Abstract
We described the molecular epidemiology of expanded-spectrum cephalosporin-resistant gram-negative bacilli (RGN) recovered from inanimate surfaces. RGN were isolated from 9% of environmental cultures. Numerous species, each with multiple unique strains, were recovered. Epidemiological links between environmental, personnel, and patient strains suggested the exogenous acquisition of RGN from the hospital environment.
The emergence of expanded-spectrum cephalosporin-resistant gram-negative bacilli (RGN) is being increasingly recognized. Colonization with RGN can occur endogenously through the emergence of ceftazidime resistance in previously susceptible gram-negative bacilli or exogenously through the cross-transmission of pathogens between patients, the environment, and/or health care workers (9). The role of each of these mechanisms in the acquisition of RGN, during a nonoutbreak period, remains to be defined. We previously described the epidemiology of RGN recovered from patients in two surgical intensive care units (SICU) (3, 4). In the present study, we describe the molecular epidemiology of RGN recovered from inanimate surfaces and the impact of environmental contamination on the acquisition of RGN, during a nonoutbreak period.
Between 15 January and 15 June 1995, a prospective study on the epidemiology of RGN was conducted in two SICU of the Beth Israel Deaconess Medical Center, West Campus. Each SICU had six single rooms and two double rooms. The patient-to-nurse ratio was 1:1, on average. Environmental and personnel specimens were obtained weekly from occupied patient rooms and nurses assigned to those rooms. Environmental surveillances were performed at unannounced times. Environmental cultures from sinks, bed rails, and mobile bedside tables were obtained by using Rodac plates containing MacConkey medium (Difco, Detroit, Mich.). Each inanimate site was cultured by imprinting a Rodac plate on four randomly selected areas. Personnel gowns were cultured by imprinting a Rodac plate on four randomly selected areas of each gowned upper extremity. Specimens from the hands of personnel were obtained by finger impressions of all five digits on MacConkey agar plates. Daily room and patient assignments for nurses were documented at the time of specimen collection. Plates obtained from the environment and personnel were incubated directly at 35°C for 48 h. The species identification of representative colonies and antimicrobial susceptibility testing were performed as previously described (3). Ceftazidime (CAZ) resistance was defined as a MIC of ≥16 μg/ml. Data on the collection and processing of patient specimens has been outlined in a previous publication (3). Molecular typing was performed by pulsed-field gel electrophoresis (PFGE) (3).
Weekly culture surveys of the environment were performed 21 times during the 5-month study period. An average of nine rooms (range, 4 to 16) were cultured per survey. Each room was screened an average of 15 times (range, 6 to 25) during the 5 months. In total, all rooms were cultured 233 times. Two hundred two cultures each were obtained from sinks, countertops, and bed rails. Fifty-four (9%) of 606 cultures were positive for RGN. Positive environmental sites were as follows: sinks (42 sites), counters, (8 sites), and bed rails (4 sites). Species and strain types for 43 isolates are presented in Tables 1 and 2. The following pathogens (with the number of isolates) were not typed: Alcaligenes spp. (two isolates), Pseudomonas sp. non-aeruginosa (one isolate), Ochrobactrum sp. (one isolate), and Agrobacterum sp. (one isolate). Six isolates were identified as non-lactose fermenters and could not be speciated further by Microscan. If only RGN of major clinical relevance (Enterobacter cloacae, Acinetobacter baumannii, Pseudomonas aeruginosa, Serratia spp., Klebsiella oxytoca, Stenotrophomonas spp.) were included in the analysis, 5% (28 of 606) of environmental cultures were positive. Analysis of environmental contamination by room revealed that 7% (16 of 233) of room surveys had at least one positive culture for clinically relevant RGN: two rooms were contaminated during 5 of the 21 weekly environmental surveys, one room was contaminated during 6 surveillances, and none was contaminated during 10 surveillances. Thus, environmental contamination by clinically relevant pathogens was detected in at least one room during 52% of weekly surveys.
TABLE 1.
Environmental, personnel, and patient isolates of RGN recovered from two SICU during a 5-month study period
| Species | No. of types/no. of isolates from:
|
||
|---|---|---|---|
| Environment | Personnel | Patientsa | |
| A. lwoffii | 6/15 | 3/3 | 1/1 |
| E. cloacae | 6/11 | 2/2 | 21/21 |
| P. aeruginosa | 5/7 | 0 | 22/22 |
| Stenotrophomonas spp. | 3/4 | 0 | 6/7 |
| Serratia spp. | 1/2 | 0 | 2/2 |
| K. oxytoca | 1/2 | 0 | 0 |
| A. baumannii | 2/2 | 0 | 9/12 |
See reference 3.
TABLE 2.
