Abstract
OBJECTIVE
Difficulty achieving preset goals (e.g., ≥5% weight loss, ≥150 min of weekly physical activity) in the yearlong National Diabetes Prevention Program (NDPP) can prompt dropout and diminish benefits. We piloted a more patient-centered NDPP adaptation (NDPP-Flex) that promotes a variety of attainable and individually tailored goals to reduce diabetes risks, along with flexibility to adjust goals each week as needed.
RESEARCH DESIGN AND METHODS
Retention, physical activity, weight, and glycated hemoglobin (HbA1c) were evaluated among diverse participants with diabetes risks who received our pilot of NDPP-Flex beginning in January and July 2018 (n = 95), with a planned comparison with standard NDPP delivery in preceding cohorts that launched between September 2016 and October 2017 (n = 245). Both the standard NDPP and NDPP-Flex interventions were 1 year in duration and implemented in phases (i.e., nonrandomized).
RESULTS
Average adjusted retention (e.g., 158.90 ± 15.20 vs. 166.71 ± 9.38 days; P = 0.674), physical activity (157.97 ± 11.91 vs. 175.64 ± 7.54 weekly min; P = 0.231), and weight loss (1.46 ± 0.38% vs. 1.90 ± 0.24%; P = 0.396) were similar between NDPP-Flex versus standard NDPP. However, NDPP-Flex participants had greater HbA1c reduction on average (0.22 ± 0.05% vs. 0.06 ± 0.03%; P = 0.018) and were more likely to have normoglycemia at follow-up (odds ratio 4.62; P = 0.013 [95% CI 1.38–15.50]) than participants in the standard NDPP.
CONCLUSIONS
An adapted, more patient-centered NDPP that focuses on flexible, self-selected goals may be a promising strategy to improve glycemia even in the absence of substantial weight loss.
Introduction
Diabetes affects 13.0% of U.S. adults, with higher prevalence among racial/ethnic minorities and individuals of low socioeconomic status (1). Another 34.5% of U.S. adults are estimated to have prediabetes (1) or elevated glycemia (e.g., glycated hemoglobin [HbA1c] 5.7–6.4%) that can progress to type 2 diabetes (2). In response, the Centers for Disease Control and Prevention (CDC) established the National Diabetes Prevention Program (NDPP) in 2010 and continues to issue updated delivery standards and curricula for dissemination (3). The NDPP seeks to translate successes from the landmark Diabetes Prevention Program (DPP) trial, in which lifestyle intervention led to 7% weight loss and 0.1% improvement in HbA1c at 1 year, reducing diabetes incidence by 58% within 3 years (4). Lifestyle intervention in the DPP trial was primarily delivered individually to participants with impaired glucose tolerance and impaired fasting glucose, who further had completed a 3-week run-in to ensure compliance (5,6). For scaling, the yearlong NDPP uses lower-cost formats (in-person group classes, online, distance-learning, or combined approaches), uses broader eligibility criteria, and does not require glycemic monitoring (3). Rather, the NDPP primarily targets ≥5% weight loss and uses frequent weight monitoring to assess progress (3). Major successes include widespread adoption (e.g., >3,000 organizations have delivered the NDPP [3]) and insurance coverage (e.g., Medicare coverage began in 2018 [7]), yet substantial challenges remain to impact diabetes prevalence (8).
Increasing effectiveness of the NDPP is a key objective to reduce diabetes prevalence (8). Concerns include that weight loss outcomes are suboptimal and that weight change alone may be misleading as an indicator of effectiveness. Nearly three-quarters of participants (71.7%) do not achieve the ≥5% weight loss target (3), and racial/ethnic minority, low-income, and younger participants lose about half as much weight as their counterparts (9–11). Previous efforts to improve NDPP effectiveness have focused on strategies to address poor attendance (3), such as partnering with health care providers for referrals and providing incentives (12). In turn, greater attendance often leads to more weight loss (3,11,13,14), but is not always sufficient (10,15,16). For example, financial incentives increased attendance but without more weight loss among Medicaid beneficiaries (16). Additional strategies to improve health outcomes, including glycemia, appear needed. Although weight loss was highly protective at first in the DPP (17), follow-up study revealed that more weight loss was paradoxically associated with higher diabetes incidence, attributed to weight regain over time (18,19). However, even a temporary return to normal glucose regulation had substantial lasting benefit, with a 56% reduction in diabetes incidence at ∼10 years compared with participants who did not attain normal glucose regulation at least once (18). Moreover, the DPP’s lifestyle intervention focused narrowly on weight loss through low-fat diet and moderate physical activity (5), which was extended to the NDPP (20). Yet, newer consensus from the American Diabetes Association (ADA) is that other lifestyle approaches (e.g., Mediterranean diet) can improve glycemia without weight loss and that interventions should be flexible to accommodate personal preferences (21).
