Abstract
Objectives.
The aim of this review is to highlight recent progress in the field of biomaterials-mediated dental pulp tissue engineering. Specifically, we aim to underscore the critical design criteria of biomaterial platforms that are advantageous for pulp tissue engineering, discuss models for preclinical evaluation, and present new and innovative multifunctional strategies that hold promise for clinical translation.
Materials and methods.
The current article is a comprehensive overview of recent progress over the last five years. In detail, we surveyed the literature in regenerative pulp biology, including novel biologic and biomaterials approaches, and those that combined multiple strategies, towards more clinically relevant models. PubMed searches were performed using the keywords: “regenerative dentistry,” “dental pulp regeneration,” “regenerative endodontics,” and “dental pulp therapy.”
Results.
Significant contributions to the field of regenerative dentistry have been made in the last five years, as evidenced by a significant body of publications. We chose exemplary studies that we believe are progressive towards clinically translatable solutions. We close this review with an outlook towards the future of pulp regeneration strategies and their clinical translation.
Conclusions.
Current clinical treatments lack functional and predictable pulp regeneration and are more focused on the treatment of the consequences of pulp exposure, rather than the restoration of healthy dental pulp.
Clinical relevance.
Clinically, there is great demand for bioinspired biomaterial strategies that are safe, efficacious, and easy to use, and clinicians are eager for their clinical translation. In particular, we place emphasis on strategies that combine favorable angiogenesis, mineralization, and functional tissue formation, while limiting immune reaction, risk of microbial infection, and pulp necrosis.
Keywords: regenerative dentistry, tissue engineering, biomaterials, pulp therapy, endodontics
Introduction
The dental pulp is a connective soft tissue surrounded by dentin, a non-elastic and non-vascularized mineralized tissue [1]. Dentin and pulp maintain an intimate relationship via odontoblasts found in the peripheral palisade pulp layer, which forms a unified tissue called the pulp-dentin complex [2]. Due to this unique configuration and the lack of a collateral blood supply, pulp tissue has relatively low compliance [1]. Therefore, inflammation is likely to lead to pulp tissue death when injured as a result of disease [3,4]. Worldwide, over 2,300,000,000 adults and 560,000,000 children are affected by caries [5]. According to the World Health Organization, it is the most common non-communicative disease in the world [6]. When left untreated, carious lesions provide a route of entry for pathogens in the pulp, and ultimately into the body through its intimate vasculature, thus posing a risk for systemic infection [7]. Methods for protecting and regenerating the dental pulp are clinically necessary to prolong the lifetime of the natural dentition [8].
Traditional dental pulp therapy involves removal of pulp tissue during endodontic treatment and sealing the pulp chamber with synthetic inert materials. While largely effective in the maintenance of masticatory function and aesthetics in the near-term, endodontically-treated teeth lose vitality and biological defense mechanisms against microbial infections, making them highly susceptible to recurrent caries and apical periodontitis [4]. These potential morbidities cause considerable loss to the quality of life [4,9]. Regenerative dentistry aims at the functional restoration and regeneration of healthy dental, oral, and craniofacial tissues [10]. The goal of regenerative dentistry, in the context of vital pulp therapy, is to restore homeostasis to the dental pulp following insult and diminish the potential for inflammatory response or loss of tooth vitality. These clinical methods take advantage of the intrinsic regenerative potential of the pulp and periapical tissues, as well as recent advances in molecular and stem cell biology, biomaterials science, and tissue engineering. Worth noting, a complete history of vital pulp therapy is beyond the scope of this review; therefore, we aim to highlight recent strides in the field of regenerative endodontics over the last five years. A historical summary of dental pulp tissue engineering is comprehensibly described in recently published reviews [11–13].
In this comprehensive multi-part review, we start by offering an analytical appraisal of biologically-based strategies for dental pulp therapy currently used in clinical practice. The second part presents key considerations for devising tissue engineering strategies and criteria for the evaluation of new vital pulp therapies. Next, we provide a critical assessment on the latest innovations in pulp-dentin complex regeneration, with specific attention to antimicrobial approaches, anti-inflammatory and pro-angiogenesis strategies, and methods to support cell-homing and/or stem cell differentiation. These critical criteria are summarized in Fig. 1. Finally, we close by highlighting recent work in the area of multifunctional approaches to engineering the pulp-dentin complex, and by addressing challenges and opportunities in the field for the effective translation of technologies and therapeutics likely to support predictable pulp-dentin complex regeneration in humans.
Figure 1.

Schematic overview of considerations for multifunctional pulp-dentin complex regeneration presented in this review. Each of the four broad categories, represented by different colors, are key design motifs that will inspire the next generation of biomaterials and vital pulp therapies. Examples of each motif are provided but are not exhaustive.
Current Clinical Standards of Dental Pulp Therapy
Two biologically-based strategies are currently used in clinics; namely, vital pulp therapy (VPT) and regenerative endodontic treatment (RET) (Fig. 2). VPT aims to preserve and maintain pulp tissue compromised of but not destroyed by extensive caries, trauma and restorative procedures, or iatrogenic reasons [14]. When the pulp becomes exposed, this healthy, mildly inflamed connective tissue is capable of self-repairing due to the presence of mesenchymal stem cell (MSC) niches laden with dental pulp stem cells (DPSCs) in the inner pulp tissue. These cells have the potential to differentiate towards many lineages, including odontoblasts, which play a key role in reparative dentinogenesis. Therefore, differentiated odontoblasts close to pulp exposure are responsible for synthesizing and depositing local collagen-rich dentin matrix upon adequate signaling [15].
Figure 2.

Graphical representation of viable pulp therapy treatment options discussed in this review. Direct pulp capping and evoked bleeding are the current standards of clinical care. Here, we present considerations and recent progress in next generation modalities of pulp-dentin tissue engineering in the form of biomaterial constructs with diverse designs and biologic activities.
The principle of VPT treatment is to stimulate the pulp-dentin complex repair via direct pulp capping (DPC). DPC is a minimally-invasive treatment in which adequate capping agents are applied on the pulp to allow its healing and protect it from additional injury [16]. The most currently employed capping agents are based on calcium hydroxide (CaOH2) and calcium silicates. Calcium hydroxide was first introduced in endodontics in 1920 and has been widely used since. Following its application in direct contact with pulp tissue, a layer of coagulation necrosis is formed. This aggressive pulp effect resulting from such alkaline capping material causes a localized inflammatory reaction that stimulates defense mechanisms and repair from resident cells present in the adjacent viable pulp tissue [15]. Repair involves vascular and inflammatory cell migration and proliferation to eliminate the irritant agent. Concomitant with the decrease in pulpal inflammation intensity, migration of MSCs and endothelial cells to the injured area is observed with time, where local differentiation of odontoblasts occur. Then, these secretory cells synthesize and deposit a collagen-rich dentin matrix that characterizes the poorly tubular tertiary dentin commonly formed beneath the necrotic layer partially replaced by dystrophic calcification [15,16,17]. Together, tertiary dentin and dystrophic calcification formed after capping the pulp tissue with CaOH2-based materials have been termed as a calcified barrier [15]. The heterogeneous calcified barrier exhibits tunnel defects that compromise adequate pulp sealing, resulting in this connective tissue being more susceptible to recurrent infections. This pathway, which is considered a defensive response rather than a regenerative event, results in the death of a number of pulp cells that would play a role in tissue repair/regeneration [15]. Therefore, clinical success is highly dependent on the healing potential of the remaining pulp tissue [2]. Such as observed for CaOH2, calcium silicate-based materials cause tissue necrosis, determining that they have similar mechanisms of action when applied on pulpal wounds [15]. Despite the thinner necrotic layer formed adjacent to calcium silicate-based materials in comparison to CaOH2, odontoblast differentiation and deposition of tertiary dentin occurs when used as capping agents [15,17]. According to Ricucci et al. [18], reparative dentin deposition following DPC represents a repair response that produces calcified scar tissue by pulpal fibroblasts.
RET encompass several strategies to restore pulp vitality by using a biological-based approach [2]. The evoked bleeding technique has been used with the aim of regenerating a pulp-like tissue in a disinfected root canal. In this strategy, the migration of stem cells from the apical papilla into root canals is mechanically induced to reach a complete and successful pulp regeneration. However, Altaii et al. [19] reported that data from animal and human studies indicate that tissues formed after applying this modality of RET are often different from physiologic dentin and pulp. In that systematic literature review, the authors described that radiographic analysis of human teeth demonstrates evidence of success by dentin walls thickening, narrowing the root apex, and increasing the root length. On the other hand, histological analysis showed the appearance of cementum- and periodontal ligament (PDL)-like tissues, rather than pulp tissue. In fact, Nosrat et al. [20] reported that stem cells may be mobilized into the root canal from different sources other than the apical papilla, including bone marrow, and that these cells do not differentiate into odontoblast-like cells. Animal studies also demonstrated the absence of an odontoblast-like cell layer and dentin-like structure, along with soft tissue [21, 22]. Mineralized tissue on the walls of the canal features cementum/bone-like tissue characteristics with irregular thickness, and the soft tissue present in the canal space is similar to PDL. Therefore, it seems that RET procedures promote biologic healing but with distortion of the healthy tissue phenotype, which may have lasting implications on the ability of the tooth to resist future insults [1,19].
An Introduction to Tissue Engineering in Dentistry
With the goal of promoting de novo dentin and pulp regeneration, leading to the establishment of tissues with physiologic structure and function as the pulp-dentin complex, researchers and clinicians are eager for next-generation techniques to improve on current VTP and RET clinical outcomes [23]. The field of tissue engineering aims to restore, repair, and replace tissues in the human body [11–13, 24,25, 26]. In designing a tissue engineering strategy, three critical components must be considered and carefully chosen: (i) a biomaterial matrix to spatially support and organize neotissue formation, (ii) an appropriate cell source capable of forming the desired tissue of either endogenous or exogenous origin, and (iii) intrinsic factors, such as growth factors, to guide cell differentiation and tissue formation toward a specific phenotype. These factors and key considerations in the context of dentin-pulp complex tissue engineering are outlined in Fig. 1.
Biomaterials-mediated tissue engineering
Biomaterial scaffolds are a critical component of any tissue engineering strategy and serve a critical role in organizing tissue regeneration. Recently, progress has been made in identifying mechanisms by which scaffold architecture influences and guides regenerative outcomes [27]. Scaffolds may be fabricated from natural or synthetic materials and take various forms incorporating hierarchical architectural features, including pores and surface textures, as reviewed in detail by Swanson and Ma [26]. In regenerative dentistry, scaffolds increase platform stability and reproducibility of tissue engineering therapies by providing a controlled environment that can be engineered towards a specific tissue. More complex scaffolds may encompass bioactive cues and/or an integrated drug delivery system to modulate specific aspects in guiding pulp-dentin complex regeneration [19]. Several platforms have been proposed, based on synthetic or natural polymers, blends of polymers and minerals, and association with therapeutic drugs or growth factors (GFs) in various forms. Key considerations include creating scaffolds with a micropatterned architecture, nanofibrillar surface, and/or enrichment with dentin/pulp components to mimic native extracellular matrix (ECM) characteristics [28].
Key considerations in scaffold architectural features include identifying those that facilitate cell proliferation and differentiation and provide provisions for infection ablation, cell chemotaxis, adequate angiogenesis into engineered constructs, managing local tissue inflammation, and reproducible outcomes. Many of these approaches draw inspiration from nature in which the tailor-made scaffold closely mimics the natural environment of the target cells and provides mechanical and biochemical cues to promote specific interactions between the cells and matrix [29]. According to Yelick and Sharpe [30], all of these aspects will facilitate the development of improved dental repair therapies, which can be exploited to an ultimate goal of functional tooth regeneration, including whole-tooth regeneration.
Here, we will first highlight recent progress in each of these areas, then describe multifunctional strategies that combine two or more of these criteria, which we deem to be the future of biomaterials-based dentin-pulp complex engineering in clinical practice. For the scope of this review, we intend to focus largely on biomaterial innovations and the intersection between materials science and integration of molecular signals, which, together, drive regeneration. Our review will encompass pre-made bulk scaffolds also referred to as cut-to-size scaffolds, and injectable systems, such as self-assembly hydrogels, photo-crosslinked hydrogels, and microspheres. We will also discuss both cell-laden and cell-free approaches.
Evaluating novel biomaterials for pulp-dentin complex engineering
Significant progress has been made in regenerative dentistry towards the development of technologies and clinical strategies to repair and regenerate pulp and dentin. Nevertheless, further advances are necessary, as several challenges still need to be addressed. A sequence of in vitro and in vivo analyses is necessary to characterize newly developed biomaterials, their safety, and efficacy, for a better understanding of key aspects related to pulp-dentin complex regeneration. Within this scope, we highlight key criteria that should be addressed in these preclinical biological studies: (i) direct interaction of a biomaterial with cells of the dental pulp to demonstrate that cell-material interactions favor cell adhesion, spreading, and proliferation within the biomaterial structure; (ii) cellular expression of the desired physiologic phenotype by cells in direct contact with the biomaterial and in defect proximity; (iii) angiogenesis within the scaffold structure, adequate for pulp regeneration; (iv) the potential for biomaterials to serve as cell-free treatments that modulate cell migration into and throughout its structure; and (v) the potential of engineered constructs to develop functional tissues with similar hierarchical architecture as native tissues. Once these criteria are met through rigorous in vitro and in vivo preclinical studies, then these technologies are ripe for consideration of human use in clinical dentistry.
In vitro and in situ strategies for evaluating regenerative strategies
Characterization of cell-material interaction by using laboratory culture techniques is the first biological analysis necessary after biomaterial development. Biomaterial structures must allow viable cells to adhere to its surface, spread throughout its porous network, and proliferate. The cells, most commonly dental pulp stem cells (DPSCs), are seeded directly onto the scaffold surface, and the constructs are cultured in vitro for several weeks and assayed at various timepoints [31,32]. At early timepoints, it is important to establish how well cells proliferate within the construct; for example, during the first week. Cell-seeded constructs should be subjected to qualitative observation of the cells in contact with scaffold structure, infiltration, and cytoskeletal organization [33,34]. It is important to determine how cells migrate throughout and populate the construct to form 3D tissues [32] (Fig. 3A-C). Second, it is critical to quantify cell differentiation and matrix deposition to demonstrate the bioactive potential of a scaffold in facilitating neotissue formation, and its capability to lead cells toward a desired phenotype either through direct response to the biomaterial environment or in combination with inductive cues. For application in dentin regeneration, demonstrating robust mineral deposition, using both qualitative and quantitative assays, is essential [31].
Figure 3.

In vitro strategies for biomaterials’ evaluation. A – Direct cell seeding onto the scaffold structure. It is important that the cells are seeded exclusively on scaffold by culturing them in a drop of culture medium (a) sufficient for scaffold absorption. After an adhesion period, the cells are cultured in complete culture medium (b). B – Cell viability assay is an important evaluation after creating a new biomaterial. Live-dead assay allows for the observation of cells at the material surface (a) and, in transversal slices, it is possible to observe viable cells throughout the whole material structure (green = viable; red = dead). C – Evaluating adhesion and the spread by F-actin staining demonstrates cytoskeleton organization according to scaffold architecture. Here it is possible to observe that the degree of porosity of a chitosan scaffold allowed for an increased cell spread. Plain chitosan scaffold with a low degree of porosity (a) results in cell aggregation (b) whereas, a macro-porous chitosan-calcium scaffold (CHCa) (c) allows the cells to spread throughout the material structure. D – Dentin disc assay. (a) A human dentin disc with standardized perforation allows scaffold adaptation (b), and the cell/scaffold/dentin construct is further cultivated (c). E – Alizarin red staining demonstrates that calcium-rich matrix was confined to scaffold structure (arrow) on a chitosan scaffold (a) whereas, the presence of a bioactive drug (simvastatin) improved deposition of calcium on the scaffold and surrounding dentin (*). F – Cell migration assay by transwell device. (a) Cells are seeded onto the transwell membrane, and after the cultivation period, cells migrate through it and adhere to the scaffold’s surface. Representative SEM images of a chitosan-collagen-calcium-aluminate (CHC-CA) surface after 24 h of incubation with a cell/transwell set. Observe the presence of cells (arrow) on the scaffold surface (*). Reproduced with permission from Soares et al. [31,32,34].
