Introduction
The National Academy of Medicine of Korea (NAMOK), established in 2004, is a non-profit organization involving erudite scholars belonging to medicine and health systems science that aims to improve public health by advancing medicine; leading modernization; and providing trusted, authoritative, and scientific advice to the government and Korean population.
NAMOK's mission is to provide objective medical advice to the Korean population and government and to increase public understanding of medicine and health systems science. NAMOK summarizes recommendations for and explains the diagnosis and treatment of coronavirus diseases 2019 (COVID-19) and the available vaccines, intensive care options, psychological matters, and policies.
Laboratory Diagnosis and Surveillance
Recommendations
○ Recommendation 1: The capacity of performing COVID-19 molecular tests and emergency diagnostic tests should be enhanced.
○ Recommendation 2: Strategies should be established for severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) variant and antibody testing.
○ Recommendation 3: The capacity of long-term diagnostic testing for infectious diseases should be enhanced by investing in diagnostic testing staff and the necessary infrastructure.
○ Recommendation 4: Research should be continued, and novel methods should be validated for diagnosing infectious diseases.
1. Current national status on COVID-19 diagnostic testing
1) Update on confirmed COVID-19 cases
The efficacy of control and preventive measures against the COVID-19 pandemic in Korea has been recognized. As of April 4, 2021, there have been 2,040 confirmed cases per million population and 33 deaths per million population in Korea; these numbers are much lesser than those reported in the USA (92,263 and 1,676, respectively), the UK (64,393 and 1,871, respectively), and Japan (3,836 and 72, respectively). In Korea, the testing, tracing, and treatment (3T) strategy has been established as a basic strategy for COVID-19 prevention and control. In this strategy, the COVID-19 diagnostic test plays a major role. Details of the COVID-19 diagnostic testing process in Korea are provided below.
2) COVID-19 diagnostic testing process implementation in Korea
- January 27, 2020: Initiation of an emergency use authorization system for COVID-19 testing.
- February 4, 2020: Final approval of COVID-19 test kits for the first time.
- February 7, 2020: Initiation of COVID-19 testing in hospitals and commercial laboratories.
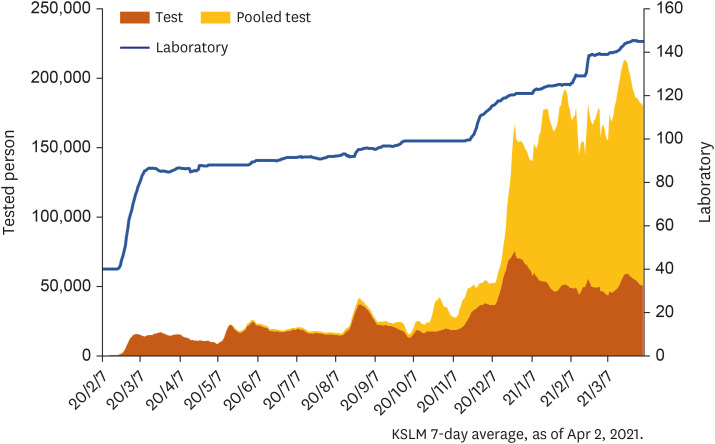
- April 1, 2021: Completion of a total of 15 million COVID-19 molecular tests for 26 million people at 146 clinical laboratories (Fig. 1).
Fig. 1. National coronavirus disease 2019 testing results.
KSLM = Korean Society for Laboratory Medicine.
Source: Korean Society for Laboratory Medicine; as of April 2, 2021.
3) Characteristics and types of COVID-19 testing methods in Korea
The COVID-19 testing methods approved in Korea are molecular, antigen, and antibody tests.
(1) Molecular test
Polymerase chain reaction (PCR) is used as the standard molecular test for COVID-19 in Korea. Despite its high cost and the need for trained laboratory staff, its high sensitivity and specificity enable accurate testing. Nasopharyngeal swabs and saliva are the most commonly used specimens.
Nasopharyngeal swabs are recommended as they show the highest positivity rate in the early phase of infection; however, a limitation is that specimen collection needs to be performed by healthcare professionals. Saliva specimens can be self-collected, but they have low sensitivity and are therefore typically used in identifying asymptomatic populations for whom specimen collection is difficult.
A pooled assay, which combines several nasopharyngeal swabs, is appropriate for testing many asymptomatic populations, and it has a sensitivity rate of 100% even after combining five to six specimens. Rapid molecular testing automatizes the testing process and shortens the testing time. Moreover, it has comparable sensitivity and specificity to conventional PCR testing. However, it is very expensive. In addition, testing multiple specimens at the same time is difficult; therefore, this method is used to quickly identify urgent, asymptomatic cases.
(2) Antigen test
The antigen test is easy to use, quick, and inexpensive. However, it has very low sensitivity when the viral load is low and has other limitations, including false positivity or false negativity as a result of cross-reaction and subjective assessment. Therefore, it is not recommended when there is sufficient capacity to perform the COVID-19 molecular test. When performing the COVID-19 antigen test, a molecular test should also be performed at the same time to validate and prevent false negativity and false positivity.
(3) Antibody test
The COVID-19 antibody test yields a high positivity rate 2–3 weeks after the infection; therefore, its use for an acute stage diagnosis is difficult. However, it is useful for confirming previous infection, diagnosing multisystem inflammatory syndrome associated with COVID-19, assessing seroprevalence, selecting antibody donors, and evaluating vaccine responses. When the prevalence of COVID-19 is low, an antibody test with a high specificity should be performed.
4) Background of the success of COVID-19 diagnostic testing in Korea
(1) Excellent human resources and policies
In Korea, medical specialists in laboratory medicine supervise and are responsible for all testing. Moreover, the integrity of testing is controlled by several policies, leading to a high accuracy of diagnostic testing.
(2) Active molecular testing market
Molecular testing devices were widely distributed in Korea after the 2009 influenza pandemic and the 2015 MERS outbreak, and the industry of in vitro diagnostic testing became active. This formed the basis for performing numerous molecular tests.
(3) Fee-for-service
Fee-for-service is a payment model used for diagnostic testing. Therefore, there is no problem related to finances when performing numerous tests.
(4) Emergency use approval system for in vitro diagnostics
The emergency use approval system for in vitro diagnostics was introduced in 2016. Consequently, COVID-19 diagnostic reagents were quickly approved in the early phase of the pandemic.
(5) Collaboration between laboratory medicine experts and disease control authorities
Experienced laboratory medicine experts actively participated in major decisions in the early phase of the COVID-19 pandemic, when there was insufficient information and rationale for controlling COVID-19. Moreover, the government was able to proactively implement mass testing very quickly.
2. Short-term pending issues and plans related to COVID-19 diagnostic testing
The COVID-19 diagnostic test has successfully played an essential role in the COVID-19 response; however, some areas need to be urgently improved.
1) Effective use of various diagnostic testing methods
It is very important to consider various COVID-19 diagnostic testing methods and select the most appropriate one for COVID-19 control and prevention. To achieve this goal, systematic collaboration among laboratory medicine experts, government authorities, and medical institutions is very important. More detailed suggestions are provided below:
(1) Discontinuation of the antigen test
The antigen test is characterized by its inability to amplify the target and can only be used for detection when the viral load is high. According to the assessment performed by the Korean Society for Laboratory Medicine, rapid antigen tests approved in Korea are thought to have 40% sensitivity.1 Some data suggest that individuals with potential infection can be detected with rapid antigen tests, but recent findings from Germany state that 20–50% of individuals with infectious potential are not detected2 with rapid antigen tests. Moreover, considering that patients who are not infectious could provide evidence for infection tracing, an antigen test is not appropriate for the current state in Korea.
In the future, if the number of COVID-19 patients rises drastically, it becomes impossible to perform testing with molecular tests only, and the prevalence surpasses 5–10%, an antigen test could be considered. Even in such cases, however, assays that achieve at least 80% sensitivity (ideally over 97% sensitivity) should be preferred.3,4
(2) Careful use of saliva specimens
The collection of nasopharyngeal swabs, the standard specimen for COVID-19 tests, requires experienced personnel and protective equipment. On the other hand, saliva specimen collection does not require additional staff; therefore, the use of saliva specimens can increase the usability of the test. However, the sensitivity of saliva specimens is lower than that of the standard specimen; the sensitivity is considerably low in asymptomatic individuals. Moreover, the use of pooled assays is difficult with saliva specimens.5 However, when nasopharyngeal specimen collection is difficult, e.g., in patients in long-term care facilities, saliva specimens may be useful.
The Korean Society for Laboratory Medicine does not recommend the use of saliva specimens at medical institutions for the diagnosis of symptomatic individuals. Even when specimen collection is difficult, the possibility of false negatives with saliva specimens should be considered carefully.6 Studies are needed to evaluate the clinical efficacy and use of various specimens other than saliva ones.
(3) Active use of pooled assays
Along with the Korea Disease Control and Prevention Agency, the Korean Society for Laboratory Medicine has developed and uses pooled assays. A pooled assay has 100% sensitivity even when pooling five specimens, and its accuracy and capability for detecting a large volume of specimens are advantages over rapid antigen tests or saliva specimen tests.7 The highest record of COVID-19 molecular tests performed per day in Korea is 100,000, and if these cases are pooled as 100%, a maximum of 500,000 individuals can be tested per day. However, this approach requires additional personnel for collecting specimens and increases fatigue in these individuals. This would necessitate adequate compensation for the staff as well as additional support. It is thought that a maximum of 1,000,000 individuals can be tested per day if additional staff can be provided. Therefore, the active use of pooled assays is needed.8
2) Increased capacity to perform diagnostic tests
In Korea, confirmed cases have continued to increase after experiencing a number of pandemic waves. Demands for COVID-19 diagnostic tests are increasing, and it is becoming difficult to continue social distancing for the long term. In order to meet these increased demands for COVID-19 diagnostic tests, we suggest the following strategies:
(1) Expansion of free screening centers
Considering the current inability to trace the transmission routes in more than 20% of confirmed COVID-19 patients, it is important to perform COVID-19 diagnostic tests, even in the absence of an epidemiological relationship, in order to identify hidden infections in the community and to prevent transmission.
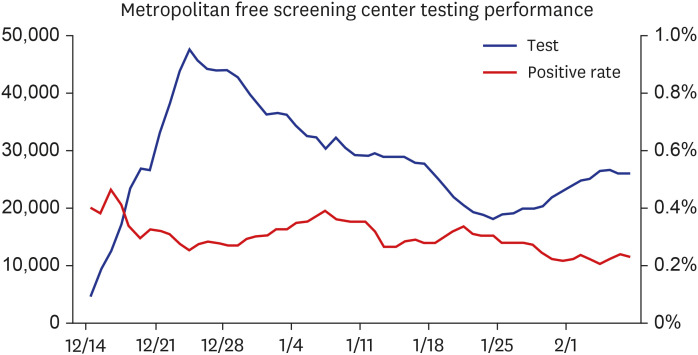
However, in terms of cost and convenience, it is difficult to expect individuals to visit hospitals and undergo testing when COVID-19 is not suspected. Therefore, the expansion of free screening centers that are easily accessible is needed so that individuals with mild symptoms can easily undergo testing. In metropolitan areas, free screening centers have been temporarily provided since the end of last year, and as of February 7, 2021, 4,699 confirmed cases have been identified (Fig. 2).
Fig. 2. Metropolitan free screening center testing performance (December 14, 2020–February 7, 2021).
It is an important accomplishment to have identified confirmed cases at an early stage through the operation of free screening centers because individuals who were not suspected to have COVID-19 could have led to mass infection if they had not been tested. Therefore, it is necessary to detect hidden infections through the expansion of free screening centers.
(2) Expansion of staff for diagnostic testing and specimen collection
Even with the expansion of screening centers, the number of tests performed cannot be increased without the expansion of laboratory capacities and the capabilities for specimen collection. Most institutions do not have sufficient staff to perform COVID-19 tests and to collect specimens. There is consensus that the number of tests needs to be increased; however, it is up to the individual institution to invest in supporting the needs, including staff expansion.8 Therefore, the reinforcement of staff expansion has been insignificant. Moreover, the existing staff members are overwhelmed and burned out from the amount of work, which affects the accuracy of the tests.9 Therefore, systematic and financial support will be needed to expand the human resources for performing the tests and collecting the specimens.
(3) Increase in the capacity of rapid molecular testing
A COVID-19 test that can provide results within 1 hour is needed for emergency cases because the COVID-19 PCR test takes at least 3 hours to complete. Moreover, the entire process, from collecting specimens to reporting results, should be automated and simple because tests need to be performed at any time in the case of emergencies when unexpected situations may occur. Therefore, different from the original PCR test, a rapid COVID-19 test should be designed to enable automation. An example of such an emergency COVID-19 test includes the Xpert Xpress COVID-19 test by Cepheid Company. This test is easy to use, and the entire process is completed within 50 minutes of inserting specimens automatically. Therefore, it can be used by individuals who are not familiar with the molecular test. In addition, depending on the devices, it can test 2–16 specimens independently. It is also usable for emergency diagnostic testing. Nine types of rapid COVID-19 molecular testing reagents have been previously approved. Of these, two types are foreign and seven are domestic (Table 1).
Table 1. Status of diagnostic testing reagent approval for COVID-19 rapid molecular testing in Korea (as of July 26, 2021).
| Manufacturing country | Approval status | Product name |
|---|---|---|
| South Korea | Regular approval | Ezplex SARS-CoV-2 Fast Kit |
| South Korea | Regular approval | LabGun COVID-19 Fast RT-PCR Kit |
| South Korea | EUA | nCov-QS |
| France | EUA | BioFire Respiratory Panel 2.1 |
| United States of America | EUA | Xpert Xpress SARS-CoV-2 |
| South Korea | EUA | iDetect SARS-CoV-2 Detection Kit |
| South Korea | EUA | A+CheQ COVID-19 High Speed RT-qPCR Detection Kit |
| South Korea | Regular approval | AQ-TOP COVID-19 Rapid Detection Kit Plus |
| South Korea | Regular approval | Real-Q Direct SARs-CoV-2 Detection Kit |
COVID-19 = coronavirus disease 2019, SARs-CoV-2 = severe acute respiratory syndrome-coronavirus-2.
However, domestic products, which are used in addition to the existing PCR machines, are not appropriate for rapid molecular testing as they are not automated and as there is insufficient information regarding their efficacy. Based on the available data, the sensitivity of domestic products is thought to be lower than that of standard PCR. All automated products used for rapid molecular testing are foreign products, and domestic products are not adequately being used as the supply and cost benefit are low. In order to prepare for various emergencies, the supply of foreign products needs to be increased temporarily and realistic testing costs need to be set to expand the capacity of COVID-19 molecular testing. Automated emergency molecular tests should be developed in the country in the long run.
