Abstract
This experiment was conducted to evaluate the effects of different levels of tannic acid (TA) on growth performance, diarrhea rate, nutrient digestibility and intestinal health in weaned piglets. A total of 180 weaned piglets (Duroc × Landrace × Yorkshire, 24 d of age, initial average BW = 7.77 ± 0.17 kg) were allotted to 5 groups (6 pigs/pen and 6 replicates/group) in a randomized complete block design according to their gender and body weight. Piglets were fed a basal diet, or the basal diet supplemented with 0.05%, 0.1%, 0.2% or 0.4% TA for 28 d. The supplementary levels of TA in the diets were obtained by adding tannalbin containing 51% TA and 40.17% protein. The results showed that, compared with the CON group, dietary TA did not affect ADFI, ADG or F:G, and linearly reduced (P < 0.01) the diarrhea rate and diarrhea index of piglets. There were no significant effects on apparent total tract digestibility (ATTD) in the 0.05%, 0.1% and 0.2% TA groups, while negative effects (P < 0.05) on apparent digestibility of crude protein and gross energy were observed in the 0.4% TA group. In addition, the nutrient digestibility of dry matter, crude protein and gross energy linearly decreased (P < 0.01) with the increase of TA dosage. Supplementation of TA increased (P < 0.05) the villus height of the duodenum and jejunum, as well as increased (P < 0.05) catalase (CAT) activity in serum. Dietary TA improved (P < 0.05) the Bacillus counts in cecal digesta. Further, TA significantly improved (P < 0.05) Bacillus counts and reduced (P < 0.05) the Escherichia coli counts in colonic digesta. The concentration of acetic acid, propionic acid, butyric acid and isovaleric acid in cecal digesta were significantly increased (P < 0.05). The mRNA expression level of zonula occludens-1 (ZO-1), zonula occludens-2 (ZO-2), and claudin-2 (CLDN-2) in the jejunum were greater (P < 0.05) in TA supplemented groups. The study showed that, compared to the control, TA prevented post-weaning diarrhea and improved intestinal health of weaned piglets, and the appropriate level of TA supplementation would be from 0.1% to 0.2%.
Keywords: Tannic acid, Weaned piglet, Post-weaning diarrhea, Intestinal health
1. Introduction
Weaning stress syndrome in piglets may lead to growth deprivation, intestinal villus atrophy, deepening crypts and an increased incidence of diarrhea due to the transition to a solid diet, environment and psychological pressure. Diarrhea is a serious problem because of the increased mortality rate resulting in huge economic losses in the swine industry (Yu et al., 2020). Antibiotic drugs and high doses of zinc oxide (ZnO) have been widely applied to alleviate post-weaning diarrhea for many years, however, the usage of antibiotics and ZnO has resulted in some adverse side effects such as environmental pollution and bacterial resistance (Mathew and Ebner, 2005; Davin et al., 2013). Plant extracts or purified plant derivatives could be potential alternatives to antibiotics, and tannic acid (TA) has received considerable attention due to its astringency and anti-diarrhea effect (Redondo et al., 2014).
Tannic acid is a naturally polyphenolic compound that can be found in different plants, including forages, shrubs, cereals, medicinal herbs and fruit species (Jezierny et al., 2012; Huang et al., 2018). Tannic acid is generally classified as hydrolysable tannic acid (HT) and condensed tannic acid (CT) due to its variable chemical structure (Buyukcapar et al., 2011). It is well known that TA can form a precipitate by binding proteins, digestive enzymes, alkaloids, polysaccharides and metal ions, and reduce nutrient digestibility in animal, therefore, TA is generally considered as an anti-nutritional factor (ANF) (Jansman, 1993; Lavin et al., 2010). However, some studies have found that appropriate doses of TA not only have a beneficial effect on the prevention of diarrhea, but also have no negative impacts on the growth performance of animals (Schiavone et al., 2008; Biagia et al., 2010). Tannic acid has various biological activities, for example, it can be an antidiarrheal, antioxidative, antibacterial, antiparasitic, antiviral and anticancer drug due to its polyphenolic hydroxyl structure (Gülçin et al., 2010; Tonda et al., 2018), and it might also be capable of improving gut health and growth performance.
There is still much controversy about the effects of TA at different concentrations in monogastric animals. We were interested in making a preliminary assessment on the effects of 0%, 0.05%, 0.1%, 0.2% and 0.4% dietary TA on growth performance and gut health in weaned pigs. Thus, the objective of our study was to evaluate the effects of different levels of TA supplementation on growth performance, diarrhea rate, nutrient digestibility, intestinal morphology and health in weaned piglets.
2. Materials and methods
2.1. Ethics statement
All experimental procedures used in this study were approved by the Institutional Animal Care and Use Committee of Sichuan Agricultural University.
2.2. Experimental animals, design, diets and housing
A total of 180 crossbred piglets (Duroc × Landrace × Yorkshire) were weaned at 21 ± 1 d. Piglets were fed the basal diet in a 5-d adaptation period. All piglets with an initial average body weight of 7.77 ± 0.17 kg, were divided into 5 dietary groups (6 pigs/pen and 6 replicates/group) in a randomized complete block design based on body weight and gender. Piglets were fed the basal diet with 0% (CON), 0.05%, 0.1%, 0.2% and 0.4% TA supplementation, respectively. These TA levels were obtained by adding tannalbin containing 51% TA and 40.17% protein. Namely, tannalbin was added to the diet for 0%, 0.1%, 0.2%, 0.4% and 0.8% at the expense of the soybean meals in the equal amount. Tannalbin was provided by Guangzhou Insighter Biotechnology Co., Ltd. (Guangzhou, China) and it contained TA that was extracted from gallnut. The composition of experimental diets was formulated according to National Research Council (NRC, 2012) recommendations to meet or exceed nutrition requirements of weaned piglets (Table 1). All pigs were housed in an environmentally-controlled room with a slatted plastic flooring and an effective mechanical ventilation system. Each pen had 2 stainless feeders and four nipple drinkers. Pigs had ad libitum access to feed and water throughout the experimental period. The animal house temperature was controlled at 25 to 28 °C and relative humidity controlled at 55% to 65%.
Table 1.
Ingredient and nutrient levels of basal diets (%, as-fed basis).
| Item | Content |
|---|---|
| Ingredients | |
| Corn | 31.00 |
| Soybean meal | 11.30 |
| Expanded soybean | 5.30 |
| Expanded corn | 29.00 |
| Soybean protein concentrate | 5.00 |
| Soybean oil | 1.50 |
| Sucrose | 3.00 |
| Plasma protein powder | 2.50 |
| Low protein whey powder | 6.00 |
| Fish meal | 2.52 |
| L-Lysine HCl | 0.40 |
| DL-Methionine | 0.15 |
| L-Threonine | 0.08 |
| L-Tryptophan | 0.02 |
| Choline chloride | 0.10 |
| Limestone | 0.90 |
| Dicalcium phosphate | 0.58 |
| NaCl | 0.40 |
| Vitamin premix1 | 0.05 |
| Mineral premix2 | 0.20 |
| Nutrient level | |
| DE, MJ/kg | 14.64 |
| CP | 18.68 |
| Ca | 0.73 |
| Total P | 0.52 |
| Available P | 0.41 |
| SID Lysine | 1.35 |
| SID Methionine | 0.43 |
| SID Methionine + Cystine | 0.74 |
| SID Threonine | 0.79 |
| SID Tryptophan | 0.22 |
DE = digestible energy; SID = standardized ileal digestible.
