Abstract
Mucociliary epithelia are composed of multiciliated, secretory and stem cells, and line various organs in vertebrates such as the respiratory tract. By means of mucociliary clearance, those epithelia provide a first line of defense against inhaled particles and pathogens. Mucociliary clearance relies on the correct composition of cell types, i.e. the proper balance of ciliated and secretory cells. A failure to generate and to maintain correct cell type composition and function results in impaired clearance and high risk to infections, such as in congenital diseases (e.g. ciliopathies) as well as in acquired diseases, including Asthma, Chronic Obstructive Pulmonary Disease (COPD) and Idiopathic Pulmonary Fibrosis (IPF). While it remains incompletely resolved how precisely cell types are specified and maintained in development and disease, many studies have revealed important mechanisms regarding the signaling control in mucociliary cell types in various species. Those studies not only provide insights into the signaling contribution to organ development and regeneration, but also highlight the remarkable plasticity of cell identity encountered in mucociliary maintenance, including frequent trans-differentiation events during homeostasis and specifically in disease. This review will summarize major findings and provide perspectives regarding the future of mucociliary research and the treatment of chronic airway diseases associated with tissue remodeling.
Keywords: Wnt, Notch, BMP, Xenopus, bronchial cells
Introduction
The importance of understanding the biology of mucociliary epithelia
A relatively small number of extremely conserved cell-cell signaling pathways is able to regulate embryonic development, adult tissue homeostasis, regeneration, and tissue remodeling associated with various diseases (Perrimon et al., 2012; Sanz-Ezquerro et al., 2017). Those pathways are being reiteratively employed and have to be precisely controlled to elicit tissue-, and context-dependent effects. Defective signaling regulation is a common reason for pathogenic physiological and morphological changes. The complexity of biological signaling regulation, the combinatorial interplay between multiple pathways, and the almost unlimited possibilities of dysregulation are making it hard to establish unambiguous links between a given change in signaling and a specific phenotype (Weng et al., 1999).
In addition to congenital defects caused by mutations in important developmental genes, many diseases develop only postnatally or during adulthood. These diseases can have predominantly genetic causes, but more often, they are promoted by environmental factors in combination with an unfavorable genetic disposition (Renz et al., 2011; Sears and Genuis, 2012). With the advent of large-scale “big data” studies of individuals and populations, we are just starting to elucidate the genetic basis of predispositions to various diseases by whole-exome and genome studies (Alyass et al., 2015; Naylor and Chen, 2010). We also learn more and more about the chemical and biological basis of various environmental factors with pathogenic potential. Nevertheless, it remains difficult to draw a clear connection between a common genetic variation, the presence of a common environmental factor, and the manifestation of a disease in an individual patient.
Even in cases where the predominant cause can be established, e.g. tobacco use and airway diseases, understanding the cause is very different from understanding the pathophysiological mechanism (Ballweg et al., 2014; Graff et al., 2012; Heijink et al., 2013; Rijt et al., 2012; Schamberger et al., 2014; Schamberger et al., 2015; Wang et al., 2018; Zhang et al., 2014; Zuo et al., 2019). Nevertheless, such a level of understanding is necessary for effective prevention, early diagnosis, and especially for establishment of successful therapies. This problem is further amplified by the fact that patients often come to the clinic only when a chronic disease is already severely impacting on their quality of life, and that definitive diagnosis requires interdisciplinary teams or specialized analytics only available in major clinics and centers. This can lead to delayed diagnosis, sometimes years after the disease onset (Hoyer et al., 2019; Smith, 2015). At this point it is even harder to establish what has initially caused a disease and which changes occurred through chronic inflammation, tissue remodeling and other secondary causes.
It is therefore extremely important to investigate not only defective patient tissue, but also to gain insights into the principle functions of genes and signaling pathways from normal development, animal models and in vitro studies employing simplified as well as organoid tissue cultures. Such studies have greatly improved our understanding of mucociliary epithelial biology over the years and have shed light onto some of the possible pathophysiological mechanisms that contribute to mucociliary dysfunction, especially relating to chronic airway diseases.
The following sections will review some important findings from basic as well as clinical research on the roles of key signaling pathways in mucociliary development and airway diseases, will summarize emerging concepts, and highlight open questions in the field.
Main Text
Mucociliary cell types, their functions, and associated diseases
Mucociliary epithelia line a wide variety of organs in animals, including the epidermis, the female reproductive tract and the airways. They usually contain stem or progenitor cells, which reside beneath the epithelial layer or at the base of the epithelium and in contact with the basement membrane, hence, they were named basal cells (BCs) (Haas et al., 2019; Rock et al., 2009; Rock et al., 2010; Zuo et al., 2015) (Figure 1). BCs can give rise to all other mucociliary cell types. Their correct regulation, proliferation and differentiation is of utmost importance for generating and maintaining correct cell type composition and function of the epithelium. Dysregulated BC behavior is found in various diseases affecting airway function, including basal cell hyperplasia, and can lead to lack of differentiated cells and to alterations in epithelial morphology (Hogan et al., 2014; Rock et al., 2010).
Figure 1: Mucociliary cell types and epithelial cell type compositions.
(A) Schematic representation of cell types in the mammalian trachea. Basal cells (BCs), multiciliated cells (MCCs) and club cells are abundant, while ionocytes and goblet cells are rare. (B) Schematic representation of cell types in the mammalian submucosal glands. Mucous cells are very abundant, while BCs, MCCs and ionocytes are missing. Additionally, myoepithelial cells function as stem cells. (C) Schematic representation of cell types in the mammalian bronchioles. MCCs and club cells are abundant and pulmonary neuroendocrine cells (PNECs) are found in clusters. BCs, ionocytes and goblet cells are less abundant than in the trachea. (D) Schematic representation of cell types in the Xenopus embryonic mucociliary epidermis. Basal cells are located in the deep cell layer. MCCs, ionocytes, small secretory cells (SSCs) are present in equal proportions, while goblet cells are more abundant. (E) Key to cell types and commonly used cell type markers.
The precise mucociliary cell type composition varies between different organisms and organs. Nevertheless, all types of mucociliary epithelia consist of multiciliated cells (MCCs) as well as one or more secretory cell type(s) (Figure 1A–D) that provide mucus.
MCCs are highly specialized and form over 100 motile cilia that project from the apical surface and that beat in a metachronal-synchronized fashion to produce directional extracellular fluid flow over the epithelium (Brooks and Wallingford, 2014; Meunier and Azimzadeh, 2016; Walentek et al., 2017). Depending on the organ, ciliary beating and mucociliary fluid flow can be employed for locomotion, to circulate water for oxygenation, or for directional transport of mucus, oocytes, symbionts and pathogens. Defects affecting MCCs or motile cilia lead to breakdown of fluid flow and of mucociliary clearance (Tilley et al., 2015). Consequently, diseases affecting cilia, collectively termed ciliopathies, frequently include respiratory manifestations, increased susceptibility to airway infections, and subfertility in females through increased rates of tubular pregnancies (Ibañez-Tallon et al., 2003; Mitchison and Valente, 2017; Roy, 2009; Zariwala et al., 2011).
Cells secreting Mucin glycoproteins, e.g. goblet cells, are another essential part of mucociliary epithelia. Mucins are long, gel-forming proteins that form a mobile layer, which is transported by ciliary motion (Corfield, 2015; Williams et al., 2006). Due to the gel-like properties of mucus, it serves as barrier against pathogen entry and the accumulation of harmful particles (Wagner et al., 2018; Zanin et al., 2016). Mucus traps and facilitates removal of pathogens, and generates spatial distance to the epithelial cell surface. Mucus composition changes in response to inflammation in the mammalian airways (Fahy and Dickey, 2010). This alters mucus structure and properties to protect the organism from pathogens. On the other hand, excessive secretion of mucus in conditions such a goblet cell hyperplasia, is also a feature of chronic lung diseases, including Chronic Obstructive Pulmonary Disease (COPD), Asthma or Idiopathic Pulmonary Fibrosis (IPF) (Fahy and Dickey, 2010; Hogan et al., 2014). In these conditions, increased Mucin secretion contributes to the pathology by means of impaired airway clearance, obstruction and enhanced pathogen accumulation.
Additional secretory cells can exist in mucociliary epithelia that regulate ion balance, secrete substances or modulate the behavior of other cells.
Ionocytes are enriched for the transmembrane proton pump vH+ATPase, encoded by ATP6-family genes. Depending on the ionocytes subtype (α or β), localization of vH+ATPase can be either apical or basolateral (Quigley et al., 2011). Together with the expression of carbonic anhydrases, vH+ATPase regulates cellular and extracellular pH (Quigley and Kintner, 2017). Ionocytes also contain other transporters, to regulate osmotic balance. In the mammalian lung, ionocytes are the major site of CFTR (Cystic Fibrosis Transmembrane Regulator) expression, an ion channel important for the hydration of the epithelial surface through chloride secretion (Montoro et al., 2018; Plasschaert et al., 2018). Mucins rely on hydration and increased pH for unfolding and gelling (Abdullah et al., 2017; Ambort et al., 2012). Thus, CFTR mutations cause Cystic fibrosis and render the resulting mucus more dense and sticky, which interferes with its removal through ciliary beating and increases the probability for airway infections.
Club cells (formerly known as Clara cells) of the mammalian lung represent another specialized secretory cell type (Rokicki et al., 2016; Zuo et al., 2020). They secrete surfactant proteins of the Secretoglobin family, most prominently Club Cell Secretory Protein (CCSP, also called CC10, CC16 or Uteroglobin). CCSP has anti-inflammatory properties and was implicated in modulating the behavior of migratory cells, but its complex bioactive functions are not fully understood to date (Mukherjee et al., 2007). Furthermore, club cells contribute to detoxification by expression of xenobiotic-metabolizing P450 cytochromes (CYPs) (Rokicki et al., 2016). They also function as facultative precursors to mucus-producing goblet cells as well as to MCCs in airway homeostasis and repair (Rao Tata and Rajagopal, 2017; Reynolds and Malkinson, 2010). These properties exemplify the range of functions that mucociliary secretory cells can have in mucociliary epithelia.
Pulmonary neuroendocrine cells (PNECs) of the mammalian lung as well as small secretory cells (SSCs) of the Xenopus embryonic mucociliary epidermis influence the function of the entire epithelium through modulating the behavior of another cell type. PNECs and SSCs both synthesize and secrete Serotonin, although PNECs release it from their basolateral surface and SSCs secrete Serotonin apically. The main function of this well-known neurotransmitter is the regulation of ciliary beating in MCCs in both systems (Dubaissi et al., 2014; König et al., 2009; Lommel, 2001; Walentek et al., 2014). The Serotonin-ciliary axis is an evolutionary ancient regulatory module frequently employed in animal hypoxia response. In the airway epithelium and the Xenopus epidermis, Serotonin was shown to increase the rate of ciliary beating (Walentek et al., 2014). A proposed mechanism for this effect is the activation of ligand-gated ion channels, which could increase calcium influx that in turn speeds up ciliary motion in MCCs (Doran, 2004; Walentek et al., 2014). In addition to Serotonin secretion, SSCs produce mucins while PNECs establish neuronal connections and express an oxygen-sensing protein complex of the NADPH oxidase (NOX) family (Cutz et al., 2013; Dubaissi et al., 2014). NOX stimulates Serotonin secretion in hypoxia conditions through promoting secretory vesicle fusion with the membrane. Hence, it is attractive to hypothesize, that in hypoxic conditions, Serotonin-producing cells release the neurotransmitter for the direct stimulation of ciliary beating to remove excess mucus and potential obstructions.
Collectively, the properties of various cell types and their interactions with each other, with non-epithelial cells as well as with the environment determine the correct function of mucociliary epithelia. Generating, maintaining and adapting mucociliary cell type composition and function are therefore extremely important to coordinate tissue-level behavior in development and disease.
One remarkable aspect of mucociliary epithelia is the degree of species- and organ-specific adaptations to a wide range of physiological needs and morphologies, while at the same time maintaining common signaling- and gene-regulatory principles. Another surprising feature of mucociliary cells is their ability to directly change fates of mature and seemingly “terminally” differentiated cells from one type to another. Such trans-differentiation behavior is relatively frequently observed in mucociliary cells, especially during developmental and pathogenic tissue remodeling.
Wnt Signaling
The Wnt signaling pathway is employed extensively during embryonic development and in adult tissue homeostasis, and its key components are conserved throughout evolution (Steinhart and Angers, 2018) (Figure 2A). Most commonly, signaling is induced by binding of secreted Wnt ligands to transmembrane Frizzled receptors, which then activate downstream cytoplasmic Dishevelled molecules. Depending on the Wnt signaling branch, e.g. the canonical Wnt/β-catenin pathway or the non-canonical Wnt/planar cell polarity (PCP) pathway, Wnt/Frizzled/Dishevelled complexes recruit various transmembrane co-receptors and cytoplasmic mediators. Through that, Wnt signaling branches can elicit distinct cellular responses, ranging from transcriptional activation to the regulation of morphogenesis and cell polarity (MacDonald et al., 2007; Semenov et al., 2007). As dysregulated Wnt signaling is established to contribute to a plethora of diseases, it is not surprising that it was also implicated in mucociliary dysfunction and airway disease (Baarsma and Königshoff, 2017; Lehmann et al., 2016).
