Abstract
Several primate species have been shown to exhibit age-related changes in cognition, brain, and behavior. However, severe neurodegenerative illnesses, such as Alzheimer's disease (AD), were once thought to be uniquely human. Recently, some chimpanzees naturally were documented to develop both neurofibrillary tangles and amyloid plaques, the main characteristics of AD pathology. In addition, like humans and other primates, chimpanzees show similar declines in cognition and motor function with age. Here, we used voxel-based morphometry to examine the relationships among gray matter volume, age, and cognition using magnetic resonance imaging scans previously acquired from chimpanzees (N = 216). We first determined the relationship between age and gray matter volume, identifying the regions that declined with age. With a subset of our sample (N = 103), we also determined differences in gray matter volume between older chimpanzees with higher cognition scores than expected for their age, and older chimpanzees with lower than expected scores. Finally, we ran a conjunction analysis to determine any overlap in brain regions between these two analyses. We found that as chimpanzees age, they lose gray matter in regions associated with cognition. In addition, cognitively healthy older chimpanzees (those performing better for their age) have greater gray matter volume in many brain regions compared with chimpanzees who underperform for their age. Finally, the conjunction analysis revealed that regions of age-related decline overlap with the regions that differ between cognitively healthy chimpanzees and those who underperform. This study provides further evidence that chimpanzees are an important model for research on the neurobiology of aging. Future studies should investigate the effects of cognitive stimulation on both cognitive performance and brain structure in aging nonhuman primates.
Keywords: aging, chimpanzee, cognition, gray matter, voxel-based morphometry
1 |. INTRODUCTION
Decades of research in humans and other primates have documented age-related changes in cognition, brain and behavior. Some loss in cognitive functions and cortical organization is considered a normal process of aging, but other decrements are attributable to specific neurodegenerative processes in humans, such as Alzheimer's disease (AD), Parkinson's disease, and Fronto-Temporal Dementia (FTD). Animal models, including nonhuman primate models, have been developed as a means of understanding the mechanisms of age-related changes in brain and cognition, as well as the development of therapeutic interventions in treating AD and related dementias (Walker & Jucker, 2017). Chimpanzees are often overlooked in studies on age-related changes in cognition and the brain, which is unfortunate for several reasons. First, studies have documented the cooccurrence of both neurofibrillary tangles (NFTs) and amyloid plaques (Aβ) in the postmortem brains of elderly chimpanzees (Edler et al., 2017; Rosen et al., 2008). The presence of both AB plaques and NFTs are two neuropathological features that are used to definitively confirm a diagnosis of AD in humans; thus, based on the available data, chimpanzees appear to be one of the few nonhuman species known to naturally develop the hallmarks of AD pathology (Arnsten et al., 2019; Edler et al., 2017; Rosen et al., 2008). Therefore, additional studies in this species could lead to important findings on the comparative biology of aging and may hold the key to understanding our own aging (Edler et al., 2018; Munger et al., 2018; Rosen et al., 2016). Moreover, given their greater genetic, neuroanatomical, behavioral, and cognitive similarities to humans compared with more distantly related primate species, studies on the neurobiology of aging in chimpanzees would likely produce results with increased translational value to humans.
Understanding the neurobiology of aging in captive chimpanzees is also germane to their short- and long-term management and welfare. Due to the 1998 National Institutes of Health (NIH) breeding moratorium and the more recent decision to retire NIH-owned apes from biomedical research, there remains at least 200 chimpanzees currently housed at the federal sanctuary. The NIH-owned population of chimpanzees is decidedly skewed toward adult and geriatric animals, as are the many other apes being retired to private sanctuaries. Therefore, there may be health, management, and welfare challenges for aging chimpanzee populations currently residing in sanctuaries that might benefit from a better understanding of the factors that foster healthy aging. Indeed, consistent with findings in other nonhuman primate species (Darusman, Call, et al., 2014; Darusman, Pandelaki, et al., 2014; Herndon et al., 1997; Joly et al., 2014; Lacreuse et al., 2014, 2020; Lai et al., 1995; Picq, 2007; Rapp & Amaral, 1989; Workman et al., 2019), cross-sectional and longitudinal data indicate that aged chimpanzees show a moderate loss in cognitive and motor functions compared with younger conspecifics (Hopkins et al., 2015, 2019, 2020; Lacreuse et al., 2014, 2018). The extent to which age-related changes in cognition observed in aged chimpanzees are attributable to “normal” aging or to neuropathology remains unknown, but the cognitive data would certainly suggest that neurobiological changes should be evident in aging chimpanzees.
To date, there have been relatively few neuroimaging studies on age-related changes in the brain of nonhuman primates (e.g., Alexander et al., 2008; Hara et al., 2012; Koo et al., 2012), and specifically chimpanzees. In rhesus macaques, Koo et al. (2012) found that cortical thickness in the somatosensory and motor cortices decreases as monkeys age. In addition, there are several studies documenting neuroanatomical changes associated with cognitive decline in rhesus monkeys (see review in Hara et al., 2012). For example, Alexander et al. (2008) used a source-based morphometry-like method to examine age-related changes in gray matter covariation networks and reported that older monkeys showed reductions in networks that included dorsal and ventral prefrontal cortex, superior temporal sulcus, and Sylvian fissure. Moreover, the gray matter weighted scores for these age-related components were associated with a decline in spatial, but not nonspatial, memory performance (Alexander et al., 2008). In chimpanzees, previous studies have also reported moderate age-related changes in overall brain volume, gyrification, gray and white matter volume, and gray matter covariation (Autrey et al., 2014; Chen et al., 2013; Herndon et al., 1999; Hopkins et al., 2018; Sherwood et al., 2011). More recently, using voxel-based morphometry (VBM), Vickery et al. (2020) found age-related gray matter decline in regions similar to those in humans. Altogether, these studies show that age-related loss in gray matter appears to be region-specific, which may have consequences for the cognitive and motor functions involved in these areas.
In this article, we present results on the relationship between gray matter volume and cognition using voxel-based morphometry (VBM) in chimpanzees. This study builds on our previous research. Specifically, Lacreuse et al. (2020) reported age-related loss in gray matter volume using VBM in a sample of 216 chimpanzees (see Figure 1). For a subsample of these chimpanzees, we have obtained a measure of general intelligence, “g,” using the Primate Cognition Test Battery (PCTB), a 13-item test that assesses different dimensions of social and physical cognition in nonhuman primates (Herrmann et al., 2007; Herrmann, Hare, et al., 2010; Herrmann, Hernandez-Lloreda, et al., 2010; Hopkins et al., 2014; Russell et al., 2011; Schmitt et al., 2011). In addition, Hopkins et al. (2020) recently showed that the overall PCTB performance score, or “g,” showed a quadratic association with age (Figure 2); thus, younger and elderly chimpanzees performed more poorly than middle-aged individuals.
FIGURE 1.
