Abstract
Background
Preclinical evidence implicates neutrophil elastase (NE) in pulmonary arterial hypertension (PAH) pathogenesis, and the NE inhibitor elafin is under early therapeutic investigation.
Research Question
Are circulating NE and elafin levels abnormal in PAH and are they associated with clinical severity?
Study Design and Methods
In an observational Stanford University PAH cohort (n = 249), plasma NE and elafin levels were measured in comparison with those of healthy control participants (n = 106). NE and elafin measurements were then related to PAH clinical features and relevant ancillary biomarkers. Cox regression models were fitted with cubic spline functions to associate NE and elafin levels with survival. To validate prognostic relationships, we analyzed two United Kingdom cohorts (n = 75 and n = 357). Mixed-effects models evaluated NE and elafin changes during disease progression. Finally, we studied effects of NE-elafin balance on pulmonary artery endothelial cells (PAECs) from patients with PAH.
Results
Relative to control participants, patients with PAH were found to have increased NE levels (205.1 ng/mL [interquartile range (IQR), 123.6-387.3 ng/mL] vs 97.6 ng/mL [IQR, 74.4-126.6 ng/mL]; P < .0001) and decreased elafin levels (32.0 ng/mL [IQR, 15.3-59.1 ng/mL] vs 45.5 ng/mL [IQR, 28.1-92.8 ng/mL]; P < .0001) independent of PAH subtype, illness duration, and therapies. Higher NE levels were associated with worse symptom severity, shorter 6-min walk distance, higher N-terminal pro-type brain natriuretic peptide levels, greater right ventricular dysfunction, worse hemodynamics, increased circulating neutrophil levels, elevated cytokine levels, and lower blood BMPR2 expression. In Stanford patients, NE levels of > 168.5 ng/mL portended increased mortality risk after adjustment for known clinical predictors (hazard ratio [HR], 2.52; CI, 1.36-4.65, P = .003) or prognostic cytokines (HR, 2.63; CI, 1.42-4.87; P = .001), and the NE level added incremental value to established PAH risk scores. Similar prognostic thresholds were identified in validation cohorts. Longitudinal NE changes tracked with clinical trends and outcomes. PAH PAECs exhibited increased apoptosis and attenuated angiogenesis when exposed to NE at the level observed in patients’ blood. Elafin rescued PAEC homeostasis, yet the required dose exceeded levels found in patients.
Interpretation
Blood levels of NE are increased while elafin levels are deficient across PAH subtypes. Higher NE levels are associated with worse clinical disease severity and outcomes, and this target-specific biomarker could facilitate therapeutic development of elafin.
Key Words: biomarkers, elastase’ inflammation’ pulmonary arterial hypertension
Abbreviations: BMPR2, bone morphogenetic protein receptor 2; HR, hazard ratio; IMT, immune modulating therapy; IQR, interquartile range; mPAP, mean pulmonary arterial pressure; NE, neutrophil elastase; PAEC, pulmonary artery endothelial cell; PAH, pulmonary arterial hypertension
FOR EDITORIAL COMMENT, SEE PAGE 1177
Pulmonary arterial hypertension (PAH) is a progressive occlusive arteriopathy that often culminates in right heart failure and death, despite available therapies. Persistent inflammation is a well-recognized feature of PAH,1,2 arising from aberrant reparative immune responses that follow a disease-provoking vascular insult.3,4 Extracellular matrix breakdown accompanies this inflammation, propagating immune cell activation and recruitment.5,6 Levels of perivascular immune cells and blood cytokines correlate with PAH clinical severity,7, 8, 9, 10 suggesting that inflammation may contribute to disease progression.
Neutrophils are among the immune cells involved and release neutrophil elastase (NE), a protease implicated in PAH arteriopathy.11 Neutrophils isolated from patients with PAH have enhanced NE release capacity12: NE localizes to pulmonary arterial smooth muscle cells and neointimal lesions in PAH lungs,13 and NE activity temporally associates with vascular remodeling in experimental pulmonary hypertension.14 NE is thought to trigger remodeling via extracellular matrix degradation, which causes release of growth factors, clustering and activation of their receptors, and ensuing migration and proliferation of smooth muscle cells and fibroblasts.15, 16, 17 NE may perpetuate inflammation by generating chemotactic elastin fragments,18 proteolytically modifying cytokines19,20 and downregulating bone morphogenetic protein receptor 2 (BMPR2) signaling.21,22
Therapies that antagonize NE have yielded favorable preclinical results.23 We found that recombinant elafin, an endogenously produced NE inhibitor, reversed experimental pulmonary hypertension and caused neointimal lesions to regress in cultured lung tissue.24 Elafin seems to amplify BMPR2 signaling and to promote normal angiogenesis.24,25 Based on these findings, we launched a phase 1 trial of elafin therapy for PAH (ClinicalTrials.gov Identifier: NCT03522935).
An increasing number of novel PAH therapies have failed in clinical trials, despite promising preclinical data. Experts call for the development of biomarkers relevant to pathobiological features, clinical disease state, and drug mechanism during initial investigation phases to inform later-phase clinical trial design.26,27 Despite preclinical evidence linking NE to PAH pathobiological features and the therapeutic potential of elafin, no study has evaluated circulating NE and elafin as clinical biomarkers. As part of the bench-to-bedside development of elafin therapy, we aimed to determine whether NE and elafin blood levels are abnormal in PAH and are associated with disease severity and outcomes. We hypothesized that NE levels would be increased and elafin levels would be deficient across PAH subtypes, with more pronounced derangements in severe disease.
Methods
Study Overview
Plasma NE and elafin levels were measured in an observational cohort study of patients with PAH and healthy control participants enrolled at Stanford University (Stanford, CA). Levels of these biomarkers (1) were evaluated across PAH subtypes in comparison with those of control participants, (2) were related to PAH clinical features and outcomes, (3) were reassessed over time to examine changes during disease progression, and (4) were correlated with ancillary blood measures (leukocyte subsets, cytokines, and BMPR2 expression). To validate observed relationships with outcomes, we analyzed existing multicenter United Kingdom (UK) data from prior PAH proteome studies. Guided by the NE and elafin blood levels found in patients, we then assessed the effects of NE-elafin imbalance on pulmonary arterial endothelial cells (PAECs) isolated from PAH lung explants.
Population and Data Collection
The primary cohort included patients with World Health Organization Group 1 PAH that underwent evaluation at Stanford and had blood collected for the Vera Moulton Wall Center biobank between 2008 and 2013 (n = 249). The study was approved by the Stanford University Institutional Review Board (protocol no., 14083), and all participants provided informed consent. PAH was diagnosed according to existing guidelines and required mean pulmonary arterial pressure (mPAP) of ≥ 25 mm Hg, pulmonary vascular resistance of > 240 dynes/sec/cm5, and wedge pressure of ≤ 15 mm Hg.28 We excluded patients with chronic lung disease, left ventricular systolic dysfunction, valvular disease, chronic infection, or any acute illness within 1 month (e-Fig 1). Control participants (n = 106) were included from the Stanford Healthy Aging Population Study, which screened volunteers for cardiovascular and immune health from 2009 through 2013 (e-Appendix 1).
NE and Elafin
Internal jugular venous samples were collected during the index right heart catheterization at Stanford, regardless of whether PAH was incident or prevalent. Plasma samples were processed immediately and stored under strict protocols (e-Appendix 2A). We obtained follow-up samples from a patient subset during routine surveillance catheterization (n = 70). NE and elafin levels were measured by enzyme-linked immunoabsorbent assay (Hycult Biotech) (e-Appendix 3A).
Clinical Data
The Stanford Pulmonary Hypertension Database was used to extract patient demographics, clinical features, and background therapies at the time of plasma collection. In patients with follow-up sampling, therapy interventions and clinical trends were captured. All patients were observed over time for the outcome of death or lung transplantation (e-Appendix 2B).
Ancillary Blood Measurements
For patients with same-day data available from prior Vera Moulton Wall Center biobank studies,29,30 we obtained blood measurements of leukocyte subset counts (CBC differential), 48 cytokines (BioPlex multiplex immunoassay; Bio-Rad), and BMPR2 messenger RNA expression (real-time quantitative reverse transcriptase polymerase chain reaction analysis; Applied Biosystems) (e-Appendix 3B, 3C).
Validation Cohorts
Patients with idiopathic PAH were included from two UK studies: a prior published cohort31 enrolled at Imperial College from 2002 through 2011 (cohort A; n = 75) and a multicenter cohort recruited from 2014 through 2018 for the UK National Cohort Study of Idiopathic and Heritable PAH (cohort B; n = 357). Aptamer-based peripheral plasma NE measurements (SomaScan; Somalogic) and survival data were obtained (e-Appendix 4).
PAEC Studies
Explanted lungs were procured from PAH transplant recipients (n = 3) and control donors (n = 3) through the Pulmonary Hypertension Breakthrough Initiative. Harvested PAECs were cultured and treated with various elastase and elafin dose combinations (e-Table 1). Under each treatment condition, we evaluated PAEC apoptosis (caspase 3/7 assay) and angiogenesis activity (tube formation) (e-Appendix 5).24,32
Statistical Analysis
Analyses were performed using R version 3.5.1 software (R Foundation for Statistical Computing) (e-Appendix 6). NE and elafin levels in PAH were compared with those of control participants via Mann-Whitney U test. Receiver operating characteristic curves assessed the PAH discriminatory power of each biomarker, and ideal discrimination cutoffs were identified (Youden’s statistic). To evaluate univariate biomarker relationships with clinical features, we applied the Spearman ρ statistic (continuous variables), the Mann-Whitney U test (binary variables), the Kruskal-Wallis test (categorical variables), and the Cuzick test (ordinal variables). We also fit median regression models adjusted for age, sex, and BMI to associate NE and elafin levels with disease severity metrics. Biomarker changes were related to clinical features over time via mixed-effects models. NE and elafin levels were correlated with leukocyte subset counts and BMPR2 expression levels in blood. Significance analysis of microarrays was implemented to ascertain differentially expressed cytokines among patients with increased NE levels.