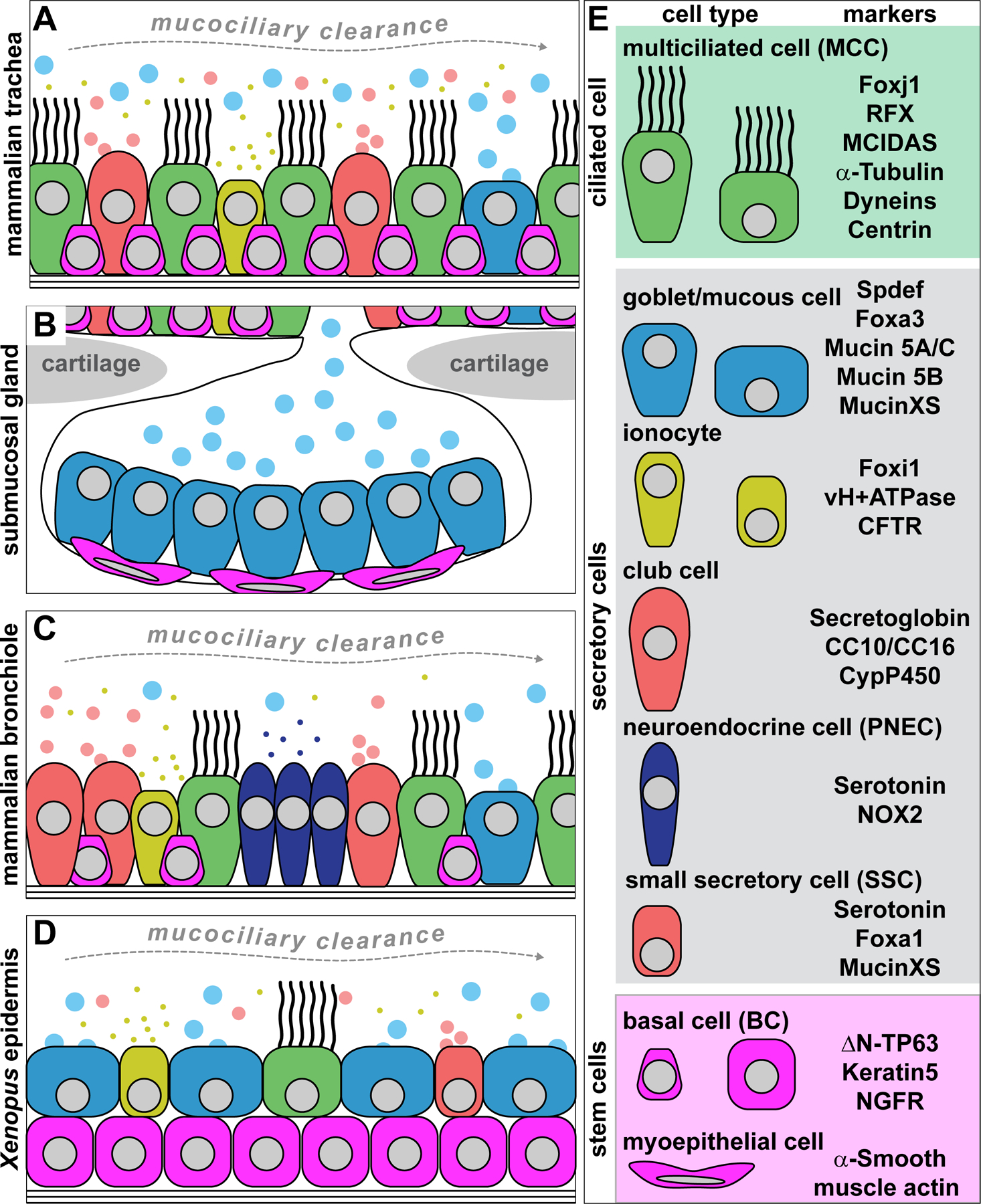
Figure 2: Wnt/β-catenin signaling requirements in mucociliary cell types.
(A) Simplified schematic representation of the canonical Wnt/β-catenin pathway. Wnt ligands bind to Frizzled receptor and recruit LRP6 co-receptor. Intracellular mediators, e.g. Dishevelled, are recruited to the ligand-receptor complex, which then leads to inhibition of GSK3β and the stabilization of β-catenin. β-catenin can then enter the nucleus, bind to TCF/LEF transcription factors, and activate Wnt target gene expression. (B) Schematic representation of cell types in the mammalian trachea and their proposed requirement levels for active Wnt/β-catenin signaling. Basal cells need high Wnt levels, while MCCs need elevated Wnt but at lower levels than basal cells. Goblet cells also require Wnt, but at low levels that heva to be precisely controlled. (C) Schematic representation of cell types in the Xenopus embryonic mucociliary epidermis and their proposed requirement levels for active Wnt/β-catenin signaling. Basal cells require high Wnt levels, while MCCs also show elevated Wnt activation. Ionocytes and small secterory cells do not seem to strictly rely on Wnt signaling, and the role for Wnt in goblet cells remains unresolved.
Many studies have addressed the complex and reiterative functions of Wnt signaling in mucociliary epithelia in different models. Collectively, these studies suggest multiple roles for canonical Wnt pathway in regulating cell fate specification and differentiation, and a role for the PCP pathway in tissue-wide and cell polarity, in particular in MCCs (Vladar and Axelrod, 2008; Walentek et al., 2017; Wallingford, 2010). The roles of Wnt/PCP in lung development and disease were recently extensively reviewed elsewhere (Vladar and Königshoff, 2020), therefore, the focus here will be on canonical Wnt functions.
Wnt/β-catenin was shown to control stemness and differentiation (Haas et al., 2019). β-catenin can bind to an alternative promoter (P2) that positively regulates the expression of the ∆N-isoform of TP63 in humans, mice and Xenopus (Kjolby and Harland, 2016; Ruptier et al., 2011). ∆N-TP63 is a transcription factor required for maintaining BCs. Lack or inactivation of ∆N-TP63 expression cause excessive differentiation of epithelial cells and a loss of BCs in mucociliary epithelia (Daniely et al., 2004; Haas et al., 2019). Interestingly, ∆N-TP63 is not strictly required for the initial establishment of mucociliary epithelia in the lung or the Xenopus epidermis. This is in line with its rather late onset of expression during mucociliary development. Nevertheless, developmental loss of ∆N-TP63 leads to abnormally increased numbers of MCCs (Daniely et al., 2004; Haas et al., 2019). Tissue culture experiments further demonstrated that knockdown of ∆N-TP63 prevents establishment of an epithelium containing goblet cells, impairs proliferation, and leads to senescence (Arason et al., 2014). These data align with the pro-proliferative role of Wnt/β-catenin and ∆N-TP63 in other epithelial systems (Arason et al., 2014; Clevers et al., 2014; Haas et al., 2019; Senoo et al., 2007).
Overactivation of Wnt/β-catenin or ∆N-TP63 prevents BCs from exiting the stem cell state, which then fail to undergo differentiation towards epithelial cell lineages (Haas et al., 2019; Mucenski et al., 2005; Reynolds et al., 2008; Schmid et al., 2017). Such effects of increased Wnt/β-catenin were reported in various models of mucociliary epithelia, and work in the Xenopus epidermis demonstrated that knockdown of ∆N-TP63 can rescue differentiation ability in the presence of high Wnt/β-catenin signaling (Haas et al., 2019). These data argue for a role of ∆N-TP63 as master regulator of BC behavior and the switch between maintenance and differentiation. Furthermore, these data support the notion that the Wnt-induced inhibition of epithelial differentiation is mediated to a significant degree by excessive ∆N-TP63 expression in BCs.
In addition to blocking differentiation, high levels of Wnt/β-catenin signaling lead to accumulation and multilayer stacking of BCs in mucociliary epithelia due to an increase in proliferation (Aros et al., 2020a; Haas et al., 2019). Increased proliferation is also normally observed during regeneration of mammalian airway epithelia after injury, when BCs transiently express Wnt ligands (Aros et al., 2020b). The source of canonical Wnt ligands is not limited to BCs themselves during epithelial repair, as it was shown that inhibition of Wnt secretion from BCs could be compensated for by ligand secreting stromal cells of the intercartilage zone in the murine trachea (Aros et al., 2020b). Abnormally high Wnt signaling, BC over-proliferation and decreased differentiation are also observed in premalignant lesions and in squamous lung cancer (Aros et al., 2020a). Similarly, single-cell RNA-sequencing (scRNA-seq) of the mouse airway revealed highly proliferative and less differentiated “hillock” cell clusters (Montoro et al., 2018). These express Krt13, a marker associated with proliferating BCs (Goldfarbmuren et al., 2020; Montoro et al., 2018). They also express Porcn, which encodes a protein required for Wnt secretion, and that is upregulated in premalignant lesions and squamous lung cancer (Aros et al., 2020a; Montoro et al., 2018). These data suggest that “hillock” cells might be similar to cells found in early stage premalignant lesions, and thereby linking them to locally upregulated Wnt signaling.
Importantly, over-proliferation and block of epithelial cell differentiation due to Wnt/β-catenin signaling can be reversed in Xenopus, human tissue culture and in mouse airway cells. In Xenopus embryos and in differentiating air-liquid interface (ALI) cultures of immortalized human BCs, increased Wnt/β-catenin signaling through inhibition of GSK3β or through application of the canonical Wnt agonist R-spondin 2 (RSPO2), respectively, prevented differentiation of epithelial cells and increased numbers of BCs. Nevertheless, differentiation could be induced simply by removal of the GSK3β inhibitor/RSPO2 even after long-term exposure to elevated Wnt signals, and the number of BCs returned to seemingly normal levels (Haas et al., 2019). Similarly, in chronic rhinosinusitis with nasal polyps that is associated with a failure of MCC differentiation, tissue overgrowth, and increased production of Wnt3a in the nasal epithelium, MCCs and ciliation could be rescued in vitro by application of a canonical Wnt inhibitor (Dobzanski et al., 2018). Furthermore, recent development of a novel canonical Wnt pathway inhibitor that acts downstream of GSK3β, WIC1, showed that its application prevented over-proliferation, decreased ∆N-TP63 expression, and induced MCC differentiation in human and mouse in vitro BC cultures with overactivated Wnt signaling (Aros et al., 2020a).
Together, these findings strongly suggest that most changes in mucociliary epithelial cell type composition and morphology associated Wnt/β-catenin signaling are due to abnormal behavior of BC and loss of their ability to correctly balance maintenance, proliferation and differentiation. Especially in diseases where loss of differentiation and an increase in BCs are observed, the use of specific inhibitors of canonical Wnt signaling offers an attractive entry point for therapeutic interventions. Understanding BC regulation by Wnt is also important regarding COPD and IPF, which were both associated with changes in Wnt signaling (Baarsma and Königshoff, 2017; Heijink et al., 2013; Shi et al., 2017; Wang et al., 2011), and for which limited treatment options are currently available.
In addition to the instructive role for Wnt/β-catenin in BCs, data support a permissive function during the differentiation of MCCs. Multiple studies have demonstrated that β-catenin signaling is required for motile cilia formation in MCCs (Aros et al., 2020b; Sun et al., 2018; Walentek et al., 2015). Studies in zebrafish and Xenopus have revealed that the master regulator of motile ciliogenesis, Foxj1, is positively regulated by β-catenin, and that decreased Wnt signaling affected ciliogenesis (Caron et al., 2012; Walentek et al., 2012). Similarly, studies in the mouse and in human tissue culture indicate that Wnt signaling can have positive effects on MCCs and ciliation (Aros et al., 2020b; Aros et al., 2020a; Schmid et al., 2017).
Different effects on different cell types could be attributed to differential functions of Wnt ligands, e.g. Wnt7a and Wnt4 in ALI cultured human airway cells (Schmid et al., 2017). On the other hand, analysis of canonical Wnt-signaling reporter activity in the Xenopus embryonic epidermis as well as in the developing mouse airway indicated that Wnt signaling levels differ between BCs and differentiating MCCs, with MCCs showing lower signaling levels than BCs. Thus, intermediate Wnt signaling levels are compatible with successful MCC differentiation from BCs, while at the same time supporting the robust expression of foxj1, as one of multiple transcription factors employed in the coordinated control of the multi-ciliogenesis program (Walentek and Quigley, 2017). Differential Wnt signaling levels between BCs and MCCs could be generated through cell type specific receptor expression levels, as differentiating cells drastically change their transcriptional program, a hypothesis also supported by scRNA-seq data (Ruiz García et al., 2019). Furthermore, apical-basal asymmetric cell division together with asymmetric distribution of the canonical Wnt co-receptor LRP6 were shown to prime basally located cells for high Wnt signaling activation during Xenopus mucociliary epidermal development, preceding ∆N-TP63 expression (Clevers et al., 2014; Huang and Niehrs, 2014). Either way, BCs are likely sensitized to percept canonical Wnt signaling and can react to the same amount of Wnt ligands by stronger downstream signaling activation than differentiated epithelial cells, including MCCs.
