Summary
Spermatogenesis-associated 5 like 1 (SPATA5L1) represents an orphan gene encoding a protein of unknown function. We report 28 bi-allelic variants in SPATA5L1 associated with sensorineural hearing loss in 47 individuals from 28 (26 unrelated) families. In addition, 25/47 affected individuals (53%) presented with microcephaly, developmental delay/intellectual disability, cerebral palsy, and/or epilepsy. Modeling indicated damaging effect of variants on the protein, largely via destabilizing effects on protein domains. Brain imaging revealed diminished cerebral volume, thin corpus callosum, and periventricular leukomalacia, and quantitative volumetry demonstrated significantly diminished white matter volumes in several individuals. Immunofluorescent imaging in rat hippocampal neurons revealed localization of Spata5l1 in neuronal and glial cell nuclei and more prominent expression in neurons. In the rodent inner ear, Spata5l1 is expressed in the neurosensory hair cells and inner ear supporting cells. Transcriptomic analysis performed with fibroblasts from affected individuals was able to distinguish affected from controls by principal components. Analysis of differentially expressed genes and networks suggested a role for SPATA5L1 in cell surface adhesion receptor function, intracellular focal adhesions, and DNA replication and mitosis. Collectively, our results indicate that bi-allelic SPATA5L1 variants lead to a human disease characterized by sensorineural hearing loss (SNHL) with or without a nonprogressive mixed neurodevelopmental phenotype.
Keywords: neurodevelopmental disorder, movement disorder, cerebral palsy, epilepsy, SPATA5L1, intellectual disability, AAA+ superfamily, ATPase, sensorineural hearing loss
Main text
Neurodevelopmental disorders (NDDs) frequently co-occur because disruption of early brain morphogenesis and connectivity can affect multiple intersecting domains of development. These disorders represent a wide spectrum of clinical manifestations, ranging from single organ (e.g., brain) pathology to embryonic lethality due to failure of vital organs, yet common, recognizable NDD phenotypes include intellectual disability, hearing loss, cerebral palsy, autism, and epilepsy. Prior work has revealed that homozygous or compound heterozygous variants in the spermatogenesis-associated 5 gene (SPATA5 [MIM: 613940]) cause an NDD syndrome1 that features microcephaly, cortical visual impairment, intellectual disability, spastic cerebral palsy, epilepsy, and sensorineural hearing loss (SNHL) (MIM: 616577). Neuroimaging features included hypomyelination in some individuals and a thin corpus callosum. Based on the domain structure, SPATA5 has been grouped into the ATPase associated with diverse activities (AAA+) protein family.2 Knockdown of Spata5 in rat cortical neurons led to abnormal mitochondrial morphology and fission/fusion ratios,3 suggesting a role in energy metabolism. In humans, SPATA5 has a paralog, spermatogenesis-associated 5 like 1 (SPATA5L1), that is 35% identical and 52% similar by Drosophila RNAi Research Center Integrative Ortholog Prediction Tool (DIOPT) alignment. Previous genome-wide association studies have found that SPATA5L1 resides within a locus associated with chronic kidney disease in a combined North American and Dutch cohort,4 which was replicated in Japanese5 and Mongolian6 cohorts. However, no Mendelian disease-associated variants have been previously reported in SPATA5L1.
Here, as part of large-scale sequencing screens of individuals with SNHL and cerebral palsy, we detected bi-allelic, predicted deleterious variants in SPATA5L1 (HGNC: 28762, GenBank: NM_024063.3). Using GeneMatcher7 services, we subsequently connected with colleagues worldwide. Together, we report 28 unique SPATA5L1 variants in 47 affected individuals from 28 (26 unrelated) families. All human subjects’ studies were performed in accordance with the ethical standards of the responsible committee on human experimentation according to institutional and national standards. Proper informed consent was obtained for all participants. Sequencing was performed at numerous centers, but all used Illumina systems and institutional pipelines based on current GATK best practices.8,9 Details regarding sequencing metrics and variant prioritization can be found in the supplemental material and methods. Among the identified variants, 25 were present in the cohort in compound heterozygous form and three were found as homozygous variants (Figure S1). Out of these three, one missense variant, c.1199C>T (p.Thr400Ile), also segregates in a compound heterozygous fashion in two other families. Most putatively damaging variants were private except for five that were detected in multiple families: c.527G>T (p.Gly176Val), c.1398T>G (p.Ile466Met), c.606_619dup14 (p.Glu207Glyfs∗25), c.1199C>T (p.Thr400Ile), and c.2066G>T (p.Gly689Val); the former two were found in both the neurologic presentation and isolated hearing loss cases, and the latter two were found only in individuals with neurologic presentation. The pathogenicity of missense variants was predicted by ³ 3 algorithms (Table S1). None of the identified variants were found in homozygous form in gnomAD.
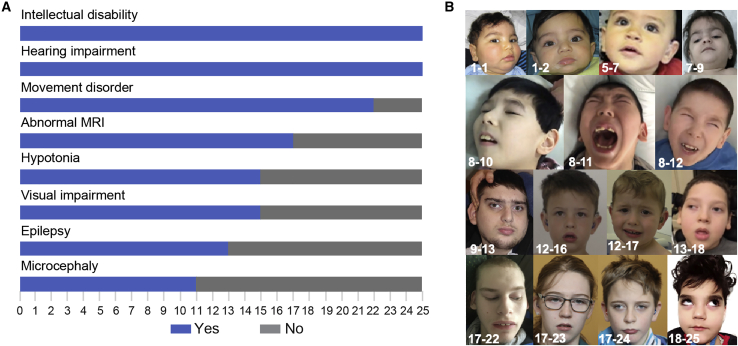
All affected individuals with bi-allelic variants in SPATA5L1 presented with mild, moderate, or severe hearing loss, and about half (25/47, 53%) also exhibited neurologic features, particularly global developmental delay/intellectual disability (seen in all individuals with neurologic involvement). Other prominent neurological findings included spastic-dystonic cerebral palsy in approximately two-thirds, epilepsy (16/25, 64%), and cortical visual impairment (15/25, 60%). Clinical features of our cohort are summarized in Table 1 and Figure 1A (and detailed in Tables S2 and S3). Operational definitions for presence/absence of NDDs can be found in the supplemental material and methods. Case video review indicated visual impairment, impaired expressive language, intellectual disability, and mixed movement disorders with resultant orthopedic complications (Videos S1, S2, S3, S4, S5, and S6).
