Abstract
OBJECTIVE
To determine the incidence and risk factors for developing proliferative diabetic retinopathy (PDR), tractional retinal detachment (TRD), and neovascular glaucoma (NVG) at 5 years after the initial diagnosis of type 2 diabetes.
RESEARCH DESIGN AND METHODS
Insured patients aged ≥18 years with newly diagnosed type 2 diabetes and 5 years of continuous enrollment were identified from a nationwide commercial claims database containing data from 2007 to 2015. The incidences of PDR, TRD, and NVG were computed at 5 years following the index diagnosis of type 2 diabetes. Associations between these outcomes and demographic, socioeconomic, and medical factors were tested with multivariable logistic regression.
RESULTS
At 5 years following the initial diagnosis of type 2 diabetes, 1.74% (1,249 of 71,817) of patients had developed PDR, 0.25% of patients had developed TRD, and 0.14% of patients had developed NVG. Insulin use (odds ratio [OR] 3.59, 95% CI 3.16–4.08), maximum HbA1c >9% or >75 mmol/mol (OR 2.10, 95% CI 1.54–2.69), renal disease (OR 2.68, 95% CI 2.09–3.42), peripheral circulatory disorders (OR 1.88, 95% CI 1.25–2.83), neurological disease (OR 1.62, 95% CI 1.24–2.11), and older age (age 65–74 years) at diagnosis (OR 1.62, 95% CI 1.28–2.03) were identified as risk factors for development of PDR at 5 years. Young age (age 18–23 years) at diagnosis (OR 0.46, 95% CI 0.29–0.74), Medicare insurance (OR 0.60, 95% CI 0.70–0.76), morbid obesity (OR 0.72, 95% CI 0.59–0.87), and smoking (OR 0.84, 95% CI 0.70–1.00) were identified as protective factors.
CONCLUSIONS
A subset of patients with type 2 diabetes develop PDR and other neovascular sequelae within the first 5 years following the diagnosis with type 2 diabetes. These patients may benefit from increased efforts for screening and early intervention.
Introduction
Diabetic eye disease is the leading cause of new blindness in those aged 20–64 years in the U.S. (1), with an estimated prevalence of vision-threatening retinopathy of 4.4–8.2% among U.S. adults with diabetes (2,3). While numerous population-based studies have shown that the risk for diabetic retinopathy (DR) increases with duration of diabetes (4–8), proliferative DR (PDR) may still occur within the first 5 years after the diagnosis of type 2 diabetes (4–6,9). Therefore, for patients with type 2 diabetes, the American Academy of Ophthalmology recommends screening fundus examinations at diagnosis and at least yearly thereafter (10).
In 2018, there were ∼34 million adults in the U.S. with diabetes, with an estimated 1.5 million people newly diagnosed with diabetes (1). Screening this growing population of patients with diabetes at guideline-recommended intervals presents a substantial public health challenge. While clinical trials have shown that up to 98% of blindness due to DR can be prevented with a combination of laser photocoagulation, intravitreal anti-vascular endothelial growth factor injections, and vitrectomy (11), if patients do not follow-up at recommended intervals, they are more likely to experience preventable vision loss or blindness (12). Given that just 30–40% of patients receive an eye examination in any of the first 5 years after the diagnosis with type 2 diabetes (13), and up to 37% of patients with type 2 diabetes have DR at diagnosis (9), a significant number of patients with newly diagnosed type 2 diabetes are at risk for preventable vision loss in the first 5 years after their initial diagnosis.
The incidence of PDR in the first 5 years after the de novo diagnosis of type 2 diabetes is not well known. Therefore, we performed a retrospective longitudinal cohort study using data from a large, nationwide commercial and Medicare Advantage claims database to determine the incidence of and risk factors for developing PDR within 5 years of a new diagnosis of type 2 diabetes in the U.S. Additionally, we report the cumulative incidence of tractional retinal detachment (TRD) and neovascular glaucoma (NVG), two vision-threatening sequelae of PDR often requiring urgent surgical intervention and their associated risk factors at 5 years following an index diagnosis of type 2 diabetes. To our knowledge, this is the first study to report the incidence and risk factors for TRD and NVG within 5 years of diagnosis of type 2 diabetes.
Research Design and Methods
Data from Optum’s deidentified Clinformatics Data Mart Database were reviewed. These deidentified data came from 47 million individuals privately insured through a single carrier at any point from 2007 to 2015. For each member, we had access to all medical and pharmacy claims that were filed with their health plan. In addition, demographic and socioeconomic data were provided. The University of Southern California Institutional Review Board determined that this study was exempt from Institutional Review Board review. This study complied with the Health Insurance Portability and Accountability Act and adhered to the tenets of the Declaration of Helsinki.
Selecting Patients
Individuals ≥18 years of age were determined to have a diagnosis of type 2 diabetes if they had any of the following: one inpatient claim with a diagnosis of type 2 diabetes, one prescription fill for diabetes medication (Supplementary Table 1) or two outpatient claims with a diagnosis of type 2 diabetes within 180 days. International Classification of Diseases, Ninth Revision (ICD-9) codes (ICD-9 codes 250.x0 or 250.x2) associated with these claims were used to identify patients with type 2 diabetes. Cases of concurrent pregnancy or gestational diabetes (ICD-9 codes 630–79, V22 or V23, V28.x, V61.6, V61.7, or 648.83), type 1 diabetes (250.x1 or 250.x3), or use of an insulin pump (V45.85, V53.91, V65.46, and 996.57) were excluded. To identify incident cases, individuals were required to have a 12-month window of continuous enrollment prior to the index type 2 diabetes diagnosis with no diabetes diagnosis or diabetes medication use. Additionally, patients were excluded if they had any diagnosis indicating the presence of diabetic eye disease prior to the index diagnosis of diabetes.
