Abstract
Group A rotaviruses, human caliciviruses, astroviruses, and adenovirus types 40 and 41 were detected by enzyme immunoassay or reverse transcription-PCR in 61, 14, 6, and 3% of stool specimens from 414 children consulting for gastroenteritis between 1995 and 1998. These data highlight the importance of caliciviruses in infantile gastroenteritis. Among these, Norwalk-like viruses belonging to genogroup II were predominant.
In industrialized countries, acute infectious diarrhea is a major cause of morbidity in infants and young children which represents a large burden in terms of medical and indirect costs (4, 12). Group A rotaviruses are recognized as the major etiologic agent (12), and astroviruses and adenovirus types 40 and 41 have been detected with prevalence rates ranging from 2.5 to 9% and from 3 to 9%, respectively, in young children with gastroenteritis (5, 8, 13). Human caliciviruses include two groups of viruses associated with gastroenteritis, the Norwalk-like and the Sapporo-like viruses. The latter have been found primarily in young children (2, 17), and excretion rates have ranged from 0.2 to 6.6% (17). Norwalk-like viruses, which are further divided into two genogroups, represented by the Norwalk virus and the Snow Mountain agent, have long been recognized as an important cause of acute gastroenteritis in adults and school-age children. Although they have been described recently in young children with gastroenteritis in industrialized countries (15, 16, 18), their relative importance in infantile severe or mild gastroenteritis compared to that of the other viruses has seldom been evaluated by sensitive new assays (23).
In France, according to data from the “Sentinelle system,” more than 3 million people (3), among them adults and children, consult a doctor every year for acute diarrhea, with an epidemic peak occurring during winter. While it has been reported that rotavirus was the most prevalent agent associated with gastroenteritis in hospitalized children (9), the prevalence of the other viruses has yet to be investigated. Moreover, there has been no report about the molecular epidemiology of such viruses.
Stool samples from 414 infants and children consulting a pediatrician in a private outpatient clinic in Dijon or the outpatients’ department of the Centre Hospitalier Universitaire for gastrointestinal symptoms were collected from December 1995 to February 1998. When hospitalization was required, stool specimens were collected within 48 h of hospitalization. The average and median ages were available for 381 of the 414 children and were 15.5 months (range, 0 to 158 months; standard deviation, 21.8) and 8 months, respectively. The 33 remaining children were under the age of 3 years. Clinical features were documented for 348 children. In addition, 50 stool samples from control subjects attending a day care center or hospitalized for a cause other than gastroenteritis were also collected in the last 3-month period of the study, from December 1997 to February 1998. For all the controls, the absence of gastrointestinal symptoms was checked during the week preceding and the week following stool sample collection.
Group A rotaviruses were detected by an enzyme immunoassay (EIA) using group-specific monoclonal antibodies (MAbs) as previously described (22). For control subjects, stools were systematically tested by both EIA and reverse transcription (RT)-PCR. RNA was extracted from 10 to 25% stool suspensions in phosphate-buffered saline with a QIA Amp Viral RNA kit (Qiagen, Hilden, Germany), and RT-PCR was performed as described by Gouvea et al. (6).
Caliciviruses were detected by RT-PCR using four primer sets in separate reactions, allowing the detection of Norwalk-like and Sapporo-like viruses. RT-PCR was performed as described previously (11). The degenerate primer NVp110 described by Le Guyader et al. (14) was used for RT together with NVp36, NVp69 (24), SR48-50-52 (1), and NI (7) were used for PCR. The PCR products were sequenced with the ABI Prism Dye Terminator Cycle Sequencing Ready Reaction Kit on an automated sequencer (model 373A DNA Sequencing System) (both from Applied Biosystems, Perkin-Elmer).
Astroviruses and adenoviruses 40 and 41 were detected with an EIA kit (IDEIA Astrovirus; Dako Diagnostics, Ltd.) (Adenoclone type 40/41 EIA; Meridian Diagnostics Inc., Cincinnati, Ohio). For astrovirus, positive samples were further confirmed by RT-PCR according to the method described by Mitchell et al. (19).