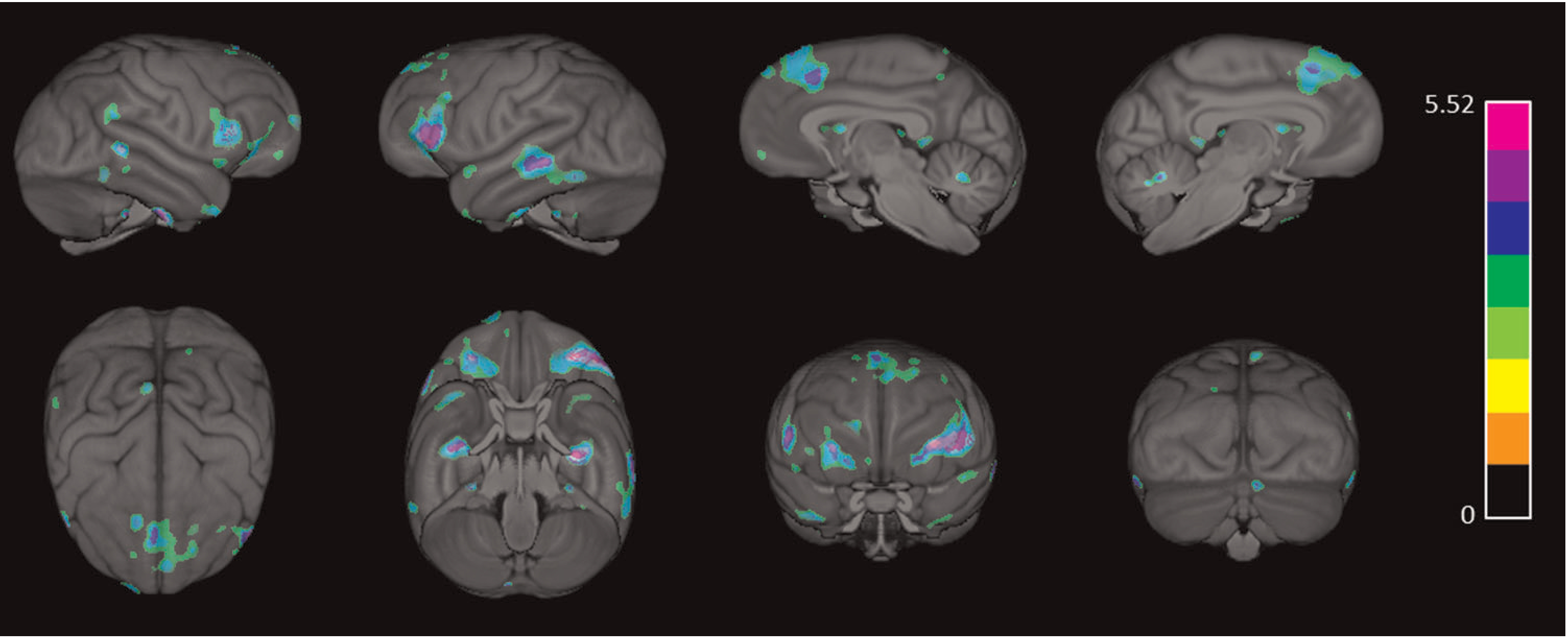
Three-dimensional surface renderings of the regions negatively associated with age (p < 0.001), indicating regions of gray matter volume loss as chimpanzees age
FIGURE 2.
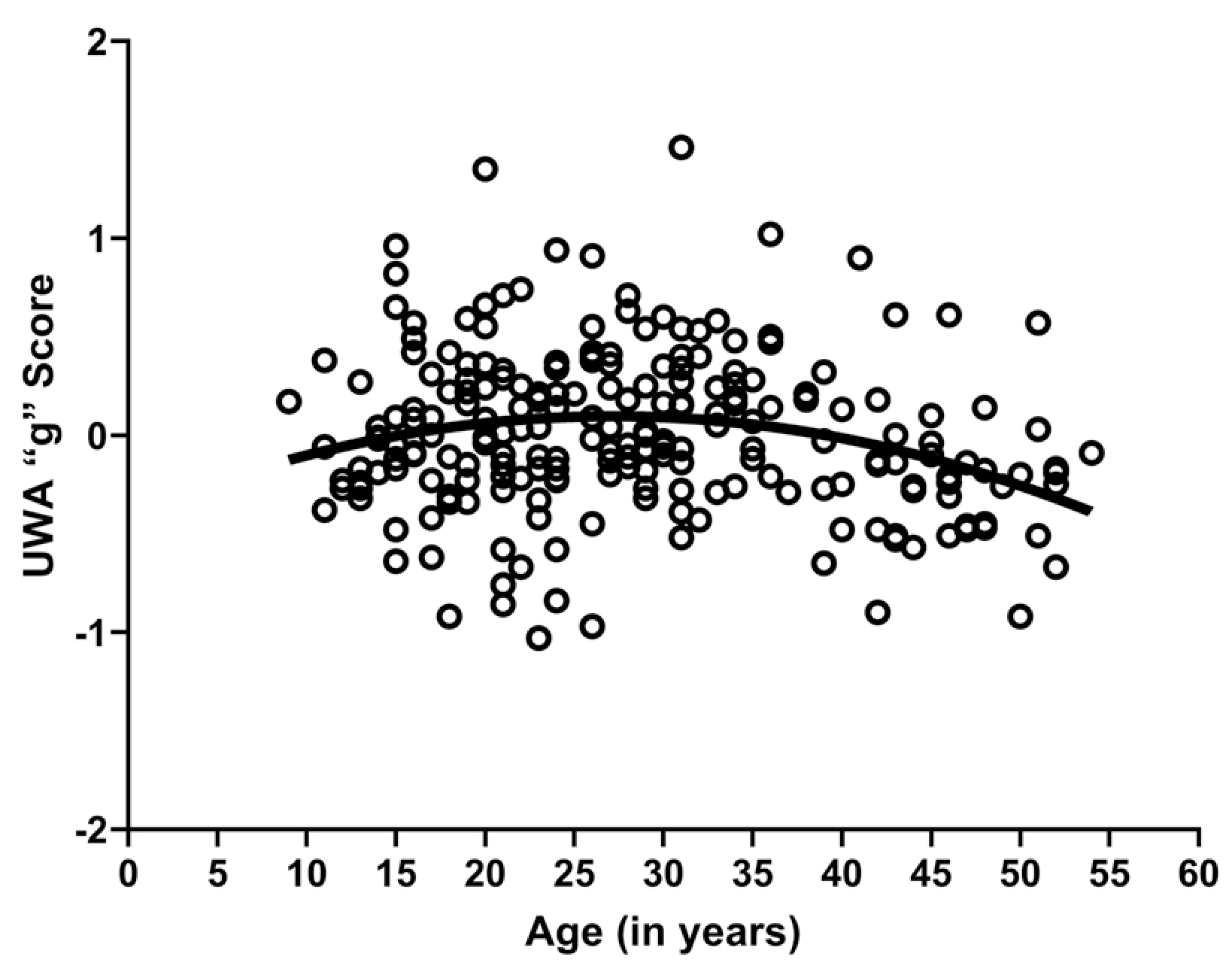
Scatter plot showing the quadratic association between age and UWA_g scores, r(222) = 0.246, p = 0.001
Building on these two separate findings, here, we tested whether the age-associated changes in “g” were associated with changes in gray matter volume after accounting for subject age. To address this question, we initially used VBM to test for the association between “g” and gray matter variation in a sample of middle-aged and elderly chimpanzees, while statistically controlling for age, sex, and scanner magnet. This initial analysis established which brain areas, if any, were associated with “g” in the older chimpanzee sample. We next performed a conjunction analysis to identify those brain regions that were associated with “g” and were also negatively associated with age. The conjunction analysis, therefore, allowed us to identify those specific brain regions that decline with age and are implicated in age-related changes in cognition.
2 |. METHODS
2.1 |. Subjects
We utilized archival in vivo magnetic resonance image (MRI) data collected from 216 captive chimpanzees (132 females and 84 males) from the National Center for Chimpanzee Care (NCCC) at The University of Texas MD Anderson Cancer Center (n = 139) or the Yerkes National Primate Research Center (YNPRC; n = 77). The chimpanzees ranged in age from 8 to 53 years (M = 26.37, SD = 10.59) at the time of scanning. All subjects were socially housed in indoor/outdoor enclosures, with 24-h access to both areas, except during cleaning. All enclosures included climbing structures, bedding, and daily environmental enrichment. Care staff fed the chimpanzees a diet of commercial primate chow and fresh produce and provided them with several daily for aging opportunities. All chimpanzees had ad libitum access to water.
2.2 |. Image acquisition and post-image processing
MRI scans were previously acquired during one of the chimpanzee's annual physical examinations (between 2009 and 2015), following YNPRC and NCCC standard procedures designed to minimize stress (for detailed acquisition information, see Hopkins & Avants, 2013; Hopkins, Latzman, et al., 2019; Mulholland et al., 2020). Seventy-seven chimpanzees (at YNPRC) were scanned using a 3.0 Tesla scanner (Siemens Trio, Siemens Medical Solutions USA, Inc.) and 139 chimpanzees (at NCCC) were scanned using a 1.5 Tesla G.E. echo-speed Horizon LX MR scanner (GE Medical Systems). All procedures were approved by the Institutional Animal Care and Use Committees at YNPRC and NCCC, followed the guidelines of the Institute of Medicine on the use of chimpanzees in research and complied with the American Society of Primatologists Principles for the Ethical Treatment of Nonhuman Primates. The MRI data are available from the National Chimpanzee Brain Resource at https://www.chimpanzeebrain.org.
