Abstract
OBJECTIVE
Previous reports of the annual incidence of type 1 diabetes (T1D) in China were conducted using retrospective hospital cases, which may not reflect the reality. This longitudinal study estimated T1D incidence in a Chinese population of 21.7 million from 2007 to 2017.
RESEARCH DESIGN AND METHODS
A population-based registry of T1D was performed by the Beijing Municipal Health Commission Information Center. Annual incidence and 95% CIs were calculated by age group and sex. The association of sex with T1D incidence and predicted new cases of T1D were assessed using Poisson regression models. Annual percentage change and average annual percentage of change were assessed using Joinpoint regression.
RESULTS
Overall, there were 6,875 individuals who developed T1D from 2007 to 2017 in this population. T1D incidence (/100,000 persons) (95% CI) significantly increased from 2.72 (2.51, 2.93) in 2007 to 3.60 (3.38, 3.78) in 2017 (P < 0.001). The T1D onset peak was in the 10–14-year-old age group. While no significant trend was found in the 0–14- and 15–29-year-old age groups, T1D incidence markedly increased from 1.87 to 3.52 in the ≥30-year-old age group (P < 0.05). The prevalence of diabetic ketoacidosis at diagnosis was highest in the 0–4-year-old age group. We predicted new cases of T1D will increase 1.57-fold over the next decade.
CONCLUSIONS
T1D incidence in this large Chinese population is higher than has been reported previously. From 2007 to 2017, although the incidence peak was in the 10–14-year age group, the T1D incidence increased sharply in adults but not in youth.
Introduction
The incidence of type 1 diabetes (T1D) varies widely (1), with high incidence in Western countries, particularly Northern Europe, and low in Asian countries, including China (1,2). According to the Diabetes Mondiale (DiaMond) Project, Chinese T1D incidence in children was 0.51/100,000 person-years from 1985 to 1994, which was one of the lowest incidences in the world (3). Weng et al. (2) reported that the estimated incidence of T1D for all ages in China was 1.01/100,000 person-years from 2010 to 2013. T1D has remained less well recognized and attracted less attention in Asia than in the West. To date, there are only five studies (1,2,4–6) of T1D incidence in the whole country or in cities in mainland China after 1994, and all are restricted to retrospective analysis of the hospital case reports. Nonregistry studies cannot be extrapolated to the Chinese population and may have underestimated the T1D incidence in China. Comprehensive population-based estimates of T1D incidence rates and trends are still limited. A registry of T1D that represents the Chinese population has not been reported.
Most studies of incidence of T1D focused on childhood and less on adulthood (7). Although most cases of T1D occurred in children, it could develop at any age (8), and it has been reported that the first onset of T1D in adults is not rare (0.69 [0.00, 1.51]/100,000 persons ≥30 years of age) in Guangdong, China (2,9).
Previous reports have shown that the incidence of T1D has increased worldwide over the past three decades (10). Recent study results suggest that environmental and behavioral factors play important roles in increasing incidence of T1D in the recent decades (11). However, there have been no studies focused on T1D incidence in the Chinese population after 2013, although China has experienced a rapidly growing economy and changing lifestyles since that time. Research on T1D in low-incidence areas such as China will help to further understand the contribution of different combinations of behavioral and environmental factors to the development of the disease.
Our study is the first population-based registry study in a Chinese population and may uncover the actual T1D incidence in China. We investigated the incidence of T1D in all age groups (0–100 years old) from 2007 to 2017. We also estimated the incidence trend by evaluating the annual percentage change (APC) and average annual percentage change (AAPC) and predicted the new cases of T1D over the next decade.
Research Design and Methods
Study Population
This registered Chinese population covered from 16.76 million (2007) to 21.71 million (2017) persons at risk in the greater Beijing area, which is made up of 16 districts (8 urban and 8 suburban areas). Beijing has an annual migrant resident population of 7.9 million (2017) from other parts of China, 5.33 million (67.5%) come from northern China and 2.57 million (32.5%) from southern China. The data used in this study were collected from the Beijing Municipal Health Commission Information Center (BMHCIC), which is a government agency in charge of the health and population in the greater Beijing area. Registry data on newly diagnosed cases of T1D were uploaded from all 153 centers that were qualified to diagnose T1D. These 153 centers are located in 8 urban and 8 suburban districts in the greater Beijing area (Supplementary Tables 1 and 2). In order to ensure the quality of medical record data, BMHCIC conducts annual supervision and inspection visits, arranges the supervision and inspection work plan of that year, and standardizes the inspection requirements (http://www.phic.org.cn/). Each year, the health committee invites experts to check the accuracy of diagnoses. Registration of all centers was compulsory, and all centers are required to upload T1D data to the information center. The main purpose of the T1D register is to calculate national statistics on related diseases, for the development of government reports, and to support the formulation and evaluation of medical policies.
The study population (the denominator for estimating incidence) was residents of all ages in the greater Beijing area from 2007 to 2017. The number of residents was drawn from annual government reports on resident population in the greater Beijing area.
