Abstract
Background
It is essential to determine the distribution of the causative microorganisms in the region and the status of local antibiotic resistance for the proper treatment of hospital-acquired pneumonia/ventilator-associated pneumonia (HAP/VAP). This study aimed to investigate the occurrence and causative strains of HAP/VAP, distribution of resistant bacteria, use of antibiotics, and the ensuing outcomes of patients in Korea.
Methods
A multicenter prospective observational cohort study was conducted among patients with HAP/VAP admitted to the medical intensive care unit of 5 tertiary referral centers between August 2012 and June 2015. Patients' demographic and clinical data were collected.
Results
A total of 381 patients were diagnosed with HAP/VAP. Their median age was 69 (59–76) years and 71% were males. A majority of the patients (88%) had late-onset (> 5 days) HAP/VAP. One-quarter of the patients (n = 99) had at least one risk factor for multidrug-resistant (MDR) pathogens, such as prior intravenous antibiotic use within the last 90 days. Microbiological specimens were mostly obtained noninvasively (87%) using sputum or endotracheal aspirates. Pathogens were identified in 235 (62%) of the 381 patients. The most common bacterial pathogen was Acinetobacter baumannii (n = 89), followed by Staphylococcus aureus (n = 52), Klebsiella pneumoniae (n = 25) and Pseudomonas aeruginosa (n = 22). Most of isolated A. baumannii (97%) and S. aureus (88%) were multidrug resistant. The most commonly used empirical antibiotic regimens were carbapenem-based antibiotics (38%), followed by extended-spectrum penicillin/β-lactamase inhibitor (34%). Glycopeptide or linezolid were also used in combination in 54% of patients. The 28-day mortality rate of the patients with HAP/VAP was 30% and the 60-day mortality was 46%. Patients who used empirical antibiotics appropriately had significantly lower mortality rates than those who did not (28-day mortality: 25% vs. 40%, P = 0.032; 60-day mortality: 41% vs. 55%, P = 0.032, respectively). Administration of appropriate empirical antibiotics (odds ratio [OR], 0.282; confidence interval [CI], 0.092–0.859; P = 0.026), Day 7 treatment failure (OR, 4.515; CI, 1.545–13.192; P = 0.006), and APACHE II score on day 1 (OR, 1.326; CI, 0.988–1.779; P = 0.012) were the factors that determined the 28-day mortality in patients with HAP who had identified bacteria as pathogens.
Conclusion
In HAP/VAP patients, there was a large burden of MDR pathogens, and their associated mortality rate was high. Proper selection of empirical antibiotics was significantly associated with the patient's prognosis; however, there was a discrepancy between major pathogens and empirical antibiotic therapy.
Keywords: Healthcare-associated Pneumonia; Ventilator-associated Pneumonia; Drug Resistance, Bacterial; Intensive Care Units; Treatment Outcome
Graphical Abstract
INTRODUCTION
Hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP) are major nosocomial infections that increase mortality and morbidity in hospitals despite improvement in antibiotic treatment and supportive care, and dissemination of preventive measures.1,2,3,4 The occurrence of HAP/VAP, especially if its pathogen is multidrug resistant, is associated with an increase in medical expenses,5,6 and the suffering of patients and their families also increases. Moreover, poorly-treated HAP/VAP is a major cause of increased mortality.1 Therefore, it is important to properly treat HAP/VAP. To do so, it is essential to determine the distribution of the causative microorganisms in the region and the status of local antibiotics resistance.7,8 However, there are still insufficient data on this in Korea.9,10
The purpose of this study was to investigate the occurrence and causative strains of HAP/VAP, the distribution of resistant bacteria, the use of antibiotics, and the ensuing outcomes of patients in tertiary medical institutions in Korea.
METHODS
Study design
This was a multicenter prospective cohort study conducted with patients with HAP/VAP admitted to the intensive care units (ICUs) in five tertiary referral centers. The demographic and clinical data of the patients were collected.
Subjects
Patients were eligible if they were admitted to the ICU between August 2012 and June 2015 and if they met all of the following criteria: 1) Admission to the adult ICU, 2) Age > 18 years old, 3) New-onset HAP or VAP, and 4) If more than one HAP or VAP was present during hospitalization, only the first event was included.
The exclusion criteria were as follows: 1) Terminal illness with patient consent for ‘Do Not Resuscitate (DNR)’, 2) Terminal solid and hematologic malignancy without an active treatment plan, 3) A history of solid organ transplantation or bone marrow transplantation, 4) Pregnancy or less than 4 weeks postpartum, 5) Ongoing treatment for acquired immune deficiency syndrome, 6) Reception of antibiotics for more than 72 hours from another hospital, and 7) Transfer from other hospitals and subsequent development of new-onset pneumonia within 48 hours.
Data collection
Data on the following demographic and clinical parameters were prospectively collected: age, gender, body mass index (BMI), co-morbidities, reason for ICU admission, place of diagnosis for HAP/VAP, type of pneumonia, risk factors for multidrug-resistant (MDR) pathogens, time of diagnosis, severity scores as Acute Physiology and Chronic Health Evaluation (APACHE) II and Sequential Organ Failure Assessment (SOFA) score, clinical response as Clinical Pulmonary Infection Score (CPIS), pathogens, the presence of colonization for pathogens and antibiotic treatment. The following clinical outcome data were collected: day 3 improvement, day 7 failure, day 28 resolution, day 60 relapse, and 28-day and 60-day mortality.
