Abstract
Chronic thromboembolic pulmonary hypertension is an underdiagnosed condition. Patients typically present with the symptoms of right heart failure. Diagnosis is usually done by radionuclide ventilation/perfusion (VQ) scan, high-quality multidetector computed tomography (CT) or pulmonary angiography at expert centers. Pulmonary endarterectomy remains the corner stone in management of chronic thromboembolic pulmonary hypertension. Deep hypothermic circulatory arrest is commonly used for the operation at most centers. In-hospital mortality ranges from 1.7 to 14.2%. Pulmonary hemorrhage, reperfusion lung injury, and right ventricular failure remain major early post-operative concerns. Five-year survival is reported to be 76 to 89%. Long-term outcome depends on residual pulmonary hypertension. Balloon pulmonary angioplasty and medical management play an adjunctive role. Here, we provide a comprehensive review on surgical management of chronic thromboembolic pulmonary hypertension.
Keywords: Pulmonary endarterectomy, Chronic thromboembolic pulmonary hypertension, Pulmonary thromboendarterectomy
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is defined as a mean pulmonary artery pressure (mPAP) of ≥25 mmHg with capillary wedge pressure < 15 mmHg by right heart catheterization together with at least one mismatched segmental perfusion defect demonstrated by radionuclide ventilation/perfusion (VQ) scan, high-quality multidetector computed tomography (CT), or pulmonary angiography [1].
CTEPH develops in up to 4% of patients within the first 2 years after acute pulmonary embolism (PE) and incidence of preceding venous thromboembolism (VTE) is found in up to 69% of patients [2]. Unfortunately, it is an underdiagnosed condition [3]. The untreated disease has a poor prognosis, proportional to the severity of pulmonary hypertension (PH).
Four decades ago, CTEPH was considered exceedingly rare, and a surgical procedure to relieve the condition—unjustified, ineffective, and dangerous. But, because of perseverance and hard work of surgeons, pulmonologists, and cardiologist, pulmonary endarterectomy (PEA) is now recognized as standard treatment for CTEPH.
Medical treatment has failed to show long-term survival advantage despite introduction of new vasodilators [4]. Surgical intervention by PEA or lung transplantation is the only effective cure. The mortality rate of untreated CTEPH is as high as 90% after 3 years. Moreover, the scarcity of available donor organs limits the use of transplantation as a first line of treatment. Moreover, PEA has the advantage of a higher survival rate compared to lung transplant (76 to 89% vs 60%) [4–14] and does not preclude transplant if the patient experiences persistent PH that does not improve with medication.
Therefore, PEA is recognized as the standard treatment for CTEPH in most patients. The procedure involves the removal of fibrous obstructive tissue from the pulmonary arteries. The subsequent degree of relief of PH is variable, and restoration of pulmonary hemodynamics to normal or near normal happens in up to 68.8 to 91.8% of cases [4, 6–12, 14–20].
In the last decade, many case series have been published from the Asia-Pacific region [10, 12, 20–23], suggesting growing enthusiasm in surgical management of CTEPH. However, high mortality due to incomplete endarterectomy and residual PH during the learning curve has been reported [7, 11, 16, 19, 23]. In this review, we discuss in detail the surgical management of CTEPH.
Preoperative evaluation
The patients with CTEPH may present with symptoms like exercise intolerance, fatigue, and dyspnea. Physical examination often reveals signs of right heart failure like jugular venous distention, hepatomegaly, ascites, and peripheral edema. Bruits may be appreciated on auscultation of the peripheral lung fields. Diagnosis is usually made on an outpatient basis and involves reasonable suspicion and multiple imaging tests.
Electrocardiogram may reveal right axis deviation, right ventricular hypertrophy, and a right bundle-branch block. Chest radiography may be normal; however, more advanced disease often shows dilation of pulmonary artery (PA) and enlargement of the right heart.
In suspected patients, the goal of transthoracic echocardiographic (TTE) examination should be determining the level of probability of PH by assessment of right heart function [24]. Echocardiography is not diagnostic for CTEPH but helps in screening the patients by estimating severity and chronicity of hemodynamic burden of PH.
If echocardiographic probability is low, alternative diagnosis should be considered. For intermediate probability, follow-up echocardiography and further investigations of PH may be considered. In patients having high probability of CTEPH, confirmation of diagnosis is done by CT angiography and VQ scan at CTEPH expert centers. Compared to CT, VQ scans (Fig. 1) have a higher sensitivity but lower specificity for detecting CTEPH. At least one perfusion defect must be present in VQ Scan to qualify as CTEPH [9].
Fig. 1.

Perfusion scan suggesting multiple perfusion defects suggesting thromboembolic disease
The first line of treatment should be lifelong anticoagulation and management of symptoms of heart failure. However, all symptomatic patients diagnosed with CTEPH must undergo presurgical evaluation as 62–82% of patients with CTEPH have a disease amenable to the surgical intervention [25–27]. Pulmonary angiography (Fig. 2) with selective catheterization of lobular branches remains the gold standard for diagnosis and assessment of surgical operability for CTEPH [28]. Characteristic angiographic findings include vascular webs, intimal irregularities, abrupt narrowing of vessels, and proximal obstruction of PA. At our hospital, patients older than 45 years routinely receive preoperative coronary angiogram. It is important to match the degree of obstructive material assessed by angiography to the pulmonary vascular resistance (PVR). Discordance might signify the presence of small vessel disease.
Fig. 2.

Pulmonary angiogram of right lung (from the same patient mentioned in Fig. 1) suggests acute cut off of right apical segmental (black arrow) and lower lobar arteries (white arrow)
Pulmonary function testing can be used to exclude other underlying pulmonary pathology. Isolated reduction in carbon monoxide–diffusing capacity with preserved lung function is the hallmark of patients with pulmonary vascular disease (i.e., PH, CTEPH).
Preoperatively, patients should be screened for deep venous thrombosis and peripheral vascular disease. CT angiogram of abdomen and pelvis is always helpful in decision-making should the patient need extracorporeal membrane oxygenation (ECMO) post-operatively. It also helps in identifying the inferior vena cava filter occlusion, which may require an additional femoral venous cannula for adequate venous return intraoperatively [29].
