Key Points
Question
Is anticoagulation superior to aspirin in reducing recurrent stroke in patients with recent embolic stroke of undetermined source (ESUS) and left ventricular (LV) dysfunction?
Findings
Among 7213 participants of the New Approach Rivaroxaban Inhibition of Factor Xa in a Global Trial vs Aspirin to Prevent Embolism in ESUS (NAVIGATE ESUS) trial, 502 (7.1%) had evidence of LV dysfunction. Participants with LV dysfunction assigned to rivaroxaban vs aspirin had a lower risk of recurrent stroke or systemic embolism compared with those without LV dysfunction.
Meaning
Rivaroxaban was superior to aspirin at reducing the risk of recurrent stroke or systemic embolism among NAVIGATE ESUS participants with LV dysfunction in this post hoc exploratory analysis.
This post hoc analysis evaluates whether anticoagulation is superior to aspirin in reducing recurrent stroke in patients with embolic stroke of undetermined source and left ventricular dysfunction.
Abstract
Importance
It is uncertain whether anticoagulation is superior to aspirin at reducing recurrent stroke in patients with recent embolic strokes of undetermined source (ESUS) and left ventricular (LV) dysfunction.
Objective
To determine whether anticoagulation is superior to aspirin in reducing recurrent stroke in patients with ESUS and LV dysfunction.
Design, Setting, and Participants
Post hoc exploratory analysis of data from the New Approach Rivaroxaban Inhibition of Factor Xa in a Global Trial vs Aspirin to Prevent Embolism in ESUS (NAVIGATE ESUS) trial, a randomized, phase 3 clinical trial with enrollment from December 2014 to September 2017. The study setting included 459 stroke recruitment centers in 31 countries. Patients 50 years or older who had neuroimaging-confirmed ESUS between 7 days and 6 months before screening were eligible. Of the 7213 NAVIGATE ESUS participants, 7107 (98.5%) had a documented assessment of LV function at study entry and were included in the present analysis. Data were analyzed in January 2021.
Interventions
Participants were randomized to receive either 15 mg of rivaroxaban or 100 mg of aspirin once daily.
Main Outcomes and Measures
The study examined whether rivaroxaban was superior to aspirin at reducing the risk of (1) the trial primary outcome of recurrent stroke or systemic embolism and (2) the trial secondary outcome of recurrent stroke, systemic embolism, myocardial infarction, or cardiovascular mortality during a median follow-up of 10.4 months. LV dysfunction was identified locally through echocardiography and defined as moderate to severe global impairment in LV contractility and/or a regional wall motion abnormality. A Cox proportional hazards model was used to assess for treatment interaction and to estimate the hazard ratios for those randomized to rivaroxaban vs aspirin by LV dysfunction status.
Results
LV dysfunction was present in 502 participants (7.1%). Of participants with LV dysfunction, the mean (SD) age was 67 (10) years, and 130 (26%) were women. Among participants with LV dysfunction, annualized primary event rates were 2.4% (95% CI, 1.1-5.4) in those assigned to rivaroxaban vs 6.5% (95% CI, 4.0-11.0) in those assigned aspirin. Among the 6605 participants without LV dysfunction, rates were similar between those assigned to rivaroxaban (5.3%; 95% CI, 4.5-6.2) vs aspirin (4.5%; 95% CI, 3.8-5.3). Participants with LV dysfunction assigned to rivaroxaban vs aspirin had a lower risk of the primary outcome (hazard ratio, 0.36; 95% CI, 0.14-0.93), unlike those without LV dysfunction (hazard ratio, 1.16; 95% CI, 0.93-1.46) (P for treatment interaction = .03). Results were similar for the secondary outcome.
Conclusions and Relevance
In this post hoc exploratory analysis, rivaroxaban was superior to aspirin in reducing the risk of recurrent stroke or systemic embolism among NAVIGATE ESUS participants with LV dysfunction.