In contrast to BCs and MCCs, the precise functions of Wnt/β-catenin in goblet, club and other secretory cells are less clear. In club cells, canonical Wnt signaling is dispensable for specification, differentiation and maintenance (Zemke et al., 2009). In fact, an increase in canonical Wnt signaling disfavors proximal airway and club cell fate during regeneration and organoid preparation (Frank et al., 2016). Instead, there is a connection between Wnt/β-catenin and the specification of goblet cells as well as with development of a mucus hyperplastic phenotype in the mammalian lung. The main sites of Mucin-secretory cell specification in the healthy mouse and human lungs are the submucosal glands, where these cells are called mucous cells (in contrast to epithelial goblet cells) (Hogan et al., 2014) (Figure 1B). Those glands are formed in the trachea and bronchi in humans and their ducts terminate between cartilage rings. Full development of mouse submucosal glands depends on Wnt3a, canonical Wnt signaling, and the transcription factor Lef-1, which in turn mediates β-catenin-dependent transcription (Driskell et al., 2004; Driskell et al., 2007; Xie et al., 2014). While this is an indirect effect of Wnt on mucous cells, it should be noted that loss of developmental Wnt signaling could in principle lead to a deficiency in mucus production.
Dysfunction of the Wnt/Frizzled co-receptor Ryk in the mouse airway epithelium was shown to decrease airway epithelial canonical Wnt activity, and to lead to goblet cell hyperplasia (Kim et al., 2019). The remodeling of the epithelium in this condition was associated with a strong increase in Mucin5A/C and 5B, in line with an over-abundance of mature goblet cells. Deletion of Ryk also caused a change in Wnt ligand expression, i.e. expression levels of Wnt2, Wnt7b, and of the canonical Wnt agonist Rspo2 were all decrease, providing an explanation for the overall decreased Wnt/β-catenin activity (but not a full loss of signaling) (Kim et al., 2019). The increase in goblet cell numbers was inversely correlated to the number of club cells. Together, these data suggest that appropriate Wnt signaling suppresses the overproduction of goblet cells, thereby promoting maintenance of club cells. This is in line with another report from the mouse airway that revealed an enrichment of goblet cell marker expression in low Wnt-positive cells over Wnt-negative or high Wnt-positive cells, while such enrichment was not found for club cell markers, and MCC markers were enriched in high Wnt-positive cells (Hu et al., 2020). Furthermore, an increase in canonical Wnt signaling during ALI differentiation of immortalized human BCs was found to increase Mucin5B expression, while the expression of the club cell marker SCGB1A1 was strongly decreased (Haas et al., 2019). On the other hand, treatment of such cultures with the canonical Wnt antagonist DKK1 did not change Mucin5B or SCGB1A1 expression, and did not result in a loss of either secretory cell type.
Although these data are not definitively conclusive regarding the roles of Wnt in secretory cells of mucociliary epithelia, they do support the idea that correctly regulated Wnt signaling is also required (directly or possibly indirectly) to control proper secretion in mucociliary epithelia, and that gain and loss of Wnt can lead to overabundance of goblet cells or to an increase in mucus production. This is in agreement with studies on patient-derived material, where COPD tissue was associated with goblet cell hyperplasia and reduced Wnt signaling (Wang et al., 2011).
In summary, a model emerges that implies an instructive and dominant role for canonical Wnt signaling in BC maintenance, proliferation and the inhibition of differentiation, while supporting a permissive function for strictly balanced and lower Wnt levels during differentiation and maintenance of MCCs and secretory cells (Figure 2B,C). Nevertheless, additional studies further clarifying the roles of Wnt signaling in secretory cells and the regulation of mucin secretion are urgently needed.
Notch Signaling
Notch signaling is another highly evolutionary conserved cell signaling pathway, but in contrast to Wnt, Notch is strictly cell-cell contact dependent (Henrique and Schweisguth, 2019) (Figure 3A). Both Notch receptors and ligands are transmembrane proteins and require processing during signaling activation. Upon Delta-like (Dll) or Jagged (Jag) ligand binding to Notch receptors, the activated extracellular portion of the complex is cleaved off from the receiving cell by Metalloproteases. Next, γ-Secretase cleaves off the cytoplasmic portion of the receptor to release the Notch intracellular domain (NICD). This allows NICD to translocate to the nucleus, where it interacts with CSL/RBPJ transcription factors. In the absence of NICD, CSL/RBPJ bind co-repressor molecules that can directly suppress transcription of target genes. Additionally, the repressive CSL/RBPJ complex also recruits chromatin modifying proteins, which cause long-term epigenetic silencing of the genomic region (Giaimo et al., 2017; Lake et al., 2014). When NICD binds to CSL/RBPJ, it displaces bound co-repressors and facilitates recruitment of co-activators (e.g. Mastermind and p300) for target gene transcription (Henrique and Schweisguth, 2019). As Notch signaling does not act through an amplifying second messenger cascade, the amount of receptor activation directly scales with the resulting level of signaling activity.
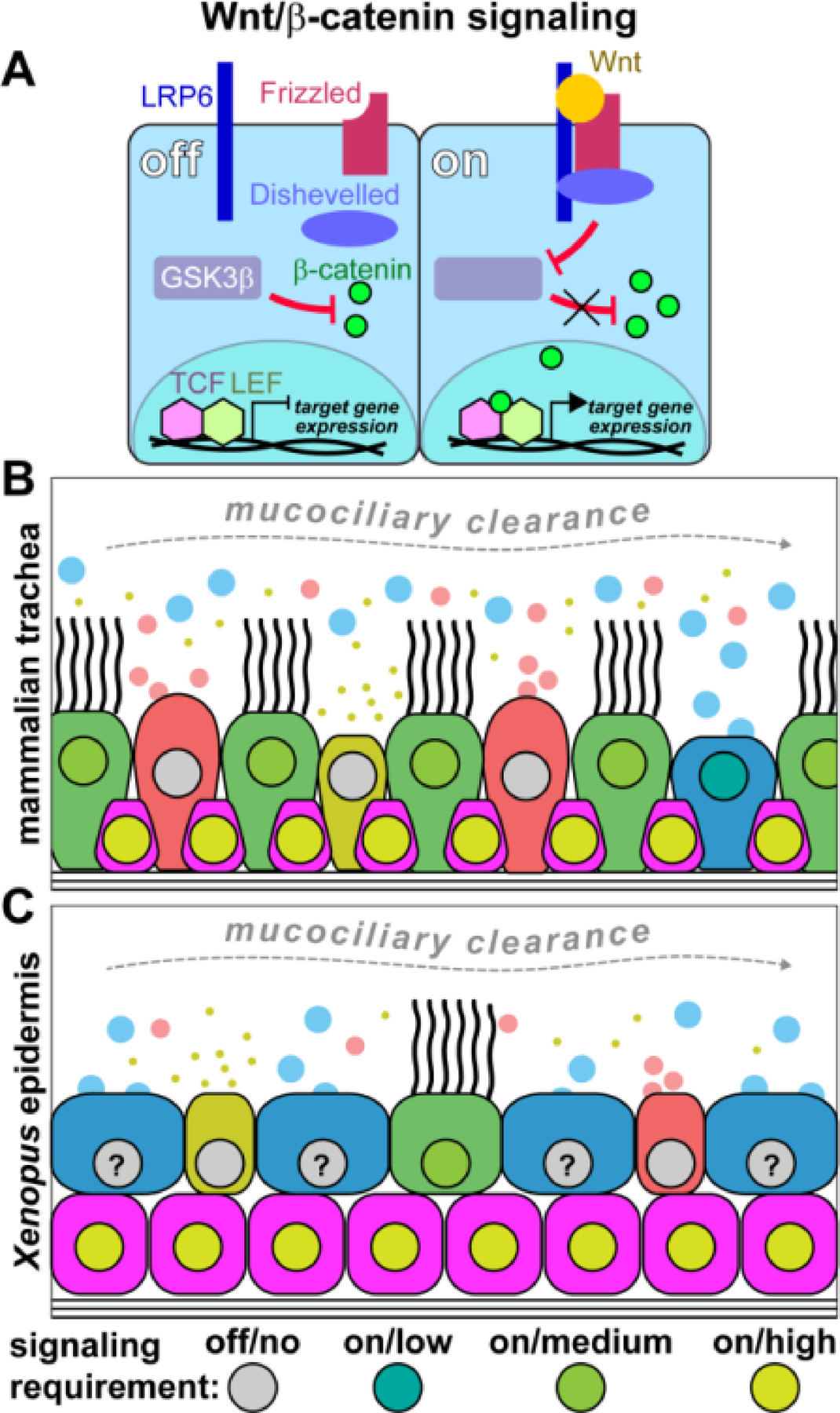
Figure 3: Notch signaling requirements in mucociliary cell types.
(A) Simplified schematic representation of the Notch pathway. Transmembrane Notch ligands from the Delta or Jagged families bind to transmembrane Notch receptors. Upon activation, Matrix metalloproteases (MMPs) cleave the extracellular portion of the complex, and γ-secretase cleaves off the Notch intracellular domain (NICD). NICD can then enter the nucleus, displace repressors from the RBPJ DNA-binding complex, and recruit transcriptional co-activators to initiate gene transcription. (B) Schematic representation of cell types in the mammalian trachea and their proposed requirement levels for active Notch signaling. Goblet, club cells and ionocytes show elevated Notch signaling, although at different levels, while MCCs and basal cells were proposed to be Notch independent. (C) Schematic representation of cell types in the Xenopus embryonic mucociliary epidermis and their proposed requirement levels for active Notch signaling. Basal and goblet cells show high Notch levels, and small secretory cells also require higher Notch levels for specification. Ionocytes and MCCs require low/no Notch signaling for specification and maintenance.
The crucial role for Notch signaling in mucociliary epithelia has been the subject of extensive studies over the past 20 years (Deblandre et al., 1999). These studies have revealed that Notch is the main signaling pathway directing cell fate decisions in mucociliary epithelial development, maintenance and disease. While seemingly simple in its signaling mechanism and being probably the most extensively studied signaling pathway in mucociliary epithelia, not all details of Notch signaling function are fully understood to date.
Notch signaling in the mammalian airway as well as in the Xenopus embryonic epidermis was shown to play a major role in the specification of MCCs and secretory cells (Rock et al., 2011; Stubbs et al., 2006; Tsao et al., 2009) (Figure 3B,C). In the Xenopus epidermis mucociliary epithelium, low Notch signaling favors specification of ionocytes and MCCs. Consequently, inhibition of NICD transcriptional activity by a dominant-negative DNA-binding mutant of the CSF/RBPJ homolog SuH (SuH-DBM) or a dominant-negative form of Mastermind leads to excessive production of ionocytes and MCCs, while overexpression of a constitutive active NICD suppresses ionocyte and MCC specification (Deblandre et al., 1999; Quigley et al., 2011; Stubbs et al., 2006; Walentek, 2018). It was further shown that overexpression of intermediate NICD levels in Xenopus increases the number of SSCs, i.e. a secretory cell type that also produces Mucins (Kurrle et al., 2020; Walentek, 2018). Similarly, data from the mouse airway demonstrated that low Notch levels lead to specification of MCCs, while high Notch levels promote the production of club secretory cells (Guseh et al., 2009; Tsao et al., 2009). Accordingly, NICD overexpression in ALI cultured human BCs also caused an overproduction of club cells and increased expression of SGB1A1 and Mucin5A/C, while simultaneously decreasing the number of MCCs and the expression of ciliated cell markers (Gomi et al., 2015). The regulation of the mucus-production master transcription factor Spdef was also shown to be positively Notch regulated (Chen et al., 2009; Chen et al., 2018; Guseh et al., 2009). Furthermore, excessive Notch signaling in the mouse airway causes mucous cell metaplasia (Guseh et al., 2009).
Nevertheless, it is not always clear if Notch-induced Mucin overexpression is due to overspecification of goblet cells, or if Mucin production is transiently upregulated in club cells, a phenomenon observed upon airway infection and inflammation (Evans et al., 2004; He et al., 2020; Méndez et al., 2019). Arguments in favor of Notch-induced overproduction of goblet cells as cause for mucous cell metaplasia are derived from studies that have shown a requirement for Notch signaling in IL-13 cytokine mediated goblet cell hyperplasia models, as well as from studies demonstrating that Spdef is required for both goblet cell specification and Mucin5A/C and 5B production (Chen et al., 2009; Chen et al., 2018; Danahay et al., 2015). Goblet cell fate can be induced by addition of IL-13, but this effect can be blocked by inhibition of Notch signaling (Danahay et al., 2015; Guseh et al., 2009; Tyner et al., 2006). IL-13 is upregulated in chronic lung disease, in mouse models for airway inflammation, and IL-13 is also known to cause an increase in goblet cells in human airway epithelial cell cultures that are usually not producing many goblet cells (Walters et al., 2013). IL-13 acts through the transcription factor STAT6, which regulates Spdef in a positive fashion and suppresses Foxj1 expression (Gomperts et al., 2007; Yu et al., 2011). Spdef, in turn, activates Mucin5A/C and 5B expression (Parker et al., 2013). Therefore, synergistic effects between IL-13/STAT6 and Notch signaling are likely, as both pathways regulate Spdef and can lead to mucous cell metaplasia. In light of these data it seems confusing that expression of Notch ligands and receptors in COPD tissue was found to be decreased, although COPD is highly correlated with mucous cell metaplasia (Tilley et al., 2009). A possible explanation could be that over the course of the disease, chronic inflammation becomes so strong that it can drive SPDEF and MUCIN5A/C, 5B expression even when Notch signaling is attenuated. Thus, further studies are urgently needed to gain more mechanistic insights into the connections between Notch signaling, goblet/mucous cells and chronic airway diseases.
