Abstract
Background
The potential effects of SARS-CoV-2 and Plasmodium falciparum co-infection on host susceptibility and pathogenesis remain unknown. We aimed to establish the prevalence of malaria and describe the clinical characteristics of SARS-CoV-2 and P falciparum co-infection in a high-burden malaria setting.
Methods
This was an exploratory prospective, cohort study of patients with COVID-19 who were admitted to hospital in Uganda. Patients of all ages with a PCR-confirmed diagnosis of SARS-CoV-2 infection who had provided informed consent or assent were consecutively enrolled from treatment centres in eight hospitals across the country and followed up until discharge or death. Clinical assessments and blood sampling were done at admission for all patients. Malaria diagnosis in all patients was done by rapid diagnostic tests, microscopy, and molecular methods. Previous P falciparum exposure was determined with serological responses to a panel of P falciparum antigens assessed using a multiplex bead assay. Additional evaluations included complete blood count, markers of inflammation, and serum biochemistries. The main outcome was overall prevalence of malaria infection and malaria prevalence by age (including age categories of 0–20 years, 21–40 years, 41–60 years, and >60 years). The frequency of symptoms was compared between patients with COVID-19 with P falciparum infection versus those without P falciparum infection. The frequency of comorbidities and COVID-19 clinical severity and outcomes was compared between patients with low previous exposure to P falciparum versus those with high previous exposure to P falciparum. The effect of previous exposure to P falciparum on COVID-19 clinical severity and outcomes was also assessed among patients with and those without comorbidities.
Findings
Of 600 people with PCR-confirmed SARS-CoV-2 infection enrolled from April 15, to Oct 30, 2020, 597 (>99%) had complete information and were included in our analyses. The majority (502 [84%] of 597) were male individuals with a median age of 36 years (IQR 28–47). Overall prevalence of P falciparum infection was 12% (95% CI 9·4–14·6; 70 of 597 participants), with highest prevalence in the age groups of 0–20 years (22%, 8·7–44·8; five of 23 patients) and older than 60 years (20%, 10·2–34·1; nine of 46 patients). Confusion (four [6%] of 70 patients vs eight [2%] of 527 patients; p=0·040) and vomiting (four [6%] of 70 patients vs five [1%] of 527 patients; p=0·014] were more frequent among patients with P falciparum infection than those without. Patients with low versus those with high previous P falciparum exposure had a increased frequency of severe or critical COVID-19 clinical presentation (16 [30%] of 53 patients vs three [5%] of 56 patients; p=0·0010) and a higher burden of comorbidities, including diabetes (12 [23%] of 53 patients vs two [4%] of 56 patients; p=0·0010) and heart disease (seven [13%] of 53 patients vs zero [0%] of 56 patients; p=0·0030). Among patients with no comorbidities, those with low previous P falciparum exposure still had a higher proportion of cases of severe or critical COVID-19 than did those with high P falciparum exposure (six [18%] of 33 patients vs one [2%] of 49 patients; p=0·015). Multivariate analysis showed higher odds of unfavourable outcomes in patients who were older than 60 years (adjusted OR 8·7, 95% CI 1·0–75·5; p=0·049).
Interpretation
Although patients with COVID-19 with P falciparum co-infection had a higher frequency of confusion and vomiting, co-infection did not seem deleterious. The association between low previous malaria exposure and severe or critical COVID-19 and other adverse outcomes will require further study. These preliminary descriptive observations highlight the importance of understanding the potential clinical and therapeutic implications of overlapping co-infections.
Funding
Malaria Consortium (USA).
Research in context.
Evidence before this study
We searched PubMed for articles published between Dec 1, 2019, and April 30, 2021, using the terms “COVID-19 OR SARS-CoV-2” AND “malaria OR P falciparum” AND “interactions OR overlap”. Additionally, we reviewed and included any relevant articles cited in the identified references. Our search found no reports of clinical interactions between SARS-CoV-2 and malaria. A few modelling studies and reports suggest that a substantial number of excess cases and deaths from malaria could occur as a consequence of the SARS-CoV-2 pandemic. The assumptions in most of these models is that SARS-CoV-2 only affects malaria by disrupting control measures with limited direct interactions between the two diseases.
Added value of this study
This preliminary descriptive investigation is to our knowledge the first to characterise potential interactions between SARS-CoV-2 infection and malaria and to describe important clinical correlates of co-infection in a setting of high malaria burden. We further investigated the potential effects of previous malaria exposure on clinical profiles and outcomes in patients with SARS-CoV-2 infection and found that low previous malaria exposure seems to be associated with more severe manifestations of COVID-19. Our findings add value to the existing literature on SARS-CoV-2 epidemiology and clinical profiles in different settings and highlight the importance of understanding the potential clinical and therapeutic implications of co-infections with other diseases such as malaria.
Implications of all the available evidence
A better understanding of the nature of interactions between SARS-CoV-2 and malaria is of great public health interest, especially in sub-Saharan Africa, given the epidemiological overlap between these diseases. These preliminary observations enhance our understanding of potential clinical interactions between SARS-CoV-2 and malaria infection. This knowledge could promote integrated approaches to investigations and disease management.
Introduction
SARS-CoV-2 has caused considerable morbidity and mortality globally, with more than 236 million cases and 4·83 million deaths reported by Oct 8, 2021.1 Since the first reported cases in Africa on Feb 14, 2020, SARS-CoV-2 has spread to all countries in the continent and has caused unprecedented socioeconomic and health-care system disruptions.2 Although sub-Saharan Africa has a relatively low global proportion of COVID-19 cases and deaths compared with other continents,3 it has a disproportionately higher burden of other infectious diseases, including malaria, HIV/AIDS, and tuberculosis.4, 5, 6 The potential effects of COVID-19 on the control of these diseases and the potential implications of any clinical interactions between COVID-19 and these diseases therefore remains a major public health concern in Africa, especially when geographical overlap results in high levels of co-infection. However, despite the global spread of SARS-CoV-2, our understanding of the epidemiology and clinical course of COVID-19 in countries with substantial burdens of other infectious diseases is limited. The magnitude of clinical overlap between these diseases and COVID-19 and the potential consequences of co-infection also remains largely unknown.
