Abstract
Introduction
Data from randomized controlled trials show that liraglutide 3.0 mg, in combination with diet and exercise, is associated with greater weight loss than diet and exercise alone in patients with obesity. In practice, the utilization of weight loss drugs is influenced by various factors, including the cost of treatment. We conducted a retrospective, observational study to assess the effectiveness of liraglutide 3.0 mg and patients' persistence on treatment, in a real-world setting.
Methods
Data were extracted from de-identified electronic medical records from an obesity management clinic in Switzerland. Changes in body weight and blood pressure were evaluated in the full cohort (N = 277, 19% of whom had undergone bariatric surgery) and subgroups who were persistent on liraglutide 3.0 mg for at least 4 months (n = 236), 7 months (n = 159), or 12 months (n = 71).
Results
Median persistence on liraglutide was 6.8 months. Median maximum dose received was 1.5 mg, and 13.7% of patients reached the maintenance dose of 3.0 mg. Mean 7-month weight change from baseline in the full cohort was −4.1 kg (95% confidence interval: −5.0, −3.2; p < 0.001; −4.2%). Weight change was −4.4 kg (−4.7%) in the ≥4-month persistence subgroup at 4 months, −5.1 kg (−5.3%) in the ≥7-month persistence subgroup at 7 months, and −7.5 kg (−7.1%) in the ≥12-month persistence subgroup at 12 months (all p < 0.001). In the full cohort, 40% and 14% of patients lost ≥5% and >10% of body weight at 7 months, respectively. Weight loss did not differ significantly according to history of bariatric surgery (p = 0.94). Diastolic blood pressure decreased (from 87.0 to 83.9 mm Hg at 7 months; p = 0.018), with no significant changes in systolic blood pressure. Approximately two-thirds of patients did not have health insurance that could cover the cost of liraglutide.
Conclusion
In a real-world setting with low insurance coverage and with most patients not reaching the recommended maintenance dose of 3.0 mg, the use of liraglutide, in combination with diet and exercise, was associated with clinically meaningful weight loss.
Keywords: Liraglutide 3.0 mg, Weight loss, Glucagon-like peptide-1 receptor agonist, Bariatric surgery, Electronic medical records
Introduction
Across Europe, the burden of obesity is growing, and an increasing number of people are likely to develop obesity (body mass index [BMI] ≥30 kg/m2) and to maintain this BMI for longer periods than in the past [1]. Pharmacotherapy is a noninvasive approach that can help people with obesity to adhere to weight loss strategies, improve quality of life, and reduce the risk of comorbidities [2]. European guidelines setting out a comprehensive strategy of obesity management recommend considering pharmacotherapy for adult patients with BMI ≥30 kg/m2 or ≥27 kg/m2 with a comorbidity [2].
Liraglutide 3.0 mg, a weight management pharmacotherapy approved by the European Medicines Agency in 2015 [3], is an acylated glucagon-like peptide-1 receptor agonist (GLP-1 RA) and a physiological regulator of appetite. It is thought to mediate weight loss in individuals with obesity by decreasing appetite and food intake, without increasing energy expenditure [4, 5]. The efficacy and safety of liraglutide 3.0 mg have been examined in an extensive phase 3a program of randomized, double-blind, placebo-controlled trials, which included >5,000 participants over a period of 1–3 years [6]. The largest of these trials (3,731 participants) assessed the efficacy of liraglutide 3.0 mg as an adjunct to a reduced calorie diet and exercise in participants with a BMI of ≥30 kg/m2 or ≥27 kg/m2 with concomitant prediabetes, dyslipidemia, or hypertension. Average weight loss associated with liraglutide 3.0 mg exceeded that associated with placebo by 5.4% after 1 year [7] and 4.3% after 3 years [8]. Furthermore, at 3 years, participants receiving liraglutide 3.0 mg had a lower average systolic blood pressure (SBP), and a smaller percentage of them were diagnosed with type 2 diabetes (T2D), relative to those receiving placebo [8].
Observational studies of patients receiving liraglutide 3.0 mg in clinical practice in Canada [9, 10, 11, 12], Spain [13], the United Arab Emirates (UAE) [14], and Italy [15] have shown significant weight loss with liraglutide 3.0 mg. Weight loss was also observed in subgroups of patients who persisted with treatment for at least 4–7 months. Less is known, however, about weight loss and persistence with liraglutide in the longer term. Furthermore, although liraglutide 3.0 mg was associated with weight loss in patients after bariatric surgery in a previous study [10], few data are available on whether such patients experience similar weight loss benefits with liraglutide 3.0 mg to those who have not undergone bariatric surgery.
