Abstract
Background:
Studies examining effects of prenatal polyunsaturated fatty acid (PUFA) intake on childhood asthma show mixed results. Inconsistencies may result from not accounting for important modifying factors such as maternal asthma or child sex. Objective: To examine whether associations between prenatal PUFA intake and childhood asthma are modified by prenatal active maternal asthma or child sex in 412 mother-child dyads.
Methods:
Energy-adjusted prenatal dietary and supplement intakes of omega-3 (n-3) and omega-6 (n-6) PUFAs were estimated using the Block98 Food Frequency Questionnaire, administered during pregnancy. Mothers reported asthma in children followed prospectively to 4.0±1.7 years. Generalized additive models with smooth terms for PUFA (n-3, n-6, n-6/n-3 ratio) effects were used to investigate associations between PUFAs and child asthma, without prespecifying the form of these relationships, including effect modification by active maternal asthma or child sex.
Results:
Among mothers (40% black, 31% Hispanic), 22% had active asthma in pregnancy; 17.5% of children developed asthma. Lower maternal n-3-PUFA intake was significantly associated with risk of childhood asthma (p-value(p)=0.03), in particular among children of mothers with active asthma and low n-3- PUFA intake(p=0.01). This inverse association was more apparent in girls (p=0.01) compared to boys (p=0.30), regardless of maternal asthma status. For n-6-PUFA and the n6/n3 ratio, there was a lower risk of childhood asthma in the mid-range of intake and increased risk at higher intake (n-6-PUFA p=0.10, n6/n3 ratio p=0.13).
Conclusion:
Consideration of factors that modify effects of prenatal PUFA intake on childhood asthma has implications for designing intervention strategies tailored to impact those at greatest risk.
Keywords: Polyunsaturated fatty acid, prenatal, maternal active asthma, childhood asthma, sex-specific effects, Developmental Origins of Health and Disease
INTRODUCTION
Asthma is a leading cause of morbidity across the lifecourse1, 2 and disproportionately affects children living in economically disadvantaged and underserved communities3. It is important to identify modifiable factors in critical windows of development to inform prevention strategies that can be targeted toward those most likely to benefit. Asthma has origins in utero4, and maternal intake of a range of micronutrients during fetal development is thought to play a role in early asthma programming5–8.
Maternal long-chain polyunsaturated fatty acid (PUFA) intake in pregnancy has been reported to have associations with child asthma, with plausible mechanisms including impacts on oxidative stress and immune disruption contributing to asthma programming (e.g., imbalance of Th1/Th2 responses). PUFAs are thought to influence asthma development and symptoms via effects on systemic inflammation, cytokine release, oxidation, and microbial composition9. The most widely studied PUFAs are omega-6 (e.g. n-6; linoleic acid) and omega-3 (e.g. n-3; alpha-linoleic acid). Omega-6 PUFAs, primarily derived from vegetable oils, are metabolized into arachidonic acid which is further metabolized into pro-inflammatory mediators (e.g., eicosanoids, thromboxanes, leukotrienes, prostaglandins). Omega-3 PUFAs, primarily derived from fish, seafood, nuts, seeds, and marine and plant oils, are the major dietary sources of eicosapentaenoic acid and docosahexaenoic acid which have anti-inflammatory properties10, 11. Over time, dietary patterns have changed, with an increase in intake of pro-inflammatory n-6 PUFAs relative to n-3 PUFAs12 from an equal balance (1:1) to as high as 30:1 in some Western cultures13. Changing patterns in PUFA intake have paralleled increases in asthma prevalence12, 14, 15.
Benefits of higher prenatal n-3 PUFAs on childhood asthma risk have been demonstrated in some observational11, 16, 17 and interventional studies18–20, however, findings are mixed, with others reporting borderline or null results21–23. Similarly, while some studies link increased prenatal n-6 PUFA intake to childhood asthma risk, results are inconsistent7, 11, 22, 23.
These disparate findings may in part be explained by lack of consideration of factors that modify the association between prenatal PUFA intake and offspring asthma24. It is established that children born to mothers with active asthma in pregnancy are at particular risk of developing asthma25–28, and that improved prenatal asthma management is protective29. However, no study has specifically considered active asthma in pregnancy as a modifier of the relationship between maternal PUFA intake and children’s asthma risk. In addition, sex-specific differences in fatty acid metabolism30 and in susceptibility to exposures in utero that impact asthma development7, 31–33, are documented.
The aim of these analyses was to examine whether associations between prenatal PUFA intake (n-3 PUFA, n-6 PUFA and n-6/n-3 ratio) and child asthma are modified by maternal prenatal active asthma or child sex. We hypothesized that lower prenatal n-3 PUFA intake and higher n-6 intake would be associated with childhood asthma and that these associations would be greatest in children born to mothers with prenatal active asthma. While we hypothesized sex-specific effects, we had no a priori hypothesis regarding whether boys or girls would be more impacted.
METHODS
Sample
Participants were from the PRogramming of Intergenerational Stress Mechanisms (PRISM) pregnancy cohort, designed to examine associations between stress and other environmental exposures on child development. This study recruited n=938 women receiving prenatal care from the Beth Israel Deaconess Medical Center and East Boston Neighborhood Health Center in Boston, MA (from March 2011-December 2013) and Mount Sinai Hospital in New York City, NY (from April 2013-March 2019). Eligibility criteria included English- or Spanish-speaking, ≥18 years of age, and single gestation pregnancy. Exclusions included maternal intake of ≥7 alcoholic drinks/week prior to pregnancy recognition or any after pregnancy recognition, and congenital abnormalities that could impact participation. Ascertainment of dietary data was added after study initiation. Analyses include n=412 mother-child dyads recruited at mean standard deviation (SD): 22.2±9.1 weeks gestation with data on prenatal PUFA intake, active maternal asthma in pregnancy and child asthma. Procedures were approved by the relevant institutions’ human studies committees; mothers provided written consent in their primary language.
Prenatal PUFA Intake
In the second trimester, women reported dietary and supplement intake over the prior three months via the semiquantitative Block98 Food Frequency Questionnaire (FFQ) administered in English and Spanish34, 35. The food list, based on the National Health and Nutrition Examination Survey III dietary recall, was modified to include a more extensive list of fish and seafood. For each food/beverage item, women were asked how often (rarely/never, daily, weekly, monthly) and how much (small, medium or large serving with portion size pictures provided) was consumed. Women also reported type and frequency of vitamins and other dietary supplements taken during pregnancy. Notably, US adults’ intake of omega-3 are higher from dietary supplements than from foods36. FFQ intake was validated in this sample using 24-hour recalls37.
FFQ data were processed as previously described37 and intake of n-6- and n-3-PUFA was calculated using methods described by Willett et al38. The usual dietary intake of PUFAs was estimated by using USDA food-composition tables. We calculated total n-6 PUFA by combining total daily intake of linoleic acid and arachidonic acid, and total marine-derived n-3 PUFAs by combining eicosopentanoic acid (EPA), docosapentanoic acid (DPA), and docosahexaenoic acid (DHA). We adjusted n-3 and n-6 PUFA levels for total energy intake using the residual method38. To use the residual method, we used expected dietary PUFA intake for the mean energy intake of the sample as a constant. We then added the mean dietary PUFA intake of the sample to the residual. The residual was derived from the regression of PUFA intake on total energy intake using the self-reported total energy intake38 derived from the FFQ. We then added supplemental PUFA intakes to energy-adjusted dietary PUFA intakes to provide total energy-adjusted intakes for n-3 and n-6 PUFAs. The n-6/n-3 ratio was calculated by dividing energy-adjusted n-6 PUFAs by energy-adjusted n-3 PUFAs.