Two overlapping cohorts of patients were selected based on length of follow-up: a 2-year cohort, with 3 years of continuous enrollment (1 pre-, 2 postdiagnosis), and a 5-year cohort, with 6 years of continuous enrollment (1 pre-, 5 postdiagnosis). All included patients had continuous enrollment, and there was no movement into and out of the health insurance plan for any of these patients. For each patient, all inpatient, outpatient, and pharmacy claims in the year prior to index and for the 2- or 5-year period after the index diagnosis were tracked, depending on the cohort. The purpose of the 2-year cohort was to compare the incidence of outcomes over time and perform a sensitivity analysis to evaluate any potential risk factors for the rare diagnoses of TRD and NVG, which may not have been apparent in the 5-year regression.
Outcome Measures
The primary outcome measure was the incidence of PDR at 5 years. Secondary outcome measures included the incidence of TRD and NVG at 5 years. As a sensitivity analysis, the incidence for these outcomes was also computed at 2 years. Patients were identified as having PDR, TRD, or NVG based on the presence of ICD-9 or Current Procedural Terminology (CPT) codes as listed in Supplementary Table 2.
Statistical Analysis
Comparisons between the subset of patients who did and did not develop PDR within 5 years of their index type 2 diabetes diagnosis were performed by using descriptive analyses (χ2 for categorical variables and t test for continuous variables). Multivariable logistic regression was used to test the association between the incidence of PDR, TRD, and NVG with the following factors: age at diagnosis, race/ethnicity, sex, education, income, Medicare insurance, medical comorbidities, insulin use, and maximum hemoglobin A1c (max HbA1c) in the 2- or 5-year period after diagnosis, depending on the cohort. Max HbA1c values were binned into ordinal categories as follows: <6.5% (48 mmol/mol), 6.5% (48 mmol/mol) to 7.5% (58 mmol/mol), 7.5% (58 mmol/mol) to 9% (75 mmol/mol), and >9% (>75 mmol/mol). The single highest HbA1c value in the period after the initial type 2 diabetes diagnosis was selected for each patient. All analyses were conducted on Unix workstations using SAS 9.4 (SAS Institute, Carey, NC) and Stata 16 (StataCorp College Station, TX) software. P ≤ 0.05 was used to determine statistical significance.
Results
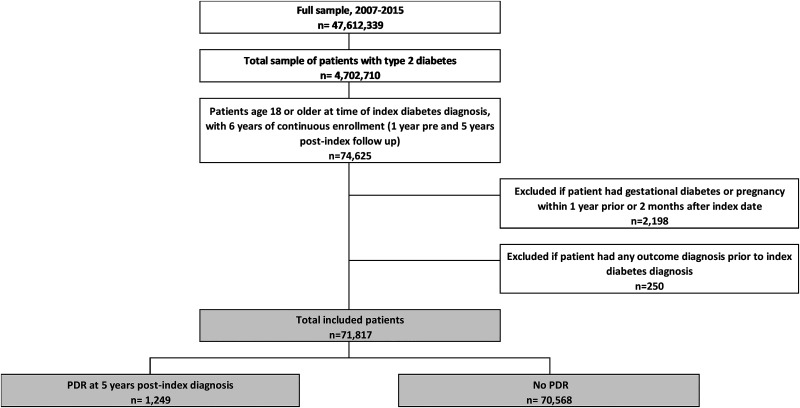
A total of 277,401 (Supplementary Fig. 1) and 71,817 (Fig. 1) patients newly diagnosed with type 2 diabetes met all criteria and were included in the 2- and 5-year cohorts, respectively. At 5 years following the diagnosis of type 2 diabetes, 1,249 of 71,817 patients (1.74%) developed PDR (Supplementary Fig. 2). Patients who developed PDR within 5 years were older on average at diagnosis (61.6 ± 12.9 vs. 60.6 ± 13.9, P < 0.001) (Table 1) and more likely to be men (54.4% vs. 50.7%, P = 0.008). Patients developing PDR were more likely to be Hispanic (17.6% vs. 14.6%, P = 0.003), and less likely to be White (62.1% vs. 65.9%, P = 0.005). There were trends toward those with PDR having lower levels of education and income than those without PDR, but these differences were not statistically significant. Patients developing PDR were more likely to have other systemic complications of type 2 diabetes, including renal disease (6.7% vs. 1.6%, P < 0.001), neurological disease (5.6% vs. 1.9%, P < 0.001), and peripheral circulatory disorders (2.2% vs. 0.6%, P < 0.001). There were no differences in the rates of comorbid hypertension, dyslipidemia, or smoking between the two groups. Morbid obesity was less common among those developing PDR (9.9% vs. 12.8%, P = 0.003). Lastly, insulin use (40.2% vs. 13.4%, P < 0.001) was far more common, and max HbA1c was higher (8.7% ± 2.4 [72 mmol/mol] vs. 7.6% ± 2.0 [60 mmol/mol], P < 0.001) among those developing PDR.
Figure 1.
Five-year attrition diagram demonstrates how patients were included and excluded in the 5-year cohort. Patients were included if they had any of the following: one inpatient claim with a diagnosis of type 2 diabetes, one prescription fill for diabetes medication, or two outpatient claims with a diagnosis of type 2 diabetes within 180 days. Only patients with an index diagnosis prior to 1 October 2010 were included to allow for 5 years of follow-up.
Table 1.
