Abstract
Eight of 9 juvenile raccoons at a rehabilitation center died without obvious prior clinical signs. Gross changes were unremarkable except for mildly distended intestines. Microscopically, crypt loss, distension, necrosis, and regeneration with intranuclear viral inclusions were observed in the small intestine, with marked lymphoid depletion and necrosis in Peyer patches and mesenteric lymph nodes. Immunohistochemistry with a canine parvovirus antibody showed intensive signals of parvoviral antigens in the crypts and lymphoid germinal centers. Metagenomic sequencing allowed assembly of a complete parvoviral genome with >99% identity to canine parvovirus 2a, as well as Salmonella enterica subsp. enterica. Also, S. enterica subsp. enterica serovar Thompson with multiple antimicrobial resistance was isolated from the intestinal contents. Concurrent infection with parvovirus and Salmonella should be included as a differential diagnosis in raccoons with sudden death.
Keywords: enteritis, parvovirus, raccoons, Salmonella
Raccoons have adapted well to urban habitats but are susceptible to several pathogens, many of which are zoonotic. Unexpected mortality raises concerns for public health and wildlife preservation. In July of 2019, a wildlife rehabilitation center in Massachusetts, USA reported the sudden deaths of 8 of 9 raccoons. The 4- to 5-mo-old raccoons were comprised of both males and females. The orphaned raccoons had been found on roads near their deceased mothers in 5 separate events in May 2019. Four days after rescue, 5 of the raccoons had been vaccinated (Fel-O-Guard Plus 3 [modified-live feline rhinotracheitis virus, calicivirus, panleukopenia virus] and Duramune Max 5 [modified canine distemper virus, adenovirus type 2, parainfluenza virus, parvovirus]; Boehringer Ingelheim). In June, all of the raccoons were vaccinated with Purevax Feline 3 (modified-live feline rhinotracheitis virus, calicivirus, panleukopenia virus; Merial) or Recombitek C6 (containing a lyophilized suspension of a recombinant canarypox vector expressing the HA and F glycoproteins of canine distemper virus, modified live adenovirus type 2, parainfluenza virus, parvovirus, and a liquid suspension of inactivated cultures of Leptospira interrogans serovars Canicola and Icterohaemorrhagiae; Merial). No obvious clinical signs were observed before the onset of sudden death at the end of July.
Five raccoons were submitted for autopsy in fair-to-poor postmortem preservation. They weighed 1.3–2.4 kg, and they had adequate body condition without evidence of trauma, fecal stains on the hair coat, or any other external lesions. On gross examination, small and large intestines were mildly to moderately distended and contained copious light-yellow, pasty-to-watery ingesta or digesta with some gas. The ileocecal lymph nodes were enlarged. Other visceral organs had no significant macroscopic findings.
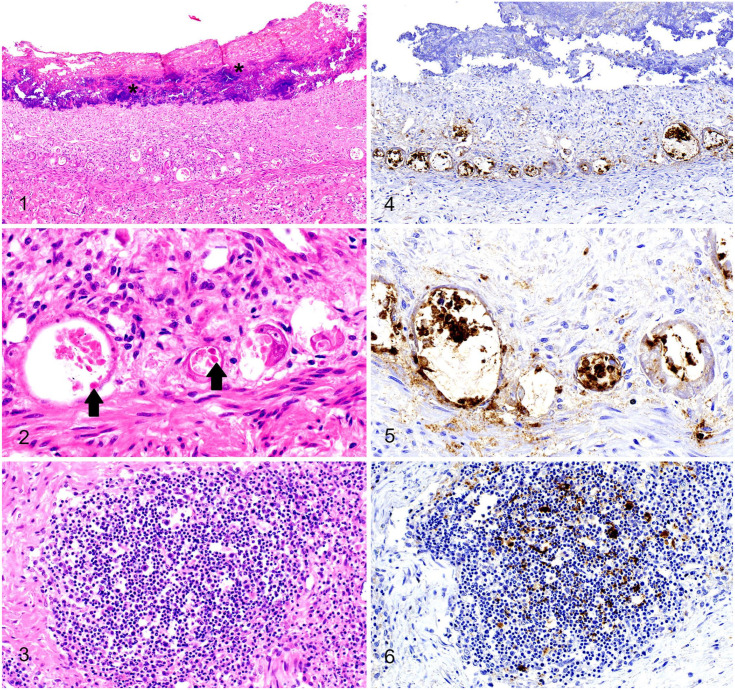
Within the intestine, there was extensive autolysis (Fig. 1), obscuring some microscopic detail. The lamina propria was collapsed with increased density of the stroma and with small numbers of lymphocytes and plasma cells. The numbers of crypts were reduced markedly and, in the few visible crypts, the lumina were widely distended with degenerate enterocytes, a few neutrophils, and necrotic debris. Enterocyte features that were observed in many crypts included attenuation, syncytia containing intranuclear viral inclusion bodies, and plump hyperplastic enterocytes that frequently were piled up (Fig. 2). Peyer patches and ileocecal lymph nodes had lymphoid necrosis and depletion (Fig. 3). Lungs were congested, with subjectively increased numbers of neutrophils in capillaries. Significant microscopic lesions were not evident in other visceral organs.