Provided per kilogram of complete diet: 17,500 IU vitamin A; 3,500 IU vitamin D3; 37.5 mg vitamin E; 4 mg vitamin K; 4.5 mg vitamin Bl; 11.5 mg vitamin B2; 6 mg vitamin B6; 0.05 mg vitamin Bl2; 2.5 mg folic acid; 50 mg nicotinamide; 0.3 mg D-biotin; 25 mg D-pantothenic acid.
Provided per kilogram of complete diet: Fe (as FeSO4•H2O), 100 mg; Cu (CuSO4•5H2O), 6 mg; Mn (as MnSO4•H2O), 4 mg; Zn (as ZnSO4•H2O), 100 mg; I (as KI), 0.14 mg; Se (as Na2SeO3·5H2O), 0.35 mg.
2.3. Sampling and measurements
All piglets were monitored for general health throughout the trial, and feed intake was recorded daily. All piglets were individually weighed at the beginning (1 d), middle (15 d) and end (29 d) of the experiment after 12 h of fasting. The average daily feed intake (ADFI), average daily gain (ADG) and feed-to-gain (F:G) ratio were calculated. The incidence and severity of piglet diarrhea were assessed by scoring fecal consistency: 0 = normal, firm feces; 1 = soft feces, possible slight diarrhea; 2 = unformed, moderately fluid feces; 3 = very watery and frothy diarrhea. Piglets were considered to be diarrheic when the diarrhea score was 2 or above. Diarrhea rate (%) = Σ (The number of pigs with diarrhea per pen × Days of diarrhea)/(Total number of piglets × 28 d) × 100. Diarrhea index = Sum of diarrhea scores of pigs per pen/(Number of piglets per pen × Total days) (Yu et al., 2020).
Fecal samples were collected on d 25 and 28 of the experiment. Fresh excrement of each pen (6 piglets) was immediately collected into a respective sealed plastic bag after defecation and then 10 mL of a 10% H2SO4 solution was evenly added to 100 g excrement to prevent nitrogen loss. At the end of 4 d period, all fecal samples of each pen were mixed thoroughly and dried at 65 °C in an oven for 72 h. After that, the samples were ground to pass through a 1-mm screen. All feed and fecal samples were measured for dry matter (method 930.15), crude protein (method 990.03), ether extract (method 920.39) and ash (method 942.05) according to procedures described by AOAC (1995). Gross energy was determined using an adiabatic oxygen bomb calorimetry (Parr Instrument Co., Moline, IL, USA). The apparent total tract digestibility (ATTD) of nutrients was calculated by means of acid-insoluble ash (AIA) as an endogenous indicator. The AIA in feed and fecal samples was measured as described by Standards Press of China (2009). The ATTD was calculated by referencing the following formula: ATTD (%) = 100 - (A1 × F2)/(A2 × F1) × 100, in which A1 = the AIA content of the feed, A2 = the AIA content of feces, F1 = the nutrient content of the feed, and F2 = the nutrient content of feces.
At the end of the trial, one piglet, with the closest to average weight per pen, was selected for collection of 10 mL of blood from the anterior vena cava to a glass vacuum tube (Bokan Bioengineering Co., Ltd, Shenyang, China) after 12 h fasting. Blood samples were then centrifuged at 3,000 × g for 15 min at 4 °C, and subsequently serum was separated and stored at −20 °C for further analysis. The same 30 piglets were then euthanized with an intravenous injection of chlorpromazine hydrochloride (3 mg/kg BW) according to a previous study (Chen et al., 2018). Subsequently, each abdomen was quickly opened to separate and obtain duodenum, jejunum, ileum, cecum and colon. About 1 cm segments of duodenum, jejunum and ileum were immediately isolated, and then fixed in 4% paraformaldehyde solution and stored to determine villus height and crypt depth. The digesta samples in the cecum and colon were collected into aseptic tubes by carefully massaging the tract for the determination of intestinal microbial DNA and volatile fatty acids (VFA). A segment of duodenum, jejunum and ileum tissue were obtained from each piglet, and then washed in 0.9% ice-cold physiological saline. The intestinal mucosa was scraped on the tray with a sterile glass microscope slide, and ice cubes were placed at the bottom of the tray to ensure a continuous low temperature. The digesta and mucosa samples were immediately frozen in liquid nitrogen and then stored at −80 °C for further analysis.
The 4% paraformaldehyde-fixed duodenum, jejunum and ileum samples were embedded in paraffin wax blocks, and consecutive sections at 5 μm thickness were stained with hematoxylin-eosin for histomorphological examination. Intestinal morphology including villus height and crypt depth was measured at 40× magnification with an Olympus CK 40 microscope (Olympus Optical Company, Shenzhen, China).
The small intestine mucosal samples were mixed with physiological saline (precooled at 4 °C) at 1:9 (wt/vol), and were then homogenized on ice. After homogenization, the homogenized buffer was centrifuged at 3,000 × g for 15 min at 4 °C to obtain supernatant for further analysis. The antioxidant parameters of serum and mucosal homogenate including malondialdehyde (MDA), catalase (CAT), total superoxide dismutase (T-SOD), glutathione peroxidase (GPx) and total antioxidant capacity (T-AOC) were measured by commercial kits (Nanjing Jiancheng Institute of Bioengineering, Jiangsu, China) according to the manufacturer's instructions. In addition, the total protein content of the homogenized samples was determined using the Bradford brilliant blue method (Jiali et al., 2018). These measurement results were displayed by using UV-VIS Spectrophotometer (UV1100, MAPADA, Shanghai, China). All samples were measured in duplicate.
Bacterial DNA was extracted from the digesta samples (approximately 0.2 g) of cecum and colon using the E.Z.N.A stool DNA kit (Omega Bio-Tek, Doraville, GA) according to the manufacturer's instructions. Primers and probes for total bacteria, Escherichia coli, Lactobacillus, Bacillus and Bifidobacterium were commercially synthesized by Invitrogen (Shanghai, China), and the specific sequences are shown in Table 2. The quantitative real-time PCR was carried out on CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Inc., Hercules, CA). The 25 μL reaction system of total bacteria includes 12.5 μL SYBR Premix EX Taq (TaKaRa, Dalian, China), 1 μL forward primer, 1 μL reverse primer, 9.5 μL nuclease-free water and 1 μL template DNA. The cycle procedure included pre-denaturation at 95 °C for 25 s, 40 cycles of denaturation at 95 °C for 5 s, annealing at 64.5 °C for 25 s, and extension at 72 °C for 60 s. The reaction system of 20 μL for Escherichia coli, Lactobacillus, Bifidobacterium and Bacillus included 0.4 μL probe, 0.6 μL forward and 0.6 μL reverse primer, 10 μL 2 × SuperReal PreMix (Tiangen, Beijing, China), 1 μL template DNA, and 7.4 μL nuclease-free water. The cycle procedure included 15 min at 95 °C and 49 cycles for 3 s at 95 °C, 25 s at annealing temperature, and 60 s at 72 °C. Each sample was run simultaneously in triplicate on the same PCR plate, and the average value of the number of copies was used for statistical analysis.
Table 2.
