ABSTRACT
The Cryptococcus gattii species complex has often been referred to as a primary pathogen due to its high infection frequency among apparently immunocompetent patients. In order to scrutinize the immune status of patients and the lineages of etiologic agents, we analyzed patient histories and the molecular types of etiologic agents from 135 global C. gattii cases. Eighty-six of 135 patients had been diagnosed as immunocompetent, although some of them had underlying medical issues, and 49 were diagnosed as immunocompromised with risk factors similar to those seen in Cryptococcus neoformans infection. We focused on the 86 apparently immunocompetent patients and were able to obtain plasma from 32 (37%) to analyze for the presence of autoantibodies against the granulocyte-macrophage colony-stimulating factor (GM-CSF) since these antibodies have been reported as a hidden risk factor for C. gattii infection. Among the 32 patients, 25 were free from any known other health issues, and 7 had various medical conditions at the time of diagnosis for cryptococcosis. Importantly, plasma from 19 (76%) of 25 patients with no recognized underlying medical condition showed the presence of GM-CSF autoantibodies, supporting this antibody as a major hidden risk factor for C. gattii infection. These data indicate that seemingly immunocompetent people with C. gattii infection warrant detailed evaluation for unrecognized immunologic risks. There was no relationship between molecular type and underlying conditions of patients. Frequency of each molecular type was related to its geographic origin exemplified by the overrepresentation of VGIV in HIV-positive (HIV+) patients due to its prevalence in Africa.
KEYWORDS: Cryptococcus gattii species complex, opportunistic pathogen, anti-GM-CSF autoantibodies, underlying medical conditions, molecular epidemiology
INTRODUCTION
Although the two etiologic agents of cryptococcosis, C. neoformans and C. gattii species complexes, share 85 to 90% genomic identity (1), their global distribution and the patient populations affected are different. C. neoformans is the dominant cause of cryptococcosis. The majority of patients affected are those with immunosuppression (2–4), and hence, C. neoformans is considered an opportunistic pathogen. Globally, C. gattii has been a significantly less frequent cause of cryptococcosis, and clinical cases have been reported more often in patients with no recognized immunosuppression (2, 5, 6). Thus, C. gattii has been regarded as a primary pathogen (5, 7–9).
According to an analysis of 2,046 human clinical and veterinary cryptococcal isolates recovered from 48 countries, 80% belonged to the C. neoformans and only 20% to the C. gattii species complex, even though the geographic distributions of the two species complexes were similar (48% C. neoformans versus 52% C. gattii) in the environment of 21 countries (10). This suggests that environmental distribution fails to explain the disparity in clinical detection.
The C. gattii species complex contains four major lineages/molecular types, and recently, two more have been described (11, 12). The four major molecular types (VGI to VGIV) have been isolated from people with and without immunosuppression, but apparent host risks vary depending on the molecular type (6). The VGIII and VGIV lineages prevalent in tropical and subtropical regions most frequently are recognized as the cause of cryptococcosis in patients with HIV/AIDS (6, 13–16) and less frequently from immunocompetent patients (6). Infections with VGI and VGII lineages have been recognized relatively more frequently in people without clear medical risks (6, 17). If the VGI and VGII lineages are primary pathogens, why are there so few cases of travel-associated cryptococcosis reported among the tourists who have visited Vancouver, where a large C. gattii outbreak due to VGII lineage occurred from 1999 to 2007 (17)?
The annual number of tourists visiting British Columbia/Vancouver, a VGII-rich environment (18), is in the several millions (www.bcstats.gov.bc.ca). To date, only five cases of Vancouver travel-associated C. gattii infections have been documented in medical literature (19–23), and no case clusters have been reported, though tourists often travel in groups.
Differences in recognized infection despite widespread exposure raises questions about relative microbial pathogenicity and host risks (24, 25). The concept that previously healthy patients (diagnosed as immunocompetent) who suffered from C. gattii meningitis (CM) have certain hidden risk factors has emerged in 2014 (24, 25). Plasma from 6 of 8 Australian and 1 Chinese previously healthy C. gattii patients contained functional antibodies against granulocyte-macrophage colony-stimulating factor (GM-CSF) (24). Since then, two reports on GM-CSF-neutralizing antibodies as a hidden risk factor for C. gattii infection in seemingly immunocompetent patients have followed (19, 26). It is understandable why GM-CSF-neutralizing antibodies predispose one to cryptococcosis since GM-CSF plays a crucial role in alveolar macrophage differentiation and development of normal innate immune function in the lung (27). Although anti-cytokine autoantibodies are increasingly recognized as important mechanisms of pathogenesis for various diseases (28), the prevalence of GM-CSF autoantibody among the healthy population is not known. It is, however, estimated to be 0.37 per 100,000 persons based on the rate of acquired pulmonary alveolar proteinosis (PAP) in which GM-CSF autoantibodies are the main cause (29).
What is not clear is the reason for more cases of C. gattii infection associated with these antibodies than with C. neoformans infection (24, 30, 31). Recently Perrineau et al. (31) and Panackal et al. (32) reported cases of central nervous system (CNS) infection caused by C. neoformans var. grubii in an HIV-seronegative patient with functional GM-CSF autoantibodies. This indicates that GM-CSF autoantibodies are more pronounced but not an exclusive risk factor for C. gattii infection. Since the routine diagnostic immunological profiling does not usually include the testing for levels of anti-cytokine autoantibodies, the presence of GM-CSF-neutralizing antibodies remains a hidden risk factor for cryptococcosis.
In this study, we analyzed microbial and host characteristics from 135 C. gattii infections representing global distribution to investigate the underlying medical conditions and determine the prevalence of GM-CSF autoantibodies among previously healthy people infected with C. gattii. We also assessed the relationship between underlying conditions and C. gattii lineages. The relationship between C. gattii molecular types and host risks was assessed further using data derived from the global cryptococcal database that incorporates multilocus sequencing type (MLST).
RESULTS AND DISCUSSION
Molecular types of C. gattii isolates from patients with known clinical background.
