Abstract
This study evaluated the effect of organic acids-essential oils blend with or without oat hulls (OH) on growth performance, organ weights, blood parameters, gut morphology, microbiota, and short-chain fatty acids (SCFA) in broiler chickens. Day-old broiler chickens were randomly allocated to 4 dietary treatments consisting of 1) a corn-soybean meal-wheat based diet (BAS), 2) BAS + 0.05% bacitracin methylene disalicylate (BMD), 3) BAS + protected organic acids-essential oils at 300 g/1,000 kg of feed (OE), and 4) BAS + protected organic acids-essential oils at 300 g/1,000 kg of feed + 3% OH (OEOH), in 8 replicate groups. Feeding was in starter (d 0 to 14), grower (d 14 to 24), and finisher (d 24 to 36) phases. Body weight (BW), feed intake (FI), feed conversion ratio (FCR), and mortality were determined weekly. On d 36, 8 chickens per treatment were sampled for blood biochemistry, organ weights, cecal SCFA production, and microbiota. Treatments had no effect on FI and FCR at all phases. Both OE and OEOH treatments reduced (P < 0.001) the body weight gain of birds at the starter phase. Birds fed the OEOH treatment had higher (P < 0.001) gizzard weight, while those offered the BMD diet showed a tendency (P = 0.08) to have higher cecal weight. Birds in the OEOH treatment recorded increased ileal villus height and villus height-to-crypt depth ratio, as well as reduced duodenal crypt depth, while birds in the OE treatment had increased jejunal villus height and villus height-to-crypt depth ratio. Both OEOH and OE treatments increased the number of goblet cells produced in the duodenum and jejunum. Treatments had no effect on SCFA concentrations. Birds in the OE treatment recorded the lowest concentration of blood urea (P = 0.05) and cholesterol (P < 0.05). Both OE and OEOH treatments increased (P < 0.05) the relative abundance of potentially beneficial bacteria in the genus Firmicutes_unclassified, Ruminococcus, Turicibacter, and Erysipelotrichaceae_unclassified, while reducing (P < 0.001) the relative abundance of potentially harmful Coprobacillus. Conclusively, both protected organic acids-essential oils blend and its combination with oat fibers show potential as tools to achieve antibiotics reduction in broiler production.
Keywords: Organic acid, Essential oil, Oat hull, Broiler chicken, Ceca microbiota
1. Introduction
To preserve the potency of antibiotics, there has been a drastic reduction in their use in food animal production. In the past, antibiotics have played a major role in promoting growth and maintaining gut functionality in broiler chickens (Mehdi et al., 2018). With the current reduction and anticipated elimination of the preventive use of medically important antibiotics in poultry production, there is a need to investigate potential alternatives so that farmers can maintain or improve productivity.
Organic acids, also known as acidifiers, occur naturally and have been used as feed preservatives to prevent contamination by bacteria and fungi (Theron and Lues, 2011). They have also been reported to suppress the growth of acid intolerant pathogenic bacteria (Dhawale, 2005) and reduce pH in the stomach, which enhances pepsin activity and increase the digestibility of nitrogen, phosphorus, and minerals (Dibner and Buttin, 2002). Organic acids have been reported to reduce bacterial production of toxic components and change the morphology of poultry intestinal wall in a way that reduced colonization of pathogens, thus preventing epithelial cell damage (Langhout, 2000). Cherington et al. (1991) and Hajati (2018) presented the mode of action of organic acids on bacteria as penetrating the cell wall of certain types of bacteria, including Escherichia coli, Salmonella spp., and Clostridium perfringens and some coliforms and disrupting their normal physiology, by inhibiting enzymatic reactions and denaturing proteins and DNA.
Essential oils are plant-derived mixtures of volatile compounds with less toxicity (Zhai et al., 2018) and have the ability to improve feed consumption and utilization in chickens (Krishan and Narang, 2014). Essential oils have been reported to possess antioxidative (Fernandez-Panchon et al., 2008), anti-inflammatory (Craig 2001), immunomodulatory (Szigeti et al., 1998), and digestion enhancement properties (Jang et al., 2007), which result in enhanced production performance and health of chickens.
The use of organic acids and essential oils as possible alternatives for antibiotics and immune modulator is gaining tremendous interest in the poultry industry (Yang et al., 2018). However, a combination of these 2 compounds may be more effective in improving gut health and modulating the immune system of broiler chickens. Recent studies that investigated the beneficial effects of protected organic acids-essential oils blends in broiler chickens have reported increased growth performance and intestinal morphology (Abdelli et al., 2020; Gao et al., 2019; Yang et al., 2018), improved intestinal integrity and barrier function (Stefanello et al., 2020; Pham et al., 2020), and increased digestive enzyme activity (Yang et al., 2018, Yang et al., 2019). To our knowledge, only a few studies (Pham et al., 2020) have investigated the effect of the association of organic acids and essential oils on gut microbiota using next-generation DNA sequencing methods. Only a few studies (Yang et al., 2019) have also reported the effect of such blends on short-chain fatty acids (SCFA), and production and blood parameters in broiler chickens.
High fiber ingredients have proven prebiotic effects, and their use in monogastric animals to modulate gut microbiota, promote gut health and growth performance has been reported (Adewole, 2020; Ndou et al., 2018). Oat (Avena sativa) hulls (OH) have high contents of lignin, an insoluble fiber (Kim et al., 2008; Jimenez-Moreno et al., 2009) and has the potential to modulate gastrointestinal microbiota and increase SCFA production, thus enhancing gut barrier integrity (Ndou et al., 2018). Short-chain fatty acids are microbial metabolites, which have been shown to exert multiple beneficial effects on mammalian energy metabolism through the complex interplay between diet, gut microbiota, and intestinal barrier, and immunity (Den Besten et al., 2013). The potential of the combinational use of organic acids and essential oils blends (having an antibacterial effect) with OH (having prebiotic effects) to promote gut microbiota of poultry has not been explored. In reality, it is more likely that a combination of additives will achieve the needed breakthroughs to eliminate or reduce the use of antibiotics in poultry production. This could be observed in the limited successes that have been recorded in most additives that were singly used. It is anticipated that this combinational approach would provide new insight into the application of non-antibiotic compounds and enhance production performance and health of chickens. Moreover, OH are currently under-utilized products, hence contributing to environmental burden (Perruzza, 2010); their use in poultry production would not only reduce feed cost but also improve ecological sustainability. Therefore, the objective of this study was to determine the effect of a protected organic acids and essential oils blend with or without OH on growth performance, organ weights, blood parameters, and ceca SCFA and microbiota in broiler chickens.
2. Materials and methods
The experimental procedures for this study were approved by Dalhousie University Animal Use and Care Committee (Ethical code 2020-011), and chickens were cared for in accordance with the guidelines of the Canadian Council on Animal Care (CCAC, 2009).
2.1. Birds and housing
Day-old broiler chickens (Ross 308) were obtained from a commercial source. Upon arrival, chicks (mixed-sex) were weighed in groups of 26 birds and assigned to floor pens (0.93 m × 2.14 m), at a stocking density of 0.076 m2/bird. The temperature in the broiler room was monitored daily and was gradually reduced from 30 to 22.6 °C from d 0 to 36. The lighting program was set to produce 18 h of light and 6 h of darkness throughout the experimental period, and illumination was gradually reduced from 20 lx on d 0 to 5 lx on d 36.
2.2. Ingredients, diets, and chemical analysis
The OH used in this study was obtained from Grain Millers, Yorton, Saskatchewan, Canada. They were received as unground coarse particle-sized. The chemical composition of the coarse OH has been reported previously by Adewole (2020). The combination of organic acids and essential oils used was provided by JEFO Nutrition Incorporation, Saint-Hyacinthe, QC, Canada as a Protected Organic Acids and Essential Oils – P(OA + EO) formula containing organic acids (fumaric, sorbic, malic, and citric acids) and essential oils (thymol, vanillin, and eugenol). The active ingredients are embedded by Jefo matrix technology, made of an emulsion of hydrogenated vegetable oil according to U.S. Patent WO2018089516 (Ferket et al., 2017). Four dietary treatments were assigned to 8 replicate pens per treatment in a completely randomized design. The dietary treatments consisted of a corn-soybean meal-wheat basal diet (BAS), BAS + 0.05% bacitracin methylene disalicylate (BMD), BAS + P(OA + EO) at 300 g/1,000 kg of feed (OE) and BAS + P(OA + EO) + 3% OH (OEOH). The BAS, BMD, and OE diets were iso-energetic and iso-nitrogenous, but the OEOH diet had reduced energy due to the addition of OH (Table 1). The reduced energy as a result of OH addition is attributable to the low energy value of oat hulls compared to their cereal components (McNab and Boorman, 2002). Mossami (2011) reported that oats with higher hull content have less metabolizable energy compared to less-hulled oats. All diets were formulated on a digestible amino acid and apparent metabolizable energy basis and met or exceeded NRC (1994) nutrient requirements for broiler chickens. A phase-feeding program that consisted of starter phase (d 0 to 14), grower phase (d 14 to 24), and finisher phase (d 24 to 36) was utilized. Diets were fed in crumbled form during the starter phase and in pelleted form during the grower and finisher phases. The ingredient and nutritional compositions of the diets in phases 1 to 3 are presented in Table 1. The diet samples were finely ground and were all analyzed for dry matter, crude protein (CP), fat, and minerals. Dry matter was determined according to AOAC (1990) method (925.09) by oven drying a 5.0 g sample at 105 °C overnight. Nitrogen content was determined using the combustion method (990.03; AOAC, 1990) with an N analyzer (Model CNS-2000; LECO Corp., St. Joseph, MO), and CP was calculated as N × 6.25. Ether extract in samples was determined after hexane extraction (Method 920.39; AOAC, 1990) in an Ankom extraction system (Macedon, NY). Samples were analyzed for minerals (Ca, Na, K, Mg, P, Mn, and Zn) after ashing at 600 °C for 12 h in a muffle furnace. Minerals were determined using inductively coupled plasma mass spectrometry (ICP-AES; Vista, Varian, Palo Alto, CA) according to the method of AOAC (2005, method 985.01).
Table 1.
Ingredient and nutrient compositions of experimental diets (as-fed basis, %, unless otherwise stated)1.