Detailed information about post-image processing can be found in Hopkins et al. (2020). Briefly, all processing was completed on a Macintosh computer running 3D Slicer 4 (www.3Dslicer.org), DWI Denoising Package for MATLAB (R2015b; Mathworks), FMRIB Software Library tools (FSL), and ANALYZE 11.0 (AnalyzeDirect). All procedures were completed twice, once for the MRI scans acquired on the 1.5 Tesla scanner and once for those acquired on the 3.0 Tesla scanner. First, we converted the raw DICOM files into NifTI format (Fedorov et al., 2012; Kikinis et al., 2014), then we subjected each image to brain extraction/skull stripping (BET in FSL; Jenkinson et al., 2005; Smith, 2002), N4ITK bias correction (Boyes et al., 2008; Tustison & Gee, 2009; Tustison, Avants, Cook, et al., 2010; Tustison, Avants, Cook, Zheng, et al., 2010; Tustison et al., 2014), and optimized nonlocal means denoising (Coupé et al., 2008). The scans were then resampled at 0.625mm isotropic voxels and aligned on the AC–PC axis before being registered to a chimpanzee template brain (Hopkins & Avants, 2013) using a 12-parameter affine linear registration (FLIRT in FSL; Jenkinson & Smith, 2001; Jenkinson et al., 2002).
Next, we ran these aligned and affine registered scans through the FSL-VBM pipeline (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLVBM). This process included (1) segmentation of each scan into gray and white matter, (2) linear registration of each scan to a standard chimpanzee template (Hopkins & Avants, 2013), (3) creation of a study-specific gray matter template (Andersson et al., 2007; Douaud et al., 2007; Smith et al., 2004), (4) nonlinear registration of each subject’s gray matter image to the study-specific template, (5) modulation of the gray matter volume by use of a Jacobian warp to correct for local expansion or contraction of gray matter within each voxel, and (6) smoothing with an isotropic Gaussian kernel with a sigma of 2 mm. Finally, we ensured that the 1.5T and 3T scans had the same orientation and voxel dimensions by reregistering each smoothed, modulated gray matter volume to the chimpanzee template brain using a six-parameter rigid body linear registration in FSL (FLIRT; Hopkins & Avants, 2013; Jenkinson & Smith, 2001; Jenkinson et al., 2002). The resulting gray matter volumes were used in subsequent analyses.
2.3 |. Primate cognition test battery
PCTB data was previously collected from chimpanzees in both colonies. The PCTB included nine physical cognition tasks and three social cognition tasks, and only subjects that completed all tasks were included in the current study. Briefly, the tasks included spatial memory, object permanence, rotation, transposition, relative numbers, causality noise, and causality visual (all measuring physical cognition), and initiation of joint attention, attentional state, and gaze following/response to joint attention (all measuring social cognition). As reported in Hopkins et al. (2020), the outcome measures for each task were converted to standardized z-scores (separately for each colony). The z-scores were then averaged across the 12 measures for each subject to create a unit weighted average (UWA) score that reflected their overall performance, or “g” (Woodley et al., 2015).
2.4 |. Statistical analyses
2.4.1 |. Age
To test for reductions in gray matter volume per voxel as chimpanzees age, we performed voxel-based morphometry (VBM) using SPM12 (https://www.fil.ion.ucl.ac.uk/spm/) (Penny et al., 2007) run in MATLAB. Voxel-based morphometry is a whole-brain, voxel-wise comparison of gray matter volume. First, we imported the smoothed gray matter volumes (generated from the FSL-VBM pipeline) for each subject and ran a one-sample t-test with the age of each chimpanzee at the time of their scan, while controlling for sex, rearing history, and scanner magnet (1.5T or 3T). A subsequent contrast (with a minimum cluster threshold of 50 voxels) was used to examine the negative correlation between age and gray matter volume per voxel (with the significance threshold set at p< 0.001, uncorrected).
2.4.2 |. Cognition and gray matter volume
Here, we examined the UWA_g scores of chimpanzees over the age of 25 when tested on the PCTB (M = 36.12, SD = 8.20, N = 126). To determine the relationship between the UWA_g scores and gray matter variation while accounting for age, we regressed age at testing, sex, and colony on the UWA_g scores and saved the residual. The colony was included as a covariate due to differences in PCTB performance between the two chimpanzee colonies. The residual reflected each subject’s UWA_g score after statistically controlling for their sex, age at the time of PCTB testing, and where they were housed when tested (NCCC or YNPRC). We then categorized the chimpanzees into two groups based on these residuals: those who performed better (positive residual, UWA_g+) or worse (negative residuals, UWA_g−) than predicted for their sex, age, and colony. Therefore, the UWA_g+ group includes chimpanzees that represent successful cognitive aging (middle aged or elderly chimpanzees who perform better than predicted for their age). Out of these 126 subjects, we had MRI data for 103 chimpanzees (73 females, 30 males; Age at scan M = 33.23, SD = 8.72). For 91 of the 103 chimpanzees, PCTB testing occurred within 5 years of MRI acquisition, but for the remaining 12 subjects, testing occurred between 6 and 16 years of MRI acquisition (overall M = −2.58, SD = 2.59). We accounted for this discrepancy in timing by statistically controlling for both the age at the time of cognitive testing when calculating the residuals to determine the UWA groups and age at the time of scan in our VBM analysis. We then performed a voxel-based morphometry analysis using SPM 12 in MATLAB. We imported the smoothed, modulated gray matter volumes for each of the 103 subjects and ran an independent samples t-test with performance group (UWA_g+ and UWA_g−) as the independent variable, while controlling for sex, age at the time of scanning, rearing history, and scanner magnet (1.5T or 3T). These variables were included as covariates because they are known to affect neuroanatomy in chimpanzees; (see below along with Bard & Hopkins, 2018; Bennett et al., in press; Hopkins, Latzman, et al., 2019). Please note, scanner magnet is confounded with colony here (all NCCC subjects were scanned with a 1.5T scanner magnet, Yerkes with a 3T). Subsequent contrasts (with a minimum cluster threshold of 50 voxels; p < 0.01) were used to compare gray matter volume per voxel between the two groups. Differences between the UWA_g+ and UWA_g- groups reveal those brain regions associated with cognition within this older chimpanzee sample.
2.4.3 |. Conjunction analysis
To determine those brain regions that were implicated in both cognitive performance and aging, we performed a conjunction analysis. For the conjunction analysis, we re-ran the VBM analyses described above (between both age and cognitive performance with the whole-brain gray matter variation) but reduced the level of significance to p < 0.05 (uncorrected). For each VBM analysis, we subsequently binarized the gray matter output volume and added these two binary volumes together. We then thresholded the volume at 2, which resulted in an image that reflected those regions associated with both age and cognitive performance. The thresholded volume was fused with the chimpanzee template brain (Hopkins & Avants, 2013) in Analyze 11.0 and we termined which regions overlapped between the two VBM analyses.
3 |. RESULTS
3.1 |. Age
As reported in Lacreuse et al. (2020), the VBM analysis revealed a significant negative correlation between age and gray matter volume (p < 0.001). As age increased, gray matter volume decreased in many regions, including (but not limited to): superior frontal cortex (bilateral), supramarginal (right), rostral middle frontal (left), pars triangularis (left), pars opercularis (bilateral), insula (bilateral), pars orbitalis (bilateral), superior and middle temporal cortex (bilateral), and the entorhinal cortex (bilateral) (see Figure 1 and Table 1 for all regions and corresponding volumes).
Table 1.
