Abstract
Pancreatic cancer produces disabling abdominal pain, and the pain medical management for pancreatic cancer is often challenging because it mainly relies on the use of narcotics (major opioids). However, opioids often provide suboptimal pain relief, and the use of opioids can lead to patient tolerance and several side effects that considerably reduce the quality of life of pancreatic cancer patients. Endosonography-guided celiac plexus neurolysis (EUS-CPN) is an alternative for pain control in patients with nonsurgical pancreatic cancer; EUS-CPN consists of the injection of alcohol and a local anesthetic into the area of the celiac plexus to achieve chemical ablation of the nerve tissue. EUS-CPN via the transgastric approach is a safer and more accessible technique than the percutaneous approach. We have reviewed most of the studies that evaluate the efficacy of EUS-CPN and that have compared the different approaches that have been performed by endosonographers. The efficacy of EUS-CPN varies from 50% to 94% in the different studies, and EUS-CPN has a pain relief duration of 4–8 wk. Several factors are involved in its efficacy, such as the onset of pain, previous use of chemotherapy, presence of metastatic disease, EUS-CPN technique, type of needle or neurolytic agent used, etc. According to this review, injection into the ganglia may be the best technique, and a good visualization of the ganglia is the best predictor for a good EUS-CPN response, although more studies are needed. However, any of the 4 different techniques could be used to perform EUS-CPN effectively with no differences in terms of complications between the techniques, but more studies are needed. The effect of EUS-CPN on pain improvement, patient survival and patient quality of life should be evaluated in well-designed randomized clinical trials. Further research also needs to be performed to clarify the best time frame in performing a EUS-CPN.
Keywords: Pancreatic cancer, Endosonography, Celiac plexus neurolysis, Opioids, Echoendoscopy
Core Tip: In this review, we analyzed the efficacy of the celiac plexus neurolysis through echoendoscopy (EUS-CPN) technique in patients with unresectable pancreatic cancer. The use of opioids for pain control are associated with numerous side effects that reduce the quality of life of pancreatic cancer patients, and the use of EUS-CPN is a safe and effective approach to pain management and allows for the reduction in the opioid doses used. There are different techniques to perform a EUS-CPN, all of which are described in this article. However, there are concerns about the efficacy of EUS-CPN (since it produces a reduction in pain for a short time), the ideal time to perform this technique is unknown, and it is also unknown whether this technique has any influence on patient survival and quality of life.
INTRODUCTION
Pancreatic cancer is one of the solid tumors with the worst prognosis. Unfortunately, it is often diagnosed at an advanced stage of the disease, and only 12%–20% of cases are resectable at the time of diagnosis. Over 50% of patients with pancreatic cancer will not survive within the first year after diagnosis, and this disease has an overall five-year survival rate under 10%[1,2].
Chronic abdominal pain is a frequent symptom in patients with advanced pancreatic cancer due to the perineural invasion of tumor cells, and pain is present in 70%–90% of the patients at diagnosis and has very complex medical management[3,4].
Pain management in patients with pancreatic cancer usually begins with the administration of nonopioid analgesics followed by opioids in refractory cases. Opioids have many adverse effects, such as nausea, constipation, urinary retention, drowsiness, and patient tolerance or dependence.
Currently, many other therapeutic alternatives have been evaluated as complementary treatments, such as celiac plexus neurolysis (CPN) with various agents, which can be administered either percutaneously or transgastrically[5,6].
Pain originating in the intra-abdominal viscera, such as the pancreas, is transmitted by the afferent nerve fibers through the celiac plexus and finally reaching the central nervous system through the posterior root of the spinal cord at the level of T12-L2. The celiac plexus is a group of nerve fibers that converge into the celiac ganglia located in the retroperitoneum and is immediately adjacent to the anterolateral wall of the aorta at the origin of the celiac trunk. Traditionally, access to the celiac plexus has been percutaneous, and it is necessary to avoid the different structures located between the skin and the celiac plexus while performing a percutaneous access to the celiac plexus[5]. However, endosonography (EUS) allows the endosonographer to perform CPN close enough to the celiac plexus through the gastric wall, which could allow a safer and more effective access. EUS-CPN was first described by Wiersema et al[6] in 1996.
EUS-CPN is performed by the injection of a neurolytic agent directly into the celiac plexus, which causes an irreversible ablation. Pure ethanol is often used as the neurolytic agent in association with a local anesthetic agent, such as bupivacaine, and nociceptive afferent nerve fibers are blocked with these agents to achieve pain reduction. EUS-CPN is performed to ameliorate pain and reduce the dose of analgesics in these patients, because the use of analgesics often causes a reduction in patient survival or quality of life.
In this review, we focused on patients with unresectable pancreatic cancer because pancreatic cancer is common and still affects a large number of cases. The options for pain management in these patients must be understood by all gastroenterologists and endoscopists. However, other pathologies, such as biliary tract tumors and patients with chronic pancreatitis, may require a CPN or celiac plexus block, respectively. Due to the large amount of evidence for the use of EUS-CPN in unresectable pancreatic cancer patients, we wanted to focus on this pathology to avoid performing such an extensive review and to focus on the management of chronic abdominal pain with this technique. We also wanted to further understand whether our interventions in this specific pathology have any impact on the survival and quality of life of patients.
INDICATIONS
EUS-CPN is performed in patients with chronic or uncontrolled abdominal pain associated with nonresectable pancreatic cancer; however, to ensure that EUS-CPN is effective, we must carefully select the patients who receive this technique. Current evidence does not precisely indicate when the best time is to perform an EUS-CPN[7].
EUS-CPN is useful in patients with uncontrolled pain or when the adverse effects of opioids reduce the patient’s quality of life. Furthermore, other causes of pain must be investigated and ruled out prior to treatment, such as carcinomatosis, liver or bone metastases and peptic ulcers, because these conditions could lead to a partial or non-response to EUS-CPN.
CONTRAINDICATIONS
EUS-CPN should not be performed in patients with resectable pancreatic tumors because this technique may be difficult to perform, and it is mandatory to discuss borderline patients within a multidisciplinary team before performing a EUS-CPN. There are no absolute contraindications, but there are certain situations where a EUS-CPN should not be performed. The contraindications of EUS-CPN are shown in Table 1.
Table 1.