Descriptive statistics comparing patients with and without PDR at 5 years after the index type 2 diabetes diagnosis
| Characteristics | PDR at 5 years | No PDR at 5 years | P value |
|---|---|---|---|
| Sample size, n (%) | 1,249 (1.74) | 70,568 (98.26) | |
| Age at diagnosis, mean (SD) years | 61.6 (12.9) | 60.6 (13.9) | 0.013 |
| Male, n (%) | 680 (54.4) | 35,757 (50.7) | 0.008 |
| Race/ethnicity, n (%) | |||
| Non-Hispanic White | 775 (62.1) | 46,492 (65.9) | 0.005 |
| Black | 176 (14.1) | 8,909 (12.6) | 0.12 |
| Hispanic | 220 (17.6) | 10,287 (14.6) | 0.003 |
| Asian | 38 (3.0) | 2,610 (3.7) | 0.22 |
| Unknown | 40 (3.2) | 2,270 (3.2) | 0.98 |
| Education, n (%) | |||
| High school diploma or less | 451 (36.1) | 23,773 (33.7) | 0.07 |
| Some college | 657 (52.6) | 38,274 (54.2) | 0.25 |
| 4 year college degree or more | 141 (11.3) | 8,521 (12.1) | 0.40 |
| Income, n (%) | |||
| <$40,000 | 335 (26.8) | 17,819 (25.3) | 0.21 |
| $40,000–$49,000 | 96 (7.7) | 5,969 (8.5) | 0.33 |
| $50,000–$99,000 | 419 (33.6) | 22,439 (31.8) | 0.19 |
| $≥100,000 | 293 (23.5) | 17,983 (25.5) | 0.10 |
| Unknown | 106 (8.5) | 6,358 (9.0) | 0.52 |
| Medicare, n (%) | 522 (41.8) | 29,413 (41.7) | 0.94 |
| Private insurance product, n (%) | |||
| Exclusive provider organization | 89 (7.1) | 5,632 (8.0) | 0.27 |
| Health maintenance organization | 498 (39.9) | 24,463 (34.7) | <0.001 |
| Indemnity | 48 (3.8) | 2,506 (3.6) | 0.58 |
| Other | 148 (11.9) | 9,147 (13.0) | 0.25 |
| Point of service | 422 (33.8) | 25,961 (36.8) | 0.029 |
| Preferred provider organization | 44 (3.5) | 2,859 (4.1) | 0.35 |
| Comorbidities, n (%) | |||
| Hypertension | 695 (55.6) | 39,124 (55.5) | 0.90 |
| Dyslipidemia | 312 (25.0) | 18,078 (25.6) | 0.61 |
| DKA/HHS | 15 (1.2) | 275 (0.4) | <0.001 |
| Renal disease | 84 (6.7) | 1,115 (1.6) | <0.001 |
| Neurological disease | 70 (5.6) | 1,336 (1.9) | <0.001 |
| Peripheral circulatory disorders | 28 (2.2) | 434 (0.6) | <0.001 |
| Morbid obesity | 124 (9.9) | 9,005 (12.8) | 0.003 |
| Smoking | 157 (12.6) | 9,938 (14.1) | 0.13 |
| Other | 63 (5.0) | 939 (1.3) | <0.001 |
| Insulin use, n (%) | 502 (40.2) | 9,445 (13.4) | <0.001 |
| Max HbA1c, mean % (SD), mmol/mol | 8.7 (2.4), 72 | 7.6 (2.0), 60 | <0.001 |
The bold P values indicate statistical significance (P < 0.05). DKA, diabetic ketoacidosis; HHS, hyperosmolar hyperglycemic state.
Multivariable logistic regression was used to identify independent factors for the development of PDR within 5 years (Table 2). The greatest independent risk factor for the development of PDR at 5 years was insulin use (odds ratio [OR] 3.59, 95% CI 3.16–4.08). Other significant risk factors included max HbA1c >9% or >75 mmol/mol (OR 2.10, 95% CI 1.64–2.69), concomitant renal disease (OR 2.68, 95% CI 2.09–3.42), peripheral circulatory disorders (OR 1.88, 95% CI 1.25–2.83), and neurological disease (OR 1.62, 95% CI 1.24–2.11). Age at diagnosis was positively correlated with the risk of developing PDR, with risk peaking in the patients diagnosed between the ages of 65 and 74 (OR 1.62, 95% CI 1.28–2.03). Younger age (18–34 years) at diagnosis (OR 0.46, 95% CI 0.29–0.74), Medicare insurance (OR 0.60, 95% CI 0.70–0.76), morbid obesity (OR 0.72, 95% CI 0.59–0.87), and smoking (OR 0.84, 95% CI 0.70–1.00) were identified as independent negatively associated factors.
Table 2.
Independent factors associated with the incidence of PDR, NVG, and TRD within the first 5 years after the index type 2 diabetes diagnosis
| PDR (n = 1,249 [1.74%]) | TRD (n = 183 [0.25%]) | NVG (n = 102 [0.14%]) | ||||
|---|---|---|---|---|---|---|
| Characteristics | OR (95% CI) | P value† | OR (95% CI) | P value† | OR (95% CI) | P value† |
| Age at diagnosis (vs. 45–54), years | ||||||
| 18–34 | 0.46 (0.29–0.74) | 0.001* | ||||
| 55–64 | 1.25 (1.05–1.48) | 0.012* | 3.66 (1.65–8.09) | 0.001* | ||
| 65–74 | 1.62 (1.28–2.03) | <0.001* | ||||
| ≥75 | 1.30 (1.00–1.68) | 0.048* | 3.97 (1.49–10.60) | 0.006* | ||
| Race/ethnicity (vs. White) | ||||||
| Black | 1.89 (1.09–3.26) | 0.023* | ||||
| Hispanic | 1.85 (1.11–3.10) | 0.019* | ||||
| Income >$100,000 (vs. $40,000–49,000) | 0.44 (0.20–0.97) | 0.041 | ||||
| Medicare | 0.60 (0.70–0.76) | <0.001* | ||||
| Smoking | 0.84 (0.70–1.00) | 0.045* | ||||
| Renal disease | 2.68 (2.09–3.42) | <0.001* | 2.46 (1.30–4.69) | 0.006* | 2.51 (1.17–5.40) | 0.018* |
| Neurological disease | 1.62 (1.24–2.11) | <0.001* | 2.90 (1.40–5.98) | 0.004 | ||
| Peripheral circulatory disorders | 1.88 (1.25–2.83) | 0.003* | ||||
| Morbid obesity | 0.72 (0.59–0.87) | 0.001* | ||||
| Insulin use | 3.59 (3.16–4.08) | <0.001* | 3.57 (2.57–4.96) | <0.001* | 2.30 (1.45–3.67) | <0.001* |
| Max HbA1c (vs. <6.5% or <48 mmol/mol) | ||||||
| 7.5% (58 mmol/mol)–9% (75 mmol/mol) | 1.59 (1.22–2.08) | 0.001* | ||||
| >9% (>75 mmol/mol) | 2.10 (1.64–2.69) | <0.001* | ||||
All variables were included in the regression analysis for each outcome, but only statistically significant results (i.e., independent factors) are displayed above for succinctness.
