Transforming growth factor beta (TGFβ) is a pleiotropic cytokine with functions related to angiogenesis, tumor suppression, and immune tolerance [1]. TGFβ is involved in tumor immune escape and is a target for cancer immune therapy [1]. We have reported that healthy donors and cancer patients harbor CD8+ cytotoxic T lymphocytes (CTLs) that are specific to TGFβ-derived epitopes and that these CD8+ CTLs kill malignant myeloid cells in a TGFβ-dependent manner [2]. We also found that T cells specific to the TGFβ-derived epitope TGFβ141–160 (REAVPEPVLLSRAELRLLRL, or TGFβ-15) that were expanded from a patient with cancer contained both CD4+ and CD8+ TGFβ-15-specific T cells. The presence of CD4+ subsets, such as Th1 and Th9 cells, is important to antitumor immunity and has been shown to increase the efficacy of PD-L1/PD-1 immune checkpoint therapy [3, 4]. Hence, we set out to characterize CD4+ T cells that were specific to TGFβ-derived epitopes.
Using magnetically activated cell sorting, we enriched CD4+ T cells from the TGFβ-15-specific culture described above, thereby establishing CD4+ T cell cultures that were specific to TGFβ-15. These T cells were characterized by fluorescence-activated cell sorting (FACS) and intracellular cytokine staining (ICS) as described previously [2]. TGFβ-15-specific CD4+ T cells produced high amounts of interferon-gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α) upon stimulation with TGFβ-15 (Fig. 1A). A pan-HLA class II-blocking assay showed that the response was HLA-II dependent (Fig. 1B), and blocking HLA-DR, DQ, and DP (Fig. 1B) showed that the response was restricted to HLA-DR. Donor genotyping identified the HLA-DRB1*15:01:01 G allele. As we wanted to investigate the ability of T cells to recognize TGFβ-expressing cells, we searched the TRON Cell Line Portal (http://celllines.tron-mainz.de/) for HLA-DR-compatible cancer cell lines and found the TGFβ-producing malignant myeloid cell line THP-1, which expresses HLA-DRB*15.
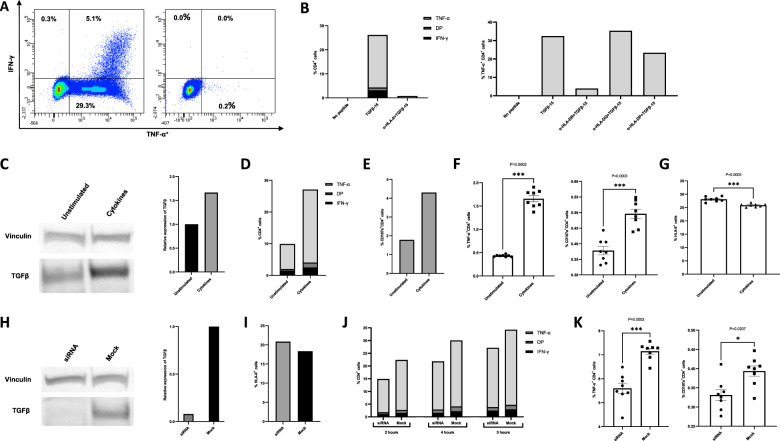
Fig. 1.
Characterization of TGFβ epitope-specific CD4+ T cells. A Intracellular cytokine staining (ICS) of the TGFβ-15-specific CD4+ T cell culture stimulated with TGFβ-15 (left) and of the unstimulated cells (right). B ICS of TGFβ-15-stimulated cells treated with a pan-HLA-II-blocking antibody (left) and HLA-DR-, DQ-, and DP-blocking antibodies (right). C Western blot analysis of lysates from THP-1 cells that were unstimulated or treated with IL-4 and TGFβ1 for 72 h (left). Relative expression of TGFβ in lysates were analyzed using ImageJ software (right). D ICS of T cells stimulated with THP-1 cells that were either untreated or treated with IL-4 and TGFβ1 for 72 h with an effector:target ratio of 3:1. E Expression of CD107a on T cells from the experiment shown in D. F Experiment performed as in D, E with 8 replicates, showing the ICS results (left) and CD107a expression (right). Error bars indicate the standard error of the mean. Statistical analysis was performed using the Mann–Whitney U-test. G FACS analysis of the expression of HLA-II by THP-1 cells that were unstimulated or stimulated with cytokines for 48 h. H Western blot analysis of lysates from THP-1 cells that were either mock transfected or transfected with TGFβ siRNA (left). Relative expression of TGFβ in lysates were analyzed using ImageJ software (right). I FACS analysis of the expression of HLA-II by THP-1 cells that were either mock transfected or transfected with TGFβ siRNA 48 h prior to analysis. J ICS performed on TGFβ-15-specific T cells stimulated with THP-1 cells that were either mock or TGFβ1 siRNA transfected 96 h before ICS. The experiment was performed using different conditions: 2 h of stimulation and an E:T of 3:1 (left); 4 h of stimulation and an E:T of 3:1 (middle); or 5 h of stimulation and an E:T of 1:1 (right). K ICS of T cells stimulated with TGFβ siRNA or mock-transfected THP-1 cells in 8 replicates. The total stimulation time was 2 h, with an E:T of 3:1. All error bars depict the standard error of the mean, and all statistical analyses were performed using the Mann–Whitney U-test. DP = cytokine double positive
