Abstract
Background:
Among patients with diabetes mellitus (diabetes) and chronic coronary disease (CCD), it is unclear if invasive management improves outcomes when added to medical therapy.
Methods:
The ISCHEMIA Trials (ISCHEMIA and ISCHEMIA CKD) randomized CCD patients to an invasive (medical therapy + angiography and revascularization if feasible) or a conservative approach (medical therapy alone with revascularization if medical therapy failed). Cohorts were combined after no trial-specific effects were observed. Diabetes was defined by history, HbA1c ≥6.5%, or use of glucose-lowering medication. The primary outcome was all-cause death or myocardial infarction (MI). Heterogeneity of effect of invasive management on death or MI was evaluated using a Bayesian approach to protect against random high or low estimates of treatment effect for patients with vs. without diabetes and for diabetes subgroups of clinical (female sex and insulin use) and anatomic features (coronary artery disease [CAD] severity or left ventricular function).
Results:
Of 5,900 participants with complete baseline data, the median age was 64 years interquartile range (IQR) [57–70], 24% were female, and the median estimated glomerular filtration was 80 ml/min/1.732 IQR [64–95]. Among the 2,553 (43%) of participants with diabetes, median percent hemoglobin A1c was 7% IQR [7–8%], and 30% were insulin treated. Participants with diabetes had a 49% increased hazard of death or MI (HR 1.49; 95% CI: 1.31–1.70, P<0.001). At median 3.1-year follow-up the adjusted event-free survival was 0.54 (95% bootstrapped CI: 0.48, 0.60) and 0.66 (95% bootstrapped CI: 0.61, 0.71) for patients with vs. without diabetes – a 12% (95% bootstrapped CI: 4%, 20%) absolute decrease in event-free survival among participants with diabetes. Female and male patients with insulin-treated diabetes had an adjusted event-free survival of 0.52 (95% bootstrapped CI: 0.42, 0.56) and 0.49 (95% bootstrapped CI: 0.42, 0.56), respectively. There was no difference in death or MI between strategies for patients with vs. without diabetes, or for clinical (female sex or insulin use) or anatomic features (CAD severity or left ventricular function) of patients with diabetes.
Conclusions:
Despite higher risk for death or MI, CCD patients with diabetes did not derive incremental benefit from routine invasive management compared with initial medical therapy alone.
Keywords: Bayesian modeling, chronic coronary disease, diabetes mellitus, heterogeneity of treatment effect, insulin treatment
Introduction
Diabetes mellitus (diabetes) is a well-established risk factor for atherosclerotic cardiovascular disease (ASCVD),1 and is a common comorbidity among patients with chronic coronary disease (CCD).2 All patients with diabetes and CCD should receive guideline-directed medical therapy, which includes comprehensive lifestyle and pharmacologic intervention, and attainment of multiple, specific risk factor goals. A common clinical question is whether patients with diabetes or high-risk diabetes subgroups and CCD derive incremental benefit from an invasive management strategy (cardiac catheterization and revascularization). Data are limited to answer this clinical question. Prior strategy trials of CCD management selected patients with and without diabetes, but only after diagnostic catheterization, and did not include many higher-risk participants, such as those with moderate or severe inducible ischemia.3–5
Diabetes mellitus is the leading cause of chronic kidney disease (CKD)6 and both diabetes and CKD portend a poor prognosis for patients with CCD.7 Prior CCD trials of patients with and without diabetes included relatively few patients with abnormal kidney function (estimated glomerular filtration rate (eGFR) <60 ml/min/1.732), and very few with advanced CKD (eGFR <30 ml/min/1.73 m2) or receiving dialysis.8 In contrast, the International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) and ISCHEMIA-Chronic Kidney Disease (CKD) (ISCHEMIA Trials) enrolled and randomized patients across the spectrum of kidney function with CCD, prior to cardiac catheterization, based on the presence of moderate or severe inducible ischemia. Approximately 45% of participants in the ISCHEMIA Trials had diabetes, providing an opportunity to test for heterogeneity of treatment effect of a routine invasive approach on a background of intensive guideline-directed medical therapy for high-risk CCD patients with diabetes compared with no diabetes. The main objective of these pooled analyses from the ISCHEMIA Trials was to analyze heterogeneity of treatment effect of a routine invasive strategy in addition to guideline-directed medical therapy on the outcome of death or myocardial infarction (MI) for patients with diabetes and high-risk diabetes subgroups compared with patients without diabetes.
Methods
Deidentified data and a data dictionary will be available starting March 30, 2022. Methods of data sharing will be determined based on the National Institutes of Health data sharing policy and in discussion with the National Institutes of Health and the National Heart, Lung, and Blood Institute program officer. IRB approval was obtained at each site, and all participants provided informed consent.
The design and primary results of the ISCHEMIA Trials have been reported.7,9–11 Patients with known or suspected CCD were selected for enrollment based on the finding of moderate or severe inducible ischemia on a stress imaging test or severe ischemia on an exercise tolerance test, as previously described.7,11 Patients meeting criteria for ischemia severity with an eGFR ≥30 ml/kg/1.732 were enrolled in the main ISCHEMIA trial, and patients with an eGFR <30 ml/kg/1.732 were enrolled in ISCHEMIA CKD. Blinded coronary computed tomography angiography (CCTA) was performed in most ISCHEMIA patients (76%) with preserved kidney function (eGFR ≥60 ml/min/1.732) with the goal of excluding from randomization patients with ≥50% left main coronary stenosis and participants with <50% stenosis of any epicardial artery.10,12 CCTA was not required in the main ISCHEMIA trial when estimated glomerular filtration rate was <60 mL/min/1.732 and was not performed in ISCHEMIA CKD because of the risk of acute kidney injury,9 or coronary anatomy was known to meet entry criteria based on prior testing.10,13 Key exclusion criteria for the ISCHEMIA Trials were acute coronary syndrome within the prior 2 months, known unprotected left main coronary stenosis >50%, left ventricular ejection fraction <35%, PCI in the prior year, unacceptable angina severity despite maximal medical therapy, ischemic stroke in the prior 6 months or any intracranial bleed, severe valvular heart disease, and heart failure exacerbation within the last 6 months or New York Heart Association class III or IV heart failure, or a contraindication to DAPT.7,11 Qualifying patients were randomized to an invasive approach ( guideline-directed medical therapy + angiography and revascularization if feasible) or a conservative approach (guideline-directed medical therapy alone with revascularization reserved for refractory symptoms on an endpoint event).
Study Population
In order to characterize the clinical outcomes of patients with diabetes with CCD across the spectrum of kidney function, these analyses combined participants from ISCHEMIA and ISCHEMIA-CKD (ISCHEMIA Trials).7,9–11 Analyses were based on participants with complete baseline data, and a comparison of included vs. excluded participants was performed (Supplemental Table S1).
Definition and Treatment of Diabetes Mellitus and Clinical Features of Diabetes
In the ISCHEMIA Trials, diabetes mellitus (diabetes) was defined by baseline medical history, glycated hemoglobin (HbA1c) ≥6.5% by laboratory testing at randomization, or recorded use of glucose-lowering medication. Diabetes treatment was categorized as none/diet-controlled, non-insulin treatment, or treatment with insulin. Sites were provided guidance on glucose control with HbA1c goals but were not provided algorithms on use of antihyperglycemic medications.10
Clinical features of diabetes defined by insulin treatment and female sex were selected because prior data suggest insulin treatment14,15 and female sex16,17 may be associated with higher risk of adverse ASCVD events for patients with CCD, and these variables were available on nearly all ISCHEMIA and ISCHEMIA-CKD participants. These subgroups were used to examine potential heterogeneity of treatment effect for the invasive or conservative treatment strategies.11
We also explored anatomic features of diabetes defined by multivessel CAD ≥50% stenosis, Duke Prognostic Index and left ventricular function, and estimated the cumulative incidence of the primary and exploratory outcomes for each of these features. A CCTA was performed in 66% (3913/5956) of all ISCHEMIA Trials participants;13 no ISCHEMIA-CKD participants received a CCTA.9 Of participants with a CCTA, 87% (3390/3913) of those studies were interpretable for multivessel CAD ≥50% stenosis. Among the 3,913 ISCHEMIA Trials participants with a CCTA, 64% (2504/3913) were interpretable for extent and severity of CAD by Duke prognostic index (“CAD severity”).18 Duke Score 6 severity of CAD was selected as a subgroup of interest because a companion ISCHEMIA analyses demonstrated potential benefit for the invasive strategy in this subset.19 Analyses of left ventricular systolic dysfunction (LVSD, ejection fraction ≥35% and <45%)20 were available in 99% (5951/5956) of patients with site-reported ejection fraction.20
Outcomes
The primary outcome was a composite of all-cause death (death) or nonfatal MI.7 The primary definition of nonprocedural infarction based on the Third Universal Definition of MI for types 1, 2, 4b and 4c in the ISCHEMIA Trials was used,21 with a higher biomarker threshold for procedural infarctions (Universal Definition of MI types 4a/5), as previously described.11,22 The individual outcomes of death, CV death, non-CV death, and fatal or non-fatal MI by the primary definition, including procedural and non-procedural MI, were examined for evidence of heterogeneity of treatment effect by diabetes status. Details of the clinical-events committee, process for event adjudication, and the definition of myocardial infarction have been previously published.7,11
Statistical Methods
Descriptive statistics
Categorical and continuously measured participant baseline characteristics were summarized and associations with diabetes were assessed using the chi-square and Kruskal-Wallis tests as appropriate. Kaplan-Meier estimates of the cumulative incidence of death or MI by diabetes and by diabetes and treatment strategy were plotted with the log rank test used to assess differences in the survival distributions. Corresponding event rates were estimated for the anatomic features of diabetes (multivessel CAD ≥50% stenosis, Duke Score 6 and LVSD [ejection fraction ≥35% and <45%]). For outcomes with competing risks, namely, CV death, non-CV death, and fatal or non-fatal MI, we estimated cumulative incidence functions accounting for competing risks, using Gray’s test to assess group-based differences. In the analysis of heterogeneity of treatment effect with respect to primary clinical features of interest (diabetes, insulin treatment and female sex), we assessed covariate balance with descriptive analyses comparing the invasive versus conservative randomized treatment strategies within diabetes strata.
Association of Death and Myocardial Infarction with Diabetes
Multivariable Cox proportional hazards regression models were fit to estimate the associations of the composite incidence of death or MI (the primary outcome for the purposes of this study) with diabetes versus no diabetes and with the subgroups defined by the clinical features of interest, after adjusting for age, treatment strategy, dialysis status, eGFR among non-dialysis patients, and ejection fraction. The model for diabetes versus no diabetes additionally adjusted for sex. To assess the proportional hazards assumption, the associations of scaled Schoenfeld residuals from the fitted model with log time were analyzed, and under a violation of proportional hazards associations in Cox models were re-evaluated with time-dependent coefficients.