Figures 1–6.
Parvoviral infection in a raccoon. Figure 1. In the jejunum, the villi were markedly atrophic and necrotic with complete loss of enterocytes and were overlaid by mats of bacterial colonies (asterisks). Crypts were either lost, ectatic, or hyperplastic. H&E. Figure 2. A distended crypt (left) was lined by attenuated epithelial cells with sloughed cells containing intranuclear viral inclusions (arrows), and other atrophied crypts (right) were lined by hypertrophic syncytial epithelial cells. H&E. Figure 3. Lymphoid necrosis and depletion in an ileocecal lymph node. H&E. Figure 4. Strong signals of parvoviral antigen were detected in jejunal crypts. Parvovirus IHC. Figure 5. Strong signals of parvoviral antigen were detected in jejunal crypt cells. Parvovirus IHC. Figure 6. Parvoviral antigens were condensed in an indistinct germinal center. Parvovirus IHC.
Immunohistochemistry (IHC) was performed with a canine/feline parvoviral monoclonal antibody (Invitrogen CPV1-2A1; Thermo Fisher). There was strong cytoplasmic and nuclear immunopositivity within the intestinal crypt epithelial cells and in fewer villus enterocytes (Fig. 4). The positive signal ranged from a few small dots to diffuse staining within the cell (Fig. 5). Peyer patches and lymph nodes also contained strong immunopositivity within follicular dendritic cells, infiltrating histiocytes or macrophages, and residual immunoblasts (Fig. 6). IHC for rabies virus (Rabies lyssavirus) and canine distemper virus (Canine morbillivirus) was negative in the brainstem and the cerebrum at the level of the hippocampus.
The first case of our series was tested by conventional PCR with canine parvovirus (CPV; Carnivore protoparvovirus) VP2-specific primers, 7 which resulted in an expected 657-bp product (spanning nucleotides 940–1670 of the VP2 gene). The sequence was >99% identical to CPV2a-like strains currently isolated from a raccoon (GenBank MF069442.1) and several reference strains of CPV2a. 4 Subsequently, metagenomic sequencing was performed on pooled intestinal homogenate collected from 5 raccoons. A complete parvoviral genome that was >99% identical to CPV2a was assembled and submitted to GenBank (MW365734). Additionally, several contigs were identified as Salmonella enterica subsp. enterica. One strain of Salmonella was isolated from pooled intestinal homogenate with enrichment procedures. 12 In antimicrobial susceptibility tests, there was resistance to amikacin, cefoxitin, clindamycin, gentamicin, and penicillin. Serotyping was performed at the National Veterinary Services Laboratories (Ames, IA, USA) with the result of S. enterica subsp. enterica ser. Thompson.
Along with rabies virus and canine distemper virus, parvovirus causes one of the most important infectious diseases of raccoons. 3 Occasionally, parvovirus can cause high mortality among unvaccinated juvenile raccoon “orphanages” in rehabilitation facilities and animal shelters 3 ; however, sudden death without expression of diarrhea is uncommon. In our cases, there were no obvious clinical signs or specific gross findings, leaving the diagnosis unclear pending microscopic examination. Although the lesions of parvoviral enteritis are well-documented, the diagnosis can be challenging, particularly in wildlife, given rapid autolysis in the intestine and the unavailability of species-specific IHC. Parvovirus has been isolated from a raccoon with enteritis; however, viral antigen distribution in tissues could not be demonstrated by IHC in a 2010 study. 9 Herein, we demonstrated the tissue distribution of parvoviral antigen and correlated it with histologic lesions. Furthermore, metagenomic sequencing enabled unbiased identification of pathogens and enabled the assembly of a complete parvoviral genome. Disruption of the intestinal mucosal barrier by parvoviral infection with concurrent enteric Salmonella, and likely other opportunistic gut bacteria, provided an explanation of unexpected sudden death.
Prior to the onset of sudden death, these raccoons were likely asymptomatic Salmonella reservoirs and effective carriers, as reported widely in epidemiologic studies.5,10 Various Salmonella serovars have been isolated from raccoons, with many isolates showing resistance to multiple antimicrobials, as noted here. Many serovars, including S. enterica ser. Thompson, matched those commonly isolated from humans with salmonellosis.5,6,10 Again, the finding of Salmonella in the raccoons of our case series raises the concern of public health risk of Salmonella transmission and dissemination, particularly in urban and suburban areas.
