Abstract
Vancomycin-sensitive, -intermediate, and -heterointermediate methicillin-resistant Staphylococcus aureus isolates were tested by using E-tests to explore the interaction of methicillin and vancomycin. For the vancomycin-intermediate and -heterointermediate strains both drugs showed antagonism at concentrations below their MICs but synergy at methicillin concentrations near the MIC. This property could be used to screen for heterointermediate S. aureus strains.
In 1997, a clinical isolate of Staphylococcus aureus (Mu50) which was resistant to vancomycin was reported from Japan (6). Previously, susceptibility to vancomycin among methicillin-resistant S. aureus (MRSA) had been universal.
Strains of S. aureus with reduced susceptibility to vancomycin reported thus far have all produced MICs in the range from 8 to 16 mg/liter and are therefore intermediately resistant by National Committee for Clinical Laboratory Standards criteria; hence, the term VISA (vancomycin-intermediate S. aureus) is used (10). Although rare, such strains have been isolated in the United States and Europe (1, 11). However, a different type of vancomycin resistance has been reported from Japan, and this appears to be much more common. Hiramatsu and colleagues described MRSA strains for which MICs are below the breakpoint (thus, these strains are sensitive by conventional criteria) but which contain bacterial cells within the populations which grow in the presence of 4 to 9 mg of vancomycin per liter (5). This heterogeneity within vancomycin-susceptible populations (termed vancomycin-heterointermediate S. aureus, or hVISA) is similar to that of MRSA, where cells expressing methicillin resistance may constitute only one in 106 to 108 cells (3, 9). hVISA strains comprised 9.3% of MRSA strains isolated in university hospitals and 1.3% of those isolated in nonuniversity hospitals in Japan (5). Heterointermediate susceptibility may be responsible for failure of vancomycin therapy, and such strains may develop into VISA strains, although this remains unclear.
We reported a strain of MRSA, with characteristics similar to those of the Japanese hVISA, which was isolated in Bristol, United Kingdom, from a patient who died of S. aureus septicemia despite 22 days of vancomycin therapy (7).
Detection of VISA is possible with standard laboratory methods using broth dilution MICs or E-tests (13), but the detection of hVISA remains difficult. The current method is laborious, irreproducible, and not suitable as a routine laboratory test (5, 15).
Reduced susceptibility to vancomycin has been reported in MRSA, and studies of the Japanese Mu50 VISA indicate both increased cell wall thickness and greater production of the penicillin-binding proteins (PBP) PBP2 and PBP2′ (6). A recent study examining the phenotypic characteristics of a laboratory-generated mutant (VM) of an MRSA strain with high-level vancomycin resistance revealed that the vancomycin resistance (MIC, 100 mg/liter) had been achieved at the expense of β-lactam resistance (methicillin MICs, 800 mg/liter for the parent strain and 1.5 mg/liter for VM) (12). This vancomycin-resistant mutant had a thickened cell wall similar to that of Mu50. Given these indications that expression of resistance to vancomycin may be associated with expression of methicillin resistance, we examined the interaction between methicillin and vancomycin. Strains of S. aureus with different levels of expression of vancomycin resistance were used with a view to designing a reproducible and sensitive method to discriminate hVISA from vancomycin-sensitive MRSA.
The strains examined were NCTC 6571 (methicillin- and vancomycin-susceptible control strain), 28 clinical isolates of vancomycin-susceptible MRSA (VSSA), 6 hVISA strains (Mu3, Smh24 [which was isolated from a patient who died despite 22 days of vancomycin treatment], 3 strains [Smh2, Smh25, and Smh26] which are derivatives of Smh1 and Smh24 [7], and Smh6250 [a strain found on screening]) (15), and 1 VISA strain (Mu50).
