Abstract
A 68-year-old man was admitted to our department because of left incomplete homonymous hemianopia accompanied by hyperglycemia. Both T2-weighted and diffusion-weighted imaging revealed a low signal intensity along the subcortex and high signal intensity along the cortex on the right parietal and occipital lobes. Furthermore, arterial spin labeling and single-photon emission computed tomography showed hyperperfusion at the right parieto-occipital lobe. However, the electroencephalography result was normal. Hyperperfusion improved after controlling the blood glucose levels; nevertheless, homonymous hemianopia remained. We suspect that the irreversible brain damage was attributable to hyperperfusion associated with long-term hyperglycemia.
Keywords: homonymous hemianopia, hyperglycemia, hyperperfusion, epilepsy, single-photon emission computed tomography
Introduction
The neurological manifestations of severe hyperglycemia generally include choreoathetosis, ballismus (1), seizures (2), and coma (3). Regarding vision loss, patients with diabetes often have visual abnormalities caused by diabetic retinopathy. Vision loss is generally classified into prechiasmal, chiasmal, or retrochiasmal deficits. The homonymous hemianopia observed in cases localized retrochiasmatically affects the right optic tract, lateral geniculate nucleus, optic radiations, or visual cortex. Hyperglycemic hemianopia has been rarely reported to cause temporary damage to the visual cortex, resulting in homonymous hemianopsia (4-18). Some reports have suggested an association of homonymous hemianopsia with epilepsy; however, the nature of the neurological impairment and neuroradiological findings in hyperglycemic hemianopia is not well known.
Magnetic resonance imaging (MRI) classically demonstrates a low signal intensity along the subcortex on T2-weighted imaging. In previous studies, visual deficits improved or disappeared after controlling blood glucose levels in all patients with homonymous hemianopia with hyperglycemia (4-18).
We herein report a case of irreversible homonymous hemianopia associated with severe hyperglycemia and cerebral hyperperfusion. Hyperperfusion improved after controlling blood glucose levels; however, homonymous hemianopia persisted. We describe the disease process and discuss its underlying mechanism.
Case Report
A 68-year-old Japanese man was admitted to our department 1 month after the sudden onset of a visual field defect on the left side. He had a history of type 2 diabetes mellitus, atrial fibrillation, and cardioembolic stroke on the territory of the left middle cerebral artery. A neurological examination revealed difficulty choosing words, which was associated with a previous stroke, and left incomplete homonymous hemianopia. Furthermore, automated perimetry revealed a visual field defect on the left side.