The outcome of time to death or transplantation from blood sampling was analyzed, and Kaplan-Meier transplant-free survival estimates were compared across biomarker quantiles. Univariate Cox regression models were fitted to assess the relationship between each biomarker and the outcome. When the assumption of linearity between biomarker levels and log hazard was not satisfied, Cox models were fitted with cubic spline functions to examine nonlinear relationships. Bootstrapped spline model estimates were used to identify the biomarker threshold beyond which mortality risk remained significantly increased. This prognostic threshold was evaluated in multivariate Cox models that adjusted for known predictors of PAH risk, including clinical parameters and cytokines. We also determined whether the prognostic threshold added incremental value to established PAH risk stratification scores. The Registry to Evaluate Early and Long-Term PAH Disease Management 2.0 calculator,33 the French Pulmonary Hypertension Registry algorithm,34 and the Comparative Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension score35 stratified low-, intermediate-, and high-risk groups (e-Appendix 6C). The predictive performance of risk score-only and biomarker-inclusive Cox regression models was compared by likelihood ratio test.
Results
The Stanford cohort with PAH (Table 1) had a median age of 49 years (interquartile range [IQR], 38-59 years) and was predominantly female (76.7%). The most common PAH subtypes were connective tissue disease associated (31.7%) and idiopathic (27.7%), followed by drugs and toxins associated (18.9%), congenital heart disease associated (15.3%), and portopulmonary hypertension (6.4%). Many participants showed New York Heart Association functional class III or IV symptoms (57.0%), and PAH was hemodynamically severe: mPAP of 50 mm Hg (IQR, 40-60 mm Hg) and pulmonary vascular resistance 821 dynes/sec/cm5 (IQR, 506-1197 dynes/sec/cm5). Most patients were either treatment naïve (35.0%) or were receiving monotherapy (28.1%).
Table 1.
Stanford Cohort Characteristics at Baseline Measurement of NE and Elafin
| Variable | Control Participants (N = 106) | All PAH (n = 249) | PAH Subgroup With Follow-up NE and Elafin Measurements (n = 70) |
|---|---|---|---|
| Age, y | 58 (48-72) | 49 (38-59) | 50 (40-58) |
| Sex | |||
| Female | 58 (54.7) | 191 (76.7) | 53 (75.7) |
| Male | 48 (45.3) | 58 (23.3) | 17 (24.3) |
| PAH cause | |||
| IPAH + HPAHa | — | 69 (27.7) | 20 (28.6) |
| D&T-associated PAH | — | 47 (18.9) | 17 (24.3) |
| CTD-associated PAH | — | 79 (31.7) | 18 (25.7) |
| PoPH | — | 16 (6.4) | 7 (10.0) |
| CHD-associated PAH | — | 38 (15.3) | 8 (11.4) |
| NYHA FC | |||
| I | — | 14 (5.6) | 5 (7.1) |
| II | — | 93 (37.3) | 33 (47.1) |
| III | — | 114 (45.8) | 24 (34.3) |
| IV | — | 28 (11.2) | 8 (11.4) |
| 6MWD, m | — | 423 (341-513) | 433 (366-512) |
| Therapy extent | |||
| Naïve | — | 87 (35.0) | 19 (27.1) |
| Monotherapy | — | 70 (28.1) | 21 (30.0) |
| Dual therapy | — | 69 (27.7) | 24 (34.3) |
| Triple therapy | — | 23 (9.2) | 6 (8.6) |
| Therapy class | |||
| PDE-5 inhibitor | — | 119 (47.8) | 36 (51.4) |
| ERA | — | 74 (29.7) | 22 (31.4) |
| Prostacyclin | — | 84 (33.7) | 29 (41.4) |
| Dlco, % predicted | — | 71 (54-87) | 73 (64-89) |
| NT-proBNP, pg/mL | — | 289 (80-1238) | 216 (75-934) |
| Hemodynamics | |||
| Right atrial pressure, mm Hg | — | 7 (5-11) | 7 (4-12) |
| mPAP, mm Hg | — | 50 (40-60) | 52 (42-60) |
| Cardiac index, L/min/m2 | — | 2.09 (1.76-2.43) | 2.07 (1.70-2.36) |
| PVR, dynes/sec/cm5 | — | 821 (506-1197) | 842 (596-1177) |
| NE, ng/mL | 97.6 (74.4-126.6) | 205.1 (123.6-387.3) | 169.9 (119.1-310.3) |
| Elafin, ng/mL | 45.5 (28.1-92.8) | 32.0 (15.3-59.1) | 42.4 (33.1-72.2) |
| NE to elafin ratio | 2.0 (0.8-3.8) | 6.2 (3.0-17.3) | 4.3 (2.0-7.2) |
Data are presented as No. (%) or median (interquartile range). Missing data: all PAH group, 6MWD (n = 5), Dlco (n = 20), RAP (n = 4), CI and PVR (n = 2); follow-up group: Dlco (n = 10). 6MWD = 6-min walk distance; APAH = associated pulmonary arterial hypertension; CHD = congenital heart disease; CTD = connective tissue disease; D&T = drugs and toxins; Dlco = diffusion capacity of lung for carbon monoxide; — = data not available for healthy control participants; ERA = endothelin receptor antagonist; HPAH = heritable pulmonary arterial hypertension; IPAH = idiopathic pulmonary arterial hypertension; mPAP = mean pulmonary arterial pressure; NE = neutrophil elastase; NT-proBNP = N-terminal B-type natriuretic peptide; NYHA FC = New York Heart Association functional class; PAH = pulmonary arterial hypertension; PDE-5 = phosphodiesterase-5; PoPH = portopulmonary hypertension; PVR = pulmonary vascular resistance.
HPAH: n = 3 patients (1.2% of cohort), who had confirmed BMPR2 mutations. Mutation status was not otherwise evaluated routinely in the cohort.
NE and Elafin Levels in Patients With PAH vs Healthy Participants
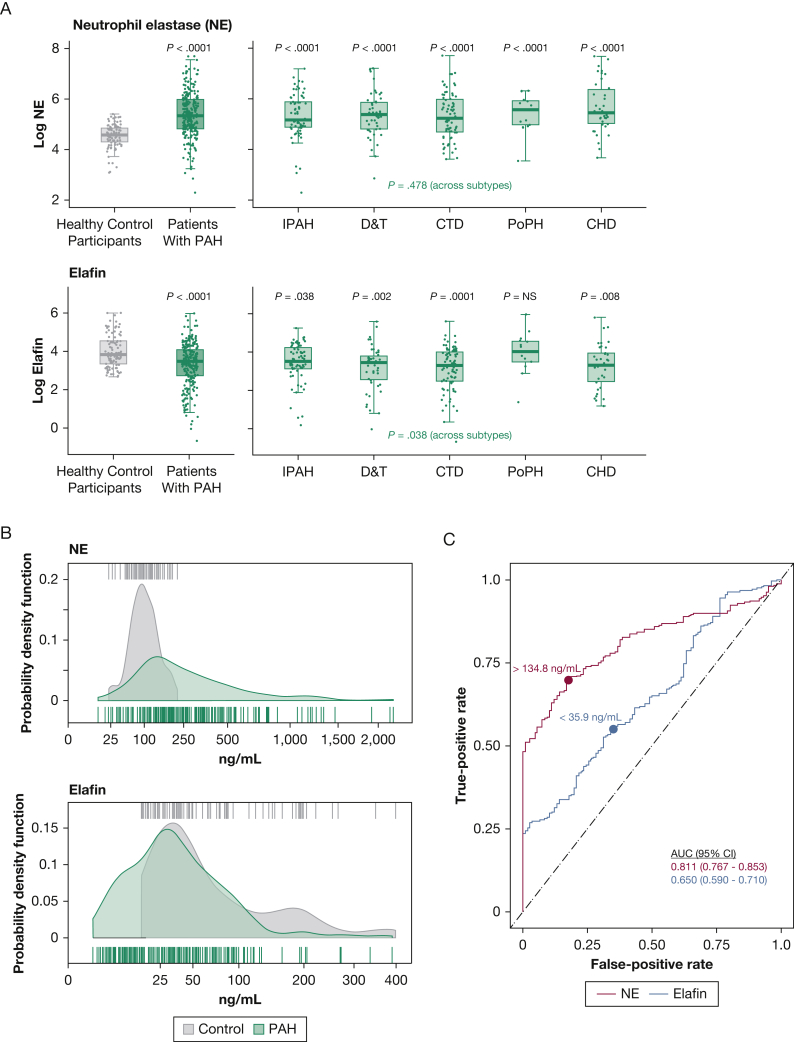
Relative to control participants, patients with PAH were observed to have increased plasma NE levels (median, 205.1 ng/mL [IQR, 123.6-387.3 ng/mL] vs 97.6 ng/mL [IQR, 74.4-126.6 ng/mL]), decreased elafin levels (median, 32.0 ng/mL [IQR, 15.3-59.1 ng/mL] vs 45.5 ng/mL [IQR, 28.1-92.8 ng/mL]), and higher NE to elafin ratio (median, 6.2 [IQR, 3.0-17.3] vs 2.0 [IQR, 0.8-3.8]; all P < .0001) (Fig 1A, 1B). The magnitude of NE level elevation was similar across PAH subtypes. Elafin was deficient for all subtypes except portopulmonary hypertension. The NE level better discriminated patients with PAH than elafin (c-statistic, 0.811 [CI, 0.767-0.853] vs 0.650 [CI, 0.590-0.710]) (Fig 1C), and ideal cutoffs were NE level of > 134.8 ng/mL (specificity, 0.83; sensitivity, 0.70) and elafin level of < 35.9 ng/mL (specificity, 0.65; sensitivity, 0.56). The NE to elafin ratio did not improve PAH discriminatory power (c-statistic, 0.808 [CI, 0.763-0.852]), and no positive or negative correlation existed between NE and elafin levels in patients with PAH or healthy participants (e-Fig 2A, 2B).