In December 2016, liraglutide 3.0 mg (Saxenda®; Novo Nordisk A/S, Copenhagen, Denmark) was approved for weight management in Switzerland as a once-daily subcutaneous injection. Although Switzerland has lower overweight and obesity rates than are recorded in many European nations, these conditions still affect a sizable proportion of the population: the self-reported rate of overweight or obesity in people older than 15 years was 41.8% according to data from 2017 [16]. In Switzerland, obesity is generally managed via an integrated approach involving nutritional counseling, and lifestyle and psychological advice [17]. A limited selection of weight loss drugs, of which liraglutide 3.0 mg is the most recent to gain approval, may be used in combination with this approach. To increase our understanding of real-world use of liraglutide 3.0 mg across diverse settings and populations, we retrospectively evaluated weight loss, other clinical outcomes and persistence on medication in patients who initiated treatment with liraglutide at a medically supervised weight management clinic in Switzerland.
Methods
Data Source
This study utilized a database containing de-identified electronic medical records (EMRs) from Adimed, an obesity clinic in Winterthur, Switzerland, that specializes in weight management and the treatment of metabolic diseases. Patients treated with liraglutide 3.0 mg at Adimed typically undergo a conservative pretreatment consisting of an individually calculated limited calorie diet and physical activity program for at least 6 months before initiating therapy. According to the prescribing information, the starting dose of 0.6 mg per day should be increased in weekly increments over 4 weeks to a recommended maintenance dose of 3.0 mg from the fifth week onward [3]. The database contained information on demographics, diagnoses, and prescriptions. To maintain the anonymity of patients, the database was de-identified by Privacy Analytics (an IQVIA company, Ottawa, ON, Canada) and EMR data on eligible patients were shared remotely, in a secure manner. Furthermore, free-text variables were not used. Patients at Adimed were asked to indicate whether they wished to opt out from the use of their electronic medical data for research purposes and were informed that their participation or lack thereof would not alter treatment. Patients who opted out of their data being used for research by Adimed were not included in this study.
Study Design and Population
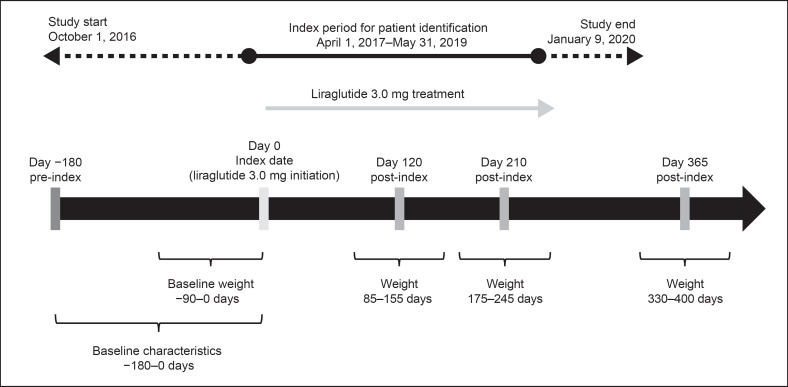
This was a retrospective, observational cohort study. Patients initiating treatment with liraglutide 3.0 mg at Adimed between April 1, 2017 and May 31, 2019 were eligible for inclusion (Fig. 1). Only patients with a BMI ≥30 kg/m2 or a BMI of ≥27 mg/m2 with at least 1 treated weight-related comorbidity, who had received at least 1 liraglutide prescription in addition to their diet and exercise programs, were included. Patients were also required to be aged ≥18 years at the index date (date of liraglutide initiation) and to have had at least 1 follow-up assessment visit within 7 months (±35 days) after the index date.
Fig. 1.
Study design.
Patients were excluded if they had been prescribed liraglutide 3.0 mg at Adimed before the index period, or if they were using GLP-1 RAs at Adimed or had used them within 1 year before the index date. The baseline period was up to 90 days pre-index for assessment of baseline weight and up to 180 days pre-index for assessment of other baseline characteristics.
Outcomes
The primary outcomes were mean absolute body weight change from baseline and percentage weight change from baseline to 4 months (120 ± 35 days), 7 months (210 ± 35 days), and 12 months (365 ± 35 days) after liraglutide initiation (Fig. 1). Secondary outcomes were the percentages of patients achieving ≥5% and >10% weight loss, persistence on liraglutide, maximum liraglutide dose reached, the percentage of patients reaching maintenance dose of 3.0 mg, and changes in SBP and diastolic blood pressure (DBP) from baseline to 7 and 12 months after liraglutide initiation.
Maximum liraglutide dose was defined as the highest dose attained by the patient between the index date and date of liraglutide discontinuation. Patients who reached the recommended maintenance dose of 3.0 mg and then reduced it thereafter were considered to have attained the maintenance dose.
Persistence on liraglutide was defined as the time (in months) on treatment between the index date and the date of liraglutide discontinuation (prescription end date). For prescriptions with the same start and end date, the end date was assumed to be missing, and for multiple complete prescriptions with the same start date, the one with the most conservative end date was used. If prescription end date was not available, prescription duration was estimated, based on dose and pack size. The maximum permissible gap between prescriptions was 60 days. Patients who did not have a discontinuation date were censored at the earliest of: date of death, loss to follow-up, or date of last clinic visit before data extraction.
