Abstract
(1) To evaluate the prevalence of severe and chronic fatigue in subjects with and without chronic disease; (2) to assess to which extent multi-morbidity contributes to severe and chronic fatigue; and (3) to identify predisposing and associated factors for severe and chronic fatigue and whether these are disease-specific, trans-diagnostic, or generic. The Dutch Lifelines cohort was used, including 78,363 subjects with (n = 31,039, 53 ± 12 years, 33% male) and without (n = 47,324, 48 ± 12 years, 46% male) ≥ 1 of 23 chronic diseases. Fatigue was assessed with the Checklist Individual Strength-Fatigue. Compared to participants without a chronic disease, a higher proportion of participants with ≥ 1 chronic disease were severely (23% versus 15%, p < 0.001) and chronically (17% versus 10%, p < 0.001) fatigued. The odds of having severe fatigue (OR [95% CI]) increased from 1.6 [1.5–1.7] with one chronic disease to 5.5 [4.5–6.7] with four chronic diseases; for chronic fatigue from 1.5 [1.5–1.6] to 4.9 [3.9–6.1]. Multiple trans-diagnostic predisposing and associated factors of fatigue were found, explaining 26% of variance in fatigue in chronic disease. Severe and chronic fatigue are highly prevalent in chronic diseases. Multi-morbidity increases the odds of having severe and chronic fatigue. Several trans-diagnostic factors were associated with fatigue, providing a rationale for a trans-diagnostic approach.
Subject terms: Fatigue, Comorbidities
Introduction
For most people, fatigue is an everyday experience. It becomes a symptom when it is an overwhelming feeling of exhaustion that interferes with the ability to function and perform activities1. Severe fatigue that persists longer than 6 months is defined as chronic2. Severe and chronic fatigue is a symptom of many non-communicable chronic diseases e.g. Parkinson’s disease (PD)3, chronic heart failure (CHF)4, chronic obstructive pulmonary disease (COPD)5, type I diabetes mellitus (T1DM)6, rheumatoid arthritis (RA)7, and inflammatory bowel disease8. Fatigue is associated with functional impairment and often reported by patients to be one of the most burdensome and challenging aspects of their disease9,10. Thus far, fatigue is mostly studied in the context of a single chronic condition. However, the proportion of patients living with two or more chronic diseases concurrently (i.e., multi-morbidity) rises11,12. This complicates our understanding of the relationship between chronic disease and fatigue.
Research comparing the prevalence of fatigue in people with and without a specific chronic disease generally found that fatigue is more prevalent in patients with a chronic disease5,8,13–15. This suggests that fatigue is linked to a specific chronic disease. Moreover, since prevalence rates of severe fatigue seem to vary across chronic diseases16, it is often assumed that fatigue is a disease-specific symptom. However, the relationship between traditional markers of disease (severity and activity) and fatigue is generally poor or even non-existent. For instance, 40% of patients with T1DM experience chronic fatigue, but fatigue is weakly associated with abnormal glucose parameters6. A similar lack of a relationship between markers of disease- and fatigue severity, has been reported in patients with multiple sclerosis (MS)14, COPD5, asthma17, systemic lupus erythematosus (SLE)18, RA19, idiopathic pulmonary fibrosis and sarcoidosis20. Hence, it seems plausible that other than the specific disease severity factors may be involved.
In identifying the factors that contribute to fatigue in chronic disease, two types can be distinguished: (1) time-independent or pre-morbid predisposing factors, that are not likely to change over time, and (2) time-dependent associated or maintaining factors, which may change over time. Knowledge about pre-morbid predisposing factors (hereafter called ‘predisposing factors’) will help identify patients at risk for developing clinically relevant levels of fatigue, while time-dependent associated factors (hereafter called ‘associated factors’) can be addressed in interventions that can reduce fatigue or help patients to manage fatigue. Predisposing socio-demographic characteristics such as a lower education21–23 and being female21,23 were found to be related to higher levels of fatigue in specific chronic diseases. Furthermore, certain personality traits are assumed to increase a person’s risk of becoming severely fatigued in response to a chronic disease. Indeed, there is evidence from research in patients with MS that there is a link between neuroticism and fatigue24–26. Besides these predisposing factors, modifiable factors such as depressed mood and anxiety may be associated with, or maintain, fatigue in specific chronic diseases6,27–30. Also, lower physical activity levels31–33, sleep disturbances7, lower body mass index (BMI)34, hyper- or hypotension35,36, pain6,28,37,38, and smoking status39,40 have been identified as factors possibly contributing to fatigue in specific chronic diseases.
Thus, fatigue in patients with a chronic illness is a complex symptom that likely involves various predisposing and associated factors. Some of the factors seem to be associated with multiple chronic diseases. Nevertheless, the above-mentioned factors have all been studied in a disease-specific context. To date, studies seldom have examined fatigue across a wide range of chronic diseases simultaneously. Only one study investigated fatigue across 15 chronic diseases, combining data from 15 clinical studies16. The results indicated that factors associated with fatigue seem mostly trans-diagnostic. Limitations however included that their sample might have been biased because of an over- or underrepresentation of fatigue cases, as data from 15 clinical studies were combined, (often) specifically designed to study fatigue. In addition, multi-morbidity and some major chronic diseases such as cardiovascular disease and chronic lung disease were not considered in that study, and a control group without chronic disease was lacking.
To date, fatigue levels have never been assessed within one large, longitudinal cohort from the general population involving presumably healthy subjects as well as patients with various chronic diseases. Such a study would enable the comparison of fatigue prevalence rates of patients with a variety of conditions and a population without chronic disease. A better understanding of the factors underlying fatigue across a wide range of conditions and healthy subjects, and knowing whether these are disease-specific, trans-diagnostic (similar for multiple chronic diseases) or generic (similar for persons with and without a chronic disease), may optimize and accelerate the development of interventions for fatigue to improve daily functioning of patients with a chronic disease. Moving away from a disease-specific focus to a trans-diagnostic approach also supports the complexity of multi-morbid chronic disease management, as disease-centered treatments will not fully address the comprehensive needs of patients with multiple chronic diseases41.
The objectives of the present study were: (1) to evaluate the prevalence of severe and chronic fatigue in subjects with and without chronic disease; (2) to assess the extent to which multi-morbidity contributes to severe and chronic fatigue; and (3) to identify possible predisposing and associated factors for severe and chronic fatigue and whether these are disease-specific, trans-diagnostic, or generic.
Methods
Study design and participants
The current study used data from the Lifelines Cohort Study42,43. Lifelines is an ongoing multi-disciplinary prospective population-based cohort study examining in a unique three-generation design the health and health-related behaviours of 167,729 persons living in the North of The Netherlands. It employs a broad range of investigative procedures in assessing the biomedical, socio-demographic, behavioural, physical and psychological factors which contribute to the health and disease of the general population, with special focus on multi-morbidity and complex genetics. The participants were recruited between 2006 and 2013, through general practitioners and self-enrollment. Participants who were unable to understand the Dutch language, were not able to fill in questionnaires, not able to visit the general practitioner, had severe mental illness (i.e. not fully capable to make rational decisions), or who had limited life expectancy (< 5 years) due to severe illness were not considered eligible. Every 5 years, participants visit a Lifelines research site for the collection of biological materials and a comprehensive physical assessment. In addition, participants are asked to fill out an extensive set of questionnaires, including questions on medical history, socioeconomic status, psychological status, environmental factors, and lifestyle. In-between these 5-year assessments, once every 1.5 years a follow-up questionnaire is administered. The Lifelines Cohort Study was conducted according to the principles of the Declaration of Helsinki and approved by the Medical Ethics Committee of the University Medical Center Groningen, the Netherlands (number 2007/152). No additional ethical approval is needed to request data collected within the regular protocol of Lifelines. All participants signed an informed consent. A detailed description of the Lifelines Cohort Study has been published elsewhere42–44.