Table 1.
Bi-allelic variants in SPATA5L1 cause a neurodevelopmental disorder featuring intellectual disability, cerebral palsy, epilepsy, and hearing loss
| Family | Family 1 | Family 2 | Family 3 | Family 4 | Family 5 | Family 6 | Family 7 | Family 8 | Family 9 |
|---|---|---|---|---|---|---|---|---|---|
| Patient | 1 (proband) | 3 (proband) | 5 | 6 | 7 | 8 | 9 | 10 (proband) | 13 |
| cDNA (GenBank: NM_024063.3) | c.1304_1305del (pat.); c.121G>C (mat.) | c.734T > A (pat.); c.1398T>G (mat.) | c.1A>T (pat.); c.2066G>T (mat.) | c.515C>A (pat.); c.196G>T (mat.) | c.1973G>A (pat.); c.2176_2177del (mat.) | c.76A>G (pat.); c.1079T>C (mat.) | c.1556C>A (hom.) | c.1199C>T (hom.) | c.1676delC (pat.); c.1682T>C (mat.) |
| Protein (GenBank: NP_076968.2) | p.Ile435Argfs∗4 (pat.); p.Ala41Pro (mat.) | p.Val245Glu (pat.); p.Ile466Met (mat.) | p.Met1? (pat.); p.Gly689Val (mat.) | p.Pro172His (pat.); p.Asp66Tyr (mat.) | p.Arg658Lys (pat.); p.Val726Lysfs∗13 (mat.) | p.Thr26Ala (pat.); p.Phe360Ser (mat.) | p.Ala519Asp (hom.) | p.Thr400Ile (hom.) | p.Ala559Glufs∗33 (pat.); p.Leu561Ser (mat.) |
| Ancestry | Iraqi | European, with Ashkenazi Jewish ancestry | American; mat. ethnicity, Italian; pat. ethnicity, Italian and Afro-American | mixed European | Spanish | African American | Turkish | Kazakh | mixed, Eastern European, and Scandinavian |
| Sex | male | male | female | male | male | female | female | female | male |
| Age at diagnosis | first year of life | 32 months | 27 months | 23 years | 4 months | 9–12 months | 5 years | 7 months | 1 month |
| Hearing impairment | + | + | + | + | + | + | + | + | + |
| Spasticity | + | − | + | − | − | − | + | + | + |
| Dystonia / hypotonia | + / − | + / + | − / + | − / N/A | − / + | N/A / − | + / − | + / + | − / + |
| Pattern | spastic quadriplegia | N/A | spastic quadriplegia | N/A | N/A | N/A | spastic-dystonic tetraparesis | spastic quadriplegia | spastic quadriplegia |
| Microcephaly (HC) | + (acq., 47 cm at 6 years) | − | + (acq., 46 cm at 3 years) | − | + (acq., 45 cm at 1 year) | − | + (27 cm at birth) | + (45 cm at 6 years) | − |
| DD / ID | + profound DD | + global DD | + global DD | + severe DD | + profound DD | + | + severe DD | + profound DD / ID | + profound DD |
| Epilepsy | + | − | − | + | − | + | + | + | + |
| Dysmorphic features | − | + | − | − | − | − | − | + | + |
| Visual impairment | cortical visual blindness | − | cortical visual impairment | − | − | N/A | severe cortical visual impairment | probable cortical blindness | severe impairment |
| MRI findings | progressive CO and CB volume loss; thin CC; periventricular T2 hyperintensities; subtle GP hyperintensity bilaterally | concern for delayed myelination | delayed myelination; thin CC | CC lipoma, otherwise normal | normal | thin CC; enlarged 4th ventricle; mega cisterna magna; possible brainstem hypoplasia; possible delayed myelination | profound cortical atrophy with predominant reduction of the white matter; cerebellum and brainstem normal | paucity of periventricular WM bilaterally with patchy confluent T2 hyperintensity; thin CC; generalized CO atrophy | normal (age 14 months); repeat MRI showed diminished cortical volume |
| Family | Family 10 | Family 11 | Family 12 | Family 13 | Family 14 | Family 15 | Family 16 | Family 17 | Family 18 |
|---|---|---|---|---|---|---|---|---|---|
| Patient | 14 | 15 | 16 (proband) | 18 | 19 | 20 | 21 | 22 (proband) | 25 |
| cDNA (GenBank: NM_024063.3) | c.190C>T; c.1826C>G | c.85T>G (hom.) | c.1199C>T (pat.); c.1090−2A>G (mat.) | c.2066G>T (pat.); c.527G>T (mat.) | c.527G>T (pat.); c.2006T>G (mat.) | c.527G>T; c.1199C>T | c.213T>G; c.1313T>C (hom.) | c.1091T>A (pat.); c.1918C>T (mat.) | c.1648_1649insC; c.2066G>T |
| Protein (GenBank: NP_076968.2) | p.Arg64Trp; p.Ser609∗ | p.Cys29Gly (hom.) | p.Thr400Ile (pat.); p.? (mat.) | p.Gly689Val (pat.); p.Gly176Val (mat.) | p.Gly176Val (pat.); p.Met669Arg (mat.) | p.Gly176Val; p.Thr400Ile | p.Phe71Leu (hom.); p.Leu438Pro (hom.) | p.Val364Glu (pat.); p.Arg640∗ (mat.) | p.Phe550Serfs∗16; p.Gly689Val |
| Ancestry | African American | Turkish | European (German) | Italian | European (German) | German | Arabian | German | Italian |
| Sex | male | male | male | male | female | male | male | male | male |
| Age at diagnosis | 3 months | 14 months | 5 years | 5 years, 6 months | 2–3 months | 15 years, 6 months | 8 weeks | 14 years, 9 months | 5 months |
| Hearing impairment | + | + | + | + | + | + | + | + | + |
| Spasticity | + | − | + | + | + | + | + | + | + |
| Dystonia / hypotonia | + / + | + / − | + / + | + / + | + / + | + / + | + / + | + / + | + / + |
| Pattern | spastic quadriplegia | quadriparesis | spastic quadriplegia | spastic quadriplegia | spastic quadriplegia | hypotonic dystonic spastic quadriplegia | hypotonic dystonic spastic quadriplegia | hypotonic dystonic spastic quadriplegia | hypotonic dystonic spastic quadriplegia |
| Microcephaly (HC) | + | + (34 cm at birth) | − | + (acq.) | + (cong.) | + (−2 SD at 1 year) | + (32 cm at birth) | − | + (48 cm at 6 years) |
| DD / ID | + profound global DD | + | + profound global DD | + severe ID | + severe ID | + profound global DD | + profound global DD | + profound global DD | + profound global DD |