Regions and corresponding volumes (>20 mm3) for each significant region from the three analyses.
| Region | Total Volume | L/R Volume |
|---|---|---|
| Age | ||
| Pars triangularis, pars opercularis, and pars orbitalis | 2433.11 | 1807.13/625.98 |
| Superior frontal cortex | 2389.89 | 1197.75/1192.14 |
| Superior and middle temporal cortex | 1599.60 | 1131.34/468.26 |
| Entorhinal cortex | 722.17 | 373.54/348.63 |
| Supramarginal cortex | 646.97 | 0/646.97 |
| Precentral cortex | 316.89 | 0/316.89 |
| Cerebellum | 171.14 | 122.07/49.07 |
| Putamen | 154.30 | 65.43/88.87 |
| Rostral middle frontal cortex | 69.34 | 0/69.34 |
| Isthmus cingulate | 56.64 | 35.40/21.24 |
| Precuneus | 39.80 | 0/39.80 |
| Insula | 35.65 | 16.85/18.8 |
| Paracentral cortex | 33.45 | 0/33.45 |
| Frontal pole | 31.98 | 0/31.98 |
| Caudate | 30.76 | 0/30.76 |
| Superior parietal cortex | 29.05 | 29.05/0 |
| Cognition (UWA_g+ > UWA_g−) | ||
| Cerebellum | 768.80 | 353.02/415.78 |
| Rostral middle frontal and pars triangularis | 513.18 | 513.18/0 |
| Superior and inferior parietal | 482.43 | 458.99/23.44 |
| Brainstem | 232.91 | 175.05/57.86 |
| Precentral cortex | 152.59 | 12.45/140.14 |
| Pars opercularis | 145.51 | 0/145.51 |
| Isthmus cingulate | 103.52 | 72.51/31.01 |
| Lingual gyrus | 102.54 | 57.13/45.41 |
| Pars orbitalis | 77.88 | 77.88/0 |
| Lateral occipital | 40.53 | 40.53/0 |
| Superior frontal | 39.55 | 39.55/0 |
| Supramarginal | 23.68 | 0/23.68 |
| Conjunction | ||
| Pars opercularis, pars triangularis, rostral middle frontal | 1334.48 | 518.31/816.17 |
| Superior parietal | 1122.07 | 996.34/125.73 |
| Cerebellum | 534.42 | 365.96/168.46 |
| Superior frontal | 180.17 | 80.32/99.85 |
| Brainstem | 151.85 | 106.69/45.16 |
| Temporal pole | 119.14 | 46.14/73.00 |
| Middle temporal cortex | 113.77 | 113.77/0 |
| Isthmus cingulate | 96.92 | 47.85/49.07 |
| Supramarginal | 58.59 | 0/58.59 |
| Putamen | 45.90 | 45.90/0 |
| Postcentral cortex | 34.42 | 34.42/0 |
3.2 |. Cognition
The residual cognition scores saved from the UWA analysis revealed that, of the 103 chimpanzees with MRI data, 54 performed worse and 49 performed better than expected for their age, sex, and colony membership. The VBM revealed significant differences in gray matter between these two cognition groups. Specifically, UWA_g+ chimpanzees had increased gray matter volume in many regions, including the prefrontal cortex, cerebellum, superior and inferior parietal cortex, brainstem, isthmus cingulate, and lingual cortex compared with the UWA_g- apes (p < 0.01 uncorrected; see Figure 3 and Table 1 for the volumes of all regions). By contrast, UWA_g-chimpanzees had higher gray matter volume in the superior frontal cortex, paracentral cortex, precuneus, inferior temporal cortex, and supramarginal cortex compared with UWA_g+ subjects (p < 0.01 uncorrected; see Figure 3).
FIGURE 3.
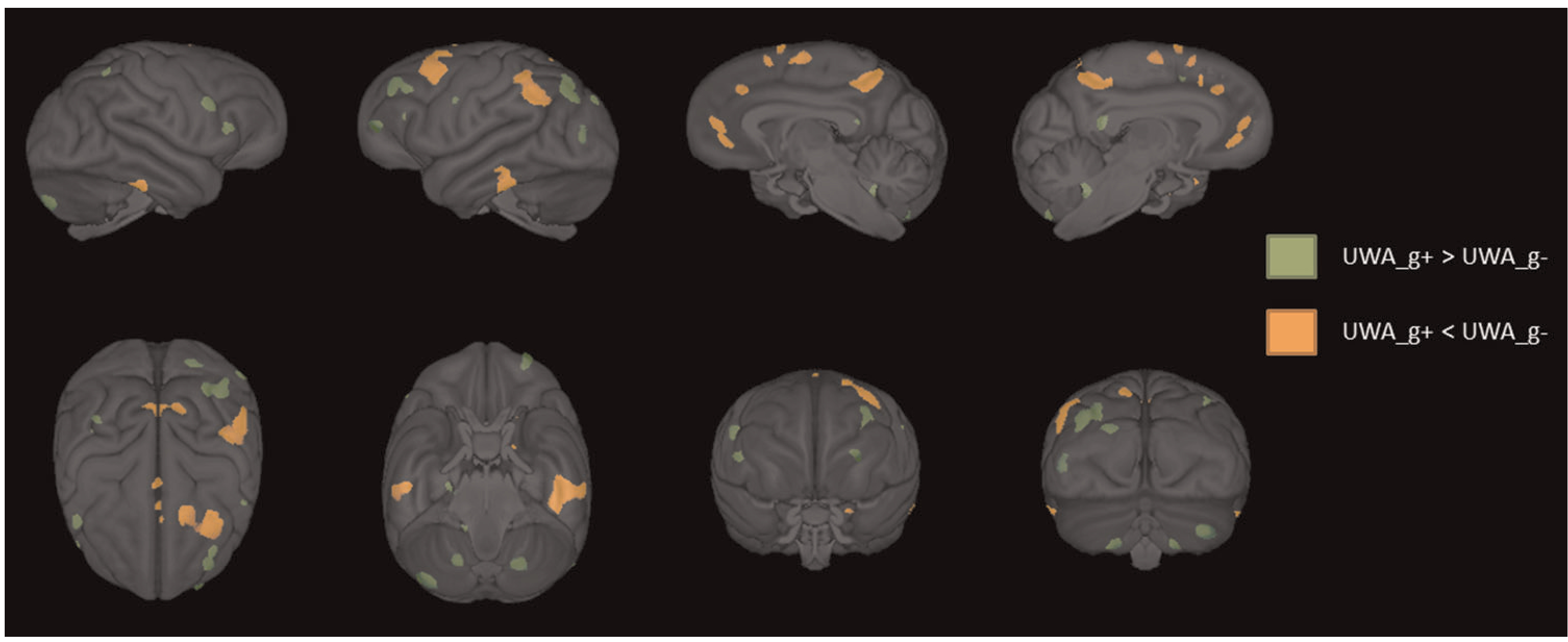
Three-dimensional surface renderings of the regions of gray matter volume that differ between those that perform better (UWA_g+; green) or worse (UWA_g−; orange) on the PCTB for their age and sex (p < 0.01). PCTB, Primate Cognition Test Battery; UWA, unit weighted average
3.3 |. Conjunction analysis
The conjunction analysis revealed that UWA_g+ chimpanzees had higher gray matter volumes than UWA_g- apes in several regions that were found to decline with increasing age. These regions included the rostral middle frontal, pars triangularis, pars opercularis, superior parietal cortex, cerebellum, and superior frontal cortex, middle temporal cortex, and temporal pole (see Figure 4). That is to say, UWA_g+ chimpanzees had significantly higher gray matter volume compared with UWA_g apes in brain regions known
FIGURE 4.
