Abstract
Aims:
G protein-coupled estrogen receptor 30 (GPR30) activation by its agonist, G1, exhibits beneficial actions in female with heart failure (HF). Recent evidence indicates its cardiovascular benefits may also include male as well. However, whether and how GPR30 activation may limit HF progression and have a salutary role in males is unknown. We hypothesized that chronic G1 treatment improves LV and cardiomyocyte function, [Ca2+]i regulation and β-adrenergic reserve, thus reversing HF progression in male.
Main Methods:
We compared left ventricle (LV) and myocyte function, [Ca2+]i transient ([Ca2+]iT) and β-AR modulation in control male mice (12/group) and isoproterenol-induced HF (150 mg/kg s.c. for 2 days). Two weeks after isoproterenol injection, HF mice received placebo, or G1 (150 μg/kg/day s.c. mini-pump) for 2 weeks.
Key Findings:
Isoproterenol-treated mice exhibited HF with preserved ejection fraction (HFpEF) at 2-weeks and progressed to HF with reduced EF (HFrEF) at 4-weeks, manifested by significantly increased LV time constant of relaxation (τ), decreased EF and mitral flow (dV/dtmax), which were accompanied by reduced myocyte contraction (dL/dtmax), relaxation (dR/dtmax) and [Ca2+]iT. Acute isoproterenol-superfusion caused significantly smaller increases in dL/dtmax, dR/dtmax and [Ca2+]iT. G1 treatment in HF increased basal and isoproterenol-stimulated increases in EF and LV contractility of EES. Importantly, G1 improved basal and isoproterenol-stimulated dL/dtmax, dR/dtmax and [Ca2+]iT to control levels and restored normal cardiac β-AR subtypes modulation.
Significance:
Chronic G1 treatment restores normal myocyte basal and β-AR-stimulated contraction, relaxation, and [Ca2+]iT, thereby reversing LV dysfunction and playing a rescue role in a male mouse model of HF.
Keywords: G protein-coupled estrogen receptor 30 (GPR30), Heart failure, Pressure-volume relation, Cardiomyocyte, [Ca2+]i regulation, β-adrenergic reserve
1. Introduction
Heart failure (HF) with preserved ejection fraction (HFpEF) is outpacing other forms of HF because of the expanding elderly population. Large cohort studies reveal that almost 50% of the HF populations have HFpEF with a comparable poor prognosis compared to HF with reduced EF (HFrEF). These worrisome epidemiological trends contrast with the uncertainties concerning the pathophysiological mechanisms underlying HFpEF, diagnostic guidelines and therapeutic strategies [1, 2]. So far, no randomized controlled trial has shown improved survival of HFpEF. There are no targeted, effective treatments available.
The G protein-coupled estrogen receptor, also known as G protein-coupled receptor 30 (GPR30), functions alongside traditional estrogen receptors, ERα and ERβ. ERα and GPR30 are expressed at similar levels in cardiac tissue from male and female rodents and humans [3–5]. GPR30 plays critical roles in the development of left ventricular (LV) dysfunction and HF [3, 6, 7]. Activation of GPR30 exhibits beneficial actions in ischemia/reperfusion injury, hypertension and HF [3, 6, 8–11]. While reports on GPR30 have highlighted mostly female sex-specific health issues, recent evidence indicates equivalent benefits in males [3, 12, 13]. It was reported that male, but not female, GPR30-deficient mice suffered from impaired cardiac function [7]. Ejection fraction (EF) and fractional shortening (FS) were both decreased in an age-dependent manner only in GPR30-knockout male mice [12]. Also, cardiomyocyte-specific GPR30-knockout leads to LV dysfunction in both sexes, but with more adverse changes in systolic function and LV structural remodeling among male knockout mice [14], which suggests a potential salutary role of GPR30 activation in male HF [7, 12]. However, the changes caused by GPR30 activation during HF progression in males have not been systematically evaluated. The functional effect of chronic GPR30 activation on single-myocyte mechanics, the dynamics of the cytosolic [Ca2+]i and contractility reserve have not been previously assessed in an integrated fashion in HF. The direct cardiac effects and cellular mechanisms of GPR30 activation-caused cardiac protective effects remain unclear. Moreover, although HFpEF is the most common form of HF, limited studies have explored the roles of GPR30 in the development and progression of diastolic dysfunction. Whether and to what extent chronic GPR30 activation can prevent the progression of HFpEF to HFrEF, or even reverse HFpEF, thereby changing the natural history of HF, remains to be critically examined.
The aim of this study was to explore the impact of chronic GPR30 activation on the progression of HFpEF. We simultaneously assessed the direct cardiac effects and underlying cellular mechanisms on the effects of early initiation of G1, a highly selective GPR30 agonist [8, 15–18], in male mice with isoproterenol-induced HF. Isoproterenol-induced HF, a time-and dose-dependent method, mimics many of the structural, functional, and hormonal changes observed in clinical HF [19–24]. In the current study, isoproterenol-treated-mice exhibited HFpEF at 2 weeks, which progressed to HFrEF at 4 weeks following the last isoproterenol injection. We assessed the hypothesis that early initiation of chronic G1 therapy could interrupt the progression of HFpEF to HFrEF and lead to regression of HFpEF in a male mouse model of isoproterenol-induced progressive HF by: (a) restoring normal LV function and structure; and (b) improving intrinsic myocyte contraction, relaxation, [Ca2+]i transient ([Ca2+]iT) and β-adrenergic reserve accompanied with reversing the abnormal cardiac β-adrenergic receptor (β-AR) subtype system modulation.
