Abstract
Neurologic diseases are common in domestic cats, and infectious agents are suspected to be the primary cause in 30–45% of cases. Among infectious etiologies, those of bacterial origin are only sporadically characterized in the literature, with few of these reports correlating gross and histologic findings with confirmatory bacteriologic identification. Here, we describe bacterial meningitis and meningoencephalomyelitis associated with Pasteurella multocida in 3 domestic cats. Purulent exudate expanding the cerebral meninges was grossly evident in 2 of the cases. In all 3 cases, histologic changes included multifocal suppurative-to-necrosuppurative meningitis and/or meningoencephalomyelitis of variable severity. Intralesional colonies of gram-negative, short rod-shaped to coccobacillary bacteria were evident histologically in only 1 case. P. multocida was confirmed by routine bacteriologic culture in all cases. Based on our cases, we hypothesize that the upper respiratory system serves as the main portal of entry for P. multocida, leading to invasion of the central nervous system and possible systemic hematogenous dissemination. A case series of meningoencephalomyelitis associated with P. multocida infection in cats has not been reported previously, to our knowledge. We also review briefly other causes of meningoencephalomyelitis in cats.
Keywords: bacterial meningoencephalomyelitis, encephalitis, feline, myelitis, neurologic signs, Pasteurella multocida
Neurologic diseases are common in cats, constituting ~10% of the referral caseload in feline patients. 9 Infectious agents are suspected to be the primary cause in 30–45% of these cases.1,9 Among infectious etiologies, bacterial infections in the central nervous system (CNS) only rarely manifest as the primary neurologic lesion; more often they develop as locoregional extension of bacterial infections affecting the middle ear and/or nasal cavity. 9 Bacterial causes of CNS infection in cats have an estimated prevalence of 1% among all neurologic diseases. 1 We describe here the gross and histopathologic features of meningitis and meningoencephalomyelitis in 3 domestic cats associated with Pasteurella multocida infection. Also, we reviewed reports of the most common differential diagnoses for meningoencephalomyelitis in cats.
A review of all feline pathology case submissions involving neurologic signs was conducted in the archives of the Louisiana Animal Disease Diagnostic Laboratory (LADDL), School of Veterinary Medicine, Louisiana State University (Baton Rouge, LA, USA), from January 2012 to July 2020 using the following search query in USALIMS: meningoencephalomyelitis, meningoencephalitis, encephalitis, myelitis, and meningitis. We retrieved 64 cases from the archives, and 55 of 64 (88%) had a defined infectious agent, including: feline infectious peritonitis virus (FIPV; 28 of 55, 51%), septicemia associated with Escherichia coli or Staphylococcus sp. (6 of 55, 11%), suspected bacterial (without bacteria identification; 3 of 55, 5%), Pasteurella multocida (3 of 55, 5%), Streptococcus canis (2 of 55, 4%), and other miscellaneous agents (13 of 55, 24%). Relevant information (signalment, clinical presentation, and postmortem findings) for the 3 cases with P. multocida were obtained from the reports (Table 1). A postmortem examination was performed in these cases within 24–72 h after death. Tissues were collected, fixed in 10% neutral-buffered formalin, sectioned at 4 μm, and stained using hematoxylin and eosin. Selected sections were subjected to a Gram stain following standard procedures. Refrigerated fresh brain samples from all cases were submitted for aerobic bacteriologic culture.
Table 1.
Signalment, clinical features, and gross lesions in 3 cats with meningitis and meningoencephalomyelitis associated with Pasteurella multocida infection.