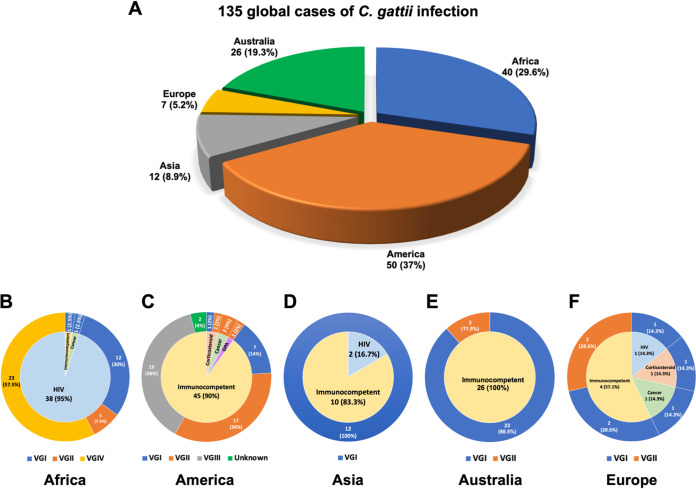
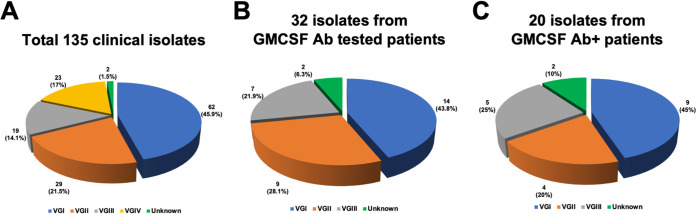
Our study included 135 C. gattii cases in which patients’ clinical history had been well documented (Table S1 in the supplemental material). They were from 5 continents, including 40 cases from Africa, 50 from North and South America, 12 from Asia, 7 from Europe, and 26 from Australia (Fig. 1A). Of these, 132 had central nervous system (CNS) infection, 2 isolated pneumonia, and 1 had a spinal epidural abscess. Of the original 135 isolates, 2 isolates from the patients SCA-2 (26) and LDS-107 (this study) had been discarded after being identified to the species level, and only 133 were available for molecular typing. We analyzed molecular types of 124 isolates in this study using the URA5 restriction fragment length polymorphism (RFLP) and matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS), and those of the remaining 9 isolates had been reported previously (24). Eighty-six (63.7%) of the 135 patients were originally considered to be lacking underlying risks (Fig. 1B to F; Table S1), and 49 (36.3%) patients were diagnosed as having recognized risks for cryptococcal infection, including HIV/AIDS, corticosteroid receipt, diabetes mellitus, cancer, or other immunosuppression. The 135 isolates represented all four major C. gattii lineages, which include 62 VGI (45.9%), 29 VGII (21.5%), 19 VGIII (14.1%), and 23 VGIV (17.0%), and 2 molecular type unknown (1.5%) (Fig. 2A). The molecular type determined by URA5 RFLP analysis and MALDI-TOF MS score agreed for all isolates tested except for one (99.2% agreement), PW-039 (Table S2; Fig. S1). The distribution of each molecular type in different geographic regions agreed with previous findings in that the VGI is not only the most frequent but is especially common in Asia, Europe, and Australia, while VGII and VGIII are most prevalent in North and South America (6, 33). The isolates of VGIV we collected were all from Africa, where VGIV is known to be the most common molecular type of C. gattii among clinical isolates, especially from people with HIV/AIDS (6, 15, 33, 34).
FIG 1.
The global distribution of 135 C. gattii infection cases. (A) Number of C. gattii infection cases in five continents. The immune status of patients determined at the time of diagnosis for cryptococcosis and the molecular types of C. gattii isolates in Africa (B), America (C), Asia (D), Australia (E), and Europe (F). The inner ring shows the immune status of C. gattii-infected patients at the time of diagnosis, and the outer ring shows the molecular types of C. gattii isolates.
FIG 2.
Molecular types of isolates obtained from patients. (A) Molecular types of total 135 clinical isolates. (B) Molecular types of 32 isolates from patients whose plasma was tested for the presence of functional GM-CSF autoantibodies. (C) Molecular types of 20 isolates from patients with anti-GM-CSF autoantibodies.
Phylogenetic analysis of the PW-039 strain. Download FIG S1, TIF file, 10.1 MB (10.1MB, tif) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
List of strains obtained from 5 continents. Download Table S1, DOCX file, 0.03 MB (32KB, docx) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Molecular type of isolates identified by MALDI-TOF MS versus URA5 RFLP. Download Table S2, DOCX file, 0.03 MB (31.3KB, docx) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
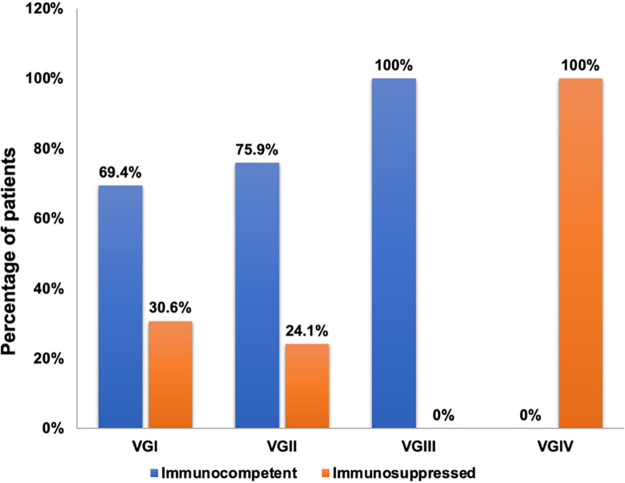
The 86 isolates from patients considered to be immunocompetent included 43 VGI (69.4% of total cases due to VGI), 22 VGII (75.9% of total cases due to VGII), and 19 VGIII (100% of total cases due to VGIII) (Fig. 3); this included no VGIV and 2 isolates of molecular type unknown. Although the distribution of molecular type across patients with and without immunosuppression is similar to the previous report by Chen et al. (6), our isolates from the patients considered immunocompetent were lower in VGI and VGII (69.4% and 75.9% versus 92.8% and 88.9%) while higher in VGIII (100% versus 75.8%) than the previous reports (6, 35) (Fig. 3). The total number of VGI isolates included in the previous report (6) was 77 from immunocompetent and 6 from immunocompromised patients, and VGII was reported from 96 immunocompetent and 12 immunosuppressed (6) compared to our isolates of 43 and 19, respectively, for VGI and 22 and 7, respectively, for VGII. Fisher’s exact test indicated the difference between the two data sets is statistically significant (P = 0.0012). The reason for this significant difference is likely due to differences in the patients’ geographic and clinical backgrounds. Although VGI is not known to be the most common molecular type in Africa (10, 33), nearly 20% of VGI isolates we collected for this study were from patients with HIV infection in South Africa (Table S1). The differences between the previous report and our data on the frequency of patients recognized as immunocompetent versus immunocompromised infected by VGII were also statistically significant (P = 0.0168). The difference can be explained by sampling difference in this study. Our collection contained more isolates of VGII from patients without recognized risks than those from immunosuppressed patients since our interest was in determining the prevalence of GM-SCF autoantibody as a hidden immune defect. A previous study (36) has shown that the patients infected by VGII in the United States are less likely to have recognized immunosuppression than those infected by VGI or VGIII. There was no significant difference between previous reports and this study in the proportion of VGIII (P = 0.7259) and VGIV (P = 0.1314) molecular types from immunocompetent versus immunosuppressed patients.
