Abstract
Background
e-Cigarette or vaping-induced lung injury (EVALI) causes a spectrum of CT lung injury patterns. Relative frequencies and associations with vaping behavior are unknown.
Research Question
What are the frequencies of imaging findings and CT patterns in EVALI and what is the relationship to vaping behavior?
Study Design and Methods
CT scans of 160 subjects with EVALI from 15 institutions were retrospectively reviewed. CT findings and patterns were defined and agreed on via consensus. The parenchymal organizing pneumonia (OP) pattern was defined as regional or diffuse ground-glass opacity (GGO) ± consolidation without centrilobular nodules (CNs). An airway-centered OP pattern was defined as diffuse CNs with little or no GGO, whereas a mixed OP pattern was a combination of the two. Other patterns included diffuse alveolar damage (DAD), acute eosinophilic-like pneumonia, and pulmonary hemorrhage. Cases were classified as atypical if they did not fit into a pattern. Imaging findings, pattern frequencies, and injury severity were correlated with substance vaped (marijuana derives [tetrahydrocannabinol] [THC] only, nicotine derivates only, and both), vaping frequency, regional geography, and state recreational THC legality. One-way analysis of variance, χ2 test, and multivariable analyses were used for statistical analysis.
Results
A total of 160 patients (79.4% men) with a mean age of 28.2 years (range, 15-68 years) with EVALI underwent CT scan. Seventy-seven (48.1%), 15 (9.4%), and 68 (42.5%) patients admitted to vaping THC, nicotine, or both, respectively. Common findings included diffuse or lower lobe GGO with subpleural (78.1%), lobular (59.4%), or peribronchovascular (PBV) sparing (40%). Septal thickening (50.6%), lymphadenopathy (63.1%), and CNs (36.3%) were common. PBV sparing was associated with younger age (P = .02). Of 160 subjects, 156 (97.5%) had one of six defined patterns. Parenchymal, airway-centered, and mixed OP patterns were seen in 89 (55.6%), 14 (8.8%), and 32 (20%) patients, respectively. Acute eosinophilic-like pneumonia (six of 160, 3.8%), DAD (nine of 160, 5.6%), pulmonary hemorrhage (six of 160, 3.8%), and atypical (four of 160, 2.5%) patterns were less common. Increased vaping frequency was associated with more severe injury (P = .008). Multivariable analysis showed a negative association between vaping for > 6 months and DAD pattern (P = .03). Two subjects (1.25%) with DAD pattern died. There was no relation between pattern and injury severity, geographic location, and state legality of recreational use of THC.
Interpretation
EVALI typically causes an OP pattern but exists on a spectrum of acute lung injury. Vaping habits do not correlate with CT patterns except for negative correlation between vaping > 6 months and DAD pattern. PBV sparing, not previously described in acute lung injury, is a common finding.
Key Words: CT scan, e-cigarette, EVALI, lung injury, vaping
Abbreviations: AEP, acute eosinophilic pneumonia; AEP-like, acute eosinophilic-like pneumonia; AFOP, acute and fibrinous organizing pneumonia; ALI, acute lung injury; CDC, Centers for Disease Control and Prevention; CN, centrilobular nodule; DAD, diffuse alveolar damage; DAH, diffuse alveolar hemorrhage; EVALI, e-cigarette or vaping-induced lung injury; GGO, ground-glass opacity; IBD, inflammatory bowel disease; IRB, institutional review board; LAD, lymphadenopathy; OP, organizing pneumonia; PBV, peribronchovascular; PCR, polymerase chain reaction; RB, respiratory bronchiolitis; THC, tetrahydrocannabinol
The use of e-cigarettes, or vaping, has skyrocketed in recent years. Although e-cigarettes were initially introduced as a safer alternative to traditional cigarettes, the recent discovery that vaping can cause acute lung injury (ALI) has proven that this option is not innocuous. Most patients who developed ALI after vaping, termed e-cigarette or vaping-induced lung injury (EVALI), were adolescents and young adults.1
From March 2019 to February 2020, EVALI hospitalized 2,807 and killed 68 people in the United States (including the District of Columbia), Puerto Rico, and the US Virgin Islands.2,3 The US Centers for Disease Control and Prevention (CDC) stopped tracking EVALI at the end of February 2020, coinciding with the rise of the COVID-19 pandemic. Although the number of EVALI cases has decreased from the fall and winter of 2019, they have not disappeared. During the COVID-19 pandemic, EVALI remains an important consideration as an underlying cause of ALI.4,5 Because both can manifest with similar clinical and imaging findings, an accurate diagnosis is important given differences in treatment and in infection prevention and control. Confounding the situation further is research suggesting increased incidence of COVID-19 in e-cigarette users6 and a positive association between statewide proportion of vapers and number of COVID-19 cases and deaths.7
Although EVALI is a clinical diagnosis, abnormalities on chest imaging are a diagnostic criterion. Various imaging patterns of EVALI on chest CT scan have been described in case reports and educational publications.8, 9, 10, 11, 12 There has been no large multicenter study designed to define EVALI’s imaging manifestations. The purpose of this study is to determine the frequency of various CT imaging findings occurring in patients with EVALI, to assess patterns of lung disease, to calculate the relative frequency of these patterns, and to determine if various clinical and vaping factors are related to imaging findings, patterns, or both.
Methods
Fellowship-trained thoracic radiologists from 15 academic institutions throughout the United States contributed cases for this retrospective review. Each coauthor obtained local institutional review board (IRB) approval based on institutional guidelines. Some local IRBs were approved for inclusion of both pediatric and adult subjects, whereas others were limited to adult subjects. No protected health information was shared between sites at any time during the study.
Initial subject selection varied by individual institution. At 14 institutions, potential patients with EVALI were initially recognized by searching radiology reports in the local radiology information system using a natural language processing program with the following key words: e-cigarette, vaping, vape, vaping, and EVALI. At 10 of these 14 institutions, clinical colleagues in the pulmonary division, toxicology division, or both were queried regarding patients with the clinical diagnosis of EVALI. At one institution, the radiology information system was not searched, and all potential patients with EVALI were located during multidisciplinary conferences with pulmonologists. After the initial selection, patients’ electronic medical records were reviewed to determine the final diagnosis. Only subjects with a final diagnosis of EVALI, as determined by the CDC guidelines, were included in the study. These inclusion criteria included e-cigarette or related product use within 90 days, lung opacities on chest CT scan or chest radiography, and absence of a plausible alternative diagnosis. Active pulmonary infection was excluded in all subjects based on various tests including negative respiratory viral panel, negative testing for other clinically indicated respiratory infections through sputum or blood cultures, urine antigen testing, BAL, and if applicable, negative testing for HIV-related opportunistic infections. Respiratory viral panels, which could be obtained by nasopharyngeal swab or BAL, varied by institution but most commonly included polymerase chain reaction (PCR) testing for subtypes of adenovirus, respiratory syncytial virus, influenza, parainfluenza, coronavirus (excluding SARS-CoV-2 subtype), metapneumovirus, and rhinovirus. These were obtained by nasopharyngeal swab. After March 1, 2020, all viral panels included PCR testing for the SARS-CoV-2 virus. Patients had to have at least two documented negative SARS-CoV-2 PCR tests at the time of evaluation for inclusion in the study.
Any subjects with an active respiratory infection, even if deemed not to be the cause of symptoms, were excluded. Patients with malignancy were excluded only if they were currently on chemotherapeutic or immunotherapy agents. Patients with connective tissue diseases were only excluded if their lung disease or current medication regimen was thought to be the cause of the lung injury by the treating clinical team. All subjects had to have a chest CT scan during the episode of ALI to be included in the study. Subjects included in this study could receive treatment as an inpatient, through an acute care provider such as the ED or urgent care clinic, or through an outpatient provider. Admission was not required for inclusion.
Chest CT scans were obtained using various contrast-enhanced and unenhanced protocols depending on clinical indication and local protocols. CT scanners with varying numbers of detectors ranging from 64 to 320 detector rows from various vendors were used depending on local availability. Because the CDC reported its first case of vaping lung injury during the week of March 31, 2019, only CT scans obtained after April 1, 2019, were included. Subjects diagnosed on or after March 1, 2020, had to undergo at least two negative COVID-19 reverse transcriptase PCR tests. All CT scans were reviewed using the thinnest section data set available. CT scans lacking slice thickness ≤ 3 mm were excluded.
After local IRB approval, each radiologist recorded relevant clinical data and imaging findings for each subject in a spreadsheet (Fig 1, Table 1). Because this was a retrospective study, clinical data from the electronic medical record was reviewed after the patient was discharged. Clinical symptoms including dyspnea/shortness of breath, cough, chest pain, abdominal pain, nausea/vomiting, and diarrhea had to be present immediately preceding or during the initial medical encounter. If a symptom was not specifically mentioned during the notes obtained during initial presentation or admission, it was considered to be absent. Similarly, fever was defined as a documented body temperature of ≥ 38 °C during initial assessment. The time between the CT scan and the patient’s initial symptoms was also recorded. All notes during the patient’s medical encounter for EVALI were reviewed to best determine the substance vaped, the duration of vaping, and the frequency of vaping.
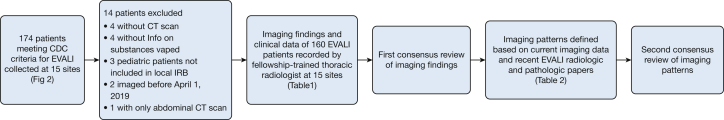
Figure 1.
Flowchart displaying method of imaging finding and pattern reviews. CDC = Centers for Disease Control and Prevention; EVALI = e-cigarette or vaping-induced lung injury; IRB = institutional review board.
Table 1.