2. Materials and Methods
2.1. Animal Model
This study was approved by the Animal Care and Use Committee of Wake Forest School of Medicine and conformed to the national Guide for the Care and Use of Laboratory Animals (NIH Publication 8th Edition, update 2011). The experimental procedures are illustrated in Figure 1. Briefly, total 47 age-matched C57BL/6 male mice (~4 months old) weighing 26~34 g (Charles River Laboratories International, Inc.) were randomly divided into control (n=12) and HF groups (n=35). HF was induced by two subcutaneous injections of isoproterenol (ISO), spaced 24 hours apart, at a dose of 150 mg/kg as documented elsewhere [21–24]. Thirty isoproterenol-treated mice survived after ISO-induction (~12% mortality within 48 hours); these included in the HF group. High doses of isoproterenol cause time-and dose-dependent structural remodeling and cardiac dysfunction that results in HF. Based on our laboratory and others previously published serial time course studies in this model [19–24], and well-characterized histology and altered LV structure and function in isoproterenol-induced HF, we designed a 4-week study. Cardiac function was assessed at the beginning of the study and weekly via transthoracic echocardiography during light isoflurane anesthesia [25]. ISO-treated animals had HFpEF onset by 2 weeks and this progressed to mid-range HFrEF by 4 weeks. To further establish the validity of early diastolic functional impairments by ISO, with maintained systolic function, 6 animals were studied 2 weeks after receiving ISO injections. Other animals were studied during a 4-week period after administration of ISO. Two weeks after receiving ISO injections, the HFpEF animals were divided into 2 subgroups (12/group) without or with 2-weeks therapeutic intervention: 1) HF, received placebo; and 2) HF/G1, received G1 treatment 150ug/kg/day s.c. via osmotic mini-pump) [Alzet®, model 1004]. The dosing protocol of G1 used was based on our initial concentration-response studies and past reports [15, 26, 27], which had been shown to activate GPR30 in vivo, but no marked effects on heart rate and end-systolic pressure. All animals were maintained in the same environment, including temperature and humidity, and had free access to chow and water.
Figure 1.
A schematization of the experimental procedures and experimental timeline.
2.2. Experimental Protocol
Two sets of experiments were conducted. First, studies were performed in the intact mice to examine systemic hemodynamics, LV contractility, LV diastolic filling dynamic, and LV arterial coupling. Second, to assess the cellular basis of LV functional responses to chronic G1 treatment, we measured cell contraction, relaxation, and [Ca2+]iT responses and β-adrenergic reserve (measured as response to acute β -adrenergic stimulation by isoproterenol) as well as β-AR subtypes (β1- and β3-ARs) responsiveness in the freshly isolated LV myocytes from the same mice.
2.3. Intact Mice Study
Determination of Hemodynamic and LV Functional Responses.
As we and others described previously [22, 23, 28, 29], the mice were anesthetized with intraperitoneal xylazine (10 mg/kg) and ketamine (50 mg/kg), then intubated. To maintain anesthesia and arterial oxygen tension, animals were mechanically-ventilated with a positive-pressure respirator (Model RSP1002, Kent Scientific Corp, Litchfield, CT) using oxygen-enriched room air with isoflurane (0.5–2%). For drug administration, a polyethylene catheter was inserted into the left external jugular vein. With adequate calibration, using our well-established closed-chest approach, a 1.2 F microtip P-V catheter (SPR-839, Millar Instruments, Austin, TX) was inserted into the right carotid artery and advanced into the LV apex. After stabilization, signals were continuously recorded at a sampling rate of 1000 samples/s using a Pressure (P)-Volume (V) conductance system (MPCU-200, Millar Instruments) with BioBench software (National Instruments, Inc). Steady-state and transient inferior vena cava occlusion at baseline were collected. As previously described, with the use of special P-V analysis program (PVAN, Millar Instruments), standard steady-state hemodynamic parameters such as heart rate, LV pressure (P), LV relaxation time constant of (τ), LV volume (V) and the peak rate of mitral flow (dV/dtmax) were measured. LV P-V relations and the slopes were derived. Effective arterial elastance (EA) was calculated as the ratio of end-systolic pressure (PES) and stroke volume (SV), and LV-arterial coupling (LVAC) was calculated as the ratio of the slope of linear PES-VES relation (EES) to EA [30].
2.4. Studies in Isolated LV Myocytes
2.4.1. Myocyte Isolation
After the hemodynamic study, the animals were deeply anesthetized and the hearts were excised and placed in ice-cold calcium-free HEPES buffer solution. By using techniques well-established in our laboratory [22, 23, 28, 31], calcium-tolerant, high-yield myocytes were obtained from each animal. Finally, cells were suspended in the modified HEPES solution (“the study buffer”) with 1.2 mM CaCl2 and stored at room temperature until ready for use. After stabilization (~ 2 hours), as we described previously, LV myocytes were counted, viability and morphology were evaluated. Within 12–14 hours, about 60–70 rod-shaped cells were randomly selected for measurement of myocyte dimensions from each experiment.
2.4.2. Myocyte Function Evaluation
2.4.2.1. Myocyte Function at Baseline and Response to a Non-Selective β-AR Agonist
As we reported previously [23, 28], freshly isolated LV myocytes were placed in superfused culture dishes. Myocyte contraction was elicited by field-stimulation (0.5 Hz) and measured with the Fluorescence and Contractility System (IonOptix, Milton, MA). First, baseline data were recorded. Then data were acquired during superfusion of a non-selective β-AR Agonist, isoproterenol (10−8 M) for 10 min and after drug washout. Changes between baseline and post-superfusion function were defined as myocyte β-adrenergic reserve.
2.4.2.2. Myocyte Functional Response to Selective β1- and β3-AR Agonists
Previously, we have shown that HF is associated with a selective cardiac β1-AR downregulation and β3-AR upregulation (unchanged β2-AR expression) with resultant a decreased β1-AR-mediated positive inotropic action and an enhanced β3-AR-coupled negative inotropic effect in the heart. This restructuring of β-AR system plays a crucial role in the decline of β-adrenergic reserve [31, 32]. To determine the contribution of the subtypes of β-AR stimulation on β-AR reserve with chronic G1 in HF, the above protocol was repeated in subsets of myocytes after myocytes were randomly exposed a selective β1-agonist, Norepinephrine (NE, 10−7 M) or a selective β3-agonist, BRL-37,344 (BRL, 10−8 M), respectively [31, 32]. Data were acquired for 8 to 10 minutes during drug exposure. The percent shortening (SA), the maximum rate of shortening (dL/dtmax), and re-lengthening (dR/dtmax) and [Ca2+]iT were obtained.
