Abstract
Trauma, corticosteroid therapy and metabolic diseases are well established aetiologies of humeral head osteonecrosis; however, there is increasing evidence that arthroscopic rotator cuff surgery may be another possible cause. One of the reasons is that there may be inadvertent damage to the arterial blood supply to the humeral head during surgical intervention. The blood supply to the humeral head displays large amounts of variation with regard to origin, course and distribution. Therefore, to shed light on the pathogenesis, the blood supply of the humeral head is reviewed together with a summary of all reported cases of osteonecrosis of the humeral head that occurred following rotator cuff repair. Inconsistencies with regard to terminologies used and contradictions concerning arterial contributions from the anterior circumflex humeral artery and the posterior circumflex humeral artery towards humeral head supply are addressed. Moreover, variations in the course of the anterior circumflex humeral artery and its branches are summarized. The vascular anatomy of the humeral head is clinically relevant due to the close relationship of these blood vessels with the surgical repair sites for rotator cuff surgery and biceps tenotomies or tenodesis procedures. Potential sites of disruption of blood supply following arthroscopic rotator cuff surgery are discussed. Detailed knowledge of the course of the arteries supplying the humeral head may help to minimize the risk of vascular injury and subsequent osteonecrosis. Given the great interindividual variations of vascular anatomy, imaging procedures preceding arthroscopic rotator cuff surgery may be advisable.
Keywords: anterior circumflex humeral artery, arcuate artery, arthroscopy, biceps tendon, osteonecrosis, shoulder joint, variations
The blood supply of the humeral head is complex and highly variable. The variations of the anterior circumflex humeral artery and its branches as well as its relationship to the long head of biceps tendon should be considered in pre‐surgical planning. Twenty‐one cases of post‐arthroscopic humeral head osteonecrosis have been reported in the literature to date and the causal relationship for this complication is still currently unknown, however, it may be linked to damage of the extra‐ and/or intraosseous blood vessels in this region.
1. INTRODUCTION
Many studies have investigated the blood supply of the humeral head, and amongst these, several contradictions and inconsistences exist. Understanding and being aware of vascular anatomy and its variations is clinically relevant for several reasons: for surgeons repairing proximal humeral fractures in order to assess the vascular injury, for physicians performing diagnostic or therapeutic procedures using angiograms of these vessels and potentially also for those inclined to develop new surgical techniques (Fontes et al., 2015; Hettrich et al., 2010).
Osteonecrosis of the humeral head, although not as common as femoral head osteonecrosis, is a serious condition leading to pain and degeneration of the shoulder joint (Cofield, 1994). It is said to be more common in men and more prevalent among younger patients (20–50 years; Harreld et al., 2009). Although the exact aetiology of humeral head osteonecrosis is still not fully understood, several traumatic and atraumatic causes have been attributed to the overall development of this condition. These include certain proximal humeral fractures (Lee & Hansen, 1981; Neer, 1972; Neer, 1990; Sarris et al., 2004), corticosteroid use (Cruess, 1976; Cruess, 1978; Cruess, 1981; Fisher et al., 1972; Hasan & Romeo, 2002; Hayes, 1989; Moran, 1962; Usher & Friedman, 1995), sickle‐cell disease (David et al., 1993; Milner et al., 1993; Poignard et al., 2012), alcohol abuse (Jacobs, 1992; Matsuo et al., 1988), dysbarism (Chryssanthou, 1978; Jones et al., 1993), Gaucher's disease (Goldblatt et al., 1988; Lebel et al., 2009; Mansat et al., 2005), other systemic diseases (Hattrup & Cofield, 2000; Sarris et al., 2004; Shakir et al., 2020), antiangiogenic therapy (Tabouret et al., 2015) and, to a lesser degree, pregnancy (Kumar et al., 2010). Humeral head osteonecrosis due to possible vascular damage following rotator cuff (RC)/shoulder repair, however, is not commonly reported in the literature as part of the aetiologies.
Osteonecrosis is essentially bone death, and in the case of humeral head osteonecrosis, the epiphysis of the proximal humerus partially or totally collapses, followed by articular disruption, functional impairment and eventual osteoarthritis of the shoulder joint (Gerber et al., 1990; Kumar et al., 2010). Treatment is dependent on the stage of disease progression (Cruess, 1978), with later stages resulting in the need for joint replacement in order to achieve functional recovery (Kumar et al., 2010). The final pathway of osteonecrosis development is accepted and defined as the disruption in the blood supply/flow to the bone (Cushner & Friedman, 1997; Harreld et al., 2009; Kumar et al., 2010; Pavelka, 2000). Additionally, there may be an already poorly vascularized area in the superomedial aspect of the humeral head (Keough et al., 2019) at the site of osteonecrosis initiation that, should either the greater tubercle or the surroundings of the bicipital tendon be damaged during surgery, could lead to even further reduction in vascular supply triggering the osteonecrosis cascade of events.
Although a direct link has not yet been established or a proven causal relationship not yet been identified, the incidence of reported osteonecrosis following arthroscopic surgery, with or without the use of anchors is increasing, and over the last two decades, 21 cases of osteonecrosis have been reported (Beauthier et al., 2010; Cho et al., 2015; Dilisio et al., 2013; Goto et al., 2015; Hattrup & Cofield, 2000; Kim et al., 2018; Magee et al., 1997). In the case of RC repair, there may be inadvertent damage to the intraosseous network within the greater tubercle with anchor placement, especially with the use of multiple anchors. Alternatively, damage at the level of the biceps tendon is conceivable when a biceps tenotomy/tenodesis is performed, due to its close relation to branches of the anterior circumflex humeral artery (ACHA). Therefore, this review serves to combine and summarize the past and current literature regarding the blood supply of this specific area to identify commonalities and areas of contradiction that may require more thorough investigation and provide a summary of the reported cases of humeral head osteonecrosis following RC repair surgery. The following databases were searched for relevant literature: Cochrane, Grey database, Ovid, EBSCOHOST, ScienceDirect, EMBASE and PUBMED. This paper will also highlight the variations of the ACHA and its branches in relation to the long head of biceps tendon. Although the cause for humeral head osteonecrosis following shoulder surgery is not known, the main concern is that caution needs to be practised when operating in this area in order to try and preserve the arterial vessels especially in high risk patients and being aware of possible variations.
2. HUMERAL HEAD VASCULARIZATION
The vascular supply of the humeral head is widely accepted as a retrograde system by the arcuate artery, a branch of the anterolateral branch of the ACHA (Brooks et al., 1993; Gerber et al., 1990; Laing, 1956; Schai et al., 1995). However, following several cadaveric selective injection studies, contributions towards the intraosseous network from the branches of the posterior circumflex humeral artery (PCHA) were also acknowledged (Duparc et al., 2001; Gerber et al., 1990; Keough et al., 2019). The course, pattern and distribution of both the ACHA and PCHA have been reported with several inconsistencies and lack of consensus as to course and degree of supply offered by each of these vessels to the humeral head.