| Case | Age | Sex | Breed | Clinical features | Gross lesions |
|---|---|---|---|---|---|
| 1 | 5 y | SF | American Shorthair | Recurrent ear infection, progressive neurologic signs (3-d course; lateral recumbency, stiff limbs, vocalization, anisocoria, nystagmus). Treated with clindamycin (10 mg/kg IV) and mannitol (0.5 g/kg IV). The cat had cardiorespiratory arrest. | CNS: opaque, white-to-green–tinged purulent exudate coating the meningeal surface of the caudodorsal aspect of the left cerebral hemisphere. |
| Kidneys: unilateral focal chronic infarct. | |||||
| 2 | 2–3 mo | SF | Mixed breed | Upper respiratory signs, ocular discharge, neurologic signs (nystagmus, paraparesis, head tilt that progressed to lateral recumbency [unknown duration]). Treated with broad-spectrum antibiotics for the respiratory signs. The cat was found dead by the owner ~1 wk after having been introduced to its new home. | Skin: periocular yellow-to-brown crusts. |
| Liver: white pinpoint foci. | |||||
| 3 | 2 mo | M | Mixed breed | Upper respiratory signs, sneezing, ocular and nasal discharge, and lethargy (unknown duration). No specific neurologic signs observed by the owner. Treated with broad-spectrum antibiotics for the respiratory signs. The cat was found dead by the owner a day after it had still been seen playing with the other kittens. | CNS: purulent exudate expanding the subdural space on the dorsal and ventrolateral aspect of the right cerebral hemisphere and interhemispheric fissure, and circumferentially around spinal cord segment C1. |
| Nasal cavity and frontal sinuses were filled with mucopurulent exudate. | |||||
| Skin: periocular and nasal dark brown crusts. | |||||
| Thymus: inconspicuous. | |||||
| Intestines: mild nematodiasis (Toxocara cati). |
CNS = central nervous system; IV = intravenous; M = intact male; SF = spayed female.
Gross findings in the CNS were similar in cases 1 and 3 but more severe in the latter, with abundant purulent exudate either partially filling the calvaria (case 1) or extensively expanding the meninges of the brain and cervical spinal cord (case 3; Fig. 1A). Additionally, the nasal cavity and the frontal sinuses of case 3 were filled with similar exudate, the eyelids and nose were coated with crusts, and the thymus was inapparent. No gross lesions were evident within the CNS in case 2 and, despite the previous history of recurrent otitis in case 1, no gross abnormalities associated with otitis were evident. No other significant gross lesions were noted in any of the animals.
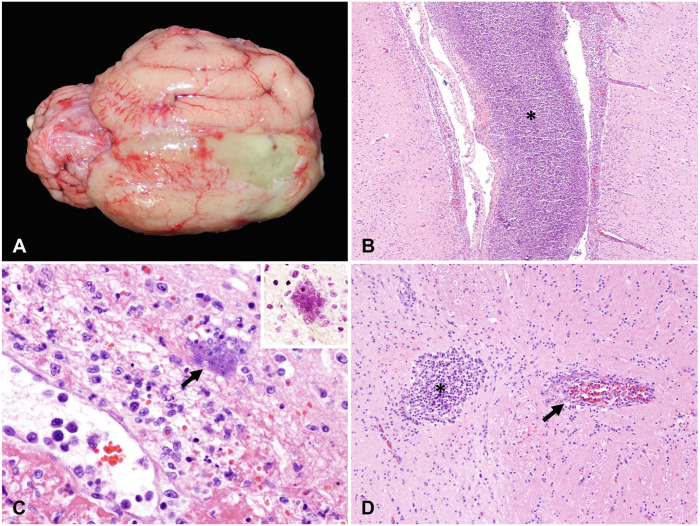
Figure 1.
Meningoencephalomyelitis caused by Pasteurella multocida in cats (case 3). A. Thick, yellow exudate expands the subdural space on the dorsal and ventrolateral aspect of the right cerebral hemisphere and interhemispheric fissure. B. Microscopically, the meninges are severely expanded by abundant necrotic cell debris, degenerate neutrophils, and fibrin (asterisk), with extension of the inflammatory process into the underlying neuroparenchyma. H&E. Original objective 10×. C. Rare-to-occasional intralesional colonies of gram-negative (inset; Gram stain) short rod-shaped to coccobacillary bacteria (arrow) are visualized. H&E. Original objective 40×. D. The central canal of the spinal cord was dilated and filled with abundant degenerate neutrophils and necrotic debris (asterisk); necrotizing vasculitis was also present (arrow). H&E. Original objective 20×.