Definitions
HAP was defined as pneumonia that occurred 48 hours or more after admission, which was not incubating at the time of admission.1 VAP was defined as pneumonia that occurred more than 48–72 hours after endotracheal intubation.1 Pneumonia was defined as new and persistent pulmonary infiltration plus at least two of following items: 1) A core body temperature ≥ 38.0°C or ≤ 36°C, 2) leukocytosis with a white blood cell count of ≥ 11,000/mm3 or leukopenia with a white blood cell count of ≤ 4,000/mm3, and 3) purulent sputum or endotracheal aspirate. As clinical response indicators, Day 3 improvement was defined as day 3 CPIS reaching ≤ 6 or decreased by ≥ 2 points with respect to day 1 CPIS. Day 7 failure was defined as day 7 CPIS not being lower than 6 or failing to decrease ≥ 2 points with respect to day 1 CPIS, or if the PaO2/FiO2 ratio was not improving between day 1 and day 7. Day 28 resolution was defined as complete patient recovery and the stoppage of all antibiotics for pneumonia within 28 days. Day 60 relapse was defined pneumonia caused by the same pathogen occurring again within 4 weeks after antibiotics were stopped due to clinical improvement. The antibiotic therapy was judged as appropriate when the causative microorganism was susceptible to one or more of the prescribed antibiotics based on the in vitro antibiotic susceptibility testing. Bacteremia was defined as at least one positive blood culture for the causative organism of pneumonia. The isolated strain was recognized as the pathogen, only in the following cases; 1) quantitative culture of bronchoalveolar lavage samples (≥ 104 colony-forming unit [CFU]/mL), 2) quantitative culture of bronchoscopic aspirates (≥ 105 CFU/mL), 3) quantitative culture of endotracheal aspirates (≥ 105 CFU/mL), 4) semi-quantitative culture of endotracheal aspirates (moderate [or 3+] or higher), or 5) a strain cultured in adequate sputum (less than 10 epithelial cells in a low power field).
Statistical analysis
Continuous variables are reported as medians (interquartile range [IQR], 25–75%) or means ± standard deviation (SD), and categorical variables are reported as frequencies (percentages). To assess the differences between the groups, data were compared using the t-test or Mann-Whitney U test for continuous variables, while the χ2 or Fisher's exact test was used to compare categorical variables. In the serial analysis of the severity score, each period was analyzed using paired t-test. For all analyses, a two-tailed P value < 0.05 was considered statistically significant. To determine the prognostic factors for 28-day mortality, univariate analysis, and multivariate analysis were performed using logistic regression. Statistical analysis was performed using IBM SPSS version 24.0 (SPSS Inc., Chicago, IL, USA).
Ethics statement
This study was approved by the Asan Medical Center Institutional Review Board (study number 2012-0595). The need for informed consent was waived by the board.
RESULTS
Patient characteristics
A total of 381 patients were diagnosed with HAP/VAP during the period. Patient characteristics at the time of diagnosis are summarized at Table 1. The median age was 69 (59–76) years, and 71% of them were male. Solid malignant tumors (37%), chronic pulmonary disease (22%), and diabetes (22%) were the most frequently encountered co-morbidities. The reason for ICU admission was mainly acute respiratory failure (60%), followed by sepsis/septic shock (15%). The most common place of diagnosis for HAP/VAP was the medical ICU (55%), followed by the general ward (32%). HAP and VAP were diagnosed in 54% and 46% of all patients, respectively. Of the 205 HAP patients, 121 (59%) were diagnosed in general ward, while the rest (41%) were diagnosed in the ICU. The mean APACHE II score on day 1 was 24 ± 7. One-quarter of HAP/VAP patients (n = 99) had prior intravenous antibiotic use within the last 90 days as a risk factor for MDR pathogens. Most of patients (88%) had late-onset (> 5 days) HAP/VAP. The median time from hospital admission to diagnosis for HAP/VAP was 11 (6–22) days. Then, the median time from mechanical ventilation to VAP was 9 (5–12) days.
Table 1. Baseline characteristics (n = 381).
| Characteristics | Values | |||
|---|---|---|---|---|
| Age, yr | 69 (59–76) | |||
| Sex, male | 270 (71) | |||
| BMI, kg/m2 | 22 ± 4 | |||
| Co-morbidities | ||||
| Malignancy | 176 (46) | |||
| Solid tumor | 141 (80) | |||
| Metastatic solid tumor | 60 (16) | |||
| Hematologic | 35 (20) | |||
| Chronic pulmonary disease | 84 (22) | |||
| Diabetes mellitus | 82 (22) | |||
| Cerebrovascular disease | 46 (12) | |||
| Congestive heart failure/ischemic heart disease | 38 (10) | |||
| Chronic kidney disease | 38 (10) | |||
| Chronic liver disease | 23 (6) | |||
| Moderate to severe liver disease | 18 (5) | |||
| Modified CCI score | 5.2 ± 2.8 | |||
| Reason for ICU admission | ||||
| Acuter respiratory failure | 230 (60) | |||
| Sepsis/septic shock | 57 (15) | |||
| Postoperative care | 18 (5) | |||
| Heart failure/acute coronary syndrome | 16 (4) | |||
| Neurologic disease | 15 (4) | |||
| Acute renal failure | 8 (2) | |||
| Acute liver failure | 5 (1) | |||
| Aspiration (macro) | 5 (1) | |||
| Others | 27 (7) | |||
| Location of diagnosis for HAP or VAP | ||||
| Medical ICU | 211 (55) | |||
| General ward | 123 (32) | |||
| Surgical ICU | 21 (6) | |||
| Cardiovascular/cardiothoracic ICU | 17 (5) | |||
| Neurologic/neurosurgical ICU | 9 (2) | |||
| Type of pneumonia | ||||
| HAP | 205 (54) | |||
| VAP | 176 (46) | |||
| Risk factors for MDR pathogens | ||||
| HAP | n = 205 | |||
| Prior intravenous antibiotic use within 90 days | 61 (30) | |||
| VAP | n = 176 | |||
| Prior intravenous antibiotic use within 90 days | 38 (22) | |||
| Septic shock at time of VAP | 48 (27) | |||
| Five or more days of hospitalization prior to the occurrence of VAP | 152 (86) | |||
| Time of diagnosis | ||||
| Five or more days of hospitalization prior to the diagnosis of HAP/VAP | 336 (88) | |||
| From hospital admission to diagnosis of HAP or VAP, days | 11 (6–22) | |||
| From mechanical ventilation to VAP, days | 9 (5–12) | |||
| Risk factors and preventive management for VAP | ||||
| Reintubation before diagnosis of pneumonia | 36 (9) | |||
| Enteral feeding | 135 (35) | |||
| Aspiration of subglottic secretion | 149 (39) | |||
| Semi-recumbent position (> 30°) | 209 (55) | |||
| APACHE II at day 1 of HAP or VAP | 24 ± 7 | |||
| Mechanical ventilation after diagnosis of HAP (n = 205) | 166 (81) | |||
Values are presented as number (%), mean ± standard deviation, or median (interquartile range).