Demographics, Vaping History, and Imaging Findings Seen in 160 Cases of e-Cigarette or Vaping Product Use-Associated Lung Injury
| Clinical Data and Vaping History | Distribution and Extent | Common Findings | Uncommon or Absent Findings | ||||
|---|---|---|---|---|---|---|---|
| Age, y | 28.2 ± 11.2 (15-68) | Craniocaudal predominance | Parenchymal sparing | BWT | 19 (11.9) | ||
| Sex | Upper | 12 (7.5) | Subpleural | 125 (78.1) | Emphysema | 12 (7.5) | |
| Men | 127 (79.4) | Lower | 46 (28.8) | Lobular | 95 (59.4) | Bronchiolar dilation | 9 (5.6) |
| Women | 33 (20.6) | Diffuse | 97 (60.6) | PBV | 64 (40) | Fissural displacement | 6 (3.7) |
| BMI (n = 128) | 26.5 ± 7 (16.4-55.8) | Patchy | 5 (3.1) | All three | 37 (23.1) | Pneumomediastinum | 6 (3.7) |
| Primary substance vaped | Axial predominance | CNs | 59 (36.9) | Reverse halo sign | 5 (3.1) | ||
| THC only | 68 (42.5) | Subpleural | 24 (15) | Distribution of CN | Prominent mosaicism | 4 (2.5) | |
| Nicotine only | 15 (9.4) | Central | 4 (2.5) | Upper predominant | 28 | Pneumothorax | 1 (0.6) |
| Both | 77 (48.1) | Diffuse | 127 (79.4) | Lower predominant | 0 | Parenchymal cysts | 1 (0.6) |
| Vaping frequency | Patchy | 5 (3.1) | Diffuse | 24 | Reticulation | 1 (0.6) | |
| Daily | 92 (57.5) | Parenchymal opacity | Patchy | 7 | Honeycombing | 0 (0) | |
| Multiple times per week | 22 (13.8) | GGO only | 82 (51.3) | Appearance of CNs | Random nodules | 0 (0) | |
| Once a week or less | 7 (4.4) | GGO > consolidation | 40 (25) | Ill-defined | 52 | Perilymphatic nodules | 0 (0) |
| Not available | 39 (24.4) | Equal mix | 38 (18.7) | Well-defined | 5 | Dilated vessels | 0 (0) |
| Vaping duration | Consolidation > GGO | 8 (5) | Both | 2 | |||
| ≥ 12 mo | 61 (38.2) | Consolidation only | 0 (0) | Septal thickening | 81 (50.6) | ||
| ≥ 6 to < 12 mo | 10 (6.2) | No. of lobes involved | Severity of septal thickening | ||||
| ≥ 1 to 6 mo | 33 (20.6) | 1 | 0 (0) | Mild to moderate | 75 | ||
| < 1 mo | 9 (5.6) | 2 | 1 (0.6) | Severe | 6 | ||
| Not available | 47 (29.4) | 3 | 0 (0) | Crazy paving | 30 (18.8) | ||
| Chest symptoms | 4 | 3 (1.9) | Pleural effusion | 35 (21.9) | |||
| Dyspnea/SOB | 131 (81.9) | 5 | 156 (97.5) | Qualitative size of pleural effusion | |||
| Cough | 104 (65) | Estimated percentage lung involved | Trace | 13 | |||
| Pain | 39 (24.4) | 0%-24% | 10 (6.2) | Small | 20 | ||
| All three | 23 (14.4) | 25%-49% | 18 (11.3) | Moderate | 2 | ||
| Hemoptysis | 12 (7.5) | 50%-74% | 47 (29.4) | Large | 0 | ||
| None | 6 (3.8) | 75%-100% | 85 (53.1) | Lymphadenopathy | 101 (63.1) | ||
| GI symptoms | Qualitative severity | Distribution of lymphadenopathy | |||||
| Nausea/vomit | 76 (47.5) | Mild | 25 (15.6) | Mediastinal | 18 | ||
| Diarrhea | 33 (20.6) | Moderate | 75 (46.9) | Hilar | 24 | ||
| Pain | 31 (19.4) | Severe | 60 (37.5) | Both | 59 | ||
| None | 68 (42.5) | Symmetrical | |||||
| Fever | 103 (63.4) | Yes | 154 (96.3) | ||||
| Symptom duration, d | 10.6 ± 29.1 (1-180) (median, 6) | No | 6 (3.7) | ||||
Values are No., No. (%), or mean ± SD (range). BWT = bronchial wall thickening; CN = centrilobular nodule; GGO = ground-glass opacity; prominent mosaicism = parenchyma demonstrates mixed areas of hypoattenuation, normal attenuation, and hyperattenuation throughout the lungs; SOB = shortness of breath; THC = tetrahydrocannabinol.
After local review, imaging findings were reviewed one-to-one via video communication software (Zoom; Zoom Video Communications) with the same thoracic radiologist (S. J. K., 12 years’ experience) using locally anonymized images and a variety of Digital Imaging and Communications in Medicine viewers based on local preference. Each imaging finding was agreed on by consensus. In instances where consensus could not be reached, the local radiologist made the final decision.
Subsequently, cases were categorized into radiologic patterns based on imaging findings (Table 2). Most patterns, including the diffuse alveolar damage (DAD) pattern, acute eosinophilic-like pneumonia (AEP-like) pattern, and diffuse alveolar hemorrhage (DAH) pattern, have been previously described with EVALI.9, 10, 11 Briefly, the DAD pattern was defined as diffuse but basilar predominant ground-glass opacity (GGO), consolidation, or both, often with lobular sparing. To meet imaging criteria for a DAD pattern, ancillary CT findings of alveolar collapse had to be present (eg, bronchial dilation, volume loss, architectural distortion, fissural displacement).13 Acute eosinophilic pneumonia (AEP) is another cause of severe ALI and can have imaging and pathologic findings that overlap with those seen in DAD.14, 15, 16 However, for this study, the main distinctions between the AEP-like and DAD patterns were findings of superimposed fluid overload in the former, including bilateral pleural effusions and pronounced interlobular septal thickening, corresponding to pulmonary edema seen on both imaging and pathology.15, 16, 17, 18, 19 Findings of volume loss were absent in the AEP-like pattern and although interlobular septal thickening could be present in the DAD pattern, it had to be qualitatively mild if present and pleural effusions were absent. The DAH pattern was defined as patchy or multifocal GGO, consolidation, or both with superimposed acinar ground-glass centrilobular nodules (CNs) filling most of the secondary lobule.9,11,19,20 Unlike other patterns, DAH could be asymmetrical. All patients with a DAH pattern had to have either bronchoscopic confirmation of DAH or witnessed hemoptysis.
Table 2.
Definition of Imaging Patterns in e-Cigarette or Vaping Product Use-Associated Lung Injury
| CT Pattern | Parenchymal Opacity | Craniocaudal Distribution | Axial Distribution | Volume Loss | CNs | Septal Thickening | Effusions |
|---|---|---|---|---|---|---|---|
| Parenchymal OP | |||||||
| Upper | GGO ≥ consolidation | Upper lung predominant | Peripheral or diffuse | No | No | ± | No or trace |
| Lower | GGO ≥ consolidation | Lower lung predominant | Peripheral or diffuse | No | No | ± | No or trace |
| Diffuse GGO | GGO > consolidation | Diffuse | Peripheral or diffuse | No | No | ± | No or trace |
| Diffuse consolidative | Consolidation ≥ GGO | Diffuse | Diffuse | No | No | ± | No or trace |
| Patchy | GGO > consolidation | Patchy | Patchy | No | No | ± | No or trace |
| Airway-centered OP | Absent or mild | Diffuse CNs | Diffuse CNs | No | Yes (dominant finding) | ± | No or trace |
| Mixed OP | GGO ≥ consolidation | Diffuse | Peripheral or diffuse | No | Yes (parenchymal opacity ≥ nodules) | ± | No or trace |
| Diffuse alveolar damage | Any mix of GGO and/or consolidation | Diffuse | Diffuse | Yes | No | ± | No or trace |
| Acute eosinophilic-like pneumonia | Any mix of GGO and/or consolidation | Diffuse | Diffuse | No | ± | Pronounced | Yes |
| Diffuse alveolar hemorrhage | Any mix of GGO and/or consolidation | Patchy and asymmetrical | Patchy or diffuse | No | Acinar ground-glass nodules | ± | No or trace |
| Atypical | Imaging pattern that does not fit into any of the listed categories | ||||||
Except for diffuse alveolar hemorrhage, which may be symmetrical or asymmetrical, all other patterns of e-cigarette or vaping product use-associated lung injury are symmetrical. For an example of acinar ground-glass nodules seen in diffuse alveolar hemorrhage, please refer to Figure 8. Airway-centered OP = diffuse craniocaudal and axial distributions related to distribution of CNs and associated parenchymal opacity is absent or mild; CN = centrilobular nodule; GGO = ground-glass opacity; OP = organizing pneumonia.
Organizing pneumonia (OP) is a common pattern of lung injury that most commonly manifests as diffuse or lower lobe predominant, bilateral, and relatively symmetrical GGO, consolidation, or both, often with areas of subpleural and lobular sparing.13 However, because of improved pathologic understanding of EVALI, it is now known that OP can manifest with airway-centered injury demonstrated as CNs on CT scan.18,21,22 Therefore, the OP pattern was subdivided into three main subtypes. If the injury was predominantly parenchymal with few or no CNs, it was classified as parenchymal OP pattern. On the other end of the spectrum, the injury could be primarily centered on the small airways manifesting as diffuse CNs with mild or no associated GGO. This was classified as airway-centered OP pattern on CT scan. The injury could also manifest as a blend of these two patterns with a mixture of CNs and parenchymal GGO and was termed a mixed OP pattern. Previously, this imaging pattern that has been termed the hypersensitivity pneumonitis (HP) pattern,11,18,19 but has been renamed as HP, has not been described pathologically with EVALI,18,21,22 which is discussed subsequently in greater detail. The parenchymal OP pattern was further divided based on craniocaudal distribution into lower lobe predominant, upper lobe predominant, or diffuse to better highlight the common and uncommon patterns seen on EVALI. The diffuse pattern was subdivided based on the extent of GGO and consolidation into either a diffuse GGO OP pattern or diffuse consolidative OP pattern. This was done because the diffuse consolidative pattern was thought to represent a more severe injury, which may be either a severe form of OP or an early exudative form of DAD. Finally, if a patient had scattered bilateral, geographic areas of OP that did not correspond to any specific axial or craniocaudal distribution, it was referred to as patchy OP pattern. This was separated from the other OP patterns both because of its unique appearance and its comparatively mild degree of lung injury.
Each case was then re-reviewed via Zoom similarly as previously detailed, this time ascribing an imaging pattern. If the pattern did not fall into any single category or if consensus could not be reached, it was defined as atypical.
Qualitative severity and patterns of lung injury in EVALI were evaluated based on geography, legality of recreational marijuana in the state of occurrence, and temporal relationship with the EVALI outbreak. For geography, the United States was divided into three zones (East, Central, and Mountain and West) to determine if regional differences were present (Fig 2). Similarly, states were divided into those where the use of recreational marijuana was legal by April 2019, which was the date of the first case of EVALI in this cohort. Finally, to determine if the imaging appearances of EVALI have changed after the initial outbreak of EVALI, comparisons were made between studies obtained before and after the CDC stopped collecting data on February 18, 2020.
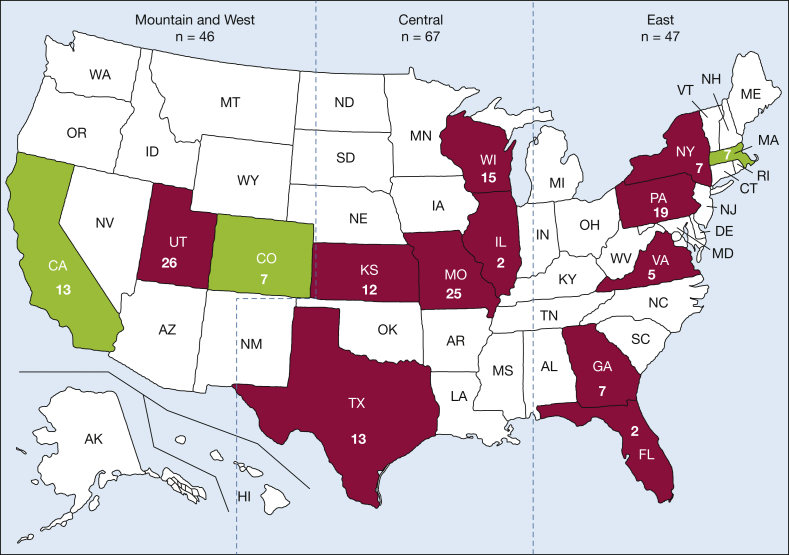
Figure 2.
