Abstract
The opposing roles of innate and adaptive immune cells in suppressing or supporting cancer initiation, progression, metastasis and response to therapy has been long debated. The mechanisms by which different monocyte and T cell subtypes affect and modulate cancer have been extensively studied. However, the role of B cells and their subtypes have remained elusive, perhaps partially due to their heterogeneity and range of actions. B cells can produce a variety of cytokines and present tumor-derived antigens to T cells in combination with co-stimulatory or inhibitory ligands based on their phenotype. Unlike most T cells, B cells can be activated by innate immune stimuli, such as endotoxin. Furthermore, the isotype and specificity of the antibodies produced by plasma cells regulate distinct immune responses, including opsonization, antibody-mediated cellular cytotoxicity (ADCC) and complement activation. B cells are shaped by the tumor environment (TME), with the capability to regulate the TME in return. In this review, we will describe the mechanisms of B cell action, including cytokine production, antigen presentation, ADCC, opsonization, complement activation and how they affect tumor development and response to immunotherapy. We will also discuss how B cell fate within the TME is affected by tumor stroma, microbiome and metabolism.
Keywords: B cells, plasma cells, cancer, anti-tumor immunity, humoral immunity, immunotherapy
1. Introduction and a short history of “Bursa of Fabricus” cells
The first description of what we know as B cells today was in 1890 with the discovery of circulating antitoxins in immunity to diphtheria and tetanus by Emil von Behring and Shibasaburo Kitasato [1,2], while working at Berlin Institute of Infectious Diseases (Robert Koch institute). Later in 1900, another alumnus of the Koch’s institute, Paul Ehrlich hypothesized that an immune cell bearing many different antibody receptors could be stimulated by binding an antigen and subsequently produce and release more of the receptor type complementary to that antigen, what we know now as immunoglobulins (Igs) [3]. Ig-producing and secreting plasma cells [4,5], that are derived from B lymphocytes [6,7]. When Paul Ehrlich was describing his side-chain theory of antibody formation, explaining that the producers of circulating antitoxins were cells with pre-formed antibody receptors [8], he also suggested that these molecules, if reactive to tumors, could play a key role in cancer therapy [9].
The first clear evidence that B cells develop within a distinct organ was provided in 1956, when Bruce Glick removed the “Bursa” of Fabricius, a hindgut lymphoid organ, from newborn chicks, which resulted in depletion of antibody production [10]. The cells were called “Bursa of Fabricus” cells or “B” cells. Finally, in the decade between 1960-1970, the separate development of functionally linked lineages of lymphocytes known as B cells and T cells was accepted as a fundamental organizing principle of the adaptive immune system in all vertebrates by Jacques Miller and Max D. Cooper who discovered the thymus, as the tissue in which “T” cells develop and the “Bursa of Fabricus” in which “B” cells develop, respectively [2,11–14]. Since then, immunologists have defined many different sublineages of the clonally diverse B and T cells and described how they interact with each other and with the innate immune system to regulate infections, cancer and the development of autoimmune and inflammatory diseases. In this review, we will give a brief summary of B cell development, their different sublineages, and how their interactions with different arms of the innate and adaptive immune systems suppress or support cancer.
2. B cell development, antibody structure and diversity
In mammals, B cells originate from the fetal liver and bone marrow [15–17]. Lymphocytes can initiate specific immune responses against antigens by generating a nearly infinite diversity of antigen receptors. This is achieved largely by a somatic recombination process known as variable, diversity, and joining V(D)J recombination. Through V(D)J recombination, the variable region of antigen receptor genes is assembled from component germline V, D, and J gene segments. There are seven different loci that are rearranged to generate the antigen receptors of T and B lymphocytes. These include the immunoglobulin (Ig) heavy chain locus (IgH) and the Ig light chain (IgL) loci (Igκ and Igλ), which encode the antigen receptor (B cell receptor, BCR) and secreted antibodies (immunoglobulins, Ig). The rearrangement of these loci is tightly controlled in a lineage-, stage-, and allele specific manner. During B cell maturation, sequential intrinsic genetic DNA sequences are rearranged in the heavy and light chain immunoglobulin loci [18]. Cells at the pro-B stage of development initiate V(D)J gene segment recombination, using recombination activating gene 1/2 (RAG1/2) endonucleases to cleave the V, D and J segments, which are then joined by non-homologous end-joining (NHEJ) to form V(D)J exons [19]. Assembly of the heavy chain locus (IgH) precedes that of the light chains loci (IgL) [18]. The rearrangements of the IgH locus itself are sequential, with DH to JH joining occurring on both alleles prior to VH to DHJH segment rearrangement. The productive assembly of the VH-DHJH variable gene regions indirectly induces progression to the next stage of B cell differentiation. In this stage, IgL chains are assembled with Igκ rearrangements generally preceding those of Igλ, generating immature B cells that express μ plus IgL chains as surface IgM [18,20]. The random insertion and deletion of nucleotides at the junction sites of the V, (D) and J gene segments create fingerprint-like sequences of the junctional regions of Ig. These sequences are specific to each lymphocyte. Surface IgM+IgD+ B cells migrate to peripheral lymphoid tissues (spleen and lymph nodes) where they engage in antigen-dependent responses including class switch recombination (CSR) and somatic hypermutations (SHM) (Figure 1). Usually, mature naïve “follicular” B cells reside in the B cell follicles (Fo B cells) of the spleen and lymph nodes and become activated upon exposure to antigen (Figure 1). Activated FO B cells interact with follicular dendritic cells (FDC) and antigen specific follicular T helper cells (TFH) and form the germinal center (GC) inside the B cell follicle, which has two regions, the dark (DZ) and light zone (LZ). The follicle is the site of affinity maturation, proliferation, and plasma cell and memory cell differentiation. CSR results in isotype switching and was thought to take place in the GC. However, new data suggest that CSR predominantly takes place before either GC or extrafollicular B cell differentiation, at the stage of the initial B cell:T cell interaction [21]. CSR is an intrachromosomal DNA rearrangement of the immunoglobulin (Ig) heavy-chain locus, which results in the deletion of μ–heavy-chain (IgM) constant region gene segment and its replacement by one of the other downstream constant region gene segments, which include γ (IgG), ε (IgE), and α (IgA) [22–24]. As a result, mature B cells express antibodies of the IgA, IgG, or IgE classes that differ in effector functions without altering their antigen specificity. Every isotype switching event also involves exposure to a particular cytokine; IL-4 regulates the IgE CSR, TGFβ induces the IgA CSR, and IFNγ usually results in IgG CSR (Figure 2). CSR and SHM are both regulated by activation-induced cytidine deaminase (AID, encoded by Aicda). Other nucleases and transcription factors involved in these processes are uracil-DNA glycosylase (UNG), apurinic-apyrimidinic endonuclease 1 (APE1), BCL6, IRF4, NF-κB, BLIMP1 and MYC [25]. The antigen-selected evolution of cumulative somatic mutations in V region genes (somatic hypermutations, SHM) is the underlaying mechanism for affinity maturation, a process that occurs primarily in the DZ of the germinal center and is needed for the generation of high affinity antibodies [2,26,27]. Moreover, in tissue-tertiary lymphoid structures (TLS), which are ectopic lymphoid organs that develop in non-lymphoid tissues at sites of chronic inflammation including tumors, antigen-specific T and B cells can undergo terminal differentiation into effector cells in the T- and B-cell-rich areas, respectively. Tumors contain variable proportions and type of TLS [28,29]. The proportion of TLS varies according to tumor types from around 10 %–20 % in HCC, and present in up to more than 80 % of tumors such as in CRC and lung squamous cell carcinoma primary tumors. In organized TLS, germinal center B cells establish close interactions with FDCs and TFH in the course of differentiation into memory B cells and PCs (Figure 1B) [29,30]. Activated B cells, plasma cells, and the antibodies produced and secreted by plasma cells can migrate to sites of inflammation and infection to regulate innate and adaptive immune responses through a wide variety of mechanisms. The cellular functions of B cells and plasma cells include: a) Secretion of soluble factor e.g. cytokines; b) Expression of stimulatory and inhibitory ligands e.g. PD-L1, CD80, CD86; c) Priming of CD4+ and CD8+ T cells through MHC-I and MHC-II expression. The humoral functions of secretory immunoglobulins include: a) Neutralization, opsonization, and antibody-dependent phagocytosis; b) Antibody-dependent cellular cytotoxicity (ADCC); c) Complement activation (Figure 3). In the following section, we will discuss each of these functions in the context of cancer, and how the TME shapes the B cell response and vice versa.
Figure 1.
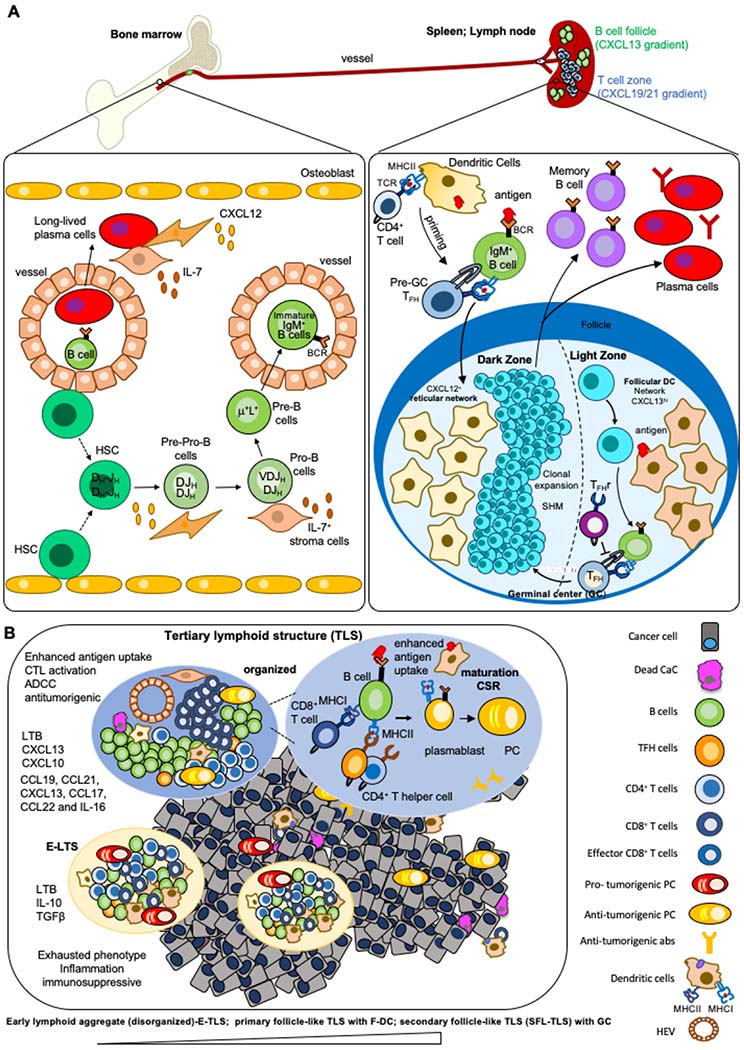
A) The development and differentiation of B cells. B cells develop in the bone marrow. During development, early B-cells similarly rearrange both of their IgH alleles before proceeding to rearrange their IgL alleles. V(D)J recombination in bone marrow pro-B cells first assembles IgH V(D)J exons leading to μ chain expression. Individual B cells express either Igκ or Igλ. Pre-pro-B cells associate with CXCL12hi reticular cells, whereas pro-B cells move towards IL-7-expressing cells like MSC and stroma cells. B cells expressing cell-surface IgM exit the bone marrow and enter the blood to reach the spleen or lymph nodes, where they mature into peripheral mature B cells. In secondary lymphoid tissues, the germinal center is built within the B cell follicles upon infection or immunization. The GC is divided into two distinct compartments. The dark zone (DZ) contains a network of CXCL12-producing reticular cells and is the site of GC B cell proliferation and somatic hypermutation (SHM). B cells follow a CXCL13 gradient to enter the light zone (LZ) through expression of CXCR5. In the LZ, B cells capture antigen presented on follicular dendritic cells (FDCs) which they internalize, process and subsequently present to T follicular helper (TFH) cells in order to undergo selection. This process is regulated by T follicular regulatory (TFr) cells which are also present in the LZ. Cytokines and soluble factors secreted by TFH define isotype CSR. Upon receiving survival signals from TFH cells, B cells re-enter the DZ for further rounds of proliferation and SHM after which they exit the GC as memory B cells or high-affinity antibody-secreting plasma cells. Long-lived plasma cells reside in the bone marrow in direct contact to bone marrow stromal cells. B) The composition and function of tertiary lymphoid structures in cancer. TLS are formed in inflamed sites upon contact between lymphoid tissue organizer IL7+ stromal cell and a lymphoid tissue inducer cell (LTi) in a CXCL13 rich environment. Tissue resident monocytic cells, TH17 or B cells can function as LTi. CCL21 and CXCL12 participate in lymphocytes recruitment, while CXCL13 and CCL19, together with adhesion molecules, control the structural organization of the forming TLS. The infiltrating immune cells form aggregates with a T cell rich zone where mature DC present MHC-Cl II peptides to CD4+T cells, promoting their activation, proliferation and differentiation into T effector cells, including TFH expressing the CXCL13 receptor, CXCR5. The TFH migrate to the CXCL13-rich B cell zone where they induce the activation, proliferation and differentiation of antigen-specific B cells into plasmablasts or their migration into follicles to form GCs where B cells undergo CSR. TLS include a series of heterogenous structures from lymphoid aggregates of T and B cells to structures including follicles of B cells (disorganized to organized). TLS range from the early loose lymphoid aggregates (disorganized, E-TLS) without FDCs to primary follicle-like TLS (PFL-TLS) containing a network of CD21+ FDCs and secondary follicle-like TLS (SFL-TLS) containing a GC including a network of CD21+CD23+ FDCs, as described for secondary lymphoid tissues above. The activation and differentiation of T cells within TLS allows for modulation of their effector function in the TME. In SFL-TLS, CD20+B cells are located within and at the periphery of GCs, similar to GCs in LN. Analysis of the BCR repertoire in follicles, compared with peripheral B cells, reveals clonal expansion, suggesting the development of an antigen-driven B-cell response occurring within TLS-GC. B cells that successfully capture antigen and receive CD40-mediated help from TFH survive and exit the GC as long-lived plasma cells or memory B cells. A fraction of the affinity matured B cells also undergo AID-dependent CSR that regulates isotype switching. Recent work suggests that this process could occur prior to differentiation of B cells into GC B cells or plasmablasts, rather than within GC as previously suggested. The question, if and at which stage of tumor-infiltrating TLSs maturation CSR appears, remains unknown.
Figure 2. Development of immunosuppressive or immunostimulating plasma cells.
