Abstract
This is a narrative review of various treatment modalities for advanced hepatocellular carcinoma (HCC), with a focus on recent updates in radiological treatments, as well as novel treatment concepts related to immune checkpoint inhibitors and combination therapies with locoregional treatments. Interventional radiologists have made efforts toward developing alternative and/or combination treatments for first-line systemic treatment of patients with advanced HCC. Locoregional treatments with or without systemic therapy may be considered in the selected patients. Various treatment modalities for advanced HCC are emerging, and several randomized controlled trials, including those of combination treatments with immunotherapy, are ongoing.
Keywords: Hepatocellular carcinoma, Locoregional therapy, Transarterial chemoembolization, Transarterial radioembolization, Immunotherapy, Combined modality therapy
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common cancer globally and the most common malignancy among primary liver cancers [1]. According to the Barcelona Clinic Liver Cancer (BCLC) staging system, advanced HCC (BCLC stage C) is defined as HCC in patients with a performance status of 1–2, Child-Pugh score A or B, macrovascular invasion (MVI), and/or extrahepatic spread (EHS) (Fig. 1) [2]. Unfortunately, almost 40% of patients have advanced stages at the time of the first diagnosis, and their prognoses are dismal [3].
Fig. 1. Schematic image of advanced HCC (BCLC stage C).
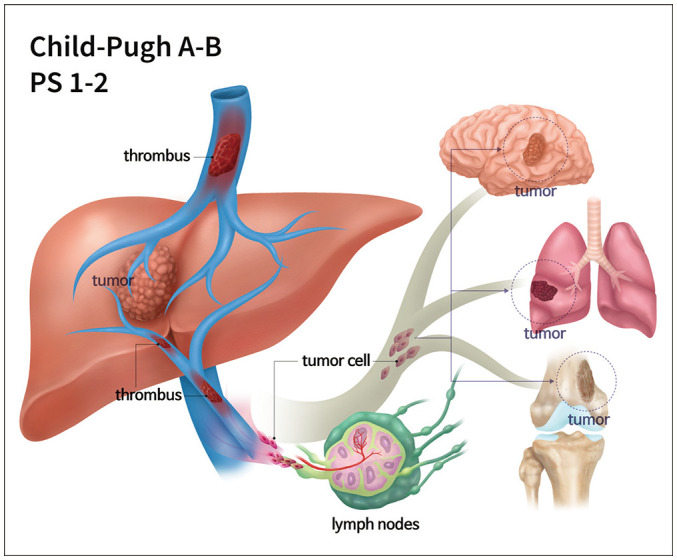
Advanced HCC (BCLC stage C) is defined as HCC in patients with performance status 1–2, Child-Pugh score A or B, macrovascular invasion, and/or extrahepatic spread. BCLC = Barcelona Clinic Liver Cancer, HCC = hepatocellular carcinoma
Sorafenib is currently the treatment of choice for patients with advanced HCC. However, its efficacy is suboptimal; it is associated with a median overall survival (OS) extension of 2–3 months [4,5]. In addition, almost 35% of patients require dose reduction and another 15% are intolerant to sorafenib and require its withdrawal. Thus, sorafenib therapy is suitable for only half of the patients with advanced HCC [6]. Lenvatinib, compared with sorafenib, was associated with non-inferior OS as first-line therapy for patients with unresectable HCC [7]. Recently, a phase-3 trial (IMbrave150) showed that the combination of atezolizumab and bevacizumab had superior OS and progression-free survival (PFS), with sorafenib as the first-line treatment for unresectable HCC (including 81% BCLC C patients) [8].
For patients with advanced HCC, several institutions have administered other locoregional therapies or combination treatments performed by interventional radiologists instead of sorafenib monotherapy. Other emerging alternative and/or combination treatments, including immune checkpoint inhibitors, have also recently been investigated for their ability to improve the OS of patients with advanced HCC. In this descriptive paper, these various treatment modalities are comprehensively reviewed, with a focus on recent updates to radiologic treatments (Table 1).
Table 1. Summary of Radiologic Treatment Options for Advanced Hepatocellular Carcinoma.
| Treatment | Number of Studies [References] | Median OS (Months) | Median PFS (Months) | Extent of MVI (mOS, Months) | EHS (mOS, Months) | Grade 3/4 AE (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| Main PV | Branch PV | HV/IVC | |||||||
| Single therapy | |||||||||
| Conventional TACE | 8 [11,14,15,25,26,27,28,29] | 6–14.9 | 3–7.1 | 10.2 | 4.0–9.0 | 5.4–38 | |||
| DEB-TACE | 3 [16,17,18] | 13.3–19.5 | 5.1 | 20 | 13.3 | ||||
| RE | 12 [33,34,35,36,38,39,40,41,42,43,44,46] | 6–45.3 | 3.4–6.5 | 4.4–9.7 | 9.9–16.9 | 5.4–7.4 | 9–48 | ||
| Sorafenib combination therapy | |||||||||
| TACE + sorafenib | 7 [52,53,54,55,56,57,58] | 7.0–16.2 | 4.8–12.6 | 3–7.8 | 13–15 | 8–16 | 12.2–35 | ||
| RFA (with TACE) + sorafenib | 2 [61,62] | 14.0–19.0 | 11.0–19.0 | 16.0 | |||||
| RE + sorafenib | 5 [65,66,67,68,69] | 8.6–18.5 | 6.5–12.3 | 21.1–64.8 | |||||
| Radiation combination therapy | |||||||||
| TACE + RT | 7 [72,73,74,75,76,77,78] | 5.9–24.2 | 6.5–10.0 | 12 | 11.7 | 6 | 9.4–19.9 | ||
| ICI combination therapy | |||||||||
| TACE/RFA + ICI | 1 [85] | 12.3 | 53.1 | ||||||
| RE + ICI | 2 [86,88] | 15.1–16.5 | 4.6–5.7 | 11–19.2 | |||||
AE = adverse event, DEB = drug eluting bead, EHS = extrahepatic spread, HAIC = hepatic arterial infusion chemotherapy, HV = hepatic vein, ICI = immune checkpoint inhibitor, IVC = inferior vena cava, mOS = median OS, MVI = major vascular invasion, OS = overall survival, PFS = progression-free survival, PV = portal vein, RE = radioembolization, RFA = radiofrequency ablation, RT = radiotherapy, TACE = transcatheter arterial chemoembolization
Transcatheter Arterial Chemoembolization
Transcatheter arterial chemoembolization (TACE) is a well-established treatment for non-resectable HCC and is used as the first-line therapy in almost half of the patients with advanced HCC [9]. Although TACE is hypothetically contraindicated in HCC with portal vein (PV) tumor thrombosis (PVTT) because of the potential risk of hepatic ischemia, several studies have verified that TACE can be safely performed in the presence of adequate collateral circulation around the involved PV [10,11]. As an alternative to conventional TACE, non-resorbable microspheres loaded with a chemotherapeutic agent, known as drug-eluting beads (DEBs), can be administered to ensure sustained and selective drug delivery to the tumor with increased local drug concentration without elevation of systemic concentration [12].