2.1. Extraosseous course and supply: The ACHA
The ACHA commonly arises from the axillary artery as a single branch (Boesmueller et al., 2014; Chen et al., 2014; Duparc et al., 2001; Fontes et al., 2015; Hettrich et al., 2010; Kasai et al., 1984; Keough et al., 2019; Meyer et al., 2005). However, several studies have highlighted variations. Common variations include an origin from a common trunk together with the PCHA (Boesmueller et al., 2014; Chen et al., 2014; Duparc et al., 2001; Fontes et al., 2015; Hettrich et al., 2010; Kasai et al., 1984; Keough et al., 2019; Meyer et al., 2005) or as a branch of the subscapular artery (Fontes et al., 2015; Keough et al., 2019). Fontes et al., (2015) observed one case where the ACHA stemmed from the profunda brachii artery. The ACHA then continues laterally, in a relatively horizontal course, deep to coracobrachialis and the short head of biceps (Kasai et al., 1984; Menck et al., 1997) towards the long head of biceps tendon located in the intertubercular groove (Figure 1). Before reaching the groove, several branches have been noted across the literature to enter and possibly supply the following structures:
The inferior and lateral parts of the glenohumeral capsule (Andary & Petersen, 2002; Duparc et al., 2001; Kasai et al., 1984) (Figure 1, branch 1)
The subscapularis muscle/tendon (de la Garza et al., 1992, Duparc et al., 2001; Figure 1, branch 1, termed subscapularis branch by the authors).
The lower part of the lesser tubercle (Duparc et al., 2001; Menck et al., 1997; Meyer et al., 2005; Figure 1, branch 1 possibly).
The tendon of latissimus dorsi and the lower end of the long head of biceps tendon (Figure 1, branch 2), termed medial descending branch by the authors (Keough, unpublished results).
Both coracobrachialis and biceps (Kasai et al., 1984; removed in Figure 1, when reflecting muscles attached to coracoid process).
A small branch (medial ascending branch) entering the medial edge of the intertubercular groove (Duparc et al., 2001; Figure 1, branch 3).
A branch coursing inferiorly from the ACHA towards the intertubercular grove and within it (Figure 1, branch 4, termed branch in the intertubercular groove by the authors (Keough, unpublished results).
FIGURE 1.
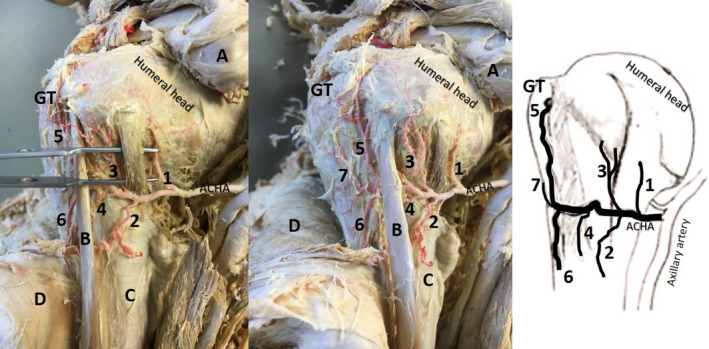
Anterior view of same left proximal shoulder (second image: slightly rotated) with exposure of the branches of the anterior circumflex humeral artery (ACHA; ethics approval: University of Pretoria 70/2017)—most common course, red latex injected. A: reflected pectoralis minor; coracobrachialis and short head of the biceps brachii; B: long head of the biceps brachii tendon; C: latissimus dorsi tendon; D: reflected deltoid; GT: greater tubercle; Pre‐tubercular branches—1: branch that supplies inferior glenohumeral capsule, subscapularis and possible lower part of lesser tubercle; 2: medial descending branch (latissimus dorsi tendon and lower long head of biceps tendon); 3: medial ascending branch (enters medial edge of intertubercular groove); 4: branch in intertubercular groove. Post‐tubercular branches—5: anterolateral (lateral ascending) branch; 6: (lateral) descending branch; 7: possible “transverse branch”
Based on the descriptions found, both Duparc et al., (2001) and de la Garza et al., (1992) refer to a “medial ascending branch”; however, they do not seem to be referring to the same vessel according to location and supply. Whereas Duparc et al., (2001) are referring to branch 3 (Figure 1), de la Garza et al., (1992) are referring to the subscapularis branch (Figure 1, branch 1) as the “medial ascending branch.”
Following the branches exiting before the intertubercular groove, most publications agree that the ACHA continues within the intertubercular groove by passing deep to the long head of biceps tendon (Gerber et al., 1996; Kasai et al., 1984; Menck et al., 1997; Meyer et al., 2005). Here, according to multiple authors, it can give off up to four branches, namely, as follows:
An anterolateral branch that courses superiorly along the lateral edge of the intertubercular groove and continues as the arcuate artery, when it enters the bone (Brooks et al., 1993; Duparc et al., 2001; Gerber et al., 1996; Hettrich et al., 2010; Kasai et al., 1984; Keough et al., 2019; Menck et al., 1997; Meyer et al., 2005; Figure 1, branch 5). Moseley and Goldie (1963) mention that before entering the humerus, the anterolateral branch gives off a small branch that runs superiorly to the rotator cuff (not labelled in Figure 1). Supply to the greater tubercle, supraspinatus and the distal part of infraspinatus have also been mentioned by Ling et al., (1990).
A descending branch (Kasai et al., 1984; Keough et al., 2019; Figure 1, branch 6).
A transverse branch (de la Garza et al., 1992; Duparc et al., 2001; Kasai et al., 1984; Figure 1, possibly branch 7).
A muscular branch to the deltoid (Kasai et al., 1984; removed in Figure 1 with the reflection of the deltoid); this branch has however also been reported as inconsistent (Hue et al., 1998).
The term anterolateral branch (branch 5 in Figure 1) is used by majority of authors (Duparc et al., 2001; Gerber et al., 1990; Keough et al., 2019; Laing, 1956). De la Garza et al., (1992), using non‐standard terminology, describe the ACHA as giving off two specific branches in this region: the transverse branch (ramus transversus), which refers to the anterolateral branch, and the lateral ascending branch (ramus ascendens lateralis), which forms an anastomosis with branches of the suprascapular artery; however, the manner and location of this anastomosis are not clearly defined. Duparc et al., (2001) also mention a lateral ascending branch, which is described as following the lateral edge of the intertubercular groove and which therefore corresponds to the anterolateral branch. Thus, the “lateral ascending branch” mentioned by de la Garza et al., (1992) does not appear to be referring to the same artery as the one described by Duparc et al., (2001). De la Garza et al., (1992) mention that the anterolateral branch (transverse branch in his terminology) also supplies the tendons of pectoralis major and the long head of biceps tendon. Other authors (Gardner et al., 2006; Kolts et al., 1994; Ling et al., 1990; Sarris et al., 2004) refer to the anterolateral branch as the “ascending branch/es” of the ACHA.
In addition, some authors have described anastomoses between these vessels. Gerber et al., (1990) mention that the anterolateral branch of the ACHA, before entering the proximal humerus, forms an anastomosis with the thoracoacromial artery. It has also been reported that branches of the PCHA may communicate dorsally with the ACHA (de la Garza et al., 1992; Duparc et al., 2001; Gerber et al., 1990). However, these anastomotic branches were not observed in other studies (Kasai et al., 1984; Keough et al., 2019; Meyer et al., 2005).