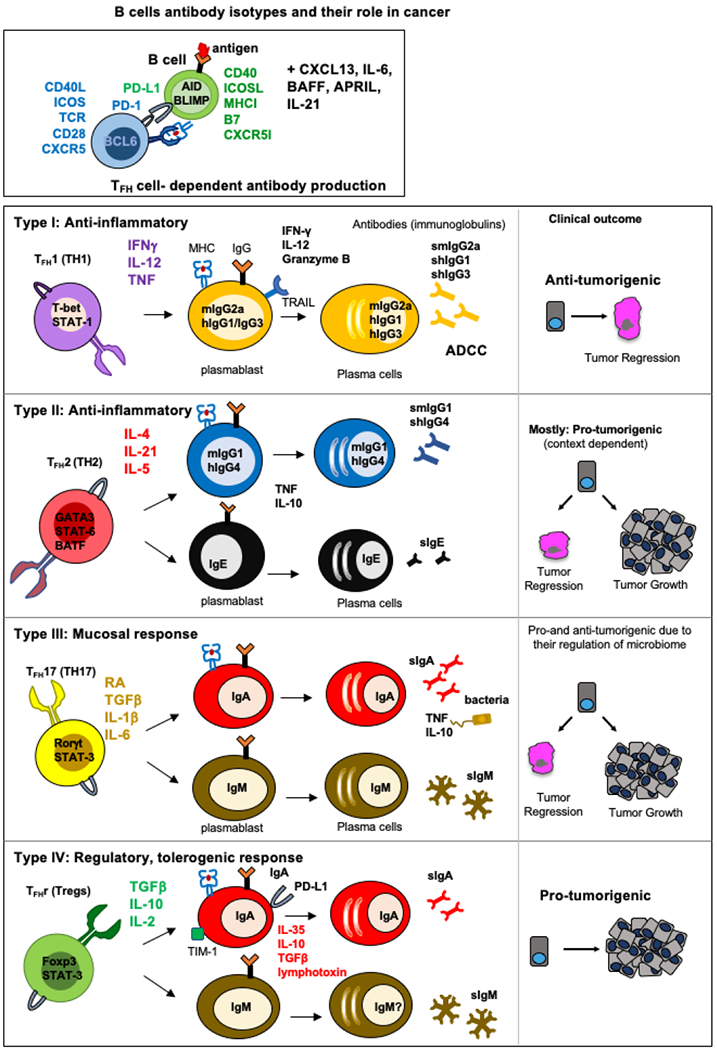
T follicular helper cell (TFH) differentiation and context-dependent TFH heterogeneity regulate B cell CSR and isotypes. Following activation of naïve CD4+ T cells, TH cells proliferate and undergo fate decisions in response to cytokines and other differentiating factors. TFH and B cell differentiation and isotypes are influenced by IL-6 and IL-21, and dependent on CD40 and ICOS signaling for expression of the transcription factor Bcl6. Cytokines including IL-12, IL-4, IL-1β, and many others, direct (A) TH1 and IgG; (B) TH2, IgG and IgE, (C) TH17, mucosal IgM and IgA and (D) regulatory cell differentiation. The microenvironment-dependent cytokines influence TFH and B cell differentiation, lineages, and isotypes, which define their fate to be pro- or anti-tumorigenic.
Figure 3. The multiple functions of B cells and their contributions to cancer.
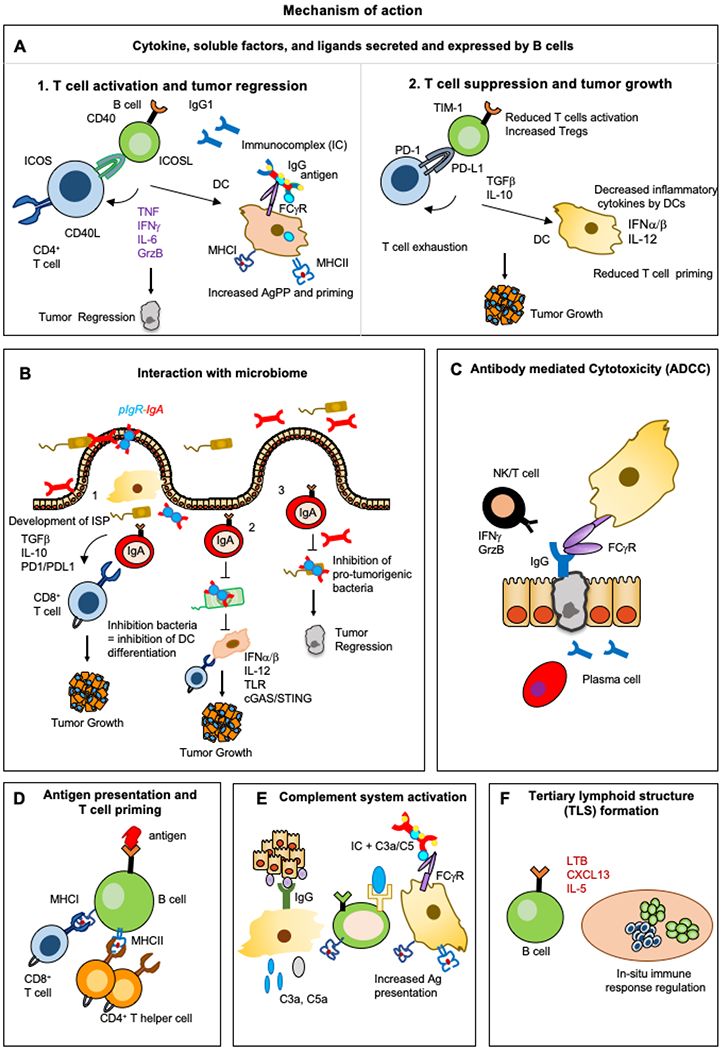
The functions of B cells and plasma cells include: A) Secretion of soluble factor and expression of ligands, opsonization; B) Interaction with microbiome; C) Antibody-dependent cellular cytotoxicity (ADCC); D) Priming of CD4+ and CD8+ T cells through MHC-I and MHC-II expression; E) Complement system activation; F) Induction of tertiary lymphoid structure (TLS) formation.
3. The multiple functions of B cells and their contributions to cancer
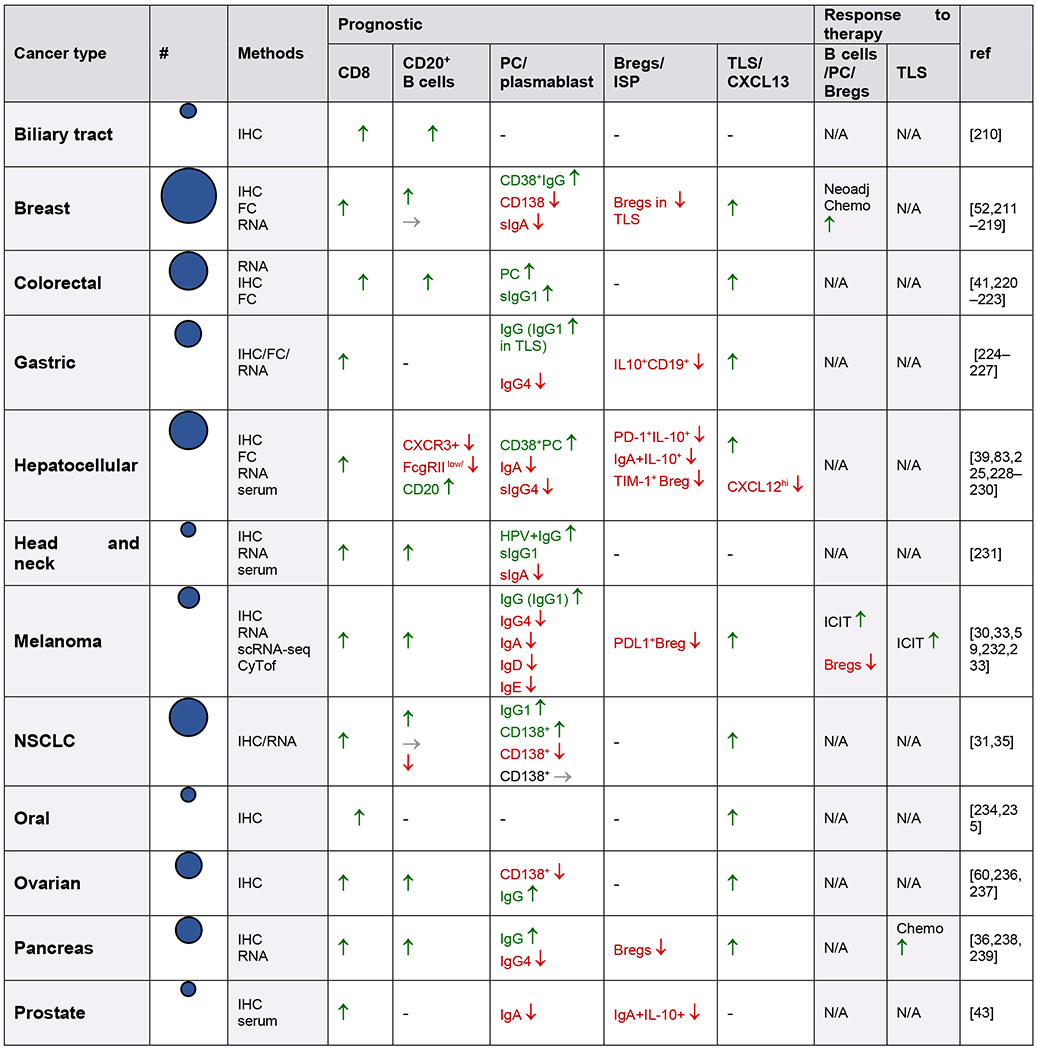
Several studies suggest that B cell presence and functionality is an important prognostic factor in cancer [31–42]. Plasma cells are present in many cancers, with the ability to produce large amounts of antibodies and cytokines [43,44]. Compared to T cells which are often scattered within tumors and sometimes in close contact with tumor cells, most B lymphocytes localize together forming clusters with varying size, cellular composition and maturation degree with T cells to from anything between a loose aggregate juxtaposing a T cell cluster in early (E)-aggregate (E-TLS) to denser organized primary follicle-like aggregates (PFL)- and secondary follicle-like (SFL)-TLS. Of the tumor-infiltrating immune cells, up to 3-30% are B and plasma cells based on cancer type, time point and the methods used, which is very similar compared to tumor-infiltrating-T cell numbers [37,40,41,45]. Immunoglobulins, dominantly IgG and IgA, specific for more than 2,000 antigens, which are often overexpressed by cancer cells, have been detected in sera of many cancer patients [46–49]. B cells and plasma cells are found scattered at tumor margins, close to cancer-associated fibroblasts (CAFs), in tumor-associated aggregates, and in unorganized clusters or organized tertiary lymphoid structures (TLS) [29,35,42,43,50]. However, tumor-infiltrating B and plasma cells have both tumor-promoting and -suppressing characteristics. This depends on their phenotype, their antibody isotype and production, the tumor type, TME, and their localization [51–53,53–56] (Figure 2,3). The Cancer Genome Atlas (TCGA) database analysis revealed that a high expression of B cell and plasma cell signature gene correlated with increased overall survival in patients with melanoma, lung adenocarcinoma, pancreatic adenocarcinoma and head and neck squamous cell carcinoma. By contrast, high levels of expression of these genes correlated with poorer clinical outcomes in patients with glioblastoma and clear cell renal cell carcinoma. High levels of immunoglobulin expression in tumors is also associated with increased survival in patients with melanoma, lung adenocarcinoma and head and neck squamous cell carcinoma, but it is associated with poor prognosis in patients with glioblastoma and renal cell carcinoma [51–53,57] (Table 1).
Table 1.
Infiltration and prognostic impact of B cells, plasma cells, serum immunoglobulins and TLS in human cancers
|
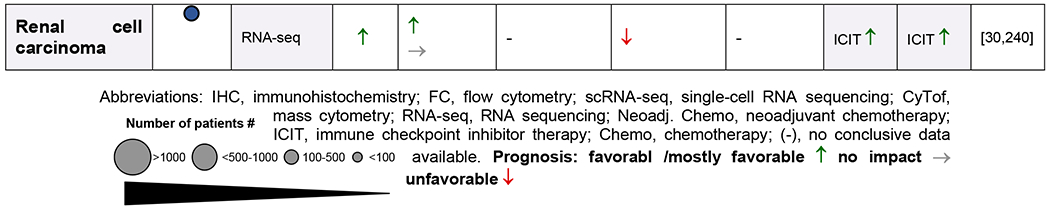
|
In particular, the cardinal role of B cells in regulation of CD8+ and CD4+ T cell responses ranges from their antitumorigenic function, including induction of cytotoxic CD8+ T cell (CTL) and CD4+ T helper cell (TH1) activation and effector function to their pro-tumorigenic function including suppression of CTLs and TH1 responses [30,32,35,42,43,58–62]. B cells and humoral immunity can also regulate anti-tumor immunity through opsonization, antibody-mediated cellular cytotoxicity, or activation of the complement system components C5a or C3a, which seem to either activate or suppress anti-tumor immunity in a context-dependent manner [62–66]. Below we will discuss different B and plasma cell effector functions and their contributions in cancer (Table 1).
3.1. Expression of soluble factors and ligands
The capability of B cells and particularly plasma cells to produce cytokines and soluble factors has long been underestimated [37,65,67–70]. In prostate cancer, newly recruited B cells promote aggressive hormone-refractory tumors by producing the proinflammatory cytokine lymphotoxin [71]. Lymphotoxin in combination with CXCL13, a chemoattractant for B lymphocytes, produced by TFH cells and fibroblasts can support lymphangiogenesis and metastasis [50,72,73]. On the other hand, The CXCL13 signature was also associated with favorable prognosis in colorectal cancer and melanoma [30,41]. In this regards, the induction of lymphangiogenesis and tertiary follicle structures supported T cell priming and improved responses to immunotherapy [30,59,74]. Recently, B cells including heterogenous populations called “regulatory B cells (Bregs)” and “immunosuppressive plasma cells (ISPC)” were shown to attenuate the development of autoimmune disease [65,68,75,76] and antitumor immunity by expressing IL-10, PD-L1, FASL, TGF-β, IL-35 and Tim-1 [42,43,66,75,75,77–79,79,80]. Of note, Tim-1, a phosphatidylserine receptor expressed on B cells, induces IL-10 production by sensing apoptotic cells [43,79]. TIM-1+ B cells have recently been shown to express a number of co-inhibitory checkpoint receptors including TIGIT, Ebi3 (a subunit of IL-27), CD39, CD73 and PD-L1. Tim-1+ B cells also highly express a set of checkpoint receptors including CTLA4, Lag3, PD-1, and Tim-3 that have been associated with CD8+ T cell exhaustion/dysfunction and Treg differentiation [79,81,82]. Inversely, B cells, particularly IgG+ cells, can express factors that activate T cells and have tumor suppressive effects like Granzyme B, TRAIL, IFNγ and IL-12 [83]. B cells can also secrete the cytokine IL-6, a cytokine with a well described pro-tumorigenic role [84,85].
3.2. Plasma cells, isotypes, immunoglobulin
As stated above, the detection of antibodies, largely IgG and IgA, specific for tumor-associated antigens in both serum and TME confirmed the existence of humoral immune responses to tumors ([47,86–89]. Antibodies of different isotypes usually operate in distinct tissues and have different effector functions. CSR affecting the BCR cytoplasmic tail alters BCR functionality and each subclass (isotype) of secreted antibody can influence B cell differentiation, survival and function by stimulating a varied immune response [90]. Antibody functionality is also determined by the constant region (Fc) that can bind to Fc receptors expressed by various cells including macrophages and dendritic cells (DC) [91]. Factors and cytokines secreted by CD4+ T helper cells in combination with co-stimulatory ligands (CD40/CD40L) and cognate cell contact selectively direct B cells to switch to specific antibody classes and subclasses (Figure 1–3).
Recently, a meta-analysis, including data from 11-14 studies with approximately 2,000 untreated cancer cases and 1,000 control subjects, evaluated the association of serum immunoglobulin classes with solid cancer and addressed the question if the immune escape of tumors is accompanied by dysregulated systemic immunoglobulin class-switching. Significantly higher serum IgA levels in patients with solid malignancies compared with healthy individuals was observed, and this further increased in patients with advanced cancer, indicating an association of IgA class-switching with solid cancer diagnosis and confirming its immunosuppressive and pro-tumorigenic role [92]. IgA serum level has also been linked to older age, male gender, metabolic syndrome, and other well-known factors (e.g. obesity, alcohol, smoking) associated with immune dysregulation, cancer risk and unfavorable prognosis [92,93]. In this meta-analysis, no association between total serum IgG levels and solid cancer was found [92]. IgG accounts for 75% of serum immunoglobulins and consists of 4 different subclasses (IgG1–IgG4), which have been shown to differ in their inflammatory and ADCC abilities. Accordingly, a lower IgG1/total IgG ratio has been reported in gynecological [94], colorectal [95], head and neck [96], and breast cancer [97], confirming its anti-tumorigenic capability. On the other hand, a raised serum IgG4/total IgG ratio has been reported in hepatocellular carcinoma [98] and melanoma, [99] and has been associated with unfavorable disease prognosis.