Randomized Controlled Trial(s)
TACE improved the survival of selected patients with unresectable HCC (including 12.5% BCLC C patients) [13]. There have been no randomized controlled trials (RCTs) comparing TACE and sorafenib in patients with advanced HCC.
Observational Studies
Pinter et al. [14] reported that TACE and sorafenib were associated with similar OS (9.2 vs. 7.4 months, respectively, p = 0.377) in patients with advanced HCC. However, candidates for TACE should still be carefully selected when PVTT is present. In a study of 331 HCC patients with segmental PVTT [15], four risk factors were associated with poor patient survival: a major tumor burden, EHS, non-regression following TACE (stable or progressive disease), and a Child-Pugh score of B. The expected median OS of HCC patients with 0, 1, or 2–4 risk factors were 29.1, 15.1, and 5.3 months, respectively. TACE may not be recommended for patients with 2–4 risk factors, owing to poor survival outcomes.
In patients with advanced HCC, the median OS associated with the use of DEB-TACE ranged from 13.3 to 19.5 months [16,17,18]. However, the comparative efficacy and survival rates of DEB-TACE and conventional TACE have not been established. Several studies have reported no significant difference between the survival outcomes associated with DEB-TACE and conventional TACE [19,20,21,22]. However, a study by Li et al. [23] demonstrated more favorable PFS and OS in the DEB-TACE group and reported that DEB-TACE was an independent predictive factor for a better objective response rate, PFS, and OS. Recently, Chu et al. [24] reported a significantly higher objective tumor regression rate in a DEB-TACE group in a subgroup analysis of BCLC C patients (17.7% vs. 10.3%, p = 0.033), although the subgroup analysis showed no significant difference in the time to progression (TTP). In addition, lesser post-procedural abdominal pain [19], fewer required treatments [20], better tolerability [21], and shorter hospital stays [22] have been widely reported in DEB-TACE groups. The current findings do not provide any evidence demonstrating the superiority of DEB-TACE or conventional TACE based on survival outcomes, although DEB-TACE may be more suitable for vulnerable patients or those anticipated to show deterioration following locoregional chemotherapy.
Jung et al. [25] found that intrahepatic tumor status was a significant predictor of survival in patients with advanced HCC, even in the case of metastasis. Several studies, including those on metastatic HCC, reported that treatment with intrahepatic TACE, compared with no therapy, was associated with improved survival [26,27]. Kirstein et al. [28] showed the non-inferiority of TACE to sorafenib based on the median OS in patients with limited EHS after propensity score matching (8 vs. 4 months, p = 0.613); however, in selected patients with low alpha fetoprotein and C-reactive protein concentrations, TACE showed a prolonged median OS of 20 months. In addition, Kim et al. [29] reported no significant difference in OS between the TACE and sorafenib subgroups of the HCC patients with EHS (p = 0.063). In the subgroup analysis, TACE was associated with better survival among younger patients and those with segmental/lobar PV invasion.
Summary
TACE for patients with advanced HCC has shown benefits in some observational studies and may be considered in selected patients who are not suitable for sorafenib, albeit the low level of evidence [6,30].
Radioembolization
Transarterial radioembolization with yttrium-90 (90Y) in resin or glass microspheres has been conducted as an alternative to TACE [31]. Radioembolization differs from TACE in that it uses a local beta radiation mechanism instead of arterial occlusion. Pretreatment technetium-99m macroaggregated albumin scan and angiography are essential for assessing lung shunt fraction and identifying vessels that may supply the gastrointestinal (GI) tract to avoid radiation pneumonitis (< 1%) and GI ulceration (< 5%). Radioembolization causes less post-embolization syndrome than TACE because the embolic effect is minimal. Other complications, including radiation-induced liver disease, radiation cholecystitis, biloma, liver abscess, and bile duct stricture, are infrequent [32].
Randomized Controlled Trial(s)
Milestone trials have compared radioembolization and sorafenib in locally advanced HCC [33,34,35]. The SIRveNIB trial [33] demonstrated no statistically significant difference in the median OS (8.8 vs. 10.0 months, p = 0.36), and the SARAH trial [34] showed comparable median OS (8.0 vs. 9.9 months, p = 0.18) or PFS. However, radioembolization was associated with significantly fewer grade 3 or 4 adverse events (AEs) in both the SIRveNIB and SARAH studies. However, the DOSISPHERE-01 trial [36] compared the personalized boosted dosimetry (≥ 205 Gy) and the standard dosimetry (120 ± 20 Gy) subgroups of locally advanced HCC patients and showed better median OS (26.6 vs. 10.7 months, p = 0.010) and higher objective response rate (71% vs. 36%, p = 0.007) in the former than in the latter group. This study may challenge the conclusions of previous RCTs of radioembolization, in which no personalized dosimetry was used.
Meta-Analysis
Patients with HCC with PVTT have shown poor response rates to sorafenib (only 2–3.3%) in two randomized trials [4,5], and several physicians prefer radioembolization treatment for HCC invading the PV. A meta-analysis of observational studies [37] revealed that radioembolization was associated with a higher pooled OS at 6 months/1 per year (76% vs. 54%/47% vs. 24%) and a longer TTP than sorafenib in the treatment of HCC with PVTT. In addition, radioembolization was associated with a lower incidence of AEs of grades higher than 3 (9% vs. 28%).
Observational Studies
Two cohort studies [38,39] demonstrated a median OS of 10–12 months for advanced HCC. A prospective phase II study reported a median OS of 13 months in patients with locally advanced HCC with PVTT [40]. The presence and extent of PVTT affect prognosis, with reported median OS durations of 9.9–16.6 months for branch PVTT and 4.4–9.7 months for main PVTT when a distinction of the PVTT extent was made [38,39,41,42,43]. Zu et al. [44] reported that the median OS of advanced HCC patients with Child-Pugh B7–9 (5.5–6.0 months) was significantly worse than those with Child-Pugh A (20.2 months).
Ablative radioembolization is intended to deliver high-dose radiation (with a target dose of greater than 190 Gy) to the liver segment or lobe where the cancer is located, inducing a maximum cytotoxic effect within the targeted area with the delivery of radioactivity to kill the tumors and adjacent normal liver parenchyma [45]. A recent study by Cardarelli-Leite et al. [46] demonstrated that ablative radioembolization was associated with a longer survival duration than conventional radioembolization in patients with advanced HCC and PVTT without EHS; ablative radioembolization was associated with a longer median OS (45.3 vs. 18.2 months, p = 0.003) and improved 4-year survival (53.9% vs. 11.2%). Neither modality was associated with toxic effects on liver function. In addition, the LEGACY study investigating high-dose radioembolization [47] showed a high objective response rate (88.3%) and better 3-year OS rate (86.6%) in patients with unresectable solitary HCC without MVI or EHS (including 39.5% BCLC C patients).
Summary
Two RCTs demonstrated that there was no significant difference in the OS after radioembolization and sorafenib treatment. Patients with locally advanced HCC and preserved liver function who have contraindications for sorafenib may be good candidates for radioembolization [6,30]. The promising results of the administration of radioembolization with boosted dose warrant further RCTs either alone or in combination with other agents [36,48].