Tissues from the CNS examined microscopically included cerebrum, cerebellum, and brainstem in all cases, and spinal cord (cervical, thoracic, and lumbar) in cases 2 and 3 (Table 2). Microscopically, cases 2 and 3 had similar histologic alterations (lesions were most severe in case 3); changes in case 1 were subtle and observed primarily in the leptomeninges. In cases 2 and 3, the subdural space and leptomeninges overlying the brain and spinal cord were multifocally to diffusely expanded by purulent exudate, comprised of necrotic cell debris, degenerate neutrophils, and fibrin (Fig. 1B). Rare-to-occasional intralesional colonies of short rod-shaped to coccobacillary bacteria were evident histologically in case 3 (Fig. 1C). The inflammatory exudate extended into and blended with the underlying neuroparenchyma. Vasculitis with fibrinoid necrosis characterized intralesional blood vessels, mainly small venules in the leptomeninges. Multifocal, occasionally extensive, areas of the underlying cortical lamina within the cerebrum and periventricular areas were characterized by rarefaction; numerous infiltrating viable and degenerate neutrophils; shrunken, angular, and hyperchromatic neurons; and blood vessels delimited by gitter cells and neutrophils. Also, in cases 2 and 3, the inflammatory exudate partially filled the lumen of the lateral ventricle, with evidence of suppurative choroid plexitis, and extended to both the molecular layer of the cerebellum and the medulla oblongata. Although in case 3 the gross changes involved mostly the right cerebral hemisphere, the histologic degree of inflammation was bilaterally similar, with unilaterally more pronounced interstitial edema within the right neuroparenchyma.
Table 2.
Brain and spinal cord lesions in 3 cats with meningitis and meningoencephalomyelitis associated with Pasteurella multocida infection.
| Case | Olfactory bulbitis | Leptomeningitis | Encephalitis | Periventricular encephalitis | Choroid plexitis | Ventriculitis | Myelitis |
|---|---|---|---|---|---|---|---|
| 1 | NA | Neu, H, MC, Mil | L, MC, Mil | U | U | U | NA |
| 2 | NA | Neu, H, Fib, VL, MC, Mod | Neu, GC, VL, MC, Mil | Neu, Fib, M, Mod | Neu, MC, Mod | Neu, H, M, Mod | Neu, Fib, VL, MC, Mar |
| 3 | Neu, Fib, B, Ext, Mar | Neu, Fib, B, VL, D, Mar | Neu, GC, Fib, VL, MC, Mod | Neu, Fib, B, MC, Mar | Neu, D, Mar | Neu, H, Fib, D, Mar | Neu, Fib, VL, MC, Mod |
B = bacteria; D = diffuse; Ext = extensive; Fib = fibrin; GC = gitter cells; H = histiocytic; L = lymphocytic; M = multifocal; Mar = marked; MC = multifocal-to-coalescing; Mil = mild; Mod = moderate; NA = not available; Neu = neutrophilic; U = unremarkable; VL = vasculitis.
Throughout all segments of the spinal cord (cervical, thoracic, and lumbar from cases 2 and 3), the central canal was filled by abundant degenerate neutrophils (Fig. 1D) that extended into the circumjacent gray matter, which had multiple blood vessels cuffed by a few neutrophils and occasionally effaced by necrotizing vasculitis. These histologic changes were most severe in case 2. All white matter tracts had multifocal mild neutrophilic infiltrates as well as digestion chambers and scattered spheroids, with no defined pattern.
Histologic changes in case 1 were limited mostly to the cerebral leptomeninges, which were expanded only mildly by interstitial edema and minimal-to-mild neutrophilic and histiocytic infiltrates. A few blood vessels at the level of the hippocampus were delimited by delicate lymphocytic cuffs. No vasculitis was observed in this case. Bacteriologic culture yielded moderate-to-heavy growth of P. multocida (exclusively) in the brain of all cases (Suppl. Table 1).