Sequence of primers and probes used for the real-time PCR analysis of microbial populations.
| Primer | Nucleotide sequence (5′-3′) | Size, bp | AT, °C |
|---|---|---|---|
| Total bacteria | F: ACTCCTACGGGAGGCAGCAG | 200 | 64.5 |
| R: ATTACCGCGGCTGCTGG | |||
| Escherichia coli | F: CATGCCGCGTGTATGAAGAA | 96 | 53.0 |
| R: CGGGTAACGTCAATGAGCAAA | |||
| P: AGGTATTAACTTTACTCCCTTCCTC | |||
| Lactobacillus | F: GAGGCAGCAGTAGGGAATCTTC | 126 | 53.0 |
| R: CAACAGTTACTCTGACACCCGTTCTTC | |||
| P: AAGAAGGGTTTCGGCTCGTAAAACTCTGTT | |||
| Bacillus | F: GCAACGAGCGCAACCCTTGA | 92 | 57.9 |
| R: TCATCCCCACCTTCCTCCGGT | |||
| P: CGGTTTGTCACCGGCAGTCACCT | |||
| Bifidobacterium | F: CGCGTCCGGTGTGAAAG | 121 | 55.0 |
| R: CTTCCCGATATCTACACATTCCA | |||
| P: ATTCCACCGTTACACCGGGAA |
PCR = polymerase chain reaction; AT = annealing temperature.
Approximately 0.7 g thawed cecal and colonic digesta samples were weighed respectively, and added to a 5-mL centrifuge tube together with 1.5 mL of ultrapure water. This was centrifuged for 15 min at 10,000 × g, then 1 mL of the supernatant was transferred to a new sterile tube and 0.2 mL 25% (wt/vol) metaphosphoric acid was added. This was further centrifuged for 10 min at 10,000 × g, then 0.3 mL of the supernatant was transferred to a new sterile tube with 0.9 mL chromatographic methanol (1:3). After being centrifuged at 3,500 × g for 5 min, the supernatant was filtered through a 0.22-μm filter to a 1.5-mL centrifuge tube for further analysis. Volatile fatty acids (acetic acid, propionic acid, butyric acid, isobutyric acid, valeric acid, and isovaleric acid) were separated and quantified in a gas chromatographic system (VARIAN CP-3800, Varian, Palo Alto, CA, USA).
Frozen jejunum and ileum mucosal samples (approximately 0.1 g) were homogenized in 1 mL Trizol reagent (TaKaRa, Dalian, China), and total RNA was extracted according to the instructions of the manufacturer. The concentration and purity of RNA were analysed spectrophotometrically (Beckman Coulter DU800; Beckman Coulter Inc., Brea, USA) at 260 and 280 nm. The ratio of OD260:OD280 (optical density) was between 1.8 and 2.0 for all samples. The integrity of RNA was measured by formaldehyde gel electrophoresis and the 28S:18S ribosomal RNA band ratio was determined as ≥ 1.8. Subsequently, reverse transcription was performed using PrimeScript RT reagent kit with gDNA Eraser (TaKaRa, Dalian, China) to obtain cDNA for all samples according to the manufacturer's instructions. The quantitative real-time PCR was performed to analyze the expression levels of zonula occludens-1 (ZO-1), zonula occludens-2 (ZO-2), occludin (OCLN), claudin-1 (CLDN-1) and claudin-2 (CLDN-2) on CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Inc., Hercules, CA). Glyceraldehyde-3 phosphate dehydrogenase (GAPDH) was chosen as the reference gene transcript. The specific primers of specific genes (primer sequences are reported in Table 3) were synthesized commercially by Invitrogen (Shanghai, China). The 10 μL reaction system included 5 μL SYBR Green (TaKaRa, Dalian, China), 0.5 μL forward primer, 0.5 μL reverse primer, 3 μL nuclease-free H2O and 1 μL cDNA. The cycle procedure included a pre-cycling stage at 95 °C for 30 s, 40 cycles of denaturization at 95 °C for 5 s, and annealing at annealing temperature for 30 s with a final extension at 72 °C for 5 min. After the reaction, the melting curve generated after PCR determination was observed to check the purity and specificity of the product. All samples were run simultaneously in triplicate on the same PCR plate, and the average value of the number of copies was used for statistical analysis.
Table 3.
Sequences for real-time PCR primers.
| Gene | Accession No. | Primer sequences (5′-3′) | Size, bp | AT, °C |
|---|---|---|---|---|
| ZO-1 | XM_005659811.1 | F: CAGCCCCCGTACATGGAGA | 114 | 55.8 |
| R: GCGCAGACGGTGTTCATAGTT | ||||
| ZO-2 | NM_001206404.1 | F: ATTCGGACCCATAGCAGACATAG | 90 | 55.8 |
| R: GCGTCTCTTGGTTCTGTTTTAGC | ||||
| OCLN | NM_001163647.2 | F: CTACTCGTCCAACGGGAAAG | 158 | 55.8 |
| R: ACGCCTCCAAGTTACCACTG | ||||
| CLDN-1 | NM_001258386.1 | F: GCCACAGCAAGGTATGGTAAC | 140 | 55.8 |
| R: AGTAGGGCACCTCCCAGAAG | ||||
| CLDN-2 | NM_001161638.1 | F: GCATCATTTCCTCCCTGTT | 156 | 55.8 |
| R: TCTTGGCTTTGGGTGGTT | ||||
| GAPDH | NM_001206359.1 | F: TGAAGGTCGGAGTGAACGGAT | 114 | 59.7 |
| R: CACTTTGCCAGAGTTAAAAGCA |
PCR = polymerase chain reaction; AT = annealing temperature; ZO-1 = zonula occludens-1; ZO-2 = zonula occludens-2; OCLN = occluding; CLDN-1 = claudin-1; CLDN-2 = claudin-2; GAPDH = glyceraldehyde-3 phosphate dehydrogenase.
2.4. Statistical analysis
The pen served as the experimental unit for growth performance, diarrhea rate and nutrient digestibility data, whereas the piglet was considered as the experimental unit for the other response criteria. All data for diarrhea evaluation were converted by arcsine square root transformation for statistics. Experimental data were preprocessed by Microsoft Excel 2010), and were then analyzed using the general linear models (GLM) procedure of SAS 9.2 (SAS Inst. Inc., Cary, NC, USA). Linear and quadratic contrasts were used to further illustrate the dose effect of TA in weaned piglets. Differences between different groups were determined using a Duncan multiple comparison. The results are shown as mean and the standard error of mean (SEM). Significance was considered at P < 0.05, and 0.05 < P < 0.1 was considered to have a trend of difference.
3. Results
3.1. Growth performance and diarrhea rate
The effects of TA supplementation in the diets on growth performance, diarrhea rate and diarrhea index are presented in Table 4, Table 5. Compared with the CON group, ADFI, ADG and F:G of piglets were not influenced (P > 0.05). The addition of 0.2% and 0.4% TA to the diet reduced (P < 0.01) the diarrhea rate and diarrhea index of weaned piglets. The decrease of diarrhea rate and diarrhea index by dietary TA was dose-dependent (P < 0.01, linear).
Table 4.