BMI = body mass index, CCI = Charlson Comorbidity Index, ICU = intensive care unit, HAP = hospital-acquired pneumonia, VAP = ventilator-acquired pneumonia, MDR = multidrug-resistant, APACHE = Acute Physiology and Chronic Health Evaluation.
Pathogens of HAP/VAP
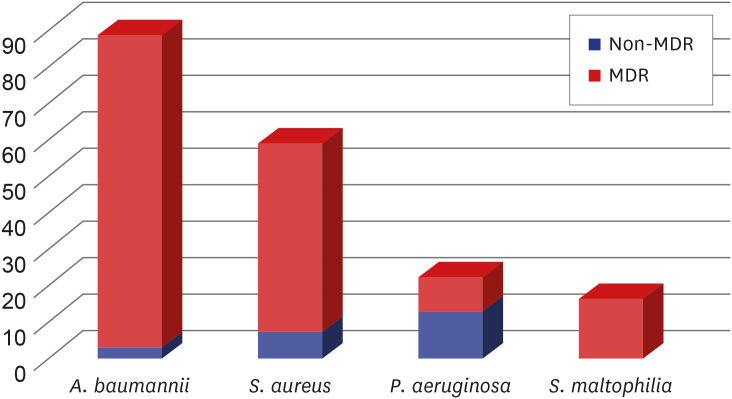
Microbiological specimens were mostly obtained noninvasively (87%) using sputum or endotracheal aspirates. Out of the 381 patients, pathogens were identified in 235 (62%). Bacteria were the most common pathogens (94%), followed by viruses (4%). Among the 267 isolated pathogens, the most common bacterial pathogen was Acinetobacter baumannii (n = 89), followed by Staphylococcus aureus (n = 52), Klebsiella pneumoniae (n = 25), and Pseudomonas aeruginosa (n = 22) (Table 2). Most of the isolated strains of A. baumannii (97%) and S. aureus (88%) were multidrug resistant (Fig. 1). The bacteremia caused by the HAP/VAP pathogens was present in 29 (12%) patients. Twenty-six (12%) patients had poly-microbial infection. The results for each strain of bacteria and the distribution of major pathogens at each center are presented in Supplementary Tables 1 and 2.
Table 2. The pathogens of HAP/VAP (n = 381).
| Pathogens | Values | ||
|---|---|---|---|
| Patients with presence of pathogens | 235 (62) | ||
| No. of pathogens, including polymicrobial infections | n = 267 | ||
| Presence of bacteremia by pathogens | 29 (12) | ||
| Patient No. with bacteria as pathogens | 221 (94) | ||
| No. of bacteria including polymicrobial infection | n = 250 | ||
| A. baumannii | 89 (36) | ||
| S. aureus | 59 (24) | ||
| K. pnemoniae | 25 (10) | ||
| P. aeruginosa | 22 (9) | ||
| S. maltophilia | 16 (6) | ||
| E. faecium | 6 (3) | ||
| E. coli | 6 (2) | ||
| E. aerogenes | 5 (2) | ||
| E. cloacae | 3 (1) | ||
| S. pneumoniae | 3 (1) | ||
| S. marcescens | 2 (1) | ||
| C. freundii | 2 (1) | ||
| A. xylosoxidans | 2 (1) | ||
| Others | 10 (4) | ||
| Patients with virus as pathogens | 12 (5) | ||
| No. of virus | n = 12 | ||
| Influenza A virus | 5 (42) | ||
| RSV | 2 (17) | ||
| PIV | 1 (8) | ||
| Adenovirus | 1 (8) | ||
| Influenza B virus | 1 (8) | ||
| Human metapneumovirus | 1 (8) | ||
| Rhinovirus | 1 (8) | ||
| Patients with bacteria + virus as pathogens | 2 (1) | ||
| Superinfection | n = 2 | ||
| A. baumannii + RSV | 1 (50) | ||
| S. pneumoniae + PIV | 1 (50) | ||
Values are presented as number (%).
HAP = hospital-acquired pneumonia, VAP = ventilator-associated pneumonia, RSV = respiratory syncytial virus, PIV = parainfluenza virus.
Fig. 1. Ratio of MDR bacteria in each major group of bacterial pathogens.
MDR = multidrug-resistant.
MDR pathogens and colonization
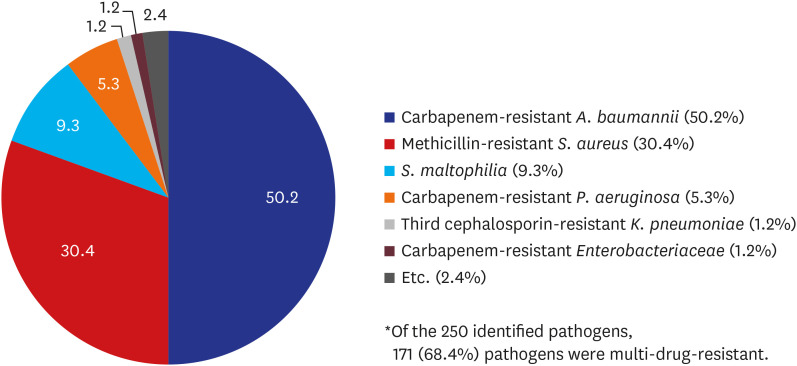
Of the 223 patients whose pathogens were confirmed to be bacteria, 158 (71%) were infected by the MDR pathogens. Out of 250 isolated strains of bacteria, there were 171 (68%) MDR pathogens. The most common MDR pathogen was A. baumannii (50%), followed by S. aureus (30%) and Stenotrophomonas maltophilia (9%) (Fig. 2). In HAP, the most common MDR bacteria were methicillin-resistant S. aureus (40%) and carbapenem-resistant A. baumannii (33%), whereas in VAP, carbapenem-resistant A. baumannii (59%) accounted for the most, followed by methicillin-resistant S. aureus (26%) (Table 3). Of the patients whose pathogens were identified, 77 (35%) had colonization of the pathogen in the upper or lower respiratory tract from 2 weeks to 2 days before the onset of HAP/VAP (Supplementary Table 3).
Fig. 2. Distributions of MDR bacteria.
MDR = multidrug-resistant.