Bayesian Modeling of Heterogeneity of Treatment Effect
In a Bayesian framework, the objective of heterogeneity of treatment effect analyses are to estimate and characterize subgroup-specific treatment effects and their associated uncertainty as opposed to hypothesis testing in a frequentist setting.23,24 As recently recommended by the Food and Drug Administration,25 a Bayesian approach may be preferred to assess heterogeneity of treatment effect in clinical trials.23,26,27
To inform the Bayesian modeling of heterogeneity of treatment effect, the proportional hazards assumption for the effect of the invasive versus conservative strategy on the primary outcome of death or MI first was examined in these combined trial analyses. In ISCHEMIA, the proportional hazards assumption was violated for the effect of randomization to the invasive vs. conservative strategy on the primary 5-component composite endpoint.11 In contrast, in ISCHEMIA-CKD the proportional hazards assumption was met in analyses of the endpoint of death or nonfatal MI.7 A Cox proportional hazards regression model of death or MI was fit to include covariates of treatment strategy, sex, age, diabetes status, dialysis status, eGFR, and ejection fraction. The score test of the null hypothesis of no association between the scaled Schoenfeld residuals for treatment strategy and log time was rejected (P<0.001) (Supplement A), thus indicating proportional hazards were violated for analyses of death or nonfatal MI in the combined ISCHEMIA Trials. Therefore, proportional hazards were not assumed in the ensuing examination of heterogeneity of treatment effect.
The Bayesian Dixon Simon28 model was adapted to a piecewise exponential non-proportional hazards setting in which the effect of randomization to invasive versus the conservative strategy in each clinical feature-based subgroup was allowed to vary over follow-up. To assess heterogeneity of treatment effect the Dixon Simon model specification included covariates for treatment strategy, the clinical features (sex, diabetes, and insulin treatment), and the interaction terms between treatment strategy and each of these clinical features. Age at randomization, dialysis status at baseline, eGFR among non-dialysis participants, and ejection fraction were included for adjustment. An exchangeable prior distribution for the interaction terms was specified that does not make assumptions about the magnitude or direction of effect for any of the prespecified clinical features. Explication of the model specification, assignment of prior distributions, computation, and convergence are available in Supplement B. As a summary of heterogeneity of treatment effect over follow-up, we estimated clinical feature-specific hazard ratios for an invasive versus conservative strategy based on the assumption of proportional hazards. This hazard ratio can be interpreted as an average over observed event times.29 In exploratory analyses, we also conducted the corresponding Bayesian heterogeneity of treatment effect analysis with respect to diabetes status and the anatomic features of multivessel CAD ≥50% stenosis, LVSD and Duke Score 6.
All analyses were conducted in R statistical software,30 with Bayesian models fit in JAGS.31
Results
Baseline characteristics
Among 5,956 participants randomized from the ISCHEMIA (N=5,179) and ISCHEMIA-CKD (N=777) trials, 56 participants were excluded for missing baseline data on diabetes treatment (n=54) or dialysis status (n=2), leaving a final analysis cohort of N=5,900 patients. (See Supplemental Table S1 for characteristics of the 56 excluded participants). Overall, 2,553 (43%) of participants had diabetes and 3,347 (57%) did not. Baseline characteristics of participants with and without diabetes are shown in Table 1. Compared with participants without diabetes, a larger proportion of those with diabetes were female, of Asian or White race, or of Hispanic or Latino ethnicity. The median (Q1, Q3) HbA1c at baseline for patients with diabetes was 7% (7, 8). The eGFR was significantly lower for participants with versus without diabetes, and approximately 17% of patients with diabetes had an eGFR <30 ml/min/1.73 m2 or required dialysis, compared with approximately 10% of patients without diabetes (Table 1). The proportion of patients requiring dialysis at baseline was greater for patients with diabetes (9%) versus without diabetes (6%) (Table 1). Patients with diabetes were also more likely to have undergone prior revascularization (percutaneous coronary intervention, PCI; coronary artery bypass graft surgery, CABG) and to have prior non-cardiac vascular disease. The burden of anginal symptoms by Seattle Angina Questionnaire was lower for participants with versus without diabetes, but patients with diabetes were more likely to have left ventricular systolic dysfunction (Table 1). In comparison to patients without diabetes, those with diabetes at baseline were significantly more likely to have an LDL-C <70 mg/dL and to be non-smokers but were less likely to have a baseline systolic blood pressure <140 mmHg (Table 1).
Table 1.
Baseline characteristics overall and by diabetes status
| Characteristic | All Participants (N=5,900) |
No Diabetes at Baseline (N=3,347) |
Diabetes at Baseline (N=2,553) |
P-value |
|---|---|---|---|---|
| Demographics | ||||
| Age at Randomization (years), Median (Q1, Q3) | 64 (57, 70) | 64 (57, 71) | 64 (58, 70) | 0.981 |
| Female sex | 1,394/5,900 (23.6%) | 765/3,347 (22.9%) | 629/2,553 (24.6%) | 0.111 |
| Race | <.001 | |||
| American Indian or Alaskan Native | 18/5,820 (0.3%) | 9/3,318 (0.3%) | 9/2,502 (0.4%) | |
| Asian | 1,647/5,820 (28.3%) | 837/3,318 (25.2%) | 810/2,502 (32.4%) | |
| Native Hawaiian or Other Pacific Islander | 16/5,820 (0.3%) | 6/3,318 (0.2%) | 10/2,502 (0.4%) | |
| Black or African American | 265/5,820 (4.6%) | 103/3,318 (3.1%) | 162/2,502 (6.5%) | |
| White | 3,861/5,820 (66.3%) | 2,356/3,318 (71.0%) | 1,505/2,502 (60.2%) | |
| Multiple Races Reported | 13/5,820 (0.2%) | 7/3,318 (0.2%) | 6/2,502 (0.2%) | |
| Hispanic or Latino ethnicity | 858/5,496 (15.6%) | 447/3,145 (14.2%) | 411/2,351 (17.5%) | <.001 |
| Diabetes Treatment | ||||
| Insulin Treated | 771/2,553 (30.2%) | - | 771/2,553 (30.2%) | |
| Non-Insulin Diabetes Medication | 1,447/2,553 (56.7%) | - | 1,447/2,553 (56.7%) | |
| None/Diet Controlled | 335/2,553 (13.1%) | - | 335/2,553 (13.1%) | |
| Cigarette Smoking | <.001 | |||
| Never Smoked | 2,555/5,895 (43.3%) | 1,376/3,345 (41.1%) | 1,179/2,550 (46.2%) | |
| Former Smoker | 2,626/5,895 (44.5%) | 1,500/3,345 (44.8%) | 1,126/2,550 (44.2%) | |
| Current Smoker | 714/5,895 (12.1%) | 469/3,345 (14.0%) | 245/2,550 (9.6%) | |
| Clinical History | ||||
| Hypertension | 4,462/5,878 (75.9%) | 2,357/3,331 (70.8%) | 2,105/2,547 (82.6%) | <.001 |
| Baseline Hemoglobin % A1c , N | 3856 | 1526 | 2330 | |
| Median (Q1, Q3) | 6 (6, 8) | 6 (6, 6) | 7 (7, 8) | <.001 |
| Prior Myocardial Infarction | 1,109/5,882 (18.9%) | 618/3,338 (18.5%) | 491/2,544 (19.3%) | 0.445 |
| Prior Percutaneous Coronary Intervention (PCI) | 1,183/5,896 (20.1%) | 642/3,344 (19.2%) | 541/2,552 (21.2%) | 0.057 |
| Prior Coronary Artery Bypass Graft (CABG) | 229/5,900 (3.9%) | 110/3,347 (3.3%) | 119/2,553 (4.7%) | 0.007 |
| Prior MI or Prior PCI or Prior CABG | 1,775/5,882 (30.2%) | 966/3,337 (28.9%) | 809/2,545 (31.8%) | 0.019 |
| eGFR among Patients not on Dialysis at Baseline, N | 5485 | 3153 | 2332 | |
| Median eGFR ml/min/1.732 (Q1, Q3) | 80 (64, 95) | 81 (66, 96) | 78 (60, 94) | <.001 |
| eGFR ml/min/1.73 m2 | <.001 | |||
| ≥60 | 4,396/5,900 (74.5%) | 2,642/3,347 (78.9%) | 1,754/2,553 (68.7%) | |
| 30–59 | 735/5,900 (12.5%) | 372/3,347 (11.1%) | 363/2,553 (14.2%) | |
| <30 or on dialysis | 769/5,900 (13.0%) | 333/3,347 (9.9%) | 436/2,553 (17.1%) | |
| On Dialysis at Baseline | 415/5,900 (7.0%) | 194/3,347 (5.8%) | 221/2,553 (8.7%) | <.001 |
| Non-Cardiac Vascular and Comorbidity History | ||||
| Prior Carotid Artery Surgery or Stent, Stroke, or Transient Ischemic Attack (TIA) | 474/5,887 (8.1%) | 232/3,340 (6.9%) | 242/2,547 (9.5%) | <.001 |
| Prior Stroke | 217/5,899 (3.7%) | 92/3,346 (2.7%) | 125/2,553 (4.9%) | <.001 |
| Prior Peripheral Vascular Disease (PAD) or Surgery or Percutaneous Procedure for PAD | 250/5,891 (4.2%) | 113/3,343 (3.4%) | 137/2,548 (5.4%) | <.001 |
| Angina & Heart Failure History | ||||
| Baseline Seattle Angina Questionnaire Angina Frequency Scale, N | 5,323 | 3,079 | 2,244 | |
| Median (25th, 75th) | 90 (70, 100) | 90 (70, 100) | 90 (70, 100) | 0.010 |
| Baseline Seattle Angina Questionnaire Angina Frequency Scale | 0.007 | |||
| Daily Angina (0–30) | 127/5,323 (2.4%) | 72/3,079 (2.3%) | 55/2,244 (2.5%) | |
| Weekly Angina (31–60) | 900/5,323 (16.9%) | 553/3,079 (18.0%) | 347/2,244 (15.5%) | |
| Monthly Angina (61–99) | 2,322/5,323 (43.6%) | 1,367/3,079 (44.4%) | 955/2,244 (42.6%) | |
| No Angina in Past Month (100) | 1,974/5,323 (37.1%) | 1,087/3,079 (35.3%) | 887/2,244 (39.5%) | |
| Participant Has Ever Had Angina | 5,179/5,900 (87.8%) | 2,984/3,347 (89.2%) | 2,195/2,553 (86.0%) | <.001 |
| New Onset of Angina Over the Past 3 Months | 966/5,610 (17.2%) | 570/3,174 (18.0%) | 396/2,436 (16.3%) | 0.094 |
| Left ventricular systolic dysfunction (LVEF ≥35%<45%) | 306/5,895 (5.2%) | 149/3,344 (4.5%) | 157/2,551 (6.2%) | 0.004 |
| Ejection Fraction*, N | 5,206 | 2,944 | 2,262 | |
| Median (25th, 75th) | 60 (55, 65) | 60 (55, 65) | 60 (54, 65) | <.001 |
| Guideline Directed Medical Therapy† | ||||
| LDL cholesterol < 70 mg/dL and on any statin | 1,815/5,615 (32.3%) | 861/3,201 (26.9%) | 954/2,414 (39.5%) | <.001 |
| Systolic blood pressure < 140 mmHg | 3,753/5,873 (63.9%) | 2,240/3,331 (67.2%) | 1,513/2,542 (59.5%) | <.001 |
| Aspirin or other anti-platelet or anti-coagulant | 5,579/5,897 (94.6%) | 3,165/3,345 (94.6%) | 2,414/2,552 (94.6%) | 0.965 |
| Non-smoker | 5,181/5,895 (87.9%) | 2,876/3,345 (86.0%) | 2,305/2,550 (90.4%) | <.001 |
Site-reported value, if available. If not available, then core-lab entered value;
,ISCHEMIA definition of optimal medical therapy.11
Stress testing and CCTA findings overall and by diabetes status are shown in Table 2. ISCHEMIA participants with diabetes were more likely to have stress nuclear imaging and less often stress echocardiography versus those without diabetes, but no difference was observed in the severity of inducible ischemia by diabetes status across these modalities (Table 2). In contrast, participants with diabetes were more likely to have multivessel or triple-vessel disease by CCTA. A larger proportion of patients with diabetes had a Duke prognostic index score of 5 or 6 compared with patients without diabetes (Table 2).
