Abstract
Background
Pathological neovascularization in neovascular age-related macular degeneration (nAMD) is the leading cause of vision loss in the elderly. Increasing evidence shows that cells of myeloid lineage play important roles in controlling pathological endothelium formation. Suppressor of cytokine signaling 3 (SOCS3) pathway has been linked to neovascularization.
Methods
We utilised a laser-induced choroidal neovascularization (CNV) mouse model to investigate the neovascular aspect of human AMD. In several cell lineage reporter mice, bone marrow chimeric mice and Socs3 loss-of-function (knockout) and gain-of-function (overexpression) mice, immunohistochemistry, confocal, and choroidal explant co-culture with bone marrow-derived macrophage medium were used to study the mechanisms underlying pathological CNV formation via myeloid SOCS3.
Findings
SOCS3 was significantly induced in myeloid lineage cells, which were recruited into the CNV lesion area. Myeloid Socs3 overexpression inhibited laser-induced CNV, reduced myeloid lineage-derived macrophage/microglia recruitment onsite, and attenuated pro-inflammatory factor expression. Moreover, SOCS3 in myeloid regulated vascular sprouting ex vivo in choroid explants and SOCS3 agonist reduced in vivo CNV.
Interpretation
These findings suggest that myeloid lineage cells contributed to pathological CNV formation regulated by SOCS3.
Funding
This project was funded by NIH/NEI (R01EY030140, R01EY029238), BrightFocus Foundation, American Health Assistance Foundation (AHAF), and Boston Children's Hospital Ophthalmology Foundation for YS and the National Institutes of Health/National Heart, Lung and Blood Institute (U01HL098166) for PZ.
Keywords: Choroidal neovascularization, Myeloid lineage, SOCS3, Pathological endothelium formation, Age-related macular degeneration
Abbreviations: nAMD, neovascular Age-related Macular Degeneration; SOCS3, Suppressor of Cytokine Signaling 3; CNV, Choroidal Neovascularization; VEGF, Vascular Endothelial Growth Factor; IL, Interleukin; EC, Endothelial Cells; STAT3, Signal Transducer and Activator of Transcription 3; pSTAT3, phosphorylated STAT3; Socs3 cKO, Socs3 myeloid-specific knockout mice; Socs3 cOE, Socs3 myeloid-specific overexpression mice; vWF, von Willebrand Factor; IBA1, Ionised calcium Binding Adaptor molecule 1; mTmG, membrane-associated Tomato red and membrane-associated Green fluorescent protein
Research in context.
Evidence before this study
Previous studies demonstrated that myeloid lineage plays a role in retinal vascular development and SOCS3 pathway has been linked to neovascularization. However, the localisation, morphology, and distribution of myeloid lineage cells around choroidal neovascularization lesion were unknown, as were how and even if myeloid lineage cell activation and recruitment around choroidal neovascularization lesion regulated by SOCS3.
Added value of this study
In this study, our findings provided better understanding of the critical barrier to identify a root cause of choroidal neovascularization in neovascular age-related macular degeneration (AMD) by investigating myeloid lineage derived immune cell activation and recruitment around choroidal neovascularization and inflammatory factor secretion. This work identified potential strategies to prevent and treat neovascular AMD by genetic overexpression or activation of SOCS3 using pharmacological approaches.
Implications of all the available evidence
These findings will broaden the concepts in this field and expand retinal vascular biology research to include modulation of immune-vascular crosstalk via SOCS3 to prevent pathological blood vessel growth.
Alt-text: Unlabelled box
1. Introduction
Choroidal neovascularization (CNV) may cause rapid and severe vision impairment in patients with neovascular age-related macular degeneration (nAMD). Current anti-vascular endothelial growth factor (VEGF) therapy can slow disease progression and may even restore some vision. However, some patients do not respond well to this therapy. There is a pressing need for new approaches for nAMD that are supplementary to anti-VEGF therapy. Understanding the underlying molecular mechanisms of CNV formation may lead to novel insights.
Inflammatory changes in immune cells, particularly myeloid lineage cells, influence pathological angiogenesis [1], [2], [3], [4]. Myeloid lineage immune cells are recruited from choroidal circulation to the eyes [5], [6], [7], which influences retinal and choroidal vascular pathology [2,8,9]. Activated myeloid cells release inflammatory mediators that stimulate ocular neovascularization [10], [11], [12], such as VEGF, interleukins (ILs), and chemokines. Chemokines can attract immune cells to ocular tissues and further activate more trafficking molecules sensed by migrating immune cells [13]. Immune cells such as microglia can influence retinal inflammation and contribute to the formation of pathological retinal neovessels [14], [15], [16]. Immune cells produce cytokines and growth factors that may interact with endothelial cells (EC). There is increasing evidence that myeloid lineage cells play roles in retinal vascular development [17,18], remodelling [19], and repair [20,21]. Although myeloid cells have been immunohistochemically characterised in mouse eyes [6], myeloid cell infiltration and inflammatory regulators in choroidal neovascular lesions have not been previously described. This information is important to understand how the inflammatory response contributes to CNV formation.
Suppressor of cytokine signalling 3 (SOCS3) is a major inflammatory mediator that controls innate and adaptive immunity, tissue inflammation, cytokine production, and macrophage polarisation [22], [23], [24]. It is essential in suppressing inflammation in disease via IL-6 and granulocyte colony-stimulating factor pathways [25], and regulates signal transducer and activator of transcription 3 (STAT3) activation using the gp130 receptor for IL-6 family. SOCS3 pathway has been linked to neovascularization [26], [27], [28], [29]. Chen aet al. reported that activation of STAT3 by myeloid deletion of SOCS3 controls laser-induced CNV [29]. Deletion of SOCS3 in Tie2-expressing cells in an oxygen-induced retinopathy mouse model of retinopathy of prematurity increases pathological neovascularization via reduced feedback inhibition of the STAT3 and mTOR pathways [26]. We previously reported that SOCS3 in neurons/glial cells suppresses pathological retinal angiogenesis by inhibiting the STAT3-mediated secretion of VEGF [27]. Suppression of SOCS3 attenuates the protective effects of retinoic-acid-receptor–related orphan receptor alpha in retinopathy in the oxygen-induced retinopathy mouse model [28]. In this study, we examined the mechanism underlying pathological CNV formation via myeloid SOCS3.
We showed that SOCS3 was significantly increased in myeloid lineage cells in a laser-induced CNV model of the neovascular aspect of human nAMD [30,31]. Myeloid Socs3 conditional knockout accelerated laser-induced CNV. Myeloid Socs3 overexpression inhibited laser-induced CNV, reduced myeloid lineage-derived macrophage/microglia recruitment onsite and pro-inflammatory factor expression in CNV. In addition, SOCS3 in myeloid cells regulated choroid explant sprouts ex vivo and SOCS3 agonist reduced in vivo CNV. These findings suggested that myeloid SOCS3 controls CNV through modulating myeloid lineage-derived macrophage/microglia and their onsite recruitment.
2. Methods
2.1. Animals
All the mice were housed in the animal facility at Boston Children's Hospital which is supplied with HEPA-filtered air and maintained at a humidity of 35% ± 4%, and operates on a 12: 12 hour light/dark cycle at 22 ± 2°C. Socs3 flox (Socs3 f/f) mice [32] (thanks to a gift from Dr. A. Yoshimura) was crossed with myeloid-specific LysM-Cre mice (Jackson Laboratory, stock # 004781) to generate myeloid-specific Socs3 knockout mice (Socs3 cKO). Socs3 OE flox (Socs3 OE) mice [33] (thanks to a gift from Dr. Allison W. Xu) were crossed with myeloid-specific LysM-Cre mice were to generate Socs3 myeloid-specific overexpression mice (Socs3 cOE). Their littermate flox/flox mice were used as control mice. Both male and female mice were used for all experiments. C57BL/6J mice (stock # 000664), mTmG (membrane-associated tomato red and membrane-associated green fluorescent protein) reporter mice (stock# 007576), and GFP mice (stock # 006567) were obtained from the Jackson Laboratory. Tie2-Cre-GFP mice and Cdh5-CreER-GFP mice were provided by Dr. William T. Pu with the permission of Dr. Ralf H. Adams [34]. The strategies of generating myeloid-specific Socs3 knockout and overexpression as well as all the reporter mice are summarized in Fig. S1.
2.2. Ethics statement
All mouse studies, animal care and monitoring were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at the Boston Children's Hospital (ref no. 19-06-3959) and all mouse experiments were performed following the guidance of The Association for Research in Vision and Ophthalmology (ARVO) for the ethical use of animals in ophthalmic and vision research.
2.3. Laser-induced CNV, choroid flat-mount, imaging, and quantification
Laser-induced CNV was induced using Micron IV Image-Guided Laser System (Phoenix) as described [35]. Mice were anesthetised using ketamine/xylazine (intraperitoneal injection) and pupils were dilated with 1% tropicamide. Four laser burns were induced in each eye. Naringenin (400mg/kg/day, gavage) and SOCS3 peptide (LKTFSSKSEYQLVVNAVRKLQESG) [36] (50ug/kg/day, i.p.) as well as their control reagents were administrated to C57BL/6J mice from Day 1 to Day 6 after laser exposure. Six-eight-week old mice per group were randomly divided into two groups: control group and treatment group. Mice were numbered with random numbers generated using the standard - RAND ()*10 function in Microsoft Excel [37]. Mice were euthanized at the indicated days after laser exposure. Eyes were collected and fixed in 4% paraformaldehyde (Fisher Scientific, AAJ19943K2) in 0·01M PBS for 1 hour. The retinal pigment epithelium-sclera-choroid complex was dissected and permeabilized with 0·2% Triton X-100 in PBS for 1 hour. Isolectin GS-IB4 (ThermoFisher Scientific, I21411, RRID: AB_23146) was used to stain the CNV lesions for phenotypical analysis. After washing with PBS, the retinal pigment epithelium-sclera-choroid complex were flat-mounted onto slides using mounting medium (Vector Labs, H-1000-10). The images were taken using AxioObserver.Z1 microscope (Zeiss) or the Zeiss 700/710 confocal microscope. Images for the CNV lesions were quantified by masked researchers using ImageJ. Exclusion criteria for laser-induced CNV lesions were used as previously described [35].