Permanent residents were defined as people whose registered address was the same as their primary address and who had stayed at their primary address for at least 6 months. People whose registered address did not match their primary address or who had lived in their primary address for <6 months were excluded from the study population. We also excluded active service men whose primary address could not be verified.
The patients were collected in series according to the name, age, identification card number, hospital case number, household registration address, and contact information. We regarded the first time the patient with T1D was hospitalized as the date of illness according to the main diagnosis.
The Ethics Committee of the Beijing Center for Disease Prevention and Control (Beijing, China) approved this study.
Case Ascertainment
The clinical diagnosis of T1D was based on the criteria of the American Diabetes Association (8) and the World Health Organization (ICD-10-CM codes) (12). Endocrinologists or pediatricians, or both, or an expert committee on T1D established the diagnosis. In addition to the diagnosis of diabetes, the following were also taken into account in the diagnosis of T1D (ICD-10-CM code E10): symptoms, signs, history, long-term application of insulin (>1 year), no other antidiabetic drug use before and 1 year after the diagnosis, absence of islet function, age of diagnosis, and/or positive autoantibodies (markers of the immune destruction of the β-cell including islet cell autoantibodies, autoantibodies to insulin, and autoantibodies to GAD). To ensure the accuracy of the diagnosis, annual inspections were conducted by BMHCIC and included a review of the above for distinguishing T1D from other types of diabetes. Uncertain cases were returned to the expert committee on T1D in the corresponding center, with final diagnosis information reported to BMHCIC. This study includes the final diagnosis data, and the laboratory information underlying these data were unavailable. Diabetic ketoacidosis (DKA) was defined based on established guidelines (13,14). DKA was diagnosed in patients with the following results: blood glucose >11.1 mmol/L or known history of diabetes, blood bicarbonate (HCO3−) <15.0 mmol/L or pH <7.3, or both, in addition to ketonuria (ICD-10-CM codes E10–111). Patients with DKA as the first symptom at the onset of T1D were registered in the database.
Statistical Analysis
Incidence rate of T1D was calculated per 100,000 person-years at risk. We assumed cases of T1D occurred in a Poisson distribution, and we calculated 95% CIs using this assumption. Poisson regression models were used to evaluate the relationship between incidence rates and the potential effect of sex. Based on a set of the most recent cases of T1D, the Poisson regression model and the Gray Model 1,1 [GM (1,1)] (15) were used to predict new cases of T1D. Time trends of T1D incidence from 2007 to 2017 were evaluated using Joinpoint 4.9.0.0 (https://surveillance.cancer.gov/joinpoint/). Joinpoint software starts with a straight line, or zero joinpoints, to describe a trend over time and tests if the addition of one or more identified join points significantly changes in the trend. We chose the Joinpoint-recommended methods, and trends were described by an APC for each segment and an AAPC for the whole study period (16). In describing trends, we use the term “increase” or “decrease” when APC or AAPC was statistically significant (P < 0.05). When there was no statistical difference in APC or AAPC, the word “stable” was used. Pearson χ2 analysis was applied to comparison of proportions difference. For all statistical analyses, we used R studio 1.383 (RStudio, Inc.) and Joinpoint software 4.9.0.0. A P value <0.05 was considered statistically significant.
Data and Resource Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
Incidence From 2007 to 2017
We identified 6,875 people with newly diagnosed T1D from 2007 to 2017: 961 were aged 0–14 years, 1,443 were aged 15–29 years, and 4,471were aged ≥30 years. A total of 3,648 (53.06%) were men (Table 1). T1D incidence (95% CI) (/100,000 persons) increased from 2.72 (2.51, 2.93) in 2007 to 3.60 (3.38, 3.78) in 2017. Between 2007 and 2017, the T1D incidence rate increased from 2.83 (2.54, 3.14) to 3.64 (3.36, 3.93) among men and from 2.60 [2.31, 2.90] to 3.52 (3.18, 3.89) (Table 1) among women. The men-to-women incidence rate ratio (95% CI) was 1.130 (1.079, 1.185) (P < 0.001) (Supplementary Table 3).
Table 1.