Child asthma
Mothers were asked about their child’s respiratory health at follow-up visits through telephone and face-to-face interviews at approximately 4-month intervals for the first 30 months, then annually. Child’s asthma status was determined based on an affirmative response to: “Has your child ever had asthma [since birth]?”, “Has a doctor or other healthcare provider ever said that your baby had asthma”, “Does your baby currently take medicine for his/her asthma?” or “Has your baby been hospitalized overnight for asthma [since birth]?”.
Maternal Active Asthma in Pregnancy
Maternal asthma history was classified based on an affirmative response to “Have you ever had asthma?” and/or “Have you ever gone to an emergency room/urgent care clinic for treatment of an asthma attack?” or “Have you ever been hospitalized overnight for asthma?”. Maternal active asthma during pregnancy was defined as mothers with a history of asthma who answered yes to “Do you still have asthma?” during any prenatal visit, or those reporting at least one of the following within the past year: emergency visit or hospitalization for asthma, asthma symptoms (wheezing, shortness of breath, cough, chest tightness, nighttime awakenings due to asthma) and/or use of asthma medications (albuterol, inhaled corticosteroids, inhaled long-acting beta agonist-corticosteroid combination medications, oral steroids or leukotriene inhibitors).
Other Covariates
Mothers reported date of birth, race/ethnicity (white/Hispanic, black/black-Hispanic, Hispanic/non-black and other), education (low[high school or less] vs high[some college or above]) and pre-pregnancy height and weight; child sex was obtained from the medical record at birth. Pre-pregnancy body mass index (BMI) was calculated as weight(kg) divided by height(m) squared. Prenatal maternal smoking was affirmative if a mother reported smoking at any time during pregnancy in any prenatal visits and/or postnatal recalls. Prenatal secondhand smoke exposure was affirmative if a mother reported that she lived with any current smokers or had exposure to secondhand smoke for more than 1 hour per week at home, work or in restaurants during pregnancy.
Analysis
We examined the distribution of sample characteristics overall, by maternal active asthma status, and by child sex. Comparisons across groups were examined using the Wilcoxon rank sum and Chi-square tests for continuous and categorical variables, respectively. Given that extreme values can be more influential in GAMs than traditional regression models, extreme PUFA values (<1st percentile and >99th percentile) were winsorized a priori to reduce the impact of potentially spurious outliers while retaining the full sample size39. We determined the Spearman’s correlation coefficient for n-6 and n-3 PUFA intake. We assessed the exposure-response relationships between PUFA intake (n-3, n-6 and n-6/n-3 ratio, considered separately) and child asthma using generalized additive models (GAMs) with a logit link (i.e. additive logistic regression). We used smooth penalized spline terms for PUFA intake to assess for the (potential nonlinear form of these) associations40. For multivariable-adjusted models, we considered covariates linked to both PUFA intake and asthma in previous research but not on the causal pathway. We formulated a Directed Acyclic Graph (eFigure 1, generated using DAGitty v3.041, available at:http://www.dagitty.net/) to determine the minimal sufficient adjustment set; this included maternal age, race/ethnicity, education, and pre-pregnancy BMI. Prenatal tobacco smoke exposures were considered in sensitivity analyses. In addition, models for n-6 PUFAs are adjusted for n-3 PUFA intake and models for n-3 PUFAs are adjusted for n-6 PUFA intake in primary analyses. Since intakes of n-6 and n-3 PUFAs were correlated (Spearman correlation coefficient=0.77, p<0.001) which may lead to unstable estimates in GAMs due to co-linearity or concurvity, we conducted sensitivity analyses considering each micronutrient (n-6 and n-3 PUFAs) separately without adjusting for the other. To examine effect modification on the association between prenatal PUFA intake and child asthma, we then stratified models by maternal active asthma status (active asthma vs no asthma history or prior asthma history that was not clinically active during the index pregnancy) or child sex. Effect modification by maternal active asthma status or child sex was also examined using factor-smooth interaction GAMs. In secondary analyses, we stratified by the combination of maternal asthma and child sex. Analyses were conducted using SAS (version 9.4; SAS Institute, Inc., Cary, NC) and R (version 3.5.1, ‘mgcv’ package; The R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Table I delineates sample characteristics. Women were primarily ethnic minorities (40.1% black, 31.3% Hispanic), 32.8% reported a high school education or less, and 22.1% had active asthma during pregnancy. Mothers with active asthma were more likely younger (median=28.1 vs 30.7 years, p=0.006) and black (56.0% vs 35.5%, p<0.001), and had a higher BMI (median=27.0 vs 23.9 mg/kg2, p<0.001), compared to those without active asthma.
Table I.
Participant Characteristics: PRISM Study
| All Children |
Maternal Active Asthma |
Child sex |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (N=412) | Yes (n=91) | No (n=321) | Boys (n=215) | Girls (n=197) | ||||||
| Maternal age at delivery | ||||||||||
| years (median, IQR) | 30.0 | (25.4–34.2) | 28.1 | (23.8–32.8) | 30.7 | (26.2–34.4) | 29.8 | (25.2–33.3) | 30.4 | (25.4–35.2) |
| Maternal pre-pregnancy BMI | ||||||||||
| kg/m2 (median, IQR) | 24.8 | (22.1–29.8) | 27.0 | (23.8–32.9) | 23.9 | (21.8–28.7) | 25.0 | (22.5–30.1) | 24.4 | (21.7–29.3) |
| Maternal education (n, %) | ||||||||||
| ≤12 years | 135 | 32.8 | 35 | 38.5 | 100 | 31.2 | 63 | 29.3 | 72 | 36.6 |
| Maternal race/ethnicity (n, %) | ||||||||||
| White | 92 | 22.3 | 10 | 11.0 | 82 | 25.6 | 43 | 20.0 | 49 | 24.9 |
| Black | 165 | 40.1 | 51 | 56.0 | 114 | 35.5 | 87 | 40.5 | 78 | 39.6 |
| Hispanic | 129 | 31.3 | 26 | 28.6 | 103 | 32.1 | 71 | 33.0 | 58 | 29.4 |
| Other | 26 | 6.3 | 4 | 4.4 | 22 | 6.9 | 14 | 6.5 | 12 | 6.1 |
| Active asthma in pregnancy (n, %) | ||||||||||
| Yes | 91 | 22.1 | -- | -- | -- | -- | 50 | 23.3 | 41 | 20.8 |
| Prenatal maternal smoking (n, %) a | ||||||||||
| Yes | 49 | 11.9 | 16 | 17.6 | 33 | 10.3 | 25 | 11.6 | 24 | 12.2 |
| Prenatal SHS exposure (n, %) b | ||||||||||
| Yes | 92 | 22.3 | 28 | 30.8 | 64 | 19.9 | 52 | 24.2 | 40 | 20.3 |
| Child asthma (n, %) | ||||||||||
| Yes | 72 | 17.5 | 28 | 30.8 | 44 | 13.7 | 44 | 20.5 | 28 | 14.2 |
| Child sex (n, %) | ||||||||||
| Boys | 215 | 52.2 | 50 | 55.0 | 165 | 51.4 | -- | -- | -- | -- |
| Child age at first report of asthma | ||||||||||
| years (median, IQR) | 3.5 | (1.6–4.9) | 3.7 | (2.0–5.0) | 3.1 | (1.6–4.7) | 3.4 | (1.5–5.0) | 3.5 | (1.8–4.5) |
| Maternal total PUFA intake c | ||||||||||
| Total n-3 PUFA grams/day (median, IQR) | 2.0 | (1.7–2.5) | 1.9 | (1.6–2.4) | 2.0 | (1.7–2.5) | 2.0 | (1.7–2.6) | 2.0 | (1.7–2.4) |
| Total n-6 PUFA grams/day (median, IQR) | 16.2 | (14.1–18.3) | 15.7 | (13.5–18.6) | 16.3 | (14.4–18.2) | 16.3 | (14.1–18.5) | 16.2 | (14.2–18.2) |
| Total n-6/n-3 ratio ratio (median, IQR) | 7.9 | (6.8–8.8) | 8.2 | (6.9–9.2) | 7.8 | (6.8–8.7) | 7.9 | (6.8–8.6) | 8.0 | (6.9–9.0) |
Mother reported smoking at any time during pregnancy in prenatal visit/postnatal recall.