Procedures
The main outcome parameter fatigue as well as the time-dependent associated factors were measured at the first follow-up measurement, which was approximately 5 years after the baseline assessment. The predisposing factors and the presence of a chronic illness were evaluated at the baseline assessment, assuming that these are time-independent. The associated and predisposing factors were selected based on evidence from existing literature, which identified these factors as likely contributors to fatigue in specific chronic diseases. Participants were included in the current study if they had provided information on the main outcome parameter fatigue and were 18 years or older.
Measures
Fatigue severity
Fatigue severity was measured using the Checklist Individual Strength (CIS)45. The CIS consists of 20 items that measure four aspects of fatigue: fatigue severity, problems with concentrating, reduction in motivation, and reduced physical activity level. The current study reports on fatigue severity using the subscale fatigue severity (CIS-Fatigue). The CIS-Fatigue consists of eight items scored on a seven-point Likert scale. The score ranges from 8 to 56, with higher scores indicating more severe fatigue. Severe (i.e. clinically relevant) fatigue is indicated by a CIS-Fatigue score ≥ 35 points, a validated cut-off score46. Chronic fatigue was defined as severe fatigue (CIS-Fatigue score ≥ 35 points) lasting at least 6 months according to self-report. Participants who experienced severe fatigue but did not report their fatigue duration were omitted from the analyses on chronic fatigue. The CIS is a standardized and validated instrument that has been used in healthy subjects46–48, and among various patient populations5,17,49,50.
Self-reported chronic disease(s)
Participants were asked to self-report the chronic diseases they have from a pre-defined list of 23 chronic medical conditions. The 23 chronic diseases were carefully selected from a broader list of chronic diseases by the authors of the current manuscript based on their non-self-limiting nature, the association with persistent and recurring health problems, and a duration in months and years, not days and weeks51. The diseases were clustered in 9 classes of chronic somatic conditions: neurological diseases (migraine, epilepsy, MS, PD, stroke); liver diseases (hepatitis, liver cirrhosis); blood disease (blood clotting disorder); endocrine and metabolic diseases (hypothyroidism or hyperthyroidism, diabetes mellitus type 1 and/or 2); circulatory diseases (thrombosis, myocardial infarction, heart valve problems, pulmonary embolism, CHF, balloon angioplasty and/or bypass surgery); respiratory disease (chronic inflammation of the throat and/or nasal cavity, COPD); inflammatory bowel disease (ulcerative colitis, Crohn’s disease); rheumatic diseases (osteoarthritis, RA); and kidney disease52.
Possible predisposing factors of fatigue
Sociodemographic factors
Sex and education level were assessed with a questionnaire. Education level was divided into low (lower secondary education or less), middle (upper secondary education), and high education (tertiary education).
Personality traits
Four facets of neuroticism were measured using the Revised NEO Personality Inventory (NEO PI-R)53. Facets of neuroticism included in the Lifelines questionnaire were: anger/hostility, self-consciousness, impulsivity, and vulnerability. Each facet is assessed with eight items, scored on a five-point Likert scale that ranges from strongly disagree to strongly agree.
Possible associated factors of fatigue
Sociodemographic factors
Self-report questionnaires were used to gather data on age, household composition (single person household, a household with two persons with or without children, a single parent household, and another composition), partner status (yes/no), number of people living in the household, and current employment status (working ≥ 12 h per week or less)54.
Anthropometry
BMI, waist circumference, resting heart rate in beats and blood pressure measurements (diastolic blood pressure (DBP) and systolic blood pressure (SBP)) were assessed by a research assistant using a standardized protocol43.
Lifestyle factors
Lifestyle factors included current smoking status (yes/no) and being involved in leisure-time sports activities (yes/no). Sports participation was assessed with the item “sports participation” of the Short Questionnaire to Assess Health-enhancing physical activity (SQUASH)55.
Mental health disorders
Depressive disorder (depressive disorder and dysthymic mood) and anxiety disorder (panic disorder with and without agoraphobia, agoraphobia without panic disorder, social phobia, and generalized anxiety disorder) were assessed using the self-report version of the Mini-International Neuropsychiatric Interview (M.I.N.I., version 5.0.0)56.
Pain
A dichotomous variable (yes/no) was created based on one question (e.g. to what extent did your locomotor apparatus pain hamper your normal activities in the past 6 months?) indicating whether bodily pain (much or very much) hampered individuals in performing their normal activities44.
Statistical analyses
Statistical analyses were performed using SPSS (V.25.0 for Windows, Chicago, IL, USA).
Prevalence
Descriptive statistics and the prevalence rates of fatigue (continuous variable), severe (CIS-Fatigue score ≥ 35, dichotomous variable), and chronic (CIS-Fatigue score ≥ 35 lasting ≥ 6 months, dichotomous variable) fatigue for participants with and without a chronic disease were reported as mean and standard deviation, median and interquartile range, or frequency and percentage, as appropriate.
Impact of multi-morbidity on the likelihood of experiencing severe and chronic fatigue
An unadjusted and adjusted (corrected for age and sex57) logistic regression was performed to determine the likelihood that participants with one to four chronic diseases were severely and chronically fatigued, compared with persons without a chronic disease.
Predisposing and associated factors of fatigue
Analyses of variance (ANOVA) were carried out to investigate whether the possible predisposing and associated factors of fatigue in chronic disease are disease-specific or trans-diagnostic, or generic. The following analyses were performed: (1) a model with only the main effect of chronic disease (model A), (2) a model with the main effect of chronic disease and the potentially predisposing and associated factors (model B), and (3) a model with the same main effects, but also with the interaction effects between chronic disease and the predisposing and associated factors on fatigue severity (model C). Fatigue was entered as a continuous variable. In the disease-specific versus trans-diagnostic model the variable “chronic disease” was entered as a categorical variable with 15 categories (each category reflecting a specific chronic disease with no intrinsic order: COPD, blood clotting disorder, stroke, angioplasty and/or bypass surgery, ulcerative colitis, thrombosis, heart valve problems, hepatitis, epilepsy, RA, diabetes mellitus type 1 and/or 2, hypothyroidism or hyperthyroidism, chronic inflammation of throat and/or nasal cavity, osteoarthritis, migraine). In the trans-diagnostic versus generic model (i.e., also associated with fatigue in subjects without chronic disease) the categorical variable “chronic disease” consisted of two categories (coded as: 0 = no chronic disease and 1 = single-morbidity). Of note, only participants with single-morbidity and chronic diseases reported by ≥ 150 participants were included in the ANOVA analyses. MS, PD, liver cirrhosis, Crohn’s disease, CHF, myocardial infarction, pulmonary embolism, and kidney disease were therefore excluded as there were less than 150 participants who suffered from these diseases. In case of a significant interaction effect, a simple linear regression was performed as post-hoc test to analyze the effect of the possible predisposing and associated variable on the fatigue severity for a specific chronic disease (the disease-specific versus trans-diagnostic model) or for subjects with and without a chronic disease in general (the trans-diagnostic versus generic model).
In addition, to explore the relation between the factors and clinically relevant levels of fatigue (severe and chronic fatigue) logistic regression analyses were performed. The logistic regression analyses did not include interaction effects. For the logistic regression models and ANOVA, the predisposing and associated variables were checked for multicollinearity by inspecting the correlation coefficients. Waist circumference and SBP were left out of the analysis since they highly correlated (r > 0.7, p < 0.05) with BMI (r = 0.83) and DBP (r = 0.72). The level of significance was set at < 0.05.