2.4.3. Simultaneous Measurement of LV Myocyte Contractile and Calcium Transient Responses
Myocytes were incubated with 10 mM indo-1-AM (Molecular Probes, Eugene, OR) and then placed in a flow-through dish. Contractile and [Ca2+]iT responses in a single cell were measured simultaneously with a dual-excitation fluorescence photo-multiplier system (IonOptix) [23, 28, 31, 32]. As described above, contractile protocol was repeated in the subsets of myocytes. Myocytes were randomly exposured to isoproterenol or a selective β1-, or β3-agonist. After stabilization, both the steady-state baseline data and the responses to β-AR and β-AR Subtype stimulation data were recorded, respectively. After myocytes were loaded with indo-1-AM, compartmentalization of the indicator in mitochondria might have occurred, thus the absolute value of [Ca2+]i was not used. Instead, we calculated relative changes in peak [Ca2+]iT before and after interventions as the ratio of the emitted fluorescence [23, 32].
2.5. Statistical Analysis
Data are presented as mean ± SD. Indices of LV function, hemodynamics, and myocyte function were compared among the treatment groups by ANOVA for the repeated measures. When the ANOVA showed significant differences, a Bonferroni adjustment was used to compare pairwise tests among each group. Treatment effects were determined by ANOVA on the outcome measures adjusted for baseline values. In each mouse, LV myocyte contraction, relaxation, and [Ca2+]iT values were averaged and treated as a single data point. The mean differences in cell dynamics and the indo-1-AM fluorescence ratios between groups were calculated. Statistical significance was considered at p<0.05.
3. Results
3.1. Animal Follow-Up and Verification of Experimental HF
Consistent with past reports by our laboratory and others [19, 22–24, 33], in the current study, following the last isoproterenol injection, mice exhibited a time-dependent cardiac dysfunction and adverse structural remodeling similar as observed in clinical HF.
HF with Preserved Ejection Fraction (HFpEF).
Two-weeks after receiving initial isoproterenol injection, mice had HFpEF onset with significantly increased LV relaxation time constant (τ) and end-diastolic pressure of LV (PED), but reduced the peak rate of mitral flow (dV/dtmax). As illustrated in Figure 2A, compared with control, there was clearly upward shift of the diastolic portion of LV P-V loop, so that early diastolic LVP was much higher. LV minimum pressure (Pmin) and LV diastolic operating stiffness of KLV (measured as the average slope of the diastolic P V trajectories) were significantly increased. Myocardial relaxation, defined by tissue Doppler-derived myocardial annular descent, or é, was reduced, and LV filling pressure, defined by early transmitral flow velocity-to-mitral annular velocity ratio (or E/e’), was increased. In contrast, as shown in Figure 2B, LV contractility of EES and MSW (the slope of stroke work-VED relation), LV ejection fraction (EF), stroke volume (SV), and fractional shortening (FS) had no significant changes. Consistently, LV myocyte peak velocity of re-lengthening (dR/dtmax) was significantly reduced 33% (Control: 108.1 ± 5.6 vs HFpEF: 72.9 ± 7.7 μm/sect). There were no significant changes in myocyte contractility of dL/dtmax (Control: 145.2 ± 5.5 vs HFpEF: 142.0 ± 9.0 μm/sec).
Figure 2.
Examples of the effects of chronic G1 in HF on LV systolic and diastolic functional responses. A. Steady-state average LV Pressure (P)-Volume (V) loops from 4 mice: one control mouse, one mouse at two weeks after initial isoproterenol injection (HFpEF), one mouse 4 weeks after isoproterenol injection (HF), and one mouse 2 weeks after isoproterenol and then received G1 treatment for 2 weeks (HF/G1). Each loop was generated by averaging the data obtained during a 10 −12-second recording period, spanning several respiratory cycles. Compared with control, 2 weeks after isoproterenol injection caused HFpEF with markedly upward shifts of the P-V loop with marked increases in LV PED and LV min, indicating impaired LV diastolic performance. After isoproterenol injection at 4 weeks, there was upward and rightward shift of P-V loop with further elevated LV PED and LV min and decreased stroke volume. In contrast, concomitant G1 prevented isoproterenol-induced abnormal upward and rightward shifts of P-V loops and the decreased SV in HF.
B. LV pressure (PES)-end-systolic volume (VES) relationships produced by inferior vena cava occlusions obtained from these same animals. The slope and position of this line provide a load-insensitive measure of LV contractility. Compared with control, 2 weeks after isoproterenol injection the LV PES-VES relations are relatively unchanged. In contrast, 4 weeks after isoproterenol injection, the LV PES-VES relations were shipped to the right with markedly reduced slope, indicating LV systolic dysfunction. Compared with control, concomitant G1-treated HF mouse had similar the LV PES-VES relations, indicating restoration of normal LV contractility.
HF with Reduced EF (HFrEF) (HF).
Four weeks after receiving the last isoproterenol injection, the cardiac indices of HFpEF were extended into a hemodynamic pattern consistent with the presence of HFrEF, with both LV systolic and diastolic dysfunction. As summarized in Table 1, compared with control, τ, and PED were further increased with about 30% decreases in dV/dtmax and stroke volume, respectively. There was upward and rightward shift of the LV P-V loop with significantly increased LV end-diastolic volume. Importantly, LV contractility of EES was reduced ~31%. EF decreased from 57% (observed in controls) to 35% (4 weeks after isoproterenol injection) (Table 1 and Figure 2). As shown in Table 1, there were no significant differences in body weight among the groups. In HF group, the surrogates of symptomatic HF, including heart weight, calculated ratio of heart weight/body weight (Control: 5.50 ± 0.29 vs. HF: 7.89 ± 0.34 mg/g), and LV myocyte Length were all significantly increased (113 ± 5 vs. 125 ± 6 μm). As shown in Table 2 and Figure 3, these LV abnormalities in HF were accompanied by intrinsic defects in LV myocyte force-generating capacity and relaxation. There were ~ 31% decreases in dL/dtmax and 35% decreases in dR/dtmax with significantly reduced the peak systolic [Ca2+]iT.
Table 1.