Mother reported living with current smokers during pregnancy or secondhand smoke (SHS) exposure for >1 hour/week at home, work or restaurants.
Sum of energy-adjusted dietary PUFA intake (adjusted for total energy intake) and PUFA supplements.
Children were followed through age 4.0±1.7 years; there was no difference in duration of follow-up by maternal asthma status (p=0.21) and no difference in maximum follow-up length (6.8 vs 6.9 years, respectively). Seventy-two (17.5%) children had asthma, with a median age at initial report of 3.5 (1.6–4.9) years. A higher proportion of boys had asthma (boys:20.5% vs girls:14.2%, p=0.10). Mothers with active asthma had lower n-3 PUFA intake (median=1.9 vs 2.0 g/day, p=0.03) and higher n-6/n-3 ratio (median=8.2 vs 7.8, p=0.04) compared to those without active asthma; there was no difference in n-6 intake by maternal asthma status (p=0.21). Examination of the proportion of children developing asthma based on tertiles of n-3 PUFA (from lowest to highest tertiles:21.1%, 13.8%, and 17.5%) and n-6 PUFA intake (from lowest to highest tertile:16.1%, 12.3%, and 24.1%), suggested non-linear associations. Maternal PUFA intakes were similar by offspring sex (all p>0.10).
Main Effects
We report associations between prenatal PUFA intakes and child asthma in the overall sample. In Figures 1–4, significant associations between PUFA intake and child asthma are demonstrated at the PUFA levels where the estimated pointwise 95% CI (shaded area) does not include zero. In Figure 1, the n-3 PUFA intake was most significantly associated with higher log odds of childhood asthma at the lowest level of n-3 intake (p-value of spline curve=0.03). For prenatal n-6 PUFA intake, there was a protective effect on child asthma in the mid-range of intake, while an increased risk of child asthma was shown at higher intake and suggested at lowest intakes; a similar U-shaped pattern was seen for associations between n-6/n-3 ratio and child asthma. However, the spline curves for n-6 PUFA and n-6/n-3 ratio did not reach statistical significance (p=0.10 and 0.13, respectively).
Figure 1. Log odds of childhood asthma across continuum of prenatal PUFA intake, overall sample.
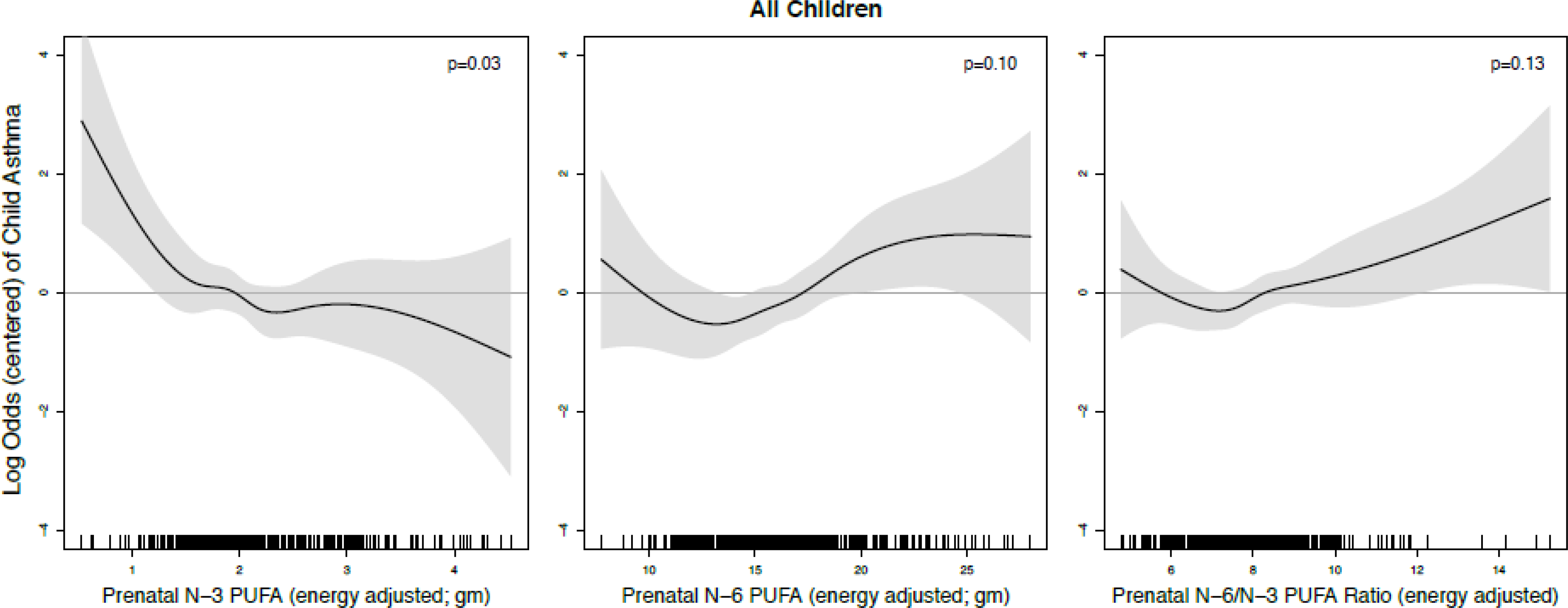
Lines reflect pointwise effect estimates in log odds. Significant associations between PUFA intake and child asthma are demonstrated at levels where the 95% CI (shaded area) does not include zero.