Given that the upper respiratory tract can serve as a portal of entry into the CNS, we evaluated the nasal cavity in cases 2 and 3. Based on the available specimens, we performed either partial (case 2) or thorough evaluation of the nasal cavity and associated structures (case 3), which included the nasal cavity, frontal sinuses, cribriform plate, olfactory nerve fascicles and olfactory bulb, as well as the middle and inner ears. The nasal epithelium was disrupted by fibrinosuppurative inflammation that also expanded the submucosa and, along with scattered colonies of coccobacillary bacteria, filled the nasal and paranasal sinus lumina. Additionally, in case 3, the olfactory neuroepithelium was extensively disrupted by similar inflammation, with frequent fibrinoid necrosis of the submucosal (nasal) blood vessels and marked osteolysis of the subjacent turbinate bone. In this cat, the nerve fascicles forming the olfactory nerve as well as the extracranial segment of the optic nerve were encircled by the inflammation, accompanied by fibrinoid necrosis of meningeal vessels, bilateral liquefactive necrosis of the olfactory bulb, and flooding of the olfactory recesses by abundant fibrinosuppurative exudate. In case 3, there was also marked bilateral suppurative otitis media; however, the inner ears were unremarkable. The eyes exhibited multifocal mild conjunctivitis and uveitis.
Additional histologic findings of significance included either mild, multifocal, portal-to-random, lymphoplasmacytic-to-suppurative hepatitis (cases 1, 2), or scattered hypertrophied (reactive) Kupffer cells, with clear cytoplasm and vesiculate nucleus, and pulmonary edema and leukocytosis (case 3). Heavy growth of P. multocida was obtained from the liver and lung of case 3, with frequent circulating clusters of gram-negative coccobacillary bacteria within hepatic sinusoids. Light growth of Staphylococcus haemolyticus was obtained from the liver and lung of case 2; no microorganisms were evident in H&E- and Gram-stained sections, however. No microorganisms were evident either in tissue sections of case 1, of which no samples other than the brain were available for bacteriologic culture. Additional ancillary testing included virus isolation (to rule out felid herpesvirus 1 [Felid alphaherpesvirus 1] and feline calicivirus; cases 2 and 3), fluorescent antibody tests for rabies virus (Rabies lyssavirus) and feline coronavirus (Alphacoronavirus 1; case 2), and reverse-transcription real-time PCR assays for eastern equine encephalitis and West Nile viruses (case 2), all of which yielded negative results. Hence, we based the diagnosis of meningitis or meningoencephalomyelitis associated with P. multocida on gross, microscopic, and bacteriologic findings.
We have described here the gross and histologic findings associated with P. multocida infecting the CNS of cats. Bacterial meningoencephalomyelitis is often a lethal disease process of challenging management given difficulties in accurate and prompt diagnosis.9,16 In another study, 3 cases of bacterial meningoencephalitis in cats were reported with identification of intralesional bacterial colonies microscopically, and further characterized by electron microscopy; however, no specific microorganism was confirmed as the causative agent. 1 Additionally, a series of 10 cases of bacterial myelitis failed to identify the microorganism(s) involved. 17 As evidenced by these studies, the role of bacterial organisms in the pathogenesis of meningoencephalitis and myelitis in cats is poorly characterized. In our cases, moderate-to-heavy growth of P. multocida was obtained from the brain of all 3 cases, unequivocally implicating P. multocida as the causative agent. In one previously reported case, the diagnosis of meningitis by P. multocida was achieved through bacteriologic culture of cerebrospinal fluid 18 ; however, no pathology analysis was performed to confirm the suspected meningoencephalomyelitis or CNS abscess and, therefore, no absolute causal relationship was demonstrated.