Contraindications of endosonography-guided celiac plexus neurolysis
TECHNIQUE
Over the years, CPN has been performed via different techniques. It was initially described in 1914 as an intraoperative procedure[8], and since then, assistance with fluoroscopy, computed tomography or abdominal ultrasonography has been utilized[5]. In 1996, Wiersema described for the first time an endosonography-guided celiac plexus neurolysis (EUS-CPN) by a transgastric approach[6]. EUS-CPN allows for a more accurate and safer technique due to the use of color Doppler to avoid vessels that could be close to the needle path. It can be performed in an outpatient setting depending on the clinical status of the patient.
STEPS
Patient medical records must be reviewed to rule out previous surgeries or anatomical abnormalities and to evaluate the radiological images to study the location of the lesion, to evaluate for any possible infiltration of the celiac trunk and to determine if there is another pathology present.
The left decubitus position is the preferred position to perform a EUS-CPN. Deep sedation is also recommended for patients undergoing a EUS-CPN along with appropriately monitored anesthesia. The breathing rate, pulse oximetry, blood pressure and heart rate of the patients must be thoroughly monitored throughout the procedure.
The administration of at least 500 mL intravenous saline solution is needed before and after the procedure to minimize the risk of hypotension, as hypotension is one of the most common adverse effects after the procedure, only second to the hyperactivity of the parasympathetic nervous system[3,9-15].
The evidence is not clear regarding the administration of prophylactic antibiotics for EUS-CPN. Infectious complications due to EUS-CPN are rare, so most of the previous studies did not use prophylactic antibiotics[11-14].
An examination with radial echoendoscopy may be initially performed to explore the celiac trunk area. Then, a linear echoendoscope is introduced until reaching the origin of the celiac trunk, which is the first large vessel of the abdominal aorta just beneath the diaphragm. The diaphragm is a structure indirectly located by the visualization of the left diaphragmatic crus, 40–45 cm distal to the superior dental arch. Immediately under the celiac trunk is the origin of the superior mesenteric artery and the myenteric plexus (Figure 1).
Figure 1.
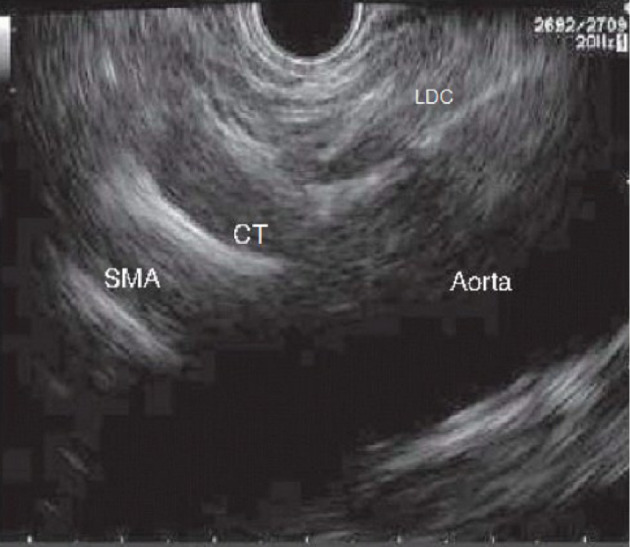
Sagittal plane of the aorta where we can see left diaphragmatic crus, celiac trunk and superior mesenteric artery emerging from Aorta. SMA: Superior mesenteric artery; LDC: Left diaphragmatic crus; CT: Celiac trunk.
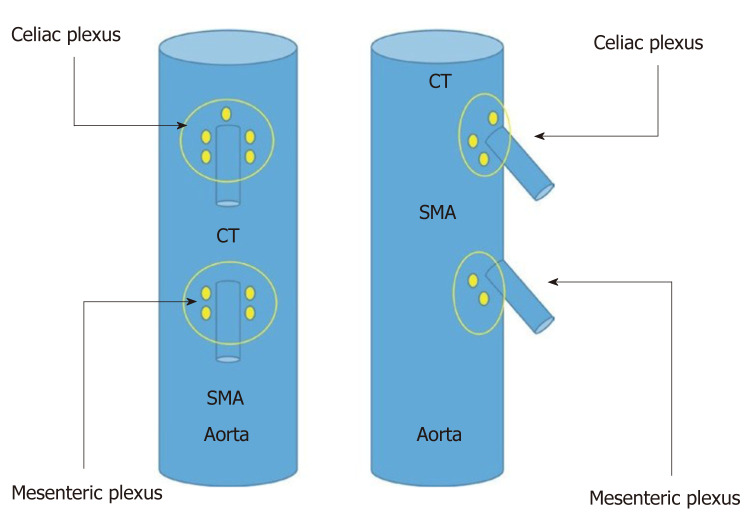
The celiac plexus is located in the anterior wall of the aorta and is on both sides of the origin of the celiac trunk, and it is sometimes 1 mm above it or can sometimes be several millimeters below it (Figure 2). To locate this area, the echoendoscope should be rotated both clockwise and counterclockwise. The puncture area must be carefully selected, and before introducing the needle, it is recommended to use color Doppler in the target area of the puncture to make sure there are no vascular structures in the path of the needle.
Figure 2.
Schematic vision (frontal and lateral) of the situation of celiac and mesenteric plexus. SMA: Superior mesenteric artery; CT: Celiac trunk.
TYPE OF NEEDLE
Any EUS needle may be used, as previous demonstrated in several studies, and these needles can range from small caliber needles, such as 25-gauge needles, to larger caliber needles, such as 19-gauge needles. Certainly, the use of a larger caliber needle will allow for an easier injection of substances.
One specific needle was designed for this technique: it is a 20-gauge needle with a dumpling pattern and conical tip [EchoTip® Ultra Celiac Plexus Neurolysis Needle, Cook Medical, Limerick (Ireland)], which allows the injection to be sprayed in a radial and uniform way and allows for adequate diffusion of the substance into the celiac plexus (Figure 3).
Figure 3.
Specific needle designed for endosonography-guided celiac plexus neurolysis (Cook Medical, Limerick, Ireland).
When the puncture area is selected, the needle must be primed with local anesthetic (usually bupivacaine or lidocaine) to avoid the injection of air into the puncture area.
Once the needle has been introduced, aspiration to confirm negative pressure must be performed to make sure that the needle was not placed into a vessel prior to injecting the substance, because the injection of these substances in a blood vessel wall or into the systemic circulation can be critical and life threatening.
