Abstract
Palm grass (Curculigo recurvata) is an ethnomedicinally important herb reported to have significant medicinal values. The present study aimed to evaluate the antidepressant and anxiolytic activities of a methanol extract of C. recurvata rhizome (Me-RCR) through different approaches. The antidepressant and anxiolytic properties of Me-RCR were assessed by using elevated plus maze (EPM), hole-board (HBT), tail suspension (TST), and forced swimming (FST) tests in Swiss Albino mice. The in-depth antioxidative potential of Me-RCR was also evaluated through DPPH radical scavenging activity, ferric-reducing power capacity, total phenolic, flavonoid, flavonol, and antioxidant content analysis. Computational investigations were performed using computer-aided methods for screening the anxiolytic, antidepressant, and antioxidative activities of the selected lead molecules. Treatment with Me-RCR (200 and 400 mg/kg, b.w.) notably increased the number of open arm entries and the time spent in the EPM test. In the HBT, Me-RCR exhibited significant anxiolytic activity at a dose of 200 mg/kg, whereas similar activity was observed at 400 mg/kg in the EPM test. Me-RCR significantly decreased the immobility time in a dose-dependent manner in both TST and FST. The IC50 for DPPH and reducing power capacity assay were found to be 18.56 and 193 μg/mL, respectively. Promising outcomes were noted for the determination of total phenolics, flavonoids, flavonols, and antioxidant capacity. In the case of computer-aided studies, nyasicoside showed promising binding energy for antidepressant and anxiolytic activities, whereas isocurculigine demonstrated promising effects as an antioxidant. Overall, these findings suggest that Me-RCR could be a favourable therapeutic candidate for the treatment of mental and psychiatric disorders, as well as a good source of antioxidants.
Keywords: Curculigo recurvata, Nyasicoside, Antidepressant, Anxiolytic, Antioxidant
Graphical abstract
Curculigo recurvata; Nyasicoside; Antidepressant; Anxiolytic; Antioxidant.
1. Introduction
Mental and psychiatric disorders are becoming extremely prevalent, pose significant psychological and socioeconomic burdens. Anxiety and depression are the main bases of impairment and self-destruction, a condition of physiological and mental illness (Islam et al., 2021; Rahman et al., 2020; Tareq et al., 2020). Depression is a widespread psychiatric disorder that significantly affects the quality of normal life. It is one of the predominant mental disorders approximately 10% of people worldwide have depression (Organization, 2017). Several studies have reported that stress is also a causative factor for anxiety and depression, leading to the increased formation of free radicals and initiating oxidative stress (Bellah et al., 2017; Hossain et al., 2020; Hossen et al., 2021a; Moni et al., 2021; Nirmal et al., 2008). Despite this, various types of medicine, such as tricyclic antidepressants, monoamine oxidase inhibitors, and selective serotonin reuptake inhibitors, are the most commonly used drugs for anxiety and depression management (Belmaker and Agam, 2008; Tareq et al., 2020). However, these classes of medications have substantial side effects (such as psychomotor impairment, dependency, and weight gain), and their usage for the treatment of anxiety and depression has become burdensome due to their adverse reactions (Pawar et al., 2011). Consequently, new medications are being sought to suppress depression and anxiety with significantly fewer adverse effects and increased efficacy, which may provide the opportunities to obtain novel drugs to extend the choice of therapies, efficiency, and protection.
Natural products are regarded as being particularly healthy by many people, plant and herb-based nutritional supplements have been widely used owing to their large variety of therapeutic and nutritional values for the management of neurological disorders (Adnan et al., 2020; Hossen et al., 2021b; Islam et al., 2021; Rahman et al., 2021a; Tareq et al., 2020). Palm grass (i.e., Curculigo recurvata W.T.Aiton), a member of the Hypoxidaceae family, is a perennial herb and is distributed widely in the Hill Tracts and marginal forests of Asian countries. The rhizome of C. recurvata (RCR) is widely used for the treatment of several ailments such as snake bites, consumptive cough, impotence, asthma, jaundice, diarrhea, colic, gonorrhea, and arthropod stings (Ahmad et al., 2020; Chifundera et al., 1991; Nie et al., 2013). Several animal and clinical studies have revealed that RCR is effective due to its hypoglycemic, antibacterial, anthelmintic, antithrombotic, and cytotoxic activities (Ahmad et al., 2020). Earlier, two novel diastereoisomeric glucosides, curculigine and isocurculigine, were isolated from this species (Chifundera et al., 1994). The total glucosides of palm grass rhizome have been found to significantly improve premenopausal syndrome (Miao et al., 2017), which is characterized by hysteria, depression, and melancholia (Shan et al., 2016). Overall, the evidence suggests that palm grass is a particularly promising but has so far only be used as a traditional medicine. Nevertheless, almost all of these ethnomedicinal statements have yet to be verified with sound scientific investigation. To date, no experimental studies have advocated the rhizome of palm grass for neuropharmacological efficiency. Hence, the present investigation aimed to evaluate the putative anxiolytic and antidepressant-like properties of the methanol extract of palm grass rhizomes.
2. Materials and methods
2.1. Chemicals & reagents
Methanol was obtained from Merck (Germany). DPPH (1,1-diphenyl-2-picrylhydrazyl) and gallic acid were purchased from Sigma-Aldrich Corporation (St. Louis, USA). Folin-Ciocalteu was procured from Merck KGaA (Darmstadt, Germany). Diazepam and imipramine HCl were purchased from Square Pharmaceuticals Ltd. (Dhaka, Bangladesh), and Tween-80 was procured from Scharlab (Sentmenat, Barcelona, Spain). All the chemicals/reagents used in this investigation were of analytical grade.
2.2. Preparation of rhizome extract
The RCR was collected from diverse parts of the Chittagong region, Bangladesh. The plants were identified by the Taxonomist and Professor Dr. Sheikh Bokhtear Uddin, Department of Botany, University of Chittagong, and a voucher specimen (accession no. 16012) has been deposited at the Department of Pharmacy, International Islamic University, Chittagong. The rhizomes were washed, dried, and finally ground to a coarse powder using a mechanical grinder (NOWAKE, Japan). Approximately 500 g of coarse powder was soaked in methanol, continually shaken, the solution was then filtered using Whatman no. 1 filter paper (Whatman International, Maidstone, Kent, UK). The filtrate was collected and evaporated using a rotary evaporator (RE200, Bibby Sterling Ltd, UK) to obtain 35.5 g of gummy crude extract (methanol extract of C. recurvata rhizome (Me-RCR)) (yield, 7.10%). Me-RCR was preserved in a refrigerator for further experiments.
2.3. Experimental animals
Male Swiss albino mice (aged 5–6 weeks, weighing 22–30 g) were collected from the animal research division of the International Centre for Diarrheal Disease and Research, Bangladesh. Then, all the animals were kept in ambient conditions with a 12:12 h dark-light cycle. Animals were permitted free access to drinking water and pellet diets ad libitum. They were acclimatized for 7 days in the laboratory environment before the study commenced. The protocol used in this study was approved by the institutional animal ethics committee, Department of Pharmacy, International Islamic University Chittagong, Bangladesh (Ref. IIUC/Pharm-78/09–18).
2.4. Acute toxicity test
The acute oral toxicity test was investigated according to the OECD guidelines and following the described method (Khan et al., 2020; OECD, 2002). Mice were divided into 7 groups (control and test) that were composed of 6 mice in each group (n = 6). Me-RCR was administered orally to the test groups at a dosage of 200–4000 mg/kg, whereas the positive control received 1% Tween 80 in distilled water. A commercial pellet feed and freshwater ad libitum were subsequently given to the mice. Experimental mice were observed for changes in behavior, adverse reactions, and fatalities for the next 3-days and subsequently the next 14 days on commencement of the experiment.
2.5. Qualitative phytochemical screening
The qualitative phytochemical analysis of Me-RCR was performed to check the presence of secondary metabolites- (i.e., alkaloids, carbohydrates, flavonoids, steroids, terpenoids, cardiac glycosides, tannins, and saponins) using standard methods (Akter et al., 2020b; Khan et al., 2020).