| Item | Production period (days of age) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 to 14 |
14 to 24 |
24 to 36 |
||||||||||
| BAS | BMD | OE | OEOH | BAS | BMD | OE | OEOH | BAS | BMD | OE | OEOH | |
| Ingredients | ||||||||||||
| Corn | 41.3 | 41.2 | 41.3 | 37.9 | 44.3 | 44.2 | 44.3 | 40.9 | 48.5 | 48.4 | 48.4 | 45.1 |
| Soybean meal | 40.2 | 40.2 | 40.2 | 40.6 | 36.5 | 36.5 | 36.5 | 36.9 | 31.5 | 31.5 | 31.5 | 31.9 |
| Wheat | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Oat hulls | – | – | – | 3.00 | – | – | 3.00 | 3.00 | – | – | 3.00 | 3.00 |
| Organic acids-essential oils | – | – | 0.03 | 0.03 | – | – | 0.03 | 0.03 | – | – | 0.03 | 0.03 |
| Animal/Vegetable fat | 3.44 | 3.47 | 3.46 | 3.46 | 4.59 | 4.63 | 4.62 | 4.62 | 5.67 | 5.70 | 5.69 | 5.69 |
| Limestone | 1.80 | 1.80 | 1.80 | 1.79 | 1.65 | 1.65 | 1.65 | 1.64 | 1.52 | 1.52 | 1.52 | 1.51 |
| Dicalcium phosphate | 1.23 | 1.23 | 1.23 | 1.24 | 1.06 | 1.06 | 1.06 | 1.07 | 0.93 | 0.93 | 0.93 | 0.94 |
| DL-Methionine Premix2 | 0.61 | 0.61 | 0.61 | 0.62 | 0.53 | 0.53 | 0.53 | 0.54 | 0.49 | 0.49 | 0.49 | 0.50 |
| Lysine HCl | 0.03 | 0.03 | 0.03 | 0.02 | – | – | – | – | 0.01 | 0.009 | 0.01 | 0.003 |
| Iodized salt | 0.40 | 0.40 | 0.40 | 0.40 | 0.37 | 0.37 | 0.37 | 0.37 | 0.38 | 0.38 | 0.38 | 0.38 |
| Pellet binding agent 3 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| BMD 110 G 4 | – | 0.05 | – | – | – | 0.05 | – | – | – | 0.05 | – | – |
| Vitamin/Mineral Premix 5, | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Calculated composition | ||||||||||||
| Crude protein | 23.0 | 23.0 | 23.0 | 23.0 | 21.5 | 21.5 | 21.5 | 21.5 | 19.5 | 19.5 | 19.5 | 19.5 |
| Metabolizable energy, kcal/kg | 3,000 | 3,000 | 3,000 | 2,909 | 3,100 | 3,100 | 3,100 | 3,009 | 3,200 | 3,200 | 3,200 | 3,109 |
| Calcium | 0.96 | 0.96 | 0.96 | 0.96 | 0.87 | 0.87 | 0.87 | 0.87 | 0.78 | 0.78 | 0.78 | 0.78 |
| Available phosphorus | 0.48 | 0.48 | 0.48 | 0.48 | 0.44 | 0.44 | 0.44 | 0.44 | 0.39 | 0.39 | 0.39 | 0.39 |
| Digestible lysine | 1.28 | 1.28 | 1.28 | 1.28 | 1.16 | 1.16 | 1.17 | 1.17 | 1.02 | 1.02 | 1.03 | 1.03 |
| Digestible methionine + Cystine | 0.95 | 0.95 | 0.95 | 0.95 | 0.87 | 0.87 | 0.87 | 0.87 | 0.80 | 0.80 | 0.80 | 0.80 |
| Sodium | 0.19 | 0.19 | 0.19 | 0.19 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 |
| Analysed composition | ||||||||||||
| Crude protein | 24.6 | 23.9 | 23.0 | 23.3 | 21.8 | 22.1 | 22.4 | 21.5 | 20.4 | 20.2 | 18.9 | 20.0 |
| Calcium | 1.08 | 1.02 | 1.10 | 1.10 | 0.92 | 1.01 | 0.98 | 0.94 | 0.83 | 0.85 | 0.84 | 0.86 |
| Total phosphorus | 0.68 | 0.68 | 0.68 | 0.70 | 0.59 | 0.63 | 0.62 | 0.61 | 0.54 | 0.54 | 0.55 | 0.56 |
| Sodium | 0.20 | 0.19 | 0.19 | 0.19 | 0.19 | 0.18 | 0.15 | 0.17 | 0.18 | 0.19 | 0.16 | 0.18 |
| Crude fat | 5.55 | 5.35 | 5.47 | 5.18 | 6.90 | 6.93 | 6.91 | 6.70 | 7.61 | 7.91 | 8.00 | 8.00 |
BAS, basal diet; BMD, antibiotic diet; OE, diet containing protected organic acids-essential oils mix at 300 g/10,00 kg of feed; OEOH, diet containing protected organic acids-essential oils mix at 300 g/1,000 kg of feed + 3% coarse oat hulls.
Supplied the following per kilogram premix: DL-methionine, 0.5 kg; wheat middlings, 0.5 kg.
Pel-stik: Uniscope, Inc., Johnstown, CO, USA.
Bacitracin methylene disalicylate (providing 55 mg/kg mixed feed); Alpharma, Inc., Fort Lee, NJ, US.
Vitamin-mineral premix contained the following per kilogram of diet: 9,750 IU vitamin A; 2,000 IU vitamin D3; 25 IU vitamin E; 2.97 mg vitamin K; 7.6 mg riboflavin; 13.5 mg DL-Ca-pantothenate; 0.012 mg vitamin B12; 29.7 mg niacin; 1.0 mg folic acid, 801 mg choline; 0.3 mg biotin; 4.9 mg pyridoxine; 2.9 mg thiamine; 70.2 mg manganese; 80.0 mg zinc; 25 mg copper; 0.15 mg selenium; 50 mg ethoxyquin; 1,543 mg wheat middlings; 500 mg ground limestone.
2.3. Sampling and measurements
On d 8 and every week thereafter, body weight (BW) and feed intake (FI) were measured for each pen, and body weight gain (BWG) and feed conversion ratio (FCR) which was corrected for mortality were calculated. On d 36, one chicken was randomly selected from each pen, individually weighed, and euthanized by electrical stunning and exsanguination. Blood samples were collected from the euthanized bird into 5-mL heparinized tubes for the determination of blood biochemistry. The weights of the bursa of Fabricius and spleen and the empty weights of gizzard and ceca were determined by trained personnel. Digesta from the pair of ceca were mixed and divided into 2 subsamples; one part was stored in plastic RNAse, and DNAse-free tubes, placed in liquid nitrogen and kept at −80 °C for analyses of the gut microbiota. The other part was placed in bio-freeze kits (Alimetric Diagnostics, Espoo, Finland) for the determination of SCFA. For intestinal morphology determination, a 0.5 cm tissue section was collected from the middle of the duodenum, jejunum, and ileum and preserved in 10% formalin.
2.3.1. Blood biochemistry analysis
Samples for blood biochemical analysis were centrifuged at 5,000 × g at 4 °C for 10 min and shipped on ice to Atlantic Veterinary College, University of Prince Edward Island Pathology Laboratory, where samples were analyzed using cobas 6,000 analyzer series.
2.3.2. Short-chain fatty acids and bacterial density
Samples were immediately preserved using BioFreeze sampling kits (Alimetrics Diagnostics Ltd., Espoo, Finland) following the recommended protocol by the manufacturer. Bacterial density and SCFA profiles were analyzed by Alimetrics Diagnostics Ltd. as described previously by Adewole (2020) and Apajalahti et al. (2019).
2.3.3. Gut morphology
Intestinal samples (duodenum, jejunum, and ileum) recovered from 8 birds per treatment were subjected to gut morphological processing using the “I See Inside” (ISI) histology methodology (Belote et al., 2018), adapted from Kraeski et al. (2017). The ISI methodology is based on a numeric score of histological alterations. For each macroscopic and microscopic alteration observed, an impact factor (IF) was defined according to the reduction of functional organ capacity, based on knowledge from the literature and background research. The IF ranges from 1 to 3, with 3 being the most impacting to organ function. The parameters evaluated by the ISI methodology were: epithelial thickness, proliferation of enterocytes, and increase of goblet cells The extent of each lesion (intensity) or the observed frequency compared to the non-affected organ was evaluated in each organ/tissue with score (S) ranging from 0 to 3: score 0 (absence of lesion or frequency), score 1 (alteration up to 25% of the area or observed frequency), score 2 (alteration ranges from 25% to 50% of the area or observed frequency), and score 3 (alteration extends to more than 50% of the area or observed frequency). To obtain the final value of the ISI index, the IF of each alteration was multiplied by the respective score number, and the results of all alterations were summed according to the formula ISI = Σ(IF × S), where IF is the impact factor, and S is the score. Additionally, other morphometric measurements including villus height (from the base of the intestinal mucosa to the tip of the villus excluding the intestinal crypt), crypt depth (from the base upward to the region of transition between the crypt and villi), and villus height-to-crypt depth ratio were evaluated (Ozdogan et al., 2014).
2.3.4. DNA extraction, PCR, sequencing, sequence processing, and quality control
Microbial DNA extraction was performed using a MoBio PowerMag Soil DNA Isolation Bead Plate, following MoBio's instructions on a KingFisher robot. Bacterial 16S rRNA genes sequencing, processing, and quality control were performed as described previously by Adewole (2020).
2.3.5. Bioinformatics analysis
The analytical flowchart for bioinformatics analysis of cecal microbiota data is presented in Appendix Fig. 1. Amplicons were sequenced with an Illumina MiSeq using the 300-bp paired-end kit (v.3). Sequences were denoised, taxonomically classified using Greengenes (v. 13_8) as the reference database, and clustered into 97% similarity operational taxonomic units (OTU) with the mothur software package (v. 1.39.5) (Schloss et al., 2009), following the recommended procedure (https://www.mothur.org/wiki/MiSeq_SOP; accessed Nov 2017). The potential for contamination was addressed by co-sequencing DNA amplified from specimens and from 3 each of template-free controls and extraction kit reagents processed the same way as the specimens. Operational taxonomic units were considered putative contaminants (and were removed) if their mean abundance in controls reached or exceeded 25% of their mean abundance in specimens. Alpha diversity was estimated with the Shannon index on raw OTU abundance tables after filtering out contaminants. The significance of diversity differences was tested by analysis of variance (ANOVA). To estimate beta diversity across samples, we excluded OTU occurring with a count of less than 3 in at least 10% of the samples and then computed Bray–Curtis indices. We visualized beta diversity, emphasizing differences across samples, using principal coordinate analysis (PCoA) ordination. Variation in community structure was assessed with permutational multivariate analyses of variance (PERMANOVA) with treatment group as the main fixed factor and using 9,999 permutations for significance testing. Statistical analysis on cecal microbiota data was conducted in the R environment.
2.4. Statistical analysis
One-way ANOVA was carried out using the mixed procedure of SAS with treatments (BAS, BMD, OE, or OEOH) as factor and the following parameters as variables: feed intake, body weight, body weight gain, feed conversion ratio, concentrations of total SCFA, acetic acid, propionic acid, butyric acid, valeric acid, lactic acid, branched chained fatty acids (BCFA), volatile fatty acids (VFA), and blood chemistry parameters. Normal probability plot of residuals was used to ascertain the normality of all datasets in the same statistical package. Datasets found to be non-normal were log base 10 transformed. Lipase dataset was inverse transformed, whereas the total bilirubin dataset failed to achieve normality with transformation. Hence a non-parametric Kruskal–Wallis test was used to test the effect of treatment on levels of blood total bilirubin acid. Following ANOVA, differences between significant means were tested using Tukey's honest significant difference (HSD) test in the same statistical package. Parametric gut morphology data were analyzed by one-way ANOVA, while non-parametric data were analyzed by Mood's median test (Mangiafico, 2016), in the same statistical package. Analyzed data are presented as means, standard error of the mean (SEM), and probability values. Values were considered statistically different at P < 0.05.
3. Results
3.1. Growth performance
The effect of all dietary treatments on the growth performance of birds is presented in Table 2. No effect of dietary treatment on FI was recorded in this study, across all phases. In the starter phase, the BWG of birds fed the BAS and BMD were not different, but they each recorded higher (P < 0.001) BWG than birds fed the OE and OEOH diets. In the grower phase, birds fed the BMD diet had the highest BWG and was significantly (P < 0.05) different from birds fed the OEOH diet; birds fed the BAS and OE diets had intermediate BWG. In the finisher phase, BWG was not affected by dietary treatments. Over the entire trial period, birds offered the BMD diet recorded the highest BWG, which was significantly higher (P = 0.01) than birds in the OEOH treatment. Birds offered the BAS, and OE treatments retained intermediate BWG values at the end of the trial. Dietary treatments recorded no significant effect on FCR in this study throughout all phases. Nonetheless, at the end of the trial period, dietary treatment OE had numerically better FCR than the other treatments.
Table 2.