In situ models using dentin discs and tooth slices, wherein cell/scaffold construct is adapted to the pulp compartment and is cultured in vitro, allows for a more realistic, complex environment, since dentin contains several growth factors that can also modulate cell differentiation [35]. Soares et al. [32] used this strategy to demonstrate the bioactive potential of a scaffold in the presence of a biologically active cue, and this in situ model allowed for observation of mineralized matrix deposition not only at the scaffold structure, but also into surrounding dentin (Fig. 3D-E), proving that the novel biomaterial formulation had an intense bioactive potential, regardless of dentin components.
Cell migration is critical for a biologically active endogenous regeneration mediated by biomaterial composition. In vitro transwell assays containing membranes with pre-established pores of uniform size are used to assess how cells infiltrate into a material. In this assay, cells are seeded onto the upper membrane surface and the biomaterial is placed at the bottom of cell culture plates. The cell/insert is positioned into a culture plate containing scaffolds or other bioactive material, and the number of migrating cells is quantified. If the scaffold is placed in intimate contact with the lower membrane surface, it is possible to observe the migration of cells through an insert membrane and adhesion onto the scaffold surface [31] (Fig. 3F-G).
One platform, i.e., artificial pulp chambers [APCs] (Fig. 4) is extensively used to evaluate in vitro the interaction of dental materials with pulp cells mimicking the clinical situation. To simulate the in vivo architecture of the pulp-dentin complex, odontoblast-like cells (e.g., MDPC-23) can be seeded onto the pulpal surface of the dentin barrier, and its occlusal side remains exposed to receive the innovative products and/or experimental therapies. After concluding this first step of the experiment, the odontoblast-like cells that remain attached to the pulpal side of the dentin barrier can be assessed regarding their viability, morphology, and phenotype [36–38]. A modified version of the APC platform was recently proposed by Soares et al. [32] to simulate in vitro a clinical situation of pulp exposure. This assembly has allowed for assessment of the chemotactic and bioactive effects of cell-free scaffolds used as capping agents. For this purpose, the authors created a 3D culture model of human dental pulp cells in self-assembly peptide hydrogel containing collagen type 1. This cell-laden collagen matrix was placed under the dentin barrier in which a central perforation was mechanically performed to simulate a pulp exposure. After adapting the set 3D cell culture/dentin barrier to the APC, the experimental cell-free scaffold was positioned into the dentin disc perforation, i.e., in direct contact with the cell-laden collagen matrix. This laboratory protocol allowed to investigate the migration and adhesion of the pulp cells from the 3D culture model to the scaffold surface. Additionally, expression of the odontoblastic phenotype of those pulp cells in contact with the scaffold could be assayed.
Figure 4.
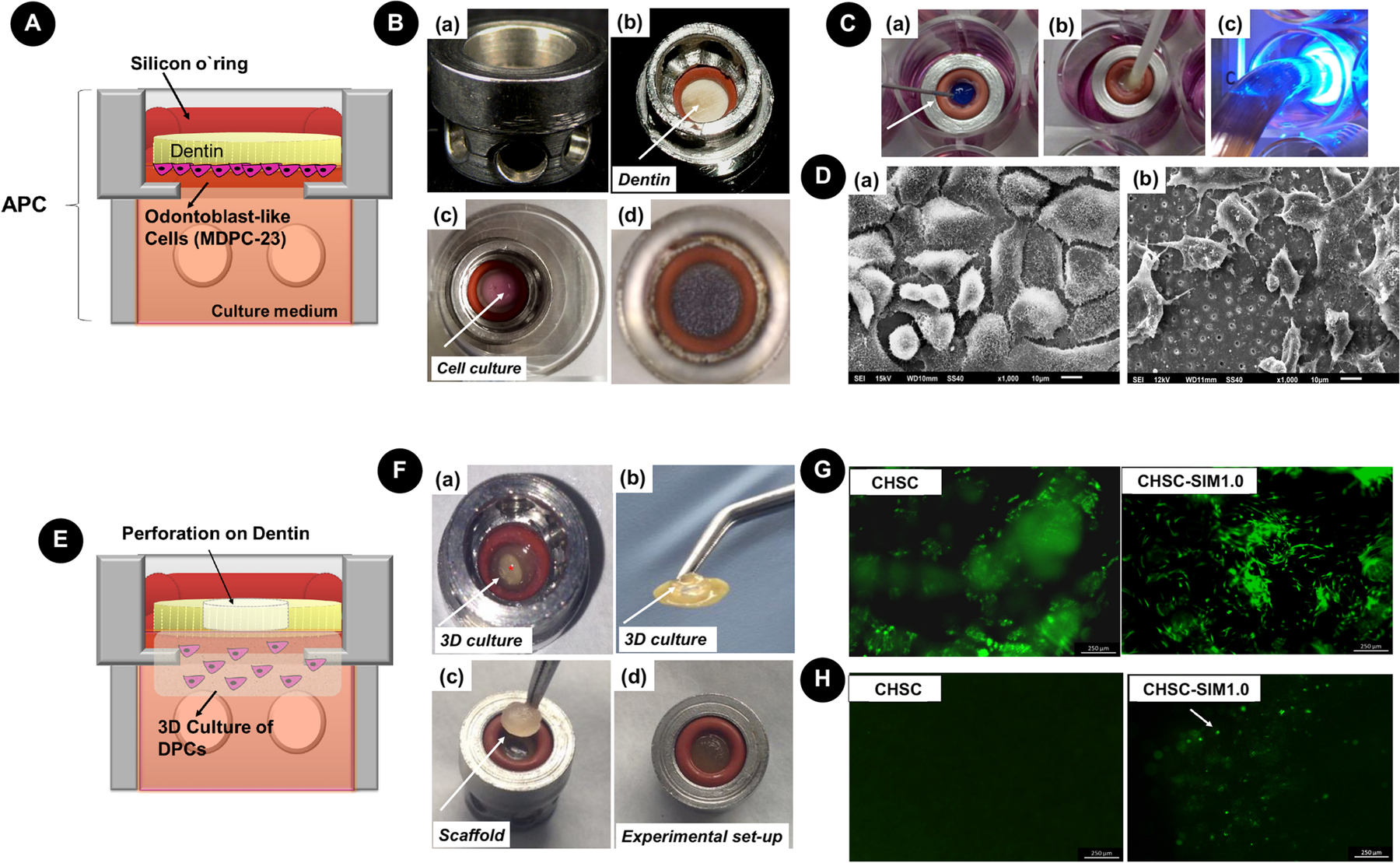
APC platform. A – Schematic representation of APC with dentin disc and odontoblast-like cells seeded onto the pulpal side. B – View APC device (a) and pulpal side of dentin (b). Seeding procedure onto dentin (c) and a view of MTT-stained cells after 24 h (d). Note that the cells are covering the entire dentin surface. C – Representation of the clinical procedure of the adhesive system application (a) phosphoric acid, (b) adhesive, (c) photopolymerization. D – SEM images of MDPC-23 cells seeded onto the dentin surface of untreated dentin (a) and from a sample with an adhesive system (b). E – Schematic representation of in vitro pulp exposure. A perforation is made on the dentin, and a 3D culture of pulp cells is established on the pulpal side of the dentin. F – View of 3D culture on APC (observe perforation in *) and detail of the dentin/3D culture (b). Scaffold is placed on the occlusal side of dentin (c) and the setup (d) is cultivated in 24-well plates. G – Live/dead images of the 3D culture in contact with a plain chitosan (CHSC) and chitosan-simvastatin (CHSC-SIM1.0), demonstrating that cells remain viable under this system. H – Live/Dead images of the scaffold surface after cultivation on this system. Note the presence of migrating cells on CHSC-SIM1.0. Reproduced with permission from Leite et al. [37] and Soares et al. [32].
Ex-vivo culture strategies
Ex-vivo tooth slices and tooth-culture models are also interesting when evaluating the potential of biomaterials to induce pulp-dentin complex regeneration in a laboratory environment [39–42]. The entire tooth-culture model has been used to evaluate the potential of biomaterials in pulp capping procedures, since in this model, pulp-capping agents can be utilized as they are employed clinically. Additionally, the pulp cells remain within their natural structural arrangement and can function in nearly similar physiological conditions as those in the human body. According to Pedano et al. [41], this model can be considered an improved cell-culture model, as it lacks immune and vascular systems but provides a cheaper, faster, and realistic tool to screen new pulp-capping formulations before performing in vivo experiments. A series of studies using this model demonstrated that it is possible to imitate the initial steps of reparative dentinogenesis of human teeth in a similar timeframe [43,44,42].
In vivo ectopic transplantation models
Using animal models to evaluate the biological activity of novel biomaterial constructs is considered essential to proving their regenerative potential. The basic analysis of constructs includes direct implantation or injection of a biomaterial (either with or without cells) into the subcutaneous tissue of immunocompromised mice. Favorable cell-material interactions, cell migration and host integration, deposition of ECM inside the scaffold structure, scaffold mineralization, expression of phenotypic markers, and early angiogenesis can be evaluated. Also, the degree of degradation and its compatibility with tissue genesis can be tested, as well as tumorigenic potential [27,45].
Pulp-dentin complex regeneration potential can be tested in direct contact with a dentin surface by using animal models with adequate subcutaneous vascularization, such as mice [46–48,23]. This methodology is also called the semiorthotopic regeneration model [49], as it uses tooth slices (1–1.5 mm) from human molars, root fragments (3–5 mm), and full-length roots (10–11 mm) from human premolars, in which dentin walls treatment is performed to simulate root canal therapy. The generation of a pulp-like tissue is frequently observed via the transplantation of precursor cells seeded in the scaffold cast within the pulp chamber/root canal space implanted in the subcutaneous pocket of immunodeficient mice [50,35]. It is possible to perform preconditioning in vitro, or simply mix the cells with scaffold at the time of implantation. This approach allows for the establishment of hierarchical organization of an engineered pulp-like tissue, including observation of odontoblast-like cells differentiation near the dentin walls, cytoplasmatic projections into dentinal tubules, pre-dentin deposition, and degree of vascularization.
Direct in vivo dental procedure models
Animal teeth can be used in vivo to evaluate dentin and pulp-dentin complex regeneration. Small and large animal models can be used, such as in the rat, ferret, mini pig, and dog. These strategies allow for the evaluation of native cells from periapical and pulp tissues in specific locations in the body, which constitute a condition that highly mimics humans. Larger animals with tooth sizes comparable to humans allow for testing, not just for orthotopic pulp regeneration, but also for clinical simulation. For a more detailed explanation of these methods, refer to an elegant review by Nakashima et al. [49].
Biomaterials-based Tissue Engineering Strategies for Pulp Regeneration
The biomaterials-based approach to pulp-dentin complex regeneration involves the classical concept of tissue engineering, in which the three essential factors, the scaffold, bioactive molecules, and transplanted cells, are working synergistically in vitro to create a functional tissue that is later implanted in the patient. Mesenchymal stem cells (MSCs) from dental origin are the most common approach to regenerating pulp tissue, more specifically, adult dental pulp stem cells (DPSCs) usually from impacted third molars, stem cells from human exfoliated deciduous teeth (SHED), and stem cells from the apical papilla (SCAP), as each is derived from pulp tissue or precursor tissue. Therefore, forming a pulp-like tissue is more predictable than using other sources, such as periodontal ligaments, bone marrow, and adipose tissue, due to its capability to differentiate into odontoblast-like cells being questionable [2]. The first studies that succeeded in creating an in vitro pulp tissue with similar architecture as vital human pulp were performed with scaffolds pre-seeded with SHEDs by using the tooth slice/scaffold model of dental pulp tissue engineering [35]. These experiments were considered a proof-of-principle, since they demonstrated the ability of SHEDs to generate a dental pulp-like tissue and differentiate into odontoblasts and endothelial cells, since a tooth slice model does not take into account the three-dimensional geometry of root canals that influence oxygen/nutrient diffusion.
Associating angiogenic cues and biologically active hydrogels
Immediate vascularization of engineered pulp tissue is essential for the success of pulp regeneration in clinical scenarios, since oxygen delivery is provided solely through the root apex. Therefore, complete vascularization of the full root space is essential for allowing pulp tissue regeneration and biological function. Injectability is considered an important characteristic for endodontic regeneration that allows scaffold adaptation into the narrow and irregular architecture of root canals, which would be difficult to obtain with pre-shaped scaffolds. The incorporation of cell binding motifs, such as RGD (arginine-glycine-aspartic acid), is also considered essential to improving cell adhesion, recognition, spread, and migration throughout the scaffold structure. In view of this, various pulp regeneration strategies have been proposed by means of: (i) injectable matrices with optimized cell-recognition for delivery of angiogenic and chemotactic cues; (ii) co-delivery of cells and biological cues, or co-delivery of two cell types to create a functional pulp tissue with optimized vascularized structure; and (iii) creating pre-vascularized biomaterials.
Self-assembly peptides (SAP) represent a class of hydrogels widely studied for endodontic regeneration due to their adequate injectability for acting as a suitable carrier for DPSCs, endothelial cells, and growth factors (GFs). In a classical study, Galler et al. [51] demonstrated the establishment of cell adhesive, enzyme-cleavable SAP nanofibers capable of stabilizing GFs’ release via heparin binding. They proposed the use of multidomain peptides (MDP) designed to display the RGD domain for cell adhesion improvement, and a matrix metalloproteinase 2 (MMP-2) sensitive cell adhesive peptide sequence to act as an enzyme cleavable site for cell-mediated degradation. The 3D structure features a fiber diameter of approximately 6 nm, mimicking the nanoscale dimensions and structure of native ECM. Additionally, the hydrogel was functionalized by heparin-bound transforming growth factor β1 (TGFβ1), fibroblast growth factor basic (FGF2), and vascular endothelial growth factor (VEGF), creating a sustained release system over 14 days capable of improving DPSCs’ behavior and differentiation and the angiogenic phenotype of human umbilical vein endothelial cells (HUVECs) seeded onto its structure. The authors tested this dual domain MDP hydrogel functionalized or not with GFs in combination or not with DPSCs in 3-mm root canal cylinders subcutaneously implanted in mice. In the presence of GFs, cell homing from mice tissue was observed, with fatty and connective tissues detected. When GFs and DPSCs were incorporated, vascularized soft connective tissue resembling dental pulp was observed, with hydrogel being replaced by connective tissue deposited by DPSCs and cells at the cell-dentin interface resembling odontoblast-like cells emitting cellular processes extended into the dentinal tubules. In the absence of GFs, DPSCs were not able to lead tissue formation.
PuraMatrix™ SAP hydrogel has been considered a versatile biomaterial for endodontic regeneration due to its injectability, presence of RGD domains, and nanofibrous architecture. According to Rosa et al. [48], it allowed for homogeneous SHED incorporation and injection into the roots of human premolars (11-mm length). Upon subcutaneous transplantation into the dorsum of immunodeficient mice, it was possible to observe the establishment of a pulp-like tissue in the full extension of the root canal, very similar to human pulp. The engineered tissues featured connective tissue containing multiple blood vessels with vessel density, a number of cells close to the predentin, and the occurrence of apoptotic cells similar to human tissue samples. Nevertheless, in the absence of cells, this hydrogel was not able to generate a pulp-like tissue. Dissanayaka et al. [52] further demonstrated that PuraMatrix™ can act as a multifunctional scaffold system to the delivery of DPSCs and co-cultures of DPSCs and endothelial cells (HUVECs) to promote the regeneration of vascularized pulp in vivo. The peptide nanofiber microenvironment supported cell survival, cell migration, and capillary network formation in the absence of exogenous GFs, with the early vascular network promoted by HUVECs due to increased VEGF expression. These cell constructs were injected into human root canals and transplanted into mouse subcutaneous tissue. Collective results showed that the combination of DPSC/HUVEC cells resulted in more pulp-like tissues and revascularization than the DPSC group.
More recently, Silva et al. [53] created a sustained release system for chemotactic and angiogenic GFs from hyaluronic acid hydrogel reinforced with cellulose nanocrystals (CNCs). CNCs were proposed to improve the hydrogel’s mechanical properties and immobilize GFs at the negatively charged surface, thus creating a sustained release system. The authors proposed an in situ gelation protocol by dual-syringe injection: one syringe containing hydrazide-functionalized hyaluronic acid (ADH-HA) solution enriched with human platelet lysate (PL); and a second syringe containing aldehyde functionalized hyaluronic acid (a-HA) with or without aldehyde-modified CNCs (a-CNCs). Hydrogels incorporating CNCs enriched with PL were produced through hydrazone cross-linking chemistry between the hydrazide/amine and aldehyde groups. A double-barrel syringe, fitted with a static mixer placed at the outlet, allowed for gel mixing, resulting in highly porous and stable hydrogels quickly formed upon cross-link reaction of the precursor components, regardless of the formulation (Fig. 5A). The incorporation of CNCs allowed for a sustained release of angiogenic growth factors from the hydrogel structure, with longer releasing times proportional to the CNCs’ concentration. 3D reconstruction based on confocal imaging showed a well-developed multidimensional network of cell-to-cell and cell-matrix contacts throughout the PL-laden hydrogel. The effect of the PL-laden hydrogel on hDPCs and endothelial cell invasion or sprouting into biomaterial structure was evaluated by embedding DPSCs/HUVECs pellets. Over time, cell invasion was significantly greater in PL-laden hydrogels in sprout length from co-culture pellets embedded in PL-laden hydrogels compared to PL-free constructs (Fig. 5B).
