Abstract
Pulmonary sclerosing pneumocytoma is a rare benign neoplasm of the lung, commonly occurs in middle-aged persons with a marked female predominance. Earlier, it was known as sclerosing hemangioma. Here, we present a case of pulmonary sclerosing pneumocytoma which was diagnosed as carcinoid of the lung, based on imaging, in a 14-year-old female. Besides radiology, the uniqueness of this case lies in the young age (14 years) of the patient. She was presented with a 3.3 × 2.5 × 2.2 cm soft tissue density mass with a tiny speck of calcification in the anterior basal segment of the lower lobe of the right lung. Based on imaging findings on fluorodeoxyglucose positron emission tomography (FDG PET) scan and DOTANOC scan, a diagnosis of carcinoid was made. We performed a video-assisted thoracoscopic right lower lobectomy. Histopathological examination showed features of pulmonary sclerosing pneumocytoma. Surgery is an established method of treatment for pulmonary sclerosing pneumocytoma. Enucleation, lobectomy, sleeve resection are possible treatment options. To define the role of adjuvant therapy, further direct evidence is required. The metastatic potential of this disease is yet to be established.
Keywords: Pulmonary sclerosing pneumocytoma, VATS, Lobectomy
Introduction
Pulmonary sclerosing pneumocytoma (PSP) is a rare benign neoplasm of the lung. It was first described by Liebow & Hubbell almost 60 years ago [1]. Earlier, it was known as sclerosing hemangioma [1]. It commonly occurs in middle-aged adults, 50 years or more, with marked female predominance [2, 3]. Most of the patients are asymptomatic, although symptoms may occur in the form of cough, chest pain, dyspnea, and hemoptysis [4]. It was previously believed to be of vascular origin, but studies have demonstrated it to be epithelial in origin [3]. The 2015 World Health Organization (WHO) lung tumor classification classifies it as an epithelial tumor [5]. On contrast-enhanced computed tomography (CECT) scan, it presents as a solitary, solid, juxtapleural mass, less than 3 cm in diameter with smooth border and calcification. Other CECT scan signs include the presence of pseudocapsule, overlying vessels, air gap, and halo sign [2, 6]. On fluorodeoxyglucose positron emission tomography (FDG PET) scan, it shows hypermetabolic activity [6]. Although it is a benign tumor, there are case reports and series which suggest that lymph node metastasis may occur [7, 8]. Here, we present a case of PSP which was misdiagnosed as carcinoid of the lung, based on imaging, in a 14-year-old female. Besides radiology, the uniqueness of this case lies in the young age (14 years) of the patient. As per our knowledge, there are only 3 other cases reported in the literature, presenting at an age of less than 20 years [2, 9].
Case report
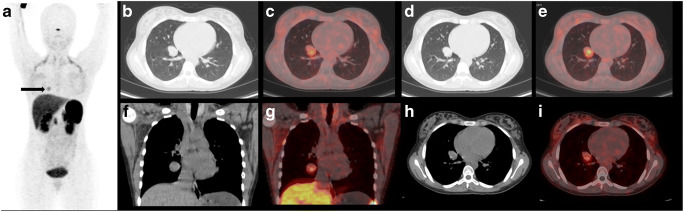
A 14-year-old female was presented with a history of fever and hemoptysis 2 years back. She was initially managed for an allergic lung condition. She experienced repeated bouts of hemoptysis associated with fever of 3–4 days duration. She had no other significant past or family history. Clinical examination was within normal limits. The initial chest X-ray showed opacity over the right lower lung. A CECT scan of the chest showed a 3.3 × 2.5 × 2.2 cm soft tissue density mass with a tiny speck of calcification in the anterior basal segment of the right lung lower lobe. The lesion showed a homogenous enhancement. Further investigation with PET CT showed an FDG avid (SUV max 4) lobular soft tissue lesion in the lower lobe of the right lung, with a tiny focus of concentric calcification. Bronchoscopy and pulmonary function test (PFT) were normal. Bronchoalveolar lavage (BAL) for acid fast bacilli (AFB) was negative. She was further investigated with Ga-68 DOTANOC PET scan (Fig. 1), which showed a moderately Ga-68 DOTANOC avid mass lesion of 2.5 × 2.2 cm, with a speck of calcification in the right lung lower lobe. Because of moderate FDG uptake in PET scan and moderate somatostatin receptor (SSTR) expression in DOTANOC scan, a diagnosis of grade II carcinoid was made. As per the diagnosis of carcinoid, made based on imaging, right lower lobectomy was planned. After all routine investigations (normal) and pre-anesthetic check-up, we performed video-assisted thoracoscopic surgery (VATS) right lower lobectomy. Intra-operatively, there was a mass of 3 × 2.5 cm size in the lower lobe of the right lung, close to the inferior pulmonary vein (IPV). There was no enlarged lymph node. The postoperative course was uneventful. She was discharged on postoperative day 3 after the removal of chest drain. Histopathological examination showed features of PSP (Fig. 2). The tumor cells were immunopositive for cytokeratin-7 (CK7) and thyroid transcription factor-1 (TTF1), while negative for chromogranin and Synaptophysin, excluding the diagnosis of carcinoid tumor. Based on the histopathological report, no adjuvant therapy was planned, and follow-up in regular intervals was advised. There is no evidence of recurrence after 8 months of follow-up.