Map of the United States showing distribution of cases from 15 institutions from 14 states with two institutions from California. Numerical values in each state correspond to the number of cases. States in green, amassing 27 e-cigarette or vaping-induced lung injury (EVALI) cases, are states where the recreational use of marijuana was legal at the start of the EVALI outbreak in April 2019. The states in red, representing 133 cases, did not have legal recreational marijuana use during the same time frame. Blue dotted lines represent division of the United States into three geographic regions (East, Central, and Mountain and West) for subgroup analysis.
The only outcomes data collected were whether the subject survived or died from EVALI. Although some subjects did undergo biopsy, pathologic diagnosis was not required for inclusion given CDC guidelines.
Statistical Analysis
All statistical analyses were performed in R software (version 3.6.3; R Foundation for Statistical Computing). To test associations between clinical and imaging variables with substance vaped and subjective severity grade measured on CT scan, we used one-way analysis of variance for continuous variables and χ2 test for tabular data. Post hoc analyses were performed using Bonferroni correction for multiple testing. Differences in the distribution of CT pattern and severity grade among states according to states where marijuana was legal vs illegal, US geographic region, and relation to COVID-19 pandemic (pandemic phase considered after February 18, 2020) were tested using χ2 test. Multivariable analyses were performed to test associations between age, sex, substance vaped, vaping frequency, vaping duration, and number of days since symptom onset with radiologic pattern and percentage of lung involved on CT scan as primary outcomes. Radiologic patterns were stratified as DAD, AEP, DAH, or OP, and logistic regression followed a one-vs-all approach. Linear regression modeling was used for evaluation of percentage of lung involved on CT scan as outcome variable. P < .05 was considered statistically significant.
Results
Subject Demographics and Vaping History
One hundred and seventy-four subjects meeting CDC criteria for EVALI were collected from 15 institutions in 14 states with two institutions in California (Fig 2). After exclusion of 14 subjects, a total of 160 subjects were included in the analysis (Fig 1, Table 1). Two patients had a history of treated malignancy but were not currently on chemotherapy or immunologic therapy. Of the 160 patients, two patients had inflammatory bowel disease (IBD), one with ulcerative colitis and the other with Crohn disease. However, neither patient had symptoms related to IBD at the time of evaluation, both were vaping tetrahydrocannabinol (THC) and nicotine derivatives up to the time of admission, and in both instances the treating clinical teams thought that the ALI was caused by vaping and not as a complication of IBD.
Of the 160 subjects, 127 were men (79.4%) and 33 were women (20.6%), with a mean age ± SD of 28.2 ± 11.2 years (median, 24; range, 15-68 years). One hundred and forty-six patients (91.3%) were admitted to the hospital for treatment and 14 (8.7%) were treated as outpatients. Seventy-seven subjects (48.1%) admitted to vaping only THC products, 15 (9.4%) admitted to vaping only nicotine products, and 68 (42.5%) admitted to vaping both. Table 3 lists substance vaped in relation to clinical information and common imaging findings.
Table 3.
Substance Vaped in Relation to Qualitative Severity and Common Imaging Findings
| Demographic or CT Variable | Substance Vaped (N = 160) |
P Valuea | ||
|---|---|---|---|---|
| THC and Nicotine (n = 68) | THC Only (n = 77) | Nicotine Only (n = 15) | ||
| Age, y | 25.5 ± 10.3 | 29.4 ± 10.9 | 33.6 ± 13.9 | NS |
| BMI, kg/m2 | 26.6 ± 6.9 | 26.1 ± 7.1 | 27.1 ± 7 | NS |
| Symptom duration | 11.4 ± 22.9 | 8.5 ± 8.9 | 17.5 ± 28.5 | NS |
| Qualitative severity (CT scan) | ||||
| Mild | 5 (7.4) | 17 (22.0) | 3 (20.0) | NS |
| Moderate | 41b(60.3) | 28 (36.4) | 6 (40.0) | .031 |
| Severe | 22 (32.3) | 32 (41.6) | 6 (40.0) | NS |
| Parenchymal attenuation | ||||
| GGO only | 35 (51.5) | 42 (54.5) | 5 (33.3) | NS |
| GGO > consolidation | 14 (20.6) | 19 (24.7) | 7 (46.7) | NS |
| Mixed | 16 (23.5) | 12 (15.6) | 2 (13.3) | NS |
| Consolidation > GGO | 3 (4.4) | 4 (5.2) | 1 (6.7) | NS |
| Parenchymal sparring | ||||
| Subpleural | 51 (75) | 63 (81.8) | 11 (73.3) | NS |
| Lobular | 41 (60.3) | 45 (58.4) | 9 (60.0) | NS |
| Peribronchovascular | 28 (41.2) | 31 (40.3) | 4 (26.7) | NS |
| Centrilobular nodules | 31 (45.6) | 24 (31.2) | 4 (26.7) | NS |
| Septal thickening | ||||
| Interlobular | 39 (57.4) | 34 (44.2) | 8 (53.3) | NS |
| Crazy paving | 16 (23.5) | 10 (13.0) | 4 (26.7) | NS |
| Lymphadenopathy | 43 (63.2) | 45 (58.4) | 12 (80) | NS |
| Pleural effusions | 13 (19.1) | 18 (23.4) | 4 (26.7) | NS |
Values are No. (%), mean ± SD, or as otherwise indicated. Bold denotes statistical significance. GGO = ground-glass opacity; NS = nonsignificant; THC = tetrahydrocannabinol.
After Bonferroni correction.
Variable with significant difference after Bonferroni correction.
CT scans of the 160 subjects were obtained between April 1, 2019, and June 30, 2020. Most (81 of 160) were diagnosed during the height of the EVALI outbreak from August 1, 2019, through October 31, 2019. However, 24 (15%) were diagnosed between February 18, 2020, through June 30, 2020, which is after the CDC stopped collected data on EVALI outbreak and coincides with the start of the COVID-19 pandemic (Fig 3). There was no difference in imaging patterns (P = .565) or qualitative severity of lung injury (P = .609) between studies obtained before and after February 18, 2020.
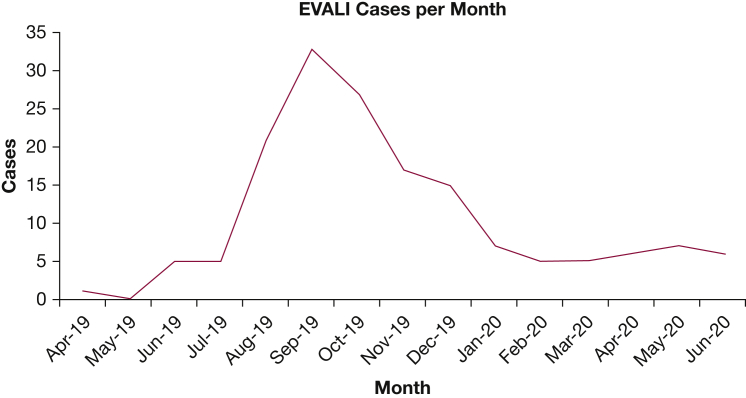
Figure 3.
Monthly breakdown of EVALI cases in the study. The slight majority of EVALI cases (81 of 160) occurred during the height of the EVALI outbreak from August 1, 2019, through October 31, 2019. However, 24 cases (15%) have been diagnosed after March 1, 2020, during the COVID-19 pandemic. EVALI = e-cigarette or vaping-induced lung injury.
In 96 subjects, both frequency and duration of vaping information were available. On logistic regression model, there was a negative association (β = −2.579 [SE,1.193], P = .03) between vaping for > 6 months and DAD pattern on CT scan compared with other CT patterns (Table 4). Patients who vaped daily had more extensive lung injury than those who vaped once per week or less (β = 1.054 [SE, 0.387], P < .008), and those who vaped multiple times per week had more severe injury than those who vaped once a week or less (β = 0.954 [SE, 0.429], P = .03). Otherwise, there were no significant associations between demographic and vaping-related variables (product, frequency, and duration) and CT findings or patterns (Table 4).
Table 4.
Multivariable Analysis of 96 Patients Including Complete Data About Vaping Habits and Demographic Covariates Against Radiologic Patterns and Percentage of Lung Involvement
| Demographic or Vaping History Variable | Dependent Variable |
||||
|---|---|---|---|---|---|
| DAD Pattern | AEP-Like Pattern | DAH Pattern | OP Pattern | % Lung Involvement | |
| Age | 0.0003 (0.04) | −0.057 (0.07) | 0.070 (0.05) | −0.012 (0.02) | −0.009 (0.01) |
| Sex (female vs male) | 0.196 (1.26) | 0.442 (1.3) | −16.912 (3,492.7) | 0.781 (0.89) | −0.117 (0.23) |
| Vaping THC | 16.608 (6,522.6) | 17.044 (17,730.4) | −3.960 (29,440.4) | −13.861 (1,455.4) | 0.237 (0.92) |
| Vaping nicotine | 17.437 (6,522.6) | −0.522 (18,521.2) | −1.826 (29,440.4) | −15,376 (1,455.4) | 0.351 (0.94) |
| Vaping THC and nicotine | −16.825 (6,522.6) | −0.417 (18,521.2) | 1.581 (29,440.4) | 15.385(1,455.4) | −0.399 (0.96) |
| Vaping multiple times per week (vs once per week or less) | 17.123 (2,452.1) | −19.316 (4,442.9) | −19,484 (6,474.4) | 1.000 (1.22) | 0.954 (0.43) (P = .03) |
| Vaping daily (vs once per week or less) | 16.098 (2,452.1) | −1,832 (1.45) | −1.892 (2.11) | 0.856 (1.04) | 1.054 (0.39) (P = .008) |
| Vaping ≥ 6 mo (vs < 6 mo) | −2.579 (1.19) (P = .03) | −0.581 (1.14) | 1.459 (1.62) | 0.984 (0.6) | −0.137 (0.2) |
| CT scan date in relation to symptom onset, d | 0.001 (0.03) | −0.016 (0.06) | −0.583 (0.55) | −0.001 (0.01) | 0.001 (0.004) |
Values are β (SE). Bold denotes statistical significance. AEP-like = acute eosinophilic-like pneumonia; DAD = diffuse alveolar damage; DAH = diffuse alveolar hemorrhage; OP = organizing pneumonia; THC = tetrahydrocannabinol.
Symptoms
Patient symptoms are listed in Table 1. There was no significant difference in chest and GI symptoms and the substance vaped or qualitative severity of injury. Similarly, there was no significant difference between severity of injury and substance vaped compared with when medical attention was sought. Interestingly, there was a significant difference in the presence of a documented fever and the substance vaped (P = .029), which was seen in 53 (68.8%) and 45 (66.2%) patients who vaped THC only and both THC and nicotine, respectively, but only in five (33.3%) of those who vaped only nicotine. However, there was no difference in fever and the severity of lung injury.
Common Imaging Findings
Most patients had axially and craniocaudally diffuse, symmetrical lung injury consisting predominantly of GGO (Table 1); however, the degree of consolidation significantly increased with more severe injury (P = .033). Twenty-five subjects’ scans (15.6%) were classified as mild, whereas 75 (46.9%) and 60 (37.5%) were qualitatively graded as either moderate or severe, respectively (Fig 4). Table 5 compares subjective severity grade to vaping history and common imaging findings.