In the 1980s, commercial animal dealers sold raccoons to hunters to restock depleted populations. Parvoviral enteritis was reported in these raccoons, and close interaction between raccoons and dogs was suggested as a serious threat to native raccoon populations. 11 Experimental inoculations demonstrated that raccoons are highly susceptible to feline panleukopenia virus and mink enteritis virus (merged into Carnivore protoparvovirus), but not the prototype of CPV, 2 suggesting that raccoons potentially serve as intermediates between domestic and wild carnivores. However, these hypotheses were not further investigated until 2010 when a phylogenetic study indicated a group of parvoviruses potentially associated with the emergence of CPV2a had been circulating in raccoons for at least 24 y. 1 More recently, a strain of parvovirus identified in a rescued raccoon was closely related to CPV2a, 9 as reported in our case. These data strongly suggest that raccoons serve as a host for parvoviral emergence, cross-species transmission, and pandemic diseases.1,8
Acknowledgments
We thank Drs. Randall Renshaw and Edward Dubovi (Cornell University) for PCR and sequencing; Jan Shivers (the University of Minnesota), Amanda Brock and Scott Goodpaster (South Dakota State University), and Linda M. Wrijil, Sarah Ducat, and Gina Scarglia (Tufts University) for the histologic sections and IHC assays; and Marlee Braun (SDSU) for Salmonella isolation.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Chun-Ming Lin  https://orcid.org/0000-0002-5721-6481
https://orcid.org/0000-0002-5721-6481
Contributor Information
Chun-Ming Lin, Animal Disease Research and Diagnostic Laboratory, South Dakota State University, Brookings, SD, USA; Department of Biomedical Sciences, Cummings School of Veterinary Medicine, Tufts University, North Grafton, MA, USA.
Benjamin Hause, Animal Disease Research and Diagnostic Laboratory, South Dakota State University, Brookings, SD, USA.
Deanna Gualtieri, North East Wildlife Animal Rehabilitation Coalition, Barre, MA, USA.
Nicholas Robinson, Department of Biomedical Sciences, Cummings School of Veterinary Medicine, Tufts University, North Grafton, MA, USA; Preclinical and Translational Development, bluebird bio, Cambridge, MA, USA.
References
- 1. Allison AB, et al. Role of multiple hosts in the cross-species transmission and emergence of a pandemic parvovirus. J Virol 2012;86:865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barker IK, et al. Response of mink, skunk, red fox and raccoon to inoculation with mink virus enteritis, feline panleukopenia and canine parvovirus and prevalence of antibody to parvovirus in wild carnivores in Ontario. Can J Comp Med 1983;47:188–197. [PMC free article] [PubMed] [Google Scholar]
- 3. Barker IK, Parrish CR. Parvovirus infections. In: Williams ES, Barker IK, eds. Infectious Diseases of Wild Mammals. 3rd ed. Iowa State University Press, 2001:131–146. [Google Scholar]
- 4. Canuti M, et al. Epidemiology and molecular characterization of protoparvoviruses infecting wild raccoons (Procyon lotor) in British Columbia, Canada. Virus Res 2017;242:85–89. [DOI] [PubMed] [Google Scholar]
- 5. Compton JA, et al. Salmonella infections in the common raccoon (Procyon lotor) in western Pennsylvania. J Clin Microbiol 2008;46:3084–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eun Y, et al. A large outbreak of Salmonella enterica serovar Thompson infections associated with chocolate cake in Busan, Korea. Epidemiol Health 2019;41:e2019002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ford J, et al. Parvovirus infection is associated with myocarditis and myocardial fibrosis in young dogs. Vet Pathol 2017;54:964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoelzer K, Parrish CR. The emergence of parvoviruses of carnivores. Vet Res 2010;41:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kapil S, et al. Isolation of a virus related to canine parvovirus type 2 from a raccoon (Procyon lotor). Vet Rec 2010;166:24–25. [DOI] [PubMed] [Google Scholar]
- 10. Lee K, et al. Prevalence of Salmonella, Yersinia and Campylobacter spp. in feral raccoons (Procyon lotor) and masked palm civets (Paguma larvata) in Japan. Zoonoses Public Health 2011;58:424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nettles VF, et al. Parvovirus infection in translocated raccoons. J Am Vet Med Assoc 1980;177:787–789. [PubMed] [Google Scholar]
- 12. Osumi T, et al. Enrichment for isolating Salmonella Choleraesuis and other Salmonella spp. from pigs. J Vet Med Sci 2003;65:949–951. [DOI] [PubMed] [Google Scholar]