Abstract
Background:
Only limited data are available that address the association between body mass index (BMI) and clinical outcomes in patients with heart failure with reduced ejection fraction who are receiving sacubitril/valsartan.
Methods:
We performed a retrospective multi-center cohort study in which we compared 3 body mass index groups (normal, overweight and obese groups) in patients with heart failure with reduced ejection fraction receiving sacubitril/valsartan. The follow-up period was at least 1 year. Propensity score weighting was performed. The primary outcomes were hospitalization for heart failure and all-cause mortality.
Results:
Of the 721 patients in the original cohort, propensity score weighting generated a cohort of 540 patients in 3 groups: normal weight (n = 78), overweight (n = 181), and obese (n = 281). All baseline characteristics were well-balanced between 3 groups after propensity score weighting. Among our results, we found no significant differences in hospitalization for heart failure (normal weight versus overweight: average hazard ratio [AHR] 1.29, 95% confidence interval [CI] = 0.76–2.20, P = 0.35; normal weight versus obese: AHR 1.04, 95% CI = 0.63–1.70, P = 0.88; overweight versus obese groups: AHR 0.81, 95% CI = 0.54–1.20, P= 0.29) or all-cause mortality (normal weight versus overweight: AHR 0.99, 95% CI = 0.59–1.67, P = 0.97; normal weight versus obese: AHR 0.87, 95% CI = 0.53–1.42, P = 0.57; overweight versus obese: AHR 0.87, 95% CI = 0.58–1.32, P = 0.52).
Conclusion:
We identified no significant associations between BMI and clinical outcomes in patients diagnosed with heart failure with a reduced ejection fraction who were treated with sacubitril/valsartan. A large-scale study should be performed to verify these results.
Keywords: obesity, sacubitril, valsartan, heart failure, obesity paradox
Introduction
Obesity has been reported to have negative effects on hemodynamics as well as on cardiac function and structure.1 Obesity also activates sympathetic nerves and the renin-angiotensin-aldosterone systems and thus significantly increases the risk of new-onset heart failure (HF).2 Nonetheless, results from several recent studies revealed among patients with HF higher BMIs was associated with improved survival compared to those with lower BMIs or cachexia.3,4 These observation contribute to the theory known as the “obesity paradox.”5 However, it is still not clear whether a reduced BMI might simply reflect the severity of HF or if obesity alone is truly a protective factor. Also, specific HF pharmacotherapy has not been fully evaluated in obese patients with HF with a reduced ejection fraction (HFrEF). To our knowledge, the only published study that addresses this issue is the post-hoc analysis of the Valsartan Heart Failure Trial (Val-He-FT). The results from this analysis, which included patients diagnosed with HFrEF who were undergoing treatment with valsartan, were consistent with obesity paradox.6 By contrast, an unpublished but nationally-presented post-hoc analysis of the Prospective Comparison of Angiotensin Neprilysin Inhibitor (ARNI) with Angiotensin Converting Enzyme Inhibitor (ACEI) to Determine Impact on Global Mortality and Morbidity in Heart Failure (the PARADIGM-HF) trial that included an evaluation of BMI and clinical outcomes in patients with HFrEF treated with sacubitril/valsartan presented results that were not consistent with the obesity paradox.7 The conclusions from post-hoc analyses of the Val-He-FT and PARADIGM-HF trials are in conflict with each other. Unfortunately, the post-hoc analysis of the PARADIGM-HF trial is only available as a conference abstract at this time. To address this important knowledge gap, we performed a real-world analysis using contemporary guideline-directed medical therapy to evaluate the relationship between BMI and clinical outcomes in patients with HFrEF treated with sacubitril/valsartan.
Methods
A retrospective, multi-center cohort study was performed in patients with HFrEF. Inpatients and outpatients aged greater than 18 years old who were diagnosed with HFrEF (EF ≤ 40%) and undergoing treatment with sacubitril/valsartan from July 2015 to December 2019 were included in this study. The follow-up period was at least 1 year. Patients who died within 1 year while undergoing treatment with sacubitril/valsartan were included. Patients undergoing treatment with sacubitril/valsartan less than 1 year and/or who were undergoing dialysis were excluded. The entire cohort was divided into 3 groups based on baseline BMIs prior to the initiation of sacubitril/valsartan: a normal weight group (BMI < 25), an overweight group (25 ≤ BMI < 30), and an obese group (30 ≤ BMI). Sacubitril/valsartan pharmacy claims were reviewed for patient dosing information. Baseline characteristics were collected from the most recent values obtained before the initial dose of sacubitril/valsartan. This study was approved by the review board of West Virginia University (WVU).