Baseline Characteristics
Disease codes in the patient records were used to identify the presence of comorbidities, based on the following definitions. Prediabetes was defined as a homeostasis model for insulin resistance (HOMA-IR) index [18] score >2.4. T2D was defined as a glycated hemoglobin concentration >6.1%, or plasma-glucose >6.9 mmol/L, or 2-h post-oral glucose load >11.1 mmol/L. Hypertension was defined as a SBP ≥140 mm Hg, a DBP ≥90 mm Hg, or a history of treatment for hypertension. Dyslipidemia was defined according to the Swiss Atherosclerosis Association (Arbeitsgruppe Lipide und Atherosklerose; AGLA) cardiovascular recommendations, based on AGLA score [19]. Patients with a history of bariatric surgery at any time in their medical records were included both in the full cohort and in the bariatric surgery subgroup in separate analyses.
Analysis Sets and Subgroups
The full cohort included all patients who met all the inclusion criteria and none of the exclusion criteria. Data were also analyzed for 3 subgroups according to the duration of persistence on liraglutide: the ≥4-month, ≥7-month, and ≥12-month persistence groups were defined as patients who persisted with liraglutide treatment for at least 4, 7, or 12 months, respectively, regardless of dosage. In addition, outcomes were compared between subgroups of patients in the full cohort with and without a history of bariatric surgery.
Statistical Analyses
Apart from time and dosing variables, data are presented as mean and standard deviation/95% confidence interval (CI) or %, and compared across groups using independent samples t test or Pearson's χ2 test. For comparisons with baseline values within the same population or subgroup, the paired samples t test was used. Data on duration of persistence on liraglutide, maintenance dose achieved, and time to achieve the maintenance dose did not meet the assumption of normality and are therefore presented as median (25th and 75th percentiles). All tests were 2-tailed, p < 0.05 was considered statistically significant, and p < 0.10 was discussed as a trend.
Results
Study Population and Baseline Characteristics
In total, 277 patients met all inclusion criteria and were included in the study (full cohort). Persistence on liraglutide for at least 4, 7, and 12 months was achieved by 236 patients (85% of the full cohort), 159 patients (57%), and 71 patients (26%), respectively.
For the ≥4-month persistence subgroup, a body weight measurement at 4 months was available for a subset of 163 patients. A body weight measurement was available at 7 months for 98 patients in the ≥7-month persistence subgroup, and at 12 months for 35 patients in the ≥12-month persistence subgroup (see online suppl. Fig. 1; for all online suppl. material, see www.karger.com/doi/10.1159/000518325).
At baseline, mean BMI in the full cohort was 36.2 kg/m2 (standard deviation: 5.9 kg/m2), and the majority of patients (83%) were women (Table 1). Hypertension was present in 70% of patients. Among all patients, 18.8% had undergone bariatric surgery before initiating liraglutide. Only 34% of patients had private insurance that could cover the costs of weight loss drugs.
Table 1.
Baseline characteristics of the study population
| Full cohort: N = 277 | |
|---|---|
| Age, years | 43.5 (12.3) |
| Women, n (%) | 231 (83.4) |
| Weight, kg | 101.9 (21.2) |
| Height, cm | 167.4 (8.7) |
| BMI, n (%) | 36.2 (5.9) |
| Overweight (BMI 25–<30 kg/m2) | 22 (7.9) |
| Obesity class I (BMI 30–<35 kg/m2) | 118 (42.6) |
| Obesity class II (BMI 35–<40 kg/m2) | 77 (27.8) |
| Obesity class III (BMI ≥40 kg/m2) | 60 (21.7) |
| History of bariatric surgery, n (%) | 52 (18.8) |
| Insurance coverage available, n (%)a | 95 (34.3) |
| Hypertension, n (%) | 194 (70.0) |
| Prediabetes, n (%) | 7 (2.5) |
| T2D, n (%) | 0 (0) |
| Dyslipidemia, n (%) | 4 (1.4) |
Data are presented as mean (SD) unless stated otherwise. BMI, body mass index; T2D, type 2 diabetes; SD, standard deviation.
Refers to private insurance covering the cost of liraglutide 3.0 mg.