Unachieved lifestyle goals may also diminish self-efficacy (a key construct of the Health Belief Model for behavior change [22]), as suggested by the premature dropout of NDPP participants who have difficulty reaching preset goals (23,24). For example, less than half of participants meet the NDPP’s preset physical activity goal (including fewer racial/ethnic minority participants) (9), and each week of goal “failure” is associated with 25% lower likelihood of returning to the next session (24). Adapting the NDPP to promote more attainable and individually tailored goals for risk reduction, plus flexibility to adjust goals over time as needed, may help increase effectiveness. The CDC’s original NDPP curriculum had the most restrictive and challenging goals, including ≥7% weight loss, ≥150 min of weekly physical activity, and ≤25% of calories from fat. By comparison, the latest curriculum (released in March 2016) does incorporate action planning to set three individualized goals at each session, albeit in addition to preset goals for ≥5% weight loss and ≥150 weekly min of physical activity (20). To inform future program delivery, we designed a more patient-centered NDPP adaptation without preset goals (NDPP-Flex). In this study, we report on our pilot of NDPP-Flex, including attendance, physical activity, weight loss, and glycemic outcomes, as compared with implementation of the standard NDPP with the latest curriculum.
Research Design and Methods
Design
We designed NDPP-Flex to align with guidelines for conducting patient-centered outcomes research (25), including through: 1) responsiveness to feedback and confirmatory evidence that preset goals deter participation; 2) developing a flexible goal-setting approach that retains other standard NDPP components and without added costs; 3) minimizing participant burden by assessing glycemic improvement through electronic health records; and 4) assessing the comparative effectiveness of NDPP-Flex versus prior delivery of the standard NDPP. The Colorado Multiple Institutional Review Board approved the program evaluation (16-1093).
Setting
Denver Health is an urban safety-net health care system that is the largest provider of Medicaid and uninsured services in Colorado through its community- and school-based clinics, specialty centers, and hospital in the Denver metropolitan area. Denver Health was an early adopter of the NDPP, receiving federal, state, and intramural funding to provide the NDPP at no cost to patients since 2013.
Participants
We included English- and Spanish-speaking adults who met CDC-established NDPP eligibility criteria, including BMI ≥25 kg/m2 (≥23 kg/m2 if Asian) and prediabetes or former diagnosis of gestational diabetes (26). Prediabetes was based on a laboratory test within the past year indicating a fasting blood glucose of 100–125 mg/dL, blood glucose of 140–199 mg/dL measured 2 h after a 75-g glucose load, or HbA1c of 5.7–6.4%. Gestational diabetes was based on past diagnosis in the medical record or self-reported. Individuals without known prediabetes or past gestational diabetes were also eligible based on a risk-screening questionnaire (27). Individuals were excluded if pregnant or known to have type 2 diabetes at enrollment.
Participants were identified primarily through provider referrals and invited to enroll in new classes that were launched every 3–6 months without fees or monetary incentives. This analysis includes participants from two cohorts of classes that began our pilot of NDPP-Flex in January and July 2018 (n = 95), with a planned comparison with five preceding cohorts of standard NDPP delivery that launched between September 2016 and October 2017 (n = 245). Selecting these comparator groups assures that both arms received the CDC’s latest NDPP curriculum (20) (delivered as standard or adapted in NDPP-Flex) and were preceded by an introductory “pre-session” 1–3 weeks before intervention, which was previously found to improve retention and weight loss (28).
Intervention
The intervention flow diagram is depicted in Fig. 1. In brief, we aimed to follow CDC guidelines in both intervention arms (standard NDPP and NDPP-Flex) by providing in-person group classes consisting of 25 hour-long sessions over 1 year (16 sessions in months 1–6 and 9 sessions in months 7–12). Classes were held in-person in English or Spanish and led by trained, bilingual lay health educators who served as lifestyle coaches. Coaches also contacted participants between sessions to support engagement and behavior change, offer make-up sessions, and provide session reminders. Further details of NDPP delivery are outlined in the CDC’s guidelines that govern features such as staffing, curricula, session planning, contacts, and data collection (26).