2.6. Antioxidative activity
2.6.1. Determination of DPPH free radical scavenging effect
The antioxidant activity of Me-RCR was assayed using the following method (Islam et al., 2013; Rahman et al., 2021b; Rashid Chowdhury et al., 2021). Three mL of 0.004% DPPH solution were combined with the sample solution at several concentrations (3–100 μg/mL). The reaction mixture was then shaken and incubated at room temperature (RT) in the dark for 15 min. Then, the absorbance was taken at 517 nm. The %inhibition of DPPH radical scavenging activity was calculated using the following equation:
| [(control absorbance−sample absorbance)/(control absorbance)] × 100 | (1) |
2.6.2. Reducing power assay
The reducing power assay of Me-RCR was assessed following the method discussed previously (Al-Araby et al., 2020; Khan et al., 2013). A 0.25 mL sample or reference solution (31.25 – 500 μg/mL) was mixed with 0.625 mL of 0.2 M phosphate buffer (pH 6.6) and 0.625 mL of 1% potassium ferricyanide (w/v). After appropriate mixing, the mixtures were incubated in a water bath for 20 min at 50 °C to complete the reaction mixture. Then, 0.625 mL of 10% TCA solution (w/v) was added to the reaction mixture and then centrifuged for 10 min at 3000 g. Finally, 1.8 mL of the supernatant was mixed with 1.8 mL of distilled water and 0.36 mL of a 0.1% ferric chloride (w/v) solution. The absorbance was measured with a spectrophotometer at 700 nm. A positive control (ascorbic acid) was used, and all tests were conducted in triplicate to maintain accuracy.
2.6.3. Assessment of total phenolic content (TPC)
The TPC of Me-RCR was determined using a spectrophotometric method described previously (Rashid Chowdhury et al., 2021; Reza et al., 2021; Uddin et al., 2015). In short, 1 mL of sample (1 mg/mL) was added to 1 mL of Folin-Ciocalteu's phenol reagent. After 5 min of incubation, 10 mL of 7% Na2CO3 solution was added to the mixture, following the addition of 13 mL of deionized distilled H2O. The mixture was set aside in a dark place for 1.30 h at RT. After incubation, the absorbance was determined at 750 nm using spectrophotometer. The TPC was calculated as milligrams of gallic acid equivalents (mg/GAE) per g of dried sample.
2.6.4. Assessment of total flavonoid content (TFC)
The TFC of Me-RCR was determined with the use of aluminum chloride (Rahman et al., 2015). Briefly, 0.5 mL of Me-RCR was added along with distilled H2O (2.5 mL) and 5% NaNO2 solution (150 μL). The mixture was vortexed for approximately 10 s and kept at RT (5 min). Then, 10% AlCl3 (300 μL), 1 mM NaOH (1 mL), and distilled H2O (550 μL) were added to this solution and mixed gently and kept at RT (15 min). The absorbance for each sample was calculated using a spectrophotometer at 510 nm. Finally, different quercetin concentrations (31.25 – 500 μg/mL) were prepared to attain the standard curve. The outcomes were presented as milligrams of quercetin equivalents (mg/QE) per g of extract.
2.6.5. Assessment of total flavonol content
The total flavanol content of Me-RCR was evaluated by a previously described method (Kabir et al., 2016). Me-RCR of 500 μg/mL was mixed with 0.5 mL of 5% aluminum chloride and 1 mL of 50 g/L C2H3NaO2 solution. To complete the reaction mixture, the prepared mixture was then incubated at RT for 150 min, and then the absorbance was taken using a spectrophotometer at 440 nm against a blank. The blank solution contained all other reagents except the extract solution. Total flavanol content was calculated as mg/g of QE by exploring an equation that was attained from the reference quercetin graph against different concentrations.
2.6.6. Total antioxidant capacity (TAC)
The total antioxidant capacity (TAC) of Me-RCR was determined with the phosphomolybdate protocol with standard ascorbic acid (Khan et al., 2016). The study was on the basis of the reduction of Mo (VI)–Mo (V) by the extract and subsequent formation of a green phosphate/Mo (V) complex at acidic pH. A 0.3 mL extract solution was added along with 3 mL of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate). The tubes that comprised the reaction solution were incubated for 90 min at 95 °C. After incubation, the samples were air-conditioned to RT, and then the absorbance was taken at 695 nm against a blank (0.3 mL of methanol).
2.7. Assessment of neuropharmacological activity
2.7.1. Study protocol
Twenty-four mice were divided into four groups (I – IV), and each group consisted of 6 mice (n = 6). The test groups (Group III and IV) received Me-RCR at the doses of 200 and 400 mg/kg, b.w., p.o. The positive control group (Group II) was treated with the standard drug diazepam (1 mg/kg, bw, i.p.) for EPM and HB, and imipramine HCl (10 mg/kg, bw, p.o.) was given for TST and FST, whereas the control group (Group I) was treated with vehicle (i.e., 1% v/v Tween-80 in saline water (10 mL/kg, b.w), respectively).
2.7.2. Anxiolytic activity
2.7.2.1. Elevated plus maze (EPM) test
The EPM consists of four arms that are subdivided into two open and two closed arms with an area of 35 cm × 5 cm and 35 cm × 5 cm × 20 cm, respectively. The two arms were interconnected with a center square of 5 cm × 5 cm that was symbolized by a plus sign and rose to a height of 25 cm from the floor. After a 30-min treatment for all groups, the mice were placed in the middle of the maze, and the head was located such that it faced toward the open arms. Throughout the 6-min test time, the number of entries in open and closed arms and time spent in open arms was noted for 5 min, wherein the first minute was kept for initial adjustment (Barua et al., 2012; Goni et al., 2020). The following equation was used to calculate the percentage of entries in the open arm:
| % of entries in open arm = Number of entries in open arm/(Number of entries in open arm + Number of entries in closed arm) | (2) |
The experimental mice of all groups (I – IV) were administered according to the experimental design mentioned in Section 2.7.1.
2.7.2.2. Hole-board test
This test was carried out according to an earlier described method (Barua et al., 2012; Goni et al., 2020). The equipment for the hole board was constructed of a matrix-patterned box comprising wood that contained 16 equidistant holes with an area of 20 cm × 40 cm. The length of the center of one hole to another hole was 10 cm, and the floor of the board was kept 25 cm from the ground. Mice were kept independently in the middle of the board after 30 min of the administration of the test doses. The number of head – dipping events was counted during 5 min of the 6-min test time, with the first minute being kept for initial adjustments. Experimental mice of all groups (I – IV) were administered according to the experimental design mentioned in Section 2.7.1.
2.7.3. Antidepressant activity
2.7.3.1. Tail suspension test (TST)
The tail suspension test (TST) was performed by the methods described previously (Khan et al., 2020; Steru et al., 1985). The mice were suspended by the tail from a ledge with adhesive tape, and the distance between the tip of the animal's tail and floor was 50 cm. Immobility time was recorded during a 6-min test for animals of all groups, with the last 4 min being considered the immobility time following the first 2-min initial adjustment. The experimental mice of all groups (I – IV) were administered according to the experimental design mentioned in Section 2.7.1.
2.7.3.2. Forced swimming test (FST)
The forced swimming test (FST) was performed using a method described earlier (Khan et al., 2020; Porsolt et al., 1977). The experimental apparatus was composed of a cylindrical glass compartment of 10 cm in diameter and 25 cm in height that contained up to 19 cm of supplied fresh water maintained at 25 ± 1 °C. The immobility time was recorded during the next 4 min of the total 6-min study time; for the first 2 min of the test, the mice showed vigorous movement that was considered to represent an initial adjustment period. When mice stopped struggling and remained motionless in the water, keeping their head above the water. They were considered immobile. The experimental mice of all groups (I – IV) were administered according to the experimental design mentioned in Section 2.7.1.
2.8. In silico molecular docking
2.8.1. Protein preparation
The structure of urate oxidase (protein data bank (PDB): 1R4U), glutathione reductase (PDB: 3GRS), human serotonin transporter (PDB id: 5I6X), and potassium channel KcsA-Fab (PDB id: 4UUJ) were downloaded in PDB format from the PDB (Berman et al., 2002). The structure was organized and refined using Schrödinger-Maestro v11.1. Using force field OPLS_2005, energy minimization was performed using the protein preparation wizard.
2.8.2. Ligand preparation
Three selected lead compounds (curculigine, isocurculigine, and nyasicoside) were collected from PubChem databases based on the prediction of activity spectra for substances. The 3D structures for these compounds were built by using the LigPrep module of Schrödinger Suite (2017) with an OPLS 2005 force field. Their ionization states were generated at pH 7.0 ± 2.0 using Epik 2.2 in Schrödinger Suite.
