Abstract
In fleshy fruits, organic acids are the main source of fruit acidity and play an important role in regulating osmotic pressure, pH homeostasis, stress resistance, and fruit quality. The transport of organic acids from the cytosol to the vacuole and their storage are complex processes. A large number of transporters carry organic acids from the cytosol to the vacuole with the assistance of various proton pumps and enzymes. However, much remains to be explored regarding the vacuolar transport mechanism of organic acids as well as the substances involved and their association. In this review, recent advances in the vacuolar transport mechanism of organic acids in plants are summarized from the perspectives of transporters, channels, proton pumps, and upstream regulators to better understand the complex regulatory networks involved in fruit acid formation.
Subject terms: Plant molecular biology, Plant signalling
Introduction
Organic acids influence fleshy fruit acidity and play an important role in regulating osmotic pressure, pH homeostasis, stress resistance, and fruit organoleptic quality1,2. While the predominant organic acid in fleshy fruits varies among species, malic and citric acids are the main acids found in most ripe fruits2–4. In general, the terms “malate” and “citrate” denote the conjugate base of malic and citric acid, respectively, but are used to represent all physiological forms of these organic acids in plants5,6.
Malate and citrate accumulation in plant cells is mostly attributed to their complicated metabolism and vacuolar storage2,7. Several pathways are involved in malate and citrate metabolism in the mesocarp cells of fleshy fruits. Among these, four typical pathways—the tricarboxylic acid cycle in the mitochondrion, the glyoxylate cycle in the glyoxysome, and citrate catabolism and decarboxylation of malate and oxaloacetate in the cytosol—are responsible for malate and citrate metabolism2. Although malate and citrate levels in fleshy fruits are altered by their metabolism7,8, it appears that their accumulation levels are largely determined by their transport from the cytosol to the vacuole2,9–11. Malate and citrate transport across the tonoplast occurs by facilitated diffusion2. This process is mediated by multiple vacuolar transporters, ion channels, and carriers, including the tonoplast dicarboxylate transporter (tDT)12,13, the channels of aluminum-activated malate transporters (ALMTs) ALMT6 and ALMT9/Ma114–18, and the vacuolar citrate/H+ symporter Cit119. Tonoplast proton pumps such as vacuolar-type H+-ATPase (V-ATPase), vacuolar-type H+-PPase (V-PPase), and P-ATPase (PH1, PH5, etc.) drive the facilitated diffusion of malate and citrate into the vacuole2,10,20,21 (Fig. 1). Malate and citrate anions are protonated upon entering an acidic vacuole, and malate and citrate are trapped in the acid form to effectively maintain their concentration gradient across the tonoplast for continuous diffusion into the vacuole2,10,22 (Fig. 1).
Fig. 1. Vacuolar proteins that are involved in the transport of organic acids.
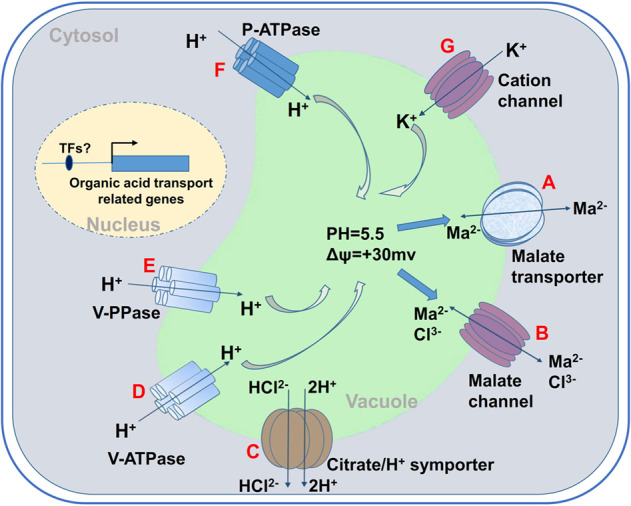
The vacuolar transport of organic acids is mainly mediated by channels, carriers, and proton pumps. Carriers catalyze either the transport of a single solute or the coupled transport of two solutes. Carrier proteins can be involved in promoting diffusion and secondary active transport of counter-electrochemical potential gradients by pro-electrochemical potential gradients. Channel proteins are another class of transporters that differ from carrier proteins in that they are simply gated membrane protein channels for diffusion. Channels act as selective holes through which molecules or ions can diffuse across the membrane. Proton pumps catalyze the coupled transport of a solute with a chemical reaction, which allows X- to utilize the energy released by the hydrolysis of ATP or PPi (ΔGATP (or PPi) <0). These mechanisms allow ionic material (X-) to pass through the biofilm and are governed by the general principle of thermodynamics that the change in free energy (ΔG1-2) of the transport reaction must be negative. During the vacuole transport of organic acids: (1) Diffusion includes simple diffusion mediated by channels (C) (used to transport some cations such as Na+, K+, and Ca2+) and facilitated diffusion mediated by carriers (A, B) (which is the main mode of transport of organic acids from the cytoplasm to the vacuole). (2) Primary active transport is mediated by three types of pumps (D, E, F) on the vacuole. Proton entry into the vacuole provides good conditions for the transport of organic acids: acidic vacuolar pH and a positive electric potential gradient. (3) Secondary active transport is mediated by symports (C), which are mainly involved in the transport of citrate out of the vacuole
Malate and citrate accumulation in mesocarp cells is under both genetic and environmental control. Many agronomic studies have investigated the effects of cultural practices, including mineral fertilization23, irrigation24–26, and thinning24,25, and environmental factors such as drought and high temperature23 on malate and citrate accumulation. However, how these effects control their accumulation is largely unknown. In this review, recent advances in the vacuolar transport mechanism of organic acids in plants have been outlined from the aspects of transporters, channels, proton pumps, and upstream regulators to enhance the understanding of the complex regulatory networks of fruit acid formation.
Vacuolar transporters and channels play a central role in organic acid transport
Vacuolar transporters and channels play a central role in malate transport
Currents of organic acids are strongly inward rectified, thus promoting the transfer of organic acids from the cytosol to the vacuole27,28. These currents through the membranes also have the characteristics of anion selectivity and activation under high membrane potential. Remarkably, vacuolar transporters and channels play a central role in organic acid transport. Malate transport to vacuoles is catalyzed by at least one transporter and several channels29. Malate channel activity is reduced after AttDT is knocked out in Arabidopsis, indicating that AttDT has malate transport activity and is an essential malate channel13.
The ALMT gene family plays a major role in malate transport. ALMT family genes encoding transmembrane proteins as anion channels perform various functions, including inorganic anion transport, aluminum resistance, mineral nutrition absorption, microbial interaction, fruit acid formation, light response, and seed development30. TaALMT1, the first identified malate transporter, is activated by the trivalent aluminum cation in acidic soil and releases the malate anion into the apoplast in wheat plants. Thus, apoplastic malate chelates aluminum cations to reduce damage to cell walls, membranes, and other cellular components31,32. Other ALMTs perform similar functions in Arabidopsis, rapeseed, rye, soybean, and alfalfa33,34. Some of them can also transport organic acids through different membranes. For example, AtALMT1 is a malate-permeable channel expressed in Arabidopsis roots that plays a vital role in resistance to aluminum by secreting malate35. AtALMT3 is a malate transporter involved in low phosphorus-induced malate secretion in Arabidopsis and is mainly located in the plasma membrane36. In rice, OsALMT4 encodes a malic acid-permeable anion channel in the plasma membrane37. Both SlALMT4 and SlALMT5 are expressed in the endoplasmic reticulum of tomato, and SlALMT5 is also expressed in the intima of tomato; both can transport malate under normal electrophysiological conditions in the cell38. In mature tomato seeds, SlALMT5 overexpression showed high concentrations of malate and citrate37. AtALMT6 present in the tonoplast is a calcium-activated malate transporter that mediates malate transport from the cytosol to the vacuole in guard cells. Its activity is regulated by vacuolar pH and the cytoplasmic malate concentration17.
Among the ALMT family members, ALMT9 is the most widely studied gene. AtALMT9 is a member of the ALMT subfamily in Arabidopsis. It is widely expressed in plant cells, such as mesophyll and guard cells, and is insensitive to cytoplasmic Ca2+, but it can be activated by cytoplasmic malate. After AtALMT9 is knocked out, the malate current in the vacuole is inhibited, indicating that AtALMT9 mainly functions as a malate channel14,15,39. VvALMT9, an AtALMT9 homolog in grape, is a vacuolar malate channel that mediates malate and tartrate accumulation in the vacuoles of grape cells. It also has a stronger ability to transport tartrate than AtALMT940. SlALMT9 is a major quantitative trait locus on chromosome 6 in tomato that is responsible for fruit malate accumulation and is considered a malate candidate gene for genotypic variation of fruit quality30. In apple, two ALMT9 homologous genes, Ma1 and Ma2, in the Ma region of the genome are the major sites contributing to malate accumulation41,42.