P. multocida is a highly versatile gram-negative rod or coccobacillus frequently associated with the normal oropharyngeal microbiota of animals, with the highest carriage rate among cats (70–90%) and dogs (20–55%). 24 It is an important cause of disease in swine (atrophic rhinitis), ruminants (pneumonia), rabbits (snuffles and septicemia), and poultry (fowl cholera). 6 In animals, descriptions of P. multocida leading to CNS lesions are uncommon and are frequently related to respiratory signs and septicemia.2,8 The majority of human cases caused by P. multocida are associated with soft tissue infections, following animal bites or scratches, and leading to the development of cellulitis and/or abscesses.16,24 In children and immunocompromised patients, septicemia, meningitis (frequently suppurative leptomeningitis), and peritonitis have been described.16,24 Immunosuppression related to stressors, such as recent changes in environment and/or diet (case 2), and inferred by thymic atrophy (case 3) could have predisposed to the development of the CNS lesions associated with P. multocida in our cases. Feline leukemia virus and feline immunodeficiency virus also play an immunocompromising role. However, the status of infection with these viruses in our cats is unknown. Although felid herpesvirus and feline calicivirus were not detected in the cases that were tested (cases 2, 3), preceding upper respiratory tract infections associated with these viruses is possible and could have acted as predisposing factors to opportunistic P. multocida infection.2,13
Key virulence factors of P. multocida identified to date include the capsule and lipopolysaccharides. Five P. multocida capsular serotypes are routinely identified based on capsule antigens (A–F). 11 The capsule is involved in bacterial avoidance of phagocytosis and resistance to complement; lipopolysaccharides are critical for bacterial survival in the host. Other virulence factors include P. multocida toxin, putative surface adhesins, and iron acquisition proteins. 11 Unfortunately, in our cases, we were unable to determine the virulence factors involved in the lesions because of limitations of microbial typing with matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry.
P. multocida can enter the host through the mucous membranes of the upper respiratory tract, conjunctiva, and cutaneous scratch or bite wounds. P. multocida is an opportunistic bacterium that can cause mucopurulent rhinitis and sinusitis. 24 Infection of the CNS can occur as result of extension of bacterial infection from neighboring tissues, such as the middle ear or the nasal cavity. 9 We hypothesize that, in our cases, P. multocida reached the CNS by direct extension from either the nasal and sinus passages (cases 2, 3) or possibly the ear (cases 1, 2). In the cat, otitis media occurs primarily as a sequela of upper respiratory tract disease, associated with bacteria translocated via the Eustachian tube into the tympanic bulla. 20 Evaluation of the ears in cases 1 and 2 was limited to the postmortem examination; however, these cats reportedly had recurrent ear infection and vestibular signs, leading to the possibility that the CNS lesions developed as extensions from ear infections. In cats with peripheral vestibular disease, inner ear disease is most often the result of extension of middle ear disease, 20 which may have gone unnoticed grossly in these cases. Although a skin wound is a plausible portal of entry for P. multocida as well, none was evident at the time of postmortem examination in our cases.
Other means of dissemination to be considered in our cats include hematogenous and retrograde parasellar venous infections. In cases of parasellar venous infection, the ventral system of dural venous sinuses (ventral cerebral veins and ventral petrosal and cavernous sinuses) drains the ventral forebrain and the face, including the nasal cavity; therefore, infective material from those areas can drain directly into this system of sinuses and spread to the neuraxis. 2 Extension of the inflammation from the olfactory recesses (case 3) and the choroid plexus (cases 2, 3) explains the presence of the exudate in the cerebral ventricular spaces in 2 of our cats. The spinal cord lesions in these animals likely occurred via cerebrospinal fluid and hematogenous dissemination. Systemic hematogenous dissemination is common in cases with bacteremia, such as observed in case 3, which had lesions in multiple tissues consistent with septicemia and from which P. multocida was also cultured from the lung and liver.
We performed a literature search for the epidemiologic and predisposing factors as well as lesions for the most common differential diagnoses of meningoencephalomyelitis in cats (Table 3). Most infectious causes of neurologic disease result in neurologic signs that are acute, progressive, and frequently related to a systemic disease process. A detailed clinical examination in addition to complementary ancillary tests is therefore essential to reach an accurate diagnosis. 9 Approximately 15–20% of neurologic disease cases in cats are caused by feline infectious peritonitis virus (FIPV; Alphacoronavirus 1) and are related to the non-effusive (or “dry”) form of the disease, seen in infected cats with marked humoral immunity but only partial cell-mediated immunity. 9 The main differential cause of bacterial meningoencephalomyelitis in cats is Streptococcus canis. This bacterium has been described as a cause of meningitis in intensively housed, closed-colony, young cats, affecting more than 150 cats during 3 time-limited outbreaks. 21 S. canis is a β-hemolytic gram-positive zoonotic bacterium associated with chronic upper respiratory infections, urogenital infection, and neonatal septicemia in cats. 8 Young cats can be predisposed to S. canis infection by primary viral infections, such as felid herpesvirus and feline calicivirus. 21 Clinical signs and gross and histologic lesions are similar in S. canis and P. multocida infections. Gram staining of tissue sections and bacteriologic culture are therefore needed to reach a presumptive and confirmatory diagnosis, respectively, in these cases.