West Virginia Clinical and Translational Science Institute provided the dataset which included the patient demographics and clinical information needed to conduct this study. Investigators also performed a retrospective chart review to collect additional patient demographic information and clinical outcomes.
The 2 primary outcomes were all-cause mortality and rehospitalization for HF. Events at WVU medicine-affiliated healthsystems that were documented in electronic records were included in our analysis. Events taking place up to 3.5 years following the index date were also recorded. If the data analyst at the WV Clinical and Translational Science Institute were unable to locate a death record in the medical electronic records, the observational medical outcomes partnership database was searched to verify ongoing survival. HF hospitalization was defined as a minimum 24-hour inpatient admission with a primary diagnosis of acute decompensated heart failure requiring an intravenous inotrope, a vasodilator and an additional diuretic.
To examine the association between BMI and hospitalization for HF or all-cause mortality, univariate survival regression analysis was performed using the Cox proportional hazards (PH) model as the assumption of PH was met (Supplemental Table 1). To balance the covariates across the 3 BMI groups, propensity scores and corresponding inverse probability of treatment weights (IPTW) were calculated. This was followed by a weighted Cox regression analysis with IPTW. Treatment weights were obtained using a generalized boosted model (GBM). In the case of 2 treatment groups, GBM fits a piecewise constant model to predict a dichotomous outcome. This model consisted of many simple regression trees that were iteratively combined to create an overall piecewise constant function. As there were 3 treatment groups in this study, the iterative fitting algorithm was applied by first creating dummy indicators for each of the 3 treatment groups. Separate GBMs were fitted to each dummy treatment group indicator to obtain the estimated propensity score for each treatment group. The estimated propensity scores from each iteration of the GBM fits were used to compute the average weights needed to estimate treatment effects. To generate propensity scores, BMI was the outcome variable, while age, brain natriuretic peptide (BNP), sacubitril/valsartan dose, serum creatinine, diabetes, ejection fraction, gender, systolic blood pressure, and prior treatment with angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) use were the predictors. These predictors were selected based on both significant baseline characteristics between the groups and the investigators’ clinical experience.
In choosing between the Cox PH model where the assumption of PH was supposed to be met and the weighted Cox regression model with a robust sandwich estimator where the assumption of PH was not supposed to be met, 2 tests were conducted via the zph.cox function from R and the 2 P-values were 0.021 and 0.004, for hospitalization for HF and all-cause mortality, respectively. Therefore, the weighted Cox regression model with a robust sandwich estimator was used in the regression analysis. In addition, from the distribution of weights used in the Cox regression models (Supplemental Figures 1 and 2), there were extremely large censoring weights after year 2 for both hospitalization for HF and all-cause mortality. Therefore, in order to remove the undue influence from those weights, the obtained weights were truncated at their 95th percentile. After truncation, the range of the normalized total weights looked consistent (Supplemental Figures 3 and 4), and the results from the weighted Cox regression were less likely to be biased. When the PH assumption was not met, instead of hazard ratio, average hazard ratio (AHR), defining each hazard relative to the sum of hazards was used in the interpretation of the results. The hazard ratios (HRs), or average hazard ratios, and their 95% confidence intervals (CIs) were calculated. The significant level was set at 0.05. All statistical analyses were performed using R (Version 4.0.5), using twang (Version 2.0), coxphw (Version 4.0.2), and survival (Version 3.2–10) packages.
Results
A total of 131 patients in normal weight group (BMI < 25), 201 patients in overweight group (25 ≤ BMI < 30), and 389 patients in obese group (30 ≤ BMI) were included in this study (Figure 1). The propensity score weighting generated a cohort of 540 patients: normal weight (n = 78), overweight (n = 181), and obese (n = 281). Baseline characteristics are shown in the Table 1. Age, sacubitril/valsartan dose, diabetes, serum creatinine, BNP, and previous ACE-I/ARB treatment were significantly different between the 3 groups (Table 1). After propensity weighting, there were no significant differences in all baseline characteristics between the 3 groups (Table 1).