ALMT9 is different from other ALMT family members, and much attention has been focused on malate transport mainly because it is the major contributor to malate accumulation; the unique C-terminal domain structure of ALMT9 determines its function15,43–45. In Arabidopsis, ALMT9 is a tetramer, and the TMa5 domain of each subunit contributes to the formation of anion channel pores. TMa1 and TMa2 are connected by salt bridges, and these special structures are related to the function of ALMT939. A 1455-bp mutation in the Ma1 open reading frame resulted in an early termination codon that truncated 84 amino acids from the C-terminus of the protein, which may be the direct cause of low acid formation in apple41. This phenomenon suggests that the C-terminal structure of ALMT9 may be closely related to its function in Arabidopsis. In comparison, apple has two ALMT homologous genes—Ma1 and Ma2. Interestingly, only the expression of Ma1, a potential gene for the fruit acidity trait, positively correlated with the fruit acidity level. The locus consists of two alleles—Ma1 and ma1. mal is a truncated protein directly associated with the low malate phenotype46. Both Ma1 and ma1 are located on the tonoplast. In plant cells, Ma1 has higher malate transport activity than ma1 because of its highly conserved C-terminal domain structure. Therefore, the highly conserved C-terminal domain structure in ALMTs is essential for the normal function of Ma1, and any truncation, either natural or artificial, significantly reduces the malate transport activity of this conserved domain16.
In addition to affecting the function of ALMT9 through changes in its C-terminal domain structure, there are other ways to determine the malate transport function of ALMT9 in plants. For example, in tomato, a 3-bp indel in the SlALMT9 promoter region led to a high malate phenotype18.
Vacuolar transporters and channels play a crucial role in citrate transport
Unlike that for malate, citrate accumulation is less affected by vacuolar storage control and is much less constrained by thermodynamic conditions. In most fleshy fruits, vacuolar uptake of citrate trianions possibly occurs by facilitated diffusion through malate channels2. It appears that the transport of citrate to the vacuole is much easier than that of malate at their optimal cytosolic concentration. This is because the thermodynamic conditions are more favorable for citrate uptake than for malate uptake at any vacuolar pH and electric potential gradient. However, the rate at which citrate passes through the malate channel under the control of cytoplasmic concentration suggests that citrate accumulation in vacuoles is mainly controlled by metabolism2,47. Citrate transport from the cytosol to the vacuole is accompanied by a large influx of protons48,49. This proton influx leads to vacuolar acidification and provides a strong driving force for increased vacuolar uptake of citrate, thereby maintaining the vacuolar buffering capacity and acidic pH environment2,19,50. This process is mainly mediated by V-ATPase48,49. Citrate transport to the vacuole is competitively inhibited by other organic acids, such as malate, because organic acids cross the tonoplast by the same channels or transporters6. Citrate accumulation and vacuolar acidification are tightly regulated throughout fruit development and vary among citrate-rich fruit varieties. Usually, lower vacuolar pH values result in higher citrate accumulation in vacuoles2,51.
Mechanisms for citrate transport from the cytosol to the vacuole have been elucidated by the manipulation of anion channels and ATP-dependent transporters5,6,19,52. AttDT may transport citrate to the vacuole; however, it is not the main tonoplast citrate carrier in Arabidopsis13. In citrus plants, the citrate transporter 1 gene CsCit1, encoding a novel vacuolar citrate/H+ symporter, mediates CitH2−- and CitH2−-dependent H+ efflux from the vacuole and maintains vacuolar acidic pH and citric acid homeostasis19. The multidrug and toxic compound extrusion (MATE) gene AtMATE, encoding an Al-activated citrate transporter in Arabidopsis, contributes to aluminum-activated root citrate exudation53. In addition, Lin et al.54 showed that the dicarboxylate carrier CitDIC and the cation/H+ exchanger CitCHX are involved not only in citrate degradation during fruit development but also in hot air-triggered citrate reduction after harvest. Nevertheless, studies on the regulation of citrate transport are largely limited in comparison with those on the regulation of malate transport.
Proton pumps provide favorable conditions for organic acid transport to vacuoles
Apart from the large number of transporters and channels in the tonoplast, proton pumps also help in the transport of organic acids from the cytosol to the vacuole. Proton pumping into the vacuole often produces an acidic vacuolar pH and positive electric potential gradient2. All three proton pumps, V-ATPase, V-PPase, and P-type ATPase, are present in the vacuoles of fruit cells.
Widely characterized V-ATPase and V-PPase in several plant species
Vacuoles contain two proton pumps—V-ATPase and V-PPase. Both proton pumps can effectively acidify the vacuolar lumen. V-ATPase and V-PPase are localized to the tonoplast, but their contributions to proton pumping vary during fruit development22,55–58. V-ATPase is the primary proton pump in vacuoles, but V-PPase has higher tonoplast activity than V-ATPase in some crassulacean acid metabolism species59. V-PPase is enriched during the early fruit developmental stage, but its function decreases and V-ATPase dominates during the fruit ripening stage2,60. Maeshima61 proposed that the high V-PPase activity in young tissues is an inhibitor of several polymerization reactions, such as RNA and starch syntheses, by scavenging more pyrophosphate (PPi). In mature fruits, PPi production may decrease since its synthesis gradually decreases, whereas ATP is constantly supplied by cellular respiration.
The main functions of V-ATPase are to transport protons to vacuoles by ATP hydrolysis and to generate pH and potential energy gradients inside and outside vacuoles to provide suitable conditions for the transport of metabolites such as organic acids. V-ATPase is a highly conserved and sophisticated complex that contains the peripheral subcomplex V1 and the membrane-bound subcomplex V062,63. Subcomplex V1 is located in the cytosol and comprises eight subunits from A to H, which are mainly responsible for ATP hydrolysis. In contrast, subcomplex V0 is embedded in the tonoplast and comprises six subunits—a, b, c′ (not present in plants), c′′, d, and e—which are mainly responsible for H+ translocation from the cytosol to the vacuolar lumen64. The functions of V-ATPase are quite diverse and have been well described. V-ATPase is involved in energizing secondary transport, vacuolar acidification, ion homeostasis, and stress tolerance in several plant species62,63,65–67.
Compared to V-ATPase, V-PPase is a homodimer of a single polypeptide that uses the energy of the PPi phosphoanhydride bond to drive proton transport across the tonoplast; unlike other proton pumps, PPi is its only source of energy68,69. Similar to V-ATPase, V-PPase performs diverse functions, such as secondary metabolite transport and stress tolerance70–73.
P-type ATPases are involved in proton transport and vacuolar acidification
P-type ATPases comprise a novel vacuolar proton pump family involved in proton transport and vacuolar acidification, distinct from V-ATPase and V-PPase. These ATPases are primary transporters that are energized by ATP hydrolysis with a series of specificities for small cations and phospholipids and are characterized structurally as having a single catalytic subunit, 8–12 transmembrane segments, N and C termini exposed to the cytoplasm, and a large central cytoplasmic domain, including the phosphorylation and ATP binding sites74,75. In plants, these ATPases are composed of five major evolutionarily associated subfamilies, P1–P5, which are classified by the ions they transport75. Among them, P3 subfamily ATPases are responsible for energizing the electrochemical gradient used as the driving force of secondary transporters74,76.
In petunia, PH5 encodes a tonoplast-localized P3A-ATPase proton pump that interacts with the P3B-ATPase PH1 complex to acidify the vacuolar lumen of petal cells, thereby affecting petal color42,76,77. Interestingly, PH5 is the only P3A subfamily ATPase that is located on the tonoplast (all others are located on the plasma membrane) and independently exhibits strong proton transport activity. In contrast, PH1 does not have proton transport activity but is necessary for maintaining proton pump activity. It can form a heteromeric pump with PH5 that hyperacidifies the central vacuole of epidermal cells in petunia petals42,77. The interaction between PH1 and PH5 can reduce the stoichiometric value of H+/ATP from 1.0 to 0.5 H+/ATP, resulting in vacuolar hyperacidification78. In addition, protein trafficking from vacuolinos to the central vacuole is impaired by the misexpression of either the PH1 or the PH5 component of the heteromeric PH1–PH5 pump79.