2.8.3. Receptor grid generation
The ligand poses bind with the calculated receptor grids. A van der Waals scaling factor of 1.00 and charge cut-off of 0.25 subjected to an OPLS 2005 force field were considered as the default factor during grid generation. A cubic box of specific dimensions centered on the active site residues (reference ligand active site) was generated for the receptor. The bounding box was set to 14 Å × 14 Å × 14 Å for docking experiments.
2.8.4. Glide standard precision (SP) ligand docking
SP ligand docking was performed using Schrödinger-Maestro v 11.1 (Bristy et al., 2020; Islam et al., 2021; Khan et al., 2020; Rahman et al., 2020) within which penalties were applied to non-cis/trans amide bonds. During docking, the van der Waals scaling factor and charge cut-off were selected at 0.80 and 0.15, respectively, for ligand atoms. The final score was calculated based on energy-minimized poses and displayed as the Glide score. The best docked pose with the lowest Glide score value was recorded for each ligand. Three parameters were mainly considered for the interpretation of the docking result; Glide score, Glide e-model, and Glide energy.
2.9. Statistical analysis
The data are presented as mean ± standard error of mean (SEM). Statistical analyses were performed using one-way ANOVA followed by Dunnett's tests. The values obtained were compared with the vehicle-treated control group and were considered statistically significant when p < 0.05.
3. Results
3.1. Phytochemical screening
Qualitative phytochemical tests revealed the presence of carbohydrates, flavonoids, tannins, saponins, and cardiac glycosides (Table 1).
Table 1.
Qualitative phytochemical screening of Me-RCR.
| Phytochemical class | Observations | Results |
|---|---|---|
| Alkaloids | Turbidity/precipitation | - |
| Carbohydrates | Formation of a purple product at the interface of the two layers | + |
| Flavonoid | Formation of yellow color, which changed to colorless on acid addition | +++ |
| Steroids | Green to pink color was absent | - |
| Terpenoids | Appearance of reddish brown-deep red color | - |
| Cardiac Glycosides | Lower reddish-brown layer and upper acetic acid layer, which turns bluish green | + |
| Tannins | Appearance of white precipitation | ++ |
| Saponins | Stable froth formation | + |
(-) = not present; (+) = present in low concentration; (++) = present in moderately high concentration; and (+++) = present in very high concentration.
3.2. In vitro antioxidant activity
3.2.1. DPPH free radical scavenging activity
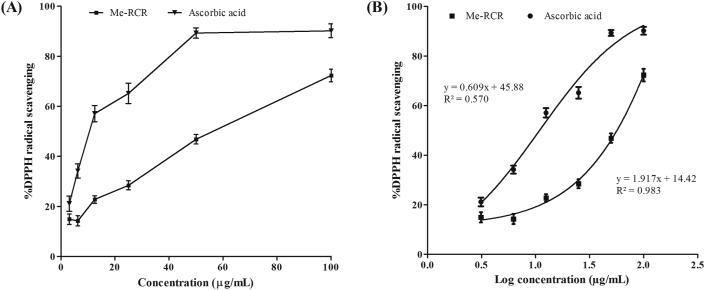
Me-RCR exhibited a concentration-dependent DPPH-scavenging capacity, which is shown in Figure 1 (A and B). The IC50 (μg/mL) value of DPPH free radical neutralizing the activity of Me-RCR was 18.56 μg/mL, indicating that Me-RCR is moderately active as compared to the standard 6.77 μg/mL (ascorbic acid) exploited in this method.
Figure 1.
DPPH radical scavenging activity of Me-RCR along with standard ascorbic acid. (A) Concentration vs. % DPPH radical scavenging and (B) Log concentration vs. % DPPH radical scavenging (IC50 was calculated from this plot). Me-RCR, methanol extract of rhizome of C. recurvata.
3.2.2. Reducing power capacity
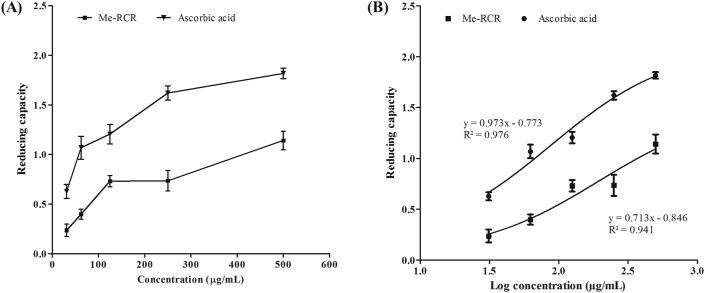
As shown in Figure 2 (A), Me-RCR showed increased absorbance of the reaction mixture with increasing concentrations of the sample solution, demonstrating that this plant has significant reducing power capacity. Regression analysis for reducing activity versus log concentration showed an IC50 value for Me-RCR of 193.0 μg/mL, which was close to that of ascorbic acid 87.35 μg/mL Figure 2 (B).
Figure 2.
Reducing capacity of Me-RCR along with standard ascorbic acid. (A) Reducing capacity vs. concentration and (B) Reducing capacity vs. Log concentration (IC50 was calculated from this plot). Me-RCR, methanol extract of rhizome of C. recurvata.
3.2.3. Determination of total phenolic content, flavonoid content, flavonols, and antioxidant capacity
In this experiment, the TPC of Me-RCR was expressed as gallic acid equivalent, and the extract was found to show 301.17 ± 2.13 mg GAE/g of dry extract (Table 2). The TFC of Me-RCR is provided in Table 1. The test was confirmed by adopting quercetin as a standard, and the results demonstrated that the extract achieved a flavonoid content of 45.47 ± 1.39 mg QE/g of dry extract. Table 1 shows the moderate amount of total flavonol quantity of the Me-RCR extract 55.40 ± 0.16 mg QE/g where quercetin was used as a standard. The TAC of the Me-RCR was determined using a phosphomolybdate assay. The study protocol was designed on the basis of the reduced ability to extract from molybdenum (VI) to molybdenum (V). The extract was found to have a maximum (315.60 ± 0.17 mg) ascorbic acid/g of extract that demonstrated a potent total antioxidant index (Table 2).
Table 2.
Spectrophotometric determination of the phytochemicals of Me-RCR.
| Phytochemicals (mg/g) | Me-RCR |
|---|---|
| Total Phenol (mg Gallic acid/g) | 301.17 ± 2.13 |
| Total Flavonoid (mg Quercetin/g) | 45.47 ± 1.39 |
| Total Flavonol (mg Quercetin/g) | 55.40 ± 0.16 |
| Total Antioxidant (mg Ascorbic acid/g) | 315.60 ± 0.17 |
Values are represented as mean ± SEM (n = 3). Me-RCR, methanol extract of rhizome of C. recurvata.
3.3. Anxiolytic activity
3.3.1. Elevated plus maze test (EPM)
In the EPM test, the Me-RCR extract-treated mice at a dose of 200 mg/kg showed a significant (p < 0.05) increase in the percentage of time spent in the open arms (50.63 ± 3.98) and the percentage of open-arm entries (53.12 ± 3.14) as compared to the control group, whereas at a dose of 400 mg/kg, the percentage of time spent in the open arms and the percentage of open-arm entries were 57.69 ± 3.17 and 54.17 ± 3.55, respectively (Table 3). On the other hand, the reference drug diazepam at a dose of 1 mg/kg produced a noticeable increase in the percentages of time spent in the open arms (79.50 ± 2.57).
Table 3.
Anxiolytic activity of Me-RCR on elevated plus maze test in mice.
| Group | Dose (mg/kg) | % of entry into open arm | % of time spent in open arm |
|---|---|---|---|
| Control | 10 mL/kg | 42.86 ± 2.23 | 38.46 ± 1.30 |
| Diazepam | 1 | 79.50 ± 2.57∗∗ | 75.47 ± 2.44∗∗ |
| Me-RCR 200 | 200 | 53.12 ± 3.14 | 50.63 ± 3.98 |
| Me-RCR 400 | 400 | 54.17 ± 3.55∗ | 57.69 ± 3.17∗ |
Values are mean ± S.E.M. ∗p < 0.05 and ∗∗p < 0.01, significantly different from control; ANOVA followed Dunnett's test (n = 10, each group). Me-RCR, methanol extract of rhizome of C. recurvata.