Effect of a protected organic acids-essential oils blend with or without oat hulls on growth performance of broiler chickens.
| Item | Treatments1 |
SEM2 | P-value | |||
|---|---|---|---|---|---|---|
| BAS | BMD | OE | OEOH | |||
| Feed intake, g/bird | ||||||
| Starter (d 1 to 14) | 542 | 548 | 522 | 519 | 10.64 | 0.1725 |
| Grower (d 14 to 28) | 1,890 | 1,857 | 1,762 | 1,768 | 40.89 | 0.0837 |
| Finisher (d 28 to 36) | 1,165 | 1,153 | 1,148 | 1,156 | 31.44 | 0.9839 |
| Overall (d 1 to 36) | 3,585 | 3,587 | 3,438 | 3,411 | 88.73 | 0.3779 |
| Body weight gain, g/bird | ||||||
| Starter (d 1 to 14) | 447a | 455a | 418b | 413b | 7.206 | 0.0004 |
| Grower (d 14 to 28) | 1,168ab | 1,177a | 1,118ab | 1,088b | 21.92 | 0.0228 |
| Finisher (d 28 to 36) | 817 | 828 | 836 | 802 | 27.59 | 0.8378 |
| Overall (d 1 to 36) | 2,449ab | 2,460a | 2,371ab | 2,303b | 35.14 | 0.0134 |
| Feed conversion ratio | ||||||
| Starter (d 1 to 14) | 1.21 | 1.21 | 1.23 | 1.24 | 0.017 | 0.3373 |
| Grower (d 14 to 28) | 1.62 | 1.58 | 1.58 | 1.63 | 0.030 | 0.5159 |
| Finisher (d 28 to 36) | 1.44 | 1.40 | 1.40 | 1.45 | 0.064 | 0.8830 |
| Overall (d 1 to 36) | 1.50 | 1.46 | 1.45 | 1.46 | 0.028 | 0.5855 |
a, b In a row, means assigned different lowercase letters are significantly different, P < 0.05 (Tukey's procedure).
BAS, basal diet; BMD, antibiotic diet; OE, diet containing 300 g of protected organic acids-essential oils blend/1,000 kg of feed; OEOH, diet containing 300 g of protected organic acids-essential oils blend/1,000 kg of feed +3% coarse oat hulls.
Standard error of means.
3.2. Organ weights
Of all relative organ (bursa, spleen, gizzard, and ceca) weights and bird slaughter weight examined in this study, only the relative empty weight of gizzard was affected by dietary treatments (Table 3). Birds fed the OEOH treatment had significantly higher (P < 0.001) gizzard weight compared to other dietary treatments. Birds fed the BMD diet showed a tendency (P = 0.08) to have higher cecal weight compared to other treatments.
Table 3.
Effect of a protected organic acids-essential oils blend with or without oat hulls on relative organ weights of broiler chickens (% BW, unless otherwise stated).
| Item | Treatments1 |
SEM2 | P-value | |||
|---|---|---|---|---|---|---|
| BAS | BMD | OE | OEOH | |||
| Slaughter weight, kg | 2.80 | 2.82 | 2.75 | 2.63 | 0.080 | 0.3559 |
| Bursa | 0.18 | 0.17 | 0.18 | 0.20 | 0.016 | 0.6352 |
| Spleen | 0.10 | 0.09 | 0.10 | 0.08 | 0.008 | 0.2431 |
| Gizzard3 | 1.17b | 1.17b | 1.19b | 1.67a | 0.073 | <0.0001 |
| Ceca4 | 0.36 | 0.42 | 0.39 | 0.34 | 0.024 | 0.0765 |
a, bIn a row, means assigned different lowercase letters are significantly different, P < 0.05 (Tukey's procedure).
BAS, basal diet; BMD, antibiotic diet; OE, diet containing 300 g of protected organic acids-essential oils blend/1,000 kg of feed; OEOH, diet containing 300 g of protected organic acids-essential oils blend/1,000 kg of feed +3% coarse oat hulls.
Standard error of means.
Empty weight of gizzard.
Empty weight of ceca.
3.3. Ceca short-chain fatty acid concentration
Dietary treatments had no significant effect on SCFA concentrations in this study (Table 4). Nonetheless, birds of the OEOH treatment recorded the highest concentrations of acetic acid, butyric acid, lactic acid, total SCFA, and volatile fatty acids (VFA). Concentrations of propionic and valeric acid were numerically higher in the BMD treatment, compared to the other groups, and BCFA concentrations were found highest in the BAS treatment. No effect of dietary treatment was also recorded for Total Eubacteria (Table 4).
Table 4.
Effect of a protected organic acids-essential oils blend with or without oat hulls on cecal short chain fatty acids (mmol/kg) and total eubacteria (16S rDNA copies/gram of sample) of broiler chickens.
| Item | Treatments1 |
SEM2 | P-value | |||
|---|---|---|---|---|---|---|
| BAS | BMD | OE | OEOH | |||
| Acetic acid | 51.5 | 51.1 | 54.9 | 59.5 | 5.622 | 0.6946 |
| Propionic acid | 4.29 | 5.26 | 4.24 | 3.87 | 0.459 | 0.2071 |
| Butyric acid | 12.2 | 12.3 | 12.1 | 12.8 | 1.466 | 0.9880 |
| Valeric acid | 0.94 | 1.06 | 0.98 | 0.81 | 0.087 | 0.2589 |
| Lactic acid | 3.00 | 1.58 | 2.98 | 3.05 | 0.914 | 0.3812 |
| Total SCFA | 74.2 | 71.3 | 77.2 | 80.3 | 7.299 | 0.8395 |
| BCFA | 2.23 | 1.78 | 1.78 | 1.85 | 0.262 | 0.5731 |
| VFA | 71.2 | 69.8 | 74.2 | 77.2 | 7.325 | 0.8905 |
| Total eubacteria | 12.0 | 11.9 | 11.8 | 12.0 | 0.019 | 0.6632 |
SCFA = short chain fatty acids; BCFA = branched chain fatty acids; VFA = volatile fatty acids.
BAS, basal diet; BMD, antibiotic diet; OE, diet containing 300 g of protected organic acids-essential oils blend/1,000 kg of feed; OEOH, diet containing 300 g of protected organic acids-essential oils blend/1,000 kg of feed +3% coarse oat hulls.
Standard error of means.
3.4. Blood parameters
The effect of dietary treatments on blood biochemical components is presented in Table 5. Birds in the OE treatment recorded the lowest levels of urea (P = 0.05) and cholesterol (P < 0.05), relative to other treatments. The levels of blood enzymes and proteins were not significantly affected by dietary treatments in this study. However, blood amylase levels in the OE treatment was at least 10% higher (P > 0.05) than the other treatments.
Table 5.
Effect of a protected organic acids-essential oils blend with or without oat hulls on blood biochemical components of broiler chickens.
| Item | Treatments1 |
SEM2 | P-value | |||
|---|---|---|---|---|---|---|
| BAS | BMD | OE | OEOH | |||
| Electrolytes and minerals, mmol/L | ||||||
| Sodium | 152 | 154 | 162 | 169 | 9.97 | 0.5941 |
| Potassium | 5.76 | 5.09 | 5.61 | 5.95 | 0.31 | 0.2572 |
| Chloride | 111 | 112 | 119 | 124 | 8.47 | 0.6493 |
| Calcium | 2.68 | 2.72 | 2.75 | 2.94 | 0.14 | 0.6026 |
| Phosphorus | 1.77 | 1.59 | 1.74 | 1.77 | 0.13 | 0.7706 |
| Magnesium | 0.83 | 0.82 | 0.73 | 0.82 | 0.06 | 0.6080 |
| Metabolites, mmol/L | ||||||
| Urea | 0.34a | 0.26ab | 0.24b | 0.29a | 0.03 | 0.0473 |
| Creatinine | 2.00 | 2.75 | 1.13 | 3.13 | 1.14 | 0.6198 |
| Glucose | 14.7 | 15.1 | 17.8 | 17.3 | 1.15 | 0.1572 |
| Cholesterol | 2.94ab | 3.41a | 2.46b | 3.29a | 0.25 | 0.0205 |
| Iron | 17.9 | 23.0 | 21.9 | 16.4 | 2.35 | 0.1685 |
| Bile acids | 17.0 | 14.0 | 18.3 | 18.3 | 3.29 | 0.7734 |
| Uric acid | 401 | 347 | 347 | 372 | 49.6 | 0.9367 |
| Total Bilirubin | 0.63 | 0.38 | 0.25 | 0.37 | 0.18 | 0.5163 |
| Enzymes, U/L | ||||||
| Amylase | 410 | 483 | 654 | 586 | 144 | 0.4234 |
| Alkaline phosphatase | 3243 | 2722 | 2972 | 3303 | 634 | 0.7807 |
| Creatine kinase | 19,820 | 19,784 | 10,917 | 19,670 | 4,011 | 0.3819 |
| Aspartate aminotransferase | 324 | 308 | 225 | 320 | 36.6 | 0.1255 |
| Gamma-glutamyl transferase | 10.0 | 11.9 | 10.5 | 14.0 | 1.57 | 0.2933 |
| Lipase | 22.0 | 17.8 | 20.0 | 20.3 | 4.15 | 0.9113 |
| Proteins, g/L | ||||||
| Total protein | 28.0 | 28.4 | 24.3 | 31.5 | 2.30 | 0.1981 |
| Albumin | 12.0 | 12.1 | 10.6 | 13.4 | 1.02 | 0.3180 |
| Globulin | 16.0 | 16.3 | 13.6 | 18.1 | 1.38 | 0.1704 |
a, b In a row, means assigned different lowercase letters are significantly different, P < 0.05 (Tukey's procedure).
BAS, basal diet; BMD, antibiotic diet; OE, diet containing 300 g of protected organic acids-essential oils blend/1,000 kg of feed; OEOH, diet containing 300 g of protected organic acids-essential oils blend/1,000 kg of feed +3% coarse oat hulls.
Standard error of means.
3.5. Gut morphology
Gut morphometric properties and intestinal health indicators in the three segments of the small intestine (duodenum, jejunum, and ileum) are presented in Table 6. In the duodenum, the antibiotic treatment recorded the lowest (P < 0.001) duodenal villus length, with other treatments been statistically similar. Duodenal crypt depth of birds in the OEOH treatment was the most shallow (P < 0.05) and was 18% shallower than those of the OE treatment. Similarly, the duodenal villus height-to-crypt depth ratio in the OEOH treatment was 23% higher (P < 0.05) than that of the antibiotic treatment, whereas other treatments expressed statistical similarity. In terms of duodenum health index, ISI total scores were not significantly different between treatments, even though OEOH treatment recorded a higher (P < 0.05) increase in goblet cells relative to other treatments. Birds in the OE treatment had the highest (P < 0.001) villus length and villus height-to-crypt depth ratio in the jejunum, compared to other treatments with respectively 44% and 46% improvement by comparison to the BAS treatment. Although crypt depth in the jejunum was not significant between treatments, crypt depth in the OEOH treatment was the most shallow (P > 0.05). Compared to other treatments, antibiotic treatment recorded numerically higher ISI total scores, attributable to significantly increased (P < 0.05) goblet cells; nonetheless, increased goblet cells were statistically similar across treatments. In the ileum, OEOH treatment recorded the highest (P < 0.001) villus height and villus height-to-crypt depth ratio, while crypt depth was found deepest (P < 0.05) in the BAS treatment, relative to the BMD and OE treatments. Birds in the OEOH treatment had intermediate crypt depth. Additionally, ileum ISI total scores were significantly higher (P < 0.001) in the OE treatment, relative to the BMD and the OEOH treatment; the BAS treatment had intermediate ISI total scores.
Table 6.