Murine IgG2a and IgG2b with their human homologs IgG1 and IgG3 usually contribute to a proinflammatory Type I immunity against intracellular pathogens in concert with TH1 and ILC1 cells and bind to proinflammatory Fc receptors, FcγR1 and FcγRIV, [100–102]. In this regard, IgG2a+ antibodies failed to eliminate tumors in a FcγRIV−/− mouse model [100]. These isotypes can effectively induce ADCC and activate the pro-inflammatory complement system [103,104], or produce Granzyme B, TRAIL and IL-12 [83]. Therefore they are associated with anti-tumor immunity and correlate with longer survival of cancer patients [38,51,53,105,106].
Murine IgG1, its human analog IgG4, and IgE usually contribute to Type II-like immunity. Murine IgG1 (hIgG4) can bind also to the inhibitory FcγRIIB with high affinity and fail to activate the pro-inflammatory complement pathway [104]. In this regard, IgG4 was shown to lack anti-tumor function and correlated with poor prognosis and induction of chronic inflammation probably by activation of tumor-associated macrophages and TAM2-like cells [89,99,107–111].
Binding of IgE to FcεRI can support the secretion of Type II cytokines like IL-4 and IL-13 by mast cells. These cytokines have a wide range of functions in tumors, being mostly pro-tumorigenic, including polarization of TH cells toward a TH2 phenotype, that lacks a strong-antitumor immunity [37,51].
Type III immunity is usually associated with protection of mucosal sites from extracellular pathogens by TH17 cells and IgA+ plasma cells. Secreted IgA+ antibodies usually bind to Polymeric immunoglobulin receptor (PIgR) to be transported through the epithelial layer [112–116]. The T cell-independent IgA CSR depends on retinoic acid and TGF-β, however TNF and inducible nitric oxide synthase (iNOS) can also attribute to their development in combination with BAFF and APRIL [113,117,118]. Intestinal secretory IgAs bind to PlgR in the mucosal layer and regulate the microbiome, which is mediated by dimeric secretory IgA that neutralizes antigens and prevents microbial adhesion to epithelial cells. IgA promotes bacterial symbiosis through protective opsonization, modification of their metabolism and epitope expression [119], which may induce immune tolerance and the maintenance of gut microbiome diversity [120]. Moreover, gut and lung microbiota can augment IgA class-switching through antigen presentation by CD103+ dendritic cells (DCs) and induction of TGFβ and IL10 [121]. Gut and skin residual CD103+ DCs are also able to induce T-cell anergy and Treg expansion by expressing aldehyde dehydrogenase and indoleamine 2,3-dioxygenase [122]. Tissue-specific immunoregulation may determine the ability of different tumors to regulate the IgA-microbiota axis in order to facilitate immune escape. A possible role of mucosal IgA in tumor immunity appears from its dynamic relationship with environmental factors, such as diet and the microbiota. Dysbiosis and changes in microbiome diversity was recently shown to influence multiple aspects of antitumor immunity, from altering the risk of developing cancer to regulating responses to immunotherapy [123–126]. In this regard, the role of mucosal IgA needs to be elucidated. IgA can also suppress tumor development in particular tumor types (e.g. colon cancer) for which a correlation between gut microbial homoeostasis and tumor growth has been established [42,127,128].
IgA and IgM can also contribute to a regulatory immunosuppressive response (Type IV, Figure 2) [42,43,75,76,112,116–118,129]. Tregs and regulatory innate lymphoid cells (ILCregs) express both IL-10 and TGF-β and support the development of immunosuppressive plasma cells (ISPC) in a T cell-dependent or -independent manner. We found a subpopulation of ISPCs that produces IgA, PD-L1, and IL-10 and strongly inhibit CTL activation in prostate cancer–bearing mice treated with the immunogenic chemotherapeutic agent oxaliplatin or in non-alcoholic steatohepatitis (NASH)-related hepatocellular carcinoma (HCC) [42,43]. Elimination of ISPCs, whose development depends on TGF-β signaling, strongly enhances the immunogenic response to low-dose oxaliplatin, resulting in tumor rejection [42,43]. Additional important features of IgA include that it does not activate the complement system and can mediate the regulatory effects of its main inducer, TGFβ [130,131]. In particular, monomeric IgA exerts inhibitory effects on many immune cell subsets via activation of FcαRI receptors [132,133] and induction of IL10 production [134], as well as by regulating proinflammatory cytokines [135]. Moreover, MDSCs were found to induce the differentiation of B cells into IgA+ plasma cells [136]. Recent high-throughput human studies involving B cell repertoire analysis confirmed that high proportions of IgA are associated with elevated cancer risk and poor prognosis in different tumor types, including melanoma, lung adenocarcinoma, bladder cancer, acute myeloid leukemia and HCC [38,51,53,137–139].
3.3. Antibody-mediated cellular cytotoxicity (ADCC)
B cells and humoral immunity have also been described to regulate anti-tumor immunity through antibody-mediated cellular cytotoxicity [140,141]. ADCC is an adaptive immune response largely mediated by NK cells through CD16 (FcγRIII) or FcγRIIC, which bind the Fc portion of IgG antibodies and trigger the lysis of targeted cells by expression of cytokines like IFNγ. Moreover, the positive correlation of IgG antibodies with good prognosis, and response to immunotherapy has been attributed partially to their ADCC capability [30,37,38,51,59]. Many therapeutic antibodies used clinically such as Rituximab (anti-CD20), cetuximab (anti-EGFR) and anti-GD2 attack cancer cells also through an ADCC dependent-mechanism [140–142]. Nevertheless, although tumor-associated Immunoglobulins were detected in the sera of many cancer patients [46,47,143], antibody-mediated cancer cell killing is usually impaired. Notably, antibody effector functions that are mediated by Fcγ receptors are also compromised during persistent infections, an effect attributed to formation of antigen/antibody immune complexes (ICs), suggesting that high concentrations of preexisting ICs can limit the effectiveness of antibody therapy in human cancer [144].
3.4. Interaction with the Complement system
The complement system can regulate B cell differentiation and also be regulated by the humoral immune system. Effective formation of GC requires the presence of T cells and a source of antigen which is retained on the surface of FDC via CD21 (complement receptor 2; CR2), CD35 (complement receptor 1; CR1) and FcR [145,146]. Moreover, antibodies can evoke complement-dependent cytotoxicity (CDC) that culminates in tumor cell elimination and regulate anti-tumor immunity by activating complement system components C5a or C3a [147,148], which can also suppress anti-tumor immunity in a context dependent manner [62,149]. However, the complement system can modulate the TME through a B cell-independent mechanism as well [146].
3.5. Antigen presentation
In addition to their role as antibody producing and secreting cells, B cells can function as professional antigen-presenting cells (APCs) for CD4+ T cells by expressing cell-surface major histocompatibility complex class II (MHCII) molecules with bound peptides that serve as α/β T cell receptor (TCR) ligands. MHCII restricted antigen presentation by B cells can play an important role in shaping the immune response, as shown for thymic B cells [150–152]. B cells can capture antigen and uptake to internalize the antigen:BCR complexes with MHCII in peptide-loading compartments, thus generating MHCII:peptide complexes followed by exocytic transport to present these complexes on their surface [150,153–157]. B cells also express co-stimulatory molecules, like CD40, CD80 and CD86. B cells’ ability to act as APC both in LN and TLS can explain the positive outcome of intratumoral B cell and T cell, in particular CD4+ T helper cells, cooperation in TLS during immunotherapy (Table 1) [29,30,35,59,60,83,158–160]. B cells can also capture immune complexes by a complement receptor–dependent mechanism from macrophages and transport the complexes to follicular dendritic cells, and later to T zone, which can enhance GC response and T cell activation [161]. However, much less is known about MHCI restricted antigen cross-presentation by B cells to prime CD8+ T cells.
Moreover, B cells can also indirectly regulate the priming of T cells through immune complexes (antigen-antibody complexes) harboring IgG-bound tumor antigens, that bind to FcγR expressed by dendritic cells and macrophages, thereby supporting phagocytosis and antigen cross-presentation and T cell priming [162–165].
4. Role of the tumor microenvironment in shaping the B cell repertoire
Regulation of hematopoietic stem cells (HSC) and B cell progenitors self-renewal and differentiation is thought to depend on their microenvironment, also called the “bone marrow” (BM) stem-cell niche [166,167]. Normal hematopoiesis requires a complex and reciprocal interaction between the BM (or fetal liver) microenvironment and HSC/ B cell progenitors [167]. The BM stem cell niche is composed of specialized cells like osteoblasts, responsible for osteogenesis (bone growth) and controlling HSC numbers and stromal cells including sinusoidal endothelial cells, supporting proliferation, differentiation, and transendothelial migration of HSC. B-cells are generated from HSC and develop in the BM. Afterwards mature B-cells migrate into the blood and reach peripheral lymphoid tissues. The development of B-cell precursors through various stages requires a coordinated interaction with BM stromal cells producing different factors in the BM niche, as well as the expression of specific transcription factors, such as Ikaros, E2A, and PAX5. In 1982, Whitlock and Witte described the growth of a B-cell precursor on BM-derived stromal cells in vitro [168]. For B-cell development several microenvironmental components have been identified, such as CXC-chemokine ligand 12 (CXCL12), FLT3 ligand, interleukin 7 (IL-7) and stem-cell factor (SCF) [167,169–171]. Different subtypes of BM stromal cells produce these factors [172,173]. Inflammatory responses in the TME are often accompanied by induction of fibrosis and recruitment of stromal cells like tumor-associated fibroblasts (CAF) and macrophages (TAM). CAFs are responsible for deposition of collagen and various extracellular matrix (ECM) components in the tumor microenvironment, where they stimulate cancer cell proliferation and angiogenesis [173,174]. CAFs and TAMs also have a critical immune function as they produce numerous cytokines and chemokines, including osteopontin (OPN), CXCL1, IL-6, CXCL12, TGF-β, IL-7, IL-1β, TN, iNOS, CCL-5, stromal-derived factor-1α (SDF-1α), and CXCL13, that are decisive for B cell differentiation, CSR, survival and effector functions, as discussed above [50,73,170,174–179]. The proinflammatory properties of TAMs and CAFs are enhanced by mediators secreted by resident immune cells [180], such as IL-1, which regulates and can be regulated by Fc receptors [181]. Activated CAFs and TAMs in the TME produce TGF-β, which inhibits NK cell and CTL activation, induces Treg and supports the IgA CSR [42,43,50,115,182,183]. CAF-derived CXCL13 mediates recruitment of B cells and lymphotoxin expression, which can lead to development of prostate and pancreatic cancers [50,71,184], but can also regulate TLS development and activation of anti-tumor responses [30,59,72,185,186]. Stromal cells and CAFs also secrete IL-6 and orchestrate B cell survival and lymphoid tissue growth and responses [187]. Most importantly, circulating TFH cells were found in tumors and tumor-infiltrating TLS that can regulate the B cell isotype via CSR by producing cytokines and soluble factors like IL-2 [188–192]. However, most tumor-infiltrating TH/TFH cells have a regulatory TrFH (IL-10, TGF-β), TH17 or TH2 (IL-4, IL-5) phenotype, which supports the development of immunosuppressive and anti-inflammatory isotypes [193]. IgA+ ISPC are derived from naïve B cells that are recruited into the TME by CAF-generated CXCL13 and CXCL12 [43,50]. Upon encounter of BCR-specific antigens, that could be bacterial-, food-, or tumor-derived, and further exposure to TGF-β and other cytokines, including IL-21 from circulatory TFH cells, lymphotoxin β (LTβ), IL-33, and IL-10, naïve IgM+ B cells undergo CSR to IgA+ plasma cells [42,43,113]. These anti-inflammatory and immunosuppressive effects are in line with the well-established homeostatic and regulatory role of IgA+ plasmocytes in mucosal immunity [112,117,194]. In this regard, an immune response coordinated by a pro-inflammatory environment and available immunogenic tumor antigens leads to the generation of enormous plasma cell clones producing high-affinity, tumor-specific IgG1 antibodies, while an immunosuppressive environment leads to B cells switching to IgA with the potential for immunosuppression.
5. Host metabolism and microbiome shape the cancer B cell repertoire
Obesity, aging and alcohol are fundamental supporters of cancer development [161], acting through induction of chronic inflammation, barrier disruption and microbiota dysbiosis [42,195–198]. Hypernutrition, alcohol consumption and aging also regulate the magnitude of B cell activity and effector function, although the underlying mechanisms remain poorly understood and have been attributed to metabolic reprograming, impairment of TFH and changes in cytokine gene expression [42,195,199–203]. Moreover, alcohol consumption, obesity and aging correlate with changes in IgA levels and diversity [204–207], which may further contribute to tumor development [42]. Mucosal or systemic microbiota exposures also shape the B cell repertoire, although its effects differs for each isotype; such as IgG and IgA. Therefore, the regulation of B cells by the microbiome and vice versa can affect the response to immunotherapy [123,208]. The development of B cells, including CSR, SHM and proliferation depends on sophisticated metabolic programing that includes anaerobic glycolysis, oxidative phosphorylation (OXPHOS), the tricarboxylic acid (TCA) cycle and nucleotide biosynthesis. Recently, it was shown that GC B cells rely primarily on fatty acid oxidation (FAO) for proliferation [200,209]. However, it remains unknown how the B cell response is affected in cancer patients with metabolic disorders, and how or if the dysregulation of the B cell response contributes to cancer development and response to therapy.
6. Summary and Future perspective
In summary, recent data have established the crucial and important roles of B cells, plasma cells and antibodies in tumor development, progression and response to therapy. A sustained and long-term protective immune response to pathogens and tumors requires the organization and orchestration of cellular and humoral adaptive immune cells in concert with innate immune cells. A proinflammatory Type I response seems to be the most protective response that inhibits tumor development, supports tumor-rejection, and responds to immunotherapy, which usually includes CTLs, TH1, IgG1+ antibodies and TLS. However, the mechanisms by which different B cell types manifest their immunosuppressive and immunosupportive effects remain poorly understood. Future work needs to be done to systematically characterize tumor-infiltrating B cells and the transcription factors that regulate their development and antigen specificity.
Acknowledgements
We thank R. K. Ngu for illustration and figure preparation and J. Huang and I. N. Bastian for discussion and editing. This work was supported by grants from the NIH U01AA027681 to S.S, and R01 AI043477, P01 CA128814, R01 CA211794 and the Tower Cancer Research Foundation to M.K.