Fig. 1.
68Ga-DOTANOC PET/CT maximum intensity projection image showing physiological radiotracer uptake (a) in the pituitary gland, liver spleen, uncinate process of the pancreas, kidney, and urinary bladder with a focal area of increased radiotracer activity in the right thoracic region in paramedian location (arrow). Computed tomography images (pulmonary window) at the level of the uptake (b and d) and fused PET/CT images (c and e) showing a well-defined moderately SSTR expressing nodular lesion in the right lung lower lobe. Corresponding coronal and axial CT images (f and h) in soft tissue window showing heterogeneous soft tissue lesion with a speck of calcification showing increased radiotracer uptake in the fused PET/CT images (g and i). (PET/CT, positron emission tomography/computed tomography; SSTR, somatostatin receptor)
Fig. 2.
a Gross examination of the right lower lobectomy specimen revealed a sub-pleural well-circumscribed tumor measuring 3.5 cm in maximum dimension. b–d Microscopic examination of the tumor revealed tumor cells to show three different patterns: hemorrhagic characterized by large blood-filled spaces (b, inset; hematoxylin & eosin, × 40), solid (b; hematoxylin & eosin, × 200), and foci of papilla formation (thin arrow, c; hematoxylin & eosin, ×100). Two types of epithelial cells were noted: the cuboidal surface cells (gray thick arrow) and the interstitial round cells (black thick arrow) (c). There were foci of sclerosis with dystrophic calcification (arrowhead) (c). Only an occasional mitotic activity was seen. Immunohistochemically, both the surface cells and the round cells were positive for thyroid transcription factor 1 (TTF-1) (d)
Discussion
PSP is a tumor of the middle-aged adult, with female predominance [2, 3]. In the current report, the patient is a 14-year-old female. The tumor is exceedingly rare in such a young age group. As per our knowledge, only three cases of PSP below the age of 20 years were reported in the literature [2, 8]. Most of the patients are asymptomatic but may present with cough, chest pain, dyspnea, hemoptysis [4]. This patient in the current study presented with hemoptysis and fever.
In our patient, CECT chest showed a homogenous mass of 3.3 cm size with well-defined border, with a focus of calcification within the tumor, which is similar to the CT findings described in the literature [2, 6].
The tumor showed FDG uptake in PET scan and SSTR expression in Ga-68 DOTANOC PET scan in the current case report corresponds with the PET scan findings described in the literature. In a study conducted by Shin et al., 17 patients showed FDG uptake in the tumor, with a mean SUV max of 1.8 [6].
In our patient, we did not perform any preoperative biopsy, as the diagnosis of carcinoid tumor was made based on FDG PET scan and Ga-68 DOTANOC scan and the patient was planned for definitive surgery, although preoperative biopsy is recommended in the literature. Serum chromogranin study was also not done. Lovrenski et al. described resection of the tumor either by open technique or by VATS as the ideal treatment for PSP. Enucleation, lobectomy, and sleeve resection are possible treatment options. They also perform an intraoperative frozen section to confirm the diagnosis [4]. Here, we performed a right lower lobectomy in our patient. Intraoperative frozen section was not performed.
Devouassoux-Shisheboran et al. published an article on 100 cases of PSP. In their study, TTF-1, cytokeratin-7, and CAM 5.2 positivity rate was 90%, 31%, and 17% respectively, although pan-cytokeratin was uniformly negative [2]. In our case report, postoperative histopathological examination revealed a diagnosis of PSP, which showed immunohistochemical positivity for TTF-1, CK-7, while negative for synaptophysin and chromogranin.
Although it is a benign tumor with an excellent prognosis, lymph node metastasis may occur. In 1986, Tanaka et al. first described the presence of lymph node metastasis [7]. The largest study published so far on PSP found lymph node metastasis in one out of 100 patients [2]. Miyagawa-Hayashino et al. reported metastasis in hilar lymph nodes in their four patients [8]. In the current case report, we did not found any enlarged and suspicious lymph nodes during surgery.