Incidence of T1D by sex and age from 2007 to 2017
| Year | All | Men | Women | 0–14 years | 15–29 years | ≥30 years | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Incidence* | Cases | Incidence* | Cases | Incidence* | Cases | Incidence* | Cases | Incidence* | Cases | Incidence* | |
| 2007 | 455 | 2.72 (2.51, 2.93) | 241 | 2.83 (2.54, 3.14) | 214 | 2.60 (2.31, 2.90) | 79 | 3.80 (3.05, 4.74) | 128 | 3.96 (3.33, 4.71) | 248 | 1.87 (1.65, 2.12) |
| 2008 | 522 | 2.95 (2.74, 3.16) | 288 | 3.20 (2.90, 3.51) | 234 | 2.69 (2.40, 2.99) | 67 | 4.17 (3.29, 5.30) | 130 | 3.09 (2.60, 3.66) | 325 | 2.74 (2.45, 3.05) |
| 2009 | 536 | 2.88 (2.68, 3.09) | 271 | 2.85 (2.58, 3.15) | 265 | 2.91 (2.63, 3.21) | 58 | 3.41 (2.63, 4.40) | 140 | 3.23 (2.74, 3.81) | 338 | 2.69 (2.42, 3.00) |
| 2010 | 585 | 2.98 (2.78, 3.19) | 309 | 3.05 (2.77, 3.34) | 276 | 2.91 (2.63, 3.21) | 64 | 3.53 (2.77, 4.51) | 159 | 3.58 (3.06, 4.18) | 362 | 2.71 (2.44, 3.00) |
| 2011 | 616 | 3.05 (2.85, 3.26) | 322 | 3.09 (2.82, 3.38) | 294 | 3.01 (2.73, 3.30) | 68 | 3.56 (2.81, 4.51) | 147 | 3.33 (2.84, 3.92) | 401 | 2.89 (2.62, 3.18) |
| 2012 | 662 | 3.20 (3.00, 3.40) | 343 | 3.21 (2.94, 3.50) | 319 | 3.19 (2.90, 3.48) | 85 | 4.17 (3.37, 5.15) | 131 | 3.08 (2.60, 3.65) | 446 | 3.09 (2.82, 3.39) |
| 2013 | 655 | 3.10 (2.90, 3.30) | 352 | 3.23 (2.96, 3.51) | 303 | 2.96 (2.69, 3.25) | 78 | 3.59 (2.87, 4.47) | 140 | 3.40 (2.88, 4.01) | 437 | 2.93 (2.67, 3.22) |
| 2014 | 623 | 2.90 (2.71, 3.09) | 343 | 3.10 (2.83, 3.38) | 280 | 2.68 (2.42, 2.96) | 85 | 3.62 (2.93, 4.48) | 115 | 2.92 (2.43, 3.50) | 423 | 2.78 (2.52, 3.05) |
| 2015 | 706 | 3.25 (3.06, 3.45) | 377 | 3.39 (3.11, 3.67) | 329 | 3.11 (2.84, 3.40) | 148 | 6.11 (5.20, 7.18) | 110 | 2.92 (2.42, 3.52) | 448 | 2.90 (2.64, 3.18) |
| 2016 | 738 | 3.40 (3.20, 3.60) | 399 | 3.59 (3.31, 3.87) | 339 | 3.20 (2.92, 3.48) | 112 | 4.27 (3.55, 5.14) | 128 | 3.66 (3.08, 4.36) | 498 | 3.20 (2.93, 3.49) |
| 2017 | 777 | 3.60 (3.38, 3.78) | 403 | 3.64 (3.36, 3.93) | 374 | 3.52 (3.18, 3.89) | 117 | 4.21 (3.51, 5.04) | 115 | 3.64 (3.03, 4.37) | 545 | 3.52 (3.23, 3.83) |
Incidence data are per 100,000 person-years (95% CI).
Incidence by Age and Sex
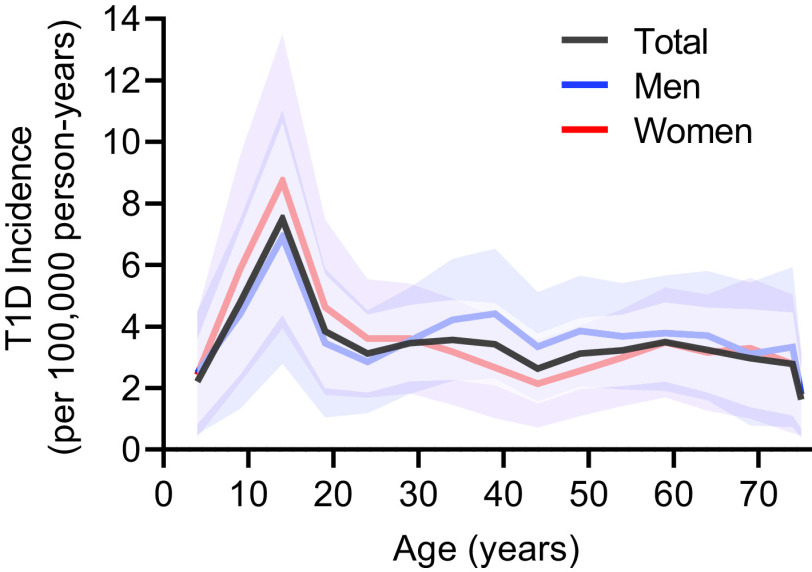
T1D incidence per 100,000 by age group is shown in Fig. 1 and Supplementary Table 4. After adjustment for the annual population change from 2007 to 2017, the incidence (95% CI) of T1D was highest among those 10–14 years old at 7.15 (5.28, 9.70) and lowest among those ≥75 years old at 1.49 (0.97, 2.29). Peak incidence was observed in the 10–14-year old group and was significantly higher than the other age groups (P < 0.001) (Fig. 1 and Supplementary Table 4). Furthermore, the men-to-women incidence rate ratio (95% CI) for those <30 years old was 0.885 (0.813, 0.952) (P = 0.002) and 1.272 (1.200, 1.350) among those ≥30 years (P < 0.001) (Supplementary Table 3). The incidence of T1D by age groups without adjusting for year was also calculated (Supplementary Table 5).