2.4. Bone marrow chimeric mice preparation
GFP bone marrow chimeric mice were prepared using GFP transgenic donor mice [38]. Recipient mice were irradiated (10·6 Gy, two doses 6 hours apart) using a 137Cs (caesium) irradiator one day before bone marrow transplant. Bone marrow suspensions were obtained by re-flushing both tibias and femurs using a 26-gauge needle and filtering through 70 μm cell strainers. A concentration of 2 × 106 cells of bone marrow cell suspension was intravenously injected into irradiated recipient mouse. Recipient mice were used to generate the laser-induced CNV model at 6-8 weeks post transplantation.
2.5. Choroid explant and bone marrow-derived macrophage medium co-culture
Choroid dissection and choroid explant culture were performed as previously described [39]. Mice were euthanized, and their eyes were collected and kept in an ice-cold culture medium. The peripheral choroid-scleral complex was cut into 1 mm2 piece, and placed on Matrigel Matrix (Corning, 356234) in 24-well plates. Fifty µL of Matrigel (a thickness of approximately 0.4 mm) was used to coat 24-well plates. The choroid complex was seeded, 500 µL of culture medium was added into each well and incubated at 37°C with 5% CO2. Bone marrow-derived macrophages were prepared from adult mice as previously described [40], and the culture medium was collected as a conditioned medium. On Day two, the conditioned medium from bone marrow-derived macrophage was added into choroid explants and replaced daily. Choroid explants were imaged using a Zeiss AxioOberver.Z1 microscope. ImageJ (National Institutes of Health) with a designed macro for SWIFT-Choroid quantification was used to quantify the sprouting area [39].
2.6. Immunohistochemistry
Mouse eyes were collected and fixed in 4% paraformaldehyde for 1 hour. Dissected choroid or frozen section was permeabilized in PBS containing 5% normal rabbit serum albumin, 2% bovine serum albumin, and 0·3% Triton X-100 for 1 hour. The following antibodies were used: isolectin IB4 (ThermoFisher Scientific, I21411, RRID: AB_23146), IBA1 (Wako, 019-19741, RRID: AB_839504), SOCS3 (Cell Signalling, 2923, RRID: AB_2255132), Collagen IV (Bio-Rad, 2150-1470, RRID: AB_2082644), vWF (Thermo Fisher, PA5-16634, RRID: AB_10982615), GFP (Abcam, ab13970, RRID: AB_300798), CD31 (MEC 13·3, BD Biosciences, 550274, RRID: AB_393571), and VEGF receptor 2 (VEGFR2) (clone Avas12, BioLegend, 136406, RRID: AB_2044067). DAPI in an anti-fade mounting medium (Vector Labs, H-1200-10) was used for nuclear staining.
2.7. RNA isolation and quantitative RT-PCR
Total RNA was extracted from mouse choroid/retina using Quick-RNA™ Miniprep Kit (Zymo Research, R1054). cDNA was synthesised using iScript™ cDNA Synthesis Kit (Bio-Rad, 1708890). Quantitative PCR (qPCR) was performed using SYBR Green qPCR Master Mix (Apex Bio, K1070). Primer sequences from 5’ to 3’ for qPCR were as follows: Il1b: GCC CAT CCT CTG TGA CTC ATG (forward); GGA GCC TGT AGT GCA GCT GTCT (reverse); Il6: TGG AGT CAC AGA AGG AGT GGC TAA G (forward); TCT GAC CAC AGT GAG GAA TGT CCA C (reverse); Tnf: TCC AGT AGA ATC CGC TCT CCT (forward); GCC ACA AGC AGG AAT GAG AAG (reverse); Iba1: ATC AAC AAG CAA TTC CTC GAT GA (forward); CAG CAT TCG CTT CAA GGA CAT A (reverse); Cd45: GAG CAG ACC CGA GAT CCA C (forward); GCA GCA CTA CCA GAA AAG GCA (reverse); Cd11b: ATG GAC GCT GAT GGC AAT ACC (forward); TCC CCA TTC ACG TCT CCC A (reverse).
2.8. Statistics
GraphPad Prism (v6·0) was used for statistical analyses. All data are representative of at least three independent experiments. Results are presented as mean ± SEM. Unpaired nonparametric Mann-Whitney Test was used for two group comparison. One-way ANOVA Tukey's Multiple Comparison Test was used for multiple group comparison. p 0·05 was considered to be statistically significant.
2.9. Role of funding source
The Funders had no role in study design, data collection, analysis and interpretation, or writing of report.
3. Results
3.1. Myeloid lineage cells contributed to the development of experimental CNV
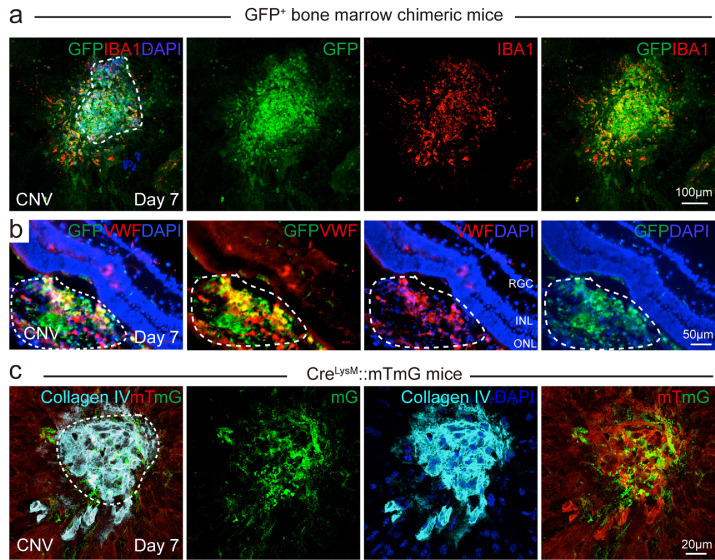
To test the presence of myeloid lineage cells in CNV, we laser induced CNV on GFP bone marrow chimeric mice (Fig. 1). GFP bone marrow-derived cells were recruited to the CNV areas (Fig. 1a), which is consistent with previous reports [41,42]. GFP positive (GFP+) bone marrow cells were quickly recruited to the CNV lesion area at 3 hours after laser exposure (Fig. S2). We stained the choroid CNV with a macrophage/microglia marker, ionised calcium binding adaptor molecule 1 (IBA1). On choroid flat mounts among these GFP+ myeloid lineage cells, there were bone marrow-derived macrophage/microglia (GFP+IBA1+ double-positive cells, yellow) around the CNV area. We also found bone marrow-derived non-macrophage/microglia cells (GFP+ IBA1− cells, green) in the centre of the CNV lesion. Retinal cross sections to examine the CNV in the subretinal space were stained with the EC marker, von Willebrand factor (vWF) (red). GFP+ bone marrow-derived cells appeared in the CNV lesion area (vWF+, red) (Fig. 1b). To trace the myeloid lineage cells in the CNV lesion area, we labelled myeloid lineage cells with GFP by generating myeloid lineage-specific mTmG report mice (CreLysM-mTmG). We laser induced CNV in CreLysM-mTmG reporter mice and myeloid cell lineage GFP+ cells were found in the laser-induced CNV lesion, labelled by EC marker Collagen IV. Many GFP+ myeloid lineage cells were located in the CNV lesion area (Fig. 1c), suggesting that myeloid lineage cells contributed to pathological neovascular formation in the CNV area. Taken together, myeloid lineage cells contributed to the pathological neovascular formation in the laser-induced CNV.
Fig. 1.
Myeloid lineage contributed to pathological endothelium formation in mice with laser-induced CNV. (a) GFP bone marrow chimeric mice were subjected to laser-induced CNV model and choroid flat mounts at Day seven after laser were stained with macrophage/microglia marker, IBA1 (red). (b) GFP bone marrow chimeric mice were subjected to laser-induced CNV and retinal cross sections at Day seven after laser were stained with EC marker vWF (red). White dotted line indicated the CNV lesion areas on retinal cross sections; (c) 8-week-old myeloid-specific LysM Cre driven mTmG reporter mice were subjected to laser-induced CNV model and choroid flat mounts at Day seven after laser were stained with EC marker Collagen IV (cyan). White dotted line indicated the CNV lesion areas on choroid flat mount. mG: membrane GFP; mT: membrane Tomato red. Scale bar: 100µm in (a), 50µm in (b), and 20µm in (c).