2.2. Variations in the branching pattern of the ACHA
Recently, seven variation patterns have been identified with regard to the ACHA's course towards and within the intertubercular groove (Figure 2), specifically its origin, branches and its relation to the long head of biceps tendon (Keough et al., 2019). Out of 50 cadaveric shoulders investigated, only 18 (36%) demonstrated the commonly “accepted” course and branching pattern of the ACHA as described above and seen in Figure 1. The remaining samples demonstrated distinct variations with regard to the location where the anterolateral and descending branches are exiting the ACHA (42%; n = 21/50; Figure 2: I). More importantly, in 22% (n = 11/50) of the samples, the anterolateral branch passed anterior to the long head of the biceps tendon (Figure 2: V–VII) and not deep to the tendon as generally described. To the authors’ knowledge, these variations have not been mentioned or studied before, and these variations may be of clinical importance in procedures that involve the long head of biceps tendon, such as tenotomies and tenodesis surgeries. The only other reference to a deviation from the acknowledged course (Kasai et al., 1984) pointed out that the ACHA was poorly developed in seven of the cases studied, to the extent that it terminated by giving off the articular branch without passing deep to biceps tendon. It may be of interest to simulate injuries or surgical interventions pertaining to the long head of the biceps tendon and to evaluate possible vascular injuries resulting from these procedures.
FIGURE 2.
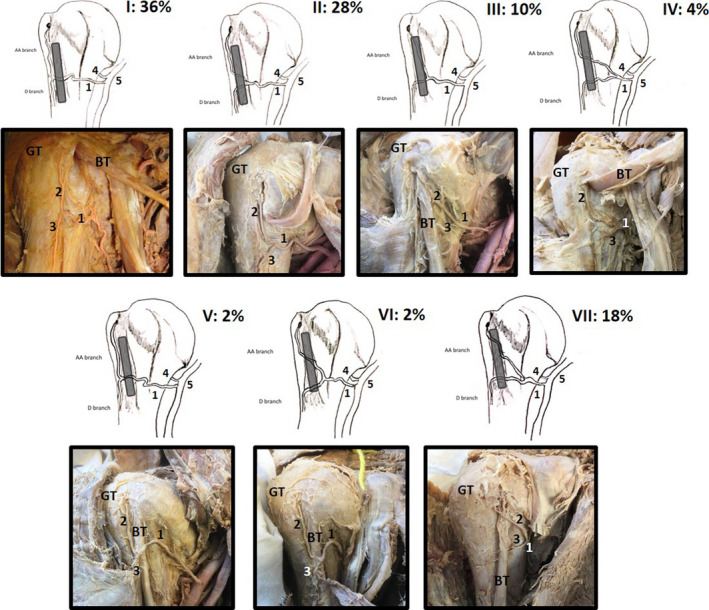
Illustrations of the anterior proximal humerus demonstrating the 7 (I–VIII) identified variations (ethics: University of Pretoria 70/2019) of the ACHA (1), its anterolateral branch (AA branch, 2) and its descending branch (D branch, 3) in relation to the long head of the biceps tendon (BT; shaded in grey). Variations I–IV illustrate the ACHA and branches lying deep to the BT; in variations V and VI, the ACHA and its branches lie anterior. Variation VII shows the anterolateral branch (2) lying anterior to the BT while the descending branch (3) lies deep to the BT (GT: greater tubercle; 4: posterior circumflex humeral artery; 5: axillary artery). All percentages represent the occurrence of these variations in a sample of 50 shoulders
Regardless of the existing variations, the one constant concerning the anterolateral branch is that it enters the proximal humerus just inferior to the transverse humeral ligament along the lateral border of the intertubercular groove and that it supplies most of the epiphysis as the arcuate artery, the largest intraosseous branch (Brooks et al., 1993; de la Garza et al., 1992; Duparc et al., 2001; Gerber et al., 1996; Keough et al., 2019; Laing, 1956; Menck et al., 1997).
2.3. Extraosseous course and supply: The PCHA
The PCHA most commonly originates as a single branch from the axillary artery (Chen et al., 2014; Duparc et al., 2001; Fontes et al., 2015; Keough et al., 2019; Menck et al., 1997) and less commonly from a common trunk shared with the ACHA, or collectively from the subscapular artery (Chen et al., 2014; Fontes et al., 2015; Kasai et al., 1984; Keough et al., 2019; Menck et al., 1997; Meyer et al., 2005). Some less common origins have also been reported, such as from the profunda brachii artery (Chen et al., 2014; Duparc et al., 2001; Fontes et al., 2015; Kasai et al., 1984), the lateral thoracic artery (Meyer et al., 2005) or from the brachial artery (Meyer et al., 2005). Together with the axillary nerve, the PCHA travels through the quadrangular space towards the posterior shoulder. Here, it has been described as having several distribution branches:
Direct branches to the inferior and posterior parts of the lateral glenohumeral joint capsule (Andary & Petersen, 2002).
A branch supplying the ventral and dorsal aspects of the shoulder joint synovial membrane (de la Garza et al., 1992). De la Garza et al., (1992) mention that at this point, the PCHA contributes towards the supply of the head together with the arcuate artery. However, the manner in which this connection is formed is not provided by the authors.
Branches perfusing the bone cartilage border similar to that observed in the femoral neck (Meyer et al., 2005).
Branches to supply the posterior aspect of the greater tubercle (Gerber et al., 1990; Keough et al., 2019; Meyer et al., 2005).
The PCHA splits into two/three branches that supply the posterior and lateral parts of the deltoid (Hue et al., 1998), as well as a branch to the upper humeral epiphysis (Duparc et al., 2001). Duparc et al., (2001) further elaborate that the branch to the upper humeral epiphysis divides into several smaller vessels penetrating the inferior, posterior and superior parts of the capsule, or the posterior and superior parts of the humeral neck and the greater tubercle. A few branches are also noted as supplying the supraspinatus, infraspinatus and teres minor tendons.
Anatomy, course and distribution pattern of the PCHA have not been studied in great detail. Specifically, concerning the origin of its branches, their names and their supply areas, there are numerous inconsistencies. Variations have also not yet been studied. It is clear from the literature presented that this area requires in‐depth investigation into both the anatomy and the potential of vascular damage.
2.4. Intraosseous supply of the humeral head
Limited studies have investigated the intraosseous blood supply of the humeral head, as it is a difficult area to infiltrate and visualize (Duparc et al., 2001; Hettrich et al., 2010; Keough et al., 2019; Moseley & Goldie, 1963). Moseley and Goldie (1963) used Schlesinger's (Schlesinger, 1957; Susheela et al., 2018) solution to inject into selected blood vessels around the shoulder to investigate the blood supply of the osseous, tendinous and muscular components of the RC. The authors mention that the arcuate artery branches course to the articular cartilage and to the zone of tendinous insertion (greater tubercle). Duparc et al., (2001) injected a series of blood vessels with barium sulphate solution and confirmed that the vascularization of the humeral head is attributed to the branches of the arcuate artery. This was again confirmed by Keough et al., (2019), who also injected a series of ACHA and PCHA vessels with a barium sulphate/gelatin solution. The arcuate artery enters the bone and forms an intraosseous anastomosis with the penetrating branches of the PCHA in the region of the greater tubercle (Duparc et al., 2001; Keough et al., 2019). These vessels then course medially, where they are distributed through the cancellous bone in the humeral head. Duparc et al., (2001) demonstrated that the PCHA infiltrated the majority of the surgical neck, the greater tubercle and humeral head (top, centre and subchondral parts) while the ACHA infiltrated the majority of the lesser tubercle and the intertubercular groove. This was also confirmed by Hettrich et al., (2010) who stated that the ACHA only contributed 34% of the blood supply to the humeral head whereas the PCHA is noted to contribute 64%. The authors further demonstrated that the ACHA mainly supplied a quadrant on the inferior aspect of the humeral head (termed medial quadrant) while the PCHA supplied the lateral, superior and inferior quadrants of the humeral head. However, Keough et al., (2019) observed a more even distribution from both the PCHA and ACHA for the humeral head. Additionally, these authors (Keough et al., 2019) noted a small area on the superomedial aspect of the humeral head that did not perfuse readily with the infused solution, leading to the assumption that this may be a poorly vascularized zone. Of note, this zone coincides with the outlined superior quadrant (Hettrich et al., 2010) and with the initial manifestation site of osteonecrosis (Figure 3).