Figure 1.
A-C, Increased NE and relative elafin deficiency across PAH subtypes. A, Box-and-whisker plots showing log-transformed NE and elafin levels in patients with PAH (overall and each subtype) compared to those in healthy control participants. Boxplots denote the median, interquartile range, and 1.5 × IQR. P values reflect comparisons of the PAH population overall vs healthy control participants (top of left panel), patients with each PAH subtype vs control participants (top of right panel), and across subtypes (bottom of right panel). B, Graphs showing the distributions of NE and elafin in patients with PAH and healthy control participants, displayed as probability density functions (smoothed histograms resulting from Gaussian kernel estimation). Rug plot hash marks above and below the probability density curves represent patient-level measures. C, Receiver operating characteristic curves displaying the PAH discriminatory power of NE and elafin. Calculated c-statistics (AUC) and ideal discrimination cutoffs (derived from Youden’s index) are indicated. AUC = area under the receiver operating characteristic curve; CHD = congenital heart disease; CTD = connective tissue disease; D&T = drugs and toxins; IPAH = idiopathic pulmonary arterial hypertension; NE = neutrophil elastase; PAH = pulmonary arterial hypertension; PoPH = portopulmonary hypertension.
NE and Elafin Levels in Relationship to Clinical Features
NE and elafin levels were independent of age in patients with PAH and healthy participants (e-Fig 3A). No sex-related NE differences were observed, although female patients with PAH exhibited more pronounced elafin deficiency (e-Fig 3B). Neither biomarker was related to race or ethnicity or to BMI (e-Fig 3C, 3D).
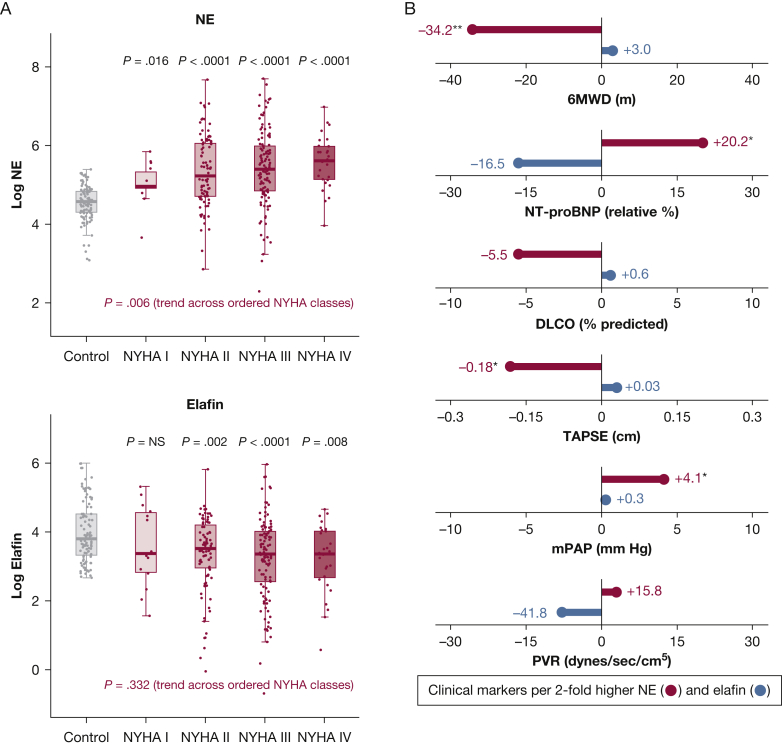
Clinical markers of disease severity were associated with NE levels, but not elafin levels. The NE level increased with more severe heart failure symptoms from New York Heart Association functional classes I to IV (Fig 2A). Higher NE levels also correlated with shorter 6-min walk distance (ρ = –0.30; P = .006), increased N-terminal pro-type brain natriuretic peptide (ρ = 0.25; P = .011), worse right ventricular function (tricuspid annular plane systolic excursion ρ = –0.23; P = .019), and higher mPAP (ρ = 0.21; P = .039) (e-Table 2). Relationships persisted after adjustment for age, sex, and BMI, where each two-fold NE elevation equated to a 6-min walk distance reduction of 34.2 m, relative N-terminal pro-type brain natriuretic peptide increase of 20.2%, tricuspid annular plane systolic excursion reduction of 0.18 cm, and mPAP increase of 4.1 mm Hg (Fig 2B).
Figure 2.
A, B, Pulmonary arterial hypertension (PAH) clinical severity metrics are associated with NE, but not elafin. A, Box-and-whisker plots showing baseline NE and elafin (log-scale) stratified by heart failure symptom severity (with increasing NYHA functional class from I to IV). B, Lollipop plot summarizing median regression analyses that related NE and elafin to baseline PAH clinical metrics. Regression models were adjusted for age, sex, and BMI. The plot shows the direction and magnitude of clinical differences associated with each two-fold elevation of NE (red) and elafin (blue). Relationships achieving statistical significance (regression coefficient P < .05) are indicated as follows: ∗P < .05, ∗∗P < .01, or ∗∗∗P < .001. 6MWD = 6-min walk distance; Dlco = diffusion capacity of lung for carbon monoxide; mPAP = mean pulmonary arterial pressure; NE = neutrophil elastase; NT-proBNP = N-terminal pro-type brain natriuretic peptide; NYHA = New York Heart Association; PVR = pulmonary vascular resistance; TAPSE = tricuspid annular plane systolic excursion.
NE and elafin levels were independent of time from PAH diagnosis (e-Fig 4), PAH treatment status (naïve, monotherapy, dual therapy, or triple therapy) (e-Fig 5), each class of background PAH therapy (phosphodiesterase-5 inhibitors, endothelin receptor antagonists, or prostanoids) (e-Fig 6), and background immune modulators (e-Fig 7).
NE and Elafin Levels as Prognostic Markers
Stanford Cohort
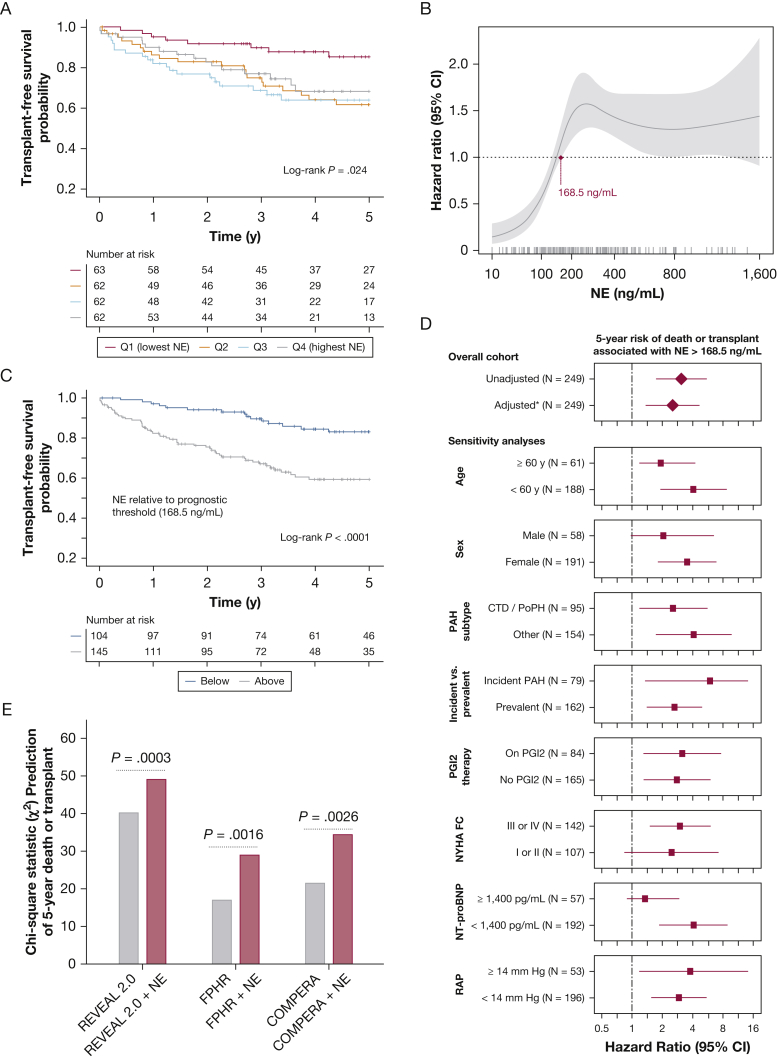
Median follow-up for outcomes was 3.4 years (IQR, 2.0-5.8 years), during which time 52 patients (20.9%) died and 19 patients (7.6%) underwent transplantation. The 5-year survival rate was more favorable in the lowest NE quartile (85.3% [CI, 76.2%-95.5%]) than higher quartiles (quartile 2, 61.8% [CI, 49.8%-76.7%]; quartile 3, 64.0% [CI, 52.2%-78.4%]; quartile 4, 66.4% [CI, 54.1%-79.3%]; P = .024) (Fig 3A). A nonlinear threshold-type relationship was observed between NE level and mortality risk, as demonstrated by a Cox regression model fitted with a cubic spline function (Fig 3B). We identified a prognostic threshold (> 168.5 ng/mL; 42nd percentile of cohort) associated with increased mortality risk (hazard ratio [HR], 3.07 [CI, 1.72-5.46]; P = .0001) (Fig 3C). This threshold retained significance in multiple sensitivity analyses and after adjustment for previously reported PAH predictors including age, sex, PAH subtype, incident (newly diagnosed) PAH status, prostacyclin treatment, functional class, N-terminal pro-type brain natriuretic peptide, and right atrial pressure (HR, 2.52 [CI, 1.36-4.65]; P = .003) (Fig 3D, e-Table 3). NE level also added incremental prognostic value to the Registry to Evaluate Early and Long-Term PAH Disease Management 2.0, French Pulmonary Hypertension Registry, and Comparative Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension PAH risk stratification scores (Fig 3E, e-Table 4). Elafin level was not associated with outcomes (e-Fig 8).
