDP: fibrin/fibrinogen degradation products, AT3: antithrombin 3, PSA: prostate-specific antigen, CYFRA: cytokeratin 19 fragment, SCC: squamous cell carcinoma, CEA: carcinoembryonic antigen, SLX: sialyl Lewis X-i antigen, NSE: neuron specific enolase, ProGRP: pro-gastrin-releasing peptide, SIL-2R: soluble interleukin-2 receptor, CRMP5: collapsin response mediator protein 5, SOX1: sex-determining region Y-related high-mobility-group box 1, zic4: zinc finger protein 4, GAD65: glutamic acid decarboxylase 65, DNER: delta/notch-like epidermal growth factor-related receptor
Figure 2.
Flow cytometry revealed that the cluster of differentiation (CD)4+ T-cells in cerebral blood flow were positive for CD3, CD5, and PD1 and negative for CD10 and CD30.
Figure 3.
Gadolinium-enhanced brain magnetic resonance imaging revealed no abnormal findings (A, B). Brain N-isopropyl-p-iodoamphetamine (123I-IMP)-single photon-emission computed tomography showing hypoperfusion (black arrow) in the left striatum compared with that in the right striatum (C) and improvement after salvage chemotherapy (D).
After four courses of salvage chemotherapy, his personality changes and the presence of chorea in the upper right and lower limbs reverted to their normal state, and asymmetric hypoperfusion of the striatum on brain 123I-IMP-SPECT improved, which suggested that the decreased cerebral blood flow (CBF) had been the cause of the chorea in this patient (Fig. 3).
Discussion
Chorea is rarely associated with malignant lymphoma, and its precise incidence is unclear. We performed a literature search in the PubMed database using the search term “chorea” and “lymphoma,” which revealed 13 reported cases of malignant lymphoma with chorea, including the present case (4-15) (Table 2). Seven and five patients were diagnosed with paraneoplastic chorea (PC) and direct invasion of lymphoma cells, respectively, and these conditions had caused chorea in those cases. However, the cause of chorea in the present case was unclear. Among the 13 chorea patients with lymphoma, 4 (cases 1, 11, 12, and our case) showed mild pleocytosis and/or slightly increased levels of protein in the CSF, although their cytology did not show the presence of lymphoma cells.
Table 2.
Summary of Reported Cases of Chorea Associated with Malignant Lymphoma.
| Case | Age/ Sex |
Histology | Cause of chorea | Autoantibody | CSF | Gadolinium-enhanced cranial MRI | Type of chorea | Response to therapy | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | N/A | NHL, HL | PNS | Not detected | Cell: 26/μL Prot: 56 mg/dL Cytology: N/A |
N/A | N/A | Some improvement after risperidone and lorazepam | N/A | 8 |
| 2 | 71/M | DLBCL | PNS | CV2/CRMP5 | N/A | Normal | Unilateral (left) | Improvement after chemotherapy, haloperidol and tetrabenazine | Alive | 9 |
| 3 | 49/F | Indolent B-cell lymphoma | PNS | CV2/CRMP5 | Normal | Normal | Generalized | Some improvement after chemotherapy | Alive | 10 |
| 4 | 75/F | NHL | PNS | CV2/CRMP5 | N/A | N/A | Generalized | No improvement after mPSL | Alive | 11 |
| 5 | 58/F | Intestinal T-cell lymphoma | PNS | N/A | N/A | Normal | Unilateral (left) | No improvement after chemotherapy | Dead | 12 |
| 6 | 70/M | Immunoblastic T-cell lymphoma | PNS | Not detected (only Hu and Yo) | Normal | Normal | Generalized | Improvement after chemotherapy | Alive | 13 |
| 7 | 67/F | HL | PNS | antibody against cytoplasmic antigen (not Hu, Yo and Ri) | Cells: 5/μL Prot: normal Cytology: normal |
Non enhancing high-intensity areas in the both caudate and putamen nuclei | Generalized | Improvement after immunoadsorption | Dead | 14 |
| 8 | 49/M | DLBCL | Direct invasion | N/A | N/A | High-intensity areas in the left cerebral peduncle, subthalamic nucleus, thalamus and internal capsule | Unilateral (right) | Improvement after chemotherapy and WBRT | N/A | 4 |
| 9 | 68/F | MF | Direct invasion | N/A | N/A | High-intensity areas in the right striatum and internal capsule | Unilateral (left) | Refusal of treatment | Dead | 5 |