His blood pressure was 136/96 mmHg, and his fundus was normal. His blood glucose and hemoglobin A1c (HbA1c) levels were 524 mg/dL and 17.7%, respectively. The urinary ketone test was weakly positive. The cerebrospinal fluid analysis revealed a normal cell count and protein levels, although the glucose level was 161 mg/dL.
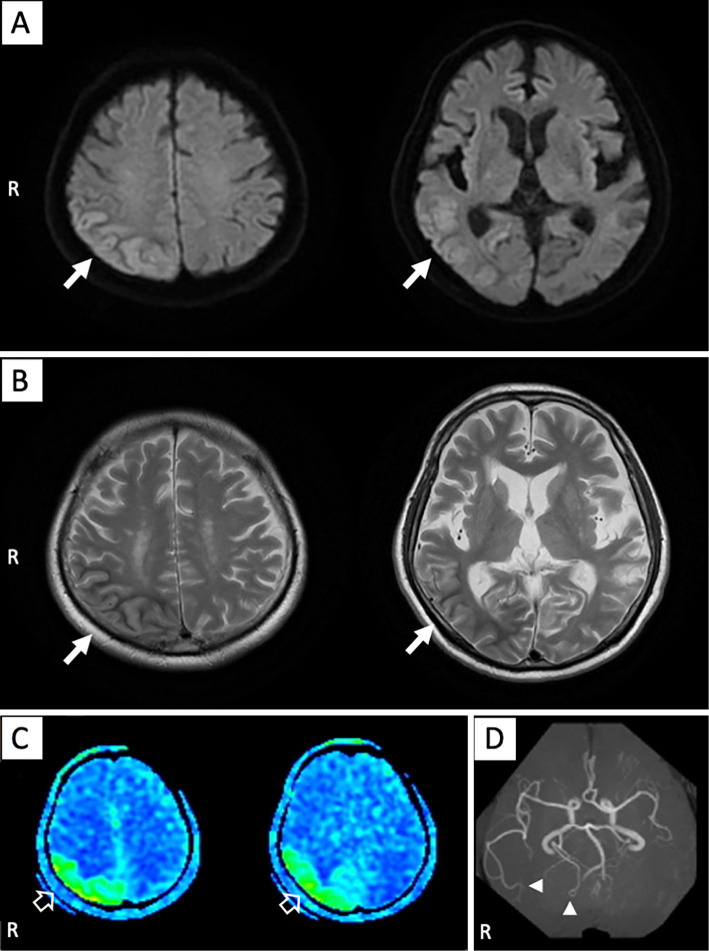
Computed tomography (CT) showed cortical swelling and narrowing of the sulci on the right parietal lobe, adjacent upper segment of the right temporal lobe, and lateral aspect of the right occipital lobe. Diffusion-weighted imaging (DWI) and T2-weighted imaging (T2WI) revealed a high cortical signal intensity and low subcortical signal intensity in the same area as described on the CT scan (Fig. 1A, B). Arterial spin labeling (ASL) revealed cerebral hyperperfusion on the same area where the abnormal signal was observed on T2WI and DWI, and magnetic resonance angiography (MRA) showed an increased signal intensity in the right middle and posterior cerebral artery, which indicated cerebral hyperperfusion (Fig. 1C, D).
Figure 1.
Magnetic resonance imaging findings at admission. (A) Diffusion-weighted imaging and (B) T2-weighted imaging revealed cortical high intensity and subcortical low-intensity signals on the right parietal lobe, adjacent upper segment of the right temporal lobe, and lateral aspect of the right occipital lobe (solid white arrows). (C) Arterial spin labeling showed high signals in the same area where the abnormal findings were observed on magnetic resonance imaging (open white arrows). (D) Magnetic resonance angiography revealed an increased signal intensity in the right middle and posterior cerebral arteries (solid white arrowheads).
We ruled out brain tumors or disruption of the blood-brain barrier (BBB) based on non-enhancement on gadolinium-enhanced MRI. In addition, Mitochondrial myopathy, Encephalopathy, Lactic Acidosis, Stroke-like episodes (MELAS) was excluded because of the elderly onset and lack of characteristic physical findings. There were no findings that prompted suspicion of venous abnormalities.
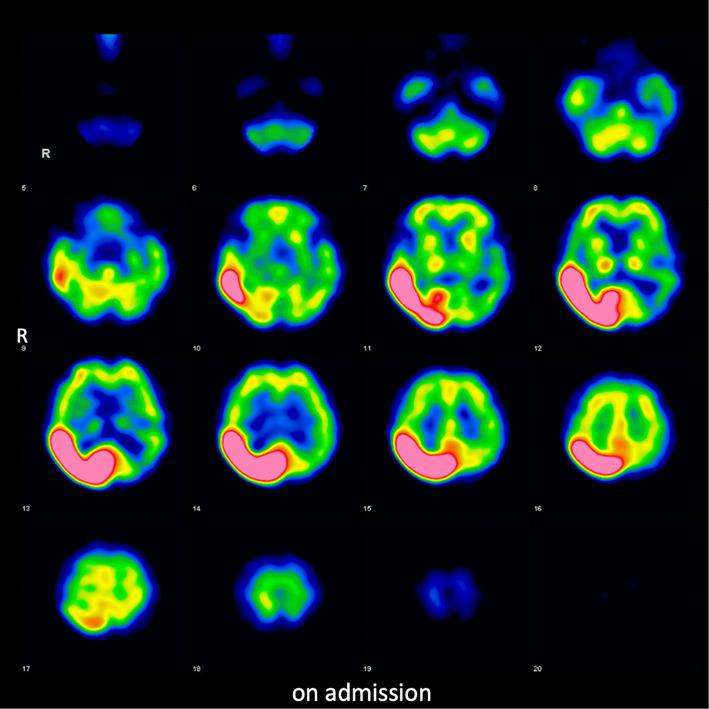
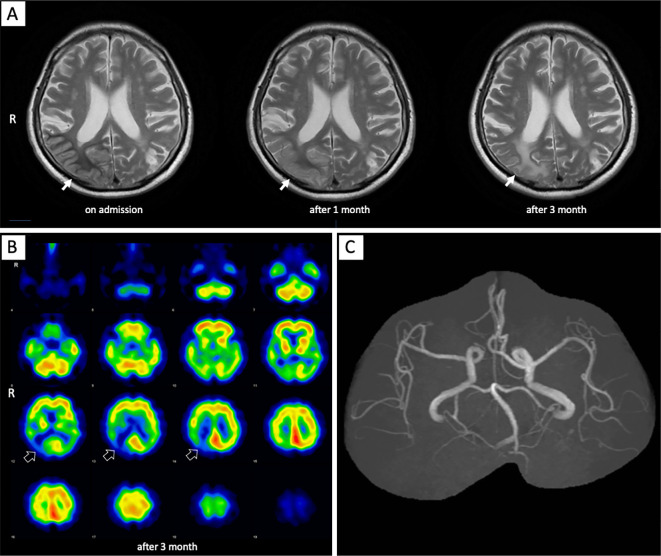