FIGURE 3.
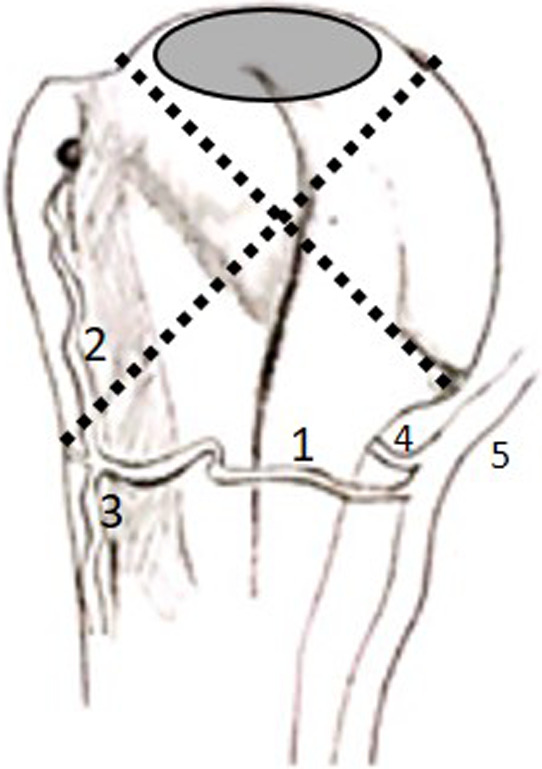
Illustration of the anterior view of the proximal humerus demonstrating the quadrants (dotted lines) outlined by Hettrich et al., (2010) and the poorly vascularized zone (grey oval) identified by Keough et al., (2019) (1: anterior circumflex humeral artery; 2: anterolateral branch; 3: descending branch; 4: posterior circumflex humeral artery; 5: axillary artery)
3. ROTATOR CUFF REPAIR SURGERY AND AVASCULAR NECROSIS
Few complications have been reported following arthroscopic RC repair using suture anchors. However, there are currently 21 cases mentioned throughout the literature that describe humeral head osteonecrosis as a direct result or directly following this surgical intervention. Whereas osteonecrosis generally occurs more commonly in males between the ages of 20 and 50 years, those cases reported in the literature of osteonecrosis following RC repair surgery are majority women over the age of 60 (Table 1), a clear deviation from the generalized incidence reported. Table 1 summarizes the current literature reporting on humeral head osteonecrosis following RC repair surgery.
TABLE 1.
Summary of cases of reported osteonecrosis of the humeral head following arthroscopic rotator cuff repair surgery
| Authors | Sample size | Sex | Age | Medical history | Side | ON cases | Sex ON cases | Time development | Biceps tenodesis/tenotomy |
|---|---|---|---|---|---|---|---|---|---|
| Magee et al., 1997 | 50 | 34 male; 16 female | 21–73 | Rotator cuff repair in 41 (9 open repair; 32 arthroscopic repair); Arthroscopic debridement in 7; open acromioplasty in 2 | Not reported | 4 | Not reported | Not reported | Not reported |
| Hattrup & Cofield, 2000 | 114 | 26 male; 88 female | 58.8 average | Rotator cuff repair | Not reported | 3 | Not reported | Not reported | Not reported |
| Beauthier et al., 2010 | 1 | Female | 67 | Arthroscopic rotator cuff repair (3 anchors) | Right | 1 | Female | 8 months | Yes (tenotomy) |
| Dilisio et al., 2013 | 3 | Female | 60–68 | Arthroscopic rotator cuff repair (1) or debridement (2) | 1 Right; 2 not reported | 3 | Female | 8 months | Yes (tenotomy) |
| Goto et al., 2015 | 1 | Female | 69 | Arthroscopic rotator cuff repair (2 anchors) | Right | 1 | Female | 3–6 months | Ruptured biceps tendon |
| Cho et al., 2015 | 1 | Female | 66 | Arthroscopic rotator cuff repair (4 anchors) | Right | 1 | Female | 7 months | Not reported |
| Kim et al., 2018 | 8 | Female | 52–74 | Arthroscopic rotator cuff repair (2–7 range) | Right | 8 | Female | Mean: 4 months | Yes (tenotomy in 7) |
To our knowledge, the first authors to report cases of osteonecrosis linked to postoperative shoulder surgery were Magee et al., (1997). They examined 50 patients that had undergone RC repair surgery (n = 41—9 open and 32 arthroscopic), arthroscopic RC debridement (n = 7) and open acromioplasty (n = 2) who presented with persistent postoperative complaints. Of the 50 patients, four were identified as having humeral head osteonecrosis. Not many details are provided, as osteonecrosis was not the focus of the main project and was only mentioned as a side note. Hattrup and Cofield (2000) examined 127 patients that were treated with replacement arthroplasty for humeral head osteonecrosis. The outcomes measured in this study revolved around the results, durability and complications of shoulder replacement for osteonecrosis; differences between total shoulder replacement and humeral head replacement were examined. Similarly to Magee et al., (1997), the authors only mentioned as a side line that three of the cases had developed osteonecrosis following RC repair. Again, no details are provided, as this was not the focus of the study. Both publications (Hattrup & Cofield, 2000; Magee et al., 1997) reported on incidental findings of humeral head osteonecrosis following RC repair surgery.
The first actual mention of a direct association between RC repair surgery and osteonecrosis was made by Beauthier et al., (2010) who reported on a single case study. The case was that of a 67‐year‐old female with a RC tear of supraspinatus that was repaired arthroscopically (using three anchors) in addition to a biceps tenotomy also performed. Eight months after surgery, the patient presented with humeral head osteonecrosis as well as cuff re‐rupture. The authors suggest that the osteonecrosis in this case may be attributed to the use of multiple anchors that may have interrupted the blood supply of the humeral head and recommend that surgeons be aware and careful in anchor localization during arthroscopic repair. It has been previously suggested that mechanical disruption of the humeral head blood supply may be a cause of idiopathic osteonecrosis (Mankin, 1992). Beauthier et al., (2010) paper is the first to report this link to surgical anchor placement in the greater tubercle. Another possible disruption site for the ACHA and its branches is not mentioned: it may be at the level of the biceps tendon due to the biceps tenotomy performed, as is demonstrated by the vascular variations presented by Keough et al., (2019).
