Summary
The clinical application of genetics and genomics to advance precision health is one of the most dynamic and promising areas of medicine. In 2020, building on nearly 15 years of work, the Roundtable on Genomics and Precision Health of the National Academies of Sciences, Engineering, and Medicine undertook a strategic planning process to assess its strengths, consider the current challenges facing the field, and set out new goals for its future work. As a result, the Roundtable has updated its vision and mission and prioritized four major areas of inquiry—innovation, dialogue, equity, and adoption—while keeping true to its founding goal of providing a neutral convening space for the diversity of stakeholders in genomics and precision health. The Roundtable is unique for its breadth of membership and is committed to fostering a new era for precision health built on decades of expanding knowledge and the emergence of new technologies. To achieve its goals, the Roundtable seeks to broaden its membership’s diversity and to engage with new audiences. Roundtable members explore how evidence-based discoveries in genomics could be adopted and used in innovative ways to better serve human health, how equitable access to genomic and precision health technologies can be ensured, and how the Roundtable and broader genomics and precision health community can communicate more effectively to inform the public regarding genomics and precision health. As a first principle, the Roundtable is working to support the overall goal that all people benefit from genomics for precision health.
The clinical application of genetics and genomics to advance precision health is one of the most dynamic and promising areas of medicine. In 2020, building on nearly 15 years of work, the Roundtable on Genomics and Precision Health of the National Academies of Sciences, Engineering, and Medicine undertook a strategic planning process to assess its strengths, consider the current challenges facing the field, and set out new goals for its future work. As a result, the Roundtable has updated its vision and mission and prioritized four major areas of inquiry—innovation, dialog, equity, and adoption—while keeping true to its founding goal of providing a neutral convening space for the diversity of stakeholders in genomics and precision health. The Roundtable is unique for its breadth of membership and is committed to fostering a new era for precision health built on decades of expanding knowledge and the emergence of new technologies. To achieve its goals, the Roundtable seeks to broaden its membership’s diversity and to engage with new audiences. Roundtable members explore how evidence-based discoveries in genomics could be adopted and used in innovative ways to better serve human health, how equitable access to genomic and precision health technologies can be ensured, and how the Roundtable and broader genomics and precision health community can communicate more effectively to inform the public regarding genomics and precision health. As a first principle, the Roundtable is working to support the overall goal that all people benefit from genomics for precision health.
Introduction
Over three decades, the growth and application of genomics knowledge has expanded exponentially. Its transformative impact touches all of biomedicine and spans research, clinical care, and consumer applications with leadership in public, private, and philanthropic sectors. As recently noted in a NEJM perspective piece celebrating the National Academy of Medicine’s 50th anniversary, progress to decode the human genome has transformed science and medicine, and “the discovery of more than 100,000 robust associations between genomic regions and common diseases has pointed to new biologic mechanisms” across specialties and generated new diagnostics, counseling options, treatments, and therapies.1
This scientific and health progress is also fueling economic growth and an entire private sector of businesses fostering research and novel applications. The once-nascent global genomics market size has grown to $17.2 billion in 2019, and one private market report projected it to reach $82.6 billion by 2027.2 Private sector organizations, too, are interwoven in the fabric of genomics advances: diagnostics and technology companies are securing large amounts of public and venture capital to build new tools, biotechnology firms are applying genomic knowledge to create new therapeutics, consumer genomics companies are leveraging large datasets to advance basic and clinical research as well as consumer-driven testing for non-clinical and clinical use, and health systems are rapidly innovating to bring clinically actionable data into health care settings to improve decision-making, operations, and outcomes. An analysis by the American Society of Human Genetics released in 2021 found that human genetics and genomics contributed $265 billion to the U.S. economy in 2019 alone, a 5-fold increase over a decade.3 Further, the report indicated that, on the basis of tax revenues from and investment in human genetics and genomics, the federal government’s overall return on investment in this space was 4.75 to 1. Despite the many recent advances in the field of genomic medicine, concerns remain about managing costs associated with research and development and ensuring that there is demonstrated clinical value, including improved patient outcomes.4 Mechanisms for gathering additional evidence to support the use of genomics innovations and assessing their potential costs and benefits will be important as the field moves forward.