NEUROLYTIC AGENT
Usually, the average injected volume of 0.25% bupivacaine is 10 to 20 mL, followed by 10 to 20 mL of 98% alcohol, although these quantities may vary slightly depending on the study. Optionally, some contrast agents can be used, even though the use of these is not clear. Ishiwatari et al[16] compared the use of phenol as compared to ethanol as a neurolytic agent and found no differences in pain control or complications.
TYPE OF APPROACHES
The different approaches for EUS-CPN are showed in Figure 4.
Figure 4.
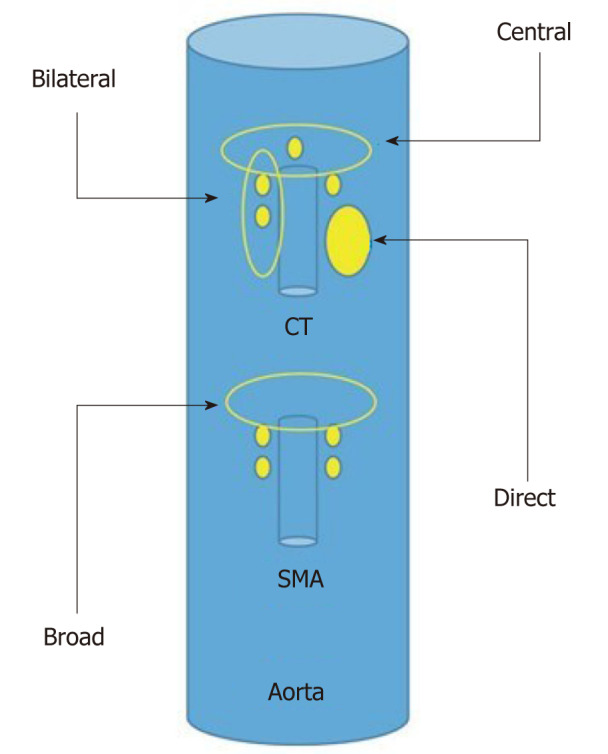
Schematic representation of the different endosonography-guided celiac plexus neurolysis approaches. SMA: Superior mesenteric artery; CT: Celiac trunk.
Bilateral approach/technique[6,17], once the celiac trunk has been located, the objective of this approach is to inject substances on both sides of it. It is recommended to make slow and rotatory clockwise movements without losing the longitudinal axis of the aorta. With these movements, we are able to see the “injection windows”, as shown in Figure 5.
Figure 5.
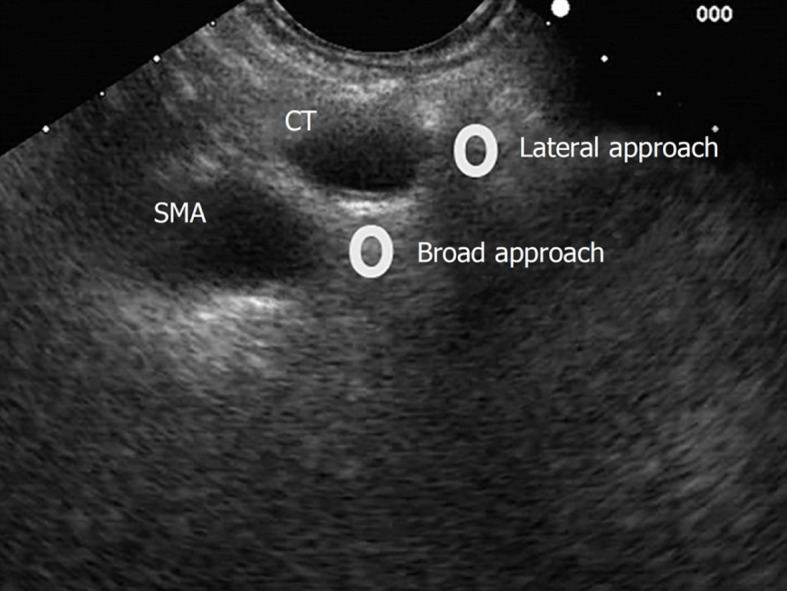
Lateral and broad approaches for endosonography-guided celiac plexus neurolysis. SMA: Superior mesenteric artery; CT: Celiac trunk.
Central approach/technique[9,10] is begun from the starting position at the origin of the celiac trunk and without losing the longitudinal axis of the aorta, the injection is performed in a cranial plane from the starting position, as shown in Figure 6.
Figure 6.
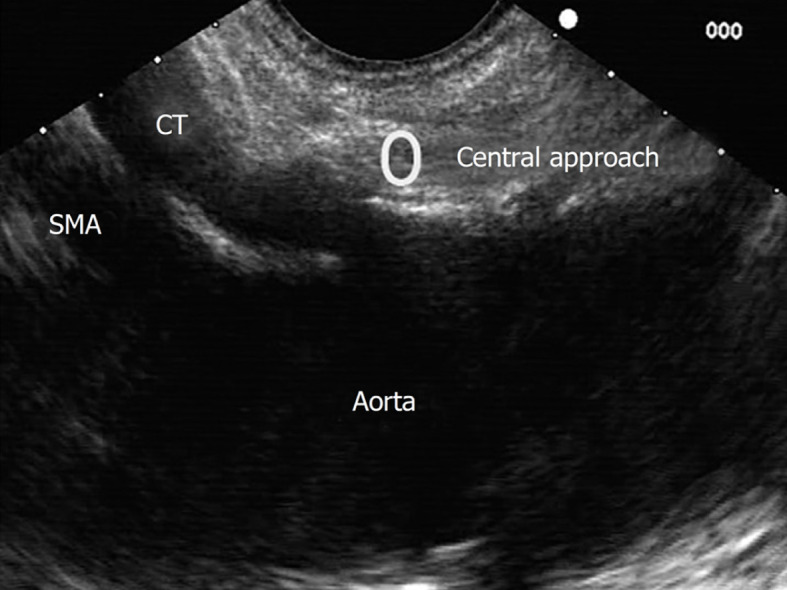
Central approach for endosonography-guided celiac plexus neurolysis. SMA: Superior mesenteric artery; CT: Celiac trunk.
Broad approach/technique was first described in 2010 by Sakamoto et al[18], and this approach is based on the injection of the substances above and on both sides of the origin of the superior mesenteric artery, without losing the longitudinal axis of the aorta, and by aiming for a broader diffusion of the neurolytic agent (Figure 5). In this technique, the needle reaches a greater depth; therefore, it is recommended to use a 25-gauge needle.