Figure 1.
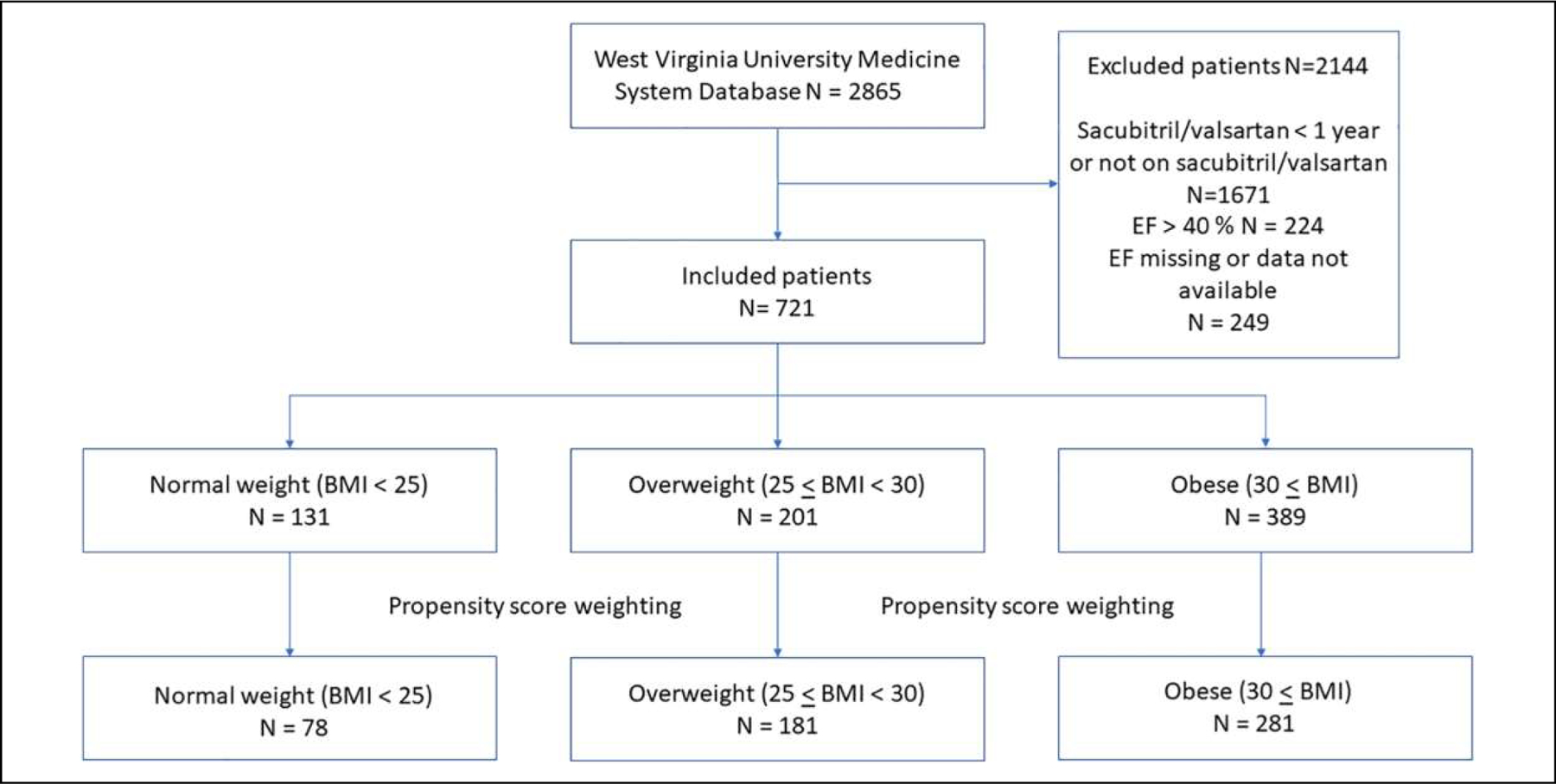
Patient selection flow diagram.
Table 1.