The potential of genomics to change the practice of medicine was recognized several years ago and is what led the National Academies of Sciences, Engineering, and Medicine (under the former Institute of Medicine) to found the Roundtable on Translating Genomic-Based Research for Health in 2007. Roundtables at the National Academies are convening activities that consist of members from across sectors and disciplines who represent government, for-profit and non-profit private sectors, patient groups, and academia. The members select challenging topics of importance and facilitate a range of discussions in a neutral setting to impact policies and programs, facilitate collaborations, and generate new ideas to shape the field. Roundtable members and the larger audience they reach (e.g., through their activities such as public workshops and perspective papers) apply the insights and lessons learned from these discussions within their own organizations and research activities. Often, the output from the Roundtable shapes policies at member organizations and in the broader community. Roundtables differ from other National Academies activities such as consensus studies, which publish recommendations or conclusions. At its inaugural meeting, the Roundtable members shared their mission “to advance the field of genomics and improve the translation of research findings to health care, education, and policy.” Some of the issues at that time included examining standards of evidence for the use of genetic/genomic tests or services in health care, the need for consistent oversight of the quality and validity of new genetic tests, concern over the commercialization of discoveries (e.g., patents and direct-to-consumer uses), adequate genetics training of health care professionals, bioethical issues of privacy and potential harms from the implementation of new genetic tools and technologies, and a recognition of the need for a high-level convening activity across stakeholder groups to foster discussions and inform the field. Since then, the Roundtable has fulfilled the role of a convener of multiple stakeholder groups to explore advances in the field by serving as a unique assembly for discussion, action, and outcomes spanning academia, industry, government, professional societies and associations, patient groups, and more. The members still share a mutual interest in addressing the issues surrounding the translation of genomics research for use in improving human health. Over the years, the group has revised its focal areas through horizon scanning and member input, but the 2020 strategic plan is the first of its kind in the Roundtable’s history; it has a formal and deliberate process to ensure we are meeting the needs of the rapidly evolving field in a more project- and output-driven way.
Impact and evolution of the Roundtable
A major impact of the Roundtable on the field has been through its 28 public workshops and companion proceedings which have covered a wide variety of topics, including generating evidence for genome-based diagnostic test and therapeutic development,4,5 integrating genomics into clinical practice,6 assessing the economics of genomic medicine,7 improving genetics education,8 applying implementation science,9 understanding disparities in access,10 and exploring the roles of consumer genomics and digital health technologies in the health care system.11,12 A full list of the workshop titles, dates, and links to the proceedings reports can be found in Table S1.
The output from the Roundtable’s activities are disseminated to a variety of audiences and through publications in JAMA, Nursing Outlook, NAM Perspectives, and the CDC’s Office of Genomics and Precision Health blog, to name a few.13,14 Our work has provided input to NIH and inspired the development of genomic medicine programs at large academic medical centers around the country, and our members have been consulted by legislative staff on Capitol Hill when questions arise about genetic technologies related to new proposed policy. The Roundtable has also been known for its “action collaboratives,” which have focused on implementing genomic medicine, population health, and advances in use of electronic medical records for genetics and genomics. (Action collaboratives are developed as ad hoc activities associated with the Roundtable on Genomics and Precision Health at the National Academies of Sciences, Engineering, and Medicine [the National Academies]. Action collaboratives do not necessarily represent the views of any one organization, the Roundtable, or the National Academies and have not been subjected to the review procedures of, nor are they a report or product of, the National Academies.) The Roundtable’s action collaboratives are ad hoc, short-term activities that stem from ideas generated from the Roundtable and often include participants with additional expertise needed for more implementation-oriented projects or products. Any products generated from these activities are independent of the National Academies and are attributed to the work of the action collaborative individuals. The Global Genomic Medicine Collaborative (G2MC), an action collaborative originally comprising 25 participating countries, launched in 2014 under the auspices of the Roundtable on Genomics and Precision Health from the Global Leaders in Genomic Medicine Summit and was incorporated as a 501(c)3 non-profit organization