More data are also needed regarding the Notch regulation of ionocytes in the mammalian lung as in contrast to the Xenopus epidermis, where this cell type was initially described, Notch inhibition was shown to negatively affect ionocyte abundance (Plasschaert et al., 2018; Quigley et al., 2011). Conversely, SSCs that share the characteristic Serotonin production and secretion with mammalian PNECs are dependent on high Notch signaling in the frog, and the opposite is true for mouse PNECs, which are increased in numbers upon developmental deletion of multiple Notch receptors in the epithelium (Kurrle et al., 2020; Morimoto et al., 2012; Walentek, 2018). In Xenopus, Notch inhibition was also shown to lead to downregulated expression of ∆N-TP63, while in the mammalian airways, Notch seems largely dispensable for BCs and elevated Notch is required for differentiation of luminal epithelial cell types (Rock et al., 2011; Sirour et al., 2011).
All these differences could be well due to species-specific aspects or potentially be related to the fact that the airway epithelium is derived from the endoderm, while the epidermal mucociliary epithelium in Xenopus is derived from the ectoderm. Additional discrepancies in studies could arise from different starting points for epithelial development in vivo and in vitro. In the airways and in the Xenopus epidermis, ∆N-TP63-positive BCs are initially not abundant during early phases of development, while in ALI culture, preparations derived from mature BC-containing airway epithelia are used, and BCs most likely represent the starting point for differentiation of all epithelial cells. All the above issues require further studies, as it seems counterintuitive to assume such fundamental differences between systems, while otherwise the principles of Notch-mediated cell fate specification are so highly conserved (Figure 3B,C).
BMP signaling
BMP signaling belongs to the TGFβ superfamily of morphogenetic signaling pathways that regulate multiple aspects of cell and tissue behavior in development and disease (Dutko and Mullins, 2011) (Figure 4A). BMP signaling activation is triggered by binding of BMP homo- or heterodimers to a combination of transmembrane type I and type II serine-threonine kinase receptors. Upon ligand binding, complexes are formed, which facilitate intermolecular auto-phosphorylation of the intracellular receptor domains. Type I receptors subsequently activate cytoplasmic Smad proteins through phosphorylation of Smad1/5/8, which allows heterodimerization with Smad4, nuclear translocation, DNA binding and transcriptional regulation of BMP target genes.
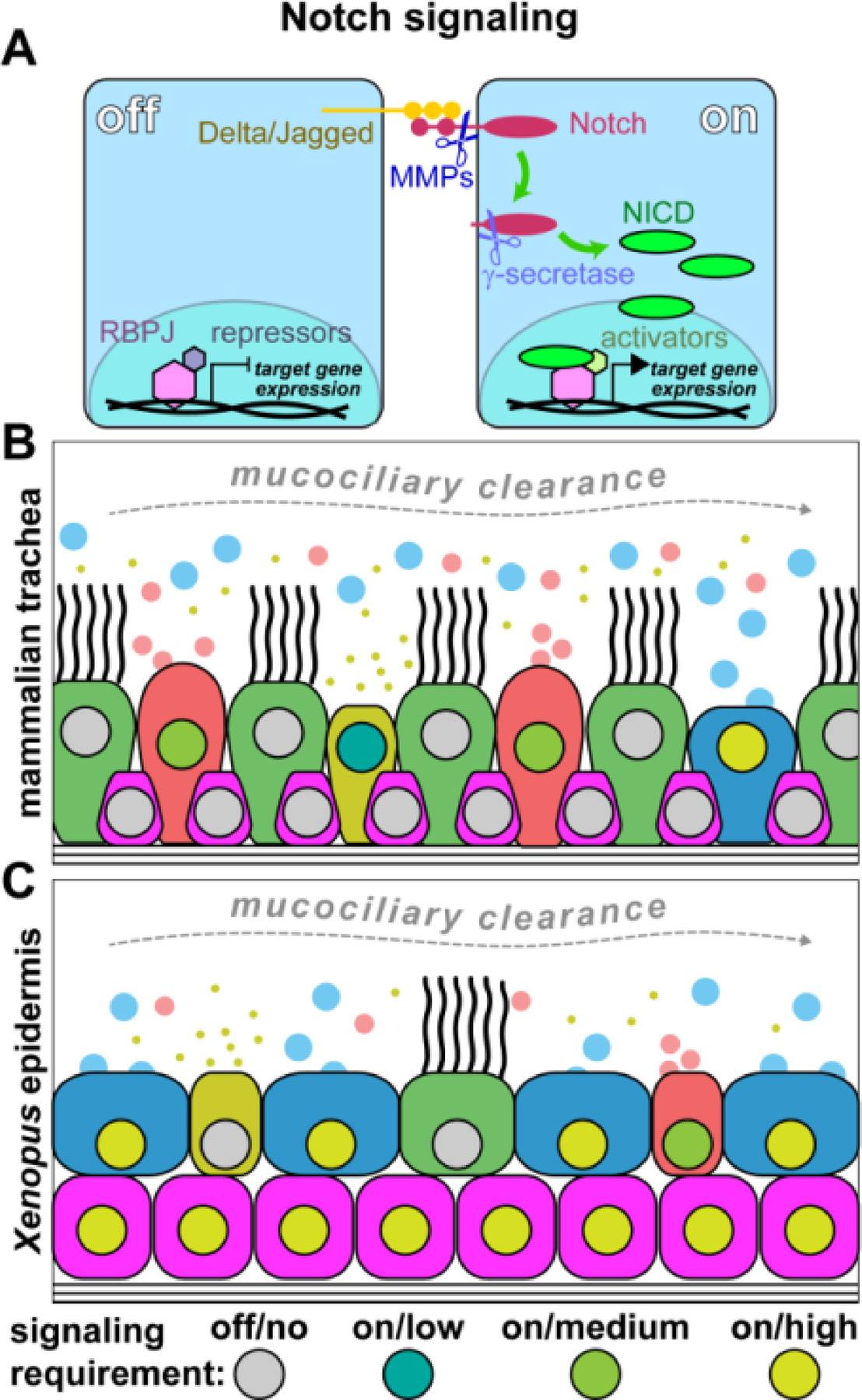
Figure 4: BMP signaling requirements in mucociliary cell types.
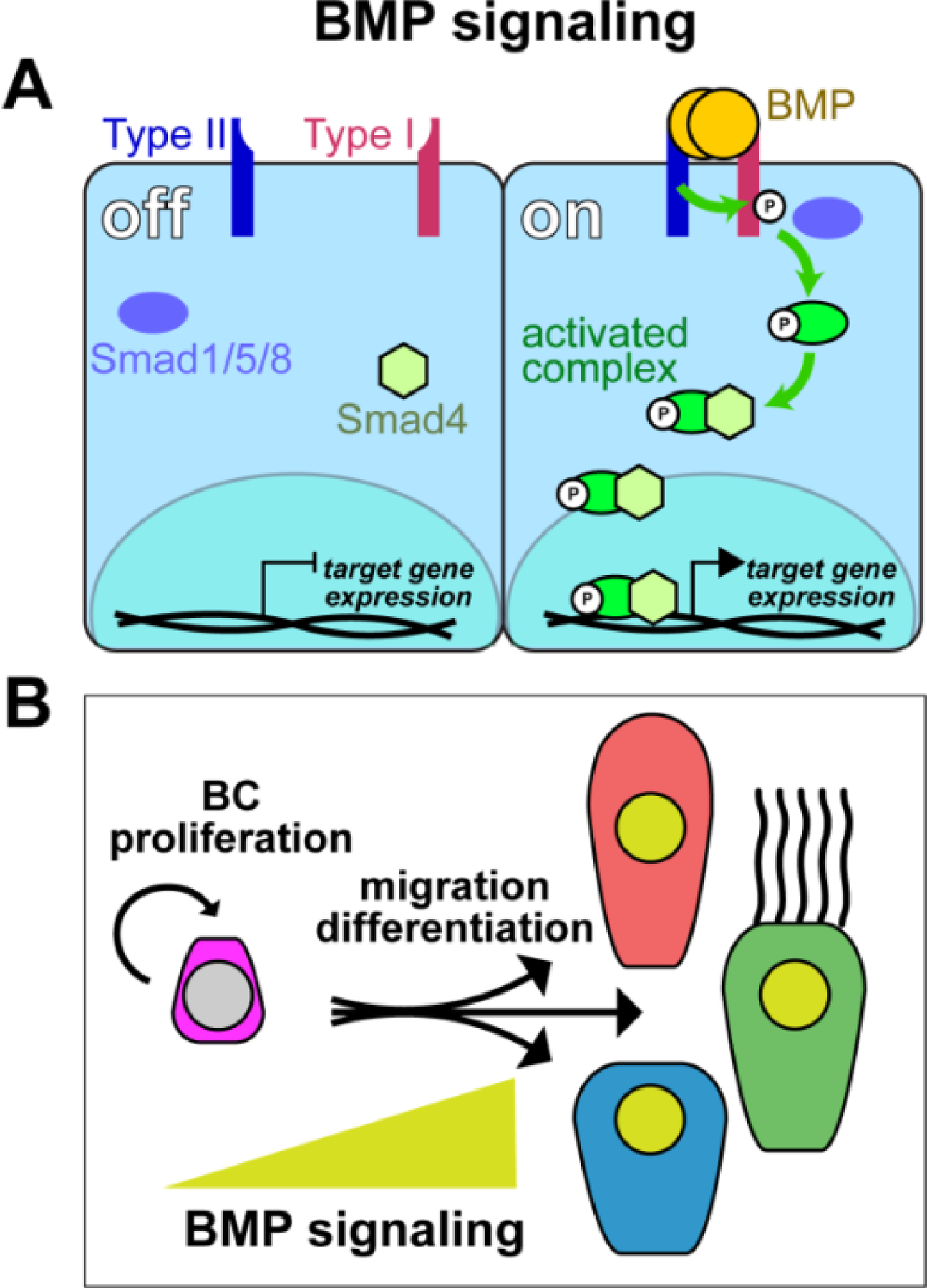
(A) Simplified schematic representation of the BMP pathway. BMP ligand dimers bind Type I/Type II transmembrane receptors. Activated Type II receptor cross-autophosphorylates Type I intracellularly, which, in turn, activates Smad proteins by phosphorylation. Activated Smad1/5/8 then binds Smad4 and the complex can enter the nucleus, bind to DNA and activate target gene expression. (B) Schematic representation of proposed BMP regulated events in vertebrate mucociliary epithelia. Yellow triangle indicates increasing BMP levels in migration and differentiated cells. Low/no BMP signaling is required in basal cells for maintenance and proliferation, while elevated BMP signaling levels are observed during migration and insertion of cell types into the epithelial cell layer during differentiation.
While BMP signaling was shown to definitively influence mucociliary epithelia, less is known about the precise functions of BMP signaling as compared to Wnt and Notch signaling.
In the developing Xenopus epidermis as well as in ALI cultures of human airway cells, BMP overactivation was shown to suppress differentiation of mature MCCs and secretory cells (Cibois et al., 2015). On the other hand, knockdown of BMPs in Xenopus increased the production of MCCs, ionocytes and SSCs, while it decreased the number of BCs (Cibois et al., 2015). Similarly, BMP signaling inhibition by application of the antagonist Noggin promoted differentiation of MCCs and Mucin5A/C-positive secretory cells in normal ALI cultured human airway cells as well as in cell cultures using Cystic Fibrosis patient cells (Cibois et al., 2015). These studies have also identified a positive role for BMP in the intercalation of progenitor cells into the epithelial lining. These data suggest that BMP signaling could have a dual role in the regulation of BC differentiation into mature cells as well as in regulating successful morphogenesis of the epithelium following cell fate specification (Figure 4B).
Inhibition of Smad-mediated signaling was further shown to allow for BC expansion in vitro, and studies of mouse tracheospheres demonstrated that BMP inhibitors promote proliferation without affecting epithelial cell fate decisions, while exogenous Bmp4 application inhibited proliferation and differentiation (Mou et al., 2016; Tadokoro et al., 2016; Zuo et al., 2019). Furthermore, BMP activity is transiently increased during airway epithelial repair in the mouse, but decreased during differentiation and regeneration of the epithelium (Tadokoro et al., 2016). Analysis of Smads in the mouse airway also showed nuclear Smad protein localization in differentiated airway cells, but not in BCs, in line with the ability of BCs to proliferate (Mou et al., 2016; Tadokoro et al., 2016). The temporal use of BMP proteins and subsequent application of BMP antagonists during differentiation was also described in protocols for the generation of airway epithelial cells from iPSCs, which further supports a dual role of BMP in proliferation and differentiation (Huang et al., 2014; Huang et al., 2015).