Results
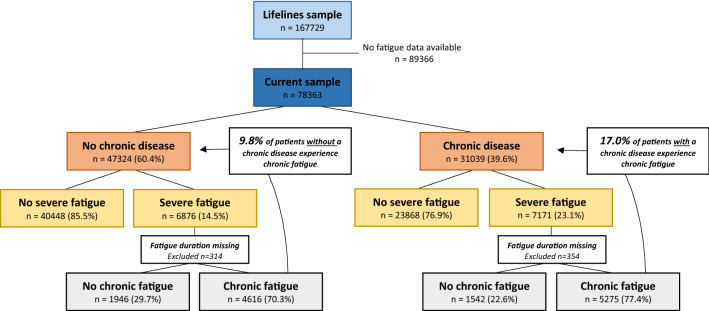
Between 2006 and 2013, 167,729 subjects were registered in the Lifelines Cohort Study, of whom 78,363 provided information on fatigue during the first follow-up measurement. Of note, 354 participants with a chronic disease and 314 without a chronic disease who experienced severe fatigue did not report their fatigue duration and were therefore omitted from the analyses on chronic fatigue. Forty percent of the total sample (n = 31,039) reported one or more of the 23 chronic diseases. Participants with one or more chronic diseases were on average 5 years older, more often female, less often completed tertiary education, less often currently employed, lived together with fewer people, had a higher BMI, a slightly higher resting heart rate, higher SBP, and a larger waist circumference. Moreover, participants with a chronic disease were less often involved in leisure-time sports activities, more often depressed and anxious, experienced substantially more bodily pain that hampers in performing activities, and scored higher on the neuroticism facets of anger/hostility, self-consciousness, impulsivity, and vulnerability. The proportion of participants that was smoking was higher in the group without chronic disease. The groups were comparable in terms of diastolic blood pressure and having a partner (Table 1). Of the 31,039 participants with one or more chronic diseases, the majority (72%, n = 22,293) had single-morbidity. See Supplemental Fig. 1 for the prevalence of chronic diseases in the sample.
Table 1.
Baseline characteristics of participants with and without a chronic disease (n = 78,363).
| No chronic illness (n = 47,324) | Chronic illness (n = 31,039) | |
|---|---|---|
| Predisposing factors | ||
| Sociodemographic factors | ||
| Male, n (%) | 21,965 (46.4) | 10,129 (32.6) |
| Education, n (%) | ||
| Low | 6607 (14.2) | 5609 (18.6) |
| Middle | 24,646 (53.1) | 16,521 (54.7) |
| High | 15,134 (32.6) | 8090 (26.8) |
| Personality traits | ||
| Neuroticism facets of anger/hostility, self-consciousness, impulsivity, and vulnerability, mean ± SD | 19.5 ± 3.1 | 20.0 ± 3.2 |
| Associated factors | ||
| Sociodemographic factors | ||
| Age, mean ± SD | 48.2 ± 12.3 | 53.2 ± 12.2 |
| Household composition, n (%) | ||
| Single person household | 4471 (10.9) | 3383 (12.8) |
| Couple household without children | 13,785 (33.5) | 11,095 (42.0) |
| Couple household with children | 20,156 (49.0) | 10,453 (39.6) |
| Single parent | 1349 (3.3) | 965 (3.7) |
| Other | 1374 (3.3) | 502 (1.9) |
| Partner, n (%) | 35,936 (82.9) | 23,572 (82.6) |
| Currently employed (≥ 12 h/week), n (%) | 32,315 (74.9) | 16,817 (59.3) |
| Nr. of people living in the house (median (IQR)) | 3 (2–4) | 2 (2–4) |
| Anthropometry | ||
| BMI, mean ± SD | 25.8 ± 4.0 | 26.6 ± 4.6 |
| Waist circumference, mean ± SD | 89.6 ± 12.1 | 91.5 ± 12.9 |
| Diastolic blood pressure, mean ± SD | 74.2 ± 9.5 | 74.2 ± 9.4 |
| Systolic blood pressure, mean ± SD | 128.1 ± 16.1 | 130.1 ± 16.8 |
| Resting heart rate, mean ± SD | 68.4 ± 11.1 | 69.5 ± 11.2 |
| Lifestyle | ||
| Currently smoking, n (%) | 7703 (16.3) | 4608 (14.9) |
| Currently engaged in leisure-time sports activities, n (%) | 24,317 (56.0) | 14,256 (49.8) |
| Mental health | ||
| Current depressive disorder, n (%) | 1245 (3.2) | 1291 (5.2) |
| Current anxiety disorder, n (%) | 2652 (6.9) | 2425 (9.8) |
| Pain | ||
| Bodily pain that hampers in performing normal activities, n (%) | 1307 (2.8) | 1915 (6.2) |
n number; SD standard deviation, IQR interquartile range, BMI body mass index.
Prevalence of severe and chronic fatigue in the general population: with and without chronic disease
Of the 78,363 participants 18% experienced severe fatigue and 13% experienced chronic fatigue. Overall, participants with one or more chronic diseases had a higher mean CIS-Fatigue score (24.8 ± 12.2 versus 21.4 ± 11.1 points, p < 0.001) and more often reported severe (23% versus 15%, p < 0.001) and chronic (17% versus 10%, p < 0.001) fatigue, compared to participants without a chronic disease (Fig. 1).
Figure 1.
Prevalence of severe and chronic fatigue in participants with and without a chronic disease. Of note, participants with severe fatigue who did not report their fatigue duration were omitted from the analyses on chronic fatigue, hence the smaller sample size of chronic fatigue versus severe fatigue.
Difference in prevalence rate of severe and chronic fatigue among chronic diseases
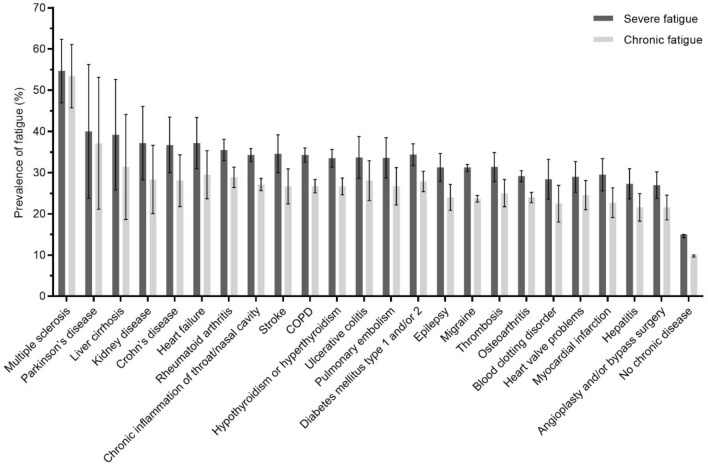
Differentiating between the 23 included chronic diseases, prevalence rates ranged from 27 to 55% for severe fatigue and from 22 to 53% for chronic fatigue (Fig. 2). The 95% confidence intervals (95% CI’s) around the mean prevalence of severe and chronic fatigue of the included diseases generally overlap, except for MS which was significantly different from the other besides PD and liver disease. Table 2 describes the prevalence rates of severe and chronic fatigue among the classes of chronic medical conditions (Table 2).
Figure 2.
Prevalence and 95% Confidence Intervals of severe and chronic fatigue across 23 chronic diseases and in subjects without a chronic disease. Of note: (1) subjects that did not report their fatigue duration were omitted from the calculation of chronic fatigue, (2) the bars represent participants with multi-morbidity, hence subjects can be represented multiple times in the analyses. COPD chronic obstructive pulmonary disease.
Table 2.