3.3.2. Hole-board test (HBT)
Me-RCR at a dose of 200 mg/kg showed significant (p < 0.05) increases in head-dipping behavior (65.60 ± 4.14). However, at a dose of 400 mg/kg, Me-RCR showed significant decreases in this behavior (57.71 ± 3.13) as compared to the control group (Table 4). Notably, the positive control diazepam also demonstrated increases in the number of head dips (78.15 ± 2.20) as compared to the control group (36.43 ± 1.50).
Table 4.
Anxiolytic activity of Me-RCR on hole-board test in mice.
| Group | Dose (mg/kg) | No. of head dipping | Latency to the first head dipping (sec) |
|---|---|---|---|
| Control | 10 mL/kg | 36.43 ± 1.50 | 10.50 ± 3.31 |
| Diazepam | 1 | 78.15 ± 2.20∗∗ | 0.50 ± 0.70∗∗ |
| Me-RCR 200 | 200 | 65.60 ± 4.14 | 14.60 ± 1.70 |
| Me-RCR 400 | 400 | 57.71 ± 3.13∗ | 4.30 ± 2.40∗ |
Values are represented as mean ± S.E.M. ∗p < 0.05 and ∗∗p < 0.01, significantly different from control; ANOVA followed Dunnett's test (n = 10, per group). Me-RCR, methanol extract of rhizome of C. recurvata.
3.4. Antidepressant activity assay
3.4.1. Tail suspension test (TST)
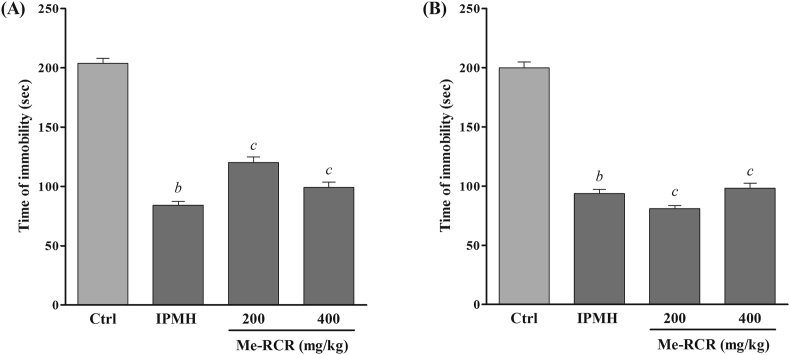
The antidepressant effect of Me-RCR on immobility time in the tail suspension test is shown in Figure 3 (A). Me-RCR at the dose of 200 mg/kg showed a significant decrease in immobility time (120.33 ± 3.70 s) as compared to the control group (191.03 ± 6 s). In contrast, Me-RCR noticeably reduced the immobility time (81.86 ± 4.50 s) at 400 mg/kg, which is slightly lower than the time (94.03 ± 3.37 s) shown by imipramine HCl (10 mg/kg).
Figure 3.
Antidepressant activity of methanol extract of rhizome of C. recurvata on tail suspension test in mice (A). Antidepressant activity of methanol extract of rhizome of C. recurvata on forced swimming test in mice (B). Mice were divided into four groups (10 for each group) that were orally administered control (1% v/v Tween-80 in saline water), imipramine HCl (10 mg/kg), Me-RCR 200 (200 mg/kg), and Me-RCR 400 (400 mg/kg), respectively. Results are expressed as mean (SEM) of immobility time (in seconds). Differences were analyzed using one-way ANOVA followed by Dunnett's test (n = 10, per group). For statistical significance, ap < 0.001, bp < 0.01, and cp < 0.05 when compared with the control group. Ctrl, Control; IPMH, Imipramine HCl; and Me-RCR, methanol extract of rhizome of C. recurvata.
3.4.2. Forced swimming test (FST)
The FST results showed that both doses (200 and 400 mg/kg) of Me-RCR produced a significant (p < 0.05) reduction of immobility time (101.87 ± 9.43 s) and (81.42 ± 5.08 s), respectively, whereas the standard drug imipramine HCl at a dose of 10 mg/kg was found to significantly (p < 0.05) decrease the immobility time (92.45 ± 3.98 s) as compared to the control. However, the observed results confirmed the dose-dependent response by Me-RCR as shown in Figure 3 (B).
3.5. In silico molecular docking for antioxidant activity
The antioxidant activities of the selected lead molecules (curculigine, isocurculigine, and nyasicoside) and standard ascorbic acid were determined. Among the compounds, isocurculigine demonstrated the highest docking score (−5.407 kcal/mol−1) binding with 1R4U protein, whereas isocurculigine showed the maximum docking score (−9.515 kcal/mol−1) with 3GRS protein. The standard ascorbic acid revealed docking scores of -4.944 kcal/mol−1 and -7.756 kcal/mol−1 with 1R4U and 3GRS proteins, respectively. The results of the docking score are summarized in Table 5, and their interactions are depicted in Figure 4, respectively.
Table 5.
Docking results of curculigine, isocurculigine, nyasicoside, and standard ascorbic acid with urate oxidase (PDB ID: 1R4U), glutathione reductase (PDB ID: 3GRS) for antioxidant activity.
| Compound name | Docking score |
Glide e-model |
Glide energy |
|||
|---|---|---|---|---|---|---|
| 1R4U | 3GRS | 1R4U | 3GRS | 1R4U | 3GRS | |
| Curculigine | -5.248 | -9.460 | -61.973 | -107.864 | -49.266 | -73.296 |
| Isocurculigine | -5.407 | -9.515 | -71.319 | -110.112 | -57.260 | -72.314 |
| Nyasicoside | -4.239 | -8.498 | -53.703 | -106.471 | -45.007 | -71.337 |
| Ascorbic acid | -4.944 | -7.756 | -37.968 | -65.904 | -26.410 | -43.207 |
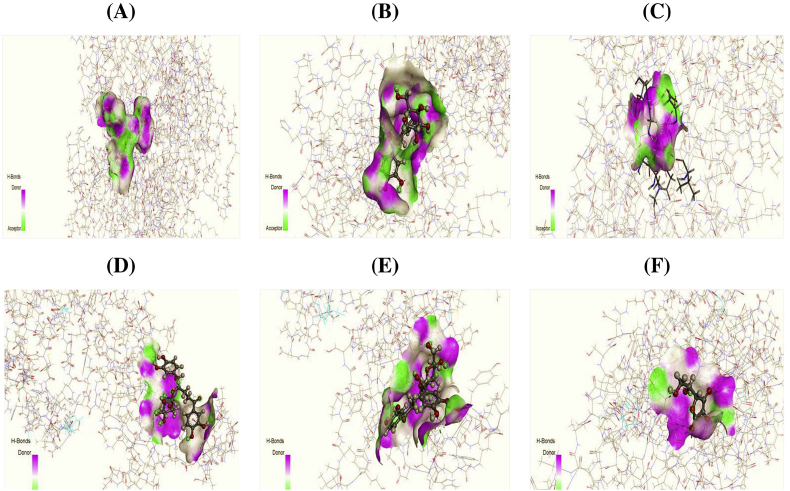
Figure 4.
3D docking interaction of antioxidative activity of curculigine (A), isocurculigine (B), and standard ascorbic acid (C) with glutathione reductase (PDB ID: 3GRS) and 3D docking interaction of antioxidative activity of curculigine (D), isocurculigine (E), and standard ascorbic acid (F) with urate oxidase (PDB ID: 1R4U).
3.6. In silico molecular docking for anxiolytic activity
Computer-aided anxiolytic activity was performed to assess the binding pattern of molecules with the amino acids present in the active pocket of the protein. In this study, the potassium channel KcsA-Fab receptor (PDB ID: 4UUJ) was used to explore the possible anxiolytic activity of Me-RCR. Among the lead molecules, nyasicoside showed the highest docking score (−3.968 kcal/mol−1) against the 4UUJ receptor, with this value being slightly lower than for the standard drug diazepam (−5.446 kcal/mol−1). The results of the docking analysis and their interactions are shown in Figure 5 (A, B, and C) and Table 6.