The regulation of pH by P3A-ATPase/P3B-ATPase exists not only in petunia but also in other angiosperms. However, independent losses of these homologs occur in many angiosperms76. PH5 homologs are found in both angiosperms and gymnosperms. These homologs have also been found in some fruits, and their mechanism of action is currently being explored. In apple, MdPH1 and MdPH5 have been identified and shown to be involved in vacuolar acidification and malate accumulation9. Their homologs, CitPH1 and CitPH5, are expressed in the fruits of sour lemon, orange, pummelo, and rangpur lime; however, their expression is significantly decreased in low acid varieties80. Additionally, a candidate gene for fruit acidity in apple, Ma10, encodes a P3A-ATPase proton pump, which promotes malate uptake into the vacuole and facilitates vacuolar acidification21.
To function, P-type ATPases either require other protein complexes or influence the activities of these protein complexes. Changes in PH1–PH5 activity caused by lower vacuolar pH triggered the collapse of the V-ATPase complex or caused other structural changes81. However, the regulatory mechanism between P-type ATPase and V-ATPase activity remains elusive. In addition, only P-type ATPase, but not V-ATPase, is used to acidify vacuoles in some specific tissues, such as petunia petals. In addition, PH1 activates PH5 through a still unknown mechanism.
Reverse transporters promote electrochemical gradient formation inside and outside vacuoles
In addition to malate transporters, channels, and proton pumps, some cation channels or cation/H+ reverse transporters (Fig. 1) also contribute to the formation of an electrochemical gradient inside and outside the vacuole. The transport of Na+ or K+ is an example of secondary transporter assistance. Na+/H+ (NHX) and K+/H+ exchangers are responsible for vacuolar alkalization, thereby changing the petal color from purple to blue82. NHX gene expression is associated with a change in vacuolar pH. For example, the Na+/H+ antiporters NHX1 and NHX2 control K+ homeostasis and vacuolar pH in Arabidopsis83. Plant NHX proteins passively exchange H+ along with K+ and Na+, consuming the H+ gradient during the process84. The protein InNHX1 mediates H+ outflow from the vacuolar lumen, and the other NHX proteins, including InNHX2, also participate in vacuolar alkalinization and changing petal color by using the K+ gradient rather than the H+ gradient in Japanese morning glory85,86. In addition, the increased expression of the MATE family gene HvAACT1 in wheat and barley was associated with increased citrate efflux from root apices87.
Upstream regulators that are involved in vacuolar transport of organic acids
Transcriptional regulation of vacuolar transport of organic acids
Regulation of organic acid transporters and proton pumps involves a complex gene regulatory network. Transcriptional regulation is one of the most common and direct ways to regulate malate follicle transporters and proton pumps. These include transcription factors such as MYB, bHLH, WRKY, and ERF family members (Table 1). These transcription factors play an important role in organic acid transport. Specifically, transcription factors can activate or inhibit the expression of transporters and proton pumps, eventually promoting or impeding the whole process of organic acid transport.
Table 1.
Upstream regulators that are involved in vacuolar transport of organic acids
| Family classification | Protein name | Positive/negative regulator | Regulatory modes | Plant species |
|---|---|---|---|---|
| MYB transcription factors | MdMYB1 | Positive | Transcriptional regulation | Malus domestica Borkh |
| MdMYB73 | Positive | Malus domestica Borkh | ||
| CrMYB73 | Positive | Citrus reticulata Blanco | ||
| VvMYB5a | Positive | Vitis vinifera | ||
| VvMYB5b | Positive | Vitis vinifera | ||
| PhPH4 | Positive | Petunia hybrid | ||
| GmPH4 | Positive | Glycine Max | ||
| WRKY transcription factors | PhPH3 | Positive | Petunia hybrid | |
| VvWRKY26 | Positive | Vitis vinifera | ||
| SlWRKY42 | Negative | Solanum lycopersicum | ||
| AtWRKY46 | Negative | Arabidopsis thaliana | ||
| bHLH transcription factors | CitAN1 | Positive | Citrus reticulata Blanco | |
| VvMYC1 | Positive | Vitis vinifera | ||
| MdCIbHLH1 | Positive | Malus domestica Borkh | ||
| MdbHLH3 | Positive | Malus domestica Borkh | ||
| MdbHLH49 | Negative | Malus domestica Borkh | ||
| ERF transcription factor | CitERF13 | Positive | Citrus reticulata Blanco | |
| WD40 protein | CitAN11 | Positive | Citrus reticulata Blanco | |
| Protein kinases | AtWNK | Positive | Post-translational modification | Arabidopsis thaliana |
| MdSOS2L1 | Positive | Malus domestica Borkh | ||
| AtPKA | Positive | Arabidopsis thaliana | ||
| MdHXK1 | Positive | Malus domestica Borkh | ||
| E3 ligase | MdSIZ1 | Positive | Malus domestica Borkh | |
| MdPUB29 | Negative | Malus domestica Borkh | ||
| MdCOP1 | Negative | Malus domestica Borkh | ||
| Small auxin-up RNA | MdSAUR37 | Positive | Malus domestica Borkh | |
| Protein phosphatase | MdPP2CH | Negative | Malus domestica Borkh | |
| BTB-BACK-TAZ domain protein | MdBT2 | Negative | Malus domestica Borkh |
The MYB transcription factor family is one of the largest transcription factor families in plants. Most members of this family, which is involved in organic acid transport, are plant-specific R2R3-MYB transcription factors, which also play important roles in plant growth and development and in biotic and abiotic responses88. In terms of fruit quality, MYB transcription factors can directly regulate the transcriptional activities of organic acid transporters and metabolic enzymes, resulting in organic acid accumulation and vacuolar acidification in fleshy fruit cells. In apple, MdMYB1, MdMYB44, and MdMYB73 are distant relatives89,90. MdMYB1, MdMYB44, and MdMYB73 can regulate the transcriptional activities of the malate transporter and proton pump to control malate accumulation and vacuolar acidification9,91,92 (Fig. 2). However, MdMYB1 and MdMYB73 are positive regulators, whereas MdMYB44 is a negative regulator. Moreover, their downstream target genes are different. The direct downstream target genes of MdMYB1 are the V-ATPase subunit genes MdVHA-B1 and MdVHA-E, V-PPase gene MdVHP1, and tDT gene MdtDT91 (Fig. 2). In contrast, MdMYB73 directly activates the expression of the V-ATPase subunit gene MdVHA-A, V-PPase gene MdVHP1, and ALMT gene MdALMT9 but not the expression of the genes MdVHA-B1, MdVHA-E, and MdtDT9 (Fig. 2). However, MdMYB44 negatively regulates malate accumulation in apple by repressing the promoter activity of the V-ATPase subunit genes MdVHA-A3 and MdVHA-D2, P-type ATPase gene Ma10, and ALMT gene MdALMT992 (Fig. 2). In citrus plants, CrMYB73, homologous to apple MdMYB73, confers an increase in citrate accumulation, but its downstream target genes are unknown93. In addition, petunia PhPH4 is an R2R3-MYB transcription factor that plays a similar role as grape VvMYB5a and VvMYB5b in the regulation of citrate accumulation; both can activate the expression of the downstream genes PH1 and PH5, thus acidifying vacuoles94–96. Similarly, the R2R3-MYB transcription factor GmPH4 is also involved in vacuolar acidification by directly regulating the expression of the P3A-type ATPase gene GmPH5 in soybean petals97.
Fig. 2. Upstream regulators that are involved in the vacuolar transport of malate and vacuolar acidification in apple parenchyma cells.