Trial Registration
ClinicalTrials.gov Identifier: NCT02313909
Introduction
Approximately 2 million ischemic strokes that occur each year worldwide lack an identifiable cause.1 These strokes are classified as cryptogenic and represent approximately 17% of all ischemic strokes.2 A subset of cryptogenic strokes often appear to be embolic and are referred to as embolic strokes of undetermined source (ESUS).3 Two recent randomized clinical trials (the New Approach Rivaroxaban Inhibition of Factor Xa in a Global Trial vs Aspirin to Prevent Embolism in ESUS [NAVIGATE ESUS] trial and the Dabigatran Etexilate for Secondary Stroke Prevention in Patients With Embolic Stroke of Undetermined Source [RESPECT-ESUS] trial) found that anticoagulation was no better than aspirin at reducing the risk of recurrent stroke and systemic embolism among all patients with ESUS.4,5 However, patients included in these trials had heterogeneous potential etiologies of ESUS,6 and anticoagulation was not an effective overarching treatment strategy for this undifferentiated population.7
There are several well-established, albeit infrequent, sources of cardiac embolism and subsequent stroke that arise from the left ventricle (LV), such as those resulting from recent acute myocardial infarction or severe LV systolic dysfunction.3,8 On the other hand, LV regional wall motion abnormalities and impaired LV contractility are commonly found in patients with ESUS and may similarly have the propensity to lead to thrombus formation, cardiac embolism, and stroke.9,10 These forms of LV dysfunction, however, are not currently considered high-risk sources of cardiac embolism. As a result, patients with stroke who have these abnormalities are considered to have ESUS and are not routinely prescribed anticoagulation.3 In this post hoc exploratory analysis, we hypothesized that anticoagulation would be superior to antiplatelet therapy in reducing the risk of cardiac embolism and subsequent stroke in patients with ESUS and evidence of LV dysfunction. We evaluated this hypothesis among the participants included in the NAVIGATE ESUS trial.
Methods
Design
This study was a post hoc exploratory analysis of the NAVIGATE ESUS trial data. NAVIGATE ESUS was an international, multicenter phase 3 trial in which patients with recent ESUS were randomly assigned (double-blind) to either 15 mg of rivaroxaban or 100 mg of aspirin once daily for the prevention of recurrent stroke. The design, rationale, baseline patient characteristics, and primary results of the trial have been previously published.4,11 Trial enrollment took place from December 2014 to September 2017, and data were analyzed in January 2021. The study setting included 459 stroke recruitment centers in 31 countries. The mean (SD) age of participants was 67 (10) years, and 4436 participants (61.5%) were male. Of the 7213 NAVIGATE ESUS participants, 7107 (98.5%) had a documented assessment of LV function at study entry and were included in the present analysis. All patients provided written informed consent before participation. The protocol was approved by the institutional review board at each trial site, and the study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Participants
Patients with ischemic stroke confirmed by cerebral imaging within 7 days to 6 months of symptom onset were eligible to participate in NAVIGATE ESUS. The qualifying ischemic stroke was considered to be ESUS if it was (1) not lacunar; (2) not associated with more than 50% luminal stenosis of the artery supplying the area of ischemia; (3) not associated with identified high-risk sources of cardiac embolism, including atrial fibrillation, LV thrombus, mechanical cardiac valve, or severe mitral stenosis; and (4) no other source of stroke was identified by the time of randomization.4 In addition, participants were required to be older than 49 years at the time of stroke. If participants were aged 50 to 59 years at the time of stroke, at least 1 additional risk factor for stroke was necessary, defined as previously reported.4
Measurements
For this post hoc exploratory analysis of NAVIGATE ESUS, all participants with a documented assessment of LV function via transthoracic echocardiogram or transesophageal echocardiogram were included. Global LV contractility was graded as normal, mildly impaired, moderate to severely impaired, or uncertain. If the global assessment of LV contractility was marked as uncertain or not reported, an LV ejection fraction less than 40% was used to define global moderate to severely impaired LV contractility. While LV ejection fraction is the most commonly used method for the assessment of LV systolic function, it is influenced by LV loading conditions. In contrast, LV intrinsic contractility is not an afterload-dependent measure and thus provides a more complete characterization of LV function.12,13 Regional wall motion abnormalities were noted as either present, absent, or not reported. Echocardiograms were performed at participating sites, and case report forms were filled out by local investigators.
In this analysis, participants were considered to have LV dysfunction if they had moderate to severely impaired global LV contractility and/or a regional wall motion abnormality. We considered any degree or type of wall motion abnormality to be indicative of LV dysfunction. These markers of LV dysfunction were selected because they had been previously found to be associated with LV thrombus formation and stroke.9,10 We defined LV dysfunction status a priori into the following 3 mutually exclusive categories: (1) normal to mildly impaired LV contractility without regional wall motion abnormalities noted, (2) normal to mildly impaired LV contractility with regional wall motion abnormalities noted, and (3) moderate to severely impaired LV contractility with or without regional wall motion abnormalities. We considered categories 2 and 3 to constitute LV dysfunction.