The second paper to report on osteonecrosis following RC surgery was Dillisio et al. (2013). The authors described three female patients aged 60–68 years that presented with osteonecrosis following arthroscopic RC debridement (n = 2) and repair (n = 1) surgery. Again, as seen in Beauthier et al., (2010) patient, in all cases, a concurrent biceps tenotomy was performed in addition to subacromial decompression, acromioplasty and distal clavicle excision. These patients presented with osteonecrosis on average 4.8 months’ post‐surgery. The authors compare their study to Beauthier et al., and and's (2010) and make an interesting comment. Two of the three patients did not have any hardware placed during surgery, thereby suggesting that the osteonecrosis is not necessarily directly linked to aberrant anchor placement alone. The authors were unable to identify a common precipitating cause of postarthroscopic osteonecrosis but do assume that there was some kind of perioperative insult that led to the disruption of humeral head blood supply. Interestingly, in all three cases, a biceps tenotomy was performed which supports the suggestion by Keough et al., (2019) that there may be a link to vascular damage at the level of the biceps tendon.
The next two papers, both in 2015, were again single case reports of osteonecrosis following arthroscopic RC repair surgery (Cho et al., 2015; Goto et al., 2015). Both cases were females (66 and 69 years old) who had, prior to surgery, fallen from a height and injured their shoulders. Goto et al., (2015) patient underwent surgery for a massive RC tear. Only two anchors were used to repair a subscapularis and infraspinatus tear. Six months following surgery, there was rapid collapse of the humeral head due to osteonecrosis. The authors reiterated that extreme care was taken to preserve the blood vessels and that the suture anchors were all placed favourably in this case. Therefore, the possible cause for osteonecrosis is attributed to the use of a metal anchor—this is the first time that the material of the anchor used has been suggested as a possible cause. The patient presented by Cho et al., (2015) developed osteonecrosis of the humeral head 7 months following arthroscopic surgery. The patient had, prior to surgery, fallen from a 1 m height and was diagnosed with an anterior shoulder dislocation accompanied by a bony Bankart lesion and a RC tear. Following the incident, the patient underwent arthroscopic Bankart and double row RC repair. A total of seven bioabsorbable suture anchors were used (three for the Bankart lesion and four for the rotator cuff tear). The authors are the first who do not fully attribute the osteonecrosis to anchor usage but instead suggest that the blood vessels may have been damaged due to prior shoulder dislocation, adding to the risk factors for development. Cho et al., (2015) recommend a more thorough and frequent radiographic follow‐up, especially in patients presenting with progressive pain and reduced range of motion. These follow‐up recommendations should especially be carried out in potential high‐risk cases such as females older than 60 years.
The most recent report on humeral head osteonecrosis following arthroscopic RC repair was by Kim et al., (2018). These authors, now being aware of the potential association of osteonecrosis and arthroscopic RC repair, investigated 24 patients from 12 institutions that had been suspected of developing humeral head osteonecrosis following arthroscopic RC repair. Only eight of the patients did not demonstrate any evidence of osteonecrosis prior to surgery and were therefore included in the outcome report. All eight patients were again female with an average age of 64 years (52–74 years range). They reported that pain developed at an average of 4 months following surgery and osteonecrosis diagnosis of all cases occurred within 12 months after surgery. One patient experienced an anterior shoulder dislocation prior to surgery, and one patient had previously undergone RC repair a year before the second index surgery. In seven of the eight patients, a biceps tenotomy was performed concurrently and the number of anchors used averaged 4.14 (2–7 range), and none of them were metal.
4. DISCUSSION
The major concern raised by the variation of the ACHA and its branches is that these vessels, specifically the anterolateral branch and its arcuate derivative, may be in danger in multiple surgical procedures, such as during surgical plating of the proximal humerus for fracture repairs, while performing a biceps tenodesis/tenotomy (suggested by Keough et al., 2019), or during placement of suture anchors for RC repair (Figure 4), the potential consequence being postoperative osteonecrosis of the humeral head.
FIGURE 4.
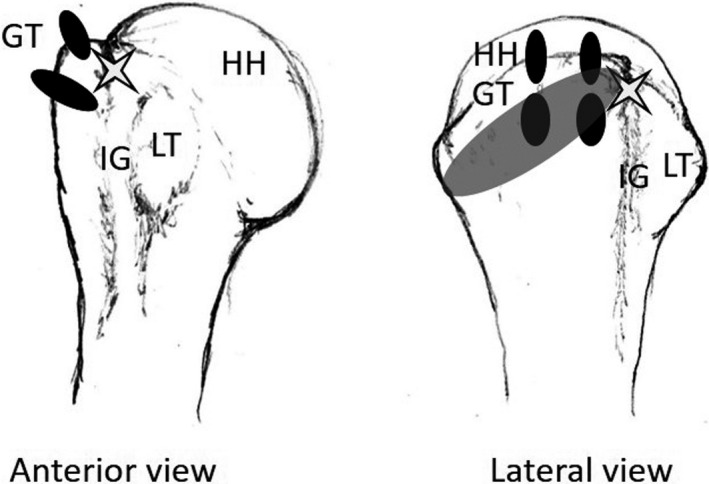
Illustration of the anterior and lateral view of the proximal humerus demonstrating the common position for double‐row suture anchor placement (black ovals) during rotator cuff repair surgery. White star, entry point for anterolateral branch of ACHA; shaded grey oval, entry point for penetrating branches of posterior circumflex humeral artery. GT, greater tubercle; HH, humeral head; IG, intertubercular groove; LT, lesser tubercle
Gerber et al., (1990) are clear on the fact that no other vessels, other than the branches of the ACHA and PCHA, enter the humeral head directly. Although inconsistent information exists in the literature concerning the vascularization of the humeral head (Duparc et al., 2001; Gerber et al., 1990; Keough et al., 2019; Laing, 1956; Moseley & Goldie, 1963; Rothman & Parke, 1965), the overall current consensus is that the humeral head is supplied via an anastomosing intraosseous network including a major contribution from the arcuate artery (from the ACHA). What is also clear is that this definitive intraosseous network receives contributions from the penetrating branches of the PCHA as well, which is evidenced by the fact that the ACHA must often be ligated medial to the bicipital groove during surgical procedures in this region, and this ligation does not necessarily result in osteonecrosis (Rowe et al., 1978).
The intraosseous network forms in the vicinity of the greater tubercle, thereby rendering it vulnerable during suture anchor, or even surgical plate, placement. The most common tendon repaired during RC surgery is the supraspinatus tendon. This tendon has a footprint area over what has been described as the most anterior portion of the greater tubercle. This surface is in close relation to the entrance site of the arcuate artery and directly overlies the area where the arcuate artery and the penetrating branches of the PCHA form the intricate intraosseous network that will eventually distribute blood to the humeral head by coursing in a medial direction and congregating in the lower two thirds of the humeral head (Keough et al., 2019; Figure 3).
