Abstract
Objective
Due to the lack of specific clinical manifestations and symptoms, it is difficult to distinguish COVID-19 from mimics. A common pitfall is to rush to make a diagnosis when encountering a patient with COVID-19-like symptoms. The present study describes a series of COVID-19 mimics using an outpatient database collected from a designated COVID-19 healthcare facility in Tokyo, Japan.
Methods
We established an emergency room (ER) tailored specifically for patients with suspected or confirmed COVID-19 called the “COVID-ER.” In this single-center retrospective cohort study, we enrolled patients who visited the COVID-ER from February 1 to September 5, 2020. The outcomes included the prevalence of COVID-19, admission, potentially fatal diseases and final diagnosis.
Results
We identified 2,555 eligible patients. The median age was 38 (interquartile range, 26-57) years old. During the study period, the prevalence of COVID-19 was 17.9% (457/2,555). Non-COVID-19 diagnoses accounted for 82.1% of all cases. The common cold had the highest prevalence and accounted for 33.0% of all final diagnoses, followed by gastroenteritis (9.4%), urinary tract infections (3.8%), tonsillitis (2.9%), heat stroke (2.6%) and bacterial pneumonia (2.1%). The prevalence of potentially fatal diseases was 14.2% (298/2,098) among non-COVID-19 patients.
Conclusion
Several potentially fatal diseases remain masked among the wave of COVID-19 mimics. It is imperative that a thorough differential diagnostic panel be considered prior to the rendering of a COVID-19 diagnosis.
Keywords: COVID-19, SARS-CoV-2, mimic, differential diagnosis, Japan
Introduction
Since the first human cases were identified in China, coronavirus disease 2019 (COVID-19) has rapidly spread throughout the world (1). Numerous studies have been published to date and the typical features of COVID-19 have now been well-documented. COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is primarily characterized by a fever, cough, fatigue, dyspnea, myalgia, diarrhea, anosmia and ageusia (2-4). Due to the lack of specific clinical manifestations and symptoms, there is currently no surefire method for distinguishing COVID-19 from other illnesses that present with a similar array of clinical spectra based on the clinical history and physical examination alone (5-7).
Given the enhanced focus on COVID-19, a common pitfall for first-line healthcare providers is to rush to a diagnosis when encountering a patient with COVID-19-like symptoms (premature closure) (8,9). Therefore, it is imperative that one be mindful of COVID-19 “mimics.” Mimics are conditions closely resembling a very distant entity-a typical example being unilateral weakness due to hypoglycemia mimicking stroke (10,11).
In any given district experiencing widespread COVID-19, the possibility of other etiologies should be carefully considered prior to arriving at a COVID-19 diagnosis. While an abundance of clinical investigations has focused on COVID-19, the assessment of COVID-19 mimics has been limited.
We herein report a series of COVID-19 mimics assessed using an outpatient database collected from a designated COVID-19 healthcare facility in Tokyo, Japan.
Materials and Methods
Setting and patients
The present was a single-center retrospective cohort study conducted at the Metropolitan Hiroo Hospital, which is located in central Tokyo and broadly accepts patients with confirmed or suspected COVID-19. Because COVID-19 is primarily transmitted amongst the population via respiratory droplets and contact routes (12,13), we incorporated zoning strategies in the hospital to prevent the spread of infection to and from medical staff or other patients (13). With increasing volumes of COVID-19 patients, our hospital established an emergency room (ER) tailored specifically for patients with suspected or confirmed COVID-19 and labelled this facility the “COVID-ER.” Outpatients who presented with a fever (≥37.5 °C), cough, fatigue, dyspnea, myalgias, diarrhea, anosmia or ageusia and a known history of close contact with COVID-19 patients were flagged. When patients presented with any of these factors, they were referred for an examination and treatment in the COVID-ER. In the present study, we enrolled patients who visited the COVID-ER from February 1 to September 5, 2020. We excluded patients with missing data and those presenting with cardiac arrest at admission.