The primary outcome of this study was the primary efficacy outcome of the trial: the composite end point of recurrent stroke or systemic embolism. The specific definitions of the components of the primary outcome based on clinical and radiographic findings have been previously described.4,11 In brief, ischemic stroke recurrence was defined as a sudden focal neurological deficit due to arterial occlusion and persisting for more than 24 hours without evidence of primary hemorrhage on brain imaging. If neurological deficits lasted less than 24 hours, evidence of acute brain infarction was necessary by neuroimaging. Hemorrhagic strokes included symptomatic nontraumatic intracerebral and subarachnoid hemorrhage. The secondary outcome of this study was the main secondary efficacy outcome of the trial; that is, the composite of first recurrent stroke, systemic embolism, myocardial infarction, or death from cardiovascular causes. The safety outcome of interest of this study was the trial primary safety outcome, specifically major bleeding according to International Society of Thrombosis and Hemostasis criteria.14 All outcomes were centrally adjudicated, and reviewers were blinded to the treatment assignments.
Statistical Analysis
Analyses were performed on the intention-to-treat population. Participant characteristics at study entry were described using means with SDs for continuous variables and proportions for discrete variables. To describe time-to-event data, annualized event rates were calculated by dividing the number of participants with an event by the total number of patient years of observation with CIs estimated by assuming a Poisson distribution. Kaplan-Meier curves are also presented. To describe the relative risk of outcome by assigned treatment, hazard ratios (HRs) and 95% CIs from the Cox proportional hazards model were computed. Heterogeneity of treatment effect across LV dysfunction groups was assessed by the statistical significance of the interaction term in the model after adjusting for main effects. A sensitivity analysis was planned a priori to adjust the model for any imbalance of baseline characteristics across assigned treatment groups. All reported P values were 2-sided, and significance was set at P < .05. Statistical analysis was done using SPSS for Windows version 27.0.1 (IBM Corp) and MedCalc Statistical Software version 19.6.4 (MedCalc Software Ltd).
Results
Description of the Cohort
Among the 7213 participants of NAVIGATE ESUS, 7107 (98.5%) had a documented assessment of LV function and were included in this exploratory analysis (Figure 1). A total of 4374 of 7107 participants with LV dysfunction (62%) were male. The mean (SD) age of participants with LV dysfunction was 67 (10) years. Of the 7107 participants in this analysis, 5504 (77%) had hypertension, and 594 (8%) had a history of coronary artery disease. Participants excluded from the analysis had similar demographic characteristics and factors associated with vascular disease compared with participants included (eTable 1 in the Supplement).
Figure 1. CONSORT Flow Diagram.
Among the 7107 participants, 502 (7%) had LV dysfunction reported. Of the 502, 405 (81%) had normal to mildly impaired LV contractility with a regional wall motion abnormality, while 97 (19%) had moderate to severely impaired LV contractility with or without a regional wall motion abnormality. Normal to mildly impaired LV contractility without a regional wall motion abnormality was observed in 6605 patients (93%). Patients with evidence of LV dysfunction were more likely to be male, have coronary artery disease, and have a history of stroke or transient ischemic attack prior to the qualifying stroke compared with those without LV dysfunction (Table 1).