Figure 1.
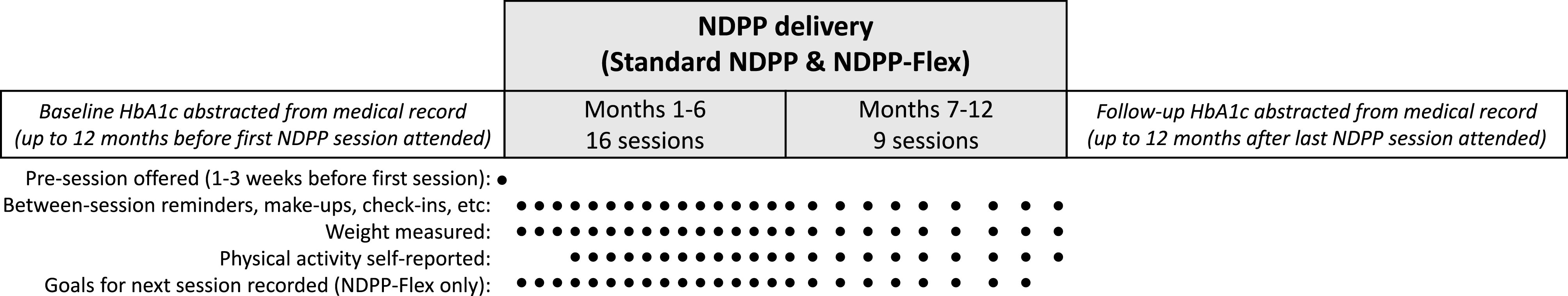
Flow diagram of NDPP delivery and data collection. Standard NDPP cohorts were launched between September 2016 and October 2017. NDPP-Flex cohorts were launched in January and July 2018.
The primary difference between the standard NDPP and NDPP-Flex is the approach to goal-setting. The standard NDPP includes two preset goals to lose ≥5% of starting weight (within 6 months and then maintained for an additional 6 months) and achieve ≥150 min of physical activity of at least moderate intensity each week (20). An additional action-planning worksheet instructs participants to set three more individualized goals at each session: “To lower your risk of diabetes…write three actions you will take. Then check off each action you complete.” The standard NDPP also promotes caloric restriction, but without a specific dietary goal. With NDPP-Flex, coaches modified delivery to: 1) de-emphasize preset goals in favor of more attainable, individually tailored goals for risk-reduction, 2) promote flexibility to adjust goals over time as needed, and 3) avoid all-or-nothing assessments of goal attainment. At each session, coaches provided an alternate goal-setting worksheet (see Supplementary Material) with a simple, fillable format to better accommodate low health literacy (e.g., limit sugary drinks to __ per day). The worksheet and protocol for NDPP-Flex was developed by a team including a dietitian, exercise physiologist, psychologist, diabetes educators, and coaches and further reviewed by a panel of patient stakeholders. Goal choices broadly included cardiovascular activity; strength-training; fruits/vegetables; sweets; fast/junk food; portion control; not eating past fullness; regular meals; water intake; sugary beverages; alcohol; and stress management, plus other write-in options. As goal “failure” can deter attendance to the next NDPP session (24), coaches encouraged participants in NDPP-Flex to set one attainable goal to focus on each week. Then, participants could choose the same, a new, or a modified goal at the next session. Rather than assessing goal attainment, coaches emphasized learning to make sustainable changes through trial and error, while continuing to collect weight and self-reported physical activity for evaluation purposes.
Measures
Demographic characteristics were collected from medical records, including age, sex, and race/ethnicity. Retention was assessed by total number of sessions attended (1–25 sessions) and duration between first and last sessions attended (1–365 days). Physical activity was based on average self-reported weekly minutes of moderate-to-vigorous activity, starting at the fourth session after activity monitoring is introduced in the curriculum (20). Weight was measured at each session on a medical-grade scale by coaches, with weight loss at 12 months based on measurements at the first and last sessions attended (i.e., last value carried forward). Baseline BMI was based on the first session weight, along with height as collected from medical records. To assess glycemic improvement, change in HbA1c from baseline to follow-up was based on laboratory results abstracted from medical records, as HbA1c was not directly collected per CDC guidelines (26). Baseline HbA1c was defined as the closest value to a participant’s first session attended within the prior 12 months. Follow-up HbA1c was based on records within 12 months after the last session attended (using values closest to 3 months after the last session attended, given HbA1c reflects average glycemia within the preceding 3 months). We further categorized follow-up HbA1c levels of <5.7% as normoglycemia, given ADA standards classify HbA1c levels of 5.7–6.4% as prediabetes and ≥6.5% as diabetes (2). Of note, patient-selected goals were not collected in the standard NDPP per CDC guidelines. However, we recorded goals in NDPP-Flex, including the number and type of goals selected at each session.