Whereas previous predictive models suggest possibly lower mortality from COVID-19 in Africa than in high-income countries,7 several modelling studies and reports indicate that a large number of excess cases and deaths from HIV/AIDs, tuberculosis, and malaria could occur as a consequence of the COVID-19 pandemic.8, 9, 10, 11, 12 However, the assumptions in some of these models that one disease does not directly influence the transmission or severity of the other and that COVID-19 only affects these diseases by disrupting control measures and affecting health systems have not been verified. Of particular interest among these infections is malaria, which causes substantial morbidity and mortality, with an estimated 229 million cases and 409 000 deaths reported globally in 2019 (>90% in sub-Saharan Africa).4 If co-infections with SARS-CoV-2 and agents such as Plasmodium falciparum should cause increased complications, then the burden of COVID-19 in low-income and middle-income countries could be substantially worse than predicted. Whether immunomodulation caused by malaria13, 14 is beneficial or harmful in the context of co-infection with SARS-CoV-2 is also unclear. Malaria-induced immunomodulation has been shown to be protective against severe manifestations of some respiratory viruses such as influenza,15 by suppressing production of cytokines and decreasing recruitment of cellular inflammatory components to the lungs, leading to milder clinical symptoms and inflammation.16 Previous malaria exposure or pre-existing infection with malaria could therefore plausibly lead to changes in susceptibility and severity of COVID-19.17 However, despite these observations, the effects of malaria and SARS-CoV-2 co-infection on host susceptibility and pathogenesis remain unclear. This preliminary descriptive investigation was conducted in this context to better characterise COVID-19 cases in a setting with a high burden of malaria, and to assess the prevalence and clinical characteristics of SARS-CoV-2 and P falciparum co-infection.
Methods
Study design and participants
This study was an exploratory, prospective, cohort study carried out from April 15, to Oct 30, 2020, at COVID-19 treatment centres in eight tertiary hospitals in Uganda. These included three hospitals in central Uganda, two hospitals in north Uganda, one hospital in east Uganda, one hospital in south Uganda, and one hospital in northwest Uganda (appendix p 2). Using consecutive sampling, we included patients with COVID-19 of all ages who were admitted to hospital, with PCR-confirmed COVID-19 diagnosis, and who had provided informed consent or assent. Written informed consent was obtained from adult patients or their next of kin (for patients who were unconscious or critically ill). Written parental or guardian consent was obtained for all children (aged <18 years) and assent was obtained from patients aged 8–17 years. If a patient could not read or write, a literate adult impartial witness (a member of the clinical team not involved in the study) was present throughout the consenting process and signed and dated the consent form, with a thumb print from the patient. Patients were followed up daily on the wards until discharge or death. The study was approved by the Mulago National Referral Hospital Research and Ethics committee and the Uganda National Council for Science and Technology. The study was also listed on the MESA Track database under the name “Evaluating the impact of COVID-19 on malaria and other infectious diseases in Uganda: Understanding comorbidities”.
Procedures
Demographic and clinical information collected included age, sex, presenting complaints, underlying medical conditions, duration of illness, clinical examination findings, diagnosis, and medications prescribed. Patient clinical outcomes and duration of treatment in hospital were also noted. Clinical classification of COVID-19 presentation as asymptomatic, mild, moderate, or severe or critical, and COVID-19 case management was done according to national guidelines.18
These COVID-19 case-management guidelines included adjunct and supportive therapy (vitamin D 500 mg twice per day for 14 days for adults and children >12 years, 125–250 mg in one or two doses per day for 14 days in children <4 years, or 250–500 mg in one or two doses for 14 days for children aged 5–12 years; zinc 20 mg once per day for 14 days; and paracetamol tablets 10–15 mg/kg every 8 h for 3–5 days) for asymptomatic, mild, moderate, and severe or critical clinical categories; oral antibiotics (azithromycin 500 mg once per day for 5 days for adults and children >12 years; or amoxicillin 500 mg every 8 h for 7 days for adults and children >12 years or 40 mg every 8 h for 7 days for children <12 years) for moderate cases; oxygen therapy, intravenous dexamethasone (6–10 mg per day), intravenous heparin and oral warfarin, and various intravenous antibiotics for severe cases; and fluid therapy, vasopressor therapy (dobutamine and epinephrine), anticoagulants, intravenous antibiotics, and ventilation for critical cases. During the study period, the case-management guidelines also included hospital treatment for at least 10–14 days, for all individuals infected with SARS-CoV-2 of all clinical categories, including asymptomatic patients diagnosed through routine screening of contacts of patients with COVID-19, or those diagnosed at border entry points. Malaria treatment was based on the results of rapid diagnostic tests (RDTs), and included artemether-lumefantrine tablets (Novartis, Basel, Switzerland) for uncomplicated malaria and intravenous artesunate (Guilin Pharmaceutical, Shanghai, China) for severe malaria, as per national guidelines.19 Blood-sample collection for laboratory assays was done at admission. Malaria diagnosis for all patients was done using three methods, which were RDTs (Paracheck; Orchid Biomedical System, Goa, India), microscopy, and molecular methods. Patients were considered to be positive for malaria if any one of these three methods gave a positive result. For microscopy, thick blood smears stained with 2% Giemsa were examined for the presence of asexual parasites in 200 fields using a 100× oil-immersion objective lens by two trained microscopists. A 20% error check was used to identify discrepancies between slide readers and all discordant results were read by a third reader (a senior microscopist).
Parasite density was estimated by counting the number of parasites per 200 white blood cells (WBCs), assuming 8000 WBCs per μL. Blood smears were considered negative if no parasites were found after reading 100 high-power fields. Molecular detection of P falciparum was done at the Molecular Laboratory at Makerere University in Kampala, Uganda, using established methods (appendix p 3).20 Extracted DNA was analysed with varATS quantitative PCR (qPCR) using 5 μL extracted DNA per assay. All qPCR outputs were analysed using the BioRad CFX Manager software, version 3.1. The sensitivity of the assay was approximately 0·02 parasites per μL of blood. Previous individual P falciparum exposure was assessed using serological responses to a panel of P falciparum antigens using a multiplex bead assay, as previously described.21 These included responses associated with cumulative malaria exposure, namely apical membrane antigen 1 (AMA-1), merozoite surface protein 1.19 (MSP1.19), and glutamate-rich protein region 2 (GLURP.R2),21, 22 and responses associated with malaria infection in the past 6 months, namely reticulocyte-binding protein homologue (Rh2.2030), gametocyte-exported protein (GEXP18), and early-transcribed membrane protein (Etramp5.Ag1).23 Patients were then ranked by the cumulative quartile score of the mean fluorescent intensities of these six predefined antigens, and those with the highest scores were assigned to high malaria-exposure groups, those with medium scores were assigned to medium malaria-exposure groups, and those with the lowest scores were assigned to low malaria-exposure groups.