Table 1. Baseline Characteristics of Patients in the NAVIGATE ESUS Trial Stratified by Left Ventricular Dysfunction.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Normal to mildly impaired contractility without regional abnormality noted (n = 6605) | Normal to mildly impaired contractility with regional abnormality noted (n = 405) | Moderate to severe impaired contractility with or without regional abnormality noted (n = 97) | |
| Age, mean (SD), y | 67 (10) | 67 (9) | 68 (10) |
| Female | 2603 (39) | 108 (27) | 22 (23) |
| Male | 4002 (61) | 297 (73) | 75 (77) |
| Region of enrollment | |||
| Canada and the US | 825 (12) | 66 (16) | 18 (19) |
| Latin America | 693 (10) | 34 (8) | 16 (16) |
| Western Europe | 2812 (43) | 156 (39) | 39 (40) |
| Eastern Europe | 1027 (16) | 72 (18) | 16 (16) |
| East Asia | 1248 (19) | 77 (19) | 8 (8) |
| Body mass index,a mean (SD) | 27 (5) | 27 (5) | 28 (6) |
| Blood pressure, mmHg | |||
| Systolic, mean (SD) | 135 (17) | 133 (17) | 132 (20) |
| Diastolic, mean (SD) | 79 (11) | 79 (11) | 78 (11) |
| Hypertension | 5107 (77) | 323 (80) | 74 (76) |
| Diabetes | 1630 (25) | 111 (27) | 36 (37) |
| Hyperlipidemia | 3645 (55) | 248 (61) | 51 (53) |
| Coronary artery disease | 422 (6) | 128 (32) | 44 (45) |
| Peripheral vascular disease | 101 (2) | 15 (4) | 3 (3) |
| Qualifying stroke, multiple lesions on imaging | 689 (10) | 50 (12) | 7 (7) |
| NIHSS score at randomization, median (IQR) | 1 (0-2) | 1 (0-2) | 1 (0-2) |
| Stroke or TIA prior to qualifying event | 1126 (17) | 83 (20) | 25 (26) |
| Current tobacco use | 1349 (20) | 88 (22) | 19 (20) |
| Assigned to rivaroxaban | 3323 (50) | 186 (46) | 43 (44) |
Abbreviations: NIHSS, National Institute of Health Stroke Scale; TIA, transient ischemic attack.
Calculated as weight in kilograms divided by height in meters squared.
Primary Efficacy Outcome
Over a median (IQR) follow-up of 10.4 (5.2-17.0) months, the primary outcome of recurrent stroke or systemic embolism occurred in 321 (4.9% per year) of the 7107 participants included in the analysis: 299 had ischemic strokes, 15 hemorrhagic strokes, 4 unknown strokes, and 3 systemic emboli.
The overall annualized rates of the primary outcome did not differ appreciably across the 3 LV dysfunction categories (Table 2). Although not prespecified in our analysis plan, because of the small number of observed events in the 2 groups having prespecified LV dysfunction (14 of 405 individuals with normal to mildly impaired contractility with regional wall motion abnormality noted and 8 of 97 individuals with moderate to severely impaired contractility with or without regional wall motion abnormality noted), these 2 groups were combined prior to performing subsequent analyses (n = 502; 22 events).
Table 2. Left Ventricular (LV) Dysfunction and Annualized Primary Event Rates by Assigned Treatmenta.
| LV dysfunction | Total | Rivaroxaban | Aspirin | |||
|---|---|---|---|---|---|---|
| No. | Rate, %/y (No. of events) | No. | Rate, %/y (No. of events) | No. | Rate, %/y (No. of events) | |
| Normal to mildly impaired contractility without regional abnormality noted | 6605 | 4.9 (299) | 3323 | 5.3 (161) | 3282 | 4.5 (138) |
| Normal to mildly impaired contractility with regional abnormality noted | 405 | 3.5 (14) | 229 | 2.4 (6) | 273 | 6.5 (16) |
| Moderate to severely impaired contractility with or without regional abnormality noted | 97 | 8.1 (8) | ||||
The primary outcome of this study was the primary efficacy outcome of the trial: the composite end point of recurrent stroke or systemic embolism.
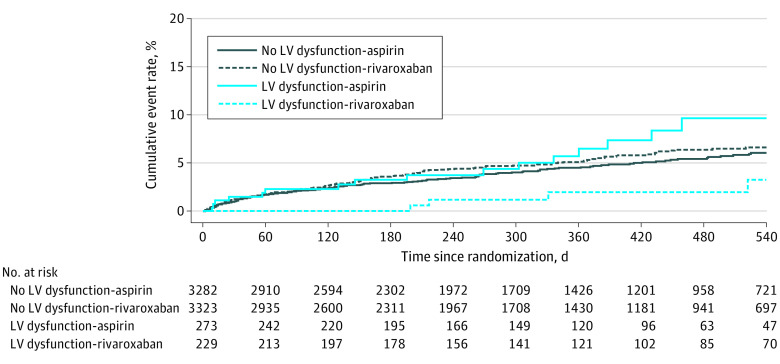
Among the 7107 participants, 167 participants assigned to rivaroxaban vs 154 participants assigned to aspirin experienced the primary outcome (5.1% vs 4.7% per year, respectively). For the 502 participants (7.1%) with LV dysfunction, rates were 2.4% per year (95% CI, 1.1-5.4) in those assigned to rivaroxaban vs 6.5% (95% CI, 4.0-11) in those assigned to aspirin (Table 2). In contrast, among the 6605 participants without LV dysfunction, annualized primary event rates were similar between those assigned to rivaroxaban (5.3% per year; 95% CI, 4.5-6.2) vs aspirin (4.5% per year; 95% CI, 3.8-5.3) (Table 2; Figure 2). This observed heterogeneity of treatment effect was statistically significant (P = .03 for interaction) after adjusting for the main effects of assigned treatment and LV dysfunction. Participants with LV dysfunction randomized to rivaroxaban vs aspirin had a significantly lower risk of the primary outcome (HR, 0.36; 95% CI, 0.14-0.93), whereas those without LV dysfunction had similar risk (HR, 1.16; 95% CI, 0.93-1.46).