(4) Implementation of automated testing devices
At present, in Korea, many steps of the COVID-19 molecular test are manually performed by medical specialists and medical technicians. Decreased manual processing through the implementation of fully automated molecular testing devices or liquid handling systems can increase the number of tests and maintain accuracy.
In other countries, fully automated molecular testing devices that can test many specimens are used for COVID-19 molecular testing. Each of these devices can test a maximum of 3,000 cases per day. The active use of automated devices has advantages, including the prevention of human errors by decreasing staff fatigue and the expansion of the number of tests by increasing the available testing times. The distribution of automated devices to central hospitals, such as national hospitals and public hospitals, will be helpful in the current COVID-19 pandemic and will prepare for other infectious disease epidemics. In terms of long-term effort, the development of domestic products, including emergency molecular tests, is needed.
A liquid handling system is a device that can accurately transfer reagents and specimens into reaction tubes. It uses general purpose reagents but is useful because many processes of the test can be automated. It is currently used in major clinical laboratories in the country, and it needs to be distributed more widely.
3. Mid-to-long-term vision and plan for COVID-19 diagnostic testing
1) Foreign introduction of viral variants and the corresponding plan
(1) Continuous reporting of novel variants
Before and after the end of 2020, diverse variants of SARS-CoV-2 have been introduced. Of these, the Alpha, Beta, Gamma, and Delta variants have been important.9,10 These variants are characterized by higher transmissibility, higher fatality, and resistance to neutralizing antibodies (Table 2). These variants are spread worldwide.
Table 2. Main variants of concern and their characteristics (source: CDC.gov).
| Variant | Spike protein variant | Countries | Characteristics |
|---|---|---|---|
| Alpha | Δ69/70, Δ144Y, (E484K), (S494P), N501Y, A570D, D614G, P681H | United Kingdom | Increased transmissibility |
| No difference in antibody neutralization | |||
| Beta | K417N, E484K, N501Y, D614G | South Africa | Increased transmissibility |
| Reduced antibody neutralization | |||
| Gamma | K417N/T, E484K, N501Y, D614G | Japan, Brazil | Reduced antibody neutralization |
| Delta | T19R, L452R, T478K, P681R, D(50N) | India, worldwide | Increased transmissibility |
| Reduced antibody neutralization |
(2) Screening test strategies for preventing the introduction of variants
Each variant has 12–17 mutations. At least three to four mutations should be confirmed to discriminate these variants. However, it is difficult to perform these tests at screening centers or clinical laboratories; therefore, strategies need to be developed for performing screening tests. The Alpha variant has N501Y and Δ69/70 mutations, while the Beta and Gamma variants have N501Y and E484K mutations. The Alpha, Beta, and Gamma variants thus have the N501Y mutation, and this can be used for screening tests.9 At present, only patients with confirmed COVID-19 with suspected variants undergo variant testing. However, a single test that can simultaneously diagnose COVID-19 and identify the N501Y mutation will be needed as a long-term plan.
(3) Surveillance using whole-genome sequencing (WGS)
Novel variants of SARS-CoV-2 will likely appear as the treatment options and vaccine availability pose selection pressure; these variants will be transmitted very quickly once social interactions resume. Mutations occur on all sites of the genome. Therefore, WGS is essential for surveillance and analysis.11 The results of SARS-CoV-2 WGS performed to date in Korea account for only 2% of all cases; this is insufficient to understand the entire aspect and extent of mutation. Therefore, a more active study of WGS will be needed for the surveillance of variant introduction.12
2) Strategies for evaluating COVID-19 vaccine efficacy using the antibody test
COVID-19 vaccination began on February 26, 2021 in Korea. The efficacy of vaccines should be evaluated objectively as it can vary depending on the vaccine type, patient characteristics, and SARS-CoV-2 variants. An important measure of efficacy is antibody assessment. The presence of protective antibodies is particularly important. However, as the protective antibody test is difficult, the binding antibody test is generally preferred. Antibody tests should have high sensitivity, specificity, and correlation to the protective antibody. The performance of COVID-19 antibody tests can vary depending on the reagents used. Therefore, appropriate strategies for the antibody test should be developed after discussion with medical specialists who have experience in laboratory medicine.
3) Strategies for the diagnosis of respiratory viruses and COVID-19 in the future
At present, almost no seasonal respiratory viral infections are circulating because of social distancing and mask use, but these infections will be back once these measures are discontinued.13 COVID-19 will also continue to occur in some areas. Therefore, seasonal respiratory viral infections and COVID-19 will need to be quickly and accurately diagnosed simultaneously in the future.14
Assays for seasonal respiratory viruses typically involve the multiplex PCR method, which detects multiple viruses at the same time; this takes longer than the current PCR test for COVID-19. Therefore, if SARS-CoV-2 is included in the multiplex PCR test, the results and reporting of COVID-19 tests will be delayed. Hence, it is more appropriate to add a rapid method that can selectively test two to three types of fatal viruses, including SARS-CoV-2 and influenza, in addition to including SARS-CoV-2 to other respiratory viruses in the multiplex assay.
4) Development of mid-to-long-term diagnostic testing methods and strategies for management
(1) Active participation of laboratory medicine specialists in policy-making
In order to make good use of infectious disease diagnostic tests as a preventative measure, the participation of laboratory medicine specialists who understand the characteristics of different diagnostic tests in the infectious disease prevention and control policy is needed. During the COVID-19 response in Korea, the Korean Society for Laboratory Medicine, which is the society of specialists in laboratory medicine, closely cooperated with the Korea Disease Control and Prevention Agency and contributed to many accomplishments. However, the cooperation with other departments was limited; therefore, the opinions of experts were not adequately addressed, which led to inaccurate information and decisions as well as inefficient use of valuable resources and time. It is imperative that experienced specialists in laboratory medicine participate in developing the infectious disease prevention and control policy to establish more efficient management strategies. An infectious disease diagnosis testing expert committee should be formed. The current infectious disease control committee of the Korea Disease Control and Prevention Agency includes 10 expert committees but does not include a diagnosis testing expert committee. As the significance of diagnostic tests has been demonstrated through the COVID-19 pandemic, there is a dire need for establishing an infectious disease diagnosis testing expert committee within the Korea Disease Control and Prevention Agency.
(2) Expansion of diagnostic testing capacity: staff, biosafety facilities, and laboratories
As discussed in the short-term pending issues, the capacity to perform infectious disease diagnostic tests needs to be expanded. This cannot be done within a short time and involves factors that require long-term preparation.
First, a professional workforce needs to be developed. The results of infectious disease diagnostic tests, including the COVID-19 test, can greatly impact patients and the society; therefore, accuracy and speed are very important. Testing for infectious diseases requires the management of various tests and interpretations, which are part of medical practice; these processes are completely different from those of tests for research purposes. The involvement of medical specialists, including laboratory medicine and medical technicians, who can perform and manage tests is needed; this investment will be a valuable resource in responding to future infectious disease outbreaks.
Second, the levels of biosafety facilities need to be improved. To perform diagnostic testing for novel infectious diseases, such as COVID-19, or for other high-risk pathogens, biosafety laboratory facilities are essential. However, most clinical laboratories in Korea have biosafety level 2 or lower equipment. Moreover, there are no regulations for such equipment and facilities. Expenses are involved in the initial establishment and maintenance of biosafety facilities, and it is difficult to expect voluntary investment. Institutional arrangements and financial support are needed to have an appropriate facility in place. The government needs to provide institutional and financial support to expand biosafety facilities in clinical laboratories.
Third, the diversification of laboratories is important. At present, 80% of COVID-19 testing is performed at commercial laboratories, with most tests being performed at the top 5 commercial laboratories. Therefore, problems at any of these centers will directly impact the overall testing capacity. To maintain the COVID-19 testing capacity, equal distribution of testing at medical center laboratories, mid-size commercial laboratories, and large-size commercial laboratories will be needed.
(3) Development of new infectious disease testing methods and long-term evaluation
PCR using nasopharyngeal specimens has been a standard diagnostic testing method for the diagnosis of respiratory viral infections since a long time. However, we have now decided to use COVID-19 PCR that was developed in a short time; this method has high credibility and is being used for COVID-19 diagnosis without significant problems. Such a decision was based on the principle that we need to rely on a device that has excellent and stable performance and could be supplied in mass amounts in case of emergencies. This decision proved to be a success.
During the COVID-19 pandemic, many novel testing methods have been introduced. While these methods have the potential to be advanced in the future, most have remained in the investigation or prototype phase and none of the products have the quality for use in clinical laboratories. Unfortunately, some researchers have suggested the use of non-verified methods for performing COVID-19 diagnostic tests immediately.
More advanced methods that can overcome the limitations of PCR will need to be developed in the long term to prepare for emergencies. However, as discussed, for using the testing methods in emergencies, such as COVID-19, the performance of these methods will need to be validated through their long-term use in the field. For this purpose, new diagnostic methods should be researched, developed, and verified as a long-term plan.
4. Summary
COVID-19 diagnostic testing has received praise and played an essential role during the COVID-19 response in Korea. However, as the pandemic has not yet ended, many short- and long-term issues need to be addressed. As described in this article, continued efforts should be made on the basis of scientific evidence and in cooperation with laboratory medicine experts.
Treatments and Vaccine Research
Recommendations
○ Recommendation 5: The transmission characteristics of SARS-CoV-2 mutants and the efficacy of vaccines should be studied.
○ Recommendation 6: Supplies of and systems for approved treatments should be established.
○ Recommendation 7: Preparations should be ongoing for a prolonged COVID-19 outbreak, and efficient treatments should be developed for patients with mild symptoms.
○ Recommendation 8: Follow-up studies on the duration of the effects of vaccines and on the long-term safety of vaccines, in addition to blocking community transmission, are needed.
○ Recommendation 9: Support for domestic vaccine and therapeutic development should be continued, and information on development and clinical applications should be disclosed.
○ Recommendation 10: The final vaccine to be used in Korea should be selected after considering the efficacy and safety of the vaccine.
○ Recommendation 11: After thorough preparation and enhancement of public confidence, the vaccination rate should be increased to achieve herd immunity.
1. COVID-19 causative virus
1) Status
Although SARS-CoV-2, which causes COVID-19, has a similar structure and 80% genetic similarity to SARS-CoV (which caused severe respiratory problems in Guangzhou, China, in 2002), it differs from SARS-CoV in terms of the disease course and pathology.15
Coronaviruses are classified as alpha-, beta-, gamma-, and deltacoronaviruses according to their genetic, structural, and pathological properties.16 Since reports of the first coronavirus, the B814 strain, in 1965, alpha- and betacoronaviruses have been found to infect humans. Alphacoronaviruses include the 229E and NL63 strains, while betacoronaviruses include the OC43 and HKU1 strains.
The SARS-CoV outbreak in China in 2002, the first MERS-CoV outbreak in Saudi Arabia in 2012, and the SARS-CoV-2 outbreak in China in 2019 were caused by betacoronaviruses. Structurally, SARS-CoV-2 is very similar to the MERS coronavirus, which caused an outbreak in Korea in 2015, but differs genetically (about 50% genetic similarity) and in terms of pathology.17
Glycosylated spike proteins cover the surface of SARS-CoV-2 virions and bind to the host cell receptor, i.e., angiotensin-converting enzyme 2 (ACE2), thereby mediating the invasion of viral cells. Two proteases (furin and TMPRSS2 proteins) facilitate SARS-CoV-2 invasion into cells by sequentially cleaving the SARS-CoV-2 spike protein.18
During the SARS-CoV-2 infection process (life cycle), the human body elicits an immune response. However, the antibodies formed in response to SARS-CoV-2 infection would be lost within 6–12 months, following which reinfection could occur.19 This suggests that the immune response to SARS-CoV-2 infection is very short.
The genetic sequence of SARS-CoV-2 may continuously change in new hosts as it is an RNA virus, similar to the influenza virus. Genetic mutations continue to occur during the replication process. As a result, new variants of concern are emerging. Genetically, seven clades (S, L, O, V, G, GH, and GR) of SARS-CoV-2 have been identified, and additional mutations continue to appear worldwide.12
Mutations related to growth characteristics can affect the clinical severity of COVID-19, while mutations in a spike protein or a protein that recognizes a receptor can affect the efficacy of vaccines by changing the antigenicity of the virus.19
2) Problems and recommendations
The number of countries where mutated viruses have been detected is increasing. Mutated viruses have been confirmed in Korea, raising concerns about the risk of mutated viruses entering from overseas (as of February 14, 2021, there were 75 in the UK, 13 in South Africa, and six in Brazil) and spreading throughout the population.
Therefore, it is necessary to analyze the characteristics of common mutants, including their propagation characteristics, severity, and degree of immune avoidance (including group immunity), in order to enable a rapid response and enhance vaccine effectiveness.
2. Development of therapeutics
1) Status
COVID-19 can be categorized as mild, severe, or critical depending on the symptoms. About 80% of patients have mild infections, and most can be isolated and monitored until they recover on their own.20 Patients with severe infections are treated with antivirals or antibiotics for secondary bacterial infections and given inhaled steroids to reduce inflammation. Patients with critical infections require ventilators, extracorporeal membrane oxygenation (ECMO), and anticoagulants (such as heparin).21 However, these are all symptomatic treatments; their efficacy should be evaluated in clinical trials. Only two treatment options have shown efficacy in clinical studies of COVID-19: remdesivir and dexamethasone. Remdesivir is used for patients who need oxygen therapy but for whom a ventilator is not needed, while dexamethasone is used for patients with critical infections who need oxygen therapy and/or a ventilator. However, these two treatment options have significant limitations for use in patients with severe or critical COVID-19.22
The symptoms of COVID-19 patients vary, and more research is needed on which drugs need to be administered, the sequence in which drugs need to be administered, which medications do not reduce natural immunity, and which medications strengthen acquired immunity, according to the patient's condition. The development of new therapeutics is necessary, in addition to conducting clinical studies on existing drugs. In Korea, clinical trials involving COVID-19 patients were planned for several drugs, including remdesivir, the antimalarial drug hydroxychloroquine, the human immunodeficiency virus (HIV) infection drug lopinavir/ritonavir (brand name, Kaletra), hydroxychloroquine derivatives, and inhaled steroids. However, most of these studies were suspended after the results in other countries were published.22
The aforementioned therapeutics are only applicable to patients with severe or critical infections. Therefore, it is necessary to develop preventive, preemptive therapies (i.e., for use before infection occurs after exposure) or therapeutics that prevent the transition from mild to severe/critical infection. Although an antibody treatment that is currently under development has shown partial efficacy in patients with mild infection, further studies are needed to assess its clinical utility.
2) Problems and recommendations
Strategies to treat and manage patients according to disease severity remain limited, and no treatment has been proven to be effective in blocking community transmission. Therefore, to prepare for a prolonged COVID-19 pandemic, the development of treatments (drug repurposing and the creation of new drugs), especially effective and easy-to-dose drugs for patients with mild infections, needs to be ongoing. This will require cooperation and support among government ministries. Developments should be freely shared among researchers, medical staff, and the public. In addition to actively supporting clinical research, approved treatments and systems to facilitate their clinical use are needed.