FIG 3.
Molecular types of the total 133 isolates obtained from patients considered to be immunocompetent or immunosuppressed at the time of diagnosis for cryptococcosis.
The prevalence of GM-CSF autoantibodies in the plasma of 32 C. gattii-infected patients.
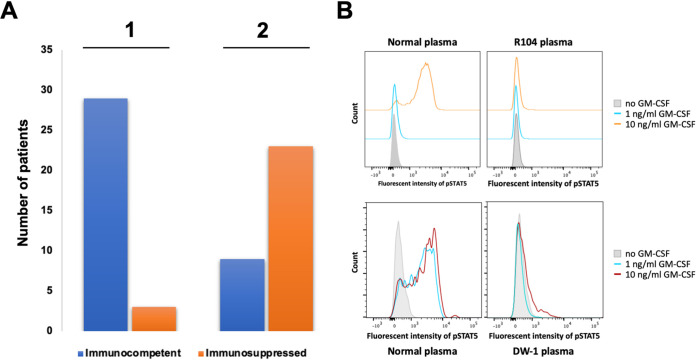
Plasma was available for detection of GM-CSF autoantibodies only from 32 of 135 C. gattii patients, which included 3 patients with known immunosuppressive conditions, such as lymphoma and receipt of corticosteroid, and 29 previously healthy with no recognized immunosuppression (Table 1; Fig. 2B). Four of the 29 previously healthy patients considered immunocompetent had other medical issues, including menometrorrhagia, increased regulatory T cells (Treg), and cerebrospinal eosinophilia, and 25 had no known comorbid conditions at the time of diagnosis for cryptococcosis. Although considered immunocompetent, 20 (69%) of those 29 patients were positive for neutralizing antibodies against the GM-CSF (Table 1; Fig. 4A and B), indicating certain immune system abnormality. Except for the patient with menometrorrhagia, all patients with other medical issues before the onset of cryptococcosis, such as increased Treg and CSF eosinophilia or history of corticosteroid therapy, were negative for GM-CSF autoantibodies. This indicates that 19 (76%) of the 25 C. gattii patients without any known other medical issue had GM-CSF autoantibody as a hidden risk factor (Table 1; Fig. 4A). Our observation also raises the question of whether increased Treg or CSF eosinophilia played any role in the patient’s susceptibility to C. gattii or they were the by-product of C. gattii infection. Corticosteroid therapy is a known risk for cryptococcosis (2, 37), although it is poorly understood whether short-term therapy for skin lesions, which does not result in clear immunosuppression, could predispose one to cryptococcosis. Figure 2C and Table 1 show that 9 of the 20 GM-CSF antibody-positive patients were infected by VGI (45%); the remaining 11 (55%) patients were infected by VGII (4, 20%), VGIII (5, 25%), and 2 (10%) by isolates of unknown molecular type. Frequency of the three molecular types, VGI to VGIII, among the cases with antibody-positive plasma reflected the geographic origin of the patients; nearly 67% of the antibody-positive VGI patients were from Australia/Asia, where VGI is the most prevalent type, and 33% were from North America (Table 1). Antibody-positive patients infected by VGII and VGIII were all from North America where VGII and VGIII are most common, except for one VGII from Australia (6, 33). The lack of VGIV among the GM-CSF antibody-positive patients is expected since the patients’ plasma for the antibody test all came from the geographic regions where cryptococcosis due to VGIV is rarely reported (6, 33). Our data clearly suggest that the presence of GM-CSF-neutralizing antibody is a hidden risk for C. gattii infection in otherwise healthy individuals and suggest that the antibody test should be included in the immunologic profiling when previously healthy patients present with C. gattii infections. It is important to detect the presence of anti-GM-CSF autoantibody since it could lead to pulmonary alveolar proteinosis (PAP), a chronic lung disease characterized by abnormalities of surfactant metabolism. PAP impairs the function of alveolar macrophages and can cause respiratory insufficiency (29). It is also noteworthy that one patient considered immunocompetent and lacking GM-CSF antibodies had a splice-site mutation in STAT3 when the whole exon of his genome was sequenced (data not shown). This suggests that exon sequencing may reveal other hidden risk factors in those seemingly immunocompetent patients infected by C. gattii.
TABLE 1.