Effects of Chronic G1 on LV Function and General Hemodynamic Variables in HF
| Control (N=6) |
HF (N=6) |
HF/G1 (N=6) |
|
|---|---|---|---|
| Heart rate (beats/min) | 633 ± 14 | 636 ± 16 | 628 ± 17 |
| LV end-diastolic pressure (mmHg) | 5.0 ± 1.1 | 10.6 ± 1.5* | 5.2 ± 1.0 |
| LV end-systolic pressure (mmHg) | 114.4 ± 3.2 | 116.9 ± 2.6 | 113.2 ± 4.7 |
| LV end-diastolic volume (μl) | 103.8 ± 4.4 | 117.8 ± 4.3* | 105.1 ± 3.9 |
| LV end-systolic volume (μl) | 45.0 ± 3.1 | 76.7 ± 2.7* | 47.7 ± 4.5 |
| Stroke volume (μl) | 58.9 ± 1.8 | 41.1 ± 2.0* | 57.4 ± 1.7 |
| Maximum dP/dt (mmHg/s) | 11,106 ± 692 | 7,726 ± 478* | 11046 ± 545 |
| Minimum dP/dt (mmHg/s) | −8,336 ± 432 | −5,514 ± 489* | −7,997 ± 492 |
| Maximum dV/dt (μl/s) | 3,692 ± 245 | 2,571 ± 282* | 3,620 ± 356 |
| Time constant of relaxation (msec) | 7.7 ± 0.3 | 11.3 ± 0.6* | 7.8 ± 0.6 |
| EA (mmHg/μl) | 1.95 ± 0.08 | 2.85 ± 0.10* | 1.97 ± 0.08 |
| SVR (mmHg/ml/min) | 3.07 ± 0.16 | 4.48 ± 0.19* | 3.14 ± 0.15 |
| EES (mmHg/μl) | 1.85 ± 0.06 | 1.28 ± 0.12* | 1.79 ± 0.05 |
| MSW (mmHg) | 103.0 ± 1.6 | 74.7 ± 2.6* | 101.5 ± 16 |
| EES/EA | 0.95 ± 0.06 | 0.45 ± 0.04* | 0.91 ± 0.03 |
Values are mean ± SD; N=number of mice.
HF: heart failure; LV: left ventricular; EA: arterial elastance; dV/dtmax: the peak rate of mitral flow; SVR: systemic vascular resistance; EES: the slope of linear PES-VES relation; MSW: the slope of SW-VED relation.
p <0.05, vs. Control
Table 2.
Effects of Chronic G1 on Myocyte Contractile Function, [Ca2+]i Transient, and β-Adrenergic Reserve in HF
| Control (N=12) | HF (N=12) | HF/G1 (N=12) | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Baseline | Isoproterenol | Baseline | Isoproterenol | Baseline | Isoproterenol | |
| Resting length (mm) | 113.4 ± 4.6 | 112.5 ± 4.9 | 124.9 ± 9.1* | 124.4 ± 8.9 | 114.3 ± 7.5 | 113.8 ± 7.5 |
| Percent of shortening (SA, %) | 9.3 ± 1.0 | 14.1 ± 1.4† | 6.2 ± 0.5* | 7.4 ± 0.7†‡ | 9.1 ± 0.4 | 13.8 ± 1.0† |
| Velocity of shortening (mm/sec) | 145.2 ± 5.5 | 233.1 ± 9.6† | 99.5 ± 3.2* | 122.8 ± 4.4†‡ | 140.6 ± 3.4 | 226.0 ± 5.0† |
| Velocity of relengthening (mm/sec) | 108.1 ± 5.6 | 169.8 ± 9.8† | 70.2 ± 3.6* | 88.5 ± 4.0†‡ | 104.7 ± 4.9 | 163.7 ± 8.9† |
| Peak systolic [Ca2+]i transient | 0.20 ± 0.01 | 0.27 ± 0.01† | 0.16 ± 0.01* | 0.18 ± 0.01†‡ | 0.20 ± 0.01 | 0.27 ± 0.01† |
Values are mean ± SD; N=number of mice. HF: heart failure.
p<0.05, vs. Control baseline.
p<0.05, Isoproterenol response vs. corresponding baseline value.
p<0.05, vs.Isoproterenol-induced percent changes in Control.
Figure 3.
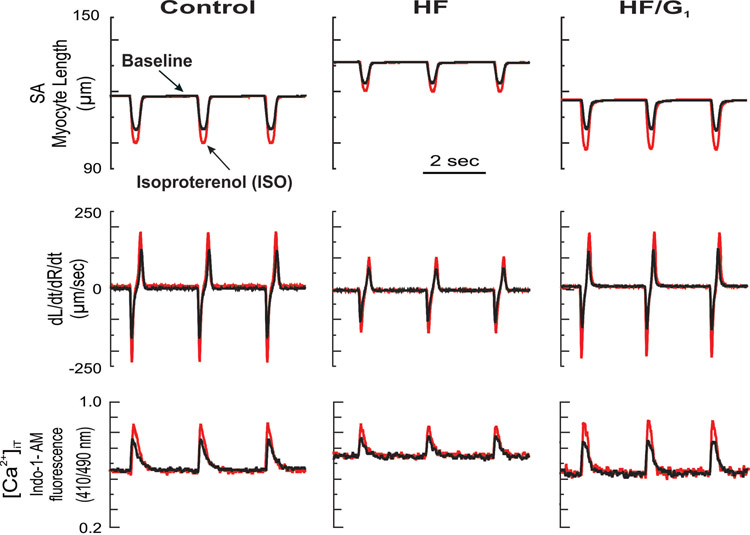
Examples of the effects of chronic G1 in HF on myocyte contractile function and [Ca2+]iT response at baseline and response to acute isoproterenol (ISO) (β-adrenergic reserve). Myocytes isolated from the LV were obtained from one mouse in each experimental group. Shown are superimposed traces of analog recordings of myocyte contractile and [Ca2+]iT responses in electrically stimulated myocytes at baseline and after acute superfusion of isoproterenol (10−8 M).