Data collection
Data were retrospectively collected from medical charts maintained by the COVID-ER. The following variables were extracted for each patient: age, sex, date of hospital visit, ambulance use, number of hospital visits during the study period, body temperature at the time of COVID-ER visit (≥37.5 °C), presence of a fever prior to COVID-ER visit (≥37.5 °C), symptoms (cough, dyspnea, sore throat, fatigue, myalgia, anosmia or ageusia, headache, chest pain, abdominal pain, nausea or vomiting, diarrhea, etc.), known history of close contact with a COVID-19 patient, the final diagnosis and hospitalization. The final diagnosis was classified as either COVID-19 or other disease. In the event that a COVID-19 patient presented with a known complication associated with COVID-19 or any other comorbidities, the patient was classified under the final diagnosis of COVID-19.
Outcomes
The outcomes included the prevalence of COVID-19, admission and potentially fatal disease. The final diagnosis was also one of the designated outcomes. The diagnosis of COVID-19 was confirmed with real-time reverse transcriptase-polymerase chain reaction (RT-PCR) for SARS-CoV-2 (14). Virtually all patients who visited the COVID-ER were administered RT-PCR tests for SARS-CoV-2 during the study period. For patients who had attempted multiple hospital visits during the study period, we only adopted the diagnosis and accounts from the most recent hospital visit. “Potentially fatal disease” was determined by the subjective judgment of two independent physicians as an illness or condition that could be fatal if overlooked. The prevalence of potentially fatal diseases was determined among non-COVID-19 patients.
In addition to the above, we endeavored to examine patients with a higher prior probability for COVID-19 at the fine scale level. A subgroup analysis was conducted that focused on the prevalence of potentially fatal disease exclusively in patients with and without a fever, anosmia or ageusia, which are the hallmark symptoms of COVID-19. A fever was defined as a body temperature at the time of COVID-ER visit ≥37.5 °C or a fever prior to the COVID-ER visit (≥37.5 °C).
Statistical analyses
Continuous variables were reported as median and interquartile range (IQR), and categorical variables were reported as number and percentage. Baseline differences between groups were evaluated using chi-squared or Fisher's exact tests for categorical variables and Wilcoxon's rank-sum tests for continuous variables. A 2-sided alpha threshold of 0.05 was considered significant. Analyses were conducted using Stata/IC 15.0 (StataCorp, College Station, USA)
Ethical statement
This study was approved by the institutional review board at Tokyo Metropolitan Hiroo Hospital. Due to the anonymous nature of the data, the requirement for informed consent was waived (approval number, JIN-32).
Results
We extracted data from 2,983 patients who visited the COVID-ER during the study period. After applying the inclusion and exclusion criteria, we identified 2,555 eligible patients (Fig. 1). Table 1 shows the patient characteristics. The median age was 38 (IQR, 26-57) years old and the prevalence of ambulance use was 22.5%. Multiple hospital visits were made by 11.9% of patients. The following baseline parameters were significantly higher in COVID-19 patients than in others: fever prior to COVID-ER visit, cough, dyspnea, fatigue, myalgia, anosmia or ageusia, and known history of close contact with a COVID-19 patient.
Figure 1.
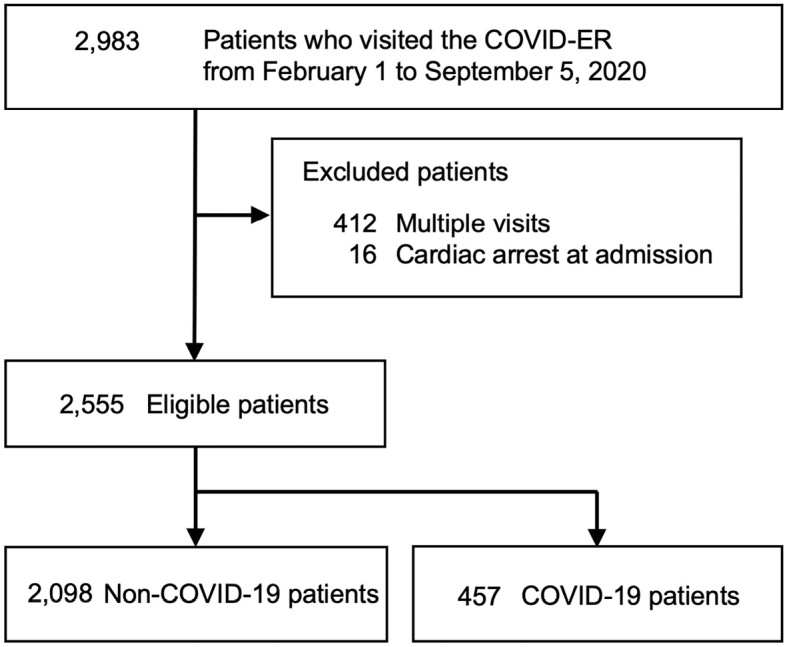
Flow of patients who visited the COVID-ER. COVID-19: coronavirus disease 2019, COVID-ER: emergency room tailored specifically for patients with suspected or confirmed COVID-19
Table 1.