3. Vaccine development
1) Status
As of February 19, 2021, 251 SARS-CoV-2 vaccines were under development globally, with 70 being at the clinical stage and 181 at the preclinical stage.23 Candidate SARS-CoV-2 vaccines at the clinical stage were developed using about 10 platforms. Approximately 87% of the vaccines are based on the protein subunit, viral vector (non-replicating), DNA, inactivated virus, and RNA platforms.
Fourteen vaccines are undergoing phase 3 clinical trials; of these, five are inactivated virus-based vaccines, four are viral vector-based vaccines (non-replicating), two are protein subunit-based vaccines, and three are RNA-based vaccines.
2) Current status of approved COVID-19 vaccines in other countries
Globally, seven vaccines in phase 3 clinical trials have been approved for emergency use: the Pfizer, Moderna, AstraZeneca, Johnson & Johnson/Janssen, Novavax, Sinopharm, and Sputnik vaccines.24 The first vaccines to be developed and used were mRNA- or viral vector-based (non-replicating) ones, but vaccines based on recombinant proteins will also likely be approved soon (Table 3).
Table 3. Severe acute respiratory syndrome-coronavirus-2 vaccines approved for emergency use by country.
| No. | Vaccine manufacturer | Platform | Inoculation | Efficacy | Stage |
|---|---|---|---|---|---|
| 1 | Pfizer/BioEntec | mRNA | Twice | 95% | Emergency use |
| 2 | Moderna | mRNA | Twice | 94% | Emergency use |
| 3 | AstraZeneca | Adenovirus vector | Twice | 62%, 82% | Emergency use |
| 4 | Janssen | Adenovirus vector | Once | 66% | Emergency use |
| 5 | Novavax | Protein subunit | Twice | 89% | Emergency use (expecting) |
| 6 | Sinopharm | Inactivated virus | Twice | Unknown | Emergency use |
| 7 | SputnikV | Adenovirus vector | Twice | Unknown | Emergency use |
3) Current status of COVID-19 vaccine approvals in Korea
In Korea, protein subunit-, DNA-, and viral vector-based (non-replicating) vaccines are undergoing clinical trials (Table 4).24
Table 4. Current status of domestic coronavirus disease 2019 vaccine development.
| No. | Vaccine manufacturer | Platform | Clinical phase |
|---|---|---|---|
| 1 | SK Bioscience | Protein subunit | Phase 3 |
| 2 | Genexine | DNA | Phase 2, 3 |
| 3 | Gene One | DNA | Phase 2a |
| 4 | Cellid | Adenovirus vector | Phase 1, 2a |
| 5 | Eubiologics | Protein subunit | Phase 1, 2a |
| 6 | HKInnoen | Protein subunit | Phase 1 |
The efficacy of the vaccines developed globally is 70–95%. Although this far exceeds the World Health Organization (WHO) recommendation (50%), the efficacies of vaccines differ among platforms. Although the efficacies of adenovirus vector- and DNA-based vaccines exceed the WHO recommendation, the efficacy of neutralizing antibodies is lower than that of mRNA-based vaccines. These differences can cause anxiety among scientists and clinicians. However, there is no evidence that the reported efficacies of vaccines reflect their actual efficacy. In addition, although the preventive efficacy of vaccines has been confirmed in clinical studies, it has not yet been evaluated at the community level. In Israel, where 48% of the population was vaccinated with the Pfizer vaccine, the vaccine efficacy was reported to be high as 92%; however, there are no reports on the efficacy of the vaccine in preventing community transmission.
Recently, with the global spread of mutations, concerns about the efficacies of developed vaccines are growing. While there appear to be no differences in their efficacies against the British mutant, the efficacies of the Moderna and Pfizer vaccines against the South Africa mutant have been reported to be 8.6 and 6.5 times lower, respectively.24 It is possible that the efficacy of vaccines will be lower against new strains as they spread globally; therefore, countermeasures are needed.
Regarding vaccine safety, the main side effects are pain in the inoculation area, seizures, and fever. Moreover, anaphylaxis occurs in about 11 per million population. This is not significantly different from the observations in the case of vaccines for other diseases.25 The most worrisome side effects of vaccines, vaccine-associated immune response disease (VAERD) and antibody-dependent enhancement (ADE), have not been seen in animal trials or phase 3 clinical trials and have rarely been observed after vaccination. However, as these side effects can appear over the long term, it is necessary to observe the vaccinated population continuously for side effects.
The worldwide vaccination rate is 4 million doses per day; this can be attributed to the low rates of vaccine production and supply. Korea has purchased vaccines from AstraZeneca, Janssen, Pfizer, and Moderna, while a contract has been signed to produce and supply the Novavax vaccine directly following technology transfer. A global vaccine supply is needed during this pandemic, but mass production has been problematic. It is necessary to ensure that vaccines are developed at the national level in preparation for a prolonged COVID-19 outbreak.
4) Further problems and recommendations
Although the short-term safety and efficacy of vaccines have been confirmed in other countries and their emergency use has been authorized, it is necessary to verify their long-term safety in follow-up studies in Korea, especially in terms of side effects (such as ADE and VAERD).
The preventive efficacy of vaccines differs according to the development platform; this can cause anxiety among scientists and clinicians. There is a need for criteria to select vaccines according to their safety and efficacy and for preparing countermeasures for situations in which the efficacy of approved vaccines is low against emerging COVID-19 mutants.
As the COVID-19 vaccines were developed at an unprecedented speed, there are concerns about their efficacy and safety. To resolve these concerns and increase public confidence in vaccines and quarantine policies, continuous efforts will be required in various fields. Thorough preparation and mock inspection are required in advance to prevent any problems in the vaccination plan, in addition to the disclosure of transparent information on vaccines. Moreover, efforts are needed to secure herd immunity by increasing the vaccination rate. The current vaccines have been reported to have preventive efficacy in clinical studies; however, the effect on blocking community transmission has not yet been evaluated and needs to be assessed in future studies.
To prepare for a prolonged COVID-19 pandemic and the emergence of other infections, it is necessary that countries have the capacity to develop their own vaccines. This will require a detailed schedule for accelerating the development of domestic vaccines and governmental measures to support global phase 3 clinical trials.
Critical Care of COVID-19 Patients
Recommendations
○ Recommendation 12: Intensive care workers need to be trained.
○ Recommendation 13: A safe critical care transport system needs to be established.
○ Recommendation 14: Dedicated hospitals for efficient intensive care should be activated.
1. Critically ill cases occurred during the last 1-year pandemic in Korea
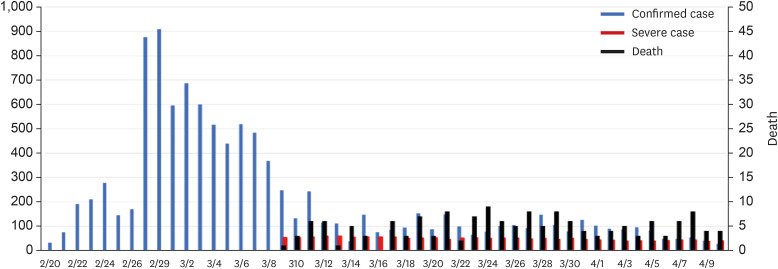
In Korea, the first COVID-19 case was confirmed on January 20, 2020, and three pandemics have occurred since then. The first pandemic spread in Daegu and Gyeongbuk areas on February 18, 2020. On February 29, the number of new confirmed cases reached a peak of 909 per day, and thereafter, for 10 days until March 10, there was an explosive number of 6,600 cases per day. Since then, the number of new cases has dropped to less than 500 per day, reducing to within 10 per day in May. The number of critically ill patients sharply increased around March 10, with about 100 people being treated in intensive care units (ICUs) every day in the next month. About 50 patients were receiving mechanical ventilation treatment, and about 10 were receiving ECMO treatment. The cumulative death toll was 248 by May 1, after the first death on February 20 (Fig. 3).
Fig. 3. Status of confirmed cases, critically ill cases, and deaths during the first coronavirus disease 2019 pandemic in Korea. There is no information on critically ill patients during the early stages of the COVID-19 outbreak in the government data. The Korean Society of Critical Care Medicine collected critical care data from March 9, focusing on training hospitals specializing in critical care medicine. In this graph, critical patients are those requiring mechanical ventilation.
Unlike the number of newly confirmed cases or deaths, the count of critically ill patients is not the number of new cases on that day. As the number of critically ill patients being treated on the same day was counted, it is difficult to count the total number of critically ill patients who were actually treated in the ICU with this data. About 4.8% of confirmed cases were reported to have been admitted to the ICU by May 2020.26
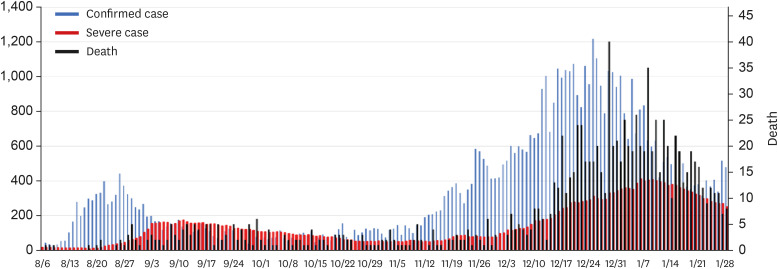
The second and third pandemics occurred in succession at short intervals. From August 14, the number of newly confirmed cases sharply increased, with about 200–400 patients per day. From September 20, the number declined to less than 100. This lasted until early November, with intermittent fluctuation of new cases. The third pandemic began in mid-November, with a steep increase in the number of new cases (over 200 per day). The number of new cases per day hit 1,216 on December 25. Since then, it gradually decreased, but there were still 300–500 new cases every day as of February 1, 2021.
The government started counting the number of critically ill patients with a severity that required beyond high-concentration oxygen treatment from July 2020. Before the second outbreak, the number of critically ill patients was maintained around 15 nationwide, but it increased after mid-August and reached 175 on September 11, gradually decreasing thereafter. However, more than 50 patients were still being treated in the ICU when the third outbreak began in mid-November. As shown in the Fig. 4, at 10 days after the number of newly confirmed patients increased, the number of critically ill patients increased. The highest number of newly confirmed cases was noted on December 25 (1,216), and on January 6, 2021, the highest number of critically ill patients reached 411. Since then, the number of new cases has been gradually decreasing, and the number of critically ill patients has also been gradually decreasing (Fig. 4).
Fig. 4. Status of confirmed cases, critically ill cases, and deaths during the second and third coronavirus disease 2019 pandemics. In this graph, critical patients are those requiring high-flow oxygen therapy, mechanical ventilation, extracorporeal membrane oxygenation, continuous renal replacement therapy, etc.
During the second and third pandemics, the cumulative death toll also rose sharply, reaching 1,425 as of February 1, 2021. From the point of view of critical care, the 2-month period from February 20, 2020 can be considered as the first pandemic period. During this period, there were 10,592 newly confirmed cases and 236 deaths, resulting in a mortality rate of 2.2%. Although the distinction between the second and third pandemics is ambiguous, referring to the slope change in the patient incidence graph, the second pandemic period can be defined as 2 months from August 15, while the third pandemic period can be defined the period from December 1, 2020 to February 1, 2021. During the second pandemic, there were 9,108 new cases and 134 deaths, with a mortality rate of 1.47%. During the third pandemic, there were 42,109 new cases and 899 deaths, with a mortality rate of 2.13%.
2. The impact of mutant viruses on the increase in critical cases in the UK
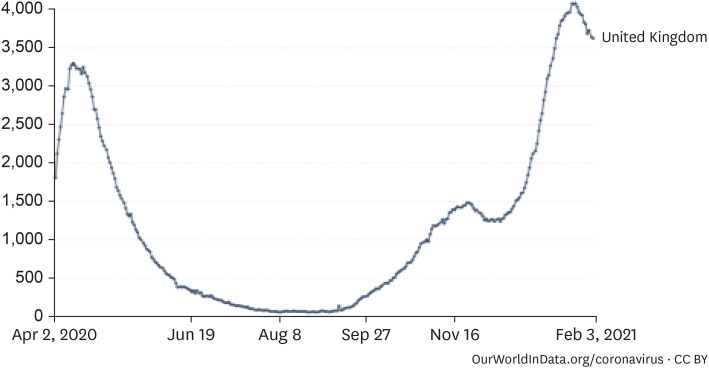
The possibility of a new outbreak caused by mutant viruses in Korea is high. The UK mutant B.1.1.7, the South African mutant B.1.135, and the Brazilian mutant P.1 are examples of mutated viruses that are generally reported to increase the number of critical cases and worsen the severity of disease. The UK is geographically located in the northern hemisphere, and the quarantine policies in the UK are comparable to those in Korea. In the UK, the rate of disease transmission by mutant viruses was estimated to have increased by 1.7 times (from 0.8 to 1.25).27 An absolute assessment is difficult because trends can vary at different times of the year. The epidemic caused by the mutant virus is known to have affected individuals since September, with a daily maximum of 60,000 new patients around mid-January, up 12 times higher than that in April. The 7-day average number of inpatients was 38,000, up 1.75 times higher than that in April, and the number inpatients in the ICU was 4,077, up 1.24 times higher than that in April. Considering the average 7-day maximum of 1,489 patients in the ICU in November, just before the mutant virus outbreak, it can be estimated that the number of patients in the ICU increased by 2.74 times because of the mutant virus (Fig. 5). The positivity rate of coronavirus antibodies reported in Korea is 0.09–0.3%, which is significantly lower than that in the UK; therefore, the mutant virus epidemic will be more disastrous and the resources needed for intensive care should be up to three times higher.
Fig. 5. Coronavirus disease 2019 inpatient trends in the UK (7-day average).27 .
Source: European CDC for EU countries, government sources for other countries.
3. Strategies for intensive care
1) Status of treatment for critically ill COVID-19 patients
Critical care for COVID-19 patients mainly involves supportive care following the treatment principles for severe pneumonia, ARDS, and septic shock. COVID-19 is an infectious disease for which a treatment modality has not yet been developed. The only goal is to allow the patient to recover through supportive therapy for organ failure. Stepwise treatment, such as oxygenation, mechanical ventilation, hemodynamic stability, renal replacement therapy, and extracorporeal membrane oxygenation, is provided. Intensive care for COVID-19 patients is mainly provided at hospitals dedicated to infectious diseases, national university hospitals, and tertiary general hospitals.