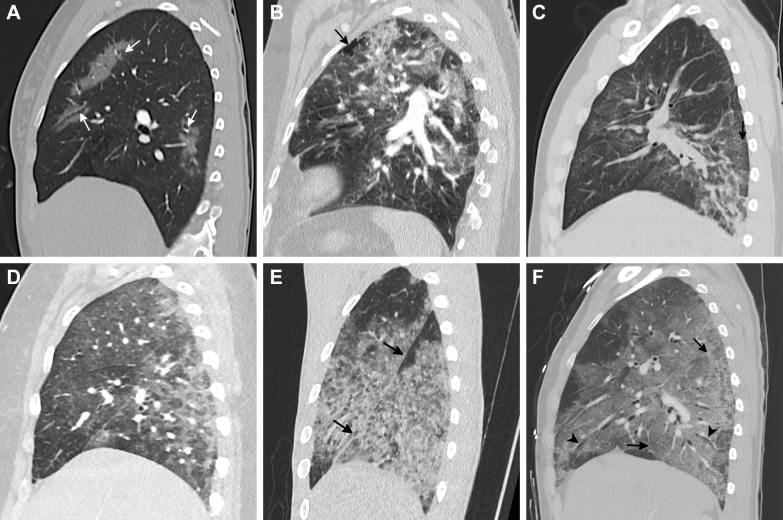
Figure 4.
A-C, Qualitative severity of e-cigarette or vaping-induced lung injury (EVALI). A, CT scan of a 40-year-old woman with a lower-lobe predominant organizing pneumonia (OP) pattern shows subpleural bands of ground-glass opacity (GGO) in the lower lobes. This injury was qualitatively graded as mild. B, CT scan of a 31-year-old man with a diffuse GGO OP pattern shows a qualitatively moderate lung injury with axially diffuse GGO, crazy-paving, and subpleural and lobular sparing. C, CT scan of a 36-year-old man who vaped both tetrahydrocannabinol and nicotine products daily shows a qualitatively severe injury with diffuse GGO with subpleural sparing. There is inferior mild displacement of the fissures (arrowhead) and areas of multifocal bronchial dilation (arrow). The patient was classified as having a diffuse alveolar damage pattern.
Table 5.
Qualitative Injury Severity in Relation to Vaping History and Common Imaging Findings
| Demographic, Vaping History, or CT Variable | Injury Severity on CT Scan (N = 160) |
P Valuea | ||
|---|---|---|---|---|
| Mild (n = 25) | Moderate (n = 75) | Severe (n = 60) | ||
| Age, y | 30.9 ± 13.4 | 27.7 ± 10.8 | 27.6 ± 10.6 | NS |
| BMI, kg/m2 | 22.7 ± 4.1 | 26.8 ± 7.4 | 27.4 ± 6.6 | NS |
| Symptom duration, d | 11.4 ± 18.1 | 11.7 ± 24.4 | 8.8 ± 7.8 | NS |
| Vaping frequency | ||||
| Daily | 12 (48.0) | 43 (57.3) | 37 (61.7) | NS |
| Multiple | 3 (25.0) | 11 (14.7) | 8 (13.3) | NS |
| Once a week | 2 (8.0) | 3 (4.0) | 2 (3.3) | NS |
| N/A | 8 (32.0) | 18 (24.0) | 13 (21.7) | |
| Vaping duration | ||||
| ≤ 6 mo | 7 (28.0) | 17 (22.7) | 18 (30.0) | NS |
| > 6 mo | 8 (32.0) | 34 (45.3) | 29 (48.3) | NS |
| N/A | 10 (40.0) | 24 (32.0) | 13 (21.7) | |
| Parenchymal attenuation | ||||
| GGO only | 22b(88.0) | 43 (57.3) | 17b(28.3) | < .001 (for both) |
| GGO > consolidation | 2 (8.0) | 16 (21.3) | 22 (36.7) | NS |
| Mixed | 1 (4.0) | 15 (20.0) | 14 (23.3) | NS |
| Consolidation > GGO | 0 (0.0) | 1 (1.3) | 7b(11.7) | .033 |
| Parenchymal sparing | ||||
| Subpleural | 17 (68.0) | 60 (80.0) | 48 (80.0) | NS |
| Lobular | 12 (48.0) | 45 (60.0) | 38 (63.3) | NS |
| Peribronchovascular | 3b(12.0) | 35 (46.7) | 26 (43.3) | .011 |
| Centrilobular nodules | 4 (16.0) | 30 (40.0) | 25 (41.7) | NS (.072) |
| Septal thickening | ||||
| Interlobular | 6b(24.0) | 40 (53.3) | 35 (58.3) | .022 |
| Crazy paving | 1 (4.0) | 18 (24.0) | 11 (18.3) | NS (.085) |
| Lymphadenopathy | 9b(36) | 53 (70.7) | 39 (65.0) | .013 |
| Pleural effusions | 2 (8) | 13 (17.3) | 20b(33.3) | .04 |
Values are mean ± SD, No. (%), or as otherwise indicated. Bold denotes statistical significance. GGO = ground-glass opacity; N/A = not available; NS = nonsignificant.
After Bonferroni correction.
Variable with significant difference after Bonferroni correction.
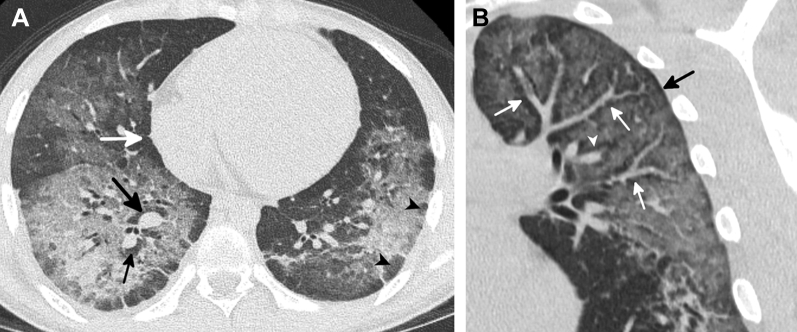
Areas of subpleural and lobular sparing occurred in most patients (Fig 5, Table 1). Presence of any type of sparing was not associated with substance vaped, and subpleural or lobular sparing was not associated with severity of injury (Table 5). There was a significant difference in the presence of peribronchovascular (PBV) sparing (Fig 4), which was seen in 64 subjects (40%), in relation to qualitative severity of lung injury on CT scan (P = .007) (Table 5), with significantly less PBV sparing in those with mild disease than those with moderate to severe injury (P = .011). Compared with those without, patients with PBV sparing were significantly younger (mean age, 25.1 ± 9.2 vs 29.9 ± 12.4 years; P = .016), an association not seen with other patterns of sparing.
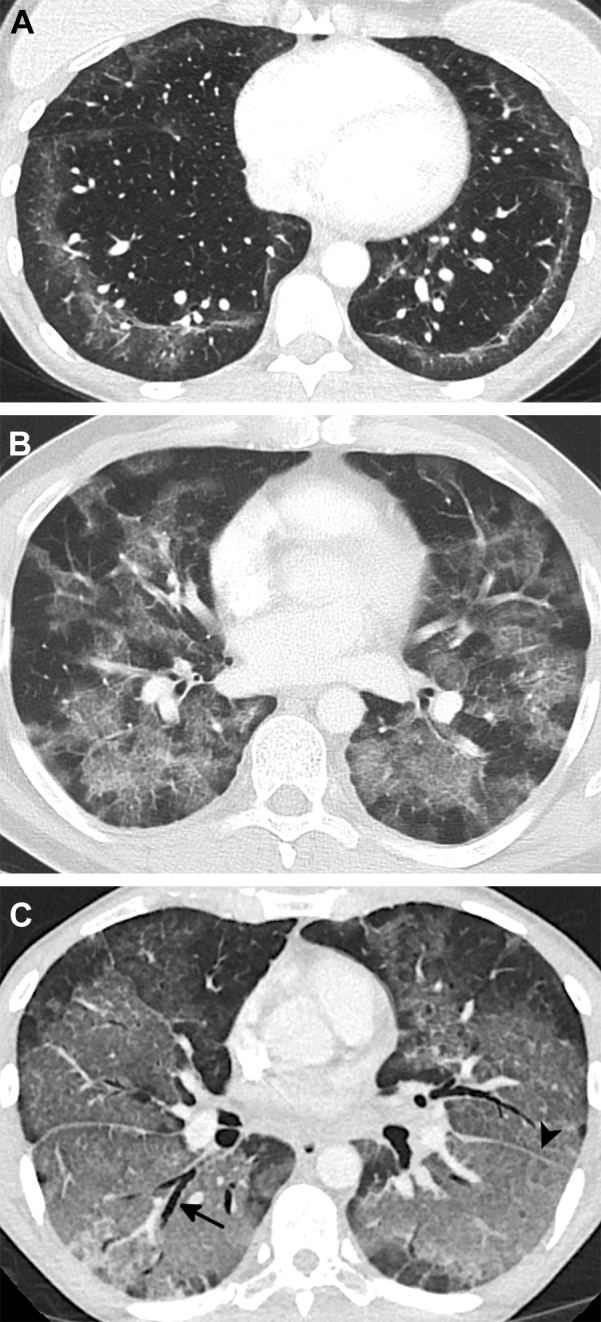
Figure 5.
A-B, Examples of patterns of sparing commonly seen in e-cigarette or vaping product use-associated lung injury. A, CT scan of a 16-year-old young man shows subpleural (white arrow) and lobular (arrowheads) areas of sparing. There is also peribronchovascular sparing around the right inferior pulmonary vein and right lower lobe basilar segmental artery (black arrows). A similar pattern of sparing was seen around numerous additional vessels. B, Coronal oblique reformatted CT image of a 39-year-old man shows pronounced sparing of the peribronchovascular interstitium surrounding the pulmonary artery branches (white arrows) and the pulmonary vein branches (arrowhead). A fine dark line around the periphery of the lung represents subpleural sparing (black arrow).
CNs were present in 59 subjects (36.9%). No other types of micronodules were present. There was no difference in the presence of CNs and the substance vaped (Table 5). Although CNs were more common in moderate and severe injuries than mild injuries, it did not reach significance (P = .072) (Table 5).
Interlobular septal thickening was a common finding occurring in 50.6% of subjects (81 of 160), whereas a crazy paving pattern, defined as GGO with superimposed interlobular septal thickening and visible intralobular lines (Fig 4B), was present in 30 (18.8%). There was a significant difference between the presence of interlobular septal thickening and injury severity (P = .013) because patients with mild injury had significantly less septal thickening than those with moderate and severe injury (P = .022). A trend toward the presence of a crazy paving pattern in more severe injury was present; however, it did not reach significance (P = .085) (Table 5). There was no difference between these findings and substance vaped (Table 4).
Pleural effusion was present in 35 subjects (21.9%) with no difference in age between subjects with (29.2 ± 11.9 years) and without (27.7 ± 11 years) effusions (P = .49). Effusions were qualitatively trace in most patients except those with the AEP-like pattern where the effusions were small to moderate. There was a significant difference between the presence of pleural effusion and qualitatively severe lung injury (P = .016) (Table 5) with effusions being significantly more common in those with severe injury (P = .04). There was no difference in effusions and the substance vaped (Table 4).
Lymphadenopathy (LAD), defined as a short axis measurement ≥ 1 cm, was a common finding occurring in 101 of 160 subjects (63.1%). There was a significant difference between severity of lung injury and LAD (P = .007) (Table 5), which was significantly less common in those with milder disease (P = .013), but not between LAD and substance vaped.