Figure 5.

A - Schematic representation of the proposed in situ crosslinking system in which the hydrazide-functionalized hyaluronic acid (ADH-HA) solution with or without PL, and aldehyde functionalized hyaluronic acid (a-HA), with or without aldehyde-modified CNCs (a-CNCs), are co-injected using a double barrel syringe. For biological assays, human dental pulp cells (hDPCs) were added to ADH-HA solution before injection in the molds. B – Cell invasion and sprouting assay. (a) (1) cell pellets on transwell are (2) overlaid by an acrylic mold for (3) polymeric solutions injected to encapsulate the cell pellet, followed by (4) cultivation up to 3 days. (b) Cell pellets within hydrogels after 3 days of incubation. Note cell invasion in the presence of PL, which was more evident for the 0.25% CNC/PL group). C - Schematic representation of the proposed strategy to engineer prevascularized full-length dental pulp-like tissue constructs. Representative longitudinal (a) and cross-sectional (b) images (Green= GelMA; Red = microchannel) (c) and (d) shows digital photographs with detail for the channel throughout GelMA. D - Confocal images of endothelialized microchannels in an OD21-laden GelMA hydrogel cultured in a full-length dental pulp-like tissue construct. Note that CD-31 positive cells were adhered to the microchannel surface; whereas, OD-21 cells adhered to dentin. Reproduced with permission from Silva et al. [53] and Athirasala et al. [58].
Other experimental self-assembly hydrogels impregnated with angiogenic cues have been proposed to enhance vascularized pulp tissue regeneration. Mu et al. [54] proposed RADA16-I, a synthetic matrix composed of periodically-repeated positively-charged arginine, hydrophobic alanine, and negatively-charged aspartic acid. This composition lead to spontaneous nanofibrous hydrogel assembly in neutral pH with 99% water content. To create a bioactive, pro-angiogenic injectable biomaterial for pulp regeneration, stem cell factors (SCF) were incorporated during the gelation process of RADA16-I at 100 ng/mL (selected by dose-response assay). This association lead to the development of a biomaterial capable of recruiting DPSCs and endothelial cells (HUVECs), promoting cellular adhesion, and migrating throughout the hydrogel structure, along with improved proliferation, differentiation, and angiogenesis by cells in contact with the construct. According to these results, SCF-RADA16-I could be used as a carrier to encapsulate DPSCs and endothelial cells into the root canal and provide a 3D environment similar to the internal pulp environment.
Another promising biomaterial with great application for endodontic regeneration is gelatin methacryloyl (GelMA) hydrogel, due to its injectability and photo-crosslinking, acquiring a natural intrinsic shape that makes the clinical translation more predictable [55]. As gelatin is a hydrolysate of collagen, resulting in significantly lower immunogenicity, a variety of bioactive motifs remain available for cell interaction, including RGD and matrix metalloproteinase (MMP) enhancing cell binding and cell-mediated matrix degradation, respectively. Of note, the incorporation of methacrylic anhydride at ~ 5% does not affect functionality of biologically active motifs. The crosslinking process occurs in the presence of a photoinitiator, which can react in the presence of UV or LED lights used in clinical practice [56]. Parameters, such as hydrogel stiffness and chemical composition, can be tailored to create a multifunctional biomaterial with designed functionalities adapted to pulp-dentin regeneration.
Khayat et al. [57] proposed the use of GelMA-encapsulated hDPSCs/HUVECs for pulpal regeneration. The authors injected GelMA with hDPSCs/HUVECs or acellular GelMA into human premolars’ root canals and photocrosslinked them by UV light. Following 4 or 8 weeks of subcutaneous implantation on nude rats, it was evident that the cellular GelMA was capable of driving formation of a vascularized and organized pulp-like tissue, features that were not detected in acellular constructs. Two important characteristics were observed on GelMA-encapsulated hDPSC/HUVECs toward pulp revitalization; namely, (i) the formation of organized and functional vasculature within a highly cellularized pulp center, and (ii) cell attachment to the inner dentin surface, with formation of cellular extensions into dentin tubules of the human tooth root segment and increased matrix deposition at the tooth root’s inner dentin layer.
Another consideration explored in recent literature is the prevascularization of biomaterial constructs for vascularized pulp tissue engineering. Athirasala et al. [58] described an innovative technique to fabricate pre-vascularized pulp-like tissue constructs in a full-length root canal. The rationale was to create a path throughout the full extension of the tooth root, from the apex to the cervical portion, by using a sacrificial fiber composed by agarose positioned in the root canal prior to loading with 15% GelMA. After hydrogel photopolymerization, the sacrificial fiber was removed (Fig. 5C). To perform a proof-of-principle experiment, the authors encapsulated odontoblast-like cell lineage OD21 cells on GelMA, and then injected the cell-laden hydrogel into EDTA-treated root canals. After removing the sacrificial fiber, endothelial colony forming cells (ECFC) were seeded inside the microchannel. These constructs were cultured in vitro for 7 days, making it possible to observe that the cells self-organized inside the hydrogel structure, with OD21 cells migrating to the dentin surface and the endothelial cells attaching to channel walls, thus forming angiogenic sprouts (Fig. 5D). According to the authors, pre-vascularized hydrogel constructs may ensure establishment of a vascular network throughout the root canal from the onset of the regenerative process, and the microchannel would allow migration of host cells from the root apex to the hydrogel structure.
Microspheres with designed topography for dual delivery function
As we have described with hydrogels, injectable solutions are highly desirable in regenerative dentistry, as they are easy to administer into small defects and conform to the anatomy of irregularly shaped defects. As an alternative to hydrogels, a promising injectable strategy for pulp-dentin complex regeneration is the use of microsphere suspensions. According to Zhang et al. [59], microspheres ranging from 100–400 μm in diameter allow fast nutrient and oxygen diffusion compared to bulk hydrogels. The superficial topography and porosity can be adjusted by different techniques to allow them to act as a drug delivery or cell delivery system at its surface, or by cell encapsulation [60].
Biodegradable nanofibrous PLLA microspheres (NF-MS) have been developed as tissue engineering scaffolds for cell and/or biological cues’ delivery, with the potential to regenerate and repair irregularly-shaped tissue defects due to their injectability and controllable biodegradability [61]. Surface topography mimics the physical architecture of collagen fibers on a nanoscale, with an average diameter of about 160 nm, thus facilitating cell adhesion, proliferation, and differentiation [62]. This architecture was fabricated by combining thermally-induced phase separation techniques with an emulsification process, allowing for a high surface area and porosity, which, in turn, facilitates cell growth, nutrient/waste exchange, and faster scaffold degradation compared to its smooth surface counterparts [63,64].
A dual injectable strategy for pulp-dentin regeneration was proposed by Wang et al. [65], with the use of PLLA NF-MS as a carrier for SCAP delivery in combination with PLGA microspheres for controlled BMP-2 delivery (BMP2 MS). After a set of in vitro experiments that selected the ideal parameters for cell attachment and differentiation, the multifunctional system composed of a BMP2 MS/NF-MS/SCAP mixture was injected into mice subcutaneous pockets. According to macro- and microscopic evaluations, angiogenesis was improved in the presence of BMP-2, along with intense mineralization throughout the collected tissue, with areas of mineralization embedded with DSPP positive cells resembling osteodentin.
A spongy architecture was also established for these PLLA nanofibrous microspheres (NF-SMS), allowing the cells to more easily enter into its structure, thus creating a more efficient cell delivery [64,66]. These NF-SMS were fabricated from a star-shaped poly(L-lactic acid)-block-polylysine (PLLA-b-PLYS) copolymer using a reverse emulsification method [60] (Fig. 6A). Kuang et al. [64] demonstrated that NF-SMS has great potential as an injectable scaffold for dentin regeneration and as a cell deliver carrier, with better outcome compared to non-porous nanofibrous (NF-MS) and solid (S-MS) microspheres. The cells were cultured in vitro using a dynamic system and higher cell density was achieved for NF-SMS. NF-SMS induced significantly higher ALP activity, calcium deposition, and gene expression of dentin sialophosphoprotein (DSPP) in comparison with the other sphere’s configuration. The sphere/cells’ constructs were subcutaneously injected into the dorsum of nude mice, and after 6 weeks, NF-SMS allowed for increased tissue deposition, a higher degree of mineralization, and a strong expression of DSPP (Fig. 6B). According to the authors, the hollow pore architecture provides more space for cells’ penetration generating bigger and denser tissues, indicating its potential to facilitate pulp-dentin complex regeneration.
Figure 6.

A - Fabrication of NF-SMS by means of emulsions self-assembled from SS-PLLA-b-PLYS, followed by phase separation and freeze-drying. The porous structure of NF-SMS allows cell loading and delivery through injection. B - Interactions of human dental pulp stem cells (hDPSCs) with NF-SMS, NF-MS, and S-MS (from top to bottom). (a-c) SEM after 24 h of cell seeding, with cells in NF-SMS attached on the surface and inner pores (d-f) Confocal images of F-actin staining (red) with a large number of cells being detected on NF-SMS. (g-i) H&E staining of constructs harvested from nude mice subcutaneous. NF-SMS had a higher degradation prolife, with abundant cells in the neo tissue. C - Schematic illustration of the synthesis of heparin-conjugated gelatin (HG) and hierarchical VEGF-loaded HG-MS. The nanofibrous PLLA microsphere (a) encapsulated gelatin nanospheres (b) containing heparin bonded VEGF (c). D – Panel of H&E and immunostaining of regenerated pulp-like tissues in the full-length root canal after in vivo implantation for nine weeks. (a-d) H&E sections for each group. In each group, 1, 2, and 3 stands for the coronal third, middle third, and apical third of every root canal, respectively. In the absence of cells, there was minimal or absence of tissue regeneration. The injection of DPSCs allowed for fibrous tissue formation at the apical third area. Pulp-like tissue regeneration fulfilled both the apical and middle third regions and reached the coronal third of the canal in the VEGF-loaded HG-MS/DPSC group, with a large number of blood vessels throughout the canals, and odontoblast-like cells aligned with the existing tubular dentin of the root. (e-h) Immunohistochemical stained images of vWF. (i-l) Immunohistochemical stained images of DSP. Strong vWF and DSP staining was observed in the VEGF-loaded HG-MS/DPSC group. Reproduced with permission from Kuang et al. [64] and Li et al. [61].
In a sequential study, Kuang et al. [66] tested NF-SMS scaffold to carry hypoxia primed hDPSCs to promote vascularized pulp-like tissue. The DPSC/NF-SMS construct was incubated in a hypoxia-bioreactor system. After 3 days in a bioreactor, the NF-SMS was injected into extracted rabbit molar pulp cavities. Four groups of rabbit molars filled solely with NF-SMS and hDPSCs, and with hypoxia- or normoxia-primed hDPSCs/NF-SMS complexes in the emptied pulp chambers, were implanted into dorsal subcutaneous pockets in mice. Additionally, the authors performed endodontic treatment on mice first molars and injected normoxia and hypoxia primed DPSC/NF-SMS constructs. The ectopic implanted tooth model indicated more blood vessels formed, along with CD31 positive cells in the hypoxia group, compared to the other three groups. Hypoxia also improved establishment of the odontoblastic layer lining at dentin walls, more intensely expressing DSPP relative to the other groups. The in-situ experiment confirmed that hDPSCs/NF-SMS could fully fill the molar canals via injection and promoted pulp-like tissue formation with intimate integration with the native dentin, and NF-SMS could undergo nearly complete degradation within 4 weeks. Hypoxia played a significant role in the vascularization and odontoblastic differentiation, and demonstrating that hypoxia-primed hDPSCs/NF-SMS effectively regenerated a vascularized pulp in an in-situ model.
Li et al. [61] described an injectable hierarchical microsphere system for full-length pulp regeneration. The controlled release of VEGF was achieved by binding this GF to heparin and further encapsulating it into heparin-conjugated gelatin (HG) nanospheres. This delivery system was then immobilized in nanofibrous biodegradable (PLLA) microspheres (MS) (Fig. 6C). The obtained hierarchical microspheres (HG-MS) acted as both a cell carrier and a controlled growth factor delivery vehicle. As a cell carrier, HG-MS is self-assembled with PLLA nanofibers that mimic the architecture of natural collagen fibers on a nanometer scale. VEGF has binding domains with heparin, and binding VEGF to heparin protects it from denaturation and proteolytic degradation, which subsequently prolongs its sustained release. Therefore, release of VEGF from HG-MS is controlled by a multiple-layer that includes binding with heparin, degradation of the HG nanosphere, and physical adsorption of the HG-MS nanofibers. To evaluate pulp-like tissue regeneration mediated by this innovative system, the authors used 13-mm human premolar roots subjected to endodontic treatment and disinfection. The construct was injected into a root canal, and a 2-mm layer of MTA was used to seal the coronal canal end, resulting in a 1 mm opening aperture at the root apex, simulating the clinical scenario. The VEGF-loaded-HG-MS group regenerated pulp-like tissue fulfilling the whole apical and middle third space, reaching to the coronal third of the canal (~ 9 mm from the apex of the root) with many blood vessels throughout the canals and odontoblast-like cells aligned with the existing tubular root dentin (Fig. 6D).
Hybrid platforms for simultaneous microbial ablation and pulp regeneration
To achieve success in the pulp-dentin regeneration process, root canal disinfection after necrotic tissue clearance is essential. Ideally, this procedure should not cause negative effects on progenitor stem cells in order to allow regenerative procedures to be effective. Nevertheless, it is known that current protocols applied in clinical scenarios consist of irrigant solution, calcium hydroxide-based cements, and/or the application of antibiotic pastes inside the root canals. Besides causing tooth discoloration, these strategies also present a high degree of cytotoxicity on precursor cells in the periapical site, which undermines their ability to regenerate tissue. Therefore, the development of multifunctional scaffolds that combine antimicrobial activity with the bioactive effects in a cell-friendly platform has been considered to be a highly translational approach over the last 10 years [10].
Evoked bleeding is a treatment option recently used after root canal decontamination, in which a blood clot is intended to act as a temporary fibrin-based scaffold inside the root canal system, allowing for the migration of undifferentiated cells from apical papilla, leading to cell differentiation and root-end [67,68]. Regrettably, it has been suggested that failure of this regenerative process may be due to the presence of residual solutions and root canal-administered drugs that interfere with cell viability/differentiation and compromise adequate pulp-dentin regeneration [69].
Next, we discuss the most recent and technologically innovative studies that proposed multifunctional scaffolds to improve pulp/dentin regeneration and promote microbial infection ablation.
Cell-friendly antibiotic-releasing nanofibers for infection ablation
In the past, many groups have demonstrated the powerful bacteriostatic effect of minocycline (MIN) applied directly within root canals [68]. However, side effects were reported, as material cytotoxicity was observed, leading to a significant reduction in the angiogenesis process when evoked bleeding was used to mediate regeneration of the pulp-dentin complex in these cases. This is due to the action mechanism of minocycline, which suppresses the growth factor production necessary to stimulate neovasculogenesis by endothelial cells in tissue regeneration [67,70].
Kamocki et al. [69] were able to incorporate Ciprofloxacin (CIP) at different concentrations into polydioxanone polymer (PDS) nanofibers obtained by electrospinning, for purposes of obtaining an efficient material that could be clinically implemented in the endodontic disinfection process without compromising the viability of undifferentiated stem cells from remnant pulp and apical papilla, thus keeping the inherent capacity to regenerate pulp tissue. Proliferation and cytotoxicity assays were performed to analyze the indirect effect of extracts containing released components from PDS-CIP nanofibers on DPSCs up to 21 days. It was possible to conclude that small concentrations of CIP (1 and 2.5 wt.%) presented in nanofibers did not have a negative effect on cell viability, in addition to their maintaining antimicrobial properties, restraining the growth of Enterococcus faecalis, and no alterations in tooth color [69].