Figure 3.
A-E, Prognostic value of NE in Stanford pulmonary arterial hypertension (PAH) cohort. A, Kaplan-Meier 5-year survival estimates by NE quartile (Q1, < 123.6 ng/mL; Q2, 123.6-205.1 ng/mL; Q3, 205.1-397.3 ng/mL; and Q4, > 397.3 ng/mL). B, Plot showing the nonlinear relationship between NE and mortality risk (solid line = hazard ratio; gray shaded area = 95% CI), which reflects bootstrapped estimates from a Cox regression model fitted with a cubic spline function. Model estimates are shown across the measured range of NE, with patient-level measurements indicated along the x-axis rug plot. The maroon point highlights the NE level beyond which mortality risk remains significantly increased (prognostic threshold > 168.5 ng/mL). C, Kaplan-Meier survival curves for subgroups stratified by this optimal NE prognostic threshold. D, Risk of death associated with NE > 168.5 ng/mL in univariate analysis, in multivariate analysis (∗adjusted for age, sex, PAH subtype, incident vs prevalent PAH, prostacyclin treatment, functional class, NT-proBNP, and right atrial pressure), and in subgroup sensitivity analyses. E, Incremental prognostic value added by NE to three validated PAH risk stratification tools: the REVEAL 2.0 calculator, the FPHR algorithm, and the COMPERA score, which each stratified patients into low-, intermediate-, and high-risk groups (e-Appendix 6). The χ2 values are shown for Cox regression models fitted with NE and each risk score (red bars) vs those fitted only with risk scores (grey bars). P values reflect the likelihood ratio test. COMPERA = Comparative Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension; CTD = connective tissue disease; FPHR = French Pulmonary Hypertension Registry; NE = neutrophil elastase; NT-proBNP = N-terminal pro-type brain natriuretic peptide; NYHA FC = New York Heart Association functional class; PGI2 = prostacyclin; PoPH = portopulmonary hypertension; Q = quartile; RAP = right atrial pressure; REVEAL = Registry to Evaluate Early and Long-Term PAH Disease Management.
UK Cohorts
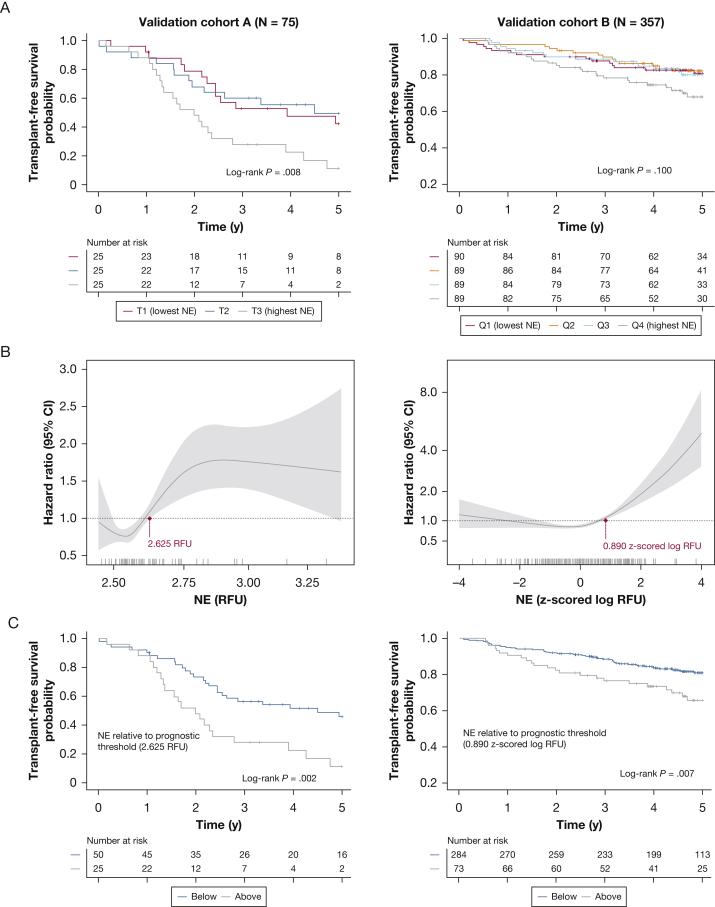
In cohort A (baseline characteristics in e-Table 5), median follow-up was 2.5 years (IQR, 1.6-4.8 years), and 56 patients (74.7%) died or underwent transplantation. In cohort B, events transpired in 78 patients (21.8%) during a median of 4.7 years (IQR, 3.6-5.4 years) of follow-up. Survival was worse in the highest NE tertile of cohort A (P = .008), and in cohort B, a trend toward worse survival was found in the highest quartile (P = .100) (Fig 4A). Nonlinear relationships between NE and survival were demonstrated in both cohorts, and prognostic thresholds above which the risk of death was increased significantly were identifiable in cohort A (HR, 2.46 [CI, 1.37-4.43]; P = .002) and cohort B (HR, 1.95 [CI, 1.20-3.20]; P = .007) (Fig 4B, 4C). Although UK SomaScan measurements (in relative fluorescent units) were not comparable with Stanford enzyme-linked immunoabsorbent assay data (in nanograms per milliliter), UK prognostic thresholds fell at higher points in measured NE distributions (67th and 79th percentiles of cohorts A and B).
Figure 4.
A-C, Prognostic value of NE in United Kingdom validation cohorts. A, Kaplan-Meier estimates of 5-year survival according to NE tertile in validation cohort A (T1, < 2.545 RFU; T2, 2.545-2.623 RFU; T3, > 2.623 RFU) and by NE quartile in cohort B (Q1, < –0.557 log RFU; Q2, –0.557 to 0.200 log RFU; Q3, 0.200-0.796 log RFU; and Q4, > 0.796 log RFU, z-scored relative to healthy control participants). B, Plots showing the nonlinear relationship between NE and mortality risk in validation cohorts A (left) and B (right), reflecting bootstrapped estimates from cubic spline Cox regression models. Prognostic thresholds are indicated (maroon points). C, Survival curves are displayed for subgroups dichotomized by the prognostic NE threshold identified in each cohort. NE = neutrophil elastase; Q = quartile; RFU = relative fluorescent unit; T = tertile.
Biomarker Changes During the Disease Course
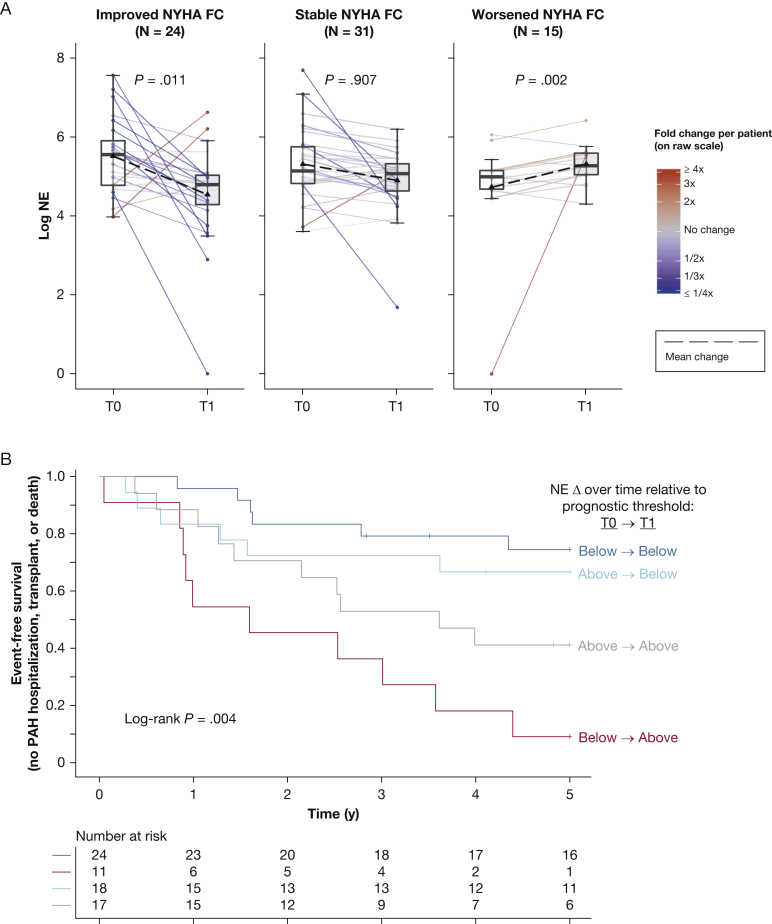
Median time to follow-up blood sampling was 0.9 years (IQR, 0.5-1.4 years) among patients who underwent reassessment (n = 70). Interval PAH therapy addition occurred in 32 patients (45.7%), and patients collectively showed modest interval improvements in 6-min walk distance, mPAP, pulmonary vascular resistance, and cardiac index (e-Table 6). Mixed models accounting for baseline patient differences demonstrated overall relative downtrends in NE levels (–39.0%; P = .023) and elafin levels (–17.2%; P = .001) (e-Fig 9), where changes were independent of age, sex, and PAH subtype (e-Table 7). NE changes were associated with New York Heart Association functional class trends, as functional class improvements corresponded to decreasing NE levels (median, –136.3 ng/mL; P = .011) and class worsening tracked with increasing NE levels (+72.8 ng/mL; P = .002) (Fig 5A). Prostacyclin initiation (n = 14/70) was associated with reductions in levels of NE (P = .035) and elafin (P = .006) (e-Figs 10, 11), but other initiated therapies did not produce significant biomarker changes.