Uncommon Findings
Uncommon findings are listed in Table 1. Twelve subjects had emphysema (Fig 5B) with a mean age of 45.8 ± 10.5 years, which was significantly older than the rest of the cohort (26.7 ± 10 years, P < .001).
Patterns
Based on imaging findings, 156 of 160 subjects (97.5%) were categorized into one of six patterns and four of 160 (2.5%) were categorized as atypical. The three most encountered CT patterns, comprising 69.4% of the entire cohort, were diffuse parenchymal OP pattern (n = 41, 25.6%), lower-lobe predominant parenchymal OP pattern (n = 38, 23.8%), and mixed OP pattern (n = 32, 20%). Imaging patterns in relation to age, substance vaped, and vaping history are shown in Table 6. There were no cases where a consensus pattern could not be agreed on between radiologists.
Table 6.
Imaging Pattern in Relation to Substance Vaped and Vaping History
| Pattern | Age (y) | Vaped Substance |
Vaping Frequency |
Vaping Duration |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| THC Only | Nicotine Only | Both | Daily | Multiple Times a Week | Once a Week or Less | N/A | ≥ 12 mo | > 6 to < 12 mo | 1-6 mo | < 1 mo | N/A | ||
| Parenchymal OP | |||||||||||||
| Upper (n = 5) | 47.2 | 3 (60) | 1 (20) | 1 (20) | 2 (40) | 0 (0) | 1 (20) | 2 (40) | 1 (20) | 0 (0) | 2 (40) | 0 (0) | 2 (40) |
| Lower (n = 38) | 26.2 | 19 (50) | 3 (7.9) | 16 (42.1) | 22 (57.9) | 5 (13.2) | 2 (5.3) | 9 (23.7) | 17 (44.7) | 2 (5.3) | 7 (18.4) | 3 (7.9) | 9 (23.7) |
| Diffuse GGO (n = 32) | 27.9 | 18 (56.3) | 3 (9.4) | 11 (34.4) | 16 (50.0) | 6 (18.8) | 1 (3.1) | 9 (28.1) | 13 (40.6) | 3 (9.4) | 5 (15.6) | 1 (3.1) | 10 (31.3) |
| Diffuse consolidative (n = 9) | 30.7 | 5 (55.6) | 0 (0) | 4 (44.4) | 7 (77.8) | 0 (0) | 0 (0) | 2 (22.2) | 5 (55.6) | 1 (11.1) | 0 (0) | 1 (11.1) | 2 (22.2) |
| Patchy (n = 5) | 30.2 | 3 (60) | 1 (20) | 1 (20.0) | 2 (40.0) | 0 (0) | 0 (0) | 3 (60) | 0 (0) | 0 (0) | 0 (0) | 1 (20.0) | 4 (80) |
| Parenchymal OP total (n = 89) | 30.5 | 48 (53.9) | 8 (9.0) | 33 (39.3) | 49 (55.0) | 11 (12.4) | 4 (4.5) | 25 (28.1) | 36 (40.4) | 6 (6.7) | 14 (15.7) | 6 (6.7) | 27 (30.3) |
| Airway-centered OP (n = 14) | 28.5 | 5 (35.7) | 1 (7.1) | 8 (57.1) | 9 (64.3) | 2 (14.3) | 0 (0) | 3 (21.4) | 4 (28.6) | 0 (0) | 2 (14.2) | 1 (7.1) | 7 (50) |
| Mixed OP (n = 32) | 24.4 | 15 (46.9) | 2 (6.2) | 15 (46.9) | 21 (65.6) | 5 (15.6) | 1 (3.1) | 5 (15.6) | 14 (43.8) | 3 (9.4) | 8 (25.0) | 0 (0) | 7 (21.9) |
| All OP total (n = 135) | 28.3 | 68 (50.4) | 11 (8.1) | 56 (41.5) | 79 (58.6) | 18 (13.3) | 5 (3.7) | 33 (24.4) | 54 (40) | 9 (6.7) | 24 (17.8) | 7 (5.2) | 41 (30.4) |
| Diffuse alveolar damage (n = 9) | 31.6 | 4 (44.9) | 1 (11.1) | 4 (44.9) | 4 (44.9) | 2 (33.3) | 0 (0) | 3 (33.3) | 1 (11.1) | 0 (0) | 5 (55.6) | 1 (11.1) | 2 (33.3) |
| AEP-like (n = 6) | 22.5 | 3 (50.0) | 0 (0) | 3 (50.0) | 3 (66.7) | 1 (16.7) | 1 (16.7) | 1 (16.7) | 2 (33.3) | 1 (16.7) | 2 (33.3) | 0 (0) | 1 (16.7) |
| Diffuse alveolar hemorrhage (n = 6) | 30.5 | 1 (16.7) | 2 (33.3) | 3 (50.0) | 4 (66.7) | 1 (16.7) | 1 (16.7) | 0 (0) | 3 (50.0) | 0 (0) | 0 (0) | 1 (16.7) | 2 (33.3) |
| Atypical (n = 4) | 39.5 | 1 (25.0) | 1 (25.0) | 2 (50.0) | 2 (50.0) | 0 (0) | 0 (0) | 2 (50) | 1 (25.0) | 0 (0) | 2 (50.0) | 0 (0) | 1 (25) |
| Total (N = 160) | 28.2 | 77 (48.1) | 15 (9.4) | 68 (42.5) | 92 (57.5) | 22 (13.8) | 7 (4.4) | 39 (24.4) | 61 (38.2) | 10 (6.2) | 33 (20.6) | 9 (5.6) | 47 (29.4) |
Values are No. (%) or as otherwise indicated. AEP-like = acute eosinophilic-like pneumonia; N/A = not available; OP = organizing pneumonia; THC = tetrahydrocannabinol.
OP Patterns
Of the 160 subjects, 135 (84.4%) had an OP pattern: 89 (55.6%) with a parenchymal OP pattern, 14 (8.8%) with an airway-centered OP pattern, and 32 (20%) with a mixed OP pattern. Those with upper lobe disease were significantly older than those with all other OP patterns (P range, .043 to < −.001) except for those with patchy disease (P = .14)
Eighty-nine subjects (55.6%) had a parenchymal OP pattern (Table 6), which was classified as patchy in five (3.1%) (Fig 6A), upper lobe in five (3.1%) (Fig 6B), lower lobe in 38 (23.8%) (Fig 6C), and diffuse in 41 (25.6%). Of the 41 diffuse OP cases, 32 (20%) were subclassified as a GGO OP pattern (Fig 6D) and nine (5.6%) as a diffuse consolidative OP pattern (Fig 6E).
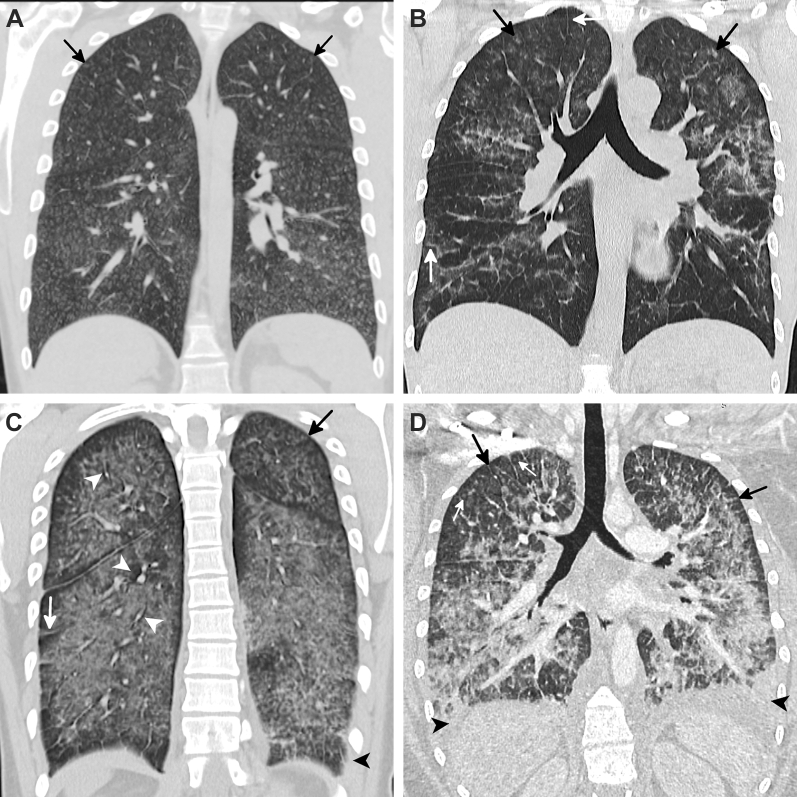
Figure 6.
A-F, Spectrum of parenchymal injury patterns in e-cigarette or vaping product use-associated lung injury. A, Sagittal reformatted CT image of a patchy organizing pneumonia (OP) pattern in a 19-year-old woman who vaped tetrahydrocannabinol (THC) daily shows a qualitatively mild injury. There are multiple areas of peribronchovascular sparing in the patchy foci of ground-glass opacity (GGO) (arrows). B, Sagittal reformatted CT image of an upper lobe predominant OP pattern in a 59-year-old woman who vaped nicotine daily. There is an associated focus of paraseptal emphysema (arrow). C, Sagittal reformatted CT image of a lower lobe predominant OP pattern in a 31-year-old man who vaped THC products multiple times per week shows a qualitatively moderate injury with a few areas of consolidation and mild septal thickening (arrow). Although the lower-lobe parenchymal OP pattern still involves all five lobes in nearly all patients, the lower lobes are more severely affected. D, Sagittal reformatted CT image of a diffuse GGO OP pattern in a 19-year-old woman who vaped THC oil daily shows a qualitatively severe injury with diffuse craniocaudal GGO with areas of subpleural and lobular sparing. E, Sagittal reformatted CT image of a diffuse consolidative OP pattern in a 19-year-old man who vaped both THC and nicotine products shows a qualitatively severe injury with a greater degree of consolidation compared with GGO. There is no fissural displacement (arrows) and no airway dilation. It is unclear whether this represents a severe form of OP or an early exudative phase of diffuse alveolar damage (DAD). F, Sagittal reformatted CT image of a DAD pattern in a 25-year-old man who vaped THC daily shows diffuse GGO with inferior displacement of the right major fissure (arrows) and lack of tapering of the subsegmental bronchi, which are mildly dilated (arrowheads). He died 3 weeks later.
The mixed OP pattern was the third most common pattern with 32 cases (20%) (Fig 7B). CNs in all 32 subjects were ill-defined and confined to the upper lobes in 26 subjects (81.3%), whereas they were diffuse in six subjects (18.8%). The mean age of 24.4 ± 8.5 years was the second youngest after those with the AEP-like pattern. Fourteen subjects (10.3%) had an airway-centered OP pattern (Fig 7A).
Figure 7.