Analysis
Between-group differences in baseline characteristics (age, sex, race/ethnicity, BMI, and HbA1c), pre-session attendance, class size, retention, physical activity, weight loss, and HbA1c improvement were assessed using paired t tests and χ2 analyses. We further analyzed the frequency of prepost HbA1c testing by group based on how many participants received tests both before and after intervention, as well as the timing of prepost measurement (i.e., number of months prior to the first NDPP session and after the last session when HbA1c was measured). We also conducted sensitivity analyses among participants with prepost HbA1c records. To minimize potential outlier influence, models included winsorized weight and HbA1c change. Multiple linear and logistic regression models then controlled for baseline characteristics (age, sex, race/ethnicity, baseline BMI, and baseline HbA1c), pre-session attendance, class language, and coach (i.e., three coaches delivered the standard NDPP, two of whom also went on to deliver NDPP-Flex), as well as retention, physical activity, and weight loss as applicable. We report descriptive statistics, including mean, SD, or SE, P values, and 95% CIs as applicable. Significance was determined by α < 0.05.
Results
Table 1 presents comparisons of between-group characteristics, with results showing that participants were similar in sex, age, race/ethnicity, baseline BMI, and baseline weight. Differences in baseline HbA1c between the standard NDPP (mean 5.89% [SD 0.28]) and NDPP-Flex (mean 5.96% [SD 0.29]) approached significance (P = 0.065), although clinically similar in presentation. Frequency and timing of prepost HbA1c testing was otherwise comparable, as was pre-session attendance and average class size.
Table 1.
Characteristics of standard NDPP and NDPP-Flex participants (N = 340)
| Standard NDPP (n = 245) | NDPP-Flex (n = 95) | P value | |
|---|---|---|---|
| Age (years) | 48.45 (12.91) | 47.54 (12.91) | 0.552 |
| Female | 196 (80.0%) | 75 (78.9%) | 0.881 |
| Race/ethnicity | |||
| Latino | 170 (70.5%) | 71 (76.3%) | 0.341 |
| Non-Hispanic Black | 25 (10.4%) | 6 (6.5%) | 0.302 |
| Non-Hispanic White | 45 (18.7%) | 15 (16.1%) | 0.637 |
| Baseline weight (kg) | 93.28 (23.69) | 91.13 (25.78) | 0.465 |
| Baseline BMI (kg/m2) | 35.50 (8.03) | 35.40 (7.89) | 0.931 |
| Baseline HbA1c (%) | 5.89 (0.28) | 5.96 (0.29) | 0.065 |
| Prepost HbA1c records available | 127 (51.8%) | 46 (48.4%) | 0.629 |
| Months before first session when baseline HbA1c measured | 3.76 (2.50) | 3.97 (2.67) | 0.630 |
| Months after last session when follow-up HbA1c measured | 5.00 (3.20) | 4.72 (3.25) | 0.618 |
| Attended pre-session prior to NDPP | 216 (88.2%) | 86 (90.5%) | 0.701 |
| Average class size (number of participants) | 15.3 (3.6) | 15.8 (5.6) | 0.846 |
Data presented as frequency (%) for categorical variables and unadjusted mean (SD) for continuous variables, with P values based on paired t tests and χ2 analyses.
Table 2 presents comparisons of program outcomes between the standard NDPP and NDPP-Flex. There were no significant differences in retention, physical activity, or weight loss. Nonetheless, adjusted models showed that NDPP-Flex participants were more likely to have normoglycemia (HbA1c <5.7%) at follow-up (odds ratio 4.62; P = 0.013 [95% CI 1.38–15.50]), with 0.22 ± 0.05% average HbA1c improvement (P = 0.018). Unadjusted differences for frequency of normoglycemia at follow-up were nonsignificant (24.2% vs. 31.7%; P = 0.171), although in a similar direction. Sensitivity analyses were consistent among participants with prepost HbA1c testing.