Effects of tannic acid (TA) supplementation on growth performance in weaned piglets.1
| Item | TA, % |
SEM |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.05 | 0.1 | 0.2 | 0.4 | ANOVA | Linear | Quadratic | ||
| Initial BW, kg | 7.77 | 7.77 | 7.77 | 7.77 | 7.77 | 0.17 | 1.000 | 0.999 | 0.995 |
| Final BW, kg | 15.26 | 15.93 | 15.40 | 15.43 | 15.20 | 0.37 | 0.680 | 0.510 | 0.675 |
| 0 to 14 d | |||||||||
| ADFI, g | 374 | 396 | 377 | 394 | 376 | 13.38 | 0.626 | 0.852 | 0.356 |
| ADG, g | 213 | 241 | 208 | 226 | 206 | 10.86 | 0.157 | 0.310 | 0.433 |
| F:G ratio | 1.78 | 1.65 | 1.81 | 1.75 | 1.83 | 0.05 | 0.188 | 0.208 | 0.733 |
| 15 to 28 d | |||||||||
| ADFI, g | 591 | 612 | 602 | 593 | 599 | 17.09 | 0.919 | 0.899 | 0.955 |
| ADG, g | 323 | 341 | 337 | 322 | 325 | 12.13 | 0.689 | 0.579 | 0.817 |
| F:G ratio | 1.84 | 1.80 | 1.79 | 1.85 | 1.85 | 0.05 | 0.805 | 0.482 | 0.756 |
| 0 to 28 d | |||||||||
| ADFI, g | 483 | 504 | 489 | 493 | 487 | 14.21 | 0.860 | 0.870 | 0.637 |
| ADG, g | 268 | 291 | 273 | 274 | 265 | 9.21 | 0.324 | 0.336 | 0.538 |
| F:G ratio | 1.81 | 1.73 | 1.80 | 1.80 | 1.84 | 0.04 | 0.338 | 0.198 | 0.647 |
ADFI = average daily feed intake; ADG = average daily gain; F:G ratio = feed-to-gain ratio.
Values represent the means of 6 pens with 6 piglets per replicate pen (n = 36) per group for body weight, and 6 pens (n = 6) per group for ADFI, ADG, and F:G ratio.
Table 5.
Effects of tannic acid (TA) supplementation on diarrhea rate and diarrhea index in weaned piglets.1
| Item | TA, % |
SEM |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.05 | 0.1 | 0.2 | 0.4 | ANOVA | Linear | Quadratic | ||
| 0 to 14 d | |||||||||
| Diarrhea rate, % | 16.47a | 15.67a | 13.49ab | 8.73b | 3.77c | 2.27 | 0.001 | <0.0001 | 0.876 |
| Diarrhea index | 0.34a | 0.34a | 0.24ab | 0.18b | 0.08c | 0.04 | 0.0003 | <0.0001 | 0.691 |
| 15 to 28 d | |||||||||
| Diarrhea rate, % | 17.86a | 11.11ab | 9.92bc | 5.95bc | 5.56c | 2.39 | 0.005 | 0.0005 | 0.087 |
| Diarrhea index | 0.35a | 0.22ab | 0.20abc | 0.12bc | 0.10c | 0.05 | 0.007 | 0.0005 | 0.166 |
| 0 to 28 d | |||||||||
| Diarrhea rate, % | 17.16a | 13.39a | 11.71ab | 7.34bc | 4.66c | 1.81 | 0.0001 | <0.0001 | 0.214 |
| Diarrhea index | 0.34a | 0.28ab | 0.22bc | 0.15cd | 0.09d | 0.03 | <0.0001 | <0.0001 | 0.192 |
a, b, c, d Within a row, means without a common superscript letter differ at P < 0.05.
Values represent the means of 6 pens (n = 6) per group.
3.2. Nutrient digestibility
As shown in Table 6, compared with the CON group, dietary TA at 0.05%, 0.1%, or 0.20% did not affect nutrient digestibility (P > 0.05). The 0.4% TA group increased (P < 0.05) digestibility of ether extract, but reduced (P < 0.05) digestibility of crude protein and gross energy. In addition, the nutrient digestibility of dry matter, crude protein, gross energy and crude fiber linearly decreased (P < 0.05, linear) with the increase of TA dosage.
Table 6.
Effects of tannic acid (TA) supplementation on nutrient digestibility in weaned piglets (%).1
| Item | TA, % |
SEM |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.05 | 0.1 | 0.2 | 0.4 | ANOVA | Linear | Quadratic | ||
| DM | 85.69 | 85.71 | 85.13 | 85.19 | 84.46 | 0.31 | 0.057 | 0.005 | 0.960 |
| CP | 79.27a | 80.07a | 79.38a | 78.53ab | 77.09b | 0.64 | 0.028 | 0.002 | 0.554 |
| GE | 85.55a | 85.60a | 85.03ab | 85.02ab | 84.26b | 0.32 | 0.043 | 0.003 | 0.990 |
| EE | 68.00b | 67.85b | 68.11b | 68.82b | 71.48a | 0.87 | 0.035 | 0.003 | 0.348 |
| Ash | 56.37 | 55.42 | 55.17 | 56.08 | 55.38 | 0.97 | 0.890 | 0.730 | 0.873 |
| CF | 35.34 | 38.61 | 33.28 | 31.88 | 28.54 | 2.95 | 0.198 | 0.034 | 0.919 |
DM = dry matter; CP = crude protein; GE = gross energy; EE = ether extract; CF = crude fiber.
a, b Within a row, means without a common superscript letter differ at P < 0.05.
Values represent the means of 6 pens (n = 6) per group.
3.3. Intestinal morphology
As shown in Table 7, compared with the CON group, the 0.4% TA group had greater (P < 0.05) villus height in the duodenum, and diets with 0.05%, 0.2% and 0.4% TA had greater (P < 0.05) villus height in the jejunum. The villus height of duodenum and jejunum was positively correlated with the TA supplemental level (P < 0.05 linear).
Table 7.
Effects of tannic acid (TA) supplementation on intestinal morphology in weaned piglets (μm).1
| Item | TA, % |
SEM |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.05 | 0.1 | 0.2 | 0.4 | ANOVA | Linear | Quadratic | ||
| Duodenum | |||||||||
| Villus height | 341.49b | 354.18b | 359.82b | 369.12b | 406.84a | 11.50 | 0.022 | 0.002 | 0.814 |
| Crypt depth | 154.95 | 148.45 | 140.29 | 153.76 | 148.56 | 11.78 | 0.908 | 0.943 | 0.813 |
| V:C ratio | 2.21 | 2.40 | 2.58 | 2.41 | 2.87 | 0.23 | 0.377 | 0.086 | 0.939 |
| Jejunum | |||||||||
| Villus height | 326.04b | 390.30a | 336.05b | 428.03a | 400.47a | 11.91 | 0.001 | 0.001 | 0.011 |
| Crypt depth | 136.99 | 161.41 | 119.98 | 148.79 | 147.95 | 8.79 | 0.066 | 0.546 | 0.715 |
| V:C ratio | 2.38 | 2.44 | 2.83 | 2.91 | 2.71 | 0.16 | 0.147 | 0.144 | 0.047 |
| Ileum | |||||||||
| Villus height | 342.86 | 333.69 | 333.53 | 355.14 | 295.61 | 19.25 | 0.322 | 0.138 | 0.235 |
| Crypt depth | 146.24 | 141.99 | 121.55 | 125.45 | 141.12 | 9.42 | 0.313 | 0.830 | 0.064 |
| V:C ratio | 2.35ab | 2.36ab | 2.82a | 2.83a | 2.10b | 0.17 | 0.046 | 0.275 | 0.007 |
V:C ratio = the ratio of villus height to crypt depth.
a, b Within a row, means without a common superscript letter differ at P < 0.05.
Values represent the means of 6 pens (n = 6) per group.
3.4. Serum and jejunal anti-oxidative properties
The effects of dietary TA supplementation on serum and jejunal anti-oxidation are shown in Table 8. Compared with the CON group, CAT activity was improved (P < 0.05) in 0.2% TA group. The CAT activity of jejunum showed a linear increasing trend with the increase of TA dosage (P = 0.075, linear).
Table 8.