Table 3. Distribution of MDR bacteria in HAP/VAP.
| Pathogens | Values | ||
|---|---|---|---|
| Patient No. with MDR bacteria as pathogens | n = 158 | ||
| Total No. of MDR pathogens | n = 171 | ||
| HAP | n = 205 | ||
| Patient No. with MDR bacteria | n = 50 | ||
| No. of identified MDR bacteria | n = 55 | ||
| Methicillin-resistant S. aureus | 22 (40) | ||
| Carbapenem-resistant A. baumannii | 18 (33) | ||
| S. maltophilia | 7 (13) | ||
| Carbapenem-resistant P. aeruginosa | 5 (9) | ||
| Carbapenem-resistant Enterobacteriaceae | 2 (4) | ||
| Others | 1 (2) | ||
| VAP | n = 176 | ||
| Patient No. with MDR bacteria | n = 108 | ||
| No. of identified MDR bacteria | n = 116 | ||
| Carbapenem-resistant A. baumannii | 68 (59) | ||
| Methicillin-resistant S. aureus | 30 (26) | ||
| S. maltophilia | 10 (9) | ||
| Carbapenem-resistant P. aeruginosa | 4 (3) | ||
| Others | 3 (3) | ||
| Carbapenem-resistant Enterobacteriaceae | 1 (1) | ||
Values are presented as number (%).
MDR = multidrug-resistant, HAP = hospital-acquired pneumonia, VAP = ventilator-associated pneumonia.
Previous antibiotics use
Within 4 weeks of onset of HAP/VAP, most patients (85%) received antibiotics for more than 48 hours, and the most common antibiotics were piperacillin/tazobactam (51%) and quinolone (56%) such as levofloxacin. In a significant number of patients, carbapenem (34%) and glycopeptide (40%) such as vancomycin were also used before the onset of HAP/VAP (Supplementary Table 3).
Antibiotic treatment
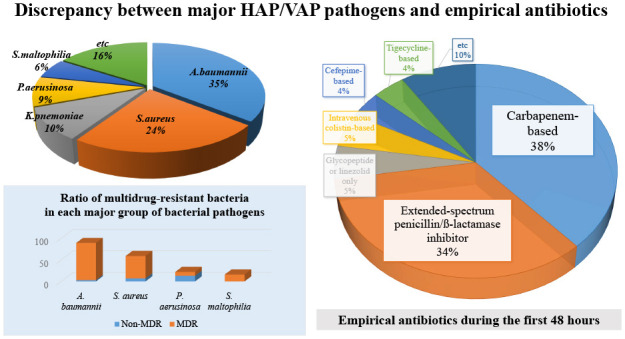
Patients enrolled in the study received empirical antibiotics for the first 48 hours after the onset of HAP/VAP. The most commonly used antibiotic regimens were carbapenem-based antibiotics (38%), followed by extended-spectrum penicillin/β-lactamase inhibitors (34%). Intravenous colistin was initiated in 19 patients (5%). Quinolones were used together with other antibiotics in 40% of patients, and glycopeptides or linezolids were also used in combination in 54% of patients (Table 4).
Table 4. Empirical and subsequent antibiotic treatments (n = 381).
| Variables | Values | ||
|---|---|---|---|
| Empirical antibiotics during the first 48 hours | |||
| Carbapenem-based | 144 (38) | ||
| Extended-spectrum penicillin/β-lactamase inhibitor | 131 (34) | ||
| Glycopeptide or linezolid only | 20 (5) | ||
| Intravenous colistin-based | 19 (5) | ||
| Cefepime-based | 15 (4) | ||
| Tigecycline-based | 14 (4) | ||
| Quinolone only | 12 (3) | ||
| Third cephalosporin-based | 9 (2) | ||
| Carbapenem + tigecycline | 8 (2) | ||
| Sulbactam-based | 4 (1) | ||
| Intravenous colistin + tigecycline | 4 (1) | ||
| Others | 1 (0.3) | ||
| Combination therapy | |||
| Quinolone | 149/369 (40) | ||
| Glycopeptide or linezolid | 196/361 (54) | ||
| Inhaled colistin | 20 (5) | ||
| Subsequent antibiotics from the first 48 hour forth to 2 weeks | n = 377a | ||
| Carbapenem-based | 137 (36) | ||
| Extended-spectrum penicillin/β-lactamase inhibitor | 109 (29) | ||
| Intravenous colistin-based | 30 (8) | ||
| Intravenous colistin + tigecycline | 20 (5) | ||
| Cefepime-based | 19 (5) | ||
| Quinolone only | 15 (4) | ||
| Glycopeptide or linezolid only | 15 (4) | ||
| Tigecycline-based | 12 (3) | ||
| Third cephalosporin-based | 11 (3) | ||
| Carbapenem + tigecycline | 2 (1) | ||
| Sulbactam-based | 2 (1) | ||
| Others | 5 (1) | ||
| Combination therapy | |||
| Quinolone | 127 (34) | ||
| Glycopeptide or linezolid | 176 (47) | ||
Values are presented as number (%).
aFour patients with subsequent antibiotics from the first 48 hour forth to 2 weeks died.
Clinical responses according to scoring systems
As a clinical response, the SOFA score on day 1 was 8 (5–11), and then showed a significant decrease over days 3, 5, and 7 (P < 0.001). The CPIS was 6 (4–7) on day 1, and it also significantly decreased over days 3, 5, and 7 (P < 0.001).
Patient outcomes
Patient who showed improvement on day 3, that is, whose CPIS scores reached 6 points or less, or fell by 2 points or more with respect to the score on day 1, accounted for 33% of all patients. The rate of day 7 treatment failure, i.e., CPIS failing to fall below 6 or failing to decrease by at least 2 points with respect to the score on day 1, or the PaO2/FiO2 ratio failing to improve even after 1 week of treatment, was 41%. Within 28 days of starting treatment, 156 (41%) patients were able to completely stop antibiotics for pneumonia. Among the 156 patients who completed antibiotic treatment for pneumonia within 28 days, out of 25 patients whose pathogens were identified, there were 13 (52%) cases of pneumonia caused by the same pathogens within 4 weeks after stopping antibiotics (day 60 Relapse). The median length of MV was 17 (8–32) days. The 28-day mortality was 30%, and the 60-day mortality was 46%.