3.2. SOCS3 was induced in myeloid lineage cells in laser-induced choroidal neovascularization eyes
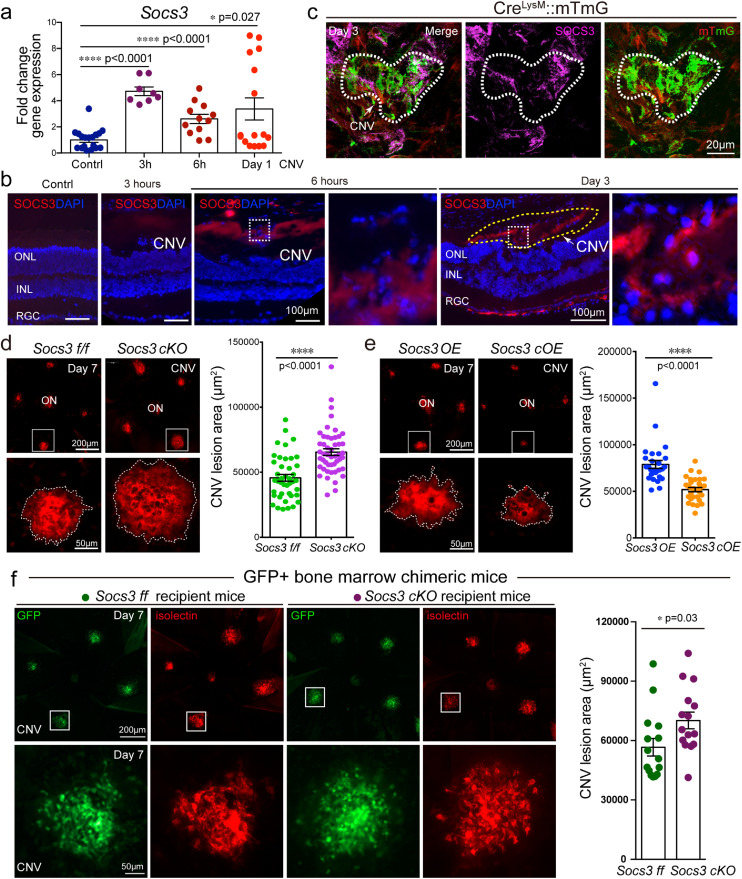
To determine the role of SOCS3 in pathological endothelium formation in CNV, the level of SOCS3 expression was examined in the laser-induced CNV model. We assessed the mRNA level of Socs3 in the CNV lesion. Since bone marrow cells can be quickly recruited to the CNV lesion (Fig. S2), we examined the expression of SOCS3 in early time points as well. The mRNA level of Socs3 was quickly induced 3 hours after the laser exposure in the choroid with CNV compared with normal choroid without laser exposure. Socs3 continued to be highly expressed at 6 hours and Day 1 after laser exposure (Fig. 2a). We evaluated the spatial expression of SOCS3 in CNV lesions using immunohistochemistry on retinal cross sections and SOCS3 was detected in the subretinal space at 6 hours after laser exposure (Fig. 2b). We also assessed SOCS3 expression in choroid flat mounts using myeloid specific mTmG reporter mice on Day three after the laser exposure. Immunohistochemistry staining showed that SOCS3 was highly upregulated in the CNV lesion (white circle) and colocalized with GFP+ myeloid lineage cells suggesting SOCS3 was induced in myeloid lineage cells in the CNV lesion (Fig. 2c).
Fig. 2.
SOCS3 was induced in myeloid cells and controlled laser-induced CNV. (a) Socs3 mRNA expression was highly induced in choroid with CNV lesions after laser exposure compared with normal choroid (n=8-20, *, p<0·05, ****, p<0·0001, unpaired nonparametric Mann-Whitney Test). (b-c) Immunohistochemistry staining on choroid flat mount of myeloid-specific report mice (CreLysM::mTmG) (c) and cross section of wild type mice (b) showed that SOCS3 (magenta in c, red in b) was induced in myeloid lineage cells labeled with GFP in CNV lesion area (white dotted line) (c) and in subretinal space (yellow dotted line) (b) at three hours, six hours or Day three after laser exposure; mG: membrane GFP; mT: membrane Tomato red. (d-e) Myeloid-specific Socs3 deficiency promoted laser-induced CNV (n=41-50 lesions) while myeloid specific Socs3 overexpression suppressed laser-induced CNV labeled by isolectin (red) at Day seven after laser exposure (n=27-31) (****, p<0·0001, unpaired nonparametric Mann-Whitney Test). (f) GFP bone marrow chimeric mice were generated using Socs3 cKO and control mice as recipient mice and laser-induced CNV were performed. The choroid flat mounts were collected on Day seven and isolectin IB4 (red) was stained for CNV lesion. Scale bar: 20µm in (c), 100µm in (b), 200µm in (d, e, and f, top panel), and 50µm in (d, e, and f, bottom panel).
3.3. SOCS3 in myeloid lineage regulated laser-induced choroidal neovascularization
To examine the role of myeloid SOCS3 in the development of laser-induced CNV, we generated myeloid lineage-specific Socs3 knockout mice (Socs3 cKO, loss-of-function) and Socs3 overexpression (Socs3 cOE, gain-of-function) mice using a myeloid-specific LysM-Cre driver (Fig. S1). The efficiency of knockout and overexpression had been validated at both mRNA (Fig. S3a and S3c) and protein levels (Fig. S3b and S3d) using mouse bone marrow derived macrophages from Socs3 cKO or Socs3 cOE mice as well as Socs3 flox/flox control mice (Socs3 f/f or Socs3 OE). Socs3 deficiency in myeloid lineage significantly increased the laser-induced CNV lesion areas by around 36% (mean ff = 44205.8, mean cKO = 67769.1) (Fig. 2d) while Socs3 overexpression in myeloid lineage significantly reduced the laser-induced CNV lesion areas (Fig. 2e) at Day seven after laser exposure. CNV lesion areas from both male and female mice were also examined separately and there was no statistical difference in the CVN lesion areas between male and female mice within group (Fig. S4a-d).
3.4. SOCS3 regulated myeloid lineage recruitment to the choroidal neovascularization lesions and production of proinflammatory factors
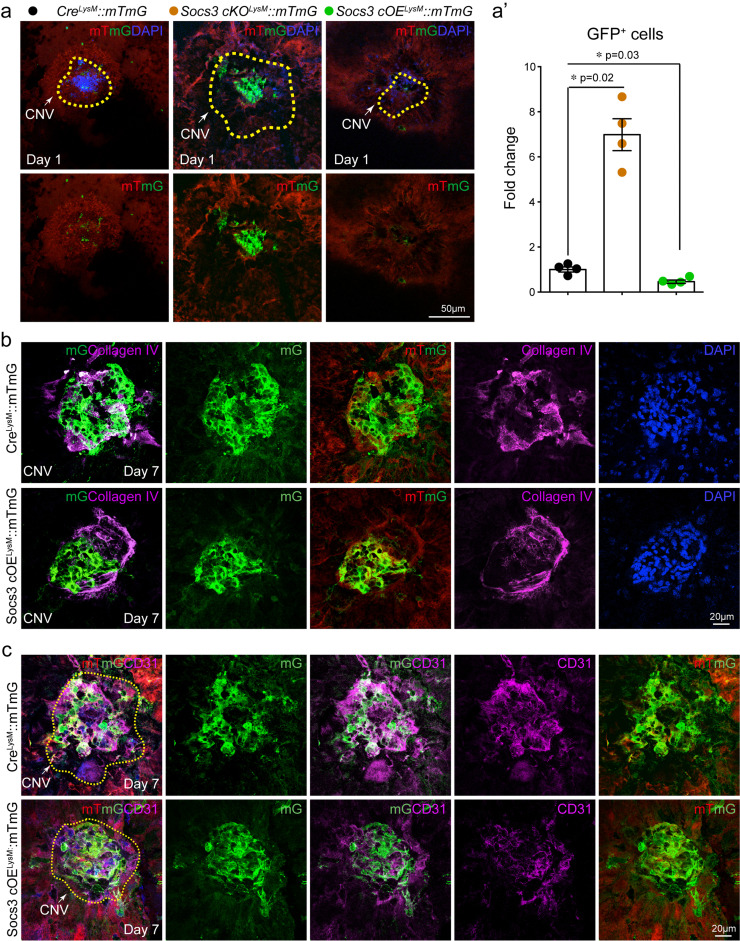
To investigate the role of myeloid SOCS3 in regulating CNV formation, we generated GFP bone marrow chimeric mice on Socs3 cKO and flox control mice using wild type GFP mice as donor mice, in which bone marrow cells from Socs3 cKO and flox control mice were replaced with wild type bone marrow cells. We laser induced CNV lesion on these mice. Wild type GFP+ bone marrow cells were recruited to the CNV lesion on both Socs3 cKO and flox control mice. Without bone marrow cells from Socs3 cKO and flox control mice, the CNV lesion areas were still significantly increased around 24% (mean ff= 56639, mean cKO=72013) in Socs3 cKO recipient mice (Fig 2f). The increase of CNV lesion rate (24%) was smaller than that in Socs3 cKO mice with Socs3 cKO bone marrow (36%). We then investigated the myeloid cells in the CNV lesion areas. We laser induced CNV in myeloid Socs3 loss-of-function (Socs3 cKOLysM::mTmG) and gain-of-function (Socs3 cOELysM::mTmG) mTmG reporter mice. Myeloid lineage cells labelled with GFP were much more abundant in the CNV lesions in Socs3 cKOLysM::mTmG mice than in CreLysM::mTmG control mice. GFP+ myeloid lineage cells within the CNV lesion area (yellow circle) was significantly increased in Socs3 cKOLysM::mTmG mice and reduced in Socs3 cOELysM::mTmG mice compared with CreLysM::mTmG control mice (Fig. 3a, 3a’), suggesting that SOCS3 may attenuate the contribution of myeloid lineage cells to CNV formation. To confirm our findings, we stained the CNV lesion with EC markers Collagen IV (Fig. 3b) and CD31 (Fig. 3c). GFP+ myeloid lineage cells were less in the CNV lesion area (labelled by Collagen IV+ or CD31+) in Socs3 overexpression mice (Socs3 cOELysM::mTmG) versus control mice (CreLysM::mTmG) (Fig. 3b). Therefore, SOCS3 overexpression reduced myeloid lineage cells appearing in the CNV lesion area.
Fig. 3.