Figure 2. Kaplan-Meier Curves for Time to Primary Outcome Event by Left Ventricular Dysfunction and Assigned Treatment.
The primary outcome of this study was the primary efficacy outcome of the trial: the composite end point of recurrent stroke or systemic embolism.
Of the 15 patients with a hemorrhagic stroke as the primary outcome, 1 occurred in the 502 patients with LV dysfunction (0.2%), while 14 occurred in the 6605 patients with normal to mildly impaired LV contractility without regional wall motion abnormality noted (0.2%). Of the 14 occurring in this latter group, 12 occurred in participants assigned to rivaroxaban and 2 occurred in participants assigned to aspirin.
Secondary Efficacy Outcome
A total of 390 of 7107 participants (5.5%) experienced a secondary outcome. Secondary event rates were similar across LV function groups with participants in the normal to mildly impaired LV contractility with a regional wall motion abnormality group and in the moderate to severely impaired LV contractility with or without a regional wall motion abnormality group having a limited number of events (Table 3). For this reason, although not prespecified in our analysis plan, we again combined the 2 LV dysfunction groups (n = 502; 35 events) before performing additional analysis.
Table 3. Left Ventricular Dysfunction and Annualized Secondary Event Rates by Treatment Strategya.
| LV dysfunction | Total | Rivaroxaban | Aspirin | |||
|---|---|---|---|---|---|---|
| No. | Rate, %/y (No. of events) | No. | Rate, %/y (No. of events) | No. | Rate, %/y (No. of events) | |
| Normal to mildly impaired contractility without regional abnormality noted | 6605 | 5.8 (355) | 3323 | 6.2 (189) | 3282 | 5.4 (166) |
| Normal to mildly impaired contractility with regional abnormality noted | 405 | 5.6 (22) | 229 | 4.9 (12) | 273 | 9.5 (23) |
| Moderate to severely impaired contractility with or without regional abnormality noted | 97 | 13.3 (13) | ||||
The secondary outcome of this study was the main secondary efficacy outcome of the trial; that is, the composite of recurrent stroke, systemic embolism, myocardial infarction, or death from cardiovascular causes.
Among the 7107 participants, 201 assigned to rivaroxaban vs 189 assigned to aspirin experienced a secondary outcome (6.1% vs 5.7% per year, respectively). For the 502 participants with LV dysfunction (7.1%), rates were 4.9% per year (95% CI, 2.8-8.6) in those assigned to rivaroxaban vs 9.5% per year (95% CI, 6.3-14) in those assigned to aspirin (Table 3). In contrast, among the 6605 participants without LV dysfunction, annualized primary event rates were similar between those assigned to rivaroxaban (6.2% per year; 95% CI, 5.4-7.1) vs aspirin (5.4% per year; 95% CI, 4.7-6.3) (Table 3). After adjusting for main effects of assigned treatment and LV dysfunction status, the P value for interaction for treatment effect was .05. Participants with LV dysfunction assigned to rivaroxaban had a lower risk of experiencing the secondary outcome compared with those assigned to aspirin (HR, 0.51; 95% CI, 0.3-1.0) whereas there was no significant difference by assigned treatment among those without LV dysfunction (HR, 1.1; 95% CI, 0.9-1.4).