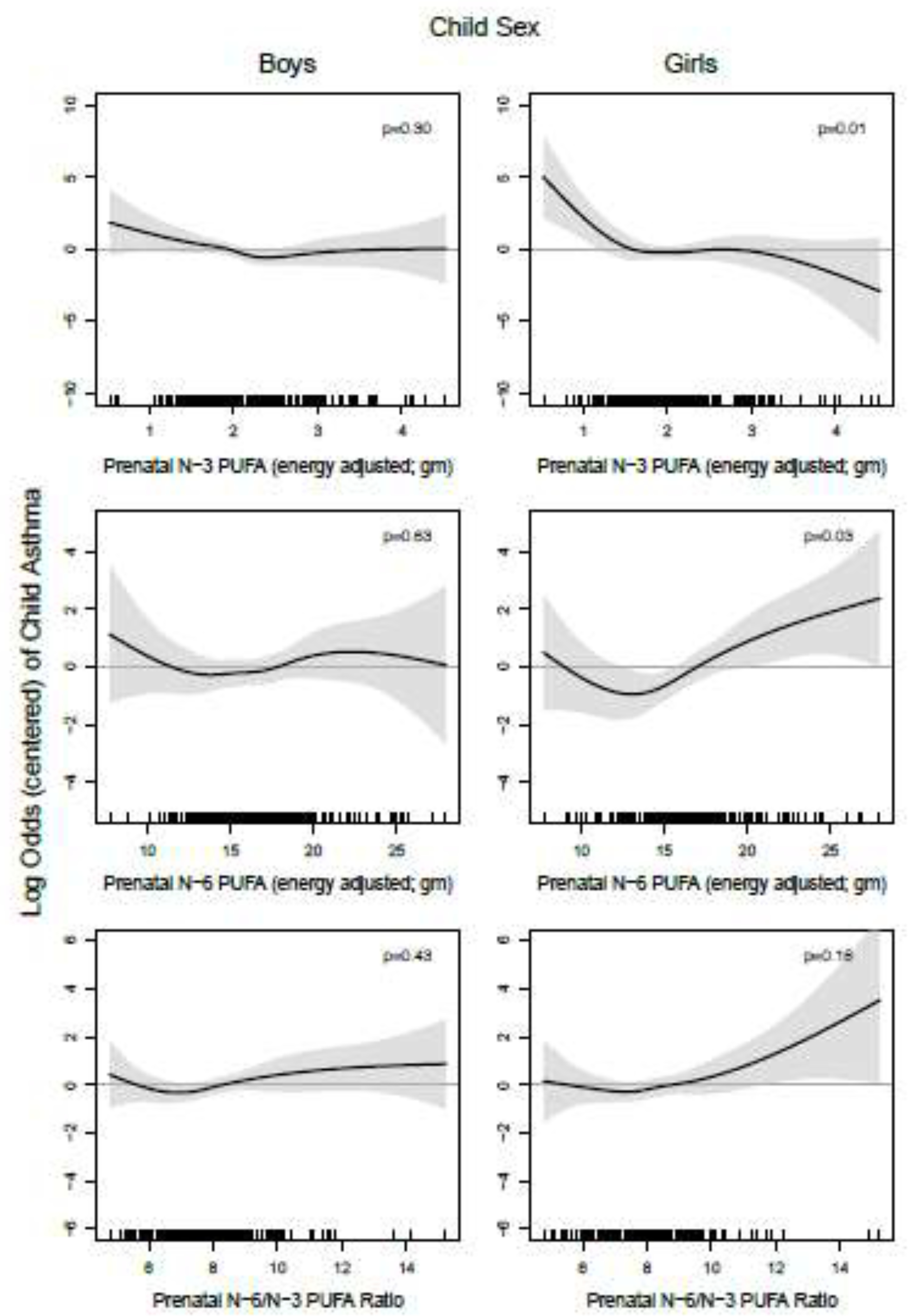
Figure 4. Log odds of childhood asthma across continuum of prenatal n-3 PUFA intake, stratified by maternal active asthma and child sex.
Line reflects pointwise effect estimates in log odds. Significant associations between PUFA intake and child asthma are demonstrated at levels where the 95% CI (shaded area) does not include zero.
Effect Modification by Maternal Active Asthma or Child Sex
Maternal active asthma in pregnancy:
We evaluated risk of childhood asthma across the continuum of maternal PUFA intakes by maternal active asthma status (Figure 2). Low n-3 PUFA intake was more strongly associated with asthma in children born to mothers with active asthma (p-value=0.01) compared to non-active asthma (p-value=0.27). Higher prenatal n-6 intake was more strongly associated with asthma in children of mothers with active asthma during pregnancy (p-value=0.04 for active asthma vs. p=0.8 for non-active asthma). Similarly, women with higher n-6/n-3 ratios were more likely to have children who developed asthma, although it did not reach statistical significance in the stratified model for children born to mothers with active asthma (p-value=0.08). Results from factor-smooth interaction GAMs reinforced the fact that maternal active asthma status during pregnancy modifies the association between prenatal PUFA indicators and child asthma (eFigure 2a) given that the 95% CI’s of the difference between curves for those with and without maternal active asthma did not uniformly contain zero.
Figure 2. Log odds of childhood asthma across continuum of prenatal PUFA intake, stratified by maternal active asthma status.
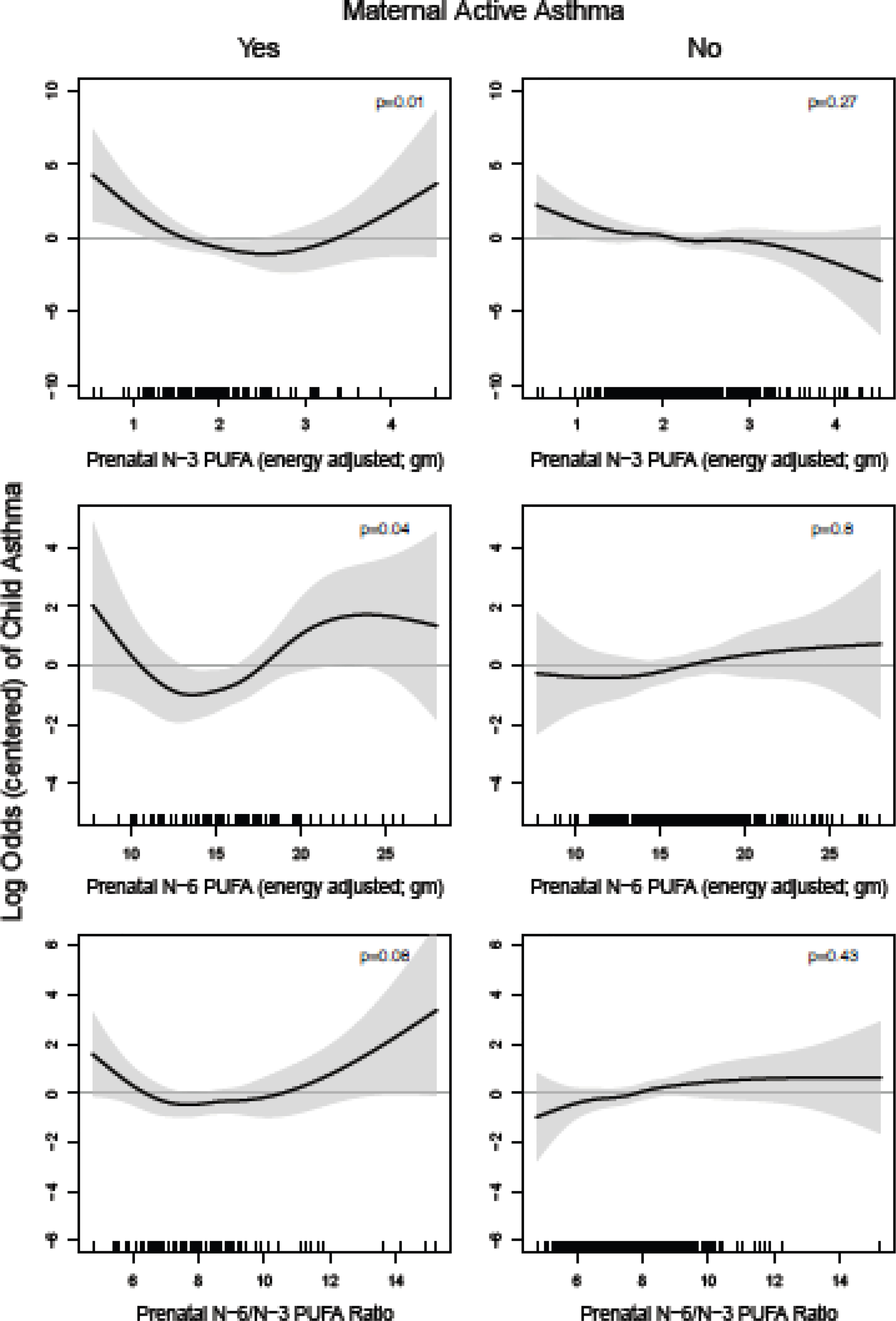
Line reflects pointwise effect estimates in log odds. Significant associations between PUFA intake and child asthma are demonstrated at levels where the 95% CI (shaded area) does not include zero.