| Epilepsy | + | + | − | + | − | + | + | + | + |
| Dysmorphic features | − | − | − | + | − | N/A | + | − | + |
| Visual impairment | − | − | mild myopia | N/A | severe impairment | − | severe impairment | severe impairment | central visual impairment |
| MRI findings | diffusely diminished CO volumes; ex vacuo dilatation LV; delayed myelination; thin CC | bilateral peritrigonal hyperintensity | diminished CO volume; periventricular WM hyperintensity | ventricle enlargement (slight); WM hyperintensity | diffuse slightly diminished CO volume; thin CC; lactate peak visualized on MRS (2 years) | mildly diminished CO volume; mildly atrophic BG; delayed myelination of the CC | mega cisterna magna; embryonic variant posterior cerebral artery | normal (11 months); slightly enlarged ventricles (19 months); no progression of ventricular enlargement (25 months); delayed myelination, thin CC (4.5 years) | brain hypomyelination; diffuse slight brain atrophy |
Abbreviations: CO, cortical; CB, cerebellar; CC, corpus callosum; DD, developmental delay; ID, intellectual disability; GP, globus pallidus; WM, white matter; BG, basal ganglia; MRS, magnetic resonance spectroscopy; mat., maternal; pat., paternal; hom., homozygous; +, clinical feature detected; −, clinical feature not observed; N/A, no information provided for clinical feature; SD, standard deviation; p, percentile; acq., acquired postnatally; cong., congenital; HC, head circumference. T2 is an MRI signal acquisition parameter.
Figure 1.
Prevalent clinical features of individuals with bi-allelic variants in SPATA5L1
(A) Bar graph illustrating the prevalence of the most relevant clinical features from the 25 individuals for whom full datasets were available from 18 families with the neurodevelopmental phenotype. Blue: individuals with the clinical feature. Gray: individuals without the clinical feature.
(B) Representative clinical features of individuals carrying bi-allelic SPATA5L1 variants with the severe neurodevelopmental phenotype, showing subtle and non-specific dysmorphic features, including downslanting palpebral fissures, bitemporal narrowing, and depressed nasal bridge.
Case 7 exhibits mild bilateral chorea of the upper limbs as a toddler.
Case 9 at age 2 years demonstrates global growth failure and hypertonia of the limbs.
Proband (case 10): An examination of case 10 at age 9 years reveals visual impairment and spastic quadriplegia with diminished head control and scissoring of the lower limbs without clear hyperreflexia. Amyotrophy of the extremities is evident, as is pes cavus. Sibling (case 11): Evaluation at age 7 years shows visual impairment with lateral-beating nystagmus. Spastic quadriplegia is noted with poor head control. Persistent glabellar tap reflex without habituation is noted as a frontal release sign. Amyotrophy of the limbs is evident and marked scoliosis and pes planus are present. Sibling (case 12): Assessment at age 5 years reveals visual impairment, spastic quadriplegia with poor head control, diffuse hyperreflexia, and equivocally positive Babinski signs. Pes planus of the feet is again seen.
Evaluation of this 10-year-old boy revealed dystonic posturing of the upper and lower limbs.
Dystonic posturing of the hand and mouth is evident while the individual is sitting.
One of the affected individual’s seizures in captured, beginning as a gelastic seizure with stereotyped laughter, followed by behavioral arrest with stereotyped blinking evolving into rhythmic saccadic eye movements, and concluding with post-ictal hyporesponsiveness.
Most individuals exhibited a movement disorder, typically spasticity (17/25, 68%), dystonia (15/25, 60%), or a combination of these two forms of hypertonia (13/25, 52%). This usually occurred in a quadriplegic or generalized distribution. More than half (17/25, 68%) of the individuals had isolated hypotonia, although these individuals tended to be younger and may not have manifested their full motor phenotype. Some individuals were reported to exhibit ataxia, while non-epileptic myoclonus was identified in one. Stereotypies were seen in several individuals as well. The degree of cognitive impairment seen in affected individuals varied from severe to profound. For severely affected individuals, hyporesponsiveness to environmental stimuli was seen. Autistic features were absent except for in two individuals. Additional neuropsychiatric features were not reported, and behavior problems were not prominent. A combination of focal and generalized seizure types was reported. Few individuals (4/25, 16%) exhibited infantile spasms, often associated with a clinical diagnosis of West syndrome. Other forms of generalized seizures included myoclonic (7/25, 28%), absence (3/25, 12%), and generalized tonic-clonic (11/25, 44%) events. Focal epilepsy (4/25, 16%), sometimes with secondary generalization, was evident in several individuals as well, and a subset demonstrated mixed focal and generalized semiologies. One individual was reported to have evidence of electrical status epilepticus in slow wave sleep (ESES), and seizures in some were intractable or described in the context of a developmental or epileptic encephalopathy.