We have previously shown that stimulation of THP-1 cells with the cytokines IL-4 and TGFβ enhances their expression of TGFβ [2]. Hence, we investigated whether increased amounts of TGFβ produced by THP-1 cells could increase their recognition by TGFβ-15-specific CD4+ T cells. Importantly, the active form of the cytokine TGFβ does not include the TGFβ-15 epitope, which is found in the N-terminal latency-associated peptide of TGFβ. Accordingly, any increased recognition of THP-1 cells was not due to the processing and presentation of the TGFβ used for stimulation. Using western blotting, we confirmed that treatment of THP-1 cells for 72 h with 200 U/ml IL-4 and 5 ng/ml TGFβ increased the expression of TGFβ (Fig. 1C). THP-1 cells that were treated with these cytokines for 72 h were then used as target cells in an ICS experiment with an effector:target (E:T) ratio of 3:1. The results showed increased expression of TNF-α, IFN-γ, and CD107a in T cells that were stimulated with cytokine-treated THP-1 cells compared with unstimulated THP-1 cells (Fig. 1D, E). An increase in TGFβ expression by THP-1 cells was already evident after 48 h of stimulation with cytokines [2], and CD4+ T cells stimulated with THP-1 cells that were treated with cytokines for 48 h also responded with increased cytokine (data not shown). The experiment was repeated with 8 replicates using another TGFβ-15-specific CD4+ T cell culture. The number of replicates allowed us to perform statistical analysis. Again, we identified significant increases in cytokine release and CD107a expression in T cells stimulated with cytokine-treated THP-1 cells (Fig. 1F). Next, we asked whether the increased T cell activation was caused by increased HLA-II expression on cytokine-treated THP-1 cells. Using FACS analysis with Fc blocking and an appropriate isotype control, we demonstrated that this was not the case (Fig. 1G). In fact, cytokine-treated THP-1 cells expressed less HLA-II than untreated THP-1 cells.
To demonstrate that the decreased expression of TGFβ induced by THP-1 cells resulted in decreased activation of TGFβ-15-specific CD4+ T cells, we transfected THP-1 cells with TGFβ siRNA [2]. THP-1 cells that were transfected with TGFβ siRNA had lower expression of TGFβ than mock-transfected THP-1 cells (Fig. 1H) and comparable levels of HLA-II expression (Fig. 1I). Accordingly, we found a difference in the activation of T cells stimulated with either mock- or siRNA-transfected THP-1 cells (Fig. 1J). The experiment was repeated with 8 replicates and showed a statistically significant decrease in the activation of T cells stimulated with siRNA-transfected THP-1 cells (Fig. 1K).
There is mounting evidence pointing to the existence of a class of self-reactive T cells, known as anti-regulatory T cells, that are not deleted in the thymus [5]. These cells can recognize and kill regulatory immune cells and cancer cells that produce immunosuppressive molecules such as indoleamine 2,3-dioxygenase 1 (IDO) [6] and programmed death ligand-1 (PD-L1) [7]. Activation of these anti-regulatory T cells through vaccination with epitopes from these immunosuppressive molecules induces an immune response against cancer cells and regulatory cells in the tumor. This response can decrease local immune suppression, enhancing the tumor-specific immune response. Therapeutic cancer vaccines against IDO and PD-L1 to enhance the anti-regulatory T-cell response have shown clinical potential in melanoma and nonsmall cell lung cancer [8, 9]. Our discovery of CD4+ and CD8+ T cells specific for TGFβ is noteworthy, as vaccination with TGFβ-derived epitopes could enhance the TGFβ-specific T cell response in cancer patients. Using the HLA-DR-compatible malignant myeloid cell line THP-1 and modulating these cells to increase or decrease intracellular TGFβ, we showed that the recognition of these cells by our TGFβ-specific CD4+ T cells was TGFβ dependent. Systemic CD4+ T cell responses to tumor antigens are important in enhancing cytotoxic CD8+ T cell immunity in patients [4]. Our discovery of TGFβ-specific CD4+ T cells is of great importance, as CD4+ TGFβ-specific T cell responses in patients could be used to enhance the responses of the TGFβ-specific CD8+ CTLs we described previously.