Figure 5.
A, B, Changes in NE show relationship with clinical outcomes over time. A, Boxplots showing log-scale NE levels at baseline (T0) and follow-up (T1) for subgroups with differing NYHA FC trends (improved, stable, and worsened). Overlying spaghetti plots display patient-level NE changes (line colors reflect raw-scale fold changes, and the black dashed line denotes average change). Mixed-effects models were fitted to NE as functions of time (T1 referenced to T0), NYHA FC trend, and their interaction. Interaction term P values are shown. B, Kaplan-Meier estimates of 5-year event-free survival (freedom from PAH hospitalization, transplantation, or death) for subgroups with differing NE changes relative to the optimal prognostic threshold (168.5 ng/mL): (1) stayed less than threshold (n = 24, dark blue), (2) increased from less than to more than threshold (n = 11, red), (3) decreased from more than to less than threshold (n = 18, light blue), or (4) remained more than threshold (n = 17, gray). PAH hospitalizations were for right heart failure, syncope, arrhythmia, hemoptysis, or therapy escalation. NE = neutrophil elastase; NYHA FC = New York Heart Association functional class; PAH = pulmonary arterial hypertension.
Analysis of NE changes relative to the established prognostic threshold (168.5 ng/mL) revealed that 5-year event-free survival was poor when the NE level increased from less than to more than the threshold (9% [CI, 2%-59%]), was intermediate if NE level remained at more than the threshold (41% [CI, 23%-73%]), and was more favorable when NE level either remained less than the threshold (75% [CI, 59%-95%]) or fell from more than to less than the threshold (67% [CI, 48%-92%]; across-group P = .004) (Fig 5B).
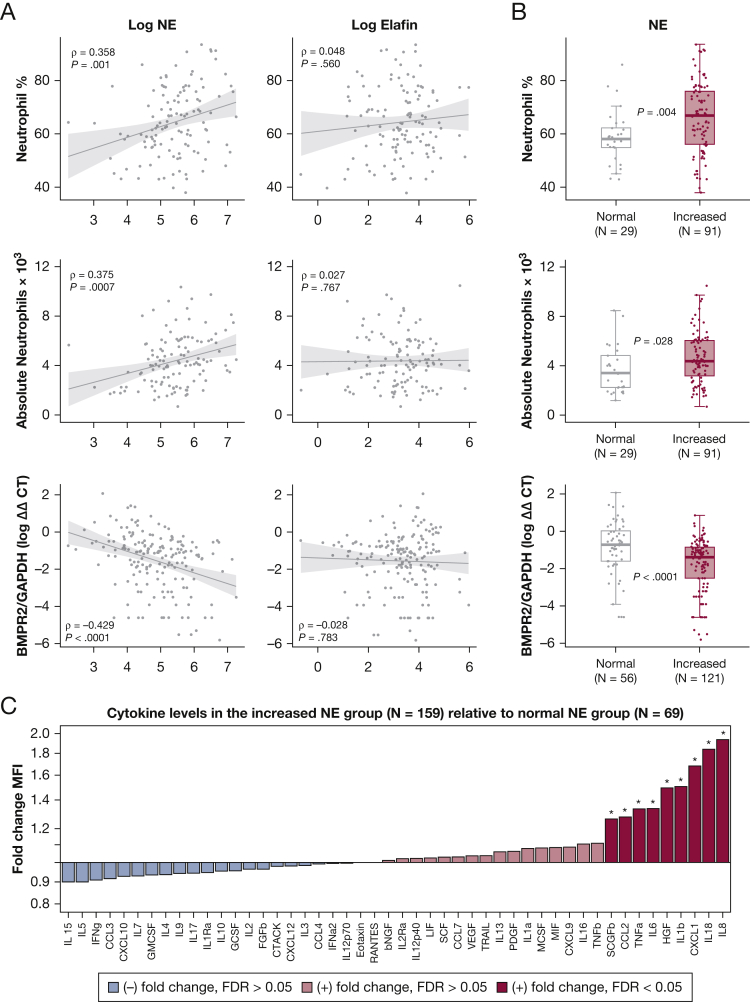
Relevant Ancillary Biological Measurements
The NE level correlated with the circulating absolute neutrophil count (ρ = 0.375, P = .0007) and neutrophil percentage (ρ = 0.368; P = .001), but not with other leukocyte subsets (Fig 6A, 6B, e-Table 8). The level of NE also correlated negatively with BMPR2 expression in blood (ρ = –0.429; P < .0001) (Fig 6A, 6B). Among patients with NE levels of more than the PAH discrimination cutoff (> 138.4 ng/mL), 9 of 48 measured cytokines were overexpressed (false-discovery rate < 0.05: IL-8, IL-18, C-X-C motif chemokine ligand 1, IL-1β, hepatocyte growth factor, IL-6, tumor necrosis factor-α, C-C motif chemokine ligand 2, and stem cell growth factor-β) (Fig 6C). In analysis of the 14 measured cytokines that have been implicated previously as prognostic PAH biomarkers,9,10,36, 37, 38, 39 IL-1β, IL-6, IL-10, and tumor necrosis factor-α were confirmed as independent predictors of survival in the cohort (e-Table 9). Even after adjustment for the effects of these cytokines, NE levels exceeding the prognostic threshold were associated with increased mortality risk (HR, 2.62; CI, 1.42-4.87; P = .001) (e-Table 10).
Figure 6.
A-C, NE is associated with ancillary biomarkers that are relevant to pathobiological features. A, Scatterplots showing the relationship of NE and elafin to circulating neutrophil counts (percentage and absolute) and BMPR2 messenger RNA expression levels in blood (real-time quantitative reverse transcriptase polymerase chain reaction measurements quantified by ΔΔCT method, relative to the GAPDH gene). Spearman ρ and associated P values are shown. Data are from patients who had measurements of neutrophil counts (n = 120/249) or BMPR2 expression (n = 177/249) on the same day as NE and elafin levels. B, Box-and-whisker plots displaying neutrophil counts and BMPR2 expression among patients with increased NE levels (> 134.8 ng/mL, the PAH discrimination cutoff from Fig 1C) vs those with normal NE levels. C, Cytokine fold-changes (immunoassay-detected MFI) in patients with increased NE levels relative to those with normal NE levels. Only same-day measurements were captured (n = 228/249). ∗Differentially expressed cytokines (false-discovery rate < 0.05 in significance analysis of microarrays). BMPR2 = bone morphogenetic protein receptor 2; bNGF = nerve growth factor beta; CCL = C-C motif chemokine ligand; CTACK = cutaneous T-cell-attracting chemokine; CXCL = C-X-C motif chemokine ligand; ΔΔCT = delta delta cycle threshold; FGFb = fibroblast growth factor basic; GAPDH = glyceraldehyde 3-phosphate dehydrogenase; GCSF = granulocyte colony stimulating factor; GMCSF = granulocyte macrophage colony stimulating factor; IFN (a2 or g) = interferon-(alpha2 or gamma); HGF = hepatocyte growth factor; IL = interleukin; IL1 (a, b, or Ra) = IL-1 (alpha, beta, or receptor antagonist); IL12 (p40 or p70) = IL-12 (subunit p40 or p70); IL2Ra = IL-2 receptor subunit alpha; LIF = leukemia inhibitory factor; MCSF = macrophage colony stimulating factor; MIF = macrophage migration inhibitory factor; MFI = median fluorescence intensity; NE = neutrophil elastase; PDGF = platelet-derived growth factor; RANTES = regulated on activation, normal T-cell expressed and secreted; SCF = stem cell factor; SCGFb = stem cell growth factor beta; TNF (a or b) = tumor necrosis factor-(alpha or beta); TRAIL = TNF-related apoptosis-inducing ligand; VEGF = vascular endothelial growth factor.