When the first pandemic occurred, the government designated 43 hospitals specializing in infectious diseases on February 21. The number increased to 67 on March 13 and mainly involved public hospitals and national university hospitals (Table 5). However, there was a significant gap in the intensive care capabilities of hospitals dedicated to infectious diseases, being only available in university hospitals and some medical institutions. Hence, tertiary general hospitals across the country, especially tertiary hospitals in Seoul and Gyeonggi Province, prepared COVID-19 critical care beds. However, there was a limitation in treating patients, mainly in Daegu and Gyeongbuk Province, because of the lack of a long-distance transport system for critically ill patients.28
Table 5. Status of hospitals designated to infectious diseases (March 13, 2020).
| Region | No. | Hospitals designated to infectious diseases |
|---|---|---|
| Total | 67 | |
| Seoul | 5 | National Medical Center, Seoul Metropolitan City Seoul Medical Center, Seoul Metropolitan City Seonam Hospital, Seoul Metropolitan City Seobuk Hospital, Boramae Medical Center |
| Busan | 1 | Busan Medical Center |
| Daegu | 7 | Daegu Medical Center, Keimyung University Dongsan Medical Center (Jung-gu), COMWEL Daegu Hospital, Daegu VHS Medical Center, Yeungnam University Medical Center, Daegu Catholic University Medical Center, Keimyung University Dongsan Medical Center (Dalseo-gu) |
| Incheon | 5 | Incheon Metropolitan City Medical Center, Inha University Hospital, Gacheon University Gil Medical Center, Incheon Red Cross Hopital, Incheon Medical Center Baengnyeong Hospital |
| Gwangju | 2 | Chonnam National University Bitgoeul Hospital, Chonnam National University Gwangju 2nd Geriatric Hospital |
| Daejeon | 4 | Armed Forces Daejeon Hospital, Central Medical Institute Silver Daejeon Hospital, Chungnam National University Hospital, Daejeon VHS Medical Center |
| Ulsan | 5 | Ulsan University Hospital, Donggang Hospital, Ulsan Metropolitan City Geriatric Hospital, Ulsanjoongang Hospital, Ulsan Medical Center |
| Sejong | 1 | NK Sejong Hospital |
| Gyeonggi | 7 | Gyeonggi Provincial Medical Center Suwon Hospital, Gyeonggi Provincial Medical Center Ansung Hospital, Gyeonggi Provincial Medical Center Icheon Hospital, Gyeonggi Provincial Medical Center Paju Hospital, Gyeonggi Provincial Medical Center Uijeongbu Hospital, Gyeonggi Provincial Medical Center Pocheon Hospital, Seongnam Citizens Medical Center |
| Gangwon | 5 | Wonju Medical Center, Gangneung Medical Center, Sokcho Medical Center, Samcheok Medical Center, Yeongwol Medical Center |
| Chungbuk | 2 | Cheongju Medical Center, Chungju Medical Center |
| Chungnam | 4 | Cheonan Medical Center, Gongju Medical Center, Seosan Medical Center, Hongseong Medical Center |
| Jeonbuk | 3 | Gunsan Medical Cente, Namwon Medical Center, Jinan Medical Center |
| Jeonnam | 3 | Mokpo Medical Center, Suncheon Medical Center, Gangjin Medical Center |
| Gyeongbuk | 6 | Pohang Medical Hospital, Gimcheon Medical Center, Andong Medical Center, Sangju Red Cross Hospital, Yeongju Red Cross Hospital, Korean Armed Forces Capital Hospital (Gyeongsan) |
| Gyeongnam | 4 | Masan Medical Center, Masan National Tuberculosis Hospital, COMWEL Changwon Hospital, Tongyeong Red Cross Hospital |
| Jeju | 3 | Jeju Medical Center, Seogwipo Medical Center, Jeju National University Hospital |
During the first pandemic, Daegu-Dongsan Hospital served as a regional hub hospital dedicated to COVID-19 as the number of patients rapidly increased in Daegu and Gyeongbuk Province. On February 21, Daegu-Dongsan Hospital quarantined the entire hospital building as a cohort to treat patients with mild-to-moderate symptoms but opened a 17-bed ICU sequentially from March 10 as the number of critically ill patients increased. Global Care, a health and medical NGO, and the Daegu Social Welfare Community Chest jointly prepared financial resources for equipment, and the Korean Society of Critical Care Medicine supported professional medical personnel. At that time, there were 18 beds for COVID-19 intensive care in Daegu and Gyeongbuk Province and 50 additional negative-pressure intensive care beds were temporarily prepared (nine in Kyungpook National University Hospital, nine in Daegu Catholic Hospital, four in Fatima Hospital, six in Yeungnam University Hospital, five in Chilgok Kyungpook National University Hospital, and 17 in Daegu-Dongsan Hospital).28 Table 6 shows the number of critically ill patients being treated at 97 medical institutions nationwide, as per the government data. As this information is aggregated by the number of hospitalized patients, the actual severity may differ. Fig. 6 shows the number of critically ill patients receiving mechanical ventilation nationwide, as per the data of the Korean Society of Critical Care Medicine, which focuses on critical care training hospitals.
Table 6. Trends in critically ill hospitalized patients (based on data from 97 institutions).
| Date | 3/30 | 3/31 | 4/1 | 4/2 | 4/3 | 4/4 | 4/5 | 4/6 | 4/7 |
|---|---|---|---|---|---|---|---|---|---|
| No. of patients | 92 | 90 | 90 | 88 | 86 | 85 | 93 | 83 | 80 |
Fig. 6. Distribution of critically ill coronavirus disease 2019 patients receiving mechanical ventilation during the first pandemic.
As the number of patients decreased after April, COVID-19 critical care beds were gradually converted to general beds from April 23. The government was planning to prepare intensive care beds according to the number of new cases, but the second pandemic started around August 15 before this was implemented. In particular, new cases surged around Seoul and Gyeonggi Province, the metropolitan area of Korea, increasing the shortage of intensive care beds. At that time, intensive care was provided mainly on state-designated isolation beds and intensive care beds at tertiary referral hospitals. This expanded intensive care beds to national university hospitals, focusing on the operational efficiency of intensive care beds. In other words, attempts were made to secure an ICU where more intensive treatment was possible by transporting patients who were not in a critical condition to sub-ICUs in public hospitals, such as the National Medical Center and Red Cross Hospital. Based on available critical care bed data compiled by the Korean Society of Critical Care Medicine and the HIRA, the metropolitan response team transferred the patients and assigned the wards. When the critical care beds in the metropolitan area reached saturation, patients were transferred to Gyeongsang-do and Jeolla-do for treatment. As intensive care beds became absolutely scarce, the government ordered tertiary referral hospitals and national university hospitals to prepare COVID-19 intensive care beds, accounting for more than 1% of their operating beds. In addition, more intensive care beds were secured by designating a hub hospital for COVID-19 (Table 7). The shortage of medical personnel at the hub hospital was met by the Disaster Medical Support Team of the Korean Medical Association.
Table 7. Status of functioning of hospital beds dedicated to coronavirus disease 2019 (press release on January 21, 2021).
| Location | Designated date | Hospital name | Severe | Moderately-severe | Moderate | |||
|---|---|---|---|---|---|---|---|---|
| Holding (n = 178) | Available (n = 104) | Holding (n = 202) | Available (n = 71) | Holding (n = 843) | Available (n = 525) | |||
| Gyeonggi Pyeongtaek | 12.12 | Bagae Hospital | 30 | 11 | 90 | 20 | 20 | 8 |
| Gyeonggi Goyang | 12.17 | National Health Insurance Service Ilsan Hospital | 12 | 3 | 10 | - | 125 | 49 |
| Gyeonggi Bucheon | 12.17 | Soonchunhyang Bucheon Hospital | 16 | 5 | 6 | 3 | - | - |
| Gyeonggi Namyangju | 12.17 | Hyundai Hospital | 25 | 20 | 18 | 6 | 64 | 64 |
| Gyeonggi Osan | 12.25 | Osan Hankook Hospital | 19 | 19 | 8 | 8 | 70 | 59 |
| Gyeonggi Seongnam | 12.28 | Seongnam Citizens Medical Center | 9 | 4 | 13 | 6 | 142 | 32 |
| Incheon Namdonggu | 12.23 | Gacheon University Gil Medical Center | 26 | 15 | - | - | 72 | 63 |
| Chungbuk Cheongju | 12.17 | Chungbuk National University Hospital | 8 | 4 | 29 | 16 | - | - |
| 12.24 | Bestian Hospital Cheongju | 10 | 7 | 10 | 4 | 90 | 69 | |
| Busan Seogu | 12.22 | Pusan National University Hospital | 17 | 12 | 8 | 1 | 80 | 25 |
| Daegu Bukgu | 12.23 | Kyungpook National University Chilgok Hospital | 6 | 4 | 10 | 7 | 180 | 156 |
2) COVID-19 critical care status in the USA
US hospitals operated 87,046 intensive care beds, accounting for 10.3% of 847,000 general beds, in mid-December. In the USA, patients were treated as acceptable in general hospitals without assigning a dedicated hospital for COVID-19. However, the surge of patients made it difficult to operate the ICU, thereby making it difficult to allocate intensive care beds for other diseases. On January 15, when the number of patients was the highest, 28,000–29,000 COVID-19 patients and 40,000 non-COVID patients were hospitalized in critical care beds. COVID-19 patients accounted for about 42% of ICU patients.
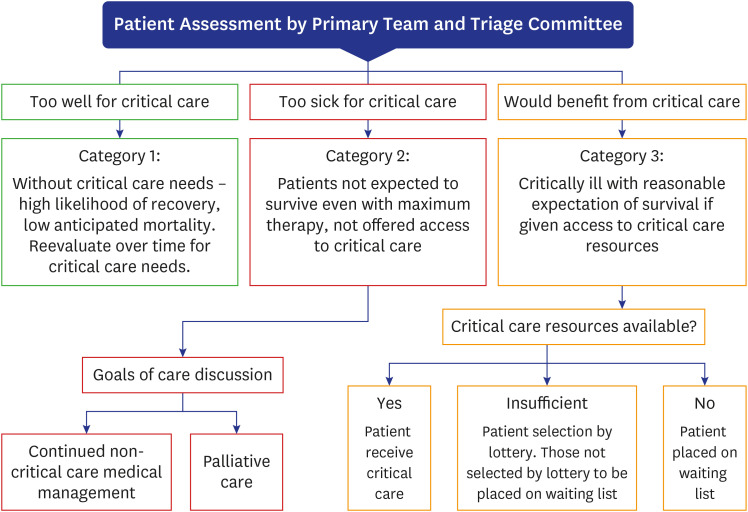
The Society of Critical Care Medicine has established an ethics committee for the allocation of intensive care beds, ventilators, and ECMO therapies and provided guidelines to suggest effective treatment options for the rapidly surging critically ill patients (Fig. 7).29
Fig. 7. Assignment guidelines for critical care beds.
Source: 2020, Society of Critical Care Medicine.
4. Ethical considerations
The COVID-19 critical care guidelines of the Korean Society of Critical Care Medicine suggest admission priorities for ICUs in the case of a shortage of treatment facilities. Step-down ICUs should be operated, and the priority for admission should be clear; this needs to be adjusted according to the situation of the hospital. The criteria for exclusion of admission include patients who do not want active intensive care. The guidelines also cover the termination of treatment of existing patients and the treatment of recoverable patients first. The ethics committee can decide terminating ventilator care in the case of imminent death, in patients who do not improve even after 3 weeks of intensive care, in patients who do not want intensive care, and in patients who are braindead.
Although it is best to provide treatment to all patients, treatment cannot save all patients. In a disaster situation, it is logical to provide intensive care to patients who can recover in a short period, considering that the lungs that have been destroyed by inflammation may not recover to their original condition in some cases. When resources are limited and the number of patients increases to exceed treatment capacity, it is most important to save as many lives as possible and maximize life expectancy. Therefore, in such a scenario, younger patients should be treated first, while older patients and co-morbid patients with a high mortality rate should be given a lower priority. Given the catastrophic nature of the pandemic, these ethical issues should lead to consultation among people at the national level in order to avoid social confusion and minimize conflicts.
5. Recommendations
The definition of intensive care beds and minimum staff standards should be established to ensure good quality of care. The mortality rate of COVID-19 is determined by the quality of intensive care and depends on how well the intensive care system works in a disaster situation. In particular, in the event of an infectious disease disaster, it is necessary to have a system that does not interfere with general intensive care, in addition to ensuring that the infection does not spread and can be safely treated. Therefore, the minimum requirement of medical personnel should be included in the bed conditions.
The biggest obstacle to building the COVID-19 intensive care system has been the difference in the understanding of intensive care beds. Among the confirmed cases, severe COVID-19 patients were defined as patients who were aged 65 years or older, had chronic underlying diseases, had special conditions (e.g., were morbidly obese, were pregnant, needed dialysis, or needed transplantation), and had less than 90% oxygen saturation in room air. In particular, patients in long-term care facilities were considered to have more severe disease and were admitted to intensive care beds first. However, these patients are high-risk groups requiring continuous monitoring and not those who need intensive care. This is different from the standard ICU admission criteria. Critical care beds for patients who need mechanical ventilation are completely different from those used for high-risk patients in terms of the need for medical personnel and equipment. There is no such distinction in intensive care beds as per the government; therefore, there is no accurate information on real intensive care beds with ventilators, monitoring equipment, and medical personnel. Of the medical institutions designated as infectious disease hospitals, few were capable of providing mechanical ventilation treatment. Therefore, if the patients’ condition deteriorated, they had to be transferred to a hospital that could manage critical care, e.g., provide mechanical ventilation.
In the process of securing intensive care beds, it was difficult to efficiently secure beds because of the lack of a concept regarding critical care beds. In addition, there was an absolute shortage of intensive care beds when the third pandemic began. In the process of urgently preparing intensive care beds, there was no consideration of essential personnel standards to ensure the quality of care. Medical resources should be secured to ensure the quality of intensive care, and measures should be prepared to efficiently operate limited resources.
Short-term and mid-to-long-term plans should be established to supply and train critical care personnel. Three to four times the usual numbers of medical personnel are needed to treat patients with infectious diseases. In particular, the lack of nursing manpower is a serious issue, and measures need to be in place to improve the working environment so that there is no loss of manpower, in addition to nurturing new manpower through education.
The third quality assessment of ICU care, in which ICU care from May to July 2019 was evaluated, shows the level of care in ICUs in Korea just before the COVID-19 pandemic. Only 16.7% of general hospitals were rated as first grade, and only 27.4% of them had six specialized equipment facilities for intensive care. An intensivist worked at 37.6% of general hospitals. On average, an intensivist and a nurse were responsible for 22.2 and 1.03 beds, respectively. Considering the three-shift work of nurses, the average number of patients handled by one nurse in an actual work situation is more than five. In order to establish a system that can provide adequate intensive care to COVID-19 patients and therefore requires three or four times the usual number of medical personnel, it is necessary to focus on the supply of human resources.