Outcomes according to the use of appropriate empirical antibiotics
The prognoses of patients differed significantly depending on whether or not they received appropriate empirical antibiotics. Of the 221 patients whose pathogens were identified as bacteria, 121 (55%) patients received appropriate empirical antibiotics. In the group that used appropriate empirical antibiotics, 42% of the patients were able to stop all antibiotics for pneumonia within day 28; however, when inappropriate used, only 21% of patients were able to stop (P = 0.001). Also, patients who used empirical antibiotics appropriately had significantly lower mortality rates than those who did not (28-day mortality: 25% vs. 40%, P = 0.032; ICU mortality: 34% vs. 48%, P = 0.033; and 60-day mortality: 41% vs. 55%, P = 0.032, respectively). There were similar results in the comparison between the two groups according to whether the antibiotic used within 2 weeks after 48 hours was appropriate for the pathogen (Supplementary Table 4).
Prognostic factors of 28-day mortality
To determine the prognostic factors for 28-day mortality, multivariate analysis was performed with the six significant factors (P < 0.05) identified during univariate analysis (Table 5). As a result, five or more days of hospitalization prior to the occurrence of HAP/VAP (odds ratio [OR], 3.945; confidence interval [CI], 1.379–8.623; P = 0.008), APACHE II score on day 1 (OR, 1.066; CI, 1.029–1.105; P < 0.001), implementation of the semi-recumbent position (> 30 degrees) (OR, 0.589; CI, 0.363–0.957; P = 0.033), and day 7 failure (OR, 2.624; CI, 1.635–4.212; P < 0.001) were prognostic factors significantly associated with 28-day mortality.
Table 5. Univariate and multivariate analysis for 28-day mortality of all HAP/VAP patients.
| Variables (n = 381) | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Age, yr | 1.009 (0.992–1.027) | 0.315 | |||
| Sex, male | 1.253 (0.779–2.015) | 0.352 | |||
| Co-morbidities | |||||
| Metastatic solid tumor | 1.711 (0.967–3.029) | 0.065 | |||
| Chronic pulmonary disease | 1.143 (0.679–1.926) | 0.615 | |||
| DM with complications | 0.935 (0.287–3.044) | 0.911 | |||
| Cerebrovascular disease | 0.708 (0.346–1.449) | 0.344 | |||
| CHF/ischemic heart disease | 0.821 (0.385–1.751) | 0.609 | |||
| Chronic kidney disease | 1.677 (0.744–3.781) | 0.217 | |||
| Mod to severe liver disease | 2.448 (0.945–6.338) | 0.065 | |||
| Modified CCI score | 1.082 (1.003–1.168) | 0.042 | |||
| Reason for ICU admission | |||||
| Acute respiratory failure | 1.247 (0.792–1.963) | 0.339 | |||
| Sepsis/septic shock | 0.995 (0.538–1.840) | 0.986 | |||
| Postoperative care | 0.454 (0.129–1.600) | 0.219 | |||
| Heart failure/ACS | 1.068 (0.362–3.146) | 0.906 | |||
| Neurologic disease | 0.349 (0.077–1.572) | 0.170 | |||
| Acute renal failure | 1.416 (0.333–6.028) | 0.638 | |||
| Acute liver failure | 3.581 (0.590–21.726) | 0.165 | |||
| Aspiration (macro) | - | - | |||
| Location of diagnosis | |||||
| Medical ICU | 1.218 (0.781–1.898) | 0.385 | |||
| General ward | 0.955 (0.596–1.529) | 0.848 | |||
| Surgical ICU | 0.374 (0.108–1.295) | 0.121 | |||
| Cardiac/Thoracic ICU | 0.975 (0.335–2.834) | 0.963 | |||
| Neuro/Neurosurgical ICU | 0.851 (0.209–3.462) | 0.821 | |||
| Risk factor for MDR organism | |||||
| Prior IV antibiotics use within 90 days | 1.165 (0.711–1.908) | 0.544 | |||
| Time of diagnosis | |||||
| Five or more days of hospitalization prior to the diagnosis of HAP/VAP | 3.945 (1.637–9.506) | 0.002 | 3.425 (1.379–8.623) | 0.008 | |
| Risk factors and preventive management for VAP | |||||
| Enteral feeding | 0.699 (0.436–1.119) | 0.136 | |||
| Semi-recumbent position (> 30 degrees) | 0.618 (0.398–0.961) | 0.033 | 0.589 (0.363–0.957) | 0.033 | |
| APACHE II at day 1 | 1.057 (1.024–1.092) | 0.001 | 1.066 (1.029–1.105) | < 0.001 | |
| Identification of pathogens | 1.286 (0.814–2.033) | 0.281 | |||
| Presence of bacteremia | 2.214 (1.041–4.708) | 0.039 | |||
| Treatment response | |||||
| Day 3 improvement | 0.553 (0.337–0.905) | 0.018 | |||
| Day 7 failure | 3.119 (1.981–4.911) | < 0.001 | 2.624 (1.635–4.212) | < 0.001 | |
HAP = hospital-acquired pneumonia, VAP = ventilator-associated pneumonia, OR = odds ratio, CI = confidence interval, DM = diabetes mellitus, CHF = congestive heart failure, CCI = Charlson Comorbidity Index, ICU = intensive care unit, ACS = acute coronary syndrome, MDR = multidrug-resistant, APACHE II = Acute Physiology and Chronic Health Evaluation II.
Appropriate empirical antibiotics as a prognostic factor for 28-day mortality
When subgroup analysis was performed on patients whose pathogens were identified as bacteria, the administration of appropriate empirical antibiotics was a significant prognostic factor for 28-day mortality (OR, 0.282; CI, 0.092–0.859; P = 0.026) in HAP patients other than VAP along with day 7 failure (OR, 4.515; CI, 1.545–13.192; P = 0.006) and the APACHE II score on day 1 (OR, 1.108; CI, 1.023–1.200; P = 0.012) (Table 6).