Characteristics of Patients Who Visited the COVID-ER.
| Variables | Total (n=2,555) |
Non-COVID-19 (n=2,098) |
COVID-19 (n=457) |
p value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, years, median (IQR) | 38 | (26-57) | 39 | (26-60) | 35 | (28-47) | 0.018 | ||||
| Sex, male | 1,435 | (56.2%) | 1,139 | (54.3%) | 296 | (64.8%) | <0.001 | ||||
| Ambulance use | 574 | (22.5%) | 545 | (26.0%) | 29 | (6.3%) | <0.001 | ||||
| Number of hospital visits | <0.001 | ||||||||||
| 1 | 2,251 | (88.1%) | 1,872 | (89.2%) | 379 | (82.9%) | |||||
| 2 | 225 | (8.8%) | 164 | (7.8%) | 61 | (13.3%) | |||||
| ≥3 | 79 | (3.1%) | 62 | (3.0%) | 17 | (3.7%) | |||||
| Hospitalization | 600 | (23.5%) | 421 | (20.1%) | 179 | (39.2%) | <0.001 | ||||
| Body temperature at the time of COVID-ER visit | 635 | (25.1%) | 543 | (26.2%) | 92 | (20.2%) | 0.008 | ||||
| Fever prior to COVID-ER visit | 1,190 | (46.6%) | 940 | (44.8%) | 250 | (54.7%) | <0.001 | ||||
| Cough | 727 | (28.5%) | 507 | (24.2%) | 220 | (48.1%) | <0.001 | ||||
| Dyspnea | 453 | (17.7%) | 349 | (16.6%) | 104 | (22.8%) | 0.002 | ||||
| Sore throat | 239 | (9.4%) | 199 | (9.5%) | 40 | (8.8%) | 0.63 | ||||
| Fatigue | 965 | (37.8%) | 756 | (36.0%) | 209 | (45.7%) | <0.001 | ||||
| Myalgia | 274 | (10.7%) | 191 | (9.1%) | 83 | (18.2%) | <0.001 | ||||
| Anosmia or ageusia | 270 | (10.6%) | 116 | (5.5%) | 154 | (33.7%) | <0.001 | ||||
| Headache | 149 | (5.8%) | 124 | (5.9%) | 25 | (5.5%) | 0.72 | ||||
| Chest pain | 45 | (1.8%) | 43 | (2.0%) | 2 | (0.4%) | 0.017 | ||||
| Abdominal pain | 63 | (2.5%) | 63 | (3.0%) | 0 | (0.0%) | <0.001 | ||||
| Nausea and vomiting | 42 | (1.6%) | 42 | (2.0%) | 0 | (0.0%) | <0.001 | ||||
| Diarrhea | 349 | (13.7%) | 293 | (14.0%) | 56 | (12.3%) | 0.33 | ||||
| Other symptoms | 159 | (6.2%) | 144 | (6.9%) | 15 | (3.3%) | 0.004 | ||||
| History of close contact with COVID-19 patient | 309 | (12.1%) | 191 | (9.1%) | 118 | (25.8%) | <0.001 | ||||
COVID-19: coronavirus disease 2019, COVID-ER: emergency room tailored specifically for patients with suspected or confirmed COVID-19, IQR: interquartile range
Data are shown as number (%) unless otherwise specified.