Table 3.
Epidemiology, predisposing factors, and lesions for the most common differential diagnosis of meningoencephalomyelitis in cats.
| Agent | Age | Predisposing factor | Remarks on brain and/or spinal cord lesions | Reference |
|---|---|---|---|---|
| Viral | ||||
| Feline peritonitis virus | Young cats (<2 y old) | Recent stress | Fibrinopyogranulomatous periventriculitis, meningitis, perivasculitis, segmental myelitis, hydrocephalus | 2 , 22 |
| Feline immunodeficiency virus | All ages can be affected | Aggressive behavior, fighting, access to outdoors, concomitant infection | Gliosis within the hilus of the dentate gyrus and granule cell axonal sprouting, lymphocytic meningoencephalomyelitis | 19 |
| Feline leukemia virus | All ages can be affected | Access to outdoors | Diffuse white-matter degeneration, myelin sheaths devoid of central axons and containing infiltrating gitter cells | 3 , 15 |
| Rabies virus | All ages can be affected | Access to outdoors, contact with the vector | Nonsuppurative meningoencephalomyelitis, neuronophagia, intracellular inclusions | 7 |
| Pseudorabies virus | All ages can be affected | Consumption of contaminated raw pork | Brainstem lesions consistent with mononuclear cuffing, pronounced proliferation of astrocytes and microglial cells, weak eosinophilic viral inclusion bodies in the nuclei of astrocytes and neurons | 12 |
| Feline herpesvirus 1 | Young cats | Access to outdoors, intensively housed | Severe nonsuppurative meningoencephalitis, chromatolysis of neurons, satellitosis, and gliosis with perivascular lymphocytic cuffing | 13 |
| Bacteria | ||||
| Streptococcus canis | All ages can be affected (kittens appear to be more susceptible) | Intensively housed, closed-colony animals | Fibrinosuppurative meningitis, thrombosis, intralesional bacterial aggregates | 8 , 21 |
| Pasteurella multocida | All ages can be affected (kittens appear to be more susceptible) | Upper respiratory infection Skin wounds |
Necrosuppurative meningoencephalomyelitis | 18 |
| Fungi | ||||
| Cryptococcus sp. | All ages can be affected | Immunocompromise | Granulomatous meningoencephalitis with intralesional fungi (“soap-bubble” appearance) | 23 |
| Parasites | ||||
| Sarcocystis neurona | Young (1–7 y old) | Use of corticosteroids, concomitant viral (FeLV) infection, chemotherapy | Pyogranulomatous meningoencephalitis with intralesional parasitic structures | 10 |
| Cytauxzoon felis | All ages can be affected | Access to outdoors, wooded environment, or tick exposure | Intravascular schizont-laden macrophages, occlusion of arterioles, venules, and capillaries by protozoal organisms, random areas of necrosis | 4 |
| Toxoplasma gondii | All ages can be affected (kittens appear to be more susceptible) | Immunosuppression, concurrent infection | Nonsuppurative encephalomyelitis, polyradiculoneuritis with intralesional protozoa | 5 |
| Cuterebra spp. | Young to middle age | Access to outdoors, summer months, upper respiratory infection | Eosinophilic meningoencephalitis with parasite tracts | 14 |
Our retrospective study unequivocally links P. multocida infection with the occurrence of bacterial meningitis and/or meningoencephalomyelitis in cats and highlights the importance of considering this microorganism among the potential etiologies in neurologic cases. Even though of rare occurrence, P. multocida infection should be considered as a differential cause of CNS infection in cats, especially in cats with associated rhinitis of sinusitis and otitis media.