Child sex:
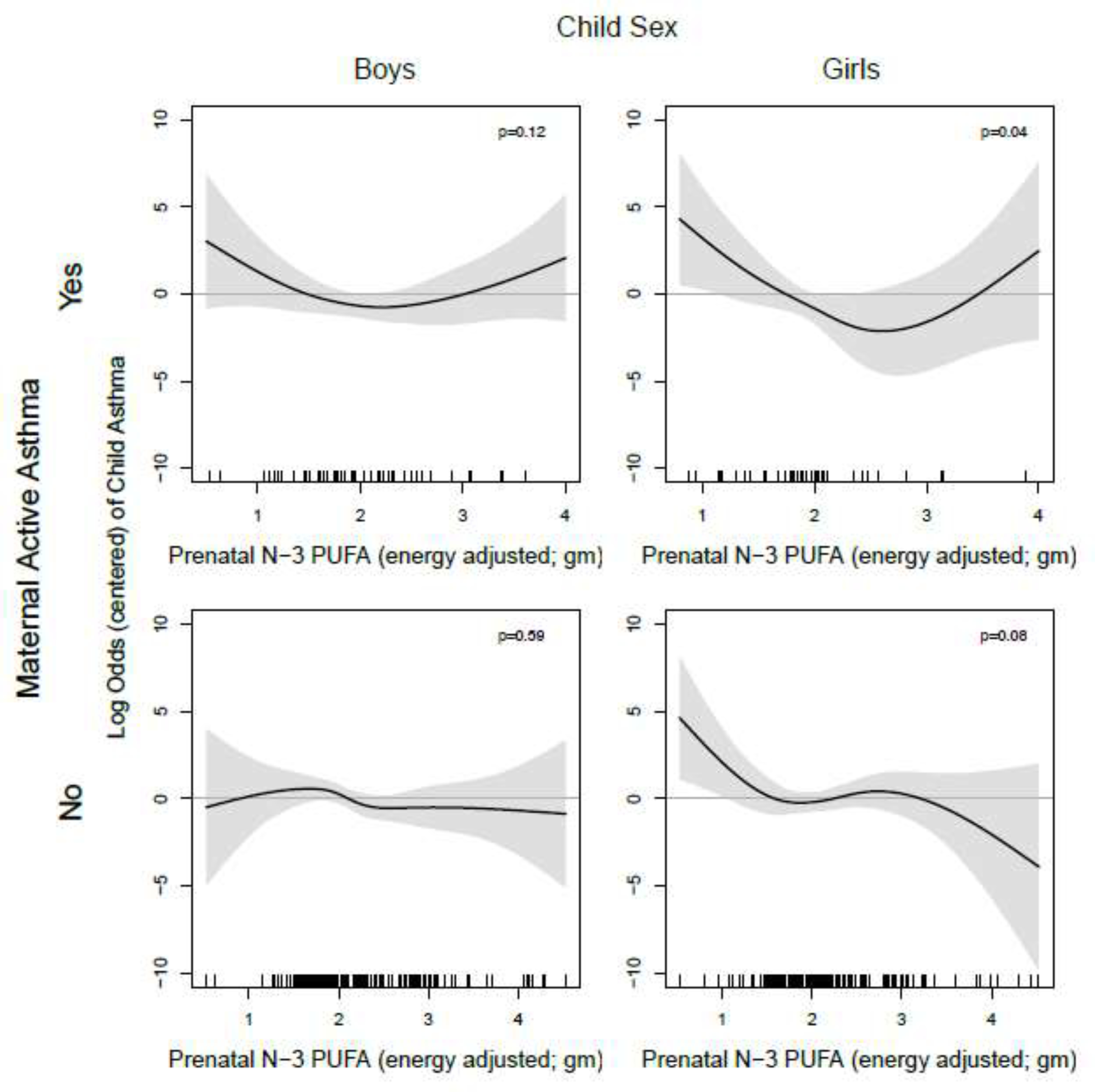
In models stratified by sex, associations between prenatal n-3 and n-6 PUFA intake and child asthma appeared to be stronger in girls compared to boys (Figure 3). The inverse association between n-3 PUFA intake and child asthma was more apparent in girls (curve p-value=0.01) compared to boys (p=0.30). For n-6 PUFA intake, the U-shape association was more apparent in girls (p-value=0.03), where there was a protective effect on child asthma in the mid-range of intake and increased risk of child asthma at higher intake and suggested at lowest intakes, compared to boys (p=0.63). Similarly, among girls, higher levels of n-6/n-3 ratio were associated with increased risk of child asthma; however, we did not find statistically significant associations for the smoothed associations of the n-6/n-3 ratio with child asthma (p=0.16 for girls, p=0.43 for boys). Results from factor-smooth interaction GAMs suggested that child sex was an effect modifier of the relationship between prenatal n-3 PUFA intake and child asthma, but not for relationships of prenatal n-6 PUFA or the n-6/n-3 ratio with child asthma (eFigure 2b).
Figure 3. Log odds of childhood asthma across the continuum of prenatal PUFA intake, stratified by child sex.
Line reflects pointwise effect estimates in log odds. Significant associations between PUFA intake and child asthma are demonstrated at levels where the 95% CI (shaded area) does not include zero.