just 2 years later.15 Displaying and Integrating Genetic Information Through the Electronic Health Record (DIGITizE) was an action collaborative also initiated in 2014 with the goal of examining how genomic information can be uniformly represented and integrated into electronic health records (EHRs) in a standards-based format. DIGITizE resulted in a number of resources, including two implementation guides, one for pharmacogenomic clinical decision support rules for HLA-B∗57:01 and TPMT variants and another for clinical decision support rules for patients carrying variants associated with Lynch syndrome. Pilot projects at academic medical centers with EHR developers and laboratories tested the pharmacogenomics implementation guide within the local EHRs. DIGITizE moved onto the HL7 FHIR Foundation to continue with its mission. The Genomics and Population Health Action Collaborative (GPHAC) was an action collaborative founded in 2015 to identify challenges and potential best practices for the widespread integration of evidence-based genomics applications in population health programs. Representatives from state public health departments, federal government, health care systems, and other non-profit and academic institutions met to collaborate and develop their ideas around new partnerships and ways to implement and measure progress of population-based screening programs.16 Additionally, with 14 years of achievement and more than 30 sponsoring members today, the Roundtable has produced dozens of publications that have been downloaded around the world and used to inform graduate classes, policy, and genomic medicine program development and implementation.
Over time, the Roundtable has evolved with the dynamics of the field of genetic testing as it became more widely implemented in clinical care and has increasingly been applied in innovative ways to expand people’s access to information about their health. For example, in 2016, the focus of the group expanded to account for the exploration and adoption of new technologies that support the notion of precision health (e.g., digital health technologies such as wearables, sensors, and electronic medical records). Embracing this shift in focus, the membership updated their identity to the Roundtable on Genomics and Precision Health (the group was founded as the Roundtable on Translating Genomic-based Research for Health in 2007).
2020 Roundtable strategic planning
In 2020, the Roundtable undertook a comprehensive strategic planning effort to assess the research and clinical landscape and chart the group’s path forward. Phases of the planning included an environmental scan of the precision medicine landscape and context in which we are working, formation of a vision statement and description of our unique role in the community (see Box 1), an analysis of strengths and weaknesses, and decisions around the group’s goals and the principles to guide our future strategies.
Box 1. Future of the Roundtable on Genomics and Precision Health.
Vision: realizing the full potential of health for all through genomics and precision health.
Mission: to bring together diverse voices to encourage innovation and actions that foster the wide adoption of and equitable access to the benefits of genomics and precision health.
Guiding principles:
-
•
creating an inclusive and optimistic environment for discussion;
-
•
learning from successes and missteps in the field;
-
•
demanding reproducible evidence-based science;
-
•
sharing trustworthy information;
-
•
embracing interdisciplinary strategies;
-
•
optimizing data privacy and security;
-
•
advancing health equity in all that we do.
Early in the strategic planning effort, Roundtable members and staff conducted a landscape analysis and gathered strategic planning information from several stakeholder groups across the field of genomics and genetics to better understand potential opportunities to advance the field and the challenges that lay ahead. This crosswalk of existing strategic plans from organizations working in the fields of genomics included, but was not limited to, the National Human Genome Research Institute (NHGRI) of the National Institutes of Health (NIH); the “All of Us” Research Program at the NIH; the American College of Medical Genetics (ACMG); the American Society of Human Genetics (ASHG); the Association for Molecular Pathology (AMP); the Centers for Disease Control and Prevention (CDC) Office of Genomics and Precision Health; Genetic Alliance; Genome Canada; Genomics England; the Global Alliance for Genomics and Health (GA4GH); and the National Society of Genetic Counselors (NSGC). This information-gathering exercise allowed the group to consider how they could best contribute to overcoming those challenges. Common themes across many of these plans included educating the genomics/precision health workforce, increasing awareness for the power of genomics/precision health, informing policy decisions, and improving data infrastructure and the evidence base. Issues related to ethical, legal, and social implications (ELSIs) and diversity, equity, and inclusion (DEI) were observed as cross-cutting elements in several of the plans as well.17 A member survey was conducted in advance of the strategic planning sessions; collection of this information provided additional context for the planning and where specific attention should be directed during the planning process.