Direct approach/technique[11] is based on the direct injection of each celiac ganglia to distribute the alcohol and anesthetic doses. Celiac ganglia are sometimes visible as hypoechoic structures, which are almond shaped, are between 2 to 20 mm and are usually located around the aorta at the origin of the celiac trunk. The right celiac ganglion is usually located 6 mm inferior to the origin of the celiac trunk, while the left celiac ganglion is located 9 mm below the origin of the celiac trunk. During the injection in the center of the ganglia, “ballonization” and an increase in volume will be seen. If this is not seen, the needle is probably misplaced.
AFTER THE PROCEDURE
Before extracting the needle, 3 mL of saline solution is injected to prevent the injection of ethanol into the path of the needle. If this injection of saline is not performed, it could result in the exacerbation of pain after the procedure. Patients should be monitored for at least two hours after the intervention, and the patient’s blood pressure should be monitored.
RESULTS
The efficacy, study design, dose and type of neurolytic agent, follow-up and complications of EUS-CPN are summarized in Table 2[18-24].
Table 2.
Endosonography-guided celiac plexus neurolysis efficacy in current literature
|
Ref.
|
Design
|
n
|
Technique
|
Neurolytic agent
|
Pain control (follow up)
|
Complications
|
| Wiersema et al[6] | Retrospective | 30 | Bilateral | 3 mL bupivacaine (0.25%) + 10 mL ethanol (98%) | 88% (10 wk) | Diarrhea 13.3%, Pain 3.3% |
| Gunaratnam et al[17] | Prospective | 58 | Bilateral | 3-6 mL bupivacaine (0.25%) + 10 mL ethanol (98%) | 78% (24 wk) | Pain 8.6% |
| Levy et al[11] | Retrospective | 17 | Direct | 8 mL bupivacaine (0.25%) + 12 mL ethanol (99%) | 94% (2-4 wk) | Hypotension 35%, pain 41% and diarrhea 16% |
| Sahai et al[9] | Prospective | 160 | Central vs Bilateral | 10 mL bupivacaine (0.5%) + 20 mL ethanol | 45.9% vs 70.5% (7 d). P < 0.05 | Bleeding 0.7% |
| Sakamoto et al[18] | Retrospective | 67 | Broad vs bilateral | 3 mL lidocaine (1%) + 9 mL ethanol (98%) | Mean VAS scores 3.9 vs 2.5 (7 d) and 4.8 vs a 3.4 (30 d) P < 0.05 | None |
| Wyse et al[7] | RCT | 48 | Bilateral vs analgesia | 10 mL bupivacaine (0.50%) + 20 mL ethanol | Likert scale reduction 28% (4 wk) + 60% (12 wk) P < 0.05 | None |
| LeBlanc et al[10] | RCT | 50 | Central vs bilateral | 20 mL lidocaine (0.75%) + 10 mL ethanol (98%) | 69% vs 81% (61.9%)(14wk) | Hypotension 2% pain 36% |
| Iwata et al[19] | Retrospective | 47 | Central, direct or bilateral | 2-3 mL bupivacaine + 20 mL ethanol | 68% (7 wk) | Hypotension 17%, diarrhea 23% and inebriation 8% |
| Ascunce et al[20] | Retrospective | 64 | Bilateral | 10 mL lidocaine (1%) + 20 mL ethanol (98%) | 50% (1 wk). OR 15.61 of response if celiac ganglia was detected | Hypotension 2%, pain 2% and diarrhea 23% |
| Wiechowska-Kozłowska et al[12] | Retrospective | 29 | Central vs bilateral | 2 mL lidocaine (2%) + 20 mL ethanol (98%) | 86% (1-2 wk) | Hypotonia 3.4%, pain 6.9% and diarrhea 10.3% |
| Téllez-Ávila et al[21] | Retrospective | 53 | Central vs bilateral | 10 mL lidocaine (1%) + 10-20 mL ethanol (98%) | 48% vs 56% (4 wk) | Transitory pain 0% vs 3% |
| Seicean et al[22] | Retrospective | 32 | Central | 10 mL lidocaine (1%) + 10-15 mL ethanol | 75% (2 wk) | None |
| Doi et al[13] | RCT | 68 | Direct vs central | 1-2 mL bupivacaine (0.25%-0.5%) + 10-20 mL ethanol | 73.5% vs 45.5% (7 d) P < 0.05 | Hypotension 2.9% vs 6%, pain 29.4% vs 21.2% and diarrhea 5.9% vs 9.1%. No diferences |
| Ishiwatari et al[16] | Retrospective | 22 | Direct or bilateral | 1-2 mL bupivacaine (0.5%) + 40-60 mL ethanol or 20-25 mL fenol | 83% (fenol) vs 69% (ethanol) (7 d) | Diarrhea 9%, hypotension 4.5%, pain 4.5% and inebriation 4.5% |
| Hao et al[23] | Retrospective | 41 | Central or direct | 10 mL bupivacaine (2%) + 20 mL ethanol | Pain < 3 mo improve 84% (3 d), 96% (7 d) and 68% (90 d). Pain > 3 mo improve 75% (3 d), 81% (7 d) and 50% (90 d) | Hypotension 4.9% |
| Minaga et al[14] | Retrospective observational | 112 | Broad ± direct | 3 mL lidocaine (1%) + 9 mL ethanol (98%) | Pain improvement 77. 7% (1 wk)+ 67.9% (4 wk) | Inebriation 8%, hypotension 4.5%, pain 3.6% and diarrhea 3.6% |
| Levy et al[24] | RCT | 110 | Direct vs bilateral | 4 mL bupivacaine (0.25%) + 20 mL ethanol (99%) | Pain improvement 46.2% vs 40.4%. No changes on quality of life | Hypotension 11.7% vs 20%, diarrhea 10% vs 12.2%. Pain 8.3% vs 44.9% (P < 0.05) |
VAS: Visual analogue scale. RCT: Randomized clinical trial.
EFFICACY OF CPN
Several studies have been performed to evaluate the efficacy of EUS-CPN. Globally, there has been a great variability shown in the efficacy of this technique for pain control associated with pancreatic cancer. The range of efficacy varies from 50% to 94% in the previous studies[6,7,9-11,13-19,23,24].