Table 2.
Stress testing and CCTA findings by overall and by diabetes status
| Characteristic | All Participants (N=5,900) |
No Diabetes at Baseline (N=3,347) |
Diabetes at Baseline (N=2,553) |
P-value |
|---|---|---|---|---|
| Qualifying Stress Test Core Lab Interpretation | ||||
| Ischemia Severity by Imaging Modality | ||||
| Stress Imaging Overall | 4,504/5,900 (76.3%) | 2,566/3,347 (76.7%) | 1,938/2,553 (75.9%) | 0.499 |
| Severity | 0.293 | |||
| Severe | 1,883/4,480 (42.0%) | 1,110/2,553 (43.5%) | 773/1,927 (40.1%) | |
| Moderate | 2,058/4,480 (45.9%) | 1,128/2,553 (44.2%) | 930/1,927 (48.3%) | |
| Mild | 317/4,480 (7.1%) | 187/2,553 (7.3%) | 130/1,927 (6.7%) | |
| None | 222/4,480 (5.0%) | 128/2,553 (5.0%) | 94/1,927 (4.9%) | |
| Nuclear | 3,020/5,898 (51.2%) | 1,653/3,347 (49.4%) | 1,367/2,551 (53.6%) | <.001 |
| Severity | 0.943 | |||
| Severe | 1,085/3,003 (36.1%) | 605/1,644 (36.8%) | 480/1,359 (35.3%) | |
| Moderate | 1,538/3,003 (51.2%) | 819/1,644 (49.8%) | 719/1,359 (52.9%) | |
| Mild | 213/3,003 (7.1%) | 128/1,644 (7.8%) | 85/1,359 (6.3%) | |
| None | 167/3,003 (5.6%) | 92/1,644 (5.6%) | 75/1,359 (5.5%) | |
| Echocardiogram | 1,229/5,898 (20.8%) | 767/3,347 (22.9%) | 462/2,551 (18.1%) | <.001 |
| Severity | 0.288 | |||
| Severe | 647/1,222 (52.9%) | 417/763 (54.7%) | 230/459 (50.1%) | |
| Moderate | 438/1,222 (35.8%) | 263/763 (34.5%) | 175/459 (38.1%) | |
| Mild | 88/1,222 (7.2%) | 51/763 (6.7%) | 37/459 (8.1%) | |
| None | 49/1,222 (4.0%) | 32/763 (4.2%) | 17/459 (3.7%) | |
| CMR | 255/5,898 (4.3%) | 146/3,347 (4.4%) | 109/2,551 (4.3%) | <.001 |
| Severity | 0.781 | |||
| Severe | 151/255 (59.2%) | 88/146 (60.3%) | 63/109 (57.8%) | |
| Moderate | 82/255 (32.2%) | 46/146 (31.5%) | 36/109 (33.0%) | |
| Mild | 16/255 (6.3%) | 8/146 (5.5%) | 8/109 (7.3%) | |
| None | 6/255 (2.4%) | 4/146 (2.7%) | 2/109 (1.8%) | |
| Exercise Tolerance Test (ETT) | 1,394/5,898 (23.6%) | 781/3,347 (23.3%) | 613/2,551 (24.0%) | <.001 |
| Severity | 0.200 | |||
| Severe | 1,176/1,338 (87.9%) | 647/748 (86.5%) | 529/590 (89.7%) | |
| Moderate | 101/1,338 (7.5%) | 64/748 (8.6%) | 37/590 (6.3%) | |
| Mild | 34/1,338 (2.5%) | 22/748 (2.9%) | 12/590 (2.0%) | |
| None | 27/1,338 (2.0%) | 15/748 (2.0%) | 12/590 (2.0%) | |
| CCTA Findings | <.001 | |||
| CCTA performed* | 3,867/5,900 (65.5%) | 2,310/3,347 (69.0%) | 1,557/2,553 (61.0%) | |
| Any Obstructive Disease ≥50% Stenosis by CCTA | 3,791/3,795 (99.9%) | 2,259/2,260 (100.0%) | 1,532/1,535 (99.8%) | 0.310 |
| Multi-vessel Disease ≥50% Stenosis by CCTA | 2,651/3,356 (79.0%) | 1,533/1,999 (76.7%) | 1,118/1,357 (82.4%) | <.001 |
| Vessels ≥50% Stenosis by CCTA | <.001 | |||
| 0 | 4/2,956 (0.1%) | 1/1,788 (0.1%) | 3/1,168 (0.3%) | |
| 1 | 691/2,956 (23.4%) | 458/1,788 (25.6%) | 233/1,168 (19.9%) | |
| 2 | 928/2,956 (31.4%) | 577/1,788 (32.3%) | 351/1,168 (30.1%) | |
| ≥3 | 1,333/2,956 (45.1%) | 752/1,788 (42.1%) | 581/1,168 (49.7%) | |
| Anatomic Severity of CAD (modified Duke Prognostic Index) | 0.003 | |||
| 6: 3-vessel severe stenosis (≥70%) or 2-vessel severe stenosis with proximal LAD | 658/2,476 (26.6%) | 369/1,505 (24.5%) | 289/971 (29.8%) | |
| 5: 2-vessel severe stenosis, 1-vessel severe proximal LAD, or 3-vessel moderate stenosis (≥50%) | 896/2,476 (36.2%) | 537/1,505 (35.7%) | 359/971 (37.0%) | |
| 4: 2-vessel moderate stenosis or 1-vessel severe stenosis other than proximal LAD | 745/2,476 (30.1%) | 480/1,505 (31.9%) | 265/971 (27.3%) | |
| 3: 1-vessel moderate stenosis (≥50%) | 177/2,476 (7.1%) | 119/1,505 (7.9%) | 58/971 (6.0%) | |
| Specific Native Vessels with ≥50% Stenosis by Interpretable CCTA | ||||
| Left Main (LM) | 40/3,803 (1.1%) | 21/2,269 (0.9%) | 19/1,534 (1.2%) | 0.353 |
| Left Anterior Descending (LAD) | 3,156/3,640 (86.7%) | 1,865/2,177 (85.7%) | 1,291/1,463 (88.2%) | 0.031 |
| Proximal LAD | 1,724/3,701 (46.6%) | 1,022/2,210 (46.2%) | 702/1,491 (47.1%) | 0.616 |
| Left Circumflex | 2,331/3,457 (67.4%) | 1,340/2,069 (64.8%) | 991/1,388 (71.4%) | <.001 |
| Right Coronary Artery | 2,285/3,324 (68.7%) | 1,338/2,002 (66.8%) | 947/1,322 (71.6%) | 0.002 |
All CCTA were from ISCHEMIA patients and none from ISCHEMIA-CKD
Duke categories 1 and 2 (non-obstructive CAD or normal arteries) and 7 (left main stenosis ≥50%) were excluded from analysis because these findings were not consistent with eligibility for randomization after CCTA.
Overall, 20.8% (615/2955) of ISCHEMIA Trials participants randomized to the conservative strategy underwent revascularization. A greater proportion of participants assigned to the conservative strategy with diabetes (22.4%, 288/1287) compared with patients without diabetes (19.6%, 327/1668) underwent revascularization (no statistical evidence of a difference, P=0.07) and was driven by the greater proportion of revascularization following a primary event in patients with diabetes (5.6%, 72/1287) compared with patients without diabetes (3.4%, 57/1668), P=0.005. In contrast, there was no difference by diabetes status for revascularization in the conservative strategy not following a primary event (Supplemental Table S2).
Among the 2,224 participants in these pooled ISCHEMIA Trials analyses assigned to the invasive strategy who underwent revascularization, 554 (25%) underwent CABG surgery and 1,670 (75%) underwent PCI. An additional 721 participants assigned to invasive were not revascularized, including 306/1266 (24%) and 415/1679 (25%), with and without diabetes, respectively (P=0.76). Of all patients in the invasive strategy those with diabetes were more likely to be revascularized by CABG (28%, 266/960) as compared with participants without diabetes (23%, 288/1,264), P=0.009. Among the subset of 1,266 patients with diabetes in the invasive strategy, 771 had available CCTA data. Of these patients, 71% (550/771) had multivessel CAD ≥50%, 29% (160/550) of whom underwent CABG. The remaining patients with diabetes and multivessel CAD by CCTA in the invasive strategy received PCI (53%, 293/550) or were not revascularized (18%, 97/550). The decision to perform PCI or CABG was determined at the site level using a heart team approach, and data were not collected on reasons for selecting a particular revascularization strategy. Among patients in the invasive strategy revascularized by CABG there was no difference in the use of internal mammary artery grafting for patients with diabetes (91.7%, 243/265) compared with those without diabetes (91.7%, 264/288).
Event-free survival by Diabetes status and Clinical Features of Diabetes
The cumulative incidence of death or MI of unadjusted Kaplan-Meier estimates demonstrated significantly lower event-free survival for patients with versus without diabetes (log rank p-value <0.0001), and by sex and insulin use among patients with diabetes (log rank p-value <0.0001) (Supplemental Figure S1a-b). Participants with diabetes exhibited higher rates of death or MI over follow-up compared with participants without diabetes, and males and females with insulin-treated diabetes showed higher rates of death or MI relative to other subgroups. The risk of nonprocedural MI was significantly greater for participants with versus without diabetes (Supplemental Figure S1c), while the risk of procedural MI did not differ regardless of diabetes status (Supplemental Figure S1d).