Kim et al. performed lobectomy with hilar and interlobar lymph node dissection in one patient. Histopathology report showed the presence of metastasis in both lymph node stations [9]. In the current case, we did not perform lymph node dissection.
There is a case report, in which the patient was treated with external beam radiotherapy (EBRT) only [10]. In another case report, the patient was treated with adjuvant chemotherapy, because of the presence of lymph node metastasis, with 2 years of recurrence-free survival [9]. In the current study, neither EBRT nor adjuvant chemotherapy was considered.
There is no specific follow-up protocol for PSP, so far. We advised our patient to follow up every 3 months with a chest X-ray. A physical examination will be done at each visit. CT scan may be advised if clinically indicated.
Conclusion
PSP is a rare disease, especially in early age groups. It can mimic carcinoid on imaging. Surgery is an established method of treatment for pulmonary sclerosing pneumocytoma.
Author contribution
All authors whose names appear on the submission:
1. Made substantial contributions to the conception or design of the work (Sunil Kumar, Amitabha Mandal, Madiwalesh Chhebbi, Sourabh Nandi, Shipra Agarwal, Sreedharan Thankarajan Arunraj); or the acquisition (Sunil Kumar, Amitabha Mandal), analysis (Madiwalesh Chhebbi, Sourabh Nandi), and interpretation of data (Sunil Kumar, Shipra Agarwal, Sreedharan Thankarajan Arunraj).
2. Drafted the work or revised it critically for important intellectual content (all authors);
3. Approved the version to be published (all authors); and
4. Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved (all authors).
Funding
None.
Declarations
Ethics approval
Not applicable being a clinical case report with no experimental therapies.
Consent to participate
Informed written consent was taken before surgery as per institutional policy.
Consent for publication
Consent for publication was taken from the mother (legal guardian) of the patient.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Amitabha Mandal, Email: amitabha.doc@gmail.com.
Sunil Kumar, Email: dr_sunilk@hotmail.com.
References
- 1.Liebow AA, Hubbell DS. Sclerosing hemangioma (histiocytoma, xanthoma) of the lung. Cancer. 1956;9:53–75. doi: 10.1002/1097-0142(195601/02)9:1<53::AID-CNCR2820090104>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 2.Devouassoux-Shisheboran M, Hayashi T, Linnoila RI, Koss MN, Travis WD. A clinicopathologic study of 100 cases of pulmonary sclerosing hemangioma with immunohistochemical studies: TTF-1 is expressed in both round and surface cells, suggesting an origin from primitive respiratory epithelium. Am J Surg Pathol. 2000;24:906–16. [DOI] [PubMed]
- 3.Illei PB, Rosai J, Klimstra DS. Expression of thyroid transcription factor-1 and other markers in sclerosing hemangioma of the lung. Arch Pathol Lab Med. 2001;125:1335–1339. doi: 10.5858/2001-125-1335-EOTTFA. [DOI] [PubMed] [Google Scholar]
- 4.Lovrenski A, Vasilijević M, Panjković M, et al. Sclerosing pneumocytoma: a ten-year experience at a Western Balkan University Hospital. Medicina (Kaunas). 2019;55:27. [DOI] [PMC free article] [PubMed]
- 5.Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E, Flieder DB, Geisinger K, Hirsch FR, Ishikawa Y, Kerr KM, Noguchi M, Pelosi G, Powell CA, Tsao MS, Wistuba I, WHO Panel The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10:1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 6.Shin SY, Kim MY, Oh SY, et al. Pulmonary sclerosing pneumocytoma of the lung: CT characteristics in a large series of a tertiary referral center. Medicine (Baltimore) 2015;94:e498. doi: 10.1097/MD.0000000000000498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka I, Inoue M, Matsui Y, Oritsu S, Akiyama O, Takemura T, Fujiwara M, Kodama T, Shimosato Y. A case of pneumocytoma (so-called sclerosing hemangioma) with lymph node metastasis. Jpn J Clin Oncol. 1986;16:77–86. [PubMed] [Google Scholar]
- 8.Miyagawa-Hayashino A, Tazelaar HD, Langel DJ, Colby TV. Pulmonary sclerosing hemangioma with lymph node metastases: report of 4 cases. Arch Pathol Lab Med. 2003;127:321–5. [DOI] [PubMed]
- 9.Kim KH, Sul HJ, Kang DY. Sclerosing hemangioma with lymph node metastasis. Yonsei Med J. 2003;44:150–4. [DOI] [PubMed]
- 10.Fayers RW, Lim TS, Havlat MF. Pulmonary sclerosing pneumocytoma (sclerosing haemangioma): radical radiation therapy. J Med Imaging Radiat Oncol. 2016;60:693–5. [DOI] [PubMed]