Microcephaly was present in about half of the affected individuals (13/25, 52%). Facial dysmorphism, assessed locally and confirmed by a trained dysmorphologist (M.C.F.) whenever possible, was noted in one-third (9/25) of affected individuals. Facial features included downslanting palpebral fissures, widow’s peak, low frontal hairline, large ears, tooth malformation, high palate, bitemporal narrowing, sparse eyebrows, depressed nasal bridge, and micrognathia as well as prominent upper lip, small chin, and mild telecanthus, evident in individual facial photographs (Figure 1B). A gestalt representation of “SPATA5L1 facies” was also constructed with the Face2Gene RESEARCH application (FDNA, Boston, MA, USA). However, this facial gestalt did not highlight any consistent dysmorphism, evidenced by the lack of a significant difference between the case and the age-, sex-, and ethnicity-matched control cohort (p = 0.223, Figure S2).
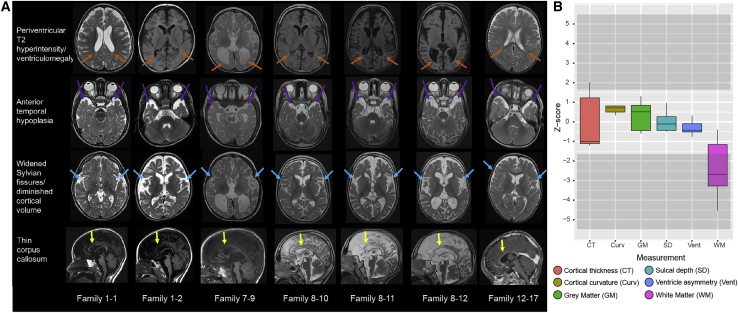
Neuroimaging findings were assessed by a board-certified neuroradiologist (P.C.). Some individuals’ brains were morphologically normal, but relatively consistent features included diminished cortical volume, periventricular leukomalacia, widened Sylvian fissures, anterior temporal hypoplasia, and hypoplastic corpus callosum (Figure 2A). More variable features included delayed myelination in toddlers, an “ears of the lynx”-like appearance, incomplete hippocampal rotation, optic nerve hypoplasia, and small pons. To further characterize the structural neuroanatomic findings, we performed a quantitative volumetric analysis with DICOM data from clinical magnetic resonance images by using a previously described method.10 This analysis revealed a significance decrease in white matter volume in SPATA5L1 cases compared to controls (Figure 2B).
Figure 2.
Neuroimaging features in individuals harboring bi-allelic predicted-deleterious variants in SPATA5L1
(A) T1 and T2/FLAIR MRI images were assessed for the presence of periventricular leukomalacia (defined as T2 hyperintensity and/or diminished white matter volume/ex vacuo ventriculomegaly evident adjacent to the ventricles); anterior temporal lobe hypoplasia; widened Sylvian fissures (characterized as diminished coverage of the insular cortex), diminished cortical volume; and thin/dysplastic corpus callosum. Ages were 4 years old (1-1, 1-2), 21 years old (7-9), 10 years old (8-10), 7 years old (8-11), 6 years old (8-12), and 1 year old (12-16).
(B) Boxplot of six structural measures quantified from brain MRI volumes, represented as Z scores, in comparison to an age-matched control cohort of typically developing children.
Intriguingly, a subset of individuals (DY1–DY11) with bi-allelic SPATA5L1 variants presented with isolated, non-syndromic hearing loss without neurological features (22/47, 47%, Figure S1, Table S2). These individuals were all of Ashkenazi Jewish decent, and in all, the missense variant c.1398T>G was identified in compound heterozygosity with various other pathogenic alleles, suggesting a hypomorphic founder allele, resulting in a partial rather than complete loss-of-protein function. According to gnomAD, the allele frequency of this variant is 0.0029 in individuals of Ashkenazi Jewish background, indicating a frequency of homozygotes of 0.8/100,000. The fact that no individuals with SNHL hearing loss and homozygosity for the c.1398T>G allele were identified further raises the question whether the variant would not lead to a clinical phenotype in homozygous form. Like the cases with neurologic involvement, the bilateral SNHL associated with isolated cases was mild to profound. There appeared to be some benefit to cochlear implants among individuals who received this intervention.
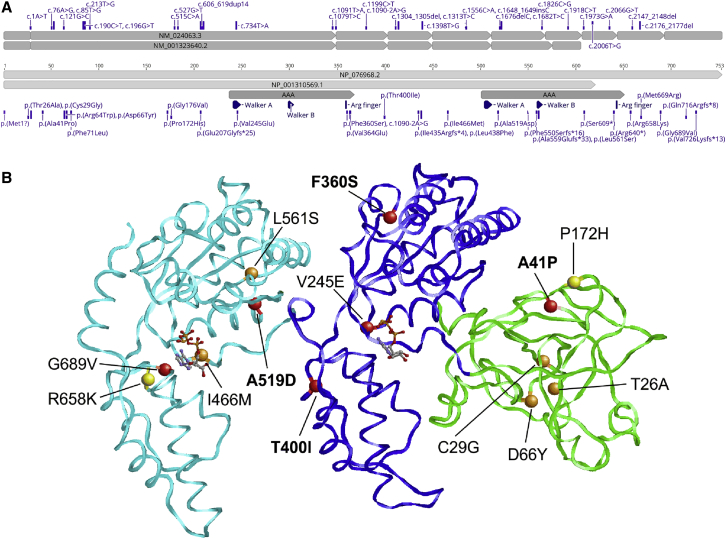
SPATA5L1 belongs to the AAA+ ATPases protein superfamily, a functionally diverse group of enzymes that hydrolyze ATP to induce changes in target substrates. Variants identified in our cohort are spread throughout the gene and protein (Figure 3A, visualized with Geneious Prime 2021.0.1). Structural effects of a subset of missense variants detected in our cohort were assessed by VIPUR11 and Missense3D12 based on a three-dimensional model of SPATA5L1. Of the 13 variants investigated, 11 variants were predicted to have a deleterious effect on protein structure (Figure 3B) via a combination of destabilizing effects, including steric clashes, loss of hydrophobic packing, loss of polar interactions, and the emergence of buried charged residues (Figure S3). All variants predicted to be deleterious are expected to destabilize domains within SPATA5L1, except for the p.Gly689Val variant, which is predicted to create steric clashes with the ATP ligand, affecting ATP-binding properties.