| 10 | 62/F | DLBCL | Direct invasion | N/A | N/A | High-intensity areas in the bilateral cerebral peduncles and internal capsule, and right pallidum, substantia nigra and subthalamic nucleus | Unilateral (left) | Improvement after chemotherapy and intrathecal MTX | N/A | 6 |
| 11 | 66/M | Immunoblastic lymphoma | Direct invasion | N/A | Cells: normal Prot: 101 mg/dL Cytology: normal |
High-intensity areas in the right thalamus, corpus callosum, and caudate nucleus | Unilateral (left) | Improvement after WBRT | Dead | 7 |
| 12 | 85/F | CLL | Direct invasion | Not detected (only Hu, Yo and Ri) | Cells: 18/μL Prot: 69 mg/dL Cytology: normal |
Subacute infarct in the left cerebellum | Unilateral (left) | Improvement after PSL and tetrabenazine | Alive | 15 |
| Our case | 72/M | Nodal PTCL with TFH phenotype | Unknown | Not detected | Cells: 18/μL Prot: 78 mg/dL Cytology: normal |
Normal | Unilateral (right) | Improvement after chemotherapy | Alive |
CSF: cerebrospinal fluid, MRI: magnetic resonance imaging, Prot: protein, NHL: non-Hodgkin lymphoma, HL: Hodgkin lymphoma, DLBCL: diffuse large B-cell lymphoma, MF: mycosis fungoides, CLL: chronic lymphocytic leukemia, PTCL: peripheral T-cell lymphoma, TFH: T-follicular helper, PNS: paraneoplastic neurological syndrome, CRMP5: collapsin response mediator protein 5, PSL: prednisolone, mPSL: methylprednisolone, MTX: methotrexate, WBRT: whole-brain radiation, N/A: not available
In patients with direct tumor invasion, lymphoma cells infiltrated in and around the basal ganglia (striatum, pallidum, substantia nigra, and subthalamic nucleus) and damaged the striatum. This event activated the excitatory neurons that project from the thalamus to the cerebral cortex and resulted in chorea-related symptoms (16). All four patients (cases 8-11) proven to have direct tumor invasion on gadolinium-enhanced head MRI had contralateral hemichorea. In contrast, among lymphoma patients with PC, hemichorea was found in two patients (cases 2 and 5). According to previous reports, the left-right asymmetry of persisting dopamine transporter (DAT) became apparent with increasing age, even in healthy individuals (17). Therefore, it was suggested that PNS may be the cause of the asymmetry-related clinical symptoms in patients with laterality in the DAT of the bilateral basal ganglia before developing chorea. Based on these results, it was difficult to determine whether the cause of chorea was PNS or direct tumor invasion based on clinical symptoms alone in the present case.
In our patient, brain SPECT showed asymmetric cerebral perfusion, suggesting a decreased CBF, but there was no clear evidence of PNS or lymphoma infiltration. Brain SPECT is mainly used for cerebrovascular diseases because it can provide information on the CBF in the whole brain. HD patients usually show decreased CBF in both caudate nuclei (18). In addition, hypoperfusion of the basal ganglia has also been found in hemichorea due to acute cerebral infarction, autoimmune diseases [e.g., systemic lupus erythematosus (SLE) or antiphospholipid syndrome (APS)], and hyperglycemia (19,20), suggesting that reduced perfusion of the basal ganglia in brain SPECT might be associated with the development of chorea. In contrast, patients with primary or secondary lymphoma in the central nervous system (CNS) show a high uptake in brain SPECT (21-23), and cases of lymphomatosis cerebri also show similar findings (24). However, in the previously reported chronic lymphocytic leukemia patient with hemichorea caused by direct lymphoma invasion (case 12), brain technetium-hexamethylpropylene amine oxime (99mTc-HMPAO)-SPECT revealed a low uptake in the right basal ganglia (15). Nakae et al. reported a thymoma patient who presented with chorea and regional hypoperfusion in the right subthalamic nucleus and pallidum on brain technetium-ethyl cysteinate dimer (99mTc-ECD)-SPECT, although the cause of chorea in this patient was PNS (25). To our knowledge, there are no studies that have used brain SPECT in patients with PC who are proven to have auto-antibodies. Therefore, further investigations are needed to elucidate this mechanism.