Baseline Characteristics Before and After Propensity Weighting.
| Original cohort groups |
Propensity weighed groups |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Normal weight (BMI < 25) (n = 131) | Overweight (25 ≤ BMI ≤ 29.9) (n = 201) | Obese (30 ≤ BMI) (n = 389) | P-value | Normal weight (BMI < 25) (n = 78) | Overweight (25 ≤ BMI ≤ 29.9) (n = 181) | Obese (30 ≤ BMI) (n = 281) | P-value | Maximum standardized effect size | |
| Age (years) | 70.70 ± 11.43 | 69.20 ± 11.55 | 61.33 ± 12.37 | <0.001 | 67.41 ± 11.80 | 66.42 ± 11.99 | 64.23 ± 12.99 | 0.052 | 0.251 |
| Male sex (%) | 71.0 | 66.7 | 69.9 | 0.638 | 69.7 | 66.8 | 69.2 | 0.601 | 0.064 |
| SBP, mm Hg | 118.46 ± 19.69 | 120.92 ± 19.21 | 122.01 ± 19.06 | 0.186 | 120.79 ± 18.40 | 120.66 ± 19.32 | 121.03 ± 19.25 | 0.866 | 0.019 |
| Sacubitril/valsartan dose (%) | 0.001 | 0.278 | |||||||
| 24/26 mg | 57.3 | 50.2 | 45.0 | 49.7 | 50.5 | 48.3 | 0.044 | ||
| 49/51 mg | 21.4 | 28.4 | 20.6 | 25.8 | 25.4 | 21.3 | 0.112 | ||
| 97/103 mg | 21.4 | 21.4 | 34.4 | 24.5 | 24.1 | 30.4 | 0.136 | ||
| Creatinine, mg/dL | 1.11 ± 0.37 | 1.23 ± 0.44 | 1.21 ± 0.46 | 0.037 | 1.13 ± 0.33 | 1.21 ± 0.45 | 1.20 ± 0.42 | 0.318 | 0.191 |
| BNP, pg/mL | 2952.41 ± 5762.82 | 1801.35 ± 4491.55 | 1223.42 ± 2718.19 | <0.001 | 1844.01 ± 4024.29 | 1625.78 ± 3918.72 | 1706.49 ± 3512.30 | 0.842 | 0.055 |
| Ejection fraction (%) | 25.46 ± 9.16 | 26.05 ± 8.47 | 26.62 ± 8.46 | 0.381 | 26.52 ± 8.17 | 25.79 ± 8.70 | 26.30 ± 8.18 | 0.943 | 0.085 |
| Diabetes (%) | 43.5 | 44.8 | 57.1 | 0.003 | 43.5 | 48.2 | 53.5 | 0.114 | 0.202 |
| BB (%) | 94.0 | 91.0 | 93.0 | 0.401 | 96.5 | 91.3 | 93.1 | 0.211 | 0.095 |
| Prior ACE-I/ARB (%) | 68.8 | 61.4 | 74.2 | 0.035 | 81.6 | 76.9 | 81.0 | 0.323 | 0.138 |
| MRA (%) | 67.0 | 68.0 | 64.0 | 0.892 | 69.8 | 66.9 | 65.4 | 0.631 | 0.028 |
| Loop diuretic (%) | 81.0 | 86.0 | 81.0 | 0.370 | 82.6 | 84.1 | 81.4 | 0.643 | 0.084 |
Abbreviations: ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, beta-blocker; BMI, body mass index; BNP, brain natriuretic peptide; MRA, mineralocorticoid receptor antagonist; SBP, systolic blood pressure.
Survival analysis revealed no significant differences in the cumulative risk of all-cause death or hospitalization for HF during the follow-up period between any of 3 BMI categories (log-rank test, P = 0.67 for hospitalization for HF and P = 0.17 for all-cause mortality; Figure 2). Our unadjusted analysis revealed all-cause mortality rates at 25.19% in the normal weight group, 22.39 % in the overweight group and 19.02% in the obese group (normal weight versus obese: HR 1.49, 95% CI = 0.99–2.24, P = 0.06; overweight versus obese: HR 1.31, 95% CI = 0.90–1.89, P = 0.16; normal weight versus overweight: HR 1.14, 95% CI = 0.73–1.79, P = 0.57; Table 2). Hospitalization rates for HF were 24.43% in the normal weight group, 21.5% in the overweight group and 23.71% in the obese group (normal weight versus obese: HR 1.15, 95% CI = 0.77–1.73, P = 0.49; overweight versus obese: HR 0.94, 95% CI = 0.65–1.35, P = 0.74; normal weight versus overweight: HR 1.23, 95% CI = 0.78–1.94, P = 0.38) (Table 2).