Prevalence of severe and chronic fatigue in classes of chronic medical conditions and no chronic disease.
| n | Severe fatigue n(%) |
Chronic fatigue n(%) |
|
|---|---|---|---|
| Neurological diseases | |||
| Including migraine | 15,505 | 3882 (25.0) | 2855 (18.4) |
| Excluding migraine | 1672 | 482 (28.8) | 369 (22.1) |
| Respiratory diseases | 7652 | 2097 (27.4) | 1574 (20.6) |
| Rheumatic diseases | 7526 | 1765 (23.5) | 1327 (17.6) |
| Endocrine and metabolic diseases | 4142 | 1086 (26.2) | 808 (19.5) |
| Circulatory diseases | 3358 | 756 (22.5) | 553 (16.5) |
| Liver diseases | 873 | 185 (21.2) | 138 (15.8) |
| Inflammatory bowel diseases | 676 | 185 (27.4) | 143 (21.2) |
| Blood diseases | 442 | 98 (22.2) | 76 (17.2) |
| Kidney disease | 144 | 45 (31.3) | 32 (22.2) |
| No chronic disease | 47,324 | 6876 (14.5) | 4616 (9.8) |
*The classes of chronic medical conditions represent participants with multi-morbidity, hence subjects can be presented multiple times in the analyses.
Impact of multi-morbidity on severe and chronic fatigue compared to participants without a chronic disease
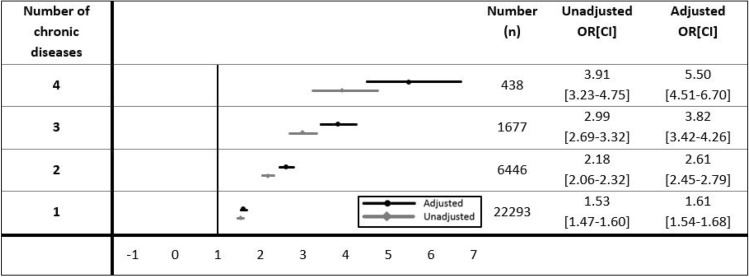
The adjusted odds ratio [95% CI] of having severe fatigue was 1.6 [1.5–1.7], 2.6 [2.5–2.8], 3.8 [3.4–4.3], and 5.5 [4.5–6.7] for one, two, three, and four chronic diseases compared to having no chronic disease, respectively (Fig. 3). Similar results were found for chronic fatigue (1.5 [1.5–1.6], 2.3 [2.2–2.5], 3.2 [2.8–3.6], and 4.9 [3.9–6.1] for one, two, three, and four chronic diseases respectively, see Online Supplemental Fig. 2). Note that the 95%CI intervals did not overlap, showing that the likelihood of having severe and chronic fatigue significantly increased with having multiple chronic diseases.
Figure 3.
Unadjusted and adjusted odds ratios and 95% CI of experiencing severe fatigue for participants with one to four chronic diseases compared to participants without a chronic disease. Reference = participants without a chronic disease. Adjusted odds ratio corrected for age and sex.
The disease-specific or trans-diagnostic relationship between fatigue severity and the predisposing and associated factors
The trans-diagnostic versus generic ANOVA model resulted in a statistically significant main effect of the presence of chronic disease, F(1, 69,007) = 691.306, p < 0.001 (model A, Online Supplemental Table 1). Adding the possible predisposing and associated variables, chronic disease remained a statistically significant predictor of fatigue severity F(1, 44,451) = 315.533, p < 0.001, though the F value decreased (model B, Online Supplemental Table 1). Similar results were found for the specific type of chronic disease (F(14, 21,670) = 10.660, p < 0.001 and F(14, 13,526) = 2.567, p = 0.001, respectively) (Table 3, model A and B).
Table 3.
The disease-specific or trans-diagnostic relationship between fatigue severity and the predisposing and associated factors.
| Disease-specific versus trans-diagnostic model | Model A | Model B | Model C | |||
|---|---|---|---|---|---|---|
| F(df) | p value | F(df) | p value | F(df) | p value | |
| Specific chronic disease* | 10.660 (14) | < 0.001 | 2.567 (14) | 0.001 | 0.814 (14) | 0.655 |
| Sex | 7.539 (1) | 0.006 | 0.909 (1) | 0.340 | ||
| Education level | 34.694 (2) | < 0.001 | 3.267 (2) | 0.038 | ||
| Household composition | 7.286 (4) | < 0.001 | 0.330 (4) | 0.858 | ||
| Partner | 0.964 (1) | 0.326 | 1.986 (1) | 0.159 | ||
| Work situation | 2.182 (1) | 0.140 | 3.514 (1) | 0.061 | ||
| Smoking | 5.228 (1) | 0.022 | 0.266 (1) | 0.606 | ||
| Depressive disorder | 324.275 (1) | < 0.001 | 64.908 (1) | < 0.001 | ||
| Anxiety disorder | 434.870 (1) | < 0.001 | 53.645 (1) | < 0.001 | ||
| Bodily pain that hampers in performing activities | 394.341 (1) | < 0.001 | 66.264 (1) | < 0.001 | ||
| Leisure-time sports activities | 302.561 (1) | < 0.001 | 64.434 (1) | < 0.001 | ||
| Age | 225.963 (1) | < 0.001 | 46.319 (1) | < 0.001 | ||
| BMI | 108.504 (1) | < 0.001 | 15.192 (1) | < 0.001 | ||
| Diastolic blood pressure | 2.607 (1) | 0.106 | 2.446 (1) | 0.118 | ||
| Resting heart rate | 22.245 (1) | < 0.001 | 20.845 (1) | < 0.001 | ||
| Neuroticism facets of anger/hostility, self-consciousness, impulsivity, and vulnerability | 865.091 (1) | < 0.001 | 148.417 (1) | < 0.001 | ||
| No. of people in household | 1.486 (1) | 0.223 | 1.725 (1) | 0.189 | ||
| Disease × sex | 0.923 (14) | 0.532 | ||||
| Disease × education level | 1.049 (28) | 0.395 | ||||
| Disease × household composition | 0.935 (54) | 0.610 | ||||
| Disease × partner | 0.950 (13) | 0.499 | ||||
| Disease × work situation | 1.351 (14) | 0.168 | ||||
| Disease × smoking | 1.343 (14) | 0.173 | ||||
| Disease x depressive disorder | 1.834 (14) | 0.029 | ||||
| Disease x anxiety disorder | 1.785 (14) | 0.035 | ||||
| Disease × bodily pain that hampers in performing activities | 1.015 (14) | 0.435 | ||||
| Disease × leisure-time sports activities | 0.362 (14) | 0.985 | ||||
| Disease × age | 1.087 (14) | 0.364 | ||||
| Disease × BMI | 1.060 (14) | 0.390 | ||||
| Disease × diastolic blood pressure | 1.364 (14) | 0.162 | ||||
| Disease × resting heart rate | 1.505 (14) | 0.100 | ||||
| Disease × neuroticism facets of anger/hostility, self-consciousness, impulsivity, and vulnerability | 0.741 (14) | 0.734 | ||||
| Disease × No. of people in household | 0.506 (14) | 0.931 | ||||
no. number, df degrees of freedom, BMI body mass index
*The factor ‘specific chronic disease’ reflects the participants with single-morbidity, entered as a categorical variable with 15 categories (each category reflecting a specific chronic disease with no intrinsic order). Model A: explores the impact of the main effect of ‘specific chronic disease’ on fatigue severity. Model B: investigates the main effects of ‘specific chronic disease’ and the predisposing and associated factors (predictors) on fatigue severity. Model C: studies the impact of the main effects plus the interaction effect between ‘specific chronic disease’ and the predisposing and associated factors on fatigue severity.