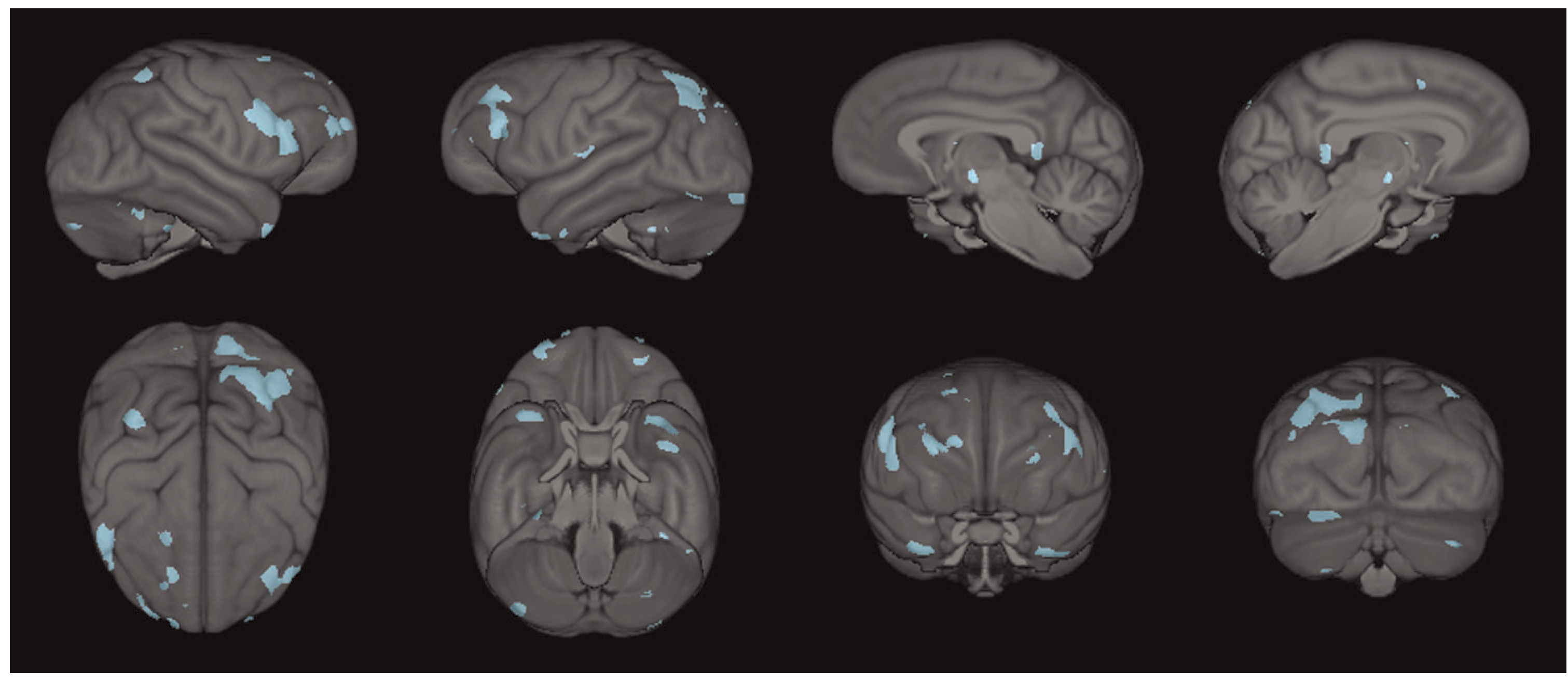
Three-dimensional surface renderings of the regions that overlap between the two voxel-based morphometry analyses; blue indicates regions of increased gray matter volume in UWA_g+ chimpanzees that typically decline with age
4 |. DISCUSSION
We found significant reductions in gray matter volume throughout the brain with increased age. This included the prefrontal cortex and middle temporal cortex, as well as the superior frontal cortex, insula, superior temporal cortex, and entorhinal cortex. The gray matter regions showing marked decline with increasing age in the chimpanzees overlap considerably with findings from both cross-sectional and longitudinal samples of healthy human subjects (Bigler et al., 2002; Lemaitre et al., 2012; Raz, 1997; Raz et al., 2005; Tisserand et al., 2004), as well as those with diseases or injuries that cause cognitive impairments (Bigler et al., 1997, 2002; Dicks et al., 2019). It is possible that these reductions in gray matter volume as chimpanzees age are the result of neuropathology; however, additional studies comparing the in vivo imaging data to pathology of postmortem brain tissue are needed.
In addition, after adjustment for age and colony, we found that chimpanzees with positive residual cognition scores (UWA_g+; or those with increased cognitive reserve; Stern et al., 2020) showed significantly higher gray matter volume than chimpanzees with negative residual scores (UWA_g−). In humans, successful cognitive aging has been consistently associated with increased global white and gray matter volume, as well as increased volume in the frontal cortex and hippocampus (Harrison et al., 2012; Kaup et al., 2011). The brain regions implicated in better cognitive performance in the chimpanzees in the current study included regions within the middle and inferior frontal gyri, supramarginal, and angulargyrus, and the cerebellum. In humans, similar brain regions have been reported to be associated with general intelligence or “g” (Haier et al., 2004, 2010; Hilger et al., 2020; Hogan et al., 2011), suggesting some homology in brain regions implicated in general cognitive functions between humans and chimpanzees. Similarly, the findings reported here are consistent with a previous study in a subset of these chimpanzees that showed that increasing cognitive performance as assessed by the PCTB was associated with larger brain volumes and increased cortical thickness (Hopkins, Li, et al., 2019).
Finally, from the conjunction analysis, we found that UWA_g+ chimpanzees had increased gray matter in regions normally associated with age-related decline compared to UWA_g− apes. These overlapping regions include the prefrontal cortex, parietal cortex, temporal cortex, and cerebellum. In a longitudinal study in humans, cognitive decline over 3 years was associated with reductions in gray matter volume in some of the same regions reported here for chimpanzees (e.g., prefrontal, temporal, and parietal cortices; Tisserand et al., 2004). Furthermore, like our UWA_g+ chimpanzees, humans with cognitive scores above average for their age seem to resist typical age-related changes in cortical volume (Harrison et al., 2012). However, it is impossible to determine the causal direction of the relationships reported here; it is possible that cognitively healthy chimpanzees (or those with higher cognitive reserve; Stern et al., 2020) are protected from some age-related gray matter loss, or that chimpanzees with less gray matter loss as they age (possibly an indication of increased brain reserve or maintenance; Cabeza et al., 2018; Stern et al., 2020) perform better on cognitive tasks. In humans, genetics (e.g., lack of the APOE ε4 allele) and other biological factors (e.g., lower IL-6, glucose, etc.), higher education, better overall health, physical functioning, and emotional wellbeing, as well as increased social support have been shown to predict successful cognitive aging among older adults (Albert et al., 1995; Barnes et al., 2007; Goveas et al., 2016; Wang et al., 2014; Yaffe et al., 2009). In addition, studies in humans show that increased cognitive stimulation, such as word games, discussion of events or people, puzzles, or other activities involving thinking and memory, may improve cognition and increase quality of life for people with dementia (Spector et al., 2003, 2010; Woods et al., 2006, 2012). However, there are some conflicting reports of the effectiveness of such stimulation on reducing cognitive decline (Kim et al., 2017); therefore, further controlled studies are needed.
It is possible that similar factors could be related to successful cognitive aging in chimpanzees. In fact, the chimpanzees included in the current study had access to regular cognitive enrichment and additional cognitive stimulation through each facility's respective enrichment and research programs. Over the years, the chimpanzees in the current study voluntarily participated in other studies of cognition, motor skill, economic games, and social learning (see Neal Webb et al., 2019, for example from NCCC). It is possible that such participation could have skewed the cognitive data reported here, and that even more differences (in both cognitive performance and gray matter decline with age) may occur in chimpanzees who do not have such cognitively enriched lives. Furthermore, Walker and Jucker (2017) suggest that environmental enrichment may be one of many protective factors that prevent nonhuman primates from the development of AD-like dementia, despite the development of amyloid and tau pathology (and neurofibrillary tangles, in the case of chimpanzees). Future research should examine the relationship between these potential protective factors (genetics, cognitive enrichment and stimulation, overall health, physical activity and functioning, and strength of social networks) and cognitive performance in aging chimpanzees. In addition, carefully controlled studies with other nonhuman primates could elucidate the effects of cognitive stimulation on successful aging and determine if there is a protective effect against age-related gray matter loss, as well as AD-like pathology.