Figure 2.
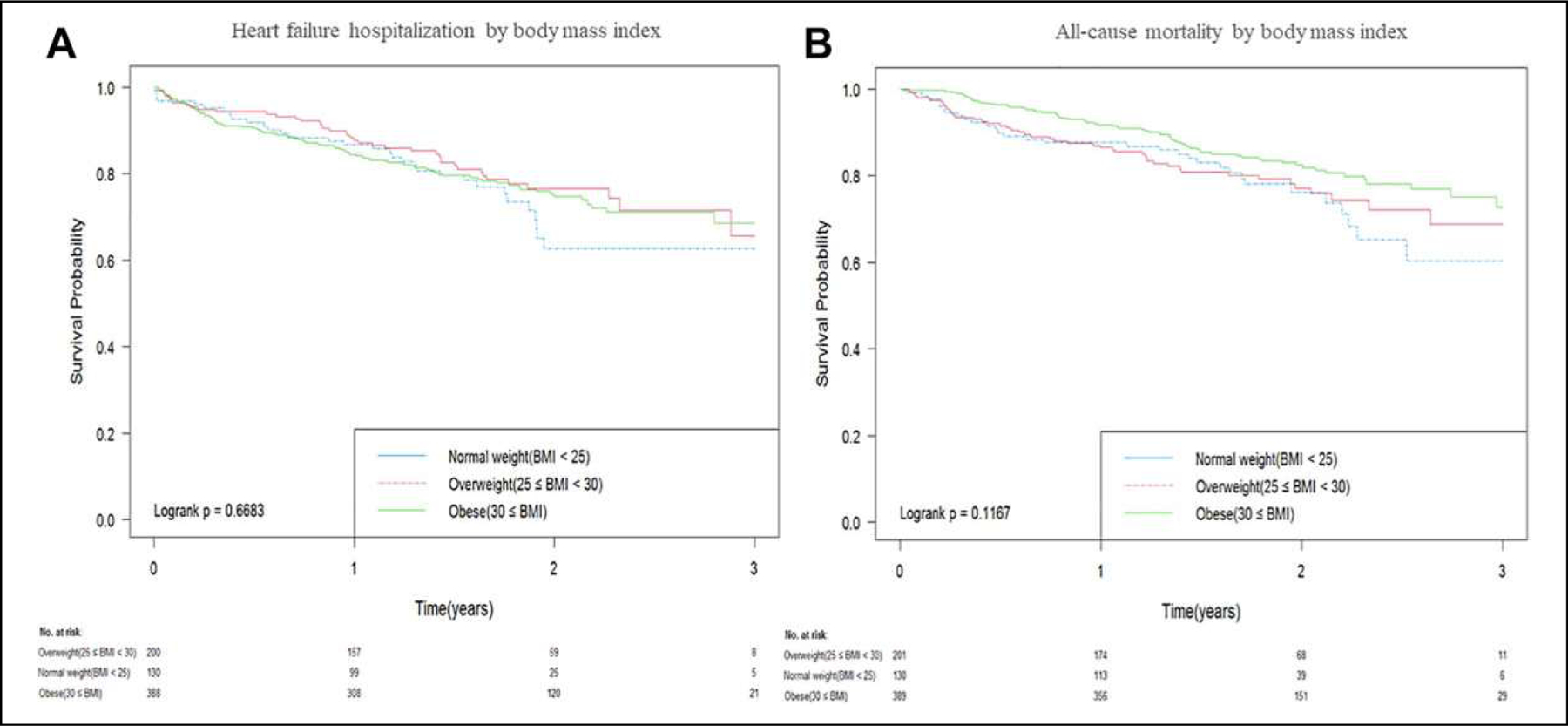
Kaplan-Meier analysis for (A) time-to-first hospitalization for HF and (B) all-cause mortality.
Table 2.