The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology and the European Respiratory Society described guidelines for risk assessment and operability [30]. Evaluation of operability should be performed by an interdisciplinary expert team including pulmonologists, cardiologists, radiologists, and surgeons [28]. Although the majority of patients with significant CTEPH will benefit from PEA, the selection of a patient remains quite subjective and difficult to standardize [24, 31]. Old age, lack of correlation between severity of PH and imaging, severe comorbidities, anatomic challenges, right heart function, and severity of PH are well accepted assessment criteria for risk profiling [9, 31].
Madani et al. [32] describe three major reasons for considering PEA: hemodynamic, alveolo-respiratory, and prophylactic. The hemodynamic goal is to prevent or ameliorate right ventricular compromise caused by PH. The respiratory objective is to improve respiratory function by the removal of a large ventilated but unperfused physiologic dead space, regardless of the severity of PH. The prophylactic goal is to prevent progressive right ventricular dysfunction or retrograde extension of the obstruction or to prevention of secondary arteriopathic changes in the remaining patent vessels.
Even though it is believed that once patient meets the operability criteria set by the multidisciplinary team, early operation is preferred to prevent long-term adverse effect on right heart function [8, 32]. International Prospective Registry analysis suggests neither delay between symptoms and PEA, most recent PE and PEA, nor the delay between CTEPH diagnosis and PEA was related to survival rates [4].
The patients who are deemed to be technically inoperable or having non-acceptable risk-benefit ratio from the operation (i.e., severe underlying parenchymal lung disease) can be managed by targeted medical therapy, balloon pulmonary angioplasty (BPA), or lung transplantation.
International prospective registry showed many surgeons/centers excluded patients having PVR more than 1500 dyn·s·cm−5 [9]. The study also found that pre-operative high PVR (>1200 dyn·s·cm−5) is a risk factor for surgery [9]. Madani et al. also suggested that high PVR (>1000 dyn·s·cm−5) is risk factor for mortality [8]. There should not be any PVR threshold or measure of right ventricle (RV) dysfunction that can be considered to preclude PEA [24] as long as the PVR correlates with imaging studies [33]. Patients with high PVR may have the most to benefit from the operation despite the higher risk [11]. Even with residual PH after surgery, they continue to enjoy survival and functional benefit after operation [6].
Advanced age per se is not a contraindication for surgery [24]. Vistarini et al. [14] found that despite a slightly higher short-term mortality (9.1% vs 5.1%) comparted to younger patients (<70 years), PEA is feasible and well tolerated for the vast majority of the elderly patients above 70. Elderly patients had significant high incidence of preoperative coronary disease, atrial fibrillation (AF), and peripheral vascular disease in their cohort [14].
The 6-min walk distance at diagnosis is an independent factor for death at 1 year after PEA [9] while obesity [34, 35] is not associated with worse outcomes. Chronicity and severity of disease, impact on physical endurance, underlying lung parenchymal disease, and surgical feasibility of thrombus burden removal are major determining risk factors for PEA [8, 24, 31, 32].
Medical management
Goals of medical management of CTEPH are prevention of recurrent VTE with lifelong anticoagulation, minimization of PVR with potential vasodilators, and improvement of symptoms of right heart failure with diuretics.
Although calcium-channel blockers, endothelin receptor blockers, and phosphodiesterase inhibitors are commonly administered [36], riociguat remains the only approved monotherapy for CTEPH patients [37]. However, several trials are going on for combination therapy and newer agents (i.e., macitentan).
As a consequence of increased use of pulmonary vasodilators, a significant delay is observed in patient referrals, even in patients who have obvious operable disease [8]. Although high pre-operative PVR (>1000 dyn·s·cm−5) is associated with worse outcomes, currently, there is no evidence to support delaying surgery to attempt optimization of PVR with vasodilators prior to PEA [24, 26] as it has minimal effect on pre-PEA hemodynamics and no effect on post-PEA outcomes/hemodynamics. We emphasize that referral to CTEPH centers of expertise for surgical treatment should not be delayed by pre-operative medical therapy.
Surgery
Similar to Jamieson’s classification, a new surgical classification was proposed by University of California San Diego (UCSD), mentioning four levels of pulmonary occlusive disease related to location of organized thrombus [3]. Level III and level IV represent presence of thrombus in segmental and subsegmental arteries, respectively, posing greatest surgical challenge (Fig. 3) [3].
Fig. 3.
Specimen from a chronic thromboembolic pulmonary hypertension (CTEPH) patient describing University of California San Diego (UCSD) classification; level 1—involving main pulmonary artery; level 2—involving lobar branches; level 3—segmental branches; level 4—subsegmental branches
The San Diego group pioneered the surgical technique with most other institutes performing the operation with minor variations [6–9, 15]. At most centers, circulatory arrest is routinely considered as it provides bloodless field and adequate visualization [6–9, 11, 15, 33]. Concerned about the possible cerebral effects of deep hypothermic circulatory arrest (DHCA), some centers have reported avoiding DHCA or recommended using multiple shorter episodes of DHCA [38] and/or antegrade cerebral perfusion [39]. But a randomized trial by Vuylsteke et al. (circulatory arrest versus cerebral perfusion during pulmonary endarterectomy surgery—PEACOG Trial, i.e., PEA COGnitive function trial) showed no difference in terms of cognitive function at 3 months [38]. Utilizing the wide variety of neurological outcome definitions, the studies reported incidence of stroke/cognitive dysfunction ranging from 0.46 to 13% [4, 7, 9, 11, 20]. Even in patients having previous history of stroke or transient ischemic attacks and in elderly patients, the surgery can be performed safely [14]. Neurological complications are more common in the patients having longer circulatory arrest times [4].
For the patients with distal disease (level III), Madani et al. have reported a modified technique [8]. The plane of dissection is started proximally within the normal PA at the level of lobar branches by separating the adventitia from the media circumferentially. Care must be taken to have full circumferential dissection without fragmentation of this fragile layer, avoiding vessel perforation. The dissection of this layer is carefully continued distally into the segmental and subsegmental branches. Care must be taken not to disrupt its continuity until the real thromboembolic material is reached. Once this material is within reach, further traction can be applied without disrupting the specimen to complete the endarterectomy into the distal branches [8]. The fragility of the layer in the proximal healthy vessels and possibility of perforation make this technique challenging. However, with experience, this layer can be safely developed and used as a guide to extend the endarterectomy distally to include segments with disease [8].