Statistical analysis was performed with EPI-INFO version 6.02 software (Centers for Disease Control and Prevention, Atlanta, Ga., and World Health Organization, Geneva, Switzerland, 1994). Cornfield’s method was used to estimate 95% confidence intervals. We used a nonparametric Kruskal-Wallis analysis of variance test to compare means when Bartlett’s chi-square test showed the variances in different samples to differ, and we used the Yates corrected chi-square test for simple analysis. All statistical analysis was performed with a level of significance of 0.05.
Among the 414 stool specimens from patients with gastrointestinal symptoms, 299 (72.2%) contained at least one of the four viruses, whereas no virus could be detected in the 115 remaining samples (27.8%). Among the 50 stool specimens from control subjects, 5 (10%) contained at least one of the four viruses and no virus could be detected in the 45 remaining samples (90%).
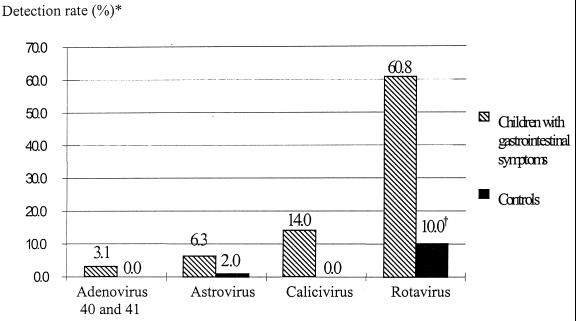
Of the 414 stool specimens from children with gastroenteritis, group A rotaviruses were detected in 252 (60.8%), human caliciviruses in 58 (14%), astroviruses in 26 (6.3%), and adenovirus types 40 and 41 in 13 (3.1%) (Fig. 1). Caliciviruses and adenovirus types 40 and 41 were never detected in stool specimens from the 50 controls (Fig. 1). Astroviruses were detected in 1 sample (2%) by both EIA and RT-PCR, and group A rotaviruses were detected in 5 samples (10%) by EIA but in 13 (26%) by RT-PCR, among which 4 samples gave equivocal results by EIA.
FIG. 1.
Percentages of stool specimens from children with gastrointestinal symptoms and from controls in which group A rotavirus, human calicivirus, astrovirus, or enteric adenovirus type 40 or 41 was detected. *, detection rates for children with symptoms add up to more than 72% (the percent virus-positive samples) because of dual infections. †, for controls, rotavirus was detected by RT-PCR as well as by EIA. Thirteen of the 50 stool specimens (26%) were positive.
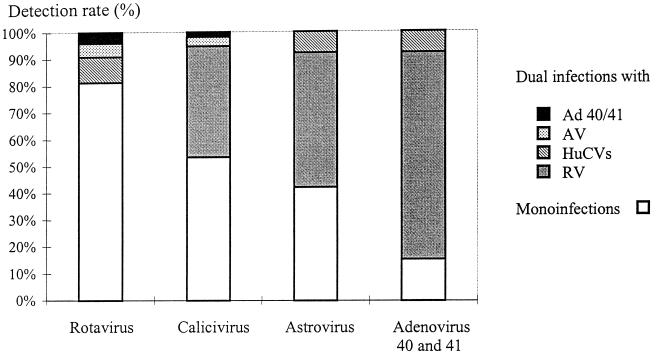
Dual infections were found in 50 of the 299 positive samples (16.7%). The majority of these (94%) were combinations of rotavirus with one of the other three viruses (Fig. 2). One dual infection of rotavirus and astrovirus was also observed among the controls.
FIG. 2.
Dual infections were found in 50 of the 299 positive samples (16.7%). Each bar represents all the positive samples for one given virus. The relative proportions of monoinfections and of dual infections with each of the other three viruses are shown.