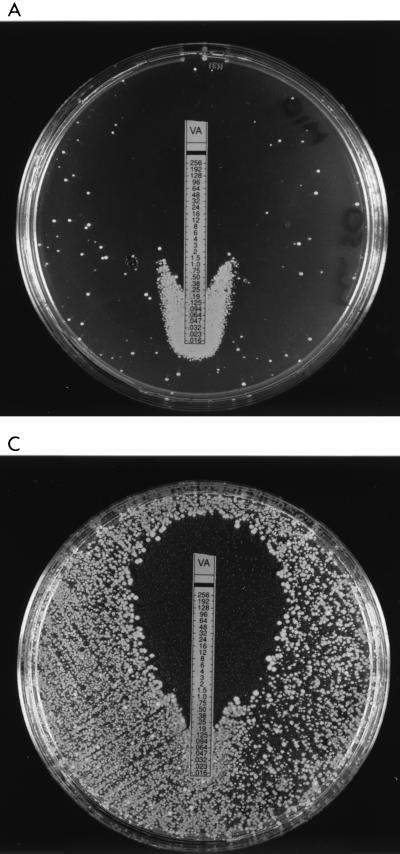
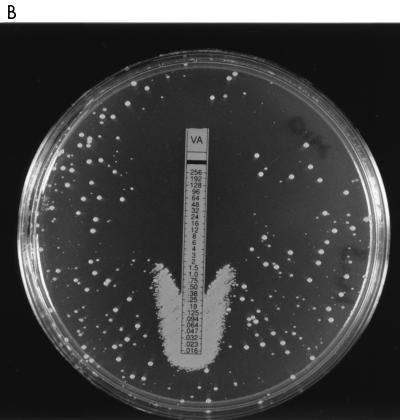
Strains were inoculated according to the E-test manufacturer’s instructions onto brain heart infusion (BHI) agar (Difco Laboratories, Detroit, Mich.) incorporating methicillin (10 mg/liter), and vancomycin E-test strips were placed on the agar. Following a 24-h incubation at 37°C, growth was observed at the bottom of the vancomycin E-test strip at much greater density than elsewhere on the plate for the VISA strain (Mu50) and the hVISA strains (Mu3, Smh2, Smh24, Smh25, Smh26, and Smh6250) but not for VSSA (Fig. 1A through C). Therefore, for hVISA and VISA there is antagonism between methicillin and vancomycin at sub-inhibitory concentrations of vancomycin and methicillin.
FIG. 1.
Vancomycin (VA) E-test strips on BHI agar containing 10 mg of methicillin inoculated with Mu50 (A), Mu3 (B), and Smh6250 (C) show antagonism at low antimicrobial concentrations.
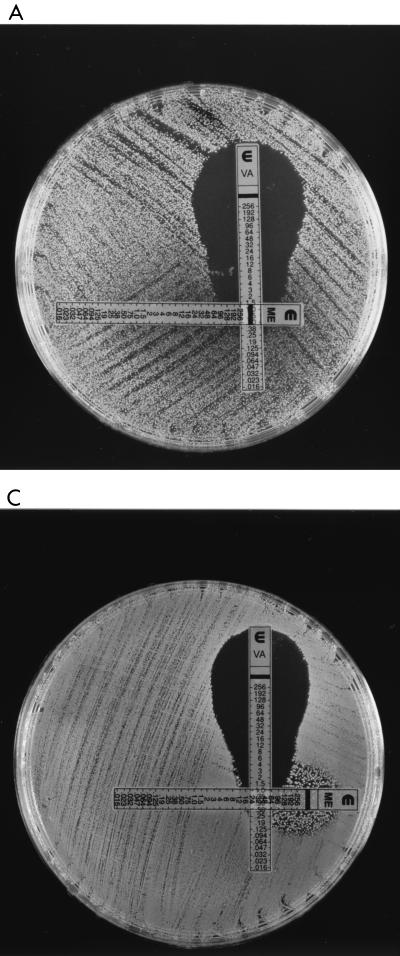
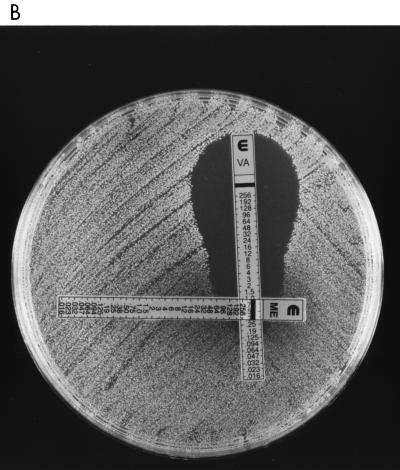
In a second experiment, vancomycin and methicillin E-test strips were placed on BHI agar with no additives at 90-degree orientation to one another to overlap at the MICs for each of the strains. When vancomycin-susceptible strains were tested, the zones of growth inhibition produced around the E-tests were normal in shape. In contrast the zones of growth inhibition for the hVISA and VISA strains were distorted, giving a “dumbbell” effect with “shoulders” of maximal distortion at vancomycin concentrations of 16, 12, and 6 mg/liter for isolates Smh6250, Smh2, and Mu3, respectively (Fig. 2A through C). This suggests that synergy exists between methicillin and vancomycin against hVISA and VISA at concentrations of methicillin above the MIC.