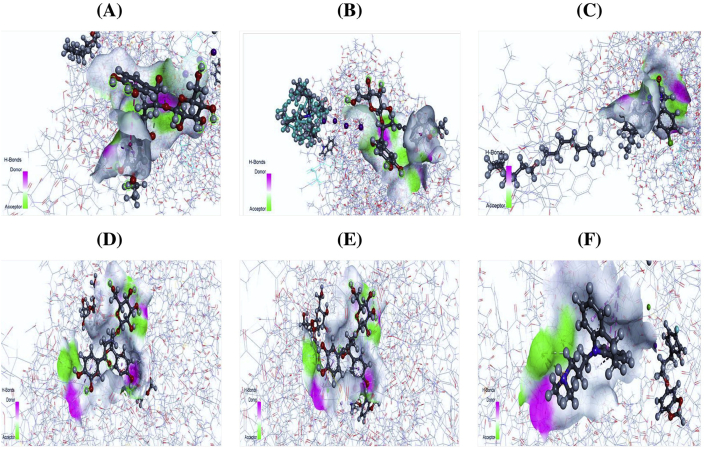
Figure 5.
3D docking interaction of anxiolytic activity of isocurculigine (A), nyasicoside (B), and standard diazepam (C) with potassium channel KcsA-Fab protein (PDB ID: 4UUJ) and 3D docking interaction of antidepressant activity of curculigine (D), nyasicoside (E), and standard imipramine HCl (F) with human serotonin transporter protein (PDB ID: 5I6X).
Table 6.
Docking results of curculigine, isocurculigine, nyasicoside, and standard diazepam with potassium channel Kcsa-Fab (PDB ID: 4UUJ) for anxiolytic activity.
| Compound name | Docking score | Glide e-model | Glide energy |
|---|---|---|---|
| Curculigine | -2.333 | -27.299 | -28.439 |
| Isocurculigine | -3.937 | -41.695 | -33.775 |
| Nyasicoside | -3.968 | -39.354 | -32.075 |
| Diazepam | -5.446 | -36.497 | -28.049 |
3.7. In silico molecular docking for antidepressant activity
A grid- and ligand-based molecular docking program was used to assess the binding patterns of molecules with the amino acids present in the active pocket of the protein. The docking study was performed for the selected chemical constituents of C. recurvata to the active site of human serotonin transporter protein (PDB ID: 5I6X) using Schrodinger suite v11.1. Among all three compounds, nyasicoside showed the highest docking score (−5.998 kcal/mol−1) (Table 7), whereas the standard drug imipramine HCl had the docking score (−4.138 kcal/mol−1). The low and negative free energy value for binding indicates a strong favorable bond with 5I6X. The docking interactions are depicted in Figure 5 (D, E, and F).
Table 7.
Docking results of curculigine, isocurculigine, nyasicoside, and standard imipramine HCl with human serotonin transporter protein (PDB ID: 5I6X) for antidepressant effect.
| Compound name | Docking score | Glide e-model | Glide energy |
|---|---|---|---|
| Curculigine | -5.589 | -57.069 | -47.092 |
| Isocurculigine | -3.937 | -41.695 | -33.775 |
| Nyasicoside | -5.998 | -63.397 | -49.398 |
| Imipramine HCl | -4.138 | -27.326 | -22.055 |
4. Discussion
Natural medicines based on plant/herbs have been widely used owing to their therapeutic efficacy since primitive ages. Natural substances are extensively used in the pharmaceutical, biopharmaceutical, and food supplement and dietary nutritional industries to prepare various natural remedies, minerals, nutritional supplements, and medicine for numerous diseases (Ahmed et al., 2021; Babar et al., 2019, 2020; Haque et al., 2017; Hossain et al., 2020). Owing to their abundant source of biologically active secondary metabolites, the significance of traditional medicines in managing a broad variety of diseases is continually increasing (Akter et al., 2020b; Haque et al., 2020; Islam et al., 2020; Rahman et al., 2021a).
Reactive oxygen species (ROS) are responsible for inflammatory neurodegenerative disorders such as anxiety and depression (Maes et al., 2011). Evidence suggests that an excessive amount of ROS initiates oxidative damage and many other fatal diseases such as CVS diseases, cancers, aging, diabetes, atherosclerosis, and depression (Ansari et al., 2017; Maria Michel et al., 2012; Nigri et al., 2004; Schetter et al., 2010). Because of the elevated amount of oxygen consumption and insufficient antioxidant protection against ROS, nerve cells become more vulnerable to oxidative damage (Halliwell, 2006; Reza et al., 2018a; Sharmin et al., 2020). However, natural antioxidants from traditional medicinal plants, vegetables, and fruits have received significant attention due to their aptitude to scavenge free radicals as well as enhance the body's own antioxidant system to prevent various disorders (Akter et al., 2020; Hossain et al., 2018; Mamun et al., 2020). In this study, Me-RCR showed significant free radical scavenging potential, which suggests that Me-RCR can prevent free radical-induced diseases, including depression.
Analogously, this plant extract encompasses the reduction of power activity, which is considered to be prominent indicator of antioxidant activity (Oliveira et al., 2008). The mechanism of the reduction of power activity is associated with the presence of a reductant, which donates hydrogen atoms after ROS breakdown. The current study showed the reducing power of Me-RCR, which is reflected in its reduction of the Fe3+-ferricyanide complex in the ferrous form, indicating the reducing activity in a dose-dependent manner.
Phenolic compounds are well recognized as being active as antioxidants because of their ability to scavenge. The TPC of the extract was revealed in the gallic acid equivalent. Gallic acid is one of the main polyphenolic compounds found in a wide variety of plants and attenuates oxidative stress (Akter et al., 2020a). In this investigation, the antioxidants and total phenolics showed linear correlations, and this concept supports the proposal that Me-RCR contains a high phenolic content and has strong antioxidant potential. In addition, the presence of a significant proportion of flavonoids and flavonols in Me-RCR also indicates that the rhizome of Curculigo recurvata is a prospective antioxidant candidate.
The results obtained from the phosphomolybdic acid assay also demonstrate that Me-RCR has a strong ability to reduce molybdenum (VI) to molybdenum (V) by donating an electron. Numerous natural products having phenols and flavonoids can cause this reduction, and the strong antioxidant effects of Me-RCR, therefore, supported. A recent study reported that flavonoids are associated with reduced chronic diseases risk, such as depression and anxiety, because of their potent antioxidant activities (Islam et al., 2014; Okarter and Liu, 2010). Recent studies have also suggested that natural antioxidative polyphenols that possess anxiolytic effects are a prime choice as neuroprotective and antiamnesic compounds (Ferdousy et al., 2017; Reza et al., 2018b). Therefore, it can be assumed that the obtained antioxidant and free radical scavenging activity of Me-RCR may be effective against depression and anxiety.
This study also aimed to investigate Me-RCR's anxiolytic-like activity by using well-known methods, (i.e., EPM and HBT). These methods are widely used owing to their accepted conditions and stimuli to induce anxiety. Drugs possessing anxiolytic effects decrease the distance for open arms and persuade the test animal to spend more time in the open arms, whereas anxiogenic effects decrease open-arm exploration by reducing both spent time and number of entries into the open arms (Griebel et al., 2000). Regarding EPM, we found that experimental animals treated with different doses of Me-RCR (200 & 400 mg/kg, p.o.) displayed a higher tendency to spend time in the open arms, an indicator of decreased anxiety, reflecting that the extract induces specific anxiolytic-like properties. In the EPM investigation, higher doses (400 mg/kg, p.o.) showed significant (p < 0.05) percentages of time spent in the open arm in a dose-dependent manner, with the standard drug diazepam (1 mg/kg, p.o.) demonstrated significant (p < 0.01) anxiolytic-like activity. Subsequently, the diminution in head-dipping behavior in HBT revealed the anxiogenic condition of experimental animals, whereas an enhancement in head dipping behavior imitates anxiolytic condition (Takeda et al., 1998). The head dipping response is an expedient index to evaluate the properties of drugs regarding central serotonergic activity in rodent tests (Takeda et al., 1998). In this study, a low dose (200 mg/kg, p.o.) of Me-RCR increased the number and extent of head-dips, whereas a high dose (400 mg/kg, p.o.) lessened the number and extent of head-dips without substantial change in locomotor actions in mice. Therefore, it appears that Me-RCR possesses a dose-dependent anxiolytic action. However, the functions of phytochemicals (i.e., flavonoids, flavonols, saponins, and tannins) with antidepressant and anxiolytic potential have been demonstrated in many herbs in traditional medicine (Howes and Houghton, 2003). In this study, strong anxiolytic activity was noted and, may be due to the presence of secondary metabolites present in this plant.