Effects of tannic acid (TA) supplementation on serum and intestinal antioxidant index in weaned piglets.1
| Item | TA, % |
SEM |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.05 | 0.1 | 0.2 | 0.4 | ANOVA | Linear | Quadratic | ||
| Serum | |||||||||
| MDA, nmoL/mL | 2.57 | 2.27 | 1.90 | 1.98 | 1.88 | 0.25 | 0.279 | 0.095 | 0.229 |
| CAT, U/mL | 12.26b | 14.20ab | 14.33ab | 17.87a | 11.56b | 1.43 | 0.039 | 0.674 | 0.004 |
| T-SOD, U/mL | 151.51 | 155.62 | 157.53 | 153.79 | 155.18 | 4.34 | 0.896 | 0.817 | 0.670 |
| GPx, U/mL | 471.91 | 521.22 | 499.00 | 490.44 | 506.80 | 19.11 | 0.464 | 0.583 | 0.785 |
| T-AOC, U/mL | 1.53 | 1.67 | 1.72 | 1.62 | 1.69 | 0.07 | 0.446 | 0.385 | 0.503 |
| Jejunum | |||||||||
| MDA, nmoL/mg prot | 0.76 | 0.68 | 0.70 | 0.73 | 0.76 | 0.11 | 0.979 | 0.756 | 0.756 |
| CAT, U/mg prot | 4.59 | 4.63 | 5.59 | 5.29 | 5.39 | 0.31 | 0.098 | 0.075 | 0.146 |
| T-SOD, U/mg prot | 74.54 | 78.26 | 82.46 | 73.95 | 77.61 | 2.74 | 0.220 | 0.972 | 0.748 |
| GPx, U/mg prot | 36.50 | 51.57 | 50.14 | 42.90 | 42.59 | 5.67 | 0.344 | 0.848 | 0.321 |
| T-AOC, U/mg prot | 0.29 | 0.39 | 0.35 | 0.39 | 0.38 | 0.03 | 0.183 | 0.185 | 0.189 |
MDA = malondialdehyde; CAT = catalase; T-SOD = total superoxide dismutase; GPx = glutathione peroxidase; T-AOC = total antioxidant capacity.
a, b Within a row, means without a common superscript letter differ at P < 0.05.
Values represent the means of 6 pens (n = 6) per group.
3.5. Intestinal digesta microbiota and microbial metabolites
As shown in Table 9, compared with the CON group, the addition of 0.05%, 0.1%, 0.2% and 0.4% TA improved (P < 0.05) Bacillus counts in cecal digesta. The Bacillus counts were improved (P < 0.01) in 0.4% TA group and the Escherichia coli counts were reduced (P < 0.05) in 0.1% TA group in colonic digesta. It was worth noting that the number of Bacillus in cecal and colon digesta linearly increased (P < 0.05, linear) with the increase of TA dosage. As shown in Table 10, compared with the CON group, dietary TA supplementation improved (P < 0.05) the concentrations of acetic acid (0.2%), propionic acid (0.2%, 0.4%), butyric acid (0.05%, 0.1%, 0.2%, 0.4%) and isovaleric acid (0.1%, 0.2%, 0.4%) in cecal digesta. The 0.4% TA group improved (P < 0.05) the concentrations of acetic acid in colon digesta. The increase of propionic acid, butyric acid and valeric acid concentrations in cecal chyme by dietary TA was dose-dependent (P < 0.05). In colonic chyme, acetic acid was linearly increased and isobutyric acid was linearly decreased with the increase of TA supplemental level (P < 0.05).
Table 9.
Effects of tannic acid (TA) supplementation on digesta microflora in weaned piglets (log10 cfu/g).1
| Item | TA, % |
SEM |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.05 | 0.1 | 0.2 | 0.4 | ANOVA | Linear | Quadratic | ||
| Cecum | |||||||||
| Total bacteria | 10.81ab | 11.05a | 10.87ab | 10.91a | 10.63b | 0.08 | 0.019 | 0.013 | 0.080 |
| Escherichia coli | 7.56 | 7.58 | 7.92 | 7.79 | 7.72 | 0.29 | 0.889 | 0.734 | 0.478 |
| Lactobacillus | 6.38 | 7.09 | 6.62 | 6.87 | 6.93 | 0.20 | 0.131 | 0.220 | 0.461 |
| Bacillus | 7.94b | 8.73a | 9.00a | 8.93a | 9.25a | 0.19 | 0.0006 | 0.0003 | 0.027 |
| Bifidobacterium | 6.01 | 6.44 | 6.60 | 6.42 | 6.56 | 0.18 | 0.175 | 0.138 | 0.211 |
| Colon | |||||||||
| Total bacteria | 11.11 | 11.04 | 10.92 | 10.98 | 11.01 | 0.05 | 0.188 | 0.411 | 0.062 |
| Escherichia coli | 7.72ab | 6.77bc | 6.64c | 7.09abc | 8.05a | 0.32 | 0.016 | 0.055 | 0.011 |
| Lactobacillus | 7.05 | 7.26 | 7.29 | 7.47 | 7.33 | 0.19 | 0.614 | 0.335 | 0.214 |
| Bacillus | 8.46b | 8.74ab | 8.44b | 8.60b | 9.05a | 0.01 | 0.008 | 0.002 | 0.173 |
| Bifidobacterium | 6.82 | 6.74 | 6.93 | 6.52 | 6.80 | 0.17 | 0.552 | 0.745 | 0.389 |
a, b Within a row, means without a common superscript letter differ at P < 0.05.
Values represent the means of 6 pens (n = 6) per group.
Table 10.
Effects of tannic acid (TA) supplementation on digesta microbial metabolites in weaned piglets (μmoL/g).1
| Item | TA, % |
SEM |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.05 | 0.1 | 0.2 | 0.4 | ANOVA | Linear | Quadratic | ||
| Cecal digesta | |||||||||
| Acetic acid | 52.11b | 51.75b | 51.36b | 68.62a | 58.89ab | 4.16 | 0.030 | 0.060 | 0.074 |
| Propionic acid | 23.93c | 25.71bc | 29.40bc | 49.58a | 41.13ab | 5.28 | 0.008 | 0.005 | 0.039 |
| Butyric acid | 4.58b | 8.12a | 9.08a | 9.78a | 11.37a | 1.46 | 0.042 | 0.006 | 0.184 |
| Isobutyric acid | 0.76 | 0.68 | 0.56 | 0.67 | 0.43 | 0.11 | 0.237 | 0.050 | 0.898 |
| Isovaleric acid | 1.65b | 2.67ab | 3.92a | 2.93a | 2.73a | 0.47 | 0.041 | 0.441 | 0.026 |
| Valeric acid | 1.12 | 1.66 | 1.80 | 2.39 | 2.24 | 0.32 | 0.070 | 0.018 | 0.081 |
| Total VFA | 80.03b | 86.03b | 89.16b | 114.58a | 102.97ab | 7.93 | 0.033 | 0.017 | 0.053 |
| Colonic digesta | |||||||||
| Acetic acid | 39.25b | 41.46b | 39.25b | 42.46b | 50.65a | 2.54 | 0.023 | 0.002 | 0.315 |
| Propionic acid | 17.59 | 17.15 | 17.74 | 18.08 | 19.91 | 1.55 | 0.756 | 0.204 | 0.724 |
| Butyric acid | 10.30 | 10.05 | 11.56 | 12.86 | 10.92 | 1.56 | 0.721 | 0.623 | 0.247 |
| Isobutyric acid | 1.45abc | 1.96a | 1.89ab | 1.33bc | 0.90c | 0.20 | 0.005 | 0.002 | 0.171 |
| Isovaleric acid | 2.73 | 3.73 | 3.73 | 3.10 | 3.19 | 0.47 | 0.516 | 0.909 | 0.458 |
| valeric acid | 1.96 | 2.44 | 2.82 | 2.49 | 2.41 | 0.35 | 0.548 | 0.659 | 0.223 |
| Total VFA | 73.27 | 76.79 | 76.99 | 80.32 | 87.97 | 5.53 | 0.419 | 0.057 | 0.966 |
VFA = volatile fatty acids.
a,b,c Values within a row lacking a common superscript are different (P < 0.05).