Persistence and Liraglutide Dosage
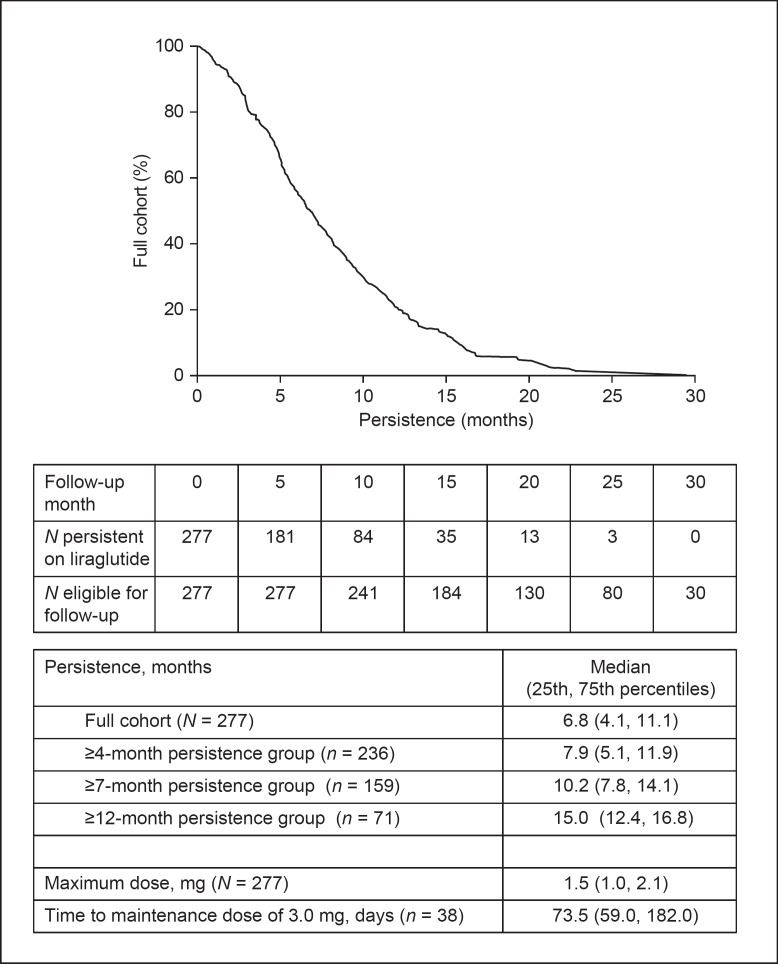
In the full cohort, the median duration of persistence on liraglutide was 6.8 months, which increased to 7.9 months in the ≥4-month persistence group, 10.2 months in the ≥7-month group, and 15.0 months in the ≥12-month group (Fig. 2). Only 38 patients (13.7%) in the full cohort reached the maintenance dose of 3.0 mg, and this was achieved after a median of 74 days (25th and 75th percentiles: 59 days, 182 days). The median maximum dose received in the full cohort was 1.5 mg (1.0 mg, 2.1 mg).
Fig. 2.
Persistence post index and maximum dose reached.
Mean Weight Loss from Baseline
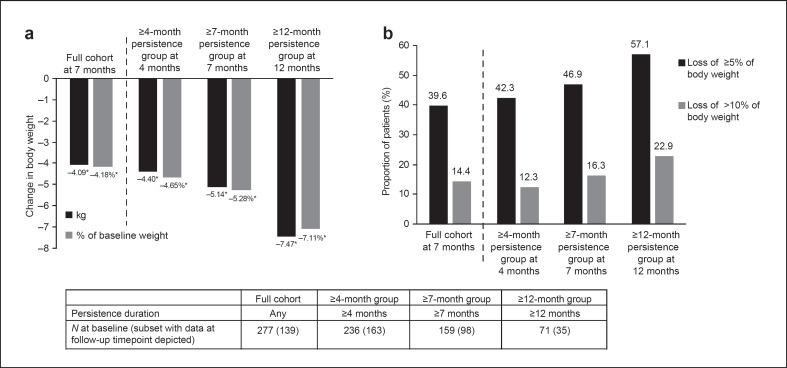
Statistically significant weight loss (p < 0.001) was achieved in all patient cohorts after treatment initiation (Fig. 3a). Mean weight change at 7 months was −4.1 kg (95% CI: −5.0, −3.2; p < 0.001) in the full cohort, equating to −4.2% of baseline body weight. In the ≥4-month persistence subgroup, mean weight change at 4 months was −4.4 kg (95% CI: −5.0, −3.8; p < 0.001; −4.7% of baseline body weight). Patients in the ≥7-month persistence subgroup had a mean weight change of −5.1 kg (95% CI: −6.2, −4.1; p < 0.001; −5.3% of baseline body weight) 7 months after treatment initiation. In the ≥12-month persistence subgroup, mean weight change was −7.5 kg (95% CI: −9.9, −5.1; p < 0.001) at 12 months, equivalent to −7.1% of baseline body weight.
Fig. 3.
Weight loss with liraglutide 3.0 mg according to persistence duration: (a) Absolute and percentage weight change from baseline, and (b) percentage of patients with ≥5% and >10% weight loss. *p < 0.001 for follow-up measurements compared with baseline by paired samples t test.
Proportion of Patients with ≥5% and >10% Weight Loss
In the full cohort, 40% of patients had lost ≥5% of their body weight at 7 months post index, and 14% had lost >10% of their body weight. The equivalent proportions for the ≥4-month persistence subgroup at 4 months were 42% and 12%, and those for the ≥7-month persistence subgroup at 7 months were 47% and 16%, respectively. In the ≥12-month persistence subgroup, 57% of patients had lost ≥5% of their body weight and 23% had lost >10% of their body weight at 12 months post index (Fig. 3b).