Circulatory arrest time must be limited to maximum 20 min at a time and reperfusion should be established for 10 min between two circulatory arrest time. While rewarming, before separating from cardiopulmonary bypass (CPB), a flexible bronchoscopy must be performed to look for bleeding in the airway.
Typically, after PEA, tricuspid regurgitation (TR) improves significantly. Surgeons usually refrain from elective correction of functional tricuspid valve regurgitation as majority of more than moderate TR improve to trivial or mild after PEA [40]. Unless there is any structural problem like chordal rupture, endocarditis or leaflet prolapse or very severe annular dilation, tricuspid valve repair can be avoided.
Post-operative care
No structured guidelines exist for postoperative care after PEA, which depends largely on personal or institutional experience [41].
Hemodynamics and fluid balance
Hemodynamics in post-PEA patients is complex. Vasoplegia and myocardial stunning often result from CPB and DHCA. Atrial arrhythmias are common after PEA. Perfusion of hypertrophied RV may require higher mean arterial pressures. Concomitantly, vasopressors can increase PVR, increasing RV strain. Vasopressors which minimally affect PVR, like vasopressin, are favored at our institution. Inotropes may be required to support the RV, while inhaled nitric oxide or epoprostenol may be used to reduce PVR. The use of pulmonary vasodilators must be judicious in the immediate post-operative period as they increase chances of pulmonary congestion and reperfusion injury. Pre-existing left ventricular (LV) dysfunction can worsen due to increase in LV preload.
Avoiding high cardiac index is preferred in immediate post-operative period as it increases the risk of pulmonary edema by exposing the pulmonary capillaries to high flow immediately after PEA. Within 24 h post-PEA, patients should return to their pre-operative weight. Overall, a net negative fluid balance should be maintained throughout the post-operative intensive care unit (ICU) stay.
In absence of structural abnormality, the presence of severe TR after PEA suggests residual PH. The focus should be on managing residual PH in these cases (i.e., reoperation, medical management, or BPA).
Ventilation
Postoperatively, care should be taken to avoid respiratory acidosis and hypoxia which can negatively affect PVR and worsen the RV failure. Tidal volume is typically set at 6–8 ml/kg and respiratory rate is set at 16–20 depending upon arterial blood gas. Although higher positive end expiratory pressure (PEEP) affects PVR, it remains the most important strategy to prevent and treat reperfusion lung injury (RLI). If immediate postoperative X-ray rules out reperfusion injury, PEEP can be reduced earlier; otherwise, PEEP is typically weaned before 2–3 h of extubation.
RLI (Fig. 4) is a localized non-cardiac pulmonary edema in revascularized lung segments. It is the result of increased vascular permeability, blunted pulmonary vasoconstriction, and circulatory overload of lung segments already perfused by collaterals [41, 42]. Severity of RLI correlates with degree of obstruction preoperatively. Clinically, it manifests as onset of sterile lung infiltrates in revascularized lung zones and presence of PaO2/FiO2 ratio of <300 within 48–72 h of surgery [41, 42]. It is usually managed with aggressive diuresis and positive pressure ventilation.
Fig. 4.
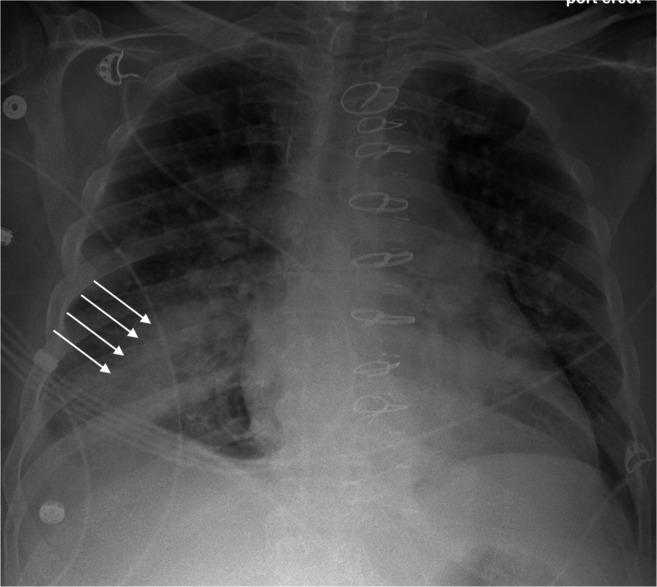
X-ray showing typical reperfusion lung injury in the form of localized pulmonary edema (white arrows, from the same patient mentioned in Figs. 1 and 2)
For severe reperfusion injury, which happens in very small number of patients, veno-venous (VV) ECMO may be considered to achieve immediate resuscitation. Methylprednisolone is found to be ineffective in preventing reperfusion injury [43].
Up to 70% of post-PEA cases are affected by PA steal [41]. It is a consequence of ventilation/perfusion mismatch where pulmonary arterial blood flow is shunted to newly endarterectomized segments. Hypoxia from V/Q mismatch is more common in distal CTEPH with predominant lower lobe involvement.
Anticoagulation
Anticoagulant strategies vary among different CTEPH centers. In general, risk of thrombosis should be stratified. The patients with high risk of thrombosis (Lupus antibody, Protein C and S deficiency) must receive early and aggressive anticoagulation therapy, once chest tube output is satisfactory. Heparin infusions can be used to bridge oral anticoagulants [3, 44]. Choice of vitamin K antagonist (VKA) or direct oral anticoagulants (DOAC) for anticoagulation is not evidence based. One single-centered study comparing DOAC vs VKAs in post-PEA patients showed lower bleeding complications but higher recurrence of VTE [44].
ECMO support
Postoperative RV failure, severe reperfusion edema and massive pulmonary hemorrhage often requires additional mechanical circulatory support in terms of ECMO. Postoperative incidence of either veno-arterial (VA) or VV ECMO support is reported to be ranging from 3.1 to 13% in various studies [7, 9, 11]. ECMO has also been considered a bridge to lung transplant strategy due to persistent PH after PEA [11]. Duration of ECMO support depends on reasons for ECMO institution and aggressiveness of the post-operative protocol. Both central and peripheral cannulations are reported in literature for VV or VA ECMO [45–47].