However, the available current literature has limitations due to the different quality of the studies (some of them are retrospective), and they differ in the injection technique, type and volume of neurolytic agent, number of patients and follow-up. In addition, the definitions for categorizing pain control vary in the different studies: improvement or resolution of pain, reduction of the Visual Analogue Scale (VAS) or Likert scale, reduction of the dose of opioids, etc.[6,7,9-11,13-19,23,24].
EUS-CPN was first performed by Wiersema et al[6] with an efficacy of 88% in 30 patients over 10 wk. In the first clinical trial, Wyse et al[7] randomized 96 patients with unresectable pancreatic cancer to either early treatment with EUS-CPN or a conventional medical treatment with analgesics and opioids. Clinical significance was observed with a reduction of 28% and 60% in the Likert scale at 4 and 10 wk of follow-up, respectively. A reduction in the dose of analgesics was also observed.
Momentary efficacy was observed in four systematic reviews and three meta-analyses. The studies demonstrated a reduction in pain in more than 50% of the patients during the 4–8 wk follow-up[15,25-27]. In addition, one of the systematic reviews concluded that pain control allowed for a reduction in the opioid dose with significantly fewer adverse effects in the treated group (P < 0.0001), but this was during the short term.
Based on this evidence, we can conclude that EUS-CPN significantly reduces the pain associated with pancreatic cancer (but does not make the pain disappear completely) and can reduce the dose of opioids[7,23,25,26]. The combination of an EUS-CPN plus analgesic opioids could be superior to opioid therapy alone[7]. However, this should be demonstrated in randomized clinical trials (RCTs) to further validate these findings[26,28].
IMPACT OF CPN ON QUALITY OF LIFE AND SURVIVAL
Current evidence supports the efficacy of CPN. However, the effect on the patient’s quality of life is controversial, and there is no effect on survival. Changes in the quality of life were measured with different QOL scores Digestive Disease Questionnaire-15[7].
On the one hand, Wyse et al[7] observed that the addition of EUS-CPN to the treatment regimen had no outcomes effect on the quality of life in patients. Lu et al[25] found in a their systematic review that EUS-CPN significantly reduced significantly the dose of opioids with a diminution of their adverse effects, but there wiwasth no differences in terms of quality of life.
On the other hand, Seicean et al[22] found little improvement in some factors associated with quality of life, such as the functional status or sleep quality, and there was no change in the acceptance of the disease and enjoyment of life.
Current evidence has not shown any clinical significance in terms of survival to recommend an EUS-CPN[7,26]. Although it has not been demonstrated that EUS-CPN significantly improves the quality of life of patients, the reduction of adverse effects associated with opioids could have some impact on the quality of life of these patients, which can be important[22,26].
PREDICTORS OF RESPONSE
CPN is usually performed as a palliative treatment in patients refractory to common analgesics. However, since Wiersema et al[6] performed the first EUS-CPN, they found that patients who had not received previous chemotherapy had significantly greater pain relief than patients who received chemotherapy.
It is known that chemotherapy improves the patient’s pain and quality of life[7,24]. Patients who received chemotherapy before EUS-CPN could be impacted by the effect of the technique. In fact, as concluded by Wyse et al[7], pain improvement was seen earlier in patients who had not received previous chemotherapy than in patients who did receive chemotherapy.
In a different study, Hao et al[23] observed a significant improvement in the pain scales of the patients who had an onset of pain earlier than 3 mo, and an improvement of pain was then observed in both the short and long terms.
The best time to perform an EUS-CPN remains unclear[7]. It could be possible that a delay in performing an EUS-CPN or its application in patients who have received other treatments for pain control could decrease the efficacy of the EUS-CPN; however, there is not enough evidence to support this theory[7,17,21].
Few studies have also compared the different techniques of EUS-CPN[9,12,14,15,23,26]. Iwata et al[19] observed that the direct invasion of the celiac plexus and the distribution of ethanol on only the left side of the artery negatively influenced pain control[13].
A retrospective study by Ascunce et al[20] evaluated the efficacy of the bilateral technique. They concluded that the direct visualization of the celiac ganglia while performing a EUS-CPN (which needed to be referenced in the endoscopic report) was a good predictor of the response (OR 15.61).
BILATERAL VS CENTRAL TECHNIQUE
As mentioned above, there are several techniques for performing a EUS-CPN. We reviewed those studies that compared the different techniques to analyze which technique may be the most effective and that had fewer adverse effects[9,13,14,18,21,24].
On the one hand, bilateral and central techniques have shown comparative outcomes in a few studies[10,25,26], and the only exception was in a study performed by Sahai et al[9] in 2009. The bilateral approach improved the pain control compared to the central technique (70.5% vs 45.9%; P < 0.05), but the effect lasted only one week.
On the other hand, in a meta-analysis published in 2009, a subgroup analysis was performed that evaluated the different approaches that were performed. The bilateral approach was more effective than the central technique in terms of pain control (84.5% vs 45.9%; P < 0.05)[15].
Finally, one more recent meta-analysis of 437 patients concluded that comparable pain control was obtained with both approaches; however, the bilateral approach significantly reduced the dose of opioids compared to the central technique[25].
GANGLIA INJECTION
Direct injection of neurolytic agents into the ganglia has been demonstrated to be effective for pain relief associated with pancreatic cancer. The rate of effectiveness has varied from 65% to 94% in different studies,[11,13,14] and one of these studies was a clinical trial. Doi et al[13] demonstrated significant pain relief with the injection directly into the ganglia compared to the central approach, but the injections were only beneficial for one week (73.5% vs 45.5%).
Despite having good results in several studies, other studies have been published that have shown some concerns regarding this technique.
Levy et al[24] published a randomized double blind clinical trial comparing direct ganglia injection to central CPN, and no differences were found in pain control or in improving the quality of life with either technique. However, the median survival was significantly higher in patients treated with direct ganglia injection (10.5 mo vs 5.6 mo), particularly for patients with nonmetastatic disease.
Recently, Koulouris et al[28] performed a systematic review and meta-analysis on the efficacy of three EUS-CPN techniques on pain control: central, bilateral and ganglia injection. Pain control was achieved in 68% of the patients at week 2 and 53% of the patients at 4 wk of follow-up. There was no difference between the techniques in terms of age, sex, tumor localization, stage or baseline pain before the intervention. Major bias could have been present in this review, because low-quality studies were included (not randomized studies), the measurement of treatment response was different, and the influence of other treatments (opioids or chemotherapy) was not evaluated in this study. However, no differences in the complications between the techniques were found.