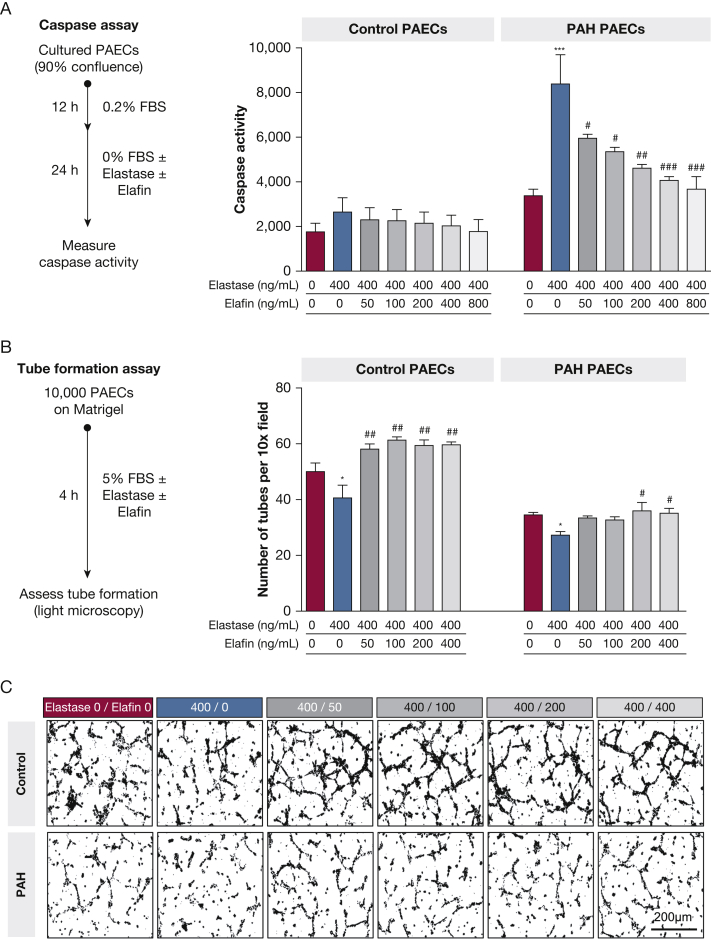
Effect of NE and Elafin Levels on PAEC Homeostasis
In studies of cultured PAECs from PAH and control lungs (e-Table 11), NE treatment (400 ng/mL) induced apoptosis (caspase activity) in PAH PAECs, but not in control PAECs (Fig 7A). Elafin prevented apoptosis in a dose-dependent manner, yet a high elafin dose (400 ng/mL) was required to normalize caspase activity. NE impaired angiogenesis (tube formation) in PAH and control PAECs (Fig 7B, 7C), although untreated PAH PAECs formed fewer tubes at baseline. A higher elafin dose was required to restore angiogenesis in PAH PAECs (200 ng/mL) than in control PAECs (50 ng/mL).
Figure 7.
A-C, NE and elafin imbalance disturbs PAEC homeostasis in PAH. PAECs were isolated from the lungs of PAH patients who were transplant recipients (n = 3) and control donors whose explants were not selected for transplantation (n = 3). These PAECs were used to study the effects of NE and elafin on apoptosis and angiogenesis. A, Apoptosis (caspase 3/7 assay): cultured PAECs were grown to 90% confluence, growth arrested (12 h), then exposed to treatment conditions (24 h). Graphs show caspase activity (mean ± SE) for control and PAH PAECs that were untreated (red), exposed to NE alone (400 ng/mL, blue), or NE plus elafin treatment (50-800 ng/mL dose series, grayscale). B, Angiogenesis (tube formation assay): PAECs were seeded on Matrigel (Corning Inc.) and exposed to the treatment conditions, and then tube formation was assessed by light microscopy. Graphs show the number of formed tubes per ×10 field. C, Corresponding light microscopy images of tube formation. Comparisons across conditions (one-way analysis of variance, Bonferroni adjustment): NE alone (blue) vs untreated (red; ∗∗∗P < .001,∗∗P < .01, ∗P < .05) or vs each NE to elafin dose ratio (gray; ###P < .001, ##P < .01, #P < .05). FBS = fetal bovine serum; NE = neutrophil elastase; PAEC = pulmonary artery endothelial cell; PAH = pulmonary arterial hypertension.
Discussion
Our work builds on prior preclinical studies which implicated NE in PAH pathogenesis and demonstrated that the NE inhibitor elafin induced disease regression.11, 12, 13, 14, 15,17,24 To the best of our knowledge, we conducted the first analysis of circulating NE and elafin in relation to PAH clinical features and outcomes. NE levels are increased across patients, associating with greater clinical disease severity and mortality risk, elevated neutrophil counts, high proinflammatory cytokine levels, and reduced BMPR2 expression in blood. A relative elafin deficiency also exists in PAH, suggesting that the excessive NE is inadequately counterbalanced. Circulating NE shows promise as an indicator of disease activity, and forthcoming elafin clinical trials should incorporate this target-specific biomarker.
We observed increased NE levels regardless of PAH subtype, which harmonizes with the subtype-independent inflammation seen in other studies.8,29 Although NE has a recognized pathogenic role in various chronic inflammatory diseases,40 we found that levels are not more elevated in connective tissue disease-associated PAH than other forms of PAH. NE is increased whether PAH is incident or prevalent, supporting the notion that neutrophils remain activated as dysregulated immunity gives rise to chronic inflammation.1,4 NE also is an indicator of clinical status because levels correlate with markers of disease severity, track with clinical trends over time, prognosticate survival, and add incremental value to established risk-stratification scores. Moreover, the NE level offers prognostic value independent of other cytokines implicated in PAH survival. Taken with preclinical data that link NE activity to PAH arteriopathy, our observations provide complementary evidence suggesting that excessive NE levels contribute to PAH progression, rather than reflecting an epiphenomenon or a nonspecific chronic inflammatory state.
NE is theorized to contribute to pathogenesis in part by perpetuating inflammation, and our data support this hypothesis. Several patients exhibited highly amplified NE levels (Fig 1B shows long-tailed distribution), which may be explained by mechanisms of positive regulation. NE excess is associated with high neutrophil counts and proinflammatory cytokines known to foster neutrophil function and survival (Fig 6C).11,19,41 NE also inversely correlates with BMPR2 expression, which is noteworthy because NE is thought to promote inflammation and vasculopathy via suppression of BMPR2 signaling.21,22,42
The effect of current PAH therapies on NE remains unclear. Baseline NE levels were independent of background therapies, yet prostacyclin initiation was associated with a significant NE downtrend over time. This could reflect a direct prostacyclin effect, because iloprost previously was shown to suppress protease release from isolated PAH neutrophils.12 Alternatively, the NE downtrend may have reflected improvement in disease severity among the prostacyclin-treated subgroup (e-Table 12), rather than a direct pharmacologic action.
In normal homeostasis, deleterious protease effects are regulated tightly by antiproteases. Therefore, increased elafin production may be expected in response to NE excess. Our paradoxical finding of elafin deficiency suggests that patients with PAH are particularly susceptible to NE-induced injury. Consistent with this finding, arterial elastic fibers are assembled poorly and are prone to degradation in PAH.43 The cause of elafin deficiency is unknown. A non-PAH study of alveolar cells suggested that NE overabundance may inhibit elafin production,44 although NE and elafin did not correlate inversely in PAH. Interestingly, women showed more pronounced elafin deficiency. Whether this sex-related difference stems from epigenetic changes, hormone influences, or other factors warrants investigation.
We anticipated higher elafin levels among patients with favorable clinical profiles, although elafin did not associate with disease severity or outcomes. Little precedence exists for biomarker studies of endogenous inhibitors used as therapeutics. However, in α-1-antitrypsin deficiency, the magnitude of α-1-antitrypsin deficit does not reliably predict the clinical phenotype.45 Possibly, elafin was not a marker of PAH clinical severity because measurement of elafin alone may inadequately capture interpatient differences in endogenous NE inhibition. In some patients, other NE inhibitors may be active or neutrophil extracellular traps could create functional elafin resistance by shielding an NE pool from inhibition.46 It is also plausible that endogenous elafin levels are too low across patients, given the significant NE excess, to yield clinically discernable inhibition. Our studies of isolated PAECs reinforce this hypothesis. NE exposure at the high level observed in patients’ blood (400 ng/mL; 75th percentile) expectedly induced PAEC apoptosis and attenuated angiogenesis. Elafin treatment rescued PAEC homeostasis, but the dose required (400 ng/mL; 1:1 NE to elafin ratio) exceeded circulating levels found in patients (n = 0/249 with elafin > 400 ng/mL; only n = 14/249 with NE to elafin ratio of ≤ 1:1). Given these PAEC findings and the relative elafin deficiency demonstrated across patients, we postulate that the observed nonlinear relationship between NE and mortality risk could be explained by a threshold phenomenon in which endogenous antiprotease activity is overwhelmed when NE exceeds a certain level. Elafin supplementation may be necessary to counteract this NE excess in PAH.
Our observational study design has limitations, although we minimized bias by enrolling consecutive patients regardless of PAH subtype, adhering to strict bio-banking protocols, using validated assays, and capturing broad phenotypic data to identify cofounding relationships. We did not measure biomarkers concomitantly in the lung; thus, it remains unknown whether circulating NE reflects activity in the pulmonary vasculature. Diseased comparators with other cardiopulmonary or inflammatory conditions were not included. If NE levels were found to be increased similarly and to track with symptom severity in left heart failure, it would signal that nonpulmonary processes may contribute significantly to circulating levels in PAH. We only measured total NE, without fractionating the biologically active and inhibited pools. We did not differentiate exosome-bound NE, which may be more pathogenic.47 Also, procedures differed between the Stanford cohort (central venous sample, enzyme-linked immunoabsorbent assay) and validation cohorts (peripheral sample, SomaScan). These differences precluded validation of the prognostic NE threshold, because SomaScan technology does not quantify absolute concentration. Despite differing cohort characteristics and biomarker platforms, the nonlinear threshold-type relationship between high NE levels and worse PAH outcomes was generalizable.