Data for C. gattii samples from patients who have thus far have been tested for the presence of anti-GM-CSF autoantibody
| Patient/isolate | Region | Source of isolate | Molecular typee | Underlying issues at diagnosis | Aba | Reference or source |
|---|---|---|---|---|---|---|
| A1 | Australia (NSW)a | CSF | VGI | None | + | 24 |
| A2 | Australia (NSW) | CSF | VGI | None | + | 24 |
| A3 | Australia (NSW) | CSF | VGI | None | + | 24 |
| A4 | Australia (NSW) | CSF | VGI | None | + | 24 |
| A5 | Australia (NSW) | CSF | VGI | None | − | 24 |
| A7 | Australia (NSW) | CSF | VGI | None | + | 24 |
| A8 | Australia (NSW) | CSF | VGII | None | + | 24 |
| A9 | Australia (NSW) | CSF | VGI | None | − | 24 |
| CZ18 | China (Shanghai) | CSF | VGI | None | + | 24 |
| SEC744 | USA (N. Carolina) | CSF | VGII | None | + | 19 |
| SCA-1 | USA (California) | Spinal abscess | VGIII | None | + | 26 |
| SCA-2 | USA (California) | Brain specimen | ?b | None | + | 26 |
| NIH-34 | USA (New Jersey) | CSF | VGIII | None | + | This study |
| NIH-435 | USA (New Jersey) | CSF | VGI | None | + | This study |
| NIH-653 | USA (Maryland) | CSF | VGI | None | − | This study |
| NIH-754 | USA (Washington DC) | CSF | VGIII | None | + | This study |
| R54 | USA (Alabama) | CSF | VGII | Corticosteroid Rxc | − | This study |
| R56 | USA (S. Carolina) | Pleural fluid | VGI | Corticosteroid Rxc | − | This study |
| R104 | USA (Oregon) | BAL fluid/lung | VGII | Menometrorrhagia | + | This study |
| R109 | USA (Oregon) | CSF | VGII | Lymphoma | − | This study |
| R141 | USA (Oregon) | CSF | VGII | None | − | This study |
| LDS-20 | Canada (Vancouver) | BAL fluid | VGII | Increased Tregsd | − | This study |
| LDS-28 | USA (Washington St.) | CSF | VGII | None | + | This study |
| LDS-36 | USA (California) | CSF | VGIII | CSF eosinophiliad | − | This study |
| LDS-49 | USA (California) | CSF | VGIII | CSF eosinophiliad | − | This study |
| LDS-58 | USA (California) | CSF | VGIII | None | + | This study |
| LDS-64 | USA (California) | CSF | VGII | None | − | This study |
| LDS-76 | Canada (Vancouver) | CSF | VGI | None | + | This study |
| LDS-96 | USA (California) | CSF | VGIII | None | + | This study |
| PCG001 | USA (California) | CSF | VGI | None | − | This study |
| DW-1 | USA (California) | CSF | VGI | None | + | This study |
| LDS-107 | USA (California) | CSF | ?b | None | + | This study |
| Total (n = 32) | 20 positive |
NSW, New South Wales, Ab, anti-GM-CSF autoantibody.
Isolates not available for molecular typing.
Patients used corticosteroid for skin condition 3 months before C. gattii infection.
Conditions found at the time of cryptococcosis diagnosis.
VGI, n = 14; VGII, n = 9; VGIII, n = 7; molecular type unknown, n = 2.
FIG 4.
Plasma samples from C. gattii patients tested for the presence of GM-CSF antibodies. (A) The immune status of 32 patients determined prior to (1) and after (2) the plasma test for the presence of GM-CSF autoantibody. (B) The functionality test of anti-GM-CSF autoantibody. Peripheral blood mononuclear cells (PBMCs) were incubated with 10% plasma from healthy volunteers (control) or GM-CSF antibody-positive patients. Cells were then unstimulated or stimulated with 1 ng/ml or 10 ng/ml GM-CSF. pSTAT5 production in PBMCs was analyzed by flow cytometry. Plasma from two patients, R104 and DW-1, tested for the presence of functional GM-CSF antibody are shown as examples.
The relationship between underlying conditions of known immunosuppression and molecular types of the C. gattii species complex.
Of the 135 patients, 49 (36.3%) had known immunosuppression. The major immunosuppression identified among patients infected by the molecular types VGI, VGII, VGIII, and VGIV were similar to those associated with C. neoformans infection, including HIV infection, cancer, corticosteroid therapy, and other immunosuppressive conditions (Table S1). There was a clear relationship between geographic origin of patients and the prevalence of C. gattii molecular type, but no clear relationship was found between C. gattii molecular type and the immunosuppressive conditions except that all VGIV isolates we studied were from HIV-associated cryptococcosis. The predominance of VGIV among the HIV-associated C. gattii cases was expected since nearly 90% of the HIV-associated C. gattii isolates we have collected were from South Africa and Botswana, where VGIV is the predominant molecular type found in AIDS patients (10, 15, 33). A strong association of VGIV with AIDS patients is supported by the fact that we found no VGIV isolates in our stock cultures from the pre-HIV era (38). The VGIV isolates are serotype C and are found mostly in tropical and subtropical regions, such as India, Sub-Saharan Africa, Mexico, and Columbia, but infrequently in temperate zones (10). Finding no VGIV infection among our 86 patients originally diagnosed as immunocompetent (Fig. 3) supports the fact that VGIV most commonly affects HIV+ patients more than any other type of immunosuppressed patients.
The prevalence of VGI (54.5%, 12/22 cases) among the isolates from South African HIV-associated C. gattii infection, however, was significantly higher than expected.
Previous surveys record no VGI infection (39) or only 27% of all C. gattii infections in South Africa (10, 40). All 13 isolates from HIV-associated C. gattii cases in Botswana were VGIV, and VGI was lacking even though the country borders with South Africa. Only three VGII and no VGIII isolates were found among the 40 isolates from C. gattii infections in Africa. This is not surprising since VGII is infrequently found in Africa (6, 33, 39, 40), and VGIII is rarely found outside the American continent (33, 35, 41, 42).
Underlying conditions of 179 patients infected by C. gattii species complex recorded in the global MLST database.
Of the 179 isolates from patients with known clinical history in the global MLST database, 47.5% (85/179) were from patients diagnosed as immunocompetent, and 52.5% (94/179) of the isolates were from those with immunosuppressive underlying conditions (Table 2). The frequency of C. gattii species complex recovered from patients recognized as immunocompetent among the 179 (https://mlst.mycologylab.org) (Table 2) was significantly lower (P < 0.0001) than in the previous report (6) or the present study. The disparity between the previous data and the MLST database on the frequency of the two types of patients is basically due to the differences in the frequency of the two groups infected by VGI and VGII lineages, but not those infected by VGIII and VGIV. The frequency of immunocompetent versus immunosuppressed patients infected by VGIII and VGIV was not significantly different (P = 0.0963 and 0.3693, respectively) between the two data sets, although the sample size was too small to be statistically significant. It is noteworthy that VGII isolates outnumber VGI isolates in the global MLST database. This is likely due to the disproportionately high number of VGII isolates being subjected to MLST analysis since it has attracted more attention than any other molecular type owing to the high-profile VGII epidemic in British Columbia and the Pacific Northwest from 1999 to 2007.