Pulmonary hemorrhage
Massive pulmonary hemorrhage preventing weaning from CPB is often a fatal complication of PEA [48–51]. It occurs when endarterectomy plane goes deep enough to perforate pulmonary artery which results in bleeding in surrounding airways. Unfortunately, the incidence of pulmonary hemorrhage is not widely reported in literature. Lopez et al. reported a 4% incidence [7]. Patients may end up having VA or VV ECMO support to overcome the crisis.
Several strategies [45, 48–50, 52, 53] have been described in literature to deal with this complication. Not one strategy fits all, and it depends on the site and nature of the bleeding, the availability of equipment and expertise, and the potential short-term and long-term advantages and disadvantages. A combination of two or more strategies may also be needed.
Outcomes
In-hospital mortality ranges from 1.7 to 14.2% [4, 6–12, 14–20]. Most studies reported significant mortality improvement in later patient cohorts [6–8, 10, 11, 17, 19] suggesting presence of a learning curve. Madani et al. reported continuous improvement in results with experience, despite patients having higher number of type III diseases and delay in referral [8].
Table 1 summarizes important studies published in last decade. Five-year survival is reported 76 to 89% [7, 8, 10, 12, 18]. The International Prospective Registry found significant survival benefit in operated patients compared to non-operated patients [4]. At our center, we have performed 280 PEA surgeries in the last 4 years with an in-hospital mortality of 4.2% and 1-year survival of 92.1% (unpublished).
Table 1.
Outcomes of pulmonary endarterectomy as reported in last decade
| Study and publication year | Type/goal of study | Duration of study | No. of patients | Age | Preop mean PAP (mmHg) | PreOp Mean PVR | PreOp cardiac index (CI)/cardiac output (CO) | PostOp PAP (mmHg) | PostOp PVR | PostOp cardiac index (CI)/cardiac output (CO) | Mortality | Residual PAH (incidence) | Bleeding requiring re-exploration | Reperfusion injury/edema | Airway bleeding | PostOp ECMO | Survival |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Freed, et al. 2011 [6] | Single-center experience with a goal of evaluating residual PAH on survival | 1997 to December 2007 | 314 | 55 (17–81) | 48 ± 12 | 805 ± 365 (dyn·s·cm−5) | 2 ± 0.7 L/min m2 (CI) | 26 ± 10 | 301 ± 232 (dyn·s·cm−5) | 2.5 ± 0.5 L/min m2 (CI) | Early mortality <3 months 14.6%; 1997–2002 mortality 25% and 2006–2007 mortality 2.7%, | 31.00% | NA | NA | NA | NA | 5 years survival 76% |
| Schölzel et al. 2011 [18] | Examining clinical worsening after PEA | May 2000 and August 2009 | 74 | 55.9 ± 13.8 | 41.3 ± 11.9 | 521 ± 264 (dyn·s·cm−5) | 4.7 ± 1.2 L/min (CO) | NA | NA | NA | 6.80% | NA | NA | NA | NA | NA | 1, 3 and 5 years survival 93%, 91% and 89% |
| van der Plas et al. 2011 [17] | Longitudinal follow-up of 6-min walk distance | January 2003 to January 2009 | 96 | 54 ± 14 | 41 ± 13 | 731 ± 403 (dyn·s·cm−5) | NA | 24.6 ± 7.2 | 422 ± 146 (dyn·s·cm−5) | NA | 10.41% | 31.20% | NA | NA | NA | NA | NA |
| Madani et al. 2012 [8] | Single-center experience comparing results after change in surgical technique—group 2 in the paper mentions 500 patients who underwent surgery with updated technique. | October 2006 to December 2010 | 500 | 51.0 ± 15.5 | 45.5 ± 11.6 | 719.0 ± 383.2 (dyn·s·cm−5) | 4.3 ± 1.4 L/min (CO) | 26.0 ± 8.4 | 253.4 ± 148.6 (dyn·s·cm−5) | 5.6 ± 1.4 L/min (CO) | 2.20% | NA | NA | NA | NA | NA | 5 years survival 82%, 10 years survival 75% |
| Yildizeli et al. 2013 [16] | Initial experience of single-center program | March 2011 and March 2012 | 49 | 47.7 ± 13.6 | 53.8 ± 14.5 | 808 ± 352 (dyn·s·cm−5) | 2.2 ± 0.7 | 28.5 ± 10.5 | 308.7 ± 91.4 (dyn·s·cm−5) | NA | 14.20% | NA | NA | NA | NA | 12.24% | NA |
| Lopez et al. 2015 [7] | Single-center experience | February 1996 to June 2014 | 106 | 53 ± 14 | 48 ± 12 | 789 ± 345 (dyn·s·cm−5) | 4.3 ± 1 L/min (CO) | 27 ± 11 | 275 ± 218 (dyn·s·cm−5) | 5.3 ± 1.4 L/min (CO) | 6.60% | 13.50% | NA | 20.00% | 4.00% | 5% | 3 years survival 90% ± 3%; 5 years survival 84% ± 5% |
| Nierlich et al. 2016 [11] | Single-center experience | 1992 to 2013 | 214 | 53.5 ± 14.9 | 51.0 ± 13.1 | 860.05 ± 386.36 (dyn·s·cm−5) | 2.3 ± 0.5 L/min m2 (CI) | 32.7 ± 11.1 | 337.6 ± 200.6 (dyn·s·cm−5) | 2.75 ± 0.8 L/min m2 (CI) | 6.50% | 27.6% had residual PVR higher than 50% of baseline | NA | NA | NA | 13% | 1 year survival 90.2% |
| International Prospective Registry Short Term (Mayer et al. 2011 [9]) and Long Term Outcomes (Delcroix et al. 2016 [4]) | Multicenter prospective registry (1 Canadian and 26 European Centers) | February 2007 to January 2009 | 386 | 60 (18–84) | 48 (17–80) | 728 (97–2880) (dyn·s·cm−5) | 2.2 (0.9–7.0) L/min m2 (CI) | NA | 248 (dyn·s·cm−5) | NA | 4.70% | 16.70% | 10.20% | 9.60% | NA | 3.10% | 1, 2, and 3 years survival 93%, 91% and 89% respectively |
| Vistarini et al. 2016 [14] | Single-center experience—comparing results between age population > 70 and < 70 | January 2008 to December 2012 | 264 | < 70 years (176 patients) | 44.3 ± 12.7 | 850.5 ± 404.3 (dyn·s·cm−5) | 2.2 ± 0.6 L/min m2 (CI) | 23.5 ± 8.6 (at 3 years) | 239.2 ± 125 (dyn·s·cm−5) | 2.7 ± 0.5 L/min m2 (CI) | 5.1% (overall 6.43%) | NA | NA | 3.40% | NA | NA | 1, 2, and 4 years survival 93%, 92%, and 91% |