CPN OVER THE MESENTERIC ARTERY (BROAD TECHNIQUE)
Few studies have evaluated the broad technique or have compared it to the other techniques. Sakamoto et al[18] compared the broad CPN technique against the bilateral technique, and this study showed that there was better pain control with the broad approach at 7 and 30 d of follow-up. There were no differences in the adverse events. Another study comparing the broad CPN technique against the broad CPN plus direct ganglia injection technique showed significantly better pain control with the combination of both techniques (OR 3.69 in the 1st week and OR 6.37 in the 1st month)[14]. Adequate pain management has been obtained by this approach of using both techniques, but more studies are needed to confirm these findings.
COMPLICATIONS
EUS-CPN is described as a safe procedure[6,7,9-11,13-19,23,24]. A total of 44% of complications have been reported, but most of them have been minor and transient. Diarrhea and interim hypotension are frequently observed due to the parasympathomimetic response. Pain exacerbation is another common adverse effect (8%) associated with ethanol injection. Transient inebriation was observed in three Japanese studies[13,14,16].
Major complications have been reported in less than 1% of patients; however, these patients frequently have fatal outcomes. Infection, bleeding, retroperitoneal abscesses, paraplegia and ischemia have been previously reported in the literature[29-34]. Usually, these complications are associated with an incorrect injection site of the neurolytic agent. EUS-CPN must be performed by expert endoscopists and at hospitals with a high volume of procedures.
NEW TECHNIQUES OF EUS-CPN
Recently, other techniques of EUS-CPN have been described with encouraging results. In 2012, Wang et al[35] achieved a EUS-CPN by the insertion of a radioactive seed, I125, directly into the celiac ganglia. Twenty-three patients were included in this study, and there was a significant reduction in pain control and the dose of opioids.
In 2015, Facciorusso et al[36] suggested in a case report that the use of an EUS-CPN associated with the injection of ethanol directly into the tumor could enhance the effects of neurolysis; however, more studies of this approach are needed to confirm the results. Recently in 2019, Bang et al[37] published that an EUS-CPN could be performed with a radiofrequency ablation of the celiac ganglia. Twelve patients were included in this study, and they compared this technique against the traditional EUS-CPN. Radiofrequency ablation obtained better results not only regarding the pain associated with pancreatic cancer, but there was also an improvement in the quality of life scales. However, more studies are needed to validate these approaches.
CONCLUSION
EUS-CPN is a safe and effective therapeutic alternative for short-term pain control in unresectable pancreatic cancer patients. It can allow for a dose reduction of opioids, which are responsible for serious adverse effects that reduce the quality of life of these patients. However, an improvement in patient survival or quality of life after using an EUS-CPN has not been demonstrated in the current literature.
The strengths of our review are the large number of studies collected (many of them are clinical trials) with an acceptable number of patients, and many studies have demonstrated favorable results in the use of EUS-CPN in these patients, even though this technique has been performed by expert endoscopists in centers with a high volume of patients. We also present a scheme for performing this technique that shows a good applicability, and most of the complications of this technique are minor and preventable. There are several techniques for performing an EUS-CPN, all of which are valid, and the most commonly used technique is the central technique, which is known by all expert endoscopists in this field and is the technique we currently perform in our centers.
Therefore, we can conclude that the best predictor for a good response could be the celiac ganglia visualization during the EUS-CPN technique. However, any of the 4 different techniques could be offered to effectively perform an EUS-CPN with no differences in complications between the techniques based on this review.
According to this review, a universal pain reduction scale should be used to design further research and to prevent heterogeneity of the results among the studies. EUS-CPN must be performed by expert endosonographers to achieve the best approach and to have a good outcome from this technique as well as to avoid serious adverse events.
Further research is needed to clarify when to perform an EUS-CPN and whether it should be included as a first-line therapy in addition to traditional medical treatment, whether it should be performed as a prevention prior to chemotherapy or if it should be reserved for patients with uncontrolled pain that is refractory to major opioids. Well-designed RCTs are required to evaluate the improvement of pain, survival and quality of life in these patients.
Footnotes
Conflict-of-interest statement: No conflicts of interest are declared.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: Sociedad Española De Patologia Digestiva; Asociación Española de Gastroenterología.
Peer-review started: March 29, 2021
First decision: June 17, 2021
Article in press: September 8, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Taira K S-Editor: Ma YJ L-Editor: A P-Editor: Zhang YL
Contributor Information
Guillermo Pérez-Aguado, Department of Gastroenterology, Complejo Hospitalario Insular Materno Infantil de Gran Canaria, Las Palmas de Gran Canaria, Las Palmas 35016, Spain. guiperez92@gmail.com.
Diego Martinez-Acitores de la Mata, Department of Gastroenterology, Endoscopy Unit, Complejo Hospitalario de Navarra CHN, Pamplona Navarra 31008, Spain.
Carlos Marra-López Valenciano, Department of Gastroenterology, Endoscopy Unit, Agencia Sanitaria Costa del Sol, Málaga 29603, Spain.
Ignacio Fernandez-Urien Sainz, Department of Gastroenterology, Endoscopy Unit, Complejo Hospitalario de Navarra CHN, Pamplona Navarra 31008, Spain.