Exploratory Analyses
We next evaluated associations between n-3 PUFAs and risk of childhood asthma stratified by both maternal active asthma status and child sex (Figure 4). Among girls born to mothers without active asthma, lower n-3 PUFA intake was significantly associated with higher risk of asthma (p-value=0.04). A similar relationship, both in pattern and magnitude of association, was observed among girls born to mothers with active asthma, although associations did not reach statistical significance (p-value=0.08). Associations between n-3 PUFA and child asthma in boys born to mothers with and without active asthma were not significant (p-value=0.12 and 0.59, respectively). Smooth effect estimates of the relationships of prenatal n-6 PUFA intake and n-6/n-3 ratio with log odds of children’s asthma, stratified by maternal asthma status and child sex, are provided in the online supplement (eFigure 3). No significant differences were evident (p-values>0.1 for all spline curves). Results of the sensitivity analysis for the n-3 PUFA model not adjusted for n-6 PUFA intake were unchanged, while the n-6 PUFA model not adjusted for n-3 PUFA intake was similar, with a slightly attenuated effect at the higher n-6 level. Sensitivity analyses including prenatal maternal smoking and secondhand smoke exposure in utero did not substantively change our findings (data not shown).
DISCUSSION
These analyses support our primary hypothesis, that mothers with lower n-3 and higher n-6 PUFA intakes prenatally were more likely to have children diagnosed with asthma. As posited, the association was stronger in children born to mothers with prenatal active asthma. Finally, when we further examined sex-specific differences, low maternal prenatal n-3 intake was most strongly associated with risk of asthma among girls. Identification of these modifying factors can guide interpretation of future observational studies, identify subgroups most likely to benefit in clinical trials, and direct research to elucidate underlying mechanisms of asthma programming in utero.
Ours is the first study to consider maternal prenatal active asthma as a modifier of associations between prenatal PUFA intake and asthma in early childhood. Maternal asthma history is more strongly associated with offspring asthma than paternal history, in particular for asthma diagnosed before age 5 years33, 42. Further, active asthma28 and asthma severity and control during pregnancy contribute to asthma risk in the developing child25, 26, 29. In a case-control study, moderate-to-severe uncontrolled asthma during pregnancy was associated with a 27% higher risk of offspring asthma compared to mild controlled asthma25. A large prospective population-based registry study (n~7000) found an increased risk of early-onset persistent asthma in children born to mothers with asthma in pregnancy categorized as mild uncontrolled (Prevalence Ratio (PR)=1.19, 95% CI=1.05–1.35), moderate-to-severe controlled (PR=1.33, 95% CI=1.09–1.63), and moderate-to-severe uncontrolled (PR=1.37, 95% CI=1.17–1.61) relative to mild controlled asthma 26. A randomized controlled trial demonstrated that improved prenatal maternal asthma control was associated with reduced risk of offspring asthma at age 4–6 years29. More active, uncontrolled asthma is associated with a shift in inflammatory mediators toward a Th2-dominated inflammatory milieu (reviewed in 6, 21) which may work in concert with higher intake of pro-inflammatory n-6 PUFAs and lower intake of more anti-inflammatory n-3 PUFAs to impact asthma risk in the developing fetus. Future research is needed to directly investigate mechanistic pathways.
Two prior studies report differences in the association between prenatal PUFA intake and offspring asthma by maternal asthma history, but neither considered active prenatal asthma. In a Southern US birth cohort comprised primarily of lower income African Americans, higher prenatal plasma levels of n-6 PUFAs were associated with increased asthma in 4 to 6 year olds born to women with a history of asthma7. In a pooled analysis of 18 birth cohorts (European and US), Stratakis et al.43 reported that higher cord blood n-3 PUFAs were protective against asthma in preschool-aged children with no parental history of asthma or hay fever43. Differences in sample composition and inclusion of asthma history, rather than active prenatal asthma, may contribute to the discrepancy with our findings. Notably, we observed that lower prenatal n-3 PUFA intake was associated with increased risk of child asthma however only at the lowest levels of n-3 PUFA intake. Similarly, the Copenhagen Prospective Studies on Asthma in Childhood (COPSAC) trial, which randomized pregnant mothers (26% with asthma history) to n-3 PUFA supplementation or placebo, found that the risk of offspring wheeze or asthma by age 3–5 years was lower in the intervention group, with the strongest beneficial effect among mothers with baseline n-3 PUFA levels in the lowest tertile18.
While relationships between n-3 PUFA intake were more linear, associations between n-6 intake and the n-6/n-3 ratio and child asthma were U-shaped. Specifically, mid-range prenatal n-6 PUFA intake was associated with reduced odds of asthma in children, but as n-6 PUFA intake and n-6/n-3 ratios increased, intake was associated with increased odds of asthma in children. Effects were again most evident among children born to mothers with active prenatal asthma. There are prior reports of non-linear relationships between PUFAs and diseases44, 45, including a prospective cohort study in Sweden reporting non-linear (inverse J-shaped) associations between n-6 PUFA levels measured at age 8 years and subsequent asthma46. While factors contributing to non-linear effects remain unclear, they may reflect underlying biologic factors or interactions with intake of other nutrients47.
We observed sex-specific differences, with low maternal n-3 PUFA intake most strongly associated with asthma risk in girls. Sex-specific differences in associations between PUFAs, maternal asthma, and childhood asthma development may be driven by underlying differences in lung development, sex hormones (e.g., estrogen), and fatty acids metabolism48–51. In the primarily African American population referenced above, Rosa et al. also observed sex-specific effects, but found the highest risk among boys born to mothers with ever asthma and a higher n-6/n-3 ratio7. The differing sociodemographic makeup, as well as consideration of ever asthma rather than active prenatal asthma, may contribute to the variable findings. Notably, prior work shows sex-specific differences in fetal growth52 and placental gene expression53 in pregnancies complicated by asthma, with girls being more impacted. In placental tissue from pregnancies complicated by asthma, Osei-Kumeh and colleagues53 found 65 differentially expressed genes involved in growth, inflammation and immune function, of which 6 genes were altered in male placentas and 59 genes in female placentas. These and other fundamental molecular mechanisms operating through the sexually dimorphic placenta should be explored in future studies.
We acknowledge both strengths and some weaknesses. We leveraged a racially/ethnically diverse urban sample and considered active asthma during pregnancy. Prenatal PUFA intake was measured via an FFQ, a validated and widely used tool to assess average dietary intake over time, including prenatally. Notably, the FFQ was validated in our study population through 24-hour dietary recalls37 and incorporates PUFA intake from both dietary sources and supplement use, an important contributor to total PUFA intake36. Moreover, because PUFA intake and maternal asthma status were reported during pregnancy, prior to the outcome of interest, we expect any misclassification to be non-differential. In addition, we used GAMs, which provided flexibility to investigate non-linear, and perhaps more biologically relevant, relationships.
It is also worth noting some limitations. We were unable to consider timing in gestation of reported PUFA intake because the FFQ was completed in the second trimester of pregnancy. However, intake has been shown to be stable prior to and across pregnancy54. Childhood asthma is self-reported which introduces the possibility of differential misclassification; however, our data does not support this. If mothers with asthma were more likely to recognize symptoms, seek care, and report asthma diagnosis in their children at an earlier age, we would expect children of women with asthma to have an earlier age at asthma diagnosis. However, in this study, asthma was diagnosed at an older age in children of mothers with active asthma in pregnancy. Unfortunately, we were unable to evaluate more detailed characteristics of maternal and child asthma, including endotypes, degree of control, and asthma medication compliance due to sample size limitations, but this will be important to consider in future studies. Finally, we cannot rule out the potential contribution of unmeasured confounders.