SOCS3 controlled myeloid origin becoming pathological endothelium. (a) 8-week-old myeloid-specific Socs3 knockout reporter mice (Socs3cKOLysM::mTmG) and overexpression reporter mice (Socs3cOELysM::mTmG) and their control reporter mice (CreLysM::mTmG) were subjected to laser-induced CNV, at Day 1 after laser exposure the choroid flat mounts were stained with DAPI. GFP intensity was quantified using Image J in (a’) (n=4, *, p<0·05, unpaired nonparametric Mann-Whitney Test). Dotted lines: CNV lesion area. (b-c) Socs3 overexpression reporter mice (Socs3cOELysM::mTmG) and their control reporter mice (CreLysM::mTmG) were subjected to laser-induced CNV, and at Day seven after laser exposure the choroid flat mounts were stained with EC markers Collagen IV (b) and CD31 (c). mG: membrane GFP; mT: membrane Tomato red. Scale bar: 50µm in (a), 20µm in (b) and (c).
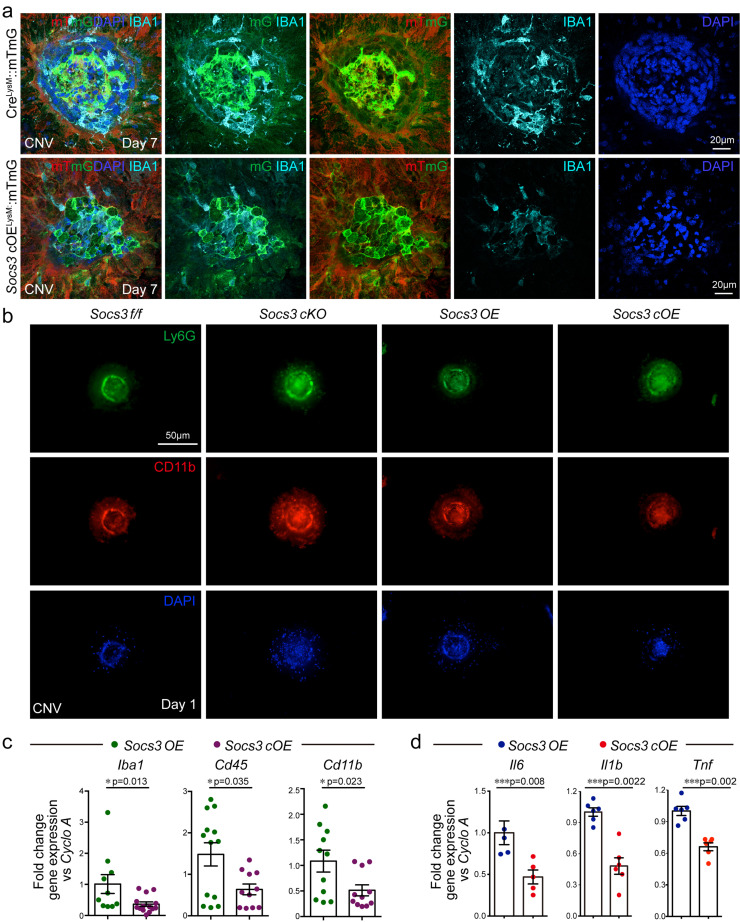
Microglia, the primary resident myeloid lineage retinal immune cells, are quickly activated after an inflammatory insult [43,44] and associated with angiogenesis [18,45]. To further assess the myeloid lineage cells in the CNV lesion, we stained myeloid lineage GFP+ cells with IBA1 staining macrophages and microglia. Myeloid lineage-derived IBA1+ macrophages/microglia were recruited to the CNV lesion area. There were fewer IBA1+ cells in Socs3 overexpression mice (Socs3 cOELysM::mTmG), suggesting that myeloid SOCS3 prevented the recruitment of myeloid lineage derived macrophages/microglia in CNV lesions (Fig. 4a). Beside macrophages and microglia, we also examined neutrophils in the CNV lesion area. Neutrophil invasion was reported as part of early inflammatory responses [46,47] to promote laser-induced CNV via the modulation of VEGF expression [46,47]. Myeloid SOCS3 deficiency enhances neutrophil activation in experimental autoimmune encephalomyelitis [48] and hematopoietic SOCS3 deficient mice exhibited enhanced neutrophilia [49]. Our results showed that at Day 1 after laser exposure, neutrophils were recruited to the CNV lesion and myeloid SOCS3 deficiency increased while myeloid SOCS3 overexpression reduced the recruitment of neutrophils to CNV lesion (Fig. 4b). Immune cell marker genes including Iba1, Cd45, and Cd11b were examined to assess the role of SOCS3 in immune cell recruitment to CNV lesions. Socs3 overexpression in myeloid lineage cells significantly reduced the expression of Iba1, Cd45, and Cd11b in the choroid with CNV (Fig. 4c), indicating that the numbers of IBA1+, CD45+, and CD11b+ myeloid lineage cells may be reduced in CNV lesions in Socs3 cOE mice. Moreover, the mRNA levels of proinflammatory mediators (Il6, Il1b and Tnf) were reduced in the choroid with CNV from Socs3 cOE mice compared with that from control mice (Fig. 4d).
Fig. 4.
SOCS3 regulated myeloid lineage recruitment to the CNV lesions. (a) 8-week-old myeloid-specific Socs3 overexpression reporter mice (Socs3cOELysM::mTmG) and their control reporter mice (CreLysM::mTmG) were subjected to laser-induced CNV, and the choroids were collected at Day seven after laser exposure and stained with macrophage/microglia marker IBA1. mG: membrane GFP; mT: membrane Tomato red. (b) 8-week-old Socs3cKO and Socs3 cOE as well as their controls were subjected to laser-induced CNV, and the choroids were collected at Day one after laser exposure and stained with Ly6G (green) and CD11b (red); (c-d) immune cell marker genes Iba1, Cd45 and Cd11b and proinflammatory cytokines including cytokines Il6, Il1b and Tnf were reduced in the CNV from Socs3 overexpression mice (n=10-14 in c, n=5-6 in d, *, p<0·05; **, p<0·01; ***, p<0·001, unpaired nonparametric Mann-Whitney Test). Scale bar: 20µm in (a), 50µm in (b).
Some GFP+ bone marrow (Fig. 1b) and GFP+ myeloid lineage cells (Fig. 1c, 3b, and 3c) were colocalized with EC markers vWF, Collagen IV, or CD31 in the CNV lesion areas, suggesting the possibility of myeloid-derived EC-like cells appearing in the CNV lesions. To further investigate the cell origins in pathological CNV lesions, we laser induced CNV in endothelial-specific Cre-driven GFP report mice (CreTie2-GFP), collected the choroid with CNV at Day seven after laser exposure, and stained the CNV lesions with Collagen IV (Fig. S5a). In the laser-induced CNV area, Collagen IV+ ECs (red) were partially colocalized with GFP+ EC lineage cells (green) suggesting that in the laser-induced CNV area, EC lineage (Collagen IV+ GFP+, yellow) is not the only cell origin of EC-like cells, and other cell lineages (Collagen IV+GFP−, red) may also contribute to pathological neovascular formation. Given that Tie2-Cre may also be expressed by a subset of immune cells, such as macrophages and microglia, we stained the choroids with isolectin for ECs and macrophage/microglia. Based on the cell morphology, GFP+ isolectin+ macrophage/microglia were found in the CNV area but there were also some isolectin+ GFP− cells present. To further validate the non-EC origin in the CNV area, we laser induced CNV on another EC-specific Cdh5-Cre driven GFP mouse line [34], and stained the CNV lesions with Collagen IV (Fig. S5b). We observed the same results, and Collagen IV+ cells (red) were only partially colocalized with GFP+ EC lineage (green) in the CNV area, suggesting that not all the Collagen IV+ ECs (red) in the CNV area are from the EC lineage. Next, we laser induced CNV on myeloid lineage-specific reporter mice and GFP+ bone marrow chimeric mice, and examined the localisation of EC markers. Myeloid lineage GFP+ cells or GFP+ bone marrow derived cells in CNV area were colocalized with EC markers including Collagen IV (Fig. 1c and 3b), CD31 (Fig. 3c), or vWF (Fig. 1b). In addition, bone marrow derived endothelial progenitor cells (EPCs) labelled with CD31 and VEGFR2 markers were found in the CNV lesion area (Fig. S5d). Therefore, myeloid lineage derived EC-likes cells may contribute to pathological CNV formation. Moreover, Socs3 overexpression reduced GFP+ Collagen IV+ (Fig. 3b) or GFP+ CD31+ (Fig. 3c) double positive myeloid lineage derived EC-like cells in the CNV area, suggesting that SOCS3 may modulate myeloid lineage-derived EC-like cells to form CNV.