FIG. 2.
Vancomycin (VA) and methicillin (ME) E-test strips on BHI agar inoculated with Smh6250 (A), Smh2 (B), and Mu50 (C) show synergy at high methicillin concentrations.
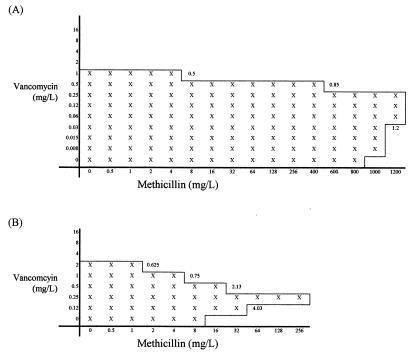
Given the contrasting results at high and low antibiotic concentrations, the interactions between methicillin and vancomycin were further studied by using the agar dilution checkerboard technique (4) and strains preincubated with or without vancomycin. Although the checkerboard method of assessing antimicrobial interactions is known to be poorly reproducible (14), the data suggest that there is significant antagonism between vancomycin and methicillin and that this can be increased by preincubation with vancomycin. Typical results for an hVISA strain are shown in Fig. 3.
FIG. 3.
Agar dilution checkerboard tests performed by using Smh2 preincubated in Trypticase soy broth without vancomycin (A) and with 8 mg of vancomycin per liter (B). Fractional inhibitory concentrations are given at appropriate points.
These data show that vancomycin and methicillin interact in ways which appear to be specific for hVISA and VISA and that do not occur with vancomycin-susceptible S. aureus (MSSA and MRSA). The mechanism of antagonism seen at low concentrations of antibiotic is unknown, but vancomycin resistance in VISA strains may be due in part to a defect in cell wall turnover leading to increased concentrations of non-cross-linked d-alanyl-d-alanine side chains, the target site for vancomycin and also the substrate for PBPs. An increase in non-cross-linked d-alanyl-d-alanine may then decrease the effectiveness of β-lactams as inhibitors of PBPs by substrate competition. Alternatively antagonism between methicillin and vancomycin may be due to induction of methicillin resistance by low concentrations of vancomycin. Vancomycin E-tests performed on methicillin-containing agar show scanty peripheral growth, suggestive of heterogeneous expression of methicillin resistance, while growth around the E-tests at low vancomycin concentrations is denser, suggesting that the proportion of cells expressing resistance has increased. The control of population structure in heterogeneous strains of MRSA likely reflects genetic constraints imposed by as yet unidentified genes (3). The fem genes are essential for methicillin resistance, and mutations in both femA and femB reduce the level of methicillin resistance to that indicating a nearly sensitive phenotype (2). Notably, the cell walls of such mutants are grossly abnormal on electron microscopy. It is possible, therefore, that the genetic changes that mediate reduced susceptibility to vancomycin also affect control elements for the expression of methicillin resistance. Consequently induction with low concentrations of vancomycin may promote a switch in population expression of methicillin resistance from heterogeneous to homogeneous or near homogeneous.
The synergy between methicillin and vancomycin at high concentrations of methicillin indicated by the dumbbell effect on E-tests may be due to inhibition of protein synthesis by methicillin. Oxacillin inhibits protein synthesis in methicillin-tolerant strains of S. aureus in a semispecific manner, suggesting the operation of a stress regulon(s) (8). Thus, high concentrations of methicillin may also cause inhibition of synthesis of proteins essential for the expression of vancomycin resistance, producing synergy.