Effect of a protected organic acids-essential oils blend with or without oat hulls on intestinal morphology in broiler chickens (data not having units are scores).
| Item | Treatments1 |
SEM | P-value | |||
|---|---|---|---|---|---|---|
| BAS | BMD | OE | OEOH | |||
| Duodenum | ||||||
| Epithelial thickness | 2 | 2 | 2 | 2 | 0.05 | 0.3988 |
| Proliferation of enterocytes | 2 | 2 | 2 | 2 | 0.05 | 0.1541 |
| Increase of goblet cells | 0b | 0b | 0b | 2a | 0.07 | 0.0020 |
| ISI total score | 11.4 | 11.5 | 11.7 | 11.5 | 0.24 | 0.9675 |
| Villus height, μm | 2,317a | 1,964b | 2,328a | 2,245a | 28.8 | 0.0000 |
| Crypt depth, μm | 113ab | 103ab | 119a | 97.9b | 1.03 | 0.0245 |
| Villus height:Crypt depth | 22.3ab | 19.4b | 21.7ab | 23.8a | 0.50 | 0.0215 |
| Jejunum | ||||||
| Epithelial thickness | 1 | 1 | 1 | 1 | 0.03 | 0.1089 |
| Proliferation of enterocytes | 1 | 1 | 1 | 1 | 0.03 | 0.8709 |
| Increase of goblet cells | 0b | 2a | 2a | 0b | 0.07 | 0.0240 |
| ISI total score | 5.80 | 7.19 | 6.58 | 6.13 | 0.22 | 0.1393 |
| Villus height, μm | 1,021b | 1,162b | 1,474a | 1,028b | 23.3 | 0.0000 |
| Crypt depth, μm | 105 | 99.6 | 105 | 94.2 | 1.96 | 0.1422 |
| Villus height:Crypt depth | 10.6b | 12.6b | 15.5a | 12.4b | 0.34 | 0.0000 |
| Ileum | ||||||
| Epithelial thickness | 1 | 1 | 1 | 1 | 0.03 | 0.1235 |
| Proliferation of enterocytes | 1 | 1 | 1 | 1 | 0.03 | 0.1073 |
| Increase of goblet cells | 0 | 0 | 0 | 0 | 0.06 | 0.4381 |
| ISI total score | 5.28ab | 4.35b | 6.60a | 4.90b | 0.42 | 0.0000 |
| Villus height, μm | 1,247b | 970c | 928c | 1,588a | 25.2 | 0.0000 |
| Crypt depth, μm | 117a | 101b | 98.9b | 113ab | 2.05 | 0.0028 |
| Villus height:Crypt depth | 11.5b | 10.5b | 9.8b | 15.4a | 0.29 | 0.0000 |
SEM = standard error of the mean; ISI = I see inside.
a, b In a row, means assigned different lowercase letters are significantly different, P < 0.05 (Tukey's procedure).
BAS, basal diet; BMD, antibiotic diet; OE, diet containing 300 g of protected organic acids-essential oils mix/1,000 kg of feed; OEOH, diet containing 300 g of protected organic acids-essential oils mix/1,000 kg of feed +3% coarse oat hulls.
3.6. Gut microbiota
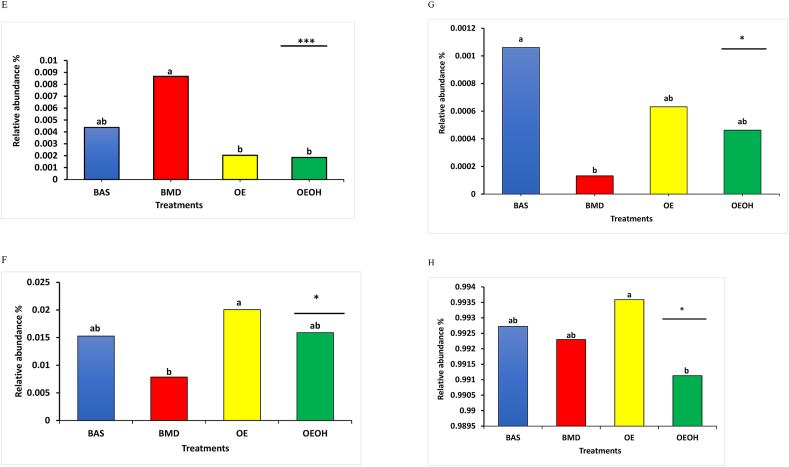
Datasets from bioinformatic analysis of cecal samples revealed 53,470 OTU at an average of 14, 285 quality-filtered reads per sample at the 97% similarity level. More information on sequence quality is presented in Appendix Fig. 2A and B. The relative abundance of the most abundant taxa are presented in Fig. 1, Fig. 2 and Appendix Figs. 3–6. Firmicutes was the most abundant (>85%) phylum across all treatment groups, and Lachnospiraceae was the most abundant family (>65%) across treatment groups. Phylum level analysis showed that treatment affected (P < 0.05) the relative abundance of Tenericutes but not the relative abundances of other bacterial phyla on d 36 (Fig. 1 B). At the genus level, the relative abundance of Firmicutes_unclassified, RF39_unclassified, Ruminococcus, Turicibacter, Erysipelotrichaceae_unclassified, and g__SMB53 general were all enhanced (P < 0.05) by OE and OEOH treatments (Fig. 2). This relative abundance was statistically similar to those of the BMD treatment, in most cases. The OE treatment increased (P < 0.05) the relative abundance of Streptococcus; however, the relative abundance of Coprobacillus was reduced (P < 0.001) in both OE and OEOH treatments (Fig. 2E and H).
Fig. 1.
Relative abundance of ceca microbial composition. (A) of the main bacteria phyla, and (B) phylum Tenericutes across the different treatments. Treatments include - BAS, basal diet; BMD, antibiotic diet; OE, diet containing 300 g of protected organic acids-essential oils blend/1,000 kg of feed; OEOH, diet containing 300 g of protected organic acids-essential oils blend/1,000 kg of feed +3% coarse oat hulls. The top of each graph assigned different lowercase letters (a,b) are significantly different. Treatment comparisons reported with (∗) indicate significance at the level P < 0.05.
Fig. 2.
Relative abundance of ceca microbial composition. (A) At the genus level, (B) comparison of the relative abundance of Firmicutes_unclassified, (C) comparison of the relative abundance of Turicibacter, (D) comparison of the relative abundance of g_SMB53, (E) comparison of the relative abundance of Coprobacillus, (F) comparison of the relative abundance of RF39_unclassified, (G) comparison of the relative abundance of Erysipelotrichaceae, and (H) comparison of the relative abundance of Streptococcus across the different treatments. Treatments include: BAS, basal diet; BMD, antibiotic diet; OE, diet containing 300 g of protected organic acids-essential oils blend/1,000 kg of feed; OEOH, diet containing 300 g of protected organic acids-essential oils blend/1,000 kg of feed +3% coarse oat hulls. The top of each graph assigned different lowercase letters (a, b) are significantly different. Treatment comparisons reported with (∗) and (∗∗∗) indicate significance at the level P < 0.05 and P < 0.001, respectively. Treatment comparisons reported with P = 0.07 indicate a statistical trend.
Alpha diversity, a measure of species richness and evenness expressed as the number of observed OTU by Shannon diversity Index, showed no significant difference between treatments (Fig. 3). Nonetheless, BAS had the highest Shannon index, 5.889 ± 0.37 (Shannon mean ± Shannon standard deviation), while the BMD treatment recorded the lowest Shannon index, 5.453 ± 0.84. The similarity between the cecal microbiota composition, based on the Bray–Curtis dissimilarities index, is graphically presented in a PCoA ordination plot (Fig. 4). Permutational analysis of variance determined significant differences (P < 0.05) in beta-diversity among treatments. Post-hoc pairwise contrast among treatments revealed significant differences and tendencies between treatments: BAS vs. OE (P = 0.05), BAS vs. OEOH (P = 0.06), and OE vs. OEOH (P = 0.06). Differential abundance testing revealed 13 differentially abundant OTU at the species level among treatments (Table 7). In the BAS treatment, species belonging to unclassified Tenericutes RF39 and unclassified Firmicutes were differentially abundant relative to other treatments. Unclassified Ruminococcaceae and Turicibacte species were differentially abundant in the BMD treatment. The OE treatments were differentially comprised of unclassified Clostridiales and Firmicutes species. A host of unclassified Firmicutes and other unclassified bacteria species were also found differentially abundant in the OEOH treatment group.
Fig. 3.
ANOVA determined no significant differences in the Shannon diversity index (P > 0.05; F value = 0.723). Cecal content was collected from 36-day-old broiler chickens. Treatments include: BAS, basal diet; BMD, antibiotic diet; OE, diet containing 300 g of protected organic acids-essential oils blend/1,000 kg of feed; OEOH, diet containing 300 g of protected organic acids-essential oils blend/1,000 kg of feed +3% coarse oat hulls.
Fig. 4.
Permutational analysis of variance (Permanova) determined significant differences (P < 0.05; R-squared = 0.1463) in beta-diversity among treatments. BAS, basal diet; BMD, antibiotic diet; OE, diet containing 300 g of protected organic acids-essential oils blend/1,000 kg of feed; OEOH, diet containing 300 g of protected organic acids-essential oils blend/1,000 kg of feed +3% coarse oat hulls. PCoA = principal coordinate analysis.
Table 7.
Effect of a protected organic acids-essential oils blend with or without oat hulls on differentially abundant bacterial species (Normalized OTU counts) between treatment groups (MetagenomeSeq, P < 0.05)1.
| Treatments2 |
P-value | |||
|---|---|---|---|---|
| BAS | BMD | OE | OEOH | |
| Firmicutes_unclassified | 0.000 | |||
| Tenericutes RF39_unclassified | 0.000 | |||
| Ruminococcaceae_unclassified | 0.000 | |||
| Bacteria_unclassified | 0.000 | |||
| Firmicutes_unclassified | 0.000 | |||
| Turicibacter_unclassified | 0.000 | |||
| Clostridiales_unclassified | 0.000 | |||
| Bacteria_unclassified | 0.000 | |||
| Bacteria _unclassified | 0.000 | |||
| Firmicutes_unclassified | 0.000 | |||
| Clostridiales_unclassified | 0.001 | |||
| Bacteria_unclassified | 0.001 | |||
| Firmicutes_unclassified | 0.002 | |||
OTU = operational taxonomic units.
Significance was set at P < 0.05.
BAS, basal diet; BMD, antibiotic diet; OE, diet containing 300 g of protected organic acids-essential oils blend/1,000 kg of feed; OEOH, diet containing 300 g of protected organic acids-essential oils blend/1,000 kg of feed +3% coarse oat hulls.
4. Discussion
Several independent studies have reported the beneficial effects of organic acids (Paul et al., 2007; Adil et al., 2010; Kamal and Ragaa, 2014), essential oils (Brenes and Roura, 2010; Franz et al., 2010), and dietary fibers (specifically moderate levels of OH) (Hetland and Svihus, 2001; Mateos et al., 2012; Scholey et al., 2020) in poultry nutrition. Here, we present for the first time the synergistic effect of a protected organic acids and essential oils formula (containing organic acids: fumaric, sorbic, malic, citric acids; and essential oils: thymol, vanillin, and eugenol) with or without OH in broiler chickens.
At the end of this trial (d 36), BWG was observed to be similar between the BAS, BMD, and the OE treatment, despite the reported growth-enhancing effect of antibiotics (Butaye et al., 2003; Castanon, 2007; Gadde et al., 2017). However, birds offered the BMD treatment had 6.4% higher BWG compared to those in the OEOH treatment in the same time period. The reduction in BWG in the OEOH treatment was unsurprising, as the OEOH treatment had a low metabolizable energy value (almost 100 kcal lower than the other treatments). Additionally, no effect of dietary treatment on feed intake was recorded in this study at all stages of the trial. Conversely, Garcia et al. (2019) showed that chickens would normally increase feed intake to compensate for the low dietary energy provided by high fiber diets. Nonetheless, Adewole et al. (2020), as in this study, has shown that a 3% OH inclusion level may not be sufficient enough to elicit a significant effect on feed intake. Regardless of the low metabolizable energy values, the OEOH treatment had no negative effect on FCR, throughout the study. Varying effects of OE and OH supplementation on growth performance are reported in the literature. Organic acids and essential oils blends have been reported to elicit superior growth performance similar to antibiotics in broiler chickens (Spais et al., 2002; Liu et al., 2017; Stefanello et al., 2020), turkey poults (Giannenas et al., 2014), and weaned piglets (Yang et al., 2019). Conversely, Wang et al. (2019) observed no effect of encapsulated organic acids and essential oils blend on body weight, mortality, average daily egg weight, and feed-to-egg ratio in laying hens, but observed a significant increase in resistance to mechanical stress for eggs as well as improvements in intestinal tract morphology and counts of bifidobacteria in cecal digesta. With respect to OH inclusion, Jiménez-Moreno et al. (2016) reported improved average daily gain and feed-to-gain ratio in broilers offered either of oat hulls, rice hulls, or sunflower hulls at 2.5% or 5% inclusion levels. Garcia et al. (2019) recently observed no effect of 3% OH feeding on growth performance in broiler chickens. The varying performance effects observed from blends of organic acids and essential oils and OH supplementation across studies can be attributable to several factors, including organic acids and essential oils mix types and doses (e.g., free or protected blends, protection type, nature of essential oils and acids, the formula of the mix, etc.), the dietary composition of basal diets, age of the bird, management and environmental conditions (Zeng et al., 2015), as well as the type and level of dietary fiber and the genetic potential of the bird (Jiménez-Moreno et al., 2016).