3.5. SOCS3 in myeloid regulated choroid sprout growth ex vivo
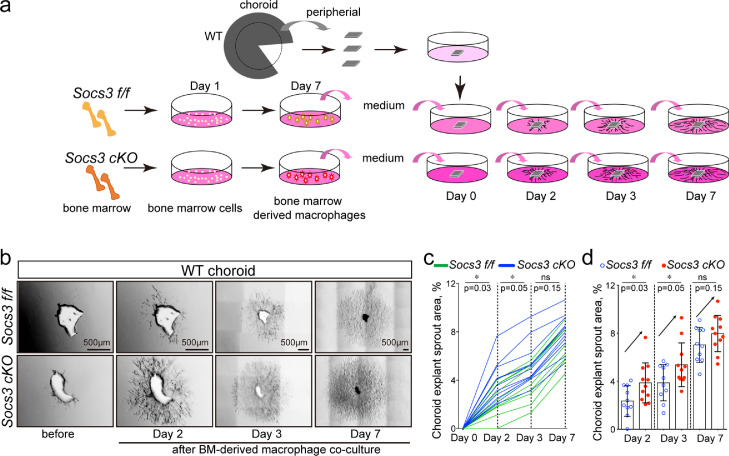
To evaluate the direct effects of myeloid SOCS3 on choroid sprouting, the choroid explant ex vivo assay was performed by coculturing sprouting choroid complexes with the conditioned medium from bone marrow-derived macrophages, which were prepared from Socs3 cKO and flox control mice. The cells were validated by macrophage marker IBA1 (Fig. S6a-S6b), and their conditioned medium was cocultured with choroid explants isolated from wild type mice (Fig. 5a). Socs3 cKO conditioned medium significantly increased choroid sprouting compared with Socs3 f/f control on Day two and three after co-culture (n=8-12, p0·05, unpaired nonparametric Mann-Whitney Test) (Fig. 5b-5d). The effects of Socs3 cKO bone marrow-derived macrophages on choroid sprouting were increased but nonsignificant on Day seven after co-culture (n=8-12, p0·05, unpaired nonparametric Mann-Whitney Test) (Fig. 5b-5d). Wild type choroid explants cocultured with conditioned medium from bone marrow-derived macrophages of Socs3 cOE and control mice were also examined. Socs3 cOE conditioned medium significantly decreased choroid sprouting compared with Socs3 OE control on Day 2 after coculture (Fig. S6c-S6d). Therefore, myeloid SOCS3 regulated choroid growth ex vivo.
Fig. 5.
Myeloid SOCS3 controlled choroid explant growth ex vivo. (a) Illustration of coculture showing peripheral choroids from wild type (WT) mice were seeded and cocultured with bone marrow (BM)-derived macrophages at 48 hours later. Bone marrow cells were collected from Socs3 cKO and flox control (Socs3 f/f) mice and cultured for seven days to derive into macrophages. The medium was collected and cocultured with choroid explants. (b) Representative images of choroid sprouts (n=8-12 per group). (c-d) Growth curve for each choroid explant (c) and choroid explant sprouting areas at Day two, three and seven were quantified using ImageJ (d) (n=8-12. *, p0·05, ns: no significance, unpaired nonparametric Mann-Whitney Test). Scale bar: 500µm in (b).
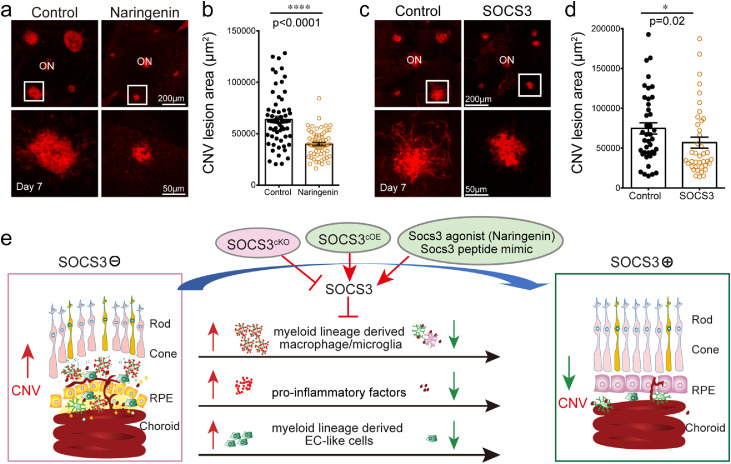
3.6. SOCS3 agonist inhibited CNV
To demonstrate the therapeutic potential of targeting the SOCS3 pathway, we examined the role of SOCS3 agonist, naringenin [50] on CNV inhibition. Naringenin can bind to the Socs3 promoter and robustly activate SOCS3 at a transcriptional level [51]. It has been examined for therapeutic potential in neurological, cardiovascular, gastrointestinal, rheumatological, metabolic, and malignant disorders [52]. Naringenin significantly induced Socs3 expression (Fig. S7) and suppressed CNV compared with control treatment (Fig. 6a and 6b). We also examined the role of SOCS3 peptide mimic [53], which binds to Jak2 and Tyk2 in vitro to mimic SOCS3 function. SOCS3 peptide significantly suppressed CNV (Fig. 6c and 6d). Taken together, targeting SOCS3 could be potential treatment of nAMD.
Fig. 6.
SOCS3 agonist inhibited laser-induced CNV. SOCS3 agonist (Naringenin) treatment (a-b) (n=62-63) lesions/group) and SOCS3 peptide mimic treatment (c-d) (n=40 lesions/group) on wild type mice significantly reduced laser-induced CNV (labeled by isolectin, red). *, p<0·05, ****, p<0·0001 (unpaired nonparametric Mann-Whitney Test). (c) A schematic diagram illustrates pathological CNV in the subretinal space controlled by SOCS3 through modulating myeloid lineage-derived macrophage/microglia recruitment, proinflammatory factor secretion, and possibly myeloid lineage-derived EC-like cells. Scale bar: 200µm in (a and c, top) and 50µm in (a and c, bottom).
4. Discussion
We identified the role of myeloid lineage cells in pathological neovascular formation controlled through SOCS3 in a laser-induced CNV model. First, we found that myeloid lineage cells contributed to CNV formation and that overexpression of SOCS3 in myeloid lineage cells inhibited CNV (Fig. 1,2, and 3). Second, we found that SOCS3 regulated myeloid lineage-derived macrophage/microglia as well as neutrophil recruitment to control CNV formation (Fig. 4). Last, we showed that myeloid SOCS3 regulated proinflammatory factors to control choroid growth (Fig. 4 and 5). In addition, we also showed the possibility that SOCS3 may regulate myeloid lineage-derived EC-like cells to form CNV pathological neovascular lesions (Fig. 1b, 1c, 3b, 3c, and S5). Together, SOCS3 overexpression in myeloid lineage cells suppressed laser-induced CNV through suppression of myeloid lineage-derived macrophage/microglia recruitment and pro-inflammatory factor secretion (Fig. 6e).
Immune dysregulation has been linked to CNV clinically and experimentally [1,2,29,54,55]. Specifically, immune cells of myeloid origin are attracted to and control experimental CNV, in part through the JAK2/STAT3/SOCS3 pathway [29]. However, the underlying mechanisms of myeloid lineage in pathological CNV formation have not been fully elucidated. Cells of the myeloid lineage include monocytes, granulocytes, and megakaryocytes. Monocytes (mononuclear circulating phagocytes) give rise to macrophages, microglia, or dendritic cells. Circulating platelets and erythrocytes are also from myeloid progenitor cells. Chen aet al [29]. studied the roles of circulating monocytes in nAMD. They found that monocytes from nAMD patients highly expressed phosphorylated STAT3 (pSTAT3) and VEGF, and that deletion of SOCS3 in myeloid cells resulted in STAT3 activation and increased CNV formation. Consistently, we also found SOCS3 deficiency in myeloid cells exacerbates CNV formation (Fig. 2d). Chen aet al. reported no difference in the percentage of different subsets of monocytes in blood between nAMD patients and controls [29]. In our study, we investigated the local cell population and cell lineage in the CNV neovascular lesion area using a genetic lineage tracing approach with myeloid lineage-specific and endothelial lineage-specific reporter mice. We showed the distribution of myeloid lineage-derived cells in CNV lesions (Fig. 1c, 3, and 4). IBA1 is a marker for macrophages and microglia, especially those activated cells. Our results showed that an abundance of IBA+ macrophage/microglia in the CNV lesion area and SOCS3 suppressed the recruitment of these cells to the CNV lesion and further reduced CNV development.
Among the GFP+IBA+ cells shown in Fig. 4a, there is also a population of GFP+IBA− cells, which are myeloid lineage-derived non-macrophage/microglia. Our data showed the possibility that myeloid lineage derived EC-like cells might be among this population of myeloid lineage-derived non-macrophage/microglia cells and could form part of the pathological CNV with EC-like properties (Fig. 1b, 1c, 3b, 3c, and S5). Previous reports showed a new EC source in pre-existing vasculature from the erythromyeloid precursors [56]. EPCs were also reported to participate in the formation of new blood vessels in various diseases [57,58]. Pro-angiogenic growth factors increase circulating levels of EPCs and promote new blood-vessel formation [59], [60], [61]. Haematopoietic stem cells in bone marrow can differentiate into cells of two primary lineages, lymphoid and myeloid, but only myeloid progenitors have the developmental potential of EPCs [62]. EPCs capable of contributing to capillary formation can be derived from bone marrow cells [61,[63], [64], [65], [66]]. Baba aet al. reported that bone marrow-derived cells can differentiate into ECs within the CNV lesion [67]. Feng aet al. recently reported [68] that there is no evidence for erythromyeloid progenitor-derived vascular endothelial cells in multiple organs during development. Our data showed that, under pathological conditions, cells of myeloid origin may be able to derive into EC-like cells and contribute to pathological CNV formation. We used several EC markers including vWF (Fig. 1b, S5c), Collagen IV (Fig. 1c, 3b, S5a, and S5b), CD31 (Fig. 3c), and isolectin (Fig. 2d, 2e, 6a, and S5a) to identify an endothelial lineage. Collagen IV was commonly used as an EC marker [69]. vWF is highly restricted to ECs but also is found on megakaryocytes [70]. CD31 is highly expressed on ECs but is also expressed on B cells, T cells, monocytes, dendritic cells, neutrophils, and macrophages [71,72]. Isolectin is expressed on both ECs [73] and microglia [74]. We identified cell types based on a few EC markers and the specificities of these EC markers. Therefore, genetic lineage tracing might give a biased view [75]. Single-cell transcriptomics technologies had been used recently for mapping cell origin and fate. Using single-cell profiling data, Prinz's group characterized several disease-associated retinal microglia and identified non-retinal microglia cell types including dendritic cells, lymphocytes, monocytes, and macrophages that are involved in CNV [76]. Single-cell transcriptomics technologies will give higher resolution to identify cell types and can further examine our hypothesis that cells of myeloid origin may be able to differentiate into EC-like cells and contribute to pathological CNV formation. Furthermore, other types of myeloid lineage derived non-macrophage/microglia are involved in CNV development, such as granulocytes, and whether they are SOCS3-dependant or not, remains to be determined, but, myeloid SOCS3 plays a role in pathological CNV formation.