Figure 1.
T1D incidence and 95% CI in age groups by sex. The black, blue, and red lines represent all cases, men, and women, respectively. The shaded regions represent the 95% CI based on a Poisson distribution.
Incidence Trends From 2007 to 2017
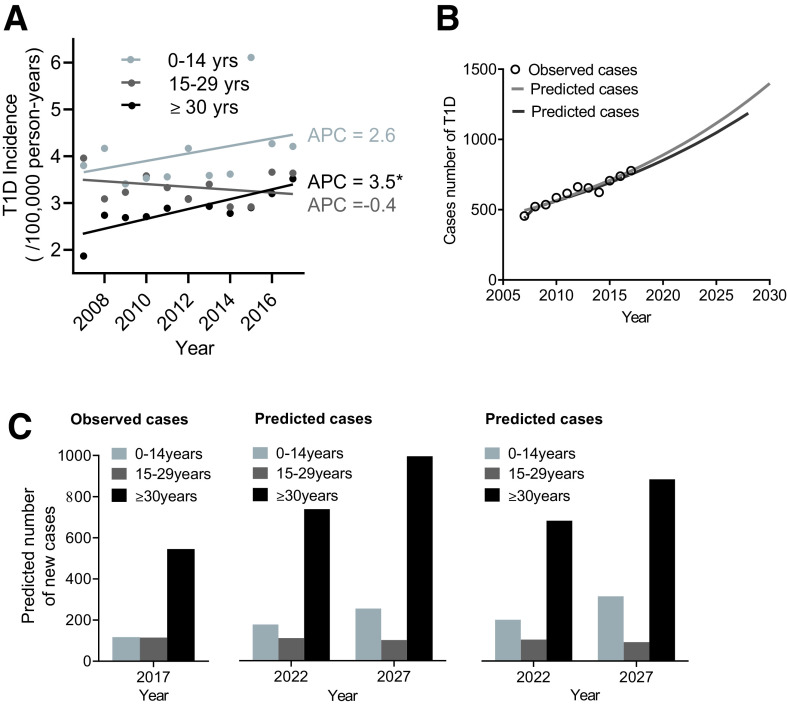
From 2007 to 2017, the T1D incidence has been increasing, with an AAPC of 2.3% (P < 0.05). Interestingly, while in the 0–14- and 15–29-year-old age groups the T1D incidence was stable (the AAPC was 2.6% and −0.4%; P > 0.05), the AAPC of the ≥30-year-old age group was 3.5% (P = 0.005), indicating that T1D incidence markedly increased in adults but not in youth from 2007 to 2017 (Table 2 and Fig. 2A). The incidence of T1D in the 15–29-year-old group was ∼2.12 times higher than that in ≥30-year-old group in 2007; however, the incidence of both the 15–29- and ≥30-year-old groups was almost the same in 2017 (Table 1). Furthermore, a total of 826 patients with latent autoimmune diabetes in adults (LADA) were included in our cases of T1D, accounting for 12.0% of all cases (6,875) and 18.5% of the ≥30-year-old group cases. After removing cases of LADA, we recalculated AAPC for all (0.9%) and for the ≥30-year-old group (3.0%), and, although the rate of increase was smaller, we still observed an increasing incidence rate of T1D in all and adults from 2007 to 2017 (P < 0.05).
Table 2.
Time trends of T1D incidence for sex and age groups from 2007 to 2017
| Groups | Number of JPs | Years | APC (95% CI) | P value | AAPC 2007–2017 (95% CI) | P value |
|---|---|---|---|---|---|---|
| Total | 2 | 2007–2014 | 1.2 (0.0, 2.3) | 0.046 | 2.3 (1.2, 3.4) | <0.001 |
| 2014–2017 | 4.9 (1.2, 8.8) | 0.018 | ||||
| Men | 2 | 2007–2014 | 1.3 (−0.6, 3.2) | 0.146 | 2.3 (0.5, 4.1) | 0.014 |
| 2014–2017 | 4.7 (−1.5, 11.2) | 0.116 | ||||
| Women | 2 | 2007–2015 | 1.3 (−1.4, 4.0) | 0.296 | 2.1 (0.7, 3.5) | 0.008 |
| 2015–2017 | 7.0 (−13.1, 31.8) | 0.454 | ||||
| 0–14 years | 1 | 2007–2017 | 2.6 (−1.2, 6.6) | 0.156 | 2.6 (−1.2,6.6) | 0.156 |
| 15–29 years | 1 | 2007–2017 | −0.4 (−2.6, 1.9) | 0.714 | −0.4 (−2.6, 1.9) | 0.714 |
| ≥30 years | 1 | 2007–2017 | 3.5 (1.4, 5.6) | 0.005 | 3.5 (1.4, 5.6) | 0.005 |
The APC/AAPC is significantly different from zero at P < 0.05. Number and times of joinpoints (JPs) in time trends were selected by Joinpoint regression model.