Impact of Bariatric Surgery
Overall, there was no significant difference in weight loss achieved at 7 months between patients in the full cohort who had a history of bariatric surgery and those who did not (p = 0.94; Table 2). This pattern was also observed in the ≥7-month persistence group, with weight change of −4.5 kg (95% CI: −7.7, −1.3), equivalent to −4.8% of baseline body weight, in patients with a history of bariatric surgery compared with −5.3 kg (95% CI: −6.4, −4.3), equivalent to −5.4% of baseline body weight, in those without a history of bariatric surgery (p = 0.16 for weight change in kilograms; p = 0.18 for weight change in %).
Table 2.
Difference in weight loss in the full cohort at 7 months post index in individuals with and without a history of bariatric surgery
| History of bariatric surgery | |||
|---|---|---|---|
| Yes | No | p valuea | |
| Baseline | n = 52 | n = 225 | |
| Weight, kg | 108.7 (22.4) | 100.3 (20.6) | 0.0035 |
| At 7 months | n = 33 | n = 106 | |
| Weight changeb | |||
| kg | −4.0 (4.9) | −4.1 (7.7) | 0.94 |
| % of baseline weight | −4.1 (7.0) | −4.2 (5.0) | 0.40 |
| Patients with weight loss, n (%) | |||
| ≥5% of baseline | 11 (33.3) | 44 (41.5) | 0.80 |
| >10% of baseline | 6 (18.2) | 14 (13.2) | 0.23 |
Data are presented as mean (SD) unless otherwise stated. SD, standard deviation.
p values are from independent samples t test or Pearson's χ2 test as appropriate.
Weight at 7 months was significantly different from baseline in both groups using paired samples t test (p ≤ 0.005).
Change in Blood Pressure
Patients receiving liraglutide experienced a decrease in their DBP at 7 months in the full cohort and in the ≥7-month persistence subgroup, and at 12 months in the ≥12-month persistence subgroup (p < 0.05 for all), but there were no statistically significant changes in SBP (Table 3). Patients who persisted on liraglutide for ≥12 months had the largest decrease in DBP (−5.4 mm Hg; 95% CI: −9.7, −1.1; p = 0.015) and a trend toward a decrease in SBP of −5.4 mm Hg (−11.7, 1.0; p = 0.093).
Table 3.
Blood pressure changes post index
| SBP (mm Hg) | DBP (mm Hg) | |
|---|---|---|
| Full cohort | ||
| Baseline (n = 274) | 135.0 (17.9) | 87.0 (11.9) |
| 7 months (n = 121) | 131.8 (16.4) | 83.9 (10.3) |
| p = 0.10 | p = 0.018 | |
| 7-month persistence group | ||
| Baseline (n = 158) | 133.9 (18.1) | 86.4 (11.0) |
| 7 months (n = 88) | 132.2 (16.1) | 84.2 (10.6) |
| p = 0.38 | p = 0.022 | |
| 12-month persistence group | ||
| Baseline (n = 71) | 134.6 (17.7) | 87.2 (11.3) |
| 12 months (n = 29) | 130.5 (15.8) | 81.2 (9.7) |
| p = 0.093 | p = 0.015 |
Data are mean (SD). p values are from paired samples t test for follow-up compared with baseline values. Statistically significant p values (<0.05) are shown in bold. DBP, diastolic blood pressure; SBP, systolic blood pressure; SD, standard deviation.
Discussion
Pharmacotherapy can form part of an effective approach to weight management in patients for whom it is indicated, but drug costs are sometimes a limiting factor. This is the 1st study to assess the use of liraglutide in Swiss clinical practice: we investigated both effectiveness of liraglutide and patients' persistence on treatment in a real-world clinical setting. In a cohort of 277 patients prescribed liraglutide at a weight loss clinic, median persistence was 6.8 months, and 26% of patients stayed on treatment for 12 months or more. Furthermore, the median maximum dose reached in the study population was 1.5 mg over a period of 10 weeks; only 14% of patients reached the recommended maintenance dose of 3.0 mg. Throughout the study period, liraglutide for weight management was not covered by the universally available basic insurance in Switzerland but could be covered by supplementary private insurance, which is available to only a fraction of the population. In the present study, nearly two-thirds of all patients had no private insurance that could cover liraglutide. Therefore, we suggest that the costs associated with dose escalation may have contributed to the relatively low maximum dose attained in this patient population.
Despite the low proportion of patients receiving the maximum dose, clinically meaningful weight loss was observed at 4, 7, and 12 months after initiation of liraglutide for weight management in all patient cohorts, regardless of persistence or maximum dose reached. However, persistence appeared to have an impact on weight loss: 7 months after initiating liraglutide, the full cohort had lost an average of 4.1 kg (4.2%) of their body weight, increasing to 5.1 kg (5.3%) in patients who had persisted on the drug for 7 months or more, and 7.5 kg (7.1%) in patients who persisted for 12 months. Together, these findings suggest that longer persistence may help more patients achieve clinically meaningful weight loss; alternatively, it could indicate that patients who lose more weight tend to persist longer on treatment.