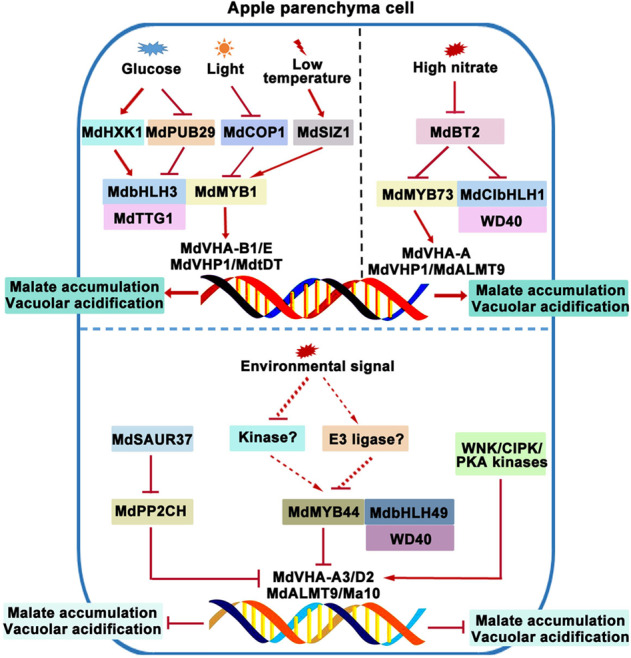
The graphs above the blue dotted line show the positive regulators of MYB-bHLH-WD40 (MBW) complexes, including MdMYB1-MdbHLH3-MdTTG1 and MdMYB73-MdCIbHLH1-WD40, that are involved in malate accumulation and vacuolar acidification; the stabilities of these two MBW complexes can be affected by posttranslational modifications, such as phosphorylation and ubiquitination, in response to environmental stimuli. The graphs below the blue dotted line reveal the negative regulators of the MBW complex or protein phosphatases and kinases involved in malate accumulation and vacuolar acidification
Plant-specific transcription factors containing WRKY domains are also important for organic acid transport and vacuolar acidification98,99. WRKY transcription factors can regulate downstream transporters by binding to specific W-box cis-elements in promoters. Fittingly, W-box cis-elements are highly enriched in the promoters of ALMT family genes, which are major contributors to malate transport and vacuolar acidification. For example, WRKY46 functions as a transcriptional repressor of ALMT1 by directly binding the specific W-box to its promoter, thus regulating aluminum-induced malate secretion in Arabidopsis100. An indel in the SlALMT9 promoter disrupts a W-box binding site, which prevents the binding of the WRKY transcription repressor SlWRKY42, thereby decreasing the repression of SlALMT9 expression and facilitating high malate accumulation in tomato18.
Apart from MYB and WRKY transcription factors, other transcription factors, such as bHLH and ERF, are also involved in the regulation of organic acid accumulation and vacuolar acidification. By analyzing the fruits of sour lemon, orange, pummelo, and rangpur lime, Strazzer et al.80 found that inactivating mutations in CitAN1, which encodes a bHLH transcription factor, led to the decreased expression of CitPH1 and CitPH5, thereby resulting in the vacuolar hyperacidification of juice vesicle cells (Fig. 3). In apple, the bHLH transcription factor MdbHLH3 directly regulates the expression of the cytosolic malate dehydrogenase gene MdcyMDH to coordinate carbohydrate allocation and malate accumulation101. The citrus transcription factor CitERF13 regulates citrate accumulation by directly activating the vacuolar proton pump gene CitVHA-c4102 (Fig. 3), whereas the CitWRKY1-CitNAC62 complex contributes to citric acid degradation in citrus fruits, potentially via the modulation of CitAco3103. An EIN3-like transcription factor is considered the regulator of ALMT1-like proteins in apple104.
Fig. 3. Upstream regulators that are involved in vacuolar acidification in petunia petal, citrus and grapevine fruit cells.
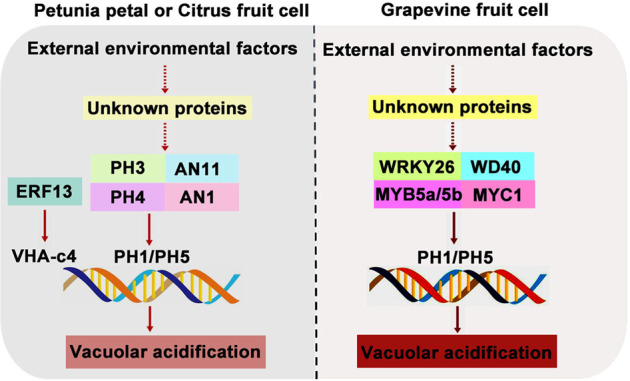
The graph to the left of the black dotted line shows the WRKY- MYB-bHLH-WD40 (WMBW) complex and ERF transcription factor that are involved in vacuolar acidification in petunia petal and citrus fruit cells. The graph to the right of the black dotted line reveals the WMBW complex that is involved in vacuolar acidification in grapevine fruit cells
Additionally, these transcription factors can form a complex and cooperate in acidifying vacuoles, a function they cannot perform independently. The MYB-bHLH-WD40 (MBW) complex performs an important function in organic acid accumulation and vacuolar acidification. As mentioned earlier, the MYB transcription factors MdMYB1, MdMYB44, and MdMYB73 affect malate accumulation and vacuolar acidification by regulating the activities of vacuolar proton pumps and malate transporters in apple9,91,92 (Fig. 2). These regulatory activities depend on MBW complex formation. MdbHLH3, MdbHLH49, and MdCIbHLH1 interact with MdMYB1, MdMYB44, and MdMYB73, respectively, and enhance corresponding MYB transcription factor activities, further regulating the activities of downstream genes, including malate transporters and vacuolar proton pumps9,91,92,105 (Fig. 2). In petunia petals, the PH4 (MYB)-AN1 (bHLH)-AN11 (WD40) complex controls vacuolar acidification by directly regulating PH5 transcription106 (Fig. 3). Similarly, CitPH1 and CitPH5 are transcriptionally activated by the CitPH4 (MYB)-CitAN1 (bHLH)-CitAN11 (WD40) complex and cause hyperacidification of citrus fruits80 (Fig. 3). Interestingly, the WRKY transcription factor forms a more complicated WMBW complex with the MBW complex to affect vacuolar acidification. For example, PH3 is a target gene of the PH4-AN1-AN11 complex; it encodes a WRKY protein that can bind to AN11 and is required for the transcriptional activation of PH5 in petunia petals as part of a feed-forward loop with the PH4-AN1-AN11 complex78,106–108 (Fig. 3). Likewise, the CitPH3 (WRKY)-CitPH4 (MYB)-CitAN1 (bHLH)-CitAN11 (WD40) complex in citrus plants regulates the expression of its target genes CitPH1 and CitPH5 to control fruit acidity and titratable acid content80 (Fig. 3). In grape, VvWRKY26 enhances the expression of the target genes VvPH1 and VvPH5 induced by the VvMYB5a/b-VvMYC1 (bHLH)-WD40 (unknown) complex94,109 (Fig. 3). Collectively, these reports suggest that the transcriptional regulation of the vacuolar transport of organic acids is a complex regulatory process in plants, especially in fleshy fruits.
Posttranslational modification mediates the vacuolar transport of organic acids
Posttranslational modification, such as phosphorylation or dephosphorylation and ubiquitination, plays an essential role in the vacuolar transport of organic acids. Remarkably, posttranslationally modified proteins can be either proton pumps and organic acid transporters or their upstream transcriptional regulators.
Protein kinases, such as WNK, CIPK, and PKA, modulate organic acid accumulation and vacuolar acidification by directly phosphorylating different V-ATPase subunits110–113 (Fig. 2). MdPP2CH of the protein phosphatase 2CH subfamily inactivated three vacuolar H+-ATPases (i.e., MdVHA-A3, MdVHA-B2, and MdVHA-D2) and one ALMT (i.e., MdALMTII) via dephosphorylation and reduced fruit malate accumulation. Its dephosphatase activity was inhibited by the small auxin-up RNA MdSAUR37 in apple114 (Fig. 2). In addition, Ucc1 (ubiquitination of citrate synthase in the glyoxylate cycle) is a recently characterized F-box protein that promotes the proteasomal degradation of citrate synthase 2 (Cit2) in the glyoxylate cycle115. Other enzymes associated with the glyoxylate cycle or the gluconeogenic pathways, such as malate dehydrogenase (Mdh2), isocitrate lyase (Icl1), and phosphoenolpyruvate carboxykinase (Pck1), are also regulated by either the vacuole import and degradation pathway or the glucose-induced degradation-deficient pathway115. These studies provide important insights into the transcriptional regulation and direct posttranslational modification of organic acid-related transporters, proton pumps, or key enzymes.
In addition to direct posttranslational modification of proton pumps and organic acid transporters in fleshy fruits, posttranslational modification of their upstream transcriptional regulators is also common. Among these modifications, the posttranslational modification of the MBW complex is the most studied in fleshy fruits, especially in apple (Fig. 2). Apple MdCOP1 ubiquitin E3 ligase interacts with MdMYB1 to regulate malate accumulation and vacuolar acidification91,116. The glucose sensor MdHXK1 and high glucose-inhibited U-box-type E3 ubiquitin ligase MdPUB29 phosphorylate and ubiquitinate MdbHLH3, respectively, affecting vacuolar acidification and malate concentration by regulating their downstream malate-associated genes in apple91,105,117,118. The BTB-BACK-TAZ domain protein MdBT2-mediated ubiquitination of MdCIbHLH1 and MdMYB73 negatively regulates malate accumulation and vacuolar acidification119,120. The small ubiquitin-like modifier E3 ligase MdSIZ1 targets MdbHLH104 to regulate plasma membrane H+-ATPase activity and proton efflux121 and acidifies vacuoles by sumoylating MdMYB1 and its downstream malate-related genes91,122.