The following factors did not show a significant interaction effect with the type of chronic disease, but did have a significant main effect on fatigue severity (model B, Table 3): female gender, lower education level, living in a single parent household, current smoker, bodily pain that hampers in performing activities, no leisure-time sports activities, younger age, higher BMI, increased resting heart rate, and having higher scores on the neuroticism domain (similar results were found for the separate facets of anger/hostility, self-consciousness, impulsivity, and vulnerability, data not shown). The main effects of the type of chronic disease and the possible predisposing and associated factors explained 26% (adjusted R2) of the variance in fatigue (model B, Table 3).
Statistically significant interaction effects were found between chronic disease and the presence of a depressive- or anxiety disorder (p < 0.05, model C, Table 3), implying that the relationship between the presence of a depressive- or anxiety disorder and fatigue severity was influenced by the type of chronic disease. In specific, the linear regression analyses indicated that having a depression was associated with more severe fatigue for all included chronic diseases, though the size of the standardized coefficient differed between the chronic diseases (from β = 0.19 in participants with blood clotting disorder to β = 0.43 in participants with heart valve problems). Having an anxiety disorder was associated with increased fatigue severity for all chronic diseases except for blood clotting disorder and stroke (Online Supplemental Table 2).
The trans-diagnostic or generic relationship between fatigue severity and the predisposing and associated factors
The trans-diagnostic versus generic ANOVA model indicated that none of the factors, apart from being currently employed and not being involved in leisure time sports activities, showed significant interaction effects with chronic disease (model C, Online Supplemental Table 1). This suggests that these factors are generic predisposing and associated factors of fatigue both in participants with and without a chronic disease. A post-hoc analysis, comparing the prevalence of the predisposing and associated factors between participants with and without a chronic disease, showed that all the generic factors that were associated with fatigue were significantly more often present or more pronounced in participants with chronic disease(s) (Table 1).
Predisposing and associated factors of clinically relevant levels of severe and chronic fatigue in participants with single- and multi-morbidity
In addition, logistic regression analyses were performed to investigate whether the predisposing and associated factors were related to clinically relevant levels of fatigue (e.g. severe and chronic fatigue) in participants with single- and multi-morbidity (Online Supplemental Table 3). Similar factors as in the ANOVA models (Table 3) were found to increase the likelihood of experiencing severe fatigue. Yet, smoking status was not significantly associated with severe fatigue, whereas being unemployed and having a lower DBP were. In addition, an inverse association compared to the ANOVA model was found for education level (e.g. the likelihood for experiencing severe fatigue was significantly increased for higher instead of lower educated people) (model A, Online Supplemental Table 3). Similar results were found for the likelihood of being chronically fatigued (model B, Online Supplemental Table 3), though being a female and being a single parent were not significantly associated with chronic fatigue.
Discussion
The present study is to the best of our knowledge the first to report prevalence rates of severe and chronic fatigue in a population-based cohort among a wide range of conditions using a validated fatigue questionnaire. The results showed that severe and chronic fatigue are common in chronic disease. The prevalence of severe and chronic fatigue was significantly higher in participants with chronic disease compared to healthy subjects. Moreover, direct comparison of prevalence rates of fatigue between chronic diseases showed remarkably similar prevalence rates of severe and chronic fatigue across chronic diseases, except for MS which was significantly higher from the other diseases besides PD and liver disease. The current study furthermore shows the impact of multi-morbidity on severe and chronic fatigue. With each additional chronic disease, the likelihood of having severe and chronic fatigue was found to increase significantly. This finding warrants the attention of healthcare professionals and policy makers, as the proportion of patients living with multiple chronic conditions rises due to the ageing population11,12.
The current study confirms that chronic disease is associated with more severe and chronic fatigue. However, most participants with a chronic disease do not experience severe or chronic fatigue. We identified several factors associated with fatigue severity in chronic disease: female gender, younger age, having a lower education level, living in a single parent household, having higher scores on neuroticism facets of anger/hostility, self-consciousness, impulsivity, and vulnerability, being a current smoker, having a higher BMI, increased resting heart rate, bodily pain that hampers in performing activities, and not being involved in leisure-time sports activities. Having a depressive disorder was also found to be associated with more severe fatigue in chronic disease, though the strength of the standardized coefficient differed among chronic diseases. This finding is in line with that of a previous study that found the same link between several factors associated with fatigue in chronic disease, albeit with a different strength16. Anxiety disorder was associated with fatigue severity in all chronic diseases, except in blood clotting disorder and stroke. In contrast, previous studies performed in post stroke patients indicated that anxiety is a common symptom, which is associated with fatigue58,59. The differences to the current findings may be attributed to the smaller sample sizes of these patient groups.
To date, the abovementioned predisposing and associated factors have often been studied in a disease-specific context6,21–32,37–39,60–62. Thus far, only one study investigated whether fatigue is a disease-specific or trans-diagnostic symptom in chronic disease16. In that study, female gender, younger age, pain and reduced physical activity were trans-diagnostically associated with fatigue severity. Reduced motivational and concentration problems, sleep disturbances, lower levels of physical functioning and lower self-efficacy concerning fatigue, which have not been evaluated in the current study, were also associated with fatigue severity across diseases. However, to the best of our knowledge the current study is the first to indicate that the predisposing and associated factors of fatigue are not only trans-diagnostic, but also generic. With the exception of employment status and being involved in sports activities, all factors were associated with fatigue in participants with a chronic disease and in healthy subjects to the same extent. Nevertheless, even though the predisposing and associated factors seem to be generic, the observation that the identified factors were more pronounced or more prevalent in chronic disease might partly explain the higher prevalence of fatigue in subjects with a chronic disease. Moving away from a disease-specific to a trans-diagnostic, or even generic focus on fatigue may help to accelerate the development of interventions for fatigue to improve daily functioning of patients with a chronic disease. That is, findings from fatigue research in one chronic disease can be generalized to other chronic diseases. Moreover, a trans-diagnostic approach also supports the complexity of multi-morbid chronic disease management, as disease-centered treatments will not fully address the comprehensive needs of patients with multiple chronic diseases. To date, the effectiveness of cognitive behavioral therapy and exercise therapy in reducing fatigue has been demonstrated in specific chronic diseases such as RA, T1DM, MS, COPD, and end-stage renal disease63–68. These interventions target factors such as physical activity which has been found to be a trans-diagnostic factor for fatigue and may, therefore, be effective for multiple chronic diseases (i.e. trans-diagnostic disease management).
Several limitations have to be considered when interpreting the results. First, the assessment of chronic disease was based on self-report which may be unreliable. Second, participants with a chronic disease are perhaps less likely to participate in studies such as Lifelines, which might have led to an underrepresentation of participants with a chronic disease, and therefore severe and chronic fatigue. Also, the exclusion of participants with low life expectancy might have biased the results. However, significant bias seems unlikely, as the prevalence rates of the chronic diseases in our study are comparable to those in the Dutch general population (Statistics Netherlands, CBS). Nevertheless, an underrepresentation of chronic diseases may also be true for general population statistics, as this is likewise based on self-report. Third, the development of new chronic diseases was re-evaluated during the first follow-up measurement. Nevertheless, not in the same manner as during baseline. Therefore, it was decided to only use the baseline information on chronic disease. Hence, no information was available about the development of new chronic diseases in the years between this assessment and the evaluation of fatigue. Also, the list of chronic diseases may not be comprehensive, as the chronicity of certain diseases (e.g., asthma) could not be determined. In addition, the measurement of fatigue may be subject to recall bias as it is does not capture diurnal variations of fatigue and is not measured in real time. Fourth, participants who were unable to understand the Dutch language were not considered eligible, which might have led to an underrepresentation of certain ethnicity groups. Fifth, the time-independent predisposing factors were not corrected for baseline fatigue, as these data were not available. It should be noted that no firm conclusions can be drawn on the direction of the association between the associated factors and fatigue. A cohort study design in which patients free of fatigue are followed over time, would provide us with in-depth information about the direction of the causality and would account for the non-response bias of patients with more profound fatigue or chronic diseases. Sixth, one should keep in mind that the current study only took a limited number of predisposing and associated factors of fatigue into account which explained 26% of the variance in fatigue. Future studies are needed to further unravel the underlying factors of fatigue in chronic disease that possibly can be addressed in interventions, thereby evaluating biological mechanisms like alterations in the hypothalamic–pituitary–adrenal (HPA) axis, autonomic nervous system, or inflammatory markers69. Then again, it should be evaluated whether these biological mechanisms are disease-specific, trans-diagnostic, or generic. Moreover, factors such as fatigue related beliefs20 which have been reported to be associated with fatigue in chronic disease should be considered.