A-D, Spectrum of lung injury associated with centrilobular nodules (CNs) in e-cigarette or vaping product use-associated lung injury. A, Coronal reformatted CT image of an airway-centered organizing pneumonia (OP) pattern in a 24-year-old man who vaped nicotine and tetrahydrocannabinol (THC) products daily shows extensive ill-defined CNs (arrows) with only a small amount of ground-glass opacity (GGO) at the bases. Subsequent biopsy showed airway-centered OP. B, Coronal reformatted CT image of a mixed OP pattern in a 40-year-old man who vaped nicotine and THC products multiple times per week shows multifocal GGO with upper lobe CNs (black arrows). Mild septal thickening is also present (white arrow). C, Coronal reformatted CT image of an acute eosinophilic-like pneumonia (AEP-like) pattern in a 33-year-old man who vaped THC products daily shows diffuse GGO with pronounced septal thickening (white arrow), subpleural sparing, and peribronchovascular sparing (white arrowheads). There are numerous upper lobe CNs (black arrow) and small pleural effusions (black arrowhead). This injury likely exists on a continuum between those seen in Figures 7A and 7B and the more typical AEP-like pattern seen in Figure 7D. D, Coronal reformatted CT image of an AEP-like pattern in a 24-year-old woman who vaped THC daily shows diffuse GGO intermixed with areas of consolidation, extensive septal thickening (arrows), moderate pleural effusions (arrowheads), and upper lobe CNs.
AEP-Like Pattern
Six subjects (3.8%), all with severe injury, were categorized as having an AEP-like pattern (Figs 7C, 7D). All patients also had underlying imaging findings similar to the mixed OP pattern with GGO and CNs. These patients were among the youngest in the cohort (mean age, 22.5 ± 6 years); however, this did not reach significance (P = .20). However, when combined with the mixed OP pattern, which shared numerous imaging findings, the mean age of the patients with these two patterns (24.5 ± 8.1 years) was significantly younger than the remainder of the cohort (28.4 ± 11.3 years, P = .0498). Four of the six cases with an AEP-like pattern underwent bronchoscopy. However, none had subsequent pathologic confirmation of AEP.
DAD Pattern
Nine subjects (5.6%) with a severe lung injury and findings of volume loss were categorized as having a DAD pattern (Figs 4C, 6F). Of all the patterns, DAD was the only pattern where relatively newer vapers were more likely to develop the injury (P = .03).
DAH Pattern
DAH occurred in six subjects (3.8%) (Fig 8). Subjects with DAH sought medical attention sooner than those with other injury patterns (2.5 ± 1.8 vs 10.9 ± 19.2 days, P = .28). Septal thickening, acinar ground-glass CNs, and asymmetrical distribution were common in DAH.
Figure 8.
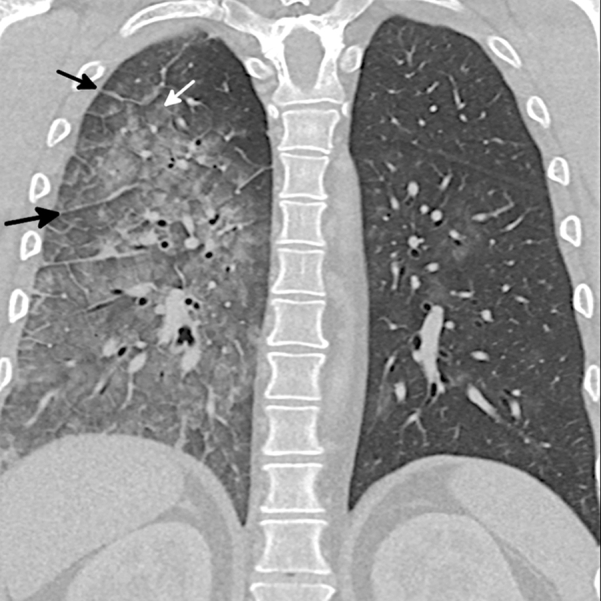
Diffuse alveolar hemorrhage (DAH) pattern in e-cigarette or vaping product use-associated lung injury. Coronal reformatted CT image of a 42-year-old man presenting with hemoptysis shows asymmetrical ground-glass opacity predominantly on the right with septal thickening (black arrows) and ill-defined ground-glass acinar nodules (white arrow) that fill most of the secondary lobule, a finding common in DAH. BAL confirmed the diagnosis, and the patient rapidly improved with steroids. DAH was the only pattern that was commonly asymmetrical.
Atypical Pattern
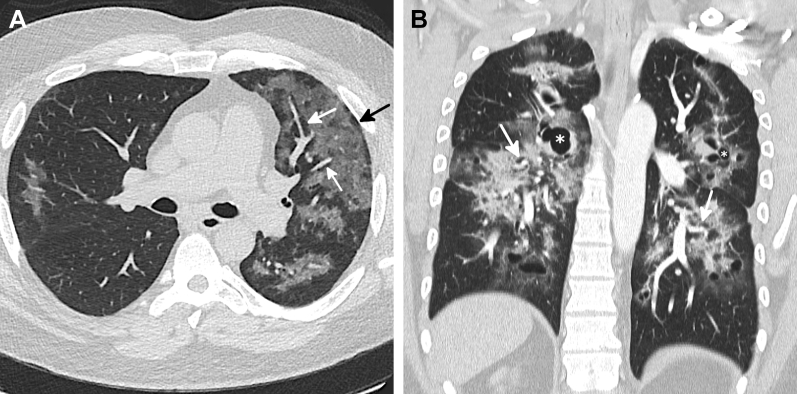
Only four subjects (2.5%) were categorized with an atypical pattern, three of which were caused by pronounced asymmetrical disease (Fig 9A). The fourth was a 36-year-old man who was symptomatic for 4 months before seeking treatment. His CT scan showed a diffuse OP pattern but with numerous parenchymal cysts measuring up to 36 mm (Fig 9B), a finding which has been reported on follow-up imaging in a patient with EVALI.11
Figure 9.
A-B, Atypical patterns in e-cigarette or vaping-induced lung injury (EVALI). A, CT image of a 49-year-old man who vaped tetrahydrocannabinol (THC) and nicotine shows common imaging findings associated with a diffuse organizing pneumonia (OP) pattern of EVALI in the left lung with diffuse ground-glass opacity (GGO) with subpleural (black arrow) and peribronchovascular (PBV) sparing (white arrows) with only minimal involvement of the right lung. B, Coronal reformatted CT image of a 36-year-old man who vaped nicotine presenting with dyspnea and cough for nearly 4 months shows a diffuse GGO OP pattern with PBV sparing (arrows). Additionally, there are numerous cystic spaces, some with surrounding foci of consolidation (asterisks), which led to the atypical classification. The cysts are likely caused by areas of parenchymal destruction and have been reported.
Regional Patterns of EVALI Based on Geography and Recreational Legal Status
After dividing the country into three zones, 47 patients lived in the Eastern region, 67 lived in the Central region, and 46 lived in the Mountain and West region (Fig 2). There was no difference in CT pattern (P = .656) or severity of lung injury (P = .129) between the geographic regions. Of the 15 sites that contributed patients for this study, four (Massachusetts, Colorado, and two in California) were in states where marijuana was recreationally legal as of April 2019. When comparing the imaging patterns (P = .227) and severity of injury (P = .118) between the 27 cases of EVALI from states where marijuana was recreationally legal with the 133 cases in states where it was illegal, there was no significant difference.
Mortality
Two subjects with a DAD pattern died during hospitalization, a 36-year-old woman and 26-year-old man who both vaped THC products daily. No other subjects died from EVALI for an overall cohort mortality rate of 1.25%.
Discussion
In this retrospective cohort study, we describe the CT imaging appearances of 160 subjects diagnosed with EVALI, the largest cohort collected to date. After detailed imaging review, it becomes clear that the toxic inhalational injury of EVALI most commonly exists along a spectrum of diffuse lung injury ranging from OP to DAD. This coincides with pathologic studies of EVALI showing a similar spectrum of ALI and sub-ALI ranging from OP to acute and fibrinous organizing pneumonia (AFOP) to DAD.8,12,21, 22, 23 AFOP, a histologic pattern of fibrin balls and OP in the alveolar spaces, can manifest with either a milder subacute course or severe fulminant course with imaging appearances indistinguishable from OP and DAD, respectively.13
Although lung injury in EVALI exists along a continuum, imaging appearances vary. This is not surprising given heterogeneity of the chemical compositions of the vaped substances, design and power of e-cigarettes affecting particle size, end user variables (eg, puff volume, duration, flow rate, vape sessions per day per week, number of vapes per session), and end user susceptibility to lung injury based on genetics and underlying pulmonary conditions.11 Given the heterogeneity, we attempted to describe common and uncommon patterns to help with recognition and diagnosis.
Nearly 70% of the cohort presented with a diffuse (25.6%), lower lobe predominant (23.8%), or mixed (20%) OP pattern, which are commonly reported patterns not only in EVALI but also are associated with a variety of acute chemical inhalation injuries.8, 9, 10, 11, 12,24, 25, 26, 27, 28, 29, 30 Although most subjects had a pattern suggestive of OP, nine subjects (5.6%) with severe diffuse lung injury and findings of alveolar collapse were classified as having DAD. Although there was no association with the DAD pattern and substance vaped, DAD was the only pattern where most patients had been vaping < 6 months. The exact reason for this is unclear; it may be related to the vaping of newer, untested products, increased experimentation, or possibly an exaggerated immunologic response to inhalation injury in naïve lungs.
Of the nine subjects with DAD, two died during hospitalization. These were the only instances of mortality in the cohort, resulting in an overall mortality rate of 1.25% (two of 160). The mortality rate in our study was lower than the 2.4% mortality reported by the CDC (68 of 2,807).2,3 In part, this may be because of the CDC counting both in-hospital and postdischarge mortality.
CNs were present in 36.3% of the subjects. In 20%, upper lobe predominant, ill-defined CNs were associated with parenchymal opacities creating a mixed pattern. Given similar imaging findings, it was initially thought that this pattern of EVALI was secondary to hypersensitivity pneumonitis. However, this has not been shown pathologically. Instead, CNs in patients with EVALI reflect bronchiolocentric injury with areas of OP or AFOP centered around the respiratory bronchioles.21,22 In most instances, OP and AFOP extend beyond the respiratory bronchiole into the surrounding alveolar spaces, leading to associated GGO or consolidation. Interestingly, very similar imaging patterns have been described in cases of toxic chemical inhalational injuries including chlorine gas25 and nitrogen dioxide,29 further supporting that EVALI is a toxic inhalational injury rather than a hypersensitivity reaction.
In approximately 10%, the injury is strikingly bronchiolocentric, creating a pattern of diffuse CNs on CT scan with little associated parenchymal opacity. Three patients in the cohort with this pattern underwent lung biopsy showing airway-centered OP or AFOP. Interestingly, this pattern of bronchiolocentric injury has been described both on imaging and pathology in patients smoking synthetic marijuana, a common contaminant in illegal and counterfeit THC vapes.31 However, although synthetic marijuana contamination may play a role, other causes are likely because one patient with this pattern admitted to vaping only nicotine products.
Septal thickening is a common finding in ALI and reflects septal edema and interstitial inflammation from pneumocyte death and capillary leakage.13 Over one-half of the subjects in the cohort had septal thickening on CT scan, which was associated with more severe disease. In six subjects, the pronounced septal thickening with pleural effusions superimposed on a qualitatively severe injury mimicked the imaging appearance of AEP, an ALI associated with smoking.32 In our study, no patient with this pattern had bronchoscopic findings to support the diagnosis of AEP. Furthermore, all subjects with this pattern had an underlying mixed OP pattern. Therefore, in most instances, this AEP-like injury likely does not actually represent AEP but rather a forme fruste of the mixed OP pattern with pronounced septal edema and interstitial inflammation. However, two patients in our study did not undergo bronchoscopic exclusion of AEP, and there have been two case reports of AEP associated with the use of e-cigarettes.33,34
Areas of subpleural and perilobular sparing are common in ALI and sub-ALI,13 and these findings were seen in 78% and 59% of subjects, respectively. Lobular sparing, especially in cases of OP, is secondary to the zonal nature of the injury.13 Subpleural sparing is likely related to lymphatic clearance given the presence of well-defined lymphatic channels along the subpleural interstitium.35 An interesting finding in this study was conspicuous PBV sparing, present in 40% of subjects. To our knowledge, this pattern of sparing has not been described in ALI or sub-ALI. This imaging finding also likely results from well-developed afferent lymphatic drainage along the pulmonary veins, bronchi, and pulmonary arteries.35 Patients with PBV sparing were significantly younger than those without, an association not seen with other patterns of sparing. Although the exact reason for this is not known, it may be related to known age-related declines in lymphatic drainage.36 Therefore, its presence may be related to the relatively young age of the cohort rather than the cause of the injury itself.