Conclusions and perspectives
Organic acids affect fruit quality and participate in the evolution and reproduction of fleshy fruits123. The transport of organic acids from the cytosol to the vacuole and their subsequent storage and potential reutilization are complex processes. Although the vacuolar transport mechanism of organic acids has been partially elucidated in some species, more studies are needed to explore and identify the transporters, proton pumps, and upstream regulators of organic acids that are responsible for organic acid accumulation and vacuolar acidification. Isolation of vacuole and tonoplast proteins, liquid chromatography–tandem mass spectrometry assays, and functional identification are effective methods to explore the mechanisms associated with organic acid accumulation and vacuolar acidification. In conclusion, further studies on the identification of organic acid-associated proteins, the regulation of their function, and the additional roles of organic acids in fruit physiology are needed in the future.
Acknowledgements
The authors would like to thank TopEdit (www.topeditsci.com) for linguistic assistance during the preparation of this manuscript. This project was supported by grants from the National Key Research and Development Program of China (2018YFD1000200); the National Natural Science Foundation of China (32122080, 31972375, 31902049); and Shandong Province (ZR2020YQ25).
Author contributions
X.Y.H., C.K.W., and Y.W.Z. investigation, writing—original draft. D.G.H. and C.H.S. visualization, writing—review & editing, supervision, funding acquisition.
Competing interests
The authors declare no competing interests.
Contributor Information
Cui-Hui Sun, Email: suncuihui@163.com.
Da-Gang Hu, Email: fap_296566@163.com.
References
- 1.Harker F, et al. Sensory interpretation of instrumental measurements 2: sweet and acid taste of apple fruit. Postharvest Biol. Tec. 2002;24:241–250. doi: 10.1016/S0925-5214(01)00157-0. [DOI] [Google Scholar]
- 2.Etienne A, Génard M, Lobit P, Mbeguié-A-Mbéguié D, Bugaud C. What controls fleshy fruit acidity? A review of malate and citrate accumulation in fruit cells. J. Exp. Bot. 2013;64:1451–1469. doi: 10.1093/jxb/ert035. [DOI] [PubMed] [Google Scholar]
- 3.Colaric M, Veberic R, Solar A, Hudina M, Stampar F. Phenolic acids, syringaldehyde, and juglone in fruits of different cultivars of Juglans regia L. J. Agric. Food Chem. 2005;53:6390–6396. doi: 10.1021/jf050721n. [DOI] [PubMed] [Google Scholar]
- 4.Osorio S, Fernie AR. Biochemistry of fruit ripening. Mol. Biol. Biochem. Fruit. Ripening. 2013;1:1–20. doi: 10.12966/bmb.06.01.2013. [DOI] [Google Scholar]
- 5.Oleski N, Mahdavi P, Bennett AB. Transport properties of the tomato fruit tonoplast: II. Citrate transport. Plant Physiol. 1987;84:997–1000. doi: 10.1104/pp.84.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rentsch D, Martinoia E. Citrate transport into barley mesophyll vacuoles—comparison with malate-uptake activity. Planta. 1991;184:532–537. doi: 10.1007/BF00197903. [DOI] [PubMed] [Google Scholar]
- 7.Sweetman C, Deluc LG, Cramer GR, Ford CM, Soole KL. Regulation of malate metabolism in grape berry and other developing fruits. Phytochemistry. 2009;70:1329–1344. doi: 10.1016/j.phytochem.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Centeno DC, et al. Malate plays a crucial role in starch metabolism, ripening, and soluble solid content of tomato fruit and affects postharvest softening. Plant Cell. 2011;23:162–184. doi: 10.1105/tpc.109.072231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu DG, et al. The R2R3‐MYB transcription factor Md MYB 73 is involved in malate accumulation and vacuolar acidification in apple. Plant J. 2017;91:443–454. doi: 10.1111/tpj.13579. [DOI] [PubMed] [Google Scholar]
- 10.Martinoia E. Vacuolar transporters–companions on a longtime journey. Plant Physiol. 2018;176:1384–1407. doi: 10.1104/pp.17.01481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ratajczak R, Lüttge U, Gonzalez P, Etxeberria E. Malate and malate-channel antibodies inhibit electrogenic and ATP-dependent citrate transport across the tonoplast of citrus juice cells. J. Plant Physiol. 2003;160:1313–1317. doi: 10.1078/0176-1617-01147. [DOI] [PubMed] [Google Scholar]
- 12.Emmerlich V, et al. The plant homolog to the human sodium/dicarboxylic cotransporter is the vacuolar malate carrier. P. Natl Acad. Sci. USA. 2003;100:11122–11126. doi: 10.1073/pnas.1832002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurth MA, et al. Impaired pH homeostasis in Arabidopsis lacking the vacuolar dicarboxylate transporter and analysis of carboxylic acid transport across the tonoplast. Plant Physiol. 2005;137:901–910. doi: 10.1104/pp.104.058453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Angeli A, Zhang J, Meyer S, Martinoia E. AtALMT9 is a malate-activated vacuolar chloride channel required for stomatal opening in Arabidopsis. Nat. Commun. 2013;4:1–10. doi: 10.1038/ncomms2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovermann P, et al. The Arabidopsis vacuolar malate channel is a member of the ALMT family. Plant J. 2007;52:1169–1180. doi: 10.1111/j.1365-313X.2007.03367.x. [DOI] [PubMed] [Google Scholar]
- 16.Li C, et al. Apple ALMT9 requires a conserved C-terminal domain for malate transport underlying fruit acidity. Plant Physiol. 2020;182:992–1006. doi: 10.1104/pp.19.01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer S, et al. Malate transport by the vacuolar AtALMT6 channel in guard cells is subject to multiple regulation. Plant J. 2011;67:247–257. doi: 10.1111/j.1365-313X.2011.04587.x. [DOI] [PubMed] [Google Scholar]
- 18.Ye J, et al. An InDel in the promoter of Al-activated malate transporter 9 selected during tomato domestication determines fruit malate contents and aluminum tolerance. Plant Cell. 2017;29:2249–2268. doi: 10.1105/tpc.17.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimada T, Nakano R, Shulaev V, Sadka A, Blumwald E. Vacuolar citrate/H+ symporter of citrus juice cells. Planta. 2006;224:472–480. doi: 10.1007/s00425-006-0223-2. [DOI] [PubMed] [Google Scholar]
- 20.Cohen S, et al. The PH gene determines fruit acidity and contributes to the evolution of sweet melons. Nat. Commun. 2014;5:1–9. doi: 10.1038/ncomms5026. [DOI] [PubMed] [Google Scholar]
- 21.Ma B, et al. A Ma10 gene encoding P‐type ATPase is involved in fruit organic acid accumulation in apple. Plant Biotechnol. J. 2019;17:674–686. doi: 10.1111/pbi.13007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinoia E, Maeshima M, Neuhaus HE. Vacuolar transporters and their essential role in plant metabolism. J. Exp. Bot. 2007;58:83–102. doi: 10.1093/jxb/erl183. [DOI] [PubMed] [Google Scholar]
- 23.Spironello A, Quaggio JA, Teixeira LAJ, Furlani PR, Sigrist JMM. Pineapple yield and fruit quality effected by NPK fertilization in a tropical soil. Rev. Bras. Frutic. 2004;26:155–159. doi: 10.1590/S0100-29452004000100041. [DOI] [Google Scholar]
- 24.Wu BH, Genard M, Lescourret F, Gomez L, Li SH. Influence of assimilate and water supply on seasonal variation of acids in peach (cv Suncrest) J. Sci. Food Agr. 2002;82:1829–1836. doi: 10.1002/jsfa.1267. [DOI] [Google Scholar]
- 25.Lechaudel M, Joas J, Caro Y, Genard M, Jannoyer M. Leaf: fruit ratio and irrigation supply affect seasonal changes in minerals, organic acids and sugars of mango fruit. J. Sci. Food Agr. 2005;85:251–260. doi: 10.1002/jsfa.1968. [DOI] [Google Scholar]
- 26.Thakur A, Singh Z. Responses of ‘Spring Bright’and ‘Summer Bright’ nectarines to deficit irrigation: fruit growth and concentration of sugars and organic acids. Sci. Hortic. 2012;135:112–119. doi: 10.1016/j.scienta.2011.12.013. [DOI] [Google Scholar]