Depression, among several other serious mental illnesses globally, is becoming increasingly common. The antidepressant-like effects of Me-RCR were estimated through behavioral models of TST and FST, which have been extensively established behavioral paradigms for analyzing antidepressants (Porsolt et al., 1977; Steru et al., 1985). In both experiments, mice were placed in an inescapable condition and their characteristic behavior was assessed in terms of immobility, which has been well established as imitating behavioral despair similar to that perceived in human depression (Porsolt et al., 1977). In the behavioral model, the administration of Me-RCR and imipramine HCl produced dissimilar immobility capacities in both TST and FST. The immobility time is abridged by treatment with antidepressants and encompasses a significant correlation between the clinical efficacy of the antidepressants and their potency in the FST and TST (Steru et al., 1985). In these latter tests, Me-RCR markedly decreased the immobility time but to a lesser extent than the reference drug, imipramine HCl. The extent of decrease in immobility has been found to be a good predictive value in the estimation of potential antidepressants (Porsolt et al., 1977). Our study demonstrated that Me-RCR had significant antidepressant-like effects as compared to the control group. Hence, it is suggested that Me-RCR may be explored for further management of depression and anxiety.
Besides experimental investigation, in silico drug design was evaluated to determine the present pharmacological potential of the major bioactive isolates/compounds of Me-RCR. The aim of this computer-based in silico investigation was to clarify the ligand-receptor complexes and verify that the experimental evidence was consistent with the in silico findings. Additionally, docking outcomes found that all three components demonstrated substantial electrostatic, van Der Waals, and hydrogen-bonding interactions between the component-receptor complex and, thus, exhibited biological properties against the selected receptor proteins. These compounds bind to receptors due to the formation of numerous interactions, lowering the hydrophilicity, which augments the hydrophobicity to keep the protein-ligand complex highly stable. The results of the study show that among the bioactive isolates, nyasicoside has a good binding capacity to both antidepressant and anxiolytic receptors, which supports the current experimental data. In addition, regarding antioxidative properties, isocurculigine showed the highest binding affinity. However, this study strongly suggests that nyasicoside might be a promising bioactive isolate due to its antidepressant and anxiolytic-like potential as well as isocurculigine for its antioxidative activity; hence, these compounds strongly recommended for next step QSAR, molecular simulation, and homology modeling.
5. Conclusion
The outcomes of this investigation revealed the potential anxiolytic and antidepressant-like effects of palm grass rhizome in behavioral models. The results further revealed the major antioxidant effects of this herb, and the outcomes of the computer-aided methods were found to agree with the experimental results. Nevertheless, to thoroughly explain the observed effects-mechanisms more in-depth analysis of the chemical constituents of this medicinal herb is required, and extensive toxicological investigations, as well as pharmacokinetic and pharmacodynamic studies, are strongly recommended before moving forward to the next phase of clinical research.
Declarations
Author contribution statement
Md. Akramul Hoque; Shabbir Ahmad; Nishan Chakrabarty; Mohammad Forhad Khan: Performed the experiments.
Mohammad Shah Hafez Kabir: Conceived and designed the experiments; Wrote the paper.
Afrina Brishti; Md. Obayed Raihan: Analyzed and interpreted the data.
A. H. M. Khurshid Alamd; Md Anwarul Haque; Mst. Samima Nasrin; Md. Areeful Haque: Analyzed and interpreted the data; wrote the paper.
A. S. M. Ali Reza: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; wrote the paper.
Funding statement
This work was supported by the Center for Research and Publication (CRP) grant (IRG 180111), International Islamic University Chittagong.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are grateful to the Department of Pharmacy, International Islamic University Chittagong for providing the facilities to conduct this research work.
References
- Adnan M., Chy M., Uddin N., Kamal A., Chowdhury K.A.A., Rahman M., Reza A., Moniruzzaman M., Rony S.R., Nasrin M. Intervention in neuropsychiatric disorders by suppressing inflammatory and oxidative stress signal and exploration of in silico studies for potential lead compounds from Holigarna caustica (Dennst.) Oken leaves. Biomolecules. 2020;10:561. doi: 10.3390/biom10040561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S., Nasrin M.S., Reza A.S.M.A., Chakrabarty N., Hoque M.A., Islam S., Hafez Kabir M.S., Tareq S.M., Alam A.H.M.K., Haque M.A., Arman M.S.I. Curculigo recurvata W.T.Aiton exhibits anti-nociceptive and anti-diarrheal effects in Albino mice and an in silico model. Anim. Mod. Experim. Med. 2020;3:169–181. doi: 10.1002/ame2.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A.A., Rahman M.A., Hossen M.A., Reza A.A., Islam M.S., Rashid M.M., Rafi M.K.J., Siddiqui M.T.A., Al-Noman A., Uddin M.N. Epiphytic Acampe ochracea orchid relieves paracetamol-induced hepatotoxicity by inhibiting oxidative stress and upregulating antioxidant genes in in vivo and virtual screening. Biomed. Pharmacother. 2021;143:112215. doi: 10.1016/j.biopha.2021.112215. [DOI] [PubMed] [Google Scholar]
- Akter N., Chowdhury F.I., Selim S., Nayan S.I., Khan F., Subhan N., Hossain H., Rahman M.M., Haque M.A., Alam M.A. Polyphenolics in ramontchi protect cardiac tissues via suppressing isoprenaline-induced oxidative stress and inflammatory responses in Long-Evans rats. J. Funct. Food. 2020;75:104250. [Google Scholar]
- Akter S., Jahan I., Khatun R., Khan M.F., Arshad L., Jakaria M., Haque M.A. Pharmacological insights into Merremia vitifolia (Burm. f.) Hallier f. leaf for its antioxidant, thrombolytic, anti-arthritic and anti-nociceptive potential. Biosci. Rep. 2020 doi: 10.1042/BSR20203022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akter S., Shah M., Tareq A.M., Nasrin M.S., Rahman M.A., Babar Z., Haque M.A., Royhan M.J., Mamun M.N., Reza A.A., Emran T.B. Pharmacological effect of methanolic and hydro-alcoholic extract of Coconut endocarp. J. Adv. Biotechnol. Exp. Ther. 2020;3:171–181. [Google Scholar]
- Al-Araby S., Rahman M.A., Chowdhury M.A., Das R., Chowdhury T., Hasan C.M.M., Afroze M., Hashem M., Hajjar D., Alelwani W. Padina tenuis (marine alga) attenuates oxidative stress and streptozotocin-induced type 2 diabetic indices in Wistar albino rats. South Afr. J. Bot. 2020;128:87–100. [Google Scholar]
- Ansari P., Uddin M., Rahman M., Abdullah-Al-Mamun M., Islam M., Ali M., Reza A. Anti-inflammatory, anti-diarrheal, thrombolytic and cytotoxic activities of an ornamental medicinal plant: Persicaria orientalis. J. Basic Clin. Physiol. Pharmacol. 2017;28:51–58. doi: 10.1515/jbcpp-2016-0023. [DOI] [PubMed] [Google Scholar]
- Babar Z., Jaswir I., Tareq A., Ali Reza A.M., Azizi W., Hafidz M., Ahfter F., Hasan M., Farhad S., Uddin M.R. In vivo anxiolytic and in vitro anti-inflammatory activities of water-soluble extract (WSE) of Nigella sativa (L.) seeds. Nat. Prod. Res. 2019:1–6. doi: 10.1080/14786419.2019.1667348. [DOI] [PubMed] [Google Scholar]