123I-IMP brain perfusion single-photon emission computed tomography (SPECT) revealed a high accumulation at the same area where cerebral hyperperfusion had been observed on ASL (Fig. 2). Because persistent monitoring with an electroencephalogram (EEG) revealed no abnormalities, including epileptic discharges, antiepileptic treatment was not provided. Magnetic resonance spectroscopy revealed a low N-acetyl-aspartate level and high choline level, thereby indicating necrotic changes. The high cortical signal intensity on DWI and high accumulation on SPECT normalized after the patient's blood glucose levels were controlled with insulin treatment; however, the subcortical abnormality in the parieto-occipital lobe on T2WI did not improve and gradually changed from low to high signal intensity three months after admission (Fig. 3A). SPECT also revealed decreased accumulation at the right parieto-occipital lobe three months after admission (Fig. 3B). The patient's left homonymous hemianopia did not improve during the clinical course.
Figure 2.
123I-IMP brain perfusion single-photon emission computed tomography revealed a high accumulation at the right parieto-occipital lobe.
Figure 3.
(A) Subcortical low-intensity signals in the right parieto-occipital lobe on T2-weighted imaging had become an iso-intensity signal at one month after admission (solid white arrow). Subcortical abnormal signals in the right parieto-occipital lobe had become a high-intensity signal at three months after admission. (B) 123I-IMP brain perfusion single-photon emission computed tomography revealed a decreased accumulation at the right parieto-occipital lobe (open white arrows). (C) The dilated right middle and posterior cerebral arteries returned to normal on magnetic resonance angiography.
Discussion
In this case of homonymous hemianopia with hyperglycemia, the following points were important: First, characteristic findings indicative of cerebral hyperperfusion were observed on several brain imaging modalities. Second, clinical findings, including epileptiform discharge on an EEG, which suggest status epilepticus, were unable to be confirmed. Third, subcortical damage on brain imaging and visual deficit did not improve during the clinical course.
The mechanisms underlying the brain damage associated with severe hyperglycemia are not fully elucidated. Intracellular dehydration, reactive oxygen species production, an altered neurotransmitter function, local cerebrovascular ischemia, or altered intracellular enzyme mechanics due to hyperglycemia are expected to result in brain cell damage (19). Furthermore, a relationship between the mechanisms underlying the brain damage associated with severe hyperglycemia and the depletion of gamma aminobutyric acid (GABA) has also been suggested. In hyperglycemic conditions, the Krebs cycle and glucose utilization are inhibited, thereby enhancing alternative pathways of energy metabolism, including conversion of GABA. In a hyperglycemic state, one of the brain energy requirements includes conversion of up to 40% of GABA supplies (20).