A safe transport system for critically ill patients should be established without the fear of transmission of infection. Sufficient oxygen supply and first aid personnel should be ensured through close communication among all medical institutions. Although patient classification is being performed at city/provincial administrative unit levels, it is necessary to systematize a transport system for critically ill patients. As critical patients do not occur constantly in any given population, an imbalance can occur depending on the region. The central government should be able to minimize the imbalance through communication with the local government’s quarantine team and the best command system.29
Intensive care should be available at regional hospitals designated to infectious diseases. In addition to intensive care beds, a semi-ICU for high-risk patients and a general bed for moderately ill patients should be operated together. Medical equipment and personnel at the base hospital should focus on treating COVID-19 patients, and a system should be established to support the insufficient manpower. It is necessary to re-evaluate the role of public hospitals as base hospitals. As most public hospitals were designated as infectious disease hospitals, they played a major role in treating patients with moderate or severe disease but had relatively little involvement in treating critically ill patients. Public hospitals, which previously had intensive care capabilities, also failed to actively participate in the intensive care of COVID-19 patients. The main reason was the lack of professional medical personnel. Despite the equipment and beds needed for intensive care, it was difficult to operate because of the lack of internal medical personnel.
It is necessary to achieve social consensus and manage ethical considerations regarding priorities for ICU admission. In the case of a shortage of intensive care resources, patients with high recovery potential and high life expectancy should be prioritized for admission. The role of the ethics committee in the allocation of limited medical resources should be defined, and social consent should be obtained.
Mental Health of COVID-19 Patients
Recommendations
○ Recommendation 15: Psychiatric issues related to COVID-19 are diverse, and in-depth domestic research is needed.
○ Recommendation 16: It is necessary to identify problems with the use of programs to heal psychiatric problems caused by COVID-19 and to develop programs that can supplement the same.
○ Recommendation 17: In order to solve psychiatric issues, systematic recommendations or suggestions are needed to prevent psychological instability as a result of COVID-19.
1. Psychiatric disorders related to COVID-19
1) Current situation
(1) Psychiatric symptoms in COVID-19 patients
Psychological stressors detected during infection and self-quarantine include fear, frustration, and boredom related to the quarantine period and infection; insufficient relief supplies; inadequate information (accusations in media and negative news reports); clinical symptoms; and anxiety about spreading the virus to others.30
According to a study,31 29.2% of infected patients complained of experiencing depressive symptoms (in comparison, 9.8% of quarantined people complained of experiencing depressive symptoms), while 96% of recovered COVID-19 patients reported experiencing posttraumatic stress symptoms. According to another online survey conducted with 770 people, the frequency of depressive symptoms was 43.1%; the exacerbating factors included severe COVID-19 (OR = 1.67, P = 0.03), family members infected with COVID-19 (OR = 1.51, P = 0.01), female gender (vs. male, OR = 0.53, P < 0.01), and the use of social media to obtain information related to COVID-19 (OR = 0.65, P < 0.01).32
In the infected population, various symptoms, such as insomnia, anxiety, poor concentration, memory loss, depression, confusion, emotional lability, change in consciousness, euphoria, aggression, auditory hallucinations, victim mentality, visual hallucinations, and suicide, have been reported (Table 8).33 This suggests the infected population has various psychiatric symptoms in comparison with the general (noninfected) public, medical staff, or mentally ill population.
Table 8. Prevalence of psychiatric symptoms in infected persons33 .
| Symptoms | Acute | Post-illness | ||||||
|---|---|---|---|---|---|---|---|---|
| Studies | Cases | Sample size | Prevalence (95% CI) | Studies | Cases | Sample size | Prevalence (95% CI) | |
| Any | 1 | 17 | 27 | 63.0% (42.8–80.4) | 1 | 0 | 4 | 0 (0.0–39.1) |
| Insomnia | 2 | 54 | 129 | 41.9% (22.5–50.5) | 4 | 34 | 280 | 12.1% (8.6–16.3) |
| Anxiety | 2 | 46 | 129 | 35.7% (27.6–44.2) | 2 | 21 | 171 | 12.3% (7.7–17.7) |
| Impaired concentration or attention | 1 | 39 | 102 | 38.2% (29.0–47.9) | 2 | 34 | 171 | 19.9% (14.2–26.2) |
| Impaired memory | 2 | 44 | 129 | 34.1% (26.2–42.5) | 3 | 44 | 233 | 18.9% (14.1–24.2) |
| Depressed mood | 2 | 42 | 129 | 32.6% (24.7–40.9) | 5 | 35 | 332 | 10.5% (7.5–14.1) |
| Confusion | 2 | 36 | 129 | 27.9% (20.5–36.0) | 1 | 1 | 621 | 0.2% (0.0–0.7) |
| Emotional lability | 1 | 30 | 102 | 29.4% (0.4–7.3) | 1 | 24 | 102 | 23.5% (15.8–32.3) |
| Altered consciousness | 1 | 17 | 82 | 20.7% (12.6–30.3) | NA | NA | NA | NA |
| Pressured speech | 1 | 21 | 102 | 20.6% (13.3–29.0) | 1 | 12 | 102 | 11.8% (6.1–18.8) |
| Euphoria | 1 | 8 | 102 | 7.8% (3.3–14.0) | 1 | 11 | 102 | 10.8% (5.4–17.6) |
| Aggression | 1 | 2 | 27 | 7.4% (0.2–21.1) | 1 | 1 | 102 | 1.0% (0.0–4.2) |
| Irritability | 1 | 5 | 102 | 4.9% (1.4–10.1) | 3 | 28 | 218 | 12.8% (8.7–17.6) |
| Auditory hallucinations | 2 | 6 | 129 | 4.7% (1.6–9.1) | 1 | 1 | 102 | 1.0% (0.0–4.2) |
| Persecutory ideas | 1 | 4 | 102 | 3.9% (0.9–8.7) | 1 | 2 | 102 | 2.0% (0.0–5.8) |
| Visual hallucinations | 1 | 2 | 102 | 2.0% (0.0–5.8) | NA | NA | NA | NA |
| Suicidality | 1 | 2 | 102 | 2.0% (0.0–5.8) | 1 | 0 | 102 | 0 (0.0–1.7) |
| Fatigue | NA | NA | NA | NA | 4 | 61 | 316 | 19.3% (15.1–23.9) |
| Frequent recall of traumatic memories | NA | NA | NA | NA | 1 | 55 | 181 | 30.4% (23.9–37.3) |
| Sleep disorder | NA | NA | NA | NA | 1 | 14 | 14 | 100% (88.0–100.0) |
| Psychotic symptoms (unspecified) | NA | NA | NA | NA | 1 | 4 | 90 | 4.4% (1.0–9.9) |
| Self-harm | NA | NA | NA | NA | 1 | 1 | 102 | 1.0% (0.0–4.2) |
CI = confidence interval.
(2) Impact on therapists
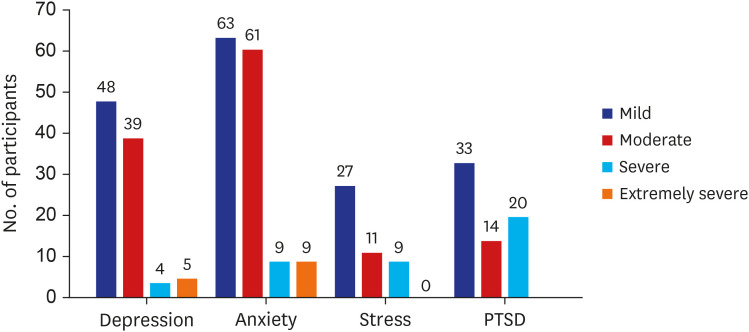
Medical workers handling COVID-19 patients also face threats to their mental health. In a study on 1,257 healthcare workers at 34 hospitals in different parts of China, 50.4% experienced depressive symptoms, 44.6% experienced anxiety symptoms, 34.0% experienced insomnia, and 71.5% experienced mental distress. In another study on 2,014 frontline nurses, 60.5% experienced moderate or severe emotional exhaustion, 42.3% experienced depersonalization, 14.3% experienced anxiety symptoms, 10.7% experienced depressive symptoms, and 91.2% experienced fear. In a systemic review and meta-analysis of 60,458 medical workers, health concerns, fear, insomnia, anxiety, and psychological distress were frequently reported. Moreover, other symptoms of depression, anxiety, posttraumatic stress disorder, and feelings of stigmatization were reported. In a COVID-19 stress questionnaire survey conducted by 309 therapists, the most frequently provided responses were “Families are worried about them” and “It’s likely that people around them are infected.” The frequency of symptoms in the order of headache, neck pain, anxiety, lethargy, and insomnia, with anxiety and depressive symptoms accounted for a large proportion (Fig. 8).34
Fig. 8. Psychiatric symptoms found among therapists.34 .
PTSD = posttraumatic stress disorder.
(3) Psychiatric effects on the general public
As social restrictions were applied in the wake of the COVID-19 outbreak, research on the general population and psychiatric symptoms was actively conducted. Social and psychological stress has increased worldwide due to concerns about infection, discomfort in daily life, and other problems in daily life. An American study involving 5,065 people confirmed that 6.9% of men and 10.1% of women experienced depression before COVID-19; this increased to 21.9% of men and 33.3% of women in April 2020. In addition, 38.8% of young people aged 18–39 years (previously, 9.0%), 26.8% of middle-aged people aged 40–59 years (previously, 8.5%), and 14.9% of older people aged over 60 years (previously, 7.9%) experienced depression.35 Overall, 75.3% of the healthy population did not report depression. Since the COVID-19 pandemic, the ratio reduced to 47.5% (around April 2020), and the social burden is likely to have increased since then.
In a Chinese study involving 56,679 people, 27.9% had depressive symptoms, 31.6% had anxiety symptoms, 29.2% had insomnia symptoms, and 24.4% had acute stress symptoms. In addition, psychological factors related to psychiatric symptoms included confirmed or suspected COVID-19 in the respondent or relatives, a risk of occupational exposure, residence in Hubei Province, experience of quarantine, and delayed return to work.36 According to a systemic review that integrated 19 studies and 93,569 participants, the frequency of psychiatric symptoms was in the order of depressive symptoms (14.6–48.3%), anxiety (6.33–50.9%), posttraumatic stress disorder symptoms (7–53.8%), psychological distress (34.43–38%), and stress (8.1–81.9%).37 Another online study on 898 people reported loneliness, COVID-19-related worries, distress tolerance, family support, and social support to be factors affecting the symptoms of depression, anxiety, and posttraumatic stress.38
(4) Psychiatric conditions found in high-risk groups
The aggravation of psychotic symptoms has been observed in patients with schizophrenia due to concerns about COVID-19. This demonstrates the possibility that psychological changes in the face of the infectious disease could influence psychopathology and cause psychotic symptoms to develop and aggravate. In addition, a study on children and adolescents with OCD reported the exacerbation of existing OCD; these exacerbated symptoms of OCD were also related to the manifestation of symptoms such as depression and anxiety and avoidance behavior. This pattern was more pronounced when the onset age was younger and there was a family history of attention deficit hyperactivity disorder. The direct threat of infection, social distancing, social isolation, and persistent obsession with hygiene can affect children and adolescents in general; this can become more intense if they have psychiatric vulnerabilities.39
In one study, the symptoms of posttraumatic stress disorder, depression, anxiety, stress and insomnia, concerns about physical health, anger, irritability, and suicidal ideation were higher after the COVID-19 pandemic in mentally ill patients than in healthy controls.40 In total, 20.9% (n = 1,434) of the patients with preexisting mental illness (n = 300) reported that their mental health status worsened after the pandemic. In another study, the mentally ill group reported higher levels of psychological distress, insomnia, depression, anxiety, and posttraumatic stress symptoms than the healthy group. Symptoms of depression and anxiety worsened after the pandemic in Alzheimer's dementia patients as well, and isolation and rapid changes in daily life and in care were found to be factors contributing to the problems.41
2) Domestic research
(1) Research on the general population
According to a survey conducted among 1,500 people aged 15 years and older in 17 metropolitan cities in Korea in June 2020, 45.7% of the subjects reported that they were “somewhat anxious or depressed,” while 1.8% said that they were “very badly so.”
In a study conducted by Professor Yoo Myeong-soon's team on 1,498 confirmed cases, contacts, or healthy people, a questionnaire survey assessing confirmed cases on a 5-point scale of fear revealed that “I am afraid of being criticized and harmed by those around me” received the highest points (3.87), while “afraid of not being cured” and “afraid of being re-infected after being cured” received lower points (2.75 and 3.46, respectively). In other words, the transfer of responsibility to and criticism of confirmed COVID-19 patients constituted the biggest fear in the patients, and the shock and social criticism caused by COVID-19 confirmation showed sequelae even after full recovery. Measuring the stress level of confirmed patients revealed that 27.3% of the total study population was highly stressed (28 points or more) and in need of immediate help. Most confirmed patients were under such great stress that they required follow-up monitoring. In addition, while 30.7% of healthy people who participated in the survey on the responsibility regarding getting COVID-19 answered “yes,” only 9.1% and 18.1% of the confirmed patients and contacts, respectively, answered “yes.” These survey results show the difference in perception between confirmed patients and healthy people in terms of responsibility regarding getting COVID-19. It can be seen that confirmed patients believed that the infection was not the patients’ fault, whereas healthy people had a relatively strong belief that the patients were to blame for their infection.
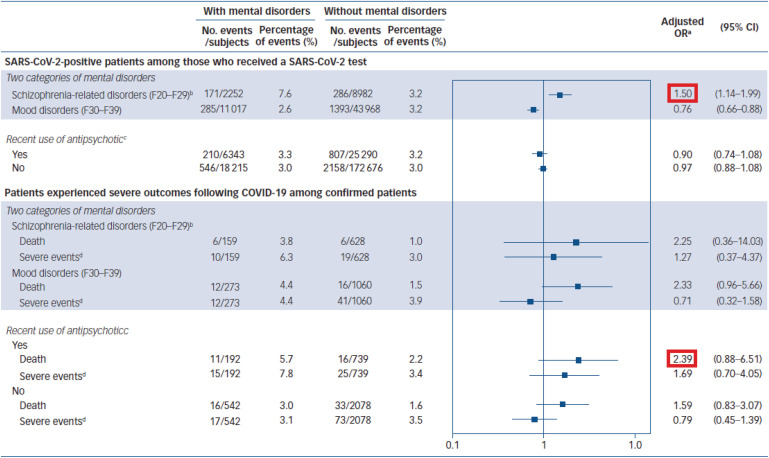
(2) Research on the mentally ill population
According to a study involving 216,418 people in Korea, the presence or absence of mental illness was not related to an increase in SARS-CoV-2 infectivity.42 However, patients with a history of mental illness were found to be more likely to have serious clinical outcomes than those without a history of mental illness.