Table 6. Univariate and multivariate analysis for 28-day survivor (n = 58) vs. non-survivor (n = 30) in HAP patients with identified bacterial pathogens.
| Variables (n = 88) | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Age, yr | 0.995 (0.958–1.033) | 0.781 | |||
| Sex, male | 1.175 (0.475–2.906) | 0.728 | |||
| Co-morbidities | |||||
| Metastatic solid tumor | 4.109 (1.096–15.409) | 0.036 | |||
| Chronic pulmonary disease | 1.200 (0.390–3.694) | 0.751 | |||
| DM with complications | - | - | |||
| Cerebrovascular disease | 0.694 (0.170–2.836) | 0.611 | |||
| CHF/ischemic heart disease | 0.251 (0.029–2.145) | 0.207 | |||
| Chronic kidney disease | 0.966 (0.084–11.099) | 0.978 | |||
| Mod to severe liver disease | 4.071 (0.354–46.838) | 0.262 | |||
| Modified CCI score | 1.297 (1.043–1.614) | 0.020 | 1.326 (0.988–1.779) | 0.060 | |
| Reason for ICU admission | |||||
| Acute respiratory failure | 0.967 (0.369–2.537) | 0.946 | |||
| Sepsis/septic shock | 1.500 (0.313–7.186) | 0.612 | |||
| Postoperative care | 0.966 (0.084–11.099) | 0.978 | |||
| Heart failure/ACS | - | - | |||
| Neurologic disease | 0.964 (0.166–5.592) | 0.986 | |||
| Acute renal failure | - | - | |||
| Acute liver failure | - | - | |||
| Aspiration (macro) | - | - | |||
| Location of diagnosis | |||||
| Medical ICU | 1.396 (0.549–3.551) | 0.483 | |||
| General ward | 1.062 (0.437–2.583) | 0.894 | |||
| Surgical ICU | - | - | |||
| Cardiac/Thoracic ICU | 1.996 (0.119–32.57) | 0.637 | |||
| Neuro/Neurosurgical ICU | 0.964 (0.166–5.592) | 0.968 | |||
| Risk factor for MDR organism | |||||
| Prior IV antibiotics use within 90 days | 1.229 (0.462–3.265) | 0.680 | |||
| Time of diagnosis | |||||
| Five or more days of hospitalization prior to the diagnosis of HAP/VAP | 2.106 (0.540–8.219) | 0.284 | |||
| Risk factors and preventive management for VAP | |||||
| Enteral feeding | 1.068 (0.352–3.240) | 0.907 | |||
| Semi-recumbent position (> 30 degrees) | 0.818 (0.324–2.065) | 0.671 | |||
| APACHE II at day 1 | 1.114 (1.036–1.198) | 0.004 | 1.108 (1.023–1.200) | 0.012 | |
| Appropriate empirical antibiotics | 0.415 (0.167–1.032) | 0.059 | 0.282 (0.092–0.859) | 0.026 | |
| Appropriate antibiotics from 48 hours to 2 weeks | 0.499 (00.190–1.310) | 0.158 | |||
| Presence of bacteremia | 0.810 (0.194–3.385) | 0.772 | |||
| Treatment response | |||||
| Day 3 improvement | 0.459 (0.180–1.170) | 0.103 | |||
| Day 7 failure | 4.952 (1.921–12.765) | 0.001 | 4.515 (1.545–13.192) | 0.006 | |
HAP = hospital-acquired pneumonia, OR = odds ratio, CI = confidence interval, DM = diabetes mellitus, CHF = congestive heart failure, CCI = Charlson Comorbidity Index, ICU = intensive care unit, ACS = acute coronary syndrome, MDR = multidrug-resistant organism, VAP = ventilator-associated pneumonia, APACHE II = Acute Physiology and Chronic Health Evaluation II.
DISCUSSION
This study aimed to identify the strain distribution and antibiotic selection of HAP/VAP patients in medical ICUs in Korea. The 28-day mortality rate of the patients with HAP/VAP was as high as 30%. The main pathogens responsible for HAP/VAP were A. baumannii and S. aureus, and most strains of A. baumannii and S. aureus were multidrug resistant. On the other hand, the empirical antibiotics, which were started within the first 48 hours of pneumonia, were mainly selected as carbapenem-based or extended-spectrum penicillin/β-lactamase inhibitor-based regimens, showing a discordance between the selection of antibiotics and the main strains. This suggests that a different standard is needed for empirical antibiotic selection in patients with HAP/VAP in domestic medical institutions.
The most commonly encountered strains in our study were A. baumannii and S. aureus. In HAP, S. aureus (22%), A. baumannii (19%), K. pneumoniae (15%), and P. aeruginosa (8%) were in that order, and in VAP, A. baumannii (46%), S. aureus (22%), P. aeruginosa (8%), and S. maltophilia (7%). In a prior study conducted in the 1990s, a multicenter surveillance of nosocomial infections was carried out in tertiary referral centers in Korea, S. aureus (45%) and P. aeruginosa (23%) were the two main strains identified in patients with nosocomial pneumonia.11 Whereas, 10 years later, in a study conducted in Asian countries (including Korea), A. baumannii emerged as a major strain responsible for nosocomial pneumonia, especially VAP.12 In terms of the distribution of MDR microorganisms, our study showed that the MDR rates of A. baumannii, S. aureus, and P. aeruginosa were 97%, 88%, and 41%, respectively. Compared to the data from the Asian countries whose rates were 82%, 82%, and 43%, respectively, the overall trends were in line with our results, although it was a little higher.12 At the same time, the distribution of these strains in the European countries or the U.S was different.4,13 In a previous study of 27 ICUs in nine countries in Europe,13 the most common bacteria in nosocomial pneumonia patients who underwent mechanical ventilation were S. aureus (32%), P. aeruginosa (23%), and A. baumannii (19%). However, when comparing the three most common strains in each participating country, A. baumannii was not included in seven western European countries, which results were in line with U.S data.4 On the other hand, in Greece and Turkey, the two remaining countries of the European study, the burden of A. baumannii was highest, in line with our results. A. baumannii is usually classified as a pathogen with low virulence.2,14 The problem is that when antibiotic resistance is acquired, the range of alternative treatment options becomes limited. In the end, it is important to prevent A. baumannii from gaining drug resistance and to prevent further spread of strains that are already resistant to antibiotics.15,16 In this regard, the role of infection control and antimicrobial stewardship in each country and each hospital is important. It could be the reason why the proportion of A. baumannii is lower in high-income countries than in middle and low-income countries. This suggests that the need for more thorough infection control should be further emphasized in Korea.