| ≥ 70 years (88 patients) | 40.3 ± 10.3 | 881 ± 415.1 (dyn·s·cm − 5) | 2.1 ± 0.5 L/min m2 (CI) | 23.2 ± 6.5 (at 3 years) | 280.7 ± 192 (dyn·s·cm − 5) | 2.5 ± 0.4 L/min m2 (CI) | 9.1% (Overall 6.43%) | NA | NA | 3.50% | NA | NA | 1, 2 and 4 years survival 88%, 86%, and 86% | ||||
| Sihag et al. 2017 [19] | Retrospective examination of learning curve | 1998 to 2016 | 134 | 54 ± 15 | NA | 639 ± 373 (dyn·s·cm−5) | 4.7 ± 1.5 L/min (CO) | NA | NA | NA | 3.70% | 8.20% | NA | NA | NA | NA | NA |
| Miwa et al. 2018 [10] | Comparison of PEA and medical management from single-center data | June 1986 to June 2016 | 159 | 56.2 ± 11.6 | 46.4 ± 10 | 10. ± 4.1 Woods | 2.6 ± 0.7 L/min m2 (CI) | NA | NA | NA | 11.94% | NA | NA | NA | NA | NA | 1, 3, 5, and 10 years survival 88.1%, 86.6%, 84.1%, and 80.6% |
| Raza et al. 2018 [15] | Single-center experience | June 2013 to December 2016 | 71 | 56 ± 16 | 45 ± 11 | 8.9 ± 4.5 (mmHg/L/min) | 2.1 ± 0.5 L/min m2 (CI) | 24 ± 8 | 2.8 ± 1.8 (mmHg/L/min) | 2.8 ± 0.5 L/min m2 (CI) | 4.20% | NA | NA | NA | NA | NA | |
| Sakurai et al. 2019 [12] | Single-center experience | 2005 to 2013 | 122 | 56 | 47 (26–73) | 832 (258–1869) (dyn·s·cm−5) | 2.28 (1.32–4.45) L/min m2 (CI) | NA | NA | NA | 7.40% | NA | NA | NA | NA | 25% | 1, 3, 5, 7, and 10 years survival 91.8%, 89.2%, 89.2%, 89.2%, and 86.1% |
| Yan et al. 2019 [20] | Single-center experience | November 2015 to December 2017 | 58 | 48.2 ± 11.6 | 49 ± 13 | 724 (538–1108) (dyn·s·cm−5) | NA | 27 (20–31) | 206 (141–284) (dyn·s·cm−5) | NA | 1.72% | 12.06% | NA | 6.89% | NA | 3.5% | NA |
Parameters of successful operation
Residual PH, post-operative RV function, and quality of life are considered major parameters to evaluate the success of the operation.
Residual PH
The best objective measurement of success is reduction in PVR. Most studies report significant reduction in PVR postoperatively [4, 6–12, 14–18, 20]. Residual PH is usually defined by the same criteria used preoperatively [6]. Incidence of residual PH ranges from 8.2 to 31.20% [6, 7, 9, 11, 17, 19, 20]. Distal disease (level III and level IV) poses the greatest surgical challenge and remains the main predictive factor for residual PH [10]. However, Sakurai et al. [12] reported that level III and IV disease does not increase risks of early or late death.
Lopez et al. identified that residual PH reduces survival on long-term follow-up in an unadjusted analysis [7]. After adjusting for perioperative mortality, they did not find significant reduction in survival in patients with residual PH [7]. Even in patients with residual PH, studies have found significant reduction in PVR [7, 11]. Similarly, Freed et al. reported that patients with residual PH had significantly compromised symptom status and functional capacity but did not have adverse medium-term survival [6]. They need medicinal support, but they enjoyed significant improvement in quality of life [6]. Similarly, van der Plas et al. found that patients having higher preoperative PVR had higher incidence of residual PH but they actually received the most benefit in terms of 6-min walk distance improvement compared to their non-residual PH counterpart [17].
Residual PH is usually managed by medicines similar to those mentioned in medical management section [6]. The patients should be evaluated by symptom status, echocardiography, and right heart catheterization at regular intervals [24]. Surgical options are redo PEA [6] or lung transplant [9], but they are limited to those who continue to have symptoms despite maximized medical therapy.
RV function
There is an immediate decrease in RV dilation and functional tricuspid regurgitation after surgery [40]. However, remodeling and functional improvement in RV is slower, and resolution of RV hypertrophy and recovery of ejection fracture takes up to 12–36 months. Pre and post tricuspid annular peak systolic excursion (TAPSE) [54], RV strain [55], and RV dimensions/ejection fraction (EF) [56] are not reliable surrogate markers of PEA success. At long-term follow-up, pulmonary flow pattern normalizes after PEA [57] resting and exercise-induced RV-EF improves, correlating with improvement in exercise capacity [58].
There is no consensus on when to repeat echocardiogram or cardiac catheterization post-PEA. Patients can be risk stratified based on preoperative measures associated with poor PEA outcomes, i.e., higher PVR. Patients with higher baseline PVR and mPAP should get echocardiography before discharge. In all patients, cardiac catheterization should be repeated 3–6 months post PEA to assess for residual persistent PH [41].
Quality of life
Although hemodynamic improvement is noticed immediately after surgery, pulmonary function, exercise capacity, and quality of life (QoL) improve slowly as pulmonary circulation continues to remodel after PEA [59]. Functional class [7, 9] and 6-min walk distance [7] are reported to improve significantly.
A prospective cohort study including 136 patients showed significant improvement in QoL 1 year after PEA with adversely affecting predictors being presence of comorbidities (such as chronic obstructive pulmonary disease and coronary artery disease) and early postoperative complications (residual PH, neurological complications, AF, and heart failure) [60]. Even in patients with residual PH, 6-min walk distance improves; actually improvement in these patients is found to be better than those without residual PH [17]. In patients with sickle cell disease, both chronic leg ulcers [61] and quality of life [62] improve drastically as PEA improves oxygenation.