In mouse lung development, BMP signaling reporter analysis revealed an absence of signaling activity during early epithelial development, but onset of reporter signal in the epithelium during stages of mucociliary differentiation (Sountoulidis et al., 2012). In the adult lung epithelium, BMP activity was attenuated and only found in few epithelial cells, possibly labeling newly differentiated cells during homeostatic epithelial cell replacement (Sountoulidis et al., 2012). In agreement with the finding that BMP signaling does not affect cell fate decisions of different epithelial cell types, BMP reporter activity was found in club cell, MCCs and PNECs (Sountoulidis et al., 2012).
BMP signaling was also implicated as positive regulator of epithelial to mesenchymal transition (EMT) during regeneration of airway epithelial lesions, in which cells at the edges of the injury site migrate to cover the affected area (McCormack et al., 2013; Molloy et al., 2008). A positive effect of BMP in cell migration could also represent a parallel to the described requirement for BMP during cell intercalation in Xenopus epidermal mucociliary development, where cells have to relocate from the deep into the epithelial layer (Cibois et al., 2015) (Figure 4B).
Together, data on BMP signaling in mucociliary development and repair indicate a need for dynamic, precise, and context-specific regulation of BMP signaling levels in BC proliferation and specification, as well as in epithelial cell differentiation and migration. While further studies into the roles of BMP signaling in chronic airway diseases are necessary, reports on the elevated state of BMP signaling during cigarette smoke-induced remodeling of the airway epithelium, associated with COPD, fit the proposed roles for BMP derived from animal models (Zuo et al., 2019). Therefore, additional data on BMP functions in human airway disease could provide important new insights for the benefit of developing treatment options for COPD patients.
Other signaling pathways in mucociliary epithelia
Additional signaling pathways likely affect mucociliary and airway functions, although perhaps by modulating cellular responses to the key signaling pathways, or by modifying Notch, Wnt and BMP signaling at the ligand, receptor or co-regulator expression levels. Unfortunately, less data are available on the functions of other pathways in mucociliary research models and in human disease. FGF and Hedgehog signaling were implicated in mucociliary epithelial BC regulation, in the differentiation of epithelial cell types during regeneration, and in COPD and IPF (Danopoulos et al., 2019; Hou et al., 2019; Peng et al., 2015; Wang et al., 2020; Yuan et al., 2018). In part, the observed effects could be traced back to subsequent changes in Wnt or Stat6 signaling, which are known to affect BCs, differentiation and mucus production more directly (Danopoulos et al., 2019; Hou et al., 2019; Peng et al., 2015; Wang et al., 2020; Yuan et al., 2018). A recent study also implicated Hedgehog signaling in COPD and possibly more directly in MCC ciliation (Belgacemi et al., 2020). Clearly, more research is needed to uncover the precise functions of other signaling pathways to promote our understanding of developmental, regenerative and disease mechanisms. Understanding the modulatory effects of other pathways on airway epithelia, even if those turn out to be indirect, could still provide attractive entry points for drug development, diagnosis and ultimately for novel therapies.
Trans-differentiation in mucociliary homeostasis and tissue remodeling
In the traditional view, a precursor or stem cell gives rise to a differentiated cell, which does not change identity after completing differentiation. This is the general textbook concept of “terminal differentiation”. This view has been challenged over the years, perhaps first, when John Gurdon was able to show that the transplantation of adult skin cell nuclei into denucleated oocytes lead to reprogramming, and that the recombined egg could give rise to a fully developed frog (GURDON, 1962). This finding was later followed up on in mammalian cells, where the use of Yamanaka’s transcription factor cocktail could reprogram cells into induced pluripotent stem cells (iPSCs) (Takahashi and Yamanaka, 2006). Since then, more and more cases of naturally or disease-induced fate changes in fully differentiated cells were reported as well (Merrell and Stanger, 2016; Wells and Watt, 2018). The direct cell fate change from one differentiated cell type into another is referred to as trans-differentiation. In most tissues trans-differentiation in not very common, but in airway mucociliary epithelia, trans-differentiation events are rather frequent (Rao Tata and Rajagopal, 2017; Rock et al., 2010) (Figure 5). To highlight some aspects of mucociliary trans-differentiation processes, relevant findings from developmental and disease models are briefly summarized here, especially because they closely relate to signaling regulation of mucociliary epithelia.
Figure 5: Mucociliary cell lineages and signaling in development, homeostasis and remodeling.
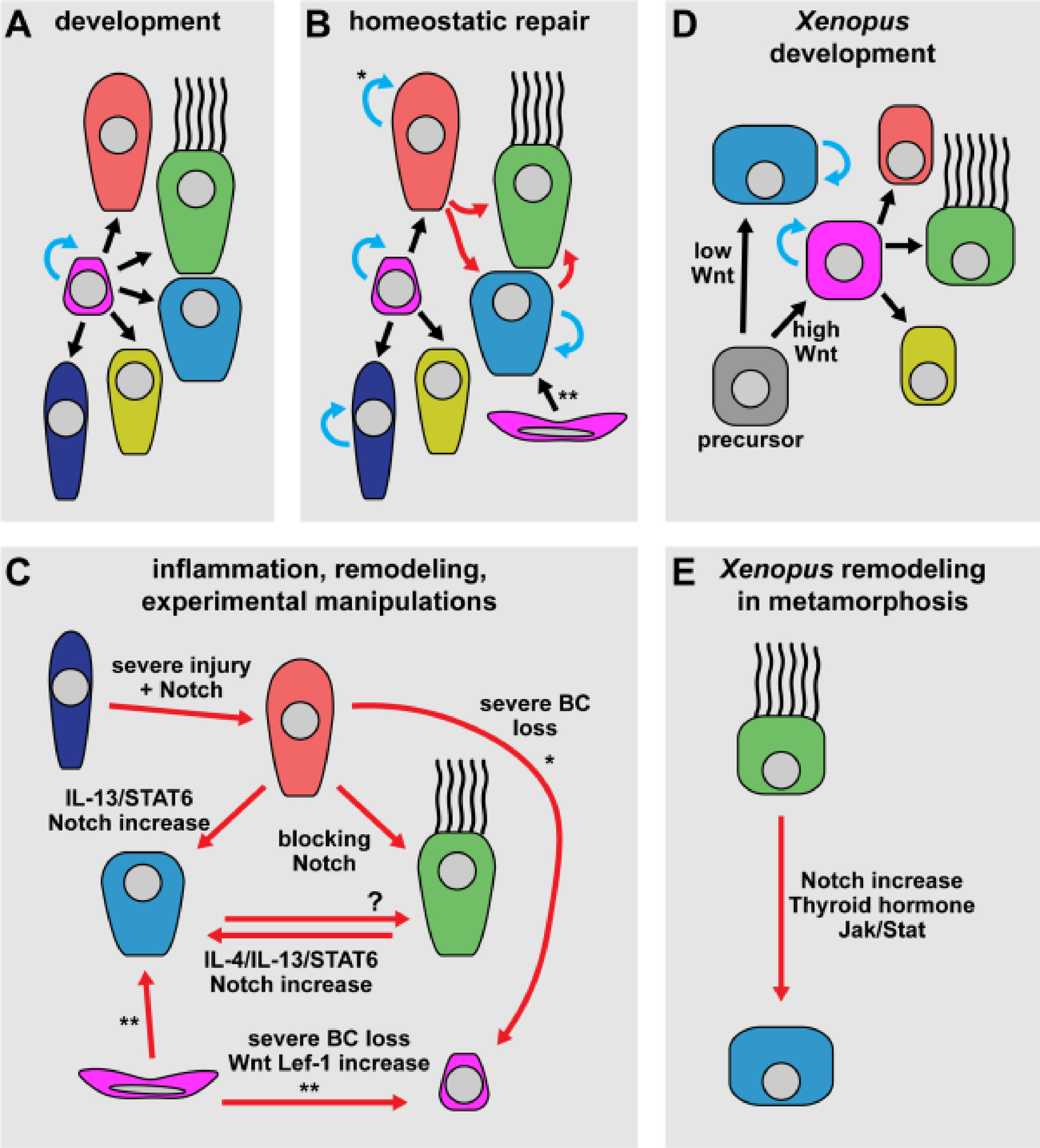
(A-E) Simplified schematic representation of mucociliary cell types (cf. Figure 1 E for legend) and their specification or trans-differentiation during normal development and homeostasis as well as in experimental models of inflammation, remodeling and severe injury. (A) In mammalian airway development, basal cells can self-renew and generate all epithelial cell types. (B) In mammalian airway homeostasis and repair, basal cells, club cells, PNECs and goblet cells can self-renew. Additionally, MCCs and goblet cell populations can be maintained by trans-differentiation from club cells. In the submucosal glands, mucous cells are generated from myoepithelial cells. (C) Trans-differentiation events in mammalian airway inflammation, tissue remodeling (e.g. in chronic airway disease), and after experimental manipulation. Club cells can trans-differentiate into MCCs, goblet cells, and can de-differentiate into basal cells upon severe basal cell loss. After severe injury, PNECs can also trans-differentiate into club cells. Trans-differentiation between MCC and goblet cell fates were reported in both directions. Myoepithelial cells in the submucosal glands can generate mucous cells, but also trans-differentiate into epithelial basal cells after severe basal cell loss. (D) Mucociliary development in the Xenopus embryonic mucociliary epidermis. All epithelial cell types are derived from precursor cells that mature into basal cells. Basal cells and goblet cells can both divide and self-renew. (E) Multiciliated to goblet cell trans-differentiation at the onset of Xenopus metamorphosis was demonstrated in the Xenopus epidermis, is triggered by increased Notch signaling and modulated by thyroid hormone and Jak/Stat signlaing. Black arrows = cell type transitions from stem/precursor cells; blue arrows = proliferation/self-renewal; red arrows = trans- and de-differentiation events. * = in the bronchiole only; ** = in the submucosal glands only.
During normal Xenopus epidermal and mammalian airway epithelial development, all cell types are thought to develop from epithelial precursors and BCs (Bilodeau et al., 2014; Collins et al., 2020; Dubaissi, 2020; Hogan et al., 2014; Walentek and Quigley, 2017) (Figure 5A,D). This seems to hold true in cases where large epithelial lesions occur in the airway epithelium (Aros et al., 2020b; Pardo-Saganta et al., 2015a). Furthermore, studies suggest that ionocytes and PNECs are derived directly from BCs in adult lung homeostasis (Rao Tata and Rajagopal, 2017) (Figure 5B). Hence, it was surprising when club cells were found to be facultative precursor cells for the majority of MCCs during mammalian airway homeostasis and repair (Hogan et al., 2014) (Figure 5B). In fact, club cells were proposed to be the stem cells of mucociliary airway epithelia, and only relatively recent studies have definitely identified multiple populations of adult stem cells along the proximal-distal axis of the mammalian lung (Reynolds and Malkinson, 2010; Rock et al., 2009; Zuo et al., 2015).
In the mouse bronchioles, club cells give rise to MCCs and maintain the club cell population through self-renewal (Rawlins et al., 2009). In the trachea, club cells also give rise to MCCs in homeostatic conditions as well as after injury, but they rarely self-renew (Rawlins et al., 2009). Hence, club cells in the trachea are likely predominantly specified from BCs in homeostatic conditions, but MCCs are replenished from epithelial club cells. Specification of MCCs from club cells is regulated by Notch signaling (Figure 5C). Notch reduction by inhibitory antibodies has shown that blocking Jag1/Jag2 leads to massive trans-differentiation of club cells into MCCs (Lafkas et al., 2015). When this treatment was terminated, club cells slowly reappeared in the epithelium. This phenomenon could be related to the specific decoration of the different mucociliary cell types by different Notch ligands, i.e. Jag1 and Jag2 were shown to be enriched in MCCs (Lafkas et al., 2015). On the other hand, Notch1 and 2 receptor blocking could elicit the same effect on mucociliary composition, and Notch1 and 2 were shown to be enriched in club cells (Lafkas et al., 2015). Further studies revealed that secretory cells, MCCs and BCs each show specific enrichment of Notch ligands and receptors (Pardo-Saganta et al., 2015b). Thus, it is attractive to speculate that this allows the mucociliary system to maintain correct cell type composition during homeostasis by providing Notch-mediated information to the surrounding epithelial cells and BCs.
Upon airway infection or allergen sensitization, club cells can also express Spdef, start Mucin production, and change their morphology towards goblet cells (Chen et al., 2009; Evans et al., 2004). Furthermore, these changes are reversible. Like in the trans-differentiation from club to ciliated cell, club to goblet cell change is Notch dependent and can be inhibited by blocking Notch signaling (Lafkas et al., 2015) (Figure 5C). Infection and allergen sensitization were shown to induce cytokine secretion, and the IL-4/IL-13/STAT6 pathway promotes Spdef expression together with Notch.