Several hypotheses concerning the mechanisms underlying the brain damage associated with severe hyperglycemia have been proposed based on the structural and anatomical aspects of the brain. A previous study hypothesized that pre-existing focal lesions, including old cerebral infarcts, may cause hemianopia with hyperglycemia (21). In our case, the patient had an old cortical infarct in the left frontal lobe; however, the new neurological symptom of left homonymous hemianopia was not caused by damage to the frontal lobe. A relationship between the embryonic type of posterior cerebral artery and posterior predominance in brain damage associated with hyperglycemia has also been suggested (4). However, no embryonic type posterior cerebral artery was observed in our case.
Hyperglycemic hemianopia has been reported to often be complicated by seizures. In previous case reports of hyperglycemic hemianopia, 20 patients underwent an EEG, and 13 patients presented with epileptiform abnormalities on an EEG (Table). It was also reported that a high signal intensity on MRA, hyperperfusion on ASL, and high accumulation on SPECT, which are also characteristics of cerebral hyperperfusion phenomenon, were observed in patients with epilepsy (22,23). In several case reports on hyperglycemic hemianopia, a high accumulation on SPECT or 18F-fluorodeoxyglucose positron emission tomography was observed due to the ictal phenomenon (8,12,18). However, other than the findings on MRA, ASL, and SPECT, there was no evidence of status epilepticus in our case. We assumed that the findings on brain imaging were associated with cerebral hyperperfusion itself, not with epileptic pathophysiology. Persistent monitoring with an EEG showed no abnormalities even without the use of antiepileptic drugs, and we could not confirm any apparent convulsions during the clinical course. Seizures in nonketotic hyperglycinemia patients may be a result of the disruption in the BBB (9). However, in our case, we were unable to identify lesions suggesting BBB disruption on gadolinium-enhanced MRI. Furthermore, the findings on brain imaging indicative of cerebral hyperperfusion disappeared after the patient's blood glucose level normalized, even without antiepileptic treatment.
Table.
Clinical Features of Homonymous Hemianopia Cases Associated with Hyperglycemia.
| Ref. No. | Age/ sex |
Side of visual loss | HbA1c (%) | Serum osmolarity (mOsm/kg) | Epileptiform discharge on EEG | Treatment for epilepsy | MRI ab. | SPECT ab. | Outcome | Recurrence |
|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 32/F | Lt. | 13.5 | n.d. | - | LEV, TPM | + | n.d. | partial improvement | + |
| 4 | 41/F | Lt. | 12.1 | n.d. | - | - | + | n.d. | remission | - |
| 5 | 39/M | Lt. | n.d. | 313 | - | n.d. | + | n.d. | partial improvement | - |
| 5 | 54/M | Lt. | n.d. | 290 | + | n.d. | + | n.d. | remission | - |
| 5 | 34/M | Rt. | n.d. | 300 | - | n.d. | + | n.d. | remission | - |
| 5 | 69/M | Rt. | n.d. | 315 | + | n.d. | + | n.d. | remission | - |
| 6 | 57/M | Rt. | 15.3 | n.d. | + | - | n.d. | n.d. | remission | - |
| 7 | 72/F | Lt. | n.d. | n.d. | n.d. | n.d. | - | n.d. | remission | - |
| 8 | 59/F | Rt. | 9.4 | 309.4 | + | DZP and LZP | + | + | remission | + |
| 9 | 65/M | Lt. | 17.4 | 313 | - | LTG | + | n.d. | remission | - |
| 10 | 67/M | Lt. | n.d. | n.d. | n.d. | n.d. | + | n.d. | remission | - |
| 11 | 61/F | Lt. | n.d. | 341 | - | - | + | n.d. | remission | - |
| 12 | 45/M | Lt. | n.d. | 293 | + | VPA, LEV and OXZ | + | + | remission | - |
| 12 | 60/M | Lt. | n.d. | 290 | + | LEV and OXZ | + | + | remission | - |
| 12 | 69/M | Rt. | n.d. | 315 | + | PHT | - | + | remission | - |
| 13 | 66/M | Rt. | 13.4 | n.d. | + | PHT and LZP | + | n.d. | remission | - |
| 14 | 53/M | Rt. | 11.4 | n.d. | + | PHT and OXZ | + | n.d. | remission | - |
| 15 | 68/M | Lt. | n.d. | n.d. | n.d. | - | - | n.d. | remission | - |
| 16 | 75/M | Lt. | n.d. | n.d. | - | n.d. | n.d. | n.d. | remission | n.d. |
| 17 | 28/M | Lt. | n.d. | 308 | + | PHT | n.d. | n.d. | remission | - |