Figure 3.
Distribution and predicted structural effects of SPATA5L1 variants
(A) Alternative splicing leads to two distinct SPATA5L1 transcripts (top), resulting in a full-length and short isoform (bottom). The variant numbering is based on the full-length transcript and isoform, GenBank: NM_024063.3 and GenBank: NP_076968.2. Non-coding and coding regions of exons are denoted by flat-edged and pointed-edged rectangles, respectively.
(B) Structural effects of a subset of variants identified in this study were evaluated with a three-dimensional model of SPATA5L1 based on the structure of the homologous ATPase p97 (PDB: 5FTN). The SPATA5L1 structure is shown in ribbon presentation, depicting the N-terminal domain (green) and two conserved ATPase domains (AAA, blue and cyan). ATP ligands are shown in stick presentation. Altered residues are highlighted as balls and labeled. Red and orange balls indicate variants that were classified as deleterious by two or one methods, respectively. Yellow balls indicate variants that were predicted to have little effect on protein structure. The variants depicted in more detail in Figure S3 are labeled in bold letters.
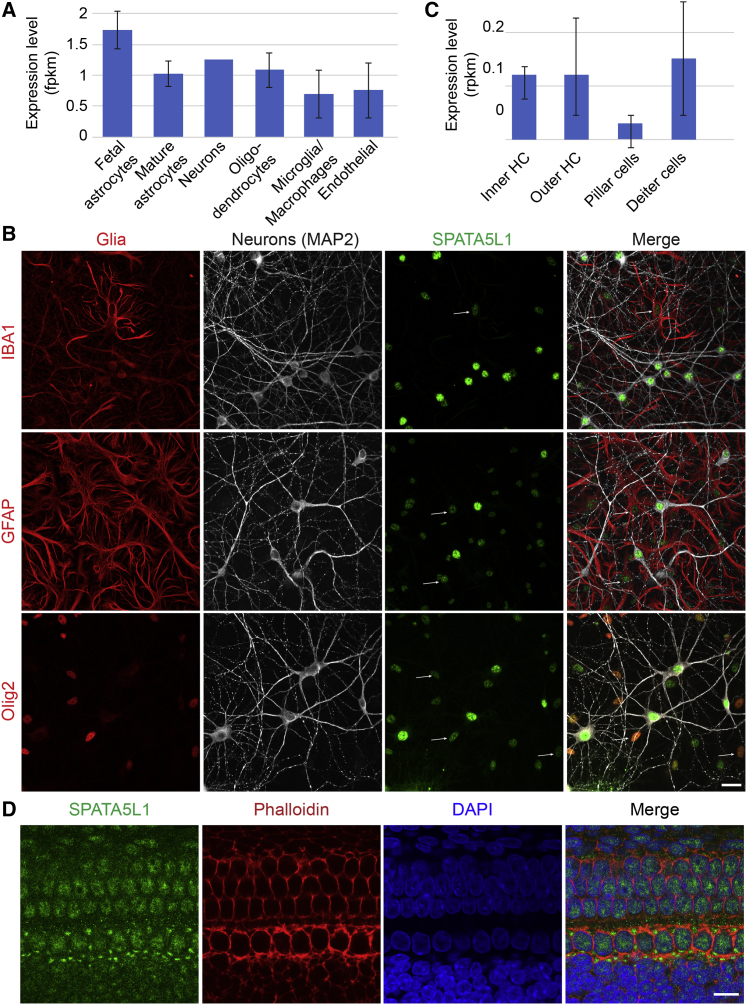
We next sought to define the typical protein localization of SPATA5L1. SPATA5L1 mRNA has been detected (albeit at low levels) in both neurons and glia, both during embryonic and adult stages of human brain development (Figure 4A). We confirmed this at the protein level in rat dissociated hippocampal cultures, identifying Spata5l1 immunoreactivity within neurons, astrocytes, oligodendrocytes, and microglia; the most prominent staining was in neurons (Figure 4B). Spata5l1 localized primarily to the nucleus. Within the ear, both inner and outer hair cells exhibit detectable levels of Spata5l1 transcript (Figure 4C). Rat whole mounts stained with a commercial antibody against Spata5l1 demonstrated that Spata5l1 is present in hair cells and pillar cells of the organ of Corti (Figure 4D), suggesting that loss of wild-type protein may lead to sensorineural hearing loss by disrupting normal function within these structures.
Figure 4.
Spata5l1 is expressed in the inner ear and the brain of rodents/humans
(A) SPATA5L1 is expressed in all cellular subtypes of the human brain (Brain RNA-Seq database).
(B) Representative immunofluorescent labeling of endogenous SPATA5L1 (green) in rat hippocampal neurons in culture highlight the co-localization of the protein within the nuclei of neurons (MAP2), as well as the nuclei of glial cells (red): microglia (IBA1), astrocytes (GFAP), and oligodendrocytes (Olig2). Signal intensity of SPATA5L1 immunolabeling suggests protein expression is higher in neurons compared to glial cells. Arrows indicate the localization of SPATA5L1 in the nuclei of glial cells. All images are projections of confocal optical section stacks. Scale bar: 25 μm.
(C) Spata5l1 is expressed at low levels in hair cells (inner and outer) as well as supporting cells (pillar and Deiter cells) in adult mice (adapted from gEAR portal). HC, hair cells.
(D) Representative immunofluorescent labeling of endogenous SPATA5L1 (green) in Sprague Dawley rat organ of Corti at E20. Immunolabeling shows SPATA5L1 is present in the hair and pillar cells of the organ of Corti. DAPI (blue) and Rhodamine Phalloidin (red) were used for counterstaining the nuclei and the cytoskeleton, respectively. All images are projections of confocal optical section stacks. Scale bar: 10 μm.