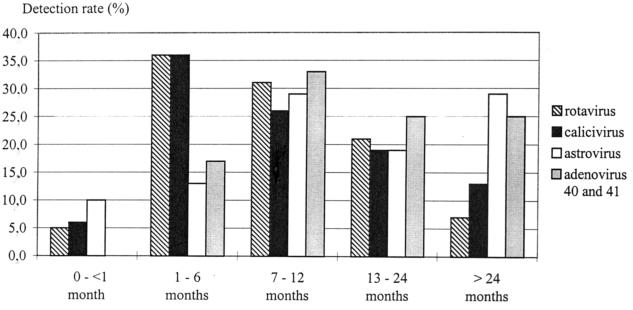
The distribution according to age groups for 381 of the 414 children is shown in Fig. 3. The average ages for rotavirus, calicivirus, astrovirus, and adenovirus type 40 and 41 infections were 11, 14.8, 34, and 15 months, respectively, while the median ages were 8, 8, 10, and 11.5 months. There was no statistically significant difference among the four viruses. Some of the rotavirus, calicivirus, and astrovirus detections, 5, 6, and 10%, respectively, occurred in neonates.
FIG. 3.
Distribution of rotavirus, calicivirus, astrovirus, and adenovirus type 40 and 41 infections by age.
Clinical features were documented for 348 children with gastroenteritis (84%). Of these, 256 were positive for virus and 92 were negative. Results are presented in Table 1. Bloody diarrhea (P < 0.001) and abdominal pain (P = 0.016) were less frequent in children with viral gastroenteritis, and vomiting (P < 0.001) was more frequent among them, than among children whose samples were negative for virus. In contrast, there was no difference in diarrhea and fever between the two populations. Then we compared clinical symptoms observed with the different viruses (Table 2). Dual infections were excluded from the analysis, and adenovirus monoinfections were excluded because of the small size of this group (two patients). Only diarrhea (P < 0.001) and fever (P = 0.049) were statistically significantly different among the three viruses; these symptoms were more frequent during rotavirus infections. Finally, there was no statistically significant difference between monoinfections and dual infections (data not shown).
TABLE 1.
Clinical features in children with and without positive viral detection
| Symptom | No. of samples/total no. of positive samplesa (%) | No. of samples/ total no. of negative samples (%) | Pb |
|---|---|---|---|
| Diarrhea | 230/256 (89.8) | 76/92 (82.6) | 0.100 |
| Bloody diarrhea | 4/256 (1.6) | 10/92 (10.9) | <0.001 |
| Vomiting | 175/256 (68.3) | 41/92 (44.6) | <0.001 |
| Fever | 169/256 (66.0) | 53/92 (57.6) | 0.189 |
| Abdominal pain | 62/256 (24.2) | 35/92 (38.0) | 0.016 |
Positive samples contained at least one of the four viruses: rotavirus, human caliciviruses, astrovirus, or adenovirus type 40 or 41.
A P value of ≤0.05 (Yates corrected χ2 test) was considered significant for differences between children with and without positive viral detection.
TABLE 2.
Clinical features with the different viruses
| Symptom | No. of samples/total no. of samples (%) with:
|
Pa | ||
|---|---|---|---|---|
| Rotavirus | Calicivirus | Astrovirus | ||
| Diarrhea | 166/177 (93.8) | 18/26 (69.2) | 6/8 (75.0) | <0.001 |
| Bloody diarrhea | 2/177 (1.1) | 1/26 (3.8) | 0/8 (0.0) | 0.518 |
| Vomiting | 123/177 (69.5) | 13/26 (50.0) | 5/8 (62.5) | 0.138 |
| Fever | 119/177 (67.2) | 17/26 (65.4) | 2/8 (25.0) | 0.049 |
| Abdominal pain | 35/177 (19.8) | 8/26 (30.8) | 4/8 (50.0) | 0.072 |
A P value of ≤0.05 (two-sided χ2 test) was considered significant for differences among rotavirus, human caliciviruses, and astrovirus.