| 17 | 67/F | Lt. | n.d. | 310 | + | PHT | n.d. | n.d. | remission | - |
| 18 | 30/M | Lt. | 13.8 | 304 | + | PHT and MDZ | + | + | remission | n.d. |
| 18 | 52/F | Lt. | 14.4 | 295 | + | PHT | + | n.d. | remission | n.d. |
| present case | 68/M | Lt. | 17.7 | 302 | - | - | + | + | no improvement | - |
Ref.: Reference, EEG: electroencephalogram, MRI: magnetic resonance imaging, SPECT: single-photon emission computed tomography, ab.: abnormality, M: male, F: female, Rt: right, Lt: left, n.d.: not demonstrated, LEV: levetiracetam, TPM: topiramate, DZP: diazepam, LZP: lorazepam, LTG: lamotrigine, VPA: valproic acid, OXZ: oxcarbazepine, PHT: phenytoin, MDZ: midazolam
In the literature, the association between neurological deficits in severe hyperglycemia and cerebral hyperperfusion has not been fully elucidated. A study reported that the vasomotor reactivity on transcranial duplex ultrasonography with CO2 decreased in patients with hyperglycemic hemianopia (4). Posterior leukoencephalopathy syndrome also affects the occipital lobe, which is attributed to autonomic dysfunction. Sympathetic nerve innervation increases cerebral vascular tone in response to blood pressure increase. Less abundant sympathetic innervation of the posterior circulation would result in posterior predominance (24). Patients with diabetes mellitus may be at risk of autoregulatory failure due to sympathetic dysautonomia and endothelial dysfunction (25). The mechanism underlying the visual deficit in patients with severe hyperglycemia may be attributed to the disruption of autoregulation in the posterior circulation.
Considering the high subcortical signal intensity on T2WI and the decreased accumulation of SPECT on the right parieto-occipital lobe several months after the onset, we believe that the damage to the right parieto-occipital lobe was irreversible. In the literature, 23 cases of hyperglycemic hemianopia have been reported; however, the visual deficits in all cases improved or disappeared after controlling blood glucose levels (Table). In our case, T2WI revealed a lesion with a low signal intensity in the subcortical white matter, which is characteristic of patients with hyperglycemic hemianopia (26,27). Lesions with a low signal intensity on T2WI at the subcortex can occur with iron deposition (28); however, our patient did not present with a low signal intensity on T2*-weighted imaging. Furthermore, the subcortical abnormalities on T2WI gradually shifted from a low to a high signal intensity.
The irreversibility in our case may have been due to the prolonged cerebral hyperperfusion or hyperglycemia itself due to late presentation (one month after the onset). In the literature, only a few reports have included information on HbA1c levels, with values exceeding 10% in most cases (Table). The degree of brain injury, including irreversibility, might be more closely correlated with long-standing hyperglycemia than to the degree of hyperglycemia in the acute setting, as shown in a previous report (18). The diagnostic criteria of hyperglycemic hyperosmolar syndrome include serum osmolality greater than 320 mOsm/L. There was only one case in which the serum osmolality was higher than this criterion (Table). In our case, the patient's serum osmolality was 302 mOsm/L. The onset of hyperglycemic hemianopia may be more strongly influenced by the duration of elevated serum osmolality and blood glucose than the hyperglycemic hyperosmolar state.
In the present case, only one side of the hemisphere was damaged. In the literature, the side of visual loss was described in all cases. Of the 23 cases, 7 involved the right side, and 16 involved the left side. There is no information concerning whether or not recurrence occurred on the same side (Table). Furthermore, we were unable to sufficiently explain why almost all cases involved laterality and the difference in the pathophysiology of other neurological manifestations, including choreoathetosis, ballismus, seizures, and coma.