There are some limitations to the current study. Here we examined age- and cognition-related gray matter decline using cross-sectional data with incongruent timing of data collection with respect to the PCTB and MRI scan collection. That is to say, the sequence and duration of time between the collection of the PCTB data and MRI scans was not consistent across subjects. However, PCTB testing occurred within 5 years of MRI acquisition for 88% of the chimpanzees in the current study, and we also accounted for this discrepancy in timing by statistically controlling for both the age at the time of scan and age at the time of cognitive testing in our analyses. Ideally, future studies would align this data collection in time to more directly illuminate the relationship between chimpanzee cognitive performance and gray matter volume, in the context of aging. However, despite incongruence in the timing of cognitive testing and MRI collection in the current study, this is the best and only opportunity to examine such questions given the current policies prohibiting in vivo MRI collection in chimpanzees for research purposes. In addition, to determine the rate of cognitive and gray matter decline with age without additional repeated in vivo imaging data, researchers could examine changes between archival MRI data and postmortem MRI data, or carryout these studies in other nonhuman primates.
In summary, we determined that certain gray matter structures display volume declines as chimpanzees age, particularly in regions associated with overall cognition. In addition, chimpanzees with preserved cognition with age have increased gray matter volume in many regions compared with their poorly aging counterparts. Furthermore, these regions overlap considerably with those that normally decline with age, indicating that chimpanzees who perform better on cognitive tasks for their age may retain gray matter volume in these regions. Altogether, the current findings, along with those on cognitive and motor decline, and AD pathology, further confirm that chimpanzees are an important model species for comparative studies of aging. Furthermore, as the captive chimpanzee population is skewed toward adult and geriatric animals, it is important to further understand the effects of aging on cognition and neurobiology, see how these age-related effects may impact the long-term care of this aged population, and determine how facilities housing chimpanzees can foster healthy aging.
ACKNOWLEDGMENTS
This study was supported in part by the National Institutes of Health: NS-42867, NS-73134, and AG-067419. The NCCC chimpanzees were supported by Cooperative Agreement U42-OD011197. The authors declare no conflicts of interest.
Footnotes
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the National Chimpanzee Brain Resource at https://www.chimpanzeebrain.org/.
REFERENCES
- Albert MS, Jones K, Savage CR, Berkman L, Seeman T, Blazer D, & Rowe JW (1995). Predictors of cognitive change in older persons: Macarthur studies of successful aging. Psychology and Aging, 10(4), 578–589. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Chen K, Aschenbrenner M, Merkley TL, Santerre-Lemmon LE, Shamy JL, Skaggs WE, Buonocore MH, Rapp PR, & Barnes CA (2008). Age-related regional network of magnetic resonance imaging gray matter in the rhesus macaque. Journal of Neuroscience, 28(11), 2710–2718. 10.1523/jneurosci.1852-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JL, Jenkinson M, & Smith S (2007). Non-linear registration aka spatial normalisation fmrib technial report tr07ja2. FMRIB Analysis Group of the University of Oxford. [Google Scholar]
- Arnsten AFT, Datta D, Leslie S, Yang ST, Wang M, & Nairn AC (2019). Alzheimer's-like pathology in aging rhesus macaques: Unique opportunity to study the etiology and treatment of alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America, 116(52), 26230–26238. 10.1073/pnas.1903671116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autrey MM, Reamer LA, Mareno MC, Sherwood CC, Herndon JG, Preuss T, Schapiro SJ, & Hopkins WD (2014). Age-related effects in the neocortical organization of chimpanzees: Gray and white matter volume, cortical thickness, and gyrification. NeuroImage, 101, 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard KA, & Hopkins WD (2018). Early socioemotional intervention mediates long-term effects of atypical rearing on structural covariation in gray matter in adult chimpanzees. Psychological Science, 29(4), 594–603. 10.1177/0956797617740685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DE, Cauley JA, Lui LY, Fink HA, McCulloch C, Stone KL, & Yaffe K (2007). Women who maintain optimal cognitive function into old age. Journal of the American Geriatrics Society, 55(2), 259–264. [DOI] [PubMed] [Google Scholar]
- Bennett A, Pierre PJ, Wesley MJ, Latzman RD, Schapiro SJ, Mareno MC, & Hopkins WD (in press). Predicting their past: Machine language learning can discriminate the brains of chimpanzees with different early-life social rearing experiences. Developmental Science. [DOI] [PMC free article] [PubMed]
- Bigler ED, Andersob CV, & Blatter DD (2002). Temporal lobe morphology in normal aging and traumatic brain injury. American Journal of Neuroradiology, 23(2), 255–266. [PMC free article] [PubMed] [Google Scholar]
- Bigler ED, Blatter DD, Anderson CV, Johnson SC, Gale SD, Hopkins RO, & Burnett B (1997). Hippocampal volume in normal aging and traumatic brain injury. American Journal of Neuroradiology, 18, 11–23. [PMC free article] [PubMed] [Google Scholar]
- Boyes RG, Gunter JL, Frost C, Janke AL, Yeatman T, Hill DLG, Bernstein MA, Thompson PM, Weiner MW, Schuff N, Alexander GE, Killiany RJ, DeCarli C, Jack CR, & Fox NC (2008). Intensity non-uniformity correction using n3 on 3-t scanners with multichannel phased array coils. NeuroImage, 39(4), 1752–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Albert M, Belleville S, Craik FIM, Duarte A, Grady CL, Lindenberger U, Nyberg L, Park DC, Reuter-Lorenz PA, Rugg MD, Steffener J, & Rajah MN (2018). Maintenance, reserve and compensation: The cognitive neuroscience of healthy ageing. Nature Reviews Neuroscience, 19(11), 701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Errangi B, Li L, Glasser MF, Westlye LT, Fjell AM, Walhovd KB, Hu X, Herndon JG, Preuss TM, & Rilling JK (2013). Brain aging in humans, chimpanzees (Pan troglodytes) and rhesus macaques (Macaca mulatta): Magnetic resonance images of macro- and microstructural changes. Neurobiology of Aging, 34, 2248–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupé P, Yger P, Prima S, Hellier P, Kervrann C, & Barillot C (2008). An optimized blockwise nonlocal means denoising filter for 3-D magnetic resonance images. IEEE Transactions on Medical Imaging, 27(4), 425–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darusman H, Call J, Sajuthi D, Schapiro SJ, Gjedde A, Kalliokoski O, & Hau J (2014). Delayed response task performance as a function of age in cynomolgus monkeys (Macaca fascicularis). Primates, 55(2), 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darusman HS, Pandelaki J, Mulyadi R, Sajuthi D, Putri IA, Kalliokoski OH, & Gjedde A (2014). Poor memory performance in aged cynomolgus monkeys with hippocampal atrophy, depletion of amyloid beta 1–42 and accumulation of tau proteins in cerebrospinal fluid. In Vivo, 28(2), 173–184. [PubMed] [Google Scholar]
- Dicks E, Vermunt L, van der Flier WM, Visser PJ, Barkhof F, Scheltens P, & Tijms BM (2019). Modeling grey matter atrophy as a function of time, aging or cognitive decline show different anatomical patterns in Alzheimer's disease. NeuroImage: Clinical, 22, 101786. 10.1016/j.nicl.2019.101786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud G, Smith S, Jenkinson M, Behrens T, Johansen-Berg H, Vickers J, James S, Voets N, Watkins K, Matthews PM, & James A (2007). Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain, 130(9), 2375–2386. [DOI] [PubMed] [Google Scholar]