To investigate the variability of the strains of human calciviruses detected over the 27-month period, the amplified products obtained from 19 specimens were sequenced and the 100-bp region located at positions 4755 to 4854 (open reading frame 1 [ORF1]) was aligned with known sequences (Table 3). Two strains were shown to belong to genogroup I and 17 were shown to belong to genogroup II of the Norwalk group of viruses. None of the 19 strains were related to Sapporo virus. Among the two genogroup I strains, one was related to Southampton virus and the other to the USC 92B strain (20). Both were found at the beginning of the study and were never detected later. The 17 genogroup II strains could be categorized in two clusters, with 8 strains related to Bristol virus and 9 related to Toronto virus. Strains belonging to the two clusters cocirculated during the whole period of the study.
TABLE 3.
Nucleotide and amino acid identities of a 100-bp regiona in 19 strains of human calicivirus detected in Dijon from December 1995 to February 1998 with known sequencesb
| Genogroup | Cluster | No. of stool specimens | Period | Nucleotide identity (%) | Amino acid identity (%) |
|---|---|---|---|---|---|
| I | Southampton virus | 1 | Dec. 1995 | 86 | 100 |
| USC 92Bc | 1 | Dec. 1995 | 91 | 96 | |
| II | Bristol virus | 8 | Jan. 1996–Dec. 1997 | 85–96 | 96–100 |
| Toronto virus | 9 | Jan. 1996–Oct. 1997 | 90–97 | 94–100 |
Positions 4755 to 4854 (ORF1) in Norwalk virus.
The sequences used were available from GenBank (Bristol virus [accession no. X76716], Toronto virus [U02030], and Southampton virus [L07418]) or from Moe et al. (20) (USC 92B).
Alignment with strain USC 92B was carried out on an 80-bp region (4755 to 4834).
In a general manner, our study shows good agreement with previous studies reporting detection rates of group A rotavirus, astrovirus, and adenovirus type 40 and 41 infections in children with gastroenteritis in industrialized countries (5, 8, 12, 13). There is a lack of studies evaluating, with sensitive, broadly reactive assays, the relative proportion of human calicivirus infections in young children compared to infections with the other three viruses. Here, we found a high frequency of calicivirus (14%) in stools from children with gastroenteritis compared to that for the controls (0%). The use of the sensitive RT-PCR and of four primer pairs for each sample, rather than a particular epidemiological situation, may explain this result. The average and median ages were 14.8 and 8 months, respectively, and were not different from those observed for the other viruses.
Sequencing of a 100-bp region of the RNA polymerase gene for 19 strains of human calicivirus showed a clear predominance of Norwalk-like viruses belonging to genogroup II (17 of 19) in Dijon during this period. Genogroup II strains have been reported in young children with gastroenteritis in different countries, including Japan (18) and Canada (15, 16), and a high prevalence of antibodies to the Mexico strain (genogroup II) was reported in London (21): more than 70% of children had antibodies to this strain by the age of 2 years, compared to 12% with antibodies to the Norwalk virus (genogroup I).
The systematic detection of the four viruses allowed us to observe a high percentage of dual infections among positive samples (16.7%), the majority of which were combinations of rotavirus and one of the other three viruses. Dual infections raise the question of whether a single virus is responsible for illness or whether two viruses act in synergy. As was previously observed (10), there was no statistically significant difference in clinical symptoms between monoinfections and dual infections.
In conclusion, this is the first report of astrovirus and human calicivirus infections in the pediatric population in France. It highlights the importance of human caliciviruses and particularly of Norwalk-like viruses in infantile gastroenteritis and conveys information about the molecular epidemiology of such viruses in France.
Acknowledgments
We thank F. le Guyader (Laboratoire de Microbiologie, Ifremer, Nantes, France) for technical advice on calicivirus detection. We are also grateful to M. Martres (Clinique Ste-Marthe, Dijon, France) and to J. F. James and J. R. Maurin (Laboratoire St-Michel, Dijon, France) for assistance with stool sample collection, to V. Boggio for inclusion of the controls to J. B. Gouyon (Service de Pédiatrie 2, CHU, Dijon, France) for permission to consult medical files, and to S. Dalle for critical reading of the manuscript.