Combined TACE and Sorafenib
The efficacy of TACE in combination with sorafenib has been investigated, as these two treatment options are anticipated to work synergistically. TACE-induced acute hypoxia in surviving tumor cells leads to the upregulation of angiogenic growth factors, which may contribute to revascularization of the tumor, local recurrence, or metastasis [49,50]. Sorafenib inhibits tumor cell proliferation by exerting antiangiogenic effects by blocking vascular endothelial growth factor (VEGF) receptor-2 and -3, and platelet-derived growth factor (PDGF) receptor tyrosine kinase [51]. However, studies on the comparative efficacy and survival rates of TACE plus sorafenib and sorafenib alone have reported conflicting results, whereas the survival benefit of combination treatment has been observed in certain subgroups.
Randomized Controlled Trial(s)
A phase III STAH trial [52] demonstrated that the combination of sorafenib and TACE (n = 170), compared with sorafenib alone (n = 169), did not improve OS in patients with advanced HCC (12.8 vs. 10.8 months, p = 0.290), although combination therapy did show significantly improved TTP (5.3 vs. 3.5 months, p = 0.003), PFS (5.2 vs. 3.6 months, p = 0.009), and tumor response rate (60.6% vs. 47.3%, p = 0.005). A post-hoc subgroup analysis revealed that the OS in the combination group receiving two or more concurrent TACE procedures was longer than that in the sorafenib alone group (18.6 vs. 10.8 months, p = 0.006). Grade 3 or 4 AEs occurred more frequently in the combination group than in the sorafenib alone group (33.3% vs. 19.8%, p = 0.006).
Observational Studies
Choi et al. [53] reported that the median OS and TTP of the TACE plus sorafenib group, compared with those of the sorafenib-only group of advanced HCC patients, improved; however, after propensity score matching (96 pairs), the improvement reduced and only TTP remained significant. In another retrospective study of advanced HCC patients with main PVTT [54], TACE plus sorafenib, compared to sorafenib, offered no significant benefit related to OS (7.0 vs. 6.0 months, p = 0.544) or TTP (3.0 vs. 3.0 months, p = 0.924). Ha et al. [55] compared the efficacies of TACE combined with sorafenib and sorafenib alone for advanced HCC. In their study, the patients were divided into three different groups (concurrent TACE with sorafenib, TACE followed by sorafenib, and sorafenib alone), and their median OS were comparable (16.2, 13.5, and 11.8 months, respectively, p = 0.13). However, among PV invasion cases, TACE administered concurrently with or before sorafenib treatment was associated with improved survival (25.7 months, p = 0.002; 14.0 months, p = 0.030, respectively) compared with sorafenib monotherapy (5.5 months). Multivariate analysis showed that sorafenib duration, TACE, and Child-Pugh scores were associated with a survival benefit. Chien et al. [56] showed that combining TACE with sorafenib resulted in better OS than sorafenib alone in advanced HCC patients with a Child-Pugh score A after propensity score matching (419 vs. 223 days, p = 0.028). Hsiao et al. [57] demonstrated that the concurrent administration of TACE and sorafenib resulted in significantly higher median OS in advanced HCC patients than sorafenib alone (14.2 vs. 7.5 months, p = 0.048).
According to the GIDEON study [58], there is a global variation in the combination of TACE with sorafenib in HCC patients; 1511 (47.2%) patients underwent sorafenib after TACE, and 325 (10.1%) underwent TACE concomitantly. The data revealed concomitant TACE to show a significant benefit in median OS in advanced HCC patients in comparison with non-concomitant TACE (15.5 vs. 8.3 months). However, this study was affected by significant heterogeneity in tumor invasiveness, metastasis patterns, and liver function across the groups.
Summary
One RCT showed that sorafenib combined with TACE did not improve OS. However, combination treatment significantly improved tumor response and secondary outcomes. The survival benefit of this combination treatment has been observed in certain subgroups in several observational studies.
Combined Radiofrequency Ablation (with TACE) and Sorafenib
Given that the cause of death in most patients with advanced HCC is intrahepatic tumor progression rather than EHS [59], debulking of the primary tumor burden is considered to show survival benefits in patients with advanced HCC [60].
Randomized Controlled Trial(s)
A western RCT in HCC patients with main PVTT and no EHS by Giorgio et al. [61] demonstrated that sorafenib combined with radiofrequency ablation (RFA) of both intraparenchymal HCC and the main PVTT showed better OS than sorafenib alone (1-, 2-, and 3-year survival rates of 60% vs. 37%, 35% vs. 0%, and 26% vs. 0%, respectively; hazard ratio [HR]: 2.87, p < 0.001). Multivariate analysis showed that the combined use of RFA and sorafenib was the only independent predictor of survival (HR: 2.89, p < 0.001).
Observational Studies
A multicenter retrospective study [62] showed that TACE-RFA combined with sorafenib was safe and effective in patients with advanced recurrent HCC after initial liver resection with PVTT involving the right or left PV or higher or EHS. This combination treatment was found to be superior to sorafenib based on the median OS (14.0 vs. 9.0 months, p < 0.001) and TTP (7.0 vs. 4.0 months, p < 0.001). Multivariate analysis showed that the treatment modality was a significant predictor of OS and TTP, and the number of intrahepatic tumors was also a prognostic factor for OS.
Summary
RFA combined with sorafenib showed better OS than sorafenib alone in patients with advanced HCC; thus, this combination may be an alternative treatment option.
Combined Radioembolization and Sorafenib
The theory behind the combined use of radioembolization and sorafenib is based on the mechanism by which sorafenib enhances the radiosensitivity of human HCC cell lines through the selective inhibition of the radiation-induced activation of vascular endothelial growth factor receptor 2 (VEGFR2) and extracellular-signal-regulated kinase pathways, thus promoting radiation-induced apoptosis [63]. However, Lewandowski et al. [64] reported that the predominant effect of adding sorafenib may be through the inhibition of PDGF and not VEGF.
Randomized Controlled Trial(s)
A SORAMIC study [65] comparing the efficacy and safety of radioembolization plus sorafenib with sorafenib alone in patients with advanced HCC found that the addition of radioembolization to sorafenib did not demonstrate significantly better OS than sorafenib alone (12.1 vs. 11.4 months, p = 0.953). However, subgroup analyses led to hypothesis-generating results related to patients with potential clinical benefits from adding radioembolization to sorafenib, with such patients possibly including non-cirrhotic HCC patients (HR: 0.46, p = 0.013), those with cirrhosis with a non-alcoholic etiology (HR: 0.63, p = 0.009), and patients aged ≤ 65 (HR: 0.65, p = 0.046). The STOP-HCC study (NCT01556490) comparing radioembolization plus sorafenib with sorafenib alone is also currently underway and the results will be available soon.