P values for all other covariates in the model, including sex, education level (4-year college vs. some college vs. high school or less), hypertension, dyslipidemia, and diabetic ketoacidosis/hyperosmolar hyperglycemic state were >0.05.
Also an independent factor at 2 years following diagnosis.
Other Neovascular Sequelae of Diabetic Eye Disease
In addition to reporting the incidence of PDR, we also studied other severe, vision-threatening neovascular sequelae of diabetic eye disease that often require urgent surgical intervention, including tractional retinal detachment (TRD) and neovascular glaucoma (NVG). Both were rare outcomes at 5 years, with just 183 patients (0.25%) developing TRD, and 102 patients (0.14%) developing NVG (Table 2). The two independent factors associated with the incidence of TRD at 5 years were insulin use (OR 3.57, 95% CI 2.57–4.96) and renal disease (OR 2.46, 95% CI 1.30–4.69). Max HbA1c >9% or >75 mmol/mol (OR 2.12, 95% CI 1.35–3.33), concomitant peripheral circulatory disorders (OR 2.57, 95% CI 1.10–6.01), and Hispanic ethnicity (OR 1.76, 95% CI 1.35–2.29) were significant independent risk factors for the incidence of TRD at 2 years, but these were not statistically significant at 5 years.
There were several independent risk factors for developing NVG at 5 years, including older age at diagnosis (55–64 years, OR 3.66, 95% CI 1.65–8.09; ≥75 years, OR 3.97, 95% CI 1.49–10.60), neurological disease (OR 2.90, 95% CI 1.40–5.98), and renal disease (OR 2.51, 95% CI 1.17–5.40). Additionally, insulin use (OR 2.30, 95% CI 1.45–3.67), Black race (OR 1.89, 95% CI 1.09–3.26), and Hispanic ethnicity (OR 1.85, 95% CI 1.11–3.10) were independent risk factors for the incidence of NVG at 5 years. Income >$100,000 was the lone protective factor (OR 0.44, 95% CI 0.20–0.97) for the development of NVG at 5 years. Max HbA1c >9% or >75 mmol/mol (OR 3.21, 95% CI 1.51–6.84) was a significant risk factor at 2 years but was not statistically significant at 5 years (OR 1.99, 95% CI 0.85–4.66).
Conclusions
This study quantified the incidence of PDR, TRD, and NVG in the first 5 years after the diagnosis of type 2 diabetes in a large, nationwide sample. Additionally, it identified potential risk factors for the development of these severe manifestations of diabetic eye disease in the early period after the initial type 2 diabetes diagnosis. To our knowledge, this is the largest study to date tracking the development of PDR longitudinally from the initial diagnosis of type 2 diabetes, and it is the first study to determine the incidence and risk factors for the development of TRD and NVG within 5 years of the initial type 2 diabetes diagnosis.
Prior longitudinal, observational studies have been critical in developing our current understanding of the epidemiology of DR (Table 3). However, most of these studies were composed of racially homogenous patient populations (4–6,14–17), with a relatively small number of patients newly diagnosed with diabetes (5,7,14–17). Additionally, most were insufficiently powered to report the 5-year cumulative incidence of PDR for patients with newly diagnosed type 2 diabetes, instead reporting the incidence of any DR, progression of DR, or rates not stratified by duration of diabetes (7,15–17). When the four studies presenting 5-year PDR incidence after the type 2 diabetes diagnosis were combined, <15 total patients developed PDR (5,6,18,19), making it difficult to extrapolate the incidence and risk factors for PDR development within 5 years of the type 2 diabetes diagnosis. Additionally, two of the four studies took place prior to 1995 (14,19), and one of the studies took place in India (6), further highlighting the need for relevant and current studies in the U.S.
Table 3.
Five-year cumulative incidence of PDR as reported in previous population-based studies and the current study
| Study population and location | Dates of enrollment | Follow-up (years)* | Age range (years) | Total, N | Patients with new DM2 diagnosis, n | Cumulative incidence of PDR within 5 years, n (%) |
|---|---|---|---|---|---|---|
| White | ||||||
| U.S. (present study) | 2007–2010 | 5 | ≥18 | 47,267 | 47,267 | 775 (1.6) |
| Blue Mountains, Australia | 1992–1994 | 5 | ≥49 | 139 | Did not report | Did not stratify results by duration of DM2 |
| Melbourne, Australia | 1992–1994 | 5 | ≥40 | 121 | 42 | Did not stratify results by duration of DM2 |
| San Luis Valley, CO | 1984–1988 | 4.8 | 20–74 | 72 | Did not report | Did not stratify results by duration of DM2 |
| Southern WI | 1980–1982 | 4 | ≥30 | 485 Insulin users | 96† | 4%‡ |
| 502 Noninsulin users | 225† | 3%‡ | ||||
| Black | ||||||
| U.S. (present study) | 2007–2010 | 5 | ≥18 | 9,085 | 9,085 | 176 (1.9) |
| Nakuru, Kenya | 2007–2008 | 6 | ≥50 | 156 | 89 | Did not stratify results by duration of DM2 |
| Barbados | 1988–1992 | 4 | 40–84 | 407 | 43 | 0 (0) |
| Hispanic | ||||||
| U.S. (present study) | 2007–2010 | 5 | ≥18 | 10,507 | 10,507 | 220 (2.1) |
| Los Angeles, CA | 2000–2003 | 4 | ≥40 | 775 | 36 | 1 (2.8) |
| San Luis Valley, CO | 1984–1988 | 4.8 | 20–74 | 172 | Did not report | Did not stratify results by duration of DM2 |
| Asian | ||||||
| U.S (present study) | 2007–2010 | 5 | ≥18 | 2,648 | 2,648 | 38 (1.4) |
| Shanghai, China | 2007 | 5 | 20–90 | 322 | 6 | Did not stratify results by duration of DM2 |
| Chennai, India | 2003–2006 | 4 | ≥40 | 958 | 244† | 2 (0.8)§ |
DM2, type 2 diabetes.