Similar to club cells, inflammation and IL-4/IL-13/STAT6 pathway activation can induce cellular changes and mucus production in MCCs through STAT6 (Tyner et al., 2006) (Figure 5C). Furthermore, MCCs were reported to loose cilia through STAT6-mediated repression of Foxj1 during trans-differentiation (Gomperts et al., 2007). These effects were co-dependent on the anti-apoptotic effect EGF signaling in MCCs (Tyner et al., 2006). Changes induced by IL-13 could be blocked by inhibition of Notch signaling, further supporting the dominant role for Notch in cell fate specification and maintenance (Danahay et al., 2015; Guseh et al., 2009; Lafkas et al., 2015). MCC to goblet cell trans-differentiation is highly controversially debated in the airway field, in part because it is hard to imagine that such an extremely differentiated cell type could change fate and remodel its highly specialized morphology. Furthermore, lineage tracing experiments in mice failed to confirm maintenance of goblet cells that were derived from MCCs, and it was observed that goblet cells can trans-differentiate into MCCs as well (Pardo-Saganta et al., 2013; Ruiz García et al., 2019; Turner et al., 2011).
In the Xenopus epidermis, MCCs are lost at the onset of metamorphosis, and MCC to goblet cell trans-differentiation was proposed in this system as well based on co-staining of cells for both cilia and mucus markers (Nishikawa et al., 1992). A recent study has provided additional evidence for MCC trans-differentiation in Xenopus, where it was demonstrated that during trans-differentiation, MCCs downregulate foxj1 gene expression, begin Mucin production as well as cilia retraction, and where loss of basal body proteins was observed in the course of the process (Tasca et al., 2021). In contrast to the inflammatory response of MCCs in the mammalian airways, this process in Xenopus was induced by an upregulation of Notch ligand expression in the mesoderm underlying the mucociliary epidermis, and cell fate change could be blocked by inhibition of Notch in this system as well (Tasca et al., 2021). Jak/Stat signaling also had a similar anti-apoptotic effect as in mammals (Figure 5C,E).(Tasca et al., 2021). Furthermore, overexpression of NICD specifically in cells committed to MCC cell fate could induce identity change to goblet cells during initial development of the epidermis (Tasca et al., 2021). Thus, these data support the possibility of MCC trans-differentiation into goblet cells in the mammalian airway, and suggest that the basis for such changes in disease could be the reactivation of an evolutionarily conserved mechanism normally employed during developmental tissue remodeling.
Equally remarkable as trans-differentiation of MCCs into goblet cells is the ability of club cells to trans-differentiate into BCs under conditions where a majority of endogenous BCs were lost (Tata et al., 2013) (Figure 5C). Ablation of more than 80% of BCs lead to in vivo dedifferentiation of club cells into BCs that could function like normal stem cells and self-renewed over an extended period of time. Similarly, BCs derived from club cells were observed in organoid-formation assays in vitro (Tata et al., 2013). Upon severe injury, myoepithelial cells lining the submucosal glands were further shown to function as stem cells for submucosal mucous cells and to migrate to the airway surface mucociliary epithelium to trans-differentiate into BCs, which then gave rise to MCCs, club cells and goblet cells (Lynch et al., 2018) (Figure 5C). This change depended on the activation of canonical Wnt signaling and Lef-1 activation, and overexpression of Lef-1 in myoepithelial cells was sufficient to induce fate change into BCs.
In summary, data from mammalian and Xenopus mucociliary epithelia have demonstrated a remarkable flexibility of cell fates and frequent trans-differentiation events during normal homeostasis, developmental tissue remodeling, airway epithelial repair upon injury, and in chronic airway diseases. In most cases, these cell fate changes could be linked to a changing signaling environment, especially in Wnt and Notch signaling, as well as to inflammation and cytokine response that likely cooperate with or alter these two pathways.
Conclusion
Extensive work has revealed many links between Wnt, Notch, BMP, and other signaling pathways to normal development as well as to pathogenesis of mucociliary epithelia, most prominently in the airways. Further understanding of signaling functions in regeneration and tissue remodeling in association with chronic lung disease will be instrumental for the future development of better treatment options for patients. Hence, additional studies are necessary to shed more light on these signaling mechanisms under normal conditions and during disease. As airway infections and chronic lung diseases are among the most common causes for death worldwide, increasing our mechanistic understanding of the signaling control of mucociliary epithelia should remain a priority in basic biological and airway pathogenesis research.
Acknowledgement
PW thanks all the members of the Walentek lab for discussions, ideas and critical comments. PW also thanks the Xenopus and mucociliary research communities for their contributions to the field as well as for communication of unpublished data, sharing of resources, and fruitful discussions on the topic auf mucociliary epithelia and airway diseases mechanisms.
Funding Sources
PW and work in the Walentek lab were supported by the NHLBI (K99HL127275) and the Deutsche Forschungsgemeinschaft (DFG) under the Emmy Noether Programme (grant WA3365/2-1) and under Germany’s Excellence Strategy (CIBSS – EXC-2189 – Project ID 390939984).
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
References [Alphabetical]
- Abdullah LH, Evans JR, Wang TT, Ford AA, Makhov AM, Nguyen K, Coakley RD, Griffith JD, Davis CW, Ballard ST, et al. (2017). Defective postsecretory maturation of MUC5B mucin in cystic fibrosis airways. JCI insight 2, e89752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alyass A, Turcotte M and Meyre D (2015). From big data analysis to personalized medicine for all: Challenges and opportunities. BMC Med. Genomics 8, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambort D, Johansson MEV, Gustafsson JK, Nilsson HE, Ermund A, Johansson BR, Koeck PJB, Hebert H and Hansson GC (2012). Calcium and pH-dependent packing and release of the gel-forming MUC2 mucin. Proc. Natl. Acad. Sci. U. S. A 109, 5645–5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arason AJ, Jonsdottir HR, Halldorsson S, Benediktsdottir BE, Bergthorsson JT, Ingthorsson S, Baldursson O, Sinha S, Gudjonsson T and Magnusson MK (2014). DeltaNp63 has a role in maintaining epithelial integrity in airway epithelium. PLoS One 9,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aros CJ, Paul MK, Pantoja CJ, Bisht B, Meneses LK, Vijayaraj P, Sandlin JM, France B, Tse JA, Chen MW, et al. (2020a). High-Throughput Drug Screening Identifies a Potent Wnt Inhibitor that Promotes Airway Basal Stem Cell Homeostasis. Cell Rep 30, 2055–2064.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aros CJ, Vijayaraj P, Pantoja CJ, Bisht B, Meneses LK, Sandlin JM, Tse JA, Chen MW, Purkayastha A, Shia DW, et al. (2020b). Distinct Spatiotemporally Dynamic Wnt-Secreting Niches Regulate Proximal Airway Regeneration and Aging. Cell Stem Cell 27, 413–429.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baarsma HA and Königshoff M (2017). “WNT-er is coming”: WNT signalling in chronic lung diseases. Thorax 72, 746–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballweg K, Mutze K, Konigshoff M, Eickelberg O and Meiners S (2014). Cigarette smoke extract affects mitochondrial function in alveolar epithelial cells. AJP Lung Cell. Mol. Physiol 307, L895–L907. [DOI] [PubMed] [Google Scholar]
- Belgacemi R, Luczka E, Ancel J, Diabasana Z, Perotin JM, Germain A, Lalun N, Birembaut P, Dubernard X, Mérol JC, et al. (2020). Airway epithelial cell differentiation relies on deficient Hedgehog signalling in COPD. EBioMedicine 51,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilodeau M, Shojaie S, Ackerley C, Post M and Rossant J (2014). Identification of a proximal progenitor population from murine fetal lungs with clonogenic and multilineage differentiation potential. Stem Cell Reports 3, 634–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks ER and Wallingford JB (2014). Multiciliated Cells. Curr. Biol 24, R973–R982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron A, Xu X and Lin X (2012). Wnt/β-catenin signaling directly regulates Foxj1 expression and ciliogenesis in zebrafish Kupffer’s vesicle. Development 139, 514–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Korfhagen TRT, Xu Y, Kitzmiller J, Wert SE, Maeda Y, Gregorieff A, Clevers H and Whitsett JA (2009). SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J. Clin. Investig 119, 2914–2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Volmer AS, Wilkinson KJ, Deng Y, Jones LC, Yu D, Bustamante-Marin XM, Burns KA, Grubb BR, O’Neal WK, et al. (2018). Role of spdef in the regulation of muc5b expression in the airways of naive and mucoobstructed mice. Am. J. Respir. Cell Mol. Biol 59, 383–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibois M, Luxardi G, Chevalier B, Thome V, Mercey O, Zaragosi L-EL, Barbry P, Pasini A, Marcet B and Kodjabachian L (2015). BMP signalling controls the construction of vertebrate mucociliary epithelia. Development 1–12. [DOI] [PubMed] [Google Scholar]
- Clevers H, Loh KM and Nusse R (2014). An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science (80-. ) 346, 1248012. [DOI] [PubMed] [Google Scholar]
- Collins C, Ventrella R and Mitchell BJ (2020). Building a ciliated epithelium: Transcriptional regulation and radial intercalation of multiciliated cells 1st ed. Elsevier Inc. [DOI] [PubMed] [Google Scholar]
- Corfield AP (2015). Mucins: A biologically relevant glycan barrier in mucosal protection. Biochim. Biophys. Acta - Gen. Subj 1850, 236–252. [DOI] [PubMed] [Google Scholar]
- Cutz E, Pan J, Yeger H, Domnik NJ and Fisher JT (2013). Recent advances and contraversies on the role of pulmonary neuroepithelial bodies as airway sensors. Semin. Cell Dev. Biol 24, 40–50. [DOI] [PubMed] [Google Scholar]
- Danahay H, Pessotti ADD, Coote J, Montgomery BEE, Xia D, Wilson A, Yang H, Wang Z, Bevan L, Thomas C, et al. (2015). Notch2 is required for inflammatory cytokine-driven goblet cell metaplasia in the lung. Cell Rep 10, 239–252. [DOI] [PubMed] [Google Scholar]
- Daniely Y, Liao G, Dixon D, Linnoila RI, Lori A, Randell SH, Oren M and Jetten AM (2004). Critical role of p63 in the development of a normal esophageal and tracheobronchial epithelium. Am. J. Physiol. - Cell Physiol 287, 171–181. [DOI] [PubMed] [Google Scholar]
- Danopoulos S, Shiosaki J and Al Alam D (2019). FGF signaling in lung development and disease: Human versus mouse. Front. Genet 10,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deblandre GA, Wettstein DA, Koyano-nakagawa N and Kintner C (1999). A two-step mechanism generates the spacing pattern of the ciliated cells in the skin of Xenopus embryos. Development 126, 4715–4728. [DOI] [PubMed] [Google Scholar]
- Dobzanski A, Khalil SM and Lane AP (2018). Nasal polyp fibroblasts modulate epithelial characteristics via Wnt signaling. Int. Forum Allergy Rhinol 8, 1412–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran SA (2004). Effect of serotonin on ciliary beating and intracellular calcium concentration in identified populations of embryonic ciliary cells. J. Exp. Biol 207, 1415–1429. [DOI] [PubMed] [Google Scholar]
- Driskell RR, Liu X, Luo M, Filali M, Zhou W, Abbott D, Cheng N, Moothart C, Sigmund CD and Engelhardt JF (2004). Wnt-responsive element controls Lef-1 promoter expression during submucosal gland morphogenesis. Am. J. Physiol. Lung Cell. Mol. Physiol 287, L752–L763. [DOI] [PubMed] [Google Scholar]
- Driskell RR, Goodheart M, Neff T, Liu X, Luo M, Moothart C, Sigmund CD, Hosokawa R, Chai Y and Engelhardt JF (2007). Wnt3a regulates Lef-1 expression during airway submucosal gland morphogenesis. Dev. Biol 305, 90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubaissi E (2020). A ‘tad’ of hope in the fight against airway disease. Biochem. Soc. Trans 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubaissi E, Rousseau K, Lea R, Soto X, Nardeosingh S, Schweickert A, Amaya E, Thornton DJ and Papalopulu N (2014). A secretory cell type develops alongside multiciliated cells, ionocytes and goblet cells, and provides a protective, anti-infective function in the frog embryonic mucociliary epidermis. Development 141, 1514–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutko JA and Mullins MC (2011). SnapShot: BMP signaling in development. Cell 145, 636–636.e2. [DOI] [PubMed] [Google Scholar]