Supplemental Material
Supplemental material, sj-pdf-1-vdi-10.1177_10406387211034484 for Meningoencephalomyelitis in domestic cats: 3 cases of Pasteurella multocida infection and literature review by Bianca S. de Cecco, Mariano Carossino, Fabio Del Piero, Nobuko Wakamatsu, Maria S. Mitchell, Natalie W. Fowlkes and Ingeborg M. Langohr in Journal of Veterinary Diagnostic Investigation
Acknowledgments
We thank members of the Histology and Immunohistochemistry sections at the Louisiana Animal Disease Diagnostic Laboratory (LADDL), School of Veterinary Medicine, Louisiana State University for their assistance.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: Our study was partially supported by the Louisiana Animal Disease Diagnostic Laboratory. Dr. Bianca S. de Cecco was supported by the Conselho Nacional de Pesquisa (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
ORCID iDs: Bianca S. de Cecco  https://orcid.org/0000-0003-4827-4011
https://orcid.org/0000-0003-4827-4011
Mariano Carossino  https://orcid.org/0000-0003-3864-5915
https://orcid.org/0000-0003-3864-5915
Ingeborg M. Langohr  https://orcid.org/0000-0001-7366-4391
https://orcid.org/0000-0001-7366-4391
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Bianca S. de Cecco, Department of Veterinary Pathology, Faculty of Veterinary Medicine, Federal University of Rio Grande do Sul, Porto Alegre, RS, Brazil
Mariano Carossino, Louisiana Animal Disease Diagnostic Laboratory, School of Veterinary Medicine, Louisiana State University, Baton Rouge, LA, USA; Department of Pathobiological Sciences, School of Veterinary Medicine, Louisiana State University, Baton Rouge, LA, USA.
Fabio Del Piero, Louisiana Animal Disease Diagnostic Laboratory, School of Veterinary Medicine, Louisiana State University, Baton Rouge, LA, USA; Department of Pathobiological Sciences, School of Veterinary Medicine, Louisiana State University, Baton Rouge, LA, USA.
Nobuko Wakamatsu, Louisiana Animal Disease Diagnostic Laboratory, School of Veterinary Medicine, Louisiana State University, Baton Rouge, LA, USA; Department of Pathobiological Sciences, School of Veterinary Medicine, Louisiana State University, Baton Rouge, LA, USA; Current address: Department of Comparative Pathobiology, Indiana Animal Disease Diagnostic Laboratory, College of Veterinary Medicine, Purdue University, West Lafayette, IN, USA.
Maria S. Mitchell, Louisiana Animal Disease Diagnostic Laboratory, School of Veterinary Medicine, Louisiana State University, Baton Rouge, LA, USA
Natalie W. Fowlkes, Louisiana Animal Disease Diagnostic Laboratory, School of Veterinary Medicine, Louisiana State University, Baton Rouge, LA, USA Department of Pathobiological Sciences, School of Veterinary Medicine, Louisiana State University, Baton Rouge, LA, USA; current address: Department of Veterinary Medicine and Surgery, Division of Basic Sciences, MD Anderson Cancer Center, The University of Texas, Houston, TX, USA.
Ingeborg M. Langohr, Louisiana Animal Disease Diagnostic Laboratory, School of Veterinary Medicine, Louisiana State University, Baton Rouge, LA, USA Department of Pathobiological Sciences, School of Veterinary Medicine, Louisiana State University, Baton Rouge, LA, USA.