Table 2.
Outcomes for delivery of the standard NDPP and NDPP-Flex (N = 340)
| Unadjusted | Covariate-adjusted | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Standard NDPP | NDPP-Flex | P value | Standard NDPP | NDPP-Flex | P value | |||||
| Mean ± SE | n | Mean ± SE | n | Mean ± SE | n | Mean ± SE | n | |||
| Main models | ||||||||||
| Duration (1–365 days) | 169.90 ± 8.59 | 245 | 170.19 ± 13.35 | 95 | 0.986 | 166.71 ± 9.38 | 206 | 158.90 ± 15.20 | 85 | 0.674 |
| Sessions attended (1–25) | 10.84 ± 0.48 | 245 | 10.67 ± 0.72 | 95 | 0.848 | 10.57 ± 0.52 | 206 | 10.27 ± 0.84 | 85 | 0.772 |
| Physical activity (weekly minutes) | 177.36 ± 7.38 | 197 | 159.90 ± 11.31 | 82 | 0.190 | 175.64 ± 7.54 | 164 | 157.97 ± 11.91 | 72 | 0.231 |
| Weight loss (%) | 1.68 ± 0.20 | 245 | 1.20 ± 0.32 | 95 | 0.214 | 1.90 ± 0.24 | 164 | 1.46 ± 0.38 | 72 | 0.353 |
| HbA1c improvement (%) | 0.06 ± 0.03 | 127 | 0.21 ± 0.05 | 46 | 0.012 | 0.06 ± 0.03 | 98 | 0.22 ± 0.05 | 40 | 0.018 |
| Normoglycemia at follow-up (%) | 24.2% | 165 | 31.7% | 60 | 0.186 | 24.2% | 99 | 35.0% | 40 | 0.013 |
| Sensitivity analyses | ||||||||||
| For those with prepost HbA1c | ||||||||||
| Duration (1–365 days) | 175.27 ± 11.99 | 127 | 171.83 ± 18.04 | 46 | 0.880 | 175.24 ± 12.44 | 122 | 195.59 ± 21.70 | 45 | 0.551 |
| Sessions attended (1–25) | 11.12 ± 0.69 | 127 | 10.98 ± 1.01 | 46 | 0.921 | 11.00 ± 0.69 | 122 | 10.64 ± 1.21 | 45 | 0.807 |
| Physical activity (weekly minutes) | 165.89 ± 8.88 | 103 | 167.09 ± 16.56 | 41 | 0.946 | 171.07 ± 8.91 | 98 | 152.73 ± 14.73 | 40 | 0.313 |
| Weight loss (%) | 1.71 ± 0.26 | 127 | 1.17 ± 0.47 | 46 | 0.303 | 2.01 ± 0.30 | 98 | 1.33 ± 0.50 | 40 | 0.267 |
| HbA1c improvement (%) | 0.06 ± 0.03 | 127 | 0.21 ± 0.05 | 46 | 0.012 | 0.06 ± 0.03 | 98 | 0.22 ± 0.05 | 40 | 0.018 |
| Normoglycemia at follow-up (%) | 25.2% | 127 | 32.6% | 46 | 0.218 | 24.5% | 98 | 35.0% | 40 | 0.013 |
Data presented as unadjusted and adjusted mean ± SE and corresponding sample size, with boldface indicating significance at P < 0.05. Weight loss and HbA1c improvement were winsorized at the 5th and 95th percentiles. Adjusted models controlled for age, sex, race/ethnicity, baseline BMI, baseline HbA1c, pre-session attendance, class language, and coach, as well as retention, physical activity, and weight loss as applicable. Physical activity was collected starting at the 4th session (when introduced in the curriculum, per delivery guidelines [20]), limiting available data. HbA1c within ±12 months of participation was assessed as available in medical records for approximately half of participants.
Post hoc analyses confirmed that NDPP-Flex participants selected 1.08 goals (SD 0.30) per session on average. NDPP-Flex participants cumulatively chose 3.28 (SD 2.15) different types of goals on average over the course of their participation. The most frequently selected goal was cardiovascular activity (selected at least once by 74.7% of participants), followed by consuming fruits/vegetables (45.3%), more water (41.1%), using a smaller plate (29.5%), and stress management (28.4%). The frequency and type of goal selected (e.g., number of times that a participant selected cardiovascular activity) did not influence glycemia. However, choosing a greater variety of goals over time (e.g., cardiovascular activity, strength training, more fruits/vegetables, and fewer sweets) affected HbA1c improvement, with each additional type of goal selected being associated with 0.06 ± 0.02% HbA1c improvement (P = 0.034).