Declaration of interests
S.S. declares no competing interests, M.K. had received research support from Jansen Pharmaceuticals, Merck and Aduro.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].von Behring E, Ueber das Zustandekommen der Diphtherie-Immunität und der Tetanus-Immunitäat bei Thieren, (2013). [PubMed] [Google Scholar]
- [2].Cooper MD, The early history of B cells, Nature Reviews Immunology. 15 (2015) 191–197. 10.1038/nri3801. [DOI] [PubMed] [Google Scholar]
- [3].Tiselius A, Kabat EA, AN ELECTROPHORETIC STUDY OF IMMUNE SERA AND PURIFIED ANTIBODY PREPARATIONS, J Exp Med. 69 (1939) 119–131. 10.1084/jem.69.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fagraeus A, The plasma cellular reaction and its relation to the formation of antibodies in vitro, Journal of Immunology (Baltimore, Md.: 1950). 58 (1948) 1–13. [PubMed] [Google Scholar]
- [5].Landsteiner K, Chase MW, Experiments on Transfer of Cutaneous Sensitivity to Simple Compounds, Proceedings of the Society for Experimental Biology and Medicine. 49 (1942) 688–690. 10.3181/00379727-49-13670. [DOI] [Google Scholar]
- [6].Gowans JL, Knight EJ, Florey HW, The route of re-circulation of lymphocytes in the rat, Proceedings of the Royal Society of London. Series B. Biological Sciences. 159 (1964) 257–282. 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- [7].Ellis ST, Gowans JL, Howard JC, The Origin of Antibody Forming Cells from Lymphocytes, The Immune Response and Its Suppression. 15 (1969)40–55. 10.1159/000386770. [DOI] [PubMed] [Google Scholar]
- [8].Ehrlich P, The Nobel Prize in Physiology or Medicine 1908, NobelPrize.Org. (n.d.). https://www.nobelprize.org/prizes/medicine/1908/ehrlich/lecture/ (accessed October 8, 2020).
- [9].Ehrlich P, On immunity with special reference to cell life., (1900). [Google Scholar]
- [10].Glick B, Chang TS, Jaap RG, The Bursa of Fabricius and Antibody Production, Poultry Science. 35 (1956) 224–225. 10.3382/ps.0350224. [DOI] [Google Scholar]
- [11].Cooper MD, Peterson RDA, Good RA, Delineation of the Thymic and Bursal Lymphoid Systems in the Chicken, Nature. 205 (1965) 143–146. 10.1038/205143a0. [DOI] [PubMed] [Google Scholar]
- [12].Cooper MD, Peterson RDA, South MA, Good RA, THE FUNCTIONS OF THE THYMUS SYSTEM AND THE BURSA SYSTEM IN THE CHICKEN, J Exp Med. 123 (1966) 75–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Miller JF, Immunological function of the thymus, Lancet (London, England). 2 (1961) 748–749. 10.1016/s0140-6736(61)90693-6. [DOI] [PubMed] [Google Scholar]
- [14].Md C, Ml S, Ra G, Restoration of gamma globulin production in agammaglobulinemic chickens., Science. 151 (1966) 471–473. 10.1126/science.151.3709.471. [DOI] [PubMed] [Google Scholar]
- [15].Raff MC, Megson M, Owen JJT, Cooper MD, Early production of intracellular IgM by B-lymphocyte precursors in mouse, Nature. 259 (1976) 224–226. 10.1038/259224a0. [DOI] [PubMed] [Google Scholar]
- [16].Owen JJ, Cooper MD, Raff MC, In vitro generation of B lymphocytes in mouse foetal liver, a mammalian “bursa equivalent,” Nature. 249 (1974) 361–363. 10.1038/249361a0. [DOI] [PubMed] [Google Scholar]
- [17].Cooper MD, Perey DY, McKneally MF, Gabrielsen AE, Sutherland DE, Good RA, A mammalian equivalent of the avian bursa of Fabricius, Lancet (London, England). 1 (1966) 1388–1391. 10.1016/s0140-6736(66)90300-x. [DOI] [PubMed] [Google Scholar]
- [18].Jung D, Giallourakis C, Mostoslavsky R, Alt FW, Mechanism and control of V(D)J recombination at the Immunoglobulin heavy chain locus, Annual Review of Immunology. 24 (2006) 541–570. 10.1146/annurev.immunol.23.021704.115830. [DOI] [PubMed] [Google Scholar]
- [19].Schatz DG, Oettinger MA, Baltimore D, The V(D)J Recombination Activating Gene, RAG-, (n.d.) 14. [DOI] [PubMed] [Google Scholar]
- [20].Hozumi N, Tonegawa S, Evidence for somatic rearrangement of immunoglobulin genes coding for variable and constant regions., Proc Natl Acad Sci U S A. 73 (1976) 3628–3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Roco JA, Mesin L, Binder SC, Nefzger C, Gonzalez-Figueroa P, Canete PF, Ellyard J, Shen Q, Robert PA, Cappello J, Vohra H, Zhang Y, Nowosad CR, Schiepers A, Corcoran LM, Toellner K-M, Polo JM, Meyer-Hermann M, Victora GD, Vinuesa CG, Class-Switch Recombination Occurs Infrequently in Germinal Centers, Immunity. 51 (2019) 337–350.e7. 10.1016/j.immuni.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Davis MM, Calame K, Early PW, Livant DL, Joho R, Weissman IL, Hood L, An immunoglobulin heavy-chain gene is formed by at least two recombinational events, Nature. 283 (1980) 733–739. 10.1038/283733a0. [DOI] [PubMed] [Google Scholar]
- [23].Sakano H, Maki R, Kurosawa Y, Roeder W, Tonegawa S, Two types of somatic recombination are necessary for the generation of complete immunoglobulin heavy-chain genes, Nature. 286 (1980) 676–683. 10.1038/286676a0. [DOI] [PubMed] [Google Scholar]
- [24].Yaoita Y, Honjo T, Deletion of immunoglobulin heavy chain genes from expressed allelic chromosome, Nature. 286 (1980) 850–853. 10.1038/286850a0. [DOI] [PubMed] [Google Scholar]
- [25].Laidlaw BJ, Cyster JG, Transcriptional regulation of memory B cell differentiation, Nature Reviews Immunology. (2020) 1–12. 10.1038/s41577-020-00446-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].McKean D, Huppi K, Bell M, Staudt L, Gerhard W, Weigert M, Generation of antibody diversity in the immune response of BALB/c mice to influenza virus hemagglutinin, PNAS. 81 (1984) 3180–3184. 10.1073/pnas.81.10.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jacob J, Kelsoe G, Rajewsky K, Weiss U, Intraclonal generation of antibody mutants in germinal centres, Nature. 354 (1991) 389–392. 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- [28].Sautès-Fridman C, Verneau J, Sun C-M, Moreira M, Chen TW-W, Meylan M, Petitprez F, Fridman WH, Tertiary Lymphoid Structures and B cells: Clinical impact and therapeutic modulation in cancer, Semin Immunol. 48 (2020) 101406. 10.1016/j.smim.2020.101406. [DOI] [PubMed] [Google Scholar]
- [29].Sautès-Fridman C, Petitprez F, Calderaro J, Fridman WH, Tertiary lymphoid structures in the era of cancer immunotherapy, Nature Reviews. Cancer. 19 (2019) 307–325. 10.1038/s41568-019-0144-6. [DOI] [PubMed] [Google Scholar]
- [30].Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, Yizhak K, Sade-Feldman M, Blando J, Han G, Gopalakrishnan V, Xi Y, Zhao H, Amaria RN, Tawbi HA, Cogdill AP, Liu W, LeBleu VS, Kugeratski FG, Patel S, Davies MA, Hwu P, Lee JE, Gershenwald JE, Lucci A, Arora R, Woodman S, Keung EZ, Gaudreau P-O, Reuben A, Spencer CN, Burton EM, Haydu LE, Lazar AJ, Zapassodi R, Hudgens CW, Ledesma DA, Ong S, Bailey M, Warren S, Rao D, Krijgsman O, Rozeman EA, Peeper D, Blank CU, Schumacher TN, Butterfield LH, Zelazowska MA, McBride KM, Kalluri R, Allison J, Petitprez F, Fridman WH, Sautès-Fridman C, Hacohen N, Rezvani K, Sharma P, Tetzlaff MT, Wang L, Wargo JA, B cells and tertiary lymphoid structures promote immunotherapy response, Nature. 577 (2020) 549–555. 10.1038/s41586-019-1922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dieu-Nosjean M-C, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, Rabbe N, Laurans L, Tartour E, de Chaisemartin L, Lebecque S, Fridman W-H, Cadranel J, Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures, Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 26 (2008) 4410–4417. 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- [32].Lund FE, Randall TD, Effector and regulatory B cells: modulators of CD4 + T cell immunity, Nature Reviews Immunology. 10(2010)236–247. 10.1038/nri2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ladányi A, Kiss J, Mohos A, Somlai B, Liszkay G, Glide K, Fejös Z, Gaudi I, Dobos J, Tímár J, Prognostic impact of B-cell density in cutaneous melanoma, Cancer Immunology, Immunotherapy: CII. 60 (2011) 1729–1738. 10.1007/s00262-011-1071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Erdag G, Schaefer JT, Smolkin ME, Deacon DH, Shea SM, Dengel LT, Patterson JW, Slingluff CL, Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma, Cancer Research. 72 (2012) 1070–1080. 10.1158/0008-5472.CAN-11-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Germain C, Gnjatic S, Tamzalit F, Knockaert S, Remark R, Goc J, Lepelley A, Becht E, Katsahian S, Bizouard G, Validire P, Damotte D, Alifano M, Magdeleinat P, Cremer I, Teillaud J-L, Fridman W-H, Sautès-Fridman C, Dieu-Nosjean M-C, Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer, American Journal of Respiratory and Critical Care Medicine. 189 (2014) 832–844. 10.1164/rccm.201309-16110C. [DOI] [PubMed] [Google Scholar]
- [36].Castino GF, Cortese N, Capretti G, Serio S, Di Caro G, Mineri R, Magrini E, Grizzi F, Cappello P, Novelli F, Spaggiari P, Roncalli M, Ridolfi C, Gavazzi F, Zerbi A, Allavena P, Marchesi F, Spatial distribution of B cells predicts prognosis in human pancreatic adenocarcinoma, Oncoimmunology. 5 (2016) e1085147. 10.1080/2162402X.2015.1085147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sharonov GV, Serebrovskaya EO, Yuzhakova DV, Britanova OV, Chudakov DM, B cells, plasma cells and antibody repertoires in the tumour microenvironment, Nature Reviews Immunology. 20 (2020) 294–307. 10.1038/S41577-019-0257-x. [DOI] [PubMed] [Google Scholar]
- [38].Hu X, Zhang J, Wang J, Fu J, Li T, Zheng X, Wang B, Gu S, Jiang P, Fan J, Ying X, Zhang J, Carroll MC, Wucherpfennig KW, Hacohen N, Zhang F, Zhang P, Liu JS, Li B, Liu XS, Landscape of B cell immunity and related immune evasion in human cancers, Nature Genetics. 51 (2019) 560–567. 10.1038/S41588-018-0339-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhang S, Liu Z, Wu D, Chen L, Xie L, Single-Cell RNA-Seq Analysis Reveals Microenvironmental Infiltration of Plasma Cells and Hepatocytic Prognostic Markers in HCC With Cirrhosis, Front Oncol. 10 (2020). 10.3389/fonc.2020.596318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Schürch CM, Bhate SS, Barlow GL, Phillips DJ, Noti L, Zlobec I, Chu P, Black S, Demeter J, Mcllwain DR, Kinoshita S, Samusik N, Goltsev Y, Nolan GP, Coordinated Cellular Neighborhoods Orchestrate Antitumoral Immunity at the Colorectal Cancer Invasive Front, Cell. 182 (2020) 1341–1359.e19. 10.1016/j.cell.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A, Bruneval P, Fridman WH, Becker C, Pagès F, Speicher MR, Trajanoski Z, Galon J, Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer, Immunity. 39 (2013) 782–795. 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- [42].Shalapour S, Lin X-J, Bastian IN, Brain J, Burt AD, Aksenov AA, Vrbanac AF, Li W, Perkins A, Matsutani T, Zhong Z, Dhar D, Navas-Molina JA, Xu J, Loomba R, Downes M, Yu RT, Evans RM, Dorrestein PC, Knight R, Benner C, Anstee QM, Karin M, Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity, Nature. 551 (2017)340–345. 10.1038/nature24302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Shalapour S, Font-Burgada J, Di Caro G, Zhong Z, Sanchez-Lopez E, Dhar D, Willimsky G, Ammirante M, Strasner A, Hansel DE, Jamieson C, Kane CJ, Klatte T, Birner P, Kenner L, Karin M, Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy, Nature. 521 (2015) 94–98. 10.1038/nature14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Dang VD, Hilgenberg E, Ries S, Shen P, Fillatreau S, From the regulatory functions of B cells to the identification of cytokine-producing plasma cell subsets, Current Opinion in Immunology. 28 (2014) 77–83. 10.1016/j.coi.2014.02.009. [DOI] [PubMed] [Google Scholar]
- [45].Durante MA, Rodriguez DA, Kurtenbach S, Kuznetsov JN, Sanchez MI, Decatur CL, Snyder H, Feun LG, Livingstone AS, Harbour JW, Single-cell analysis reveals new evolutionary complexity in uveal melanoma, Nat Commun. 11 (2020) 496. 10.1038/s41467-019-14256-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Blankenstein T, Coulie PG, Gilboa E, Jaffee EM, The determinants of tumour immunogenicity, Nature Reviews. Cancer 12 (2012) 307–313. 10.1038/nrc3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Schreiber H, Ward PL, Rowley DA, Stauss HJ, Unique tumor-specific antigens, Annual Review of Immunology. 6 (1988) 465–483. 10.1146/annurev.iy.06.040188.002341. [DOI] [PubMed] [Google Scholar]
- [48].Gerstl B, Eng LF, Bigbee JW, Tumor-associated immunoglobulins in pulmonary carcinoma, Cancer Research. 37 (1977) 4449–4455. [PubMed] [Google Scholar]
- [49].Streets AJ, Brooks SA, Dwek MV, Leathern AJ, Identification, purification and analysis of a 55 kDa lectin binding glycoprotein present in breast cancer tissue, Clinica Chimica Acta; International Journal of Clinical Chemistry. 254 (1996) 47–61. 10.1016/0009-8981(96)06363-2. [DOI] [PubMed] [Google Scholar]
- [50].Ammirante M, Shalapour S, Kang Y, Jamieson CAM, Karin M, Tissue injury and hypoxia promote malignant progression of prostate cancer by inducing CXCL13 expression in tumor myofibroblasts, Proc. Natl. Acad. Sci. U.S.A 111 (2014) 14776–14781. 10.1073/pnas.1416498111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Bolotin DA, Poslavsky S, Davydov AN, Frenkel FE, Fanchi L, Zolotareva OI, Hemmers S, Putintseva EV, Obraztsova AS, Shugay M, Ataullakhanov RI, Rudensky AY, Schumacher TN, Chudakov DM, Antigen receptor repertoire profiling from RNA-seq data, Nature Biotechnology. 35 (2017) 908–911. 10.1038/nbt.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Iglesia MD, Vincent BG, Parker JS, Hoadley KA, Carey LA, Perou CM, Serody JS, Prognostic B-cell signatures using mRNA-seq in patients with subtype-specific breast and ovarian cancer, Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 20 (2014) 3818–3829. 10.1158/1078-0432.CCR-13-3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Isaeva OI, Sharonov GV, Serebrovskaya EO, Turchaninova MA, Zaretsky AR, Shugay M, Chudakov DM, Intratumoral immunoglobulin isotypes predict survival in lung adenocarcinoma subtypes, Journal for Immunotherapy of Cancer. 7 (2019)279. 10.1186/s40425-019-0747-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lohr M, Edlund K, Botling J, Hammad S, Hellwig B, Othman A, Berglund A, Lambe M, Holmberg L, Ekman S, Bergqvist M, Pontén F, Cadenas C, Marchan R, Hengstler JG, Rahnenführer J, Micke P, The prognostic relevance of tumour-infiltrating plasma cells and immunoglobulin kappa C indicates an important role of the humoral immune response in non-small cell lung cancer, Cancer Letters. 333 (2013) 222–228. 10.1016/j.canlet.2013.01.036. [DOI] [PubMed] [Google Scholar]