- Babar Z.U.M., Jaswir I., Maifiah M.H.M., Ismail S., Raus R.A., Tareq A.M., Ahfter F., Faraque A., Reza A.A., Sayeed M.A. The thrombolytic and cytotoxic effects of Nigella sativa (L.) seeds: the prophetic medicine. Int. J. Halal Res. 2020;2:70–77. [Google Scholar]
- Barua C.C., Talukdar A., Begum S.A., Borah P., Lahkar M. Anxiolytic activity of methanol leaf extract of Achyranthes aspera Linn in mice using experimental models of anxiety. Indian J. Pharmacol. 2012;44:63. doi: 10.4103/0253-7613.91869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellah S.F., Islam M.N., Karim M.R., Rahaman M.M., Nasrin M.S., Rahman M.A., Reza A.A. Evaluation of cytotoxic, analgesic, antidiarrheal and phytochemical properties of Hygrophila spinosa (T. Anders) whole plant. J. Basic Clin. Physiol. Pharmacol. 2017;28:185–190. doi: 10.1515/jbcpp-2016-0103. [DOI] [PubMed] [Google Scholar]
- Belmaker R., Agam G. Major depressive disorder. N. Engl. J. Med. 2008;358:55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- Berman H.M., Battistuz T., Bhat T., Bluhm W.F., Bourne P.E., Burkhardt K., Feng Z., Gilliland G.L., Iype L., Jain S. The protein data bank. Acta Crystallogr. Sect. D Biol. Crystallogr. 2002;58:899–907. doi: 10.1107/s0907444902003451. [DOI] [PubMed] [Google Scholar]
- Bristy T.A., Barua N., Montakim Tareq A., Sakib S.A., Etu S.T., Chowdhury K.H., Jyoti M.A., Aziz M., Ibn A., Reza A. Deciphering the pharmacological properties of methanol extract of Psychotria calocarpa leaves by in vivo, in vitro and in silico approaches. Pharmaceuticals. 2020;13:183. doi: 10.3390/ph13080183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chifundera K., Messana I., Galeffi C., De Vicente Y. Research on african medicinal plants-XXV-the (1R, 2S) absolute configuration of nyasicoside. Its occurrence in curculigorecurvata. Tetrahedron. 1991;47:4369–4374. [Google Scholar]
- Chifundera K., Palazzino G., Messana I., Ping L., Galeffi C., Cannarsa G. Norlignan glucosides from Curculigo recurvata. Phytochemistry. 1994;35:1343–1348. [Google Scholar]
- Ferdousy S., Rahman M.A., Al-Amin M.M., Aklima J., Chowdhury J.K.H. Antioxidative and neuroprotective effects of Leea macrophylla methanol root extracts on diazepam-induced memory impairment in amnesic Wistar albino rat. Clin. Phytosci. 2017;2:17. [Google Scholar]
- Goni O., Khan M.F., Rahman M.M., Hasan M.Z., Kader F.B., Sazzad N., Sakib M.A., Romano B., Haque M.A., Capasso R. Pharmacological insights on the antidepressant, anxiolytic and aphrodisiac potentials of Aglaonema hookerianum Schott. J. Ethnopharmacol. 2020;268:113664. doi: 10.1016/j.jep.2020.113664. [DOI] [PubMed] [Google Scholar]
- Griebel G., Belzung C., Perrault G., Sanger D.J. Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology. 2000;148:164–170. doi: 10.1007/s002130050038. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress and neurodegeneration: where are we now? J. Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- Haque M.A., Jantan I., Bukhari S.N.A. Tinospora species: an overview of their modulating effects on the immune system. J. Ethnopharmacol. 2017;207:67–85. doi: 10.1016/j.jep.2017.06.013. [DOI] [PubMed] [Google Scholar]
- Haque M.A., Reza A.A., Nasrin M.S., Rahman M.A. Pleurotus highking mushrooms potentiate antiproliferative and antimigratory activity against triple-negative breast cancer cells by suppressing Akt signaling. Integr. Cancer Ther. 2020;19 doi: 10.1177/1534735420969809. 1534735420969809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain K.H., Rahman M.A., Taher M., Tangpong J., Hajjar D., Alelwani W., Makki A.A., Reza A.A. Hot methanol extract of Leea macrophylla (Roxb.) manages chemical-induced inflammation in rodent model. J. King Saud Univ. Sci. 2020 [Google Scholar]
- Hossain M.S., Reza A.A., Rahaman M.M., Nasrin M.S., Rahat M.R.U., Islam M.R., Uddin M.J., Rahman M.A. Evaluation of morning glory (Jacquemontia tamnifolia (L.) Griseb) leaves for antioxidant, antinociceptive, anticoagulant and cytotoxic activities. J. Basic Clin. Physiol. Pharmacol. 2018;29:291–299. doi: 10.1515/jbcpp-2017-0042. [DOI] [PubMed] [Google Scholar]
- Hossen M.A., Ali Reza A., Amin M.B., Nasrin M.S., Khan T.A., Rajib M.H.R., Tareq A.M., Haque M.A., Rahman M.A., Haque M.A. Bioactive metabolites of Blumea lacera attenuate anxiety and depression in rodents and computer-aided model. Food Sci. Nutr. 2021 doi: 10.1002/fsn3.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossen M.A., Reza A.A., Ahmed A.A., Islam M.K., Jahan I., Hossain R., Khan M.F., Maruf M.R.A., Haque M.A., Rahman M.A. Pretreatment of Blumea lacera leaves ameliorate acute ulcer and oxidative stress in ethanol-induced Long-Evan rat: a combined experimental and chemico-biological interaction. Biomed. Pharmacother. 2021;135:111211. doi: 10.1016/j.biopha.2020.111211. [DOI] [PubMed] [Google Scholar]
- Howes M.-J.R., Houghton P.J. Plants used in Chinese and Indian traditional medicine for improvement of memory and cognitive function. Pharmacol. Biochem. Behav. 2003;75:513–527. doi: 10.1016/s0091-3057(03)00128-x. [DOI] [PubMed] [Google Scholar]
- Islam M.N., Tasnim H., Arshad L., Haque M.A., Tareq S.M., Kamal A.M., Rahman M.M., Reza A.S.A., Chowdhury K.A.A., Tareq A.M. Stem extract of Albizia richardiana exhibits potent antioxidant, cytotoxic, antimicrobial, anti-inflammatory and thrombolytic effects through in vitro approach. Clin. Phytosci. 2020;6:1–9. [Google Scholar]
- Islam M.R., Reza A., Chawdhury K.A.A., Uddin J., Farhana K. Evaluation of in vitro antioxidant activity and cytotoxicity of methanolic extract of Sida cordata leaves. Int. J. Biol. Pharmaceut. Res. 2014;5:196–200. [Google Scholar]
- Islam N., Khan M.F., Khatun M.R., Nur S., Hanif N.B., Kulsum U., Arshad L., Lyzu C., Cacciola N.A., Capasso R. Neuropharmacological insights of African oil palm leaf through experimental assessment in rodent behavioral model and computer-aided mechanism. Food Biosci. 2021:100881. [Google Scholar]
- Islam S., Nasrin S., Khan M.A., Hossain A.S., Islam F., Khandokhar P., Mollah M.N.H., Rashid M., Sadik G., Rahman M.A.A. Evaluation of antioxidant and anticancer properties of the seed extracts of Syzygium fruticosum Roxb. growing in Rajshahi, Bangladesh. BMC Compl. Alternative Med. 2013;13:142. doi: 10.1186/1472-6882-13-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabir M.S.H., Hossain M.M., Kabir M.I., Ahmad S., Chakrabarty N., Rahman M.A., Rahman M.M. Antioxidant, antidiarrheal, hypoglycemic and thrombolytic activities of organic and aqueous extracts of Hopea odorata leaves and in silico PASS prediction of its isolated compounds. BMC Compl. Alternative Med. 2016;16:1–13. doi: 10.1186/s12906-016-1461-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.A., Rahman A.A., Islam S., Khandokhar P., Parvin S., Islam M.B., Hossain M., Rashid M., Sadik G., Nasrin S. A comparative study on the antioxidant activity of methanolic extracts from different parts of Morus alba L.(Moraceae) BMC Res. Notes. 2013;6:24. doi: 10.1186/1756-0500-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.A., Rahman M.M., Sardar M.N., Arman M.S.I., Islam M.B., Khandakar M.J.A., Rashid M., Sadik G., Alam A.K. Comparative investigation of the free radical scavenging potential and anticancer property of Diospyros blancoi (Ebenaceae) Asi. Pacif. J. Trop. Biomed. 2016;6:410–417. [Google Scholar]
- Khan M.F., Kader F.B., Arman M., Ahmed S., Lyzu C., Sakib S.A., Tanzil S.M., Zim A.I.U., Imran M.A.S., Venneri T. Pharmacological insights and prediction of lead bioactive isolates of Dita bark through experimental and computer-aided mechanism. Biomed. Pharmacother. 2020;131:110774. doi: 10.1016/j.biopha.2020.110774. [DOI] [PubMed] [Google Scholar]