Values represent the means of 6 pens (n = 6) per group.
3.6. The mRNA expression of tight junction protein
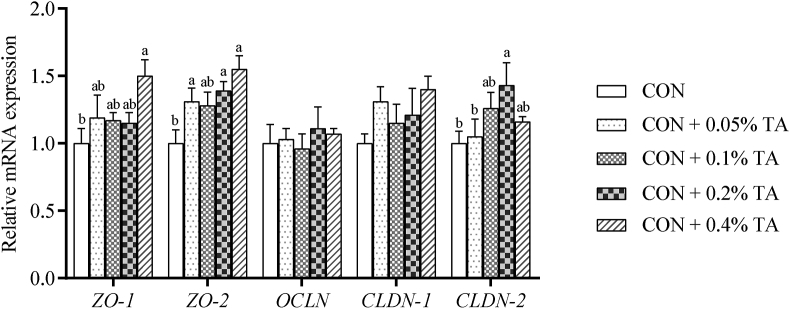
As shown in Fig. 1, compared with the CON group, the mRNA expression levels of ZO-1 (0.4%), ZO-2 (0.05%, 0.2%, 0.4%) and CLDN-2 (0.2%) were improved (P < 0.05) in the jejunum.
Fig. 1.
Effects of tannic acid (TA) on mRNA expression levels of tight junction proteins in jejunal mucosa of weaned piglets. Each bar represents the average expression level with 6 independent replications. CON, piglets receiving a basal diet; CON + 0.05% TA, piglets receiving basal diet supplemented with 0.05% TA; CON + 0.1% TA, piglets receiving basal diet supplemented with 0.1% TA; CON + 0.2% TA, piglets receiving basal diet supplemented with 0.2% TA; CON + 0.4% TA, piglets receiving basal diet supplemented with 0.4% TA. ZO-1 = zonula occludens-1, ZO-2 = zonula occludens-2, OCLN = occludin, CLDN-1 = claudin-1, CLDN-2 = claudin-2. a, b Mean values with different superscript letters were significantly different (P < 0.05).
4. Discussion
Tannic acid is an excellent astringent that has impressive anti-diarrhea effects (Song et al., 2017; Girard et al., 2018). Tannic acid can significantly increase dry matter content and reduce moisture content in feces (Schiavone et al., 2008; Rezar and Salobir, 2014). Tannic acid exhibits antidiarrheal activity against castor oil-induced diarrhea in mice through alleviating diarrhea symptoms and inhibiting small intestinal motility and secretion (Song et al., 2017). Tannic acid significantly reduced the incidence rate of diarrhea and the duration of diarrhea in an experimental ETEC F4 challenge model (Girard et al., 2018). Our study found that addition of TA significantly reduced diarrhea rate and diarrhea index of weaned piglets, and there was a dose-dependent relationship between the decrease of diarrhea rate and index and the increase of TA supplementation. It is speculated that TA coagulates proteins on the mucosal surface of the gastrointestinal tract, reduces inflammatory exudates and the flow rate of chyme, slows intestinal peristalsis, and presents an antidiarrheal effect (Tonda et al., 2018). As an ANF, diets rich in TA are generally considered unfavorable to the growth performance and nutrient digestibility of monogastric animals due to its strong affinity with proteins, digestive enzymes, and polysaccharides (Viveros et al., 2011; Ebrahim et al., 2015). However, many scholars have observed that the addition of 0% to 1% TA had no effect on growth performance and nutrient digestibility in monogastric animals (Štukelj et al., 2010; Rezar and Salobir, 2014). So as of now, there is still some controversy about the impact of TA on the growth of monogastric animals. This study found that adding 0.05% to 0.4% TA to the diet had no negative effect on the growth performance of weaned piglets. The 0.05%, 0.1% and 0.2% TA groups had no adverse effects on nutrient digestibility of weaned piglets. The 0.4% TA group significantly reduced the digestibility of crude protein and gross energy, but significantly increased the digestibility of ether extract. Tannic acid is an ANF, but it has the effect of improving intestinal health. These 2 opposite effects may be one of the reasons why TA showed no significant effect on growth performance in this experiment. The improvement in ether extract digestibility in 0.4% TA group may be related to the interaction between TA and lipase, but the mechanism is still unclear and further research is needed. In summary, higher doses of TA have been shown to have anti-nutritional effects, such as reduced nutrient utilization, while relatively appropriate doses have been shown to improve intestinal health, such as reduced diarrhea.
The gastrointestinal tract (GIT) plays an important role in the digestion, absorption and immunity of animals. Small intestinal morphology including the villus height, crypt depth and their ratio, is one of the main indicators reflecting the health status of piglets’ intestines (Fei et al., 2013). Studies have found that TA can significantly reduce crypt depth of weaned piglets' ileum (Biagia et al., 2010) and increased villus height, villus circumference and mucosal thickness of male pigs' duodenum (Bilić-Šobot et al., 2016). In our experiment, the addition of TA to the diet significantly increased the villus height of the duodenum and jejunum. The small intestinal crypt has secretory function, so reducing crypt depth is beneficial to alleviate post-weaning diarrhea (Biagia et al., 2010). There was a quadratic regression relationship between the amount of dietary TA addition and ileal crypt depth in our trial, which may be one of the reasons why 0.1% - 0.2% TA exhibits an anti-diarrheal effect. The results of this experiment showed that the addition of TA to the diet has a positive effect on intestinal morphology development.
Reactive oxygen species (ROS) are by-products of cellular metabolism and essential participants in cell signaling and regulation (Tezel, 2006). Oxidative stress is generally thought to be an imbalance between an increase in ROS and a decrease in activity in antioxidant mechanisms, which can cause damage to cells and tissues (Droge, 2002). Tannic acid is an effective natural antioxidant component, as it can combine with free-radicals forming resonance-stabilized phenoxy radicals (Gülçin et al., 2010; Dalle Zotte et al., 2018). Previous studies have found that TA can significantly improve the antioxidant capacity of diabetic rats, and of rabbits under high ambient temperature (Hua et al., 2011; Wei et al., 2011). Superoxide dismutase, GPx and CAT are 3 important antioxidant enzymes in the animal body. They are the main components of the antioxidant defense mechanism, which can protect cells from damage caused by excessive concentration of ROS, and reduce oxidative stress (Weydert and Cullen, 2009). Glutathione peroxidase and CAT activities are often related because they degrade the same kind of substrates (Faria et al., 2007). (Ye et al., 2016) found that TA can significantly increase the activity of CAT and GPx in the liver of Brandt's voles. In our experiment, the diets with TA supplementation increased CAT activity in serum and jejunum mucosa. Our experimental results demonstrated that TA has a beneficial effect on improving the antioxidant capacity of weaned pigs. There is still a lack of research on the effect of TA on antioxidant properties of piglets, and this experiment did not reflect the effect of TA on other antioxidant capacities, which may be related to animal species.