Complete blood count and serum biochemistries, including liver and renal function tests, were done using standardised procedures at the Case Hospital Clinical Laboratory in Kampala, Uganda. Although HIV clinical staging was not documented, HIV testing was done using Ministry of Health protocols.24 Testing for C-reactive protein (CRP) was done with the Selectra ProXL immunofluorescence analyser (ELITechGroup, Puteaux, France) and supporting kits (MISPA i3; Agape Diagnostics Switzerland, Cham, Switzerland). Serum cytokines, including interleukin (IL)-2, IL-6, IL-7, IL-8, IL-10, transforming growth factor β (TGF-β1), and tumour-necrosis factor α (TNF-α) were tested with the BD FACSCalibur flow cytometer (BD Bioscience, Mississauga, ON, Canada) and human T-helper cell (Th) 1 and Th2 cytokines kit (Bio-Techne, Abingdon, UK) following the manufacturers' instructions. Result outputs for each of these laboratory assays included normal value ranges, from which the thresholds for elevation of the parameters was determined. Laboratory personnel were masked to patient clinical status to avoid potential bias.
Outcomes
The main outcome was prevalence of P falciparum infection in this cohort of patients infected with SARS-CoV-2. Secondary outcomes included patient clinical characteristics, association between P falciparum infection, previous malaria exposure, and COVID-19 clinical characteristics (including signs and symptoms at presentation and clinical classification as asymptomatic, mild, moderate, or severe or critical), COVID-19 clinical outcomes (including favourable outcomes, defined as discharge in good condition, unfavourable outcomes, defined as death, discharged with sequelae, or requiring intensive care), and predictors of unfavourable outcomes.
Statistical analysis
As this study was exploratory, a convenience sample of 600 participants selected from an available population of approximately 800 patients with SARS-CoV-2 infection admitted to hospital (75% of the sampling frame) was included in this preliminary investigation. Categorisation of serological responses was done as in Achan and colleagues,22 with highest and lowest deciles used to differentiate high and low malaria exposure. Descriptive statistics were computed for continuous variables (age and laboratory parameters, including complete blood count, liver-function and renal function profiles, cytokine concentrations, and individual P falciparum antibody responses) and categorical variables (sex, age categories 0–20 years, 21–40 years, 41–60 years, and >60 years, marital status, education level, occupation, patient signs and symptoms, COVID-19 severity, comorbidities, P falciparum infection status, previous malaria exposure category, and COVID-19 clinical outcomes). Medians with IQRs were calculated for skewed data distributions and means with SDs for normally distributed data. Proportions and 95% CIs were computed for categorical variables. Fisher's exact test was used to evaluate the significance of the differences in proportions. Statistical comparisons between subgroups of continuous variables (laboratory parameters, including complete blood counts and serum biochemistries) were evaluated with t tests, ANOVAs, Mann-Whitney U tests, and Kruskal-Wallis tests where appropriate. For immunological analyses, differences were assessed by comparing mean values between previous malaria exposure groups using either a two-tailed Student's t test or non-parametric equivalents, with main comparisons of interest being between groups with low previous exposure to P falciparum or high previous exposure. To better understand the relation between previous malaria exposure and COVID-19 clinical presentation and outcomes, we further categorised the patients into two groups, those with comorbidities and those without comorbidities. One-way ANOVAs were done to compare mean individual P falciparum antibody responses (Kruskal-Wallis test) across the illness gradient associated with COVID-19 symptoms, with adjusted multiple comparisons against the symptomatic category. Mean (SD) cytokine concentrations according to COVID-19-severity groups, presence of malaria co-infection, amount of previous malaria exposure, and clinical outcomes were compared using a linear-regression approach to estimate the regression coefficient and 95% CI. A forward-fitting logistics regression model was used to establish predictors of unfavourable outcomes by estimating the crude and adjusted odds ratio (OR) and 95% CI, with parameters that had a p<0·1 at bivariate analysis (age categories, sex, current malaria infection, previous malaria infection, diabetes, and heart disease) included in the multivariate model. Kaplan-Meier survival analyses were used to estimate recovery rates and comparisons done using log-rank tests. p values lower than 0·05 based on a two-sided hypothesis were considered significant. When data were missing, the numbers involved are stated and no attempt was made to impute missing values. Data analyses were done using STATA version 15.0.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Of 600 patients admitted to hospital with SARS-CoV-2 infection and enrolled and followed up from April 15, to Oct 30, 2020, we included 597 (>99%) with complete information in our analyses (appendix p 4). Patient characteristics at admission are summarised (table 1 ). The majority were male individuals, reflecting the COVID-19 patient profile in Uganda. Median age was 36 years (IQR 28–47), with 46 (8%) of 597 patients older than 60 years and 23 (4%) aged 20 years or less. 169 (38%) of 450 patients were long-distance truck drivers and 22 (5%) were health-care workers. Comorbidities were reported by 233 (39%) of 597 patients, with hypertension, diabetes, and HIV being the most prevalent. The most common symptoms at presentation included cough, runny nose, fever, headache, shortness of breath, and chest pain (Table 1, Table 2 ). Median temperature was 36·5°C (IQR 36·3–36·7) and only 26 (5%) of 545 patients had a documented temperature of at least 37·5°C. Overall, of 597 patients, 255 (43%) were asymptomatic, 233 (39%) had mild symptoms, 45 (8%) had moderate symptoms, 48 (8%) had severe symptoms, and 16 (3%) had critical COVID-19 symptoms. Mean duration of hospital stay was 17·4 days (SD 4·6), and 115 (21%) of 553 patients were treated in hospital for more than 21 days. The majority of patients were discharged in good general condition. Nine (2%) of 597 patients died.
Table 1.
Baseline demographic characteristics of patients with COVID-19 in Uganda
| Overall (n=597) | |
|---|---|
| Age categories* | |
| 0–20 years | 23 (4%) |
| 21–40 years | 355 (60%) |
| 41–60 years | 163 (28%) |
| >60 years | 46 (8%) |
| Age, years | |
| Mean (SD) | 38·2 (13) |
| Median (IQR) | 36 (28–47) |
| Sex | |
| Male | 502 (84%) |
| Female | 95 (16%) |
| Ethnicity | |
| Black | 587 (98%) |
| Asian | 10 (2%) |
| Marital status† | |
| Single | 98 (22%) |
| Married | 327 (74%) |
| Separated | 13 (3%) |
| Education‡ | |
| None | 26 (10%) |
| Primary | 76 (28%) |
| Secondary | 95 (35%) |
| University | 47 (17%) |
| Other tertiary | 28 (10%) |
| Occupation§ | |
| Unemployed | 10 (2%) |
| Farmer | 12 (3%) |
| Health worker | 22 (5%) |
| Armed forces | 17 (4%) |
| Professionals | 41 (9%) |
| Business | 24 (5%) |
| Truck driver | 169 (38%) |
| Other | 160 (36%) |
| Comorbidities | |
| Tuberculosis | 3 (1%) |
| Diabetes | 49 (8%) |
| Asthma or chronic obstructive pulmonary disease | 9 (2%) |
| Obesity | 16 (3%) |
| Heart disease | 23 (4%) |
| HIV | 35 (6%) |
| Hypertension | 149 (25%) |
Data are n (%) unless otherwise specified.