Pankajakshan et al. [71] incorporated PDS nanofibers with triple antibiotic (TAP - MET, MIN, and CIP), and applied them for 3 days onto dentin specimens containing dual species biofilm (Actinomyces naeslundii and E. faecalis). It was possible to detect bacterial elimination that was in the same range as TAP solution. DPSCs seeded onto dentin surfaces pretreated with PDS-TAP and TAP adhered well to PDS-TAP-treated surfaces but not to TAP-treated surfaces. Karczewski et al. [72] created PDS nanofibers’ incorporated clindamycin (CLIN) and clindamycin-modified triple antibiotic paste (CLIN-m), thus eliminating MIN from the composition (Fig. 7). CLIN was the drug of choice in this study to bring forward the broad-spectrum bacteriostatic effect over microorganisms related to persistent endodontic infection. As a result, the effectively obtained materials inhibited the growth of A. naeslundii, E. faecalis, Aggregatibacter actinomycetemcomitans, and Fusobacterium nucleatum, with CLIN-m showing the best results over time, comparable to CLIN and chlorhexidine (CHX). Also, these nanofibers did not promote tooth pigmentation even after 3 weeks of incubation. Besides, high cell viability was observed for DPSCs in contact with CLIN nanofibers (70%) compared with CLIN-m (50%) throughout the 28 day-trial period. Collectively, these results demonstrated that loading antibiotics into nanofibrous mats minimize toxicity to dental pulp cells compared to protocols traditionally employed in daily clinical practice and it may be potentially applied as a mediator of the endodontic regeneration process.
Figure 7.
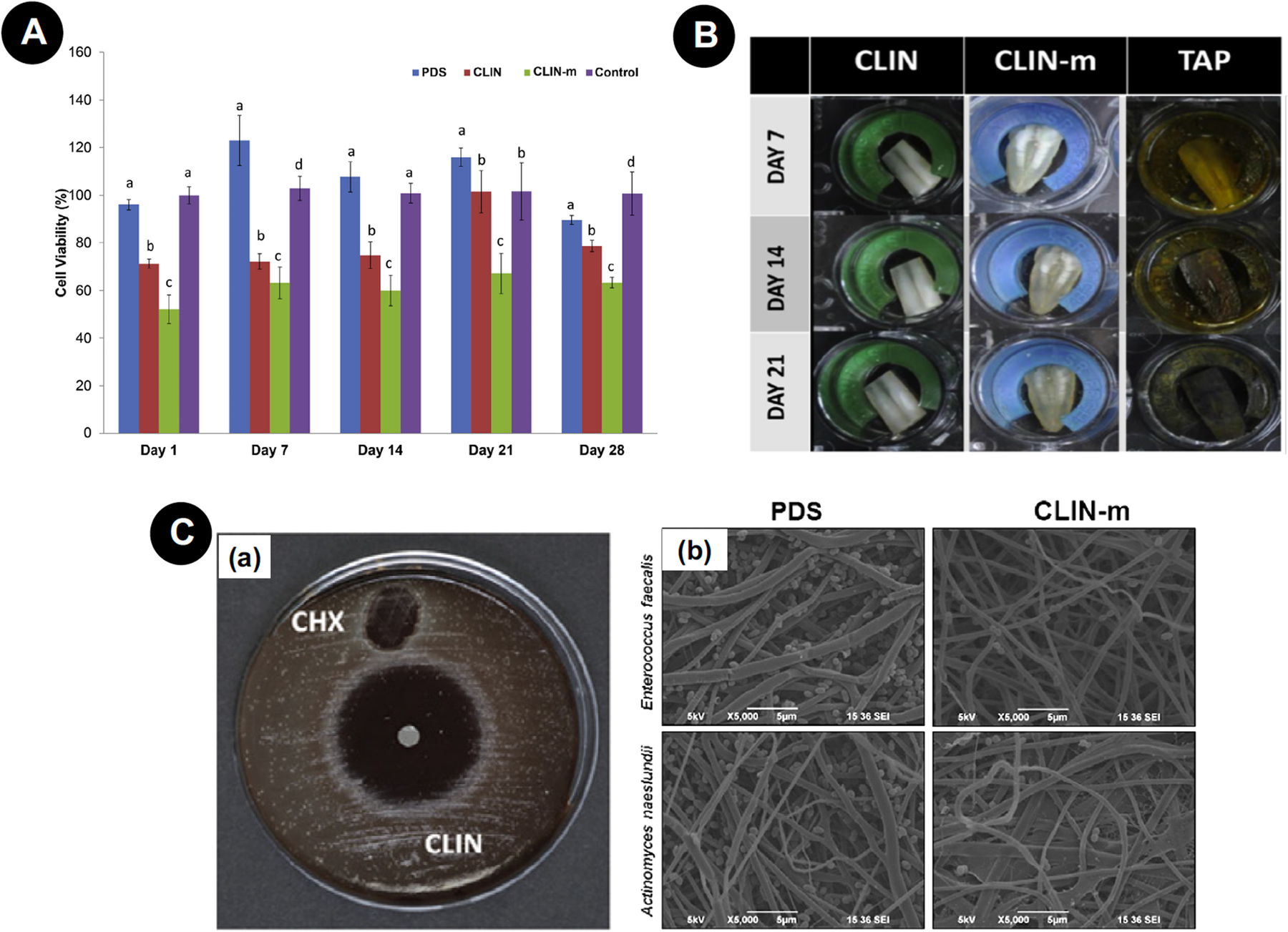
A - Human DPSC viability in response to extracts collected from nanofibers on 1, 3, 7, 14, 21, and 28 days. Significant difference is denoted with a different letter (*P < .05) when compared with the control. Statistical analyses were compared with the same-day results. B - Representative macrophotographs showing human dentin color stability/change after 1, 7, 14, and 21 days of exposure to CLIN and CLIN-m nanofibers and TAP paste. C - (a) Representative blood agar plate showing growth inhibition zones of CLIN-containing fibers and the control (0.12% CHX) against F. nucleatum. (b) Representative SEM micrographs showing growth inhibition of A. naeslundii and E. faecalis on CLIN-m electrospun nanofibers when compared with PDS. Reproduced with permission from Karczewski et al. [72].
Aimed at a translational approach, Bottino et al. [73] created, via electrospinning, 3D tubular PDS constructs loaded with triple (MET, CIP and MINO) antibiotics (~ 2.5 mg/mL each) with the intention of promoting dentin disinfection, resolution of periapical lesions, and stimulating apexification via mineralized tissue deposition in immature permanent teeth in combination with RET (Fig. 8A). In vitro studies were first performed to evaluate the antimicrobial efficacy of constructs in intimate contact up to 7 days with the root canal space of dentin tooth slices infected by A. naeslundii. The results have shown a 99.1–99.94% reduction in bacterial contamination for the tubular 3D constructs containing antibiotics. From these data, this construct was considered appropriate for in vivo testing in immature premolars of canines (dog) with incomplete periapical closure and periapical disease, combined with evoked bleeding treatment. A drug delivery system with low dosages of three antibiotics created in this study was capable of decreasing microorganisms involved in persistent endodontic infection (Fig. 8B). This biomaterial successfully promoted the osteo/odontoblastic phenotype expression by mesenchymal stem cells from apical papilla, which enabled the mineralized matrix deposition to guide root formation and apex closure. Accordingly, similar results were obtained for the construct evaluated in this study and TAP paste; but in a positive way, the tubular 3D TAP-nanofibers’ group demonstrated a significant reduction in negative side effects. Similarly, the authors observed defense mechanism preservation, complete root formation, and less dentin weakness, aside from the restoration of tooth function with this treatment regimen (Fig. 8C-D).
Figure 8.

A - (a) TA-3DC and SEM micrograph (×5000) of the triple antibiotic-eluting nanofibers obtained via electrospinning. (b) Image showing the smooth fitting of the TA-3DC inside the root canal and the method used (CLSM) to verify its disinfection ability against A. naeslundii biofilm. B - (a) CLSM images of 7-day A. naeslundii biofilm growth inside dentinal tubules (control) and treated for 7 days with (b) TAP solution or (c) tubular 3D triple antibiotic-eluting construct (TA-3DC). C - Images showing the disinfection procedure performed for the in vivo study (a) TAP paste loaded into a plastic syringe and injected into the canals, (b) tubular 3D electrospun fibers adapted inside canal space (c) tubular 3D triple antibiotic-eluting construct (TA-3DC) group prepared to fit in the tooth prepared. D - (a) Hematoxylin-eosin stained micrograph of extracted infected tooth treated with TAP solution, showing a thin bridge of apical osteodentin with early periapical bone destruction. (b) Hematoxylin-eosin stained micrograph of extracted infected tooth treated with the tubular 3D triple antibiotic-eluting construct, showing thick layer of regenerated osteodentin restoring the outline of the root apex. The osteodentin demonstrates cellular inclusions and resembles a combination of bone, osteoid, predentin, and dentin. Reproduced with permission from Bottino et al. [73].
Dual function hydrogels with regenerative and antimicrobial function
The integration of nanofibers containing antimicrobial agents into highly biocompatible injectable hydrogels has shown significant clinical promise in regenerative endodontics. Ribeiro et al. [74] designed a hybrid hydrogel based on ciprofloxacin (CIP)-releasing nanofibers for microbial ablation. The authors used β-cyclodextrin (β-CD) to improve CIP solubility and facilitate drug diffusion through a hydrogel structure (Fig. 9A). PDS nanofibrous mats loaded with CIP or CIP/β-CD inclusion complex (IC) were processed into short nanofibers via cryo-cutting and then embedded in GelMA to obtain an injectable hybrid antimicrobial hydrogel. A slight reduction in cell viability, around 25–35%, was noted for DPSCs treated with extracts collected from both hydrogels at 3 and 7 days (Fig. 9B). Moreover, 2.5% GelMA-CIP/β-CD-IC-SF demonstrated growth inhibition for E. faecalis in planktonic form, proving the antimicrobial effect of inclusion complex β-CD incorporated on hydrogel structure ensured by higher surface area supplied by short fibers (SF) . Furthermore, to address the clinical application of developed biomaterials, human dentin slices were incubated for 7-days with E. faecalis to form an organized biofilm. Then, CIP and CIP/β-CD-IC hydrogels were applied over the dentin specimens, followed by 1-week’s incubation to evaluate the antimicrobial effectiveness. A significant decrease in the number of live bacteria was observed in the 2.5% GelMA-CIP/β-CD-IC-SF group (Fig. 9C). These results suggest the effective design of a cell-friendly drug delivery hydrogel system with potential therapeutic application in controlling endodontic infections.
Figure 9.

A - Representative macroscopic image of the homogeneous injectability distribution of the embedded short nanofibers within the hydrogel. B - Viability of DPSC cells after 24 h of cell exposure with eluates collected at days 1, 3, and 7 from GelMA-based hydrogels. Same letters above the bar columns indicate nonsignificant differences compared with the results on the same day (p > 0.05). C - Representative SEM images of antimicrobial activity for (a) infected dentin (control) and after treatment for 14 days with (b) 2.5% GelMA-PDS-CIP/β-CD-IC-SF (10 µm). Reproduced with permission from Ribeiro et al. [74].
In another study, Ribeiro et al. [75] synthesized an MMP-responsive hydrogel with antimicrobial activity based on GelMA modification with halloysite clay nanotubes (HNT) loaded with chlorhexidine (CHX). Briefly, halloysite clay nanotubes’ (HNT) were functionalized with 10% and 20% CHX, which was then incorporated into GelMA at 2.5% and 5.0%, respectively (Fig. 10A). To determine the sustained antimicrobial effect of the engineered hydrogels, agar diffusion assays were performed against several bacteria. Additionally, hydrogel biocompatibility was established in vitro on DPSCs and in vivo by subcutaneous implantation in rats. GelMA containing 5% HNT/CHX-loaded showed a greater halo inhibition zone for all tested microorganisms and demonstrated high antimicrobial effects in direct contact with E. faecalis and in microcosms’ biofilm. The group GelMA hydrogel with 5% HNT-CHX10% reached a minimal cytotoxicity effect in vitro after 28 days (~ 70%) (Fig. 10B-C). In vivo analysis also demonstrated that this biomaterial exhibited low immunogenicity at 7 and 14 days with minimal inflammatory cell infiltration (Fig. 10D), demonstrating the great potential of this hydrogel for pulp-like dentin regeneration in infected root canals.
Figure 10.
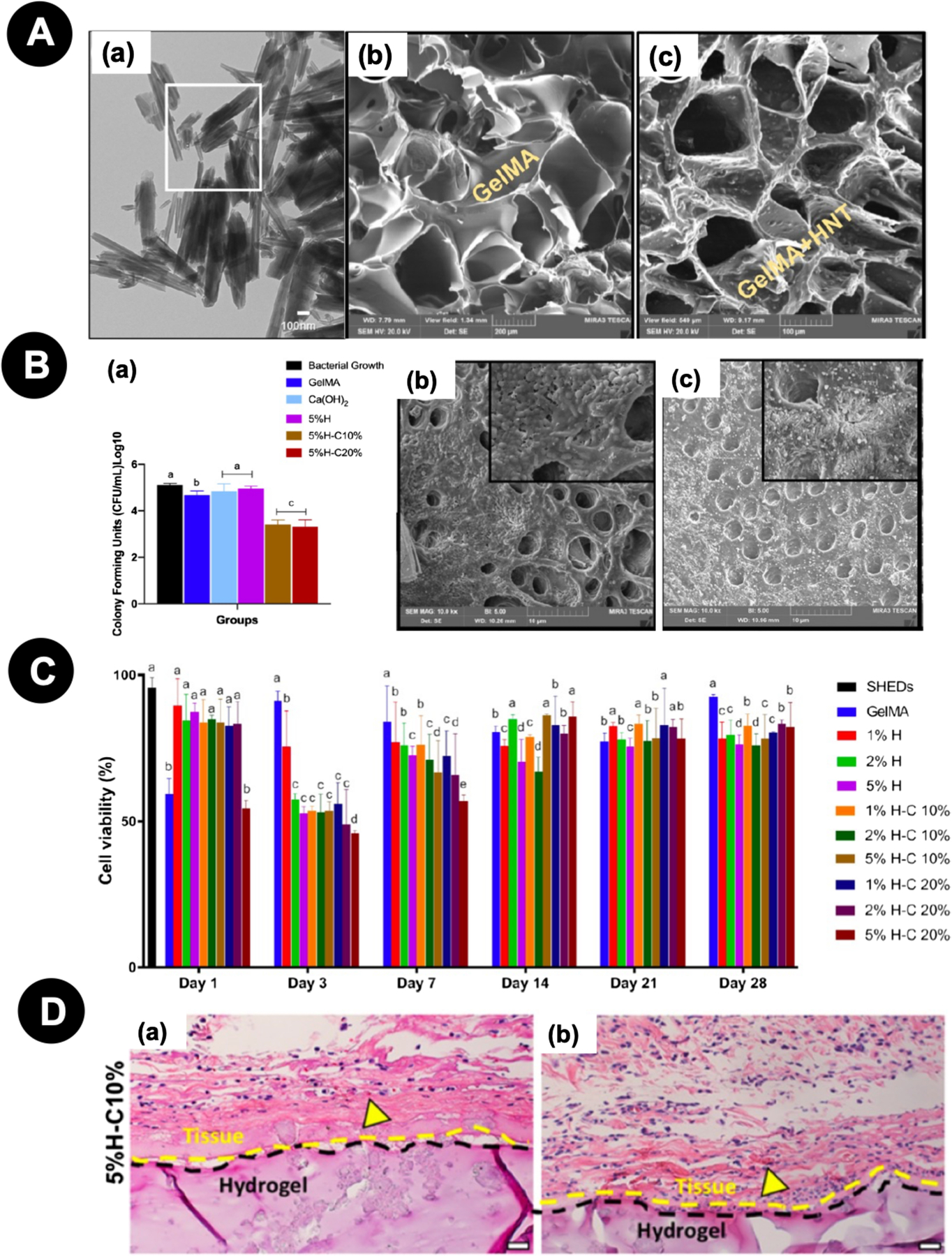
A - (a) Representative TEM micrograph of the as-received HNTs. (b) SEM micrograph of a cross-section of GelMA hydrogel and (c) GelMA modified with CHX-loaded nanotubes (5% H− C10%). B - Microcosm biofilm model. (a) CHX-loaded GelMA-based hydrogels (5%H−C10% and 5%H−C20%) significantly reduced bacterial numbers compared to all other groups. SEM micrographs of bacterial biofilm on the dentin surface of (b) pristine GelMA and (c) GelMA-5%H− C10%. C - Cell viability (%) of SHEDs in response to aliquots at day 1, 3, 7, 14, 21, and 28 from GelMA-based hydrogels modified or not with 1, 2, and 5% of halloysite nanotubes and CHX-loaded nanotubes (H−C10% and H−C20%). Distinct letters indicate statistically significant differences between the groups in the same day when compared with the control (SHED). D - Representative H&E staining images of 5%H−C10% after 7 and 14 days in vivo (scale bar = 200 μm). Yellow arrowheads indicate the presence of numerous blood vessels containing murine erythrocytes. Reproduced with permission from Ribeiro et al. [75].