In conclusion, the high prevalence rates of severe and chronic fatigue in chronic disease emphasize the clinical relevance of assessing fatigue in patients with a chronic disease. Screening for fatigue is of particular importance in patients with multi-morbidity, as the results of the current study indicate that the severity of fatigue increases in patients with multiple chronic diseases. Moreover, it appears that the predisposing and associated factors of fatigue are likely to be trans-diagnostic or even (mostly) generic for the general population. Several, predisposing and associated factors were identified. This provides direction to the development of trans-diagnostic interventions for fatigue and has also clinical implications for multi-morbid chronic disease management. Future studies are needed to further examine the underlying factors of fatigue in chronic disease and evaluate trans-diagnostic interventions in randomized clinical trials in samples with patients with different chronic diseases.
Supplementary Information
Author contributions
A.M.J.B., H.K., J.G.M.R. formulated the research questions and applied for the use of Lifelines data. Y.M.J.G., A.M.J.B. and H.K. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analyses. Y.M.J.G., A.M.J.B., D.A.J., M.A.S., H.K. interpreted the findings and drafted the manuscript. F.L., J.W.R.T. assisted in the statistical interpretation of the data. All authors critically revised and reviewed the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. Y.M.J.G., A.M.J.B., H.K., D.A.J., M.A.S. affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned (and, if relevant, registered) have been explained.
Funding
The Lifelines Biobank initiative has been made possible by subsidy from the Dutch Ministry of Health, Welfare and Sport, the Dutch Ministry of Economic Affairs, the University Medical Center Groningen (UMCG the Netherlands), University Groningen and the Northern Provinces of the Netherlands. Y.M.J.G. is financially supported by Lung Foundation Netherlands grant 4.1.16.085. The funding organizations had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Data availability
We are not permitted to share individual data from the Dutch Lifelines study. Information on applying for access to the Dutch Lifelines data is available at https://www.lifelines.nl/researcher/how-to-apply.
Competing interests
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf. Y.M.J.G., A.M.J.B., Z.E., M.V.H., C.B., J.B.P., M.A.G.S., F.L., J.W.R.T., M.S.Y.T., J.H.V., S.E.G., A.W.V., R.J.H.C.G.B., M.B., A.M.W.J.S., J.G.M.R. and H.K. have nothing to disclose. D.A.J. reports personal fees from Novartis, personal fees from Boehringer Ingelheim, personal fees from AstraZeneca, outside the submitted work. M.A.S. reports grants from Lung Foundation Netherlands, grants from Stichting Astma Bestrijding, grants and personal fees from Boehringer Ingelheim, and grants and personal fees from AstraZeneca, outside the submitted work.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-00337-z.
References
- 1.Ream E, Richardson A. Fatigue in patients with cancer and chronic obstructive airways disease: A phenomenological enquiry. Int. J. Nurs. Stud. 1997;34(1):44–53. doi: 10.1016/S0020-7489(96)00032-6. [DOI] [PubMed] [Google Scholar]
- 2.Korenromp IH, Meeus M, Bleijenberg G. Dutch language area definition of chronic fatigue. Ned. Tijdschr. Geneeskd. 2012;156(16):A4403. [PubMed] [Google Scholar]
- 3.Alves G, Wentzel-Larsen T, Larsen JP. Is fatigue an independent and persistent symptom in patients with Parkinson disease? Neurology. 2004;63(10):1908–1911. doi: 10.1212/01.WNL.0000144277.06917.CC. [DOI] [PubMed] [Google Scholar]
- 4.Evangelista LS, Moser DK, Westlake C, Pike N, Ter-Galstanyan A, Dracup K. Correlates of fatigue in patients with heart failure. Prog. Cardiovasc. Nurs. 2008;23(1):12–17. doi: 10.1111/j.1751-7117.2008.07275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goertz YMJ, Spruit MA, Van’t Hul AJ, Peters JB, Van Herck M, Nakken N, et al. Fatigue is highly prevalent in patients with COPD and correlates poorly with the degree of airflow limitation. Therap. Adv. Respir. Dis. 2019;13:1753466619878128. doi: 10.1177/1753466619878128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goedendorp MM, Tack CJ, Steggink E, Bloot L, Bazelmans E, Knoop H. Chronic fatigue in type 1 diabetes: Highly prevalent but not explained by hyperglycemia or glucose variability. Diabetes Care. 2014;37(1):73–80. doi: 10.2337/dc13-0515. [DOI] [PubMed] [Google Scholar]
- 7.van Hoogmoed D, Fransen J, Bleijenberg G, van Riel P. Physical and psychosocial correlates of severe fatigue in rheumatoid arthritis. Rheumatology (Oxford) 2010;49(7):1294–1302. doi: 10.1093/rheumatology/keq043. [DOI] [PubMed] [Google Scholar]
- 8.Jelsness-Jørgensen LP, Bernklev T, Henriksen M, Torp R, Moum BA. Chronic fatigue is more prevalent in patients with inflammatory bowel disease than in healthy controls. Inflamm. Bowel Dis. 2011;17(7):1564–1572. doi: 10.1002/ibd.21530. [DOI] [PubMed] [Google Scholar]
- 9.Jaime-Lara RB, Koons BC, Matura LA, Hodgson NA, Riegel B. A qualitative metasynthesis of the experience of fatigue across five chronic conditions. J Pain Sympt. Manage. 2019;2:25. doi: 10.1016/j.jpainsymman.2019.12.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gelauff JM, Kingma EM, Kalkman JS, Bezemer R, van Engelen BGM, Stone J, et al. Fatigue, not self-rated motor symptom severity, affects quality of life in functional motor disorders. J. Neurol. 2018;265(8):1803–1809. doi: 10.1007/s00415-018-8915-7. [DOI] [PubMed] [Google Scholar]
- 11.Marengoni A, Rizzuto D, Wang HX, Winblad B, Fratiglioni L. Patterns of chronic multimorbidity in the elderly population. J. Am. Geriatr. Soc. 2009;57(2):225–230. doi: 10.1111/j.1532-5415.2008.02109.x. [DOI] [PubMed] [Google Scholar]
- 12.Pefoyo AJ, Bronskill SE, Gruneir A, Calzavara A, Thavorn K, Petrosyan Y, et al. The increasing burden and complexity of multimorbidity. BMC Public Health. 2015;15:415. doi: 10.1186/s12889-015-1733-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belza BL. Comparison of self-reported fatigue in rheumatoid arthritis and controls. J. Rheumatol. 1995;22(4):639–643. [PubMed] [Google Scholar]
- 14.Krupp LB, Alvarez LA, LaRocca NG, Scheinberg LC. Fatigue in multiple sclerosis. Arch. Neurol. 1988;45(4):435–437. doi: 10.1001/archneur.1988.00520280085020. [DOI] [PubMed] [Google Scholar]
- 15.Franssen PM, Bultmann U, Kant I, van Amelsvoort LG. The association between chronic diseases and fatigue in the working population. J. Psychosom. Res. 2003;54(4):339–344. doi: 10.1016/S0022-3999(02)00395-1. [DOI] [PubMed] [Google Scholar]