10 missing age.
154 missing marital status.
325 missing education information.
147 missing occupation information.
Table 2.
Association between clinical parameters among patients with SARS-CoV-2 and P falciparum infection and previous malaria exposure
| Overall (n=597) |
Patients with COVID-19 by malaria infection and exposure category |
|||||||
|---|---|---|---|---|---|---|---|---|
| Current malaria infection |
Previous malaria exposure |
|||||||
| Malaria (n=70) | No malaria (n=527) | Fisher's exact p value | Low (n=53) | Medium (n=418) | High (n=56) | Fisher's exact p value | ||
| Presence of signs and symptoms | ||||||||
| Fever | 121 (20%) | 15 (21%) | 106 (20%) | 0·75 | 17 (32%) | 92 (22%) | 6 (11%) | 0·024 |
| Cough | 198 (33%) | 19 (27%) | 179 (34%) | 0·34 | 27 (51%) | 150 (36%) | 13 (23%) | 0·012 |
| Runny nose | 129 (22%) | 11 (16%) | 118 (22%) | 0·22 | 9 (17%) | 103 (25%) | 10 (18%) | 0·30 |
| Shortness of breath | 98 (16%) | 13 (19%) | 85 (16%) | 0·61 | 23 (43%) | 71 (17%) | 3 (5%) | 0·0010 |
| Muscle pains | 22 (4%) | 3 (4%) | 19 (4%) | 0·73 | 0 | 20 (5%) | 1 (2%) | 0·23 |
| Confusion | 12 (2%) | 4 (6%) | 8 (2%) | 0·040 | 1 (2%) | 9 (2%) | 0 | 0·72 |
| Headache | 112 (19%) | 16 (23%) | 96 (18%) | 0·33 | 13 (25%) | 81 (19%) | 11 (20%) | 0·67 |
| Sore throat | 49 (8%) | 2 (3%) | 47 (9%) | 0·10 | 5 (9%) | 38 (9%) | 4 (7%) | 0·92 |
| Chest pain | 84 (14%) | 8 (11%) | 76 (14%) | 0·59 | 17 (32%) | 61 (15%) | 4 (7%) | 0·0010 |
| Diarrhoea | 20 (3%) | 4 (6%) | 16 (3%) | 0·28 | 3 (6%) | 16 (4%) | 1 (2%) | 0·53 |
| Vomiting | 9 (2%) | 4 (6%) | 5 (1%) | 0·014 | 0 | 7 (2%) | 2 (4%) | 0·43 |
| Fatigue | 17 (3%) | 1 (1%) | 16 (3%) | 0·71 | 3 (6%) | 13 (3%) | 0 | 0·21 |
| Red eyes | 4 (1%) | 0 | 4 (1%) | 1·00 | 1 (2%) | 3 (1%) | 0 | 0·39 |
| COVID-19 severity | ||||||||
| Asymptomatic | 255 (43%) | 36 (51%) | 219 (42%) | 0·084 | 13 (25%) | 155 (37%) | 32 (57%) | 0·0010 |
| Mild | 233 (39%) | 18 (26%) | 215 (41%) | 0·084 | 17 (32%) | 183 (44%) | 20 (36%) | 0·0010 |
| Moderate | 45 (8%) | 6 (9%) | 38 (7%) | 0·084 | 7 (13%) | 35 (8%) | 1 (2%) | 0·0010 |
| Severe or critical | 64 (11%) | 10 (14%) | 54 (10%) | 0·084 | 16 (30%) | 45 (11%) | 3 (5%) | 0·0010 |
| Comorbidities | ||||||||
| Tuberculosis | 3 (1%) | 0 | 3 (1%) | 1·00 | 0 | 3 (1%) | 0 | 1·00 |
| Diabetes | 49 (8%) | 7 (10%) | 42 (8%) | 0·64 | 12 (23%) | 28 (7%) | 2 (4%) | 0·0010 |
| Chronic obstructive pulmonary disease | 1 (<1%) | 1 (1%) | 0 | 0·12 | 0 | 1 (<1%) | 0 | 1·00 |
| Asthma | 8 (1%) | 0 (0%) | 8 (2%) | 1·00 | 4 (8%) | 4 (1%) | 0 | 0·014 |
| Obesity | 16 (3%) | 3 (4%) | 13 (3%) | 0·42 | 5 (9%) | 10 (2%) | 1 (2%) | 0·031 |
| Heart disease | 23 (4%) | 2 (3%) | 21 (4%) | 1·00 | 7 (13%) | 14 (3%) | 0 | 0·0030 |
| HIV | 35 (6%) | 5 (7%) | 30 (6%) | 0·59 | 1 (2%) | 21 (5%) | 4 (7%) | 0·43 |
| Hypertension | 149 (25%) | 11 (16%) | 138 (26%) | 0·057 | 20 (38%) | 100 (24%) | 12 (21%) | 0·085 |
| Clinical outcomes | ||||||||
| Discharged in good condition | 479 (80%) | 57 (81%) | 422 (80%) | 0·56 | 32 (60%) | 334 (80%) | 48 (86%) | 0·094 |
| Discharged with sequelae | 7 (1%) | 0 | 7 (1%) | 0·56 | 0 | 6 (1%) | 0 | 0·094 |
| Admitted to intensive-care unit | 31 (5%) | 4 (6%) | 27 (5%) | 0·56 | 7 (13%) | 22 (5%) | 2 (4%) | 0·094 |
| Died | 9 (2%) | 2 (3%) | 7 (1%) | 0·56 | 2 (4%) | 7 (2%) | 0 | 0·094 |
The overall prevalence of P falciparum infection was 12% (95% CI 9·4–14·6; 70 of 597 patients; table 3 ), whereas the prevalence established by RDT or microscopy alone was 4% (2·6–5·9; 24 of 597 patients). The highest overall prevalence of P falciparum infection was observed in the age groups of 0–20 years and older than 60 years. Median parasite density among patients who were positive for P falciparum was 104 parasites per μL (IQR 32–1010). Patients with P falciparum co-infection had a higher frequency of confusion and vomiting than those without malaria (table 2). There were no differences in sex (p=0·52), occupation (p=0·51), fever (p=0·75), headache (p=0·33), or duration of hospital stay (p=0·95) between patients with SARS-CoV-2 infection with and without P falciparum co-infection. Mean platelet count (216 400, SD 10 500, vs 240 100, 3900; p=0·042) and serum albumin concentrations (38·8, SD 1·4, vs 42·1, 0·4; p=0·0080) were significantly lower in patients with SARS-CoV-2 and P falciparum co-infection than in those without (appendix p 5). Overall, patients who were positive for P falciparum had a lower prevalence of liver-function abnormalities and lower median liver-enzyme concentrations than those who were P falciparum-negative, with significant differences in the proportions of patients with elevated gamma-glutamyl transferase (four [7%] of 58 patients vs 81 [18%] of 456 patients; p=0·036) and total protein (41 [71%] of 58 patients vs 367 [83%] of 440 patients; p=0·018; appendix p 5). Kaplan-Meier survival analyses showed no significant differences in probability of unfavourable outcomes among patients with and without P falciparum infection (log rank p=0·55; appendix p 6).