TABLE 2.
Available metadata from MLST database on C. gattii
| Molecular type | No. of isolates in DBa | No. of isolates from known clinical background | No. (%) immunocompetent (previously healthy) | No. (%) immunocompromised |
Underlying issues other than HIV (no. of patients) | |
|---|---|---|---|---|---|---|
| HIV+ | Other | |||||
| VGI | 354 | 40 | 20 (50) | 11 (27.5) | 9 (22.5) | Cirrhosis (1), SLE (3), tumor-like structure (1), pregnancy (1), CML (1), diabetes (1), CD4 lymphopenia (1) |
| VGII | 862 | 112 | 55 (49.1) | 52 (46.4) | 5 (4.5) | SLE (1), diabetes (2), alcoholic (1), MLS (1) |
| VGIII | 210 | 15 | 7 (46.6) | 1 (6.6) | 7 (46.6) | Hepatoma (1), diabetes (1), depression (1), hypertension (1), paracoccidioidomycosis (1), strongyloidiasis (1), malnutrition (1), immunosuppressed (4) |
| VGIV | 28 | 12 | 3 (25) | 8 (66.6) | 1 (8.3) | Diabetes (1) |
| Total | 1454 | 179 | 85 (47.4) | 72 (40.2) | 22 (12.2) | |
DB, database; SLE, systemic lupus erythematosus; CML, chronic myelogenous leukemia; MLS, microphthalmia syndrome.
The frequency of VGIII from immunocompetent patients in the global MLST database was lower than the number we observed in the present study. We speculate that such disparity in the frequency of VGIII isolates from immunocompetent patients between the MLST database and our study is likely due to the difference in the isolation era of the VGIII isolates. Unlike the previous report and the global MLST database, a large portion of our VGIII isolates were from the pre-HIV era in which the number of the immunosuppressed population was lower.
As Table 2 shows, the most common underlying immunosuppression in patients infected by the C. gattii species complex was HIV infection, regardless of the molecular types of the etiologic agent. Although the number of patients infected by VGIII and VGIV in the MLST database with clinical information is too small (15 and 12, respectively), diabetes was commonly identified as an underlying condition in infection by all four lineages. Underlying conditions, such as systemic lupus erythematosus (SLE), were found among patients infected by VGI and VGII. Other underlying conditions, such as chronic myelogenous leukemia (CML), CD4 lymphopenia, and cirrhosis, were found among VGI patients. These data indicate that the underlying immunosuppressive conditions which predispose patients to infections by the C. gattii and C. neoformans species complex overlap and there is no unique condition that predisposes patients only to the C. gattii species complex.
The important finding in the present study, however, is that the seemingly immunocompetent people with anti-GMCSF autoantibodies in their plasma are more often infected by C. gattii species complex than C. neoformans species complex.
Conclusion.
It is important to recognize the C. gattii species complex as an opportunistic pathogen. Since GM-CSF-neutralizing antibodies are one of the most common hidden underlying conditions in previously healthy C. gattii patients, the antibody test should be implemented in C. gattii patients with no known immunosuppression. This test is especially important for patient management because patients with GM-CSF antibody may eventually develop further complications, such as pulmonary alveolar proteinosis (30).
The C. gattii patients considered immunocompetent and negative for the GM-CSF antibodies could carry other hidden risk factors, which may be revealed by whole-exon sequencing of the patient’s genome.
The known immunosuppressive underlying conditions that predispose patients to C. gattii infection are no different from those to C. neoformans infection except that the presence of anti-GM-CSF autoantibodies in plasma may pose more risk for infection by C. gattii species complex than the C. neoformans species complex.
MATERIALS AND METHODS
Clinical data and samples.
Clinical data from 135 global cases of C. gattii infection were retrieved from studies approved by local institutional review boards. Isolates (n = 124) recovered from cerebrospinal fluid (CSF), bronchoalveolar lavage fluid (BAL fluid)/lung tissue, or extrapulmonary sources were available for determination/confirmation of molecular types in our laboratory. Isolates were streaked on YPD agar (glucose 2%, yeast extract 1%, and peptone 2%) to test for their purity. Purified isolates were stored in 25% glycerol at −80°C until use. The species identity of each strain was confirmed by culturing on CGB media (43) in addition to testing for urease production (44), melanin production (45), and growth at 37°C.
Clinical data correlating with infections characterized to MLST type was obtained from the cryptococcal database (https://mlst.mycologylab.org). Clinical data were available for 179 of a total of 1,454 patients whose isolates had been characterized by MLST (Table 2).
Identification of molecular types.
Molecular types (VGI to VGIV) of the isolates were determined via URA5 gene restriction fragment length polymorphism (RFLP) analysis (34) and MALDI-TOF MS (46). DNA from each culture was isolated (47), and the URA5 gene was amplified using two PCR primers, URA5 (5′-ATGTCCTCCCAAGCCCTCGACTCCG-3′) and SJ01 (5′-TTAAGACCTCTGAACACCGTACTC-3′). The PCR products were double digested with Sau96I and HhaI and were separated by 3% agarose gel electrophoresis (12) to compare with the URA5 RFLP patterns of WM179 (VGI), WM178 (VGII), WM161 (VGIII), and WM779 (VGIV). For MALDI-TOF MS analysis, MALDI target plates of the isolates were prepared as previously described (46). MALDI-TOF MS was performed on the MicroFlex LT mass spectrometer and analyzed by Biotyper 3.1 software (Bruker Daltonics, Inc.). Spectra were acquired over a mass/charge (m/z) ratio of 2,000 to 20,000. Spots were measured using 250 laser shots at 60 Hz in groups of 50 shots per sampling area. Both the Bruker and previously developed NIH custom databases included 22 C. gattii strains representing VGI to VGIV. The best of two scores was recorded, and a cutoff of ≥1.80 was considered acceptable.
Evaluation of anti-GM-CSF autoantibodies in patient’s plasma.