- 16.Menting J, Tack CJ, Bleijenberg G, Donders R, Droogleever Fortuyn HA, Fransen J, et al. Is fatigue a disease-specific or generic symptom in chronic medical conditions? Health Psychol. 2018;37(6):530–543. doi: 10.1037/hea0000598. [DOI] [PubMed] [Google Scholar]
- 17.Van Herck M, Spruit MA, Burtin C, Djamin R, Antons J, Goertz YMJ, et al. Fatigue is highly prevalent in patients with asthma and contributes to the burden of disease. J. Clin. Med. 2018;7:12. doi: 10.3390/jcm7020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krupp LB, LaRocca NG, Muir J, Steinberg AD. A study of fatigue in systemic lupus erythematosus. J. Rheumatol. 1990;17(11):1450–1452. [PubMed] [Google Scholar]
- 19.Huyser BA, Parker JC, Thoreson R, Smarr KL, Johnson JC, Hoffman R. Predictors of subjective fatigue among individuals with rheumatoid arthritis. Arthritis Rheum. 1998;41(12):2230–2237. doi: 10.1002/1529-0131(199812)41:12<2230::AID-ART19>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 20.Bloem AEM, Mostard RLM, Stoot N, Vercoulen JH, Peters JB, Janssen DJA, et al. Severe fatigue is highly prevalent in patients with IPF or sarcoidosis. J. Clin. Med. 2020;9:4. doi: 10.3390/jcm9041178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mollaoglu M, Ustun E. Fatigue in multiple sclerosis patients. J. Clin. Nurs. 2009;18(9):1231–1238. doi: 10.1111/j.1365-2702.2008.02733.x. [DOI] [PubMed] [Google Scholar]
- 22.Lerdal A, Celius EG, Moum T. Fatigue and its association with sociodemographic variables among multiple sclerosis patients. Multiple Scler. (Houndmills, Basingstoke, England). 2003;9(5):509–514. doi: 10.1191/1352458503ms943oa. [DOI] [PubMed] [Google Scholar]
- 23.Mollaoglu M. Fatigue in people undergoing hemodialysis. Dialysis Transplant. 2009;38(6):216–220. doi: 10.1002/dat.20330. [DOI] [Google Scholar]
- 24.Merkelbach S, Konig J, Sittinger H. Personality traits in multiple sclerosis (MS) patients with and without fatigue experience. Acta Neurol. Scand. 2003;107(3):195–201. doi: 10.1034/j.1600-0404.2003.02037.x. [DOI] [PubMed] [Google Scholar]
- 25.Penner IK, Bechtel N, Raselli C, Stocklin M, Opwis K, Kappos L, et al. Fatigue in multiple sclerosis: Relation to depression, physical impairment, personality and action control. Multiple Scler. (Houndmills, Basingstoke, England). 2007;13(9):1161–1167. doi: 10.1177/1352458507079267. [DOI] [PubMed] [Google Scholar]
- 26.Schreiber H, Lang M, Kiltz K, Lang C. Is personality profile a relevant determinant of fatigue in multiple sclerosis? Front. Neurol. 2015;6:2. doi: 10.3389/fneur.2015.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falk K, Patel H, Swedberg K, Ekman I. Fatigue in patients with chronic heart failure—a burden associated with emotional and symptom distress. Eur. J. Cardiovas. Nurs. 2009;8(2):91–96. doi: 10.1016/j.ejcnurse.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Jump RL, Robinson ME, Armstrong AE, Barnes EV, Kilbourn KM, Richards HB. Fatigue in systemic lupus erythematosus: Contributions of disease activity, pain, depression, and perceived social support. J. Rheumatol. 2005;32(9):1699–1705. [PubMed] [Google Scholar]
- 29.Kroencke DC, Lynch SG, Denney DR. Fatigue in multiple sclerosis: Relationship to depression, disability, and disease pattern. Multiple Scler. (Houndmills, Basingstoke, Engl.) 2000;6(2):131–136. doi: 10.1177/135245850000600213. [DOI] [PubMed] [Google Scholar]
- 30.Kentson M, Todt K, Skargren E, Jakobsson P, Ernerudh J, Unosson M, et al. Factors associated with experience of fatigue, and functional limitations due to fatigue in patients with stable COPD. Ther. Adv. Respir. Dis. 2016;10(5):410–424. doi: 10.1177/1753465816661930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andersson M, Stridsman C, Ronmark E, Lindberg A, Emtner M. Physical activity and fatigue in chronic obstructive pulmonary disease—a population based study. Respir. Med. 2015;109(8):1048–1057. doi: 10.1016/j.rmed.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Da Costa D, Dritsa M, Bernatsky S, Pineau C, Menard HA, Dasgupta K, et al. Dimensions of fatigue in systemic lupus erythematosus: Relationship to disease status and behavioral and psychosocial factors. J. Rheumatol. 2006;33(7):1282–1288. [PubMed] [Google Scholar]
- 33.O'Sullivan D, McCarthy G. An exploration of the relationship between fatigue and physical functioning in patients with end stage renal disease receiving haemodialysis. J. Clin. Nurs. 2007;16(11c):276–284. doi: 10.1111/j.1365-2702.2007.01965.x. [DOI] [PubMed] [Google Scholar]
- 34.Williams BA. The clinical epidemiology of fatigue in newly diagnosed heart failure. BMC Cardiovasc. Disord. 2017;17(1):122. doi: 10.1186/s12872-017-0555-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Erickson SR, Williams BC, Gruppen LD. Perceived symptoms and health-related quality of life reported by uncomplicated hypertensive patients compared to normal controls. J. Hum. Hypertens. 2001;15(8):539–548. doi: 10.1038/sj.jhh.1001236. [DOI] [PubMed] [Google Scholar]
- 36.Harbison JA, Walsh S, Kenny RA. Hypertension and daytime hypotension found on ambulatory blood pressure is associated with fatigue following stroke and TIA. QJM Mon. J. Assoc. Physicians. 2009;102(2):109–115. doi: 10.1093/qjmed/hcn146. [DOI] [PubMed] [Google Scholar]
- 37.Belza BL, Henke CJ, Yelin EH, Epstein WV, Gilliss CL. Correlates of fatigue in older adults with rheumatoid arthritis. Nurs. Res. 1993;42(2):93–99. doi: 10.1097/00006199-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Wolfe F, Hawley DJ, Wilson K. The prevalence and meaning of fatigue in rheumatic disease. J. Rheumatol. 1996;23(8):1407–1417. [PubMed] [Google Scholar]
- 39.Kahraman T, Ozdogar AT, Abasiyanik Z, Ozakbas S. Associations between smoking and walking, fatigue, depression, and health-related quality of life in persons with multiple sclerosis. Acta Neurol. Belg. 2020;20:20. doi: 10.1007/s13760-020-01341-2. [DOI] [PubMed] [Google Scholar]
- 40.Stridsman C, Mullerova H, Skar L, Lindberg A. Fatigue in COPD and the impact of respiratory symptoms and heart disease—a population-based study. COPD. 2013;10(2):125–132. doi: 10.3109/15412555.2012.728642. [DOI] [PubMed] [Google Scholar]
- 41.Hopman P, Schellevis FG, Rijken M. Health-related needs of people with multiple chronic diseases: Differences and underlying factors. Quali. Life Res. 2016;25(3):651–660. doi: 10.1007/s11136-015-1102-8. [DOI] [PubMed] [Google Scholar]
- 42.Stolk RP, Rosmalen JG, Postma DS, de Boer RA, Navis G, Slaets JP, et al. Universal risk factors for multifactorial diseases: LifeLines: A three-generation population-based study. Eur. J. Epidemiol. 2008;23(1):67–74. doi: 10.1007/s10654-007-9204-4. [DOI] [PubMed] [Google Scholar]
- 43.Scholtens S, Smidt N, Swertz MA, Bakker SJ, Dotinga A, Vonk JM, et al. Cohort Profile: LifeLines, a three-generation cohort study and biobank. Int. J. Epidemiol. 2015;44(4):1172–1180. doi: 10.1093/ije/dyu229. [DOI] [PubMed] [Google Scholar]