Table 3.
Prevalence of P falciparum infection and previous malaria exposure in the study cohort
| n/N | Percentage (95% CI) | ||
|---|---|---|---|
| P falciparum infection* | 70/597 | 12·0% (9·4–14·6) | |
| P falciparum infection by age* | |||
| 0–20 years | 5/23 | 22·0% (8·7–44·8) | |
| 21–40 years | 36/365 | 10·0% (6·7–12·8) | |
| 41–60 years | 20/163 | 12·0% (8·0–18·3) | |
| >60 years | 9/46 | 20·0% (10·2–34·1) | |
| Previous malaria exposure† | |||
| Low | 53/527 | 10·0% (7·8–12·9) | |
| Medium | 418/527 | 79·0% (75·6–82·6) | |
| High | 56/527 | 11·0% (8·3–13·6) | |
Defined as positive by either rapid diagnostic test, microscopy, or molecular methods (PCR).
Malaria exposure data were missing for 70 participants because of insufficient blood sample volume.
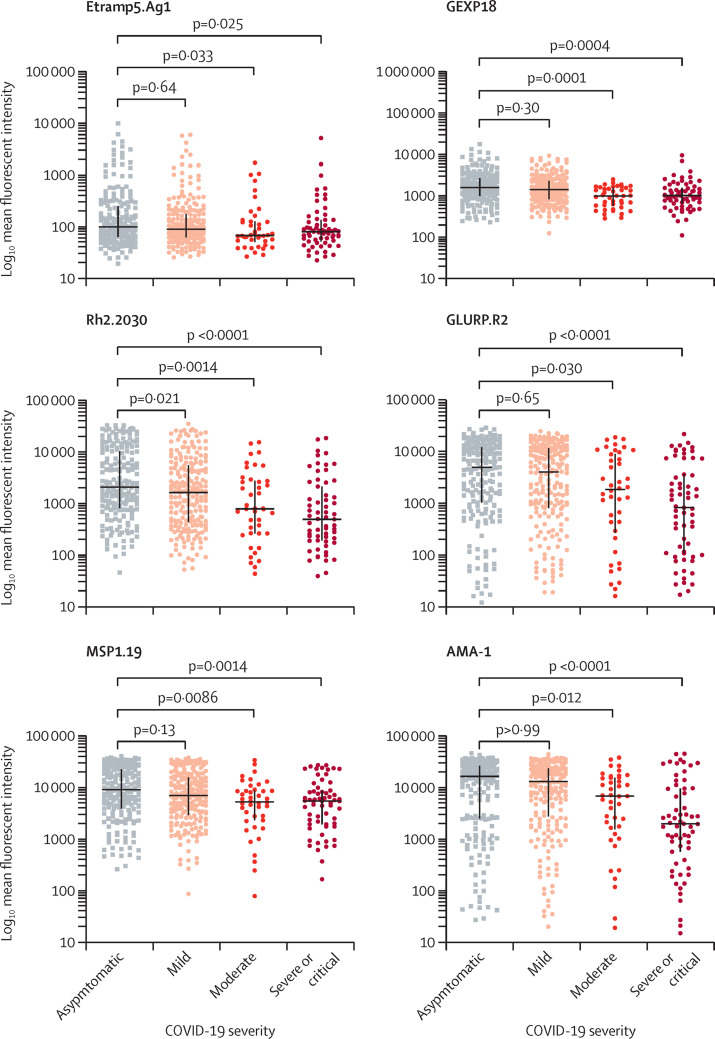
The majority of patients had medium previous P falciparum exposure, with approximately equal proportions having low and high previous exposure (table 3). Data for 70 participants were not available because of insufficient blood samples. Patients with low previous malaria exposure were older (mean age 45·8 years, SD 12·7) than those with medium exposure (37·6 years, 13·1) and high exposure (37·5 years, 12·7; p=0·0010). The majority of patients in the group with low previous malaria exposure were aged 41–60 years (24 [45%] of 53 patients) and 21–40 years (18 [34%] of 53 patients), with only nine (17%) of 53 patients older than 60 years (appendix p 7). A similar distribution was also seen in the groups with medium and high previous malaria exposure. The group with low previous malaria exposure had a higher frequency of fever, cough, shortness of breath, and chest pain than those with high previous malaria exposure (table 2). Patients with low previous malaria exposure were more likely to present with severe or critical COVID-19 (p=0·0010) and have a higher burden of comorbidities, including diabetes, asthma, obesity, and heart disease than those with high previous malaria exposure. Two (4%) of 53 participants in the low-exposure group, seven (2%) of 418 in the medium-exposure group, and no patients in the high-exposure group died; the difference in mortality between the high-exposure and low-exposure groups was not significant (table 2). Median antibody responses to individual P falciparum antigens were significantly lower in patients with severe or critical COVID-19 than in those who were asymptomatic, but the differences were modest for Etramp5.Ag1 (figure ). An overall significant mean rank difference was found within the four groups (p<0·0001 for all six antigens).
Figure.
Dot plots comparing individual Plasmodium falciparum antibody responses in participants with different severities of COVID-19
513 patients with COVID-19 were included in this one-way ANOVA comparing mean ranks (Kruskal-Wallis test) across the illness spectrum associated with COVID-19 symptoms (195 asymptomatic, 213 mild, 42 moderate, and 63 severe or critical cases). An overall significant mean-rank difference was found within the four groups (p<0·0001 for all six antigens). Horizontal lines (whiskers) represent median (IQR) fluorescent intensities. p values from adjusted multiple comparisons between categories of COVID-19 severity and the asymptomatic group are shown. AMA-1=apical membrane antigen 1. Etramp5.Ag1=early-transcribed membrane protein. GEXP18=gametocyte-exported protein. GLURP.R2=glutamate-rich protein region 2. MSP1.19=merozoite surface protein 1.19. Rh2.2030=reticulocyte-binding protein homologue.