While the mechanisms responsible for the different interactions between methicillin and vancomycin at different concentrations are still unknown, the antagonism displayed when a vancomycin E-test is placed on BHI agar containing 10 mg of methicillin per liter is consistent for hVISA and VISA isolates but not seen with VSSA, and it may therefore form the basis of a screening test for these strains. Further work is required to investigate the effects of using different agars and/or the addition of NaCl on the interactions between vancomycin and methicillin in S. aureus strains with reduced susceptibility to vancomycin.
REFERENCES
- 1.Centers for Disease Control. Staphylococcus aureus with reduced susceptibility to vancomycin—United States, 1997. Morbid Mortal Weekly Rep. 1997;46:765–766. [PubMed] [Google Scholar]
- 2.Chambers H F. Methicillin resistance in staphylococci: molecular and biochemical basis and clinical implications. Clin Microbiol Rev. 1997;10:781–791. doi: 10.1128/cmr.10.4.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Lencastre H, Figueiredo A M S, Tomasz A. Genetic control of population structure in heterogeneous strains of methicillin resistant Staphylococcus aureus. Eur J Clin Microbiol Infect Dis. 1993;12(Suppl. 1):S13–S18. doi: 10.1007/BF02389872. [DOI] [PubMed] [Google Scholar]
- 4.Eliopoulos E M, Moellering R C. Antimicrobial combinations. In: Lorian V, editor. Antibiotics in laboratory medicine. 4th ed. Baltimore, Md: The Williams and Wilkins Co.; 1996. pp. 330–396. [Google Scholar]
- 5.Hiramatsu K, Aritaka N, Hanaki H, Kawasaki S, Hosoda Y, Hori S. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet. 1997;350:1670–1673. doi: 10.1016/S0140-6736(97)07324-8. [DOI] [PubMed] [Google Scholar]
- 6.Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover F C. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother. 1997;40:135–136. doi: 10.1093/jac/40.1.135. [DOI] [PubMed] [Google Scholar]
- 7.Howe R A, Bowker K B, Walsh T R, Feest T G, MacGowan A P. Vancomycin resistant Staphylococcus aureus. Lancet. 1998;351:602. doi: 10.1016/S0140-6736(05)78597-4. [DOI] [PubMed] [Google Scholar]
- 8.Jablonski P E, Mychajlonka M. Oxacillin-induced inhibition of protein and RNA synthesis in a tolerant Staphylococcus aureus isolate. J Bacteriol. 1988;170:1831–1836. doi: 10.1128/jb.170.4.1831-1836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthews P R, Stewart P R. Resistance heterogeneity in methicillin-resistant Staphylococcus aureus. FEMS Microbiol Lett. 1984;22:161–166. [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard. NCCLS publication M7-A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 11.Ploy M C, Grelaud C, Martin C, de Lumley L, Denis F. First clinical isolate of vancomycin-intermediate Staphylococcus aureus in a French hospital. Lancet. 1998;351:1212. doi: 10.1016/s0140-6736(05)79166-2. [DOI] [PubMed] [Google Scholar]
- 12.Sieradzki K, Tomasz A. Inhibition of cell wall turnover and autolysis by vancomycin in a highly vancomycin-resistant mutant of Staphylococcus aureus. J Bacteriol. 1997;179:2557–2566. doi: 10.1128/jb.179.8.2557-2566.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tenover F C, Lancaster M V, Hill B C, Steward C D, Stocker S A, Hancock G A, O’Hara C M, Clark N C, Hiramatsu K. Characterization of staphylococci with reduced susceptibilities to vancomycin and other glycopeptides. J Clin Microbiol. 1998;36:1020–1027. doi: 10.1128/jcm.36.4.1020-1027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wootton M, Hedges A J, Bowker K E, Holt H A, Reeves D S, MacGowan A P. A critical assessment of the agar dilution chequerboard technique for studying in vitro antimicrobial interactions using a representative β-lactam, aminoglycoside, and fluoroquinolone. J Antimicrob Chemother. 1995;35:569–576. doi: 10.1093/jac/35.5.569. [DOI] [PubMed] [Google Scholar]
- 15.Wootton M, Howe R A, Walsh T R, MacGowan A P. Abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1998. Methodological factors in the detection of heterogeneously vancomycin-resistant Staphylococcus aureus (h-VRSA), abstr. C-139; p. 108. [Google Scholar]