Death or MI occurred in 19% (485/2,553) of diabetes participants versus 12% (402/3,347) of non-diabetes participants (Figure 1). In multivariable Cox proportional hazard models, males and females with insulin-treated diabetes showed an increased hazard of death or MI compared with the referent group of males without diabetes (Figure 1). At median 3.1-year follow-up, event-free survival was 0.54 (95% bootstrapped CI: 0.48, 0.60) and 0.66 (95% bootstrapped CI: 0.61, 0.71) for patients with vs. without diabetes – with a 12% (95% bootstrapped CI: 4%, 20%) absolute decrease in survival free from death or MI among participants with diabetes (Figure 2a). The hazard of death or MI differed among diabetes-based subgroups (likelihood ratio P <0.001), with the lowest adjusted event-free survival among participants with insulin-treated diabetes (Figure 2b). At median follow-up, female and male patients with insulin-treated diabetes had an adjusted event-free survival of 0.52 (95% bootstrapped CI: 0.42, 0.56) and 0.49 (95% bootstrapped CI: 0.42, 0.56), respectively.
Figure 1. Multivariable Cox proportional hazard ratios for death or myocardial infarction for ISCHEMIA Trials participants with diabetes and clinical features of diabetes.
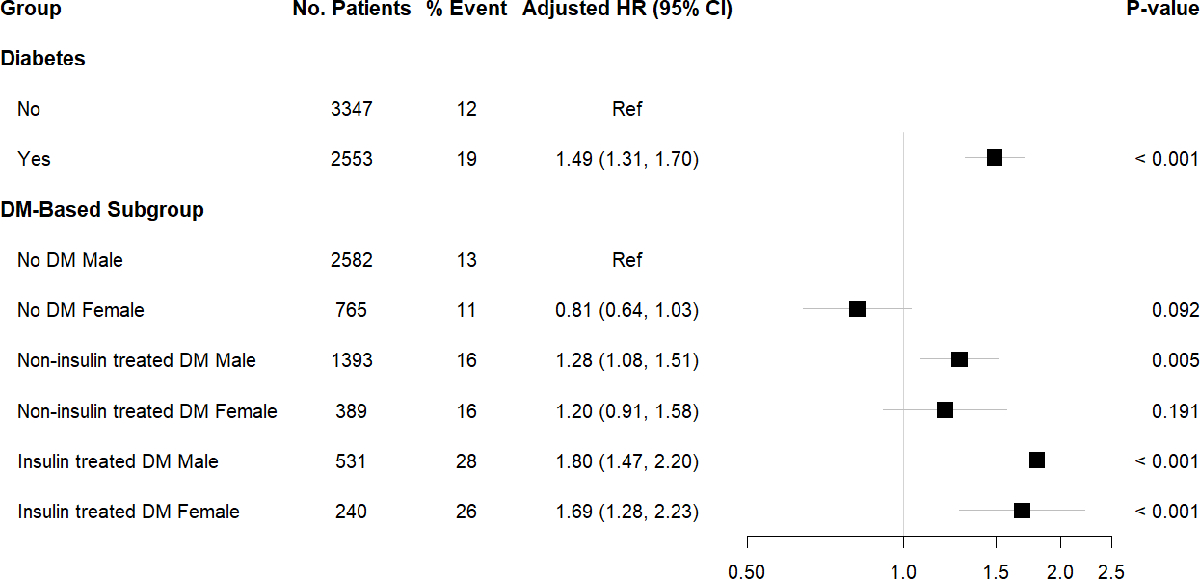
Abbreviations: HR, hazard ratio; CI, confidence interval; Ref, referent. DM, diabetes mellitus.
P-value for comparison of patients with DM to no DM, and for clinical features of diabetes with referent group defined as male patients without DM.
Figure 2. Multivariable Cox proportional hazard survival curves.
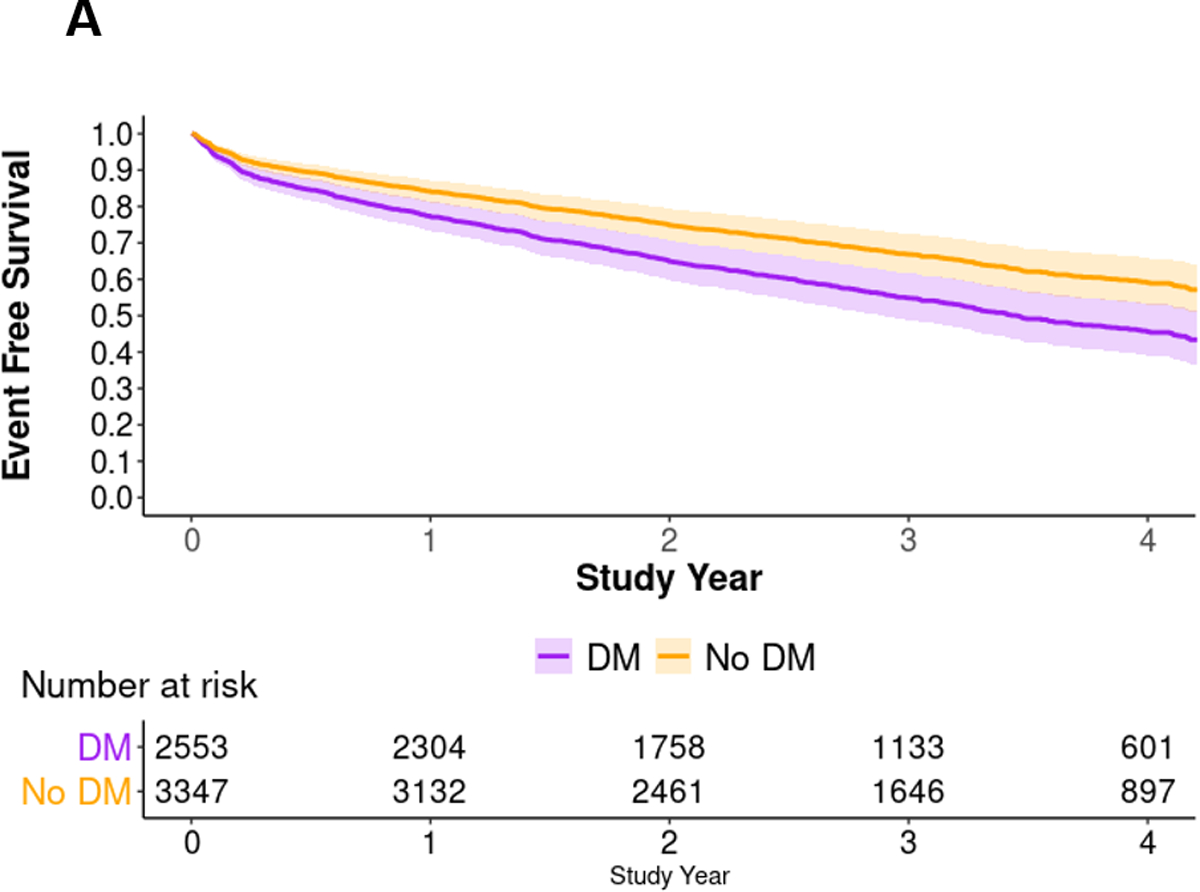
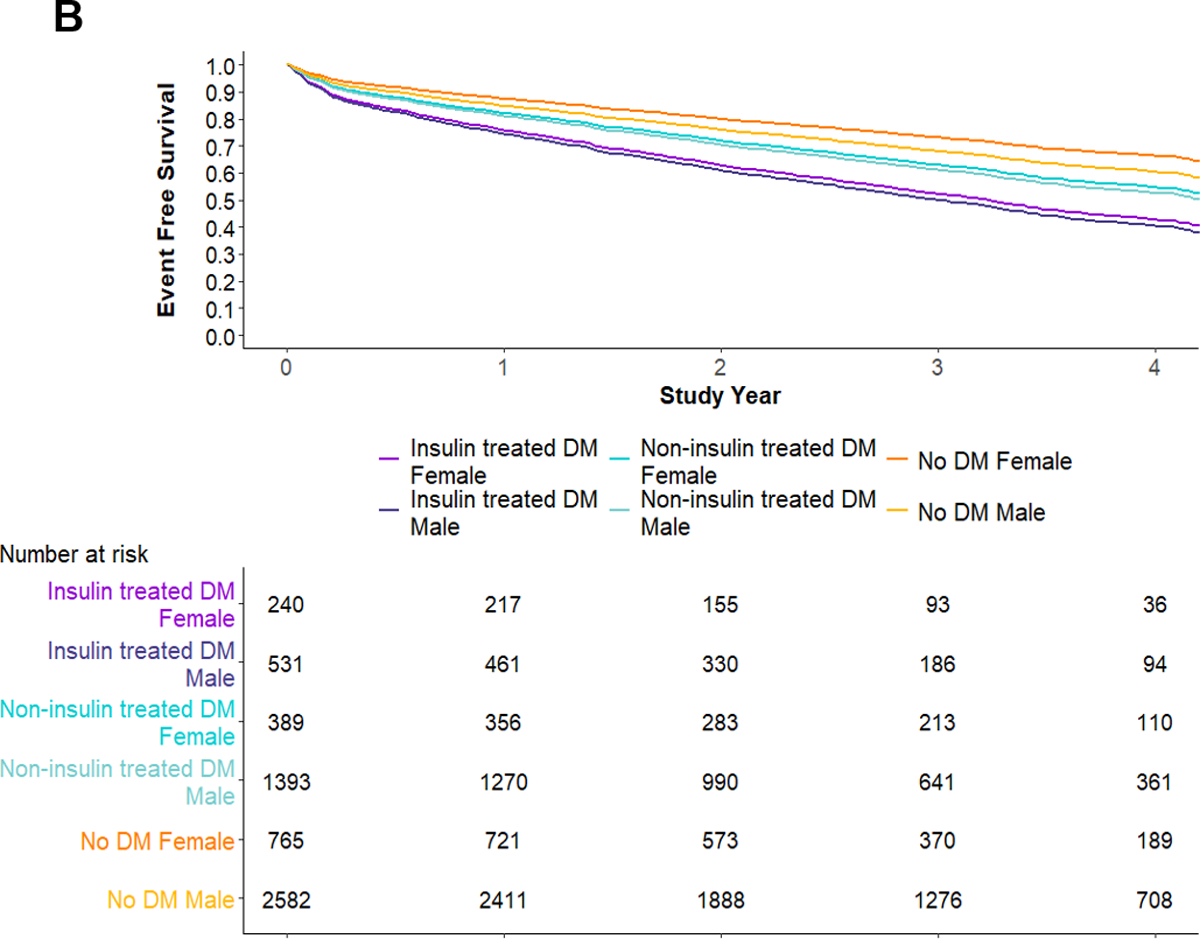
A) Multivariable Cox proportional hazard survival curves by diabetes status. B) Multivariable Cox proportional hazard survival curves by clinical features of diabetes. Abbreviations: DM, diabetes mellitus.
In assessing the proportional hazards assumption (Supplement C), there was no evidence of a time-varying association with diabetes versus no diabetes. For clinical features of diabetes, specifically males and females with insulin treated diabetes, the proportional hazards assumption was violated. A Cox model with time-varying coefficients indicated hazard ratios of greater magnitude later versus earlier in follow-up (Supplement C, Supplemental Table SC1). Sensitivity analyses of ISCHEMIA and ISCHEMIA-CKD separately demonstrated that the associations of death or MI with diabetes and diabetes clinical features were consistent between both ISCHEMIA and ISCHEMIA-CKD (Supplemental Figure S2a-b).