Gut microbiota and their metabolites are closely involved in host physiology, such as nutritional status, metabolism and stress response (Sekirov et al., 2010). Gut microbiota disruption is considered as one of the important reasons leading to post-weaning diarrhea and enteric infections (Gresse et al., 2017). E. coli is one of the important pathogenic causes of diarrhea after weaning (Fairbrother et al., 2005). The proliferation of beneficial bacteria such as Lactobacillus, Bacillus, Bifidobacterium, etc. is conducive to improving intestinal health. Previous studies have found that diets supplemented with TA can reduce the number of E. coli and increase the number of Lactobacilli in the gut of monogastric animals (Jamroz et al., 2009; Brus et al., 2013). Our results showed that, the addition of TA to the diet significantly improved Bacillus counts in cecal digesta. Further, the addition of TA significantly improved Bacillus counts and significantly reduced the E. coli counts in colon digesta. The results of this study are similar to those of the predecessors, indicating that adding TA to the diet can effectively reduce the number of harmful bacteria, increase the number of beneficial bacteria, and improve the intestinal microbial homeostasis, however, the mechanism by which dietary TA affects intestinal bacteria still remains elusive.
Volatile fatty acids are indicators of bacterial activity in the large intestine. Acetic acid, propionic acid and butyric acid are the most abundant in VFA, and these VFA account for about 90% of the total acid production in the hindgut of monogastric animals (Dongsheng et al., 2019). The results of this experiment showed that dietary TA supplementation increased the concentrations of acetic acid, propionic acid, butyric acid, isovaleric acid, valeric acid in cecal digesta. The reason for this phenomenon may be that dietary TA supplementation reduces the digestion and absorption of nutrients in the small intestine, resulting in more nutrients flowing to the large intestine and more intensive bacterial fermentation (Barszcz et al., 2011). An increase in beneficial bacteria also seems to reinforce this behavior. In fact, increasing concentrations of TA linearly reduced concentrations of isobutyric acid in cecal and colon digesta, which indicates that TA may reduce the proteolytic reaction of intestinal bacteria because isobutyric acid is a metabolite deriving from protein bacterial catabolism.
Intestinal epithelial tight junction proteins are closely related to intestinal barrier and intestinal inflammatory diseases (Wang et al., 2015). It and intestinal morphology are important factors affecting intestinal barrier function (Tan et al., 2018). Our study found that the addition of TA to the diet significantly improved the mRNA expression level of ZO-1, ZO-2 and CLDN-2 in jejunum. This indicates that TA can up-regulate the mRNA expression level of tight junction proteins, thereby improving the paracellular barrier.
5. Conclusions
In summary, the present study demonstrated that TA (0.05% to 0.4%) significantly reduced the diarrhea rate and diarrhea index without altering growth performance, and improved intestinal integrity and morphology, hindgut microbial homeostasis and fermentation in weaned piglets. This study suggests that, compared to the control, the most appropriate level of TA supplementation would be 0.1% to 0.2%.
Author contribution
Yanyan Song: Methodology, Investigation, Data curation, Writing-Original draft. Yong Luo: Methodology, Writing-Review & editing. Bing Yu: Methodology, Investigation. Jun He: Investigation, Resources. Ping Zheng: Investigation, Formal analysis. Xiangbing Mao: Investigation. Zhiqing Huang: Data curation. Junqiu Luo: Investigation. Yuheng Luo: Validation. Hui Yan: Writing-Review & editing. Quyuan Wang: Project administration. Huifen Wang: Investigation. Jie Yu: Methodology, Writing-Review & editing, Funding acquisition. Daiwen Chen: Conceptualization, Writing-Review & editing, Funding acquisition.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgement
This study was supported by National Key Research and Development Project (2016YFD0501204), Sichuan Provincial Transformation Program for Agricultural Science and Technology Achievements (2020NZZJ005), the earmarked fund for China Agriculture Research System of MOF and MARA (CARS-35), and funded in part by Guangzhou Insighter Biotechnology Co., Ltd. (GuangZhou, China).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Contributor Information
Daiwen Chen, Email: dwchen@sicau.edu.cn.
Jie Yu, Email: yujie@sicau.edu.cn.
References
- Barszcz M., Taciak M., Skomiał J. A dose-response effects of tannic acid and protein on growth performance, caecal fermentation, colon morphology, and β-glucuronidase activity of rats. J Anim Feed Sci. 2011;20:613–625. [Google Scholar]
- Biagia G., Cipollini I., Paulicks B.R., Roth F.X. Effect of tannins on growth performance and intestinal ecosystem in weaned piglets. Arch Anim Nutr. 2010;64:121–135. doi: 10.1080/17450390903461584. [DOI] [PubMed] [Google Scholar]
- Bilić-Šobot D., Kubale V., Prevolnik P.M., Fazarinc G. Effect of hydrolysable tannins on intestinal morphology, proliferation and apoptosis in entire male pigs. Arch Anim Nutr. 2016;70:378. doi: 10.1080/1745039X.2016.1206735. [DOI] [PubMed] [Google Scholar]
- Brus M., Dolinšek J., Cencič A., Škorjanc D. Effect of chestnut (Castanea sativa Mill.) wood tannins and organic acids on growth performance and faecal microbiota of pigs from 23 to 127 d of age. Bulg J Agric Sci. 2013;19:841–847. [Google Scholar]
- Buyukcapar H., Atalay A., Kamalak A. Growth performance of Nile tilapia (Oreochromis niloticus) fed with diets containing different levels of hydrolysable and condensed tannin. J Agric Sci Technol. 2011;13:1045–1051. [Google Scholar]
- Chen J., Yu B., Chen D., Huang Z., Mao X., Zhen P. Dietary chlorogenic acid improves growth performance of weaned pigs through maintaining antioxidant capacity and intestinal digestion and absorption function. J Anim Sci. 2018;96:1108–1118. doi: 10.1093/jas/skx078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalle Zotte A., Cullere M., Tasoniero G., Gerencsér Z., Szendrő Z., Novelli E. Supplementing growing rabbit diets with chestnut hydrolyzable tannins: effect on meat quality and oxidative status, nutrient digestibilities, and content of tannin metabolites. Meat Sci. 2018;146:101–108. doi: 10.1016/j.meatsci.2018.08.007. [DOI] [PubMed] [Google Scholar]
- Davin R., Manzanilla E.G., Klasing K.C., Pérez J. Effect of weaning and in-feed high doses of zinc oxide on zinc levels in different body compartments of piglets. J Anim Physiol Anim Nutr. 2013;97:6–12. doi: 10.1111/jpn.12046. [DOI] [PubMed] [Google Scholar]
- Dongsheng C., Seidu A., Cai W., Qin G.X., Atiba E.M., Jiang H. Effects of Astragalus membranaceus fiber on growth performance, nutrient digestibility, microbial composition, VFA production, gut pH, and immunity of weaned pigs. Microbiology. 2019;8 doi: 10.1002/mbo3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Ebrahim R., Liang J.B., Jahromi M.F., Shokryazdan P., Ebrahimi M., Chen W.L. Effects of tannic acid on performance and fatty acid composition of breast muscle in broiler chickens under heat stress. Ital J Anim Sci. 2015;14:3956. [Google Scholar]