Observational Studies
Kaseb et al. [66] conducted a phase 2 study of advanced HCC patients (including patients with metastasis) that showed that the combination of radioembolization and sorafenib was tolerable, and it was associated with improved OS and PFS (18.5 and 12.3 months) compared with previous reports evaluating sorafenib alone. The combination of radioembolization and sorafenib for advanced HCC patients is reported to be well-tolerated, with the median OS durations reported by several investigations [67,68,69] ranging from 8.6–12.4 months, which are longer than those reported for sorafenib alone in similarly designed reports.
Summary
The combined use of radioembolization and sorafenib, compared with sorafenib alone, did not result in a significant improvement in OS. However, this combination treatment may be an option for selected patients with advanced HCC.
Combined TACE and Radiotherapy
The efficacy of TACE combined with radiotherapy (RT) has been investigated, as it is expected to result in better outcomes in advanced HCC patients. The rationale for this combination therapy is that reducing PVTT with RT can inhibit tumor growth in blood vessels and preserve proper portal venous blood flow to prevent the deterioration of liver function, limit intrahepatic tumor spread, and promote subsequent treatments of primary tumors [70]. In addition, RT may potentially boost the effects of TACE by causing regression of the arteriovenous shunt around the PVTT [71].
Randomized Controlled Trial(s)
Yoon et al. [72] conducted an RCT comparing the efficacies of TACE plus RT (n = 45) and sorafenib (n = 45) in 90 patients with locally advanced HCC with MVI. At 12 weeks, the PFS rate was significantly higher in the TACE plus RT group than in the sorafenib group (86.7% vs. 34.3%, p < 0.001). The TACE plus RT group demonstrated a significantly higher radiologic response rate at week 24 (15 [33.3%] vs. 1 [2.2%], p < 0.001) and significantly improved median TTP (31.0 vs. 11.7 weeks, p < 0.001), PFS (30.0 vs. 11.3 weeks, p < 0.001), and OS (55.0 vs. 43.0 weeks, p = 0.04) compared with the sorafenib group. The AEs of grades 3–4 in the two groups were similar (p = 0.18), and no patients in the TACE plus RT group discontinued treatment because of hepatic decompensation.
Observational Studies
Chung et al. [73] reported a median OS of 12 months in patients with HCC with the main PVTT treated with TACE plus RT. Kim et al. [74] evaluated the efficacy of TACE plus RT as a first-line treatment in 639 patients with HCC and MVI. The median OS was 10.7 months, with 1- and 2-year survival rates of 46.5% and 23.9%, respectively. For HCC with inferior vena cava tumor thrombosis, a prospective study [75] evaluated the effects of TACE plus RT and TACE alone in a historical control group. The results showed that the median OS was significantly higher in the TACE plus RT group than in the TACE group (11.7 vs. 4.7 months, p < 0.01).
Kim et al. [76] compared the efficacy of TACE with or without RT with that of sorafenib alone for advanced HCC with PVTT. In the propensity score-matched analysis, the TACE plus RT group demonstrated longer OS and TTP than the group that received TACE alone (102 pairs; 11.4 vs. 7.4 months, p = 0.023; 8.7 vs. 3.6 months, p < 0.001, respectively) or sorafenib alone (30 pairs; 8.2 vs. 3.2 months, p < 0.001; 5.1 vs. 1.6 months, p < 0.001, respectively). Shen et al. [77] evaluated the survival outcomes of TACE plus RT and TACE plus sorafenib in advanced HCC patients with MVI. After propensity score matching, TACE plus RT provided improved OS (24.2 vs. 8.4 months, p = 0.007) and PFS (10.0 vs. 3.5 months, p < 0.001) compared with TACE plus sorafenib. However, a recent study by Chu et al. [78] comparing TACE plus RT (n = 203) with TACE plus sorafenib (n = 104) in advanced HCC with PVTT demonstrated conflicting results. The median OS and PFS in the two groups were not significantly different after propensity score matching (n = 87). However, in a subgroup analysis of non-metastatic advanced HCC patients, TACE plus RT showed better OS (HR: 1.42; 95% confidence interval [CI]: 1.00–2.03; p = 0.05) and PFS (HR: 1.35; 95% CI: 0.98–1.86; p = 0.071) than TACE plus sorafenib, with borderline statistical significance.
Summary
One RCT and several observational studies have shown that TACE plus RT could be considered a first-line treatment option for patients with locally advanced HCC [30].
Immunotherapy
Immune checkpoint inhibitors have demonstrated promising benefits for the treatment of patients who are intolerant to or have progressed under approved multikinase inhibitors in recent phase II clinical trials. In the CheckMate040 study [79], nivolumab, a programmed cell death protein 1 (PD-1) immune checkpoint inhibitor, provided a median OS of 15.0 months (95% CI: 9.6–20.2 months) in the dose-escalation phase. The KEYNOTE-224 study [80] showed a comparable result for median OS with pembrolizumab (12.9 months; 95% CI: 9.7–15.5 months).
Interventional radiological treatments including TACE, radioembolization, and ablation can increase tumor immunogenicity by stimulating a pro-immune inflammatory response and releasing tumor-associated antigens, which can lead to an increase in the systemic antitumor immune response, including tumor-infiltrating cytotoxic CD8+ T cells [81], thus providing a solid rationale for the combination treatment with immunotherapy. Furthermore, immunotherapy has an advantage in that it does not require liver metabolism [82]. In several preclinical studies, the combination of locoregional treatments with immune checkpoint inhibitors demonstrated an increased antitumor immune response [83]. Recently, Craciun et al. [84] compared intra-tumor immune infiltrates in surgical specimens after preoperative treatment with TACE or radioembolization. Significantly increased recruitment/activation of intra-tumor immune cells (tumor-infiltrating lymphocytes, and CD4+ and CD8+ T cells) was observed in the radioembolization group compared to the groups that underwent TACE or no preoperative treatment. The authors suggested that radioembolization is a better option than TACE in combination with an immune checkpoint inhibitor.
Currently, several studies investigating the efficacy of combinations of various immunotherapies with TACE, radioembolization, or ablation are underway, and the role of combination treatment using immunotherapy in advanced HCC patients should be determined in the foreseeable future.
Combined Immunotherapy and TACE or RFA
Duffy et al. [85] evaluated the efficacy of combining tremelimumab (anti-CTLA-4 monoclonal antibody) with TACE or ablation in 32 patients with advanced HCC. Most (75%) of the patients were intolerant of sorafenib or progressed on it previously, and all patients had evidence of progressive disease at enrollment. The median OS and TTP were 12.3 months (95% CI: 9.3–15.4) and 7.4 months (95% CI: 4.7–19.4), respectively. The majority of patients experienced a marked reduction in HCV load, objective tumor responses outside of the embolized or ablated zone, and infiltration of intratumoral CD8+ T cells.
To date, multiple trials of TACE plus nivolumab (NCT03143270, NCT03572582, NCT04268888), TACE plus pembrolizumab (NCT03397654, NCT03099564), and ablation plus nivolumab (NCT03383458) are recruiting patients. In addition, a trial of TACE plus durvalumab and bevacizumab (NCT03778957) is currently recruiting patients, as the literature proposes a synergistic effect for immunotherapy and anti-angiogenic therapy.