Although the CDC stopped tracking EVALI in February 2020, the disease has not disappeared. In this cohort, 15% of cases occurred after the start of the COVID-19 pandemic, a finding echoed in recent publications.5 Given that COVID-19 pneumonia also causes ALI, COVID-19 pneumonia and EVALI have overlapping imaging manifestations. Although distinguishing these entities may not always be possible, some imaging findings are discriminatory. The presence of CNs, seen in 35.6% of the subjects with EVALI, is uncommon with COVID-19-related pneumonia.37 Additionally, PBV sparing, occurring in 40% of the subjects, has not yet been described with COVID-19 pneumonia. LAD occurs in > 60% of EVALI CT scans. Although early studies suggested LAD is quite rare in COVID-19-related pneumonia,38, 39, 40 a 5.4% incidence was reported in a recent large meta-analysis.41 Given the different treatment paradigms for the two, accurate distinction, if possible, is important to optimize patient care.
Numerous additional manifestations of vaping-associated lung disease were described early in the outbreak but were not used in this research. These included, but were not limited to, hypersensitivity pneumonitis,9,10 respiratory bronchiolitis (RB),42 and giant cell interstitial pneumonia.9,43 With improved radiologic-pathologic correlation of disease, it is now known that many of these are likely not part of the spectrum of lung injury in EVALI. In addition to hypersensitivity pneumonitis, which has been previously discussed, RB is another cause of upper lobe predominant ground-glass CNs on CT scan.44 This radiologic pattern with pathologic confirmation has only been described in one case report.42 Unfortunately, the patient in this case report also smoked 10 traditional cigarettes per day in addition to vaping. It is known that pathologic RB occurs in a very large percentage of active smokers and may persist for years after a patient has quit smoking.45,46 Therefore, the findings in this case were likely caused by the patient’s current smoking habits rather than vaping. To our knowledge, pathologic RB has not been shown in vapers who have never smoked cigarettes.
In one case report, giant cell interstitial pneumonia caused by hard metal pneumoconiosis was discovered on biopsy in a patient who vaped marijuana.43 The patient had slowly progressive shortness of breath over many months and her CT scan showed fibrotic lung disease composed of GGO, reticulation, and traction bronchiectasis, which remained stable for > 2 years. Analysis of her vape fluid revealed high levels of cobalt, which was confirmed to be the cause of the lung injury after detailed testing. Although this chronic, fibrotic injury is distinct from the more acute and subacute cases of EVALI, it highlights two important points. First, the chronic lung injuries that may be associated with vaping have yet to be fully recognized and need to be researched. Second, the heterogeneity of substances put into vaping liquids suggests that unrecognized imaging and pathologic patterns will likely be encountered in the future.
The presence of lipoid pneumonia in EVALI is a topic of debate given the presence of lipid-laden macrophages and vitamin E acetate on BAL specimens in a large percentage of patients with EVALI.1,47,48 However, as previously discussed, lipid-laden macrophages are common in numerous causes of ALI, the areas of parenchymal fat attenuation commonly associated with but not diagnostic of exogenous lipoid pneumonia on CT scan have not been seen with EVALI, and to date no pathologic case of EVALI has shown histology evidence of classic exogenous lipoid pneumonia.11,18,21,49 Nonetheless, given that exogenous lipoid pneumonia typically occurs because of the aspiration of large droplets of oil, whereas in vaping there is vaporization and inhalation of oil microparticles, some have argued that this injury represents a new variant of lipoid pneumonia.50
Given the known association of vaping synthetic cannabinoids with the development of airway-centered OP,31 one could expect to see an increased incidence of the mixed and airway-centered OP patterns in states where recreational marijuana is illegal, given the known lacing of black market vapes with these substances.51 Similarly, one could expect to see regional differences in imaging patterns based on local availability of supply. However, there was no significant difference in imaging patterns or severity of injury when states were divided based on the legal status of recreational marijuana or geography. Unfortunately, the brand of substance vaped and location of purchase was unknown in most situations, and we cannot confirm that substances vaped in a recreationally legal state were purchased from a state-regulated marijuana dispensary nor do we know the chemical composition of any vape purchased. Similarly, in states where marijuana is recreationally illegal, THC derivatives may have been purchased legally for medical use or possibly purchased from a licensed dispensary in a legal state and transported across state borders. Despite this, given the limited responses of the lung and airways to inhalational injury and marked heterogeneity of the chemical compositions seen in vapes, one should be aware of all potential patterns irrespective of legality and geography.
Our study has several limitations. First, EVALI is a diagnosis of exclusion. It is possible that some subjects may have had an undiagnosed underlying infection or inflammatory condition. However, all patients were treated at a tertiary academic center, underwent an extensive workup to exclude other causes of lung injury, and met CDC criteria for EVALI. Although we describe various imaging patterns with EVALI and attempt to associate them with known pathologic entities, most of the cases lacked pathologic confirmation. This is because once the clinical diagnosis of EVALI is established, most subjects rapidly improve with supportive therapy and possible corticosteroids, negating the need for biopsy. Our study is also subject to image-based selection bias because there may be imaging manifestations of EVALI yet to be recognized. Some of our imaging parameters, including severity of injury and degree of lung involvement, were qualitatively assessed. EVALI manifests along a spectrum of diffuse lung injury, and different pathologic patterns of injury, sometimes with similar imaging patterns, may coexist. Therefore, although all findings and patterns were agreed on by consensus among fellowship-trained thoracic radiologists, patients with EVALI may have imaging patterns with overlapping findings. Because imaging patterns were agreed on by consensus, kappa values and other statistics assessing level of agreement were not measured. However, there were no instances where a consensus could not be reached. Vaping history including substances vaped, frequency, and duration were extracted from the patients’ electronic medical record. It is unclear if all subjects were being truthful regarding this history. Regarding geography, the country was divided longitudinally into three zones, and this may not truly represent regional differences in patterns and severity. Although we do not think this to be the case, the inclusion of more sites in various states may have revealed regional differences. We only evaluated the initial chest CT scan. Although many subjects underwent subsequent imaging, analysis of those findings was beyond our scope. We also did not directly compare imaging findings of EVALI with those of COVID-19 pneumonia. Both final points are avenues for further research.
Interpretation
Previous literature has suggested that imaging findings in patients with EVALI may result from a broad range of underlying pathologic mechanisms. The results presented in this paper confirm that most patients with EVALI have CT imaging findings along a spectrum ranging from OP to DAD, which can mimic causes of ALI because of various causes. These include diffuse or lower lobe predominant GGO with or without consolidation that may demonstrate areas of subpleural or lobular sparing. As such, vaping exposure should be included in the clinical history of all patients with CT findings of ALI without another known cause, particularly if they are young and otherwise healthy. Furthermore, some patients in this EVALI cohort demonstrated CT findings less commonly seen in other etiologies of ALI including superimposed upper lobe predominant CNs and PBV sparing. When these findings are present in conjunction with the more typically seen manifestations of ALI, suspicion for EVALI should be elevated.
Take-home Points.
Study Question: What are the frequencies of imaging findings and CT patterns in e-cigarette or vaping-induced lung injury (EVALI) and what is the relationship to vaping behavior?
Results: Our multicenter study shows that most patients with EVALI have CT imaging findings along a spectrum ranging from organizing pneumonia to diffuse alveolar damage which can mimic causes of acute lung injury because of various causes. These include diffuse or lower lobe predominant ground-glass opacity with or without consolidation that often demonstrate areas of subpleural, or lobular, or peribronchovascular sparing. Upper lobe predominant centrilobular nodules are also common. Increased frequency of vaping was associated with more extensive lung injury, and newer vapers have an increased likelihood of developing diffuse alveolar damage compared with those who have been vaping > 6 months.
Interpretation: Vaping exposure should be included in the clinical history of all patients with CT findings of acute lung injury without another known cause, particularly if they are young and otherwise healthy.
Acknowledgments
Author contributions: S. J. K. is the guarantor of the content of the manuscript, including the data and analysis. All authors have provided substantial contributions to the following: conception or design of the work, or the acquisition, analysis, or interpretation of data for the work; drafting of the work or revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Financial/nonfinancial disclosures: None declared.
Other contributions: We thank Howard Mann, MBBCh, Professor of Radiology at the University of Utah, for his assistance with this paper.
Footnotes
FUNDING/SUPPORT:The authors have reported to CHEST that no funding was received for this study.