In another study, however, out of 230,565 people tested for COVID-19, 33,653 (14.6%) had a mental illness; 928 (2.76%) of them tested positive for COVID-19. Of the 928 people who tested positive, 56 (6.03%) died; schizophrenia-related diseases were reported to be associated with COVID-19 positivity (OR, 1.50; 95% CI, 1.14–1.99). Among the confirmed COVID-19 patients, those with mental disorders had a significantly higher mortality rate than those without (OR, 1.99; 95% CI, 1.15–3.43) (Fig. 9). The study revealed that the presence or absence of mental illness may affect mortality from COVID-19 and that people with schizophrenia-related disorders may be more susceptible to infection.43
Fig. 9. Underlying psychiatric disorders, use of antipsychotics, and SARS-CoV-2.43 .
OR = odd ratio, CI = confidence interval, SARS-CoV-2 = severe acute respiratory syndrome-coronavirus-2, COVID-19 = coronavirus diseases 2019.
2. Corona blue prevention and treatment measures
1) Review of the psychological prevention protocol of the trauma center of the National Center for Mental Health
(1) Tips for good mental health (Fig. 10)
Fig. 10. Seven tips to keep your mind healthy through coronavirus diseases 2019.
Source: The Trauma Center of the National Center for Mental Health.
① Accept the changes in daily life due to the COVID-19 quarantine. Recognize that feeling depressed or anxious is not an abnormal emotion. Instead, it is a normal process for adjusting to the environment.
② Too much information or uncertain news aggravates anxiety and stress; therefore, do not worry too much. It is important to listen to accurate information and practice quarantine guidelines well.
③ Enhance the immune system of the body and mind by getting sufficient sleep and maintaining a regular lifestyle. In particular, it is important to go to bed and wake up at regular times for maintaining mental health.
④ Have a hobby or leisure time. Recharging brings joy to the mind and accumulates energy to overcome difficult situations.
⑤ Keep the body and mind healthy through moderate physical activity, such as walking. It is possible to engage in physical activity outside while observing quarantine guidelines, such as the use of a mask.
⑥ Continue communication with family, friends, and people around you through phone or e-mail, even if you do not see each other often. Sharing your mind is important for reducing depression and gaining psychological support.
⑦ If it is difficult, do not try to handle the condition on your own. It is necessary to seek professional help through the psychological counseling hotline (1577-0199). You can easily and conveniently check your mental health status and get information through mobile applications, such as Mental Health Self-Examination, Mind Program, and Magical Soothing.
(2) The psychological support operating system
The Integrated Psychological Support Group for the New Coronavirus consists of the National Trauma Center, national mental health facilities, and metropolitan and municipal mental health welfare centers. These facilities cover confirmed cases and their families, quarantined persons, and bereaved families in the case of death. The five national mental health facilities respond to confirmed cases and their families by region, and public health centers and municipal mental health welfare centers respond to those quarantined. The National Trauma Center establishes and operates a detailed psychological support plan and conducts Integrated Psychological Support Group meetings, monitoring the status of COVID-19 and distributing the list of psychological support recipients (confirmed patients and their families) provided by public health centers to the relevant national mental health facilities. The center provides other counseling forms and various materials, financial and technical support for disaster mental health services (provision of promotional materials, such as Integrated Psychological Support Group Guide and Infectious Disease Stress Mind Care Guide, COVID-19 Psychological Support Guidelines, psychological stabilization supplies, mental health assessment sheets, and high-risk group selection criteria, in addition to disaster mental health service education), psychological support-related publicity and media response, and advice on high-risk cases.
2) Review of response plans of Seoul and other local governments
The Seoul Metropolitan Government's Mental Health Checkup and Counseling Support is a system that covers the costs of examinations and consultations at mental health facilities for citizens aged 19 years or older. It is being implemented in cooperation with 202 mental health units in Seoul. Citizens can check participating health facilities at their local public health center or on the “Mental Health Mind Talk” in Blue Touch on the Mental Health Information website, and make a reservation by phone. In addition, the Mental Health Counseling Hotline, Seoul Mental Health Welfare Center, regional mental health welfare centers, and Seoul Psychological Support Center provide services. Various content items are provided as psychological support for COVID-19 depression (Autumn Mind Prescription, Sebasi—Time to Change the World, Koala—Live Cast Talking About What COVID-19 Tells Us, Expert Webinar, Overcoming COVID-19 Stress, How to Get Along With Children During COVID-19, Love of Life Heart-to-Heart Broadcasting, and Special Program to Prevent Suicides in the Unemployed or Jobless Households or Single-Person Households). In addition, the Seoul Metropolitan Office of Education is proposing methods for psychological stability, such as producing and distributing the Heart Friend Package for checking the psychology or raising companion plants. Moreover, face-to-face and noncontact counseling sessions are provided by reorganizing the blended counseling system that combines online and offline counseling. Daegu City has installed five smart gardens for citizens and medical staff who are exhausted from prolonged COVID-19. Here, they can rest and relax in indoor green spaces, and the number of the gardens is scheduled to increase to 25 by May 2021. The city expects healing, rest, and contemplation through the project. Gwangju City plans to reorganize the psychological support system, expand and strengthen the services, conduct a campaign to recover the minds of citizens to overcome corona blue, upgrade mental health self-examination and 24/7 psychological counseling, provide free support for the high-mental health risk group in connection with Mental Health Specialist, and continuously provide psychological support services and psychological healing information for psychological quarantine. Exhausted medical staff members are included in the target group to provide psychological support for each stage, e.g., burnout prevention education, mental health counseling reservation support, and noncontact counseling by a mental health doctor. Gwangju City has also decided to expand and upgrade psychological support services, including the provision of additional batches of mental health guides and psychological stabilization kits consisting of self-examination questionnaires for confirmed patients and quarantined persons.
3) Suggesting the most appropriate psychological prevention measures for the future
Most mental health management strategies developed so far have been programs such as individual-level stress management and cognitive behavioral therapy. However, as mental health can be influenced by not only individual factors but also environmental factors, appropriate environmental support is required. For this purpose, efforts should be made to strengthen the resources or coping strategies that individuals have.
To focus on the mental health of people, it is necessary for the various municipal and provincial governments mentioned above to promote the psychological prevention protocol and programs that can help with mental health issues caused by COVID-19 and encourage visits to professional mental health facilities. In addition, psychological problems caused by isolation, such as social distancing, can be improved by maintaining social relationships with people. Therefore, it is necessary to propose a method of maintaining social relationships through the use of information and communication technology by noncontact. VR or applications allow individuals to share their experiences, thereby strengthening social connections.
The organization to which the COVID-19 response team belongs should establish a policy to manage job demands and maximize the resource structure for the prevention of psychological issues with medical staff. Establishing an effective crisis management system to prepare for disasters can minimize inefficient job demands. During the disaster response phase, managers and employees must actively manage their own stress. However, focusing on work can make it difficult for people to judge their stress levels. Professional mental health support should be provided when the problem is beyond what can be handled by individual coping strategies. Adequate rest and compensation should be provided after the disaster is over. Moreover, work adjustments should be made to help recover from burnout, if necessary. Stress should be managed through training or management programs on burnout.
It is necessary for individuals to promote awareness about meeting their basic needs well, taking care of themselves, and performing periodic self-inspection at all stages before, during, and after a disaster. To this end, it is necessary to be familiar with the symptoms of stress or signs of mental health deterioration, to develop strategies for reducing stress, and to obtain advice from experts in professional mental health facilities.
Policy and Legislation
Recommendations
○ Recommendation 18: The government should set and manage patient outbreak suppression targets by region and period based on the existing three quarantine measures consisting of testing, tracking, and quarantining individuals with suspected infection.
○ Recommendation 19: People should strictly comply with three actions, i.e., wearing a mask, washing hands, and following coughing etiquettes, to prevent the spread of COVID-19.
○ Recommendation 20: People should maintain distance between each other and minimize the time spent in a 3C environment (i.e., closed space, close contact, and crowded place) to reduce the possibility of transmitting COVID-19.
○ Recommendation 21: The government should evaluate the risk level in various environments based on evidence and continuously improve social distancing guidelines accordingly.
○ Recommendation 22: The government should strengthen the facilitating factors of nonpharmacological interventions, make efforts to remove obstacles, and induce desirable behaviors in people through active risk communication.
○ Recommendation 23: The government should strengthen the capabilities of the healthcare system, including personnel, facilities, and equipment, to provide affordable treatment to COVID-19 patients.
○ Recommendation 24: The government should embark on the long-term enhancement of public health capacity, strengthening of public–private cooperation, and development of a new future healthcare delivery model.
○ Recommendation 25: As the variants that have recently spread globally have affected the speed of transmission, disease severity, and vaccine effectiveness, the surveillance network should be expanded, rapid contact tracing and isolation should be performed, and rapid effective vaccine roll-out should be prioritized in order to prevent the variants from entering Korea and causing a new epidemic.
1. Current situation and progress
1) Status of occurrence44
The COVID-19 outbreak was first reported as a cluster of pneumonia of unknown cause in Wuhan City, Hubei Province, China, on December 31, 2019, following which the virus spread worldwide. Accordingly, on March 11, 2020, the WHO declared a COVID-19 pandemic, which is underway worldwide.
In the Republic of Korea, a novel coronavirus infection case was first discovered during the quarantine stage on January 19, 2020. Korea has since experienced three waves of pandemics (as of April 12, 2021, 109,559 confirmed cases and 1,768 deaths have been reported).
According to the WHO,45 at the beginning of March 2021, the British variant had spread to 106 countries, the South African variant to 56 countries, and the Brazilian variant to 29 countries (Fig. 11).
Fig. 11. Coronavirus diseases 2019 situation.45 .
2) Republic of Korea's COVID-19 response
(1) First epidemic wave
The first wave of the COVID-19 epidemic in the Republic of Korea occurred among religious groups in Daegu and Gyeongbuk Province. As the number of confirmed COVID-19 cases increased, the government set the crisis alert of the Framework Act on Management of Disaster and Safety to the interest level (January 3), caution level (January 20), alert level (January 27), and severe level (February 23).
During this period, the Central Disaster Management Headquarter and the Central Disease Control Headquarter strengthened the infectious disease crisis management response system and set the foundation for the quarantine and treatment systems related to COVID-19. A special border control procedure was introduced (February 4) to prevent any further inflow of COVID-19 into the country. In order to prevent community transmission through the early detection of patients, the emergency use of diagnostic reagents was approved and various types of screening clinics (drive-in and walking) were operated. In addition, healthcare facilities were established according to the severity of the patients in response to the rapid increase in the number of COVID-19 cases by introducing community-based isolation facilities (so-called residential treatment centers).
Sachs et al.46 conducted a survey to evaluate the initial responses (March–May 2020) of 33 OECD countries to COVID-19. The aim of the survey was to evaluate the performance in terms of infection control and prevention. As per their report, the successful measures implemented by the Republic of Korea include an excellent public health system, the rapid development of diagnostic kits for pharmaceutical and biological companies, sharing of information with the public using information and communication technology, contact tracing, sharing of virus transmission areas, and the implementation of distance education.
(2) Second epidemic wave
In the period before the second COVID-19 outbreak, the infection spread among specific groups using multi-use facilities, such as entertainment establishments, logistics centers, and multi-level religious facilities. The second wave was attributed to the Liberation Day protests in downtown Seoul and the existing group infections. The infection spread around the facilities where this occurred.
After the first wave of COVID-19, the Korea Centers for Disease Control and Prevention (KCDC) was promoted to the Korea Disease Control and Prevention Agency (September 12, 2020). The classification has been subdivided into five stages from the existing three stages.
(3) Third epidemic wave
The first and second epidemics spread in a specific group, but the third epidemic showed a sporadically increasing number of infected cases in daily life, e.g., on contact with family and acquaintances, in the metropolitan area. On December 25, 2020, the number of infected people exceeded the healthcare capacity. In particular, the highest daily number of newly confirmed cases was recorded (1,235), making it difficult to treat critically ill patients.
In response to this, social distancing in the metropolitan area was continuously raised to level 1.5 (November 19, 2020), level 2 (November 24, 2020), and level 2.5 (December 8, 2020) for five or more people. Special measures, e.g., the prohibition of gatherings, were implemented during the year end and New Year holidays. In the future, the government plans to reduce the five steps of social distancing to four steps.
In addition, preemptive diagnostic tests were expanded and the number of treatment beds for COVID-19 patients was increased. From February 26, 2021, vaccination with the AstraZeneca vaccine started for inpatients and workers under the age of 65 years in elderly group facilities, such as nursing homes. The government is preparing for the fourth epidemic that is likely to occur in the future and is preparing a sustainable COVID-19 response strategy considering changes in the situation related to COVID-19. Furthermore, it is necessary to set a target for suppressing the number of cases by region and period and to prepare specific management plans.
3) Main activities of the NAMOK
The NAMOK held an online joint forum in response to COVID-19 in collaboration with the Korean Federation of Science and Technology Societies and the Korean Academy of Science and Technology (Table 9).
Table 9. The status of online forum in response to COVID-19 (as of March 2021).
| Category | Date | Topics |
|---|---|---|
| 1 | Mar 12, 2020 (Thu) | 1. Interim inspection of the COVID-19 situation from scientific and technological points of view |
| 2 | Apr 3, 2020 (Fri) | 2. COVID-19 pandemic: critical care practices and solutions |
| Apr 10, 2020 (Fri) | 3. Major mental health-related issues and future measures to prepare for the COVID-19 pandemic | |
| Apr 17, 2020 (Fri) | 4. How far has COVID-19 therapeutic and vaccine development progressed? | |
| May 8, 2020 (Fri) | 5. Reorganization of the medical system in preparation for the second COVID-19 waves | |
| Jun 19, 2020 (Fri) | 6. COVID-19 experience in Daegu and Gyeongbuk areas and response plans | |
| Jul 9, 2020 (Thu) | 7. Living with COVID-19 | |
| Aug 7, 2020 (Fri) | 8. Clinical and epidemiological characteristics of COVID-19 and herd immunity | |
| Aug 21, 2020 (Fri) | 9. Urgent discussion on preparedness for the second wave of the COVID-19 pandemic | |
| Sep 25, 2020 (Fri) | 10. Current status on the development of COVID-19 therapeutics | |
| Oct 22, 2020 (Thu) | 11. COVID-19 diagnostic tests for successful K-quarantine | |
| 3 | Nov 9, 2020 (Mon) | 12. Prediction of and effective response to COVID-19 |
| Nov 28, 2020 (Sat) | 13. Urgent meeting with experts in response to the third wave of the COVID-19 pandemic | |
| Dec 8, 2020 (Tue) | 14. Influenza co-infection in the COVID-19 pandemic | |
| Dec 9, 2020 (Wed) | 15. Critical care issues and reform in the surge of COVID-19 | |
| Jan 29, 2021 (Fri) | 16. COVID-19 vaccine update | |
| Feb 26, 2021 (Fri) | 17. Is COVID-19 a prelude to a fourth pandemic wave? |
COVID-19 = coronavirus disease 2019.