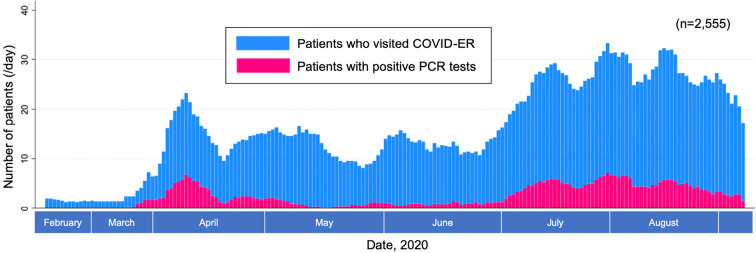
Fig. 2 shows the daily trends in the total number of patients visiting the COVID-ER along with the total number of patients diagnosed with COVID-19 (positive RT-PCR test). During the entire study period, the prevalence of COVID-19 was 17.9% (457/2,555).
Figure 2.
Daily trend in the number of patients who visited the COVID-ER and patients with positive PCR tests. Moving average of 7 days (including the 3 days before and after the index date) applied. COVID-19: coronavirus disease 2019, COVID-ER: emergency room tailored specifically for patients with suspected or confirmed COVID-19, PCR: polymerase chain reaction for COVID-19
Table 2 shows a breakdown of the final diagnoses. Non-COVID-19 diagnoses accounted for 82.1% of all cases after excluding all but the most recent diagnosis for patients attempting multiple hospital visits. The common cold had the highest prevalence and accounted for 33.0% of all final diagnoses. The subsequently most common diagnoses were gastroenteritis (9.2%), urinary tract infection (3.8%), tonsillitis (2.9%), heat stroke (2.6%) and bacterial pneumonia (2.1%). Multiple conditions and illnesses were flagged as potentially fatal disease, typically including myocardial infarction, stroke, bacteremia, epiglottitis, peritonsillar abscess, pneumonia, meningitis, infectious endocarditis, intestinal perforation, aortic dissection, hyperthyroidism, pulmonary thromboembolism, tuberculosis, acquired immunodeficiency syndrome, bacterial arthritis and diabetic ketoacidosis. The prevalence of potentially fatal diseases was 14.2% (298/2,098) among non-COVID-19 patients.
Table 2.
Breakdown of the Final Diagnoses Rendered in the COVID-ER.
| Final diagnosis | n | (%) |
|---|---|---|
| Common cold | 844 | (33.0) |
| COVID-19 | 457 | (17.9) |
| Gastroenteritis | 236 | (9.2) |
| No symptom/Sick contact | 146 | (5.7) |
| Urinary tract infection | 96 | (3.8) |
| Tonsillitis | 75 | (2.9) |
| Heat stroke | 66 | (2.6) |
| Bacterial pneumonia | 53 | (2.1) |
| Poisoning/Over dose | 52 | (2.0) |
| Cancer | 36 | (1.4) |
| Dermatological disease | 33 | (1.3) |
| Aspiration pneumoniae | 32 | (1.3) |
| Psychological disorder/Hyperventilation | 31 | (1.2) |
| Trauma | 29 | (1.1) |
| Seizure | 27 | (1.1) |
| Heart failure | 22 | (0.9) |
| Myocardial infarction/Angina | 22 | (0.9) |
| Stroke | 22 | (0.9) |
| Others | 21 | (0.8) |
| Migraine | 20 | (0.8) |
| Otolaryngological illness | 18 | (0.7) |
| Atypical pneumoniae | 16 | (0.6) |
| Biliary tract infection | 15 | (0.6) |
| Appendicitis/Diverticulitis | 14 | (0.6) |
| Asthma attack | 14 | (0.6) |
| Bacteremia | 11 | (0.4) |
| Epiglottitis | 11 | (0.4) |
| Gynecologic illness | 11 | (0.4) |
| Interstitial pneumonia | 8 | (0.3) |
| Acute hepatitis/Cirrhosis | 7 | (0.3) |
| Anaphylaxis | 7 | (0.3) |
| Bowel obstruction | 7 | (0.3) |
| COPD | 7 | (0.3) |
| Fungal/Viral pneumoniae | 7 | (0.3) |
| Peritonsillar abscess | 7 | (0.3) |
| Pneumothorax/Pneumomediastinum | 7 | (0.3) |
| Atrial fibrillation | 6 | (0.2) |
| Acute pancreatitis | 5 | (0.2) |
| Collagen vascular disease | 5 | (0.2) |
| Meningitis | 5 | (0.2) |
| Infectious endocarditis | 4 | (0.2) |
| Intestinal perforation | 4 | (0.2) |
| Lung abscess | 4 | (0.2) |
| Necrotizing fasciitis | 4 | (0.2) |
| Aortic dissection | 3 | (0.1) |
| Exanthema subitum | 3 | (0.1) |
| Hyperthyroidism | 3 | (0.1) |
| Pulmonary thromboembolism | 3 | (0.1) |
| Tuberculosis | 3 | (0.1) |