Heterogeneity of treatment effect by Diabetes status and Clinical Features of Diabetes
Supplemental Tables 3 and 4a-c present participant baseline characteristics by treatment strategy and diabetes status, and within each clinical feature-based subgroup. In Figure 3, unadjusted Kaplan-Meier estimators of the cumulative incidence of death or MI by the invasive versus conservative strategy are shown separately for participants with and without diabetes. Over a median 3.1-year follow-up, there was no evidence of heterogeneity of treatment effect for the invasive vs. conservative strategy on the outcome of death or MI for patients with diabetes (log rank p=0.75) or without diabetes (log rank p=0.76). There was no evidence of heterogeneity of treatment effect among the clinical features of diabetes, although violation of proportional hazards was observed for some clinical features (Supplemental Figures 3a-f).
Figure 3. Kaplan-Meier estimate of the cumulative incidence of death or MI by the invasive vs. conservative strategy, stratified by diabetes status.
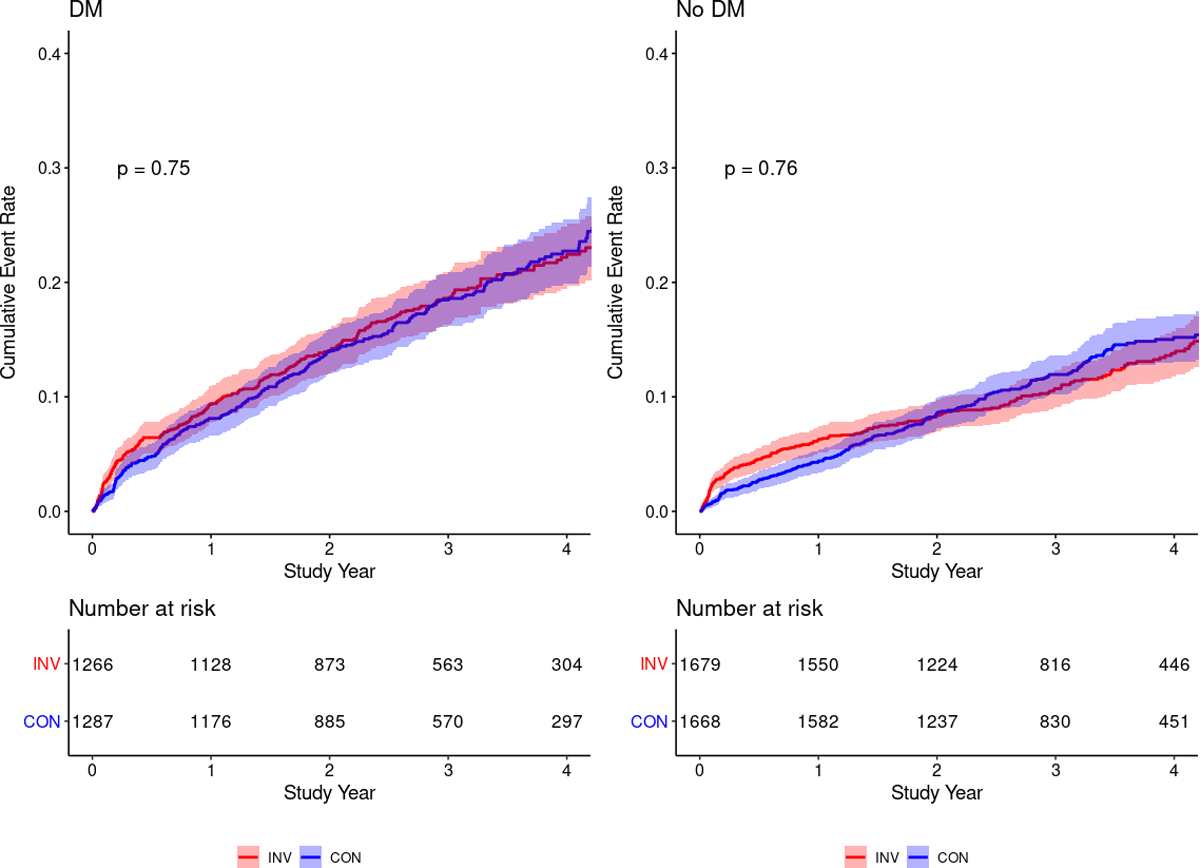
Abbreviations: INV, invasive; CON, conservative; DM, diabetes mellitus.
Figure 4 shows the estimated hazard ratios for the clinical feature-specific treatment effects over follow-up according to yearly time intervals. Over all intervals of follow-up time there was no evidence of heterogeneity of treatment effect for the invasive vs. conservative strategy by diabetes status, or among the clinical features of diabetes: The hazard ratios of individual diabetes clinical features closely align with the hazard ratio for the overall treatment effect – with the 95% credible intervals for the clinical feature-specific estimates overlapping the overall treatment effect. At less than 1 year of follow-up, the overall treatment effect favored the conservative strategy: invasive was associated with 1.34 times the hazard of death or MI (95% CrI: 1.09, 1.62). Between year 1 and year 2, the overall treatment effect favored the invasive strategy, with a 30% reduction in the hazard of death or MI (HR=0.70; 95% CrI: 0.52, 0.90). After 2 years, there was no evidence of a treatment effect of an invasive vs. conservative strategy on death or MI. Consistent findings were observed when participants were limited to the ISCHEMIA trial alone (excluding ISCHEMIA-CKD, Supplemental Figure S4a).11 Heterogeneity of treatment effect was not observed in ISCHEMIA-CKD, and in no time interval was there a treatment effect of an invasive vs. conservative strategy on death or MI (Supplemental Figure S4b).
Figure 4. Diabetes and clinical feature-specific treatment effects over each year of study follow-up. Vertical gray bar is the overall treatment effect and the associated gray shading corresponds to the 95% credible interval. Vertical dashed red bar is at 1 for the null value.
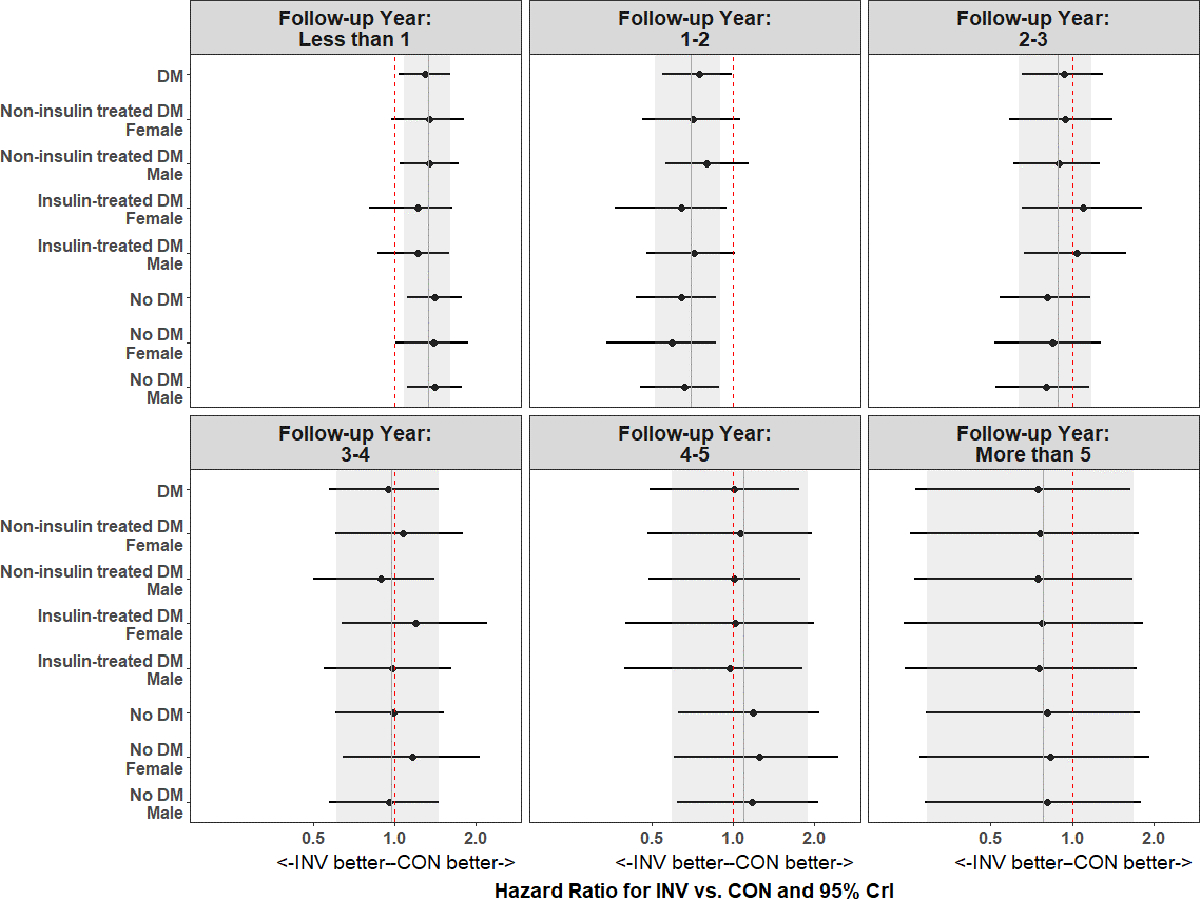
Abbreviations: CrI, credible interval.
Supplemental Figure 5 displays the summary estimated hazard ratios for diabetes and clinical feature-specific treatment effects assuming proportional hazards over the complete study follow-up period. The overall treatment effect was nearly identical for the invasive as compared with the conservative strategy for patients with diabetes (hazard ratio (HR) 1.00, 95% Credible Interval (CrI) 0.87–1.17) versus without diabetes (HR 1.01, 95% CrI 0.86–1.17). There was no evidence of heterogeneity of treatment effect of the invasive versus the conservative strategy over study follow-up by clinical features of diabetes. Summary estimated hazard ratios were similar for all participants in the ISCHEMIA Trials, and when ISCHEMIA and ISCHEMIA-CKD were considered separately.
Corresponding cumulative incidence of death, CV death, non-CV death and fatal and non-fatal MI by treatment strategy stratified by diabetes status was consistent with the primary outcome of death or MI (Supplemental Figures 6a-d). The incidence of non-CV death was increased among patients without diabetes assigned to the invasive versus the conservative strategy (P=0.04), (Supplemental Figure 6c).
Exploratory Analysis of Anatomic Features of Diabetes
There was an increased risk of death or MI for participants with multivessel CAD ≥50% stenosis (no statistical evidence of a difference, P=0.07) and without diabetes (P<0.001) (Supplemental Figure S7a). Duke Score 6 severity of CAD and LVSD conferred a significantly increased risk of death or MI regardless of diabetes status (Supplemental Figure S7b-c).