Combined Immunotherapy and Radioembolization
A retrospective study [86] including 26 patients with advanced (n = 21) or aggressive intermediate stage HCC demonstrated the efficacy of combined immune checkpoint inhibitor therapy (nivolumab and ipilimumab plus nivolumab) within 3 months of radioembolization. From the first radioembolization, the median OS and PFS were 16.5 months (95% CI: 6.6–26.4) and 5.7 months (95% CI: 4.3–7.1), respectively. Nine patients (35%) maintained disease control, and one patient had a complete response on imaging that was pathologically confirmed after liver explantation. The combination treatment resulted in limited treatment-related toxicity. In addition, a case report [87] from another institution showed that combining nivolumab with radioembolization in an advanced HCC patient with MVI successfully bridged the patient to surgery. The pathological report showed negative margins with a complete pathological response. A phase II nonrandomized trial [88] combining nivolumab and radioembolization in Asian patients with advanced HCC (n = 36) showed an encouraging overall response rate of 31%, with median PFS and OS of 4.6 months (95% CI: 2.3–8.4) and 15.1 months (95% CI: 7.8-not evaluable), respectively. Only 11% of the patients showed grade 3–4 AEs. Marinelli et al. [89] evaluated the efficacy and safety of combining nivolumab with TACE or radioembolization in 17 patients with advanced HCC. The median OS and TTP were 11.3 months and 7.9 months, respectively. No AEs of grades 3–4 attributable to immunotherapy were observed. Furthermore, trials evaluating radioembolization plus nivolumab (NCT03380130, NCT03033446, NCT02837029) and radioembolization plus pembrolizumab (NCT03099564) are currently recruiting patients.
CONCLUSION
Interventional radiologists have made efforts toward developing alternative and/or combination treatments for first-line systemic treatment for patients with advanced HCC. Locoregional treatments with or without systemic therapy may be considered in the selected patients. However, all RCTs compared locoregional therapy alone or in combination with systemic therapy to sorafenib alone, and the shift of the standard systemic therapy to atezolizumab plus bevacizumab can influence the interpretation of the results of previous studies. In addition, in the BCLC staging system, advanced HCC includes heterogeneous patient populations; therefore, subclassifications for accurate prognosis prediction are needed, which should also be appropriate for interpreting clinical trials and comparing treatment modalities. Various treatment modalities for advanced HCC continue to evolve, and several RCTs, including those of combination treatments with immunotherapy, are ongoing.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Jin Hyoung Kim, Pyeong Hwa Kim.
- Data curation: Gun Ha Kim.
- Formal analysis: Gun Ha Kim, Jin Hyoung Kim.
- Investigation: all authors.
- Methodology: Gun Ha Kim, Jin Hyoung Kim, Pyeong Hwa Kim.
- Resources: Gun Ha Kim, Pyeong Hwa Kim.
- Software: Gun Ha Kim.
- Supervision: Jin Hyoung Kim.
- Visualization: Gun Ha Kim, Jin Hyoung Kim.
- Writing—original draft: Gun Ha Kim.
- Writing—review & editing: Jin Hyoung Kim, Pyeong Hwa Kim, Hee Ho Chu, Dong Il Gwon, Heung-Kyu Ko.
References
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Cabibbo G, Enea M, Attanasio M, Bruix J, Craxì A, Cammà C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010;51:1274–1283. doi: 10.1002/hep.23485. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 5.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 6.Chen LT, Martinelli E, Cheng AL, Pentheroudakis G, Qin S, Bhattacharyya GS, et al. Pan-Asian adapted ESMO clinical practice guidelines for the management of patients with intermediate and advanced/relapsed hepatocellular carcinoma: a TOS-ESMO initiative endorsed by CSCO, ISMPO, JSMO, KSMO, MOS and SSO. Ann Oncol. 2020;31:334–351. doi: 10.1016/j.annonc.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 8.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 9.Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35:2155–2166. doi: 10.1111/liv.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kothary N, Weintraub JL, Susman J, Rundback JH. Transarterial chemoembolization for primary hepatocellular carcinoma in patients at high risk. J Vasc Interv Radiol. 2007;18:1517–1526. doi: 10.1016/j.jvir.2007.07.035. quiz 1527. [DOI] [PubMed] [Google Scholar]
- 11.Kim JH, Yoon HK, Kim SY, Kim KM, Ko GY, Gwon DI, et al. Transcatheter arterial chemoembolization vs. chemoinfusion for unresectable hepatocellular carcinoma in patients with major portal vein thrombosis. Aliment Pharmacol Ther. 2009;29:1291–1298. doi: 10.1111/j.1365-2036.2009.04016.x. [DOI] [PubMed] [Google Scholar]
- 12.Nouri YM, Kim JH, Yoon HK, Ko HK, Shin JH, Gwon DI. Update on transarterial chemoembolization with drug-eluting microspheres for hepatocellular carcinoma. Korean J Radiol. 2019;20:34–49. doi: 10.3348/kjr.2018.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 14.Pinter M, Hucke F, Graziadei I, Vogel W, Maieron A, Königsberg R, et al. Advanced-stage hepatocellular carcinoma: transarterial chemoembolization versus sorafenib. Radiology. 2012;263:590–599. doi: 10.1148/radiol.12111550. [DOI] [PubMed] [Google Scholar]
- 15.Kim JH, Shim JH, Yoon HK, Ko HK, Kim JW, Gwon DI. Chemoembolization related to good survival for selected patients with hepatocellular carcinoma invading segmental portal vein. Liver Int. 2018;38:1646–1654. doi: 10.1111/liv.13719. [DOI] [PubMed] [Google Scholar]
- 16.Ray CE, Jr, Brown AC, Green TJ, Winston H, Curran C, Kreidler SM, et al. Survival outcomes in patients with advanced hepatocellular carcinoma treated with drug-eluting bead chemoembolization. AJR Am J Roentgenol. 2015;204:440–447. doi: 10.2214/AJR.14.12844. [DOI] [PubMed] [Google Scholar]
- 17.Kalva SP, Pectasides M, Liu R, Rachamreddy N, Surakanti S, Yeddula K, et al. Safety and effectiveness of chemoembolization with drug-eluting beads for advanced-stage hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2014;37:381–387. doi: 10.1007/s00270-013-0654-7. [DOI] [PubMed] [Google Scholar]
- 18.Prajapati H, Kim H. Development of the new prognostic staging system and proposal of treatment plans based on the multivariate survival analyses (MVA) after doxorubicin drug eluting beads trans-arterial chemoembolization (DEB TACE) in patients with BCLC C (advanced) hepatocellular carcinoma (HCC) J Vasc Interv Radiol. 2016;27:S127–S128. [Google Scholar]