Epidemiological links between environmental, personnel, and patient strains of RGN from two SICU
| Species and strain | No. of isolates (site[s]a)
|
||
|---|---|---|---|
| Environment | Personnel | Patients | |
| A. lwoffii | |||
| AW-1 | 10 (all S) | 1 (G) | 0 |
| AW-2 to AW-6 | 1 (C, B, S, S, S) | 0 | 0 |
| AW-7 and AW-8 | 0 | 1 (H) | 0 |
| E. cloacae | |||
| EC-1 | 3 (B, S, S) | 1 (G) | 1 (O) |
| EC-2 | 1 (S) | 0 | 0 |
| EC-3 | 4 (all S) | 0 | 0 |
| EC-4 | 1 (B) | 0 | 1 (R) |
| EC-5 | 1 (S) | 0 | 1 (R) |
| EC-6 | 1 (S) | 0 | 1 (R) |
| EC-7 | 0 | 1 (H) | 0 |
| P. aeruginosa | |||
| PA-1 | 1 (S) | 0 | 0 |
| PA-2 | 2 (all S) | 0 | 1 (O) |
| PA-3 | 1 (S) | 0 | 0 |
| PA-4 | 2 (S, T) | 0 | 0 |
| PA-5 | 1 (S) | 0 | 0 |
| Stenotrophomonas spp. | |||
| S-1 | 1 (B) | 0 | 0 |
| S-2 | 1 (S) | 0 | 1 (R) |
| S-3 | 2 (all S) | 0 | 0 |
B, bed rail; T, table; S, sink; G, gown; H, hand; O, oropharynx; R, rectum.
A total of 284 personnel cultures were obtained from SICU nurses (142 each from gowns and hands), of which five (2%) were positive for RGN (three originating from gowns and two from hands) (Table 1). Of the 333 patients admitted during the study period, 86 (25%) were colonized with RGN. Data on patient isolates and typing profiles have been reported separately (3, 4).
Epidemiological links between patients, personnel, and environmental surfaces are presented in Table 2 (Fig. 1 and 2). Stenotrophomonas sp. strain S-2 was recovered from a patient for the first time from the fourth serial surveillance culture, 15 days after his admission. Strain S-2 was also recovered from the sink of the patient’s room, 26 days prior to the isolation of S-2 from the patient. E. cloacae EC-6 and P. aeruginosa PA-2 continued to be isolated from environmental surfaces 13 and 5 days, respectively, after patients colonized with the identical strain were discharged from the SICU. Two strains were recovered from inanimate surfaces over prolonged periods: Acinetobacter lwoffii AW-1 for 98 days, E. cloacae EC-1 for 89 days, and Stenotrophomonas sp. strain S-2 for 30 days. A. lwoffii AW-3 and AW-4 were isolated from the same room (counter and sink) on the same day.
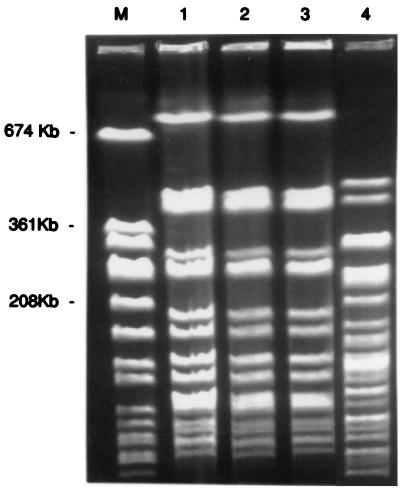
FIG. 1.
PFGE types of ceftazidime-resistant E. cloacae isolates. Lane M, Staphylococcus aureus ATCC 8325 size marker; lanes 1, 2, and 3, EC-1 strain isolated from environmental, personnel, and patient cultures, respectively, with indistinguishable PFGE profiles; lane 4, EC-3 strain isolated from environmental cultures only, with a distinct PFGE profile.
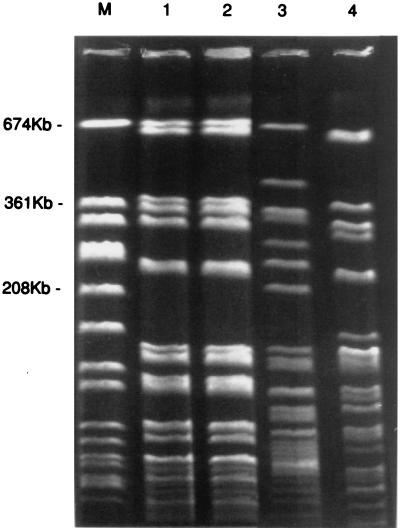
FIG. 2.
PFGE types of ceftazidime-resistant Stenotrophomonas sp. isolates. Lane M, S. aureus ATCC 8325 size marker; lanes 1 and 2, S-2 strain isolated from a patient and an environmental culture, respectively, with indistinguishable PFGE profiles; lanes 3 and 4, S-1 and S-3 strains, respectively, isolated from environmental cultures, with different PFGE profiles.