- 27.Cerana R, Giromini L, Colombo R. Malate-regulated channels permeable to anions in vacuoles of Arabidopsis thaliana. Funct. Plant Biol. 1995;22:115–121. doi: 10.1071/PP9950115. [DOI] [Google Scholar]
- 28.Epimashko S, Meckel T, Fischer‐Schliebs E, Lüttge U, Thiel G. Two functionally different vacuoles for static and dynamic purposes in one plant mesophyll leaf cell. Plant J. 2004;37:294–300. doi: 10.1046/j.1365-313X.2003.01958.x. [DOI] [PubMed] [Google Scholar]
- 29.Carter C, et al. The vegetative vacuole proteome of Arabidopsis thaliana reveals predicted and unexpected proteins. Plant Cell. 2004;16:3285–3303. doi: 10.1105/tpc.104.027078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, Zhou M. The ALMT gene family performs multiple functions in plants. Agronomy. 2018;8:20. doi: 10.3390/agronomy8020020. [DOI] [Google Scholar]
- 31.Ligaba A, Katsuhara M, Ryan PR, Shibasaka M, Matsumoto H. The BnALMT1 and BnALMT2 genes from rape encode aluminum-activated malate transporters that enhance the aluminum resistance of plant cells. Plant Physiol. 2006;142:1294–1303. doi: 10.1104/pp.106.085233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sasaki T, et al. A wheat gene encoding an aluminum‐activated malate transporter. Plant J. 2004;37:645–653. doi: 10.1111/j.1365-313X.2003.01991.x. [DOI] [PubMed] [Google Scholar]
- 33.Chen Q, et al. Overexpression of MsALMT1, from the aluminum-sensitive Medicago sativa, enhances malate exudation and aluminum resistance in tobacco. Plant Mol. Biol. Rep. 2013;31:769–774. doi: 10.1007/s11105-012-0543-2. [DOI] [Google Scholar]
- 34.Liang C, et al. Low pH, aluminum, and phosphorus coordinately regulate malate exudation through GmALMT1 to improve soybean adaptation to acid soils. Plant Physiol. 2013;161:1347–1361. doi: 10.1104/pp.112.208934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobayashi Y, et al. Characterization of AtALMT1 expression in aluminum-inducible malate release and its role for rhizotoxic stress tolerance in Arabidopsis. Plant Physiol. 2007;145:843–852. doi: 10.1104/pp.107.102335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maruyama H, Sasaki T, Yamamoto Y, Wasaki J. AtALMT3 is involved in malate efflux induced by phosphorus deficiency in Arabidopsis thaliana root hairs. Plant Cell Physiol. 2019;60:107–115. doi: 10.1093/pcp/pcy190. [DOI] [PubMed] [Google Scholar]
- 37.Liu J, Zhou M, Delhaize E, Ryan PR. Altered expression of a malate-permeable anion channel, OsALMT4, disrupts mineral nutrition. Plant Physiol. 2017;175:1745–1759. doi: 10.1104/pp.17.01142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sasaki T, et al. Two members of the aluminum-activated malate transporter family, SlALMT4 and SlALMT5, are expressed during fruit development, and the overexpression of SlALMT5 alters organic acid contents in seeds in tomato (Solanum lycopersicum) Plant Cell Physiol. 2016;57:2367–2379. doi: 10.1093/pcp/pcw157. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J, Baetz U, Krügel U, Martinoia E, De Angeli A. Identification of a probable pore-forming domain in the multimeric vacuolar anion channel AtALMT9. Plant Physiol. 2013;163:830–843. doi: 10.1104/pp.113.219832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Angeli A, et al. The vacuolar channel VvALMT9 mediates malate and tartrate accumulation in berries of Vitis vinifera. Planta. 2013;238:283–291. doi: 10.1007/s00425-013-1888-y. [DOI] [PubMed] [Google Scholar]
- 41.Bai Y, et al. A natural mutation-led truncation in one of the two aluminum-activated malate transporter-like genes at the Ma locus is associated with low fruit acidity in apple. Mol. Genet. Genomics. 2012;287:663–678. doi: 10.1007/s00438-012-0707-7. [DOI] [PubMed] [Google Scholar]
- 42.Verweij W, et al. An H+ P-ATPase on the tonoplast determines vacuolar pH and flower colour. Nat. Cell Biol. 2008;10:1456–1462. doi: 10.1038/ncb1805. [DOI] [PubMed] [Google Scholar]
- 43.Ligaba A, Kochian L, Piñeros M. Phosphorylation at S384 regulates the activity of the TaALMT1 malate transporter that underlies aluminum resistance in wheat. Plant J. 2009;60:411–423. doi: 10.1111/j.1365-313X.2009.03964.x. [DOI] [PubMed] [Google Scholar]
- 44.Furuichi T, et al. An extracellular hydrophilic carboxy‐terminal domain regulates the activity of TaALMT1, the aluminum‐activated malate transport protein of wheat. Plant J. 2010;64:47–55. doi: 10.1111/j.1365-313X.2010.04309.x. [DOI] [PubMed] [Google Scholar]
- 45.Mumm P, et al. C-terminus-mediated voltage gating of Arabidopsis guard cell anion channel QUAC1. Mol. Plant. 2013;6:1550–1563. doi: 10.1093/mp/sst008. [DOI] [PubMed] [Google Scholar]
- 46.Ma, B. et al. Genes encoding aluminum‐activated malate transporter II and their association with fruit acidity in apple. Plant Genome8, plantgenome2015.03.0016 (2015). [DOI] [PubMed]
- 47.Gout E, Bligny R, Pascal N, Douce R. 13C nuclear magnetic resonance studies of malate and citrate synthesis and compartmentation in higher plant cells. J. Biol. Chem. 1993;268:3986–3992. doi: 10.1016/S0021-9258(18)53568-7. [DOI] [PubMed] [Google Scholar]
- 48.Aprile A, et al. Expression of the H+-ATPase AHA10 proton pump is associated with citric acid accumulation in lemon juice sac cells. Funct. Integr. Genomics. 2011;11:551–563. doi: 10.1007/s10142-011-0226-3. [DOI] [PubMed] [Google Scholar]
- 49.Müller ML, Irkens-Kiesecker U, Kramer D, Taiz L. Purification and reconstitution of the vacuolar H+-ATPases from lemon fruits and epicotyls. J. Biol. Chem. 1997;272:12762–12770. doi: 10.1074/jbc.272.19.12762. [DOI] [PubMed] [Google Scholar]
- 50.Müller M, Taiz L. Regulation of the lemon-fruit V-ATPase by variable stoichiometry and organic acids. J. Membr. Sci. 2002;185:209. doi: 10.1007/s00232-001-0124-z. [DOI] [PubMed] [Google Scholar]
- 51.Echeverria E, Burns JK. Vacuolar acid hydrolysis as a physiological mechanism for sucrose breakdown. Plant Physiol. 1989;90:530–533. doi: 10.1104/pp.90.2.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Canel C, Bailey-Serres JN, Roose ML. In vitro [14C] citrate uptake by tonoplast vesicles of acidless citrus juice cells. J. Am. Soc. Hortic. Sci. 1995;120:510–514. doi: 10.21273/JASHS.120.3.510. [DOI] [Google Scholar]
- 53.Liu J, Magalhaes JV, Shaff J, Kochian LV. Aluminum‐activated citrate and malate transporters from the MATE and ALMT families function independently to confer Arabidopsis aluminum tolerance. Plant J. 2009;57:389–399. doi: 10.1111/j.1365-313X.2008.03696.x. [DOI] [PubMed] [Google Scholar]
- 54.Lin Q, et al. Involvement of CitCHX and CitDIC in developmental-related and postharvest-hot-air driven citrate degradation in citrus fruits. PloS ONE. 2015;10:e0119410. doi: 10.1371/journal.pone.0119410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Drozdowicz YM, Rea PA. Vacuolar H+ pyrophosphatases: from the evolutionary backwaters into the mainstream. Trends Plant Sci. 2001;6:206–211. doi: 10.1016/S1360-1385(01)01923-9. [DOI] [PubMed] [Google Scholar]
- 56.Hedrich R, Kurkdjian A, Guern J, Flügge U. Comparative studies on the electrical properties of the H+ translocating ATPase and pyrophosphatase of the vacuolar‐lysosomal compartment. EMBO J. 1989;8:2835–2841. doi: 10.1002/j.1460-2075.1989.tb08430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sze H, Li X, Palmgren MG. Energization of plant cell membranes by H+-pumping ATPases: regulation and biosynthesis. Plant Cell. 1999;11:677–689. doi: 10.1105/tpc.11.4.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Terrier N, Deguilloux C, Sauvage FX, Martinoia E, Romieu C. Proton pumps and anion transport in Vitis vinifera: the inorganic pyrophosphatase plays a predominant role in the energization of the tonoplast. Plant Physiol. Biochem. 1998;36:367–377. doi: 10.1016/S0981-9428(98)80078-8. [DOI] [Google Scholar]