To combine bioactivity and its antimicrobial effects, Wang et al. [76] proposed association of natural extracellular matrix hydrogels (ECMs) incorporated or not with mesoporous silicate-calcium glass nanospheres doped with Ag ions (Ag-BG). For this, extracts were obtained from the hydrogels and applied for 24 h in contact with Streptococcus mutans / Lactobacillus casei and DPSCs to respectively evaluate the antimicrobial and cytotoxic potential. In vivo pulp/dentin formation was investigated after co-culture DPSCs in hydrogels, followed by their implantation in the dorsum of immunodeficient mice. The Ag-BG/ECM extracts’ treatment resulted in a lack of bacterial growth, cell viability preservation (~ 80%), and cell proliferation over time without affecting DPSCs’ morphology. After 21 days of cell contact with Ag-BG/ECM, the group demonstrated increased expression of odontogenic differentiation markers and mineralized matrix deposition, in addition to immuno-positive DSP and VEGF tissue labeling, confirming the odontoblast-like phenotype and pulp/dentin-like tissue formation after an 8-week implantation.
Development of cell-homing biomaterial platforms
Cell-homing, also known as in-situ tissue engineering or the cell-free approach, has gained increased attention recently as a regenerative strategy, since clinical translation is more predictable. Largely, this is due to significant manufacturing and regulatory considerations for a biomaterial-only or biomaterial and drug-only approach, rather than considering complications with stem cell sourcing, manufacturing, sterilization, and handling. This process uses a scaffold to deliver bioactive cues to recruit resident stem cells and induce their differentiation toward tissue neogenesis. This strategy is highly feasible for stem cell-rich dental pulp, given its variety of stem cell sources in close proximity. Therefore, creating a scaffold capable of promoting deposition of a dentin barrier by resident pulp cells, in the absence of toxicity to remnant pulp, is the goal of current studies. A multifunctional approach for dentin cell-homing encompasses studies that incorporate two or more of the following strategies: (1) create a biopolymer to mimic the improvement of dentin ECM for cell recognition and boost cell differentiation; (2) establish an organized and interconnected macro-porous network to allow for cell ingrowth, adequate vascularization, and needed cell-cell interactions; (3) modulate surface topography to mimic the nanofibrous architecture of native ECM; and (4) release bioactive cues to modulate several cell phenomena, as chemotaxis, differentiation, angiogenesis, and inflammation.
Mineral chemoattractant encourages odontoblast genesis
Blends of the mineral phase with polymeric matrices have been proposed as a biomimetic strategy for dentin cell-homing aimed at improving cell recognition at scaffold structure due to mineral content and promoting a controlled release of ions to create a pro-mineralization gradient. Studies have demonstrated that hydroxyapatite, calcium silicate, calcium phosphate, calcium aluminate, magnesium phosphate, and fluorapatite, solely as scaffolds or in polymeric blends, induce a high mineralization potential [77–79,31,32,34]. Nevertheless, as discussed earlier, there is need for a structured macro-architecture to allow for better interaction of scaffolds with resident cells, allowing them to migrate inside the scaffold architecture. With this goal, Soares et al. [34] developed a strategy to create a chitosan scaffold containing calcium complexed to its structure (CHCa scaffold), with highly organized round macro-pore architecture and an interconnected network. The authors proposed the association of chitosan as a naturally derived polymer that resembles glycosaminoglycans, in association with a mineral phase to improve cell recognition and differentiation, with a designed pore architecture to improve cell ingrowth and neo-tissue-genesis. Ca(OH)2 aqueous dispersion was incorporated into the chitosan solution to modulate both the degree of porosity and chemical composition. This process, in combination with a gradual freezing protocol, gave rise to a highly porous scaffold containing calcium complexed within its structure, generating a controlled Ca2+ release system [32]. An 86.89% degree of porosity and a 202.1 μm pore size was achieved, which allowed DPSCs to spread throughout, also leading them to be more proliferative and overexpress odontogenic markers compared to those seeded onto plain chitosan.
Another interesting mineral-rich formulation was proposed by Soares et al. [31]. The authors developed a tricomponent biomaterial, based on chitosan, collagen, and calcium-aluminate (AlCa), that gave rise to a scaffold with improved chemotaxis, cell-material interaction, and odontogenic differentiation. The CHC-AlCa scaffold was able to enhance the potential of DPCs to deposit mineralized matrix, along with increased cell proliferation and the overexpression of DMP-1 (dentin matrix acidic phosphoprotein 1), DSPP (dentin sialophosphoprotein), and ALP (alkaline phosphatase). The chemoattractant potential was evaluated by means of the transwell assay, in which DPCs were seeded onto the upper membrane (8 µm pore size) and cell-free scaffold was placed in direct contact with the bottom surface. Effective cell migration was detected in the presence of plain CHC and CHC-AlCa scaffold in comparison to control samples (no scaffold) with AlCa-supplemented scaffold featuring significantly higher cell migration. More interesting, the cells migrated through a transwell membrane and adhered onto the CHC-AlCa surface within 24 h [31]. Therefore, CHC-CA promoted odontogenic precursor cell mobilization and allowed for a more intense expression of secreted odontoblastic markers, culminating with deposition of a significant amount of calcium-rich matrix. Sequential studies demonstrated that incorporating 1% AlCa suspension in chitosan scaffolds, using the same fabrication route as described by Soares et al. [34], promoted spatial organization of the macro-porous architecture, thus allowing DPCs to effectively migrate inside it and maintain the chemotactic and bioactive potentials [80,81].
Precise control of specific pore sizes and organization can be achieved by 3D printing. Therefore, it is possible to formulate bioink to contain chemical and biological cues in association with highly controllable microarchitectures to more precisely modulate cell fate. Dubey et al. [82] proposed the development of a bioink with a dual strategy: 1) self-assembly peptide hydrogel to simulate the nanofiber structure of native ECM; and 2) the incorporation of amorphous magnesium phosphate (AMP) particles as a bioactive cue to trigger osteo/odontogenic differentiation DPSCs (Fig. 11A). The authors created a printable 3D matrix with a 500 µm pore grid structure in which AMP particles were well dispersed throughout the printed construct. This bioink allowed printing of the homogeneous encapsulated viable DPSCs displaying an elongated morphology (Fig. 11B). To test the potential of this bioink as a cell-homing strategy for mineralized tissue regeneration, cell-free bioprinted ECM and ECM/AMP scaffolds were implanted on critical defects in rat calvaria (Fig. 11C). ECM and ECM/AMP promoted bone ingrowth from the defect margin towards the scaffold’s center, and the edges of regenerated bone that appear continuous with the surrounding bone after 4 and 8 weeks. The AMP-loaded bioprinted scaffold featured higher bone volume and density, demonstrating that this bioink significantly improved bone formation in comparison to plain ECM scaffold. Histological sections confirmed these results, with the defect area in the ECM/AMP group covered with dense, regenerated bone tissue that was far more extensive than the ECM or control group. AMP particles degraded fast from hydrogel, and these releasing bioactive cues improved the migration of cells from surrounding tissue into the defect, resulting in enhanced bone formation.
Figure 11.

A – (a) ECM/AMP bioink formulation process; (b) Schematic representation of ECM/AMP bioink printing and representative images of composites with and without cells. B – Live/Dead assay for DPSCs’ printed with ECM and ECM/AMC composites after 1, 3, and 5 days (green – live cells; red – dead cells; arrows indicate dead cells). Note that DPSCs show an elongated morphology in AMP-modified constructs. C – Critical sized bilateral defect procedure performed in rats (a) The bioprinted construct was placed onto the PTFE membrane that served as a carrier for the adaptation (c) of printed construct on a prepared defect (b), followed by closure (d). D - Representative Micro-CT and histological analysis of empty defects and those filled with ECM or ECM/1.0AMP composites at 4 weeks (a) and 8 weeks (b). Calvarial defects that were left empty did not heal spontaneously for the duration of the study. In contrast, bone healing was gradually achieved when the defects were filled with either ECM or ECM/1.0AMP, with the latter featuring significantly higher bone volume at both time-points. H&E and Masson’s trichrome staining after 4 weeks indicates healing of the defects with new bone formation restricted to an area close to the border of the defects. After 8 weeks, healing of the defects with new bone formation restricted to the area close to the border of the defects, with the ECM and ECM/1.0AMP showing thicker bone formation compared to 4 weeks. Connective tissue: CT, osteoblast: OB, new bone: NB, blood vessel: BV, osteocytes: OC, woven bone (blue): WB, lamellar bone (red): LB. Reproduced with permission from Dubey et al. [82].
Scaffolds incorporating small molecule and biologic factors for guided regeneration
To improve the endogenous regenerative potential of engineered biomaterials, bioactive cues can be used to direct progenitor or stem cells to the injury site and aid the healing of damaged tissues. This strategy can be achieved by dual release of different molecules or by means of pleiotropic drugs. The release of bioactive substances for direct influence on the behavior of ingrowing cells on the scaffold structure is considered a hallmark of tissue engineering applications. This technique has the potential to increase the amount and quality of neotissue genesis in a shorter period of time [32,33].
The use of pleiotropic drugs allows for a simplistic and effective strategy for a multifunctional approach. Simvastatin (SIM) has been used, as this drug acts as an inducer of osteo/odontogenesis and is a chemoattractant, anti-inflammatory, and pro-angiogenic agent. The incorporation of low-dosage simvastatin on chitosan scaffolds proved to be a good combination as a direct pulp capping biomaterial. Soares et al. [32] demonstrated that chitosan scaffolds capable of releasing 0.05–0.1 µm SIM improved the chemotactic and odontogenic potential of DPSCs. The drug was incorporated onto the scaffold by adsorption at a specific concentration selected by a dose-response assay. Therefore, cells were cultivated onto SIM-loaded scaffolds and remained viable at the surface and inside scaffold structures. The mineralizing potential was demonstrated by adapting the cell/scaffold construct in perforations made in human dentin discs, with calcium-rich matrix being deposited onto the scaffold and surrounding dentin in the presence of SIM [31,32]. To evaluate the potential of these scaffolds as a cell-free approach for dentin regeneration, an artificial pulp chamber (APC) model containing a 3D culture of DPCs was established [32]. It was shown that CHSCSIM1.0 and CHSC-SIM0.5 induced the migration and adhesion of viable DPCs from 3D culture to the scaffold’s surface, and cells in 3D culture exhibited increased gene expression of ALP, Col1, DMP-1, and DSPP in the absence of osteogenic supplementation in the culture medium. All the cell phenomenon described were more intensely mediated by CHSC-SIM1.0.
Li et al. [83] proposed the creation of a bilayer chitosan membrane incorporated with TGF-β1-containing chitosan microspheres as a pulp capping biomaterial. The top side of the membrane consisted of a dense chitosan film to protect pulp from bacterial invasion and interact with lining dental material. In intimate connection, a bioactive layer was established. This layer was composed of a highly macroporous (83% porosity) chitosan structure with a pore opening of around 151 µm to allow for adequate cell infiltration. The authors managed to incorporate this porous surface with microspheres encapsulated with TGF-β1 (MS-TGF), which were thoroughly dispersed on the surface and inside the macropores. The potential of this innovative biomaterial to induce dentinogenesis was tested in experimentally exposed pulps in a dog model. The chitosan membrane + MS-TGF group promoted substantial reparative dentin formation with numerous visible dentinal tubule lines, and the odontoblasts were arranged in a palisade pattern with columnar cell bodies at the reparative pulp-dentin interface. This feature was superior to CaOH2 cement that leads to irregular reparative dentin formation without the presence of dentinal tubules. In the chitosan membrane group without TGF, irregular or absent hard tissue formation was observed.
A combination of the mineral phase with bioactive drugs has also been proposed to boost cell differentiation. Cassiano et al. [81] incorporated SIM on CH-AlCa scaffolds by immersion into 0.5–1.0 µM SIM solutions and observed a synergistic effect mediated by the AlCa mineral phase and SIM on the odontogenic differentiation of DPCs seeded onto scaffold structures. Bordini et al. [80] proposed the incorporation of 1α,25-dihydroxyvitamin D3 (1α,25VD) with CH-AlCa scaffold aimed at improvement of the chemotactic and bioactive potentials. The authors selected the ideal dosage to be incorporated by means of a dose-response assay and found that 1 nM improved both desired cell parameters. A synergistic potential of CH-AlCa and 1 nM 1α,25VD was detected in vitro by improvement of cell proliferation, chemotaxis (transwell assay), and the expression of odontoblastic markers ALP, DMP-1, DSPP, and calcium-rich matrix deposition. This positive effect is a result of activation of the different pathways in which calcium ions that present in the scaffolds interact directly with the cells, and 1α,25VD regulates calcium and phosphate through the ion channel of the cell membrane, resulting in a synergistic effect on cell differentiation. Daghrery et al. [84] proposed the association of nanofibrous PLGA mats containing dexamethasone (DEX)/cyclodextrin inclusion complex (IC) as a pulp capping biomaterial. The authors managed to improve the release time of DEX by beta-cyclodextrin (β-CD) due to the improvement of drug aqueous solubility. Low-dosage sustained DEX release provided by electrospun PLGA/DEX-CD-IC was capable of improving SHEDs differentiation in comparison to PLGA/DEX nanofibers.
Aimed at dentin regeneration, a particularly important strategy is to modulate the inflammatory phenotype of dental pulp cells, rescuing the regenerative potential in challenging situations. Also, improving angiogenesis into biomaterial structure from surrounding tissues is essential for allowing effective neo-tissue-genesis. Soares et al. [33] proposed the association of a highly porous nanofibrous PLLA (NF-PLLA) scaffold with SIM at bioactive dosage (0.1 μM) to improve the regenerative potential of DPSCs under a degenerative inflammatory stimulus and also to favor angiogenesis. The cell/biomaterial constructs were cultured in the presence/absence of LPS at a concentration capable of reducing around 50% deposition of mineralized matrix by DPCs. It was possible to observe that NF-PLLA/SIM partially recovered the odontogenic differentiation of DPCs under LPS treatment, minimized the expression of pro-inflammatory mediators by the pulp cells, and improved the angiogenic potential of endothelial cells at a distance and inside the scaffold. Cell constructs treated in vitro with LPS for 14 days were implanted onto nude mice subcutaneous tissue, and it was noticeable that the deposition of collagenous rich-matrix and mineralization was greatly impaired by LPS treatment, and that, in the presence of SIM, this negative effect was reverted, allowing cells to feature a regenerative potential.
Niu et al. [85] created a microsphere system based on chitosan and PLA for dual release of hydrophobic and hydrophilic molecules aimed at controlling inflammation and inducing odontogenic differentiation of DPSCs. As most anti-inflammatory drugs are hydrophobic and potent odontogenic growth factors are hydrophilic, it remains a great challenge to co-deliver these two types of therapeutics. The authors synthesized chitosan-graft-poly(lactic acid) (CS-g-PLA) copolymers and developed micelle-in-microsphere assemblies and used them to simultaneously co-deliver hydrophobic and hydrophilic biomolecules with distinct release profiles. The hydrophobic steroid drug fluocinolone acetonide (FA) was selected to relieve inflammation and hydrophilic BMP-2 as a bioactive cue for mineralization. This delivery system provides an innovative solution to the need for co-delivery of biochemically distinct molecules to promote dentin regeneration in an inflammatory environment. Hydrophobic FA molecules were encapsulated into the hydrophobic cores of CS-g-PLA copolymeric micelle-like nanospheres. Hydrophilic BMP-2 molecules were then introduced, and micellar emulsion was crosslinked into microspheres. Hydrophilic biomolecules were, therefore, primarily distributed in hydrophilic interspaces between the crosslinked micelle-like nanospheres and the outer CS layer of micelles. BMP-2 was released at a faster rate to promote dentinogenesis at an early stage of treatment. FA was released in a sustained manner to inhibit inflammation during the entire healing process. Cumulative release reached 90% after 2 weeks. The authors observed that dual delivery recovery of the expressions of odontogenic markers on LPS-stimulated DPSCs in a degree higher than single delivery groups demonstrated the synergistic effect of FA and BMP-2 co-delivery.