3.2. LV Systolic and Diastolic Function in HF: Effects of Chronic GPR30 Activation
As summarized in Table 1 and displayed in Figure 2, there were no differences in heart rate and LV end-systolic pressure (PES) among the groups. In HF animals, LV end-diastolic pressure (PED), LV end-systolic volume (VES) and LV end-diastolic volume (VED) were all significantly increased and accompanied by significant reductions in LV dV/dtmax and stroke volume (SV). The slopes of LV P-V relations of EES, load-insensitive measures of LV contractile performance, were decreased by 31%. The P-V relations shifted to the right and ratio of LV arterial coupling (EES/EA) was reduced by ~53 %, indicating significant systolic function impairment. By contrary, LV systolic and diastolic function and general hemodynamics were similar between the control and HF/G1 groups. Chronic treatment with G1 prevented HF-caused decreased LV contractility of EES, EF, SV, and the abnormal upward and rightward shifts of LV P-V loops (Figure 2A). τ, PED, VED, SV, dV/dtmax and EES/EA as well as the ratio of heart weight/body weight (5.75 ± 0.64 mg/g) were all close to the control values.
3.3. Myocyte Function, β-AR Reserve, and [Ca2+]iT in HF: Effects of Chronic GPR30 Activation
3.3.1. Cardiomyocyte Contraction, Relaxation, and [Ca2+]iT at Baseline
Basal cell contractile function and [Ca2+]iT responses in LV myocytes are summarized in Table 2 and displayed in Figures 3 and 4A. Compared with controls, in HF, LV myocyte dL/dtmax, dR/dtmax and [Ca2+]iT were significantly decreased 31%, 35% and 20%, respectively. After G1 treatment, LV myocyte SA, dL/dtmax, dR/dtmax, and [Ca2+]iT recovered to control values. In addition, myocyte length and the length-width ratio were normalized.
Figure 4.
(A) LV myocyte functional performance of dL/dtmax, dR/dtmax, and [Ca2+]iT at baseline, and (B) β-adrenergic reserve, calculated as % changes from baseline after acute isoproterenol stimulation. Data are shown as mean (± SD). N=12/group. * p<0.05 vs. Control group.
3.3.2. Myocyte Functional Responses to β-AR, and β-AR Subtype Stimulation
3.3.2.1. Effects of Isoproterenol.
Compared with controls, functional performance of LV myocyte in HF mice was impaired at baseline (Table 2 and Figures 3 and 4A). Further, the ability of acute β-adrenergic agonist isoproterenol to increase myocyte contractility was also significantly reduced. As displayed in Figures 3 and 4B, compared with control myocytes, in HF myocytes, acute isoproterenol-superfusion-induced increases in SA, dL/dtmax and dR/dtmax, as well as [Ca2+]iT were all significantly lower, demonstrating a decline in β-adrenergic reserve. G1 treatment restored normal β-adrenergic reserve in HF myocytes.
3.3.2.2. Effects of Norepinephrine or BRL-37344.
As shown in Figure 5, compared with control myocytes, in HF myocytes, β1-AR stimulation with norepinephrine caused significantly less increases in the normalized percent changes of myocyte contractility dL/dtmax (Control: 61% ± 4% vs HF: 24% ± 2%), dR/dtmax, and [Ca2+]iT, indicating β1-AR desensitization. In contrast, β3-AR stimulation with BRL-37344 produced significantly greater decreases in the normalized percent changes of dL/dtmax (Control: 9% ± 1% vs HF: 16% ± 2%), dR/dtmax, and [Ca2+]iT, demonstrating enhanced β3-AR-mediated negative modulation. Importantly, G1 treatment reversed HF produced contract changes of β1-AR and β3-AR-stimulated inotropic responses. As presented in Figure 5, in G1-treated myocytes, Norepinephrine or BRL-37344 caused changes in dL/dtmax, dR/dtmax, and [Ca2+]iT were similar as that in normal control myocytes, respectively, indicating restoration of cardiac β-AR subtypes (β1- and β3-ARs) normal responsiveness.
Figure 5.
The effects of chronic G1 in HF on LV myocyte functional responses to β1-AR or β3-agonists. Group means of the changes on dL/dtmax, dR/dtmax, and [Ca2+]iT in response to norepinephrine stimulation, a selective β1-AR agonist (10−7M), or BRL-37,344, a selective β3-agonist (10−8 M). Values shown mean (± SD). * p<0.05, norepinephrine or BRL-37,344-induced changes vs corresponding baselines; † p<0.05, norepinephrine or BRL-37,344-induced changes among groups.
4. Discussion
We show here, for the first time, that chronic GPR30 activation reverses HFpEF LV diastolic dysfunction and prevents the progression of HFpEF to HFrEF. The normalization of LV systolic and diastolic function performance in G1-treated HF is associated with preservation of normal intrinsic LV myocyte contraction, relaxation, [Ca2+]iT and β-adrenergic reserve accompanied by the restoration of normal cardiac β-AR subtypes (β1- and β3 -AR ) modulation. These data provide evidence and important insights that chronic GPR30 activation is able to rescue or reverse HFpEF, suggesting GPR30 agonist may provide significant benefits in HF therapy.
4.1. LV Functional Performance and Chronic GPR30 Activation
In the current study, 2-weeks after isoproterenol injection, animals had HFpEF characterized by significantly impaired diastolic function, but without systolic dysfunction (Figure 2). These observations are consistent with previously well-characterized histology, LV function and structure alterations in isoproterenol-induced HF by others and our serial time course studies in this model [19–21, 23, 24]. Grimm et al [20] reported that myocardial injury was associated with increases in right atrial pressure, LV filling pressure and LV hypertrophy two weeks after isoproterenol (150 mg/kg) application. Teerlink et al [19] clearly described the histology, and LV structure and function alterations in male Wistar rats receiving two subcutaneous isoproterenol injections of either 85 mg/kg or 170 mg/kg at 2 and 6 weeks after injection. They reported that histological resolution of the damage by both doses to the myocardium occurred by 2 weeks; there was no evidence for any further increase in this pathology score with respect to time (2 to 16 weeks). Further, Brooks and Conrad [21] showed myocardial injury and LV diastolic dysfunction associated with hypertrophy of surviving myocytes and increased myocardial fibrosis 14 days after the last isoproterenol injection. LV diastolic dysfunction was evidenced by decreased LV compliance, an upward shift in the diastolic relationship, with normal LV systolic function. Isoproterenol produces a time-and dose-dependent impairment of cardiac function and structural remolding that results primarily in diastolic dysfunction within two weeks. In agreement, in the present study, without treatment, at 4 weeks after receiving isoproterenol, HFpEF was further preceded to HFrEF, with both LV systolic and diastolic dysfunction.