While encouraged by progress in the genomics and precision health field, the Roundtable members remained focused on the profound societal implications and use of genomic knowledge, as well as honest and sometimes difficult reflection on the field’s shortcomings and challenges and related, important choices for society, public policy, and private sector responsibility and stewardship. Open questions and challenges remain around the following:
-
•
how to encourage and facilitate data collection, use, and sharing while maintaining rigor for due privacy and human rights goals;18
-
•
deep shortcomings in the diversity of research populations and resulting disparities and importance of focusing on health equity and applicability of tools such as polygenic risk scores;
-
•
the legacy of genetics and genomics research and its misuse or misapplication, especially in relationship to underrepresented or marginalized populations;
-
•
root causes underlying gaps in adoption of evidence-based clinical practices in genetics and genomics in health care and public health systems; and
-
•
approaches for enhancing diversity in the genomics workforce.
Strategic planning discussions illuminated the challenge of focusing on genomics and precision health while also acknowledging the interface of genomics with many other facets of medicine, health, and society. Roundtable members recognized the role of the social determinants of health (i.e., the environmental conditions where people are born, live, learn, work, play, worship, and age)19 in contributing to improving population health outcomes.20 The strategic planning conversations among Roundtable members surfaced many topics—such as the interplay between genomics and the social determinants of health and the role of digital technologies, privacy policies, and data management, among others. The group considered these important topics on the basis of timeliness, membership interest, and the potential ability of the Roundtable’s actions to advance the subject area for the good of the biomedical community. While there were many important topics raised, certain topics were chosen as fruitful areas for future collaboration with other stakeholder groups that were already focused on those challenges, such as the National Academies’ Roundtable on Population Health Improvement.
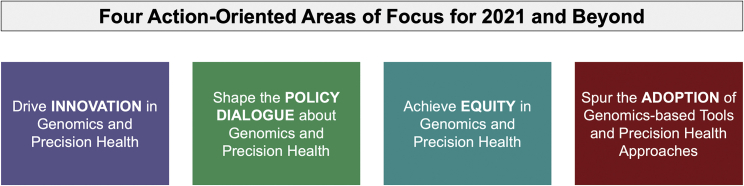
Not surprisingly, with the diverse spectrum of expertise on the Roundtable, more than a dozen potential areas of emphasis emerged from the strategic planning efforts, so Roundtable members used a logic model in combination with a discussion-driven process to consider where the group’s resources would have the most impact over the next few years. Ultimately, the group decided to focus on and prioritize four specific action-oriented areas for 2021, which are listed in Figure 1.
Figure 1.
Areas of focus for the Genomics Roundtable
Areas of focus
Following the information gathering stage and throughout 2020, the Roundtable members took stock of the group’s progress, and potential, to foster this vital, transformative community and ensure broad and equitable access to genomic benefits. Over 12 months, Roundtable members assessed the group’s progress, identified areas of shared interest and unique opportunity, and renewed their commitment to be a leading forum for discussion—and for action. The updated vision and mission of the Roundtable can be found below in Box 1.
Innovation
The goal of this workstream is to consider the barriers and facilitators of innovation for genomics-based diagnostics, risk assessment tools, and therapies such that policies, tools, and pathways can be developed to enhance innovation in genomics and precision health. The initial focus will be on case studies from the past decade to provide best practices and lessons learned that can then be used to promote innovative approaches that can accelerate commercialization and integration to drive impact of genomics on precision health. In this regard, the Roundtable could also provide a venue for discussing the potential value of genomics innovations to enable the field to move forward more efficiently and effectively.