Driving cell differentiation by favorable texture cues
Others have demonstrated that physical and structural properties of the extracellular matrix onto which cells adhere can determine stem cell fate decisions. Therefore, to achieve dentin regeneration in a close relationship with native tissues, inducing precursor cells to polarize and remain longitudinally aligned with dentin tubules in the pulp-dentin interface would be necessary. Ha et al. [86] proposed to modulate GelMA stiffness and microarchitecture to lead cells into an odontogenic phenotype in the absence of additional cues. First, the authors observed that SCAPs seeded onto stiffer GelMA by increasing their concentration to 10%−15% featured significantly higher cell proliferation and odontogenic differentiation, demonstrating that cell commitment to an odontoblast lineage differentiation is dependent on substrate stiffness. To create micropatterned hydrogels, they designed a mold via 3D printing in which GelMA was dispersed and photo-crosslinked with UV light, creating 60 and 120 µm alternating parallel geometries. Both patterned geometries guided cell alignment along the length, with cells featuring highly elongated cell bodies indicative of odontoblast-like processes. These elongated cells presented higher ALP activity in comparison to non-patterned hydrogels in the absence of odontogenic inductor on culture medium. These data demonstrated that pulp cell precursors are mechanoresponsive, and morphological changes are mediated by substrate microarchitecture direct cell differentiation toward odontoblasts. Therefore, substrate mechanics and geometry may inform cell response and potentially assist in the regeneration of dentin-pulp tissues.
A dual approach to achieving highly organized and vascularized pulp-like tissue regeneration was proposed by Pankajakshan et al. [87], modulating DPSC phenotype into odontoblasts or endothelial cells via hydrogel stiffness and GF incorporation. This innovative technology proposed that a single-cell population could be used to target both endothelial and odontogenic differentiation by using the concentric injection technique in which DPSCs’ transplantation using a stiffer matrix along with BMP-2 would be applied in intimate contact with dentin, and a more compliant matrix containing VEGF would be injected in the root center to allow for vascularized pulp regeneration (Fig. 12A-B). Two hydrogel stiffnesses (235 and 800 Pa) were fabricated by varying collagen concentration. GFs were incorporated prior to gelation, resulting in a slow-release pattern. The effect of stiffness on the cytoskeletal organization and cell shape was evident, in which stiffer collagen (800 Pa) decreased the cell spreading and actin fiber organization, while compliant (235 Pa) collagen supported the growth of elongated DPSCs (Fig. 12C). These mechanical cues induced osteogenic and endothelial differentiation, respectively, and these effects were further enhanced by the addition of specific GFs. According to the authors, these hydrogels could be optimized for delivery and fate regulation of DPSCs in a spatially oriented fashion to form dentin and vascularized pulp at the appropriate locations when concentrically injected into the root canal system, leading to dentin and pulp regeneration in the appropriate locations.
Figure 12.

A - Schematic illustration of the proposed highly-tunable injectable oligomeric collagen-fibril matrix system (a) tooth slice, (b) concentrically injected collagen matrices (the low-stiffness matrix is purposely dyed in red), and (c) interface adaptation between the two matrices evidenced by reflectance microscopy. B – Proposed model schematics in which the DPSC-laden VEGF-incorporated 235Pa collagen matrix is intended to be at the pulp core to allow for vascularized soft tissue regeneration, and DPSC-laden BMP-2-incorporated 800Pa collage matrix contacts the dentin surface to induce odontoblastic differentiation. C – Actin filaments staining (red) of DPSCs seeded onto collagen matrices at different conditions. Overall, it is possible to observe that cell spread differs according to matrix stiffness. Elongated cells being observed on the 235 Pa matrix being increased in the presence of VEGF incorporated into hydrogel or supplemented into culture medium. DPSCs cultured within Oligomer 800 Pa demonstrated decreased spreading, featuring cytoplasmic projections all around the cell periphery, regardless on BMP-2 incorporation/supplementation. Reproduced with permission from Pankajakshan et al. [87].
In a recent study, Dubey et al. [88] proposed a multifunctional approach to achieving a stable platform for mineralized tissue regeneration by associating three strategies: 1) utilizing bioactive inorganic particles to modulate cell fate, 2) using a natural hydrogel with high crosslink density and functional motifs to promote cell interaction, and 3) incorporating a nanofibrous mesh with well-controlled 3D architecture to enhance cell recognition and biomechanical properties. The authors associated amorphous magnesium phosphate (AMP) with photo-crosslinked gelatin methacrylate (GelMA) and incorporated a designed porous poly(ε-caprolactone) (PCL) fibrous mesh. This nanoarchitecture was favorable for MSCs’ attachment, with several cells adhering and spreading onto the nanofiber structure and extending their cellular processes into and around the melt-electrowritten PCL fibers (Fig. 13A-B). The hybrid fiber-hydrogel membrane was created by incorporating this mesh onto GelMA, with consecutive mesh layers increasing mechanical behavior. The PCL mesh and AMP incorporated into GelMA caused a synergistic bioactive effect on MSCs by increasing cell proliferation and osteogenic differentiation in vitro. After implantation of cell-free biomaterials on calvaria defects in rats, the authors concluded that a fiber-GelMA combination had a better outcome towards bone regeneration than plain GelMA, with AMP particles improving this outcome in a dose-dependent fashion (Fig. 13C-D).
Figure 13.

A - (a) SEM image of MEW PCL mesh shows the well-aligned (0–90 °-oriented junctions) fibrous 3D architecture. (b) SEM image of hMSCs-MEW PCL mesh interaction after 3 days of culture (white arrows). (c) Actin filaments (red) of hMSCs-MEW PCL mesh interaction. B – View of PCL mesh before (b) and after (b) GelMA incorporation. (b-c) SEM image showing hydrogel phase uniformly infiltrated into the pores of the polymer mesh. C - Bone defects with or without (sham) membrane were scanned by micro-CT (G=GelMA; GP=GelMA/PCL; GA5%=GelMA/AMP5%; GPA=GelMA/PCL/AMP5%). Note that both PCL mesh and AMP increased mineralization in comparison to GelMA, and a synergistic effect being observed. G- Masson’s trichrome-stained sections of implanted membrane after 8 weeks, in which is possible to observe the higher amount of new bone (Nb) in the presence of AMP and PCL in comparison to plain GelMA. St: soft tissue; Sd: GelMA; Nb: new bone. Reproduced with permission from Dubey et al. [88].
Recapitulating in vivo cell-cell communication through controlled release of biologic factors
The use of exosomes from DPSCs and SCAP, in association with scaffolds and stem cells, with the aim of recapitulating the paracrine stimulation to mediate tissue regeneration, has been considered promising for pulp and dentin regeneration. Exosomes are rich in RNA material, thus playing an important role in the transference of mRNA and miRNA, increasing proliferative potential, and featuring immunomodulatory and pro-angiogenic effects [89]. Previous work has shown that exosomes from DPSCs [90,91], SCAP [92], and Hertwig’s epithelial root sheath cells [92] positively modulate pulp-like tissue regeneration and angiogenesis both in vitro and in tooth root models in vivo. An innovative strategy described by Swanson et al. [45] created a self-assembling synthetic PLGA-based triblock copolymer vehicle with tunable degradation properties to encapsulate and control release dentinogenic exosomes as regenerative pulp capping biomaterial (Fig 14A-B). Exosomes were collected from human DPSCs (DPSC-EXO) and murine odontoblast-like cells’ lineage (MDPC-EXO). Poly- (lactic-co-glycolic acid) block, poly- (ethylene glycol) block, and poly- (lactic go-glycolic acid) block (PLGA-PEG-PLGA), an amphiphilic triblock copolymer, was used to encapsulate exosomes and form exosome-loaded microspheres (EXO-MS). PEG is the hydrophilic segment of the PLGA-PEG-PLGA copolymer that was designed to interface with aqueous droplets containing exosomes inside the hydrophobic PLGA body of a microsphere driven by hydrophilic/hydrophobic self-assembly. A formulation capable of promoting sustained DPSC-EXO release up to 10 weeks was selected (Fig. 14C) and a series of in vitro and in vivo analyses were performed to prove the integrality and bioactivity of exosomes eluted from polymer microspheres. A rat pulp-capping model was performed without cell transplantation, in which EXO-loaded microspheres were applied onto exposed pulps, followed by glass ionomer cement restoration (Fig. 14D). The sustained local release of DPSC-EXO and MDPC-EXO at the pulp interface resulted in more complete dentin healing and strong dentin bridge formation after six weeks in vivo. This platform is of interest, because it harnesses natural cell-cell communication methods without requiring stem cell transplantation.
Figure 14.
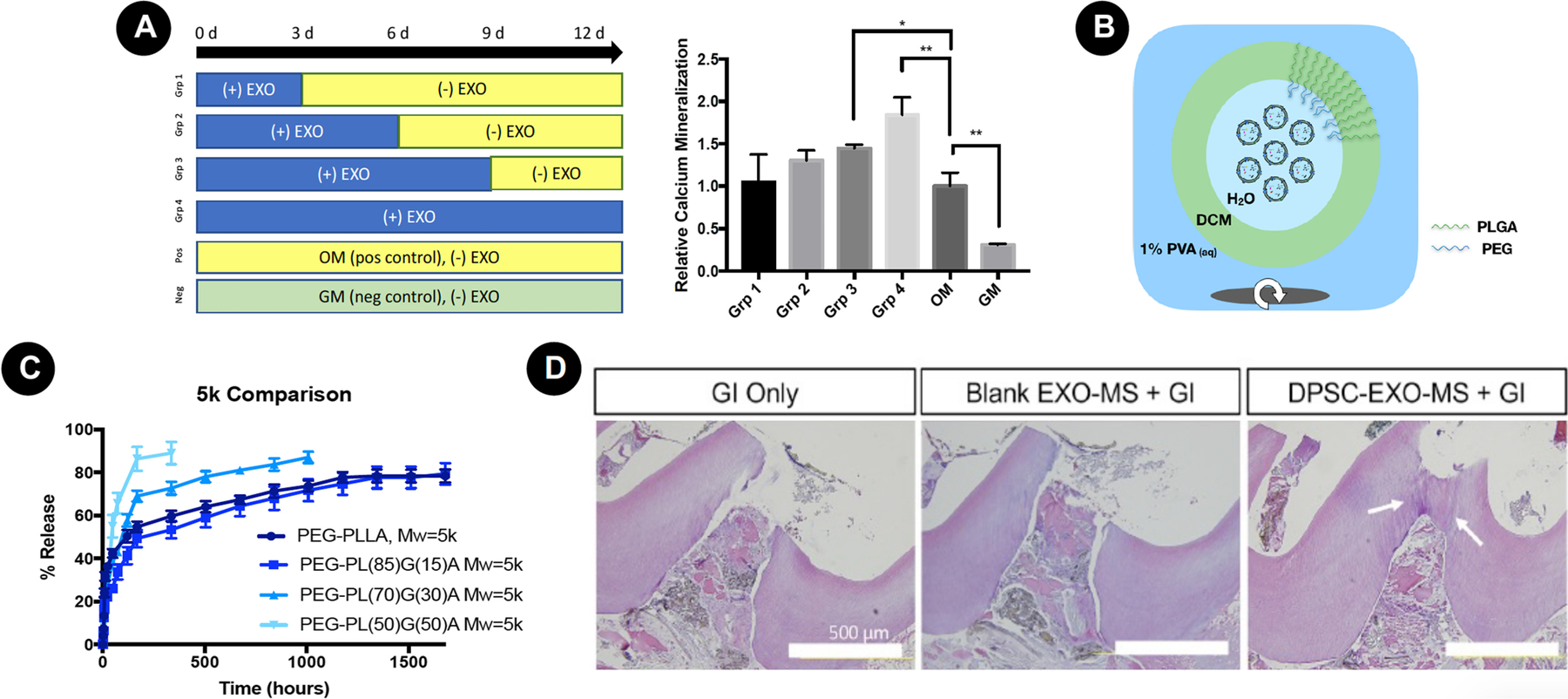
A - Sustained exposure to odontogenic exosomes caused increased mineralization compared to a single exposure. B - Designed polymer vector that encapsulated exosomes by self-assembly, with a synthetic biodegradable copolymer. C - Controlled release of intact exosomes over the course of 10 weeks, depending on copolymer composition. D – This release was sufficient to cause tertiary dentin formation in a rat pulpotomy model, superior to treatment with glass ionomer resin, alone. Reproduced with permission from Swanson et al. [45].
Multifunctional, biomimetic approaches to pulp-dentin complex regeneration
Several biological factors are natively sequestered into tissue ECM; they can induce several cell phenomena important to regeneration. In view of this, the use of naturally derived tissues and factors has been explored to recapitulate natural regenerative phenomenon by modulating the phenotype of endogenous stem cells and guiding them toward deposition of functional tissues. For pulp-dentin regeneration, the following strategies have been explored: 1) Human-treated dentin matrix (hTDM) [93] [94]; 2) Solubilized dentin matrix proteins (DMP) [95–100]; and 3) Decellularized pulp tissue (DPT). These biological cues have been associated with scaffolds to compose multifunctional biomaterials capable of recapitulating several cell phenomena close to natural tissues.
Similarly, laboratory-made biomaterials can be loaded with biologic factors to mimic the native sequestration of growth factors into the ECM. A hybrid biomimetic strategy based on creating a dual ECM scaffold to promote vascularized pulp tissue was proposed by Huang et al. [101]. They first created a DPSCs-laden chitosan-collagen hydrogel construct, and after 2 weeks of in vitro cultivation, a pulp-ECM scaffold was obtained by decellularization protocol. To create a dual ECM scaffold, HUVECs were seeded onto decellularized pulp ECM scaffolds for 1 week, followed by decellularization and lyophilization. The rationale was to enrich the scaffold with ECM proteins expressed by the cells seeded onto its structure. They were able to demonstrate the presence of proteins required for MSC differentiation and angiogenesis on dual ECM scaffolds, such as fibronectin, DMP1, dentin phosphophoryn (DPP), dentin sialoprotein (DSP), BMP2, vonWillebrand factor (vWF), VEGF, and bFGF. Dual ECM scaffolds positively regulated the gene expression levels of pro-odontogenic growth factors, transcription factors, and ECM proteins, along with pro-angiogenic ones when MSCs from pulp and bone marrow were cultivated in vitro onto scaffold structures. After subcutaneous implantation of tooth root slices containing pulp ECM and dual ECM cell-laden scaffolds into mice, it was clear that cells in both ECM scaffolds had increased odontogenic markers’ expression in comparison to plain collagen scaffolds, with dual ECM leading to increased angiogenesis.
By using a similar perspective, Athirasala et al. [102] proposed the creation of a dentin-derived bioink for 3D bioprinting of cell-laden scaffolds applied for regenerative dentistry. They created a blend of printable alginate (3% w/v) hydrogel with soluble and insoluble fractions of the dentin matrix to create natural and cytocompatible microenvironments for tissue regeneration in association with controllable microarchitecture provided by 3D bioprinting. The authors first created suitable bioink-containing insoluble dentin proteins, optimizing printability in association with improved DPSCs’ viability and proliferation. By doing this, they were capable of introducing RGD domains from human dentin in alginate, thus improving cell adhesion. The authors then added different concentrations of soluble dentin matrix molecules to this Alg-Dent bioink to create a cell-laden construct with improved odontogenic differentiation of encapsulated SCAPs. Taken together, these results show proof of the enhanced odontogenic potential for hydrogel blends enriched with DMPs. However, the authors highlight that the specific strategy to 3D bioprint dental pulp tissue with adequate clinical translation is still elusive.
Conclusions
Over the last five years, significant progress has been achieved in terms of the evaluation, validation, and translation of regenerative dentistry technologies toward their application in clinical practice. In this review, we have highlighted numerous examples of technology that we believe has translational potential in the next decade. These approaches draw inspiration from natural healing processes, are bioactive, and induce tissue-specific and cell-level phenotypes required for regeneration. We ponder multifunctional approaches that combine multiple critical considerations that pose the most promising future therapies, given that regenerative procedures will most often be performed in diseased teeth with potential infection and inflammation, which must be managed as part of the therapeutic approach.
Figure 15.