Several limitations associated with the present study warrant mention. First, we were unable to completely rule out non-convulsive status epilepticus despite normal findings on continuous EEG monitoring. The diagnosis of epilepsy in patients with parieto-occipital lobe lesions is sometimes difficult. Second, it might be difficult to generalize the findings in our case due to the late presentation and prolonged symptoms.
We encountered a case of irreversible homonymous hemianopia associated with severe hyperglycemia. This study indicated that the brain imaging findings were associated with the cerebral hyperperfusion state itself, not with epileptic pathophysiology. To obtain more detailed pathophysiology of the neurological manifestations associated with severe hyperglycemia, a large prospective study should be conducted.
Author's disclosure of potential Conflicts of Interest (COI).
Ryo Itabashi: Honoraria, Bayer, Eisai, Bristol-Myers-Squibb, Daiichi-Sankyo, Nippon Boehringer-Ingelheim, Takeda, Otsuka Pharmaceutical, Tanabe-Mitsubishi Parma, Pfizer, GE Healthcare Japan, Stryker, Medtronic and Johnson and Johnson; Research funding, Nippon Boehringer-Ingelheim.
Financial support
Ryo Itabashi: Tohoku Fukushi University.
References
- 1. Oh SH, Lee KY, Im JH, Lee MS. Chorea associated with non-ketotic hyperglycemia and hyperintensity basal ganglia lesion on T1-weighted brain MRI study: a meta-analysis of 53 cases including four present cases. J Neurol Sci 200: 57-62, 2002. [DOI] [PubMed] [Google Scholar]
- 2. Hennis A, Corbin D, Fraser H. Focal seizures and non-ketotic hyperglycaemia. J Neurol Neurosurg Psychiatry 55: 195-197, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guisado R, Arieff AI. Neurologic manifestations of diabetic comas: correlation with biochemical alterations in the brain. Metabolism 24: 665-679, 1975. [DOI] [PubMed] [Google Scholar]
- 4. Strowd RE, Wabnitz A, Balakrishnan N, Craig J, Tegeler CH. Clinical reasoning: acute-onset homonymous hemianopia with hyperglycemia: seeing is believing. Neurology 82: e129-e133, 2014. [DOI] [PubMed] [Google Scholar]
- 5. Lavin PJ. Hyperglycemic hemianopia: a reversible complication of non-ketotic hyperglycemia. Neurology 65: 616-619, 2005. [DOI] [PubMed] [Google Scholar]
- 6. Johnson SF, Loge RV. Palinopsia due to nonketotic hyperglycemia. West J Med 148: 331-332, 1988. [PMC free article] [PubMed] [Google Scholar]
- 7. Freedman KA, Polepalle S. Transient homonymous hemianopia and positive visual phenomena in nonketotic hyperglycemic patients. Am J Ophthalmol 137: 1122-1124, 2004. [DOI] [PubMed] [Google Scholar]
- 8. Wang CP, Hsieh PF, Chen CC, et al. Hyperglycemia with occipital seizures: images and visual evoked potentials. Epilepsia 46: 1140-1144, 2005. [DOI] [PubMed] [Google Scholar]
- 9. Kim DW, Moon Y, Gee Noh H, Choi JW, Oh J. Blood-brain barrier disruption is involved in seizure and hemianopsia in nonketotic hyperglycemia. Neurologist 17: 164-166, 2011. [DOI] [PubMed] [Google Scholar]
- 10. Mitchell JP, Yancy A, Saint Louis L, Rosberger DF. Reversible hyperglycemic homonymous hemianopia. J Natl Med Assoc 101: 373-376, 2009. [DOI] [PubMed] [Google Scholar]
- 11. Guez A, Obadia M, Lafitte F, Tin SN, Héran F, Gout O. Magnetic resonance spectroscopy findings in a case of hyperglycaemic hemianopia. Rev Neurol (Paris) 166: 737-740, 2010. [DOI] [PubMed] [Google Scholar]