Of importance, chronic administration of G1 completely reversed the HFpEF progression in this mouse model of progressive HF. In HF/G1 group, the major indices of LV systolic and diastolic functional performance and general hemodynamics (EF, PED, τ, SV and dV/dtmax) were all restored to control values (Table 1). To avoid the potentially confounding effects of G1-induced changes in loading conditions on conventional measures of LV performance, LV contractile performance was evaluated in the pressure-volume plane. Chronic G1 significantly increased LV contractility (measured as EES and MSW) and the EES/EA ratio (Table1 and Figure 2B). Chronic G1 prevented the classic systolic HF-induced rightward and upward shifts of LV P-V loops (Figure 2A) and increased heart weight/body weight ratios.
Although the effects of G1 on HF progress in males have not been previously investigated, our present observation of chronic G1 restoration of normal LV systolic and diastolic function in HF is supported by previous studies in GPR30-deficient male mice [7, 12, 14]. Chronic G1 treatment attenuates salt-induced diastolic dysfunction and myocyte hypertrophy without changes in blood pressure [34]. G1 showed the ability to attenuate HF in female OVX Sprague-Dawley rats [15]. G1 pretreatment reduces infarct size and preserves cardiac function in isolated hearts from male and female rats exposed to ischemia-reperfusion [34].
4.2. Myocyte Function, [Ca2+]i Regulation β-Adrenergic Reserve and Chronic GPR30 Activation
What is the mechanism of the restoration of normal LV systolic and diastolic functional performance in HF after chronic G1 treatment? Since the protective effects are present in the freshly-isolated single LV myocytes, this beneficial action is not due to extracardiac factors such as alterations of heart rate, fibrosis and loading conditions, but it is directly attributable to changes of LV myocytes.
We found that in isoproterenol-induced HF, LV chamber abnormalities were paralleled with progressive LV myocyte dysfunction with significantly depressed myocyte contractility (dL/dtmax), relaxation rates (dR/dtmax) and [Ca2+]iT. There was a maladaptive remodeling of LV myocyte shape. These changes were normalized and myocyte β-AR desensitization reversed after G1 treatment. Normalization of basal and β-AR stimulated Ca2+ handling may be the primary driver for reversal of HF-caused intrinsic defects of myocyte force-generating capacity and relaxation after chronic G1 treatment. Recovering normal [Ca2+]i regulation by chronic G1 may be the key mechanism for reversal of HF-caused intrinsic defects of myocytes and rescuing HFpEF.
Growing evidence has shown that estrogen alters cardiovascular gene expression of β-adrenergic receptors (AR) and calcium (Ca2+)-handling proteins [35]. We have shown previously that in HF, increased sympathetic nervous system was associated with cardiac β1-AR downregulation, but β3-AR upregulation. β1-AR-stimulated-GS-coupled positive inotropic effects reduced, while β3-AR-stimulated-Gi-coupled negative modulation on LV, and myocyte contraction, relaxation as well as [Ca2+]i regulation were enhanced. This contract changes between cardiac β1-AR and β3-AR are responsible for the β-AR desensitization in HF [31–33]. In the current study, G1 treatment caused improved β-adrenergic reserve is likely due to the restoration of normal β-AR subtypes modulation. Notably, Kang et al.[15] showed that the ability of GPR30-agonist G1 to reduce isoproterenol-induced HF in female OVX Sprague-Dawley rats was achieved through the expression of β1- and β2-ARs. We and others have reported that HF, as well as GPR30-deficiency, decreased LV myocyte sarcoplasmic reticulum Ca2+ ATPase 2a (SERCA2a) expression and activity with increased sarcolemmal Na+- Ca2+ exchange expression accompanied by increased SR Ca2+ leak with defective Ca2+ removal. Of note, GPR30 improves LV lusitropy in models of hypertension and aging. This is largely attributable to GPR30-related increase in cardiac Ca2+ mobilization by increasing the expression and activity of SERCA2a [3, 36]. A large body of evidence indicates that increased oxidative stress contributes directly to cellular damage, impaired [Ca2+]i regulation and remodeling during HF, whereas GPR30 activation was sufficient to protect against myocardial death, cardiac apoptosis and adverse LV remodeling [3, 6]. The ability of GPR30 activation to counteract pathologic oxidation may be another important mechanism by which G1 treatment could ameliorate HF. We propose that in the current study, G1 reverses HF-caused contrast changes in cardiac β1-AR and β3-AR expression and activity and downregulation of SERCA2a, thereby leading to restoration of normal SERCA2a activity and [Ca2+]i regulation. These changes may be the key molecular mechanism for repairing LV and myocyte contractile defects, restoration of cardiac β-AR responsiveness and reversal of HFpEF and prevention of its progression. Clearly, further studies on the molecular basis for chronic GPR30 activation in HF are urgently needed.
4.3. Study Limitations
Some limitations should be considered in interpreting our data. First, we used a mouse model of isoproterenol-induced HF. Although pathologic changes in isoproterenol-treated rats resemble those of myocardial infarction [19–21, 23], and isoproterenol-induced HF mimics many structural, functional and neurohormonal changes of clinical HF [20], we cannot ascertain that these results are applicable to clinical HF or HF from other causes such as pure pressure overloaded cardiomyopathy or pure volume overload cardiomyopathy. Second, in the current investigation, G1 was initiated at the onset of HFpEF. Although the isoproterenol-induced HFpEF is consistent with previously well-characterized histological findings, LV structure and function alterations from others [19–24], we do not know if late initiation of G1 is capable of achieving the same efficacy given that dynamic nature (time dependency) of isoproterenol-induced HF. Finally, it is important to state that the current study did not address the molecular mechanisms underlying the therapy/rescue actions of chronic GPR30 activation on cardiac function and cardiac reserve in mice with HFpEF (as well as the transition to HFrEF) and will require further investigations. More insights will be gained from ongoing investigations in our laboratory to elucidate these mechanisms.