Tooth-on-a-chip platform. A – Fabrication involves (a) a positive PMMA mold in which the PDMS polymer is poured, creating the chip template (b). A dentin fragment (0.5 mm) is positioned (c) to create two chambers, (d) the pulp side in red and the cavity side in green. B – Live cell image on chip. Observe real-time assembly of SCAPs seeded on the chip, in direct contact with dentin. Application of HEMA caused the appearance of dead cells (pink) that were observed in real-time, as well. C – F-actin staining (red) of cells on the chip after treatment on the cavity side. (a) 1 day. (b) 7 days. Note that it is possible to observe the cytotoxic effect of treatments of assembled SCAPs. D – Gelatinolytic activity on the cavity side (a/b), and the pulp side (c/d). With this assay, it was possible to observe the interaction of MMPs from dentin and cells on the hybrid layer on the dentin surface. Reproduced with permission from França et al. [104].
Future Outlook.
Next generation strategies for reliably regenerating the pulp-dentin complex in the clinical setting are critically important for advancing oral and systemic health, particularly when enabling individuals to maintain permanent dentition throughout their lifetime. A tissue engineering approach that combines innovative development of biomaterials, inductive growth factors, and both endogenous/exogenous cell sources, has provided enthusing performance in terms of inducing physiologic dental pulp and reparative dentin formation in a predictable fashion. The ideal biomaterial construct integrates motifs that promote cell recruitment, proliferation, and differentiation, as well as angiogenesis, while minimizing inflammation and ablating bacterial infection.In the near further, one can expect a significant improvement in the field of regenerative dentistry. Some foreseeable advancements, which will augment these current technologies, include tooth-on-a-chip models for high-throughput assessment, as well as 3D bioprinting.
Organ-on-a-chip models have been explored over the last ten years by Ingber and others to develop high throughput, animal-free models of physiology, and disease [103]. Until recently, no models of the tooth were available. França et al. [104] proposed a tooth-on-a-chip platform to evaluate biomaterials in a biomimetic environment, simulating the pulp-dentin complex in vitro (Fig. 15). The microdevice was composed of two parallel channels, two perfusable chambers, and a central groove to hold a dentin fragment. This configuration mimics hierarchical architecture of the pulp-dentin complex. The fully assembled microdevice replicates the interface of dentin with dental pulp on one side and dental material with dentin on the other side, and it represents a new perspective for understanding the ways that biomaterials and regenerative strategies may interface with these tissues in a high-throughput model. To prove its functionality, the authors tested the effect of HEMA, phosphoric acid, and an adhesive system. It was possible to perform a real-time observation of biomaterial cytotoxicity on the cavity side, as well as cell organization at the pulpal side. Besides cell architecture, quantitative analysis of cell viability and cell-dentin gelatinolytic activity could be assayed, demonstrating great versatility of this platform. This type of device represents an important step forward for engineered tissues in vitro and holds great potential for tissue engineering analysis. We believe these devices have significant potential in accelerating the development of novel biomaterials and regenerative dentistry strategies; they may decrease the need for the extensive animal models, particularly early in the development.
Recently, Campos et al. [105] proposed a hand-held bioprinting strategy for in situ fabrication, which demonstrates a significant step towards the clinical realization of 3D bioprinting in routine dental practice. A cell-laden collagen-based bioink was printed via a series of single drops of bioink dispensed directly into human root canals. The authors demonstrated that these bioprinted gel matrices successfully facilitate vascular tube formation towards the regeneration of dental pulp. While this technology is in early development, we believe it serves the field as a stimulating example of translational strategies for exciting bioprinting and materials-processing technology.
A key concern with tissue engineered modalities is a lack of scalable processing, particularly in light of challenges associated with stem cell manufacturing. While research laboratories are optimized for handling biologic products, dental offices do not regularly have the cell culture equipment or storage needed for clinical translation of these products. Therefore, it is critical that academics consider the challenge faced by future industry partners in scaling these laborious procedures. Scalability and manufacturing considerations must be appraised early in the development of new technologies for regenerative dentistry. While not exhaustively reviewed in this work, custom-made three-dimensional constructs, including cells and/or inductive cues in combination with biomaterials, are increasingly fabricated by 3D printing, particularly as the cost of this technology has decreased and resolution has improved dramatically. In many aspects of dentistry, including regenerative dentistry, the 3D printing of pulp-dentin regenerative materials may become the standard of care.
Acknowledgements
Figures (Figures 1 and 2) in this manuscript were partially created with Bio Render.
Funding: The work was supported by the National Institutes of Health/National Institute for Dental and Craniofacial Research (NIH/NIDCR, grants F30-DE029359 to WBS and R01DE026578 to MCB), the São Paulo Research Foundation – FAPESP (grant 2016/15674–5), and by Coordination for the Improvement of Higher Education Personnel – CAPES (Finance Code 001). The content of this review is solely the responsibility of the authors and does not necessarily represent the official view of the funding sources. We apologize to colleagues whose work we could not discuss due to space limitations.
Footnotes
Compliance with Ethical Standards
Conflict of Interest: All authors involved in this study declare that they have no conflict of interest.
Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent For this type of study, formal consent is not required.
REFERENCES
- 1.Schmalz G, Smith AJ (2014) Pulp development, repair, and regeneration: challenges of the transition from traditional dentistry to biologically based therapies. J Endod 40 (4 Suppl):S2–5. [DOI] [PubMed] [Google Scholar]
- 2.Orti V, Collart-Dutilleul PY, Piglionico S, Pall O, Cuisinier F, Panayotov I (2018) Pulp Regeneration Concepts for Nonvital Teeth: From Tissue Engineering to Clinical Approaches. Tissue Eng Part B Rev 24 (6):419–442. [DOI] [PubMed] [Google Scholar]
- 3.Adnan S, Ullah R (2018) Top-cited Articles in Regenerative Endodontics: A Bibliometric Analysis. J Endod 44 (11):1650–1664. [DOI] [PubMed] [Google Scholar]
- 4.Morotomi T, Washio A, Kitamura C (2019) Current and future options for dental pulp therapy. Jpn Dent Sci Rev 55 (1):5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Organization WH (2017) Oral Health https://www.who.int/publications/i/item/WHO-NMH-NHD-17.12. Accessed 19 November 2020
- 6.Organization WH (2003) Dental diseases and oral health https://www.who.int/oral_health/publications/en/orh_fact_sheet.pdf. Accessed 19 November 2020
- 7.Li X, Kolltveit KM, Tronstad L, Olsen I (2000) Systemic diseases caused by oral infection. Clin Microbiol Rev 13 (4):547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albuquerque MT, Valera MC, Nakashima M, Nor JE, Bottino MC (2014) Tissue-engineering-based strategies for regenerative endodontics. J Dent Res 93 (12):1222–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Itoh Y, Sasaki JI, Hashimoto M, Katata C, Hayashi M, Imazato S (2018) Pulp Regeneration by 3-dimensional Dental Pulp Stem Cell Constructs. J Dent Res 97 (10):1137–1143. [DOI] [PubMed] [Google Scholar]
- 10.Bottino MC, Pankajakshan D, Nor JE (2017) Advanced Scaffolds for Dental Pulp and Periodontal Regeneration. Dent Clin North Am 61 (4):689–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yildirim S, Fu SY, Kim K, Zhou H, Lee CH, Li A, Kim SG, Wang S, Mao JJ (2011) Tooth regeneration: a revolution in stomatology and evolution in regenerative medicine. Int J Oral Sci 3 (3):107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bansal R, Jain A (2015) Current overview on dental stem cells applications in regenerative dentistry. J Nat Sci Biol Med 6 (1):29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer NG, Munchow EA, Tamerler C, Bottino MC, Aparicio C (2020) Harnessing biomolecules for bioinspired dental biomaterials. J Mater Chem B 8 (38):8713–8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bjorndal L, Simon S, Tomson PL, Duncan HF (2019) Management of deep caries and the exposed pulp. Int Endod J 52 (7):949–973. [DOI] [PubMed] [Google Scholar]
- 15.de Souza Costa CA, Hebling J, Scheffel DL, Soares DG, Basso FG, Ribeiro AP (2014) Methods to evaluate and strategies to improve the biocompatibility of dental materials and operative techniques. Dent Mater 30 (7):769–784. [DOI] [PubMed] [Google Scholar]
- 16.Hanna SN, Perez Alfayate R, Prichard J (2020) Vital Pulp Therapy an Insight Over the Available Literature and Future Expectations. Eur Endod J 5 (1):46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medina-Fernandez I, Celiz AD (2019) Acellular biomaterial strategies for endodontic regeneration. Biomater Sci 7 (2):506–519. [DOI] [PubMed] [Google Scholar]
- 18.Ricucci D, Loghin S, Lin LM, Spangberg LS, Tay FR (2014) Is hard tissue formation in the dental pulp after the death of the primary odontoblasts a regenerative or a reparative process? J Dent 42 (9):1156–1170. [DOI] [PubMed] [Google Scholar]
- 19.Altaii M, Richards L, Rossi-Fedele G (2017) Histological assessment of regenerative endodontic treatment in animal studies with different scaffolds: A systematic review. Dent Traumatol 33 (4):235–244. [DOI] [PubMed] [Google Scholar]
- 20.Nosrat A, Kolahdouzan A, Khatibi AH, Verma P, Jamshidi D, Nevins AJ, Torabinejad M (2019) Clinical, Radiographic, and Histologic Outcome of Regenerative Endodontic Treatment in Human Teeth Using a Novel Collagen-hydroxyapatite Scaffold. J Endod 45 (2):136–143. [DOI] [PubMed] [Google Scholar]
- 21.Yoo C, Vines JB, Alexander G, Murdock K, Hwang P, Jun HW (2014) Adult stem cells and tissue engineering strategies for salivary gland regeneration: a review. Biomater Res 18:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saoud TM, Zaazou A, Nabil A, Moussa S, Aly HM, Okazaki K, Rosenberg PA, Lin LM (2015) Histological observations of pulpal replacement tissue in immature dog teeth after revascularization of infected pulps. Dent Traumatol 31 (3):243–249. [DOI] [PubMed] [Google Scholar]
- 23.Huang GT, Garcia-Godoy F (2014) Missing Concepts in De Novo Pulp Regeneration. J Dent Res 93 (8):717–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langer R, Vacanti JP (1993) Tissue engineering. Science 260 (5110):920–926. [DOI] [PubMed] [Google Scholar]
- 25.Ma PX (2008) Biomimetic materials for tissue engineering. Adv Drug Deliv Rev 60 (2):184–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swanson WB, Ma PX (2020) 1.4.7 - Textured and Porous Biomaterials. In: Wagner WR, Sakiyama-Elbert SE, Zhang G, Yaszemski MJ (eds) Biomaterials Science (Fourth Edition). Academic Press, pp 601–622. [Google Scholar]
- 27.Gupte MJ, Swanson WB, Hu J, Jin X, Ma H, Zhang Z, Liu Z, Feng K, Feng G, Xiao G, Hatch N, Mishina Y, Ma PX (2018) Pore size directs bone marrow stromal cell fate and tissue regeneration in nanofibrous macroporous scaffolds by mediating vascularization. Acta Biomater 82:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mousa M, Evans ND, Oreffo ROC, Dawson JI (2018) Clay nanoparticles for regenerative medicine and biomaterial design: A review of clay bioactivity. Biomaterials 159:204–214. [DOI] [PubMed] [Google Scholar]
- 29.Galler KM, Widbiller M (2017) Perspectives for Cell-homing Approaches to Engineer Dental Pulp. J Endod 43 (9S):S40–S45. [DOI] [PubMed] [Google Scholar]
- 30.Yelick PC, Sharpe PT (2019) Tooth Bioengineering and Regenerative Dentistry. J Dent Res 98 (11):1173–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soares DG, Rosseto HL, Scheffel DS, Basso FG, Huck C, Hebling J, de Souza Costa CA (2017) Odontogenic differentiation potential of human dental pulp cells cultured on a calcium-aluminate enriched chitosan-collagen scaffold. Clin Oral Investig 21 (9):2827–2839. [DOI] [PubMed] [Google Scholar]
- 32.Soares DG, Anovazzi G, Bordini EAF, Zuta UO, Silva Leite MLA, Basso FG, Hebling J, de Souza Costa CA (2018a) Biological Analysis of Simvastatin-releasing Chitosan Scaffold as a Cell-free System for Pulp-dentin Regeneration. J Endod 44 (6):971–976 e971. [DOI] [PubMed] [Google Scholar]
- 33.Soares DG, Zhang Z, Mohamed F, Eyster TW, de Souza Costa CA, Ma PX (2018b) Simvastatin and nanofibrous poly(l-lactic acid) scaffolds to promote the odontogenic potential of dental pulp cells in an inflammatory environment. Acta Biomater 68:190–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soares DG, Bordini EAF, Cassiano FB, Bronze-Uhle ES, Pacheco LE, Zabeo G, Hebling J, Lisboa-Filho PN, Bottino MC, de Souza Costa CA (2020) Characterization of novel calcium hydroxide-mediated highly porous chitosan-calcium scaffolds for potential application in dentin tissue engineering. J Biomed Mater Res B Appl Biomater 108 (6):2546–2559. [DOI] [PubMed] [Google Scholar]
- 35.Sakai VT, Cordeiro MM, Dong Z, Zhang Z, Zeitlin BD, Nor JE (2011) Tooth slice/scaffold model of dental pulp tissue engineering. Adv Dent Res 23 (3):325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.da Fonseca Roberti Garcia L, Pontes EC, Basso FG, Hebling J, de Souza Costa CA, Soares DG (2016) Transdentinal cytotoxicity of resin-based luting cements to pulp cells. Clin Oral Investig 20 (7):1559–1566. [DOI] [PubMed] [Google Scholar]
- 37.Leite M, Costa CAS, Duarte RM, Andrade AKM, Soares DG (2018) Bond Strength and Cytotoxicity of a Universal Adhesive According to the Hybridization Strategies to Dentin. Braz Dent J 29 (1):68–75. [DOI] [PubMed] [Google Scholar]
- 38.Leite ML, Soares DG, de Oliveira Duque CC, Bordini EAF, Anovazzi G, Basso FG, Spolidorio DMP, Hebling J, de Souza Costa CA (2019) Positive influence of simvastatin used as adjuvant agent for cavity lining. Clin Oral Investig 23 (9):3457–3469. [DOI] [PubMed] [Google Scholar]
- 39.Murray PE, Lumley PJ, Ross HF, Smith AJ (2000) Tooth slice organ culture for cytotoxicity assessment of dental materials. Biomaterials 21 (16):1711–1721. [DOI] [PubMed] [Google Scholar]
- 40.Laurent P, Camps J, About I (2012) Biodentine(TM) induces TGF-beta1 release from human pulp cells and early dental pulp mineralization. Int Endod J 45 (5):439–448. [DOI] [PubMed] [Google Scholar]
- 41.Pedano MS, Li X, Jeanneau C, Ghosh M, Yoshihara K, Van Landuyt K, About I, Van Meerbeek B (2019) Survival of human dental pulp cells after 4-week culture in human tooth model. J Dent 86:33–40. [DOI] [PubMed] [Google Scholar]
- 42.Li J, Rao Z, Zhao Y, Xu Y, Chen L, Shen Z, Bai Y, Lin Z, Huang Q (2020) A Decellularized Matrix Hydrogel Derived from Human Dental Pulp Promotes Dental Pulp Stem Cell Proliferation, Migration, and Induced Multidirectional Differentiation In Vitro. J Endod 46 (10):1438–1447.e1435. [DOI] [PubMed] [Google Scholar]
- 43.Tecles O, Laurent P, Aubut V, About I (2008) Human tooth culture: a study model for reparative dentinogenesis and direct pulp capping materials biocompatibility. J Biomed Mater Res B Appl Biomater 85 (1):180–187. [DOI] [PubMed] [Google Scholar]