- Evans CM, Williams OW, Tuvim MJ, Nigam R, Mixides GP, Blackburn MR, DeMayo FJ, Burns AR, Smith C, Reynolds SD, et al. (2004). Mucin is produced by clara cells in the proximal airways of antigen-challenged mice. Am. J. Respir. Cell Mol. Biol 31, 382–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahy JV and Dickey BF (2010). Airway Mucus Function and Dysfunction. N. Engl. J. Med 363, 2233–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DB, Peng T, Zepp JA, Snitow M, Vincent TL, Penkala IJ, Cui Z, Herriges MJ, Morley MP, Zhou S, et al. (2016). Emergence of a Wave of Wnt Signaling that Regulates Lung Alveologenesis by Controlling Epithelial Self-Renewal and Differentiation. Cell Rep 17, 2312–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaimo BD, Oswald F and Borggrefe T (2017). Dynamic chromatin regulation at Notch target genes. Transcription 8, 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarbmuren KC, Jackson ND, Sajuthi SP, Dyjack N, Li KS, Rios CL, Plender EG, Montgomery MT, Everman JL, Bratcher PE, et al. (2020). Dissecting the cellular specificity of smoking effects and reconstructing lineages in the human airway epithelium. Nat. Commun 11,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomi K, Arbelaez V, Crystal RG and Walters MS (2015). Activation of NOTCH1 or NOTCH3 Signaling Skews Human Airway Basal Cell Differentiation toward a Secretory Pathway. PLoS One 10, e0116507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomperts BN, Kim LJ, Flaherty SA and Hackett BP (2007). IL-13 regulates cilia loss and foxj1 expression in human airway epithelium. Am. J. Respir. Cell Mol. Biol 37, 339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff JW, Powers LS, Dickson AM, Kim J, Reisetter AC, Hassan IH, Kremens K, Gross TJ, Wilson ME and Monick MM (2012). Cigarette smoking decreases global microRNA expression in human alveolar macrophages. PLoS One 7, e44066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GURDON JB (1962). The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. J. Embryol. Exp. Morphol 10, 622–640. [PubMed] [Google Scholar]
- Guseh JS, Bores SA, Stanger BZ, Zhou Q, Anderson WJ, Melton DA and Rajagopal J (2009). Notch signaling promotes airway mucous metaplasia and inhibits alveolar development. Development 136, 1751–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M, Gómez Vázquez JL, Sun DI, Tran HT, Brislinger M, Tasca A, Shomroni O, Vleminckx K and Walentek P (2019). ΔN-Tp63 Mediates Wnt/β-Catenin-Induced Inhibition of Differentiation in Basal Stem Cells of Mucociliary Epithelia. Cell Rep 28, 3338–3352.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Cai S, Feng H, Cai B, Lin L, Mai Y, Fan Y, Zhu A, Huang H, Shi J, et al. (2020). Single-cell analysis reveals bronchoalveolar epithelial dysfunction in COVID-19 patients. Protein Cell 11, 680–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijink IH, de Bruin HG, van den Berge M, Bennink LJC, Brandenburg SM, Gosens R, van Oosterhout AJ and Postma DS (2013). Role of aberrant WNT signalling in the airway epithelial response to cigarette smoke in chronic obstructive pulmonary disease. Thorax 68, 709–16. [DOI] [PubMed] [Google Scholar]
- Henrique D and Schweisguth F (2019). Mechanisms of notch signaling: A simple logic deployed in time and space. Dev 146,. [DOI] [PubMed] [Google Scholar]
- Hogan BLM, Barkauskas CE, Chapman HA, Epstein JA, Jain R, Hsia CCW, Niklason L, Calle E, Le A, Randell SH, et al. (2014). Repair and Regeneration of the Respiratory System: Complexity, Plasticity, and Mechanisms of Lung Stem Cell Function. Cell Stem Cell 15, 123–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Z, Wu Q, Sun X, Chen H, Li Y, Zhang Y, Mori M, Yang Y, Que J and Jiang M (2019). Wnt/Fgf crosstalk is required for the specification of basal cells in the mouse trachea. Development 146, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer N, Prior TS, Bendstrup E, Wilcke T and Shaker SB (2019). Risk factors for diagnostic delay in idiopathic pulmonary fibrosis. Respir. Res 20, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Ng-Blichfeldt JP, Ota C, Ciminieri C, Ren W, Hiemstra PS, Stolk J, Gosens R and Königshoff M (2020). Wnt/β-catenin signaling is critical for regenerative potential of distal lung epithelial progenitor cells in homeostasis and emphysema. Stem Cells [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y and Niehrs C (2014). Polarized Wnt signaling regulates ectodermal cell fate in Xenopus. Dev. Cell 29, 250–7. [DOI] [PubMed] [Google Scholar]
- Huang SXL, Islam MN, O’Neill J, Hu Z, Yang Y-G, Chen Y-W, Mumau M, Green MD, Vunjak-Novakovic G, Bhattacharya J, et al. (2014). Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat. Biotechnol 32, 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SXL, Green MD, de Carvalho AT, Mumau M, Chen Y-W, D’Souza SL and Snoeck H-W (2015). The in vitro generation of lung and airway progenitor cells from human pluripotent stem cells. Nat. Protoc 10, 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibañez-Tallon I, Heintz N, Omran H, Ibanez-Tallon I, Heintz N and Omran H (2003). To beat or not to beat: roles of cilia in development and disease. Hum. Mol. Genet 12, 27R–35. [DOI] [PubMed] [Google Scholar]
- Kim HT, Yin W, Nakamichi Y, Panza P, Grohmann B, Buettner C, Guenther S, Ruppert C, Kobayashi Y, Guenther A, et al. (2019). WNT/RYK signaling restricts goblet cell differentiation during lung development and repair. Proc. Natl. Acad. Sci. U. S. A 116, 25697–25706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjolby RAS and Harland RM (2016). Genome-wide identi fi cation of Wnt / β -catenin transcriptional targets during Xenopus gastrulation. Dev. Biol 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König P, Krain B, Krasteva G, Kummer W and Ko P (2009). Serotonin increases cilia-driven particle transport via an acetylcholine-independent pathway in the mouse trachea. PLoS One 4, e4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurrle Y, Kunesch K, Bogusch S and Schweickert A (2020). Serotonin and MucXS release by small secretory cells depend on Xpod, a SSC specific marker gene. Genesis 58, 1–9. [DOI] [PubMed] [Google Scholar]
- Lafkas D, Shelton A, Chiu C, De Leon Boenig G, Chen Y, Stawicki SS, Siltanen C, Reichelt M, Zhou M, Wu X, et al. (2015). Therapeutic antibodies reveal Notch control of transdifferentiation in the adult lung. Nature 528, 127–131. [DOI] [PubMed] [Google Scholar]
- Lake RJ, Tsai PF, Choi I, Won KJ and Fan HY (2014). RBPJ, the Major Transcriptional Effector of Notch Signaling, Remains Associated with Chromatin throughout Mitosis, Suggesting a Role in Mitotic Bookmarking. PLoS Genet 10,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M, Baarsma HA and Königshoff M (2016). WNT signaling in lung aging and disease. In Annals of the American Thoracic Society, pp. S411–S416. [DOI] [PubMed] [Google Scholar]
- Lommel A (2001). Pulmonary neuroendocrine cells (PNEC) and neuroepithelial bodies (NEB): chemoreceptors and regulators of lung development. Paediatr. Respir. Rev 171–176. [DOI] [PubMed] [Google Scholar]
- Lynch TJ, Anderson PJ, Rotti PG, Tyler SR, Crooke AK, Choi SH, Montoro DT, Silverman CL, Shahin W, Zhao R, et al. (2018). Submucosal Gland Myoepithelial Cells Are Reserve Stem Cells That Can Regenerate Mouse Tracheal Epithelium. Cell Stem Cell 22, 653–667.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald BBT, Semenov MMV and He X (2007). SnapShot: Wnt/β-catenin signaling. Cell 131, 1204–1205. [DOI] [PubMed] [Google Scholar]
- McCormack N, Molloy EL and O’Dea S (2013). Bone morphogenetic proteins enhance an epithelial-mesenchymal transition in normal airway epithelial cells during restitution of a disrupted epithelium. Respir. Res 14, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez A, Rojas DA, Ponce CA, Bustamante R, Beltrán CJ, Toledo J, García-Angulo VA, Henriquez M and Vargas SL (2019). Primary infection by Pneumocystis induces Notch-independent Clara cell mucin production in rat distal airways. PLoS One 14, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrell AJ and Stanger BZ (2016). Adult cell plasticity in vivo: De-differentiation and transdifferentiation are back in style. Nat. Rev. Mol. Cell Biol 17, 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier A and Azimzadeh J (2016). Multiciliated Cells in Animals. Cold Spring Harb. Perspect. bBology 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison HM and Valente EM (2017). Motile and non-motile cilia in human pathology: from function to phenotypes. J. Pathol 241, 294–309. [DOI] [PubMed] [Google Scholar]
- Molloy E, Adams A, Moore JB, Masterson J, Madrigal-Estebas L, Mahon B and O’Dea S (2008). BMP4 induces an epithelial-mesenchymal transition-like response in adult airway epithelial cells. Growth Factors 26, 12–22. [DOI] [PubMed] [Google Scholar]
- Montoro DT, Haber AL, Biton M, Vinarsky V, Lin B, Birket SE, Yuan F, Chen S, Leung HM, Villoria J, et al. (2018). A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature 560, 319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto M, Nishinakamura R, Saga Y and Kopan R (2012). Different assemblies of Notch receptors coordinate the distribution of the major bronchial Clara, ciliated and neuroendocrine cells. Development 139, 4365–4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou H, Vinarsky V, Tata PR, Brazauskas K, Choi SH, Crooke AK, Zhang B, Solomon GM, Turner B, Bihler H, et al. (2016). Dual SMAD Signaling Inhibition Enables Long-Term Expansion of Diverse Epithelial Basal Cells. Cell Stem Cell 19, 217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucenski ML, Nation JM, Thitoff AR, Besnard V, Xu Y, Wert SE, Harada N, Taketo MM, Stahlman MT and Whitsett JA (2005). Beta-catenin regulates differentiation of respiratory epithelial cells in vivo. Am. J. Physiol. Lung Cell. Mol. Physiol 289, L971–L979. [DOI] [PubMed] [Google Scholar]
- Mukherjee AB, Zhang Z and Chilton BS (2007). Uteroglobin: A steroid-inducible immunomodulatory protein that founded the Secretoglobin superfamily. Endocr. Rev 28, 707–725. [DOI] [PubMed] [Google Scholar]
- Naylor S and Chen JY (2010). Unraveling human complexity and disease with systems biology and personalized medicine. Per. Med 7, 275–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa S, Hirata J and Sasaki F (1992). Fate of ciliated epidermal cells during early development of Xenopus laevis using whole-mount immunostaining with an antibody against chondroitin 6-sulfate proteoglycan and anti-tubulin: transdifferentiation or metaplasia of amphibian epidermis. Histochemistry 98, 355–358. [DOI] [PubMed] [Google Scholar]
- Pardo-Saganta A, Law BM, Gonzalez-Celeiro M, Vinarsky V and Rajagopal J (2013). Ciliated cells of pseudostratified airway epithelium do not become mucous cells after ovalbumin challenge. Am. J. Respir. Cell Mol. Biol 48, 364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo-Saganta A, Law BM, Tata PR, Villoria J, Saez B, Mou H, Zhao R and Rajagopal J (2015a). Injury induces direct lineage segregation of functionally distinct airway basal stem/progenitor cell subpopulations. Cell Stem Cell 16, 184–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo-Saganta A, Tata PR, Law BM, Saez B, Chow RDW, Prabhu M, Gridley T and Rajagopal J (2015b). Parent stem cells can serve as niches for their daughter cells. Nature 523, 597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JC, Thavagnanam S, Skibinski G, Lyons J, Bell J, Heaney LG and Shields MD (2013). Chronic IL9 and IL-13 Exposure Leads to an Altered Differentiation of Ciliated Cells in a Well-Differentiated Paediatric Bronchial Epithelial Cell Model. PLoS One 8,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng T, Frank DB, Kadzik RS, Morley MP, Rathi KS, Wang T, Zhou S, Cheng L, Lu MM and Morrisey EE (2015). Hedgehog actively maintains adult lung quiescence and regulates repair and regeneration. Nature 526, 578–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrimon N, Pitsouli C and Shilo BZ (2012). Signaling mechanisms controlling cell fate and embryonic patterning. Cold Spring Harb. Perspect. Biol 4,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plasschaert LW, Žilionis R, Choo-Wing R, Savova V, Knehr J, Roma G, Klein AM and Jaffe AB (2018). A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature 560, 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley IK and Kintner C (2017). Rfx2 Stabilizes Foxj1 Binding at Chromatin Loops to Enable Multiciliated Cell Gene Expression. PLoS Genet 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley IK, Stubbs JL and Kintner C (2011). Specification of ion transport cells in the Xenopus larval skin. Development 138, 705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao Tata P and Rajagopal J (2017). Plasticity in the lung: Making and breaking cell identity. Dev 144, 755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F and Hogan BLM (2009). The Role of Scgb1a1+ Clara Cells in the Long-Term Maintenance and Repair of Lung Airway, but Not Alveolar, Epithelium. Cell Stem Cell 4, 525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renz H, Autenrieth IB, Brandtzæg P, Cookson WO, Holgate S, Von Mutius E, Valenta R and Haller D (2011). Gene-environment interaction in chronic disease: A European Science Foundation Forward Look. J. Allergy Clin. Immunol 128, S27–S49. [DOI] [PubMed] [Google Scholar]