With regards to the relative weight of assessed organs in this study, birds in the OEOH treatment recorded the highest gizzard weight, while birds of the BMD treatment tended to have higher ceca weight. No effect of dietary treatments was found for the relative weight of the bursa and spleen. This result reaffirms a recent report from our laboratory, which revealed that the delivery of free choice OH to broiler chickens significantly improved the relative weight of the gizzard (Adewole et al., 2020). Consistent with results from the current study, several studies with similar or higher OH inclusion levels have reported an increase in the relative weight of gizzard in broilers and pullets (Hetland et al., 2003; González-Alvarado et al., 2008, González-Alvarado et al., 2010; García et al., 2019). An enlarged gizzard positively influences nutrient digestibility by increasing feed retention time, feed grinding rigor, digestive enzyme secretion, and feed-enzyme contact time (Amerah et al., 2007; González-Alvarado et al., 2008; Svihus, 2011; Scholey et al., 2020). It is not entirely clear why the potentially enhanced nutrient digestibility associated with the OEOH treatment did not translate into a significant growth performance effect in this study. However, it is most likely that birds in this treatment did not consume enough feed to meet their nutritional requirement, which could have resulted in a lower BWG. The propensity of antibiotic treatment to increase ceca weight is not surprising, as intestinal and ceca enlargement are often consequences of selective antimicrobial inhibition (Franti et al., 1972; Tayeri et al., 2018). Increased ceca weight is associated with increased ceca fermentation activity and microbial activity (Oyarzabal and Conner, 1996; Khaksar et al., 2008). The lack of dietary treatment effect on the relative weight of lymphoid organs (bursa and spleen) observed in this study could be attributed to the controlled experimental conditions in which birds were raised. Hence, there was no strain on their immune system. Dietary organic acids and essentials oils blends delivered in challenge trials have yielded better immune responses (Emami et al., 2017).
Asides bird management and pathological condition, diet has been shown to influence gut morphometric properties in poultry birds (Cowieson et al., 2017). In the current study, both OE and OEOH treatments exerted beneficial effects on the gut morphology of assessed birds. Birds in the OEOH treatment had significantly reduced duodenal crypt depth and significantly increased villus height and villus height-to-crypt depth ratio in the ileum. Birds in the OE treatment had significantly increased villus height and villus height-to-crypt depth ratio in the jejunum. Although the jejunum has been described as the main site of intestinal absorption (Zeinali et al., 2017), the ileum has also been reported to play a substantial role in starch digestion and absorption, especially in fast-growing broiler chickens (Svihus, 2014). A deeper crypt is often indicative of a higher rate of enterocyte cell renewal (Ząbek et al., 2020) and faster tissue turnover (Berrocoso et al., 2017), which results in an increased nutrient requirement for intestinal maintenance and, ultimately, poor bird performance. Also, increased villus height and villus height-to-crypt depth ratio is indicative of increased epithelial cell turnover and a well-differentiated intestinal mucosa, suggesting increased digestive and absorptive capacity (Fan et al., 1997; Jeurissen et al., 2002). In agreement with our results, other studies have reported that both essential oil and dietary fibers reduced duodenal crypt depth and increased villus height and villus height-to-crypt depth ratio in the ileum (Sarikhan et al., 2010; Basmacioğlu-Malayoğlu et al., 2016; He et al., 2017; Masouri et al., 2017). Additionally, Giannenas et al. (2018) have shown that feeding broilers herbal additives could increase villus height, especially in the jejunum. The active ingredients of most essential oils are reported to stimulate the secretion of endogenous digestive enzymes and balance the gut microbial ecosystem by pathogen exclusion through competition for epithelial binding sites (Chiang et al., 2010; Ghazanfari et al., 2014), hence contributing to the observed positive effect on gut morphology. With regards to dietary fibers, their type, inclusion levels, and physiochemical properties dictate their influence on gut morphology (Montagne et al., 2003). Insoluble fibers, as in this study, often results in enhanced gut morphometric properties (Rezaei et al., 2011; Rahmatnejad and Saki, 2016). Furthermore, in this study, both OEOH and OE significantly increased the number of goblet cells produced in the duodenum and jejunum, respectively. This is consistent with the results of Jamroz et al. (2006), Reisinger et al. (2011), and Rezaei et al. (2011), who all observed an increased number of goblet cells when birds were offered different essential oil blends and dietary insoluble fibers, respectively. Goblet cells are major secretory cells responsible for mucin production (McCauley and Guasch, 2015). Mucin produced by the goblet cells serves several essential functions in the gut, including lubricating intestinal surfaces, trapping and neutralizing bacteria, detoxifying heavy metal binding, interacting with the intestinal immune system, and acting as a diffusion barrier for nutrients and macromolecules (Forstner and Forstner, 1994). Additionally, glycoproteins secreted by goblet cells can also prevent pathogens and other harmful substances from contacting the intestinal epithelial cells (Round et al., 2012). Despite these benefits, the proliferation of goblet cells has been associated with inflammation (Sanches, 2019). Birds with significantly increased goblet cell numbers may have increased capacity to provide more mucin layer protective functions in the gut. Furthermore, according to the ISI histology methodology utilized in this study, the OE and the BAS (negative control) treatments had statistically similar and significant ISI total scores in the ileum, relative to other treatments. The ISI methodology is expected to improve on the deficiencies associated with current linear measures of gut villi and crypts dimensions, hence providing an adequate measure of gut mucosa status. Although lower ISI total scores have been described as indicators of better intestinal health (Cardinal et al., 2019), it is possible that the higher ISI total scores observed in the BAS and OE treatments are indicative of constant basal intestinal inflammatory and proliferative responses. This is normal even in control birds, as in this study, raised under clean experimental conditions (Sanches, 2019). This basal enteritis effect is further reaffirmed by the uncompromised growth performance results obtained in these treatments relative to other treatments.
Bacterial fermentation of dietary fibers in the ceca often results in SCFA production. These SCFA play essential roles in maintaining intestinal functionality and integrity (Meimandipour et al., 2010). In this study, dietary treatments had no significant effect on SCFA concentrations. Nonetheless, OEOH treatment numerically increased concentrations of total SCFA, VFA, acetic acid, butyric acid, and lactic acid. This is consistent with our previous study (Adewole et al., 2020), which reported no significant effect of 3% OH on SCFA concentration in broiler chickens. Contrarily; Leung et al. (2018) recorded higher acetic acid and total SCFA concentration in broiler breeder hens fed OH (33% of diets), relative to the control diet. SCFA concentrations in birds are affected by several factors including, fermentation site (which changes with age), dietary fiber type and amount, microbiota composition, and the age of the bird (Meimandipour et al., 2011; Walugembe et al., 2015; Sun et al., 2020).
The OE treatment exerted a hypocholesterolemic effect in broiler chickens in this study. The cholesterol-lowering effect of essential oils and dietary acidifiers are well-substantiated in the literature (Elson et al., 1989; Craig, 1999; Abdo, 2004; Essa Al-Mashhadani et al., 2011; Özek et al., 2011; Kamal and Ragaa, 2014; Ali, 2016; Matty and Hassan, 2020). This hypocholesterolemic effect is associated with the inhibition of the hepatic 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase activity, a key regulatory enzyme involved in cholesterol synthesis (Qureshi et al., 1988; Lee et al., 2004). Additionally, the OE diet significantly reduced plasma urea levels in the current study. Urea is a protein metabolite, and its level in the blood indicates the immune status of the bird. Increased blood urea levels are often correlated with toxic or disease stress conditions in birds (Tekce and Gül, 2017). Despite the limited report on urea levels in broiler chickens in the literature, both essential oil and organic acids have been reported to reduce serum levels of biogenic urea (Abdel-Fattah et al., 2008; Windisch et al., 2008; Tekce and Gül, 2017), which are in agreement with the results of the current study. This result could imply enhanced protein, and amino acid utilization in birds fed the OE diet, as urea are products of protein metabolism (Kamal and Ragaa, 2014). Furthermore, blood electrolytes and mineral levels were within the normal physiological range for broiler chickens (Clinical Diagnostic Division, 1990) and were not affected by dietary treatments. Similarly, levels of evaluated blood enzymes in this study were also not affected by dietary treatments. These enzymes are indicative of the bird's liver function (Sun et al., 2019). Our results on evaluated blood enzymes and blood proteins are indicative of the healthy physiological state of all birds, as values were in the range of published values for healthy broiler chickens (Ilo et al., 2019).
It is becoming increasingly evident that gut microbiota is an important determinant of host physiology and health status (Sekirov et al., 2010; Nielsen et al., 2013). Cecal microbiota profile was majorly (>85%) dominated by Firmicutes, Proteobacteria, Tenericutes, and Actinobacteria at the phylum level, in this study (Fig. 1 A). Similar to antibiotic treatment, OEOH treatment reduced the relative abundance of Tenericutes compared to the OE treatment (Fig. 1 B). In agreement with our results, Das et al. (2020) have recently reported the tendency of a fiber-containing feed treatment using 2% blueberry pomace supplemented diet to reduce the relative abundance of Tenericutes in the cecum of broiler chickens. Tenericutes were negatively correlated with cumulative feed efficiency in their study. Tenericutes are also implicated in mycoplasma pathology in chickens. At the genus level, the relative abundances of Firmicutes_unclassified, Ruminococcus, Turicibacter, and Erysipelotrichaceae_unclassified were increased by both OE and OEOH treatments (Fig. 2). Firmicutes are associated with polysaccharide degradation, butyrate production, and enhanced nutrient absorption capacity (Louis et al., 2014; Meehan and Beiko, 2014). Ruminococcus is also correlated with polysaccharide degradation and butyrate production (Gong et al., 2007; Xiao et al., 2017). Turicibacter contributes to host metabolic and homeostatic mechanisms via cholesterol metabolism (Li et al., 2017; Hoffman and Margolis, 2020). Abdelli et al. (2020) recently showed that the targeted release of microencapsulated organic acids and essential oils blend increases the abundance of Erysipelotrichaceae in NE challenged broilers. Erysipelotrichaceae are also linked to butyrate production (De Maesschalck et al., 2014). The OE treatment increased the relative abundance of Streptococcus compared to OEOH treatment in this study (Fig. 2H). Although Streptococcus might be associated with poultry infection (Sekizaki et al., 2008), depending on the specific strain, they are also capable of reducing gut pathogen load (Roto et al., 2015). Coprobacillus have been referred to as “intestinal dysbiosis associated genera” (Janssens et al., 2018; Adhikari, 2019). Both OE and OEOH treatments in our study reduced the relative abundance of Coprobacillus. Similar to our results (Fig. 2C), Adhikari (2019) reported an increase in the genus Coprobacillus in non-supplemented control birds in their study.
Also, no difference in α-diversity was found between treatments in this study (Fig. 3). Both Pham et al. (2020), who researched a dietary encapsulated essential oil and organic acids mixture (though formulation differs from this study), and Bortoluzzi et al. (2017) who researched a sodium butyrate additive, have equally reported no difference in α-diversity. Conversely, permutational analysis of variance indicated that OE diet modified cecal microbiota diversity, with OEOH diet showing a tendency to elicit a similar response (Fig. 4). Pham et al. (2020) recently reported the potential of the organic acids and essentials oils blend they tested to modify the intestinal bacterial community profiles of broiler chickens. Furthermore, Firmicutes unclassified species were found differentially abundant in birds belonging to both the OE and OEOH treatments in this study. Birds of the OE treatment recorded differentially abundant Clostridiales_unclassified species. A positive correlation between body weight and Clostridia has been reported in broiler chickens offered essential oil supplemented diet (Betancourt et al., 2019). Overall, both OE and OEOH supplemented diet seemed to stimulate the growth of potentially beneficial intestinal microorganisms, whilst also inhibiting the proliferation of pathogenic bacteria.