Certain studies have longer follow-up, but for the purposes of identifying the 5-year incidence of PDR, the data interval closest to this time period was chosen. For studies with variable follow-up, the median follow-up is reported.
These patients had 0–4 years of DM2 at baseline, making average duration >5 years at follow-up.
Exact n not provided.
Data reported were severe non-PDR or PDR.
The current study also highlighted racial health disparities in the incidence of PDR (Table 3) compared with prior studies, which were generally racially homogeneous. For the White population, the Wisconsin Epidemiological Study of Diabetic Retinopathy (WESDR) reported PDR incidence rates of 3% for those not using insulin and 4% for those using insulin at 4–8 years following diagnosis (19). Our study found lower rates of early PDR in White patients, with just 1.6% of patients developing PDR at 5 years. In Black patients, 1.9% developed PDR at 5 years in our study. For comparison, the Barbados Eye Study found 0 of 43 patients had developed PDR at 4 years after diagnosis (14). Hispanic patients had the highest incidence of PDR at 5 years in our study, at 2.1%. The Los Angeles Latino Eye Study (LALES) also found high rates at 2.8% (5). Lastly, we found the lowest 5-year incidence of PDR among ethnic groups in Asian patients, at 1.4%. This was similar to the findings by Raman et al. (6), who also reported low rates at 0.8% at 4–8 years following the type 2 diabetes diagnosis among patients in Chennai, India. Asians comprise a diverse ethnic group; therefore, further studies are warranted to investigate differences in development of DR among subsets of the Asian population.
As all patients in our study were continuously insured and thus had presumable access to health care, our results may help stratify patients in the U.S. for their risk of early development of PDR based on ethnicity, with Hispanic and Black patients having higher rates of early progression to PDR. In addition to type 2 diabetes being >60% more prevalent in the Black and Hispanic populations compared with the White population (20), Black and Hispanic patients have been shown to have higher HbA1c levels, higher blood pressure, and higher LDL cholesterol compared with White patients (21). These disparities in cardiovascular factors associated with diabetes suggest better glycemic control and management of comorbidities is crucial for this population, and taken together with our finding of a higher incidence of PDR in the early period after diagnosis, suggest a need for earlier and more widespread screening for vision-threatening complications of pathologic neovascularization among Black and Hispanic patients (22). While high rates of type 2 diabetes among Hispanics may be partly due to genetic/epigenetic factors (23), many health care disparities, such as in access to care, contribute to higher rates of progression of diabetic eye disease (24). Some studies suggest Hispanic and Black individuals are less likely to use eye care services (2,13). The findings in this study highlight the importance of improving access to care for these populations as well as improving health care literacy in order to emphasize the importance of routine diabetic eye examinations and glycemic control to prevent vision-threatening complications of diabetes.
Aside from race/ethnicity, we found several independent risk factors for the development of PDR at 5 years, including insulin use (OR 3.59, 95% CI 3.16–4.08), max HbA1c >9% or >75 mmol/mol (OR 2.10, 95% CI 1.64–2.69), older age at diagnosis (OR 1.62, 95% CI 1.28–2.03), comorbid renal disease (OR 2.68, 95% CI 2.09–3.42), peripheral circulatory disorders (OR 1.88, 95% CI 1.25–2.83), and neurological disease (OR 1.62, 95% CI 1.24–2.11). The presence of other microvascular comorbidities highlights two factors that may relate to more rapid development of PDR after the initial type 2 diabetes diagnosis: a delayed type 2 diabetes diagnosis and poor glycemic control. Unlike patients with type 1 diabetes, patients with type 2 diabetes often have asymptomatic hyperglycemia for years prior to diagnosis, leading to an average delay in diagnosis of 4–6 years following disease onset (25). With this knowledge, the risk factors identified in our study may represent those individuals not only with worse control of disease but who also had a longer duration of undiagnosed type 2 diabetes. This relationship between increasing patient age and potential longer duration of undiagnosed diabetes may represent a chronological or survival bias in our study. For example, patients diagnosed with diabetes at older ages may have had a longer duration of undiagnosed diabetes and may therefore be at a higher risk of complications such as PDR in the early period after diagnosis. Furthermore, while our sample did include a number of patients >65, we did not have data for patients who transitioned to Medicare not via Medicare Advantage. Thus, the older patients included in our sample may not be representative of all patients >65 years of age. Despite this, our results showing increased age is associated with higher rates of retinopathy are in line with prior landmark studies. The UK Prospective Diabetes Study (UKPDS) reported the highest rates for progression of retinopathy in those diagnosed at age ≥58 (9), and Zhang et al. (2) found the highest prevalence of retinopathy in those >65 years.
Similar to retinopathy, renal disease, peripheral circulatory disorders, and neurological complications are signs of end-organ damage of diabetes. Patients with any of these risk factors early on in their disease course should be monitored closely for the impending development of PDR. For renal disease specifically, studies have found that microalbuminuria, one of the earliest signs of diabetic nephropathy, may be predictive of manifest DR (26). Furthermore, recent studies indicate that treating end-stage renal disease with initiating hemodialysis may also improve outcomes in DR (27).