- 59.Haschke HP, Grötsch S, Lüttge U. Proton transporting enzymes at the tonoplast of leaf cells of the CAM plant Kalanchoe daigremontiana III. Regulation of the ATPase. J. Plant Physiol. 1988;132:604–607. doi: 10.1016/S0176-1617(88)80262-1. [DOI] [Google Scholar]
- 60.Terrier N, Sauvage FX, Ageorges A, Romieu C. Changes in acidity and in proton transport at the tonoplast of grape berries during development. Planta. 2001;213:20–28. doi: 10.1007/s004250000472. [DOI] [PubMed] [Google Scholar]
- 61.Maeshima M. Vacuolar H+-pyrophosphatase. Biochim. Biophys. Acta Biomembr. 2000;1465:37–51. doi: 10.1016/S0005-2736(00)00130-9. [DOI] [PubMed] [Google Scholar]
- 62.Hu DG, et al. Conserved vacuolar H+-ATPase subunit B1 improves salt stress tolerance in apple calli and tomato plants. Sci. Hortic. 2015;197:107–116. doi: 10.1016/j.scienta.2015.09.019. [DOI] [Google Scholar]
- 63.Schumacher K, Krebs M. The V-ATPase: small cargo, large effects. Curr. Opin. Plant Biol. 2010;13:724–730. doi: 10.1016/j.pbi.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 64.Sze H, Schumacher K, Müller ML, Padmanaban S, Taiz L. A simple nomenclature for a complex proton pump: VHA genes encode the vacuolar H+-ATPase. Trends Plant Sci. 2002;7:157–161. doi: 10.1016/S1360-1385(02)02240-9. [DOI] [PubMed] [Google Scholar]
- 65.Formica, M. et al. V-ATPase controls tumor growth and autophagy in a Drosophila model of gliomagenesis. Autophagy 1–11 (2021). [DOI] [PMC free article] [PubMed]
- 66.Marshansky V, Futai M. The V-type H+-ATPase in vesicular trafficking: targeting, regulation and function. Curr. Opin. Cell Biol. 2008;20:415–426. doi: 10.1016/j.ceb.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marshansky V, Rubinstein JL, Grüber G. Eukaryotic V-ATPase: novel structural findings and functional insights. Biochim. Biophys. Acta Biomembr. 2014;1837:857–879. doi: 10.1016/j.bbabio.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 68.Gaxiola RA, Palmgren MG, Schumacher K. Plant proton pumps. FEBS Lett. 2007;581:2204–2214. doi: 10.1016/j.febslet.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 69.Rea PA, et al. Vacuolar H+-translocating pyrophosphatases: a new category of ion translocase. Trends Biochem. Sci. 1992;17:348–353. doi: 10.1016/0968-0004(92)90313-X. [DOI] [PubMed] [Google Scholar]
- 70.Baltscheffsky M, Schultz A, Baltscheffsky H. H+-PPases: a tightly membrane-bound family. FEBS lett. 1999;457:527–533. doi: 10.1016/S0014-5793(99)90617-8. [DOI] [PubMed] [Google Scholar]
- 71.Gao, M. Y., Liang, J., Zhong, R. & Di-an, N. Loss-of-function of vacuolar-type H + pyrophosphatase gene lead to reduce in stomatal aperture and density. in IOP Conference Series: Earth and Environmental Science Vol. 657 012024 (IOP Publishing, 2021).
- 72.Hussain SB, et al. Type I H+-pyrophosphatase regulates the vacuolar storage of sucrose in citrus fruit. J. Exp. Bot. 2020;71:5935–5947. doi: 10.1093/jxb/eraa298. [DOI] [PubMed] [Google Scholar]
- 73.Jiang YT, et al. Two tonoplast proton pumps function in Arabidopsis embryo development. N. Phytol. 2020;225:1606–1617. doi: 10.1111/nph.16231. [DOI] [PubMed] [Google Scholar]
- 74.Axelsen CNPKB, Harper JF, Palmgren MG. Evolution of plant P-type ATPases. Front. Plant Sci. 2012;3:31. doi: 10.3389/fpls.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Axelsen KB, Palmgren MG. Evolution of substrate specificities in the P-type ATPase superfamily. J. Mol. Evol. 1998;46:84–101. doi: 10.1007/PL00006286. [DOI] [PubMed] [Google Scholar]
- 76.Li Y, et al. Evolution of tonoplast P‐ATP ase transporters involved in vacuolar acidification. N. Phytol. 2016;211:1092–1107. doi: 10.1111/nph.14008. [DOI] [PubMed] [Google Scholar]
- 77.Faraco M, et al. Hyperacidification of vacuoles by the combined action of two different P-ATPases in the tonoplast determines flower color. Cell Rep. 2014;6:32–43. doi: 10.1016/j.celrep.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 78.Eisenach C, Baetz U, Martinoia E. Vacuolar proton pumping: more than the sum of its parts? Trends Plant Sci. 2014;19:344–346. doi: 10.1016/j.tplants.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 79.Faraco M, et al. A tonoplast P3B-ATPase mediates fusion of two types of vacuoles in petal cells. Cell Rep. 2017;19:2413–2422. doi: 10.1016/j.celrep.2017.05.076. [DOI] [PubMed] [Google Scholar]
- 80.Strazzer P, et al. Hyperacidification of citrus fruits by a vacuolar proton-pumping P-ATPase complex. Nat. Commun. 2019;10:1–11. doi: 10.1038/s41467-019-08516-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schnitzer D, Seidel T, Sander T, Golldack D, Dietz KJ. The cellular energization state affects peripheral stalk stability of plant vacuolar H+-ATPase and impairs vacuolar acidification. Plant Cell Physiol. 2011;52:946–956. doi: 10.1093/pcp/pcr044. [DOI] [PubMed] [Google Scholar]
- 82.Fukada-Tanaka S, Inagaki Y, Yamaguchi T, Saito N, Iida S. Colour-enhancing protein in blue petals. Nature. 2000;407:581–581. doi: 10.1038/35036683. [DOI] [PubMed] [Google Scholar]
- 83.Bassil E, et al. The Arabidopsis Na+/H+ antiporters NHX1 and NHX2 control vacuolar pH and K+ homeostasis to regulate growth, flower development, and reproduction. Plant Cell. 2011;23:3482–3497. doi: 10.1105/tpc.111.089581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bassil E, Blumwald E. The ins and outs of intracellular ion homeostasis: NHX-type cation/H+ transporters. Curr. Opin. Plant. Biol. 2014;22:1–6. doi: 10.1016/j.pbi.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 85.Yamaguchi T, et al. Genes encoding the vacuolar Na+/H+ exchanger and flower coloration. Plant Cell Physiol. 2001;42:451–461. doi: 10.1093/pcp/pce080. [DOI] [PubMed] [Google Scholar]
- 86.Ohnishi M, et al. Characterization of a novel Na+/H+ antiporter gene InNHX2 and comparison of InNHX2 with InNHX1, which is responsible for blue flower coloration by increasing the vacuolar pH in the Japanese morning glory. Plant Cell Physiol. 2005;46:259–267. doi: 10.1093/pcp/pci028. [DOI] [PubMed] [Google Scholar]
- 87.Zhou G, Delhaize E, Zhou M, Ryan PR. The barley MATE gene, HvAACT1, increases citrate efflux and Al3+ tolerance when expressed in wheat and barley. Ann. Bot. 2013;112:603–612. doi: 10.1093/aob/mct135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu W, Dubos C, Lepiniec L. Transcriptional control of flavonoid biosynthesis by MYB–bHLH–WDR complexes. Trends Plant Sci. 2015;20:176–185. doi: 10.1016/j.tplants.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 89.Cao ZH, Zhang SZ, Wang RK, Zhang RF, Hao YJ. Genome wide analysis of the apple MYB transcription factor family allows the identification of MdoMYB121 gene confering abiotic stress tolerance in plants. PLoS ONE. 2013;8:e69955. doi: 10.1371/journal.pone.0069955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao Q, et al. Overexpression of MdbHLH104 gene enhances the tolerance to iron deficiency in apple. Plant Biotechnol. 2016;14:1633–1645. doi: 10.1111/pbi.12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hu DG, et al. MdMYB1 regulates anthocyanin and malate accumulation by directly facilitating their transport into vacuoles in apples. Plant Physiol. 2016;170:1315–1330. doi: 10.1104/pp.15.01333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jia D, et al. Genetic variation in the promoter of an R2R3− MYB transcription factor determines fruit malate content in apple (Malus domestica Borkh.) Plant Physiol. 2021;186:549–568. doi: 10.1093/plphys/kiab098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li SJ, et al. CrMYB73, a PH-like gene, contributes to citric acid accumulation in citrus fruit. Sci. Hortic. 2015;197:212–217. doi: 10.1016/j.scienta.2015.09.037. [DOI] [Google Scholar]