5. Conclusion
The OE treatment reduced plasma cholesterol and urea levels in broiler chickens. Both OE and OEOH treatment improved gut morphology and positively influenced birds’ gut health by shifting gut microbiota diversity and stimulating the growth of potentially beneficial intestinal microorganisms. An additional beneficial effect on gut health was observed with the use of OEOH treatment, as evidenced by the increased relative weight of the gizzard. However, this benefit did not translate into increased growth performance effect in birds offered the OEOH treatment. More studies are needed to determine the optimum dietary fiber inclusion level (beyond the 3% OH utilized in this study) that could elicit a superior growth performance effect. Conclusively, this study shows that both protected organic acids-essential oils blend and its combination with oat fibers show potential as an important strategy to achieve antibiotics reduction in broiler production.
Author contributions
Deborah I. Adewole: Conceptualization, Methodology, Software, Writing – original draft preparation, writing - reviewing & editing, Funding acquisition, Supervision. Samson Oladokun: Investigation, Data curation, Writing – original draft preparation, writing - reviewing & editing. Elizabeth Santin: Resources, Investigation, writing - reviewing & editing.
Conflict of interest
The authors declare the following competing interest(s): Elizabeth Santin is a co-author in this manuscript. She is an employee at Jefo Nutrition Inc. that provided the organic acids-essential oils blend for this work.
Acknowledgments
The Authors acknowledge Janice MacIsaac and Jamie Fraser for diet formulation and preparation and Sarah Macpherson and Krista Budgell for animal care. Appreciation goes to Grain Millers, Saskatoon, SK, Canada for supplying the oat hulls used in this study. This work was financially supported by Dalhousie University, Agriculture and Agri-Food Canada (Pan Atlantic Program), and Chicken Farmers of Nova Scotia.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aninu.2021.02.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Abdel-Fattah S.A., El-Sanhoury M.H., El-Mednay N.M., Abdel-Azeem F. Thyroid activity, some blood constituents, organs morphology and performance of broiler chicks fed supplemental organic acids. Int J Poultry Sci. 2008;7:215–222. [Google Scholar]
- Abdelli N., Pérez J.F., Vilarrasa E., Luna I.C., Melo-Duran D., D'angelo M., Solà-Oriol D. Targeted-release organic acids and essential oils improve performance and digestive function in broilers under a necrotic enteritis challenge. Animals. 2020;10:259. doi: 10.3390/ani10020259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdo Z.M. Efficacy of acetic acid in improving the utilization of low protein-low energy broiler diets. Egypt Poult Sci. 2004;24:123–141. [Google Scholar]
- Adewole D. Effect of dietary supplementation with coarse or extruded oat hulls on growth performance, blood biochemical parameters, ceca microbiota and short chain fatty acids in broiler chickens. Animals. 2020;10:1429. doi: 10.3390/ani10081429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adewole D., MacIsaac J., Fraser G., Rathgeber B. Effect of oat hulls incorporated in the diet or fed as free choice on growth performance, carcass yield, gut morphology and digesta short chain fatty acids of broiler chickens. Sustainability. 2020;12:3744. [Google Scholar]
- Adhikari B. University of Arkansas; Fayetteville: 2019. Investigation of microbiota in health and disease of poultry. [Doctoral degree thesis Dissertation] [Google Scholar]
- Adil S., Banday T., Bhat G.A., Mir M.S., Rehman M. Effect of dietary supplementation of organic acids on performance, intestinal histomorphology, and serum biochemistry of broiler chicken. Vet Med Int. 2010 doi: 10.4061/2010/479485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali A. Effect of some organic acids and essential oils as feed additives on growth performance, immune response and carcass quality of Japanese quail. Alexandria J Vet Sci. 2016;51:68. [Google Scholar]
- Amerah A.M., Ravindran V., Lentle R.G., Thomas D.G. Feed particle size: implications on the digestion and performance of poultry. World’s Poult Sci J. 2007;63:439–455. [Google Scholar]
- Aoac . 18th ed. AOAC Int.; Gaithersburg, MD: 2005. Official methods of analysis of AOAC International. [Google Scholar]
- Aoac . 15th ed. Assoc. Off. Anal. Chem.; Washington, DC: 1990. Official methods of analysis. [Google Scholar]
- Apajalahti J., Vienola K., Raatikainen K., Holder V., Moran C.A. Conversion of branched-chain amino acids to corresponding isoacids-an in vitro tool for estimating ruminal protein degradability. Front Vet Sci. 2019;6:311. doi: 10.3389/fvets.2019.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basmacioğlu-Malayoğlu H., Ozdemir P., Bağriyanik H.A. Influence of an organic acid blend and essential oil blend, individually or in combination, on growth performance, carcass parameters, apparent digestibility, intestinal microflora and intestinal morphology of broilers. Br Poultry Sci. 2016;57:227–234. doi: 10.1080/00071668.2016.1141171. [DOI] [PubMed] [Google Scholar]
- Belote B.L., Tujimoto-Silva A., Hummelgen P.H., Sanches A.W.D., Wammes J.C.S., Hayashi R.M. Histological parameters to evaluate intestinal health on broilers challenged with Eimeria and Clostridium perfringens with or without enramycin as growth promoter. Poultry Sci. 2018;97:2287–2294. doi: 10.3382/ps/pey064. [DOI] [PubMed] [Google Scholar]
- Berrocoso J.D., Kida R., Singh A.K., Kim Y.S., Jha R. Effect of in ovo injection of raffinose on growth performance and gut health parameters of broiler chicken. Poultry Sci. 2017;96:1573–1580. doi: 10.3382/ps/pew430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancourt L., Hume M., Rodríguez F., Nisbet D., Sohail M.U., Afanador-Tellez G. Effects of Colombian oregano essential oil (Lippia origanoides Kunth) and Eimeria species on broiler production and cecal microbiota. Poultry Sci. 2019;98:4777–4786. doi: 10.3382/ps/pez193. [DOI] [PubMed] [Google Scholar]
- Bortoluzzi C., Pedroso A.A., Mallo J.J., Puyalto M., Kim W.K., Applegate T.J. Sodium butyrate improved performance while modulating the cecal microbiota and regulating the expression of intestinal immune-related genes of broiler chickens. Poultry Sci. 2017;96:3981–3993. doi: 10.3382/ps/pex218. [DOI] [PubMed] [Google Scholar]
- Brenes A., Roura E. Essential oils in poultry nutrition: main effects and modes of action. Anim Feed Sci Technol. 2010;158:1–14. [Google Scholar]
- Butaye P., Devriese L.A., Haesebrouck F. Antimicrobial growth promoters used in animal feed: effects of less well known antibiotics on gram-positive bacteria. Clin Microbiol Rev. 2003;16:175–188. doi: 10.1128/CMR.16.2.175-188.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadian Council on Animal Care . Canadian Council on Animal Care; Ottawa, ON, Canada: 2009. Guidelines on: the care and use of farm animals in research, teaching and testing. [Google Scholar]
- Cardinal K.M., Moraes M.L.D., Andretta I., Schirmann G.D., Belote B.L., Barrios M.A. Growth performance and intestinal health of broilers fed a standard or low-protein diet with the addition of a protease. R Bras Zootec. 2019;48 [Google Scholar]
- Castanon J.I.R. History of the use of antibiotic as growth promoters in European poultry feeds. Poultry Sci. 2007;86:2466–2471. doi: 10.3382/ps.2007-00249. [DOI] [PubMed] [Google Scholar]
- Cherrington C.A., Hinton M., Mead G.C., Chopra I. vol. 32. Academic Press; 1991. Organic acids: chemistry, antibacterial activity and practical applications; pp. 87–108. (Advances in microbial physiology). [DOI] [PubMed] [Google Scholar]
- Chiang G., Lu W.Q., Piao X.S., Hu J.K., Gong L.M., Thacker P.A. Effects of feeding solid-state fermented rapeseed meal on performance, nutrient digestibility, intestinal ecology and intestinal morphology of broiler chickens. Asian Austral J Anim. 2010;23:263–271. [Google Scholar]
- Clinical Diagnostic Division . Rochester; New York: 1990. Veterinary reference guide. [Google Scholar]
- Cowieson A.J., Zaefarian F., Knap I., Ravindran V. Interactive effects of dietary protein concentration, a mono-component exogenous protease and ascorbic acid on broiler performance, nutritional status and gut health. Anim Prod Sci. 2017;57:1058–1068. [Google Scholar]
- Craig W.J. Health-promoting properties of common herbs. Am J Clin Nutr. 1999;70:491s–499s. doi: 10.1093/ajcn/70.3.491s. [DOI] [PubMed] [Google Scholar]
- Craig W.J. In: Vegetables, fruits, and herbs in health promotion. Watson R.R., editor. CRC Press; Boca Raton, Florina, USA: 2001. Herbal remedies that promote health and prevent disease; pp. 179–204. [Google Scholar]
- Das Q., Islam M.R., Lepp D., Tang J., Yin X., Mats L., Liu H., Ross K., Kennes Y.M., Yacini H., Warriner K., Marcone M.F., Diarra M.S. Gut microbiota, blood metabolites, and spleen immunity in broiler chickens fed berry pomaces and phenolic-enriched extractives. Front Vet Sci. 2020;7:150. doi: 10.3389/fvets.2020.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maesschalck C., Van Immerseel F., Eeckhaut V., De Baere S.D., Cnockaert M., Croubels S., Haesebrouck F., Ducatelle R., Vandamme P. Faecalicoccus acidiformans gen. nov., Sp. nov., Isolated from the chicken caecum, and reclassification of Streptococcus pleomorphus (barnes et al. 1977), Eubacterium biforme (eggerth 1935) and Eubacterium cylindroides (cato et al. 1974) as Aecalicoccus pleomorphus comb. nov., Holdemanella biformis gen. nov., Comb. nov. and faecalitalea cylindroides gen. nov., Comb. nov., Respectively, within the family erysipelotrichaceae. Int J Syst Evol Microbiol. 2014;64:3877–3884. doi: 10.1099/ijs.0.064626-0. [DOI] [PubMed] [Google Scholar]
- Den Besten G., van Eunen K., Groen A.K., Venema K., Reijngoud D.J., Bakker B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawale A. Better eggshell quality with a gut acidifier. Poultry Int. 2005;44:18–21. [Google Scholar]
- Dibner J.J., Buttin P. Use of organic acids as a model to study the impact of gut microflora on nutrition and metabolism. J Appl Poultry Res. 2002;11:453–463. [Google Scholar]
- Elson C.E., Underbakke G.L., Hanson P., Shrago E., Wainberg R.H., Qureshi A.A. Impact of lemongrass oil, an essential oil, on serum cholesterol. Lipids. 1989;24:677–679. doi: 10.1007/BF02535203. [DOI] [PubMed] [Google Scholar]
- Emami N.K., Daneshmand A., Naeini S.Z., Graystone E.N., Broom L.J. Effects of commercial organic acid blends on male broilers challenged with E. coli K88: performance, microbiology, intestinal morphology, and immune response. Poultry Sci. 2017;96:3254–3326. doi: 10.3382/ps/pex106. [DOI] [PubMed] [Google Scholar]
- Essa Al-Mashhadani B.H., Al-Jaff K., Farhan Y.M., AL-Mashhadani H.E. Effect of anise, thyme essential oils and their mixture (eom) on broiler performance and some physiological traits. Egypt. Poultry Sci. 2011;31:481–489. [Google Scholar]
- Fan Y.K., Croom J., Christensen V.L., Black B.L., Bird A.R., Daniel L.R. Jejunal glucose uptake and oxygen consumption in Turkey poults selected for rapid growth. Poultry Sci. 1997;76:1738–1745. doi: 10.1093/ps/76.12.1738. [DOI] [PubMed] [Google Scholar]