- Fairbrother J.M., Nadeau E., Gyles C.L. Escherichia coli in postweaning diarrhea in pigs: an update on bacterial types, pathogenesis, and prevention strategies. Anim Health Res Rev. 2005;6:17–39. doi: 10.1079/ahr2005105. [DOI] [PubMed] [Google Scholar]
- Faria A., Monteiro R., Mateus N., Azevedo I., Calhau C. Effect of pomegranate ( Punica granatum ) juice intake on hepatic oxidative stress. Eur J Nutr. 2007;46:271–278. doi: 10.1007/s00394-007-0661-z. [DOI] [PubMed] [Google Scholar]
- Fei H., Liang H., Yue X., Xuemei D., Yuheng L., Shiping B. Effects of high nutrient intake on the growth performance, intestinal morphology and immune function of neonatal intra-uterine growth-retarded pigs. Br J Nutr. 2013;110:1819–1827. doi: 10.1017/S0007114513001232. [DOI] [PubMed] [Google Scholar]
- Gülçin İ., Huyut Z., Elmastaş M., Aboul-Enein H.Y. Radical scavenging and antioxidant activity of tannic acid. Arab J Chem. 2010;3:43–53. [Google Scholar]
- Girard M., Thanner S., Pradervand N., Hu D., Ollagnier C., Bee G. Hydrolysable chestnut tannins for reduction of postweaning diarrhea: efficacy on an experimental ETEC F4 model. PloS One. 2018;13 doi: 10.1371/journal.pone.0197878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresse R., Chaucheyras-Durand F., Fleury M.A., Forano E., Blanquet-Diot S. Gut microbiota dysbiosis in postweaning piglets: understanding the keys to health. Trends Microbiol. 2017;25:851–873. doi: 10.1016/j.tim.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Hua W.L., Xiao F.D., Jian M.T., Zhang Q.I. A comparative study of growth performance and antioxidant status of rabbits when fed with or without chestnut tannins under high ambient temperature. Anim Feed Sci Technol. 2011;164 0-95. [Google Scholar]
- Huang Q., Liu X., Zhao G., Hu T., Wang Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim Nutr. 2018;4:137–150. doi: 10.1016/j.aninu.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamroz D., Wiliczkiewicz A., Skorupińska J., Orda J., Kuryszko J., Tschirch H. Effect of sweet chestnut tannin (SCT) on the performance, microbial status of intestine and histological characteristics of intestine wall in chickens. Br Poultry Sci. 2009;50:687–699. doi: 10.1080/00071660903191059. [DOI] [PubMed] [Google Scholar]
- Jansman A.J. Tannins in feedstuffs for simple-stomached animals. Nutr Res Rev. 1993;6:209–236. doi: 10.1079/NRR19930013. [DOI] [PubMed] [Google Scholar]
- Jezierny D., Mosenthin R., Bauer E. The use of grain legumes as a protein source in pig nutrition: a review. Anim Feed Sci Technol. 2012;157:111–128. [Google Scholar]
- Jiali C., Bing Y., Daiwen C., Zhiqing H., Xiangbing M., Ping Z. Chlorogenic acid improves intestinal barrier functions by suppressing mucosa inflammation and improving antioxidant capacity in weaned pigs. J Nutr Biochem. 2018;59:84–92. doi: 10.1016/j.jnutbio.2018.06.005. [DOI] [PubMed] [Google Scholar]
- Lavin S.R., Chen Z.S., Abrams S.A. Effect of tannic acid on iron absorption in straw-colored fruit bats (Eidolon helvum) Zoo Biol. 2010;29:335–343. doi: 10.1002/zoo.20258. [DOI] [PubMed] [Google Scholar]
- Mathew A.G., Ebner P.D. 2005. Issues of drug use and antibiotic resistance in pig production. [Google Scholar]
- Redondo L.M., Chacana P.A., Dominguez J.E., Fernandez Miyakawa M.E. Perspectives in the use of tannins as alternative to antimicrobial growth promoter factors in poultry. Front Microbiol. 2014;5:118. doi: 10.3389/fmicb.2014.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezar V., Salobir J. Effects of tannin-rich sweet chestnut (Castanea sativa mill.) wood extract supplementation on nutrient utilisation and excreta dry matter content in broiler chickens. Eur Poult Sci. 2014;78:1–10. [Google Scholar]
- Schiavone A., Guo K., Tassone S., Gasco L., Hernandez E., Denti R. Effects of a natural extract of chestnut wood on digestibility, performance traits, and nitrogen balance of broiler chicks. Poultry Sci. 2008;87:521–527. doi: 10.3382/ps.2007-00113. [DOI] [PubMed] [Google Scholar]
- Sekirov I., Russell S.L., Antunes L., Finlay B. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- Song X., Yu L., Yin Z., Xie J., Yang J., Lin J. Preparation of Galla Chinensis oral solution as well as its stability, safety, and antidiarrheal activity evaluation. Evid base Compl Alternative Med. 2017:1851459. doi: 10.1155/2017/1851459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Štukelj M., Valencak Z., Krsnik M., Svete A.N. The effect of the combination of acids and tannin in diet on the performance and selected biochemical, haematological and antioxidant enzyme parameters in grower pigs. Acta Vet Scand. 2010;52:19. doi: 10.1186/1751-0147-52-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X., Sun Z., Zhou C., Huang Z., Tan L., Xun P. Effects of dietary dandelion extract on intestinal morphology, antioxidant status, immune function and physical barrier function of juvenile golden pompano Trachinotus ovatus. Fish Shellfish Immunol. 2018;73:197. doi: 10.1016/j.fsi.2017.12.020. [DOI] [PubMed] [Google Scholar]
- Tezel G. Oxidative stress in glaucomatous neurodegeneration: mechanisms and consequences. Prog Retin Eye Res. 2006;25:490–513. doi: 10.1016/j.preteyeres.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonda R.M., Rubach J.K., Lumpkins B.S., Mathis G.F., Poss M.J. Effects of tannic acid extract on performance and intestinal health of broiler chickens following coccidiosis vaccination and/or a mixed-species Eimeria challenge. Poultry Sci. 2018;97:3031–3042. doi: 10.3382/ps/pey158. [DOI] [PubMed] [Google Scholar]
- Viveros A., Chamorro S., Pizarro M., Arija I., Centeno C., Brenes A. Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poultry Sci. 2011;90:566–578. doi: 10.3382/ps.2010-00889. [DOI] [PubMed] [Google Scholar]
- Wang Z., Li R., Tan J., Peng L., Wang P., Xiong H. Syndecan-1 acts in synergy with tight junction through stat 3 signaling to maintain intestinal mucosal barrier and prevent bacterial translocation. Inflamm Bowel Dis. 2015;21:1894–1907. doi: 10.1097/MIB.0000000000000421. [DOI] [PubMed] [Google Scholar]
- Wei H.F., Wei Y.H., Xiang-Jun L. The effect of tannic acid on antioxidant capacity in diabetic rats and non-enzymatic glycation of protein in vitro. Chin J Lab Diagnosis. 2011;15:977–979. [Google Scholar]
- Weydert C.J., Cullen J.J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat Protoc. 2009;5:51–66. doi: 10.1038/nprot.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M.H., Nan Y.L., Ding M.M., Hu J.B., Liu Q., Wei W.H. Effects of dietary tannic acid on the growth, hepatic gene expression, and antioxidant enzyme activity in Brandt's voles ( Microtus brandti ) Comp Biochem Physiol B Biochem Mol Biol. 2016;196–197:19–26. doi: 10.1016/j.cbpb.2016.01.011. [DOI] [PubMed] [Google Scholar]
- Yu J., Song Y., Yu B., He J., Chen D. Tannic acid prevents post-weaning diarrhea by improving intestinal barrier integrity and function in weaned piglets. J Anim Sci Biotechnol. 2020;11:87. doi: 10.1186/s40104-020-00496-5. [DOI] [PMC free article] [PubMed] [Google Scholar]