Among patients with comorbidities, the group with low previous malaria exposure had a significantly higher frequency of shortness of breath than the group with high previous malaria exposure (14 [70%] of 20 patients vs one [14%] of seven patients; p=0·047), and although not statistically significant, differences were also present in the proportion of patients with severe or critical COVID-19 (ten [50%] of 20 patients vs two [29%] of seven patients; p=0·55) and unfavourable outcomes (eight [40%] of 20 patients vs two [29%] of seven patients; p=0·48; appendix p 8). Among those with no comorbidities, the group with low previous malaria exposure had a higher frequency of fever (11 [33%] of 33 patients vs four [8%] of 49 patients; p=0·019), shortness of breath (nine [27%] of 33 patients vs two [4%] of 49 patients; p=0·0050), chest pain (eight [24%] of 33 patients vs two [4%] of 49 patients; p=0·018), and severe or critical COVID-19 (six [18%] of 33 patients vs one [2%] of 49 patients; p=0·015) than patients in the group with high previous malaria exposure (appendix p 9).
There were no significant differences in cytokine concentrations among patients who were positive and negative for P falciparum, except for TNF-α, which was significantly higher in patients who were positive for P falciparum (5·4 pg/mL vs 5·8 pg/mL; regression coefficient 0·6, 95% CI 0·3 to 1·0; p=0·0010; appendix pp 10–11). Patients with medium and high previous malaria exposure had significantly lower concentrations of IL-7 (regression coefficient −2·9, −4·9 to −0·9, p=0·0040, and regression coefficient −5·6, −8·2 to −3·0, p=0·0010) and TGF-β1 (regression coefficient −34 813·9, −65344·4 to −4283·4, p=0·026, and regression coefficient −85 439·2, p=0·0010) than those with low malaria exposure (appendix pp 10–11). Cytokine concentrations also varied with severity of COVID-19, with higher IL-6 concentrations in patients with moderate (10·8 pg/mL; regression coefficient 9·3, 5·5 to 13·1; p=0·0010) and severe or critical COVID-19 (13·0 pg/mL; regression coefficient 11·5, 8·2 to 14·7; p=0·0010) and higher IL-7 concentrations among patients with moderate (12·4 pg/mL; regression coefficient 6·5, 4·4 to 8·5; p=0·0010) and severe or critical COVID-19 (13·9 pg/mL; regression coefficient 7·7, 5·9 to 7·1; p=0·0010) than in asymptomatic patients. TNF-α (5·4 pg/mL; regression coefficient 0·6, 0·2 to 1·0; p=0·0040) and TGF-β1 (192 485 pg/mL; regression coefficient 30 028·2, 162·9 to 59893·5; p=0·049) were also higher among patients with severe or critical COVID-19 than asymptomatic patients. IL-8 concentrations were lowest among patients with mild COVID-19 (28·4 pg/mL; regression coefficient −24·7, −43·2 to −6·3; p=0·0090) and did not significantly vary with P falciparum infection, previous malaria exposure, or clinical outcomes. No significant elevation in IL-2 was observed in this patient population. Unfavourable outcomes were associated with high concentrations of IL-6 (20·1 pg/mL; regression coefficient 17·3, 11·2 to 23·3; p=0·0010), TNF-α (5·8 pg/mL; regression coefficient 0·9, 0·1 to 1·8; p=0·029) and IL-10 (160 pg/mL; regression coefficient 149·1, 84·5 to 213·6); p=0·0010; appendix pp 10–11).
Overall, 73 (12%) of 597 patients had an unfavourable outcome and being older than 60 years was associated with significantly higher odds of unfavourable outcomes. Among patients with diabetes, the adjusted OR for unfavourable outcomes was 2·1 (0·9–5·0; p=0·077; table 4 ).
Table 4.
Clinical factors associated with unfavourable outcomes among patients with COVID-19
| Patients with unfavourable COVID-19 outcomes*(n=73) | Patients discharged in good condition (n=524) | Crude OR (95% CI) | Adjusted OR (95% CI) | p value | |
|---|---|---|---|---|---|
| Age categories* | |||||
| 0–20 years | 2 (3%) | 21 (4%) | Ref | Ref | .. |
| 21–40 years | 32 (44%) | 323 (62%) | 1·0 (0·2–4·6) | 1·8 (0·2–14·0) | 0·58 |
| 41–60 years | 18 (25%) | 145 (28%) | 1·3 (0·3–6·0) | 1·9 (0·2–15·8) | 0·53 |
| >60 years | 19 (26%) | 27 (5%) | 7·4 (1·5–35·3) | 8·7 (1·0–75·5) | 0·049 |
| Sex | |||||
| Male | 59 (81%) | 443 (85%) | Ref | Ref | .. |
| Female | 14 (19%) | 81 (15%) | 1·3 (0·7–2·4) | 0·9 (0·4–1·9) | 0·82 |
| Current malaria infection | |||||
| Negative | 65 (89%) | 462 (88%) | Ref | Ref | .. |
| Positive | 8 (11%) | 62 (12%) | 0·9 (0·4–2·0) | 0·8 (0·3–2·0) | 0·66 |
| Previous malaria exposure | |||||
| Moderate | 53 (73%) | 365 (70%) | Ref | Ref | .. |
| Low | 10 (14%) | 43 (8%) | 1·6 (0·8–3·4) | 1·1 (0·5–2·5) | 0·84 |
| High | 6 (8%) | 50 (10%) | 0·8 (0·3–2·0) | 1·0 (0·4–2·6) | >0·99 |
| Diabetes | |||||
| No | 57 (78%) | 488 (93%) | Ref | Ref | .. |
| Yes | 16 (22%) | 33 (6%) | 4·2 (2·2–8·0) | 2·1 (0·9–5·0) | 0·077 |
| Heart disease | |||||
| No | 64 (88%) | 501 (96%) | Ref | Ref | .. |
| Yes | 7 (10%) | 16 (3%) | 0·3 (0·1–0·7) | 0·5 (0·2–1·5) | 0·22 |
OR=odds ratio. Ref=reference.
Defined as death, discharge with sequelae, or requiring intensive care.