This analysis highlights the importance of considering active maternal asthma as a modifier of the relationship between prenatal PUFA intake and childhood asthma risk. Consideration of factors that modify associations between prenatal PUFA intake on respiratory disease in children has implications for designing intervention strategies tailored to impact those at greatest risk.
Supplementary Material
Funding source:
The PRogramming of Intergenerational Stress Mechanisms (PRISM) cohort has been supported under US National Institute of Health (NIH) grants R01 HL095606, R01 HL114396, R21 ES021318, R21HD080359 and R01 ES030302. Guidance on nutritional intake measurement and analyses were supported through NIH grants P30 ES023515 and UL1 TR001363, respectively. During the preparation of this manuscript, KC and RJW were supported by R01 HL132338 and WC was supported by T32HD049311.
Abbreviations/acronyms
- PUFA
Polyunsaturated Fatty Acid
- SD
standard deviation
- FFQ
Food Frequency Questionnaire
- BMI
body mass index
- kg
kilograms
- m
meters
- GAMs
generalized additive models
- n-3
Omega-3
- n-6
Omega-6
- PR
Prevalence Ratio
- CI
Confidence Interval
- IQR
Interquartile range
- SHS
Secondhand smoke
Footnotes
Conflict of interest: none
Clinical trial registration: N/A
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Julie D. Flom, Kravis Children’s Hospital, Department of Pediatrics, Division of Pediatric Allergy & Immunology, Icahn School of Medicine at Mount Sinai, 1425 Madison Avenue, New York, NY 10029.
Yueh-Hsiu Mathilda Chiu., Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, 1236 Park Avenue, New York, NY 10026.
Whitney Cowell, Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, 1236 Park Avenue, New York, NY 10026.
Srimathi Kannan, Department of Metabolism, Endocrinology, and Diabetes, University of Michigan, 24 Frank Lloyd Wright Drive, Dominos Farms, Lobby C, Suite G1500, Ann Arbor, Michigan 48019.
Harish B. Ganguri, University of Cumberlands, Williamsburg, Kentucky, 40769.
Brent A. Coull, Harvard TH Chan School of Public Health, Harvard University, 655 Huntington Avenue, Building II, Boston, MA 02115.
Rosalind J. Wright, Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, 1236 Park Avenue, New York, NY 10026; Institute for Exposomic Research, Icahn School of Medicine at Mount Sinai, 1236 Park Avenue, New York, NY 10026.
Kecia Carroll, Department of Pediatrics, Vanderbilt University Medical Center, 2146 Belcourt Avenue, 2nd Floor, Nashville, TN, 37232.
References
- 1.Ferrante G, La Grutta S. The Burden of Pediatric Asthma. Front Pediatr. 2018;6:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant T, Brigham EP, McCormack MC. Childhood Origins of Adult Lung Disease as Opportunities for Prevention. J Allergy Clin Immunol Pract. 2020;8:849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Federico MJ, McFarlane AE 2nd, Szefler SJ, Abrams EM. The Impact of Social Determinants of Health on Children with Asthma. J Allergy Clin Immunol Pract. 2020;8:1808–1814. [DOI] [PubMed] [Google Scholar]
- 4.Duijts L Fetal and infant origins of asthma. Eur J Epidemiol. 2012;27:5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Mason J, Portnoy JM. Immunologic Strategies for Prevention of Asthma. J Allergy Clin Immunol Pract. 2020;8:834–847. [DOI] [PubMed] [Google Scholar]
- 6.Pham MN, Bunyavanich S. Prenatal Diet and the Development of Childhood Allergic Diseases: Food for Thought. Curr Allergy Asthma Rep. 2018;18:58. [DOI] [PubMed] [Google Scholar]
- 7.Rosa MJ, Hartman TJ, Adgent M, et al. Prenatal polyunsaturated fatty acids and child asthma: Effect modification by maternal asthma and child sex. J Allergy Clin Immunol. 2020;145:800–807 e804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Best KP, Gold M, Kennedy D, Martin J, Makrides M. Omega-3 long-chain PUFA intake during pregnancy and allergic disease outcomes in the offspring: a systematic review and meta-analysis of observational studies and randomized controlled trials. Am J Clin Nutr. 2016;103:128–143. [DOI] [PubMed] [Google Scholar]
- 9.Venter C, Meyer RW, Nwaru BI, et al. EAACI position paper: Influence of dietary fatty acids on asthma, food allergy, and atopic dermatitis. Allergy. 2019;74:1429–1444. [DOI] [PubMed] [Google Scholar]
- 10.Calder PC, Kremmyda LS, Vlachava M, Noakes PS, Miles EA. Is there a role for fatty acids in early life programming of the immune system? P Nutr Soc. 2010;69:373–380. [DOI] [PubMed] [Google Scholar]
- 11.Lee-Sarwar K, Kelly RS, Lasky-Su J, et al. Dietary and plasma polyunsaturated fatty acids are inversely associated with asthma and atopy in early childhood. J Allergy Clin Immunol Pract. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer BJ, Mann NJ, Lewis JL, Milligan GC, Sinclair AJ, Howe PR. Dietary intakes and food sources of omega-6 and omega-3 polyunsaturated fatty acids. Lipids. 2003;38:391–398. [DOI] [PubMed] [Google Scholar]
- 13.Simopoulos AP. Fatty acid omega-3 polyunsaturated. In: Benjamin C, ed. Encyclopedia of human nutrition. 2nd ed 2005:205–219. [Google Scholar]
- 14.Black PN, Sharpe S. Dietary fat and asthma: Is there a connection? Eur Respir J. 1997;10:6–12. [DOI] [PubMed] [Google Scholar]
- 15.Kim JH, Ellwood PE, Asher MI. Diet and asthma: looking back, moving forward. Respir Res. 2009;10:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stratakis N, Gielen M, Margetaki K, et al. PUFA status at birth and allergy-related phenotypes in childhood: a pooled analysis of the Maastricht Essential Fatty Acid Birth (MEFAB) and RHEA birth cohorts. Brit J Nutr. 2018;119:202–210. [DOI] [PubMed] [Google Scholar]
- 17.Lumia M, Luukkainen P, Tapanainen H, et al. Dietary fatty acid composition during pregnancy and the risk of asthma in the offspring. Pediat Allerg Imm-Uk. 2011;22:827–835. [DOI] [PubMed] [Google Scholar]
- 18.Bisgaard H, Stokholm J, Chawes BL, et al. Fish Oil-Derived Fatty Acids in Pregnancy and Wheeze and Asthma in Offspring. New Engl J Med. 2016;375:2530–2539. [DOI] [PubMed] [Google Scholar]
- 19.Hansen S, Strom M, Maslova E, et al. Fish oil supplementation during pregnancy and allergic respiratory disease in the adult offspring. J Allergy Clin Immunol. 2017;139:104–111 e104. [DOI] [PubMed] [Google Scholar]
- 20.Olsen SF, Osterdal ML, Salvig JD, et al. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. American Journal of Clinical Nutrition. 2008;88:167–175. [DOI] [PubMed] [Google Scholar]
- 21.Gunaratne AW, Makrides M, Collins CT. Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. Cochrane Database Syst Rev. 2015:CD010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rucci E, den Dekker HT, de Jongste JC, et al. Maternal fatty acid levels during pregnancy, childhood lung function and atopic diseases. The Generation R Study. Clin Exp Allergy. 2016;46:461–471. [DOI] [PubMed] [Google Scholar]