- 44.Li X, Pedano MS, Camargo B, Hauben E, De Vleeschauwer S, Chen Z, De Munck J, Vandamme K, Van Landuyt K, Van Meerbeek B (2018) Experimental tricalcium silicate cement induces reparative dentinogenesis. Dent Mater 34 (9):1410–1423. [DOI] [PubMed] [Google Scholar]
- 45.Swanson WB, Gong T, Zhang Z, Eberle M, Niemann D, Dong R, Rambhia KJ, Ma PX (2020) Controlled release of odontogenic exosomes from a biodegradable vehicle mediates dentinogenesis as a novel biomimetic pulp capping therapy. J Control Release 324:679–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang GT, Yamaza T, Shea LD, Djouad F, Kuhn NZ, Tuan RS, Shi S (2010) Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng Part A 16 (2):605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iohara K, Imabayashi K, Ishizaka R, Watanabe A, Nabekura J, Ito M, Matsushita K, Nakamura H, Nakashima M (2011) Complete pulp regeneration after pulpectomy by transplantation of CD105+ stem cells with stromal cell-derived factor-1. Tissue Eng Part A 17 (15–16):1911–1920. [DOI] [PubMed] [Google Scholar]
- 48.Rosa V, Zhang Z, Grande RH, Nor JE (2013) Dental pulp tissue engineering in full-length human root canals. J Dent Res 92 (11):970–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakashima M, Iohara K, Bottino MC, Fouad AF, Nor JE, Huang GT (2019) Animal Models for Stem Cell-Based Pulp Regeneration: Foundation for Human Clinical Applications. Tissue Eng Part B Rev 25 (2):100–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, Smith AJ, Nor JE (2008) Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod 34 (8):962–969. [DOI] [PubMed] [Google Scholar]
- 51.Galler KM, Hartgerink JD, Cavender AC, Schmalz G, D’Souza RN (2012) A customized self-assembling peptide hydrogel for dental pulp tissue engineering. Tissue Eng Part A 18 (1–2):176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dissanayaka WL, Hargreaves KM, Jin L, Samaranayake LP, Zhang C (2015) The interplay of dental pulp stem cells and endothelial cells in an injectable peptide hydrogel on angiogenesis and pulp regeneration in vivo. Tissue Eng Part A 21 (3–4):550–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silva CR, Babo PS, Gulino M, Costa L, Oliveira JM, Silva-Correia J, Domingues RMA, Reis RL, Gomes ME (2018) Injectable and tunable hyaluronic acid hydrogels releasing chemotactic and angiogenic growth factors for endodontic regeneration. Acta Biomater 77:155–171. [DOI] [PubMed] [Google Scholar]
- 54.Mu X, Shi L, Pan S, He L, Niu Y, Wang X (2020) A Customized Self-Assembling Peptide Hydrogel-Wrapped Stem Cell Factor Targeting Pulp Regeneration Rich in Vascular-Like Structures. ACS Omega 5 (27):16568–16574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiao S, Zhao T, Wang J, Wang C, Du J, Ying L, Lin J, Zhang C, Hu W, Wang L, Xu K (2019) Gelatin Methacrylate (GelMA)-Based Hydrogels for Cell Transplantation: an Effective Strategy for Tissue Engineering. Stem Cell Rev Rep 15 (5):664–679. [DOI] [PubMed] [Google Scholar]
- 56.Monteiro N, Thrivikraman G, Athirasala A, Tahayeri A, França CM, Ferracane JL, Bertassoni LE (2018) Photopolymerization of cell-laden gelatin methacryloyl hydrogels using a dental curing light for regenerative dentistry. Dent Mater 34 (3):389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khayat A, Monteiro N, Smith EE, Pagni S, Zhang W, Khademhosseini A, Yelick PC (2017) GelMA-Encapsulated hDPSCs and HUVECs for Dental Pulp Regeneration. J Dent Res 96 (2):192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Athirasala A, Lins F, Tahayeri A, Hinds M, Smith AJ, Sedgley C, Ferracane J, Bertassoni LE (2017) A Novel Strategy to Engineer Pre-Vascularized Full-Length Dental Pulp-like Tissue Constructs. Sci Rep 7 (1):3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang R, Xie L, Wu H, Yang T, Zhang Q, Tian Y, Liu Y, Han X, Guo W, He M, Liu S, Tian W (2020) Alginate/laponite hydrogel microspheres co-encapsulating dental pulp stem cells and VEGF for endodontic regeneration. Acta Biomater 113:305–316. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Z, Marson RL, Ge Z, Glotzer SC, Ma PX (2015) Simultaneous Nano- and Microscale Control of Nanofibrous Microspheres Self-Assembled from Star-Shaped Polymers. Adv Mater 27 (26):3947–3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li X, Ma C, Xie X, Sun H, Liu X (2016) Pulp regeneration in a full-length human tooth root using a hierarchical nanofibrous microsphere system. Acta Biomater 35:57–67. [DOI] [PubMed] [Google Scholar]
- 62.Ma PX, Zhang R (1999) Synthetic nano-scale fibrous extracellular matrix. J Biomed Mater Res 46 (1):60–72. [DOI] [PubMed] [Google Scholar]
- 63.Wang X, Song G, Lou T (2010) Fabrication and characterization of nano composite scaffold of poly(L-lactic acid)/hydroxyapatite. J Mater Sci Mater Med 21 (1):183–188. [DOI] [PubMed] [Google Scholar]
- 64.Kuang R, Zhang Z, Jin X, Hu J, Gupte MJ, Ni L, Ma PX (2015) Nanofibrous spongy microspheres enhance odontogenic differentiation of human dental pulp stem cells. Adv Healthc Mater 4 (13):1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang W, Dang M, Zhang Z, Hu J, Eyster TW, Ni L, Ma PX (2016) Dentin regeneration by stem cells of apical papilla on injectable nanofibrous microspheres and stimulated by controlled BMP-2 release. Acta Biomater 36:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuang R, Zhang Z, Jin X, Hu J, Shi S, Ni L, Ma PX (2016) Nanofibrous spongy microspheres for the delivery of hypoxia-primed human dental pulp stem cells to regenerate vascularized dental pulp. Acta Biomater 33:225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sabrah AH, Yassen GH, Liu WC, Goebel WS, Gregory RL, Platt JA (2015) The effect of diluted triple and double antibiotic pastes on dental pulp stem cells and established Enterococcus faecalis biofilm. Clin Oral Investig 19 (8):2059–2066. [DOI] [PubMed] [Google Scholar]
- 68.Sotomil JM, Munchow EA, Pankajakshan D, Spolnik KJ, Ferreira JA, Gregory RL, Bottino MC (2019) Curcumin-A Natural Medicament for Root Canal Disinfection: Effects of Irrigation, Drug Release, and Photoactivation. J Endod 45 (11):1371–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kamocki K, Nor JE, Bottino MC (2015) Dental pulp stem cell responses to novel antibiotic-containing scaffolds for regenerative endodontics. Int Endod J 48 (12):1147–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Albuquerque MT, Evans JD, Gregory RL, Valera MC, Bottino MC (2016) Antibacterial TAP-mimic electrospun polymer scaffold: effects on P. gingivalis-infected dentin biofilm. Clin Oral Investig 20 (2):387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pankajakshan D, Albuquerque MT, Evans JD, Kamocka MM, Gregory RL, Bottino MC (2016) Triple Antibiotic Polymer Nanofibers for Intracanal Drug Delivery: Effects on Dual Species Biofilm and Cell Function. J Endod 42 (10):1490–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Karczewski A, Feitosa SA, Hamer EI, Pankajakshan D, Gregory RL, Spolnik KJ, Bottino MC (2018) Clindamycin-modified Triple Antibiotic Nanofibers: A Stain-free Antimicrobial Intracanal Drug Delivery System. J Endod 44 (1):155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bottino MC, Albuquerque MTP, Azabi A, Munchow EA, Spolnik KJ, Nor JE, Edwards PC (2019) A novel patient-specific three-dimensional drug delivery construct for regenerative endodontics. J Biomed Mater Res B Appl Biomater 107 (5):1576–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ribeiro JS, Daghrery A, Dubey N, Li C, Mei L, Fenno JC, Schwendeman A, Aytac Z, Bottino MC (2020) Hybrid Antimicrobial Hydrogel as Injectable Therapeutics for Oral Infection Ablation. Biomacromolecules 21 (9):3945–3956. [DOI] [PubMed] [Google Scholar]
- 75.Ribeiro JS, Bordini EAF, Ferreira JA, Mei L, Dubey N, Fenno JC, Piva E, Lund RG, Schwendeman A, Bottino MC (2020) Injectable MMP-Responsive Nanotube-Modified Gelatin Hydrogel for Dental Infection Ablation. ACS Appl Mater Interfaces 12 (14):16006–16017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang YY, Chatzistavrou X, Faulk D, Badylak S, Zheng L, Papagerakis S, Ge L, Liu H, Papagerakis P (2015) Biological and bactericidal properties of Ag-doped bioactive glass in a natural extracellular matrix hydrogel with potential application in dentistry. Eur Cell Mater 29:342–355. [DOI] [PubMed] [Google Scholar]
- 77.Qu T, Jing J, Jiang Y, Taylor RJ, Feng JQ, Geiger B, Liu X (2014) Magnesium-containing nanostructured hybrid scaffolds for enhanced dentin regeneration. Tissue Eng Part A 20 (17–18):2422–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Okamoto M, Matsumoto S, Sugiyama A, Kanie K, Watanabe M, Huang H, Ali M, Ito Y, Miura J, Hirose Y, Uto K, Ebara M, Kato R, Yamawaki-Ogata A, Narita Y, Kawabata S, Takahashi Y, Hayashi M (2020) Performance of a Biodegradable Composite with Hydroxyapatite as a Scaffold in Pulp Tissue Repair. Polymers (Basel) 12 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Soares DG, Rosseto HL, Basso FG, Scheffel DS, Hebling J, Costa CA (2016) Chitosan-collagen biomembrane embedded with calcium-aluminate enhances dentinogenic potential of pulp cells. Braz Oral Res 30 (1):e54. [DOI] [PubMed] [Google Scholar]
- 80.Bordini EAF, Cassiano FB, Silva ISP, Usberti FR, Anovazzi G, Pacheco LE, Pansani TN, Leite ML, Hebling J, de Souza Costa CA, Soares DG (2017) Synergistic potential of 1alpha,25-dihydroxyvitamin D3 and calcium-aluminate-chitosan scaffolds with dental pulp cells. Clin Oral Investig 24 (2):663–674. [DOI] [PubMed] [Google Scholar]
- 81.Cassiano FB, Soares DG, Bordini EAF, Anovazzi G, Hebling J, Costa CAS (2020) Simvastatin-Enriched Macro-Porous Chitosan-Calcium-Aluminate Scaffold for Mineralized Tissue Regeneration. Braz Dent J 31 (4):385–391. [DOI] [PubMed] [Google Scholar]
- 82.Dubey N, Ferreira JA, Malda J, Bhaduri SB, Bottino MC (2020) Extracellular Matrix/Amorphous Magnesium Phosphate Bioink for 3D Bioprinting of Craniomaxillofacial Bone Tissue. ACS Appl Mater Interfaces 12 (21):23752–23763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li F, Liu X, Zhao S, Wu H, Xu HH (2014) Porous chitosan bilayer membrane containing TGF-beta1 loaded microspheres for pulp capping and reparative dentin formation in a dog model. Dent Mater 30 (2):172–181. [DOI] [PubMed] [Google Scholar]
- 84.Daghrery A, Aytac Z, Dubey N, Mei L, Schwendeman A, Bottino MC (2020) Electrospinning of dexamethasone/cyclodextrin inclusion complex polymer fibers for dental pulp therapy. Colloids Surf B Biointerfaces 191:111011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Niu X, Liu Z, Hu J, Rambhia KJ, Fan Y, Ma PX (2016) Microspheres Assembled from Chitosan-Graft-Poly(lactic acid) Micelle-Like Core-Shell Nanospheres for Distinctly Controlled Release of Hydrophobic and Hydrophilic Biomolecules. Macromol Biosci 16 (7):1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ha M, Athirasala A, Tahayeri A, Menezes PP, Bertassoni LE (2020) Micropatterned hydrogels and cell alignment enhance the odontogenic potential of stem cells from apical papilla in-vitro. Dent Mater 36 (1):88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pankajakshan D, Voytik-Harbin SL, Nor JE, Bottino MC (2020) Injectable Highly Tunable Oligomeric Collagen Matrices for Dental Tissue Regeneration. ACS Appl Bio Mater 3 (2):859–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dubey N, Ferreira JA, Daghrery A, Aytac Z, Malda J, Bhaduri SB, Bottino MC (2020) Highly tunable bioactive fiber-reinforced hydrogel for guided bone regeneration. Acta Biomater 113:164–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cooper LF, Ravindran S, Huang CC, Kang M (2019) A Role for Exosomes in Craniofacial Tissue Engineering and Regeneration. Front Physiol 10:1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang CC, Narayanan R, Alapati S, Ravindran S (2016) Exosomes as biomimetic tools for stem cell differentiation: Applications in dental pulp tissue regeneration. Biomaterials 111:103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xian X, Gong Q, Li C, Guo B, Jiang H (2018) Exosomes with Highly Angiogenic Potential for Possible Use in Pulp Regeneration. J Endod 44 (5):751–758. [DOI] [PubMed] [Google Scholar]
- 92.Zhang S, Yang Y, Jia S, Chen H, Duan Y, Li X, Wang S, Wang T, Lyu Y, Chen G, Tian W (2020) Exosome-like vesicles derived from Hertwig’s epithelial root sheath cells promote the regeneration of dentin-pulp tissue. Theranostics 10 (13):5914–5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen J, Cui C, Qiao X, Yang B, Yu M, Guo W, Tian W (2017) Treated dentin matrix paste as a novel pulp capping agent for dentin regeneration. J Tissue Eng Regen Med 11 (12):3428–3436. [DOI] [PubMed] [Google Scholar]
- 94.Holiel AA, Mahmoud EM, Abdel-Fattah WM, Kawana KY (2020) Histological evaluation of the regenerative potential of a novel treated dentin matrix hydrogel in direct pulp capping. Clin Oral Investig [DOI] [PubMed] [Google Scholar]
- 95.Salehi S, Cooper P, Smith A, Ferracane J (2016) Dentin matrix components extracted with phosphoric acid enhance cell proliferation and mineralization. Dent Mater 32 (3):334–342. [DOI] [PubMed] [Google Scholar]
- 96.Zhang R, Cooper PR, Smith G, Nor JE, Smith AJ (2011) Angiogenic activity of dentin matrix components. J Endod 37 (1):26–30. [DOI] [PubMed] [Google Scholar]
- 97.Smith JG, Smith AJ, Shelton RM, Cooper PR (2015) Dental Pulp Cell Behavior in Biomimetic Environments. J Dent Res 94 (11):1552–1559. [DOI] [PubMed] [Google Scholar]
- 98.Galler KM, Widbiller M, Buchalla W, Eidt A, Hiller KA, Hoffer PC, Schmalz G (2016) EDTA conditioning of dentine promotes adhesion, migration and differentiation of dental pulp stem cells. Int Endod J 49 (6):581–590. [DOI] [PubMed] [Google Scholar]
- 99.Widbiller M, Eidt A, Hiller KA, Buchalla W, Schmalz G, Galler KM (2017) Ultrasonic activation of irrigants increases growth factor release from human dentine. Clin Oral Investig 21 (3):879–888. [DOI] [PubMed] [Google Scholar]
- 100.Widbiller M, Driesen RB, Eidt A, Lambrichts I, Hiller KA, Buchalla W, Schmalz G, Galler KM (2018a) Cell Homing for Pulp Tissue Engineering with Endogenous Dentin Matrix Proteins. J Endod 44 (6):956–962 e952. [DOI] [PubMed] [Google Scholar]
- 101.Huang KH, Chen YW, Wang CY, Lin YH, Wu YA, Shie MY, Lin CP (2018) Enhanced Capability of Bone Morphogenetic Protein 2-loaded Mesoporous Calcium Silicate Scaffolds to Induce Odontogenic Differentiation of Human Dental Pulp Cells. J Endod 44 (11):1677–1685. [DOI] [PubMed] [Google Scholar]
- 102.Athirasala A, Tahayeri A, Thrivikraman G, Franca CM, Monteiro N, Tran V, Ferracane J, Bertassoni LE (2018) A dentin-derived hydrogel bioink for 3D bioprinting of cell laden scaffolds for regenerative dentistry. Biofabrication 10 (2):024101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang JW, Shen YC, Lin KC, Cheng SJ, Chen SL, Chen CY, Kumar PV, Lin SF, Lu HE, Chen GY (2020) Organ-on-a-Chip: Opportunities for Assessing the Toxicity of Particulate Matter. Front Bioeng Biotechnol 8:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.França CM, Tahayeri A, Rodrigues NS, Ferdosian S, Puppin Rontani RM, Sereda G, Ferracane JL, Bertassoni LE (2020) The tooth on-a-chip: a microphysiologic model system mimicking the biologic interface of the tooth with biomaterials. Lab Chip 20 (2):405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Duarte Campos DF, Zhang S, Kreimendahl F, Kopf M, Fischer H, Vogt M, Blaeser A, Apel C, Esteves-Oliveira M (2020) Hand-held bioprinting for de novo vascular formation applicable to dental pulp regeneration. Connect Tissue Res 61 (2):205–215. [DOI] [PubMed] [Google Scholar]