| AIDS | 2 | (0.1) |
| Anemia | 2 | (0.1) |
| Bacterial arthritis | 2 | (0.1) |
| Diabetic ketoacidosis | 2 | (0.1) |
| Gout | 2 | (0.1) |
| Kawasaki disease | 2 | (0.1) |
| Odontogenic infection | 2 | (0.1) |
| Adrenal insufficiency | 1 | (0) |
| Malignant syndrome | 1 | (0) |
| Total | 2,555 | (100) |
AIDS: acquired immunodeficiency syndrome, COPD: chronic obstructive pulmonary disease, COVID-19: coronavirus disease 2019, COVID-ER: emergency room tailored specifically for patients with suspected or confirmed COVID-19
The results of a subgroup analysis conducted on the prevalence of potentially fatal disease in patients with and without a fever, anosmia or ageusia are provided in Table 3. The prevalence of potentially fatal disease did not significantly differ between patients with and without these symptoms.
Table 3.
Subgroup Analysis on the Prevalence of Potentially Fatal Disease in Non-COVID-19 Patients with and without Fever, Anosmia or Ageusia.
| Non-COVID-19 patients (n=2,098) | Patients with fever, anosmia or ageusia (n=1,055) |
Patients without fever, anosmia or ageusia (n=1,043) |
p value |
|---|---|---|---|
| Potentially fatal disease, n (%) | 157(14.9) | 141(13.5) | 0.37 |
COVID-19: coronavirus disease 2019
Discussion
The present study documented an aggregate of final diagnoses rendered in the “COVID-ER.” Even in the midst of the COVID-19 pandemic in Tokyo, 82.1% of patients presenting with COVID-19-like symptoms were diagnosed with non-COVID-19 illnesses. The prevalence of potentially fatal diseases that should not be overlooked accounted for 14.2% of non-COVID-19 patients.
COVID-19 fails to present with specific clinical manifestations or symptoms, making the differential diagnosis of COVID-19 mimics essential yet challenging. In our study, we focused on dissecting COVID-19 mimics identified in the COVID-ER at the fine scale level. The common cold, gastroenteritis and tonsillitis accounted for approximately half of all the final diagnoses rendered in the COVID-ER. These results emphasize the notion that even in the COVID-19 era, common diseases remain common. On the opposite end of the spectrum, 14.2% of non-COVID-19 patients in the COVID-ER were diagnosed with potentially fatal diseases. It is important to note that while common diseases remain prevalent in the age of COVID-19, primary care physicians should avoid anchoring biases in favor of COVID-19 diagnoses and instead include potentially life-threatening conditions in the differential diagnosis for patients presenting with COVID-19-like symptoms.
Previous studies have shown mixed results concerning common diseases in the emergency department prior to the COVID-19 pandemic (15). Trauma, poisoning, circulatory diseases, respiratory diseases and stroke were among the most common conditions encountered in emergency departments in Japan (16). Our study showed similar frequencies in these conditions with the exception of trauma and poisoning, as the COVID-ER was selectively biased to cater to patients with COVID-19-like symptoms.
A subgroup analysis of patients with a higher prior probability for COVID-19 (patients with a fever, anosmia or ageusia) revealed that the prevalence of potentially fatal disease was similar compared with patients with a lower prior probability for COVID-19. No matter how closely a patient's symptoms mimic COVID-19, potentially fatal diseases aside from COVID-19 still appear at a given frequency.