Figure 2.
Time trends of T1D incidence and predicted cases of T1D using a Poisson regression model and GM (1,1). A: Time trends of T1D incidence by age group. Circles and lines represent T1D incidence and incidence trends for different age groups (light gray: 0–14 years; dark gray: 15–29 years; black: ≥30 years) from 2007 to 2017. *The APC is significantly different from zero (P < 0.05). B: Observed and predicted cases of T1D in the future years. The circles represent the observed cases of T1D. The light gray and dark gray lines represent the predicted cases of T1D using a Poisson regression model and GM (1,1), respectively. C: Cases of T1D by age group in 2017 (left) and predicted cases by age group in 2022 and 2027 using Poisson regression model (middle) and GM (1,1) model (right).
Prediction of New Cases of T1D
Assuming the same trend continues, we predict that new cases of T1D will increase to 973 cases by 2022 and will increase 1.57-fold (1,220 cases) over the next 10 years (by 2027) compared with 777 cases (by 2017) (Fig. 2B and Supplementary Table 6). The GM (1,1) was also used to predict future value of new cases of T1D, with similar results as with the Poisson regression (Fig. 2B and Supplementary Table 7). We also predicted the distribution of new cases by age group (Fig. 2C). The percentage of new cases in the three different age groups in 2027 will be 18.80% (0–14 years), 7.43% (15–29 years), and 73.77% (≥30 years).
Prevalence of DKA at Onset of T1D
T1D with DKA as the first symptom as a percentage of all incident T1D is described in Supplementary Fig. 1A. Of the 6,875 patients with available data, 31.10% presented with DKA at diagnosis. The prevalence of DKA was stable in all age groups over the time period studied (P > 0.05). After adjustment for year, the percentage is the highest in the 0–4-year-old group, regardless of sex (Supplementary Fig. 1B). The proportion of DKA at diagnosis decreased with age from 52.52% in patients aged 0–4 years to 28.81% in patients aged ≥15 years (Supplementary Fig. 1B). Considering 2012 as the midpoint in the considered time range, no significant change in the DKA prevalence was shown between the first 5 years and last 5 years of surveillance (χ2 = 0.23; P = 0.63). There was no significant difference in DKA prevalence between men and women.
Incidence by Season and Region
Supplementary Fig. 2 shows the percentage of incident cases each month. While the greatest percentages of cases were diagnosed in July and December, no significant difference was found between months. No significant difference in T1D incidence was found between urban and suburban centers in our study.
Conclusions
Overall, the T1D incidence went from 2.72 to 3.60/100,000 person-years (for individuals 0–100 years of age) in the 21.7 million (2017) Chinese individuals evaluated from 2007 to 2017, which was more than previously recognized. The T1D incidence rapidly increased during this period. This increase partly reflects the further improvement of disease information management. BMHCIC provided robust data for our current study. Furthermore, our study also shows that T1D incidence increased overall and in those ≥30 years of age, but not in younger age groups. We also found a higher prevalence of DKA at diagnosis in younger patients during our study period.
We confirmed that the T1D incidence remains low in the Chinese population; however, it is higher than in previous reports. An earlier study estimated the T1D incidence was 1.01 (0.18, 1.84) in China and 0.91 (0.84, 0.97) in Beijing from 2010 to 2013 (2), which was lower than in studies in other parts of Asia (17). Our study, using data from 153 centers, indicated that previous figures may be underestimates. A possible reason for this difference is that previous estimates were based on <30 hospitals or centers in Beijing and identified 1,255 (for China as a whole) and 185 (for Beijing) newly diagnosed cases of T1D per year. In contrast, our analysis included data from all of the hospitals that were qualified to diagnose T1D (∼625 newly diagnosed with T1D per year from 153 centers in the greater Beijing area). The greater Beijing area is a metropolis with permanent residents from all over the country. It has an average annual migrant resident population of 7.94 million (2017), 5.36 million (67.5%) from northern China, and 2.58 million (32.5%) from southern China, indicating that the collection of ethnic groups within Beijing is a better representation of the ethnic diversity of China.
The geographical variation of T1D incidence is well established. The SEARCH for Diabetes in Youth (SEARCH) study in the U.S. pointed out that the incidence rate of T1D in the 0–19-year-old population was 25.5/100,000 person-years from 2002 to 2009 (11). In the period of 2000–2006, the incidence rate of T1D in children aged 0–14 years in Australia was 21.6/100,000 person-years (18), compared with 62.5/100,000 person-years from 2006 to 2011 in Finland (19). In contrast, the reported incidence rate of T1D in Asia was relatively low. Childhood-onset T1D was reported to be 2.25/100,000 persons in Japan from 2005 to 2010 and 1.36/100,000 person-years in Korea from 1995 to 2000 (17). From 2003 to 2008, the annual incidence of T1D in childhood in Taiwan was 5.3/100,000 person-years (20). Little has been understood about the incidence of T1D in mainland Chinese populations. We showed that T1D incidence increased from 2.72 to 3.60/100,000 person-years in a Chinese population from 2007 to 2017, which was close to what was reported for Japan.