The aetiology and pathogenesis of humeral head osteonecrosis following RC repair surgery are currently unknown and under‐investigated, with 21 known cases being reported in the literature since 1997. Several authors have suggested that there may be perioperative injury to either the ACHA or the anterolateral branch of the ACHA by poor suture anchor placement or by the use of multiple anchors (Cho et al., 2015; Goto et al., 2015). However, Cho et al., (2015) make a valid point in saying that proximal humeral fractures account for the majority of cases associated with damage of the ACHA and osteonecrosis following these fractures is not very common. This leads to the assumption that damage of the ACHA by suture anchors cannot be the sole cause of humeral head osteonecrosis. Based on the varying course and relationship of the ACHA and its branches to the long head of biceps tendon, Keough et al., (2019) suggest that there may be additional damage to these vessels at the level of the biceps tendon; during concurrent tenotomy or biceps tenodesis procedures performed. Therefore, it is possible that multiple factors are at play with the development of postarthroscopic humeral head osteonecrosis, including either damage to the anterolateral branch at the level of the biceps tendon or damage to the intraosseous network in the greater tubercle, or both concurrently.
Additionally, the superomedial aspect of the humeral head has also been noted to be almost void of large intraosseous blood vessels and may receive its supply via a more indirect or diffusive route (Keough et al., 2019). This also suggests that damage to the main intraosseous network may compromise the blood flow to this already high risk zone (Keough et al., 2019), which corresponds to the initial starting location of osteonecrosis (Figure 3). This poorly vascularized area may be more susceptible to perioperative damage to the intraosseous network and/or damage to the ACHA and its anterolateral branch, which as described above is fairly variable in origin, course and branching pattern. Although the study did not provide evidence that due to the reduced intraosseous supply to this high risk area is directly linked to osteonecrosis, it was noted that this area is almost always the initial site for development of osteonecrosis.
5. CONCLUSION
This review illustrates that blood supply of the humeral head is complex and variable and humeral head osteonecrosis, although not common, is a potential complication of RC repair. Vascular injury plays an important role in the pathogenesis. During surgical procedures, special care should be taken not to injure the ACHA and its anterolateral branch, which is located close to the long head of biceps tendon. Variations of the ACHA and its branches should be taken into account during surgical repair of the RC. One way to reduce the risk of vascular injury has been suggested by Cho et al., (2015), that is, to increase routine radiographs post‐operatively, especially in high risk cases, in order to detect vascular damage at a very early stage. This would include women over 60 years of age with dominant hand involvement and surgical interventions requiring additional procedures performed at the shoulder during typical RC repair (e.g., biceps tenotomy/tenodesis, acromioplasty and debridement). Ultimately, knowledge of vascular variations is a crucial element for surgeons to be aware of and should form a firm part of the surgical planning process in order to reduce postoperative complications.
CONFLICT OF INTEREST
No conflict of interest has been declared by the authors.
AUTHOR CONTRIBUTIONS
Conceptualization, methodology, and data curation was conducted by the corresponding author, Natalie Keough. Preparing and writing the origical draft presentation, reviewing and editing the manuscript, and final proof reading and visualisation was conducted by both authors, Natalie Keough and Dietrich Lorke. All authors have read and agreed to the published version of the manuscript.
AKNOWLEDGEMENTS
This publication was supported by the National Research Foundation (No. NRF‐N01515‐120410).
Keough, N. & Lorke, D.E. (2021) The humeral head: A review of the blood supply and possible link to osteonecrosis following rotator cuff repair. Journal of Anatomy, 239, 973–982. 10.1111/joa.13496
REFERENCES
- Andary, J.L. & Petersen, S.A. (2002) The vascular anatomy of the glenohumeral capsule and ligaments: an anatomic study. Journal of Bone and Joint Surgery. American Volume, 84, 2258–2265. 10.2106/00004623-200212000-00020 [DOI] [PubMed] [Google Scholar]
- Beauthier, V. , Sanghavi, S. , Roulot, E. & Hardy, P. (2010) Humeral head osteonecrosis following arthroscopic rotator cuff repair. Knee Surgery, Sports Traumatology, Arthroscopy, 18, 1432–1434. 10.1007/s00167-009-1016-5 [DOI] [PubMed] [Google Scholar]
- Boesmueller, S. , Fialka, C. & Pretterklieber, M.L. (2014) The arterial supply of the tendon of the long head of the biceps brachii in the human: a combined anatomical and radiological study. Ann Anat, 196, 449–455. 10.1016/j.aanat.2014.08.006 [DOI] [PubMed] [Google Scholar]
- Brooks, C.H. , Revell, W.J. & Heatley, F.W. (1993) Vascularity of the humeral head after proximal humeral fractures. An anatomical cadaver study. Journal of Bone and Joint Surgery. British Volume, 75, 132–136. 10.1302/0301-620X.75B1.8421010 [DOI] [PubMed] [Google Scholar]
- Chen, Y.‐X. , Zhu, Y.i. , Wu, F.‐H. , Zheng, X. , Wangyang, Y.‐F. , Yuan, H. et al. (2014) Anatomical study of simple landmarks for guiding the quick access to humeral circumflex arteries. BMC Surgery, 14, 39. 10.1186/1471-2482-14-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, H.I.K. , Cho, H.L. , Hwang, T.H. , Wang, T.H. & Cho, H. (2015) Rapidly progressive osteonecrosis of the humeral head after arthroscopic bankart and rotator cuff repair in a 66‐year old woman: a case report. Clinics in Shoulder and Elbow, 18, 167–171. 10.5397/cise.2015.18.3.167 [DOI] [Google Scholar]
- Chryssanthou, C.P. (1978) Dysbaric osteonecrosis. Etiological and pathogenetic concepts. Clinical Orthopaedics and Related Research, 94–106. 10.1097/00003086-197801000-00009 [DOI] [PubMed] [Google Scholar]
- Cofield, R.H. (1994) Osteonecrosis. In: Friedman, R.J. (Ed.) Arthroplasty of the Shoulder. New York: Thieme Medical Publishers, pp. 170–182. [Google Scholar]