The precise function of SPATA5L1 in brain, inner ear, and other tissues is currently unknown. However, the sequence similarity and overlapping clinical manifestations in individuals harboring putatively loss-of-function or protein-damaging variants in SPATA5 and SPATA5L1 led us to speculate as to a potential redundancy between the two proteins. Given the proposed role of SPATA5 in mitochondrial function,1 we assessed oxidative phosphorylation (OXPHOS) in primary fibroblasts from affected individuals 2-3, 2-4, 7-9 (i.e., individuals 3 and 4 from family 2 and individual 9 from family 7) in comparison to passage and age-matched controls by using the Seahorse XF96 assay. These assays indicated no impairment of OXPHOS in affected fibroblasts (Figure S4), suggesting that despite the degree of clinical overlap seen in affected individuals, the two proteins may have divergent functions. Indeed, a mitochondrial localization for SPATA5L1 is not predicted via MitoMiner13 and it is absent from the MitoCarta3.0 human and mouse inventory.14 However, alterations to mitochondrial biogenesis remain possible, and we cannot exclude disruption to mitochondrial morphology and dynamics (as observed in SPATA5-deficient neurons).3
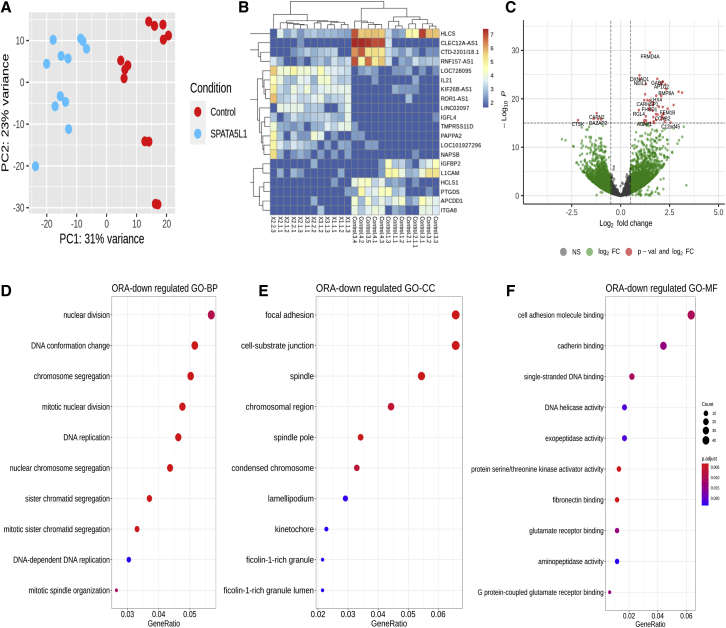
Next, we turned to an unbiased transcriptome approach to try to distinguish SPATA5L1 fibroblast cell lines from controls. The analysis was performed in the same affected (plus 1-1 and 4-6, i.e., individual 1 from family 1 and individual 6 from family 4) cell lines utilized for the Seahorse study and in passage and age-matched controls. Principal-component analysis from RNA sequencing (RNA-seq) data indicated that individuals harboring bi-allelic SPATA5L1 variants could be distinguished from controls on the basis of their differentially expressed genes (Figure 5A). This provided proof of principle data supporting the p.Ala41Pro, p.Val245Glu, p.Ile435fs, p.Ile466Met, and p.Ala519Asn variants as bona fide disease-associated variants. These findings allowed us to pool these data for subsequent analyses. Significantly upregulated networks were not identified. However, several significantly downregulated genes were identified (Figures 5B and 5C). These genes converged on several hubs (Figures 5D–5F), pointing to a role for SPATA5L1 in mitosis (mitotic nuclear division, sister chromatid segregation, mitotic spindle organization, kinetochores) and DNA replication (DNA conformation change, single-stranded DNA binding, DNA helicase activity). Adhesion receptors, which connect cell-substrate junctions (Figure 5E) and include fibronectin-binding (Figure 5F) integrins (i.e., ITGA8; Figure 5B), cadherins (Figure 5F), and immunoglobulin superfamily members (i.e., L1CAM; Figure 5B), were significantly downregulated. Members of the AAA+ ATPases protein superfamily have known roles in mitosis, DNA replication, metabolism, and repair processes.15 For example, cytoplasmic dynein plays a role in mammalian mitotic spindle formation,16 while WRNIP1 protects stalled replication forks from degradation.17 Our transcriptome analyses posit a potential role for SPATA5L1 in mitosis and DNA replication, however additional studies are required for assessment of this possibility.
Figure 5.
Analysis of gene expression patterns in SPATA5L1 fibroblasts by RNA-seq reveals differential expression of DNA replication and mitosis-related genes
(A) Principal-component analysis plot shows a differential clustering of SPATA5L1 samples (n = 4) from control samples (n = 4).
(B) Transcriptomic heatmap of the top 20 differentially expressed genes (top ten with fold change > 1.5 and p < 0.05 and bottom 10 with fold change < 0.5 and p < 0.05). Red/yellow colors represent highly expressed genes and blue colors represent under-expressed genes for these 20 genes in the respective case and control samples. The legend corresponds to expression values.
(C) Volcano plot highlighting genes with large fold changes that are either significantly upregulated or downregulated between SPATA5L1 and control samples. Log2 fold change of normalized counts (red dots indicate genes with p < 10−16).
(D) Over-representation analysis (ORA) for downregulated genes (log2 fold change < −1, p < 0.05) for GO-BP (Gene Ontology Biological Processes).
(E) Over-representation analysis (ORA) for downregulated genes (log2 fold change < −1, p < 0.05) for GO-CC (Gene Ontology Cellular Components).
(F) Over-representation analysis (ORA) for downregulated genes (log2 fold change < −1, p < 0.05) for GO-CC and GO-MF (Gene Ontology Molecular Functions).