- [55].Stockert E, Jäger E, Chen YT, Scanlan MJ, Gout I, Karbach J, Arand M, Knuth A, Old LJ, A survey of the humoral immune response of cancer patients to a panel of human tumor antigens, The Journal of Experimental Medicine. 187(1998) 1349–1354. 10.1084/jem.187.8.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, Nair VS, Xu Y, Khuong A, Hoang CD, Diehn M, West RB, Plevritis SK, Alizadeh AA, The prognostic landscape of genes and infiltrating immune cells across human cancers, Nature Medicine. 21 (2015) 938–945. 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Mose LE, Selitsky SR, Bixby LM, Marron DL, Iglesia MD, Serody JS, Perou CM, Vincent BG, Parker JS, Assembly-based inference of B-cell receptor repertoires from short read RNA sequencing data with V’DJer, Bioinformatics. 32 (2016) 3729–3734. 10.1093/bioinformatics/btw526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Schultz KR, Klarnet JP, Gieni RS, HayGlass KT, Greenberg PD, The role of B cells for in vivo T cell responses to a Friend virus-induced leukemia, Science. 249 (1990) 921–923. 10.1126/science.2118273. [DOI] [PubMed] [Google Scholar]
- [59].Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, Johansson I, Phung B, Harbst K, Vallon-Christersson J, van Schoiack A, Lövgren K, Warren S, Jirström K, Olsson H, Pietras K, Ingvar C, Isaksson K, Schadendorf D, Schmidt H, Bastholt L, Carneiro A, Wargo JA, Svane IM, Jönsson G, Tertiary lymphoid structures improve immunotherapy and survival in melanoma, Nature. 577 (2020) 561–565. 10.1038/S41586-019-1914-8. [DOI] [PubMed] [Google Scholar]
- [60].Kroeger DR, Milne K, Nelson BH, Tumor-Infiltrating Plasma Cells Are Associated with Tertiary Lymphoid Structures, Cytolytic T-Cell Responses, and Superior Prognosis in Ovarian Cancer, Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 22 (2016) 3005–3015. 10.1158/1078-0432.CCR-15-2762. [DOI] [PubMed] [Google Scholar]
- [61].Qin Z, Richter G, Schüler T, Ibe S, Cao X, Blankenstein T, B cells inhibit induction of T cell-dependent tumor immunity, Nature Medicine. 4 (1998)627–630. 10.1038/nm0598-627. [DOI] [PubMed] [Google Scholar]
- [62].Medler TR, Murugan D, Horton W, Kumar S, Cotechini T, Forsyth AM, Leyshock P, Leitenberger JJ, Kulesz-Martin M, Margolin AA, Werb Z, Coussens LM, Complement C5a Fosters Squamous Carcinogenesis and Limits T Cell Response to Chemotherapy, Cancer Cell. 34 (2018) 561–578.e6. 10.1016/j.ccell.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Gunderson AJ, Kaneda MM, Tsujikawa T, Nguyen AV, Affara NI, Ruffell B, Gorjestani S, Liudahl SM, Truitt M, Olson P, Kim G, Hanahan D, Tempera MA, Sheppard B, Irving B, Chang BY, Varner JA, Coussens LM, Bruton Tyrosine Kinase-Dependent Immune Cell Cross-talk Drives Pancreas Cancer, Cancer Discov. 6 (2016)270–285. 10.1158/2159-8290.CD-15-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Markiewski MM, DeAngelis RA, Benencia F, Ricklin-Lichtsteiner SK, Koutoulaki A, Gerard C, Coukos G, Lambris JD, Modulation of the antitumor immune response by complement, Nature Immunology. 9 (2008) 1225–1235. 10.1038/ni.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Mauri C, Menon M, The expanding family of regulatory B cells, Int Immunol. 27 (2015) 479–486. 10.1093/intimm/dxv038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Pylayeva-Gupta Y, Das S, Handler JS, Hajdu CH, Coffre M, Koralov SB, Bar-Sagi D, IL35-Producing B Cells Promote the Development of Pancreatic Neoplasia, Cancer Discov. 6 (2016) 247–255. 10.1158/2159-8290.CD-15-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Mauri C, Menon M, Human regulatory B cells in health and disease: therapeutic potential, J Clin Invest. 127 (2017) 772–779. 10.1172/JCI85113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM, B cells regulate autoimmunity by provision of IL-10, Nature Immunology. 3 (2002) 944–950. 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- [69].Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, Johansson I, Phung B, Harbst K, Vallon-Christersson J, van Schoiack A, Lövgren K, Warren S, Jirstrom K, Olsson H, Pietras K, Ingvar C, Isaksson K, Schadendorf D, Schmidt H, Bastholt L, Carneiro A, Wargo JA, Svane IM, Jönsson G, Author Correction: Tertiary lymphoid structures improve immunotherapy and survival in melanoma, Nature. 580 (2020) E1. 10.1038/s41586-020-2155-6. [DOI] [PubMed] [Google Scholar]
- [70].Yuen GJ, Demissie E, Pillai S, B lymphocytes and cancer: a love-hate relationship, Trends Cancer. 2 (2016) 747–757. 10.1016/j.trecan.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Ammirante M, Luo J-L, Grivennikov S, Nedospasov S, Karin M, B-cell-derived lymphotoxin promotes castration-resistant prostate cancer, Nature. 464 (2010) 302–305. 10.1038/nature08782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Lu TT, Browning JL, Role of the Lymphotoxin/LIGHT System in the Development and Maintenance of Reticular Networks and Vasculature in Lymphoid Tissues, Front. Immunol 5 (2014). 10.3389/fimmu.2014.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Litsiou E, Semitekolou M, Galani IE, Morianos I, Tsoutsa A, Kara P, Rontogianni D, Bellenis I, Konstantinou M, Potaris K, Andreakos E, Sideras P, Zakynthinos S, Tsoumakidou M, CXCL13 production in B cells via Toll-like receptor/lymphotoxin receptor signaling is involved in lymphoid neogenesis in chronic obstructive pulmonary disease, American Journal of Respiratory and Critical Care Medicine. 187 (2013) 1194–1202. 10.1164/rccm.201208-1543OC. [DOI] [PubMed] [Google Scholar]
- [74].Schrama D, thor Straten P, Fischer WH, McLellan AD, Bröcker E-B, Reisfeld RA, Becker JC, Targeting of Lymphotoxin-α to the Tumor Elicits an Efficient Immune Response Associated with Induction of Peripheral Lymphoid-like Tissue, Immunity. 14 (2001) 111–121. 10.1016/S1074-7613(01)00094-2. [DOI] [PubMed] [Google Scholar]
- [75].Shen P, Roch T, Lampropoulou V, O’Connor RA, Stervbo U, Hilgenberg E, Ries S, Dang VD, Jaimes Y, Daridon C, Li R, Jouneau L, Boudinot P, Wilantri S, Sakwa I, Miyazaki Y, Leech MD, McPherson RC, Wirtz S, Neurath M, Hoehlig K, Meinl E, Grützkau A, Grün JR, Horn K, Kühl AA, Dörner T, Bar-Or A, Kaufmann SHE, Anderton SM, Fillatreau S, IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases, Nature. 507 (2014) 366–370. 10.1038/nature12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Rojas OL, Pröbstel A-K, Porfilio EA, Wang AA, Charabati M, Sun T, Lee DSW, Galicia G, Ramaglia V, Ward LA, Leung LYT, Najafi G, Khaleghi K, Garcillán B, Li A, Besla R, Naouar I, Cao EY, Chiaranunt P, Burrows K, Robinson HG, Allanach JR, Yam J, Luck H, Campbell DJ, Allman D, Brooks DG, Tomura M, Baumann R, Zamvil SS, Bar-Or A, Horwitz MS, Winer DA, Mortha A, Mackay F, Prat A, Osborne LC, Robbins C, Baranzini SE, Gommerman JL, Recirculating Intestinal IgA-Producing Cells Regulate Neuroinflammation via IL-10, Cell. 176 (2019) 610–624.e18. 10.1016/j.cell.2018.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Katz SI, Parker D, Turk JL, B-cell suppression of delayed hypersensitivity reactions, Nature. 251 (1974) 550–551. 10.1038/251550a0. [DOI] [PubMed] [Google Scholar]
- [78].Lindner S, Dahlke K, Sontheimer K, Hagn M, Kaltenmeier C, Barth TFE, Beyer T, Reister F, Fabricius D, Lotfi R, Lunov O, Nienhaus GU, Simmet T, Kreienberg R, Müller P, Schrezenmeier H, Jahrsdörfer B, Interleukin 21-lnduced Granzyme B-Expressing B Cells Infiltrate Tumors and Regulate T Cells, Cancer Res. 73 (2013)2468–2479. 10.1158/0008-5472.CAN-12-3450. [DOI] [PubMed] [Google Scholar]
- [79].Xiao S, Brooks CR, Sobel RA, Kuchroo VK, Tim-1 is essential for induction and maintenance of IL-10 in regulatory B cells and their regulation of tissue inflammation, J Immunol. 194 (2015) 1602–1608. 10.4049/jimmunol.1402632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Xiao S, Bod L, Pochet N, Kota SB, Hu D, Madi A, Kilpatrick J, Shi J, Ho A, Zhang H, Sobel R, Weiner HL, Strom TB, Quintana FJ, Joller N, Kuchroo VK, Checkpoint Receptor TIGIT Expressed on Tim-1 + B Cells Regulates Tissue Inflammation, Cell Rep. 32 (2020) 107892. 10.1016/j.celrep.2020.107892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Cherukuri A, Mohib K, Rothstein DM, Regulatory B cells: TIM-1, transplant tolerance, and rejection, Immunol Rev. 299 (2021) 31–44. 10.1111/imr.12933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Yeung MY, Ding Q, Brooks CR, Xiao S, Workman CJ, Vignali DAA, Ueno T, Padera RF, Kuchroo VK, Najafian N, Rothstein DM, TIM-1 signaling is required for Maintenance and Induction of regulatory B cells, Am J Transplant. 15 (2015) 942–953. 10.1111/ajt.13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Shi J-Y, Gao Q, Wang Z-C, Zhou J, Wang X-Y, Min Z-H, Shi Y-H, Shi G-M, Ding Z-B, Ke A-W, Dai Z, Qiu S-J, Song K, Fan J, Margin-infiltrating CD20(+) B cells display an atypical memory phenotype and correlate with favorable prognosis in hepatocellular carcinoma, Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 19 (2013) 5994–6005. 10.1158/1078-0432.CCR-12-3497. [DOI] [PubMed] [Google Scholar]
- [84].Arkatkar T, Du SW, Jacobs HM, Dam EM, Hou B, Buckner JH, Rawlings DJ, Jackson SW, B cell-derived IL-6 initiates spontaneous germinal center formation during systemic autoimmunity, J Exp Med. 214 (2017) 3207–3217. 10.1084/jem.20170580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Taniguchi K, Karin M, IL-6 and related cytokines as the critical lynchpins between inflammation and cancer, Semin Immunol. 26 (2014) 54–74. 10.1016/j.smim.2014.01.001. [DOI] [PubMed] [Google Scholar]
- [86].Zhou F, Molecular Mechanisms of IFN-γ to Up-Regulate MHC Class I Antigen Processing and Presentation, International Reviews of Immunology. 28 (2009) 239–260. 10.1080/08830180902978120. [DOI] [PubMed] [Google Scholar]
- [87].Chiaruttini G, Mele S, Opzoomer J, Crescioli S, Ilieva KM, Lacy KE, Karagiannis SN, B cells and the humoral response in melanoma: The overlooked players of the tumor microenvironment, Oncoimmunology. 6 (2017) e1294296. 10.1080/2162402X.2017.1294296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Reuschenbach M, von Knebel Doeberitz M, Wentzensen N, A systematic review of humoral immune responses against tumor antigens, Cancer Immunol Immunother. 58 (2009) 1535. 10.1007/s00262-009-0733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Tan T-T, Coussens LM, Humoral immunity, inflammation and cancer, Current Opinion in Immunology. 19 (2007)209–216. 10.1016/j.coi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- [90].Higgins BW, McHeyzer-Williams LJ, McHeyzer-Williams MG, Programming Isotype-Specific Plasma Cell Function, Trends in Immunology. 40 (2019) 345–357. 10.1016/j.it.2019.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Vidarsson G, Dekkers G, Rispens T, IgG subclasses and allotypes: from structure to effector functions, Frontiers in Immunology. 5 (2014) 520. 10.3389/fimmu.2014.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Peppas I, George G, Sollie S, Josephs DH, Hammar N, Walldius G, Karagiannis SN, Hemelrijck MV, Association of Serum Immunoglobulin Levels with Solid Cancer: A Systematic Review and Meta-analysis, Cancer Epidemiol Biomarkers Prev. 29 (2020) 527–538. 10.1158/1055-9965.EPI-19-0953. [DOI] [PubMed] [Google Scholar]
- [93].Gonzalez-Quintela A, Alende R, Gude F, Campos J, Rey J, Meijide LM, Fernandez-Merino C, Vidal C, Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities, Clinical & Experimental Immunology. 151 (2008) 42–50. 10.1111/j.1365-2249.2007.03545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Schauenstein E, Lahousen M, Weblacher M, Steinschifter W, Estelberger W, Schauenstein K, Selective decrease in serum immunoglobulin G1. A tissue nonspecific tumor marker detecting early stages of gynecologic malignant disease with high efficiency, Cancer. 78 (1996) 511–516. . [DOI] [PubMed] [Google Scholar]
- [95].Schauenstein E, Rabl H, Steinschifter W, Hirschmann C, Estelberger W, Schauenstein K, Selective decrease of serum immunoglobulin G1 as a marker of malignant transformation in colorectal tissue, Cancer. 79 (1997) 1482–1486. [PubMed] [Google Scholar]
- [96].Anderhuber W, Steinschifter W, Schauenstein E, Gotschuli A, Habermann W, Fischer M, Felsner P, Schauenstein K, The IgG1/G2 subclass shift - a sensitive, tissue non-specific marker for malignancy. Diagnostic performance with squamous cell carcinoma of the head and neck, British Journal of Cancer. 79 (1999) 1777–1781. 10.1038/sj.bjc.6690283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Saito H, Miyatani K, Kono Y, Murakami Y, Kuroda H, Matsunaga T, Fukumoto Y, Takano S, Osaki T, Fujiwara Y, Decreased Serum Concentration of Total IgG Is Related to Tumor Progression in Gastric Cancer Patients, Yonago Acta Med. 60 (2017) 119–125. [PMC free article] [PubMed] [Google Scholar]
- [98].Wu J, Ma X-L, Tian L, Zhang C-Y, Wang B-L, Hu Y-Y, Gao X-H, Shen M-N, Peng Y-F, Pan B-S, Zhou J, Fan J, Yang X-R, Guo W, Serum IgG4:IgG Ratio Predicts Recurrence of Patients with Hepatocellular Carcinoma after Curative Resection, Journal of Cancer. 8 (2017) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Karagiannis P, Gilbert AE, Josephs DH, AN N, Dodev T, Saul L, Correa I, Roberts L, Beddowes E, Koers A, Hobbs C, Ferreira S, Geh JLC, Healy C, Harries M, Acland KM, Blower PJ, Mitchell T, Fear DJ, Spicer JF, Lacy KE, Nestle FO, Karagiannis SN, IgG4 subclass antibodies impair antitumor immunity in melanoma, The Journal of Clinical Investigation. 123 (2013) 1457–1474. 10.1172/JCI65579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Nimmerjahn F, Ravetch JV, Divergent Immunoglobulin G Subclass Activity Through Selective Fc Receptor Binding, Science. 310 (2005) 1510–1512. 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- [101].Bruhns P, Jönsson F, Mouse and human FcR effector functions, Immunological Reviews. 268 (2015) 25–51. 10.1111/imr.12350. [DOI] [PubMed] [Google Scholar]