- 23.Standl M, Demmelmair H, Koletzko B, Heinrich J. Cord blood LC-PUFA composition and allergic diseases during the first 10 yr. Results from the LISAplus study. Pediat Allerg Imm-Uk. 2014;25:344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nurmatov U, Nwaru BI, Devereux G, Sheikh A. Confounding and effect modification in studies of diet and childhood asthma and allergies. Allergy. 2012;67:1041–1059. [DOI] [PubMed] [Google Scholar]
- 25.Martel MJ, Rey E, Beauchesne MF, et al. Control and severity of asthma during pregnancy are associated with asthma incidence in offspring: two-stage case-control study. Eur Respir J. 2009;34:579–587. [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Agerbo E, Schlunssen V, Wright RJ, Li J, Munk-Olsen T. Maternal asthma severity and control during pregnancy and risk of offspring asthma. J Allergy Clin Immunol. 2018;141:886–892 e883. [DOI] [PubMed] [Google Scholar]
- 27.Mirzakhani H, Carey VJ, Zeiger R, et al. Impact of parental asthma, prenatal maternal asthma control, and vitamin D status on risk of asthma and recurrent wheeze in 3-year-old children. Clin Exp Allergy. 2019;49:419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lebold KM, Jacoby DB, Drake MG. Inflammatory mechanisms linking maternal and childhood asthma. J Leukoc Biol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morten M, Collison A, Murphy VE, et al. Managing Asthma in Pregnancy (MAP) trial: FENO levels and childhood asthma. J Allergy Clin Immunol. 2018;142:1765–1772 e1764. [DOI] [PubMed] [Google Scholar]
- 30.Lohner S, Fekete K, Marosvolgyi T, Decsi T. Gender differences in the long-chain polyunsaturated fatty acid status: systematic review of 51 publications. Ann Nutr Metab. 2013;62:98–112. [DOI] [PubMed] [Google Scholar]
- 31.Lee A, Leon Hsu HH, Mathilda Chiu YH, et al. Prenatal fine particulate exposure and early childhood asthma: Effect of maternal stress and fetal sex. J Allergy Clin Immunol. 2018;141:1880–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bose S, Chiu YM, Hsu HL, et al. Prenatal Nitrate Exposure and Childhood Asthma. Influence of Maternal Prenatal Stress and Fetal Sex. Am J Respir Crit Care Med. 2017;196:1396–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim RH, Kobzik L, Dahl M. Risk for asthma in offspring of asthmatic mothers versus fathers: a meta-analysis. PLoS One. 2010;5:e10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–469. [DOI] [PubMed] [Google Scholar]
- 35.Siega-Riz AM, Bodnar LM, Savitz DA. What are pregnant women eating? Nutrient and food group differences by race. Am J Obstet Gynecol. 2002;186:480–486. [DOI] [PubMed] [Google Scholar]
- 36.Papanikolaou Y, Brooks J, Reider C, Fulgoni VL 3rd. U.S. adults are not meeting recommended levels for fish and omega-3 fatty acid intake: results of an analysis using observational data from NHANES 2003–2008. Nutr J. 2014;13:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brunst KJ, Kannan S, Ni YM, Gennings C, Ganguri HB, Wright RJ. Validation of a Food Frequency Questionnaire for Estimating Micronutrient Intakes in an Urban US Sample of Multi-Ethnic Pregnant Women. Matern Child Health J. 2016;20:250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–1228S; discussion 1229S-1231S. [DOI] [PubMed] [Google Scholar]
- 39..In: Kotz S, Read CB, Balakrishnan N, Vidakovic B, eds. Encyclopedia of Statistical Sciences, 2nd Edition. Hoboken, NJ: Wiley; 2005. [Google Scholar]
- 40.Wood SN. Generalized additive models: An introduction with R: Chapman & Hall/CRC; 2006. [Google Scholar]
- 41.Textor J, van der Zander B, Gilthorpe MS, Liskiewicz M, Ellison GT. Robust causal inference using directed acyclic graphs: the R package ‘dagitty’. Int J Epidemiol. 2016;45:1887–1894. [DOI] [PubMed] [Google Scholar]
- 42.Litonjua AA, Carey VJ, Burge HA, Weiss ST, Gold DR. Parental history and the risk for childhood asthma. Does mother confer more risk than father? Am J Respir Crit Care Med. 1998;158:176–181. [DOI] [PubMed] [Google Scholar]
- 43.Stratakis N, Roumeliotaki T, Oken E, et al. Fish and seafood consumption during pregnancy and the risk of asthma and allergic rhinitis in childhood: a pooled analysis of 18 European and US birth cohorts. Int J Epidemiol. 2017;46:1465–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsuoka YJ, Sawada N, Mimura M, et al. Dietary fish, n-3 polyunsaturated fatty acid consumption, and depression risk in Japan: a population-based prospective cohort study. Transl Psychiatry. 2017;7:e1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bork CS, Veno SK, Lundbye-Christensen S, et al. Adipose tissue content of alpha-linolenic acid and the risk of ischemic stroke and ischemic stroke subtypes: A Danish case-cohort study. PLoS One. 2018;13:e0198927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magnusson J, Ekstrom S, Kull I, et al. Polyunsaturated fatty acids in plasma at 8 years and subsequent allergic disease. J Allergy Clin Immunol. 2018;142:510–516 e516. [DOI] [PubMed] [Google Scholar]
- 47.National Academies of Sciences E, and Medicine. Guiding principles for developing Dietary Reference Intakes based on chronic disease. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- 48.Almqvist C, Worm M, Leynaert B, working group of GALENWPG. Impact of gender on asthma in childhood and adolescence: a GA2LEN review. Allergy. 2008;63:47–57. [DOI] [PubMed] [Google Scholar]
- 49.Decsi T, Kennedy K. Sex-specific differences in essential fatty acid metabolism. American Journal of Clinical Nutrition. 2011;94:1914s–1919s. [DOI] [PubMed] [Google Scholar]
- 50.Liptzin DR, Landau LI, Taussig LM. Sex and the lung: Observations, hypotheses, and future directions. Pediatr Pulmonol. 2015;50:1159–1169. [DOI] [PubMed] [Google Scholar]
- 51.Childs CE, Romeu-Nadal M, Burdge GC, Calder PC. Gender differences in the n-3 fatty acid content of tissues. Proc Nutr Soc. 2008;67:19–27. [DOI] [PubMed] [Google Scholar]
- 52.Clifton VL. Sexually dimorphic effects of maternal asthma during pregnancy on placental glucocorticoid metabolism and fetal growth. Cell Tissue Res. 2005;322:63–71. [DOI] [PubMed] [Google Scholar]
- 53.Osei-Kumah A, Smith R, Jurisica I, Caniggia I, Clifton VL. Sex-specific differences in placental global gene expression in pregnancies complicated by asthma. Placenta. 2011;32:570–578. [DOI] [PubMed] [Google Scholar]
- 54.Crozier SR, Robinson SM, Godfrey KM, Cooper C, Inskip HM. Women’s Dietary Patterns Change Little from Before to During Pregnancy. J Nutr. 2009;139:1956–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