Primary Safety Outcome
A major bleeding event occurred in 83 of the 7107 participants. Nearly 95% (n = 78) of events occurred in participants without evidence of LV dysfunction (55 assigned to rivaroxaban and 23 assigned to aspirin) with the remaining 5 occurring in participants with evidence of LV dysfunction (5 assigned to rivaroxaban and 0 assigned to aspirin).
Sensitivity Analysis
Baseline characteristics of participants with LV dysfunction appeared similar across assigned treatment groups with the exception that those randomized to rivaroxaban were less likely to have diabetes (23% vs 35%; eTable 2 in the Supplement). After adjusting for the main effects of diabetes in addition to assigned treatment and LV dysfunction status in a Cox proportional hazard model, the heterogeneity of treatment effect with LV dysfunction remained (P = .04 for interaction). Among participants with LV dysfunction, after adjusting for diabetes, rivaroxaban remained associated with a lower risk of the primary outcome (HR, 0.35; 95% CI, 0.14-0.9).
Discussion
In this exploratory analysis of the NAVIGATE ESUS trial, rivaroxaban was associated with a reduced risk of recurrent stroke or systemic embolism compared with aspirin among the 7% of participants with reported LV dysfunction.
The results of this study should be considered in the context of prior studies evaluating the optimal antithrombotic therapy for patients with ESUS. Two randomized clinical trials have concluded that anticoagulation is not superior to antiplatelet therapy among all patients with ESUS4,5; however, the etiologies of ESUS in these trials were heterogeneous, and the risk of recurrent stroke among patients without sources of cardiac embolism may not necessarily be reduced by anticoagulation.7 Certain rare forms of LV dysfunction, such as recent acute myocardial infarction or severe LV systolic dysfunction, are considered to be high-risk cardioembolic sources of stroke that respond to anticoagulation.3,8,15,16 Other more common forms of LV dysfunction, including impaired LV contractility or regional wall motion abnormalities, are not currently considered high-risk sources of cardiac embolism and yet appear to be associated with LV thrombus formation and recurrent stroke.9,10 A previously published subgroup analysis of NAVIGATE ESUS reported that rivaroxaban was not superior to aspirin in participants with any type of LV disease. In that analysis, LV disease was liberally defined to include diastolic dysfunction and LV hypertrophy, neither of which has been found to be associated with cardiac embolism, and 36% of participants met this broad definition.6 Rather than evaluating all types of LV disease, we tested the hypothesis that specific forms of LV dysfunction previously found to be associated with LV thrombus formation and stroke would respond to anticoagulation.9,10 In this context, our results provide novel data suggesting that ESUS patients with evidence of impaired LV contractility and/or regional wall motion abnormalities may benefit from anticoagulation.
Impaired LV contractility and/or regional wall motion abnormalities may be an independent risk factor for recurrent ischemic stroke. Impaired LV contractility and regional wall motion abnormalities are prevalent in patients with stroke.9,17 In NAVIGATE ESUS, 7% of participants had evidence of this type of LV dysfunction. Other studies have suggested that these common forms of LV dysfunction may pose a relatively similar risk of thrombus formation and stroke compared with more severe but rarer forms of LV dysfunction, such as acute myocardial infarction or severely depressed ejection fraction (less than 30%).18 A prior study evaluating the use of cardiac magnetic resonance imaging in patients with stroke found that 40% of LV thrombi were detected in patients who had an ejection fraction greater than 30% and that most of these thrombi were not visualized by standard transthoracic echocardiogram.10 Another study demonstrated that regional wall motion abnormalities were independently associated with a 1.7-fold increased risk of recurrent stroke.9 Taken together, these data suggest that certain forms of LV dysfunction, including impaired LV contractility and regional wall motion abnormalities may be associated with LV thrombus formation and recurrent ischemic stroke.