References
- 1.Layden J.E., Ghinai I., Pray I. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin - final report. N Engl J Med. 2020;382(10):903–916. doi: 10.1056/NEJMoa1911614. [DOI] [PubMed] [Google Scholar]
- 2.Werner A.K., Koumans E.H., Chatham-Stephens K. Hospitalizations and deaths associated with EVALI. N Engl J Med. 2020;382(17):1589–1598. doi: 10.1056/NEJMoa1915314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention Outbreak of lung injury associated with the use of e-cigarette, or vaping, products. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html
- 4.Ansari-Gilani K., Petraszko A.M., Gilkeson R.C. COVID-19 pneumonia versus EVALI, distinguishing the overlapping CT features in the COVID-19 era. Heart Lung. 2020;49(6):885–886. doi: 10.1016/j.hrtlng.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armatas C., Heinzerling A., Wilken J.A. Notes from the field: e-cigarette, or vaping, product use-associated lung injury cases during the COVID-19 response - California, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25):801–802. doi: 10.15585/mmwr.mm6925a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaiha S.M., Cheng J., Halpern-Felsher B. Association between youth smoking, electronic cigarette use, and COVID-19. J Adolesc Health. 2020;67(4):519–523. doi: 10.1016/j.jadohealth.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li D., Croft D.P., Ossip D.J., Xie Z. The association between statewide vaping prevalence and COVID-19. Prev Med Rep. 2020;20:101254. doi: 10.1016/j.pmedr.2020.101254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bin Saeedan M., MacMurdo M.G., Mukhopadhyay S., Choi H., Parkar N., Ghosh S. Radiologic review with pathology correlation of e-cigarette or vaping product use-associated lung injury. J Thorac Imaging. 2020;35(5):277–284. doi: 10.1097/RTI.0000000000000526. [DOI] [PubMed] [Google Scholar]
- 9.Henry T.S., Kanne J.P., Kligerman S.J. Imaging of vaping-associated lung disease. N Engl J Med. 2019;381(15):1486–1487. doi: 10.1056/NEJMc1911995. [DOI] [PubMed] [Google Scholar]
- 10.Henry T.S., Kligerman S.J., Raptis C.A., Mann H., Sechrist J.W., Kanne J.P. Imaging findings of vaping-associated lung injury. AJR Am J Roentgenol. 2020;214(3):498–505. doi: 10.2214/AJR.19.22251. [DOI] [PubMed] [Google Scholar]
- 11.Kligerman S., Raptis C., Larsen B. Radiologic, pathologic, clinical, and physiologic findings of electronic cigarette or vaping product use-associated lung injury (EVALI): evolving knowledge and remaining questions. Radiology. 2020;294(3):491–505. doi: 10.1148/radiol.2020192585. [DOI] [PubMed] [Google Scholar]
- 12.MacMurdo M., Lin C., Saeedan M.B. e-cigarette or vaping product use-associated lung injury: clinical, radiologic, and pathologic findings of 15 cases. Chest. 2020;157(6):e181–e187. doi: 10.1016/j.chest.2020.01.030. [DOI] [PubMed] [Google Scholar]
- 13.Kligerman S.J., Franks T.J., Galvin J.R. From the radiologic pathology archives: organization and fibrosis as a response to lung injury in diffuse alveolar damage, organizing pneumonia, and acute fibrinous and organizing pneumonia. Radiographics. 2013;33(7):1951–1975. doi: 10.1148/rg.337130057. [DOI] [PubMed] [Google Scholar]
- 14.Tazelaar H.D., Linz L.J., Colby T.V., Myers J.L., Limper A.H. Acute eosinophilic pneumonia: histopathologic findings in nine patients. Am J Respir Crit Care Med. 1997;155(1):296–302. doi: 10.1164/ajrccm.155.1.9001328. [DOI] [PubMed] [Google Scholar]
- 15.Jeong Y.J., Kim K.I., Seo I.J. Eosinophilic lung diseases: a clinical, radiologic, and pathologic overview. Radiographics. 2007;27(3):617–637. doi: 10.1148/rg.273065051. [DOI] [PubMed] [Google Scholar]
- 16.Johkoh T., Muller N.L., Akira M. Eosinophilic lung diseases: diagnostic accuracy of thin-section CT in 111 patients. Radiology. 2000;216(3):773–780. doi: 10.1148/radiology.216.3.r00se01773. [DOI] [PubMed] [Google Scholar]
- 17.De Giacomi F., Vassallo R., Yi E.S., Ryu J.H. Acute eosinophilic pneumonia. causes, diagnosis, and management. Am J Respir Crit Care Med. 2018;197(6):728–736. doi: 10.1164/rccm.201710-1967CI. [DOI] [PubMed] [Google Scholar]
- 18.Panse P.M., Feller F.F., Butt Y.M. Radiologic and pathologic correlation in EVALI. AJR Am J Roentgenol. 2020;215(5):1057–1064. doi: 10.2214/AJR.20.22836. [DOI] [PubMed] [Google Scholar]
- 19.Aberegg S.K., Cirulis M.M., Maddock S.D. Clinical, bronchoscopic, and imaging findings of e-cigarette, or vaping, product use-associated lung injury among patients treated at an academic medical center. JAMA Netw Open. 2020;3(11) doi: 10.1001/jamanetworkopen.2020.19176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lichtenberger J.P., III, Digumarthy S.R., Abbott G.F., Shepard J.A., Sharma A. Diffuse pulmonary hemorrhage: clues to the diagnosis. Curr Probl Diagn Radiol. 2014;43(3):128–139. doi: 10.1067/j.cpradiol.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Butt Y.M., Smith M.L., Tazelaar H.D. Pathology of vaping-associated lung injury. N Engl J Med. 2019;381(18):1780–1781. doi: 10.1056/NEJMc1913069. [DOI] [PubMed] [Google Scholar]
- 22.Mukhopadhyay S., Mehrad M., Dammert P. Lung biopsy findings in severe pulmonary illness associated with e-cigarette use (vaping) Am J Clin Pathol. 2020;153(1):30–39. doi: 10.1093/ajcp/aqz182. [DOI] [PubMed] [Google Scholar]
- 23.Reagan-Steiner S., Gary J., Matkovic E. Pathological findings in suspected cases of e-cigarette, or vaping, product use-associated lung injury (EVALI): a case series. Lancet Respir Med. 2020;8(12):1219–1232. doi: 10.1016/S2213-2600(20)30321-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu H.H., Tzao C., Chang W.C. Zinc chloride (smoke bomb) inhalation lung injury: clinical presentations, high-resolution CT findings, and pulmonary function test results. Chest. 2005;127(6):2064–2071. doi: 10.1378/chest.127.6.2064. [DOI] [PubMed] [Google Scholar]
- 25.Akira M., Suganuma N. Acute and subacute chemical-induced lung injuries: HRCT findings. Eur J Radiol. 2014;83(8):1461–1469. doi: 10.1016/j.ejrad.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 26.Kim K.I., Kim C.W., Lee M.K. Imaging of occupational lung disease. Radiographics. 2001;21(6):1371–1391. doi: 10.1148/radiographics.21.6.g01nv011371. [DOI] [PubMed] [Google Scholar]
- 27.Lee E., Seo J.H., Kim H.Y. Toxic inhalational injury-associated interstitial lung disease in children. J Korean Med Sci. 2013;28(6):915–923. doi: 10.3346/jkms.2013.28.6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shin H.J., Chang J.S., Ahn S. Acute respiratory distress syndrome and chemical burns after exposure to chlorine-containing bleach: a case report. J Thorac Dis. 2017;9(1):E17–E20. doi: 10.21037/jtd.2017.01.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka N., Emoto T., Matsumoto T., Matsunaga N., Tsuruta R., Lynch D.A. Inhalational lung injury due to nitrogen dioxide: high-resolution computed tomography findings in 3 patients. J Comput Assist Tomogr. 2007;31(5):808–811. doi: 10.1097/rct.0b013e318032e8ed. [DOI] [PubMed] [Google Scholar]
- 30.Girvin F., Naidich D. CT features of electronic-cigarette or vaping-associated lung injury (EVALI); our experience during the recent outbreak. BJR Case Rep. 2020;6(3):20200027. doi: 10.1259/bjrcr.20200027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berkowitz E.A., Henry T.S., Veeraraghavan S., Staton G.W., Jr., Gal A.A. Pulmonary effects of synthetic marijuana: chest radiography and CT findings. AJR Am J Roentgenol. 2015;204(4):750–757. doi: 10.2214/AJR.14.13138. [DOI] [PubMed] [Google Scholar]
- 32.Shorr A.F., Scoville S.L., Cersovsky S.B. Acute eosinophilic pneumonia among US military personnel deployed in or near Iraq. JAMA. 2004;292(24):2997–3005. doi: 10.1001/jama.292.24.2997. [DOI] [PubMed] [Google Scholar]
- 33.Arter Z.L., Wiggins A., Hudspath C., Kisling A., Hostler D.C., Hostler J.M. Acute eosinophilic pneumonia following electronic cigarette use. Respir Med Case Rep. 2019;27:100825. doi: 10.1016/j.rmcr.2019.100825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thota D., Latham E. Case report of electronic cigarettes possibly associated with eosinophilic pneumonitis in a previously healthy active-duty sailor. J Emerg Med. 2014;47(1):15–17. doi: 10.1016/j.jemermed.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 35.Okada Y., Ito M., Nagaishi C. Anatomical study of the pulmonary lymphatics. Lymphology. 1979;12(3):118–124. [PubMed] [Google Scholar]
- 36.Jakic B., Kerjaschki D., Wick G. Lymphatic capillaries in aging. Gerontology. 2020;66(5):419–426. doi: 10.1159/000508459. [DOI] [PubMed] [Google Scholar]
- 37.Simpson S., Kay F.U., Abbara S. Radiological Society of North America Expert Consensus Statement on reporting chest CT findings related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA - secondary publication. J Thorac Imaging. 2020;35(4):219–227. doi: 10.1097/RTI.0000000000000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernheim A., Mei X., Huang M. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020;295(3):200463. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guan C.S., Lv Z.B., Yan S. Imaging features of coronavirus disease 2019 (COVID-19): evaluation on thin-section CT. Acad Radiol. 2020;27(5):609–613. doi: 10.1016/j.acra.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salehi S., Abedi A., Balakrishnan S., Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020;215(1):87–93. doi: 10.2214/AJR.20.23034. [DOI] [PubMed] [Google Scholar]
- 41.Ojha V., Mani A., Pandey N.N., Sharma S., Kumar S. CT in coronavirus disease 2019 (COVID-19): a systematic review of chest CT findings in 4410 adult patients. Eur Radiol. 2020;30(11):6129–6138. doi: 10.1007/s00330-020-06975-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flower M., Nandakumar L., Singh M., Wyld D., Windsor M., Fielding D. Respiratory bronchiolitis-associated interstitial lung disease secondary to electronic nicotine delivery system use confirmed with open lung biopsy. Respirol Case Rep. 2017;5(3) doi: 10.1002/rcr2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fels Elliott D.R., Shah R., Hess C.A. Giant cell interstitial pneumonia secondary to cobalt exposure from e-cigarette use. Eur Respir J. 2019;54(6):1901–1922. doi: 10.1183/13993003.01922-2019. [DOI] [PubMed] [Google Scholar]
- 44.Attili A.K., Kazerooni E.A., Gross B.H., Flaherty K.R., Myers J.L., Martinez F.J. Smoking-related interstitial lung disease: radiologic-clinical-pathologic correlation. Radiographics. 2008;28(5):1383–1396. doi: 10.1148/rg.285075223. [DOI] [PubMed] [Google Scholar]
- 45.Fraig M., Shreesha U., Savici D., Katzenstein A.L. Respiratory bronchiolitis: a clinicopathologic study in current smokers, ex-smokers, and never-smokers. Am J Surg Pathol. 2002;26(5):647–653. doi: 10.1097/00000478-200205000-00011. [DOI] [PubMed] [Google Scholar]
- 46.Cottin V., Streichenberger N., Gamondes J.P., Thevenet F., Loire R., Cordier J.F. Respiratory bronchiolitis in smokers with spontaneous pneumothorax. Eur Respir J. 1998;12(3):702–704. doi: 10.1183/09031936.98.12030702. [DOI] [PubMed] [Google Scholar]
- 47.Blount B.C., Karwowski M.P., Shields P.G. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382(8):697–705. doi: 10.1056/NEJMoa1916433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maddock S.D., Cirulis M.M., Callahan S.J. Pulmonary lipid-laden macrophages and vaping. N Engl J Med. 2019;381(15):1488–1489. doi: 10.1056/NEJMc1912038. [DOI] [PubMed] [Google Scholar]
- 49.Panse P.M., Feller F.F., Butt Y.M. Pulmonary injury resulting from vaping or e-cigarette use: imaging appearances at presentation and follow-up. Radiol Cardiothorac Imaging. 2020;2(4) doi: 10.1148/ryct.2020200081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davidson K.R., Fox D.L. More on the pathology of vaping-associated lung injury. N Engl J Med. 2020;382(4):388. doi: 10.1056/NEJMc1914980. [DOI] [PubMed] [Google Scholar]
- 51.Tai H., Swartz M.D., Marsden D., Perry C.L. The future of substance abuse now: relationships among adolescent use of vaping devices, marijuana, and synthetic cannabinoids. Subst Use Misuse. 2021;56(2):192–204. doi: 10.1080/10826084.2020.1849305. [DOI] [PubMed] [Google Scholar]