Adoption
The goal of this workstream is to understand barriers and facilitators to the adoption of genomics as a tool for improved health and to use this knowledge to accelerate the appropriate adoption of evidence-based practices in genomics and precision health. Initial work will include gathering information from existing genomic medicine programs where widespread integration of genomics has been undertaken in order to identify gaps in adoption, root causes underlying these gaps, and potentially generalizable solutions. From this initial work, the adoption group will engage with the broad community of stakeholders represented on the Roundtable to develop activities that might serve to facilitate advancement in equitable access to genomic and precision health technologies, including informing policy deliberations, educational efforts, health technology standards development, and funding prioritization. The end goal of the group is to help ensure that health care and public health systems have a well-developed and clear path for adoption of genomic and precision health technologies in order to benefit the widest number of individuals possible.
Equity
The goal of this workstream is to foster action related to underrepresentation of diverse populations and inequities in genomic research, workforce, and access to genomic services by people who need them. The initial approach will involve an introspective view on the processes and practices the Roundtable employs to achieve its mission as well as learning from the external genomics communities broadly grappling with equity challenges. The aim of the group’s activities will be to offer opportunities for researchers, clinicians, and others to consider how to more fully represent underserved groups in genomics research and also to reduce the genomic and precision medicine divide in health care.
Shaping the dialogue
The goal of this workstream is to accelerate the dissemination of actionable knowledge to shape practice and increase public awareness and to inform and influence how decisions are made across the Roundtable’s broad stakeholder community. One approach that this group will take is to implement an infrastructure within and across the redefined workstreams for the purpose of increasing awareness, transparency, and accountability to the Roundtable’s commitment to action. Success of the Roundtable is dependent on effective communication and dialog with our community of participants, researchers, clinicians, industry, and policy makers.
Plan implementation
With the working group themes finalized in the fall of 2020, members chose their areas of interest to commit to, and co-facilitators were chosen for each group and asked to work with the members to develop quarterly plans and metrics for assessing progress for 2021. The equity group began their work as early as November 2020, hosting a series of educational sessions for the Roundtable members on systemic racism and diversity needs of the field. In early 2021, all of the new working groups convened to take action around their quarterly goals—some of which are to collect information to inform their steps toward papers (such as this one) or public discussions.
A path to impact
The Roundtable is poised to impact the field of genomics and precision health over the next decade. This cannot be accomplished without the engagement of the broadest possible community of stakeholders and experts both in the United States and globally. Realizing the full potential of Genomics and Precision Health will require your engagement, your partnership, and your voice in our work. For our work to be as impactful as we aspire it to be, we call on you to join us in the debate and dialogue, the strategies and policy agenda, and ultimately in its implementation. To learn more about how to connect with the Roundtable and its members, please visit the group’s website (available in the web resources section below).
Acknowledgments
The authors would like to thank Vence Bonham (NIH/NHGRI), Nikoletta Sidiropoulos (University of Vermont Medical Center), and Catherine Wicklund (Northwestern University) for their careful review of the paper and thoughtful comments and edits.
Declaration of interests
Geoffrey S. Ginsburg reports the following: consulting for Konica-Minolta and Fabric Genomics and ownership interest in Peer Medical, Origin Commercial Advisors, Predigen, MeTree&You, and Coprata. He receives royalties from Elsevier.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ajhg.2021.08.015.