In this study, 19% of patients in the full analysis cohort had undergone bariatric surgery before initiating liraglutide. In a subgroup analysis, a similar magnitude of weight loss was observed in these patients and in the subgroup without a history of bariatric surgery. There is limited published evidence on liraglutide use in this subgroup of patients compared with those without a history of bariatric surgery; however, our results reflect the findings of a previous study conducted in the UAE, which found no difference in percentage weight loss between subgroups with and without bariatric surgery [14]. Taken together, these data suggest patients who have already experienced substantial weight loss due to bariatric surgery, may derive further weight loss benefit from obesity pharmacotherapy. This has been demonstrated previously for other weight loss drugs used in clinical practice [20]. Liraglutide has also been shown to facilitate weight loss in patients who experience insufficient weight loss or significant weight regains post-bariatric surgery, regardless of the type of surgery that they had undergone [10]. Detailed information on type of bariatric surgery was not available for our study cohort; therefore, we were not able to examine this factor. We speculate that the subcutaneous administration and central mode of action of liraglutide mean that its bioavailability and efficacy are not compromised in patients who have undergone bariatric surgery; however, this requires further study.
A consistent significant reduction in DBP was observed in the full cohort and the 7- and 12-month persistence subgroups. A nonsignificant trend for SBP reduction was also observed at 12 months in patients with 12 months' persistence or more. These findings are aligned with previous published data: reductions in both SBP and DBP were observed in the Satiety and Clinical Adiposity–Liraglutide Evidence in individuals with and without diabetes (SCALE) Obesity and Prediabetes trial [7], as well as in other real-world studies of liraglutide use [13, 15]. The findings of our study are also in line with data from a previous systematic review, showing that weight loss is associated with reduction of SBP and DBP independent of the type of weight loss intervention [21]. In one real-world study, weight loss with liraglutide was associated with a reduction in SBP, but not DBP [9]. There is evidence that the magnitude of blood pressure reduction is proportional to the amount of weight lost, independent of the type of weight loss intervention: in a meta-analysis of 25 randomized controlled trials of weight reduction, each kilogram of body weight loss was associated with a 0.92 mm Hg reduction in DBP and a 1.05 mm Hg reduction in SBP [22].
Compared with previous real-world studies, average weight loss was slightly lower in our study. Patients persistent on liraglutide lost an average of 4.4 kg at 4 months in the current study, compared with an average weight loss of 7.0 kg at 4 months, and 6.4 kg at 3–6 months in real-world studies in Canada [9] and Spain [13], respectively. The fraction of the current population achieving 5% and 10% weight loss (47% and 16% at 7 months in patients persistent on treatment, respectively) was also lower than that observed in other real-world studies. For example, 69% and 30% of patients studied in the UAE achieved 5% and 10% weight loss, respectively, at 7 months [14], and the equivalent percentages in Italy were 70% and 23% at 6 months in those persistent on liraglutide [15]. This may result from differences in adherence to the associated diet or lifestyle changes across studies, or the fact that most patients in the present study did not reach the recommended liraglutide maintenance dose of 3.0 mg, and therefore may not have gained the full potential benefit from the drug.
Alternatively, the disparities between study results may be linked to differences in baseline characteristics, although a previous real-world study has indicated that the percentage weight loss with liraglutide is largely independent of baseline obesity class [11]. Mean baseline weight in the study population was 101.9 kg, which is somewhat lower than in previous real-world studies of liraglutide used in Spain (105.1 kg) [13] and Canada (114.8 kg) [9]. Overall, 50% of the study cohort had a BMI of 35 kg/m2 or less, compared with 19%, 20%, 23%, and 38% of patients in real-world studies in Spain [13], Italy [15], Canada [9], and UAE [14], respectively. This may reflect the lower prevalence of overweight and obesity in Switzerland than these countries [16], or a propensity to utilize weight loss drugs at less severe degrees of obesity.
Access to a high-quality longitudinal database of de-identified EMR data was a key strength of this study. This allowed us to examine the specific target population of patients receiving liraglutide in a single specialized weight management clinic, with a high degree of confidence that all patients had received the same diet and exercise interventions at baseline. The range of data captured in these records meant that, in addition to the primary outcome of absolute weight loss from baseline, we could also examine persistence, maximum dosage, and changes in blood pressure. Furthermore, available information on insurance coverage allowed us to speculate on the underlying reasons for dosing and persistence patterns.
The lack of a control group in this study means that it is not possible to differentiate the impact of liraglutide plus diet and exercise from the effects of diet and exercise alone. Furthermore, as this is an observational study, unmeasured confounding and selection bias cannot be ruled out. In studies using EMRs, some characteristics that are likely to affect clinical outcomes, such as sociodemographical and lifestyle factors, are not captured in the data source. To maintain anonymity of patient records in our study, free-text variables in the data set were not used, and therefore some relevant information could not be captured, including reasons for liraglutide discontinuation, such as the occurrence of side effects. For the bariatric surgery subgroup, data on type of surgery and time from surgery to liraglutide initiation were not available, limiting the interpretation of the results for these patients. A common limitation of prescriptions data is the possibility that patients have not taken their medication as directed, which can affect estimates of treatment benefit and persistence. Owing to the low maximum dose received by the study population, comparisons with previous observational and interventional studies may be impaired, and the results may not be generalizable to all populations. Importantly, however, our results indicate that liraglutide can provide weight loss benefits even when maximum dose is not reached.