Hospitalization and Mortality Before and After Propensity Score Weighting.
| Pre | Hazard ratio | 95% CI | P-value | |
|---|---|---|---|---|
| Hospitalization | Normal weight vs. Obese weight | 1.15 | 0.77–1.73 | 0.49 |
| Overweight vs. Obese | 0.94 | 0.65–1.35 | 0.74 | |
| Normal weight vs. Overweight | 1.23 | 0.78–1.94 | 0.38 | |
| All-cause mortality | Normal weight vs. Obese | 1.49 | 0.99–2.24 | 0.06 |
| Overweight vs. Obese | 1.31 | 0.90–1.89 | 0.16 | |
| Normal weight vs. Overweight | 1.14 | 0.73–1.79 | 0.57 | |
| Post | Average hazard ratio | 95% CI | P-value | |
|
| ||||
| Hospitalization | Normal weight vs. Obese | 1.04 | 0.63–1.70 | 0.88 |
| Overweight vs. Obese | 0.81 | 0.54–1.20 | 0.29 | |
| Normal weight vs. Overweight | 1.29 | 0.76–2.20 | 0.35 | |
| All-cause mortality | Normal weight vs. Obese | 0.87 | 0.53–1.42 | 0.57 |
| Overweight vs. Obese | 0.87 | 0.58–1.32 | 0.52 | |
| Normal weight vs. Overweight | 0.99 | 0.59–1.67 | 0.97 | |
Abbreviation: CI, confidence interval.
Analysis of this dataset after propensity score weighting also revealed no significant differences in hospitalization rates for HF (normal weight versus obese: AHR 1.04, 95% CI = 0.63–1.70, P = 0.88; overweight versus obese: AHR 0.81, 95% CI = 0.54–1.20, P = 0.29; normal weight versus overweight: AHR 1.29, 95% CI = 0.76–2.20, P = 0.35) or all-cause mortality (normal weight versus obese weight groups: AHR 0.87, 95% CI = 0.53–1.42, P = 0.57; overweight versus obese groups: AHR 0.87, 95% CI = 0.58–1.32, P = 0.52; normal weight versus overweight: AHR 0.89, 95% CI = 0.59–1.67, P = 0.97).
Discussion
To the best of our knowledge, this is the first real-world study that evaluated the associations between BMI and clinical outcomes in patients with HFrEF who were undergoing treatment with sacubitril/valsartan. Our results revealed no significant differences in hospitalization for HF or all-cause mortality in patients that were assigned to 1 of the 3 standard BMI groups.
Interestingly, our real-world study did not reproduce the results of the post-hoc analysis of the Val-He-FT trial which showed that a higher BMI was associated with a lower mortality rate in patients with HFrEF undergoing a treatment with valsartan.6 Similar to the post-hoc analysis of the Val-He-FT trial, we controlled for the impact of potential prognostic predictors, including BNP, EF, serum creatinine and systolic blood pressure by propensity score weighting. However, the results of our study did not support the obesity paradox for sacubitril/valsartan therapy. These seemingly contradictory results might be related to differences in the analyses (e.g., propensity score weighting in our study versus multivariate Cox proportional hazards model in the post-hoc analysis of Val-He-FT trial), drug therapies (sacubitril/valsartan versus valsartan), and the nature of the data source (e.g., real-world data versus findings from a randomized controlled trial). Although the findings have not yet been fully published, results of the post-hoc analysis of the PARADIGM-HF trial were also not consistent with the obesity paradox. The results of the PARADIGM-HF trial, which evaluated the association between BMI and clinical outcomes in patients with HFrEF receiving either enalapril or sacubitril/valsartan, are consistent with the results of our real-world study.7
It is well-known in clinical practice that a higher BMI is associated with a higher mortality rate in the general population.8,9 By contrast, several reports focused on patients with HF suggested an inverse relationship between these factors, as they found that lower BMIs were associated with a higher mortality rate in this specific patient cohort.4,5 The similar obesity paradox was also evident in a cohort of patients diagnosed with atrial fibrillation who were undergoing treatment with direct oral anticoagulants.10–12 The obesity paradox did not hold true for the patients in our study; this may be due to persistent benefits from sacubitril/valsartan regardless of BMI. Although further investigation will be needed, this is the first study that has revealed no association between BMI and clinical outcomes in patients with HFrEF who were treated with sacubitril/valsartan.