- Edler MK, Sherwood CC, Meindl RS, Hopkins WD, Ely JJ, Erwin JM, Mufson EJ, Hof PR, & Raghanti MA (2017). Aged chimpanzees exhibit pathologic hallmarks of Alzheimer's disease. Neurobiology of Aging, 59, 107–120. 10.1016/j.neurobiolaging.2017.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edler MK, Sherwood CC, Meindl RS, Munger EL, Hopkins WD, Ely JJ, Erwin JM, Perl DP, Mufson EJ, Hof PR, & Raghanti MA (2018). Microglia changes associated to Alzheimer's disease pathology in aged chimpanzees. Journal of Comparative Neurology, 526(18), 2921–2936. 10.1002/cne.24484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, Bauer C, Jennings D, Fennessy F, Sonka M, Buatti J, Aylward S, Miller JV, Pieper S, & Kikinis R (2012). 3D slicer as an image computing platform for the quantitative imaging network. Magnetic Resonance Imaging, 30(9), 1323–1341. 10.1016/j.mri.2012.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goveas JS, Rapp SR, Hogan PE, Driscoll I, Tindle HA, Smith JC, Kesler SR, Zaslavsky O, Rossom RC, Ockene JK, Yaffe K, Manson JE, Resnick SM, & Espeland MA (2016). Predictors of optimal cognitive aging in 80+ women: The women's health initiative memory study. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 71(Suppl 1), S62–S71. 10.1093/gerona/glv055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haier RJ, Jung RE, Yeo RA, Head K, & Alkire MT (2004). Structural brain variation and general intelligence. NeuroImage, 23(1), 425–433. 10.1016/j.neuroimage.2004.04.025 [DOI] [PubMed] [Google Scholar]
- Haier RJ, Schroeder DH, Tang C, Head K, & Colom R (2010). Gray matter correlates of cognitive ability tests used for vocational guidance. BMC Research Notes, 3, 206. 10.1186/1756-0500-3-206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Rapp PR, & Morrison JH (2012). Neuronal and morphological bases of cognitive decline in aged rhesus monkeys. Age, 34(5), 1051–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison TM, Weintraub S, Mesulam MM, & Rogalski E (2012). Superior memory and higher cortical volumes in unusually successful cognitive aging. Journal of the International Neuropsychological Society: JINS, 18(6), 1081–1085. 10.1017/S1355617712000847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon JG, Moss MB, Rosene DL, & Killiany RJ (1997). Patterns of cognitive decline in aged rhesus monkeys. Behavioural Brain Research, 87,25–34. [DOI] [PubMed] [Google Scholar]
- Herndon JG, Tigges J, Anderson DC, Klumpp SA, & McClure HM (1999). Brain weight throughout the life span of the chimpanzee. The Journal of Comparative Neurology, 409, 567–572. [PubMed] [Google Scholar]
- Herrmann E, Call J, Hernandez-Lloreda MV, Hare B, & Tomasello M (2007). Humans have evolved specialized skills of social cognition: The cultural intelligence hypothesis. Science, 317, 13601366. [DOI] [PubMed] [Google Scholar]
- Herrmann E, Hare B, Call J, & Tomasello M (2010). Differences in the cognitive skills of bonobos and chimpanzees. PLoS One, 5(8), e12438. 10.1371/journal.pone.0012438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann E, Hernandez-Lloreda MV, Call J, Hare B, & Tomasello M (2010). The structure of individual differences in the cognitive abilities of children and chimpanzees. Psychological Science, 21(1), 102–110. 10.1177/0956797609356511 [DOI] [PubMed] [Google Scholar]
- Hilger K, Winter NR, Leenings R, Sassenhagen J, Hahn T, Basten U, & Fiebach CJ (2020). Predicting intelligence from brain gray matter volume. Brain Structure & Function, 225(7), 2111–2129. 10.1007/s00429-020-02113-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan MJ, Staff RT, Bunting BP, Murray AD, Ahearn TS, Deary IJ, & Whalley LJ (2011). Cerebellar brain volume accounts for variance in cognitive performance in older adults. Cortex, 47(4), 441–450. 10.1016/j.cortex.2010.01.001 [DOI] [PubMed] [Google Scholar]
- Hopkins WD, & Avants BB (2013). Regional and hemispheric variation in cortical thickness in chimpanzees (Pan troglodytes). Journal of Neuroscience, 33, 5241–5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Latzman RD, Mareno MC, Schapiro SJ, Gomez-Robles A, & Sherwood CC (2018). Heritability of gray matter structural covariation and tool use skills in chimpanzees (Pan troglodytes): A source-based morphometry and quantitative genetic analysis. Cerebral Cortex, 29, 3702–3711. 10.1093/cercor/bhy250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Latzman RD, Mareno MC, Schapiro SJ, Gómez-Robles A, & Sherwood CC (2019). Heritability of gray matter structural covariation and tool use skills in chimpanzees (Pan troglodytes): A source-based morphometry and quantitative genetic analysis. Cerebral Cortex, 29(9), 3702–3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Li X, & Roberts N (2019). More intelligent chimpanzees (Pan troglodytes) have larger brains and increased cortical thickness. Intelligence, 74(18–24), 18–24. [Google Scholar]
- Hopkins WD, Mareno MC, Neal Webb SJ, Schapiro SJ, Raghanti MA, & Sherwood CC (2020). Age-related changes in chimpanzee (Pan troglodytes) cognition: Cross-sectional and longitudinal analyses. American Journal of Primatology, 83(3), e23214. 10.1002/ajp.23214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Mareno MC, & Schapiro SJ (2019). Further evidence of left hemisphere dominance in motor skill by chimpanzees on a tool use task. Journal of Comparative Psychology, 133(4), 512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Mulholland MM, Reamer LA, Mareno MC, & Schapiro SJ (2020). The role of early social rearing, neurological and genetic factors on individual differences in mutual eye gaze among captive chimpanzees. Scientific Reports, 10, 1–10. https://doi. org/10.1038/s41598–020-64051-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Reamer L, Mareno MC, & Schapiro SJ (2015). Genetic basis for motor skill and hand preference for tool use in chimpanzees (Pan troglodytes). Proceedings of the Royal Society: Biological Sciences B, 282(1800), 20141223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Russell JL, & Schaeffer J (2014). Chimpanzee intelligence is heritable. Current Biology, 24(14), 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, & Smith S (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17(2), 825–841. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Pechaud M, & Smith S (2005). Bet2: Mr-based estimation of brain, skull and scalp surfaces. Eleventh Annual Meeting of the Organization for Human Brain Mapping, 17, 167. [Google Scholar]
- Jenkinson M, & Smith S (2001). A global optimisation method for robust affine registration of brain images. Medical Image Analysis, 5(2), 143–156. [DOI] [PubMed] [Google Scholar]
- Joly M, Ammersdorfer S, Schmidtke D, & Zimmermann E (2014). Touchscreen-based cognitive tasks reveal age-related impairment in a primate aging model, the grey mouse lemur (Microcebus murinus). PLoS One, 9(10), e109393. 10.1371/journal.pone.0109393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaup AR, Mirzakhanian H, Jeste DV, & Eyler LT (2011). A review of the brain structure correlates of successful cognitive aging. The Journal of Neuropsychiatry and Clinical Neurosciences, 23(1), 6–15. 10.1176/jnp.23.1.jnp6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikinis R, Pieper SD, & Vosburgh KG (2014). 3D slicer: A platform for subject-specific image analysis, visualization, and clinical support, Intraoperative imaging and image-guided therapy (pp. 277–289). Springer Science & Business Media. [Google Scholar]
- Kim K, Han JW, So Y, Seo J, Kim YJ, Park JH, Lee SB, Lee JJ, Jeong HG, Kim TH, & Kim KW (2017). Cognitive stimulation as a therapeutic modality for dementia: A meta-analysis. Psychiatry Investigation, 14(5), 626–639. 10.4306/pi.2017.14.5.626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo BB, Schettler SP, Murray DE, Lee JM, Killiany RJ, Rosene DL, Kim DS, & Ronen I (2012). Age-related effects on cortical thickness patterns of the rhesus monkey brain. Neurobiology of Aging, 33, 200.e223–200e.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacreuse A, Parr L, Chennareddi L, & Herndon JG (2018). Age-related decline in cognitive flexibility in female chimpanzees. Neurobiology of Aging, 72, 83–88. 10.1016/j.neurobiolaging.2018.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacreuse A, Raz N, Schmidtke D, Hopkins WD, & Herndon JG (2020). Age-related decline in executive function as a hallmark of cognitive ageing in primates: An overview of cognitive and neurobiological studies. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 375(1811), 20190618. 10.1098/rstb.2019.0618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacreuse A, Russell JL, Hopkins WD, & Herndon JG (2014). Cognitive and motor aging in female chimpanzees. Neurobiology of Aging, 35(3), 623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai ZC, Moss MB, Killiany RJ, Rosene DL, & Herndon JG (1995). Executive system dysfunction in the aged monkey: Spatial and object reversal learning. Neurobiology of Aging, 16(6), 947–954. 10.1016/0197-4580(95)02014-4 [DOI] [PubMed] [Google Scholar]