With regard to the auto-antibodies associated with PC, anti-CV2/collapsin response mediator protein 5 (CRMP5) antibody was most frequently detected (3). First reported by Honnorat et al. in 1996 (26), anti-CV2 antibody was later proven to target an intracellular protein called CRMP5 expressed in the human brain in areas such as the cerebral cortex, hippocampus, cerebellum, and thalamus (27,28). CRMP-5 IgG is a neuronal-autoantibody produced during an immune response to small-cell lung cancer and, rarely, thymoma and is not found in the blood of healthy individuals (29). However, auto-antibodies were not detected in up to 20% of PC patients (3), suggesting the importance of alternative approaches, such as brain SPECT, to identifying PC patients without auto-antibodies.
In conclusion, we encountered a case of nodal PTCL with TFH phenotype in a patient who developed chorea during treatment. Localized basal ganglia hypoperfusion in brain SPECT may reflect the cause of chorea in lymphoma patients. Further investigations with similar cases are warranted.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Hermann A, Walker RH. Diagnosis and treatment of chorea syndromes. Curr Neurol Neurosci Rep 15: 514, 2015. [DOI] [PubMed] [Google Scholar]
- 2. Graus F, Ariño H, Dalmau J. Paraneoplastic neurological syndromes in Hodgkin and non-Hodgkin lymphomas. Blood 123: 3230-3238, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vigliani MC, Honnorat J, Antoine JC, et al. Chorea and related movement disorders of paraneoplastic origin: the PNS EuroNetwork experience. J Neurol 258: 2058-2068, 2011. [DOI] [PubMed] [Google Scholar]
- 4. Noda K, Hattori N, Okuma Y. Primary central nervous system lymphoma presenting as choreoathetosis. BMJ Case Rep 2014: 1-2, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hengstman GJD, van Rossum MM, van der Kerkhof PCM, Bloem BR. Chorea due to mycosis fungoides metastasis. J Neurooncol 73: 87-88, 2005. [DOI] [PubMed] [Google Scholar]
- 6. Tan EK, Chan LL, Auchus AP, Wong MC. Reversible choreoathetosis in primary cerebral lymphoma: clinicoradiologic correlation. Eur Neurol 50: 53-54, 2003. [DOI] [PubMed] [Google Scholar]
- 7. Poewe WH, Kleedorfer B, Willeit J, Gerstenbrand F. Primary CNS lymphoma presenting as a choreic movement disorder followed by segmental dystonia. Mov Disord 3: 320-325, 1988. [DOI] [PubMed] [Google Scholar]
- 8. O'Toole O, Lennon VA, Ahlskog JE, et al. Autoimmune chorea in adults. Neurology 80: 1133-1144, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nevison S, Rizek P. Anti-CV2-associated paraneoplastic hemichorea secondary to diffuse large B-cell lymphoma. Can J Neurol Sci 46: 480-481, 2019. [DOI] [PubMed] [Google Scholar]
- 10. Samii A, Dahlen DD, Spence AM, Maronian NC, Kraus EE, Lennon VA. Paraneoplastic movement disorder in a patient with non-Hodgkin's lymphoma and CRMP-5 autoantibody. Mov Disord 18:12: 1556-1558, 2003. [DOI] [PubMed] [Google Scholar]
- 11. Vernino S, Tuite P, Adler CH, et al. Paraneoplastic chorea associated with CRMP-5 neuronal antibody and lung carcinoma. Ann Neurol 51: 625-630, 2002. [DOI] [PubMed] [Google Scholar]
- 12. Kitiyakara T, Jackson M, Gorard DA. Refractory coeliac disease, small-bowel lymphoma and chorea. J R Soc Med 95: 133-134, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nuti A, Ceravolo R, Salvetti S, Gambaccini G, Bonuccelli U, Capochiani E. Paraneoplastic choreic syndrome during non-Hodgkin's lymphoma. Mov Disord 15: 350-352, 2000. [DOI] [PubMed] [Google Scholar]