PEA is potentially curative for majority of patients with CTEPH. However, Schölzel et al. reported that clinical worsening occurred in almost 19% of the patients within 4 years after the surgical treatment [18]. Patients, having a very distal disease, severe intractable residual PH, and poor quality of life, may be considered candidates for bilateral lung transplant.
Hybrid approach
Use of BPA as monotherapy, or in conjunction with medical therapy, is also currently being studied extensively [10]. Use of BPA in CTEPH management in addition to medical management is being studied [63]. A study by Miwa et al. reported comparable survival to PEA using BPA and medications in their most recent cohort of CTEPH population [10].
Conclusion
PEA remains the corner stone in management of CTEPH. High-volume centers have described a substantial improvement in outcomes during the last decade despite delayed referrals. PEA remains the best treatment for CTEPH in suitable patients. Medical management and BPA play a supportive role.
Funding
None.
Declarations
Research involving human participants and/or animals
Not applicable as per institutional ethical committee as the scientific information presented in paper does not fall into category of clinical trial or usage of experimental modalities.
Informed consent
Obtained.
Conflicts of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lang IM, Pesavento R, Bonderman D, Yuan JX. Risk factors and basic mechanisms of chronic thromboembolic pulmonary hypertension: a current understanding. Eur Respir J. 2013;41:462–8. [DOI] [PubMed]
- 2.Bonderman D, Lang IM. Risk factors for chronic thromboembolic pulmonary hypertension. Textbook of Pulmonary Vascular Disease. Springer; 2011. p. 1253–9.
- 3.Madani MM. Surgical treatment of chronic thromboembolic pulmonary hypertension: pulmonary thromboendarterectomy. Methodist Debakey Cardiovasc J. 2016;12(4):213–218. doi: 10.14797/mdcj-12-4-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delcroix M, Lang I, Pepke-Zaba J, et al. Long-term outcome of patients with chronic thromboembolic pulmonary hypertension: results from an International Prospective Registry. Circulation. 2016;133:859–71. [DOI] [PubMed]
- 5.Chambers DC, Cherikh WS, Harhay MO, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult lung and heart-lung transplantation Report-2019; Focus theme: Donor and recipient size match. J Heart Lung Transplant. 2019;38:1042–55. 10.1016/j.healun.2019.08.001. [DOI] [PMC free article] [PubMed]
- 6.Freed DH, Thomson BM, Berman M, et al. Survival after pulmonary thromboendarterectomy: effect of residual pulmonary hypertension. J Thorac Cardiovasc Surg. 2011;141:383–7. 10.1016/j.jtcvs.2009.12.056. [DOI] [PubMed]
- 7.Lopez Gude MJ, Perez de la Sota E, Forteza Gil A, et al. Pulmonary thromboendarterectomy in 106 patients with chronic thromboembolic pulmonary hypertension. Arch Bronconeumol. 2015;51:502–8. 10.1016/j.arbres.2014.11.012. [DOI] [PubMed]
- 8.Madani MM, Auger WR, Pretorius V, et al. Pulmonary endarterectomy: recent changes in a single institution's experience of more than 2,700 patients. Ann Thorac Surg. 2012;94:97–103. [DOI] [PubMed]
- 9.Mayer E, Jenkins D, Lindner J, et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an International Prospective Registry. J Thorac Cardiovasc Surg. 2011;141:702–10. 10.1016/j.jtcvs.2010.11.024. [DOI] [PubMed]
- 10.Miwa H, Tanabe N, Jujo T, et al. Long-term outcome of chronic thromboembolic pulmonary hypertension at a single Japanese pulmonary endarterectomy Center. Circ J. 2018;82:1428–1436. doi: 10.1253/circj.CJ-17-1242. [DOI] [PubMed] [Google Scholar]
- 11.Nierlich P, Hold A, Ristl R. Outcome after surgical treatment of chronic thromboembolic pulmonary hypertension: dealing with different patient subsets. A single-centre experience. Eur J Cardiothorac Surg. 2016;50:898–906. doi: 10.1093/ejcts/ezw099. [DOI] [PubMed] [Google Scholar]
- 12.Sakurai Y, Takami Y, Amano K, et al. Predictors of outcomes after surgery for chronic thromboembolic pulmonary hypertension. Ann Thorac Surg. 2019;108:1154–1161. doi: 10.1016/j.athoracsur.2019.03.100. [DOI] [PubMed] [Google Scholar]
- 13.Scholzel B, Snijder R, Morshuis W, Saouti N, Plokker T, Post M. Clinical worsening after pulmonary endarterectomy in chronic thromboembolic pulmonary hypertension. Neth Heart J. 2011;19:498–503. doi: 10.1007/s12471-011-0203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vistarini N, Morsolini M, Klersy C, et al. Pulmonary endarterectomy in the elderly: safety, efficacy and risk factors. J Cardiovasc Med (Hagerstown). 2016;17:144–151. doi: 10.2459/JCM.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 15.Raza F, Vaidya A, Lacharite-Roberge A-S, et al. Initial clinical and hemodynamic results of a regional pulmonary thromboendarterectomy program. J Cardiovasc Surg (Torino) 2018;59:428–437. doi: 10.23736/S0021-9509.17.10188-6. [DOI] [PubMed] [Google Scholar]
- 16.Yıldızeli B, Taş S, Yanartaş M, et al. Pulmonary endarterectomy for chronic thrombo-embolic pulmonary hypertension: an institutional experience. Eur J Cardiothorac Surg. 2013;44:e219–ee27. doi: 10.1093/ejcts/ezt293. [DOI] [PubMed] [Google Scholar]
- 17.van der Plas MN, Surie S, Reesink HJ, van Steenwijk RP, Kloek JJ, Bresser P. Longitudinal follow-up of six-minute walk distance after pulmonary endarterectomy. Ann Thorac Surg. 2011;91:1094–1099. doi: 10.1016/j.athoracsur.2010.11.061. [DOI] [PubMed] [Google Scholar]