- Cruess, R.L. (1976) Steroid‐induced avascular necrosis of the head of the humerus. Natural history and management. Journal of Bone and Joint Surgery. British Volume, 58, 313–317. 10.1302/0301-620X.58B3.956247 [DOI] [PubMed] [Google Scholar]
- Cruess, R.L. (1978) Experience with steroid‐induced avascular necrosis of the shoulder and etiologic considerations regarding osteonecrosis of the hip. Clinical Orthopaedics and Related Research, 86–93. 10.1097/00003086-197801000-00008 [DOI] [PubMed] [Google Scholar]
- Cruess, R.L. (1981) Steroid‐induced osteonecrosis: a review. Canadian Journal of Surgery, 24, 567–571. [PubMed] [Google Scholar]
- Cushner, M.A. & Friedman, R.J. (1997) Osteonecrosis of the humeral head. Journal of American Academy of Orthopaedic Surgeons, 5, 339–346. 10.5435/00124635-199711000-00006 [DOI] [PubMed] [Google Scholar]
- David, H.G. , Bridgman, S.A. , Davies, S.C. , Hine, A.L. & Emery, R.J. (1993) The shoulder in sickle‐cell disease. Journal of Bone and Joint Surgery. British Volume, 75, 538–545. 10.1302/0301-620X.75B4.8331106 [DOI] [PubMed] [Google Scholar]
- de la Garza, O. , Lierse, W. & Steiner, D. (1992) Anatomical study of the blood supply in the human shoulder region. Acta Anat (Basel), 145, 412–415. [DOI] [PubMed] [Google Scholar]
- Desforges, J.F. & Mankin, H.J. (1992) Nontraumatic necrosis of bone (osteonecrosis). New England Journal of Medicine, 326, 1473–1479. 10.1056/NEJM199205283262206 [DOI] [PubMed] [Google Scholar]
- Dilisio, M.F. , Noble, J.S. , Bell, R.H. & Noel, C.R. (2013) Postarthroscopic humeral head osteonecrosis treated with reverse total shoulder arthroplasty. Orthopedics, 36, e377–e380. 10.3928/01477447-20130222-30 [DOI] [PubMed] [Google Scholar]
- Duparc, F. , Muller, J.M. & Freger, P. (2001) Arterial blood supply of the proximal humeral epiphysis. Surgical and Radiologic Anatomy, 23, 185–190. 10.1007/s00276-001-0185-9 [DOI] [PubMed] [Google Scholar]
- Fisher, D.E. , Bickel, W.H. , Holley, K.E. & Ellefson, R.D. (1972) Corticosteroid‐induced aseptic necrosis. II. Experimental study. Clinical Orthopaedics and Related Research, 84, 200–206. 10.1097/00003086-197205000-00033 [DOI] [PubMed] [Google Scholar]
- Fontes, E.B. , Precht, B.L.C. , Andrade, R.C.L. , Fernandes, R.M.P. & Cisne, R. (2015) Output relations of humeral circumflex arteries and its variations. International Journal of Morphology, 33, 1171–1175. 10.4067/S0717-95022015000300058 [DOI] [Google Scholar]
- Gardner, M.J. , Voos, J.E. , Wanich, T. , Helfet, D.L. & Lorich, D.G. (2006) Vascular implications of minimally invasive plating of proximal humerus fractures. Journal of Orthopaedic Trauma, 20, 602–607. 10.1097/01.bot.0000246412.10176.14 [DOI] [PubMed] [Google Scholar]
- Gerber, C. , Lambert, S.M. & Hoogewoud, H.M. (1996) Absence of avascular necrosis of the humeral head after post‐traumatic rupture of the anterior and posterior humeral circumflex arteries. A case report. Journal of Bone and Joint Surgery. American Volume, 78, 1256–1259. 10.2106/00004623-199608000-00018 [DOI] [PubMed] [Google Scholar]
- Gerber, C. , Schneeberger, A.G. & Vinh, T.S. (1990) The arterial vascularization of the humeral head. An anatomical study. Journal of Bone and Joint Surgery. American Volume, 72, 1486–1494. 10.2106/00004623-199072100-00009 [DOI] [PubMed] [Google Scholar]
- Goldblatt, J. , Sacks, S. , Dall, D. & Beighton, P. (1988) Total hip arthroplasty in Gaucher's disease. Long‐term prognosis. Clinical Orthopaedics and Related Research., 94–98. [PubMed] [Google Scholar]
- Goto, M. , Gotoh, M. , Mitsui, Y. , Okawa, T. , Higuchi, F. & Nagata, K. (2015) Rapid collapse of the humeral head after arthroscopic rotator cuff repair. Knee Surgery, Sports Traumatology, Arthroscopy, 23, 514–516. 10.1007/s00167-013-2790-7 [DOI] [PubMed] [Google Scholar]
- Harreld, K.L. , Marker, D.R. , Wiesler, E.R. , Shafiq, B. & Mont, M.A. (2009) Osteonecrosis of the humeral head. Journal of American Academy of Orthopaedic Surgeons, 17, 345–355. 10.5435/00124635-200906000-00003 [DOI] [PubMed] [Google Scholar]
- Hasan, S.S. & Romeo, A.A. (2002) Nontraumatic osteonecrosis of the humeral head. Journal of Shoulder and Elbow Surgery, 11, 281–298. 10.1067/mse.2002.124347 [DOI] [PubMed] [Google Scholar]
- Hattrup, S.J. & Cofield, R.H. (2000) Osteonecrosis of the humeral head: results of replacement. Journal of Shoulder and Elbow Surgery, 9, 177–182. 10.1067/mse.2000.105126 [DOI] [PubMed] [Google Scholar]
- Hayes, J.M. (1989) Arthroscopic treatment of steroid‐induced osteonecrosis of the humeral head. Arthroscopy, 5, 218–221. 10.1016/0749-8063(89)90175-8 [DOI] [PubMed] [Google Scholar]
- Hettrich, C.M. , Boraiah, S. , Dyke, J.P. , Neviaser, A. , Helfet, D.L. & Lorich, D.G. (2010) Quantitative assessment of the vascularity of the proximal part of the humerus. Journal of Bone and Joint Surgery. American Volume, 92, 943–948. 10.2106/JBJS.H.01144 [DOI] [PubMed] [Google Scholar]
- Hue, E. , Gagey, O. , Mestdagh, H. , Fontaine, C. , Drizenko, A. & Maynou, C. (1998) The blood supply of the deltoid muscle. Application to the deltoid flap technique. Surgical and Radiologic Anatomy, 20, 161–165. [PubMed] [Google Scholar]
- Jacobs, B. (1992) Alcoholism‐induced bone necrosis. New York State Journal of Medicine, 92, 334–338. [PubMed] [Google Scholar]
- Jones, J.P. Jr , Ramirez, S. & Doty, S.B. (1993) The pathophysiologic role of fat in dysbaric osteonecrosis. Clinical Orthopaedics and Related Research, 256–264. 10.1097/00003086-199311000-00042 [DOI] [PubMed] [Google Scholar]
- Kasai, T. , Suzuki, T. , Fukushi, T. , Kodama, M. & Chiba, S. (1984) Two circumflex humeral arteries in the arm, with special reference to their communications. Okajimas Folia Anatomica Japonica, 61, 347–353. 10.2535/ofaj1936.61.5_347 [DOI] [PubMed] [Google Scholar]
- Keough, N. , de Beer, T. , Uys, A. & Hohmann, E. (2019) An anatomical investigation into the blood supply of the proximal humerus: surgical considerations for rotator cuff repair. JSES Open Access, 3, 320–327. 10.1016/j.jses.2019.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J.K. , Jeong, H.J. , Shin, S.‐J. , Yoo, J.C. , Rhie, T.‐Y. , Park, K.‐J. et al. (2018) Rapid progressive osteonecrosis of the humeral head after arthroscopic rotator cuff Surgery. Arthroscopy, 34, 41–47. 10.1016/j.arthro.2017.06.051 [DOI] [PubMed] [Google Scholar]