- Maes M., Galecki P., Chang Y.S., Berk M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro) degenerative processes in that illness. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2011;35:676–692. doi: 10.1016/j.pnpbp.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Mamun F., Rahman M.M., Zamila M., Subhan N., Hossain H., Hasan S.R., Alam M.A., Haque M.A. Polyphenolic compounds of litchi leaf augment kidney and heart functions in 2K1C rats. J. Funct. Food. 2020;64:103662. [Google Scholar]
- Maria Michel T., Pulschen D., Thome J. The role of oxidative stress in depressive disorders. Curr. Pharmaceut. Des. 2012;18:5890–5899. doi: 10.2174/138161212803523554. [DOI] [PubMed] [Google Scholar]
- Miao M., Tian S., Bai M., Weiyun X. Total glucosides of Curculigo rhizome to perimenopausal period mice model. Pak. J. Pharm. Sci. 2017;30 [PubMed] [Google Scholar]
- Moni J.N.R., Adnan M., Tareq A.M., Kabir M., Reza A., Nasrin M., Chowdhury K.H., Sayem S.A.J., Rahman M.A., Alam A. Therapeutic potentials of Syzygium fruticosum fruit (seed) reflect into an array of pharmacological assays and prospective receptors-mediated pathways. Life. 2021;11:155. doi: 10.3390/life11020155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Y., Dong X., He Y., Yuan T., Han T., Rahman K., Qin L., Zhang Q. Medicinal plants of genus Curculigo: traditional uses and a phytochemical and ethnopharmacological review. J. Ethnopharmacol. 2013;147:547–563. doi: 10.1016/j.jep.2013.03.066. [DOI] [PubMed] [Google Scholar]
- Nigri G.R., Kossodo S., Waterman P., Fungaloi P., LaMuraglia G.M. Free radical attenuation prevents thrombosis and enables photochemical inhibition of vein graft intimal hyperplasia. J. Vasc. Surg. 2004:843–849. doi: 10.1016/j.jvs.2003.11.028. [DOI] [PubMed] [Google Scholar]
- Nirmal J., Babu C.S., Harisudhan T., Ramanathan M. Evaluation of behavioural and antioxidant activity of Cytisus scoparius Link in rats exposed to chronic unpredictable mild stress. BMC Compl. Alternative Med. 2008;8:15. doi: 10.1186/1472-6882-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD T.N. OECD Publishing; 2002. 423: Acute Oral Toxicity–OECD Guideline for the Testing of Chemicals Section 4. [Google Scholar]
- Okarter N., Liu R.H. Health benefits of whole grain phytochemicals. Crit. Rev. Food Sci. Nutr. 2010;50:193–208. doi: 10.1080/10408390802248734. [DOI] [PubMed] [Google Scholar]
- Oliveira I., Sousa A., Ferreira I.C., Bento A., Estevinho L., Pereira J.A. Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) green husks. Food Chem. Toxicol. 2008;46:2326–2331. doi: 10.1016/j.fct.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Organization W.H. World Health Organization; 2017. Depression and Other Common Mental Disorders: Global Health Estimates. [Google Scholar]
- Pawar V.S., Anup A., Shrikrishna B., Shivakumar H. Antidepressant–like effects of Acorus calamus in forced swimming and tail suspension test in mice. Asi. Pacif. J. Trop. Biomed. 2011;1:S17–S19. [Google Scholar]
- Porsolt R.D., Le Pichon M., Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Rahman J., Tareq A.M., Hossain M., Sakib S.A., Islam M.N., Ali M., Uddin A., Hoque M., Nasrin M., Emran T.B. Biological eValuation, DFT calculations and molecular docking studies on the antidepressant and cytotoxicity activities of Cycas pectinata. Buch.-Ham. Compounds. Pharmaceuticals. 2020;13:232. doi: 10.3390/ph13090232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M., Uddin M., Reza A., Tareq A.M., Emran T.B., Simal-Gandara J. Ethnomedicinal value of antidiabetic plants in Bangladesh: a comprehensive review. Plants. 2021;10:729. doi: 10.3390/plants10040729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M.M., Islam M.B., Biswas M., Alam A.K. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res. Notes. 2015;8:621. doi: 10.1186/s13104-015-1618-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M.M., Reza A.M.A., Khan M.A., Sujon K.M., Sharmin R., Rashid M., Sadik M.G., Reza M.A., Tsukahara T., Capasso R. Unfolding the apoptotic mechanism of antioxidant enriched-leaves of Tabebuia pallida (Lindl.) Miers in EAC cells and mouse model. J. Ethnopharmacol. 2021:114297. doi: 10.1016/j.jep.2021.114297. [DOI] [PubMed] [Google Scholar]
- Rashid Chowdhury M.M., Tareq A.M., Sayeed M.A., Haque M.A. Vitex peduncularis boosted anxiolytic, antidepressant, and antioxidant properties in albino mice and in silico model. J. Herbs, Spices, Med. Plants. 2021;27:57–67. [Google Scholar]
- Reza A.A., Haque M.A., Sarker J., Nasrin M.S., Rahman M.M., Tareq A.M., Khan Z., Rashid M., Sadik M.G., Tsukahara T. Antiproliferative and antioxidant potentials of bioactive edible vegetable fraction of Achyranthes ferruginea Roxb. in cancer cell line. Food Sci. Nutr. 2021 doi: 10.1002/fsn3.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reza A.A., Hossain M.S., Akhter S., Rahman M.R., Nasrin M.S., Uddin M.J., Sadik G., Alam A.K. In vitro antioxidant and cholinesterase inhibitory activities of Elatostema papillosum leaves and correlation with their phytochemical profiles: a study relevant to the treatment of Alzheimer’s disease. BMC Compl. Alternative Med. 2018;18:123. doi: 10.1186/s12906-018-2182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reza A.A., Nasrin M.S., Alam A.K. 2018. Phytochemicals, Antioxidants, and Cholinesterase Inhibitory Profiles of Elatostema Papillosum Leaves: an Alternative Approach for Management of Alzheimer's Disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schetter A.J., Heegaard N.H., Harris C.C. Inflammation and cancer: interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis. 2010;31:37–49. doi: 10.1093/carcin/bgp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan C., Shuo T., Ming B., Shaoyan L., Jiaojiao J., Mingsan M. Effects of Curculigo orchioides total glucosides in mouse perimenopause model of related organization and organs morphology. Bangladesh J. Pharmacol. 2016;11:72–81. [Google Scholar]
- Sharmin S., Sifat T., Azad A., Reza A.A., Islam M.K., Sayeed M.A. Preliminary phyto-pharmacological investigation of Duabanga Grandiflora (Roxb. Ex DC) Walpers. Banglad. Pharmaceut. J. 2020;23:54–60. [Google Scholar]
- Steru L., Chermat R., Thierry B., Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Takeda H., Tsuji M., Matsumiya T. Changes in head-dipping behavior in the hole-board test reflect the anxiogenic and/or anxiolytic state in mice. Eur. J. Pharmacol. 1998;350:21–29. doi: 10.1016/s0014-2999(98)00223-4. [DOI] [PubMed] [Google Scholar]
- Tareq A.M., Farhad S., Uddin A.N., Hoque M., Nasrin M.S., Uddin M.M.R., Hasan M., Sultana A., Munira M.S., Lyzu C. Chemical profiles, pharmacological properties, and in silico studies provide new insights on Cycas pectinata. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e04061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tareq A.M., Sohel M., Uddin M., Mahmud M.H., Hoque M., Reza A.A., Nasrin M.S., Kader F.B., Emran T.B. Possible neuropharmacological effects of Apis cerana indica beehive in the Swiss Albino mice. J. Adv. Biotechnol. Exp. Ther. 2020;3:128–134. [Google Scholar]
- Uddin M.N., Afrin R., Uddin M.J., Uddin M.J., Alam A., Rahman A.A., Sadik G. Vanda roxburghii chloroform extract as a potential source of polyphenols with antioxidant and cholinesterase inhibitory activities: identification of a strong phenolic antioxidant. BMC Compl. Alternative Med. 2015;15:195. doi: 10.1186/s12906-015-0728-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.