Our study also noted multiple surprising factors that may be protective against the development of PDR, including younger age at diagnosis, Medicare insurance, morbid obesity, and smoking. The lower rates among patients in the 18–34 age-group suggests they were diagnosed closer to the onset of their type 2 diabetes, with less time to develop DR. Prior studies have found high rates of DR in patients diagnosed at younger ages (4), but this observation may be confounded by a longer duration of type 2 diabetes after the initial diagnosis. While the 18- to 34-year-old patients in our study had a fairly low risk for developing PDR during the early period after the initial type 2 diabetes diagnosis in our study, their lifetime risk of developing DR increases with type 2 diabetes disease duration. Patients with Medicare had lower rates of PDR in our study. Sloan et al. (28) also found an exceedingly low 6-year incidence of PDR (0.11–0.15%) among patients newly diagnosed with diabetes and Medicare insurance.
Morbid obesity was also protective in our study. Prior studies examining the association between PDR and obesity have demonstrated conflicting results, with some studies finding a protective effect (4,29–31), and others identifying obesity as a risk factor for DR (32,33). A recent meta-analysis found no association between BMI and DR (34). We chose to study morbid obesity rather than obesity, as prior studies have found greater sensitivity of ICD-9 coding for more advanced levels of obesity, because lower levels of obesity are often underreported (35). Lim et al. (31) attributed the protective effect of obesity to increased levels of C-reactive protein, which is proangiogenic and may improve retinal perfusion during preproliferative stages of DR. Unfortunately, our data set did not include metabolic markers other than HbA1c.
Lastly, we found smoking to be slightly protective against the development of PDR. Early studies did not find a significant association between smoking and DR (36), although a recent meta-analysis suggested smoking was protective against PDR in patients with type 2 diabetes, postulating that lower systemic blood pressure in smokers may be responsible (37). While our study may support the conclusion of this recent meta-analysis, the association had only borderline significance (P = 0.045) despite our large sample size, suggesting any potential effect of smoking on development of PDR is likely small.
In addition to reporting the incidence and risk factors for early development of PDR, our study was unique in reporting the incidence of NVG and TRD at 5 years following the diagnosis with type 2 diabetes. We found extremely low rates of these complications, with just 1 in 400 patients developing TRD and 1 in 700 patients developing NVG at 5 years. TRD and NVG are both end-stage manifestations of DR, which take many years of uncontrolled hyperglycemia to develop. Developing TRD and NVG so soon after the type 2 diabetes diagnosis likely reflects many prior years of undiagnosed type 2 diabetes. Similar to the risk factors for PDR, we found patients using insulin and those with renal disease were at higher risk for developing TRD and NVG. Additionally, there were socioeconomic factors that were associated with risk for NVG, including Black race (OR 1.89, 95% CI 1.09–3.26) and Hispanic ethnicity (OR 1.85, 95% CI 1.11–3.10). Lastly, patients making >$100,000 were less likely to develop NVG (OR 0.44, 95% CI 0.20–0.97). As a result, efforts should be made to increase screening efforts in Black and Hispanic patients as well as patients with lower levels of income. Ultimately, the small numbers of patients developing TRD and NVG at 5 years likely limited the ability to detect other associations.
Our study has several limitations. It was retrospective in nature; therefore, our study did not have predetermined intervals for eye examinations or standardized grading of fundus photos at screening examinations. Rather, our study relied on diagnoses submitted via medical claims data to measure our end points. While this method is highly cost-effective and allows for rapid study of much larger groups of patients than are feasible with prospective studies, the quality of the data are potentially lower than that for rigorously controlled, prospective studies. For example, other than identifying the presence of certain medical comorbidities (e.g., hypertension, dyslipidemia, renal disease) by codified diagnoses, we were unable to compare discrete clinical observations to assess the relationship between good or poor control of comorbidities (e.g., blood pressure, estimated glomerular filtration rate, microalbuminuria) and development of PDR, TRD, or NVG. While these represent limitations to claims-based types of studies as compared with studies with medical record review, a prior validation study found good concordance between the diagnosis of DR in clinic notes and those detected in a claims-based analysis as a proof of concept of this research strategy (38). Additionally, we aimed to include only patients with incident diabetes, using the method of Borkar et al. (39) to augment diagnosis ICD-9 codes with National Drug Code prescription codes in the identification and exclusion of patients with established type 2 diabetes. We believe that by applying these criteria in our study, we dramatically reduced the risk of misclassifying patients as newly diagnosed, but the risk is likely not entirely eliminated. For instance, one example of a false positive would be a patient with established type 2 diabetes who did not see a physician or fill any prescriptions for diabetes medication in the year prior to the index diagnosis. This may result in an increase in the observed incidence of PDR and other neovascular sequelae of diabetic eye disease in our study. On the other hand, another example of a false positive would be a patient who was prescribed metformin off-label for prediabetes. This may have decreased the observed incidence of PDR and other neovascular sequelae in our study.
Another limitation is that while all other variables were available for 90–100% of patients, HbA1c laboratory values were only available for 48% of patients at 5 years. This is due to the claims-based nature of our study. For this reason, we chose to use maximum HbA1c, because monitoring for trends in HbA1c was not feasible. Despite this limitation, this still left >34,000 patients with HbA1c data at 5 years. Additionally, given our patients were not mandated to follow-up at strict intervals, we may have potentially missed patients with undiagnosed PDR. This likely led to an underestimation of the incidence of PDR at 5 years in our study.