5. Conclusions
Chronic G1 treatment restores LV systolic and diastolic function accompanied with the preservation of normal intrinsic myocyte contraction, relaxation, [Ca2+]iT and β-adrenergic reserve, thereby completely reverses HFpEF progression in a male mouse model of progressive HF. These data provide new insights and strong evidence that chronic GPR30 activation is able and sufficient to rescue HFpEF in males, and support the view that GPR30 plays critical roles in cardiac health in both females and males.
Acknowledgments
This work was supported in part by the National Institutes of Health grants R01AG049770 (HJC), R01AG033727 (LG), R01HL074318 (CPC) and National Natural Science Foundation of China 81900358 (XWZ).
ABBREVIATIONS
- β-AR
β-adrenergic receptor
- [Ca2+]iT
calcium transient
- dL/dtmax
the maximum rate of myocyte shortening
- dR/dtmax
the maximum rate of myocyte re-lengthening
- dV/dtmax
the peak rate of mitral flow
- EA
arterial elastance
- E/e’
early transmitral flow velocity-to-mitral annular velocity ratio
- P
Pressure
- V
Volume
- EES
the slope of linear PES-VES relation
- EES/EA
ratio of LV-arterial coupling
- EF
ejection fraction
- FS
fractional shortening
- GPER
G protein-coupled estrogen receptor
- GPR30
G protein-coupled receptor 30
- HF
heart failure
- HFpEF
HF with preserved ejection fraction
- HFrEF
HF with reduced ejection fraction
- LV
left ventricle
- LVAC
LV-arterial coupling
- MSW
the slope of stroke work-VED relation
- PES
end-systolic pressure
- PED
end-systolic pressure
- Pmin
LV minimum pressure
- SA
myocyte percent shortening
- SERCA2a
sarcoplasmic reticulum Ca2+ ATPase 2a
- SV
stroke volume
- τ
LV time constant of relaxation
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- 1.Tomasoni D, et al. , Heart failure in the last year: progress and perspective. ESC Heart Fail. 2020;7: p. 3505–3530.DOI: 10.1002/ehf2.13124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfeffer MA, Shah AM, and Borlaug BA, Heart Failure With Preserved Ejection Fraction In Perspective. Circ Res, 2019. 124(11): p. 1598–1617. DOI: 10.1161/circresaha.119.313572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Groban L, et al. , Female Heart Health: Is GPER the Missing Link? Front Endocrinol (Lausanne), 2019. 10: p. 919. DOI: 10.3389/fendo.2019.00919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hutson DD, et al. , Estrogen receptor profiles across tissues from male and female Rattus norvegicus. Biol Sex Differ. 2019. 10(1): p. 4. DOI: 10.1186/s13293-019-0219-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel VH, et al. , G-protein coupled estrogen receptor 1 expression in rat and human heart: Protective role during ischaemic stress. Int J Mol Med, 2010. 26(2): p. 193–9. DOI: 10.3892/ijmm_00000452 [DOI] [PubMed] [Google Scholar]
- 6.Aryan L, et al. , The Role of Estrogen Receptors in Cardiovascular Disease. Int J Mol Sci. 2020. 21(12). DOI: 10.3390/ijms21124314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delbeck M, et al. , Impaired left-ventricular cardiac function in male GPR30-deficient mice. Mol Med Rep, 2011. 4(1): p. 37–40. DOI: 10.3892/mmr.2010.402 [DOI] [PubMed] [Google Scholar]
- 8.Pei H, et al. , G Protein-Coupled Estrogen Receptor 1 Inhibits Angiotensin II-Induced Cardiomyocyte Hypertrophy via the Regulation of PI3K-Akt-mTOR Signalling and Autophagy. Int J Biol Sci, 2019. 15(1): p. 81–92. DOI: 10.7150/ijbs.28304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fredette NC, Meyer MR, and Prossnitz ER, Role of GPER in estrogen-dependent nitric oxide formation and vasodilation. J Steroid Biochem Mol Biol, 2018. 176: p. 65–72. DOI: 10.1016/j.jsbmb.2017.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Machuki JO, et al. , Estrogen regulation of cardiac cAMP-L-type Ca(2+) channel pathway modulates sex differences in basal contraction and responses to β(2)AR-mediated stress in left ventricular apical myocytes. Cell Commun Signal. 2019. 17(1): p. 34. DOI: 10.1186/s12964-019-0346-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogola BO, et al. , G Protein-Coupled Estrogen Receptor Protects From Angiotensin II-Induced Increases in Pulse Pressure and Oxidative Stress. Front Endocrinol (Lausanne), 2019. 10: p. 586. DOI: 10.3389/fendo.2019.00586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meoli L, et al. , Sex- and age-dependent effects of Gpr30 genetic deletion on the metabolic and cardiovascular profiles of diet-induced obese mice. Gene, 2014. 540(2): p. 210–6. DOI: 10.1016/j.gene.2014.02.036 [DOI] [PubMed] [Google Scholar]
- 13.Wang X, et al. , GPR 30 reduces myocardial infarct area and fibrosis in female ovariectomized mice by activating the PI3K/AKT pathway. Life Sci, 2019. 226: p. 22–32. DOI: 10.1016/j.lfs.2019.03.049 [DOI] [PubMed] [Google Scholar]