It is recognized that weight loss has significant health benefits even in patients who do not reach a BMI below 25 kg/m2 [23, 24]. A previous review examining the magnitude of weight loss required for therapeutic benefit in different obesity-related conditions indicates that 3–10% weight loss is beneficial for prevention of T2D, while 5–10% weight loss helps to lower blood pressure and elicits improvements in osteoarthritis, gastroesophageal reflux disease, and stress incontinence [24]. Therefore, the results of our study can be interpreted in the broader context of the patient benefits, reductions in comorbidities, and improvement in health-related quality of life that can be achieved via weight loss. In particular, the availability of 12-month data suggests that weight loss, and its associated benefits, may be sustained in the longer term. Future longitudinal real-world studies, examining pharmacotherapy as part of an overall, patient-centered long-term approach to weight management, and assessing comorbidities during follow-up, will be valuable to gauge these potential benefits. Additionally, despite the benefits of remaining on liraglutide observed in the current study, future comparative studies would aid in quantifying the effectiveness of liraglutide in clinical practice.
Our results show that use of liraglutide in combination with diet and exercise is associated with clinically meaningful weight loss in patients with obesity, or with overweight and an obesity-related comorbidity, despite most patients not reaching the recommended dosage of 3.0 mg. These results corroborate previous studies [9, 13, 14, 15], provide confirmatory evidence for the use of liraglutide in clinical practice, and can be used to support treatment decisions by clinicians and patients.
Statement of Ethics
The study design and protocol were approved by the Cantonal Ethics Committee, Zurich (Die Kantonale Ethikkommission Zürich; approval number: BASEC No. 2019-02363). All the procedures were in accordance with the requirements set out in the international standards for epidemiological studies, as recorded in the International Guidelines for Ethical Review of Epidemiological Studies and with the Helsinki Declaration of 1964, as revised in 2013. For this type of study, individual consent was not required.
Conflict of Interest Statement
C.L.H. is an employee of Novo Nordisk A/S. M.G.S.A. is an employee of Novo Nordisk Pharma AG. G.L. and N.J. are employees of IQVIA, who received funding from Novo Nordisk A/S for this analysis. S.M. and U.E. have participated in Novo Nordisk symposia, and have received travel grants from Novo Nordisk.
Funding Sources
This study was funded by Novo Nordisk A/S.
Author Contributions
C.L.H., M.G.S.A., S.M., and U.E. designed the study. G.L. and N.J. conducted the data analysis. All authors contributed to data interpretation and critical revision of the manuscript. All authors agreed to be accountable for all aspects of this work.
Data Availability Statement
All data analyzed during this study are included in this article and its online suppl. files. Further inquiries can be directed to the corresponding author. Online suppl. information is available at Obesity Facts' website.
Supplementary Material
Supplementary data
Acknowledgments
The authors acknowledge the medical writing assistance of Oxford PharmaGenesis (funded by Novo Nordisk).
References
- 1.Blundell JE, Baker JL, Boyland E, Blaak E, Charzewska J, de Henauw S, et al. Variations in the prevalence of obesity among European countries, and a consideration of possible causes. Obes Facts. 2017;10((1)):25–37. doi: 10.1159/000455952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yumuk V, Tsigos C, Fried M, Schindler K, Busetto L, Micic D, et al. European guidelines for obesity management in adults. Obes Facts. 2015;8((6)):402–24. doi: 10.1159/000442721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Medicines Agency . Saxenda Summary of Product Characteristics. Available from: https://www.ema.europa.eu/en/documents/product-information/saxenda-epar-product-information_en.pdf Accessed 2021 May 18. [Google Scholar]
- 4.Tronieri JS, Wadden TA, Walsh O, Berkowitz RI, Alamuddin N, Gruber K, et al. Effects of liraglutide on appetite, food preoccupation, and food liking: results of a randomized controlled trial. Int J Obes. 2020;44((2)):353–61. doi: 10.1038/s41366-019-0348-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes. 2014;38((6)):784–93. doi: 10.1038/ijo.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christensen RM, Juhl CR, Torekov SS. Benefit-risk assessment of obesity drugs: focus on glucagon-like peptide-1 receptor agonists. Drug Saf. 2019;42((8)):957–71. doi: 10.1007/s40264-019-00812-7. [DOI] [PubMed] [Google Scholar]