- [102].Bruhns P, lannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, Daëron M, Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses, Blood. 113 (2009) 3716–3725. 10.1182/blood-2008-09-179754. [DOI] [PubMed] [Google Scholar]
- [103].Tao MH, Smith RI, Morrison SL, Structural features of human immunoglobulin G that determine isotype specific differences in complement activation., J Exp Med. 178 (1993) 661–667. 10.1084/jem.178.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Klaus GG, Pepys MB, Kitajima K, Askonas BA, Activation of mouse complement by different classes of mouse antibody., Immunology. 38 (1979) 687–695. [PMC free article] [PubMed] [Google Scholar]
- [105].Hamanaka Y, Suehiro Y, Fukui M, Shikichi K, Imai K, Hinoda Y, Circulating anti-MUC1 IgG antibodies as a favorable prognostic factor for pancreatic cancer, International Journal of Cancer. 103 (2003) 97–100. 10.1002/ijc.10801. [DOI] [PubMed] [Google Scholar]
- [106].Fremd C, Stefanovic S, Beckhove P, Pritsch M, Lim H, Wallwiener M, Heil J, Golatta M, Rom J, Sohn C, Schneeweiss A, Schuetz F, Domschke C, Mucin 1-specific B cell immune responses and their impact on overall survival in breast cancer patients, Oncolmmunology. 5 (2016) e1057387. 10.1080/2162402X.2015.1057387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Harada K, Nakanuma Y, Cholangiocarcinoma with respect to IgG4 Reaction, International Journal of Hepatology. 2014 (2014) 803876. 10.1155/2014/803876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Neut Kolfschoten M van der, Schuurman J, Losen M, Bleeker WK, Martínez-Martínez P, Vermeulen E, den Bleker TH, Wiegman L, Vink T, Aarden LA, De Baets MH, van de Winkel JGJ, Aalberse RC, Parren PWHI, Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange, Science (New York, N.Y.). 317 (2007) 1554–1557. 10.1126/science.1144603. [DOI] [PubMed] [Google Scholar]
- [109].Barbera-Guillem E, May KF, Nyhus JK, Nelson MB, Promotion of tumor invasion by cooperation of granulocytes and macrophages activated by anti-tumor antibodies, Neoplasia (New York, N.Y.). 1 (1999) 453–460. 10.1038/sj.neo.7900054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Andreu P, Johansson M, Affara NI, Pucci F, Tan T, Junankar S, Korets L, Lam J, Tawfik D, DeNardo DG, Naldini L, de Visser KE, De Palma M, Coussens LM, FcRgamma activation regulates inflammation-associated squamous carcinogenesis, Cancer Cell. 17 (2010) 121–134. 10.1016/j.ccr.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].de Visser KE, Korets LV, Coussens LM, De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent, Cancer Cell. 7 (2005)411–423. 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- [112].Macpherson AJ, Yilmaz B, Limenitakis JP, Ganal-Vonarburg SC, IgA Function in Relation to the Intestinal Microbiota, Annual Review of Immunology. 36 (2018) 359–381. 10.1146/annurev-immunol-042617-053238. [DOI] [PubMed] [Google Scholar]
- [113].Cerutti A, The regulation of IgA class switching, Nat Rev Immunol. 8 (2008) 421–434. 10.1038/nri2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Fagarasan S, Kinoshita K, Muramatsu M, Ikuta K, Honjo T, In situ class switching and differentiation to IgA-producing cells in the gut lamina propria, Nature. 413 (2001)639–643. 10.1038/35098100. [DOI] [PubMed] [Google Scholar]
- [115].Stavnezer J, Kang J, The surprising discovery that TGF beta specifically induces the IgA class switch, Journal of Immunology (Baltimore, Md.: 1950). 182 (2009) 5–7. 10.4049/jimmunol.182.1.5. [DOI] [PubMed] [Google Scholar]
- [116].Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO, A dominant, coordinated T regulatory cell-lgA response to the intestinal microbiota, Proceedings of the National Academy of Sciences of the United States of America. 106 (2009) 19256–19261. 10.1073/pnas.0812681106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Mantis NJ, Rol N, Corthésy B, Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut, Mucosal Immunology. 4(2011)603–611. 10.1038/mi.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Tezuka H, Abe Y, Iwata M, Takeuchi H, Ishikawa H, Matsushita M, Shiohara T, Akira S, Ohteki T, Regulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cells, Nature. 448 (2007) 929–933. 10.1038/nature06033. [DOI] [PubMed] [Google Scholar]
- [119].Nakajima A, Vogelzang A, Maruya M, Miyajima M, Murata M, Son A, Kuwahara T, Tsuruyama T, Yamada S, Matsuura M, Nakase H, Peterson DA, Fagarasan S, Suzuki K, IgA regulates the composition and metabolic function of gut microbiota by promoting symbiosis between bacteria, Journal of Experimental Medicine. 215 (2018)2019–2034. 10.1084/jem.20180427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Kawamoto S, Maruya M, Kato LM, Suda W, Atarashi K, Doi Y, Tsutsui Y, Qin H, Honda K, Okada T, Hattori M, Fagarasan S, Foxp3(+) T cells regulate immunoglobulin a selection and facilitate diversification of bacterial species responsible for immune homeostasis, Immunity. 41 (2014) 152–165. 10.1016/j.immuni.2014.05.016. [DOI] [PubMed] [Google Scholar]
- [121].Ruane D, Chorny A, Lee H, Faith J, Pandey G, Shan M, Simchoni N, Rahman A, Garg A, Weinstein EG, Oropallo M, Gaylord M, Ungaro R, Cunningham-Rundles C, Alexandropoulos K, Mucida D, Merad M, Cerutti A, Mehandru S, Microbiota regulate the ability of lung dendritic cells to induce IgA class-switch recombination and generate protective gastrointestinal immune responses, J Exp Med. 213 (2016) 53–73. 10.1084/jem.20150567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Audiger C, Rahman MJ, Yun TJ, Tarbell KV, Lesage S, The Importance of Dendritic Cells in Maintaining Immune Tolerance, J Immunol. 198 (2017)2223–2231. 10.4049/jimmunol.1601629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, Cogdill AP, Zhao L, Hudgens CW, Hutchinson DS, Manzo T, de Macedo MP, Cotechini T, Kumar T, Chen WS, Reddy SM, Sloane RS, Galloway-Pena J, Jiang H, Chen PL, Shpall EJ, Rezvani K, Alousi AM, Chemaly RF, Shelburne S, Vence LM, Okhuysen PC, Jensen VB, Swennes AG, McAllister F, Sanchez EMR, Zhang Y, Chatelier EL, Zitvogel L, Pons N, Austin-Breneman JL, Haydu LE, Burton EM, Gardner JM, Sirmans E, Hu J, Lazar AJ, Tsujikawa T, Diab A, Tawbi H, Glitza IC, Hwu WJ, Patel SP, Woodman SE, Amaria RN, Davies MA, Gershenwald JE, Hwu P, Lee JE, Zhang J, Coussens LM, Cooper ZA, Futreal PA, Daniel CR, Ajami NJ, Petrosino JF, Tetzlaff MT, Sharma P, Allison JP, Jenq RR, Wargo JA, Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients, Science. 359 (2018) 97–103. 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Kalaora S, Nagler A, Nejman D, Alon M, Barbolin C, Barnea E, Ketelaars SLC, Cheng K, Vervier K, Shental N, Bussi Y, Rotkopf R, Levy R, Benedek G, Trabish S, Dadosh T, Levin-Zaidman S, Geller LT, Wang K, Greenberg P, Yagel G, Peri A, Fuks G, Bhardwaj N, Reuben A, Hermida L, Johnson SB, Galloway-Pena JR, Shropshire WC, Bernatchez C, Haymaker C, Arora R, Roitman L, Eilam R, Weinberger A, Lotan-Pompan M, Lotem M, Admon A, Levin Y, Lawley TD, Adams DJ, Levesque MP, Besser MJ, Schachter J, Golani O, Segal E, Geva-Zatorsky N, Ruppin E, Kvistborg P, Peterson SN, Wargo JA, Straussman R, Samuels Y, Identification of bacteria-derived HLA-bound peptides in melanoma, Nature. 592 (2021) 138–143. 10.1038/s41586-021-03368-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Helmink BA, Khan MAW, Hermann A, Gopalakrishnan V, Wargo JA, The microbiome, cancer, and cancer therapy, Nature Medicine. 25 (2019)377–388. 10.1038/s41591-019-0377-7. [DOI] [PubMed] [Google Scholar]
- [126].Sims TT, El Alam MB, Karpinets TV, Dorta-Estremera S, Hegde VL, Nookala S, Yoshida-Court K, Wu X, Biegert GWG, Delgado Medrano AY, Solley T, Ahmed-Kaddar M, Chapman BV, Sastry KJ, Mezzari MP, Petrosino JF, Lin LL, Ramondetta L, Jhingran A, Schmeler KM, Ajami NJ, Wargo J, Colbert LE, Klopp AH, Gut microbiome diversity is an independent predictor of survival in cervical cancer patients receiving chemoradiation, Communications Biology. 4 (2021) 1–10. 10.1038/s42003-021-01741-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Malik A, Sharma D, Zhu Q, Karki R, Guy CS, Vogel P, Kanneganti T-D, IL-33 regulates the IgA-microbiota axis to restrain IL-1α-dependent colitis and tumorigenesis, The Journal of Clinical Investigation. 126 (2016)4469–4481. 10.1172/JCI88625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu G-Y, Osterreicher CH, Hung KE, Datz C, Feng Y, Fearon ER, Oukka M, Tessarollo L, Coppola V, Yarovinsky F, Cheroutre H, Eckmann L, Trinchieri G, Karin M, Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth, Nature. 491 (2012)254–258. 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Fritz JH, Rojas OL, Simard N, McCarthy DD, Hapfelmeier S, Rubino S, Robertson SJ, Larijani M, Gosselin J, Ivanov II, Martin A, Casellas R, Philpott DJ, Girardin SE, McCoy KD, Macpherson AJ, Paige CJ, Gommerman JL, Acquisition of a multifunctional IgA + plasma cell phenotype in the gut, Nature. 481 (2012) 199–203. 10.1038/nature10698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Dedobbeleer O, Stockis J, van der Woning B, Coulie PG, Lucas S, Cutting Edge: Active TGF-β1 Released from GARP/TGF-β1 Complexes on the Surface of Stimulated Human B Lymphocytes Increases Class-Switch Recombination and Production of IgA, J Immunol. 199 (2017) 391–396. 10.4049/jimmunol.1601882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Konkel JE, Chen W, Balancing acts: the role of TGF-β in the mucosal immune system, Trends Mol Med. 17 (2011)668–676. 10.1016/j.molmed.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Mkaddem SB, Christou I, Rossato E, Berthelot L, Lehuen A, Monteiro RC, IgA, IgA Receptors, and Their Anti-inflammatory Properties, in: Daeron M, Nimmerjahn F (Eds.), Fc Receptors, Springer International Publishing, Cham, 2014: pp. 221–235. 10.1007/978-3-319-07911-0_10. [DOI] [PubMed] [Google Scholar]
- [133].Ben Mkaddem S, Rossato E, Heming N, Monteiro RC, Anti-inflammatory role of the IgA Fc receptor (CD89): From autoimmunity to therapeutic perspectives, Autoimmunity Reviews. 12 (2013) 666–669. 10.1016/j.autrev.2012.10.011. [DOI] [PubMed] [Google Scholar]
- [134].Pilette C, Detry B, Guisset A, Gabriels J, Sibille Y, Induction of interleukin-10 expression through Fca receptor in human monocytes and monocyte-derived dendritic cells: role of p38 MAPKinase, Immunology & Cell Biology. 88 (2010) 486–493. 10.1038/icb.2009.120. [DOI] [PubMed] [Google Scholar]
- [135].Saha C, Das M, Patil V, Stephen-Victor E, Sharma M, Wymann S, Jordi M, Vonarburg C, Kaveri SV, Bayry J, Monomeric Immunoglobulin A from Plasma Inhibits Human Th17 Responses In Vitro Independent of FcαRI and DC-SIGN, Front. Immunol 8 (2017). 10.3389/fimmu.2017.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Xu X, Meng Q, Erben U, Wang P, Glauben R, KUhl AA, Wu H, Ma CW, Hu M, Wang Y, Sun W, Jia J, Wu X, Chen W, Siegmund B, Qin Z, Myeloid-derived suppressor cells promote B-cell production of IgA in a TNFR2-dependent manner, Cellular & Molecular Immunology. 14 (2017) 597–606. 10.1038/cmi.2015.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Zhang J, Hu X, Wang J, Sahu AD, Cohen D, Song L, Ouyang Z, Fan J, Wang B, Fu J, Gu S, Sade-Feldman M, Hacohen N, Li W, Ying X, Li B, Liu XS, Immune receptor repertoires in pediatric and adult acute myeloid leukemia, Genome Medicine. 11 (2019) 73. 10.1186/s13073-019-0681-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Welinder C, Jirström K, Lehn S, Nodin B, Marko-Varga G, Blixt O, Danielsson L, Jansson B, Intratumour IgA1 is common in cancer and is correlated with poor prognosis in bladder cancer, Heliyon. 2 (2016) e00143. 10.1016/j.heliyon.2016.e00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Fedirko V, Tran HQ, Gewirtz AT, Stepien M, Trichopoulou A, Aleksandrova K, Olsen A, Tjønneland A, Overvad K, Carbonnel F, Boutron-Ruault M-C, Severi G, Kühn T, Kaaks R, Boeing H, Bamia C, Lagiou P, Grioni S, Panico S, Palli D, Tumino R, Naccarati A, Peeters PH, Bueno-de-Mesquita HB, Weiderpass E, Castaño JMH, Barricade A, Sánchez M-J, Dorronsoro M, Quirós JR, Agudo A, Sjöberg K, Ohlsson B, Hemmingsson O, Werner M, Bradbury KE, Khaw K-T, Wareham N, Tsilidis KK, Aune D, Scalbert A, Romieu I, Riboli E, Jenab M, Exposure to bacterial products lipopolysaccharide and flagellin and hepatocellular carcinoma: a nested case-control study, BMC Medicine. 15 (2017). 10.1186/s12916-017-0830-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Lo Nigro C, Macagno M, Sangiolo D, Bertolaccini L, Aglietta M, Merlano MC, NK-mediated antibody-dependent cell-mediated cytotoxicity in solid tumors: biological evidence and clinical perspectives, Ann Transl Med. 7(2019). 10.21037/atm.2019.01.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Wang W, Erbe AK, Hank JA, Morris ZS, Sondel PM, NK Cell-Mediated Antibody-Dependent Cellular Cytotoxicity in Cancer Immunotherapy, Front. Immunol 6 (2015). 10.3389/fimmu.2015.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Kurai J, Chikumi H, Hashimoto K, Yamaguchi K, Yamasaki A, Sako T, Touge H, Makino H, Takata M, Miyata M, Nakamoto M, Burioka N, Shimizu E, Antibody-dependent cellular cytotoxicity mediated by cetuximab against lung cancer cell lines, Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 13 (2007) 1552–1561. 10.1158/1078-0432.CCR-06-1726. [DOI] [PubMed] [Google Scholar]
- [143].Dai L, Tsay J-CJ, Li J, Yie T-A, Munger JS, Pass H, Rom WN, Zhang Y, Tan EM, Zhang J-Y, Autoantibodies against tumor-associated antigens in the early detection of lung cancer, Lung Cancer (Amsterdam, Netherlands). 99 (2016) 172–179. 10.1016/j.lungcan.2016.07.018. [DOI] [PubMed] [Google Scholar]