In summary, just under 2% of patients with type 2 diabetes developed PDR within 5 years of the diagnosis. Numerous risk factors for early development of PDR were identified in this study, which may highlight groups of patients with longer duration of undiagnosed diabetes or worse control of diabetes following diagnosis. This study underscores the importance of adhering to guideline-recommended fundus screening at the time of diagnosis of type 2 diabetes and at least yearly thereafter, especially for patients with a history of insulin use, renal disease, max HbA1c >9% (>75 mmol/mol), peripheral circulatory disorders, neurological disease, and older age at diagnosis. Additionally, the higher incidence of PDR at 5 years among Black and Hispanic patients may suggest a delay in the diagnosis of type 2 diabetes in these populations. Several strategies to improve adherence to screening at-risk populations have previously demonstrated success, including patient education programs (40), teleretinal screening programs (41), and provider financial incentives (42). Our data highlight a need to target these and other efforts toward specific subsets of the population with type 2 diabetes who are at risk for developing vision loss from PDR and its sequelae.
Article Information
Funding. This study was supported by National Institutes of Health National Eye Institute grant P30EY029220 and by a Research to Prevent Blindness, New York, NY, unrestricted Departmental Grant.
The sponsor or funding organizations had no role in the design or conduct of this research.
Duality of Interest. S.A.S. is a consultant for Precision Health Economics LLC, a life sciences industry consulting firm. B.C.T. is a consultant for Advanced Clinical and an advisory board member for Mallinckrodt. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. W.S.G. created all initial figures and tables. W.S.G., B.Y.X., S.A.S., B.C.T., and K.L. designed the study. W.S.G. and B.C.T. drafted the first version of the manuscript. J.L. edited the manuscript, tables, and figures. B.Y.X. and S.A.S critically reviewed multiple versions of the manuscript. B.C.T. designed the analysis plan and critically reviewed multiple versions of the manuscript. B.C.T. and K.L. performed the data analysis. All authors contributed to the interpretation of the results and review of the manuscript. B.C.T. is the guarantor of this work, and as such, had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the virtual meeting of the Association for Research in Vision and Ophthalmology 2020 Annual Meeting, 3–7 May 2020, and an abstract was published in Invest Ophthalmol Vis Sci 2020,61:1898.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.15141843.
References
- 1. Centers for Disease Control and Prevention . National Diabetes Statistics Report 2020: Estimates of Diabetes and Its Burden in the United States. Accessed 4 August 2021. Available from: https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
- 2. Zhang X, Saaddine JB, Chou C-F, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA 2010;304:649–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kempen JH, O’Colmain BJ, Leske MC, et al.; Eye Diseases Prevalence Research Group . The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol 2004;122:552–563 [DOI] [PubMed] [Google Scholar]
- 4. Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol 1984;102:527–532 [DOI] [PubMed] [Google Scholar]
- 5. Varma R, Choudhury F, Klein R, Chung J, Torres M; Los Angeles Latino Eye Study Group . Four-year incidence and progression of diabetic retinopathy and macular edema: the Los Angeles Latino Eye Study. Am J Ophthalmol 2010;149:752–61.e1, 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Raman R, Ganesan S, Pal SS, Gella L, Kulothungan V, Sharma T. Incidence and progression of diabetic retinopathy in urban India: Sankara Nethralaya-Diabetic Retinopathy Epidemiology and Molecular Genetics Study (SN-DREAMS II), report 1. Ophthalmic Epidemiol 2017;24:294–302 [DOI] [PubMed] [Google Scholar]
- 7. Tudor SM, Hamman RF, Baron A, Johnson DW, Shetterly SM. Incidence and progression of diabetic retinopathy in Hispanics and non-Hispanic whites with type 2 diabetes. San Luis Valley Diabetes Study, Colorado. Diabetes Care 1998;21:53–61 [DOI] [PubMed] [Google Scholar]
- 8. Jee D, Lee WK, Kang S. Prevalence and risk factors for diabetic retinopathy: the Korea National Health and Nutrition Examination Survey 2008-2011. Invest Ophthalmol Vis Sci 2013;54: 6827–6833 [DOI] [PubMed] [Google Scholar]
- 9. Stratton IM, Kohner EM, Aldington SJ, et al. UKPDS 50: risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia 2001;44:156–163 [DOI] [PubMed] [Google Scholar]
- 10. American Academy of Opthalmology . AAO PPP Retina/Vitreous Committee, Hoskins Center for Quality Eye Care. Diabetic Retinopathy PPP 2019. Accessed 20 May 2020. Available from https://www.aao.org/preferred-practice-pattern/diabetic-retinopathy-ppp
- 11. Wong TY, Sun J, Kawasaki R, et al. Guidelines on diabetic eye care: the International Council of Ophthalmology recommendations for screening, follow-up, referral, and treatment based on resource settings. Ophthalmology 2018;125:1608–1622 [DOI] [PubMed] [Google Scholar]
- 12. Sloan FA, Grossman DS, Lee PP. Effects of receipt of guideline-recommended care on onset of diabetic retinopathy and its progression. Ophthalmology 2009;116:1515–1521, 1521.e1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gange WS, Xu BY, Lung K, et al. Rates of eye care and diabetic eye disease among insured patients with newly-diagnosed type 2 diabetes. Ophthalmol Retina 2021;5:160–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leske MC, Wu S-Y, Hennis A, Nemesure B, Hyman L; Barbados Eye Studies Group . Incidence of diabetic retinopathy in the Barbados Eye Studies. Ophthalmology 2003;110:941–947 [DOI] [PubMed] [Google Scholar]
- 15. McCarty DJ, Fu CL, Harper CA, Taylor HR, McCarty CA. Five-year incidence of diabetic retinopathy in the Melbourne Visual Impairment Project. Clin Exp Ophthalmol 2003;31:397–402 [DOI] [PubMed] [Google Scholar]