Discussion
This study reports the prevalence and describes the clinical characteristics of SARS-CoV-2 and P falciparum co-infection, and assesses the effects of previous malaria exposure on COVID-19 clinical presentation and outcomes in a cohort of individuals positive for SARS-CoV-2 who were admitted to hospitals in Uganda. To our knowledge, this study is the first to characterise potential interactions between SARS-CoV-2 and P falciparum infection. In this cohort of patients with COVID-19 admitted to hospital, the overall prevalence of malaria was 12%, and low previous malaria exposure was associated with more severe COVID-19 and higher probability of adverse outcomes. The prevalence of malaria in this cohort is similar to population-level regional estimates in the country, as shown by studies that used molecular methods for malaria diagnosis.25 However, although malaria-disease burden in malaria-endemic settings such as Uganda is largely concentrated in infants and young children, the substantial burden of P falciparum infection among patients with COVID-19 who were older than 60 years of age in this study is intriguing and requires further investigation.
Given the important symptom overlap between COVID-19 and malaria, the higher frequency of confusion and vomiting and the distinct laboratory profiles of patients with SARS-CoV-2 and P falciparum infection are important clinical correlates that should trigger investigations for malaria and ensure integrated management. The higher prevalence of HIV among patients with COVID-19 and malaria, although non-significant, requires further evaluation to better understand the clinical implications of all three co-infections at the individual patient level. The observation of similar clinical outcomes in patients with COVID-19 with and without P falciparum co-infection is encouraging and could suggest that immunomodulation caused by malaria13, 14, 15 might not be deleterious. The association between low previous malaria exposure and severe or critical COVID-19 along with a trend towards a higher probability of unfavourable outcomes is novel. However, patients with low previous malaria exposure were also older and had a higher burden of comorbidities. Low malaria exposure in the older age group might be caused by antibody decay due to long-term interruption of exposure to blood-stage infection that could result from more consistent use of exposure-reducing interventions, such as bednets. Given that both age and comorbidities are associated with severe or critical COVID-19 and adverse outcomes,26, 27, 28 the correlation between previous malaria exposure and COVID-19 clinical presentation and outcomes will require further study. However, findings among patients with COVID-19 without comorbidities indicate that the association between low previous malaria exposure and more severe manifestations of COVID-19 remains even in the absence of comorbidities. This observation is important because it could imply that malaria exposure might have a role in the pathogenesis of COVID-19 in these settings and other similar high-malaria-burden settings.
Although cytokine profiles in patients with COVID-19 in setting of low malaria burden have been well characterised,29, 30, 31, 32 data from high-burden settings are scarce. In our study, TNF-α concentrations were higher among patients with P falciparum infection, IL-7 and TGF-β1 concentrations were higher among patients with low malaria exposure, and IL-6, IL-10, TNF-α, and TGF-β1 concentrations were higher among patients with more severe manifestations and adverse outcomes. Our findings concur with accumulating evidence that suggests that severity of COVID-19 is associated with an increase in cytokines and chemokines, including IL-2, IL-7, IL-10, TNF-α, CRP, and IL-6, with IL-6 being highly correlated with mortality.33, 34 With the exception of TNF-α, P falciparum infection did not significantly affect cytokine concentrations in our study population. This finding could suggest a blunting of cytokine and chemokine responses to malaria that has been shown to occur in older children and adults residing in endemic areas, which might be caused by repeated malaria exposure.35 These findings are further supported by the relatively normal cytokine profiles among patients in this study who had high previous malaria exposure. Therefore, the unique balance between proinflammatory and anti-inflammatory cytokines could explain the clinical observations among patients with SARS-CoV-2 and P falciparum co-infection.
The overall mortality in our study population of 2% is significantly lower than that in Europe, China, and Egypt,33, 34, 36 but is similar to that reported in other malaria-endemic settings, such as Burkina Faso and Kenya.36 Predictors of unfavourable outcomes such as age and high IL-6 concentrations are consistent with those previously described.37, 38
This study had some limitations, including a small sample size of patients who were positive for P falciparum, and the absence of information on SARS-CoV-2 viral load and radiology; for these reasons, this study is primarily an exploratory and preliminary investigation. Despite this fact, the strengths of our study include the prospective design, broad eligibility criteria, small loss to follow-up, and robust malaria diagnostic and immunology assays.
In conclusion, the prevalence of P falciparum infection among patients with COVID-19 treated in hospital in this setting of high malaria transmission was 12%. Although patients with COVID-19 with P falciparum infection had a higher frequency of confusion and vomiting, co-infection with malaria did not seem deleterious. The association between low previous malaria exposure and severe or critical COVID-19 and adverse outcomes will require further study. These preliminary observations highlight the importance of understanding the potential clinical and therapeutic implications of such overlapping co-infections in similar settings with high malaria burden.
Data sharing
All data requests should be submitted to the corresponding author (JA) for consideration. Requests will be assessed for scientific rigour before being granted. Data will be anonymised and securely transferred. Patient-level data will be made available within 6 months of publication. Related documents will be available on request. A data-sharing agreement might be required.
Declaration of interests
HA and FN are members of the Mulago Hospital Research and Ethics but did not participate in decisions pertaining to this study. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This project was funded by the US Malaria Consortium. We would like to thank the health-care workers at all the COVID-19 treatment centres for their contribution to the study. We extend special gratitude to the patients for their contributions to the study. We acknowledge the immense support from Charles Nelson, and the Malaria Consortium administrative team in Uganda and the UK. We would also like to thank the laboratory teams at the Molecular Laboratory at Makerere University and the Case Hospital Clinical Laboratory.
Contributors
JA and JKT conceived and designed the study. JA, AS, IS, HA, and FN were involved in study implementation and data collection. JA, HW, and IS did the statistical analysis. JA drafted the first version of the manuscript. JA, JKT, AS, HW, CD, and IS had access to and verified the study data. All authors contributed to data review and interpretation and finalised and approved the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.WHO. Coronavirus disease (COVID-19) dashboard. 2021. httwvid19.who.int/ (accessed Oct 8, 2021).