- [144].Wieland A, Shashidharamurthy R, Kamphorst AO, Han J-H, Aubert RD, Choudhury BP, Stowell SR, Lee J, Punkosdy GA, Shlomchik MJ, Selvaraj P, Ahmed R, Antibody effector functions mediated by Fcγ-receptors are compromised during persistent viral infection, Immunity. 42 (2015) 367–378. 10.1016/j.immuni.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Carroll MC, Complement and Humoral Immunity, Vaccine. 26 (2008) I28–I33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Reis ES, Mastellos DC, Ricklin D, Mantovani A, Lambris JD, Complement in cancer: untangling an intricate relationship, Nature Reviews Immunology. 18 (2018) 5–18. 10.1038/nri.2017.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Taylor RP, Lindorfer MA, Cytotoxic mechanisms of immunotherapy: Harnessing complement in the action of anti-tumor monoclonal antibodies, Seminars in Immunology. 28 (2016) 309–316. 10.1016/j.smim.2016.03.003. [DOI] [PubMed] [Google Scholar]
- [148].Derer S, Beurskens FJ, Rosner T, Peipp M, Valerius T, Complement in antibody-based tumor therapy, Critical Reviews in Immunology. 34 (2014) 199–214. 10.1615/critrevimmunol.2014009761. [DOI] [PubMed] [Google Scholar]
- [149].Lu Y, Zhao Q, Liao J-Y, Song E, Xia Q, Pan J, Li Y, Li J, Zhou B, Ye Y, Di C, Yu S, Zeng Y, Su S, Complement Signals Determine Opposite Effects of B Cells in Chemotherapy-Induced Immunity, Cell. 180 (2020) 1081–1097.e24. 10.1016/j.cell.2020.02.015. [DOI] [PubMed] [Google Scholar]
- [150].Yamano T, Nedjic J, Hinterberger M, Steinert M, Koser S, Pinto S, Gerdes N, Lutgens E, Ishimaru N, Busslinger M, Brors B, Kyewski B, Klein L, Thymic B Cells Are Licensed to Present Self Antigens for Central T Cell Tolerance Induction, Immunity. 42 (2015) 1048–1061. 10.1016/j.immuni.2015.05.013. [DOI] [PubMed] [Google Scholar]
- [151].Lu F-T, Yang W, Wang Y-H, Ma H-D, Tang W, Yang J-B, Li L, Ansari AA, Lian Z-X, Thymic B cells promote thymus-derived regulatory T cell development and proliferation, Journal of Autoimmunity. 61 (2015) 62–72. 10.1016/j.jaut.2015.05.008. [DOI] [PubMed] [Google Scholar]
- [152].Perera J, Huang H, The development and function of thymic B cells, Cell Mol Life Sci. 72 (2015) 2657–2663. 10.1007/S00018-015-1895-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Adler LN, Jiang W, Bhamidipati K, Millican M, Macaubas C, Hung S, Mellins ED, The Other Function: Class ll-Restricted Antigen Presentation by B Cells, Front. Immunol 8 (2017). 10.3389/fimmu.2017.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Constant SL, B Lymphocytes as Antigen-Presenting Cells for CD4+ T Cell Priming In Vivo, The Journal of Immunology. 162 (1999) 5695–5703. [PubMed] [Google Scholar]
- [155].Ron Y, Sprent J, T cell priming in vivo: a major role for B cells in presenting antigen to T cells in lymph nodes., The Journal of Immunology. 138 (1987) 2848–2856. [PubMed] [Google Scholar]
- [156].Rivera A, Chen CC, Ron N, Dougherty JP, Ron Y, Role of B cells as antigen-presenting cells in vivo revisited: antigen-specific B cells are essential for T cell expansion in lymph nodes and for systemic T cell responses to low antigen concentrations, International Immunology. 13 (2001) 1583–1593. 10.1093/intimm/13.12.1583. [DOI] [PubMed] [Google Scholar]
- [157].Rubtsov AV, Rubtsova K, Kappler JW, Jacobelli J, Friedman RS, Marrack P, CD11c-Expressing B Cells Are Located at the T Cell/B Cell Border in Spleen and Are Potent APCs, Journal of Immunology (Baltimore, Md.: 1950). 195 (2015) 71–79. 10.4049/jimmunol.1500055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Nielsen JS, Sahota RA, Milne K, Kost SE, Nesslinger NJ, Watson PH, Nelson BH, CD20+ tumor-infiltrating lymphocytes have an atypical CD27-memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer, Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 18 (2012) 3281–3292. 10.1158/1078-0432.CCR-12-0234. [DOI] [PubMed] [Google Scholar]
- [159].Bruno TC, Ebner PJ, Moore BL, Squalls OG, Waugh KA, Eruslanov EB, Singhal S, Mitchell JD, Franklin WA, Merrick DT, McCarter MD, Palmer BE, Kern JA, Slansky JE, Antigen-Presenting Intratumoral B Cells Affect CD4+ TIL Phenotypes in Non-Small Cell Lung Cancer Patients, Cancer Immunology Research. 5 (2017) 898–907. 10.1158/2326-6066.CIR-17-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Rossetti RAM, Lorenzi NPC, Yokochi K, F. Rosa MBS de, Benevides L, Margarido PFR, Baracat EC, Carvalho JP, Villa LL, Lepique AP, B lymphocytes can be activated to act as antigen presenting cells to promote anti-tumor responses, PloS One. 13 (2018) e0199034. 10.1371/journal.pone.0199034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Phan TG, Grigorova I, Okada T, Cyster JG, Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells, Nature Immunology. 8 (2007) 992–1000. 10.1038/ni1494. [DOI] [PubMed] [Google Scholar]
- [162].Carmi Y, Spitzer MH, Linde IL, Burt BM, Prestwood TR, Perlman N, Davidson MG, Kenkel JA, Segal E, Pusapati GV, Bhattacharya N, Engleman EG, Allogeneic IgG combined with dendritic cell stimuli induce antitumour T-cell immunity, Nature. 521 (2015) 99–104. 10.1038/nature14424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Baker K, Rath T, Flak MB, Arthur JC, Chen Z, Glickman JN, Zlobec I, Karamitopoulou E, Stachler MD, Odze RD, Lencer WI, Jobin C, Blumberg RS, Neonatal Fc receptor expression in dendritic cells mediates protective immunity against colorectal cancer, Immunity. 39 (2013) 1095–1107. 10.1016/j.immuni.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Rafiq K, Bergtold A, Clynes R, Immune complex-mediated antigen presentation induces tumor immunity, The Journal of Clinical Investigation. 110 (2002) 71–79. 10.1172/JCI15640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Platzer B, Stout M, Fiebiger E, Antigen cross-presentation of immune complexes, Frontiers in Immunology. 5 (2014) 140. 10.3389/fimmu.2014.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Morrison SJ, Spradling AC, Stem cells and niches: mechanisms that promote stem cell maintenance throughout life, Cell. 132 (2008) 598–611. 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Melchers F, Checkpoints that control B cell development, J Clin Invest. 125 (2015) 2203–2210. 10.1172/JCI78083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Whitlock CA, Witte ON, Long-term culture of B lymphocytes and their precursors from murine bone marrow., Proc Natl Acad Sci U S A. 79 (1982) 3608–3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Fistonich C, Zehentmeier S, Bednarski JJ, Miao R, Schjerven H, Sleckman BP, Pereira JP, Cell circuits between B cell progenitors and IL-7+ mesenchymal progenitor cells control B cell development, J Exp Med. 215 (2018)2586–2599. 10.1084/jem.20180778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Barinov A, Luo L, Gasse P, Meas-Yedid V, Donnadieu E, Arenzana-Seisdedos F, Vieira P, Essential role of immobilized chemokine CXCL12 in the regulation of the humoral immune response, Proc Natl Acad Sci U S A. 114 (2017) 2319–2324. 10.1073/pnas.1611958114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Tokoyoda K, Egawa T, Sugiyama T, Choi B-I, Nagasawa T, Cellular Niches Controlling B Lymphocyte Behavior within Bone Marrow during Development, Immunity. 20 (2004) 707–718. 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- [172].Nagasawa T, Microenvironmental niches in the bone marrow required for B-cell development, Nat Rev Immunol. 6 (2006) 107–116. 10.1038/nri1780. [DOI] [PubMed] [Google Scholar]
- [173].Moser K, Tokoyoda K, Radbruch A, MacLennan I, Manz RA, Stromal niches, plasma cell differentiation and survival, Curr Opin Immunol. 18 (2006)265–270. 10.1016/j.coi.2006.03.004. [DOI] [PubMed] [Google Scholar]
- [174].Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA, Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion, Cell. 121 (2005) 335–348. 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- [175].Bhowmick NA, Neilson EG, Moses HL, Stromal fibroblasts in cancer initiation and progression, Nature. 432 (2004) 332–337. 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Kazanietz MG, Durando M, Cooke M, CXCL13 and Its Receptor CXCR5 in Cancer: Inflammation, Immune Response, and Beyond, Front. Endocrinol 10 (2019). 10.3389/fendo.2019.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Cosgrove J, Novkovic M, Albrecht S, Pikor NB, Zhou Z, Onder L, Mörbe U, Cupovic J, Miller H, Alden K, Thuery A, O’Toole P, Pinter R, Jarrett S, Taylor E, Venetz D, Heller M, Uguccioni M, Legler DF, Lacey CJ, Coatesworth A, Polak WG, Cupedo T, Manoury B, Thelen M, Stein JV, Wolf M, Leake MC, Timmis J, Ludewig B, Coles MC, B cell zone reticular cell microenvironments shape CXCL13 gradient formation, Nature Communications. 11 (2020) 3677. 10.1038/s41467-020-17135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P, Tumor-Associated Macrophages as Treatment Targets in Oncology, Nat Rev Clin Oncol. 14 (2017) 399–416. 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Shalapour S, Karin M, Pas de Deux: Control of Anti-Tumor Immunity by Cancer-Associated Inflammation, Immunity. 51 (2019) 15–26. 10.1016/j.immuni.2019.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Erez N, Truitt M, Olson P, Arron ST, Hanahan D, Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner, Cancer Cell. 17 (2010) 135–147. 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- [181].Vogelpoel LTC, Baeten DLP, de Jong EC, den Dunnen J, Control of cytokine production by human fc gamma receptors: implications for pathogen defense and autoimmunity, Front Immunol. 6 (2015) 79. 10.3389/fimmu.2015.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Carlsen HS, Baekkevold ES, Johansen F-E, Haraldsen G, Brandtzaeg P, B cell attracting chemokine 1 (CXCL13) and its receptor CXCR5 are expressed in normal and aberrant gut associated lymphoid tissue, Gut. 51 (2002) 364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Batlle E, Massagué J, Transforming Growth Factor-β Signaling in Immunity and Cancer, Immunity. 50 (2019) 924–940. 10.1016/j.immuni.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Lee K, Spata M, Bayne LJ, Buza EL, Durham AC, Allman D, Vonderheide RH, Simon MC, Hif1α deletion reveals pro-neoplastic function of B cells in pancreatic neoplasia, Cancer Discov. 6 (2016) 256–269. 10.1158/2159-8290.CD-15-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Tang H, Zhu M, Qiao J, Fu Y-X, Lymphotoxin signalling in tertiary lymphoid structures and immunotherapy, Cellular & Molecular Immunology. 14 (2017) 809–818. 10.1038/cmi.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Petitprez F, de Reyniès A, Keung EZ, Chen TW-W, Sun C-M, Calderaro J, Jeng Y-M, Hsiao L-P, Lacroix L, Bougoüin A, Moreira M, Lacroix G, Natario I, Adam J, Lucchesi C, Laizet YH, Toulmonde M, Burgess MA, Bolejack V, Reinke D, Wani KM, Wang W-L, Lazar AJ, Roland CL, Wargo JA, Italiano A, Sautès-Fridman C, Tawbi HA, Fridman WH, B cells are associated with survival and immunotherapy response in sarcoma, Nature. 577 (2020) 556–560. 10.1038/s41586-019-1906-8. [DOI] [PubMed] [Google Scholar]
- [187].Nagasaki T, Hara M, Nakanishi H, Takahashi H, Sato M, Takeyama H, Interleukin-6 released by colon cancer-associated fibroblasts is critical for tumour angiogenesis: anti-interleukin-6 receptor antibody suppressed angiogenesis and inhibited tumour-stroma interaction, Br J Cancer. 110 (2014) 469–478. 10.1038/bjc.2013.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Gu-Trantien C, Willard-Gallo K, Tumor-infiltrating follicular helper T cells, Oncoimmunology. 2 (2013). 10.4161/onci.26066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, Ravoet M, Le Buanec H, Sibille C, Manfouo-Foutsop G, Veys I, Haibe-Kains B, Singhal SK, Michiels S, Rothé F, Salgado R, Duvillier H, Ignatiadis M, Desmedt C, Bron D, Larsimont D, Piccart M, Sotiriou C, Willard-Gallo K, CD4+ follicular helper T cell infiltration predicts breast cancer survival, J Clin Invest. 123 (2013) 2873–2892. 10.1172/JCI67428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Nurieva RI, Liu Z, Gangadharan A, Bieerkehazhi S, Zhao Y-Z, Alekseev A, Sahoo A, Function of T follicular helper cells in anti-tumor immunity, The Journal of Immunology. 202 (2019) 138.18–138.18. [Google Scholar]
- [191].Hollern DP, Xu N, Thennavan A, Glodowski C, Garcia-Recio S, Mott KR, He X, Garay JP, Carey-Ewend K, Marron D, Ford J, Liu S, Vick SC, Martin M, Parker JS, Vincent BG, Serody JS, Perou CM, B Cells and T Follicular Helper Cells Mediate Response to Checkpoint Inhibitors in High Mutation Burden Mouse Models of Breast Cancer, Cell. 179 (2019) 1191–1206.e21. 10.1016/j.cell.2019.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Crotty S, T Follicular Helper Cell Biology: A Decade of Discovery and Diseases, Immunity. 50 (2019) 1132–1148. 10.1016/j.immuni.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Song H, Liu A, Liu G, Wu F, Li Z, T follicular regulatory cells suppress Tfh-mediated B cell help and synergistically increase IL-10-producing B cells in breast carcinoma, Immunol Res. 67 (2019) 416–423. 10.1007/si2026-019-09090-y. [DOI] [PubMed] [Google Scholar]