The present study demonstrated a significant difference in survival rate depending on whether the causative organisms were susceptible to the empirical antibiotics used within 48 hours of HAP/VAP. The results of this study are similar to those of previous ones.17,18,19,20 In particular, in our study, the administration of adequate empirical antibiotics was a major determinant of the 28-day survival in HAP patients. The appropriate use of empirical antibiotics could help improve patients' prognosis; however, in reality, antibiotics are usually not administered properly as intravenous colistin was used as an empirical antibiotic in only about 5% of patients. If we look at the reasons, in the ICU setting, potentially-resistant microorganisms were the main strains responsible for HAP/VAP in the present study. Considering the previously known local microbiological epidemiology,12 it is appropriate to use empirical antibiotics targeting these multidrug resistant bacteria; however, the main reason for not doing so is the lack of investigation or understanding of local epidemiologic data, and, more importantly, the concerns about the unnecessary adverse effect and potential toxicity of antibiotics and the emergence of resistance.21 In particular, for critically-ill patients who often suffer from renal insufficiency, physicians could hesitate to use potentially nephrotoxic antibiotics before the identification of the culprit microorganisms. Third, there is the issue of insurance. In Korea, insurance coverage for colistin is difficult if carbapenem-resistant microorganisms are not isolated. This will also be another reason why medical staff are hesitant to use it as an empirical antibiotic. Considering these aspects, this might be a difficult problem to solve, at least until drugs that can cover these bacteria without adversely affecting organs will be readily available.
Treatment response was evaluated on day 3, day 7, and day 28 in this study. Two-thirds of patients did not show a decrease in CPIS within 72 hours after the start of treatment, while 41% of patients had treatment failure after 1 week of treatment. Only 41% of all the patients completed treatment for pneumonia within 28 days; the remaining 59% either died or are still receiving antibiotics. We investigated whether the treatment response on day 3 and day 7 was associated with patients' 28-day mortality and found that day 3 improvement and day 7 failure were significantly associated with 28-day mortality in univariate analysis; however, in multivariate analysis, only day 7 treatment failure was significantly associated with 28-day mortality (P < 0.001). Of the 157 patients with treatment failure on day 7 (i.e., no decrease in CPIS or no improvement in the P/F ratio), pneumonia was not completely resolved in 124 (79%) on day 28; their 28-day mortality was 54%, while their 60-day mortality was 73%. Considering the previous literature that looked at the treatment response up to day 5 as an indicator of late treatment failure,22 day 7 treatment failure was sufficient to evaluate the treatment response, and this study confirmed that it has a significant correlation with the patient's prognosis. Thus, when the patients do not improve within 7 days, taking into account the possibility of continuing deterioration, more careful attention should be paid to whether antibiotics are appropriately selected, whether sufficient supportive care is provided, and to prevent additional VAP. This study revealed that the implementation of the semi-recumbent position (> 30 degrees) was an important factor associated with a lower 28-day mortality. The semi-recumbent position has already been identified as a method of preventing VAP in several studies.23,24,25 Our results suggested that the sufficient application of this method reduces the risk of additional pneumonia in mechanically-ventilated HAP/VAP patients, which led to better outcomes.
Although this was a prospective multicenter study, it had some limitations. First, the five medical centers that participated in the study consisted of only tertiary hospitals, which is not representative of all patients with HAP/VAP in Korea. Second, in patients who started empirical therapy with broad-spectrum antibiotics, there is a possibility that the adverse effects of the antibiotics used might have affected the patients; however, this has not been specifically investigated. Third, since we only recruited HAP/VAP patients who were admitted to the ICU, not all HAPs that occurred in the hospitals were recruited, as HAPs that occurred and improved in the general ward were not investigated.
In this study, the strains of bacteria responsible for HAP/VAP in tertiary referral hospitals in Korea were associated with a huge burden of MDR bacteria. Proper selection of empirical antibiotics was significantly associated with an improvement the patient's prognosis. In contrast, it was demonstrated that there was a discrepancy between initial empirical antibiotic selection and major strains. In selecting antibiotics, the adverse effects of drugs should be considered important. Future studies based on these domestic microbiologic data are needed to establish criteria for selecting appropriate antibiotics considering all of these factors. In addition, a prompt judgment of the treatment response and prevention of additional pneumonia should not be neglected.
Footnotes
Funding: This work was supported by a grant from the Korean Healthcare Technology R&D Project, Ministry for Health and Welfare, Republic of Korea (grant number HI12C0756).
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Hong SB, Chong YP.
- Data curation: Chang Y, Jeon K, Lee SM, Cho YJ, Kim YS, Chong YP.
- Formal analysis: Chang Y, Jeon K, Hong SB, Chong YP.
- Investigation: Chang Y, Jeon K, Lee SM, Cho YJ, Kim YS, Chong YP, Hong SB.
- Methodology: Chang Y, Jeon K, Hong SB, Chong YP.
- Writing - original draft: Chang Y, Jeon K.
- Writing - review & editing: Chang Y, Jeon K, Lee SM, Cho YJ, Kim YS, Chong YP, Hong SB.