- Ferket P.R., Fontaine J., Bunod J.D. North Carolina State Univ., as signee. US Pat; 2017. Encapsulation of nutritional and/or compounds for controlled release and enhancing their bioavailability by limiting chemical or microbial exposure. No. WO2018089516. [Google Scholar]
- Fernandez-Panchon M.S., Villano D., Troncoso A.M., Garcia-Parrilla M.C. Antioxidant activity of phenolic compounds: from in vitro results to in vivo evidence. Crit Rev Food Sci Nutr. 2008;48:649–671. doi: 10.1080/10408390701761845. [DOI] [PubMed] [Google Scholar]
- Forstner J.F., Forstner G.G. In: Physiology of the gastrointestinal tract. Johnson L.R., editor. Raven Press; New York, NY: 1994. Gastrointestinal mucus; pp. 1255–1283. [Google Scholar]
- Franti C.E., Julian L.M., Adler H.E., Wiggins A.D. Antibiotic growth promotion: effects of zinc bacitracin and oxytetracycline on the digestive, circulatory, and excretory systems of New Hampshire cockerels. Poultry Sci. 1972;51:1137–1145. doi: 10.3382/ps.0511137. [DOI] [PubMed] [Google Scholar]
- Franz C., Baser K.H.C., Windisch W. Essential oils and aromatic plants in animal feeding - a European perspective. A review. Flavour Fragrance J. 2010;25:327–340. [Google Scholar]
- Gadde U., Kim W.H., Oh S.T., Lillehoj H.S. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review. Anim Health Res Rev. 2017;18:26–45. doi: 10.1017/S1466252316000207. [DOI] [PubMed] [Google Scholar]
- Gao Y.Y., Zhang X.L., Xu L.H., Peng H., Wang C.K., Bi Y.Z. Encapsulated blends of essential oils and organic acids improved performance, intestinal morphology, cecal microflora, and jejunal enzyme activity of broilers. Czech J Anim Sci. 2019;64:189–198. [Google Scholar]
- Garcia J., Fondevila G., Camara L., Scappaticcio R.E., Aguirre L., Mateos G.G. Influence of egg weight and inclusion of oat hulls in the diet on digestive tract traits and growth performance of brown pullets reared under stress conditions. Poultry Sci. 2019;98:5767–5777. doi: 10.3382/ps/pez370. [DOI] [PubMed] [Google Scholar]
- Ghazanfari S., Adib Moradi M., Mahmoodi Bardzardi M. Intestinal morphology and microbiology of broiler chicken fed diets containing myrtle (Myrtus communis) essential oil supplementation. Iran J Appl Anim Sci. 2014;4:549–554. [Google Scholar]
- Giannenas I., Bonos E., Skoufos I., Tzora A., Stylianaki I., Lazari D., Tsinas A., Christaki E., Florou-Paneri P. Effect of herbal feed additives on performance parameters, intestinal microbiota, intestinal morphology and meat lipid oxidation of broiler chickens. Br Poultry Sci. 2018;59:545–553. doi: 10.1080/00071668.2018.1483577. [DOI] [PubMed] [Google Scholar]
- Giannenas I., Papaneophytou C.P., Tsalie E., Pappas I., Triantafillou E., Tontis D., Kontopidis G.A. Dietary supplementation of benzoic acid and essential oil compounds affects buffering capacity of the feeds, performance of Turkey poults and their antioxidant status, pH in the digestive tract, intestinal microbiota and morphology. Asian-Australas J Anim Sci. 2014;27:225–236. doi: 10.5713/ajas.2013.13376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J., Si W., Forster R.J., Huang R., Yu H., Yin Y., Yang C., Han Y. 16S rRNA gene-based analysis of mucosa-associated bacterial community and phylogeny in the chicken gastrointestinal tracts: from crops to ceca. FEMS Microbiol Ecol. 2007;59:147–157. doi: 10.1111/j.1574-6941.2006.00193.x. [DOI] [PubMed] [Google Scholar]
- González-Alvarado J.M., Jiménez-Moreno E., González-Sánchez D., Lázaro R., Mateos G.G. Effect of inclusion of oat hulls and sugar beet pulp in the diet on productive performance and digestive traits of broilers from 1 to 42 days of age. Anim Feed Sci Technol. 2010;162:37–46. [Google Scholar]
- González-Alvarado J.M., Jiménez-Moreno E., Valencia D.G., Lázaro R., Mateos R.R. Effects of fiber source and heat processing of the cereal on the development and pH of the gastrointestinal tract of broilers fed diets based on corn or rice. Poultry Sci. 2008;87:1779–1795. doi: 10.3382/ps.2008-00070. [DOI] [PubMed] [Google Scholar]
- Hajati H. Application of organic acids in poultry nutrition. Int J Avian Wildl. 2018;3:324–329. [Google Scholar]
- He X., Hao D., Liu C., Zhang X., Xu D., Xu X., Wang J., Wu R. Effect of supplemental oregano essential oils in diets on production performance and relatively intestinal parameters of laying hens. Am J Biochem Mol Biol. 2017;7:73. [Google Scholar]
- Hetland H., Svihus B., Krogdahl A. Effects of oat hulls and wood shavings on digestion in broilers and layers fed diets based on whole or ground wheat. Br Poultry Sci. 2003;44:275–282. doi: 10.1080/0007166031000124595. [DOI] [PubMed] [Google Scholar]
- Hetland H., Svihus B. Effect of oat hulls on performance, gut capacity and feed passage time in broiler chickens. Br Poultry Sci. 2001;42:354–361. doi: 10.1080/00071660120055331. [DOI] [PubMed] [Google Scholar]
- Hoffman J.M., Margolis K.G. Building community in the gut: a role for mucosal serotonin. Nat Rev Gastroenterol Hepatol. 2020;17:6–8. doi: 10.1038/s41575-019-0227-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilo S., Maduneme F.C., Ogbu O.C., Okonkwo M.N. Haematological and biochemical characteristics of broiler finisher fed different feed forms (pelleted and mash) J Agric Sustain. 2019;12:175–184. [Google Scholar]
- Jamroz D., Wertelecki T., Houszka M., Kamel C. Influence of diet type on the inclusion of plant origin active substances on morphological and histochemical characteristics of the stomach and jejunum walls in chicken. J Anim Physiol Anim Nutr (Berl) 2006;90:255–268. doi: 10.1111/j.1439-0396.2005.00603.x. [DOI] [PubMed] [Google Scholar]
- Jang I.S., Ko Y.H., Kang S.Y., Lee C.Y. Effect of a commercial essential oil on growth performance, digestive enzyme activity and intestinal microflora population in broiler chickens. Anim Feed Sci Technol. 2007;134:304–315. [Google Scholar]
- Janssens Y., Nielandt J., Bronselaer A., Debunne N., Verbeke F., Wynendaele E., Van Immerseel F., Vandewynckel Y.P., De Tre G., De Spiegeleer B. Disbiome database: linking the microbiome to disease. BMC Microbiol. 2018;18:1–6. doi: 10.1186/s12866-018-1197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeurissen S.H., Lewis F., Van der Klis J.D., Mroz Z., Rebel J.M., Ter Huurne A.A. Parameters and techniques to determine intestinal health of poultry as constituted by immunity, integrity, and functionality. CIIM. 2002;3:1–14. [PubMed] [Google Scholar]
- Jiménez-Moreno E., De Coca-Sinova A., González-Alvarado J.M., Mateos G.G. Inclusion of insoluble fiber sources in mash or pellet diets for young broilers. Effects on growth performance and water intake. Poultry Sci. 2016;95:41–52. doi: 10.3382/ps/pev309. [DOI] [PubMed] [Google Scholar]
- Jiménez-Moreno E., González-Alvarado J.M., de Coca-Sinova A., Lazaro R., Mateos G.G. Effects of source of fibre on the development and pH of the gastrointestinal tract of broilers. Anim Feed SciTechnol. 2009;154:93–101. 2009. [Google Scholar]
- Kamal A.M., Ragaa N.M. Effect of dietary supplementation of organic acids on performance and serum biochemistry of broiler chicken. Nat Sci. 2014;12:38–45. [Google Scholar]
- Khaksar V., Golifian A., Kermanshahi H., Movasseghiand A.R., Jamshidi A. Effect of prebiotic Fennacto® on gut development and performance of broiler chickens fed diet low in Digestible Amino Acids. J Anim Vet Adv. 2008;7:251–257. [Google Scholar]
- Kim J.C., Mullan B.P., Hampson D.J., Pluske J.R. Addition of oat hulls to an extruded rice-based diet for weaner pigs ameliorates the incidence of diarrhoea and reduces indices of protein fermentation in the gastrointestinal tract. Br J Nutr. 2008;99:1217–1225. doi: 10.1017/S0007114507868462. [DOI] [PubMed] [Google Scholar]
- Kraeski A.L., Hayashi R.M., Sanchez A., Almeida G.C., Santin E. Effect of aflatoxin experimental ingestion and Eimeira vaccine challenges on intestinal histopathology and immune cellular dynamic of broilers: applying an Intestinal Health Index. Poultry Sci. 2017;96:1078–1087. doi: 10.3382/ps/pew397. [DOI] [PubMed] [Google Scholar]
- Krishan G., Narang A. Use of essential oils in poultry nutrition: a new approach. J Adv Vet Anim Res. 2014;1:156–162. [Google Scholar]
- Langhout P. New additives for broiler chickens. World poultry. 2000;16:22–27. [Google Scholar]
- Lee K.W., Everts H., Beynen A.C. Essential oils in broiler nutrition. Int J Poultry Sci. 2004;3:738–752. [Google Scholar]
- Leung H., Arrazola A., Torrey S., Kiarie E. Utilization of soy hulls, oat hulls, and flax meal fiber in adult broiler breeder hens. Poultry Sci. 2018;97:1368–1372. doi: 10.3382/ps/pex434. [DOI] [PubMed] [Google Scholar]
- Li Q., Wu T., Liu R., Zhang M., Wang R. Soluble dietary fiber reduces trimethylamine metabolism via gut microbiota and Co-regulates host AMPK pathways. Mol Nutr Food Res. 2017;61:1700473. doi: 10.1002/mnfr.201700473. [DOI] [PubMed] [Google Scholar]
- Liu Y., Yang X., Xin H., Chen S., Yang C., Duan Y., Yang X. Effects of a protected inclusion of organic acids and essential oils as antibiotic growth promoter alternative on growth performance, intestinal morphology and gut microflora in broilers. Anim Sci J. 2017;88:1414–1424. doi: 10.1111/asj.12782. [DOI] [PubMed] [Google Scholar]
- Louis P., Hold G.L., Flint H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12:661–672. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- Mangiafico S.S. Rutgers Cooperative Extension; New Brunswick, NJ: 2016. Summary and analysis of extension program evaluation in R, version 1.15. 0.https://rcompanion.org/handbook/ [Google Scholar]
- Masouri L., Salari S., Sari M., Tabatabaei S., Masouri B. Effect of feed supplementation with Satureja khuzistanica essential oil on performance and physiological parameters of broilers fed on wheat-or maize-based diets. Br Poultry Sci. 2017;58:425–434. doi: 10.1080/00071668.2017.1327701. [DOI] [PubMed] [Google Scholar]
- Mateos G.G., Jiménez-Moreno E., Serrano M.P., Lázaro R.P. Poultry response to high levels of dietary fiber sources varying in physical and chemical characteristics. J Appl Poultry Res. 2012;21:156–174. [Google Scholar]
- Matty H.N., Hassan A.A. Effect of supplementation of encapsulated organic acid and essential oil Gallant+® on some physiological parameters of Japanese quails. Iraqi J Vet Sci. 2020;34:181–188. [Google Scholar]
- McCauley H.A., Guasch G. Three cheers for the goblet cell: maintaining homeostasis in mucosal epithelia. Trends Mol Med. 2015;21:492–503. doi: 10.1016/j.molmed.2015.06.003. [DOI] [PubMed] [Google Scholar]
- McNab J.M., Boorman K.N. CABI publishing; 2002. Poultry feedstuffs: supply, composition and nutritive value. [Google Scholar]