In addition, GFP−IBA+ macrophages/microglia were also detected within the CNV lesion area. We suspect that the recombination efficiency of LysM-Cre was imperfect. The deletion efficiency of floxed target genes driven by LysM-Cre is 83%–98% in macrophages [77]. LysM-Cre has been used to target myeloid cells in the retinal angiogenesis models in several research laboratories [4,78,79]. A recent report [80] found that the specificity of LysM-Cre recombination in microglia within the central nervous system including the retina was less than 40%. Therefore, GFP−IBA+ macrophages/microglia within the CNV lesion could potentially be of myeloid origin but were not labelled with GFP driven by LysM-Cre due to low deletion efficiency. Despite LysM-Cre modulating SOCS3 expression in less than 40% of the microglia population, our results still showed significant phenotypical changes and strengthened our conclusion that myeloid SOCS3 plays a critical role in controlling CNV. In summary, this study demonstrated that myeloid SOCS3 controlled pathological CNV formation by modulating myeloid lineage-derived macrophage/microglia recruitment to CNV lesions and controlled myeloid lineage-derived macrophage/microglia secretion of proinflammatory factors. Targeting SOCS3 might be a novel way to control pathological CNV.
Myeloid SOCS3 controls the CNV formation potentially through modulating proinflammatory mediators including Il6, Il1b and Tnf as showed in Fig. 4d. In addition, SOCS3 deficiency attenuates the response of macrophages to IFN-β signalling [81], and IFN-β treatment can inhibit laser-induced CNV [82]. Therefore, myeloid SOCS3 may also potentially control CNV lesion via IFN-β signalling.
Naringenin was selected from a high-throughput screen of small molecules capable of inducing the expression of the minimal human SOCS3 gene promoter (a 1.7kb fragment of the human SOCS3 promoter) in a cell-based dual- luciferase reporter assay [51]. The library consists of 1040 U.S.A. Food and Drug Administration-approved compounds of known medicinal benefit. From the screen, two structurally related flavanoid compounds produced a robust (approximately 4-fold) induction of SOCS3 promoter activity. One of the two compounds is 5,7-dihydroxy- 2-(4-hydroxyphenyl) chroman-4-one (Naringenin). Naringenin can specifically bind to the Socs3 promoter and robustly activate SOCS3 at a transcriptional level [51]. But whether it may also bind to other gene promoters remains to be determined. Naringenin elicits broad biological effects on human health as summarized in a review [83], and it is very likely it may be involved in multiple signal pathways under different pathological conditions. In Fig. 2e, the reduction of the CNV lesion area in Socs3 cOE group (mean=52600) was 28% compared with lesions in the Socs3 OE group (mean=73500). In Fig. 6b, the reduction of the CNV lesion area in Naringenin treated group (mean=39230) was 36% compared with that in control group (mean=61600). The CNV reduction rate in Naringenin group is higher than that in Socs3cOE group, suggesting that Naringenin may play other roles in addition to the induction of SOCS3 expression in myeloid lineage cells. Potentially, Naringenin's regulatory role in other signaling pathways such as the VEGF/KDR pathway [84] could contribute to further CNV reduction. Additionally, SOCS3 activation by Naringenin in other cell types, such as in ECs and neurons could plausibly increase CNV reduction. This would be consistent with previous articles, which reported that SOCS3 deficiency increased retinopathy [26,27]. SOCS3 peptide mimetic was designed based on the X-ray structure of mouse SOCS3, JAK2 kinase domain and gp130 [53]. The agonist can specifically bind to Jak2 and Tyk2 to mimic SOCS3 function. This SOCS3 mimetic peptide has demonstrated efficacy with tumor suppression in a human breast tumor xenograft mouse model with intraperitoneal injection daily, and shows a lack of observable toxicity in intestine, liver, and kidney of treated animals [85]. Our data in Fig. 6d showed 24% reduction of the CNV lesion area in SOCS3 peptide group (mean=74786) compared with that in control group (mean=56884), which is lower compared with that in both Naringenin and Socs3 cOE group. SOCS3 peptide mimic has the possibility of targeting the cell types other than myeloid lineage cells. Due to the limitation of peptide nucleic acid therapy, such as poor intracellular delivery and rapid clearance [86], there were variations on the efficacy of SOCS3 peptide mimetic although it significantly inhibited the CNV lesions. The off-target effects of Naringenin and SOCS3 mimetic need to be further investigated by treating CNV in myeloid-specific Socs3 cKO mice as well as wild type with CNV for comparison.
5. Contributors
Tianxi Wang: study design, data collection, data analysis, data interpretation, preparing the figures, writing the manuscript; Pingzhu Zhou: study design, data collection, data analysis, data interpretation, reviewing the manuscript; Xuemei Xie: study design, data collection, data analysis, data interpretation, reviewing the manuscript; Yohei Tomita: data collection, data analysis, data interpretation; reviewing the manuscript; Steve Cho: data collection, data analysis, data interpretation; reviewing the manuscript; Demetrios Tsirukis: data collection, reviewing the manuscript; Enton Lam: data collection, reviewing the manuscript; Hongbo Robert Luo: study supervision and reviewing the final version of manuscript; William T. Pu: study supervision, providing mouse lines and material support, reviewing the final version of manuscript; Ye Sun: study design, data analysis, data interpretation, preparing the figures, writing the manuscript, study supervision, reviewing the final version of manuscript. Tianxi Wang and Ye Sun had full access to all the data in the study and have verified all the data. All authors have read and approved the final version of the manuscript.
Declaration of Competing Interest
All authors declare that they have no conflict of interests.
Acknowledgments
Acknowledgements
We thank Dr. Lois E.H. Smith for her advice and critical review of the manuscript, and thank Drs. Yan Gong, Chi-Hsiu Liu and Raffael Liegl for their excellent technical assistance. This work was supported by the National Institutes of Health/National Eye Institute (R01EY030140, R01EY029238), BrightFocus Foundation, American Health Assistance Foundation (AHAF), and Children's Hospital Ophthalmology Foundation for Ye Sun, and the National Institutes of Health/National Heart, Lung and Blood Institute (U01HL098166) for Pingzhu Zhou. The funding sources had no role in determining how the research was conducted.
Data sharing statements
All the data supporting the conclusions of this study are included within the article and supplementary data. All the other data and materials are available upon request to the corresponding author.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2021.103632.
Appendix. Supplementary materials
References
- 1.Ambati J, Atkinson JP, Gelfand BD. Immunology of age-related macular degeneration. Nat Rev Immunol. 2013;13(6):438–451. doi: 10.1038/nri3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cherepanoff S, McMenamin P, Gillies MC, Kettle E, Sarks SH. Bruch's membrane and choroidal macrophages in early and advanced age-related macular degeneration. Br J Ophthalmol. 2010;94(7):918–925. doi: 10.1136/bjo.2009.165563. [DOI] [PubMed] [Google Scholar]
- 3.Lechner J, Chen M, Hogg RE, Toth L, Silvestri G, Chakravarty U. Alterations in Circulating Immune Cells in Neovascular Age-Related Macular Degeneration. Sci Rep. 2015;5:16754. doi: 10.1038/srep16754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dejda A, Mawambo G, Cerani A, Miloudi K, Shao Z, Daudelin JF. Neuropilin-1 mediates myeloid cell chemoattraction and influences retinal neuroimmune crosstalk. J Clin Invest. 2014;124(11):4807–4822. doi: 10.1172/JCI76492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang P, de Vos AF, Kijlstra A. Macrophages and MHC class II positive cells in the choroid during endotoxin induced uveitis. Br J Ophthalmol. 1997;81(5):396–401. doi: 10.1136/bjo.81.5.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMenamin PG. Dendritic cells and macrophages in the uveal tract of the normal mouse eye. Br J Ophthalmol. 1999;83(5):598–604. doi: 10.1136/bjo.83.5.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forrester JV, Xu H, Kuffova L, Dick AD, McMenamin PG. Dendritic cell physiology and function in the eye. Immunol Rev. 2010;234(1):282–304. doi: 10.1111/j.0105-2896.2009.00873.x. [DOI] [PubMed] [Google Scholar]