References
- 1.Bilimoria KY, Bentrem DJ, Ko CY, Ritchey J, Stewart AK, Winchester DP, Talamonti MS. Validation of the 6th edition AJCC Pancreatic Cancer Staging System: report from the National Cancer Database. Cancer. 2007;110:738–744. doi: 10.1002/cncr.22852. [DOI] [PubMed] [Google Scholar]
- 2.Sirri E, Castro FA, Kieschke J, Jansen L, Emrich K, Gondos A, Holleczek B, Katalinic A, Urbschat I, Vohmann C, Brenner H. Recent Trends in Survival of Patients With Pancreatic Cancer in Germany and the United States. Pancreas. 2016;45:908–914. doi: 10.1097/MPA.0000000000000588. [DOI] [PubMed] [Google Scholar]
- 3.Mekaroonkamol P, Willingham FF, Chawla S. Endoscopic management of pain in pancreatic cancer. JOP. 2015;16:33–40. doi: 10.6092/1590-8577/2890. [DOI] [PubMed] [Google Scholar]
- 4.Caraceni A, Portenoy RK. Pain management in patients with pancreatic carcinoma. Cancer. 1996;78:639–653. doi: 10.1002/(SICI)1097-0142(19960801)78:3<639::AID-CNCR45>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 5.Noble M, Gress FG. Techniques and results of neurolysis for chronic pancreatitis and pancreatic cancer pain. Curr Gastroenterol Rep. 2006;8:99–103. doi: 10.1007/s11894-006-0004-x. [DOI] [PubMed] [Google Scholar]
- 6.Wiersema MJ, Wiersema LM. Endosonography-guided celiac plexus neurolysis. Gastrointest Endosc. 1996;44:656–662. doi: 10.1016/s0016-5107(96)70047-0. [DOI] [PubMed] [Google Scholar]
- 7.Wyse JM, Carone M, Paquin SC, Usatii M, Sahai AV. Randomized, double-blind, controlled trial of early endoscopic ultrasound-guided celiac plexus neurolysis to prevent pain progression in patients with newly diagnosed, painful, inoperable pancreatic cancer. J Clin Oncol. 2011;29:3541–3546. doi: 10.1200/JCO.2010.32.2750. [DOI] [PubMed] [Google Scholar]
- 8. Kappis M. Erfahrungen mit Lokalansthesie bei Bauchoperationen. Verh Dsch Ges Cire 1914; 43: 87. [Google Scholar]
- 9.Sahai AV, Lemelin V, Lam E, Paquin SC. Central vs. bilateral endoscopic ultrasound-guided celiac plexus block or neurolysis: a comparative study of short-term effectiveness. Am J Gastroenterol. 2009;104:326–329. doi: 10.1038/ajg.2008.64. [DOI] [PubMed] [Google Scholar]
- 10.LeBlanc JK, Al-Haddad M, McHenry L, Sherman S, Juan M, McGreevy K, Johnson C, Howard TJ, Lillemoe KD, DeWitt J. A prospective, randomized study of EUS-guided celiac plexus neurolysis for pancreatic cancer: one injection or two? Gastrointest Endosc. 2011;74:1300–1307. doi: 10.1016/j.gie.2011.07.073. [DOI] [PubMed] [Google Scholar]
- 11.Levy MJ, Topazian MD, Wiersema MJ, Clain JE, Rajan E, Wang KK, de la Mora JG, Gleeson FC, Pearson RK, Pelaez MC, Petersen BT, Vege SS, Chari ST. Initial evaluation of the efficacy and safety of endoscopic ultrasound-guided direct Ganglia neurolysis and block. Am J Gastroenterol. 2008;103:98–103. doi: 10.1111/j.1572-0241.2007.01607.x. [DOI] [PubMed] [Google Scholar]
- 12.Wiechowska-Kozłowska A, Boer K, Wójcicki M, Milkiewicz P. The efficacy and safety of endoscopic ultrasound-guided celiac plexus neurolysis for treatment of pain in patients with pancreatic cancer. Gastroenterol Res Pract. 2012;2012:503098. doi: 10.1155/2012/503098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doi S, Yasuda I, Kawakami H, Hayashi T, Hisai H, Irisawa A, Mukai T, Katanuma A, Kubota K, Ohnishi T, Ryozawa S, Hara K, Itoi T, Hanada K, Yamao K. Endoscopic ultrasound-guided celiac ganglia neurolysis vs. celiac plexus neurolysis: a randomized multicenter trial. Endoscopy. 2013;45:362–369. doi: 10.1055/s-0032-1326225. [DOI] [PubMed] [Google Scholar]
- 14.Minaga K, Kitano M, Sakamoto H, Miyata T, Imai H, Yamao K, Kamata K, Omoto S, Kadosaka K, Sakurai T, Nishida N, Chiba Y, Kudo M. Predictors of pain response in patients undergoing endoscopic ultrasound-guided neurolysis for abdominal pain caused by pancreatic cancer. Therap Adv Gastroenterol. 2016;9:483–494. doi: 10.1177/1756283X16644248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puli SR, Reddy JB, Bechtold ML, Antillon MR, Brugge WR. EUS-guided celiac plexus neurolysis for pain due to chronic pancreatitis or pancreatic cancer pain: a meta-analysis and systematic review. Dig Dis Sci. 2009;54:2330–2337. doi: 10.1007/s10620-008-0651-x. [DOI] [PubMed] [Google Scholar]
- 16.Ishiwatari H, Hayashi T, Yoshida M, Ono M, Masuko H, Sato T, Miyanishi K, Sato Y, Takimoto R, Kobune M, Miyamoto A, Sonoda T, Kato J. Phenol-based endoscopic ultrasound-guided celiac plexus neurolysis for East Asian alcohol-intolerant upper gastrointestinal cancer patients: a pilot study. World J Gastroenterol. 2014;20:10512–10517. doi: 10.3748/wjg.v20.i30.10512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gunaratnam NT, Sarma AV, Norton ID, Wiersema MJ. A prospective study of EUS-guided celiac plexus neurolysis for pancreatic cancer pain. Gastrointest Endosc. 2001;54:316–324. doi: 10.1067/mge.2001.117515. [DOI] [PubMed] [Google Scholar]
- 18.Sakamoto H, Kitano M, Kamata K, Komaki T, Imai H, Chikugo T, Takeyama Y, Kudo M. EUS-guided broad plexus neurolysis over the superior mesenteric artery using a 25-gauge needle. Am J Gastroenterol. 2010;105:2599–2606. doi: 10.1038/ajg.2010.339. [DOI] [PubMed] [Google Scholar]
- 19.Iwata K, Yasuda I, Enya M, Mukai T, Nakashima M, Doi S, Iwashita T, Tomita E, Moriwaki H. Predictive factors for pain relief after endoscopic ultrasound-guided celiac plexus neurolysis. Dig Endosc. 2011;23:140–145. doi: 10.1111/j.1443-1661.2010.01046.x. [DOI] [PubMed] [Google Scholar]