Secondary stroke prevention guidelines remain noncommittal regarding the treatment of patients who have had stroke and who have LV dysfunction, including depressed LV ejection fraction, impaired LV contractility, and regional wall motion abnormalities.19,20 As far back as 2012, the double-blind, multicenter Warfarin and Aspirin in Patients With Heart Failure and Sinus Rhythm (WARCEF) study demonstrated a reduced risk of ischemic stroke, albeit offset by an increased risk of major hemorrhage, for participants with an ejection fraction less than 35% who were assigned to warfarin vs aspirin.21 A recent meta-analysis of 5 randomized clinical trials (including WARCEF) of patients with heart failure with an ejection fraction less than 35% to 40% demonstrated that anticoagulation was superior to aspirin and placebo at reducing risk of ischemic stroke risk during follow-up (HR, 0.63; 95% CI, 0.49-0.81).22 Despite the heightened propensity for thrombus formation in patients with depressed LV ejection fraction, the American Heart Association/American Stroke Association Guidelines and Canadian Stroke Best Practice Recommendations are equivocal with regard to the optimal secondary stroke prevention therapy in this population, likely owing to the lack of level 1 evidence from a dedicated trial of secondary stroke prevention in patients with LV dysfunction and excess reported rates of major bleeding.19,20 To our knowledge, there are no recommendations or guidelines regarding use of anticoagulation for patients with impaired LV contractility or segmental wall motion abnormalities. Additionally, the high risk of bleeding found in older trials, such as WARCEF, may be offset by the use of novel anticoagulants, which have a more favorable risk-benefit profile compared with warfarin.23 The results of this study may therefore provide the rationale and justification for a randomized clinical trial evaluating the efficacy of anticoagulation compared with antiplatelet therapies in reducing recurrent stroke risk in patients with recent stroke and evidence of LV dysfunction.
Limitations
There are several limitations to this study. Most importantly, this was an exploratory analysis in the setting of a negative overall trial, and our results should be considered hypothesis generating. In addition, determination of LV dysfunction was not standardized across all participating sites, and there was no requirement to document echocardiographic parameters. Furthermore, we lacked granular data on the presence or extent of myocardial scar, which may serve as a nidus for thrombus formation and affect the propensity of cardiac embolism. We may also have been limited in our ability to detect associations because of the relatively small number of participants with LV dysfunction (7%).
Conclusions
In this analysis, we found that rivaroxaban, a factor Xa inhibitor, was superior to aspirin in reducing recurrent stroke or systemic embolism among patients with recent ESUS and evidence of LV dysfunction. A dedicated secondary stroke prevention trial in patients with LV dysfunction should be considered to evaluate the efficacy and safety of anticoagulation to prevent cardiac embolism and subsequent stroke.
eTable 1. Baseline characteristics of participants included and excluded from analysis
eTable 2. Baseline characteristics of patients with left ventricular dysfunction by assigned treatment
References
- 1.Krishnamurthi RV, Feigin VL, Forouzanfar MH, et al. ; Global Burden of Diseases, Injuries, Risk Factors Study 2010 (GBD 2010); GBD Stroke Experts Group . Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health. 2013;1(5):e259-e281. doi: 10.1016/S2214-109X(13)70089-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hart RG, Catanese L, Perera KS, Ntaios G, Connolly SJ. Embolic stroke of undetermined source: a systematic review and clinical update. Stroke. 2017;48(4):867-872. doi: 10.1161/STROKEAHA.116.016414 [DOI] [PubMed] [Google Scholar]
- 3.Hart RG, Diener HC, Coutts SB, et al. ; Cryptogenic Stroke/ESUS International Working Group . Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13(4):429-438. doi: 10.1016/S1474-4422(13)70310-7 [DOI] [PubMed] [Google Scholar]
- 4.Hart RG, Sharma M, Mundl H, et al. ; NAVIGATE ESUS Investigators . Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med. 2018;378(23):2191-2201. doi: 10.1056/NEJMoa1802686 [DOI] [PubMed] [Google Scholar]
- 5.Diener HC, Sacco RL, Easton JD, et al. ; RE-SPECT ESUS Steering Committee and Investigators . Dabigatran for prevention of stroke after embolic stroke of undetermined source. N Engl J Med. 2019;380(20):1906-1917. doi: 10.1056/NEJMoa1813959 [DOI] [PubMed] [Google Scholar]