Conclusions
In order to improve effectiveness of the NDPP for diverse populations, we evaluated a more patient-centered adaptation, NDPP-Flex, that promotes attainable and individually tailored goals to reduce diabetes risks, along with flexibility to adjust goals over time as needed. This study included relatively younger (48 vs. 57 mean years nationally [23]) and more racial/ethnic minority participants (82% vs. 45% nationally [23]) who usually benefit less from standard delivery of the NDPP (3,23). Compared with the standard NDPP, NDPP-Flex did not increase retention, weight loss, or physical activity, but resulted in greater glycemic improvement (0.2% mean HbA1c improvement) and over fourfold likelihood of normoglycemia, which is considered key to diabetes prevention irrespective of weight (18). By comparison, intensive lifestyle intervention in the DPP trial yielded 0.1% mean HbA1c improvement after 1 year (4) and twofold likelihood of normoglycemia at follow-up versus placebo (18). Alternatively, NDPP-Flex may benefit disadvantaged populations by improving glycemia without requiring adherence to preset goals for lifestyle change or completing a full year of intervention. Retention was 170 days in both the standard and adapted approaches, compared with 96 days when previously delivering the NDPP without pre-sessions (28). Longer retention may require removing socioeconomic barriers (e.g., lack of transportation) (29) and expanding delivery of distance-learning programs upon further study (3).
This pilot study has limitations and lacks generalizability. The study design was nonrandomized, although the similarity of baseline characteristics between groups may support outcome comparisons. Without testing for impaired glucose tolerance and impaired fasting glucose as in the original DPP trial (6), there may be other unknown differences in metabolic risk profiles at baseline. Nonetheless, measuring glycemic improvement both linearly (total change in HbA1c) and dichotomously (normal vs. hyperglycemia) may mitigate this concern, as individuals with higher baseline risk may likely attain greater HbA1c improvement after intervention, whereas participants with lower baseline risk may more likely have normoglycemia at follow-up. Although half of participants lacked prepost laboratory testing of HbA1c, obtaining HbA1c values through medical records remains a relative strength given that glycemic outcomes are understudied in previous NDPP evaluations. Optional HbA1c reporting is newly added to the CDC’s revised NDPP delivery guidelines that were released in May 2021 (30), which may help expand evaluation of glycemic outcomes, as well as support ADA recommendations for annual screening (31). Moreover, the revised guidelines newly allow NDPP participants to focus on glycemic improvement without weight loss and define ≥0.2% HbA1c improvement as a successful outcome, coinciding with the average improvement in NDPP-Flex. In contrast, mean HbA1c improvement in our delivery of the standard NDPP was only 0.06%, suggesting that NDPP-Flex may be a preferred approach. Given NDPP-Flex was designed to follow existing CDC guidelines as much as possible, NDPP-Flex participants still received the latest CDC-developed curriculum and may have remained influenced, positively or negatively, by its prescriptive content focusing on weight loss. These participants may have also benefited from reporting goals (i.e., increasing accountability [32]), whereas the standard curriculum does not instruct coaches to collect goals.
A randomized trial of NDPP-Flex appears warranted to confirm findings and underlying mechanisms. For example, glycemic improvement has been linked to self-efficacy and perceived control (33,34), which may result from more patient-centered goal-setting. Goal variety also appeared to improve glycemia in this study. More research on goal-setting to improve glycemia is needed to conclusively inform best practices (35,36), although assessing achievability may be a foremost consideration (32). Above all, the NDPP has relied on the extensive collaboration of cross-sector stakeholders to establish commendable successes in its first decade (7). Our data suggest that further improvements in the NDPP are possible and may improve impact of this landmark intervention. Concurrent efforts also remain needed to improve other aspects of NDPP delivery, such as more screening to identify and refer at-risk individuals, expanded program access, and greater overall uptake (37–39). If successful upon further study, NDPP-Flex could contribute to these collective efforts with a relatively simple adaptation for use by the many organizations delivering the NDPP to help reduce diabetes prevalence and disparities nationwide.