- 44.Klijs B, Scholtens S, Mandemakers JJ, Snieder H, Stolk RP, Smidt N. Representativeness of the LifeLines Cohort Study. PloS One. 2015;10(9):e0137203. doi: 10.1371/journal.pone.0137203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vercoulen JH, Swanink CM, Fennis JF, Galama JM, van der Meer JW, Bleijenberg G. Dimensional assessment of chronic fatigue syndrome. J. Psychosom. Res. 1994;38(5):383–392. doi: 10.1016/0022-3999(94)90099-X. [DOI] [PubMed] [Google Scholar]
- 46.Worm-Smeitink M, Gielissen M, Bloot L, van Laarhoven HWM, van Engelen BGM, van Riel P, et al. The assessment of fatigue: Psychometric qualities and norms for the Checklist individual strength. J. Psychosom. Res. 2017;98:40–46. doi: 10.1016/j.jpsychores.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 47.Beurskens AJ, Bultmann U, Kant I, Vercoulen JH, Bleijenberg G, Swaen GM. Fatigue among working people: Validity of a questionnaire measure. Occup. Environ. Med. 2000;57(5):353–357. doi: 10.1136/oem.57.5.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bultmann U, de Vries M, Beurskens AJ, Bleijenberg G, Vercoulen JH, Kant I. Measurement of prolonged fatigue in the working population: Determination of a cutoff point for the checklist individual strength. J. Occup. Health Psychol. 2000;5(4):411–416. doi: 10.1037/1076-8998.5.4.411. [DOI] [PubMed] [Google Scholar]
- 49.Peters JB, Heijdra YF, Daudey L, Boer LM, Molema J, Dekhuijzen PN, et al. Course of normal and abnormal fatigue in patients with chronic obstructive pulmonary disease, and its relationship with domains of health status. Patient Educ. Couns. 2011;85(2):281–285. doi: 10.1016/j.pec.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 50.Repping-Wuts H, Fransen J, van Achterberg T, Bleijenberg G, van Riel P. Persistent severe fatigue in patients with rheumatoid arthritis. J. Clin. Nurs. 2007;16(11c):377–383. doi: 10.1111/j.1365-2702.2007.02082.x. [DOI] [PubMed] [Google Scholar]
- 51.Goodman RA, Posner SF, Huang ES, Parekh AK, Koh HK. Defining and measuring chronic conditions: Imperatives for research, policy, program, and practice. Prev. Chronic Dis. 2013;10:E66. doi: 10.5888/pcd10.120239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Janssen DJA, Rechberger S, Wouters EFM, Schols J, Johnson MJ, Currow DC, et al. Clustering of 27,525,663 death records from the United States based on health conditions associated with death: An example of big health data exploration. J. Clin. Med. 2019;8:7. doi: 10.3390/jcm8070922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Costa, P. & McCrae, R. Revised NEO Personality Inventory (NEO PI-R) and the Five Factor Inventory (NEO-FFI): Professional manual. FL: Odessa; 1992.
- 54.Netherlands, S. International definition of unemployment. https://www.cbs.nl/en-gb/news/2007/29/dutch-unemployment-rate-lowest-in-eu/international-definition-of-unemployment.
- 55.Wendel-Vos GC, Schuit AJ, Saris WH, Kromhout D. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J. Clin. Epidemiol. 2003;56(12):1163–1169. doi: 10.1016/S0895-4356(03)00220-8. [DOI] [PubMed] [Google Scholar]
- 56.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (MINI): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- 57.Abad-Díez JM, Calderón-Larrañaga A, Poncel-Falcó A, Poblador-Plou B, Calderón-Meza JM, Sicras-Mainar A, et al. Age and gender differences in the prevalence and patterns of multimorbidity in the older population. BMC Geriatr. 2014;14:75. doi: 10.1186/1471-2318-14-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Galligan NG, Hevey D, Coen RF, Harbison JA. Clarifying the associations between anxiety, depression and fatigue following stroke. J. Health Psychol. 2016;21(12):2863–2871. doi: 10.1177/1359105315587140. [DOI] [PubMed] [Google Scholar]
- 59.Delva I, Lytvynenko N, Delva M. Factors associated with post-stroke fatigue within the first 3 month after stroke. Georgian Med. News. 2017;267:38–42. [PubMed] [Google Scholar]
- 60.Warren RE, Deary IJ, Frier BM. The symptoms of hyperglycaemia in people with insulin-treated diabetes: Classification using principal components analysis. Diabetes Metab. Res. Rev. 2003;19(5):408–414. doi: 10.1002/dmrr.396. [DOI] [PubMed] [Google Scholar]
- 61.Tang WR, Yu CY, Yeh SJ. Fatigue and its related factors in patients with chronic heart failure. J. Clin. Nurs. 2010;19(1–2):69–78. doi: 10.1111/j.1365-2702.2009.02959.x. [DOI] [PubMed] [Google Scholar]
- 62.Liu HE. Fatigue and associated factors in hemodialysis patients in Taiwan. Res. Nurs. Health. 2006;29(1):40–50. doi: 10.1002/nur.20109. [DOI] [PubMed] [Google Scholar]
- 63.Li LSK, Butler S, Goldstein R, Brooks D. Comparing the impact of different exercise interventions on fatigue in individuals with COPD: A systematic review and meta-analysis. Chron. Respir. Dis. 2019;16:1479973119894855. doi: 10.1177/1479973119894855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Asano M, Finlayson ML. Meta-analysis of three different types of fatigue management interventions for people with multiple sclerosis: Exercise, education, and medication. Multiple Scler. Int. 2014;2014:798285. doi: 10.1155/2014/798285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao QG, Zhang HR, Wen X, Wang Y, Chen XM, Chen N, et al. Exercise interventions on patients with end-stage renal disease: A systematic review. Clin. Rehabil. 2019;33(2):147–156. doi: 10.1177/0269215518817083. [DOI] [PubMed] [Google Scholar]
- 66.Heine M, van de Port I, Rietberg MB, van Wegen EE, Kwakkel G. Exercise therapy for fatigue in multiple sclerosis. Cochrane Database Syst. Rev. 2015;9:Cd009956. doi: 10.1002/14651858.CD009956.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prothero L, Barley E, Galloway J, Georgopoulou S, Sturt J. The evidence base for psychological interventions for rheumatoid arthritis: A systematic review of reviews. Int. J. Nurs. Stud. 2018;82:20–29. doi: 10.1016/j.ijnurstu.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 68.Menting J, Tack CJ, van Bon AC, Jansen HJ, van den Bergh JP, Mol M, et al. Web-based cognitive behavioural therapy blended with face-to-face sessions for chronic fatigue in type 1 diabetes: A multicentre randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5(6):448–456. doi: 10.1016/S2213-8587(17)30098-0. [DOI] [PubMed] [Google Scholar]
- 69.Matura LA, Malone S, Jaime-Lara R, Riegel B. A Systematic review of biological mechanisms of fatigue in chronic illness. Biol. Res. Nurs. 2018;20(4):410–421. doi: 10.1177/1099800418764326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We are not permitted to share individual data from the Dutch Lifelines study. Information on applying for access to the Dutch Lifelines data is available at https://www.lifelines.nl/researcher/how-to-apply.