- Lemaitre H, Goldman AL, Sambataro F, Verchinski BA, Meyer-Lindenberg A, Weinberger DR, & Mattay VS (2012). Normal age-related brain morphometric changes: Nonuniformity across cortical thickness, surface area and gray matter volume? Neurobiology of Aging, 33(617), e671–e679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland MM, Navabpour SV, Mareno MC, Schapiro SJ, Young LJ, & Hopkins WD (2020). Avpr1a variation is linked to gray matter covariation in the social brain network of chimpanzees. Genes, Brain and Behavior, 19, e12631. 10.1111/gbb.12631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger EL, Edler MK, Hopkins WD, Ely JJ, Erwin JM, Perl DP, Mufson EJ, Hof PR, Sherwood CC, & Raghanti MA (2018). Astrocytic changes with aging and alzheimer's disease-type pathology in chimpanzees. Journal of Comparative Neurology, 527, 1179–1195. 10.1002/cne.24610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal Webb SJ, Hau J, & Schapiro SJ (2019). Relationships between captive chimpanzee (Pan troglodytes) welfare and voluntary participation in behavioural studies. Applied Animal Behaviour Science, 214, 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny WD, Friston KJ, Ashburner JT, Kiebel SJ, & Nichols TE (Eds.), Statistical parametric mapping: The analysis of functional brain images. Elsevier. [Google Scholar]
- Picq JL (2007). Aging affects executive functions and memory in mouse lemur primates. Experimental Gerontology, 42(3), 223–232. 10.1016/j.exger.2006.09.013 [DOI] [PubMed] [Google Scholar]
- Rapp PR, & Amaral DG (1989). Evidence for task-dependent memory dysfunction in the aged monkey. The Journal of Neuroscience, 9, 3568–3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N (1997). Selective aging of the human cerebral cortex observed in vivo: Differential vulnerability of the prefrontal gray matter. Cerebral Cortex, 7, 268–282. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, & Acker JD (2005). Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cerebral Cortex, 15(11), 1676–1689. [DOI] [PubMed] [Google Scholar]
- Rosen RF, Farberg AS, Gearing M, Dooyema J, Long MP., Anderson DC., Davis-Turak J., Coppola G., Geschwind DH., Paré JF., Duong TQ., Hopkins WD., Preuss TM., & Walker LC. (2008). Tauopathy with paired helical filaments in an aged chimpanzee. Journal of Comparative Neurology, 509, 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen RF, Tomidokoro Y, Farberg AS, Dooyema J, Ciliax B, Preuss TM, Neubert TA, Ghiso JA, LeVine H, & Walker LC (2016). Comparative pathobiology of beta-amyloid and the unique susceptibility of humans to Alzheimer's disease. Neurobiology of Aging, 44, 185–196. 10.1016/j.neurobiolaging.2016.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JL, Lyn H, Schaeffer JA, & Hopkins WD (2011). The role of socio-communicative rearing environments in the development of social and physical cognition in apes. Developmental Science, 14(6), 1459–1470. 10.1111/j.1467-7687.2011.01090.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt V, Pankau B, & Fischer J (2011). Old world monkeys compare to apes in the primate cognition test battery. PLoS One, 7(4), e32024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood CC, Gordon AD, Allen JS, Phillips KA, Erwin JM, Hof PR, & Hopkins WD (2011). Aging of the cerebral cortex differs between humans and chimpanzees. Proceedings of the National Academy of Sciences of the United States of America, 108, 13029–13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM (2002). Fast robust automated brain extraction. Human Brain Mapping, 17(3), 143–155. 10.1002/hbm.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, & Matthews PM (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23, S208–S219. [DOI] [PubMed] [Google Scholar]
- Spector A, Orrell M, & Woods B (2010). Cognitive stimulation therapy (CST): Effects on different areas of cognitive function for people with dementia. International Journal of Geriatric Psychiatry, 25(12), 1253–1258. [DOI] [PubMed] [Google Scholar]
- Spector A, Thorgrimsen L, Woods B, Royan L, Davies S, Butterworth M, & Orrell M (2003). Efficacy of an evidence-based cognitive stimulation therapy programme for people with dementia: Randomised controlled trial. The British Journal of Psychiatry, 183(3), 248–254. [DOI] [PubMed] [Google Scholar]
- Stern Y, Arenaza-Urquijo EM, Bartrés-Faz D, Belleville S, Cantilon M, Chetelat G, Ewers M, Franzmeier N, Kempermann G, Kremen WS, Okonkwo O, Scarmeas N, Soldan A, Udeh-Momoh C, Valenzuela M, Vemuri P, & Vuoksimaa E (2020). Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer's & Dementia, 16(9), 1305–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisserand DJ, van Boxtel MPJ, Pruessner JC, Hofman P, Evans AC, & Jolles J (2004). A voxel-based morphometric study to determine individual differences in gray matter density associated with age and cognitive change over time. Cerebral Cortex, 14, 966–973. [DOI] [PubMed] [Google Scholar]
- Tustison N, Avants BB, Cook PA, & Gee JC (2010). N4itk: Improved n3 bias correction with robust b-spline approximation. Paper presented at the Proceedings of the ISBI. [DOI] [PMC free article] [PubMed]
- Tustison N, & Gee J (2009). N4itk: Nick's n3 ITK implementation for mri bias field correction. Insight Journal, 9. [Google Scholar]
- Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, & Gee JC (2010). N4itk: Improved n3 bias correction. IEEE Transactions on Medical Imaging, 29(6), 1310–1320. 10.1109/TMI.2010.2046908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustison NJ, Cook PA, Klein A, Song G, Das SR, Duda JT, Kandel BM, van Strien N, Stone JR, Gee JC, & Avants BB (2014). Large-scale evaluation of ants and freesurfer cortical thickness measurements. NeuroImage, 99, 166–179. 10.1016/j.neuroimage.2014.05.044 [DOI] [PubMed] [Google Scholar]
- Vickery S, Hopkins WD, Sherwood CC, Schapiro SJ, Latzman RD, Caspers S, Gaser C, Eickhoff SB, Dahnke R, & Hoffstaedter F (2020). Chimpanzee brain morphometry utilizing standardized MRI preprocessing and macroanatomical annotations. eLife, 9, e60136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LC, & Jucker M (2017). The exceptional vulnerability of humans to Alzheimer's disease. Trends in Molecular Medicine, 23(6), 534–545. 10.1016/j.molmed.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Luo X, Barnes D, Sano M, & Yaffe K (2014). Physical activity and risk of cognitive impairment among oldest-old women. The American Journal of Geriatric Psychiatry, 22(11), 1149–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodley MA, Fernandes HBF, & Hopkins WD (2015). The more G-loaded, the more heritable, evolvable, and phenotypically variable: Homology with humans in chimpanzee cognitive abilities. Intelligence, 50, 159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods B, Aguirre E, Spector AE, & Orrell M (2012). Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database of Systematic Reviews, 15(2):CD005562. [DOI] [PubMed] [Google Scholar]
- Woods B, Thorgrimsen L, Spector A, Royan L, & Orrell M (2006). Improved quality of life and cognitive stimulation therapy in dementia. Aging and Mental Health, 10(3), 219–226. [DOI] [PubMed] [Google Scholar]
- Workman KP, Healey B, Carlotto A, & Lacreuse A (2019). One-year change in cognitive flexibility and fine motor function in middle-aged male and female marmosets (Callithrix jacchus). American Journal of Primatology, 81(2), e22924. 10.1002/ajp.22924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Fiocco AJ, Lindquist K, Vittinghoff E, Simonsick EM, Newman AB, Satterfield S, Rosano C, Rubin SM, Ayonayon HN, & Harris TB (2009). Predictors of maintaining cognitive function in older adults: The health ABC study. Neurology, 72(23), 2029–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]