- 19.Golfieri R, Giampalma E, Renzulli M, Cioni R, Bargellini I, Bartolozzi C, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer. 2014;111:255–264. doi: 10.1038/bjc.2014.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kloeckner R, Weinmann A, Prinz F, Pinto dos Santos D, Ruckes C, Dueber C, et al. Conventional transarterial chemoembolization versus drug-eluting bead transarterial chemoembolization for the treatment of hepatocellular carcinoma. BMC Cancer. 2015;15:465. doi: 10.1186/s12885-015-1480-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Massani M, Stecca T, Ruffolo C, Bassi N. Should we routinely use DEBTACE for unresectable HCC? cTACE versus DEBTACE: a single-center survival analysis. Updates Surg. 2017;69:67–73. doi: 10.1007/s13304-017-0414-3. [DOI] [PubMed] [Google Scholar]
- 22.Gomes AS, Monteleone PA, Sayre JW, Finn RS, Sadeghi S, Tong MJ, et al. Comparison of triple-drug transcatheter arterial chemoembolization (TACE) with single-drug TACE using doxorubicin-eluting beads: long-term survival in 313 patients. AJR Am J Roentgenol. 2017;209:722–732. doi: 10.2214/AJR.17.18219. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Wu F, Duan M, Zhang G. Drug-eluting bead transarterial chemoembolization (TACE) vs conventional TACE in treating hepatocellular carcinoma patients with multiple conventional TACE treatments history: a comparison of efficacy and safety. Medicine (Baltimore) 2019;98:e15314. doi: 10.1097/MD.0000000000015314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu HH, Gwon DI, Kim JH, Ko GY, Shin JH, Yoon HK. Drug-eluting microsphere versus cisplatin-based transarterial chemoembolization for the treatment of hepatocellular carcinoma: propensity score–matched analysis. AJR Am J Roentgenol. 2020;215:745–752. doi: 10.2214/AJR.19.21669. [DOI] [PubMed] [Google Scholar]
- 25.Jung SM, Jang JW, You CR, Yoo SH, Kwon JH, Bae SH, et al. Role of intrahepatic tumor control in the prognosis of patients with hepatocellular carcinoma and extrahepatic metastases. J Gastroenterol Hepatol. 2012;27:684–689. doi: 10.1111/j.1440-1746.2011.06917.x. [DOI] [PubMed] [Google Scholar]
- 26.Bettinger D, Spode R, Glaser N, Buettner N, Boettler T, Neumann-Haefelin C, et al. Survival benefit of transarterial chemoembolization in patients with metastatic hepatocellular carcinoma: a single center experience. BMC Gastroenterol. 2017;17:98. doi: 10.1186/s12876-017-0656-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee IC, Huo TI, Huang YH, Chao Y, Li CP, Lee PC, et al. Transarterial chemoembolization can prolong survival for patients with metastatic hepatocellular carcinoma: a propensity score matching analysis. Hepatol Int. 2012;6:753–762. doi: 10.1007/s12072-011-9322-7. [DOI] [PubMed] [Google Scholar]
- 28.Kirstein MM, Voigtländer T, Schweitzer N, Hinrichs JB, Marquardt J, Wörns MA, et al. Transarterial chemoembolization versus sorafenib in patients with hepatocellular carcinoma and extrahepatic disease. United European Gastroenterol J. 2018;6:238–246. doi: 10.1177/2050640617716597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim J, Sinn DH, Choi MS, Kang W, Gwak GY, Paik YH, et al. Hepatocellular carcinoma with extrahepatic metastasis: are there still candidates for transarterial chemoembolization as an initial treatment? PLoS One. 2019;14:e0213547. doi: 10.1371/journal.pone.0213547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korean Liver Cancer Association (KLCA); National Cancer Center (NCC) 2018 Korean Liver Cancer Association-National Cancer Center Korea practice guidelines for the management of hepatocellular carcinoma. Korean J Radiol. 2019;20:1042–1113. doi: 10.3348/kjr.2019.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salem R, Mazzaferro V, Sangro B. Yttrium 90 radioembolization for the treatment of hepatocellular carcinoma: biological lessons, current challenges, and clinical perspectives. Hepatology. 2013;58:2188–2197. doi: 10.1002/hep.26382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riaz A, Lewandowski RJ, Kulik LM, Mulcahy MF, Sato KT, Ryu RK, et al. Complications following radioembolization with yttrium-90 microspheres: a comprehensive literature review. J Vasc Interv Radiol. 2009;20:1121–1130. doi: 10.1016/j.jvir.2009.05.030. quiz 1131. [DOI] [PubMed] [Google Scholar]
- 33.Chow PK, Gandhi M, Tan SB, Khin MW, Khasbazar A, Ong J, et al. SIRveNIB: selective internal radiation therapy versus sorafenib in Asia-Pacific patients with hepatocellular carcinoma. J Clin Oncol. 2018;36:1913–1921. doi: 10.1200/JCO.2017.76.0892. [DOI] [PubMed] [Google Scholar]
- 34.Vilgrain V, Pereira H, Assenat E, Guiu B, Ilonca AD, Pageaux GP, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1624–1636. doi: 10.1016/S1470-2045(17)30683-6. [DOI] [PubMed] [Google Scholar]
- 35.Gramenzi A, Golfieri R, Mosconi C, Cappelli A, Granito A, Cucchetti A, et al. Yttrium-90 radioembolization vs sorafenib for intermediate-locally advanced hepatocellular carcinoma: a cohort study with propensity score analysis. Liver Int. 2015;35:1036–1047. doi: 10.1111/liv.12574. [DOI] [PubMed] [Google Scholar]
- 36.Garin E, Tselikas L, Guiu B, Chalaye J, Edeline J, de Baere T, et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol. 2021;6:17–29. doi: 10.1016/S2468-1253(20)30290-9. [DOI] [PubMed] [Google Scholar]
- 37.Kim PH, Choi SH, Kim JH, Park SH. Comparison of radioembolization and sorafenib for the treatment of hepatocellular carcinoma with portal vein tumor thrombosis: a systematic review and meta-analysis of safety and efficacy. Korean J Radiol. 2019;20:385–398. doi: 10.3348/kjr.2018.0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sangro B, Carpanese L, Cianni R, Golfieri R, Gasparini D, Ezziddin S, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011;54:868–878. doi: 10.1002/hep.24451. [DOI] [PubMed] [Google Scholar]
- 39.Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, et al. Radioembolization for hepatocellular carcinoma using yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Mazzaferro V, Sposito C, Bhoori S, Romito R, Chiesa C, Morosi C, et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology. 2013;57:1826–1837. doi: 10.1002/hep.26014. [DOI] [PubMed] [Google Scholar]
- 41.Kulik LM, Carr BI, Mulcahy MF, Lewandowski RJ, Atassi B, Ryu RK, et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008;47:71–81. doi: 10.1002/hep.21980. [DOI] [PubMed] [Google Scholar]