Frozen plasma was available from 32 people with C. gattii infection: 29 people had no known immunosuppression, and 3 had lymphoma (n = 1) and/or had received corticosteroids (n = 2). Among the 32, 12 cases have already been reported (Table 1), and 20 cases were from the present study. The presence of anti-GM-CSF autoantibodies in plasma samples was tested using previously described particle-based methodology (24, 30). Briefly, fluorescent magnetic beads (Bio-Rad) were covalently coupled to 2.5 μg of human GM-CSF (R&D Systems). Beads were transferred into magnet 96-well plate and incubated for 30 min with diluted plasma (1:100) on microplate shaker, washed, and incubated with streptavidin-phycoerythrin (Bio-Rad)-labeled goat anti-human IgG (eBioscience) for an additional 30 min. The plate was then run on Bio-Plex (Bio-Rad) instrument. Normal plasma and GM-CSF autoantibody-positive plasma were used as negative and positive controls, respectively. The antibodies’ neutralizing functionality was evaluated by flow cytometry to detect the phosphorylation of STAT5 in normal peripheral blood monocytes (PBMCs) after stimulation with GM-CSF in presence of the patient’s plasma as described (24, 30).
Statistics.
Fisher’s exact test (Prism 8; GraphPad Software, San Diego, CA) was used to determine if there is a significant difference between the previous data and our data for the frequency of each molecular type from patients with immunosuppression versus those with no known immunosuppression. A P value of <0.05 was considered significant.
ACKNOWLEDGMENTS
This work was supported by the Division of Intramural Research (DIR), NIAID, NIH.
Public Health Service Grants (A173896 and A193257) supported the collection of isolates from Botswana by J.R.P.
Footnotes
This article is a direct contribution from Kyung J. Kwon-Chung, a Fellow of the American Academy of Microbiology, who arranged for and secured reviews by James Kronstad, University of British Columbia, and Gary Huffnagle, University of Michigan-Ann Arbor.
Citation Yang D-H, England MR, Salvator H, Anjum S, Park Y-D, Marr KA, Chu LA, Govender NP, Lockhart SR, Desnos-Ollivier M, Chen S, Halliday C, Kan A, Chen J, Wollenberg KR, Zelazny A, Perfect JR, Chang YC, Bennett JE, Holland SM, Meyer W, Williamson PR, Kwon-Chung KJ. 2021. Cryptococcus gattii species complex as an opportunistic pathogen: underlying medical conditions associated with the infection. mBio 12:e02708-21. https://doi.org/10.1128/mBio.02708-21.
Contributor Information
Kyung J. Kwon-Chung, Email: jkchung@niaid.nih.gov.
Tamara L. Doering, Washington University School of Medicine
REFERENCES
- 1.Kavanaugh LA, Fraser JA, Dietrich FS. 2006. Recent evolution of the human pathogen Cryptococcus neoformans by intervarietal transfer of a 14-gene fragment. Mol Biol Evol 23:1879–1890. doi: 10.1093/molbev/msl070. [DOI] [PubMed] [Google Scholar]
- 2.Kwon-Chung KJ, Fraser JA, Doering TL, Wang Z, Janbon G, Idnurm A, Bahn YS. 2014. Cryptococcus neoformans and Cryptococcus gattii, the etiologic agents of cryptococcosis. Cold Spring Harb Perspect Med 4:a019760. doi: 10.1101/cshperspect.a019760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dromer F, Mathoulin S, Dupont B, Laporte A, French Cryptococcosis Study Group . 1996. Epidemiology of cryptococcosis in France: a 9-year survey (1985–1993). Clin Infect Dis 23:82–90. doi: 10.1093/clinids/23.1.82. [DOI] [PubMed] [Google Scholar]
- 4.Williamson PR, Jarvis JN, Panackal AA, Fisher MC, Molloy SF, Loyse A, Harrison TS. 2017. Cryptococcal meningitis: epidemiology, immunology, diagnosis and therapy. Nat Rev Neurol 13:13–24. doi: 10.1038/nrneurol.2016.167. [DOI] [PubMed] [Google Scholar]
- 5.Sorrell T, Chen SCA, Phillips P, Marr KA. 2011. Clinical perspectives on Cryptococcus neoformans and Cryptococcus gattii: implications for diagnosis and management. P 595–606. In Heitman J, Kozel TR, Kwon-Chung KJ, Perfect JR, Casadevall A (ed), Cryptococcus: from human pathogen to model yeast. ASM Press, Washington, DC. [Google Scholar]
- 6.Chen SC, Meyer W, Sorrell TC. 2014. Cryptococcus gattii infections. Clin Microbiol Rev 27:980–1024. doi: 10.1128/CMR.00126-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seaton RA, Naraqi S, Wembri JP, Warrell DA. 1996. Predictors of outcome in Cryptococcus neoformans var. gattii meningitis. QJM 89:423–428. doi: 10.1093/qjmed/89.6.423. [DOI] [PubMed] [Google Scholar]
- 8.Chen SC, Slavin MA, Heath CH, Playford EG, Byth K, Marriott D, Kidd SE, Bak N, Currie B, Hajkowicz K, Korman TM, McBride WJ, Meyer W, Murray R, Sorrell TC, Australia and New Zealand Mycoses Interest Group (ANZMIG)-Cryptococcus Study . 2012. Clinical manifestations of Cryptococcus gattii infection: determinants of neurological sequelae and death. Clin Infect Dis 55:789–798. doi: 10.1093/cid/cis529. [DOI] [PubMed] [Google Scholar]
- 9.Andreou M, Cogliati M, Kolonitsiou F, Stroumpos C, Stamouli V, Ravazoula P, Siagris D, Papadogeorgaki H, Christofidou M, Lekkou A. 2020. Cryptococcus gattii infection in an immunocompetent host in Greece. Med Mycol Case Rep 27:1–3. doi: 10.1016/j.mmcr.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer W, Gilgado F, Ngamskulrungroj P, Trilles L, Hagen F, Castaneda E, Boekhout T. 2011. Molecular typing of the Cryptococcus neoformans/Cryptococcus gattii species complex, p 327–357. In Heitman J, Kozel TR, Kwon-Chung KJ, Perfect JR, Casadevall A (ed), Cryptococcus: from human pathogen to model yeast. ASM Press, Washington, DC. [Google Scholar]