- 20.Ascunce G, Ribeiro A, Reis I, Rocha-Lima C, Sleeman D, Merchan J, Levi J. EUS visualization and direct celiac ganglia neurolysis predicts better pain relief in patients with pancreatic malignancy (with video) Gastrointest Endosc. 2011;73:267–274. doi: 10.1016/j.gie.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 21.Téllez-Ávila FI, Romano-Munive AF, Herrera-Esquivel Jde J, Ramírez-Luna MA. Central is as effective as bilateral endoscopic ultrasound-guided celiac plexus neurolysis in patients with unresectable pancreatic cancer. Endosc Ultrasound. 2013;2:153–156. doi: 10.7178/eus.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seicean A, Cainap C, Gulei I, Tantau M, Seicean R. Pain palliation by endoscopic ultrasound-guided celiac plexus neurolysis in patients with unresectable pancreatic cancer. J Gastrointestin Liver Dis. 2013;22:59–64. [PubMed] [Google Scholar]
- 23.Hao SJ, Xu WJ, Di Y, Yao L, Yang F, Jiang YJ, Li J, Jin C, Zhong L, Fu DL. How to improve the efficacy of endoscopic ultrasound-guided celiac plexus neurolysis in pain management in patients with pancreatic cancer: analysis in a single center. Surg Laparosc Endosc Percutan Tech. 2014;24:31–35. doi: 10.1097/SLE.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levy MJ, Gleeson FC, Topazian MD, Fujii-Lau LL, Enders FT, Larson JJ, Mara K, Abu Dayyeh BK, Alberts SR, Hallemeier CL, Iyer PG, Kendrick ML, Mauck WD, Pearson RK, Petersen BT, Rajan E, Takahashi N, Vege SS, Wang KK, Chari ST. Combined Celiac Ganglia and Plexus Neurolysis Shortens Survival, Without Benefit, vs Plexus Neurolysis Alone. Clin Gastroenterol Hepatol. 2019;17:728–738.e9. doi: 10.1016/j.cgh.2018.08.040. [DOI] [PubMed] [Google Scholar]
- 25.Lu F, Dong J, Tang Y, Huang H, Liu H, Song L, Zhang K. Bilateral vs. unilateral endoscopic ultrasound-guided celiac plexus neurolysis for abdominal pain management in patients with pancreatic malignancy: a systematic review and meta-analysis. Support Care Cancer. 2018;26:353–359. doi: 10.1007/s00520-017-3888-0. [DOI] [PubMed] [Google Scholar]
- 26.Arcidiacono PG, Calori G, Carrara S, McNicol ED, Testoni PA. Celiac plexus block for pancreatic cancer pain in adults. Cochrane Database Syst Rev. 2011:CD007519. doi: 10.1002/14651858.CD007519.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaufman M, Singh G, Das S, Concha-Parra R, Erber J, Micames C, Gress F. Efficacy of endoscopic ultrasound-guided celiac plexus block and celiac plexus neurolysis for managing abdominal pain associated with chronic pancreatitis and pancreatic cancer. J Clin Gastroenterol. 2010;44:127–134. doi: 10.1097/MCG.0b013e3181bb854d. [DOI] [PubMed] [Google Scholar]
- 28.Koulouris AI, Alexandre L, Hart AR, Clark A. Endoscopic ultrasound-guided celiac plexus neurolysis (EUS-CPN) technique and analgesic efficacy in patients with pancreatic cancer: A systematic review and meta-analysis. Pancreatology. 2021;21:434–442. doi: 10.1016/j.pan.2020.12.016. [DOI] [PubMed] [Google Scholar]
- 29.Mittal MK, Rabinstein AA, Wijdicks EF. Pearls & oy-sters: Acute spinal cord infarction following endoscopic ultrasound-guided celiac plexus neurolysis. Neurology. 2012;78:e57–e59. doi: 10.1212/WNL.0b013e318248df51. [DOI] [PubMed] [Google Scholar]
- 30.Fujii L, Clain JE, Morris JM, Levy MJ. Anterior spinal cord infarction with permanent paralysis following endoscopic ultrasound celiac plexus neurolysis. Endoscopy. 2012;44 Suppl 2 UCTN:E265–E266. doi: 10.1055/s-0032-1309708. [DOI] [PubMed] [Google Scholar]
- 31.Minaga K, Kitano M, Imai H, Miyata T, Kudo M. Acute spinal cord infarction after EUS-guided celiac plexus neurolysis. Gastrointest Endosc. 2016;83:1039–40; discussion 1040. doi: 10.1016/j.gie.2015.10.044. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed HM, Friedman SE, Henriques HF, Berk BS. End-organ ischemia as an unforeseen complication of endoscopic-ultrasound-guided celiac plexus neurolysis. Endoscopy. 2009;41 Suppl 2:E218–E219. doi: 10.1055/s-0029-1214941. [DOI] [PubMed] [Google Scholar]
- 33.Gimeno-García AZ, Elwassief A, Paquin SC, Sahai AV. Fatal complication after endoscopic ultrasound-guided celiac plexus neurolysis. Endoscopy. 2012;44 Suppl 2 UCTN:E267. doi: 10.1055/s-0032-1309709. [DOI] [PubMed] [Google Scholar]
- 34.Jang HY, Cha SW, Lee BH, Jung HE, Choo JW, Cho YJ, Ju HY, Cho YD. Hepatic and splenic infarction and bowel ischemia following endoscopic ultrasound-guided celiac plexus neurolysis. Clin Endosc. 2013;46:306–309. doi: 10.5946/ce.2013.46.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang KX, Jin ZD, Du YQ, Zhan XB, Zou DW, Liu Y, Wang D, Chen J, Xu C, Li ZS. EUS-guided celiac ganglion irradiation with iodine-125 seeds for pain control in pancreatic carcinoma: a prospective pilot study. Gastrointest Endosc. 2012;76:945–952. doi: 10.1016/j.gie.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 36.Facciorusso A, Maso MD, Barone M, Muscatiello N. Echoendoscopic ethanol ablation of tumor combined to celiac plexus neurolysis improved pain control in a patient with pancreatic adenocarcinoma. Endosc Ultrasound. 2015;4:342–344. doi: 10.4103/2303-9027.170428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bang JY, Sutton B, Hawes RH, Varadarajulu S. EUS-guided celiac ganglion radiofrequency ablation vs celiac plexus neurolysis for palliation of pain in pancreatic cancer: a randomized controlled trial (with videos) Gastrointest Endosc. 2019;89:58–66.e3. doi: 10.1016/j.gie.2018.08.005. [DOI] [PubMed] [Google Scholar]