- 42.Memon K, Kulik L, Lewandowski RJ, Mulcahy MF, Benson AB, Ganger D, et al. Radioembolization for hepatocellular carcinoma with portal vein thrombosis: impact of liver function on systemic treatment options at disease progression. J Hepatol. 2013;58:73–80. doi: 10.1016/j.jhep.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spreafico C, Sposito C, Vaiani M, Cascella T, Bhoori S, Morosi C, et al. Development of a prognostic score to predict response to yttrium-90 radioembolization for hepatocellular carcinoma with portal vein invasion. J Hepatol. 2018;68:724–732. doi: 10.1016/j.jhep.2017.12.026. [DOI] [PubMed] [Google Scholar]
- 44.Zu Q, Schenning RC, Jahangiri Y, Tomozawa Y, Kolbeck KJ, Kaufman JA, et al. Yttrium-90 radioembolization for BCLC stage C hepatocellular carcinoma comparing child-pugh A versus B7 patients: are the outcomes equivalent? Cardiovasc Intervent Radiol. 2020;43:721–731. doi: 10.1007/s00270-020-02434-4. [DOI] [PubMed] [Google Scholar]
- 45.Riaz A, Gates VL, Atassi B, Lewandowski RJ, Mulcahy MF, Ryu RK, et al. Radiation segmentectomy: a novel approach to increase safety and efficacy of radioembolization. Int J Radiat Oncol Biol Phys. 2011;79:163–171. doi: 10.1016/j.ijrobp.2009.10.062. [DOI] [PubMed] [Google Scholar]
- 46.Cardarelli-Leite L, Chung J, Klass D, Marquez V, Chou F, Ho S, et al. Ablative transarterial radioembolization improves survival in patients with HCC and portal vein tumor thrombus. Cardiovasc Intervent Radiol. 2020;43:411–422. doi: 10.1007/s00270-019-02404-5. [DOI] [PubMed] [Google Scholar]
- 47.Salem R, Johnson GE, Kim E, Riaz A, Bishay V, Boucher E, et al. Yttrium-90 radioembolization for the treatment of solitary, unresectable HCC: the LEGACY study. Hepatology. 2021 Mar; doi: 10.1002/hep.31819. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim HC, Choi JW, Lee M, Kim YJ, Paeng JC, Chung JW. Yttrium-90 radioembolization for hepatocellular carcinoma: virtual tumor absorbed dose as a predictor of complete response. Anticancer Res. 2021;41:2625–2635. doi: 10.21873/anticanres.15043. [DOI] [PubMed] [Google Scholar]
- 49.Sergio A, Cristofori C, Cardin R, Pivetta G, Ragazzi R, Baldan A, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914–921. doi: 10.1111/j.1572-0241.2007.01712.x. [DOI] [PubMed] [Google Scholar]
- 50.Shim JH, Park JW, Kim JH, An M, Kong SY, Nam BH, et al. Association between increment of serum VEGF level and prognosis after transcatheter arterial chemoembolization in hepatocellular carcinoma patients. Cancer Sci. 2008;99:2037–2044. doi: 10.1111/j.1349-7006.2008.00909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129–3140. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PubMed] [Google Scholar]
- 52.Park JW, Kim YJ, Kim DY, Bae SH, Paik SW, Lee YJ, et al. Sorafenib with or without concurrent transarterial chemoembolization in patients with advanced hepatocellular carcinoma: the phase III STAH trial. J Hepatol. 2019;70:684–691. doi: 10.1016/j.jhep.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 53.Choi GH, Shim JH, Kim MJ, Ryu MH, Ryoo BY, Kang YK, et al. Sorafenib alone versus sorafenib combined with transarterial chemoembolization for advanced-stage hepatocellular carcinoma: results of propensity score analyses. Radiology. 2013;269:603–611. doi: 10.1148/radiol.13130150. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y, Fan W, Wang Y, Lu L, Fu S, Yang J, et al. Sorafenib with and without transarterial chemoembolization for advanced hepatocellular carcinoma with main portal vein tumor thrombosis: a retrospective analysis. Oncologist. 2015;20:1417–1424. doi: 10.1634/theoncologist.2015-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ha Y, Lee D, Shim JH, Lim YS, Lee HC, Chung YH, et al. Role of transarterial chemoembolization in relation with sorafenib for patients with advanced hepatocellular carcinoma. Oncotarget. 2016;7:74303–74313. doi: 10.18632/oncotarget.11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chien SC, Chen CY, Cheng PN, Liu YS, Cheng HC, Chuang CH, et al. Combined transarterial embolization/chemoembolization-based locoregional treatment with sorafenib prolongs the survival in patients with advanced hepatocellular carcinoma and preserved liver function: a propensity score matching study. Liver Cancer. 2019;8:186–202. doi: 10.1159/000489790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hsiao P, Hsieh KC, Chen YS, Hsu CC, Lo GH, Li YC, et al. Sorafenib with concurrent multiple-line therapies improves overall survival in advanced stage hepatocellular carcinoma. Medicine (Baltimore) 2019;98:e16074. doi: 10.1097/MD.0000000000016074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Geschwind JF, Kudo M, Marrero JA, Venook AP, Chen XP, Bronowicki JP, et al. TACE treatment in patients with sorafenib-treated unresectable hepatocellular carcinoma in clinical practice: final analysis of GIDEON. Radiology. 2016;279:630–640. doi: 10.1148/radiol.2015150667. [DOI] [PubMed] [Google Scholar]
- 59.Uka K, Aikata H, Takaki S, Shirakawa H, Jeong SC, Yamashina K, et al. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol. 2007;13:414–420. doi: 10.3748/wjg.v13.i3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Stefano G, Farella N, Scognamiglio U, Liorre G, Calabria G, Ascione T, et al. Sorafenib after RFA in HCC patients: a pilot study. Hepatogastroenterology. 2015;62:261–263. [PubMed] [Google Scholar]
- 61.Giorgio A, Merola MG, Montesarchio L, Merola F, Santoro B, Coppola C, et al. Sorafenib combined with radio-frequency ablation compared with sorafenib alone in treatment of hepatocellular carcinoma invading portal vein: a western randomized controlled trial. Anticancer Res. 2016;36:6179–6183. doi: 10.21873/anticanres.11211. [DOI] [PubMed] [Google Scholar]
- 62.Peng Z, Chen S, Wei M, Lin M, Jiang C, Mei J, et al. Advanced recurrent hepatocellular carcinoma: treatment with sorafenib alone or in combination with transarterial chemoembolization and radiofrequency ablation. Radiology. 2018;287:705–714. doi: 10.1148/radiol.2018171541. [DOI] [PubMed] [Google Scholar]
- 63.Yu W, Gu K, Yu Z, Yuan D, He M, Ma N, et al. Sorafenib potentiates irradiation effect in hepatocellular carcinoma in vitro and in vivo. Cancer Lett. 2013;329:109–117. doi: 10.1016/j.canlet.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 64.Lewandowski RJ, Andreoli JM, Hickey R, Kallini JR, Gabr A, Baker T, et al. Angiogenic response following radioembolization: results from a randomized pilot study of yttrium-90 with or without sorafenib. J Vasc Interv Radiol. 2016;27:1329–1336. doi: 10.1016/j.jvir.2016.03.043. [DOI] [PubMed] [Google Scholar]
- 65.Ricke J, Klümpen HJ, Amthauer H, Bargellini I, Bartenstein P, de Toni EN, et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J Hepatol. 2019;71:1164–1174. doi: 10.1016/j.jhep.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 66.Kaseb AO, Abdel-Wahab R, Murthy R, Hassan M, Raghav K, Xiao L, et al. A phase II study of sorafenib and yttrium-90 glass microspheres for advanced hepatocellular carcinoma, BCLC stage C. Ann Oncol. 2017;28:242 [Google Scholar]