Our clinical, radiologic, genomic, and transcriptomic evidence support the existence of a mixed neurodevelopmental syndrome with hearing loss due to bi-allelic variants in SPATA5L1. The pathogenicity of the variants we identified is supported by their rarity (many private variants), predicted deleteriousness by multiple algorithms, consistent phenotype in affected individuals, and RNA-seq validation of several variants. Although we did identify individual loss-of-function (premature stop, frameshift, start-loss, stop-loss, or canonical splice site) alleles, we did not identify bi-allelic loss-of-function variants, suggesting that perhaps human knockout genotypes might show reduced viability. We were not able to clearly identify any firm genotype-phenotype correlations. Our morphologic neuroimaging analyses revealed thin corpus callosum, diminished cortical volume, open opercula, and anterior temporal hypoplasia in several individuals, while quantitative morphometry indicated that white matter volume was significantly diminished in multiple members of the cohort. When observed, microcephaly correlated with reduced white matter volume (as observed in affected individuals 10-2, 12-1, 13-1).
Although SPATA5L1 is an orphan gene, our transcriptomic studies provide some clues as to its function. Adhesion receptors collectively play a major role in the control of cell-extracellular matrix (fibronectin-integrin and immunoglobulin superfamily members) and cell-cell interactions (cadherin family members). These interactions in turn integrate cell growth/migration and proliferation on the basis of environmental cues such as contact inhibition. Fibronectin-integrin binding is known to be mediated through focal adhesions, intracellular cytoskeleton/signaling hubs that transmit extracellular cues through phosphorylation events (i.e., protein serine-threonine kinase activator activity) and ultimately control DNA replication and mitosis. Although neuroimaging in our affected individuals did not indicate malformations of cortical development that would suggest abnormalities of neuronal migration, the diminished cortical volumes and microcephaly seen may reflect impairment of neuronal cell division during brain development.
In conclusion, we present evidence that rare coding variants and loss-of-function alleles in SPATA5L1 lead to hearing loss and a mixed neurodevelopmental disorder that features microcephaly, global developmental delay/intellectual disability, spastic-dystonic cerebral palsy (some children presented with hypotonia), and focal or generalized epilepsy. Although our studies support a role for SPATA5L1 in DNA replication, further experimental studies will be required to support or refute this hypothesis.
Acknowledgments
The authors are grateful to the participants and their families, without whose support this work would not have been possible. K.Mc., A.B., A.C., M.J.G.S., R.E.P., and R.E.S. are employees of GeneDx. We thank Minerva Contreras and Thomas Blanpied for assisting with neuronal culture and the CIBR platform (UMSOM, Baltimore, MD, USA). This work was supported in part by R01NS107428 (S.R.), 1R01NS106298 (M.C.K.), and Cerebral Palsy Alliance Research Foundation PG07217 award to W.M. and M.C.K. Portions of this work were also funded by the Fondazione Bambino Gesù (Vite Coraggiose), the Italian Ministry of Health (Ricerca 5 × 1000) to M.T.A., and in part by Telethon Undiagnosed Diseases Program (TUDP, GSP15001). H.L. receives support from the Canadian Institutes of Health Research (Foundation Grant FDN-167281), the Canadian Institutes of Health Research and Muscular Dystrophy Canada (Network Catalyst Grant for NMD4C), the Canada Foundation for Innovation (CFI-JELF 38412), and the Canada Research Chairs Program (Canada Research Chair in Neuromuscular Genomics and Health, 950-232279). B.V. is funded by intramural funding (fortüne) at the University of Tübingen (2545-1-0) and the Ministry of Science, Research, and Art Baden-Württemberg. R.H. is supported by NEI intramural funds. W.K.C. receives support from the JPB Foundation and SFARI. S.C.J. is supported by a K99/R00 Pathway to Independence Award (K99HL143036 and R00HL143036-02) and the Clinical and Translational Research Funding Program award (CTSA1405). This project was funded in part by The Foundation for Barnes-Jewish Hospital and their generous donors and by the NIH/National Center for Advancing Translational Sciences grant UL1TR002345. Several families were enrolled as part of the SYNaPS Study Group collaboration funded by The Wellcome Trust and strategic award (Synaptopathies) funding (WT093205 MA and WT104033AIA). This research was conducted as part of the Queen Square Genomics group at University College London, supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre. A.P.L.M. was supported by a NHMRC Early Career Research Fellowship (GNT1156820). S.B.’s contributions were funded by a Cerebral Palsy Alliance Research Foundation Career Development Award. C.v.E., M.A.C., and A.H.M. were supported by Australian National Health and Medical Research Council Project Grant (1099163). J.G. was supported by Australian National Health and Medical Research Council Fellowship (1041920) and Channel 7 CRF Chair for the Prevention of Childhood Disability. C.v.E. was supported by The Hospital Research Foundation Mid-Career Fellowship. C.v.E., M.A.C., A.H.M., and J.G. were supported by infrastructure funding from the Tenix Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of interests
The authors declare no competing interests.
Published: October 7, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ajhg.2021.08.003.
Contributor Information
Saima Riazuddin, Email: sriazuddin@som.umaryland.edu.
Michael C. Kruer, Email: kruerm@arizona.edu.
Data and code availability
Variants identified in this study have been submitted to ClinVar (accession numbers pending). Original data are available from the authors upon reasonable request.