Our research revealed the rapid growth trend of T1D in those >30 years of age from 2007 to 2017 (AAPC was 3.5%). In contrast with childhood-onset T1D, adult-onset T1D has a longer duration of symptoms, a lower incidence of DKA, and a better residual islet β-cell function or slow decline rate (21,22). Using the existing data, it is impossible to directly determine the cause of this increase. Although diabetes in both young and adult patients is described as an autoimmune disease in nature, the etiological determinants are still being studied in children, but not in adults. Further investigation is needed to determine relevant factors. This phenomenon has also been reported in previous studies. Weng et al. (2) found that, in their study, most of the new cases of T1D occurred in adulthood, and ∼65.3% of the newly diagnosed cases were participants >20 years old. Another study (17) reported that there was no increase in the incidence of childhood-onset T1D in Japan, which was consistent with our results. Our results provide clues that the etiology of adult T1D is different from that of children. In adult cases, the incidence rate increased faster, indicating that some early and late-onset factors may be different.
The increase in T1D among Chinese individuals has not been documented previously, and, as mentioned above, an earlier study of incidence of T1D in Beijing did not show an increase over time (23). We found an average annual increase of 2.3% (P < 0.05) overall from 2007 to 2017. Such a rapid increase is consistent with those observed in other populations, with an average annual increase in recent years of 3.9% in Europe, 5.3% in the U.S., and 2.8% in Australia (24). This rising incidence of T1D is a global phenomenon (24). Of note, it is reported that environmental factors may have a larger role than genetic factors in increasing the incidence of T1D (25). Thus, it is likely that changes in infrastructure, westernization of lifestyles, and changes in environmental factors may underlie the increase in incidence of T1D. Rapidly changing environmental risks, such as childhood obesity (26), nutrition (19) and viral infections (27), have been reported to be associated with the rising incidence of diabetes. Some of these risk factors have been reported in China, accompanied by a rapid increase in type 2 diabetes (23).
The sex difference by age groups is worthy of further study. Two international studies showed that the overall sex ratio of T1D incidence rate in children is approximately equal. Based on previous reports, T1D incidence is slightly higher in boys in high incidence areas (among populations of European descent) and higher in girls in low incidence areas (e.g., Asia and Africa) (2,28). In our study, the incidence of T1D was slightly higher in girls aged 0–14 years (P = 0.064). Several reports (29–31) indicated an excess in men among adults in populations of European origin. Moreover, Weng et al. (2) also found that the incidence of T1D in the population among adults was greater in men than in women. We found that the T1D incidence in this Chinese population was higher in women <30 years and higher in men >30 years. Different effects of environmental risk factors and lifestyle on incidence of T1D in women and men are possible explanations for this difference (29), and, given that boys reach puberty later than girls, it has been suggested that physiological changes at puberty may act as triggers for diabetes (32).
The SEARCH study recently reported that the prevalence of DKA at or near diagnosis in youth with T1D was 35.3% in 2010 and 40.6% in 2016 (33). Consistent with this and earlier reports from SEARCH, in our study, we found 31.10% of all cases of T1D presented with DKA, and younger age was associated with higher prevalence of DKA at T1D diagnosis (33,34). DKA is associated with acute complications and mortality (33), and the high prevalence of DKA at diagnosis emphasizes the need for urgent public health preventive measures to decrease the frequency of DKA at the time of T1D diagnosis (35). An Italian study showed that an education program focused on DKA markedly decreased its prevalence at T1D onset (9,36). Public health preventive measures should be emphasized, especially targeting preschool patients who are at the highest risk, by including educational interventions in kindergartens and preschools in order to prevent this leading cause of acute mortality in youth with T1D.
There are limitations to our investigation. Like most of the epidemiological studies in T1D, the diagnosis was a clinical one, and the determination of adult-onset T1D was more difficult because, in practice, it maybe mistakenly diagnosed as type 2 diabetes. In our study, long-term application of insulin (>1 year), no other antidiabetic drugs use before and 1 year after the diagnosis, absence of islet function, age of diagnosis, and/or positive autoantibodies were also taken into account in the diagnosis of T1D. To ensure the accuracy of the diagnosis, annual inspections were conducted by BMHCIC and included a review of the factors listed above for distinguishing T1D from other types of diabetes. All patients with newly diagnosed T1D are required to have a standardized treatment in a hospital that is qualified to diagnose T1D, although it is also possible that our definition of cases of T1D may have been too restrictive, because some individuals with T1D may be diagnosed in hospitals that were not qualified to do the test. However, it is unlikely that diagnosis at a hospital that was not included in the registry had much of an effect on our results, as these diagnoses generally occurred just before the patient was transferred to a qualified facility where the diagnosis would have been captured. Although submission to the registry is mandated by the government and regularly monitored, data may have been incomplete in the first year of the registry. To evaluate a potential impact on our conclusions, we removed the 2007 data and recalculated the APC and AAPC (Supplementary Table 8) and still observed an increase in T1D incidence over the time period (AAPC 2.2%; P < 0.05). Also, we included cases of LADA in which β-cell failure occurs slowly instead of limiting to acute-onset T1D. Although the rate of increase was smaller after removing cases of LADA, we still observed an increasing incidence rate of T1D in the full study population and in adults from 2007 to 2017 (P < 0.05).