- 18.Schölzel B, Snijder R, Morshuis W, Saouti N, Plokker T, Post M. Clinical worsening after pulmonary endarterectomy in chronic thromboembolic pulmonary hypertension. Neth Heart J. 2011;19:498–503. doi: 10.1007/s12471-011-0203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sihag S, Le B, Witkin AS, et al. Quantifying the learning curve for pulmonary thromboendarterectomy. J Cardiothorac Surg. 2017;12:121. doi: 10.1186/s13019-017-0686-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan S, Lou S, Zhu J, et al. Perfusion strategy and mid-term results of 58 consecutive pulmonary endarterectomy. Perfusion. 2019;34:475–481. doi: 10.1177/0267659119831518. [DOI] [PubMed] [Google Scholar]
- 21.Gu S, Liu Y, Su P-x, Zhai Z-g, Yang Y-h, Wang C. Pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension: preliminary exploration in China. Chin Med J (Engl) 2010;123:979–983. [PubMed] [Google Scholar]
- 22.Luo W-C, Huang S-C, Lin Y-H, et al. Pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension--A single-center experience in Taiwan. J Formos Med Assoc. 2015;114:1197–1203. doi: 10.1016/j.jfma.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Oh SJ, Bok JS, Hwang HY, Kim K-H, Kim KB, Ahn H. Clinical outcomes of thromboendarterectomy for chronic thromboembolic pulmonary hypertension: 12-year experience. Korean J Thorac Cardiovasc Surg. 2013;46:41–48. doi: 10.5090/kjtcs.2013.46.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 25.Fares WH, Heresi GA. Chronic thromboembolic pulmonary hypertension: a worldwide view of how far we have come. Lung. 2016;194:483–485. doi: 10.1007/s00408-016-9863-6. [DOI] [PubMed] [Google Scholar]
- 26.Pepke-Zaba J, Delcroix M, Lang I, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation. 2011;124:1973–1981. doi: 10.1161/CIRCULATIONAHA.110.015008. [DOI] [PubMed] [Google Scholar]
- 27.Machuca T, de Perrot M. When to refer a patient with chronic thromboembolic pulmonary hypertension for pulmonary endarterectomy. Can J Cardiol. 2015;31:509–514. doi: 10.1016/j.cjca.2015.01.042. [DOI] [PubMed] [Google Scholar]
- 28.Kim NH, Delcroix M, Jenkins DP, et al. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol. 2013;62:D92–D99. doi: 10.1016/j.jacc.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 29.Chloros T, Burt C, Dunning J. The role of intraoperative transesophageal echocardiography in identifying a fenestrated occlusion of the inferior vena cava during pulmonary thromboendarterectomy. J Cardiothorac Vasc Anesth. 2018;32:1329–1332. doi: 10.1053/j.jvca.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Galie N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Respir J. 2015;46:903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 31.Madani M, Mayer E, Fadel E, Jenkins DP. Pulmonary endarterectomy. Patient selection, technical challenges, and outcomes. Ann Am Thorac Soc. 2016;13:S240–S247. doi: 10.1513/AnnalsATS.201601-014AS. [DOI] [PubMed] [Google Scholar]
- 32.Madani MM, Higgins JR. Pulmonary Thromboendarterectomy: Springer International Publishing; 2020. p. 717–26.
- 33.Jamieson SW. Re: Outcome after surgical treatment of chronic thromboembolic pulmonary hypertension: dealing with different patient subsets. A single-centre experience. Eur J Cardiothorac Surg. 2016;50:907–908. doi: 10.1093/ejcts/ezw145. [DOI] [PubMed] [Google Scholar]
- 34.Matthews DT, Le CN, Robbins IM, et al. Severity of pulmonary hypertension and obesity are not associated with worse functional outcomes after pulmonary thromboendarterectomy. Pulm Circ. 2016;6:174–180. doi: 10.1086/685736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandes TM, Auger WR, Fedullo PF, et al. Baseline body mass index does not significantly affect outcomes after pulmonary thromboendarterectomy. Ann Thorac Surg. 2014;98:1776–1781. doi: 10.1016/j.athoracsur.2014.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shenoy V, Anton JM, Collard CD, Youngblood SC. Pulmonary thromboendarterectomy for chronic thromboembolic pulmonary hypertension. Anesthesiology. 2014;120:1255–1261. doi: 10.1097/ALN.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 37.Simonneau G, D'Armini AM, Ghofrani H-A, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension: a long-term extension study (CHEST-2) Eur Respir J. 2015;45:1293–1302. doi: 10.1183/09031936.00087114. [DOI] [PubMed] [Google Scholar]
- 38.Vuylsteke A, Sharples L, Charman G, et al. Circulatory arrest versus cerebral perfusion during pulmonary endarterectomy surgery (PEACOG): a randomised controlled trial. Lancet. 2011;378:1379–1387. doi: 10.1016/S0140-6736(11)61144-6. [DOI] [PubMed] [Google Scholar]
- 39.Lafci G, Tasoglu I, Ulas MM, Yalcinkaya A, Cagli K. Pulmonary endarterectomy: with use of moderate hypothermia and antegrade cerebral perfusion without circulatory arrest. Tex Heart Inst J. 2012;39:65–67. [PMC free article] [PubMed] [Google Scholar]
- 40.Ishida K, Masuda M, Imamaki M, Katsumata M, Maruyama T, Miyazaki M. Improvement of tricuspid regurgitation after pulmonary thromboendarterectomy. Asian Cardiovasc Thorac Ann. 2010;18:229–233. doi: 10.1177/0218492310367684. [DOI] [PubMed] [Google Scholar]
- 41.Kratzert WB, Boyd EK, Saggar R, Channick R. Critical care of patients after pulmonary thromboendarterectomy. J Cardiothorac Vasc Anesth. 2019;33:3110–3126. doi: 10.1053/j.jvca.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 42.Fernandes TM, Poch DS, Papamatheakis DG, et al. Pre-operative determinants of reperfusion lung injury after pulmonary thromboendarterectomy. J Heart Lung Transplant. 2016;35:S175-S176. 10.1016/j.healun.2016.01.487.