There were several limitations to this study. First, we did not have access to some of the clinical data, including device therapies (implantable cardioverter defibrillator or cardiac resynchronization therapy), and the New York Heart Association functional classifications or American College of Cardiology/American Heart Association staging. Second, we were unable to estimate the sample size because we had no previous data that addressed this research aim. Thus, the possibility of type 2 errors cannot be excluded completely. Third, these real-world data are based on retrospective study results which can only show association between BMI and clinical outcomes, not causality. Future prospective studies might be designed to address this point. Fourth, we could not access a cause of each death event. Thus, we used all-cause mortality as an outcome, and cardiovascular mortality rate was not reported. Finally, the BMI values recorded at enrollment were used for patient assignments to 1 of 3 specific BMI groups. However, patients rarely maintained the same BMI throughout the entire follow-up. Some of the patients in our study might have been categorized into multiple different BMI groups at different times during the follow-up period, given the degree of weight fluctuation as well as original weights that were close to the cut-off values between BMI categories.
Conclusion
The study revealed no significant associations between BMI and hospitalization for HF or all-cause mortality in patients diagnosed with HFrEF who were treated with sacubitril/valsartan. Further investigation with a larger scale study will be needed to verify these results.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number 5U54GM104942-04. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Received support from the National Institute of General Medical Sciences of the National Institutes of Health under Award Number 5U54GM104942-04.
Footnotes
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article. George Sokos was a speaker for Novartis Pharmaceutical sponsored presentation.
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Ortega-Loubon C, Fernández-Molina M, Singh G, Correa R. Obesity and its cardiovascular effects. Diabetes Metab Res Rev 2019; 35(4):e3135. [DOI] [PubMed] [Google Scholar]
- 2.Horwich TB, Fonarow GC, Clark AL. Obesity and the obesity paradox in heart failure. Prog Cardiovasc Dis 2018;61(2): 151–156. [DOI] [PubMed] [Google Scholar]
- 3.Güder G, Frantz S, Bauersachs J, et al. Reverse epidemiology in systolic and nonsystolic heart failure: cumulative prognostic benefit of classical cardiovascular risk factors. Circ Heart Fail 2009; 2(6):563–571. [DOI] [PubMed] [Google Scholar]
- 4.Davos CH, Doehner W, Rauchhaus M, et al. Body mass and survival in patients with chronic heart failure without cachexia: the importance of obesity. J Card Fail 2003;9(1):29–35. [DOI] [PubMed] [Google Scholar]
- 5.Lavie CJ, Osman AF, Milani RV, Mehra MR. Body composition and prognosis in chronic systolic heart failure: the obesity paradox. Am J Cardiol 2003;91(7):891–894. [DOI] [PubMed] [Google Scholar]
- 6.Cicoira M, Maggioni AP, Latini R, et al. Body mass index, prognosis and mode of death in chronic heart failure: results from the Valsartan Heart Failure Trial. Eur J Heart Fail 2007;9(4): 397–402. [DOI] [PubMed] [Google Scholar]
- 7.Mogensen UM, Jhund P, Køber L, et al. Abstract 17986: Is there really an ‘obesity paradox’ in heart failure? An analysis of PARA-DIGM-HF. Circulation 2016;134(suppl 1):A17986. [Google Scholar]
- 8.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW Jr.Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med 1999;341(15):1097–1105. [DOI] [PubMed] [Google Scholar]
- 9.Allison DB, Gallagher D, Heo M, Pi-Sunyer FX, Heymsfield SB.Body mass index and all-cause mortality among people age 70 and over: the longitudinal study of aging. Int J Obes Relat Metab Disord 1997;21(6):424–431. [DOI] [PubMed] [Google Scholar]
- 10.Sandhu RK, Ezekowitz J, Andersson U, et al. The ‘obesity paradox’ in atrial fibrillation: observations from the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial. Eur Heart J 2016;37(38): 2869–2878. [DOI] [PubMed] [Google Scholar]
- 11.Tittl L, Endig S, Marten S, Reitter A, Beyer-Westendorf I, Beyer-Westendorf J. Impact of BMI on clinical outcomes of NOAC therapy in daily care—results of the prospective Dresden NOAC registry (NCT01588119). Int J Cardiol 2018;262:85–91. [DOI] [PubMed] [Google Scholar]
- 12.Proietti M, Guiducci E, Cheli P, Lip GY. Is there an obesity paradox for outcomes in atrial fibrillation? A systematic review and meta-analysis of non-vitamin K antagonist oral anticoagulant trials. Stroke 2017;48(4):857–866. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