- 14. Batchelor TT, Platten M, Palmer-Toy DE, et al. Chorea as a paraneoplastic complication of Hodgkin's disease. J Neurooncol 36: 185-190, 1998. [DOI] [PubMed] [Google Scholar]
- 15. Sheen VL, Asimakopoulos F, Heyman E, Henderson G, Feske SK. Hemichorea as a presentation of recurrent non-Hodgkin's lymphoma. J Neurol 249: 1746-1748, 2002. [DOI] [PubMed] [Google Scholar]
- 16. Obeso JA, Rodríguez-Oroz MC, Rodríguez M, Arbizu J, Giménez-Amaya JM. The basal ganglia and disorders of movement: pathophysiological mechanisms. News Physiol Sci 17: 51-55, 2002. [DOI] [PubMed] [Google Scholar]
- 17. Van Dyck CH, Seibyl JP, Malison RT, et al. Age-related decline in dopamine transporters: analysis of striatal subregions, nonlinear effects, and hemispheric asymmetries. Am J Geriatr Psychiatry 10: 36-43, 2002. [PubMed] [Google Scholar]
- 18. Ehrlich DJ, Walker RH. Functional neuroimaging and chorea: a systematic review. J Clin Mov Disord 4: 1-15, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim JS, Lee KS, Lee KH, et al. Evidence of thalamic disinhibition in patients with hemichorea: semiquantitative analysis using SPECT. J Neurol Neurosurg Psychiatry 72: 329-333, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nordal EB, Nielsen J, Marhaug G. Chorea in juvenile primary antiphospholipid syndrome. Reversible decreased circulation in the basal ganglia visualised by single photon emission computed tomography. Scand J Rheumatol 28: 324-327, 1999. [DOI] [PubMed] [Google Scholar]
- 21. Shinoda J, Yano H, Murase S, Yoshimura S, Sakai N, Asano T. High 123I-IMP retention on SPECT image in primary central nervous system lymphoma. J Neurooncol 61: 261-265, 2003. [DOI] [PubMed] [Google Scholar]
- 22. Kitanaka C, Eguchi T, Kokubo T. Secondary malignant lymphoma of the central nervous system with delayed high uptake on 123I-IMP single-photon emission computerized tomography: case report. J Neurol Surg 76: 871-873, 1992. [DOI] [PubMed] [Google Scholar]
- 23. Suess E, Malessa S, Ungersböck K, et al. Technetium-99m-d,1-hexamethylpropyleneamine oxime (HMPAO) uptake and glutathione content in brain tumors. J Nucl Med 32: 1675-1681, 1991. [PubMed] [Google Scholar]
- 24. Murakami T, Yoshida K, Segawa M, et al. A case of lymphomatosis cerebri mimicking inflammatory diseases. BMC Neurol 16: 1-5, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakae Y, Ikeda S, Yamamoto R, Tanaka F, Johkura K. Hemichorea in a thymoma patient without anti-CRMP-5 antibody. Neurol Sci 35: 629-630, 2014. [DOI] [PubMed] [Google Scholar]
- 26. Honnorat J, Antoine JC, Derrington E, Aguera M, Belin MF. Antibodies to a subpopulation of glial cells and a 66 kDa developmental protein in patients with paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry 61: 270-278, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fukada M, Watakabe I, Yuasa-Kawada J, et al. Molecular characterization of CRMP5, a novel member of the collapsin response mediator protein family. J Biol Chem 275: 37957-37965, 2000. [DOI] [PubMed] [Google Scholar]
- 28. Brot S, Malleval C, Benetollo C, et al. Identification of a new CRMP5 isoform present in the nucleus of cancer cells and enhancing their proliferation. Exp Cell Res 319: 588-599, 2013. [DOI] [PubMed] [Google Scholar]
- 29. Yu Z, Kryzer TJ, Griesmann GE, Kim KK, Benarroch EE, Lennon VA. CRMP-5 neuronal autoantibody: marker of lung cancer and thymoma-related autoimmunity. Ann Neurol 49: 146-154, 2001. [PubMed] [Google Scholar]