- 16. Cikamatana L, Mitchell P, Rochtchina E, Foran S, Wang JJ. Five-year incidence and progression of diabetic retinopathy in a defined older population: the Blue Mountains Eye Study. Eye (Lond) 2007;21:465–471 [DOI] [PubMed] [Google Scholar]
- 17. Bastawrous A, Mathenge W, Wing K, et al. The incidence of diabetes mellitus and diabetic retinopathy in a population-based cohort study of people age 50 years and over in Nakuru, Kenya. BMC Endocr Disord 2017;17:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Klein R, Klein BEK, Moss SE, Linton KLP. The Beaver Dam Eye Study. Retinopathy in adults with newly discovered and previously diagnosed diabetes mellitus. Ophthalmology 1992;99:58–62 [DOI] [PubMed] [Google Scholar]
- 19. Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. X. Four-year incidence and progression of diabetic retinopathy when age at diagnosis is 30 years or more. Arch Ophthalmol 1989;107:244–249 [DOI] [PubMed] [Google Scholar]
- 20. Golden SH, Yajnik C, Phatak S, Hanson RL, Knowler WC. Racial/ethnic differences in the burden of type 2 diabetes over the life course: a focus on the USA and India. Diabetologia 2019;62:1751–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Walker RJ, Strom Williams J, Egede LE. Influence of race, ethnicity and social determinants of health on diabetes outcomes. Am J Med Sci 2016;351:366–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang X, Bullard KM, Gregg EW, et al. Access to health care and control of ABCs of diabetes. Diabetes Care 2012;35:1566–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chande AT, Rishishwar L, Conley AB, Valderrama-Aguirre A, Medina-Rivas MA, Jordan IK. Ancestry effects on type 2 diabetes genetic risk inference in Hispanic/Latino populations. BMC Med Genet 2020;21(Suppl. 2):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Varma R, Mohanty SA, Deneen J, Wu J; LALES Group . Burden and predictors of undetected eye disease in Mexican-Americans: the Los Angeles Latino Eye Study. Med Care 2008;46:497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Porta M, Curletto G, Cipullo D, et al. Estimating the delay between onset and diagnosis of type 2 diabetes from the time course of retinopathy prevalence. Diabetes Care 2014;37:1668–1674 [DOI] [PubMed] [Google Scholar]
- 26. Chang CH, Chuang LM. Microalbuminuria and diabetic retinopathy in type 2 diabetic patients: from risk association to risk prediction. J Diabetes Investig 2013;4:42–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Takamura Y, Matsumura T, Ohkoshi K, et al. Functional and anatomical changes in diabetic macular edema after hemodialysis initiation: one-year follow-up multicenter study. Sci Rep 2020;10:7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sloan FA, Belsky D, Ruiz D Jr, Lee P. Changes in incidence of diabetes mellitus-related eye disease among US elderly persons, 1994-2005. Arch Ophthalmol 2008;126:1548–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. West KM, Erdreich LJ, Stober JA. A detailed study of risk factors for retinopathy and nephropathy in diabetes. Diabetes 1980;29:501–508 [DOI] [PubMed] [Google Scholar]
- 30. Klein R, Klein BE, Moss SE. Is obesity related to microvascular and macrovascular complications in diabetes? The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Intern Med 1997;157:650–656 [PubMed] [Google Scholar]
- 31. Lim LS, Tai ES, Mitchell P, et al. C-reactive protein, body mass index, and diabetic retinopathy. Invest Ophthalmol Vis Sci 2010;51:4458–4463 [DOI] [PubMed] [Google Scholar]
- 32. Dowse GK, Humphrey AR, Collins VR, et al. Prevalence and risk factors for diabetic retinopathy in the multiethnic population of Mauritius. Am J Epidemiol 1998;147:448–457 [DOI] [PubMed] [Google Scholar]
- 33. Chaturvedi N; The WHO Multinational Study of Vascular Disease in Diabetes . Mortality risk by body weight and weight change in people with NIDDM. Diabetes Care 1995;18:766–774 [DOI] [PubMed] [Google Scholar]
- 34. Zhou Y, Zhang Y, Shi K, Wang C. Body mass index and risk of diabetic retinopathy: a meta-analysis and systematic review. Medicine (Baltimore) 2017;96:e6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lloyd JT, Blackwell SA, Wei II, Howell BL, Shrank WH. Validity of a claims-based diagnosis of obesity among Medicare beneficiaries. Eval Health Prof 2015;38:508–517 [DOI] [PubMed] [Google Scholar]
- 36. Moss SE, Klein R, Klein BEK. Cigarette smoking and ten-year progression of diabetic retinopathy. Ophthalmology 1996;103:1438–1442 [DOI] [PubMed] [Google Scholar]
- 37. Cai X, Chen Y, Yang W, Gao X, Han X, Ji L. The association of smoking and risk of diabetic retinopathy in patients with type 1 and type 2 diabetes: a meta-analysis. Endocrine 2018;62:299–306 [DOI] [PubMed] [Google Scholar]
- 38. Bearelly S, Mruthyunjaya P, Tzeng JP, et al. Identification of patients with diabetic macular edema from claims data: a validation study. Arch Ophthalmol 2008;126:986–989 [DOI] [PubMed] [Google Scholar]
- 39. Borkar DS, Sobrin L, Hubbard RA, Kempen JH, VanderBeek BL. Techniques for improving ophthalmic studies performed on administrative databases. Ophthalmic Epidemiol 2019;26: 147–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brunisholz KD, Briot P, Hamilton S, et al. Diabetes self-management education improves quality of care and clinical outcomes determined by a diabetes bundle measure. J Multidiscip Healthc 2014;7:533–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Daskivich LP, Vasquez C, Martinez C Jr, Tseng CH, Mangione CM. Implementation and evaluation of a large-scale teleretinal diabetic retinopathy screening program in the Los Angeles County Department of Health Services. JAMA Intern Med 2017;177:642–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hatef E, Vanderver BG, Fagan P, Albert M, Alexander M. Annual diabetic eye examinations in a managed care Medicaid population. Am J Manag Care 2015;21:e297–e302 [PubMed] [Google Scholar]