Web resources
Brain RNA-Seq, http://www.brainrnaseq.org/
BrainSpan Atlas of the Developing Human Brain, https://www.brainspan.org/
gEAR portal, https://umgear.org/
MARRVEL, http://marrvel.org/
MutationTaster, http://mutationtaster.org/
OMIM, https://omim.org/
PolyPhen-2, http://genetics.bwh.harvard.edu/pph2/
Provean, http://provean.jcvi.org
Supplemental information
References
- 1.Tanaka A.J., Cho M.T., Millan F., Juusola J., Retterer K., Joshi C., Niyazov D., Garnica A., Gratz E., Deardorff M. Mutations in SPATA5 Are Associated with Microcephaly, Intellectual Disability, Seizures, and Hearing Loss. Am. J. Hum. Genet. 2015;97:457–464. doi: 10.1016/j.ajhg.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y., Black J., Kisiel N., Kulesz-Martin M.F. SPAF, a new AAA-protein specific to early spermatogenesis and malignant conversion. Oncogene. 2000;19:1579–1588. doi: 10.1038/sj.onc.1203442. [DOI] [PubMed] [Google Scholar]
- 3.Puusepp S., Kovacs-Nagy R., Alhaddad B., Braunisch M., Hoffmann G.F., Kotzaeridou U., Lichvarova L., Liiv M., Makowski C., Mandel M. Compound heterozygous SPATA5 variants in four families and functional studies of SPATA5 deficiency. Eur. J. Hum. Genet. 2018;26:407–419. doi: 10.1038/s41431-017-0001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Köttgen A., Glazer N.L., Dehghan A., Hwang S.J., Katz R., Li M., Yang Q., Gudnason V., Launer L.J., Harris T.B. Multiple loci associated with indices of renal function and chronic kidney disease. Nat. Genet. 2009;41:712–717. doi: 10.1038/ng.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kubo Y., Imaizumi T., Ando M., Nakatochi M., Yasuda Y., Honda H., Kuwatsuka Y., Kato S., Kikuchi K., Kondo T. Association between kidney function and genetic polymorphisms in atherosclerotic and chronic kidney diseases: A cross-sectional study in Japanese male workers. PLoS ONE. 2017;12:e0185476. doi: 10.1371/journal.pone.0185476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park H., Kim H.J., Lee S., Yoo Y.J., Ju Y.S., Lee J.E., Cho S.I., Sung J., Kim J.I., Seo J.S. A family-based association study after genome-wide linkage analysis identified two genetic loci for renal function in a Mongolian population. Kidney Int. 2013;83:285–292. doi: 10.1038/ki.2012.389. [DOI] [PubMed] [Google Scholar]
- 7.Sobreira N., Schiettecatte F., Valle D., Hamosh A. GeneMatcher: a matching tool for connecting investigators with an interest in the same gene. Hum. Mutat. 2015;36:928–930. doi: 10.1002/humu.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKenna A., Hanna M., Banks E., Sivachenko A., Cibulskis K., Kernytsky A., Garimella K., Altshuler D., Gabriel S., Daly M., DePristo M.A. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van der Auwera G.A., Carneiro M.O., Hartl C., Poplin R., Del Angel G., Levy-Moonshine A., Jordan T., Shakir K., Roazen D., Thibault J. From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr. Protoc. Bioinformatics. 2013;43:11.10.1–11.10.33. doi: 10.1002/0471250953.bi1110s43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pagnozzi A.M., Dowson N., Doecke J., Fiori S., Bradley A.P., Boyd R.N., Rose S. Identifying relevant biomarkers of brain injury from structural MRI: Validation using automated approaches in children with unilateral cerebral palsy. PLoS ONE. 2017;12:e0181605. doi: 10.1371/journal.pone.0181605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baugh E.H., Simmons-Edler R., Müller C.L., Alford R.F., Volfovsky N., Lash A.E., Bonneau R. Robust classification of protein variation using structural modelling and large-scale data integration. Nucleic Acids Res. 2016;44:2501–2513. doi: 10.1093/nar/gkw120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ittisoponpisan S., Islam S.A., Khanna T., Alhuzimi E., David A., Sternberg M.J.E. Can Predicted Protein 3D Structures Provide Reliable Insights into whether Missense Variants Are Disease Associated? J. Mol. Biol. 2019;431:2197–2212. doi: 10.1016/j.jmb.2019.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith A.C., Robinson A.J. MitoMiner v3.1, an update on the mitochondrial proteomics database. Nucleic Acids Res. 2016;44(D1):D1258–D1261. doi: 10.1093/nar/gkv1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rath S., Sharma R., Gupta R., Ast T., Chan C., Durham T.J., Goodman R.P., Grabarek Z., Haas M.E., Hung W.H.W. MitoCarta3.0: an updated mitochondrial proteome now with sub-organelle localization and pathway annotations. Nucleic Acids Res. 2021;48:D1541–D1547. doi: 10.1093/nar/gkaa1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snider J., Thibault G., Houry W.A. The AAA+ superfamily of functionally diverse proteins. Genome Biol. 2008;9:216. doi: 10.1186/gb-2008-9-4-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaisberg E.A., Koonce M.P., McIntosh J.R. Cytoplasmic dynein plays a role in mammalian mitotic spindle formation. J. Cell Biol. 1993;123:849–858. doi: 10.1083/jcb.123.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leuzzi G., Marabitti V., Pichierri P., Franchitto A. WRNIP1 protects stalled forks from degradation and promotes fork restart after replication stress. EMBO J. 2016;35:1437–1451. doi: 10.15252/embj.201593265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Case 7 exhibits mild bilateral chorea of the upper limbs as a toddler.
Case 9 at age 2 years demonstrates global growth failure and hypertonia of the limbs.
Proband (case 10): An examination of case 10 at age 9 years reveals visual impairment and spastic quadriplegia with diminished head control and scissoring of the lower limbs without clear hyperreflexia. Amyotrophy of the extremities is evident, as is pes cavus. Sibling (case 11): Evaluation at age 7 years shows visual impairment with lateral-beating nystagmus. Spastic quadriplegia is noted with poor head control. Persistent glabellar tap reflex without habituation is noted as a frontal release sign. Amyotrophy of the limbs is evident and marked scoliosis and pes planus are present. Sibling (case 12): Assessment at age 5 years reveals visual impairment, spastic quadriplegia with poor head control, diffuse hyperreflexia, and equivocally positive Babinski signs. Pes planus of the feet is again seen.
Evaluation of this 10-year-old boy revealed dystonic posturing of the upper and lower limbs.
Dystonic posturing of the hand and mouth is evident while the individual is sitting.
One of the affected individual’s seizures in captured, beginning as a gelastic seizure with stereotyped laughter, followed by behavioral arrest with stereotyped blinking evolving into rhythmic saccadic eye movements, and concluding with post-ictal hyporesponsiveness.
Data Availability Statement
Variants identified in this study have been submitted to ClinVar (accession numbers pending). Original data are available from the authors upon reasonable request.