- Reynolds SD and Malkinson AM (2010). Clara cell: Progenitor for the bronchiolar epithelium. Int. J. Biochem. Cell Biol 42, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SD, Zemke AC, Giangreco A, Brockway BL, Teisanu RM, Drake JA, Mariani T, Di PYP, Taketo MM and Stripp BR (2008). Conditional Stabilization of β-Catenin Expands the Pool of Lung Stem Cells. Stem Cells 26, 1337–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijt S. H. Van, Keller IE, John G, Kohse K, Yildirim AOÖ, Eickelberg O, Meiners S, van Rijt SH, Keller IE, John G, et al. (2012). Acute cigarette smoke exposure impairs proteasome function in the lung. AJP Lung Cell. Mol. Physiol 303, L814–L823. [DOI] [PubMed] [Google Scholar]
- Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH and Hogan BLM (2009). Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci U S A 106, 12771–12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, Randell SH and Hogan BLMM (2010). Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis. Model. Mech 3, 545–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, Gao X, Xue Y, Randell SH, Kong YY and Hogan BLMM (2011). Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell 8, 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokicki W, Rokicki M, Wojtacha J and Dzeljijli A (2016). The role and importance of club cells (Clara cells) in the pathogenesis of some respiratory diseases. Kardiochirurgia i Torakochirurgia Pol 13, 26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S (2009). The motile cilium in development and disease: emerging new insights. Bioessays 31, 694–9. [DOI] [PubMed] [Google Scholar]
- Ruiz García S, Deprez M, Lebrigand K, Cavard A, Paquet A, Arguel M-J, Magnone V, Truchi M, Caballero I, Leroy S, et al. (2019). Novel dynamics of human mucociliary differentiation revealed by single-cell RNA sequencing of nasal epithelial cultures. Development 146, dev.177428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruptier C, De Gaspéris A, Ansieau S, Granjon A, Tanière P, Lafosse I, Shi H, Petitjean A, Taranchon-Clermont E, Tribollet V, et al. (2011). TP63 P2 promoter functional analysis identifies Β-catenin as a key regulator of Δnp63 expression. Oncogene 30, 4656–4665. [DOI] [PubMed] [Google Scholar]
- Sanz-Ezquerro JJ, Münsterberg AE and Stricker S (2017). Editorial: Signaling pathways in embryonic development. Front. Cell Dev. Biol 5, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schamberger AC, Mise N, Jia J, Genoyer E, Yildirim AÖ, Meiners S and Eickelberg O (2014). Cigarette Smoke–Induced Disruption of Bronchial Epithelial Tight Junctions Is Prevented by Transforming Growth Factor-β. Am. J. Respir. Cell Mol. Biol 50, 1040–1052. [DOI] [PubMed] [Google Scholar]
- Schamberger AC, Staab-Weijnitz C. a., Mise-Racek N and Eickelberg O (2015). Cigarette smoke alters primary human bronchial epithelial cell differentiation at the air-liquid interface. Sci. Rep 5, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid A, Sailland J, Novak L, Baumlin N, Fregien N and Salathe M (2017). Modulation of Wnt signaling is essential for the differentiation of ciliated epithelial cells in human airways. FEBS Lett 591, 3493–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears ME and Genuis SJ (2012). Environmental determinants of chronic disease and medical approaches: Recognition, avoidance, supportive therapy, and detoxification. J. Environ. Public Health 2012,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenov MV, Habas R, Macdonald BT and He X (2007). SnapShot: Noncanonical Wnt Signaling Pathways. Cell 131, 1378. [DOI] [PubMed] [Google Scholar]
- Senoo M, Pinto F, Crum CP and McKeon F (2007). p63 Is Essential for the Proliferative Potential of Stem Cells in Stratified Epithelia. Cell 129, 523–536. [DOI] [PubMed] [Google Scholar]
- Shi J, Li F, Luo M, Wei J and Liu X (2017). Distinct Roles of Wnt/ β-Catenin Signaling in the Pathogenesis of Chronic Obstructive Pulmonary Disease and Idiopathic Pulmonary Fibrosis. Mediators Inflamm 2017,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirour C, Hidalgo M, Bello V, Buisson N, Darribere T and Moreau N (2011). Dystroglycan is involved in skin morphogenesis downstream of the Notch signaling pathway. Mol. Biol. Cell 22, 2957–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CR (2015). Diagnosis in chronic obstructive pulmonary disease - “too little, too late?” Chron. Respir. Dis 12, 281–283. [DOI] [PubMed] [Google Scholar]
- Sountoulidis A, Stavropoulos A, Giaglis S, Apostolou E, Monteiro R, Chuva de Sousa Lopes SM, Chen H, Stripp BR, Mummery C, Andreakos E, et al. (2012). Activation of the canonical bone morphogenetic protein (BMP) pathway during lung morphogenesis and adult lung tissue repair. PLoS One 7,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhart Z and Angers S (2018). Wnt signaling in development and tissue homeostasis. Development 145, 1–8. [DOI] [PubMed] [Google Scholar]
- Stubbs JL, Davidson L, Keller R and Kintner C (2006). Radial intercalation of ciliated cells during Xenopus skin development. Development 133, 2507–15. [DOI] [PubMed] [Google Scholar]
- Sun DI, Tasca A, Haas M, Baltazar G, Harland RM, Finkbeiner WE and Walentek P (2018). Na+/H+ Exchangers Are Required for the Development and Function of Vertebrate Mucociliary Epithelia. Cells Tissues Organs 205, 279–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadokoro T, Gao X, Hong CC, Hotten D and Hogan BLMM (2016). BMP signaling and cellular dynamics during regeneration of airway epithelium from basal progenitors. Dev 143, 764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K and Yamanaka S (2006). Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 126, 663–676. [DOI] [PubMed] [Google Scholar]
- Tasca A, Helmstädter M, Brislinger M, Haas M, Mitchell BJ and Walentek P (2021). Notch signaling induces either apoptosis or cell fate change in multiciliated cells during mucociliary tissue remodeling. Dev. Cell 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata PR, Mou H, Pardo-Saganta A, Zhao R, Prabhu M, Law BM, Vinarsky V, Cho JL, Breton S, Sahay A, et al. (2013). Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature 503, 218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley AE, Harvey BG, Heguy A, Hackett NR, Wang R, O’Connor TP and Crystal RG (2009). Down-regulation of the notch pathway in human airway epithelium in association with smoking and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med 179, 457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley AE, Walters MS, Shaykhiev R and Crystal RG (2015). Cilia dysfunction in lung disease. Annu. Rev. Physiol 77, 379–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao P-N, Vasconcelos M, Izvolsky KI, Qian J, Lu J and Cardoso WV (2009). Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development 136, 2297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J, Roger J, Fitau J, Combe D, Giddings J, Heeke G. Van and Jones CE (2011). Goblet cells are derived from a FOXJ1-expressing progenitor in a human airway epithelium. Am. J. Respir. Cell Mol. Biol 44, 276–84. [DOI] [PubMed] [Google Scholar]
- Tyner JW, Kim EY, Ide K, Pelletier MR, Roswit WT, Morton JD, Battaile JT, Patel AC, Patterson GA, Castro M, et al. (2006). Blocking airway mucous cell metaplasia by inhibiting EGFR antiapoptosis and IL-13 transdifferentiation signals. J. Clin. Invest 116, 309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladar EK and Axelrod JD (2008). Dishevelled links basal body docking and orientation in ciliated epithelial cells. Trends Cell Biol 18, 517–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladar EK and Königshoff M (2020). Noncanonical Wnt planar cell polarity signaling in lung development and disease. Biochem. Soc. Trans 48, 231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner CE, Wheeler KM and Ribbeck K (2018). Mucins and Their Role in Shaping the Functions of Mucus Barriers. Annu. Rev. Cell Dev. Biol 34, 189–215. [DOI] [PubMed] [Google Scholar]
- Walentek P (2018). Manipulating and Analyzing Cell Type Composition of the Xenopus Mucociliary Epidermis. In Methods in Molecular Biology, pp. 251–263. [DOI] [PubMed] [Google Scholar]
- Walentek P and Quigley IK (2017). What we can learn from a tadpole about ciliopathies and airway diseases: Using systems biology in Xenopus to study cilia and mucociliary epithelia. Genesis 55, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walentek P, Beyer T, Thumberger T, Schweickert A and Blum M (2012). ATP4a is required for Wnt-dependent Foxj1 expression and leftward flow in Xenopus left-right development. Cell Rep 1, 516–27. [DOI] [PubMed] [Google Scholar]
- Walentek P, Bogusch S, Thumberger T, Vick P, Dubaissi E, Beyer T, Blum M and Schweickert A (2014). A novel serotonin-secreting cell type regulates ciliary motility in the mucociliary epidermis of Xenopus tadpoles. Development 141, 1–8. [DOI] [PubMed] [Google Scholar]
- Walentek P, Beyer T, Hagenlocher C, Müller C, Feistel K, Schweickert A, Harland RMRM and Blum M (2015). ATP4a is required for development and function of the Xenopus mucociliary epidermis - a potential model to study proton pump inhibitor-associated pneumonia. Dev. Biol 408, 292–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walentek P, Boutin C and Kodjabachian L (2017). Planar Cell Polarity in Ciliated Epithelia. In Cell Polarity in Development and Disease, pp. 177–209. [Google Scholar]
- Wallingford JB (2010). Planar cell polarity signaling, cilia and polarized ciliary beating. Curr. Opin. Cell Biol 22, 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters MS, Gomi K, Ashbridge B, Moore M. a S., Arbelaez V, Heldrich J, Ding B-S, Rafii S, Staudt MR and Crystal RG (2013). Generation of a human airway epithelium derived basal cell line with multipotent differentiation capacity. Respir. Res 14, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Ahmed J, Wang G, Hassan I, Strulovici-Barel Y, Hackett NR and Crystal RG (2011). Down-regulation of the canonical Wnt β-catenin pathway in the airway epithelium of healthy smokers and smokers with COPD. PLoS One 6, e14793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liu J, Zhou J-S, Huang H-Q, Li Z-Y, Xu X-C, Lai T-W, Hu Y, Zhou H-B, Chen H-P, et al. (2018). MTOR Suppresses Cigarette Smoke–Induced Epithelial Cell Death and Airway Inflammation in Chronic Obstructive Pulmonary Disease. J. Immunol 200, 2571–2580. [DOI] [PubMed] [Google Scholar]
- Wang X, Xu C, Ji J, Cai Y, Shu Y, Chao Y, Wu X, Zou C, Wu X and Tang L (2020). IL-4/IL-13 upregulates Sonic hedgehog expression to induce allergic airway epithelial remodeling. Am. J. Physiol. Cell. Mol. Physiol 318, L888–L899. [DOI] [PubMed] [Google Scholar]
- Wells JM and Watt FM (2018). Diverse mechanisms for endogenous regeneration and repair in mammalian organs. Nature 557, 322–328. [DOI] [PubMed] [Google Scholar]
- Weng G, Bhalla US and Iyengar R (1999). Complexity in biological signaling systems. Science (80-. ) 284, 92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams OW, Sharafkhaneh A, Kim V, Dickey BF and Evans CM (2006). Airway mucus: From production to secretion. Am. J. Respir. Cell Mol. Biol 34, 527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Lynch TJ, Liu X, Tyler SR, Yu S, Zhou X, Luo M, Kusner DM, Sun X, Yi Y, et al. (2014). Sox2 modulates Lef-1 expression during airway submucosal gland development. AJP Lung Cell. Mol. Physiol 306, L645–L660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Li Q, Kolosov VP, Perelman JM and Zhou X (2011). Interleukin-13 induces mucin 5AC production involving STAT6/SPDEF in human airway epithelial cells. Cell Commun. Adhes 17, 83–92. [DOI] [PubMed] [Google Scholar]
- Yuan T, Volckaert T, Chanda D, Thannickal VJ and De Langhe SP (2018). Fgf10 Signaling in Lung Development, Homeostasis, Disease, and Repair After Injury. Front. Genet 9, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanin M, Baviskar P, Webster R and Webby R (2016). The Interaction between Respiratory Pathogens and Mucus. Cell Host Microbe 19, 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zariwala M. a, Omran H and Ferkol TW (2011). The emerging genetics of primary ciliary dyskinesia. Proc. Am. Thorac. Soc 8, 430–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemke AC, Teisanu RM, Giangreco A, Drake J. a, Brockway BL, Reynolds SD and Stripp BR (2009). beta-Catenin is not necessary for maintenance or repair of the bronchiolar epithelium. Am. J. Respir. Cell Mol. Biol 41, 535–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Gallup M, Zlock L, Chen YTF, Finkbeiner WE and McNamara N. a. (2014). Pivotal role of MUC1 glycosylation by cigarette smoke in modulating disruption of airway adherens junctions in vitro. J. Pathol 234, 60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo W, Zhang T, Wu DZA, Guan SP, Liew AA, Yamamoto Y, Wang X, Lim SJ, Vincent M, Lessard M, et al. (2015). P63 + Krt5 + distal airway stem cells are essential for lung regeneration. Nature 517, 616–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo WL, Yang J, Strulovici-Barel Y, Salit J, Rostami M, Mezey JG, O’Beirne SL, Kaner RJ and Crystal RG (2019). Exaggerated BMP4 signalling alters human airway basal progenitor cell differentiation to cigarette smoking-related phenotypes. Eur. Respir. J 53,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo WL, Rostami MR, LeBlanc M, Kaner RJ, O’Beirne SL, Mezey JG, Leopold PL, Quast K, Visvanathan S, Fine JS, et al. (2020). Dysregulation of club cell biology in idiopathic pulmonary fibrosis. PLoS One 15, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]