- 8.Karlstetter M, Scholz R, Rutar M, Wong WT, Provis JM, Langmann T. Retinal microglia: just bystander or target for therapy? Prog Retin Eye Res. 2015;45:30–57. doi: 10.1016/j.preteyeres.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Crespo-Garcia S, Reichhart N, Hernandez-Matas C, Zabulis X, Kociok N, Brockmann C. In vivo analysis of the time and spatial activation pattern of microglia in the retina following laser-induced choroidal neovascularization. Exp Eye Res. 2015;139:13–21. doi: 10.1016/j.exer.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Grigsby JG, Cardona SM, Pouw CE, Muniz A, Mendiola AS, Tsin AT. The role of microglia in diabetic retinopathy. J Ophthalmol. 2014;2014 doi: 10.1155/2014/705783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talia DM, Deliyanti D, Agrotis A, Wilkinson-Berka JL. Inhibition of the Nuclear Receptor RORgamma and Interleukin-17A Suppresses Neovascular Retinopathy: Involvement of Immunocompetent Microglia. Arterioscler Thromb Vasc Biol. 2016;36(6):1186–1196. doi: 10.1161/ATVBAHA.115.307080. [DOI] [PubMed] [Google Scholar]
- 12.Vecino E, Rodriguez FD, Ruzafa N, Pereiro X, Sharma SC. Glia-neuron interactions in the mammalian retina. Prog Retin Eye Res. 2016;51:1–40. doi: 10.1016/j.preteyeres.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Schmid MC, Varner JA. Myeloid cell trafficking and tumor angiogenesis. Cancer Lett. 2007;250(1):1–8. doi: 10.1016/j.canlet.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kambhampati SP, Clunies-Ross AJ, Bhutto I, Mishra MK, Edwards M, McLeod DS. Systemic and Intravitreal Delivery of Dendrimers to Activated Microglia/Macrophage in Ischemia/Reperfusion Mouse Retina. Invest Ophthalmol Vis Sci. 2015;56(8):4413–4424. doi: 10.1167/iovs.14-16250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grunin M, Hagbi-Levi S, Chowers I. The role of monocytes and macrophages in age-related macular degeneration. Adv Exp Med Biol. 2014;801:199–205. doi: 10.1007/978-1-4614-3209-8_26. [DOI] [PubMed] [Google Scholar]
- 16.Chambers SE, O’Neill CL, O’Doherty TM, Medina RJ, Stitt AW. The role of immune-related myeloid cells in angiogenesis. Immunobiology. 2013;218(11):1370–1375. doi: 10.1016/j.imbio.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Stefater JA, 3rd, Ren S, Lang RA, Duffield JS. Metchnikoff's policemen: macrophages in development, homeostasis and regeneration. Trends Mol Med. 2011;17(12):743–752. doi: 10.1016/j.molmed.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stefater JA, 3rd, Lewkowich I, Rao S, Mariggi G, Carpenter AC, Burr AR. Regulation of angiogenesis by a non-canonical Wnt-Flt1 pathway in myeloid cells. Nature. 2011;474(7352):511–515. doi: 10.1038/nature10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang RA, Bishop JM. Macrophages are required for cell death and tissue remodeling in the developing mouse eye. Cell. 1993;74(3):453–462. doi: 10.1016/0092-8674(93)80047-i. [DOI] [PubMed] [Google Scholar]
- 20.Ritter MR, Banin E, Moreno SK, Aguilar E, Dorrell MI, Friedlander M. Myeloid progenitors differentiate into microglia and promote vascular repair in a model of ischemic retinopathy. J Clin Invest. 2006;116(12):3266–3276. doi: 10.1172/JCI29683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Binet F, Mawambo G, Sitaras N, Tetreault N, Lapalme E, Favret S. Neuronal ER stress impedes myeloid-cell-induced vascular regeneration through IRE1alpha degradation of netrin-1. Cell Metab. 2013;17(3):353–371. doi: 10.1016/j.cmet.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Qin H, Holdbrooks AT, Liu Y, Reynolds SL, Yanagisawa LL, Benveniste EN. SOCS3 deficiency promotes M1 macrophage polarization and inflammation. J Immunol. 2012;189(7):3439–3448. doi: 10.4049/jimmunol.1201168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spence S, Fitzsimons A, Boyd CR, Kessler J, Fitzgerald D, Elliott J. Suppressors of cytokine signaling 2 and 3 diametrically control macrophage polarization. Immunity. 2013;38(1):66–78. doi: 10.1016/j.immuni.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 24.Arnold CE, Whyte CS, Gordon P, Barker RN, Rees AJ, Wilson HM. A critical role for SOCS3 in promoting M1 macrophage activation and function in vitro and in vivo. Immunology. 2013 doi: 10.1111/imm.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ushiki T, Huntington ND, Glaser SP, Kiu H, Georgiou A, Zhang JG. Rapid Inflammation in Mice Lacking Both SOCS1 and SOCS3 in Hematopoietic Cells. PLoS One. 2016;11(9) doi: 10.1371/journal.pone.0162111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stahl A, Joyal JS, Chen J, Sapieha P, Juan AM, Hatton CJ. SOCS3 is an endogenous inhibitor of pathologic angiogenesis. Blood. 2012;120(14):2925–2929. doi: 10.1182/blood-2012-04-422527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun Y, Ju M, Lin Z, Fredrick TW, Evans LP, Tian KT. SOCS3 in retinal neurons and glial cells suppresses VEGF signaling to prevent pathological neovascular growth. Sci Signal. 2015;8(395):ra94. doi: 10.1126/scisignal.aaa8695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun Y, Liu CH, SanGiovanni JP, Evans LP, Tian KT, Zhang B. Nuclear receptor RORalpha regulates pathologic retinal angiogenesis by modulating SOCS3-dependent inflammation. Proc Natl Acad Sci U S A. 2015;112(33):10401–10406. doi: 10.1073/pnas.1504387112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen M, Lechner J, Zhao J, Toth L, Hogg R, Silvestri G. STAT3 Activation in Circulating Monocytes Contributes to Neovascular Age-Related Macular Degeneration. Curr Mol Med. 2016;16(4):412–423. doi: 10.2174/1566524016666160324130031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwak N, Okamoto N, Wood JM, Campochiaro PA. VEGF is major stimulator in model of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2000;41(10):3158–3164. [PubMed] [Google Scholar]
- 31.Miller H, Miller B, Ishibashi T, Ryan SJ. Pathogenesis of laser-induced choroidal subretinal neovascularization. Invest Ophthalmol Vis Sci. 1990;31(5):899–908. [PubMed] [Google Scholar]
- 32.Yasukawa H, Ohishi M, Mori H, Murakami M, Chinen T, Aki D. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol. 2003;4(6):551–556. doi: 10.1038/ni938. [DOI] [PubMed] [Google Scholar]
- 33.Reed AS, Unger EK, Olofsson LE, Piper ML, Myers MG, Jr., Xu AW. Functional role of suppressor of cytokine signaling 3 upregulation in hypothalamic leptin resistance and long-term energy homeostasis. Diabetes. 2010;59(4):894–906. doi: 10.2337/db09-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature. 2010;465(7297):483–486. doi: 10.1038/nature09002. [DOI] [PubMed] [Google Scholar]
- 35.Gong Y, Li J, Sun Y, Fu Z, Liu CH, Evans L. Optimization of an Image-Guided Laser-Induced Choroidal Neovascularization Model in Mice. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0132643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madonna S, Scarponi C, Morelli M, Sestito R, Scognamiglio PL, Marasco D. SOCS3 inhibits the pathological effects of IL-22 in non-melanoma skin tumor-derived keratinocytes. Oncotarget. 2017;8(15):24652–24667. doi: 10.18632/oncotarget.15629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao S, Kang R, Deng T, Luo L, Wang J, Li E. Comparison of two cannulation methods for assessment of intracavernosal pressure in a rat model. PLoS One. 2018;13(2) doi: 10.1371/journal.pone.0193543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holl EK. Generation of bone marrow and fetal liver chimeric mice. Methods Mol Biol. 2013;1032:315–321. doi: 10.1007/978-1-62703-496-8_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shao Z, Friedlander M, Hurst CG, Cui Z, Pei DT, Evans LP. Choroid sprouting assay: an ex vivo model of microvascular angiogenesis. PLoS One. 2013;8(7):e69552. doi: 10.1371/journal.pone.0069552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weischenfeldt J, Porse B. Bone Marrow-Derived Macrophages (BMM): Isolation and Applications. CSH Protoc. 2008;2008:prot5080. doi: 10.1101/pdb.prot5080. pdb. [DOI] [PubMed] [Google Scholar]
- 41.Hou HY, Wang YS, Xu JF, Wang YC, Liu JP. The dynamic conduct of bone marrow-derived cells in the choroidal neovascularization microenvironment. Curr Eye Res. 2006;31(12):1051–1061. doi: 10.1080/02713680601100459. [DOI] [PubMed] [Google Scholar]
- 42.Tomita M, Yamada H, Adachi Y, Cui Y, Yamada E, Higuchi A. Choroidal neovascularization is provided by bone marrow cells. Stem Cells. 2004;22(1):21–26. doi: 10.1634/stemcells.22-1-21. [DOI] [PubMed] [Google Scholar]
- 43.Santos AM, Calvente R, Tassi M, Carrasco MC, Martin-Oliva D, Marin-Teva JL. Embryonic and postnatal development of microglial cells in the mouse retina. J Comp Neurol. 2008;506(2):224–239. doi: 10.1002/cne.21538. [DOI] [PubMed] [Google Scholar]
- 44.Chen L, Yang P, Kijlstra A. Distribution, markers, and functions of retinal microglia. Ocul Immunol Inflamm. 2002;10(1):27–39. doi: 10.1076/ocii.10.1.27.10328. [DOI] [PubMed] [Google Scholar]
- 45.Checchin D, Sennlaub F, Levavasseur E, Leduc M, Chemtob S. Potential role of microglia in retinal blood vessel formation. Invest Ophthalmol Vis Sci. 2006;47(8):3595–3602. doi: 10.1167/iovs.05-1522. [DOI] [PubMed] [Google Scholar]
- 46.Zhou J, Pham L, Zhang N, He S, Gamulescu MA, Spee C. Neutrophils promote experimental choroidal neovascularization. Mol Vis. 2005;11:414–424. [PubMed] [Google Scholar]
- 47.Lambert V, Lecomte J, Hansen S, Blacher S, Gonzalez ML, Struman I. Laser-induced choroidal neovascularization model to study age-related macular degeneration in mice. Nat Protoc. 2013;8(11):2197–2211. doi: 10.1038/nprot.2013.135. [DOI] [PubMed] [Google Scholar]