Article Information
Funding. The NDPP at Denver Health was funded by the Amendment 35 Cancer, Cardiovascular Disease and Pulmonary Disease Grant Program administered by the Colorado Department of Public Health and Environment, and an award from America’s Health Insurance Plans in partnership with the Centers for Disease Control and Prevention. Additional support was provided by Denver Health, including the Denver Health Office of Research Pilot Award Program. Manuscript preparation was supported in part by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health (R01DK119478). The Colorado Multiple Institutional Review Board approved this program evaluation project (16-1093).
The contents of this publication are the sole responsibility of the authors and do not represent official views of any organization.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. N.D.R. conceived the study, conducted the data analysis, and wrote the manuscript. K.A.S., P.G.K. and L.P. participated in writing and interpretation. All authors critically reviewed the manuscript, as well as read and approved the final submitted version. N.D.R. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
See accompanying article, p. 2457.
This article contains supplementary material online at https://doi.org/10.2337/figshare.14994912.
This article is featured in a podcast available at https://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1. Centers for Disease Control and Prevention . National Diabetes Statistics Report, 2020: Estimates of Diabetes and Its Burden in the United States. Atlanta, GA, U.S. Department of Health and Human Services, 2020 [Google Scholar]
- 2. American Diabetes Association . 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021;44(Suppl. 1):S15–S33 [DOI] [PubMed] [Google Scholar]
- 3. Gruss SM, Nhim K, Gregg E, Bell M, Luman E, Albright A. Public health approaches to type 2 diabetes prevention: the US National Diabetes Prevention Program and beyond. Curr Diab Rep 2019;19:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Knowler WC, Barrett-Connor E, Fowler SE, et al.; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Diabetes Prevention Program (DPP) Research Group . The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care 2002;25:2165–2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. The Diabetes Prevention Program . The Diabetes Prevention Program. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care 1999;22:623–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burd C, Gruss S, Albright A, Zina A, Schumacher P, Alley D. Translating knowledge into action to prevent type 2 diabetes: Medicare expansion of the National Diabetes Prevention Program lifestyle intervention. Milbank Q 2020;98:172–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ritchie ND. Solving the puzzle to lasting impact of the National Diabetes Prevention Program. Diabetes Care 2020;43:1994–1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ely EK, Gruss SM, Luman ET, et al. A national effort to prevent type 2 diabetes: participant-level evaluation of CDC’s National Diabetes Prevention Program. Diabetes Care 2017;40:1331–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ritchie ND, Sauder KA, Phimphasone-Brady P, Amura CR. Rethinking the National Diabetes Prevention Program for low-income whites. Diabetes Care 2018;41:e56–e57 [DOI] [PubMed] [Google Scholar]
- 11. Sauder KA, Ritchie ND, Crowe B, Cox E, Hudson M, Wadhwa S. Participation and weight loss in online National Diabetes Prevention Programs: a comparison of age and gender subgroups. Transl Behav Med 2021;11:342–350 [DOI] [PubMed] [Google Scholar]
- 12. Nhim K, Gruss SM, Porterfield DS, et al. Using a RE-AIM framework to identify promising practices in National Diabetes Prevention Program implementation. Implement Sci 2019;14:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ritchie ND, Christoe-Frazier L, McFann KK, Havranek EP, Pereira RI. Effect of the National Diabetes Prevention Program on weight loss for English- and Spanish-speaking Latinos. Am J Health Promot 2018;32:812–815 [DOI] [PubMed] [Google Scholar]
- 14. Ritchie ND, Sauder KA, Fabbri S. Reach and effectiveness of the National Diabetes Prevention Program for young women. Am J Prev Med 2017;53:714–718 [DOI] [PubMed] [Google Scholar]
- 15. Ritchie ND, Baucom KJW, Sauder KA. Benefits of participating with a partner in the National Diabetes Prevention Program. Diabetes Care 2020;43:e20–e21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. VanEpps EM, Troxel AB, Villamil E, et al. Effect of process- and outcome-based financial incentives on weight loss among prediabetic New York Medicaid patients: a randomized clinical trial. Am J Health Promot 2019;33:372–380 [DOI] [PubMed] [Google Scholar]