- 14.Wang H, et al. , Cardiomyocyte-specific deletion of the G protein-coupled estrogen receptor (GPER) leads to left ventricular dysfunction and adverse remodeling: A sex-specific gene profiling analysis. Biochim Biophys Acta Mol Basis Dis, 2017. 1863(8): p. 1870–1882. DOI: 10.1016/j.bbadis.2016.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang S, et al. , Chronic activation of the G protein-coupled receptor 30 with agonist G-1 attenuates heart failure. PLoS One, 2012. 7(10): p. e48185. DOI: 10.1371/journal.pone.0048185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitcomb V, et al. , Regulation of beta adrenoceptor-mediated myocardial contraction and calcium dynamics by the G protein-coupled estrogen receptor 1. Biochem Pharmacol, 2020. 171: p. 113727. DOI: 10.1016/j.bcp.2019.113727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng Y, et al. , Activation of G protein-coupled oestrogen receptor 1 at the onset of reperfusion protects the myocardium against ischemia/reperfusion injury by reducing mitochondrial dysfunction and mitophagy. Br J Pharmacol. 2017. 174(23): p. 4329–4344. DOI: 10.1111/bph.14033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Z, et al. , Role of estrogen in diastolic dysfunction. Am J Physiol Heart Circ Physiol, 2014. 306(5): p. H628–40. DOI: 10.1152/ajpheart.00859.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teerlink JR, Pfeffer JM, and Pfeffer MA, Progressive ventricular remodeling in response to diffuse isoproterenol-induced myocardial necrosis in rats. Circ Res, 1994. 75(1): p. 105–13. DOI: 10.1161/01.res.75.1.105 [DOI] [PubMed] [Google Scholar]
- 20.Grimm D, et al. , Effects of beta-receptor blockade and angiotensin II type I receptor antagonism in isoproterenol-induced heart failure in the rat. Cardiovasc Pathol, 1999. 8(6): p. 315–23. DOI: 10.1016/s1054-8807(99)00021-6 [DOI] [PubMed] [Google Scholar]
- 21.Brooks WW and Conrad CH, Isoproterenol-induced myocardial injury and diastolic dysfunction in mice: structural and functional correlates. Comp Med, 2009. 59(4): p. 339–43. [PMC free article] [PubMed] [Google Scholar]
- 22.Li T, et al. , Reversal of angiotensin-(1–12)-caused positive modulation on left ventricular contractile performance in heart failure: Assessment by pressure-volume analysis. Int J Cardiol, 2020. 301: p. 135–141. DOI: 10.1016/j.ijcard.2019.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, et al. , Cellular basis of angiotensin-(1–7)-induced augmentation of left ventricular functional performance in heart failure. Int J Cardiol, 2017. 236: p. 405–412. DOI: 10.1016/j.ijcard.2017.01.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki M, et al. , Altered inotropic response of endothelin-1 in cardiomyocytes from rats with isoproterenol-induced cardiomyopathy. Cardiovasc Res, 1998. 39(3): p. 589–99. DOI: 10.1016/s0008-6363(98)00166-7 [DOI] [PubMed] [Google Scholar]
- 25.Groban L, et al. , Progressive diastolic dysfunction in the female mRen(2). Lewis rat: influence of salt and ovarian hormones. J Gerontol A Biol Sci Med Sci, 2008. 63(1): p. 3–11. DOI: 10.1093/gerona/63.1.3 [DOI] [PubMed] [Google Scholar]
- 26.Wang H, et al. , Activation of GPR30 attenuates diastolic dysfunction and left ventricle remodelling in oophorectomized mRen2.Lewis rats. Cardiovasc Res, 2012. 94(1): p. 96–104. DOI: 10.1093/cvr/cvs090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindsey SH, et al. , Estrogen receptor GPR30 reduces oxidative stress and proteinuria in the salt-sensitive female mRen2.Lewis rat. Hypertension, 2011. 58(4): p. 665–71. DOI: 10.1161/hypertensionaha.111.175174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shao Q, et al. , Overexpression myocardial inducible nitric oxide synthase exacerbates cardiac dysfunction and beta-adrenergic desensitization in experimental hypothyroidism. Int J Cardiol, 2016. 204: p. 229–41. DOI: 10.1016/j.ijcard.2015.11.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He Q, Cheng J, and Wang Y, Chronic CaMKII inhibition reverses cardiac function and cardiac reserve in HF mice. Life Sci, 2019. 219: p. 122–128. DOI: 10.1016/j.lfs.2019.01.010 [DOI] [PubMed] [Google Scholar]
- 30.Segers P, et al. , Conductance catheter-based assessment of arterial input impedance, arterial function, and ventricular-vascular interaction in mice. Am J Physiol Heart Circ Physiol, 2005. 288(3): p. H1157–64. DOI: 10.1152/ajpheart.00414.2004 [DOI] [PubMed] [Google Scholar]
- 31.Cheng HJ, et al. , Upregulation of functional beta(3)-adrenergic receptor in the failing canine myocardium. Circ Res, 2001. 89(7): p. 599–606. DOI: 10.1161/hh1901.098042 [DOI] [PubMed] [Google Scholar]
- 32.Morimoto A, et al. , Endogenous beta3-adrenoreceptor activation contributes to left ventricular and cardiomyocyte dysfunction in heart failure. Am J Physiol Heart Circ Physiol, 2004. 286(6): p. H2425–33. DOI: 10.1152/ajpheart.01045.2003 [DOI] [PubMed] [Google Scholar]
- 33.Cheng H-J, et al. , Abstract 14636: Upregulation of Cardiac β3-Adrenergic Signaling Promotes Heart Failure Progression: Evidence from Chronic β3-Adrenoceptor (AR) deficiency or β3-AR Blockade Mice. Circulation, 2014. 130(suppl_2): p. A14636–A14636. DOI: doi: 10.1161/circ.130.suppl_2.14636 [DOI] [Google Scholar]
- 34.Jessup JA, et al. , Attenuation of salt-induced cardiac remodeling and diastolic dysfunction by the GPER agonist G-1 in female mRen2.Lewis rats. PLoS One, 2010. 5(11): p. e15433. DOI: 10.1371/journal.pone.0015433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Machuki JO, et al. , Molecular pathways of oestrogen receptors and β-adrenergic receptors in cardiac cells: Recognition of their similarities, interactions and therapeutic value. Acta Physiol (Oxf), 2018. 222(2). DOI: 10.1111/apha.12978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alencar AK, et al. , Effect of Age, Estrogen Status, and Late-Life GPER Activation on Cardiac Structure and Function in the Fischer344×Brown Norway Female Rat. J Gerontol A Biol Sci Med Sci, 2017. 72(2): p. 152–162. DOI: 10.1093/gerona/glw045 [DOI] [PMC free article] [PubMed] [Google Scholar]