TGFβ is produced not only by cancer cells but also by several regulatory cells in the tumor microenvironment, including Tregs, tumor-associated macrophages, and cancer-associated fibroblasts [1]. Hence, the induction of a synergistic CD4+ and CD8+ TGFβ-specific T cell response by therapeutic cancer vaccination with one or several TGFβ-derived epitopes might result in the specific recognition of cancer cells, cancer-associated fibroblasts, and other regulatory immune cells in the tumor microenvironment. This recognition could revert local immune suppression, induce a proinflammatory environment and push the balance toward antitumor immunity, thereby improving responses to other therapies.
Acknowledgements
This project was supported by Danish Health Authority grant “Empowering Cancer Immunotherapy in Denmark” (grant number 4-1612-236/8), the Copenhagen University Hospital, Herlev, and Gentofte and through a research funding agreement between IO Biotech ApS and the National Center for Cancer Immune Therapy (CCIT-DK). We greatly appreciate the tremendous technical support from Merete Jonassen.
Author contributions
REJM performed experiments, interpreted the data, and wrote the manuscript; MOH performed experiments, interpreted the data, and wrote the manuscript; and MHA conceived the project, interpreted the data, and wrote the manuscript.
Competing interests
MHA has developed an invention based on the use of TGFβ for vaccinations. The rights of the invention have been transferred to Copenhagen University Hospital Herlev, according to the Danish Law of Public Inventions at Public Research Institutions. The capital region has licensed the rights to the company IO Biotech ApS. The patent application was filed by IO Biotech ApS. MHA is board member, consultant and shareholder in IO Biotech. The other authors declare no competing financial interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Batlle E, Massague J. Transforming growth factor-b signaling in immunity and cancer. Immunity. 2019;50:924–40. doi: 10.1016/j.immuni.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holmström MO, Mortensen R, Pavlidis AM, Martinenaite E, Weis-Banke SE, Aaboe-Jørgensen M, et al. Cytotoxic T cells isolated from healthy donors and cancer patients kill TGFβ-expressing cancer cells in a TGFβ-dependent manner. Cell Mol Immunol. 2021;18:415–26. doi: 10.1038/s41423-020-00593-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chandwaskar R, Awasthi A. Emerging Roles of Th9 Cells as an Anti-tumor Helper T Cells. Int Rev Immunol. 2019;38:204–11. doi: 10.1080/08830185.2019.1648453. [DOI] [PubMed] [Google Scholar]
- 4.Zuazo M, Arasanz H, Bocanegra A, Fernandez G, Chocarro L, Vera R, et al. Systemic CD4 Immunity as a Key Contributor to PD-L1/PD-1 Blockade Immunotherapy Efficacy. Front Immunol. 2020;11:586907. doi: 10.3389/fimmu.2020.586907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersen MH. Anti-regulatory T cells. Semin Immunopathol. 2017;39:317–26. doi: 10.1007/s00281-016-0593-x. [DOI] [PubMed] [Google Scholar]
- 6.Sørensen RB, Hadrup SR, Svane IM, Hjortsø MC, Thor Straten P, Andersen MH. Indoleamine 2,3-dioxygenase specific, cytotoxic T cells as immune regulators. Blood. 2011;117:2200–10. doi: 10.1182/blood-2010-06-288498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munir S, Andersen GH, Met Ö, Donia M, Frøsig TM, Larsen SK, et al. HLA-restricted CTL that are specific for the immune checkpoint ligand PD-L1 occur with high frequency in cancer patients. Cancer Res. 2013;73:1764–76. doi: 10.1158/0008-5472.CAN-12-3507. [DOI] [PubMed] [Google Scholar]
- 8.Svane I-M, Kjeldsen JW, Lorentzen CL, Martinenaite E, Andersen MH. Clinical efficacy and immunity of combination therapy with nivolumab and IDO/PD-L1 peptide vaccine in patients with metastatic melanoma: a phase I/II trial. Ann Oncol. 2020;31:S1176. doi: 10.1016/j.annonc.2020.08.2278. [DOI] [Google Scholar]
- 9.Iversen TZ, Engell-Noerregaard L, Ellebaek E, Andersen R, Larsen SK, Bjoern J, et al. Long-lasting disease stabilization in the absence of toxicity in metastatic lung cancer patients vaccinated with an epitope derived from indoleamine 2,3 dioxygenase. Clin Cancer Res. 2014;20:221–32. doi: 10.1158/1078-0432.CCR-13-1560. [DOI] [PubMed] [Google Scholar]