The findings of this study depict a complex pattern of environmental contamination by RGN during a nonoutbreak period. A variety of species, each with multiple unique strains, were recovered from inanimate surfaces. Rooms were contaminated by more than one species of RGN, and different strains were recovered from the same environmental site on the same day. Although environmental contamination was not extensive, epidemiological links between the environment, patients, and health care workers were detected throughout the study period. Importantly, an environmental source was directly linked to the exogenous acquisition of CAZ-resistant Stenotrophomonas spp. by one patient. The inability to identify similar genotypes from other patients or nurses further strengthens the link between an environmental source and the acquisition of CAZ-resistant Stenotrophomonas spp.
Infrequent environmental contamination by colonized patients has also been shown for vancomycin-resistant enterococci, antibiotic-susceptible gram-negative organisms, and Candida spp., in contrast to Clostridium difficile, which has been implicated in widespread environmental contamination (2, 7, 8, 10). The extent of environmental contamination in this study, however, may have been underestimated, as only a portion of SICU rooms were surveyed each week.
The survival time of these gram-negative organisms on inanimate surfaces was significantly longer than previously reported (1, 5). As the majority of isolates were recovered from sinks, replication in a moist environment may explain their prolonged survival (6). Reintroduction into the environment is unlikely to explain their persistent recovery, since similar strains were not identified from other patients or personnel despite extensive surveillance. These findings suggest that the potential for the cross-transmission of RGN from an environmental source is always present even in a setting where RGN are endemic. The recovery of RGN from inanimate surfaces persisted even after colonized patients were discharged from the SICU, suggesting that the environment should be considered as a source of RGN even when patients cannot be identified with these resistant pathogens.
In conclusion, this study highlights the complexity of the molecular epidemiology of RGN in the environment during nonoutbreak periods. The detection of several epidemiological links between environmental surfaces, nurses, and patients and the prolonged survival of RGN in the environment underscore the potential significance of environmental contamination in the cross-transmission of RGN in a setting where RGN are endemic. The results of this study may be helpful in emphasizing to health care workers the importance of infection control practices.
REFERENCES
- 1.Allen K D, Green H T. Hospital outbreak of multi-resistant Acinetobacter anitratus: an airborne mode of spread? J Hosp Infect. 1987;9:110–119. doi: 10.1016/0195-6701(87)90048-x. [DOI] [PubMed] [Google Scholar]
- 2.Bonten M J M, Hayden M K, Nathan C, van Voorhis J, Matushek M, Slaughter S, Rice T, Weinstein R A. Epidemiology of colonisation of patients and environment with vancomycin-resistant enterococci. Lancet. 1996;348:1615–1619. doi: 10.1016/S0140-6736(96)02331-8. [DOI] [PubMed] [Google Scholar]
- 3.D’Agata E, Venkataraman L, DeGirolami P, Samore M. Molecular epidemiology of acquisition of ceftazidime-resistant gram-negative bacilli in a nonoutbreak setting. J Clin Microbiol. 1997;35:2602–2605. doi: 10.1128/jcm.35.10.2602-2605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Agata E, Venkataraman L, DeGirolami P, Burke P, Eliopoulos G, Karchmer A W, Samore M. Multidrug resistant gram-negative bacilli: epidemiology and risk factors for colonization. Crit Care Med. 1999;27:1090–1095. doi: 10.1097/00003246-199906000-00026. [DOI] [PubMed] [Google Scholar]
- 5.Getchell-White S I, Donowitz L G, Groschel H M D. The inanimate environment of an intensive care unit as a potential source of nosocomial bacteria: evidence for long survival of Acinetobacter calcoaceticus. Infect Control Hosp Epidemiol. 1989;10:402–407. doi: 10.1086/646061. [DOI] [PubMed] [Google Scholar]
- 6.Gould I M. Risk factors for acquisition of multiply drug-resistant gram-negative bacteria. Eur J Clin Microbiol Infect Dis. 1994;13(Suppl. 1):S30–S38. doi: 10.1007/BF02390682. [DOI] [PubMed] [Google Scholar]
- 7.McGowan J E., Jr Environmental factors in nosocomial infection—a selective focus. Rev Infect Dis. 1981;3:760–769. doi: 10.1093/clinids/3.4.760. [DOI] [PubMed] [Google Scholar]
- 8.Samore M H, Venkataraman L, DeGirolami P C, Arbeit R D, Karchmer A W. Clinical and molecular epidemiology of sporadic and clustered cases of nosocomial Clostridium difficile diarrhea. Am J Med. 1996;100:32–40. doi: 10.1016/s0002-9343(96)90008-x. [DOI] [PubMed] [Google Scholar]
- 9.Sanders C C, Sanders W E. Clinical importance of inducible β-lactamases in gram-negative bacteria. Eur J Clin Microbiol. 1987;6:435–437. doi: 10.1007/BF02013106. [DOI] [PubMed] [Google Scholar]
- 10.Vazquez J A, Dembry L M, Sanchez V, Vazquez M A, Sobel J D, Dmuchowski C, Zervos M J. Nosocomial Candida glabrata colonization: an epidemiologic study. J Clin Microbiol. 1998;36:421–426. doi: 10.1128/jcm.36.2.421-426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]