Web resources
Global Genomic Medicine Collaborative (G2MC), https://g2mc.org/
Roundtable on Genomics and Precision Health, https://www.nationalacademies.org/our-work/roundtable-on-genomics-and-precision-health
Supplemental information
References
- 1.Collins F.S., Doudna J.A., Lander E.S., Rotimi C.N. Human Molecular Genetics and Genomics - Important Advances and Exciting Possibilities. N. Engl. J. Med. 2021;384:1–4. doi: 10.1056/NEJMp2030694. [DOI] [PubMed] [Google Scholar]
- 2.Fortune Business Insights . 2020. Pharmaceutical – Genomics Market Analysis.https://www.fortunebusinessinsights.com/industry-reports/genomics-market-100941 [Google Scholar]
- 3.American Society of Human Genetics (ASHG) 2021. The Economic Impact and Functional Applications of Human Genetics and Genomics.https://www.ashg.org/wp-content/uploads/2021/05/ASHG-TEConomy-Impact-Report-Final.pdf [Google Scholar]
- 4.IOM . The National Academies Press; Washington, DC: 2012. Genome-Based Therapeutics: Targeted Drug Discovery and Development: Workshop Summary. [PubMed] [Google Scholar]
- 5.IOM . The National Academies Press; Washington, DC: 2011. Generating Evidence for Genomic Diagnostic Test Development: Workshop Summary. [PubMed] [Google Scholar]
- 6.IOM . The National Academies Press; Washington, DC: 2012. Integrating Large-Scale Genomic Information into Clinical Practice: Workshop Summary. [PubMed] [Google Scholar]
- 7.IOM . The National Academies Press; Washington, DC: 2013. The Economics of Genomic Medicine: Workshop Summary. [PubMed] [Google Scholar]
- 8.IOM . The National Academies Press; Washington, DC: 2015. Improving Genetics Education in Graduate and Continuing Health Professional Education: Workshop Summary. [PubMed] [Google Scholar]
- 9.NASEM . The National Academies Press; Washington, DC: 2016. Applying an Implementation Science Approach to Genomic Medicine: Workshop Summary. [PubMed] [Google Scholar]
- 10.NASEM . The National Academies Press; Washington, DC: 2018. Understanding Disparities in Access to Genomic Medicine: Proceedings of a Workshop. [PubMed] [Google Scholar]
- 11.NASEM . The National Academies Press; Washington, DC: 2020. Exploring the Current Landscape of Consumer Genomics: Proceedings of a Workshop. [PubMed] [Google Scholar]
- 12.NASEM . The National Academies Press; Washington, DC: 2020. The Role of Digital Health Technologies in Drug Development: Proceedings of a Workshop. [PubMed] [Google Scholar]
- 13.Chambers D.A., Feero W.G., Khoury M.J. Convergence of Implementation Science, Precision Medicine, and the Learning Health Care System: A New Model for Biomedical Research. JAMA. 2016;315:1941–1942. doi: 10.1001/jama.2016.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams J.K., Feero W.G., Leonard D.G.B., Coleman B. Implementation science, genomic precision medicine, and improved health: A new path forward? Nurs. Outlook. 2017;65:36–40. doi: 10.1016/j.outlook.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 15.Ginsburg G.S. A Global Collaborative to Advance Genomic Medicine. Am. J. Hum. Genet. 2019;104:407–409. doi: 10.1016/j.ajhg.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray M.F., Evans J.P., Angrist M., Chan K., Uhlmann W., Doyle D.L., Fullerton S.M., Ganiats T., Hagenkord J., Imhof S. A Proposed Approach for Implementing Genomics-Based Screening Programs for Healthy Adults. NAM Perspectives. 2018 Discussion Paper, National Academy of Medicine, Washington, DC. [Google Scholar]
- 17.Green E.D., Gunter C., Biesecker L.G., Di Francesco V., Easter C.L., Feingold E.A., Felsenfeld A.L., Kaufman D.J., Ostrander E.A., Pavan W.J. Strategic vision for improving human health at The Forefront of Genomics. Nature. 2020;586:683–692. doi: 10.1038/s41586-020-2817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meagher K.M., Cadigan R.J., Henderson G.E., Juengst E. 2019. Public Health Genomics, Biobanking, and Ethics. Oxford Handbooks Online.https://www.oxfordhandbooks.com/view/10.1093/oxfordhb/9780190245191.001.0001/oxfordhb-9780190245191-e-57 [Google Scholar]
- 19.U.S. Department of Health and Human Services (HHS) 2021. Healthy People 2030: Social Determinants of Health.https://health.gov/healthypeople/objectives-and-data/social-determinants-health [Google Scholar]
- 20.Braveman P., Gottlieb L. The social determinants of health: it’s time to consider the causes of the causes. Public Health Rep. 2014;129(Suppl 2):19–31. doi: 10.1177/00333549141291S206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.