- 48.Yan Z, Yang W, Parkitny L, Gibson SA, Lee KS, Collins F. Deficiency of Socs3 leads to brain-targeted EAE via enhanced neutrophil activation and ROS production. JCI Insight. 2019;5 doi: 10.1172/jci.insight.126520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Croker BA, Metcalf D, Robb L, Wei W, Mifsud S, DiRago L. SOCS3 is a critical physiological negative regulator of G-CSF signaling and emergency granulopoiesis. Immunity. 2004;20(2):153–165. doi: 10.1016/s1074-7613(04)00022-6. [DOI] [PubMed] [Google Scholar]
- 50.Wu LH, Lin C, Lin HY, Liu YS, Wu CY, Tsai CF. Naringenin Suppresses Neuroinflammatory Responses Through Inducing Suppressor of Cytokine Signaling 3 Expression. Mol Neurobiol. 2016;53(2):1080–1091. doi: 10.1007/s12035-014-9042-9. [DOI] [PubMed] [Google Scholar]
- 51.Wiejak J, Dunlop J, Mackay SP, Yarwood SJ. Flavanoids induce expression of the suppressor of cytokine signalling 3 (SOCS3) gene and suppress IL-6-activated signal transducer and activator of transcription 3 (STAT3) activation in vascular endothelial cells. Biochem J. 2013;454(2):283–293. doi: 10.1042/BJ20130481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rani N, Bharti S, Krishnamurthy B, Bhatia J, Sharma C, Kamal MA. Pharmacological Properties and Therapeutic Potential of Naringenin: A Citrus Flavonoid of Pharmaceutical Promise. Curr Pharm Des. 2016;22(28):4341–4359. doi: 10.2174/1381612822666160530150936. [DOI] [PubMed] [Google Scholar]
- 53.La Manna S, Lee E, Ouzounova M, Di Natale C, Novellino E, Merlino A. Mimetics of suppressor of cytokine signaling 3: Novel potential therapeutics in triple breast cancer. Int J Cancer. 2018;143(9):2177–2186. doi: 10.1002/ijc.31594. [DOI] [PubMed] [Google Scholar]
- 54.Raychaudhuri S, Iartchouk O, Chin K, Tan PL, Tai AK, Ripke S. A rare penetrant mutation in CFH confers high risk of age-related macular degeneration. Nat Genet. 2011;43(12):1232–1236. doi: 10.1038/ng.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kumar A, Zhao L, Fariss RN, McMenamin PG, Wong WT. Vascular associations and dynamic process motility in perivascular myeloid cells of the mouse choroid: implications for function and senescent change. Invest Ophthalmol Vis Sci. 2014;55(3):1787–1796. doi: 10.1167/iovs.13-13522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Plein A, Fantin A, Denti L, Pollard JW, Ruhrberg C. Erythro-myeloid progenitors contribute endothelial cells to blood vessels. Nature. 2018;562(7726):223–228. doi: 10.1038/s41586-018-0552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park S, Tepper OM, Galiano RD, Capla JM, Baharestani S, Kleinman ME. Selective recruitment of endothelial progenitor cells to ischemic tissues with increased neovascularization. Plast Reconstr Surg. 2004;113(1):284–293. doi: 10.1097/01.PRS.0000091169.51035.A5. [DOI] [PubMed] [Google Scholar]
- 58.Takakura N, Watanabe T, Suenobu S, Yamada Y, Noda T, Ito Y. A role for hematopoietic stem cells in promoting angiogenesis. Cell. 2000;102(2):199–209. doi: 10.1016/s0092-8674(00)00025-8. [DOI] [PubMed] [Google Scholar]
- 59.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18(14):3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kalka C, Masuda H, Takahashi T, Gordon R, Tepper O, Gravereaux E. Vascular endothelial growth factor(165) gene transfer augments circulating endothelial progenitor cells in human subjects. Circ Res. 2000;86(12):1198–1202. doi: 10.1161/01.res.86.12.1198. [DOI] [PubMed] [Google Scholar]
- 61.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5(4):434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 62.Bailey AS, Willenbring H, Jiang S, Anderson DA, Schroeder DA, Wong MH. Myeloid lineage progenitors give rise to vascular endothelium. PNAS. 2006;103(35):13156–13161. doi: 10.1073/pnas.0604203103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 64.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105(1):71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bhattacharya V, McSweeney PA, Shi Q, Bruno B, Ishida A, Nash R. Enhanced endothelialization and microvessel formation in polyester grafts seeded with CD34(+) bone marrow cells. Blood. 2000;95(2):581–585. [PubMed] [Google Scholar]
- 66.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85(3):221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 67.Baba T, Miyazaki D, Inata K, Uotani R, Miyake H, Sasaki SI. Role of IL-4 in bone marrow driven dysregulated angiogenesis and age-related macular degeneration. eLife. 2020;9 doi: 10.7554/eLife.54257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feng T, Gao Z, Kou S, Huang X, Jiang Z, Lu Z. No Evidence for Erythro-Myeloid Progenitor-Derived Vascular Endothelial Cells in Multiple Organs. Circ Res. 2020;127(10):1221–1232. doi: 10.1161/CIRCRESAHA.120.317442. [DOI] [PubMed] [Google Scholar]
- 69.Schmidt D, von Hochstetter AR. The use of CD31 and collagen IV as vascular markers. A study of 56 vascular lesions. Pathol Res Pract. 1995;191(5):410–414. doi: 10.1016/S0344-0338(11)80727-2. [DOI] [PubMed] [Google Scholar]
- 70.Sadler JE. von Willebrand factor. J Biol Chem. 1991;266(34):22777–22780. [PubMed] [Google Scholar]
- 71.Clement M, Fornasa G, Guedj K, Ben Mkaddem S, Gaston AT, Khallou-Laschet J. CD31 is a key coinhibitory receptor in the development of immunogenic dendritic cells. PNAS. 2014;111(12):E1101–E1110. doi: 10.1073/pnas.1314505111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Merchand-Reyes G, Robledo-Avila FH, Buteyn NJ, Gautam S, Santhanam R, Fatehchand K. CD31 Acts as a Checkpoint Molecule and Is Modulated by FcgammaR-Mediated Signaling in Monocytes. J Immunol. 2019;203(12):3216–3224. doi: 10.4049/jimmunol.1900059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alroy J, Goyal V, Skutelsky E. Lectin histochemistry of mammalian endothelium. Histochemistry. 1987;86(6):603–607. doi: 10.1007/BF00489554. [DOI] [PubMed] [Google Scholar]
- 74.Boscia F, Esposito CL, Casamassa A, de Franciscis V, Annunziato L, Cerchia L. The isolectin IB4 binds RET receptor tyrosine kinase in microglia. J Neurochem. 2013;126(4):428–436. doi: 10.1111/jnc.12209. [DOI] [PubMed] [Google Scholar]
- 75.Kester L, van Oudenaarden A. Single-Cell Transcriptomics Meets Lineage Tracing. Cell stem cell. 2018;23(2):166–179. doi: 10.1016/j.stem.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 76.Wieghofer P, Hagemeyer N, Sankowski R, Schlecht A, Staszewski O, Amann L. Mapping the origin and fate of myeloid cells in distinct compartments of the eye by single-cell profiling. EMBO J. 2021;40(6) doi: 10.15252/embj.2020105123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8(4):265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 78.Vahatupa M, Cordova ZM, Barker H, Aittomaki S, Uusitalo H, Jarvinen TAH. Furin deficiency in myeloid cells leads to attenuated revascularization in a mouse-model of oxygen-induced retinopathy. Exp Eye Res. 2018;166:160–167. doi: 10.1016/j.exer.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 79.Liyanage SE, Fantin A, Villacampa P, Lange CA, Denti L, Cristante E. Myeloid-Derived Vascular Endothelial Growth Factor and Hypoxia-Inducible Factor Are Dispensable for Ocular Neovascularization–Brief Report. Arterioscler Thromb Vasc Biol. 2016;36(1):19–24. doi: 10.1161/ATVBAHA.115.306681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wieghofer P, Knobeloch KP, Prinz M. Genetic targeting of microglia. Glia. 2015;63(1):1–22. doi: 10.1002/glia.22727. [DOI] [PubMed] [Google Scholar]
- 81.Akhtar LN, Qin H, Muldowney MT, Yanagisawa LL, Kutsch O, Clements JE. Suppressor of cytokine signaling 3 inhibits antiviral IFN-beta signaling to enhance HIV-1 replication in macrophages. J Immunol. 2010;185(4):2393–2404. doi: 10.4049/jimmunol.0903563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Luckoff A, Caramoy A, Scholz R, Prinz M, Kalinke U, Langmann T. Interferon-beta signaling in retinal mononuclear phagocytes attenuates pathological neovascularization. EMBO Mol Med. 2016;8(6):670–678. doi: 10.15252/emmm.201505994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Salehi B, Fokou PVT, Sharifi-Rad M, Zucca P, Pezzani R, Martins N. The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharmaceuticals (Basel) 2019;12(1) doi: 10.3390/ph12010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li Q, Wang Y, Zhang L, Chen L, Du Y, Ye T. Naringenin exerts anti-angiogenic effects in human endothelial cells: Involvement of ERRalpha/VEGF/KDR signaling pathway. Fitoterapia. 2016;111:78–86. doi: 10.1016/j.fitote.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 85.La Manna S, Lee E, Ouzounova M, Di Natale C, Novellino E, Merlino A. Mimetics of Suppressor of cytokine signalling 3: novel potential therapeutics in triple breast cancer. Int J Cancer. 2018 doi: 10.1002/ijc.31594. [DOI] [PubMed] [Google Scholar]
- 86.Quijano E, Bahal R, Ricciardi A, Saltzman WM, Glazer PM. Therapeutic Peptide Nucleic Acids: Principles, Limitations, and Opportunities. Yale J Biol Med. 2017;90(4):583–598. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.