- 11.Hagen F, Khayhan K, Theelen B, Kolecka A, Polacheck I, Sionov E, Falk R, Parnmen S, Lumbsch HT, Boekhout T. 2015. Recognition of seven species in the Cryptococcus gattii/Cryptococcus neoformans species complex. Fungal Genet Biol 78:16–48. doi: 10.1016/j.fgb.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Farrer RA, Chang M, Davis MJ, van Dorp L, Yang DH, Shea T, Sewell TR, Meyer W, Balloux F, Edwards HM, Chanda D, Kwenda G, Vanhove M, Chang YC, Cuomo CA, Fisher MC, Kwon-Chung KJ. 2019. A new lineage of Cryptococcus gattii (VGV) discovered in the Central Zambezian Miombo Woodlands. mBio 10:e02306-19. doi: 10.1128/mBio.02306-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byrnes EJ, III, Bartlett KH, Perfect JR, Heitman J. 2011. Cryptococcus gattii: an emerging fungal pathogen infecting humans and animals. Microbes Infect 13:895–907. doi: 10.1016/j.micinf.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris J, Lockhart S, Chiller T. 2012. Cryptococcus gattii: where do we go from here? Med Mycol 50:113–129. doi: 10.3109/13693786.2011.607854. [DOI] [PubMed] [Google Scholar]
- 15.Litvintseva AP, Thakur R, Reller LB, Mitchell TG. 2005. Prevalence of clinical isolates of Cryptococcus gattii serotype C among patients with AIDS in Sub-Saharan Africa. J Infect Dis 192:888–892. doi: 10.1086/432486. [DOI] [PubMed] [Google Scholar]
- 16.Nyazika TK, Hagen F, Meis JF, Robertson VJ. 2016. Cryptococcus tetragattii as a major cause of cryptococcal meningitis among HIV-infected individuals in Harare, Zimbabwe. J Infect 72:745–752. doi: 10.1016/j.jinf.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 17.Galanis E, Macdougall L, Kidd S, Morshed M, British Columbia Cryptococcus gattii Working Group . 2010. Epidemiology of Cryptococcus gattii, British Columbia, Canada, 1999–2007. Emerg Infect Dis 16:251–257. doi: 10.3201/eid1602.090900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kidd SE, Chow Y, Mak S, Bach PJ, Chen HM, Hingston AO, Kronstad JW, Bartlett KH. 2007. Characterization of environmental sources of the human and animal pathogen Cryptococcus gattii in British Columbia, Canada, and the Pacific Northwest of the United States. Appl Environ Microbiol 73:1433–1443. doi: 10.1128/AEM.01330-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Applen Clancey S, Ciccone EJ, Coelho MA, Davis J, Ding L, Betancourt R, Glaubiger S, Lee Y, Holland SM, Gilligan P, Sung J, Heitman J. 2019. Cryptococcus deuterogattii VGIIa infection associated with travel to the Pacific Northwest outbreak region in an anti-granulocyte-macrophage colony-stimulating factor autoantibody-positive patient in the United States. mBio 10:e02733-18. doi: 10.1128/mBio.02733-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bauer M, Wickenhauser C, Haak A, Pazaitis N, Siebolts U, Mawrin C, Strauss C, Rickerts V, Stoevesandt D, Cornely OA, Meis JF, Hagen F. 2018. Case report: a fatal case of cryptococcosis in an immunocompetent patient due to Cryptococcus deuterogattii (AFLP6/VGII). JMM Case Rep 5:e005168. doi: 10.1099/jmmcr.0.005168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagen F, van Assen S, Luijckx GJ, Boekhout T, Kampinga GA. 2010. Activated dormant Cryptococcus gattii infection in a Dutch tourist who visited Vancouver Island (Canada): a molecular epidemiological approach. Med Mycol 48:528–531. doi: 10.3109/13693780903300319. [DOI] [PubMed] [Google Scholar]
- 22.Johannson KA, Huston SM, Mody CH, Davidson W. 2012. Cryptococcus gattii pneumonia. CMAJ 184:1387–1390. doi: 10.1503/cmaj.111346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindberg J, Hagen F, Laursen A, Stenderup J, Boekhout T. 2007. Cryptococcus gattii risk for tourists visiting Vancouver Island, Canada. Emerg Infect Dis 13:178–179. doi: 10.3201/eid1301.060945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saijo T, Chen J, Chen SC, Rosen LB, Yi J, Sorrell TC, Bennett JE, Holland SM, Browne SK, Kwon-Chung KJ. 2014. Anti-granulocyte-macrophage colony-stimulating factor autoantibodies are a risk factor for central nervous system infection by Cryptococcus gattii in otherwise immunocompetent patients. mBio 5:e00912-14. doi: 10.1128/mBio.00912-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwon-Chung KJ, Saijo T. 2015. Is Cryptococcus gattii a primary pathogen? J Fungi (Basel) 1:154–167. doi: 10.3390/jof1020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crum-Cianflone NF, Lam PV, Ross-Walker S, Rosen LB, Holland SM. 2017. Autoantibodies to granulocyte-macrophage colony-stimulating factor associated with severe and unusual manifestations of Cryptococcus gattii infections. Open Forum Infect Dis 4:ofx211. doi: 10.1093/ofid/ofx211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shibata Y, Berclaz PY, Chroneos ZC, Yoshida M, Whitsett JA, Trapnell BC. 2001. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity 15:557–567. doi: 10.1016/s1074-7613(01)00218-7. [DOI] [PubMed] [Google Scholar]
- 28.Browne SK, Holland SM. 2010. Immunodeficiency secondary to anticytokine autoantibodies. Curr Opin Allergy Clin Immunol 10:534–541. doi: 10.1097/ACI.0b013e3283402b41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trapnell BC, Whitsett JA, Nakata K. 2003. Pulmonary alveolar proteinosis. N Engl J Med 349:2527–2539. doi: 10.1056/NEJMra023226. [DOI] [PubMed] [Google Scholar]
- 30.Rosen LB, Freeman AF, Yang LM, Jutivorakool K, Olivier KN, Angkasekwinai N, Suputtamongkol Y, Bennett JE, Pyrgos V, Williamson PR, Ding L, Holland SM, Browne SK. 2013. Anti-GM-CSF autoantibodies in patients with cryptococcal meningitis. J Immunol 190:3959–3966. doi: 10.4049/jimmunol.1202526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perrineau S, Guery R, Monnier D, Puel A, Lanternier F. 2020. Anti-GM-CSF autoantibodies and Cryptococcus neoformans var. grubii CNS vasculitis. J Clin Immunol 40:767–769. doi: 10.1007/s10875-020-00796-5. [DOI] [PubMed] [Google Scholar]