In summary, although T1D incidence in this Chinese population was relatively low, more new cases of T1D occurred each year than were previously recognized. T1D annual incidence increased over time and a marked increase in incidence was found in adults but not in youth, suggesting that precipitating factors of adult-onset T1D may differ from those of youth. This distinction may provide etiological clues and facilitate the planning of appropriate health services.
Article Information
Acknowledgments. The authors thank participants and staff of BMHCIC for the valuable contributions.
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official view of BMHCIC.
Funding. This research was supported by grants from the National Natural Science Foundation of China (81930019, 8151101058, 81400824, and 81471014) and National Key R&D Program of China (2017YFC0909600) to J.-K.Y. This research was supported in part by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.-K.Y. conceived the idea for the study, supported the study, wrote the manuscript, and designed the experiments. C.L. and M.-N.G. collected the epidemiological and clinical data and processed statistical data. C.L. wrote the manuscript. Y.-C.Y. helped to draw and analyze part of the tables and figures. Y.-C.Y., Z.X., G.-J.C., A.R.B., L.H., J.-P.Z., and K.E. partially helped with the interpretation of the results, design of experiments, and proofing of the manuscript. All authors read and approved the final version. J.-K.Y. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.15073935.
References
- 1. Karvonen M, Viik-Kajander M, Moltchanova E, Libman I, LaPorte R, Tuomilehto J. Incidence of childhood type 1 diabetes worldwide. Diabetes Mondiale (DiaMond) Project Group. Diabetes Care 2000;23:1516–1526 [DOI] [PubMed] [Google Scholar]
- 2. Weng J, Zhou Z, Guo L, et al.; T1D China Study Group . Incidence of type 1 diabetes in China, 2010-13: population based study. BMJ 2018;360:j5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang Z, Wang K, Li T, et al. Childhood diabetes in China. Enormous variation by place and ethnic group. Diabetes Care 1998;21:525–529 [DOI] [PubMed] [Google Scholar]
- 4. Wu HB, Zhong JM, Hu RY, et al. Rapidly rising incidence of type 1 diabetes in children and adolescents aged 0-19 years in Zhejiang, China, 2007 to 2013. Diabet Med 2016;33:1339–1346 [DOI] [PubMed] [Google Scholar]
- 5. Zhang H, Xia W, Yu Q, et al. Increasing incidence of type 1 diabetes in children aged 0-14 years in Harbin, China (1990-2000). Prim Care Diabetes 2008;2:121–126 [DOI] [PubMed] [Google Scholar]
- 6. Gong C, Meng X, Jiang Y, Wang X, Cui H, Chen X. Trends in childhood type 1 diabetes mellitus incidence in Beijing from 1995 to 2010: a retrospective multicenter study based on hospitalization data. Diabetes Technol Ther 2015;17:159–165 [DOI] [PubMed] [Google Scholar]
- 7. Chiang JL, Kirkman MS, Laffel LM; Type 1 Diabetes Sourcebook Authors . Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care 2014;37:2034–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2013;36(Suppl. 1):S67–S74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang D, Deng H, Luo G, et al. Demographic and clinical characteristics of patients with type 1 diabetes mellitus: a multicenter registry study in Guangdong, China. J Diabetes 2016;8:847–853 [DOI] [PubMed] [Google Scholar]
- 10. Mayer-Davis EJ, Lawrence JM, Dabelea D, et al.; SEARCH for Diabetes in Youth Study . Incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. N Engl J Med 2017;376:1419–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lawrence JM, Imperatore G, Dabelea D, et al.; SEARCH for Diabetes in Youth Study Group . Trends in incidence of type 1 diabetes among non-Hispanic white youth in the U.S., 2002-2009. Diabetes 2014;63:3938–3945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539–553 [DOI] [PubMed] [Google Scholar]
- 13. Wolfsdorf JI, Glaser N, Agus M, et al. ISPAD Clinical Practice Consensus Guidelines 2018: diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes 2018;19(Suppl. 27):155–177 [DOI] [PubMed] [Google Scholar]
- 14. Kitabchi AE, Umpierrez GE, Murphy MB, Kreisberg RA. Hyperglycemic crises in adult patients with diabetes: a consensus statement from the American Diabetes Association. Diabetes Care 2006;29:2739–2748 [DOI] [PubMed] [Google Scholar]