There was no evidence of heterogeneity of treatment effect for the invasive compared with the conservative strategy for death or MI among patients with multivessel CAD ≥50% stenosis, CAD severity by Duke Score 6, or LVSD stratified by diabetes, among patient subsets defined by receipt of CCTA, a CCTA interpretable for Duke Prognostic Index, or site reported ejection fraction, respectively (Supplemental Figures 8a-c).
Supplemental Figure 9 shows the estimated hazard ratios for anatomic features of diabetes including multivessel CAD ≥50%, Duke Score 6 and LVSD stratified by DM over yearly intervals of follow-up time. At less than 1 year, the overall treatment did not differ between an invasive and conservative strategy. Between year 1 and year 2, the overall treatment effect favored the invasive strategy, with a nearly 50% reduction in the hazard of death or MI (HR 0.51, 95% CrI 0.28–0.81). After 2 years, there was no evidence of a treatment effect of an invasive vs. conservative strategy on death or MI for any anatomic features of diabetes. There was also no heterogeneity of treatment effect observed for death or MI when proportional hazards were assumed for anatomic features of diabetes over the complete study follow-up period (Supplemental Figure 10): The overall hazard ratio of the invasive versus the conservative strategy was 0.93 (95% CrI 0.74–1.17). The anatomic feature-specific hazard ratios align with that of the overall treatment effect for these features – with the 95% credible intervals overlapping the overall treatment effect.
Additional exploratory analyses of the cumulative incidence of death, CV death, non-CV death and fatal and non-fatal MI by treatment strategy for anatomic features of multivessel CAD ≥50% stenosis, Duke Score 6 and LVSD stratified by diabetes status were also consistent with the primary outcome of death or MI (Supplemental Figures 11-14). Patients with diabetes and Duke Score 6 CAD randomized to invasive had an increased incidence of non-CV death (P=0.02) compared to the conservative strategy (Supplemental Figure 13b), and a decrease in fatal or non-fatal MI (no statistical evidence of a difference, P=0.07) (Supplemental Figure 14b).
Discussion
In these pooled analyses of the ISCHEMIA and ISCHEMIA-CKD trials, baseline diabetes was present in 43% of participants with CCD and site-assessed moderate or severe ischemia. Patients with diabetes had higher rates of death or MI than those without diabetes, with the highest rates observed among patients with insulin-treated diabetes. However, Bayesian assessment of heterogeneity of treatment effect showed no difference in death or MI between the invasive or conservative strategies for patients with or without diabetes or in high-risk subgroups of patients with diabetes, during a median 3.1 year follow-up; study results were consistent across both the ISCHEMIA and ISCHEMIA-CKD trials. The current study confirms the higher risk of death or MI for CCD patients with diabetes, extends this to those with moderate or severe ischemia, and provides important new insights about the use of a routine invasive approach for CCD patients with diabetes across the spectrum of kidney function.
Over the last 15 years several major trials have investigated whether an invasive or a conservative strategy is the preferred approach for management of patients with CCD and diabetes who receive guideline-directed medical therapy.3,4,32 Approximately 20–30% of patients in prior CCD trials had diabetes.33 In the COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) trial, patients with diabetes comprised 33% (766/2,287) of those randomized.3 Those patients derived no additional benefit from PCI in addition to guideline-directed medical therapy compared with guideline-directed medical therapy alone on the primary outcome of death or nonfatal MI (HR=0.99; 95% CI 0.73, 1.32). There were two CCD strategy trials that enrolled only patients with diabetes. In BARI 2D (Bypass Angioplasty Revascularization Investigation 2 Diabetes), among patients assigned to the PCI stratum, the rate of death or a composite of death, MI or stroke over 5 years did not differ between patients randomized to PCI and guideline-directed medical therapy compared with those randomized to guideline-directed medical therapy alone (23% vs. 21%, P>0.1).4 In a separate but higher risk stratum, patients in BARI 2D treated with CABG had significantly fewer major ASCVD events than those receiving guideline-directed medical therapy alone, driven by a reduction in non-fatal MI (7.4% vs. 14.6%, P<0.05).4,34 A recent patient-level pooled-analysis including these trials and the Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) trial compared guideline-directed medical therapy to revascularization and demonstrated an incremental benefit of guideline-directed medical therapy and revascularization by CABG but not PCI on the occurrence of death, MI and stroke in CCD patients with diabetes, driven by a reduction in MI.33 Our exploratory findings of a potential reduction in MI with an invasive approach in addition to guideline-directed medical therapy in patients with diabetes and Duke Score 6 severity of CAD align with these prior observations, and recent findings from ISCHEMIA.19
In ISCHEMIA and ISCHEMIA-CKD, 26% and 15% of participants revascularized in the invasive treatment arm received CABG, respectively.7,11 Among patients with diabetes and multivessel CAD ≥50% stenosis in ISCHEMIA (CCTA data on CAD severity was not available in ISCHEMIA-CKD), 29% underwent surgical revascularization, a proportion comparable to the 32% of participants in BARI 2D thought suitable for CABG.4 The selection for CABG compared with PCI in ISCHEMIA (as in BARI 2D) was non-randomized, limiting the ability to make comparisons of revascularization strategies on cardiovascular outcomes for patients with diabetes and multivessel CAD. Additional comparisons between participants with diabetes in ISCHEMIA and those of BARI 2D are limited in part by differences in the populations of these trials, in particular the inclusion of patients with kidney disease in ISCHEMIA-CKD,9 and exclusion of patients with a creatinine >2 mg/dL in BARI 2D.4
A strength of the current report is the inclusion of CCD patients with diabetes across the spectrum of kidney function, including 13% with severe CKD (eGFR <30 ml/min/1.73m2) or on hemodialysis from ISCHEMIA-CKD. While CCD patients with comorbid diabetes and CKD were at highest risk of death or MI, there was no evidence of heterogeneity of treatment effect for an invasive vs. conservative strategy in this patient subgroup. A recent analysis highlighted the increased risk of adverse ASCVD events for CCD patients with comorbid diabetes and CKD from major CCD trials.8 However, prior trials included few participants with severe CKD or patients on dialysis. The consistency of these results for both the ISCHEMIA and ISCHEMIA-CKD trials contributes important data to guide the management of high-risk CCD patients with comorbid diabetes and CKD.
In addition to CCD patients with diabetes and CKD, there was no heterogeneity of treatment effect for an invasive vs. conservative approach for other potential high-risk subgroups of CCD patients with diabetes, including those patients requiring insulin, and for female CCD patients with diabetes. Consistent with prior studies,14,15 CCD patients with diabetes requiring insulin were at greater risk of adverse ASCVD outcomes than patients with non-insulin treated diabetes. At baseline, patients with diabetes were more likely to be at trial goal for LDL-C (LDL <70 mg/dL and on any statin) but less likely to have systolic blood pressure <140 mmHg. At 1 year, as previously shown, participants with diabetes remained less likely to attain systolic blood pressure <140 mmHg but attainment of of LDL-C goals did not differ compared with patients without diabetes.35 These findings suggest that the increased risk of adverse CV events in insulin-treated CCD patients with diabetes persists despite risk factor goal attainment and indicates a continued unmet need to improve ASCVD outcomes in this high-risk cohort. Exploratory analyses applying a Bayesian approach to death or MI for patients with diabetes and anatomic features including multivessel CAD ≥50% stenosis, Duke Score 6 CAD severity or LVSD did not show a benefit of an initial invasive compared with a conservative approach. Unadjusted analyses demonstrating potential increased non-CV death in patients without diabetes, or reduction in MI in patients with diabetes and Duke Score 6 CAD severity with an initial invasive approach should be viewed as hypothesis generating. The proportion of patients with LVSD in the ISCHEMIA Trials was small (≈5%). Patients with LVSD had an increased risk of death or MI regardless of diabetes status, without evidence of heterogeneity of treatment effect for an initial invasive compared with a conservative approach. Additional data are needed for high-risk patients with CCD and comorbid diabetes and LVSD.
Observational studies and meta-analyses have often reported that diabetes is a particularly strong risk factor for cardiovascular events among women.36–39 More recent analyses suggest this may no longer be the case, perhaps due to more uniform application of preventive strategies in both sexes.17 The current analyses are consistent with results from BARI-2D, demonstrating that women with diabetes were not at higher risk of events than men with diabetes.40 The lack of difference in ASCVD outcomes for female compared with male patients with diabetes may be related to the use of management protocols to improve risk factor goal attainment for all patients with CCD in these trials; however, in both ISCHEMIA and BARI-2D, optimization of medical therapy was less successful for women.35,41
Overall the pattern of non-proportional hazards for death and MI in ISCHEMIA Trials patients with and without diabetes was consistent with previously reported results from ISCHEMIA11 and ISCHEMIA-CKD.7 In ISCHEMIA, the opposing trends in procedural and non-procedural infarctions resulted in the lack of proportionality for the primary and key secondary outcomes,11 consistent with observations from these analyses of non-proportionality for patients with and without diabetes for death and MI. The current analyses were also consistent for the individual outcomes of death, CV death, or fatal and non-fatal MI when considered individually, demonstrating that the primary composite outcome of death or MI used herein is consistent with its individual components, and also with CV death.42
The present findings should be interpreted considering certain limitations. First, ISCHEMIA-CKD participants and those in ISCHEMIA with an eGFR <60 ml/min/1.73 m2 were not required to undergo a pre-randomization CCTA,7,11 leading to 57% and 42% of the pooled ISCHEMIA Trials (ISCHEMIA and ISCHEMIA-CKD) cohort for these analyses with a CCTA interpretable for number of disease coronary arteries and Duke prognostic index, respectively. Therefore, power is limited to determinate potential heterogeneity of treatment effect for an invasive approach in these subsets of patients with diabetes and available anatomic data. We also lack data on reasons PCI versus CABG was selected for diabetes patients with multivessel CAD. The decision to perform PCI or CABG was determined at the site level using a heart team approach, and data were not collected on reasons for selecting a particular revascularization strategy. Other limitations include the lack of data on measures of diabetes severity associated with ASCVD outcomes including duration of diabetes,43 or presence of microalbuminuria.44 It is also unknown if ISCHEMIA participants had type 1 or type 2 diabetes, although data from patients with and without ASCVD from the Heart Protection Study45 and Cholesterol Treatment Trialists’ Collaborators46 suggest 90–95% of ISCHEMIA patients likely had type 2 diabetes. Follow-up for patients in ISCHEMIA CKD was less than ISCHEMIA,7,11 so event rates after 3 years reflect ISCHEMIA more than ISCHEMIA CKD. Data are also lacking on changes in diabetes control over time or initiation of newer glucose lowering medications shown to reduce ASCVD events,47 although use of these newer agents was likely minimal over the trial duration. Approximately two-thirds of ISCHEMIA Trials participants were of White race, and data on CCD management and outcomes remains more limited for participants with and without diabetes who are of Non-White race, including patients of Black or African American race, or those of Hispanic or Latino ethnicity. Finally, the results of these analyses do not apply to patients excluded from the ISCHEMIA Trials, including those with progressive angina, recent acute coronary syndromes, left main disease or left ventricular dysfunction with an ejection fraction <35%.