- 43.Kerr KM, Auger WR, Marsh JJ, et al. Efficacy of methylprednisolone in preventing lung injury following pulmonary thromboendarterectomy. Chest. 2012;141:27–35. doi: 10.1378/chest.10-2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bunclark K, Newnham M, Chiu Y-D, et al. A multicenter study of anticoagulation in operable chronic thromboembolic pulmonary hypertension. J Thromb Haemost. 2020;18:114–22. 10.1111/jth.14649. [DOI] [PubMed]
- 45.Guth S, Wiedenroth CB, Wollenschlager M, et al. Short-term venoarterial extracorporeal membrane oxygenation for massive endobronchial hemorrhage after pulmonary endarterectomy. J Thorac Cardiovasc Surg. 2018;155:643–649. doi: 10.1016/j.jtcvs.2017.09.045. [DOI] [PubMed] [Google Scholar]
- 46.Edemskiy A, Chernyavskiy M, Tarkova A, Chernyavskiy A. Central extracorporeal membrane oxygenation for treatment of reperfusion oedema following pulmonary thromboendarterectomy: a case report. J Cardiothorac Surg. 2016;11:76. doi: 10.1186/s13019-016-0476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Donahoe L, Granton J, McRae K, et al. Role of extracorporeal life support after pulmonary endarterectomy: a single-centre experience. Interact Cardiovasc Thorac Surg. 2016;23:74–78. doi: 10.1093/icvts/ivw075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shetty DP, Nair HC, Shetty V, Punnen J. A novel treatment for pulmonary hemorrhage during thromboendarterectomy surgery. Ann Thorac Surg. 2015;99:e77–e78. doi: 10.1016/j.athoracsur.2014.11.060. [DOI] [PubMed] [Google Scholar]
- 49.Yildizeli B, Arslan O, Tas S, et al. Management of massive pulmonary hemorrhage following pulmonary endarterectomy. Thorac Cardiovasc Surg. 2014;62:89–91. doi: 10.1055/s-0032-1324711. [DOI] [PubMed] [Google Scholar]
- 50.Reddy S, Rajanbabu BB, Kumar NKS, Rajani I. Temporary clamping of branch pulmonary artery for pulmonary hemorrhage after endarterectomy. Ann Thorac Surg. 2013;96:1459–1461. doi: 10.1016/j.athoracsur.2012.12.067. [DOI] [PubMed] [Google Scholar]
- 51.Morsolini M, Azzaretti A, Orlandoni G, D’Armini AM. Airway bleeding during pulmonary endarterectomy: the “bubbles” technique. J Thorac Cardiovasc Surg. 2013;145:1409–1410. doi: 10.1016/j.jtcvs.2012.12.045. [DOI] [PubMed] [Google Scholar]
- 52.Kolnikova I, Kunstyr J, Lindner J, et al. Extracorporeal membrane oxygenation used in a massive lung bleeding following pulmonary endarterectomy. Prague Med Rep. 2012;113:299–302. doi: 10.14712/23362936.2015.14. [DOI] [PubMed] [Google Scholar]
- 53.Mangukia C, Forfia P, Vaidya A, et al. Percutaneous coil embolization to manage pulmonary artery hemorrhage after distal endarterectomy. JTCVS Techniques. 2020;4:147–9. 10.1016/j.xjtc.2020.08.050. [DOI] [PMC free article] [PubMed]
- 54.Wong DJ, Sampat U, Gibson MA, et al. Tricuspid annular plane systolic excursion in chronic thromboembolic pulmonary hypertension before and after pulmonary thromboendarterectomy. Echocardiography. 2016;33:1805–1809. doi: 10.1111/echo.13364. [DOI] [PubMed] [Google Scholar]
- 55.Marston N, Brown JP, Olson N, et al. Right ventricular strain before and after pulmonary thromboendarterectomy in patients with chronic thromboembolic pulmonary hypertension. Echocardiography. 2015;32:1115–1121. doi: 10.1111/echo.12812. [DOI] [PubMed] [Google Scholar]
- 56.Cronin B, O'Brien EO, Gu W, Banks D, Maus T. Intraoperative 3-dimensional echocardiography-derived right ventricular volumetric analysis in chronic thromboembolic pulmonary hypertension patients before and after pulmonary thromboendarterectomy. J Cardiothorac Vasc Anesth. 2019;33:1498–1503. doi: 10.1053/j.jvca.2018.09.038. [DOI] [PubMed] [Google Scholar]
- 57.Han QJ, Contijoch F, Forfia PR, Han Y. Four-dimensional flow magnetic resonance imaging visualizes drastic changes in the blood flow in a patient with chronic thromboembolic pulmonary hypertension after pulmonary thromboendarterectomy. Eur Heart J. 2016;37:2802. doi: 10.1093/eurheartj/ehw064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Waziri F, Mellemkjaer S, Clemmensen TS, et al. Long-term changes of resting and exercise right ventricular systolic performance in patients with chronic thromboembolic pulmonary hypertension following pulmonary thromboendarterectomy - A two-dimensional and three-dimensional echocardiographic study. Echocardiography. 2019;36:1656–1665. doi: 10.1111/echo.14456. [DOI] [PubMed] [Google Scholar]
- 59.Triantafyllidi H, Katogiannis K, Mayer E, Anthi A, Orfanos S, Lekakis J. The time difference between clinical improvement and exercise tolerance increase following pulmonary thromboendarterectomy. Int J Cardiol. 2016;222:267–269. doi: 10.1016/j.ijcard.2016.07.244. [DOI] [PubMed] [Google Scholar]
- 60.Kamenskaya O, Klinkova A, Loginova I, et al. Determinants of health-related quality of life 1 year after pulmonary thromboendarterectomy. Ann Vasc Surg. 2018;51:254–261. doi: 10.1016/j.avsg.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 61.Agrawal A, Shah R, Bacchetta MD, Talwar A. Successful pulmonary thromboendarterectomy in a patient with sickle cell disease and associated resolution of a leg ulcer. Lung India. 2018;35:73–77. doi: 10.4103/lungindia.lungindia_47_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marques MB, Wille KM, Ren Z, Sheth M, McGiffin DC. Successful pulmonary thromboendarterectomy in a patient with sickle cell disease treated with a single preoperative red blood cell exchange. Transfusion. 2014;54:1901–1902. doi: 10.1111/trf.12651. [DOI] [PubMed] [Google Scholar]
- 63.Wang W, Wen L, Song Z, Shi W, Wang K, Huang W. Balloon pulmonary angioplasty vs riociguat in patients with inoperable chronic thromboembolic pulmonary hypertension: A systematic review and meta-analysis. Clin Cardiol. 2019;42:741–752. doi: 10.1002/clc.23212. [DOI] [PMC free article] [PubMed] [Google Scholar]