- 15. Hu YC. A genetic-algorithm-based remnant grey prediction model for energy demand forecasting. PLoS One 2017;12:e0185478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000;19:335–351 [DOI] [PubMed] [Google Scholar]
- 17. Onda Y, Sugihara S, Ogata T, Yokoya S, Yokoyama T; Type 1 Diabetes (T1D) Study Group . Incidence and prevalence of childhood-onset type 1 diabetes in Japan: the T1D study. Diabet Med 2017;34:909–915 [DOI] [PubMed] [Google Scholar]
- 18. Catanzariti L, Faulks K, Moon L, Waters AM, Flack J, Craig ME. Australia’s national trends in the incidence of type 1 diabetes in 0-14-year-olds, 2000-2006. Diabet Med 2009;26:596–601 [DOI] [PubMed] [Google Scholar]
- 19. Harjutsalo V, Sund R, Knip M, Groop PH. Incidence of type 1 diabetes in Finland. JAMA 2013;310:427–428 [DOI] [PubMed] [Google Scholar]
- 20. Lu CL, Shen HN, Chen HF, Li CY. Epidemiology of childhood type 1 diabetes in Taiwan, 2003 to 2008. Diabet Med 2014;31:666–673 [DOI] [PubMed] [Google Scholar]
- 21. Karjalainen J, Salmela P, Ilonen J, Surcel HM, Knip M. A comparison of childhood and adult type I diabetes mellitus. N Engl J Med 1989;320:881–886 [DOI] [PubMed] [Google Scholar]
- 22. Howson JM, Rosinger S, Smyth DJ, Boehm BO; ADBW-END Study Group . Genetic analysis of adult-onset autoimmune diabetes. Diabetes 2011; 60:2645–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu M, Wang Z, Sun X, Chen Y, Zhang Q. Rapid increase in the incidence of clinically diagnosed type 2 diabetes in Chinese in Harbin between 1999 and 2005. Prim Care Diabetes 2007;1:123–128 [DOI] [PubMed] [Google Scholar]
- 24. DIAMOND Project Group . Incidence and trends of childhood type 1 diabetes worldwide 1990-1999. Diabet Med 2006;23:857–866 [DOI] [PubMed] [Google Scholar]
- 25. Kubin M, Tossavainen P, Hannula V, Lahti S, Hautala N, Falck A. Prevalence of retinopathy in Finnish children and adolescents with type 1 diabetes: a cross-sectional population-based retrospective study. Arch Dis Child 2011;96:963–968 [DOI] [PubMed] [Google Scholar]
- 26. Winkler C, Krumsiek J, Buettner F, et al. Feature ranking of type 1 diabetes susceptibility genes improves prediction of type 1 diabetes. Diabetologia 2014;57:2521–2529 [DOI] [PubMed] [Google Scholar]
- 27. Craig ME, Nair S, Stein H, Rawlinson WD. Viruses and type 1 diabetes: a new look at an old story. Pediatr Diabetes 2013;14:149–158 [DOI] [PubMed] [Google Scholar]
- 28. Park Y, Wintergerst KA, Zhou Z. Clinical heterogeneity of type 1 diabetes (T1D) found in Asia. Diabetes Metab Res Rev 2017;33:e2907. [DOI] [PubMed] [Google Scholar]
- 29. Blohmé G, Nyström L, Arnqvist HJ, et al. Male predominance of type 1 (insulin-dependent) diabetes mellitus in young adults: results from a 5-year prospective nationwide study of the 15-34-year age group in Sweden. Diabetologia 1992;35:56–62 [DOI] [PubMed] [Google Scholar]
- 30. Kyvik KO, Nystrom L, Gorus F, et al. The epidemiology of type 1 diabetes mellitus is not the same in young adults as in children. Diabetologia 2004;47:377–384 [DOI] [PubMed] [Google Scholar]
- 31. Pundziute-Lyckå A, Dahlquist G, Nyström L, et al.; Swedish Childhood Diabetes Study Group . The incidence of type I diabetes has not increased but shifted to a younger age at diagnosis in the 0-34 years group in Sweden 1983-1998. Diabetologia 2002;45:783–791 [DOI] [PubMed] [Google Scholar]
- 32. Forga Llenas L, Goñi Iriarte MJ, Cambra Contin K, Ibáñez Beroiz B, Chueca Guendulain M, Berrade Zubiri S. Incidence and temporal trends of childhood type 1 diabetes between 1975 and 2012 in Navarre (Spain). Gac Sanit 2015;29:51–54 [DOI] [PubMed] [Google Scholar]
- 33. Jensen E, Stafford J, Saydah S, et al. Increase in prevalence of diabetic ketoacidosis at diagnosis among youth with type 1 diabetes: the SEARCH for Diabetes in Youth Study. Diabetes Care 2021; 44:1573–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dabelea D, Rewers A, Stafford JM, et al.; SEARCH for Diabetes in Youth Study Group . Trends in the prevalence of ketoacidosis at diabetes diagnosis: the SEARCH for diabetes in youth study. Pediatrics 2014;133:e938–e945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am 2010;39:481–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vanelli M, Chiari G, Ghizzoni L, Costi G, Giacalone T, Chiarelli F. Effectiveness of a prevention program for diabetic ketoacidosis in children. An 8-year study in schools and private practices. Diabetes Care 1999;22:7–9 [DOI] [PubMed] [Google Scholar]