This work was supported by the Centre Hospitalier Universitaire of Dijon, France.
REFERENCES
- 1.Ando T, Monroe S S, Gentsch J R, Jin Q, Lewis D C, Glass R I. Detection and differentiation of antigenically distinct small round-structured viruses (Norwalk-like viruses) by reverse transcription-PCR and southern hybridization. J Clin Microbiol. 1995;33:64–71. doi: 10.1128/jcm.33.1.64-71.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiba S, Sakuma Y, Kogasaka R, Akihara M, Horino K, Nakao T, Fukui S. An outbreak of gastroenteritis associated with calicivirus in an infant home. J Med Virol. 1979;4:249–254. doi: 10.1002/jmv.1890040402. [DOI] [PubMed] [Google Scholar]
- 3.Flahault A, Dréau H, Farran N, Carrat F, Chauvin P, Massari V, Letrillard L, Retel O, Toubiana L, Dangoumau L, Desenclos J C, Le Quellec Nathan M, Valleron A J l’Ensemble des Médecins Sentinelles. Epidémiologie des maladies transmissibles en médecine libérale: bilan du réseau “Sentinelles” en 1996. Bull Epidémiol Hebdom. 1997;33:149–151. [Google Scholar]
- 4.Glass R I, Kilgore P E, Holman R C, Jin S, Smith J C, Woods P A, Clarke M J, Ho M S, Gentsch J R. The epidemiology of rotavirus diarrhea in the United States: surveillance and estimates of disease burden. J Infect Dis. 1996;174(Suppl. 1):S5–S11. doi: 10.1093/infdis/174.supplement_1.s5. [DOI] [PubMed] [Google Scholar]
- 5.Glass R I, Noel J, Mitchell D, Herrmann J E, Blacklow N R, Pickering L K, Dennehy P, Ruiz-Palacios G, de Guerrero M L, Monroe S S. The changing epidemiology of astrovirus-associated gastroenteritis: a review. Arch Virol. 1996;12(Suppl.):287–300. doi: 10.1007/978-3-7091-6553-9_31. [DOI] [PubMed] [Google Scholar]
- 6.Gouvea V, Glass R I, Woods P, Taniguchi K, Clarck H F, Forrester B, Fang Z Y. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1990;28:276–282. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green J, Gallimore C I, Norcott J P, Lewis D, Brown D W G. Broadly reactive reverse transcriptase polymerase chain reaction (RT-PCR) for the diagnosis of SRSV-associated gastroenteritis. J Med Virol. 1995;47:392–398. doi: 10.1002/jmv.1890470416. [DOI] [PubMed] [Google Scholar]
- 8.Grimwood K, Carzino R, Barnes G L, Bishop R F. Patients with enteric adenovirus gastroenteritis admitted to an Australian pediatric teaching hospital from 1981 to 1992. J Clin Microbiol. 1995;33:131–136. doi: 10.1128/jcm.33.1.131-136.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hebert J P, Caillet R. Etiologie des gastro-entérites aiguës infantiles en pratique hospitalière courante. Méd Malad Infect. 1984;6:342–346. doi: 10.1016/S0399-077X(84)80070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrmann J E, Taylor D N, Echeverria P, Blacklow N R. Astroviruses as a cause of gastroenteritis in children. N Engl J Med. 1991;324:1757–1760. doi: 10.1056/NEJM199106203242501. [DOI] [PubMed] [Google Scholar]
- 11.Jiang X, Wang J, Graham D Y, Estes M K. Detection of Norwalk virus in stool by polymerase chain reaction. J Clin Microbiol. 1992;30:2529–2534. doi: 10.1128/jcm.30.10.2529-2534.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kapikian A Z. Viral gastroenteritis. In: Evans A S, Kaslow R A, editors. Viral infections of humans: epidemiology and control. 4th ed. New York, N.Y: Plenum Medical Book Company; 1997. pp. 285–343. [Google Scholar]