References
- 1. Bradshaw JM, et al. A retrospective study of 286 cases of neurological disorders of the cat. J Comp Pathol 2004;131:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cantile C, Youssef S. Nervous system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 6th ed. Vol. 1. Elsevier, 2016:250–406. [Google Scholar]
- 3. Carmichael KP, et al. Feline leukemia virus-associated myelopathy in cats. Vet Pathol 2002;39:536–545. [DOI] [PubMed] [Google Scholar]
- 4. Clarke LL, Rissi DR. Neuropathology of natural Cytauxzoon felis infection in domestic cats. Vet Pathol 2015;52:1167–1171. [DOI] [PubMed] [Google Scholar]
- 5. Dubey JP. Toxoplasmosis—a waterborne zoonosis. Vet Parasitol 2004;126:57–72. [DOI] [PubMed] [Google Scholar]
- 6. Ewers C, et al. Virulence genotype of Pasteurella multocida strains isolated from different hosts with various disease status. Vet Microbiol 2006;114:304–317. [DOI] [PubMed] [Google Scholar]
- 7. Greene CE. Rabies and other lyssavirus infections. In: Greene CE, ed. Infectious Diseases of the Dog and Cat. 4th ed. Saunders Elsevier, 2012:179–198. [Google Scholar]
- 8. Greene CE, Prescott JF. Gram-positive bacterial infections. In: Greene CE, ed. Infectious Diseases of the Dog and Cat. 4th ed. Saunders Elsevier, 2012:325–333. [Google Scholar]
- 9. Gunn-Moore D, Reed N. CNS disease in the cat: current knowledge of infectious causes. J Feline Med Surg 2011;13:824–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hammerschmitt ME, et al. First molecular characterization of Sarcocystis neurona causing meningoencephalitis in a domestic cat in Brazil. Parasitol Res 2020;119:675–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harper M, et al. Pasteurella multocida pathogenesis: 125 years after Pasteur. FEMS Microbiol Lett 2006;265:1–10. [DOI] [PubMed] [Google Scholar]
- 12. Henke D, Vandevelde M. Pseudorabies. In: Greene CE, ed. Infectious Diseases of the Dog and Cat. 4th ed. Saunders Elsevier, 2012:198–201. [Google Scholar]
- 13. Hora AS, et al. Felid herpesvirus 1 as a causative agent of severe nonsuppurative meningoencephalitis in a domestic cat. J Clin Microbiol 2013;51:676–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. James FMK, Poma R. Neurological manifestations of feline cuterebriasis. Can Vet J 2010;51:213–215. [PMC free article] [PubMed] [Google Scholar]
- 15. Levy JK. FeLV and non-neoplastic FeLV-related disease. In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine. 5th ed. WB Saunders, 2000:424–432. [Google Scholar]
- 16. Lloret A, et al. Pasteurella multocida infection in cats: ABCD guidelines on prevention and management. J Feline Med Surg 2013;15:570–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marioni-Henry K, et al. Prevalence of diseases of the spinal cord of cats. J Vet Intern Med 2004;18:851–858. [DOI] [PubMed] [Google Scholar]
- 18. Messer JS, et al. Meningoencephalomyelitis caused by Pasteurella multocida in a cat. J Vet Intern Med 2006;20:1033–1036. [DOI] [PubMed] [Google Scholar]
- 19. Mitchell TW, et al. Axonal sprouting in the hippocampus of cats infected with feline immunodeficiency virus (FIV). J Acquir Immune Defic Syndr Hum Retrovirol 1998;17:1–8. [DOI] [PubMed] [Google Scholar]
- 20. Njaa BL, Wilcock BP. The ear and eye. In: Zachary JF, McGavin MD, eds. Pathologic Basis of Veterinary Disease. 5th ed. Elsevier Mosby, 2012:1153–1165. [Google Scholar]
- 21. Pesavento PA, et al. Fatal Streptococcus canis infections in intensively housed shelter cats. Vet Pathol 2007;44:218–221. [DOI] [PubMed] [Google Scholar]
- 22. Rissi DR. A retrospective study of the neuropathology and diagnosis of naturally occurring feline infectious peritonitis. J Vet Diagn Invest 2018;30:392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sykes JE, et al. Clinical signs, imaging features, neuropathology, and outcome in cats and dogs with central nervous system cryptococcosis from California. J Vet Intern Med 2010;24:1427–1438. [DOI] [PubMed] [Google Scholar]
- 24. Zurlo JJ. Pasteurella species. In: Mandell GL, et al., eds. Mandell, Douglas and Bennet’s Principle and Practice of Infectious Disease. 7th ed. Churchill Livingstone Elsevier, 2009:2939–2942. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-vdi-10.1177_10406387211034484 for Meningoencephalomyelitis in domestic cats: 3 cases of Pasteurella multocida infection and literature review by Bianca S. de Cecco, Mariano Carossino, Fabio Del Piero, Nobuko Wakamatsu, Maria S. Mitchell, Natalie W. Fowlkes and Ingeborg M. Langohr in Journal of Veterinary Diagnostic Investigation