- Kolts, I. , Tillmann, B. & Lullmann‐Rauch, R. (1994) The structure and vascularization of the biceps brachii long head tendon. Annals of Anatomy ‐ Anatomischer Anzeiger, 176, 75–80. 10.1016/S0940-9602(11)80420-6 [DOI] [PubMed] [Google Scholar]
- Kumar, V. , White, A.D. & Venkateswaran, B. (2010) Atraumatic osteonecrosis of the humeral head associated with pregnancy. Shoulder and Elbow, 2, 188–190. 10.1111/j.1758-5740.2010.00074.x [DOI] [Google Scholar]
- Laing, P.G. (1956) The arterial supply of the adult humerus. Journal of Bone and Joint Surgery. American Volume, 38‐A, 1105–1116. 10.2106/00004623-195638050-00013 [DOI] [PubMed] [Google Scholar]
- Lebel, E. , Phillips, M. , Elstein, D. , Zimran, A. & Itzchaki, M. (2009) Poor results of drilling in early stages of juxta‐articular osteonecrosis in 12 joints affected by Gaucher disease. Acta Orthopaedica, 80, 201–204. 10.3109/17453670902930032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, C.K. & Hansen, H.R. (1981) Post‐traumatic avascular necrosis of the humeral head in displaced proximal humeral fractures. Journal of Trauma, 21, 788–791. 10.1097/00005373-198109000-00006 [DOI] [PubMed] [Google Scholar]
- Ling, S.C. , Chen, C.F. & Wan, R.X. (1990) A study on the vascular supply of the supraspinatus tendon. Surgical and Radiologic Anatomy, 12, 161–165. 10.1007/BF01624517 [DOI] [PubMed] [Google Scholar]
- Magee, T.H. , Gaenslen, E.S. , Seitz, R. , Hinson, G.A. & Wetzel, L.H. (1997) MR imaging of the shoulder after surgery. AJR. American Journal of Roentgenology, 168, 925–928. 10.2214/ajr.168.4.9124141 [DOI] [PubMed] [Google Scholar]
- Mansat, P. , Huser, L. , Mansat, M. , Bellumore, Y. , Rongières, M. & Bonnevialle, P. (2005) Shoulder arthroplasty for atraumatic avascular necrosis of the humeral head: nineteen shoulders followed up for a mean of seven years. Journal of Shoulder and Elbow Surgery, 14, 114–120. 10.1016/j.jse.2004.06.019 [DOI] [PubMed] [Google Scholar]
- Matsuo, K. , Hirohata, T. , Sugioka, Y. , Ikeda, M. & Fukuda, A. (1988) Influence of alcohol intake, cigarette smoking, and occupational status on idiopathic osteonecrosis of the femoral head. Clinical Orthopaedics and Related Research, 115–123. 10.1097/00003086-198809000-00021 [DOI] [PubMed] [Google Scholar]
- Menck, J. , Döbler, A. & Döhler, J.R. (1997) Vaskularisation des Humerus. Langenbecks Archiv für Chirurgie, 382, 123–127. 10.1007/BF02498662 [DOI] [PubMed] [Google Scholar]
- Meyer, C. , Alt, V. , Hassanin, H. , Heiss, C. , Stahl, J.‐P. , Giebel, G. et al. (2005) The arteries of the humeral head and their relevance in fracture treatment. Surgical and Radiologic Anatomy, 27, 232–237. 10.1007/s00276-005-0318-7 [DOI] [PubMed] [Google Scholar]
- Milner, P.F. , Kraus, A.P. , Sebes, J.I. , Sleeper, L.A. , Dukes, K.A. , Embury, S.H. et al. (1993) Osteonecrosis of the humeral head in sickle cell disease. Clinical Orthopaedics and Related Research, 136–143. 10.1097/00003086-199304000-00018 [DOI] [PubMed] [Google Scholar]
- Moran, T.J. (1962) Cortisone‐induced alterations in lipid metabolism. Morphologic and serologic observations in rabbits. Archives of Pathology, 73, 300–312. [PubMed] [Google Scholar]
- Moseley, H.F. & Goldie, I. (1963) The arterial pattern of the rotator cuff of the shoulder. Journal of Bone and Joint Surgery. British Volume, 45, 780–789. [PubMed] [Google Scholar]
- Neer, C.S. 2nd (1972) Anterior acromioplasty for the chronic impingement syndrome in the shoulder: a preliminary report. Journal of Bone and Joint Surgery. American Volume, 54, 41–50. 10.2106/00004623-197254010-00003 [DOI] [PubMed] [Google Scholar]
- Neer, C.S. 2nd (1990) Cuff tears, biceps lesions and impingement. In Neer, C.S. , 2nd (ed) Shoulder reconstruction, pp. 41–142. Philadelphia: Saunders. [Google Scholar]
- Pavelka, K. (2000) Osteonecrosis. Baillieres Best Pract Res Clin Rheumatol, 14, 399–414. 10.1053/berh.2000.0072 [DOI] [PubMed] [Google Scholar]
- Poignard, A. , Flouzat‐Lachaniette, C.‐H. , Amzallag, J. , Galacteros, F. & Hernigou, P. (2012) The natural progression of symptomatic humeral head osteonecrosis in adults with sickle cell disease. Journal of Bone and Joint Surgery. American Volume, 94, 156–162. 10.2106/JBJS.J.00919 [DOI] [PubMed] [Google Scholar]
- Rothman, R.H. & Parke, W.W. (1965) The vascular anatomy of the rotator cuff. Clinical Orthopaedics and Related Research, 41, 176–186. [PubMed] [Google Scholar]
- Rowe, C.R. , Patel, D. & Southmayd, W.W. (1978) The Bankart procedure: a long‐term end‐result study. Journal of Bone and Joint Surgery. American Volume, 60, 1–16. 10.2106/00004623-197860010-00001 [DOI] [PubMed] [Google Scholar]
- Sarris, I. , Weiser, R. & Sotereanos, D.G. (2004) Pathogenesis and treatment of osteonecrosis of the shoulder. Orthopedic Clinics of North America, 35, 397–404. 10.1016/j.ocl.2004.03.004 [DOI] [PubMed] [Google Scholar]
- Schai, P. , Imhoff, A. & Preiss, S. (1995) Comminuted humeral head fractures: a multicenter analysis. Journal of Shoulder and Elbow Surgery, 4, 319–330. 10.1016/S1058-2746(95)80015-8 [DOI] [PubMed] [Google Scholar]
- Schlesinger, M.J. (1957) New radiopaque mass for vascular injection. Laboratory Investigation, 6, 1–11. [PubMed] [Google Scholar]
- Shakir, I. , Kim, A. & Salazar, D. (2020) Avascular necrosis of the humeral head in a patient with methylenetetrahydrofolate reductase 1 gene polymorphism: a case report. JBJS Case Connector, 10(e19), 00486. 10.2106/JBJS.CC.19.00486 [DOI] [PubMed] [Google Scholar]
- Susheela, A.T. , Saffitz, J.E. & Cohen, S.I. (2018) Monroe J. Schlesinger's radiographic visualization of the coronary arteries in postmortem hearts and its clinical applications. American Journal of Cardiology, 122, 521–523. 10.1016/j.amjcard.2018.04.034 [DOI] [Google Scholar]
- Tabouret, T. , Gregory, T. , Dhooge, M. , Brezault, C. , Mir, O. , Dréanic, J. et al. (2015) Long term exposure to antiangiogenic therapy, bevacizumab, induces osteonecrosis. Investigational New Drugs, 33, 1144–1147. 10.1007/s10637-015-0283-x [DOI] [PubMed] [Google Scholar]
- Usher, B.W. Jr & Friedman, R.J. (1995) Steroid‐induced osteonecrosis of the humeral head. Orthopedics, 18, 47–51. 10.3928/0147-7447-19950101-10 [DOI] [PubMed] [Google Scholar]