- 13.Kim K H, Yang J M, Joo S I, Cho Y G, Glass R I, Cho Y J. Importance of rotavirus and adenovirus types 40 and 41 in acute gastroenteritis in Korean children. J Clin Microbiol. 1990;28:2279–2284. doi: 10.1128/jcm.28.10.2279-2284.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Guyader F, Estes M K, Hardy M E, Neill F H, Green J, Brown D W G, Atmar R L. Evaluation of a degenerate primer for the PCR detection of human calciviruses. Arch Virol. 1996;141:2225–2235. doi: 10.1007/BF01718228. [DOI] [PubMed] [Google Scholar]
- 15.Levett P N, Gu M, Luan B, Fearon M, Stubberfield J, Jamieson F, Petric M. Longitudinal study of molecular epidemiology of small round-structured viruses in a pediatric population. J Clin Microbiol. 1996;34:1497–1501. doi: 10.1128/jcm.34.6.1497-1501.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lew J F, Petric M, Kapikian A Z, Jiang X, Estes M K, Green K Y. Identification of minireovirus as a Norwalk-like virus in pediatric patients with gastroenteritis. J Virol. 1994;68:3391–3396. doi: 10.1128/jvi.68.5.3391-3396.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matson D O, Estes M K, Glass R I, Bartlett A V, Penaranda M, Calomeni E, Tanaka T, Nakata S, Chiba S. Human calicivirus-associated diarrhea in children attending day care centers. J Infect Dis. 1989;159:71–78. doi: 10.1093/infdis/159.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuno S, Sawada R, Kimura K, Suzuki H, Yamanishi S, Shinozaki K, Sugieda M, Hasegawa A. Sequence analysis of SRSV in fecal specimens from an epidemic of infantile gastroenteritis, October to December 1995, Japan. J Med Virol. 1997;52:377–380. [PubMed] [Google Scholar]
- 19.Mitchell D K, Monroe S S, Jiang X, Matson D, Glass R I, Pickering L K. Virologic features of an astrovirus diarrhea outbreak in a day care center revealed by reverse transcriptase-polymerase chain reaction. J Infect Dis. 1995;172:1437–1444. doi: 10.1093/infdis/172.6.1437. [DOI] [PubMed] [Google Scholar]
- 20.Moe C L, Gentsch J, Ando T, Grohmann G, Monroe S S, Jiang X, Wang J, Estes M K, Seto Y, Humphrey C, Stine S, Glass R I. Application of PCR to detect Norwalk virus in fecal specimens from outbreaks of gastroenteritis. J Clin Microbiol. 1994;32:642–648. doi: 10.1128/jcm.32.3.642-648.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parker S P, Cubitt W D, Jiang X. Enzyme immunoassay using baculovirus-expressed human calicivirus (Mexico) for the measurement of IgG responses and determining its seroprevalence in London, UK. J Med Virol. 1995;46:194–200. doi: 10.1002/jmv.1890460305. [DOI] [PubMed] [Google Scholar]
- 22.Pothier P, Drouet E. Development and evaluation of a rapid one-step ELISA for rotavirus detection in stool specimens using monoclonal antibodies. Ann Inst Pasteur Virol. 1987;13:285–295. [Google Scholar]
- 23.Ueda Y, Nakaya S, Takagi M, Ushijima H. Diagnosis and clinical manifestations of diarrheal virus infections in Maizuru area from 1991 to 1994—especially focused on small round structured viruses. J Jpn Assoc Infect Dis. 1996;70:1092–1097. doi: 10.11150/kansenshogakuzasshi1970.70.1092. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Jiang X, Madore H P, Gray J, Desselberger U, Ando T, Seto Y, Oishi I, Lew J F, Green K Y, Estes M K. Sequence diversity of small round-structured viruses in the Norwalk virus group. J Virol. 1994;68:5982–5990. doi: 10.1128/jvi.68.9.5982-5990.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]