SUPPLEMENTARY MATERIALS
The distribution of poly-microbial infection
Distribution of major pathogens of HAP/VAP at each center
Colonization and previous antibiotics use before the occurrence of HAP/VAP
Outcomes according to appropriate antibiotics used from after 48 hours to 2 weeks
References
- 1.American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 2.Falagas ME, Bliziotis IA, Siempos II. Attributable mortality of Acinetobacter baumannii infections in critically ill patients: a systematic review of matched cohort and case-control studies. Crit Care. 2006;10(2):R48. doi: 10.1186/cc4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agrafiotis M, Siempos II, Ntaidou TK, Falagas ME. Attributable mortality of ventilator-associated pneumonia: a meta-analysis. Int J Tuberc Lung Dis. 2011;15(9):1154–1163. doi: 10.5588/ijtld.10.0498. [DOI] [PubMed] [Google Scholar]
- 4.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370(13):1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zilberberg MD, Nathanson BH, Sulham K, Fan W, Shorr AF. A novel algorithm to analyze epidemiology and outcomes of carbapenem resistance among patients with hospital-acquired and ventilator-associated pneumonia: a retrospective cohort study. Chest. 2019;155(6):1119–1130. doi: 10.1016/j.chest.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 6.Safdar N, Dezfulian C, Collard HR, Saint S. Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med. 2005;33(10):2184–2193. doi: 10.1097/01.ccm.0000181731.53912.d9. [DOI] [PubMed] [Google Scholar]
- 7.Beardsley JR, Williamson JC, Johnson JW, Ohl CA, Karchmer TB, Bowton DL. Using local microbiologic data to develop institution-specific guidelines for the treatment of hospital-acquired pneumonia. Chest. 2006;130(3):787–793. doi: 10.1378/chest.130.3.787. [DOI] [PubMed] [Google Scholar]
- 8.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JK, Lee J, Park YS, Lee CH, Yim JJ, Yoo CG, et al. Clinical manifestations of pneumonia according to the causative organism in patients in the intensive care unit. Korean J Intern Med. 2015;30(6):829–836. doi: 10.3904/kjim.2015.30.6.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwak YG, Choi JY, Yoo H, Lee SO, Kim HB, Han SH, et al. Korean National Healthcare-associated Infections Surveillance System, intensive care unit module report: summary of data from July 2014 through June 2015. Korean J Healthc Assoc Infect Control Prev. 2016;21(2):37–49. [Google Scholar]
- 11.Kim JM, Park ES, Jeong JS, Kim KM, Kim JM, Oh HS, et al. Multicenter surveillance study for nosocomial infections in major hospitals in Korea. Am J Infect Control. 2000;28(6):454–458. doi: 10.1067/mic.2000.107592. [DOI] [PubMed] [Google Scholar]
- 12.Chung DR, Song JH, Kim SH, Thamlikitkul V, Huang SG, Wang H, et al. High prevalence of multidrug-resistant nonfermenters in hospital-acquired pneumonia in Asia. Am J Respir Crit Care Med. 2011;184(12):1409–1417. doi: 10.1164/rccm.201102-0349OC. [DOI] [PubMed] [Google Scholar]
- 13.Koulenti D, Lisboa T, Brun-Buisson C, Krueger W, Macor A, Sole-Violan J, et al. Spectrum of practice in the diagnosis of nosocomial pneumonia in patients requiring mechanical ventilation in European intensive care units. Crit Care Med. 2009;37(8):2360–2368. doi: 10.1097/CCM.0b013e3181a037ac. [DOI] [PubMed] [Google Scholar]
- 14.Lambert ML, Suetens C, Savey A, Palomar M, Hiesmayr M, Morales I, et al. Clinical outcomes of health-care-associated infections and antimicrobial resistance in patients admitted to European intensive-care units: a cohort study. Lancet Infect Dis. 2011;11(1):30–38. doi: 10.1016/S1473-3099(10)70258-9. [DOI] [PubMed] [Google Scholar]
- 15.Higgins PG, Dammhayn C, Hackel M, Seifert H. Global spread of carbapenem-resistant Acinetobacter baumannii . J Antimicrob Chemother. 2010;65(2):233–238. doi: 10.1093/jac/dkp428. [DOI] [PubMed] [Google Scholar]
- 16.Villar M, Cano ME, Gato E, Garnacho-Montero J, Miguel Cisneros J, Ruíz de Alegría C, et al. Epidemiologic and clinical impact of Acinetobacter baumannii colonization and infection: a reappraisal. Medicine (Baltimore) 2014;93(5):202–210. doi: 10.1097/MD.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kollef KE, Schramm GE, Wills AR, Reichley RM, Micek ST, Kollef MH. Predictors of 30-day mortality and hospital costs in patients with ventilator-associated pneumonia attributed to potentially antibiotic-resistant gram-negative bacteria. Chest. 2008;134(2):281–287. doi: 10.1378/chest.08-1116. [DOI] [PubMed] [Google Scholar]
- 18.Zilberberg MD, Nathanson BH, Sulham K, Fan W, Shorr AF. Multidrug resistance, inappropriate empiric therapy, and hospital mortality in Acinetobacter baumannii pneumonia and sepsis. Crit Care. 2016;20(1):221. doi: 10.1186/s13054-016-1392-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zilberberg MD, Shorr AF, Micek ST, Vazquez-Guillamet C, Kollef MH. Multi-drug resistance, inappropriate initial antibiotic therapy and mortality in Gram-negative severe sepsis and septic shock: a retrospective cohort study. Crit Care. 2014;18(6):596. doi: 10.1186/s13054-014-0596-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rello J, Ulldemolins M, Lisboa T, Koulenti D, Mañez R, Martin-Loeches I, et al. Determinants of prescription and choice of empirical therapy for hospital-acquired and ventilator-associated pneumonia. Eur Respir J. 2011;37(6):1332–1339. doi: 10.1183/09031936.00093010. [DOI] [PubMed] [Google Scholar]
- 21.Kalanuria AA, Ziai W, Mirski M. Ventilator-associated pneumonia in the ICU. Crit Care. 2014;18(2):208. doi: 10.1186/cc13775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torres A, Sibila O, Ferrer M, Polverino E, Menendez R, Mensa J, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA. 2015;313(7):677–686. doi: 10.1001/jama.2015.88. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Li X, Yang Z, Tang X, Yuan Q, Deng L, et al. Semi-recumbent position versus supine position for the prevention of ventilator-associated pneumonia in adults requiring mechanical ventilation. Cochrane Database Syst Rev. 2016;2016(1):CD009946. doi: 10.1002/14651858.CD009946.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coppadoro A, Bellani G, Foti G. Non-pharmacological interventions to prevent ventilator-associated pneumonia: a literature review. Respir Care. 2019;64(12):1586–1595. doi: 10.4187/respcare.07127. [DOI] [PubMed] [Google Scholar]
- 25.Park SY, Lee HB. Prevention and management of ventilator-associated pneumonia. Korean J Med. 2014;86(5):537–545. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The distribution of poly-microbial infection
Distribution of major pathogens of HAP/VAP at each center
Colonization and previous antibiotics use before the occurrence of HAP/VAP
Outcomes according to appropriate antibiotics used from after 48 hours to 2 weeks