- 32.Panackal AA, Rosen LB, Uzel G, Davis MJ, Hu G, Adeyemo A, Tekola-Ayele F, Lisco A, Diachok C, Kim JD, Shaw D, Sereti I, Stoddard J, Niemela J, Rosenzweig SD, Bennett JE, Williamson PR. 2017. Susceptibility to cryptococcal meningoencephalitis associated with idiopathic CD4(+) lymphopenia and secondary germline or acquired defects. Open Forum Infect Dis 4:ofx082. doi: 10.1093/ofid/ofx082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cogliati M. 2013. Global molecular epidemiology of Cryptococcus neoformans and Cryptococcus gattii: an atlas of the molecular types. Scientifica (Cairo) 2013:675213. doi: 10.1155/2013/675213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyer W, Castaneda A, Jackson S, Huynh M, Castaneda E, IberoAmerican Cryptococcal Study Group . 2003. Molecular typing of IberoAmerican Cryptococcus neoformans isolates. Emerg Infect Dis 9:189–195. doi: 10.3201/eid0902.020246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Byrnes EJ, Li W, Ren P, Lewit Y, Voelz K, Fraser JA, Dietrich FS, May RC, Chaturvedi S, Chatuverdi S, Chaturvedi V, Chatuverdi V, Heitman J. 2011. A diverse population of Cryptococcus gattii molecular type VGIII in Southern Californian HIV/AIDS patients. PLoS Pathog 7:e1002205. doi: 10.1371/journal.ppat.1002205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris JR, Lockhart SR, Debess E, Marsden-Haug N, Goldoft M, Wohrle R, Lee S, Smelser C, Park B, Chiller T. 2011. Cryptococcus gattii in the United States: clinical aspects of infection with an emerging pathogen. Clin Infect Dis 53:1188–1195. doi: 10.1093/cid/cir723. [DOI] [PubMed] [Google Scholar]
- 37.Casadevall A, Perfect JR. 1998. Cryptococcus neoformans. ASM Press, Washington, DC. [Google Scholar]
- 38.Pharkjaksu S, Kwon-Chung KJ, Bennett JE, Ngamskulrungroj P. 2020. Population diversity and virulence characteristics of Cryptococcus neoformans/C. gattii species complexes isolated during the pre-HIV-pandemic era. PLoS Negl Trop Dis 14:e0008651. doi: 10.1371/journal.pntd.0008651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Govender NP, Mitchell TG, Litventseva A, Miglia K. 2011. Cryptococcosis in Africa, p 269–285. In Heitman J, Kozel TR, Kwon-Chung KJ, Perfect JR, Casadevall A (ed), Cryptococcus: from human pathogen to model yeast. ASM Press, Washington, DC. [Google Scholar]
- 40.Naicker SD, Govender NP. 2017. Molecular typing of clinical Cryptococcus gattii isolates from South Africa using multi locus sequence typing. Abstract Gmb3. In 10th International conference on Cryptococcus and Cryptococcosis, Foz do Iguacu, Brazil, [Google Scholar]
- 41.Firacative C, Roe CC, Malik R, Ferreira-Paim K, Escandon P, Sykes JE, Castanon-Olivares LR, Contreras-Peres C, Samayoa B, Sorrell TC, Castaneda E, Lockhart SR, Engelthaler DM, Meyer W. 2016. MLST and whole-genome-based population analysis of Cryptococcus gattii VGIII links clinical, veterinary and environmental strains, and reveals divergent serotype specific sub-populations and distant ancestors. PLoS Negl Trop Dis 10:e0004861. doi: 10.1371/journal.pntd.0004861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gonzalez GM, Casillas-Vega N, Garza-Gonzalez E, Hernandez-Bello R, Rivera G, Rodriguez JA, Bocanegra-Garcia V. 2016. Molecular typing of clinical isolates of Cryptococcus neoformans/Cryptococcus gattii species complex from Northeast Mexico. Folia Microbiol (Praha) 61:51–56. doi: 10.1007/s12223-015-0409-8. [DOI] [PubMed] [Google Scholar]
- 43.Kwon-Chung KJ, Polacheck I, Bennett JE. 1982. Improved diagnostic medium for separation of Cryptococcus neoformans var. neoformans (serotypes A and D) and Cryptococcus neoformans var. gattii (serotypes B and C). J Clin Microbiol 15:535–537. doi: 10.1128/jcm.15.3.535-537.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh A, Panting RJ, Varma A, Saijo T, Waldron KJ, Jong A, Ngamskulrungroj P, Chang YC, Rutherford JC, Kwon-Chung KJ. 2013. Factors required for activation of urease as a virulence determinant in Cryptococcus neoformans. mBio 4:e00220-13. doi: 10.1128/mBio.00220-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Polacheck I, Hearing VJ, Kwon-Chung KJ. 1982. Biochemical studies of phenoloxidase and utilization of catecholamines in Cryptococcus neoformans. J Bacteriol 150:1212–1220. doi: 10.1128/jb.150.3.1212-1220.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Firacative C, Trilles L, Meyer W. 2012. MALDI-TOF MS enables the rapid identification of the major molecular types within the Cryptococcus neoformans/C. gattii species complex. PLoS One 7:e37566. doi: 10.1371/journal.pone.0037566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sionov E, Lee H, Chang YC, Kwon-Chung KJ. 2010. Cryptococcus neoformans overcomes stress of azole drugs by formation of disomy in specific multiple chromosomes. PLoS Pathog 6:e1000848. doi: 10.1371/journal.ppat.1000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic analysis of the PW-039 strain. Download FIG S1, TIF file, 10.1 MB (10.1MB, tif) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
List of strains obtained from 5 continents. Download Table S1, DOCX file, 0.03 MB (32KB, docx) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Molecular type of isolates identified by MALDI-TOF MS versus URA5 RFLP. Download Table S2, DOCX file, 0.03 MB (31.3KB, docx) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.