Although common diseases accounted for the majority of cases presenting to the COVID-ER during this pandemic, a variety of potentially fatal conditions lay hidden amongst COVID-19 mimics. A good hypothetical case would be a patient complaining of dyspnea initially being diagnosed with COVID-19 due to an anchoring bias - premature closure - when in reality the patient was suffering from a pulmonary thromboembolism (17). Premature closure is the failure to continue considering reasonable alternatives after an initial diagnosis is reached and is one of the most common causes of diagnostic error in internal medicine (8). Physicians should enlist the use of differential diagnoses in order to avoid misdiagnosing potentially life-threatening illnesses as they routinely did in the pre-COVID-19 era.
Several limitations associated with the present study warrant mention. First, the study was conducted in a single facility. In addition to this, our hospital is a tertiary emergency care center and regularly treats patients transported via emergency medical services. The percentage of PCR-positive tests in our facility was higher than the average in the greater Tokyo Metropolitan area during the study period (18,19). Therefore, our study is skewed in favor of including patients with a higher prior probability of COVID-19. As a result, the outcome may not be universally applicable to other institutions and geographical locations. Second, the definition of “potentially fatal diseases” in the present study was based on the subjective assessment of two independent physicians with no strict or otherwise validated criteria adopted.
Conclusions
Several potentially fatal diseases remain masked among the wave of COVID-19 mimics. It is thus imperative that a thorough differential diagnostic panel be considered prior to the rendering of a COVID-19 diagnosis.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors wish to express their deepest appreciation to the entire hospital staff involved in providing medical care to COVID-19 patients during an unprecedented and challenging period.
References
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382: 727-733, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395: 507-513, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus disease 2019 case surveillance - United States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep 69: 759-765, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497-506, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Emperador D, Dittrich S, Domen J, et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19 disease. Cochrane 7: 1-96, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arons MM, Hatfield KM, Reddy SC, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med 382: 2081-2090, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wei WE, Li Z, Chiew CJ, Yong SE, Toh MP, Lee VJ. Presymptomatic transmission of SARS-CoV-2-Singapore. Morb Mortal Wkly Rep 69: 411-415, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med 165: 1493-1499, 2005. [DOI] [PubMed] [Google Scholar]
- 9. Kumar B, Kanna B, Kumar S. The pitfalls of premature closure: clinical decision-making in a case of aortic dissection. BMJ Case Rep 2011: bcr0820114594, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yong AW, Morris Z, Shuler K, Smith C, Wardlaw J. Acute symptomatic hypoglycaemia mimicking ischaemic stroke on imaging: a systemic review. BMC Neurol 12: 139, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moulin S, Leys D. Stroke mimics and chameleons. Curr Opin Neurol 32: 54-59, 2019. [DOI] [PubMed] [Google Scholar]
- 12. Lu J, Gu J, Li K, et al. COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg Infect Dis 26: 1628-1631, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bahl P, Doolan C, de Silva C, Chughtai AA, Bourouiba L, MacIntyre CR. Airborne or droplet precautions for health workers treating coronavirus disease 2019?. J Infect Dis. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Patel A, Jernigan DB. Initial public health response and interim clinical guidance for the 2019 novel coronavirus outbreak - United States. MMWR Morb Mortal Wkly Rep 69: 140-146, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Søvsø MB, Hermansen SB, Færk E, et al. Diagnosis and mortality of emergency department patients in the North Denmark region. BMC Health Serv Res 18: 1-9, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. The statistics of the emergency medical care, 2015. Saiseikai Kumamoto Hospital [Internet]. [cited 2020 Oct 1]. Available from: http://sk-kumamoto.jp/issue/kyukyutokei2015.pdf.
- 17. Coleman JJ, Manavi K, Marson EJ, Botkai AH, Sapey E. COVID-19: to be or not to be; that is the diagnostic question. Postgrad Med J 96: 392-398, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nakajima M, Yamamoto Y, Kaszynski RH, et al. A comparison on the percentage of polymerase chain reaction positivity for SARS-CoV-2 between public health center referrals and direct walk-in patients: a single center retrospective analysis in Tokyo. J Infect Chemother 27: 852-856, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tokyo Metropolitan Government [Internet]. [cited 2021 Mar 18]. Available from: https://stopcovid19.metro.tokyo.lg.jp/cards/positive-rate/.