- [194].Gutzeit C, Magri G, Cerutti A, Intestinal IgA production and its role in host-microbe interaction, Immunological Reviews. 260 (2014)76–85. 10.1111/imr.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Shalapour S, Karin M, Cruel to Be Kind: Epithelial, Microbial, and Immune Cell Interactions in Gastrointestinal Cancers, Annu. Rev. Immunol 38 (2020) 649–671. 10.1146/annurev-immunol-082019-081656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Todoric J, Di Caro G, Reibe S, Henstridge DC, Green CR, Vrbanac A, Ceteci F, Conche C, McNulty R, Shalapour S, Taniguchi K, Meikle PJ, Watrous JD, Moranchel R, Najhawan M, Jain M, Liu X, Kisseleva T, Diaz-Meco MT, Moscat J, Knight R, Greten FR, Lau LF, Metallo CM, Febbraio MA, Karin M, Fructose stimulated de novo lipogenesis is promoted by inflammation, Nature Metabolism. (2020) 1–12. 10.1038/s42255-020-0261-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Fane M, Weeraratna AT, How the ageing microenvironment influences tumour progression, Nature Reviews Cancer. 20 (2020) 89–106. 10.1038/s41568-019-0222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Ma H-Y, Yamamoto G, Xu J, Liu X, Karin D, Kim JY, Alexandrov LB, Koyama Y, Nishio T, Benner C, Heinz S, Rosenthal SB, Liang S, Sun M, Karin G, Zhao P, Brodt P, Mckillop IH, Quehenberger O, Dennis E, Saltiel A, Tsukamoto H, Gao B, Karin M, Brenner DA, Kisseleva T, IL-17 signaling in steatotic hepatocytes and macrophages promotes hepatocellular carcinoma in alcohol-related liver disease, Journal of Hepatology. 72 (2020) 946–959. 10.1016/j.jhep.2019.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Kosaraju R, Guesdon W, Crouch MJ, Teague HL, Sullivan EM, Karlsson EA, Schultz-Cherry S, Gowdy K, Bridges LC, Reese LR, Neufer PD, Armstrong M, Reisdorph N, Milner JJ, Beck M, Shaikh SR, B Cell Activity Is Impaired in Human and Mouse Obesity and Is Responsive to an Essential Fatty Acid upon Murine Influenza Infection, The Journal of Immunology. 198 (2017) 4738–4752. 10.4049/jimmunol.1601031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Weisel FJ, Mullett SJ, Eisner RA, Menk AV, Trivedi N, Luo W, Wikenheiser D, Hawse WF, Chikina M, Smita S, Conter LJ, Joachim SM, Wendell SG, Jurczak MJ, Winkler TH, Delgoffe GM, Shlomchik MJ, Germinal center B cells selectively oxidize fatty acids for energy while conducting minimal glycolysis, Nature Immunology. 21 (2020) 331–342. 10.1038/s41590-020-0598-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Pasala S, Barr T, Messaoudi I, Impact of Alcohol Abuse on the Adaptive Immune System, Alcohol Res. 37 (2015) 185–197. [PMC free article] [PubMed] [Google Scholar]
- [202].Ma S, Wang C, Mao X, Hao Y, B Cell Dysfunction Associated With Aging and Autoimmune Diseases, Front. Immunol 10(2019). 10.3389/fimmu.2019.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Khan S, Chan YT, Revelo XS, Winer DA, The Immune Landscape of Visceral Adipose Tissue During Obesity and Aging, Frontiers in Endocrinology. 11 (2020). 10.3389/fendo.2020.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].McPherson S, Henderson E, Burt AD, Day CP, Anstee QM, Serum immunoglobulin levels predict fibrosis in patients with non-alcoholic fatty liver disease, Journal of Hepatology. 60 (2014) 1055–1062. 10.1016/j.jhep.2014.01.010. [DOI] [PubMed] [Google Scholar]
- [205].van de Wiel A, van Hattum J, Schuurman H-J, Kater L, Immunoglobulin A in the diagnosis of alcoholic liver disease, Gastroenterology. 94 (1988) 457–462. 10.1016/0016-5085(88)90437-4. [DOI] [PubMed] [Google Scholar]
- [206].Lindner C, Wahl B, Föhse L, Suerbaum S, Macpherson AJ, Prinz I, Pabst O, Age, microbiota, and T cells shape diverse individual IgA repertoires in the intestine, The Journal of Experimental Medicine. 209 (2012) 365. 10.1084/jem.20111980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Nagafusa H, Sayama K, Age-related chemokine alterations affect IgA secretion and gut immunity in female mice, Biogerontology. 21 (2020) 609–618. 10.1007/s10522-020-09877-9. [DOI] [PubMed] [Google Scholar]
- [208].H. T, O. G, W. J, J. Rr, Uncovering the role of the gut microbiota in immune checkpoint blockade therapy: A mini-review, Seminars in Hematology. 57 (2020). 10.1053/j.seminhematol.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Choi S-C, Morel L, Immune metabolism regulation of the germinal center response, Experimental & Molecular Medicine. 52 (2020) 348–355. 10.1038/s12276-020-0392-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Goeppert B, Frauenschuh L, Zucknick M, Stenzinger A, Andrulis M, Klauschen F, Joehrens K, Warth A, Renner M, Mehrabi A, Hafezi M, Thelen A, Schirmacher P, Weichert W, Prognostic impact of tumour-infiltrating immune cells on biliary tract cancer, British Journal of Cancer. 109 (2013) 2665–2674. 10.1038/bjc.2013.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Gu-Trantien C, Migliori E, Buisseret L, de Wind A, Brohée S, Garaud S, Noël G, Chi VLD, Lodewyckx J-N, Naveaux C, Duvillier H, Goriely S, Larsimont D, Willard-Gallo K, CXCL13-producing TFH cells link immune suppression and adaptive memory in human breast cancer, (2017). 10.1172/jci.insight.91487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Mahmoud SMA, Lee AHS, Paish EC, Macmillan RD, Ellis IO, Green AR, The prognostic significance of B lymphocytes in invasive carcinoma of the breast, Breast Cancer Res Treat. 132 (2012) 545–553. 10.1007/s10549-011-1620-1. [DOI] [PubMed] [Google Scholar]
- [213].Yeong J, Lim JCT, Lee B, Li H, Chia N, Ong CCH, Lye WK, Putti TC, Dent R, Lim E, Thike AA, Tan PH, Iqbal J, High Densities of Tumor-Associated Plasma Cells Predict Improved Prognosis in Triple Negative Breast Cancer, Front. Immunol 9(2018). 10.3389/fimmu.2018.01209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Ishigami E, Sakakibara M, Sakakibara J, Masuda T, Fujimoto H, Hayama S, Nagashima T, Sangai T, Nakagawa A, Nakatani Y, Otsuka M, Coexistence of regulatory B cells and regulatory T cells in tumor-infiltrating lymphocyte aggregates is a prognostic factor in patients with breast cancer, Breast Cancer. 26 (2019) 180–189. 10.1007/s12282-018-0910-4. [DOI] [PubMed] [Google Scholar]
- [215].Iglesia MD, Parker JS, Hoadley KA, Serody JS, Perou CM, Vincent BG, Genomic Analysis of Immune Cell Infiltrates Across 11 Tumor Types, J Natl Cancer Inst. 108 (2016). 10.1093/jnci/djw144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Liu X, Tsang JYS, Hlaing T, Hu J, Ni Y-B, Chan SK, Cheung SY, Tse GM, Distinct Tertiary Lymphoid Structure Associations and Their Prognostic Relevance in HER2 Positive and Negative Breast Cancers, The Oncologist. 22 (2017) 1316–1324. 10.1634/theoncologist.2017-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Martinet L, Garrido I, Girard J-P, Tumor high endothelial venules (HEVs) predict lymphocyte infiltration and favorable prognosis in breast cancer, Oncolmmunology. 1 (2012) 789–790. 10.4161/onci.19787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Martinet L, Garrido I, Filleron T, Guellec SL, Bellard E, Fournie J-J, Rochaix P, Girard J-P, Human Solid Tumors Contain High Endothelial Venules: Association with T-and B-Lymphocyte Infiltration and Favorable Prognosis in Breast Cancer, Cancer Res. 71 (2011) 5678–5687. 10.1158/0008-5472.CAN-11-0431. [DOI] [PubMed] [Google Scholar]
- [219].Lee M, Heo S-H, Song IH, Rajayi H, Park HS, Park IA, Kim Y-A, Lee H, Gong G, Lee HJ, Presence of tertiary lymphoid structures determines the level of tumor-infiltrating lymphocytes in primary breast cancer and metastasis, Modern Pathology. 32 (2019) 70–80. 10.1038/s41379-018-0113-8. [DOI] [PubMed] [Google Scholar]
- [220].Di Caro G, Bergomas F, Grizzi F, Doni A, Bianchi P, Malesci A, Laghi L, Allavena P, Mantovani A, Marchesi F, Occurrence of tertiary lymphoid tissue is associated with T-cell infiltration and predicts better prognosis in early-stage colorectal cancers, Clin Cancer Res. 20 (2014) 2147–2158. 10.1158/1078-0432.CCR-13-2590. [DOI] [PubMed] [Google Scholar]
- [221].Posch F, Silina K, Leibl S, Mündlein A, Moch H, Siebenhüner A, Samaras P, Riedl J, Stotz M, Szkandera J, Stöger H, Pichler M, Stupp R, van den Broek M, Schraml P, Gerger A, Petrausch U, Winder T, Maturation of tertiary lymphoid structures and recurrence of stage II and III colorectal cancer, Oncolmmunology. 7 (2018) e1378844. 10.1080/2162402X.2017.1378844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Berntsson J, Eberhard J, Nodin B, Leandersson K, Larsson AH, Jirström K, Expression of programmed cell death protein 1 (PD-1) and its ligand PD-L1 in colorectal cancer: Relationship with sidedness and prognosis, Oncoimmunology. 7 (2018). 10.1080/2162402X.2018.1465165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Edin S, Kaprio T, Hagström J, Larsson P, Mustonen H, Böckelman C, Strigård K, Gunnarsson U, Haglund C, Palmqvist R, The Prognostic Importance of CD20 + B lymphocytes in Colorectal Cancer and the Relation to Other Immune Cell subsets, Scientific Reports. 9 (2019) 19997. 10.1038/s41598-019-56441-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Jiang H, Kang B, Huang X, Yan Y, Wang S, Ye Y, Shen Z, Cancer IgG, a potential prognostic marker, Promotes colorectal cancer progression, Chin J Cancer Res. 31 (2019) 499–510. 10.21147/j.issn.1000-9604.2019.03.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Lin Q, Tao P, Wang J, Ma L, Jiang Q, Li J, Zhang G, Liu J, Zhang Y, Hou Y, Lu W, Xue R, Tong H, Tumor-associated tertiary lymphoid structure predicts postoperative outcomes in patients with primary gastrointestinal stromal tumors, Oncoimmunology. 9 (2020). 10.1080/2162402X.2020.1747339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Murakami Y, Saito H, Shimizu S, Kono Y, Shishido Y, Miyatani K, Matsunaga T, Fukumoto Y, Ashida K, Sakabe T, Nakayama Y, Fujiwara Y, Increased regulatory B cells are involved in immune evasion in patients with gastric cancer, Scientific Reports. 9 (2019) 13083. 10.1038/s41598-019-49581-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Yamakoshi Y, Tanaka H, Sakimura C, Deguchi S, Mori T, Tamura T, Toyokawa T, Muguruma K, Hirakawa K, Ohira M, Immunological potential of tertiary lymphoid structures surrounding the primary tumor in gastric cancer, International Journal of Oncology. 57 (2020) 171–182. 10.3892/ijo.2020.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Liu R-X, Wei Y, Zeng Q-H, Chan K-W, Xiao X, Zhao X-Y, Chen M-M, Ouyang F-Z, Chen D-P, Zheng L, Lao X-M, Kuang D-M, Chemokine (C-X-C motif) receptor 3-positive B cells link interleukin-17 inflammation to protumorigenic macrophage polarization in human hepatocellular carcinoma, Hepatology. 62 (2015) 1779–1790. 10.1002/hep.28020. [DOI] [PubMed] [Google Scholar]
- [229].Calderaro J, Petitprez F, Becht E, Laurent A, Hirsch TZ, Rousseau B, Luciani A, Amaddeo G, Derman J, Charpy C, Zucman-Rossi J, Fridman WH, Sautès-Fridman C, Intra-tumoral tertiary lymphoid structures are associated with a low risk of early recurrence of hepatocellular carcinoma, J Hepatol. 70 (2019) 58–65. 10.1016/j.jhep.2018.09.003. [DOI] [PubMed] [Google Scholar]
- [230].Zhang Z, Ma L, Goswami S, Ma J, Zheng B, Duan M, Liu L, Zhang L, Shi J, Dong L, Sun Y, Tian L, Gao Q, Zhang X, Landscape of infiltrating B cells and their clinical significance in human hepatocellular carcinoma, Oncolmmunology. 8 (2019) e1571388. 10.1080/2162402X.2019.1571388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].van Herpen CML, van der Voort R, van der Laak JAWM, Klasen IS, de Graaf AO, van Kempen LCL, de Vries IJM, Boer TD, Dolstra H, Torensma R, van Krieken JH, Adema GJ, Mulder PHMD, Intratumoral rhIL-12 administration in head and neck squamous cell carcinoma patients induces B cell activation, International Journal of Cancer. 123 (2008)2354–61. 10.1002/ijc.23756. [DOI] [PubMed] [Google Scholar]
- [232].Selitsky SR, Mose LE, Smith CC, Chai S, Hoadley KA, Dittmer DP, Moschos SJ, Parker JS, Vincent BG, Prognostic value of B cells in cutaneous melanoma, Genome Medicine. 11 (2019) 36. 10.1186/s13073-019-0647-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Garg K, Maurer M, Griss J, Brüggen M-C, Wolf IH, Wagner C, Willi N, Mertz KD, Wagner SN, Tumor-associated B cells in cutaneous primary melanoma and improved clinical outcome, Human Pathology. 54 (2016) 157–164. 10.1016/j.humpath.2016.03.022. [DOI] [PubMed] [Google Scholar]
- [234].Wirsing AM, Ervik IK, Seppola M, Uhlin-Hansen L, Steigen SE, Hadler-Olsen E, Presence of high-endothelial venules correlates with a favorable immune microenvironment in oral squamous cell carcinoma, Modern Pathology. 31 (2018)910–922. 10.1038/s41379-018-0019-5. [DOI] [PubMed] [Google Scholar]
- [235].Li K, Guo Q, Zhang X, Dong X, Liu W, Zhang A, Li Y, Yan J, Jia G, Zheng Z, Tang W, Pan L, An M, Zhang B, Liu S, Fu B, Oral cancer-associated tertiary lymphoid structures: gene expression profile and prognostic value, Clinical & Experimental Immunology. 199 (2020) 172–181. 10.1111/cei.13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Milne K, Köbel M, Kalloger SE, Barnes RO, Gao D, Gilks CB, Watson PH, Nelson BH, Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors, PloS One. 4 (2009) e6412. 10.1371/journal.pone.0006412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Montfort A, Pearce O, Maniati E, Vincent BG, Bixby L, Böhm S, Dowe T, Wilkes EH, Chakravarty P, Thompson R, Topping J, Cutillas PR, Lockley M, Serody JS, Capasso M, Balkwill FR, A Strong B-cell Response Is Part of the Immune Landscape in Human High-Grade Serous Ovarian Metastases, Clin Cancer Res. 23 (2017) 250–262. 10.1158/1078-0432.CCR-16-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Kuwabara S, Tsuchikawa T, Nakamura T, Hatanaka Y, Hatanaka KC, Sasaki K, Ono Μ, Umemoto K, Suzuki T, Sato O, Hane Y, Nakanishi Y, Asano T, Ebihara Y, Kurashima Y, Noji T, Murakami S, Okamura K, Shichinohe T, Hirano S, Prognostic relevance of tertiary lymphoid organs following neoadjuvant chemoradiotherapy in pancreatic ductal adenocarcinoma, Cancer Science. 110 (2019) 1853–1862. 10.1111/cas.14023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Hiraoka N, Ino Y, Yamazaki-ltoh R, Kanai Y, Kosuge T, Shimada K, Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic cancer, Br J Cancer. 112 (2015) 1782–1790. 10.1038/bjc.2015.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Remark R, Alifano M, Cremer I, Lupo A, Dieu-Nosjean M-C, Riquet M, Crozet L, Ouakrim H, Goc J, Cazes A, Fléjou J-F, Gibault L, Verkarre V, Régnard J-F, Pagès O-N, Oudard S, Mlecnik B, Sautès-Fridman C, Fridman W-H, Damotte D, Characteristics and Clinical Impacts of the Immune Environments in Colorectal and Renal Cell Carcinoma Lung Metastases: Influence of Tumor Origin, Clin Cancer Res. 19 (2013) 4079–4091. 10.1158/1078-0432.CCR-12-3847. [DOI] [PubMed] [Google Scholar]