- Meehan C.J., Beiko R.G. A phylogenomic view of ecological specialization in the lachnospiraceae, a family of digestive tract-associated bacteria. Genome Biol. Evol. 2014;6:703–713. doi: 10.1093/gbe/evu050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdi Y., Létourneau-Montminy M.P., Gaucher M.L., Chorfi Y., Suresh G., Rouissi T., Brar S.K., Cote C., Godbout S. Use of antibiotics in broiler production: global impacts and alternatives. Anim Nutr. 2018;4:170–178. doi: 10.1016/j.aninu.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meimandipour A., Shuhaimi M., Soleimani A.F., Azhar K., Hair-Bejo M., Kabeir B.M., Javanmard A., Muhammad Anas O., Yazid A.M. Selected microbial groups and short-chain fatty acids profile in a simulated chicken cecum supplemented with two strains of Lactobacillus. Poultry Sci. 2010;89:470–476. doi: 10.3382/ps.2009-00495. [DOI] [PubMed] [Google Scholar]
- Meimandipour A., Soleimanifarjam A., Azhar K., Hair-Bejo M., Shuhaimi M., Nateghi L., Abd Manap M.Y. Age effects on short chain fatty acids concentrations and pH values in the gastrointestinal tract of broiler chickens. Arch. fur Geflugelkd. 2011;75:164–168. [Google Scholar]
- Montagne L., Pluske J.R., Hampson D.J. A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim Feed Sci Technol. 2003;108:95–117. [Google Scholar]
- Mossami A. Swedish University of Agricultural Science, Department of Animal Nutrition and Management; Kurskod: 2011. Effects of different inclusions of oat hulls on performance, carcass yield and gut development in broiler chickens. Master thesis, Sveriges Lantbruksuniversitet, Institutionen för husdjurensut fodringochvård. [Google Scholar]
- National Research Council . 9th rev. Natl. Acad. Press.; Washington, DC: 1994. Nutrient requirements of poultry. [Google Scholar]
- Ndou S.P., Tun H.M., Kiarie E., Walsh M.C., Khafipour E., Nyachoti C.M. Dietary supplementation with flaxseed meal and oat hulls modulates intestinal histomorphometric characteristics, digesta-and mucosa-associated microbiota in pigs. Sci Rep. 2018;8:1–15. doi: 10.1038/s41598-018-24043-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen T., Qin J., Prifti E., Hildebrand F., Falony G., Almeida M., Arumugam M., Batto J., Kennedy S., Leonard P. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- Oyarzabal O.A., Conner D.E. Application of direct-fed microbial bacteria and fructooligosaccharides for Salmonella control in broilers during feed withdrawal. Poultry Sci. 1996;75:186–190. doi: 10.3382/ps.0750186. [DOI] [PubMed] [Google Scholar]
- Ozdogan M., Topal E., Paksuz E.P., Kirkan S. Effect of different levels of crude glycerol on the morphology and some pathogenic bacteria of the small intestine in male broilers. Animal. 2014;8:36–42. doi: 10.1017/S1751731113001833. [DOI] [PubMed] [Google Scholar]
- Özek K., Wellmann K.T., Ertekin B., Tanm B. Effects of dietary herbal essential oil mixture and organic acid preparation on laying traits, gastrointestinal tract characteristics, blood parameters and immune response of laying hens in a hot summer season. J Anim Feed Sci. 2011;20:575–586. [Google Scholar]
- Paul S.K., Halder G., Mondal M.K., Samanta G. Effect of organic acid salt on the performance and gut health of broiler chicken. J Poultry Sci. 2007;44:389–395. [Google Scholar]
- Perruzza A.L. Master’s Thesis, University of Toronto; Toronto, ON, Canada: 2010. Exploring pretreatment methods and enzymatic Hydrolysis of oat hulls. [Google Scholar]
- Pham V.H., Kan L., Huang J., Geng Y., Zhen W., Guo Y., Abbas W., Wang Z. Dietary encapsulated essential oils and organic acids mixture improves gut health in broiler chickens challenged with necrotic enteritis. J Anim Sci Biotechnol. 2020;11:1–18. doi: 10.1186/s40104-019-0421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi A.A., Din Z.Z., Reed Mangels W., Elson C.E. Inhibition of hepatic mevalonate biosynthesis by the monoterpene, d-limonene. J Agric Food Chem. 1988;36:1220–1224. [Google Scholar]
- Rahmatnejad E., Saki A.A. Effect of dietary fibres on small intestine histomorphology and lipid metabolism in young broiler chickens. J Anim Physiol Anim Nutr (Berl) 2016;100:665–672. doi: 10.1111/jpn.12422. [DOI] [PubMed] [Google Scholar]
- Reisinger N., Steiner T., Nitsch S., Schatzmayr G., Applegate T.J. Effects of a blend of essential oils on broiler performance and intestinal morphology during coccidial vaccine exposure. J Appl Poultry Res. 2011;20:272–283. [Google Scholar]
- Rezaei M., Karimi Torshizi M.A., Rouzbehan Y. The influence of different levels of micronized insoluble fiber on broiler performance and litter moisture. Pout. Sci. 2011;90:2008–2012. doi: 10.3382/ps.2011-01352. [DOI] [PubMed] [Google Scholar]
- Roto S.M., Rubinelli P.M., Ricke S.C. An introduction to the avian gut microbiota and the effects of yeast-based prebiotic-type compounds as potential feed additives. Front Vet Sci. 2015;2:28. doi: 10.3389/fvets.2015.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round A.N., Rigby N.M., Garcia de la Torre A., Macierzanka A., Mills E.C., Mackie A.R. Lamellar structures of MUC2-rich mucin: a potential role in governing the barrier and lubricating functions of intestinal mucus. Biomacromolecules. 2012;13:3253–3261. doi: 10.1021/bm301024x. 2012. [DOI] [PubMed] [Google Scholar]
- Sanches A.W.D. Universidade Federal do Paraná; Brazil: 2019. The avian microscopic enteritis under the" I See Inside"(ISI) methodology: new perspectives on a missed subject. [Doctoral degree thesis Dissertation] [Google Scholar]
- Sarikhan M., Shahryar H.A., Gholizadeh B., Hosseinzadeh M.H., Beheshti B., Mahmoodnejad A. Effects of insoluble fiber on growth performance, carcass traits and ileum morphological parameters on broiler chick males. Int J Agric Biol. 2010;12:531–536. [Google Scholar]
- Schloss Patrick D., Westcott Sarah L., Ryabin Thomas, Hall Justine R., Hartmann Martin, Hollister Emily B., Lesniewski Ryan A. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;23:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholey D.V., Marshall A., Cowan A.A. Evaluation of oats with varying hull inclusion in broiler diets up to 35 days. Poultry Sci. 2020;99:2566–2572. doi: 10.1016/j.psj.2019.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekirov I., Russell S.L., Caetano L., Antunes M., Finlay B.B. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- Sekizaki T., Nishiya H., Nakajima S., Nishizono M., Kawano M., Okura M., Takamatsu D., Nishino H., Ishiji T., Osawa R. Endocarditis in chickens caused by subclinical infection of Streptococcus gallolyticus subsp. gallolyticus. Avian Dis. 2008;52:183–186. doi: 10.1637/8048-070307-Case. [DOI] [PubMed] [Google Scholar]
- Spais A.B., Giannenas I.A., Florou-Paneri P., Christaki E., Botsoglou N.A. Effect of Genex, a feed additive containing organic acids and herb extracts, on the performance of broiler chickens. J. Hell. Vet Med Soc. 2002;53:247–256. [Google Scholar]
- Stefanello C., Rosa D.P., Dalmoro Y.K., Segatto A.L., Vieira M.S., Moraes M.L., Santin E. Protected blend of organic acids and essential oils improves growth performance, nutrient digestibility, and intestinal health of broiler chickens undergoing an intestinal challenge. Front Vet Sci. 2020;6:491. doi: 10.3389/fvets.2019.00491. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B., Hou L., Yang Y. Effects of altered dietary fiber on the gut microbiota, short-chain fatty acids and cecum of chickens during different growth periods. Preprints. 2020:2020020109. [Google Scholar]
- Sun S.S., Wang K., Ma K., Bao L., Liu H.W. An insoluble polysaccharide from the sclerotium of Poria cocos improves hyperglycemia, hyperlipidemia and hepatic steatosis in ob/ob mice via modulation of gut microbiota. Chin J Nat Med. 2019;17:3–14. doi: 10.1016/S1875-5364(19)30003-2. [DOI] [PubMed] [Google Scholar]
- Svihus B. Function of the digestive system. J Appl Poultry Res. 2014;23:306–314. [Google Scholar]
- Svihus B. The gizzard: function, influence of diet structure and effects on nutrient availability. World Poult. Sci. J. 2011;67:207–223. [Google Scholar]
- Szigeti G., Palfi V., Nagy B., Ine E., Nagy G. New type of immune stimulant to increase antibody production generated by viral and bacterial vaccines. Magy Allatorv Lapja. 1998;120:719–721. [Google Scholar]
- Tayeri V., Seidavi A., Asadpour L., Phillips C.J.C. A comparison of the effects of antibiotics, probiotics, synbiotics and prebiotics on the performance and carcass characteristics of broilers. Vet Res Commun. 2018;42:195–207. doi: 10.1007/s11259-018-9724-2. 2018. [DOI] [PubMed] [Google Scholar]
- Tekce E., Gul M. Effects of origanum syriacum essential oil on blood parameters of broilers reared at high ambient heat. Rev Bras Ciência Avícola. 2017;19:655–662. [Google Scholar]
- Theron M.M., Lues J.F.R. CRC Press; 2011. Mechanisms of microbial inhibition. Organic acids and food preservation; pp. 117–150. [Google Scholar]
- Walugembe M., Hsieh J.F.C., N J., Koszewski N.J., Lamont S.J., Persia M.E., Rothschild M.F. Effects of dietary fiber on cecal short-chain fatty acid and cecal microbiota of broiler and laying-hen chicks. Poultry Sci. 2015;94:2351–2359. doi: 10.3382/ps/pev242. [DOI] [PubMed] [Google Scholar]
- Wang H., Liang S., Li X., Yang X., Long F., Yang X. Effects of encapsulated essential oils and organic acids on laying performance, egg quality, intestinal morphology, barrier function, and microflora count of hens during the early laying period. Poultry Sci. 2019;98:6751–6760. doi: 10.3382/ps/pez391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windisch W., Schedle K., Plitzner C., Kroismayr A. Use of phytogenic products as feed additives for swine and poultry. J Anim Sci. 2008;86:E140–E148. doi: 10.2527/jas.2007-0459. [DOI] [PubMed] [Google Scholar]
- Xiao Y., Xiang Y., Zhou W., Chen J., Li K., Yang H. Microbial community mapping in intestinal tract of broiler chicken. Poultry Sci. 2017;96:1387–1393. doi: 10.3382/ps/pew372. [DOI] [PubMed] [Google Scholar]
- Yang X., Liu Y., Yan F., Yang C., Yang X. Effects of encapsulated organic acids and essential oils on intestinal barrier, microbial count, and bacterial metabolites in broiler chickens. Poultry Sci. 2019;98:2858–2865. doi: 10.3382/ps/pez031. [DOI] [PubMed] [Google Scholar]
- Yang X., Xin H., Yang C., Yang X. Impact of essential oils and organic acids on the growth performance, digestive functions and immunity of broiler chickens. Anim. Nutr. 2018;4:388–393. doi: 10.1016/j.aninu.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ząbek K., Szkopek D., Michalczuk M., Konieczka P. Dietary phytogenic combination with hops and a mixture of a free butyrate acidifier and gluconic acid maintaining the health status of the gut and performance in chickens. Animals. 2020;10:1335. doi: 10.3390/ani10081335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeinali S., Ghazanfari S., Ebrahimi M.A. Mucin2 gene expression in the chicken intestinal goblet cells are affected by dietary essential oils. Bulg J Agric Sci. 2017;23:134–141. [Google Scholar]
- Zeng Z., Zhang S., Wang H., Piao X. Essential oil and aromatic plants as feed additives in non-ruminant nutrition: a review. J Anim Sci Biotechnol. 2015:1–10. doi: 10.1186/s40104-015-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai H., Liu H., Wang S., Wu J., Kluenter A.M. Potential of essential oils for poultry and pigs. Anim Nutr. 2018;4:179–186. doi: 10.1016/j.aninu.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.