- 67.Chow PK, Poon DY, Khin MW, Singh H, Han HS, Goh AS, et al. Multicenter phase II study of sequential radioembolization-sorafenib therapy for inoperable hepatocellular carcinoma. PLoS One. 2014;9:e90909. doi: 10.1371/journal.pone.0090909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Salman A, Simoneau E, Hassanain M, Chaudhury P, Boucher LM, Valenti D, et al. Combined sorafenib and yttrium-90 radioembolization for the treatment of advanced hepatocellular carcinoma. Curr Oncol. 2016;23:e472–e480. doi: 10.3747/co.23.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mahvash A, Murthy R, Odisio BC, Raghav KP, Girard L, Cheung S, et al. Yttrium-90 resin microspheres as an adjunct to sorafenib in patients with unresectable hepatocellular carcinoma. J Hepatocell Carcinoma. 2016;3:1–7. doi: 10.2147/JHC.S62261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zeng ZC, Fan J, Tang ZY, Zhou J, Qin LX, Wang JH, et al. A comparison of treatment combinations with and without radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombus. Int J Radiat Oncol Biol Phys. 2005;61:432–443. doi: 10.1016/j.ijrobp.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 71.Hsu HC, Chen TY, Chiu KW, Huang EY, Leung SW, Huang YJ, et al. Three-dimensional conformal radiotherapy for the treatment of arteriovenous shunting in patients with hepatocellular carcinoma. Br J Radiol. 2007;80:38–42. doi: 10.1259/bjr/55395102. [DOI] [PubMed] [Google Scholar]
- 72.Yoon SM, Ryoo BY, Lee SJ, Kim JH, Shin JH, An JH, et al. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: a randomized clinical trial. JAMA Oncol. 2018;4:661–669. doi: 10.1001/jamaoncol.2017.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chung SR, Kim JH, Yoon HK, Ko GY, Gwon DI, Shin JH, et al. Combined cisplatin-based chemoembolization and radiation therapy for hepatocellular carcinoma invading the main portal vein. J Vasc Interv Radiol. 2015;26:1130–1138. doi: 10.1016/j.jvir.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 74.Kim YJ, Jung J, Joo JH, Kim SY, Kim JH, Lim YS, et al. Combined transarterial chemoembolization and radiotherapy as a first-line treatment for hepatocellular carcinoma with macroscopic vascular invasion: necessity to subclassify Barcelona Clinic Liver Cancer stage C. Radiother Oncol. 2019;141:95–100. doi: 10.1016/j.radonc.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 75.Koo JE, Kim JH, Lim YS, Park SJ, Won HJ, Sung KB, et al. Combination of transarterial chemoembolization and three-dimensional conformal radiotherapy for hepatocellular carcinoma with inferior vena cava tumor thrombus. Int J Radiat Oncol Biol Phys. 2010;78:180–187. doi: 10.1016/j.ijrobp.2009.07.1730. [DOI] [PubMed] [Google Scholar]
- 76.Kim GA, Shim JH, Yoon SM, Jung J, Kim JH, Ryu MH, et al. Comparison of chemoembolization with and without radiation therapy and sorafenib for advanced hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. J Vasc Interv Radiol. 2015;26:320–329.e6. doi: 10.1016/j.jvir.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 77.Shen L, Xi M, Zhao L, Zhang X, Wang X, Huang Z, et al. Combination therapy after TACE for hepatocellular carcinoma with macroscopic vascular invasion: stereotactic body radiotherapy versus sorafenib. Cancers (Basel) 2018;10:516. doi: 10.3390/cancers10120516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chu HH, Kim JH, Shim JH, Yoon SM, Kim PH, Alrashidi I. Chemoembolization plus radiotherapy versus chemoembolization plus sorafenib for the treatment of hepatocellular carcinoma invading the portal vein: a propensity score matching analysis. Cancers (Basel) 2020;12:1116. doi: 10.3390/cancers12051116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 81.Hickey RM, Kulik LM, Nimeiri H, Kalyan A, Kircher S, Desai K, et al. Immuno-oncology and its opportunities for interventional radiologists: immune checkpoint inhibition and potential synergies with interventional oncology procedures. J Vasc Interv Radiol. 2017;28:1487–1494. doi: 10.1016/j.jvir.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 82.Giannini EG, Aglitti A, Borzio M, Gambato M, Guarino M, Iavarone M, et al. Overview of immune checkpoint inhibitors therapy for hepatocellular carcinoma, and the ITA. LI. CA cohort derived estimate of amenability rate to immune checkpoint inhibitors in clinical practice. Cancers (Basel) 2019;11:1689. doi: 10.3390/cancers11111689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Slovak R, Ludwig JM, Gettinger SN, Herbst RS, Kim HS. Immuno-thermal ablations - boosting the anticancer immune response. J Immunother Cancer. 2017;5:78. doi: 10.1186/s40425-017-0284-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Craciun L, de Wind R, Demetter P, Lucidi V, Bohlok A, Michiels S, et al. Retrospective analysis of the immunogenic effects of intra-arterial locoregional therapies in hepatocellular carcinoma: a rationale for combining selective internal radiation therapy (SIRT) and immunotherapy. BMC Cancer. 2020;20:135. doi: 10.1186/s12885-020-6613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545–551. doi: 10.1016/j.jhep.2016.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhan C, Ruohoniemi D, Shanbhogue KP, Wei J, Welling TH, Gu P, et al. Safety of combined yttrium-90 radioembolization and immune checkpoint inhibitor immunotherapy for hepatocellular carcinoma. J Vasc Interv Radiol. 2020;31:25–34. doi: 10.1016/j.jvir.2019.05.023. [DOI] [PubMed] [Google Scholar]
- 87.Wehrenberg-Klee E, Goyal L, Dugan M, Zhu AX, Ganguli S. Y-90 radioembolization combined with a PD-1 inhibitor for advanced hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2018;41:1799–1802. doi: 10.1007/s00270-018-1993-1. [DOI] [PubMed] [Google Scholar]
- 88.Tai WMD, Loke KSH, Gogna A, Tan SH, Ng DCE, Hennedige TP, et al. A phase II open-label, single-center, nonrandomized trial of Y90-radioembolization in combination with nivolumab in Asian patients with advanced hepatocellular carcinoma: CA 209–678. J Clin Oncol. 2020;38:4590 [Google Scholar]
- 89.Marinelli B, Cedillo M, Pasik SD, Charles D, Murthy S, Patel RS, et al. Safety and efficacy of locoregional treatment during immunotherapy with nivolumab for hepatocellular carcinoma: a retrospective study of 41 interventions in 29 patients. J Vasc Interv Radiol. 2020;31:1729–1738.e1. doi: 10.1016/j.jvir.2020.07.009. [DOI] [PubMed] [Google Scholar]