2. Response status analysis
1) Conceptual framework of approach
In order to minimize the health damage caused by COVID-19, health interventions aimed at suppression and mitigation and the use of effective drugs and vaccines are important (OECD, 2020).47
Suppression strategies aim to prevent outbreaks by minimizing the risk of transmission of the virus from an infected person to a noninfected one. Such strategies could include the early detection of infected people, tracing contacts of infected people, and quarantining them. Mitigation strategies aim to slow disease transmission and reduce the surge in healthcare needs. Both these strategies could include nonpharmaceutical measures, such as social distancing, a complete social lockdown, and improvements in personal hygiene and environmental hygiene.
According to the results of previous studies, the simultaneous application of suppression and mitigation measures rather than individual policies is the most effective approach to reduce the impact of infectious diseases. Along with these suppression and mitigation measures, a strategy is needed to strengthen the capacity of the healthcare system in order to provide an appropriate level of medical care to COVID-19 patients.
2) Performance outcome and limitations
(1) Performance outcome
From the early stage of the COVID-19 outbreak, the Republic of Korea rapidly introduced the so-called 3T (test, trace, and treatment) strategy, which involves expanding the testing capacity, tracing the movement of confirmed patients, and promptly isolating and treating confirmed patients. Moreover, the appropriate use of information and communication technology has been encouraged to identify infected patients at an early stage. The government is trying to prevent further spread and inflow from overseas.48
Resources for the diagnosis and treatment of COVID-19 could be saved and efficiently used by taking appropriate measures to change the healthcare systems, e.g., the operation of a screening test center (mobile vehicle or walking type) with a new sample collection method and the introduction of a residential treatment center for asymptomatic or mild patients.
Among OECD countries, this has relatively successfully reduced the economic damage caused by COVID-19 and balanced strict suppression policies (mortality) and GNP loss, while controlling the epidemic through continuous testing and efficient epidemic control (Sachs et al.46). It is judged that people's voluntary participation in pandemic control has played a key role in achieving these results.
(2) Limitations
Despite the efforts of healthcare authorities and the public, the number of confirmed cases of community infection continues to increase because of the characteristic of COVID-19, i.e., the asymptomatic nature of infection. Before the first COVID-19 outbreak, the average number of confirmed cases was less than 10 per day, but after the first epidemic, it was around 30 per day. This number increased to around 90 per day after the second epidemic, and now, the baseline is rising to around 400 per day. If a fourth wave occurs, the number of confirmed COVID-19 cases will be expected to increase significantly.
As high-intensity social distancing is prolonged, socioeconomic damage is increasing and social acceptability is decreasing. Social distancing is associated with mental side effects, such as shrinking social and economic activities, feelings of isolation and depression, and increased social distrust, in addition to the deterioration of national health status due to decreased physical activity.
As public medical institutions are mainly in charge of treating COVID-19 patients, there is a possibility that this could negatively impact the treatment of general patients (especially those from a vulnerable class). In addition, the government's emergency response plan, such as the delayed mobilization of private hospital beds during the third epidemic, may hamper the appropriate treatment of critically ill patients.
As the intensive work of epidemiological investigation personnel and medical personnel engaged in COVID-19 response and treatment is prolonged, they are burned out and their work efficiency is decreasing.
Due to social distancing, various social welfare facilities are not functioning properly and are restricted in terms of use by children, the elderly, and the disabled. Moreover, there is a care gap for the socially vulnerable population. In addition, in economically vulnerable groups, there are individuals who continue working in order to maintain their livelihood despite having symptoms and being suspected of having COVID-19 because there are no appropriate protective measures (such as sickness allowance), thereby posing a risk of causing an epidemic.
In addition, as the goal of the current measures is not clear, it is necessary to set a general goal and specific targets for preventing the occurrence of new cases in the future and to mobilize specific policy measures to achieve it.
3. General recommendations
1) Nonpharmacological interventions
(1) Although COVID-19 vaccination has started, strong nonpharmaceutical interventions must be continued to slow the spread of the virus. The main nonpharmacological interventions are as follows:
① Persons suspected of being infected or being exposed to the virus shall be tested as soon as possible, and support for quarantine at home or in a supervised facility for a certain period (10–14 days) shall be provided. A confirmed infected person is supposed to be isolated for a set period at a treatment facility (medical institution, residential treatment center, etc.).
② When a confirmed case is detected, the source of infection must be quickly identified through an epidemiological investigation, and appropriate measures must be taken.
③ All individuals must maintain physical distance (2 m or more) to reduce the possibility of droplet transmission and wear a mask when they are with other people in an indoor space (especially in a confined, close-contact, crowded environment). If the epidemiological investigation and follow-up measures are delayed, the number of infected persons and contacts will increase.
④ If possible, events that require the gathering of a lot of people should be switched to non-face-to-face ones or held outdoors. If a meeting is unavoidably held indoors, adequate ventilation should be maintained, all participants should be checked for symptoms (such as temperature measurement) before entering, and personal contact information should be obtained. In addition, all participants must observe physical distancing, wear a mask, wash hands, and follow coughing etiquettes. Large-scale events (such as weddings, funerals, religious activities, political gatherings, cultural events, and sports events) should be canceled, postponed, or rescheduled in accordance with the social distancing guidelines of the quarantine authorities.
a. Risk assessment should be performed using existing research results and epidemiological investigation information, and social distancing guidelines should be improved accordingly.
b. A suitable atmosphere should be created for managers and users to voluntarily participate in the control of infectious diseases at various facilities and places (e.g., organization and activation of autonomous inspection groups in shopping malls or markets).
c. The flexible social distance policy should be applied on the premise of voluntary participation of managers and users due to deregulation, and strong punishments should be in place for the occurrence and spread of confirmed cases at the facility in the future.
d. Alternatives should be in place to handle the exhaustion of healthcare workers caused by the prolonged corona pandemic and the spread of a noncooperative atmosphere caused by the suppression policy (e.g., realizing compensation for damage caused by business restrictions).
⑤ Strict suppression measures, such as the limitation of movement, business restrictions, and border control, may be considered to contain the outbreak to a controllable level with nonpharmacological interventions if the number of patients significantly exceeds the treatment facility’s capacity. However, movement and work restrictions (shutdowns or lockdowns) are interim measures to reduce community transmission, thereby alleviating overburden on the healthcare system and freeing up the time necessary for the expansion of nonpharmacological interventions. If disease transmission can be suppressed, it should be mitigated immediately.
⑥ Governments should take multipronged measures to promote behavioral changes in order to ensure long-term compliance with nonpharmacological interventions by the public.49 These include establishing and cracking down on rules, providing persuasion and incentives, providing education and training on how to behave, setting an example, and creating an environment conducive to action.
⑦ Government communications must always be clear, consistent, and evidence based in order to be trusted. Moreover, the government must be able to effectively communicate with all people (including foreigners residing in the Republic of Korea).50 Measures should be taken to prevent the spread of inaccurate or unverified information among the public so that the effectiveness of nonpharmacological interventions is not diminished.
(2) Nonpharmacological interventions should be designed on the basis of strong data analysis and modeling, strong evidence, and situational considerations, in addition to the use of behavioral science approaches. These interventions should be revised in consideration of new evidence as it emerges. For decision-making based on the evidence, systematic collection and analysis of epidemiological data, clinical data, and socioeconomic activity data are essential. The role of the government in inducing behavioral changes in a desirable direction involves the identification of key behaviors, measurement and monitoring of key behaviors, designing and evaluation of interventions based on the understanding of factors driving and hindering key behaviors, and effective communication.49
(3) The government should make an effort to remove obstacles by checking whether there are socially vulnerable groups for which it is difficult to implement nonpharmaceutical interventions for various reasons.
(4) Recently, undesirable phenomena and mistakes, such as the relaxation of social distancing and nonwearing of masks due to the increase in vaccination rates, are occurring in some countries. These should be pointed out.
2) Mass vaccination
(1) Securing a vaccine
The government should make an effort to secure safe and effective amounts of vaccines necessary for mass immunization of the entire population in order to control the spread of COVID-19 and achieve herd immunity as soon as possible.
(2) Safety and effectiveness
① Information on the safety and effectiveness of vaccines (relative effectiveness of various vaccines, protection periods and immune pathways, side effects, data of subgroups, etc.) in countries where vaccine campaigns have begun is being collected and disclosed transparently; this information should be used for policy decision-making.
② Efforts should be made to determine the safety and efficacy of existing vaccines against COVID-19 variants.
(3) Priority of vaccination
① Prioritization of vaccination should be established through social consensus.
② Proper measures should be taken to handle the decrease in vaccine access for the socially vulnerable population.
(4) Strategy of vaccination
① A specific vaccination strategy should be prepared on the basis of the characteristics of the vaccine and clinical trial data.
② Vaccinations should be appropriately performed through preconsultation with private medical institutions.
③ Guidelines for medical personnel should be developed so that vaccination can be appropriately performed, and the guidelines should be revised and supplemented through various simulations.
④ It must be possible to maintain physical distance between medical personnel and inoculated persons during the preinterview, physical examination, inoculation, and postobservation processes.
⑤ A computerized system should be used for the systematic management of targeted persons.
⑥ A contingency plan should be prepared in the case of unexpected deviations.
(5) Improving vaccination rate
① When a new mutant with a larger basic reproduction rate is dominant, the target vaccination rate necessary to achieve herd immunity should be raised.
② Various measures to increase the vaccination rate should be in place (e.g., restrictions on movement/work for individuals who are not vaccinated, communication with the public, customized communication with each group, inoculation and promotion using celebrities, increasing the number of vaccination sites, mobile vaccination, and notice of the second inoculation).
③ A system that can quickly respond to adverse reactions that may occur after vaccination should be established.
④ It should be remembered that “no one is safe until everyone is safe.”
3) Strengthening the healthcare system
(1) Local governments (public health centers, large city/provincial authorities, and the central government) should strengthen their capabilities to identify the source of infection, block community transmission through thorough contact tracing, and conduct epidemiological investigations of cluster outbreaks. During the epidemiological investigation, personal privacy or confidentiality should not be unreasonably infringed because of the use of personal information for contact tracing.
(2) Sufficient testing capacity must be secured in preparation for an increase in the number of confirmed cases and contacts.
(3) The capacity to provide appropriate treatment to COVID-19 patients should be secured. Expanding the capacity to treat patients can ease the level of social distancing.
① Patient classification and transport system
A triage system that can classify confirmed COVID-19 patients according to their disease severity and transport them to a treatment facility suitable to them must be maintained.
② Securing resources
a. Hospital beds
A residential treatment center should be secured and prepared to treat asymptomatic or mild patients when the number of infected cases increases.
Preparations should be in place for the conversion of public medical institutions to COVID-19-dedicated hospitals by region.
Private hospitals that can participate voluntarily should be designated as hospitals exclusively for COVID-19 patient care, and sufficient compensation should be given to participating hospitals.
An ICU that can take care of critically ill patients must be secured (in parallel with manpower training).
b. Manpower
A system that can mobilize public health doctors or military medical personnel (military doctors, nursing officers, medics, etc.) should be in place in cases of emergency.
Specialized personnel from tertiary general hospitals should support hospitals dedicated to COVID-19 so that intensive care can be provided appropriately. For this purpose, organized support from related societies (the Korean Society of Critical Care Medicine, Korean Tuberculosis and Respiratory Association, Korean Society of Infectious Diseases, etc.) is required.
The cooperative system for resource mobilization with medical personnel groups (the Korean Medical Association, Korean Hospital Association, Korean Nursing Association, etc.) should be maintained and strengthened.
c. Equipment and medical materials
Various medical materials, such as personal protective equipment and artificial respirators, needed for medical treatment should be properly supplied.
(4) Special efforts to minimize the gap in the treatment of other patients due to the treatment of COVID-19 patients
Various efforts to maintain the continuity of chronic disease management are required (e.g., non-face-to-face treatment).
Emergency patient care should be facilitated.
(5) Dealing with mental health problems caused by COVID-19
Mental problems related to COVID-19 should be addressed.
Mental problems related to social isolation should be addressed.
Mental problems related to economic hardships should be addressed.
4) Preparing for the post-COVID-19 healthcare system
(1) Strengthening the capacity of public medical care
Beds: A public hospital that will serve as a base medical institution in each region should be newly established or expanded.
Manpower: A plan to secure excellent manpower in public hospitals should be prepared.
(2) Review of the possibility of developing and introducing a new healthcare delivery model
The possibility of transformation from the current system that provides segmented medical care to a community-based integrated medical system that provides comprehensive medical care should be reviewed. This requires a comprehensive review of patient registration, providing organizations, and payment systems (e.g., Trust-based Cooperative Health System Reform Incentive Payment).51
Conclusion
NAMOK COVID-19 committee calls for urgent recommendations in the pandemic COVID-19 response. COVID-19 effects deeply on human living everyday and everywhere. The multilateral system must be supported to work effectively and systematically to deliver the knowledge of SARS-CoV-2, the effective surveillance and precise application of diagnostics to COVID-19, the rapid development and effective distribution of vaccines and therapeutics, the flexible support of medical facilities for patients, the identification and healing programs of psychiatric problems from COVID-19, and the comprehensive establishment and application of policy besides of the personal and social preventive implementation. The other factors not mentioned in this recommendation should be included for the resolution of pandemic COVID-19 as soon as possible.
ACKNOWLEDGMENTS
NAMOK COVID-19 Committee members are Hyoung-Shik Shin* (Department of Infectious Diseases, Daejeon Eulji Medical Center, Eulji University, Daejeon, Korea), Hyesook Park* (Department of Preventive Medicine, Graduate Program in System Health Science and Engineering, Ehwa University College of Medicine, Seoul, Korea), Jun Soo Kwon* (Department of Psychiatry, Seoul National University College of Medicine, Seoul, Korea), Hyun Namgoong* (GreenCross Company, Yongin, Korea), Seong-Jun Kim* (Korea Research Institute of Chemical Technology, Daejeon, Korea), June Myung Kim* (Department of Infectious Diseases, Yonsei University College of Medicine, Seoul, Korea), Kyong Ran Peck* (Division of Infectious Diseases, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea), Kyungwon Lee* (Seoul Clinical Laboratories Academy, Yongin, Korea), Jong-koo Lee* (Department of Family Medicine, Seoul National University College of Medicine, Seoul, Korea), JinHan Lee* (DONG-A ILBO Newspaper, Seoul, Korea), Hee Chul Han* (Department of Physiology, Korea University College of Medicine, Seoul, Korea), SungJin Hong* (Department of Anesthesiology, Catholic University of Korea College of Medicine, Seoul, Korea), Byung-Joo Park* (Department of Preventive Medicine, Seoul National University College of Medicine, Seoul, Korea), Tae Hwan Lim* (Department of Radiology, University of Ulsan College of Medicine, Ulsan, Korea), Eung Soo Hwang* (Department of Microbiology and Immunology, Seoul National University College of Medicine, Seoul, Korea), Jun Hee Woo* (Department of Infectious Diseases, Uijeongbu Eulji Medical Center, Eulji University, Uijeongbu, Korea), Dong-hyun Kim (Department of Preventive Medicine, Hallym University College of Medicine, Chuncheon, Korea), Je-hyeong Kim (Department of Pulmonology, Korea University College of Medicine, Ansan, Korea), Yoon Yung Nam (Department of Adult Psychiatry, National Center for Mental Health, Seoul, Korea), Sanghoon Oh (Department of Psychiatry, Uijeongbu Eulji Medical Center, Eulji University College of Medicine, Uijeongbu, Korea), Suk-Joon Yoon (Department of Preventive Medicine, Korea University College of Medicine, Seoul, Korea), SangWon Han (Department of Pediatric Urology, Yonsei University College of Medicine, Seoul, Korea), Ki Ho Hong (Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea), Hyukmin Lee (Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea), Chulhun Chang (Department of Laboratory Medicine, Pusan National University School of Medicine, Pusan, Korea), Kye Chul Kwon (Department of Laboratory Medicine, Chungnam University School of Medicine, Daejeon, Korea), and Suk Kyung Hong (Department of Acute Care Surgery, University of Ulsan College of Medicine, Seoul, Korea).