Conducted as part of the bench-to-beside translation of elafin therapy, our study demonstrated that the target-specific biomarker NE is increased across PAH subtypes and is associated with clinical severity and outcomes. Given its relevance to drug mechanism, pathobiological features, and clinical disease state, NE is a biomarker that could aid therapeutic development of elafin. Experts cite the need for more efficient PAH trials of novel therapies, calling for biomarker-driven strategies to maximize knowledge gain and to reduce participant risk exposure.26,27 In our current phase 1 trial evaluating the safety and tolerability of elafin (ClinicalTrials.gov Identifier: NCT03522935), we are measuring NE levels to provide pharmacodynamic insights. Because no PAH cohort has received elafin to date, we have not yet tested our hypothesis that NE levels may predict treatment response. NE-stratified randomization, adaptive learning, or a split-phase design could be implemented in the next trial phase to identify responders based on biomarker levels. This information could be used to inform cohort enrichment and to increase power in subsequent trials.48 We speculate that elafin responses may be variable given the wide NE distribution observed in PAH. Among some patients, NE levels are normal and neutrophils may be less involved in pathogenesis. Conversely, others have amplified NE levels and more severe disease. Trials of therapies targeting inflammation especially may benefit from biomarker-driven strategies, because a recent study identified PAH immune phenotypes with distinct inflammatory profiles that stratify subgroups with different clinical risk.29 Our observational study was not designed to establish NE as a prognostic biomarker or surrogate end point with immediate clinical applicability, because large-scale prospective validation would be required. However, based on our findings, NE warrants attention in the context of future PAH studies aiming to investigate immune-targeting therapies, to identify disease endotypes, or to develop novel risk assessment approaches.
Take-home Points.
Study question: Are blood levels of neutrophil elastase (NE) and the NE inhibitor elafin abnormal and associated with clinical features in PAH?
Results: There is a circulating imbalance of increased NE and deficient elafin across PAH subtypes, and NE levels associate with disease severity, prognosticate long-term outcomes, and add incremental value to established clinical risk stratification scores.
Interpretation: Circulating NE is a promising PAH biomarker which could aid the development of elafin and other novel therapies directed at inflammation, on account of its drug target relevance, its suspected mechanistic role in PAH pathogenesis, and its association with clinical disease severity and progression.
Acknowledgments
Author contributions: A. J. S. is the author responsible for all content of the manuscript. A. J. S., M. R., and R. T. Z. prepared the manuscript. A. J. S., M. B.-R., R. D. B., M. R. N., M. R., and R. T. Z. designed the study. P. A. D. R., A. H., and F. H. had central roles in sample acquisition and clinical data extraction. E. S. facilitated experimental assays and K. M. conducted cell culture experiments. C. J. R., E. M. S., S. G., M. R. W., and N. W. M. contributed data for validation cohorts. A. J. S., M. R., and R. T. Z. analyzed the study. K. M., S. T., M. B.-R., F. H., and M. R. N. helped with data interpretation and advised on statistical analysis. A. J. S., K. M., C. J. R., S. T., F. H., E. S., M. B.-R., S. G., M. R. W., N. W. M., M. R. N., M. R., and R. T. Z. participated in manuscript revision.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: M. R. and R. T. Z. lead an investigator-initiated phase 1 clinical trial evaluating the safety and tolerability of subcutaneous Elafin. C. J. R. received personal fees from United Therapeutics and Jannsen Pharmaceuticals outside the submitted research. E. S. served as a scientific advisor for Selten Pharma, Inc., outside the submitted work. None declared (A. J. S., S. T., P. A. D. R., A. H., F. H., M. B.-R., R. D. B., S. G., M. R. W., N. W. M., M. R. N.).
Roleofsponsors: Beyond the financial support granted, none of the funding sources provided any input or contribution to the development of this research or manuscript.
∗British Heart Foundation/Medical Research Council UK PAH Consortium Collaborators: Investigators and coordinators from all 10 UK study sites allowed for our inclusion of validation cohort data. University of Cambridge (Cambridge, England), organizing center: Nicholas W. Morrell (primary investigator), Stefan Gräf (lead data scientist), Emilia M. Swietlik (clinical phenotyping lead), and Carmen M. Treacy and Jennifer M. Martin (cohort coordinators). Freeman Hospital (Newcastle, England): James Lordan (primary investigator) and Alan Greenhalgh and Debbie Shipley (coordinators). Golden Jubilee National Hospital (Glasgow, Scotland): Colin Church (primary investigator) and Val Irvine and Fiona Johnston (coordinators). Imperial College Healthcare (London, England): Martin R. Wilkins (primary investigator), Christopher J. Rhodes (coinvestigator), and Souad Ali (coordinator). Royal Papworth Hospital (Cambridge, England): Joanna Pepke-Zaba (primary investigator) and Gary Polwarth (coordinator). Royal Brompton Hospital (London, England): S. John Wort (primary investigator) and Rosa Da Costa (coordinator). Royal Free London Hospital (London, England): Gerry Goghlan (primary investigator) and Tani Ngcozana and Ivy Wanjiku (coordinators). Royal Hallamshire Hospital (Sheffield, England): David Kiely (primary investigator) and Sara Walker and Kathryn Birchall (coordinators). Royal United Hospitals NHS Foundation Trust (Bath, England): Robert Mackenzie-Ross (primary investigator) and Oliver Griffiths (coordinator). Great Ormond Street Hospital (London, England): Shahin Moledina (primary investigator) and Lynsay MacDonald and Eleni Tamvaki (coordinators).
Othercontributions: The authors thank the Vera Moulton Wall Center for Pulmonary Vascular Disease at Stanford for the multimodal support, in particular the leadership team (Jeffery Feinstein, MD, Mark Krasnow, JD, and coauthor Mark Nicolls, MD); the Vera Moulton Wall Center biobank research coordinators, including Jordan Burgess, BS, Audrey Inglis, BA, Alex Yacob, BS, and Devon Kelley, MS; Carlos Milla, MD, and his laboratory colleagues for facilitating enzyme-linked immunoabsorbent assay quantification of biomarkers; all Pulmonary Hypertension Breakthrough Initiative investigators (e-Appendix 7), whose work permitted us to perform studies of cultured pulmonary artery endothelial cells; and the Stanford Adult Pulmonary Hypertension Service, namely, current and former clinicians (Yon Sung, MD, Kristina Kudelko, MD, Vinicio de Jesus Perez, MD, Julie Lai, MD, Adam Andruska, MD, Cyrus Kholdani, MD, John Faul, MD, Jeremy Feldman, MD, David Poch, MD, Ali Khan, MD, Karla de la Cruz, MD, Richard Wells, MD, Lana Melendres-Grove, MD, Shigeki Saito, MD, Olga Fortenko, MD, Nathan Brunner, MD, and Krithika Ramachandran, MD) and nurse practitioners and physician assistants (Juliana Liu, NP, Jordan Sloan, PA, Evelyn Lopez, NP, Jaclyn Doyle, NP, Aileen Lin, NP, and Janine Martin, RN).
Additional information: The e-Appendixes, e-Figures, and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
∗Collaborators from the British Heart Foundation/Medical Research Council UK PAH Consortium are listed in the Acknowledgments.
FUNDING/SUPPORT: This study was supported primarily by the National Institutes of Health (NIH) [Grant P01 HL108797]. A. J. S. is supported by the National Heart, Lung, and Blood Institute of the NIH [Grant 1K23HL151892] and the American Thoracic Society Foundation/Pulmonary Hypertension Association Research Fellowship. E. S. is supported by the National Heart, Lung, and Blood Institute of the NIH. K. M. received grant support from the Japan Heart Foundation/Bayer Yakuhin Research Grant Abroad and the Uehara Memorial Foundation during conduct of the study. E. M. S. received grant support from the British Heart Foundation and Medical Research Council during conduct of the study. Data were shared from the UK National Cohort Study of Idiopathic and Heritable PAH, which is supported by the National Institute of Health Research BioResource, the British Heart Foundation [Grant SP/12/12/29836], and the UKMedical Research Council [Grant MR/K020919/1]. The study used human lung tissue obtained through the Pulmonary Hypertension Breakthrough Initiative, which is supported by the National Institutes of Health [Grant R24 HL123767] and the Cardiovascular and Medical Research and Education Foundation.