- 2.UN Impact of COVID-19 in Africa. 2020. https://unsdg.un.org/sites/default/files/2020-05/Policy-brief-Impact-of-COVID-19-in-Africa.pdf
- 3.WHO Coronavirus disease 2019 (COVID-19): situation reports. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports
- 4.WHO . World Health Organization; Geneva: 2020. World malaria report. [Google Scholar]
- 5.WHO . World Health Organization; Geneva: 2019. Global tuberculosis report. [Google Scholar]
- 6.WHO HIV/AIDS. 2019. https://www.afro.who.int/health-topics/hivaids
- 7.Ghisolfi S, Almås I, Sandefur JC, von Carnap T, Heitner J, Bold T. Predicted COVID-19 fatality rates based on age, sex, comorbidities and health system capacity. BMJ Glob Health. 2020;5 doi: 10.1136/bmjgh-2020-003094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO . World Health Organization; Geneva: 2020. The potential impact of health service disruptions on the burden of malaria: a modelling analysis for countries in sub-Saharan Africa. [Google Scholar]
- 9.Sherrard-Smith E, Hogan AB, Hamlet A, et al. The potential public health consequences of COVID-19 on malaria in Africa. Nat Med. 2020;26:1411–1416. doi: 10.1038/s41591-020-1025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jewell BL, Smith JA, Hallett TB. Understanding the impact of interruptions to HIV services during the COVID-19 pandemic: a modelling study. EClin Med. 2020;26 doi: 10.1016/j.eclinm.2020.100483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jewell BL, Mudimu E, Stover J, et al. Potential effects of disruption to HIV programmes in sub-Saharan Africa caused by COVID-19: results from multiple mathematical models. Lancet HIV. 2020;7:e629–e640. doi: 10.1016/S2352-3018(20)30211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alene KA, Wangdi K, Clements ACA. Impact of the COVID-19 pandemic on tuberculosis control: an overview. Trop Med Infect Dis. 2020;5:E123. doi: 10.3390/tropicalmed5030123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coban C, Ishii KJ, Kawai T, et al. Toll-like receptor 9 mediates innate immune activation by the malaria pigment hemozoin. J Exp Med. 2005;201:19–25. doi: 10.1084/jem.20041836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frosch AE, John CC. Immunomodulation in Plasmodium falciparum malaria: experiments in nature and their conflicting implications for potential therapeutic agents. Expert Rev Anti Infect Ther. 2012;10:1343–1356. doi: 10.1586/eri.12.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson MG, Breiman RF, Hamel MJ, et al. Influenza and malaria coinfection among young children in western Kenya, 2009–2011. J Infect Dis. 2012;206:1674–1684. doi: 10.1093/infdis/jis591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards CL, Zhang V, Werder RB, et al. Coinfection with blood-stage plasmodium promotes systemic type I interferon production during pneumovirus infection but impairs inflammation and viral control in the lung. Clin Vaccine Immunol. 2015;22:477–483. doi: 10.1128/CVI.00051-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gutman JR, Lucchi NW, Cantey PT, et al. Malaria and parasitic neglected tropical diseases: potential syndemics with COVID-19? Am J Trop Med Hyg. 2020;103:572–577. doi: 10.4269/ajtmh.20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ministry of Health Uganda . Ministry of Health Uganda; Kampala: 2020. National guidelines for management of COVID-19. [Google Scholar]
- 19.Ministry of Health Uganda . Ministry of Health Uganda; Kampala: 2016. National guidelines for management of common conditions. [Google Scholar]
- 20.Hofmann N, Mwingira F, Shekalaghe S, Robinson LJ, Mueller I, Felger I. Ultra-sensitive detection of Plasmodium falciparum by amplification of multi-copy subtelomeric targets. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu L, Hall T, Ssewanyana I, et al. Optimisation and standardisation of a multiplex immunoassay of diverse Plasmodium falciparum antigens to assess changes in malaria transmission using sero-epidemiology. Wellcome Open Res. 2020;4:26. doi: 10.12688/wellcomeopenres.14950.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Achan J, Reuling IJ, Yap XZ, et al. Serologic markers of previous malaria exposure and functional antibodies inhibiting parasite growth are associated with parasite kinetics following a Plasmodium falciparum controlled human infection. Clin Infect Dis. 2020;70:2544–2552. doi: 10.1093/cid/ciz740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helb DA, Tetteh KK, Felgner PL, et al. Novel serologic biomarkers provide accurate estimates of recent Plasmodium falciparum exposure for individuals and communities. Proc Natl Acad Sci USA. 2015;112:e4438–e4447. doi: 10.1073/pnas.1501705112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ministry of Health . 4th edn. Ministry of Health; Kampala: 2016. National HIV testing services policy and implementation guidelines Uganda. [Google Scholar]
- 25.Andolina C, Rek JC, Briggs J, et al. Sources of persistent malaria transmission in a setting with effective malaria control in eastern Uganda: a longitudinal, observational cohort study. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00072-4. published online June 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nachega JB, Ishoso DK, Otokoye JO, et al. Clinical characteristics and outcomes of patients hospitalized for COVID-19 in Africa: early insights from the Democratic Republic of the Congo. Am J Trop Med Hyg. 2020;103:2419–2428. doi: 10.4269/ajtmh.20-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Estiri H, Strasser ZH, Murphy SN. Individualized prediction of COVID-19 adverse outcomes with MLHO. Sci Rep. 2021;11 doi: 10.1038/s41598-021-84781-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mesas AE, Cavero-Redondo I, Álvarez-Bueno C, et al. Predictors of in-hospital COVID-19 mortality: a comprehensive systematic review and meta-analysis exploring differences by age, sex and health conditions. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu YCZ, Huang F, Yang Y, et al. Elevated plasma levels of selective cytokines in COVID-19 patients reflect viral load and lung injury. Natl Sci Rev. 2020;7:1003–1011. doi: 10.1093/nsr/nwaa037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burke H, Freeman A, Cellura DC, et al. Inflammatory phenotyping predicts clinical outcome in COVID-19. Respir Res. 2020;21:245. doi: 10.1186/s12931-020-01511-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merza MY, Hwaiz RA, Hamad BK, Mohammad KA, Hama HA, Karim AY. Analysis of cytokines in SARS-CoV-2 or COVID-19 patients in Erbil city, Kurdistan region of Iraq. PLoS One. 2021;16 doi: 10.1371/journal.pone.0250330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bilinski A, Emanuel EJ. COVID-19 and excess all-cause mortality in the US and 18 comparison countries. JAMA. 2020;324:2100–2102. doi: 10.1001/jama.2020.20717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giacomelli A, Ridolfo AL, Milazzo L, et al. 30-day mortality in patients hospitalized with COVID-19 during the first wave of the Italian epidemic: a prospective cohort study. Pharmacol Res. 2020;158 doi: 10.1016/j.phrs.2020.104931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farrington L, Vance H, Rek J, et al. Both inflammatory and regulatory cytokine responses to malaria are blunted with increasing age in highly exposed children. Malar J. 2017;16:499. doi: 10.1186/s12936-017-2148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.John Hopkins University Mortality analyses. 2021. https://coronavirus.jhu.edu/data/mortality
- 37.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data requests should be submitted to the corresponding author (JA) for consideration. Requests will be assessed for scientific rigour before being granted. Data will be anonymised and securely transferred. Patient-level data will be made available within 6 months of publication. Related documents will be available on request. A data-sharing agreement might be required.