- 6.Ntaios G, Pearce LA, Veltkamp R, et al. ; NAVIGATE ESUS Investigators . Potential embolic sources and outcomes in embolic stroke of undetermined source in the NAVIGATE-ESUS trial. Stroke. 2020;51(6):1797-1804. doi: 10.1161/STROKEAHA.119.028669 [DOI] [PubMed] [Google Scholar]
- 7.Kamel H, Merkler AE, Iadecola C, Gupta A, Navi BB. Tailoring the approach to embolic stroke of undetermined source: a review. JAMA Neurol. 2019;76(7):855-861. doi: 10.1001/jamaneurol.2019.0591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial: TOAST trial of org 10172 in acute stroke treatment. Stroke. 1993;24(1):35-41. doi: 10.1161/01.STR.24.1.35 [DOI] [PubMed] [Google Scholar]
- 9.Choi JY, Cha J, Jung JM, et al. Left ventricular wall motion abnormalities are associated with stroke recurrence. Neurology. 2017;88(6):586-594. doi: 10.1212/WNL.0000000000003588 [DOI] [PubMed] [Google Scholar]
- 10.Takasugi J, Yamagami H, Noguchi T, et al. Detection of left ventricular thrombus by cardiac magnetic resonance in embolic stroke of undetermined source. Stroke. 2017;48(9):2434-2440. doi: 10.1161/STROKEAHA.117.018263 [DOI] [PubMed] [Google Scholar]
- 11.Hart RG, Sharma M, Mundl H, et al. Rivaroxaban for secondary stroke prevention in patients with embolic strokes of undetermined source: design of the NAVIGATE ESUS randomized trial. Eur Stroke J. 2016;1(3):146-154. doi: 10.1177/2396987316663049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain P, Hayward CS. Left ventricular ejection fraction under continuous-flow mechanical support. Circ Heart Fail. 2020;13(9):e007427. doi: 10.1161/CIRCHEARTFAILURE.120.007427 [DOI] [PubMed] [Google Scholar]
- 13.Monge García MI, Jian Z, Settels JJ, et al. Determinants of left ventricular ejection fraction and a novel method to improve its assessment of myocardial contractility. Ann Intensive Care. 2019;9(1):48. doi: 10.1186/s13613-019-0526-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. doi: 10.1111/j.1538-7836.2005.01204.x [DOI] [PubMed] [Google Scholar]
- 15.Andreotti F, Testa L, Biondi-Zoccai GG, Crea F. Aspirin plus warfarin compared to aspirin alone after acute coronary syndromes: an updated and comprehensive meta-analysis of 25,307 patients. Eur Heart J. 2006;27(5):519-526. doi: 10.1093/eurheartj/ehi485 [DOI] [PubMed] [Google Scholar]
- 16.Rothberg MB, Celestin C, Fiore LD, Lawler E, Cook JR. Warfarin plus aspirin after myocardial infarction or the acute coronary syndrome: meta-analysis with estimates of risk and benefit. Ann Intern Med. 2005;143(4):241-250. doi: 10.7326/0003-4819-143-4-200508160-00005 [DOI] [PubMed] [Google Scholar]
- 17.Ramasamy S, Yaghi S, Salehi Omran S, et al. Association between left ventricular ejection fraction, wall motion abnormality, and embolic stroke of undetermined source. J Am Heart Assoc. 2019;8(9):e011593. doi: 10.1161/JAHA.118.011593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hays AG, Sacco RL, Rundek T, et al. Left ventricular systolic dysfunction and the risk of ischemic stroke in a multiethnic population. Stroke. 2006;37(7):1715-1719. doi: 10.1161/01.STR.0000227121.34717.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021;52(7):e364-e467. doi: 10.1161/STR.0000000000000375 [DOI] [PubMed] [Google Scholar]
- 20.Wein T, Lindsay MP, Cote R, et al. Canadian stroke best practice recommendations: secondary prevention of stroke, sixth edition practice guidelines, update 2017. Int J Stroke. 2018;13(4):420-443. doi: 10.1177/1747493017743062 [DOI] [PubMed] [Google Scholar]
- 21.Homma S, Thompson JL, Pullicino PM, et al. ; WARCEF Investigators . Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med. 2012;366(20):1859-1869. doi: 10.1056/NEJMoa1202299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beggs SAS, Rørth R, Gardner RS, McMurray JJV. Anticoagulation therapy in heart failure and sinus rhythm: a systematic review and meta-analysis. Heart. 2019;105(17):1325-1334. doi: 10.1136/heartjnl-2018-314381 [DOI] [PubMed] [Google Scholar]
- 23.Vinogradova Y, Coupland C, Hill T, Hippisley-Cox J. Risks and benefits of direct oral anticoagulants versus warfarin in a real world setting: cohort study in primary care. BMJ. 2018;363:k4413. doi: 10.1136/bmj.k2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Baseline characteristics of participants included and excluded from analysis
eTable 2. Baseline characteristics of patients with left ventricular dysfunction by assigned treatment