Conclusion
In the ISCHEMIA Trials, CCD patients with diabetes had worse ASCVD outcomes than patients without diabetes, especially patients with insulin-treated diabetes. At a median of 3.1 years of follow-up, the adjusted absolute difference in survival free from death or MI was 12% lower for participants with versus without diabetes. However, there was no incremental benefit from an initial invasive strategy for death or MI, or for any clinical (sex or insulin use) or anatomic (CAD severity or LVSD) features of patients with diabetes. Future analyses will characterize the effects of a conservative or invasive approach on health status outcomes for ISCHEMIA Trials patients with diabetes.
Supplementary Material
Clinical Perspective.
What is new?
Among patients with chronic coronary disease (CCD) from ISCHEMIA and ISCHEMIA-CKD, an initial invasive versus conservative approach did not reduce death or myocardial infarction (D/MI) for patients with or without diabetes.
Patients with insulin-treated diabetes had reduced event-free survival compared with non-insulin treated patients. Despite this elevated risk, an initial invasive approach did not reduce D/MI compared with a conservative approach.
Although a greater proportion of patients with versus without diabetes had multivessel CAD, severe CAD or left ventricular systolic dysfunction (LVSD), an initial invasive vs. a conservative approach for D/MI in these subsets was without benefit.
What are the clinical implications?
Despite their increased risk of cardiovascular events, CCD patients with diabetes and moderate or severe ischemia did not experience a lower risk of D/MI from an initial invasive approach when added to guideline-directed medical therapy.
These results did not differ by sex or by insulin use.
Multivessel CAD, severe CAD and LVSD were more common among CCD patients with versus without diabetes and were each associated with an increased risk of cardiovascular events that was similar between an initial invasive compared with a conservative approach.
Tweet summary:
ISCHEMIA Trials patients with vs. without diabetes had increased risk of CV events without benefit of initial invasive compared with conservative management.
Acknowledgements:
Funding Sources:
NIH grants U01HL105907, U01HL105462, U01HL105561, U01HL105565
Other Support: This project was supported in part by Clinical Translational Science Award Nos. 11UL1 TR001445 and UL1 TR002243 from the National Center for Advancing Translational Sciences and by grants from Arbor Pharmaceuticals LLC and Astra Zeneca Pharmaceuticals LP. Devices or medications were provided by Abbott Vascular (previously St. Jude Medical, Inc); Medtronic, Inc.; Phillips (previously Volcano Corporation); and Omron Healthcare, Inc.; medications provided by Amgen Inc; Arbor Pharmaceuticals, LLC; AstraZeneca Pharmaceuticals, LP; Espero Pharmaceuticals; Merck Sharp & Dohme Corp. and Sunovion Pharmaceuticals
Non-standard Abbreviations and Acronyms
- ASCVD
Atherosclerotic cardiovascular disease
- CABG
Coronary artery bypass graft
- CCD
Chronic coronary disease
- CCTA
Coronary computed tomography angiography
- CI
Confidence interval
- CrI
Credible interval
- CV
Cardiovascular
- EF
Ejection fraction
- eGFR
Estimated glomerular filtration rate
- HbA1c
Hemoglobin A1c
- HF
Heart failure
- HR
Hazard ratio
- IQR
Interquartile range
- LVEF
left ventricular ejection fraction
- MI
myocardial infarction
- NYHA
New York Heart Association
- PCI
percutaneous coronary intervention
- UA
unstable angina
Footnotes
This work was presented as an abstract at the American College of Cardiology Meetings, May 16th2021.
Clinical Trial Registration—URL: http://www.clinicaltrials.gov. Unique identifier: NCT01471522
Twitter handle: @jdnewmanMD
Publisher's Disclaimer: Disclaimer: Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences, the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the Department of Health and Human Services.
Disclosures
Dr. Jonathan D. Newman reports grants from National Heart, Lung and Blood Institute during the conduct of the study.
Dr. Rebecca Anthopolos reports grants from National Heart, Lung and Blood Institute during the conduct of the study.
Dr. G.B. John Mancini reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study; grants and personal fees from Amgen, grants and personal fees from Sanofi, grants and personal fees from Boerhinger Ingelheim, grants and personal fees from Astra Zeneca, grants and personal fees from Bayer, grants and personal fees from Janssen, grants and personal fees from Novo Nordisk, grants from Novartis, grants and personal fees from HLS Therapeutics, outside the submitted work.
Dr. Sripal Bangalore reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study; grants and personal fees from Abbott Vascular, personal fees from Biotronik, personal fees from Pfizer, personal fees from Amgen, personal fees from Reata, outside the submitted work.
Dr. Harmony Reynolds reports grants from National Heart, Lung and Blood Institute during the conduct of the study; non-financial support from Abbott Vascular, non-financial support from BioTelemetry, non-financial support from Siemens, outside the submitted work.
Dennis F. Kunichoff reports grants from National Heart, Lung and Blood Institute during the conduct of the study.
Dr. Roxy Senior reports grants from National Heart, Lung and Blood Institute, during the conduct of the study.
Dr. Jesus Peteiro reports grants from National Heart, Lung and Blood Institute, during the conduct of the study.
Dr. Balram Bhargava reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study.
Dr. Pallav Garg reports grants from National Heart, Lung and Blood Institute during the conduct of the study.
Dr. Jorge Escobedo reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study.
Dr. Rolf Doerr reports grants from National Heart, Lung and Blood Institute, during the conduct of the study.
Dr. Tomasz Mazurek reports grants from National Heart, Lung and Blood Institute, during the conduct of the study.
Jose Gonzalez-Juanatey reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study.
Dr. Grzegorz Gajos reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study.
Dr. Carlo Briguori reports grants from National Heart, Lung and Blood Institute, during the conduct of the study.
Dr. Hong Cheng reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study.
Dr. Andras Vertes reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study.
Dr. Sandeep Mahajan reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study.
Dr. Luis A. Guzman reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study.
Dr. Matyas Keltai reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study.
Dr. Aldo P. Maggioni reports grants from National Heart, Lung and Blood Institute, during the conduct of the study; personal fees from Bayer, personal fees from Fresenius, personal fees from Novartis, outside the submitted work.
Dr. Gregg W. Stone reports grants and personal fees from the National Heart, Lung, and Blood Institute during the conduct of the study; personal fees from Terumo, Amaranth, and Shockwave; personal fees and other from Valfix; personal fees from TherOx, Reva, Vascular Dynamics, Robocath, HeartFlow, Gore, Ablative Solutions, Matrizyme, Miracor, Neovasc, V-wave, Abiomed, Claret, and Sirtex; personal fees and other from Ancora and Qool Therapeutics; other from Cagent, Applied Therapeutics, Biostar family of funds, and MedFocus family of funds; personal fees and other from SpectraWave; personal fees from MAIA Pharmaceuticals; personal fees and other from Orchestra Biomed; other from Aria; personal fees from Vectorious; and other from Cardiac Success, outside the submitted work.
Dr. Jeffrey S. Berger reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study; grants from Astrazeneca, other from Jansen, other from Amgen, grants from AHA, outside the submitted work.
Dr. Yves D. Rosenberg reports employment by the National Heart, Lung, and Blood Institute during the conduct of the study.
Dr. William E. Boden reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study; grants from Abbvie, grants from Amarin, grants from Amgen, personal fees from Amgen, personal fees from Cleveland Clinic Clinical Coordinating Center, personal fees from Janssen, outside the submitted work.
Dr. Bernard R. Chaitman reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study; personal fees from Merck, NovoNordisk, Lilly, Johnson and Johnson, Daiichi Sankyo, Imbria, Xylocor, Sanofi, Tricida, and Xylocor, outside the submitted work.
Dr. Jerome L. Fleg reports employment by the National Heart, Lung, and Blood Institute during the conduct of the study.
Dr. Judith S. Hochman is PI for the ISCHEMIA trial for which, in addition to support by National Heart, Lung, and Blood Institute grant, devices and medications were provided Medtronic, Inc.; Abbott Vascular, Inc (formerly St. Jude Medical, Inc).; Royal Philips NV (formerly Volcano Corporation); Arbor Pharmaceuticals, LLC; AstraZeneca Pharmaceuticals, LP; Merck Sharp & Dohme Corp.; Omron Healthcare, Inc, Sunovion Pharmaceuticals, Inc; Espero BioPharma; and Amgen Inc; and financial donations from Arbor Pharmaceuticals LLC and AstraZeneca Pharmaceuticals LP.
Dr. David J. Maron reports grants from National Heart, Lung and Blood Institute during the conduct of the study.