Supplementary Data
References
- 1.Rabinovitch M., Guignabert C., Humbert M., Nicolls M.R. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res. 2014;115(1):165–175. doi: 10.1161/CIRCRESAHA.113.301141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huertas A., Perros F., Tu L. Immune dysregulation and endothelial dysfunction in pulmonary arterial hypertension: a complex interplay. Circulation. 2014;129(12):1332–1340. doi: 10.1161/CIRCULATIONAHA.113.004555. [DOI] [PubMed] [Google Scholar]
- 3.Tamosiuniene R., Tian W., Dhillon G. Regulatory T cells limit vascular endothelial injury and prevent pulmonary hypertension. Circ Res. 2011;109(8):867–879. doi: 10.1161/CIRCRESAHA.110.236927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicolls M.R., Voelkel N.F. The roles of immunity in the prevention and evolution of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2017;195(10):1292–1299. doi: 10.1164/rccm.201608-1630PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Humbert M., Morrell N.W., Archer S.L. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43(12 suppl S):13S–24S. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 6.Thenappan T., Chan S.Y., Weir E.K. Role of extracellular matrix in the pathogenesis of pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol. 2018;315(5):H1322–H1331. doi: 10.1152/ajpheart.00136.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savai R., Pullamsetti S.S., Kolbe J. Immune and inflammatory cell involvement in the pathology of idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186(9):897–908. doi: 10.1164/rccm.201202-0335OC. [DOI] [PubMed] [Google Scholar]
- 8.Stacher E., Graham B.B., Hunt J.M. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186(3):261–272. doi: 10.1164/rccm.201201-0164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soon E., Holmes A.M., Treacy C.M. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation. 2010;122(9):920–927. doi: 10.1161/CIRCULATIONAHA.109.933762. [DOI] [PubMed] [Google Scholar]
- 10.Cracowski J.L., Chabot F., Labarere J. Proinflammatory cytokine levels are linked to death in pulmonary arterial hypertension. Eur Respir J. 2014;43(3):915–917. doi: 10.1183/09031936.00151313. [DOI] [PubMed] [Google Scholar]
- 11.Taylor S., Dirir O., Zamanian R.T., Rabinovitch M., Thompson A.A.R. The role of neutrophils and neutrophil elastase in pulmonary arterial hypertension. Front Med (Lausanne) 2018;5:217. doi: 10.3389/fmed.2018.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rose F., Hattar K., Gakisch S. Increased neutrophil mediator release in patients with pulmonary hypertension—suppression by inhaled iloprost. Thromb Haemost. 2003;90(6):1141–1149. doi: 10.1160/TH03-03-0173. [DOI] [PubMed] [Google Scholar]
- 13.Kim Y.M., Haghighat L., Spiekerkoetter E. Neutrophil elastase is produced by pulmonary artery smooth muscle cells and is linked to neointimal lesions. Am J Pathol. 2011;179(3):1560–1572. doi: 10.1016/j.ajpath.2011.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu L., Wigle D., Hinek A. The endogenous vascular elastase that governs development and progression of monocrotaline-induced pulmonary hypertension in rats is a novel enzyme related to the serine proteinase adipsin. J Clin Invest. 1994;94(3):1163–1171. doi: 10.1172/JCI117432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson K., Rabinovitch M. Exogenous leukocyte and endogenous elastases can mediate mitogenic activity in pulmonary artery smooth muscle cells by release of extracellular-matrix bound basic fibroblast growth factor. J Cell Physiol. 1996;166(3):495–505. doi: 10.1002/(SICI)1097-4652(199603)166:3<495::AID-JCP4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 16.Tu L., Dewachter L., Gore B. Autocrine fibroblast growth factor-2 signaling contributes to altered endothelial phenotype in pulmonary hypertension. Am J Respir Cell Mol Biol. 2011;45(2):311–322. doi: 10.1165/rcmb.2010-0317OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cowan K.N., Jones P.L., Rabinovitch M. Elastase and matrix metalloproteinase inhibitors induce regression, and tenascin-C antisense prevents progression, of vascular disease. J Clin Invest. 2000;105(1):21–34. doi: 10.1172/JCI6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Senior R.M., Griffin G.L., Mecham R.P. Chemotactic responses of fibroblasts to tropoelastin and elastin-derived peptides. J Clin Invest. 1982;70(3):614–618. doi: 10.1172/JCI110654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alfaidi M., Wilson H., Daigneault M. Neutrophil elastase promotes interleukin-1beta secretion from human coronary endothelium. J Biol Chem. 2015;290(40):24067–24078. doi: 10.1074/jbc.M115.659029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valenzuela-Fernandez A., Planchenault T., Baleux F. Leukocyte elastase negatively regulates stromal cell-derived factor-1 (SDF-1)/CXCR4 binding and functions by amino-terminal processing of SDF-1 and CXCR4. J Biol Chem. 2002;277(18):15677–15689. doi: 10.1074/jbc.M111388200. [DOI] [PubMed] [Google Scholar]
- 21.Burton V.J., Ciuclan L.I., Holmes A.M., Rodman D.M., Walker C., Budd D.C. Bone morphogenetic protein receptor II regulates pulmonary artery endothelial cell barrier function. Blood. 2011;117(1):333–341. doi: 10.1182/blood-2010-05-285973. [DOI] [PubMed] [Google Scholar]
- 22.Li W., Hoenderos K., Salmon R.M. Neutrophil and redox dependent proteolysis of bone morphogenetic protein 9: potential role in the pathogenesis of pulmonary arterial hypertension. Thorax. 2013;68 A146-A146. [Google Scholar]
- 23.Cowan K.N., Heilbut A., Humpl T., Lam C., Ito S., Rabinovitch M. Complete reversal of fatal pulmonary hypertension in rats by a serine elastase inhibitor. Nat Med. 2000;6(6):698–702. doi: 10.1038/76282. [DOI] [PubMed] [Google Scholar]
- 24.Nickel N.P., Spiekerkoetter E., Gu M. Elafin reverses pulmonary hypertension via caveolin-1-dependent bone morphogenetic protein signaling. Am J Respir Crit Care Med. 2015;191(11):1273–1286. doi: 10.1164/rccm.201412-2291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sa S., Gu M., Chappell J. Induced pluripotent stem cell model of pulmonary arterial hypertension reveals novel gene expression and patient specificity. Am J Respir Crit Care Med. 2017;195(7):930–941. doi: 10.1164/rccm.201606-1200OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sitbon O., Gomberg-Maitland M., Granton J. Clinical trial design and new therapies for pulmonary arterial hypertension. Eur Respir J. 2019;53(1):1801908. doi: 10.1183/13993003.01908-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grieve A.P., Chow S.C., Curram J. Advancing clinical trial design in pulmonary hypertension. Pulm Circ. 2013;3(1):217–225. doi: 10.4103/2045-8932.109933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simonneau G., Gatzoulis M.A., Adatia I. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 suppl):D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 29.Sweatt A.J., Hedlin H.K., Balasubramanian V. Discovery of distinct immune phenotypes using machine learning in pulmonary arterial hypertension. Circ Res. 2019;124(6):904–919. doi: 10.1161/CIRCRESAHA.118.313911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sweatt A., Wells R., Purington N. BMPR2 expression is reduced in blood across PAH subtypes but does not reflect disease severity. Am J Respir Crit Care Med. 2018;197(9):A2449. [Google Scholar]
- 31.Rhodes C.J., Wharton J., Ghataorhe P. Plasma proteome analysis in patients with pulmonary arterial hypertension: an observational cohort study. Lancet Respir Med. 2017;5(9):717–726. doi: 10.1016/S2213-2600(17)30161-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diebold I., Hennigs J.K., Miyagawa K. BMPR2 preserves mitochondrial function and DNA during reoxygenation to promote endothelial cell survival and reverse pulmonary hypertension. Cell Metab. 2015;21(4):596–608. doi: 10.1016/j.cmet.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benza R.L., Gomberg-Maitland M., Elliott C.G. Predicting survival in patients with pulmonary arterial hypertension: the REVEAL risk score calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies. Chest. 2019;156(2):323–337. doi: 10.1016/j.chest.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Boucly A., Weatherald J., Savale L. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J. 2017;50(2):1700889. doi: 10.1183/13993003.00889-2017. [DOI] [PubMed] [Google Scholar]
- 35.Hoeper M.M., Kramer T., Pan Z. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J. 2017;50(2):1700740. doi: 10.1183/13993003.00740-2017. [DOI] [PubMed] [Google Scholar]
- 36.Heresi G.A., Aytekin M., Newman J., Dweik R.A. CXC-chemokine ligand 10 in idiopathic pulmonary arterial hypertension: marker of improved survival. Lung. 2010;188(3):191–197. doi: 10.1007/s00408-010-9232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCullagh B.N., Costello C.M., Li L. Elevated plasma CXCL12alpha is associated with a poorer prognosis in pulmonary arterial hypertension. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0123709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joshi A.A., Davey R., Rao Y., Shen K., Benza R.L., Raina A. Association between cytokines and functional, hemodynamic parameters, and clinical outcomes in pulmonary arterial hypertension. Pulm Circ. 2018;8(3) doi: 10.1177/2045894018794051. 2045894018794051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amsallem M., Sweatt A.J., Arthur Ataam J. Targeted proteomics of right heart adaptation to pulmonary arterial hypertension. Eur Respir J. 2021;57(4):2002428. doi: 10.1183/13993003.02428-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doring G. The role of neutrophil elastase in chronic inflammation. Am J Respir Crit Care Med. 1994;150(6 pt 2):S114–S117. doi: 10.1164/ajrccm/150.6_Pt_2.S114. [DOI] [PubMed] [Google Scholar]
- 41.Martin C., Burdon P.C., Bridger G., Gutierrez-Ramos J.C., Williams T.J., Rankin S.M. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003;19(4):583–593. doi: 10.1016/s1074-7613(03)00263-2. [DOI] [PubMed] [Google Scholar]
- 42.Long L., Ormiston M.L., Yang X. Selective enhancement of endothelial BMPR-II with BMP9 reverses pulmonary arterial hypertension. Nat Med. 2015;21(7):777–785. doi: 10.1038/nm.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tojais N.F., Cao A., Lai Y.J. Codependence of bone morphogenetic protein receptor 2 and transforming growth factor-beta in elastic fiber assembly and its perturbation in pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol. 2017;37(8):1559–1569. doi: 10.1161/ATVBAHA.117.309696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reid P.T., Marsden M.E., Cunningham G.A., Haslett C., Sallenave J.M. Human neutrophil elastase regulates the expression and secretion of elafin (elastase-specific inhibitor) in type II alveolar epithelial cells. FEBS Lett. 1999;457(1):33–37. doi: 10.1016/s0014-5793(99)01004-2. [DOI] [PubMed] [Google Scholar]
- 45.Torres-Duran M., Lopez-Campos J.L., Barrecheguren M. Alpha-1 antitrypsin deficiency: outstanding questions and future directions. Orphanet J Rare Dis. 2018;13(1):114. doi: 10.1186/s13023-018-0856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kolaczkowska E., Jenne C.N., Surewaard B.G. Molecular mechanisms of NET formation and degradation revealed by intravital imaging in the liver vasculature. Nat Commun. 2015;6:6673. doi: 10.1038/ncomms7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Genschmer K.R., Russell D.W., Lal C. Activated PMN exosomes: pathogenic entities causing matrix destruction and disease in the lung. Cell. 2019;176(1-2):113–126 e115. doi: 10.1016/j.cell.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Freidlin B., Korn E.L. Biomarker enrichment strategies: matching trial design to biomarker credentials. Nat Rev Clin Oncol. 2014;11(2):81–90. doi: 10.1038/nrclinonc.2013.218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.