- 12. Stayman A, Abou-Khalil BW, Lavin P, Azar NJ. Homonymous hemianopia in nonketotic hyperglycemia is an ictal phenomenon. Neurol Clin Pract 3: 392-397, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Putta SL, Weisholtz D, Milligan TA. Occipital seizures and subcortical T2 hypointensity in the setting of hyperglycemia. Epilepsy Behav Case Rep 2: 96-99, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nissa Z, Siddiqi SA, Abdool SA. Occipital seizures and persistent homonymous hemianopia with T2 hypointensity on MRI in nonketotic hyperglycemia. Epilepsy Behav Case Rep 6: 3-5, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Taban M, Naugle RI, Lee MS. Transient homonymous hemianopia and positive visual phenomena in patients with nonketotic hyperglycemia. Arch Ophthalmol 125: 845-847, 2007. [DOI] [PubMed] [Google Scholar]
- 16. Brazis PW, Lee AG, Graff-Radford N, Desai NP, Eggenberger ER. Homonymous visual field defects in patients without corresponding structural lesions on neuroimaging. J Neuroophthalmol 20: 92-96, 2000. [DOI] [PubMed] [Google Scholar]
- 17. Harden CL, Rosenbaum DH, Daras M. Hyperglycemia presenting with occipital seizures. Epilepsia 32: 215-220, 1991. [DOI] [PubMed] [Google Scholar]
- 18. Hung WL, Hsieh PF, Lee YC, Chang MH. Occipital lobe seizures related to marked elevation of hemoglobin A1C: report of two cases. Seizure 19: 359-362, 2010. [DOI] [PubMed] [Google Scholar]
- 19. Kodl CT, Seaquist ER. Cognitive dysfunction and diabetes mellitus. Endocr Rev 29: 494-511, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guisado R, Arieff AI. Neurologic manifestations of diabetic comas: correlation with biochemical alterations in the brain. Metabolism 24: 665-679, 1975. [DOI] [PubMed] [Google Scholar]
- 21. Siddiqi ZA, VanLandingham KE, Husain AM. Reflex seizures and non-ketotic hyperglycemia: an unresolved issue. Seizure 11: 63-66, 2002. [DOI] [PubMed] [Google Scholar]
- 22. Kim BS, Lee ST, Yun TJ, et al. Capability of arterial spin labeling MR imaging in localizing seizure focus in clinical seizure activity. Eur J Radiol 85: 1295-1303, 2016. [DOI] [PubMed] [Google Scholar]
- 23. Magistretti PL, Uren RF, Parker JA, Royal HD, Front D, Kolodny GM. Monitoring of regional cerebral blood flow by single photon emission tomography of I123-N-isopropyl-iodoamphetamine in epileptics. Ann Radiol (Paris) 26: 68-71, 1983. [PubMed] [Google Scholar]
- 24. Ay H, Buonanno FS, Schaefer PW, et al. Posterior leukoencephalopathy without severe hypertension: utility of diffusion-weighted MRI. Neurology 51: 1369-1376, 1998. [DOI] [PubMed] [Google Scholar]
- 25. Aaron IV, Raelene EM, Braxton DM, Roy F. Diabetic autonomic neuropathy. Diabetes Care 26: 1553-1579, 2003. [DOI] [PubMed] [Google Scholar]
- 26. Raghavendra S, Ashalatha R, Thomas SV, Kesavadas C. Focal neuronal loss, reversible subcortical focal T2 hypointensity in seizures with a nonketotic hyperglycemic hyperosmolar state. Neuroradiology 49: 299-305, 2007. [DOI] [PubMed] [Google Scholar]
- 27. Chen CCI, Chai JW, Wu CH, Chen WS, Hung HC, Lee SK. Neuroimaging in seizure patients associated with nonketotic hyperglycemia. Neuroradiol J 24: 215-220, 2011. [DOI] [PubMed] [Google Scholar]
- 28. Seo DW, Na DG, Na DL, et al. Subcortical hypointensity in partial status epilepticus associated with nonketotic hyperglycemia. J Neuroimaging 13: 259-263, 2003. [PubMed] [Google Scholar]