- 17. Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006;29:2102–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Perreault L, Pan Q, Mather KJ, Watson KE, Hamman RF; Diabetes Prevention Program Research Group . Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: results from the Diabetes Prevention Program Outcomes Study. Lancet 2012;379:2243–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Knowler WC, Fowler SE, Hamman RF, et al.; Diabetes Prevention Program Research Group . 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374:1677–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Centers for Disease Control and Prevention . National Diabetes Prevention Program. Accessed 7 August 2017. Available from https://www.cdc.gov/diabetes/prevention/lifestyle-program/curriculum.html
- 21. Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care 2019;42:731–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Champion VL, Skinner CS. The health belief model. In Health Behavior and Health Education. 4th ed. Glanz K, Rimer BK, Viswanath K, Eds. San Francisco, CA, Jossey-Bass, 2008, pp. 45–65. [Google Scholar]
- 23. Cannon MJ, Masalovich S, Ng BP, et al. Retention among participants in the National Diabetes Prevention Program Lifestyle Change Program, 2012-2017. Diabetes Care 2020;43: 2042–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ritchie ND, Carroll JK, Holtrop JS, Havranek EP. Effects of physical activity goal attainment on engagement and outcomes in the National Diabetes Prevention Program. Transl Behav Med 2018;8:932–937 [DOI] [PubMed] [Google Scholar]
- 25. Patient-Centered Outcomes Research Institute . Patient-Centered Outcomes Research, 2013. Accessed 11 June 2021. Available from https://www.pcori.org/research-results/about- our-research/patient-centered-outcomes- research
- 26. Centers for Disease Control and Prevention . Diabetes Prevention Recognition Program: Standards and Operating Procedures, 2018. Accessed 12 June 2021. Available from https://idph.iowa.gov/Portals/1/userfiles/187/DPRP%20Standards.pdf
- 27. Centers for Disease Control and Prevention . CDC Prediabetes Screening Test. Accessed 6 August 2018. Available from https://www.cdc.gov/diabetes/prevention/pdf/prediabetestest.pdf
- 28. Ritchie ND, Kaufmann PG, Gritz RM, Sauder KA, Holtrop JS. Presessions to the National Diabetes Prevention Program may be a promising strategy to improve attendance and weight loss outcomes. Am J Health Promot 2019;33:289–292 [DOI] [PubMed] [Google Scholar]
- 29. Ritchie N, Phimphasone-Brady P, Sauder K, Amura C. Perceived barriers and potential solutions to engagement in the National Diabetes Prevention Program. ADCES Practice 2021;9:16–20 [Google Scholar]
- 30. Center for Disease Control and Prevention . Diabetes Prevention Recognition Program: Standards and Operating Procedures, 2021. Accessed 12 June 2021. Available from https://www.cdc.gov/diabetes/prevention/pdf/dprp-standards.pdf
- 31. American Diabetes Association . 3. Prevention or delay of type 2 diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021;44(Suppl. 1):S34–S39 [DOI] [PubMed] [Google Scholar]
- 32. Hawkes RE, Warren L, Cameron E, French DP. An evaluation of goal setting in the NHS England Diabetes Prevention Programme. Psychol Health 2021;1:1–20 [DOI] [PubMed] [Google Scholar]
- 33. Gonzalez JS, Shreck E, Psaros C, Safren SA. Distress and type 2 diabetes-treatment adherence: a mediating role for perceived control. Health Psychol 2015;34:505–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Indelicato L, Dauriz M, Santi L, et al. Psychological distress, self-efficacy and glycemic control in type 2 diabetes. Nutr Metab Cardiovasc Dis 2017;27:300–306 [DOI] [PubMed] [Google Scholar]
- 35. Fredrix M, McSharry J, Flannery C, Dinneen S, Byrne M. Goal-setting in diabetes self-management: a systematic review and meta-analysis examining content and effectiveness of goal-setting interventions. Psychol Health 2018;33:955–977 [DOI] [PubMed] [Google Scholar]
- 36. Miller CK, Bauman J. Goal setting: an integral component of effective diabetes care. Curr Diab Rep 2014;14:509. [DOI] [PubMed] [Google Scholar]
- 37. Ackermann RT, O’Brien MJ. Evidence and challenges for translation and population impact of the Diabetes Prevention Program. Curr Diab Rep 2020;20:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bergman M. Expanding diabetes prevention: obstacles and potential solutions. Am J Prev Med 2019;57:853–857 [DOI] [PubMed] [Google Scholar]
- 39. Ritchie ND, Baucom KJW, Sauder KA. Current perspectives on the impact of the National Diabetes Prevention Program: building on successes and overcoming challenges. Diabetes Metab Syndr Obes 2020;13:2949–2957 [DOI] [PMC free article] [PubMed] [Google Scholar]