- 7.Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373((1)):11–22. doi: 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- 8.le Roux CW, Astrup A, Fujioka K, Greenway F, Lau DCW, Van Gaal L, et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet. 2017;389((10077)):1399–409. doi: 10.1016/S0140-6736(17)30069-7. [DOI] [PubMed] [Google Scholar]
- 9.Wharton S, Liu A, Pakseresht A, Nørtoft E, Haase CL, Mancini J, et al. Real-world clinical effectiveness of liraglutide 3.0 mg for weight management in Canada. Obesity. 2019;27((6)):917–24. doi: 10.1002/oby.22462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wharton S, Kuk JL, Luszczynski M, Kamran E, Christensen RAG. Liraglutide 3.0 mg for the management of insufficient weight loss or excessive weight regain post-bariatric surgery. Clin Obes. 2019;9((4)):e12323. doi: 10.1111/cob.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wharton S, Haase CL, Kamran E, Liu A, Mancini J, Neish D, et al. Weight loss and persistence with liraglutide 3.0 mg by obesity class in the real-world effectiveness study in Canada. Obes Sci Pract. 2020;6((4)):439–44. doi: 10.1002/osp4.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wharton S, Haase CL, Kamran E, Liu A, Mancini J, Neish D, et al. Real-world persistence with liraglutide 3.0 mg for weight management and the SaxendaCare® patient support program. Obes Sci Pract. 2020;6((4)):382–9. doi: 10.1002/osp4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorgojo-Martinez JJ, Basagoiti-Carreno B, Sanz-Velasco A, Serrano-Moreno C, Almodovar-Ruiz F. Effectiveness and tolerability of orlistat and liraglutide in patients with obesity in a real-world setting: the XENSOR study. Int J Clin Pract. 2019;73((11)):e13399. doi: 10.1111/ijcp.13399. [DOI] [PubMed] [Google Scholar]
- 14.Suliman M, Buckley A, Al Tikriti A, Tan T, le Roux CW, Lessan N, et al. Routine clinical use of liraglutide 3 mg for the treatment of obesity: outcomes in non-surgical and bariatric surgery patients. Diabetes Obes Metab. 2019;21((6)):1498–501. doi: 10.1111/dom.13672. [DOI] [PubMed] [Google Scholar]
- 15.Ferrari F, Fierabracci P, Salvetti G, Jaccheri R, Vitti J, Scartabelli G, et al. Weight loss effect of liraglutide in real-life: the experience of a single Italian obesity center. J Endocrinol Invest. 2020;43((12)):1779–85. doi: 10.1007/s40618-020-01334-1. [DOI] [PubMed] [Google Scholar]
- 16.Organisation for Economic Co-operation and Development (OECD) Overweight or obese population. 2019. Available from: https://data.oecd.org/healthrisk/overweight-or-obese-population.htm Accessed 2021 May 18.
- 17.Swiss Consensus for Obesity Management Adiposity-consensus 2016. (in German). Available from: https://www.sgedssed.ch/fileadmin/user_upload/1_ueber_uns/15_ASEMO/2017_05_30_consensus_FINAL_d.pdf Accessed 2021 May 18.
- 18.Nakai Y, Nakaishi S, Kishimoto H, Seino Y, Nagasaka S, Sakai M, et al. The threshold value for insulin resistance on homeostasis model assessment of insulin sensitivity. Diabet Med. 2002;19((4)):346–7. doi: 10.1046/j.1464-5491.2002.00712_3.x. [DOI] [PubMed] [Google Scholar]
- 19.Swiss Atherosclerosis Association Swiss Atherosclerosis Association (AGLA/GSLA) cardiovascular recommendations 2018. Available from: https://www.gsla.ch/atherosclerose/prevention-de-latherosclerose/diagnostic-et-valeurs-cibles-lors-de-dyslipidemie Accessed 2021 May 18.
- 20.Nor Hanipah Z, Nasr EC, Bucak E, Schauer PR, Aminian A, Brethauer SA, et al. Efficacy of adjuvant weight loss medication after bariatric surgery. Surg Obes Relat Dis. 2018;14((1)):93–8. doi: 10.1016/j.soard.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Zomer E, Gurusamy K, Leach R, Trimmer C, Lobstein T, Morris S, et al. Interventions that cause weight loss and the impact on cardiovascular risk factors: a systematic review and meta-analysis. Obes Rev. 2016;17((10)):1001–11. doi: 10.1111/obr.12433. [DOI] [PubMed] [Google Scholar]
- 22.Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2003;42((5)):878–84. doi: 10.1161/01.HYP.0000094221.86888.AE. [DOI] [PubMed] [Google Scholar]
- 23.Lean ME, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391((10120)):541–51. doi: 10.1016/S0140-6736(17)33102-1. [DOI] [PubMed] [Google Scholar]
- 24.Cefalu WT, Bray GA, Home PD, Garvey WT, Klein S, Pi-Sunyer FX, et al. Advances in the science, treatment, and prevention of the disease of obesity: reflections from a diabetes care editors' expert forum. Diabetes Care. 2015;38((8)):1567–82. doi: 10.2337/dc15-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
All data analyzed during this study are included in this article and its online suppl. files. Further inquiries can be directed to the corresponding author. Online suppl. information is available at Obesity Facts' website.