References:
- 1.Cavender MA, Steg PG, Smith SC, Eagle K, Ohman EM, Goto S, Kuder J, Im K, Wilson PWF, Bhatt DL, et al. Impact of Diabetes Mellitus on Hospitalization for Heart Failure, Cardiovascular Events, and Death. Circulation 2015;132:923–931. [DOI] [PubMed] [Google Scholar]
- 2.Arnold Suzanne V, Bhatt Deepak L, Barsness Gregory W, Beatty Alexis L, Deedwania Prakash C, Inzucchi Silvio E, Kosiborod Mikhail, Leiter Lawrence A., Lipska Kasia J., Newman Jonathan D., et al. Clinical Management of Stable Coronary Artery Disease in Patients With Type 2 Diabetes Mellitus: A Scientific Statement From the American Heart Association. Circulation 2020;141:e779–e806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, et al. Optimal Medical Therapy with or without PCI for Stable Coronary Disease. New England Journal of Medicine 2007;356:1503–1516. [DOI] [PubMed] [Google Scholar]
- 4.BARI 2D Study Group, Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, Orchard TJ, Chaitman BR, Genuth SM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009;360:2503–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, Yang M, Cohen DJ, Rosenberg Y, Solomon SD, et al. Strategies for Multivessel Revascularization in Patients with Diabetes. New England Journal of Medicine 2012;367:2375–2384. [DOI] [PubMed] [Google Scholar]
- 6.United States Renal Data System. 2020 USRDS annual data report: Epidemiology of kidney disease in the United States National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2020. [cited 2021 Feb 26]; Available from: https://www.usrds.org/annual-data-report/ [Google Scholar]
- 7.Bangalore S, Maron DJ, O’Brien SM, Fleg JL, Kretov EI, Briguori C, Kaul U, Reynolds HR, Mazurek T, Sidhu MS, et al. Management of Coronary Disease in Patients with Advanced Kidney Disease. N Engl J Med 2020;382:1608–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farkouh ME, Sidhu MS, Brooks MM, Vlachos H, Boden WE, Frye RL, Hartigan P, Siami FS, Bittner VA, Chaitman BR, et al. Impact of Chronic Kidney Disease on Outcomes of Myocardial Revascularization in Patients With Diabetes. J Am Coll Cardiol 2019;73:400–411. [DOI] [PubMed] [Google Scholar]
- 9.Bangalore S, Maron DJ, Fleg JL, O’Brien SM, Herzog CA, Stone GW, Mark DB, Spertus JA, Alexander KP, Sidhu MS, et al. International Study of Comparative Health Effectiveness with Medical and Invasive Approaches- Chronic Kidney Disease (ISCHEMIA-CKD): Rationale and Design. Am Heart J 2018;205:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maron DJ, Hochman JS, O’Brien SM, Reynolds HR, Boden WE, Stone GW, Bangalore S, Spertus JA, Mark DB, Alexander KP, et al. International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) Trial: Rationale and Design. American Heart Journal 2018;201:124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O’Brien SM, Boden WE, Chaitman BR, Senior R, López-Sendón J, Alexander KP, et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. New England Journal of Medicine 2020; 382:1395–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mancini GBJ, Leipsic J, Budoff MJ, Hague CJ, Min JK, Stevens SR, Reynolds HR, O’Brien SM, Shaw LJ, Manjunath CN, et al. Coronary CT Angiography Followed by Invasive Angiography in Patients With Moderate or Severe Ischemia-Insights From the ISCHEMIA Trial. JACC Cardiovasc Imaging 2021;S1936–878X(20)31019–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hochman JS, Reynolds HR, Bangalore S, O’Brien SM, Alexander KP, Senior R, Boden WE, Stone GW, Goodman SG, Lopes RD, et al. Baseline Characteristics and Risk Profiles of Participants in the ISCHEMIA Randomized Clinical Trial. JAMA Cardiology 2019;4:273–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bangalore S, Bhagwat A, Pinto B, Goel PK, Jagtap P, Sathe S, Arambam P, Kaul U. Percutaneous Coronary Intervention in Patients With Insulin-Treated and Non–Insulin-Treated Diabetes Mellitus: Secondary Analysis of the TUXEDO Trial. JAMA Cardiol 2016;1:266. [DOI] [PubMed] [Google Scholar]
- 15.Dangas GD, Farkouh ME, Sleeper LA, Yang M, Schoos MM, Macaya C, Abizaid A, Buller CE, Devlin G, Rodriguez AE, et al. Long-term outcome of PCI versus CABG in insulin and non-insulin-treated diabetic patients: results from the FREEDOM trial. J Am Coll Cardiol 2014;64:1189–1197. [DOI] [PubMed] [Google Scholar]
- 16.Peters SAE, Huxley RR, Sattar N, Woodward M. Sex Differences in the Excess Risk of Cardiovascular Diseases Associated with Type 2 Diabetes: Potential Explanations and Clinical Implications. Current Cardiovascular Risk Reports 2015;9:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright AK, Kontopantelis E, Emsley R, Buchan I, Mamas MA, Sattar N, Ashcroft DM, Rutter MK. Cardiovascular Risk and Risk Factor Management in Type 2 Diabetes Mellitus. Circulation 2019;139:2742–2753. [DOI] [PubMed] [Google Scholar]
- 18.Min JK, Shaw LJ, Devereux RB, Okin PM, Weinsaft JW, Russo DJ, Lippolis NJ, Berman DS, Callister TQ. Prognostic Value of Multidetector Coronary Computed Tomographic Angiography for Prediction of All-Cause Mortality. J Am Coll Cardiol 2007;50:1161–1170. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds HR. Outcomes in the ISCHEMIA Trial Based on Coronary Artery Disease and Ischemia Severity. Circulation 2021. [In Press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopes Renato D, Alexander Karen P, Stevens Susanna R, Reynolds Harmony R, Stone Gregg W, Piña Ileana L, Rockhold Frank W, Elghamaz Ahmed, Lopez-Sendon Jose Luis, Farsky Pedro S., et al. Initial Invasive Versus Conservative Management of Stable Ischemic Heart Disease in Patients With a History of Heart Failure or Left Ventricular Dysfunction. Circulation 2020;142:1725–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Infarction JETF for the UD of M, Katus HA, Lindahl B, Morrow DA, et al. Third Universal Definition of Myocardial Infarction. Circulation 2012;126:2020–2035. [DOI] [PubMed] [Google Scholar]
- 22.Chaitman Bernard R, Alexander Karen P, Cyr Derek D, Berger Jeffrey S, Reynolds Harmony R, Bangalore Sripal, Boden William E., Lopes Renato D., Demkow Marcin, Piero Perna Gian, et al. Myocardial Infarction in the ISCHEMIA Trial. Circulation 2021;143:790–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henderson NC, Louis TA, Wang C, Varadhan R. Bayesian analysis of heterogeneous treatment effects for patient-centered outcomes research. Health Serv Outcomes Res Method 2016;16:213–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones HE, Ohlssen DI, Neuenschwander B, Racine A, Branson M. Bayesian models for subgroup analysis in clinical trials. Clin Trials 2011;8:129–143. [DOI] [PubMed] [Google Scholar]
- 25.Impact Story: Using innovative statistical approaches to provide the most reliable treatment outcomes information to patients and clinicians FDA. 2020. [cited 2020 Jul 24]; Available from: https://www.fda.gov/drugs/regulatory-science-action/impact-story-using-innovative-statistical-approaches-provide-most-reliable-treatment-outcomes
- 26.Bittl John A, He Yulei. Bayesian Analysis: A Practical Approach to Interpret Clinical Trials and Create Clinical Practice Guidelines. Circulation: Cardiovascular Quality and Outcomes 2017;10:e003563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alosh M, Fritsch K, Huque M, Mahjoob K, Pennello G, Rothmann M, Russek-Cohen E, Smith F, Wilson S, Yue L. Statistical Considerations on Subgroup Analysis in Clinical Trials. Statistics in Biopharmaceutical Research 2015;7:286–303. [Google Scholar]
- 28.Dixon DO, Simon R. Bayesian Subset Analysis. Biometrics 1991;47:871–881. [PubMed] [Google Scholar]
- 29.Schemper M, Wakounig S, Heinze G. The estimation of average hazard ratios by weighted Cox regression. Statistics in Medicine 2009;28:2473–2489. [DOI] [PubMed] [Google Scholar]
- 30.Team RC. R: A Language and Environment for Statistical Computing [Internet] 2018. Available from: https://www.R-project.org/
- 31.Plummer M JAGS: A Program for Analysis of Bayesian Graphical Models using Gibbs Sampling 3rd International Workshop on Distributed Statistical Computing (DSC 2003); Vienna, Austria. 2003;124. [Google Scholar]
- 32.Sripal Bangalore, Maron David J, Stone Gregg W, Hochman Judith S. Routine Revascularization Versus Initial Medical Therapy for Stable Ischemic Heart Disease. Circulation 2020;142:841–857. [DOI] [PubMed] [Google Scholar]
- 33.Mancini GBJ, Farkouh ME, Brooks MM, Chaitman BR, Boden WE, Vlachos H, Hartigan PM, Siami FS, Sidhu MS, Bittner V, et al. Medical Treatment and Revascularization Options in Patients With Type 2 Diabetes and Coronary Disease. Journal American College Cardiology 2016;68:985–995. [DOI] [PubMed] [Google Scholar]
- 34.Bittl JA. Percutaneous Coronary Interventions in the Diabetic Patient. Circulation: Cardiovascular Interventions 2015;8. [DOI] [PubMed] [Google Scholar]
- 35.Newman Jonathan D, Alexander Karen P, Gu Xiangqiong, O’Brien Sean M., Boden William E., Govindan Sajeev C., Senior Roxy, Moorthy Nagaraja, Rezende Paulo C., Demkow Marcin, et al. Baseline Predictors of Low-Density Lipoprotein Cholesterol and Systolic Blood Pressure Goal Attainment After 1 Year in the ISCHEMIA Trial. Circulation: Cardiovascular Quality and Outcomes 2019;12:e006002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peters SAE, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia 2014;57:1542–1551. [DOI] [PubMed] [Google Scholar]
- 37.Peters SAE, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet 2014;383:1973–1980. [DOI] [PubMed] [Google Scholar]
- 38.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ 2006;332:73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gnatiuc L, Herrington WG, Halsey J, Tuomilehto J, Fang X, Kim HC, De Bacquer D, Dobson AJ, Criqui MH, Jacobs DR Jr. Sex-specific relevance of diabetes to occlusive vascular and other mortality: a collaborative meta-analysis of individual data from 980 793 adults from 68 prospective studies. The lancet Diabetes & endocrinology 2018;6:538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tamis-Holland JE, Lu J, Korytkowski M, Magee M, Rogers WJ, Lopes N, Mighton L, Jacobs AK, BARI 2D Study Group. Sex differences in presentation and outcome among patients with type 2 diabetes and coronary artery disease treated with contemporary medical therapy with or without prompt revascularization: a report from the BARI 2D Trial (Bypass Angioplasty Revascularization Investigation 2 Diabetes). J Am Coll Cardiol 2013;61:1767–1776. [DOI] [PubMed] [Google Scholar]
- 41.Magee MF, Tamis-Holland JE, Lu J, Bittner VA, Brooks MM, Lopes N, Jacobs AK, Group B 2D S. Sex, Prescribing Practices and Guideline Recommended, Blood Pressure, and LDL Cholesterol Targets at Baseline in the BARI 2D Trial. International Journal of Endocrinology 2015;2015:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lauer MS, Blackstone EH, Young JB, Topol EJ. Cause of death in clinical research: Time for a reassessment? Journal of the American College of Cardiology 1999;34:618–620. [DOI] [PubMed] [Google Scholar]
- 43.Fox CS, Sullivan L, D’Agostino RB, Wilson PWF. The Significant Effect of Diabetes Duration on Coronary Heart Disease Mortality: The Framingham Heart Study. Diabetes Care 2004;27:704–708. [DOI] [PubMed] [Google Scholar]
- 44.Bakris GL, Molitch M. Microalbuminuria as a risk predictor in diabetes: the continuing saga. Diabetes Care 2014;37:867–875. [DOI] [PubMed] [Google Scholar]
- 45.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:7–22.12114036 [Google Scholar]
- 46.Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy of cholesterol-lowering therapy in 18 686 people with diabetes in 14 randomised trials of statins: a meta-analysis. The Lancet 2008;371:117–125. [DOI] [PubMed] [Google Scholar]
- 47.Wilcox T, Block CD, Schwartzbard AZ, Newman JD. Diabetic Agents, From Metformin to SGLT2 Inhibitors and GLP1 Receptor Agonists: JACC Focus Seminar. J Am Coll Cardiol 2020;75:1956–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ibrahim JG, Chen M-H, Sinha D. Bayesian Survival Analysis Springer Science & Business Media; 2013. [Google Scholar]
- 49.Gelman A, Carlin JB, Stern HS, Rubin DB. Bayesian Data Analysis Chapman & Hall/CRC. Inc, New York. 2004. [Google Scholar]
- 50.R: The R Project for Statistical Computing [Internet] [cited 2021. Mar 9]; Available from: https://www.r-project.org/
- 51.Plummer M JAGS: A Program for Analysis of Bayesian Graphical Models Using Gibbs Sampling 2003;10. [Google Scholar]
- 52.Su YS; Yajima M R2jags: Using R to Run ‘JAGS’. R Package Version 0.5–7 Available online: https://CRAN.R-project.org/package=R2jags [cited 2021. Mar 9].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