- 94.Amato A, et al. The MYB 5‐driven MBW complex recruits a WRKY factor to enhance the expression of targets involved in vacuolar hyper‐acidification and trafficking in grapevine. Plant J. 2019;99:1220–1241. doi: 10.1111/tpj.14419. [DOI] [PubMed] [Google Scholar]
- 95.Cavallini E, et al. Functional diversification of grapevine MYB5a and MYB5b in the control of flavonoid biosynthesis in a petunia anthocyanin regulatory mutant. Plant Cell Physiol. 2014;55:517–534. doi: 10.1093/pcp/pct190. [DOI] [PubMed] [Google Scholar]
- 96.Kasajima I, Sasaki K. A chimeric repressor of petunia PH4 R2R3-MYB family transcription factor generates margined flowers in torenia. Plant Signal. Behav. 2016;11:e1177693. doi: 10.1080/15592324.2016.1177693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sundaramoorthy, J. et al. A P3A-type ATPase and an R2R3-MYB transcription factor are involved in vacuolar acidification and flower coloration in soybean. Front. Plant Sci.11, 580085 (2020). [DOI] [PMC free article] [PubMed]
- 98.Eulgem T, Rushton PJ, Robatzek S, Somssich IE. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000;5:199–206. doi: 10.1016/S1360-1385(00)01600-9. [DOI] [PubMed] [Google Scholar]
- 99.Rushton PJ, Somssich IE, Ringler P, Shen QJ. WRKY transcription factors. Trends Plant Sci. 2010;15:247–258. doi: 10.1016/j.tplants.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 100.Ding ZJ, Yan JY, Xu XY, Li GX, Zheng SJ. WRKY 46 functions as a transcriptional repressor of ALMT 1, regulating aluminum‐induced malate secretion in Arabidopsis. Plant J. 2013;76:825–835. doi: 10.1111/tpj.12337. [DOI] [PubMed] [Google Scholar]
- 101.Yu, J. Q. et al. The apple bHLH transcription factor MdbHLH3 functions in determining the fruit carbohydrates and malate. Plant Biotechnol. J.10.1111/pbi.13461 (2020). [DOI] [PMC free article] [PubMed]
- 102.Li SJ, et al. The Citrus transcription factor, CitERF13, regulates citric acid accumulation via a protein-protein interaction with the vacuolar proton pump, CitVHA-c4. Sci. Rep. 2016;6:1–10. doi: 10.1038/s41598-016-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li SJ, et al. Citrus CitNAC62 cooperates with CitWRKY1 to participate in citric acid degradation via up-regulation of CitAco3. J. Exp. Bot. 2017;68:3419–3426. doi: 10.1093/jxb/erx187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bai Y, Dougherty L, Cheng L, Xu K. A co-expression gene network associated with developmental regulation of apple fruit acidity. Mol. Genet. Genomics. 2015;290:1247–1263. doi: 10.1007/s00438-014-0986-2. [DOI] [PubMed] [Google Scholar]
- 105.Xie XB, et al. The bHLH transcription factor MdbHLH3 promotes anthocyanin accumulation and fruit colouration in response to low temperature in apples. Plant Cell Environ. 2012;35:1884–1897. doi: 10.1111/j.1365-3040.2012.02523.x. [DOI] [PubMed] [Google Scholar]
- 106.Verweij W, et al. Functionally similar WRKY proteins regulate vacuolar acidification in petunia and hair development in Arabidopsis. Plant Cell. 2016;28:786–803. doi: 10.1105/tpc.15.00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Quattrocchio F, et al. PH4 of petunia is an R2R3 MYB protein that activates vacuolar acidification through interactions with basic-helix-loop-helix transcription factors of the anthocyanin pathway. Plant Cell. 2006;18:1274–1291. doi: 10.1105/tpc.105.034041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Spelt C, Quattrocchio F, Mol J, Koes R. ANTHOCYANIN1 of petunia controls pigment synthesis, vacuolar pH, and seed coat development by genetically distinct mechanisms. Plant Cell. 2002;14:2121–2135. doi: 10.1105/tpc.003772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hichri I, et al. The basic helix-loop-helix transcription factor MYC1 is involved in the regulation of the flavonoid biosynthesis pathway in grapevine. Mol. Plant. 2010;3:509–523. doi: 10.1093/mp/ssp118. [DOI] [PubMed] [Google Scholar]
- 110.Dechant R, et al. Cytosolic pH is a second messenger for glucose and regulates the PKA pathway through V‐ATPase. EMBO J. 2010;29:2515–2526. doi: 10.1038/emboj.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hong-Hermesdorf A, Brüx A, Grüber A, Grüber G, Schumacher K. A WNK kinase binds and phosphorylates V-ATPase subunit C. FEBS Lett. 2006;580:932–939. doi: 10.1016/j.febslet.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 112.Hu DG, Sun CH, Sun MH, Hao YJ. MdSOS2L1 phosphorylates MdVHA-B1 to modulate malate accumulation in response to salinity in apple. Plant Cell Rep. 2016;35:705–718. doi: 10.1007/s00299-015-1914-6. [DOI] [PubMed] [Google Scholar]
- 113.Liu J, Ji Y, Zhou J, Xing D. Phosphatidylinositol 3-kinase promotes V-ATPase activation and vacuolar acidification and delays methyl jasmonate-induced leaf senescence. Plant Physiol. 2016;170:1714–1731. doi: 10.1104/pp.15.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jia D, et al. Apple fruit acidity is genetically diversified by natural variations in three hierarchical epistatic genes: MdSAUR37, MdPP2CH and MdALMTII. Plant J. 2018;95:427–443. doi: 10.1111/tpj.13957. [DOI] [PubMed] [Google Scholar]
- 115.Nakatsukasa K, Okumura F, Kamura T. Proteolytic regulation of metabolic enzymes by E3 ubiquitin ligase complexes: lessons from yeast. Crit. Rev. Biochem. Mol. Biol. 2015;50:489–502. doi: 10.3109/10409238.2015.1081869. [DOI] [PubMed] [Google Scholar]
- 116.Li YY, et al. MdCOP1 ubiquitin E3 ligases interact with MdMYB1 to regulate light-induced anthocyanin biosynthesis and red fruit coloration in apple. Plant Physiol. 2012;160:1011–1022. doi: 10.1104/pp.112.199703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hu DG, et al. Glucose sensor MdHXK1 phosphorylates and stabilizes MdbHLH3 to promote anthocyanin biosynthesis in apple. PLoS Genet. 2016;12:e1006273. doi: 10.1371/journal.pgen.1006273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hu DG, et al. The regulatory module MdPUB29‐MdbHLH3 connects ethylene biosynthesis with fruit quality in apple. N. Phytol. 2019;221:1966–1982. doi: 10.1111/nph.15511. [DOI] [PubMed] [Google Scholar]
- 119.Zhang QY, et al. BTB-TAZ domain protein MdBT2 modulates malate accumulation and vacuolar acidification in response to nitrate. Plant Physiol. 2020;183:750–764. doi: 10.1104/pp.20.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang QY, et al. BTB-BACK-TAZ domain protein MdBT2-mediated MdMYB73 ubiquitination negatively regulates malate accumulation and vacuolar acidification in apple. Hortic. Res. 2020;7:1–12. doi: 10.1038/s41438-019-0222-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhou LJ, et al. The SUMO E3 ligase MdSIZ1 targets MdbHLH104 to regulate plasma membrane H+-ATPase activity and iron homeostasis. Plant Physiol. 2019;179:88–106. doi: 10.1104/pp.18.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhou LJ, et al. The small ubiquitin‐like modifier E3 ligase MdSIZ1 promotes anthocyanin accumulation by sumoylating MdMYB1 under low‐temperature conditions in apple. Plant Cell Environ. 2017;40:2068–2080. doi: 10.1111/pce.12978. [DOI] [PubMed] [Google Scholar]
- 123.Liao L, et al. Unraveling a genetic roadmap for improved taste in the domesticated apple. Mol. Plant. 2021;9:1454–1471. doi: 10.1016/j.molp.2021.05.018. [DOI] [PubMed] [Google Scholar]