*Byline authors.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Lee JK, Han HC, Park B-J, Woo JH.
- Data curation: Shin HS, Park H, Namgoong H, Peck KR, Lee JK, Lee J, Hong S.
- Formal analysis: Shin HS, Park H, Kim S-J, Kim JM, Peck KR, Lee K, Han HC, Lim TH.
- Investigation: Park H, Kwon JS, Kim S-J, Peck KR, Lee J, Han HC, Hong S, Hwang ES.
- Methodology: Shin HS, Park H, Kwon JS, Namgoong H, Lee J, Hong S.
- Project administration: Lee JK, Lim TH, Woo JH, Hwang ES.
- Resources: Shin HS, Kim SJ, Kim JM, Lee K, Lee JK, Lee J, Han HC, Hong S, Park BJ.
- Software: Park H, Kwon JS, Kim SJ, Lee K, Lee JK, Lee J, Hong S, Park BJ.
- Supervision: Kim JM, Lee JK, Lim TH, Woo JH, Hwang ES.
- Validation: Shin HS, Peck KR, Lee K, Han HC, Hong S, Park BJ.
- Visualization: Lee J.
- Writing - original draft: Shin HS, Park H, Kwon JS.
- Writing - review & editing: Woo JH, Hwang ES.
References
- 1.Korean Society for Laboratory Medicine. [Updated 2021]. [Accessed August 5, 2021]. https://www.kslm.org/popup/popup_201222_02.html.
- 2.Kohmer N, Toptan T, Pallas C, Karaca O, Pfeiffer A, Westhaus S, et al. The comparative clinical performance of four SARS-CoV-2 rapid antigen tests and their correlation to infectivity in vitro. J Clin Med. 2021;10(2):328. doi: 10.3390/jcm10020328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Antigen-detection in the Diagnosis of SARS-CoV-2 Infection Using Rapid Immunoassays. Geneva, Switzerland: WHO; 2020. [Google Scholar]
- 4.European Centre for Disease Prevention and Control. Options for the Use of Rapid Antigen Tests for COVID-19 in the EU/EEA and the UK. 19 November 2020. Stockholm, Sweden: ECDC; 2020. [Google Scholar]
- 5.Barat B, Das S, De Giorgi V, Henderson DK, Kopka S, Lau AF, et al. Pooled saliva specimens for SARS-CoV-2 testing. J Clin Microbiol. 2021;59(3):e02486–e02420. doi: 10.1128/JCM.02486-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Korean Society for Laboratory Medicine COVID-19 Response Task Force, Korea Disease Control and Prevention Agency Infectious Disease Diagnosis and Analysis Bureau. Guidelines for the Laboratory Diagnosis of COVID-19 in Korea. Vol. 4. [Updated 2020]. [Accessed August 5, 2021]. https://www.kslm.org/
- 7.Kim SY, Lee J, Sung H, Lee H, Han MG, Yoo CK, et al. Pooling upper respiratory specimens for rapid mass screening of COVID-19 by real-time RT-PCR. Emerg Infect Dis. 2020;26(10):2469–2472. doi: 10.3201/eid2610.201955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Academy of Medicine of Korea and The Korean Federation of Science and Technology Societies 16th Online Joint Forum. COVID-19 diagnosis test for successful K quarantine. December 22, 2020. [Updated 2020]. [Accessed August 5, 2021]. https://youtu.be/MucEP9Iqx6Q.
- 9.Korea Disease Control and Prevention Agency. Final judgment of false-positive results for 3 suspected cases of COVID-19 in Gwangju and Chungnam. [Updated 2020]. [Accessed August 5, 2021]. https://kdca.go.kr/board/board.es?mid=a20501000000&bid=0015&list_no=367517&cg_code=&act=view&nPage=1.
- 10.The New York Times. C.D.C. warns the new virus variant could fuel huge spikes in Covid-19 cases. [Updated 2021]. [Accessed August 5, 2021]. https://www.nytimes.com/2021/01/15/health/covid-cdc-variant.html.
- 11.Nextstrain. Genomic epidemiology of novel coronavirus - Global subsampling. [Updated 2021]. [Accessed September 2, 2021]. https://nextstrain.org/ncov/global.
- 12.GISAID. GISAID comments on the discussion of different types or clades of the hCoV-19 virus and their origin. [Updated 2020]. [Accessed February 21, 2021]. https://www.gisaid.org/references/statements-clarifications/different-types-or-clades-of-the-virus-and-their-origin/#c501.
- 13.Lee H, Lee H, Song KH, Kim ES, Park JS, Jung J, et al. Impact of public health interventions on seasonal influenza activity during the SARS-CoV-2 outbreak in Korea. Clin Infect Dis. 2021;73(1):e132–e140. doi: 10.1093/cid/ciaa672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang L, Yan Y, Wang L. Coronavirus disease 2019: coronaviruses and blood safety. Transfus Med Rev. 2020;34(2):75–80. doi: 10.1016/j.tmrv.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hussein HA, Hassan RYA, Chino M, Febbraio F. Point-of-care diagnostics of COVID-19: From current work to future perspectives. Sensors (Basel) 2020;20(15):4289. doi: 10.3390/s20154289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korea Disease Control and Prevention Agency. Starting the Development of New Coronavirus Analysis and Inspection Methods. Cheongju, Korea: Korea Disease Control and Prevention Agency; 2020. [Google Scholar]
- 17.Hoffmann M, Kleine-Weber H, Pöhlmann S. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol Cell. 2020;78(4):779–784.e5. doi: 10.1016/j.molcel.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368(6493):860–868. doi: 10.1126/science.abb5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim D, Lee JY, Yang JS, Kim JW, Kim VN, Chang H. The architecture of SARS-CoV-2 transcriptome. Cell. 2020;181(4):914–921.e10. doi: 10.1016/j.cell.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heo JY. Clinical and epidemiological characteristics of coronavirus disease 2019 in the early stage of outbreak. Korean J Med. 2020;95(2):67–73. [Google Scholar]
- 21.National Academy of Medicine of Korea and The Korean Federation of Science and Technology Societies 4th Online Joint Forum. How far has COVID-19 therapeutic and vaccine development progressed? April 17, 2020. [Updated 2020]. [Accessed March 2, 2021]. https://www.youtube.com/watch?v=1XgnQUcWIts.
- 22.National Academy of Medicine of Korea and The Korean Federation of Science and Technology Societies 10th Online Joint Forum. Current status on the development of COVID-19 therapeutics. September 25, 2020. [Updated 2020]. [Accessed March 2, 2021]. https://www.youtube.com/watch?v=o_RU75W4xP8.
- 23.World Health Organization. The COVID-19 candidate vaccine landscape and tracker. [Updated 2021]. [Accessed February 21, 2021]. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
- 24.Jung J. Preparing for the coronavirus disease (COVID-19) vaccination: Evidence, plans, and implications. J Korean Med Sci. 2021;36(7):e59. doi: 10.3346/jkms.2021.36.e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimabukuro T, Nair N. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine. JAMA. 2021;325(8):780–781. doi: 10.1001/jama.2021.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sung HK, Kim JY, Heo J, Seo H, Jang YS, Kim H, et al. Clinical course and outcomes of 3,060 patients with coronavirus disease 2019 in Korea, January–May 2020. J Korean Med Sci. 2020;35(30):e280. doi: 10.3346/jkms.2020.35.e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eurosurveillance Editorial Team. Updated rapid risk assessment from ECDC on the risk related to the spread of new SARS-CoV-2 variants of concern in the EU/EEA - first update. Euro Surveill. 2021;26(3):2101211. doi: 10.2807/1560-7917.ES.2021.26.3.2101211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Academy of Medicine of Korea and The Korean Federation of Science and Technology Societies 6th Online Joint Forum. COVID-19 experience in Daegu and Gyeongbuk areas and response plans. June 19, 2020. [Updated 2020]. [Accessed July 19, 2021]. https://www.youtube.com/watch?v=Xn6VTRtXOZs&t=133s.
- 29.Emanuel EJ, Persad G, Upshur R, Thome B, Parker M, Glickman A, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382(21):2049–2055. doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- 30.Pfefferbaum B, North CS. Mental health and the Covid-19 pandemic. N Engl J Med. 2020;383(6):510–512. doi: 10.1056/NEJMp2008017. [DOI] [PubMed] [Google Scholar]
- 31.Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: Systematic review of the current evidence. Brain Behav Immun. 2020;89:531–542. doi: 10.1016/j.bbi.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma YF, Li W, Deng HB, Wang L, Wang Y, Wang PH, et al. Prevalence of depression and its association with quality of life in clinically stable patients with COVID-19. J Affect Disord. 2020;275:145–148. doi: 10.1016/j.jad.2020.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rogers JP, Chesney E, Oliver D, Pollak TA, McGuire P, Fusar-Poli P, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chew NW, Lee GK, Tan BY, Jing M, Goh Y, Ngiam NJ, et al. A multinational, multicentre study on the psychological outcomes and associated physical symptoms amongst healthcare workers during COVID-19 outbreak. Brain Behav Immun. 2020;88:559–565. doi: 10.1016/j.bbi.2020.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ettman CK, Abdalla SM, Cohen GH, Sampson L, Vivier PM, Galea S. Prevalence of depression symptoms in US adults before and during the COVID-19 pandemic. JAMA Netw Open. 2020;3(9):e2019686. doi: 10.1001/jamanetworkopen.2020.19686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Shi L, Que J, Lu Q, Liu L, Lu Z, et al. The impact of quarantine on mental health status among general population in China during the COVID-19 pandemic. Mol Psychiatry. 2021 doi: 10.1038/s41380-021-01019-y. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiong J, Lipsitz O, Nasri F, Lui LM, Gill H, Phan L, et al. Impact of COVID-19 pandemic on mental health in the general population: a systematic review. J Affect Disord. 2020;277:55–64. doi: 10.1016/j.jad.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu CH, Zhang E, Wong GT, Hyun S, Hahm HC. Factors associated with depression, anxiety, and PTSD symptomatology during the COVID-19 pandemic: Clinical implications for U.S. young adult mental health. Psychiatry Res. 2020;290:113172. doi: 10.1016/j.psychres.2020.113172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nissen JB, Højgaard DR, Thomsen PH. The immediate effect of COVID-19 pandemic on children and adolescents with obsessive compulsive disorder. BMC Psychiatry. 2020;20(1):511. doi: 10.1186/s12888-020-02905-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hao F, Tan W, Jiang L, Zhang L, Zhao X, Zou Y, et al. Do psychiatric patients experience more psychiatric symptoms during COVID-19 pandemic and lockdown? A case-control study with service and research implications for immunopsychiatry. Brain Behav Immun. 2020;87:100–106. doi: 10.1016/j.bbi.2020.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El Haj M, Altintas E, Chapelet G, Kapogiannis D, Gallouj K. High depression and anxiety in people with Alzheimer’s disease living in retirement homes during the covid-19 crisis. Psychiatry Res. 2020;291:113294. doi: 10.1016/j.psychres.2020.113294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee SW, Yang JM, Moon SY, Yoo IK, Ha EK, Kim SY, et al. Association between mental illness and COVID-19 susceptibility and clinical outcomes in South Korea: a nationwide cohort study. Lancet Psychiatry. 2020;7(12):1025–1031. doi: 10.1016/S2215-0366(20)30421-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeon HL, Kwon JS, Park SH, Shin JY. Association of mental disorders with SARS-CoV-2 infection and severe health outcomes: nationwide cohort study. Br J Psychiatry. 2021 doi: 10.1192/bjp.2020.251. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization. Republic of Korea. [Updated 2021]. [Accessed April 12, 2021]. https://covid19.who.int/region/wpro/country/kr.
- 45.World Health Organization. Weekly epidemiological update – 2 March 2021. [Updated 2021]. [Accessed March 5, 2021]. https://www.who.int/publications/m/item/weekly-epidemiological-update---2-march-2021.
- 46.Sachs J, Schmidt-Traub G, Kroll C, Lafortune G, Fuller G, Woelm F. The sustainable development goals and COVID-19. Sustainable development report 2020. [Updated 2021]. [Accessed March 8, 2021]. https://s3.amazonaws.com/sustainabledevelopment.report/2020/2020_sustainable_development_report.pdf.
- 47.OECD. Flattening the COVID-19 peak: containment and mitigation policies. [Updated 2020]. [Accessed March 8, 2021]. https://www.oecd.org/coronavirus/policy-responses/flattening-the-covid-19-peak-containment-and-mitigation-policies-e96a4226.
- 48.The Government of the Republic of Korea. Flattening the curve on COVID-19: How Korea responded to a pandemic using ICT. [Updated 2020]. [Accessed March 8, 2021]. http://overseas.mofa.go.kr/gr-en/brd/m_6940/view.do?seq=761548.
- 49.Lee JK, Bullen C, Ben Amor Y, Bush SR, Colombo F, Gaviria A, et al. Institutional and behaviour-change interventions to support COVID-19 public health measures: a review by the Lancet Commission Task Force on public health measures to suppress the pandemic. Int Health. 2021;13(5):399–409. doi: 10.1093/inthealth/ihab022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.World Health Organization. Communicating and managing uncertainty in the COVID-19 pandemic: a quick guide. [Updated 2020]. [Accessed March 8, 2021]. https://www.who.int/docs/default-source/searo/whe/coronavirus19/managing-uncertainty-in-covid-19-a-quick-guide.pdf?sfvrsn=270e4ac8_4.
- 51.Oh J. Roles of insurance for a sustainable society amid disasters. Living with COVID-19 (2020.7.9.) [Updated 2020]. [Accessed March 8, 2021]. https://www.youtube.com/watch?v=vUoO5d2GI4w.