Key Points
Question
Can nonfatal myocardial infarction be validated as a surrogate end point for all-cause or cardiovascular mortality in the treatment and prevention of coronary artery disease?
Findings
In this meta-analysis of 144 randomized clinical trials with data from 1 211 897 patients, surrogacy between nonfatal myocardial infarction and all-cause or cardiovascular mortality was not noted.
Meaning
Results of this meta-analysis suggest that treatments that reduce nonfatal myocardial infarction cannot be assumed to reduce all-cause or cardiovascular mortality.
Abstract
Importance
Although nonfatal myocardial infarction (MI) is associated with an increased risk of mortality, evidence validating nonfatal MI as a surrogate end point for all-cause or cardiovascular (CV) mortality is lacking.
Objective
To examine whether nonfatal MI may be a surrogate for all-cause or CV mortality in patients with or at risk for coronary artery disease.
Data Sources
In this meta-analysis, PubMed was searched from inception until December 31, 2020, for randomized clinical trials of interventions to treat or prevent coronary artery disease reporting mortality and nonfatal MI published in 3 leading journals.
Study Selection
Randomized clinical trials including at least 1000 patients with 24 months of follow-up.
Data Extraction and Synthesis
Trial-level correlations between nonfatal MI and all-cause or CV mortality were assessed for surrogacy using the coefficient of determination (R2). The criterion for surrogacy was set at 0.8. Subgroup analyses based on study subject (primary prevention, secondary prevention, mixed primary and secondary prevention, and revascularization), era of trial (before 2000, 2000-2009, and 2010 and after), and follow-up duration (2.0-3.9, 4.0-5.9, and ≥6.0 years) were performed.
Main Outcomes and Measures
All-cause or CV mortality and nonfatal MI.
Results
A total of 144 articles randomizing 1 211 897 patients met the criteria for inclusion. Nonfatal MI did not meet the threshold for surrogacy for all-cause (R2 = 0.02; 95% CI, 0.00-0.08) or CV (R2 = 0.11; 95% CI, 0.02-0.27) mortality. Nonfatal MI was not a surrogate for all-cause mortality in primary (R2 = 0.01; 95% CI, 0.001-0.26), secondary (R2 = 0.03; 95% CI, 0.00-0.20), mixed primary and secondary prevention (R2 = 0.001; 95% CI, 0.00-0.08), or revascularization trials (R2 = 0.21; 95% CI, 0.002-0.50). For trials enrolling patients before 2000 (R2 = 0.22; 95% CI, 0.08-0.36), between 2000 and 2009 (R2 = 0.02; 95% CI, 0.00-0.17), and from 2010 and after (R2 = 0.01; 95% CI, 0.00-0.09), nonfatal MI was not a surrogate for all-cause mortality. Nonfatal MI was not a surrogate for all-cause mortality in randomized clinical trials with 2.0 to 3.9 (R2 = 0.004; 95% CI, 0.00-0.08), 4.0 to 5.9 (R2 = 0.06; 95% CI, 0.001-0.16), or 6.0 or more years of follow-up (R2 = 0.30; 95% CI, 0.01-0.55).
Conclusions and Relevance
The findings of this meta-analysis do not appear to establish nonfatal MI as a surrogate for all-cause or CV mortality in randomized clinical trials of interventions to treat or prevent coronary artery disease.
This meta-analysis of randomized clinical trials examines the use of myocardial infarction as a surrogate for all-cause or cardiovascular mortality in patients with or at risk for cardiovascular disease.
Introduction
In the 19th century, acute myocardial infarction (MI) resulting from coronary thrombosis was believed to be universally fatal. However, by the beginning of the next century, it was recognized that survival from acute MI was possible although still associated with significant mortality.1 However, as mortality rates decreased with advances in acute MI management in the second half of the 20th century, the evaluation of new therapies required a different standard.2 However, as mortality rates decreased with advances in acute MI management in the second half of the 20th century, the evaluation of new therapies became necessary using a different standard.2 In the early 1990s, Braunwald and colleagues3 suggested that combining mortality with nonfatal complications might better inform the overall outcomes of experimental therapies, accelerate the pace of evaluating proposed innovations, and minimize the number of patients exposed to ineffective therapies. Nonfatal MI was subsequently incorporated as an end point in landmark studies of acute coronary syndromes and ultimately in almost all studies of treatment or prevention of coronary artery disease based on the assumption that nonfatal MI was a surrogate for mortality and that preventing nonfatal MI would reduce mortality—a belief that endures.4,5,6,7,8,9,10 However, the use of nonfatal MI as a surrogate for mortality has been questioned for not meeting accepted standards for surrogacy.10,11,12,13,14
The assertion of surrogacy of one end point for another requires 3 levels of evidence.15 First, there must be biological plausibility. Although biological plausibility is necessary, it is not sufficient to establish surrogacy.16 Second, observational or epidemiologic studies demonstrating a consistent association between the surrogate and the final outcome are required. The highest level of proof is trial-level surrogacy in which, across many trials, treatments that improve the surrogate end point also improve the ultimate end point of interest. Establishing trial-level surrogacy requires an analysis of all randomized clinical trials (RCTs), with each trial serving as a unique data point. There is abundant evidence demonstrating that the association between nonfatal MI and all-cause or cardiovascular (CV) mortality meets the first 2 levels of evidence but, to our knowledge, the third and most stringent level of proof has never been assessed. We therefore performed an analysis of RCTs reported over the past approximately 50 years to test the null hypothesis that nonfatal MI is not a surrogate for all-cause or CV mortality by assessing the correlation between the treatment effect of an intervention on nonfatal MI and the treatment effect of the same intervention on all-cause or CV mortality.
Methods
PubMed was searched from inception until December 31, 2020, for RCTs of interventions to treat or prevent the clinical manifestations of coronary artery disease published in The New England Journal of Medicine, JAMA, or The Lancet that reported all-cause mortality and nonfatal MI outcomes (eAppendix in the Supplement). The search was limited to these journals because trials published in the highest-impact journals have higher methodological quality, larger sample sizes, and lower risk of bias.17 This report follows the Preferred Reporting Items for Systematic Reviews and Meta-analyses protocol (PRISMA-P) and was registered with PROSPERO (CRD42020172341).18
Included studies required randomization to an investigational treatment or treatment strategy or a placebo or active control. Investigational treatments or strategies included any pharmacological, imaging, revascularization, or lifestyle intervention aimed at the primary or secondary prevention of coronary artery disease. To maximize precision of the reported treatment effects and the number of events, the inclusion criteria required a minimum sample size of at least 1000 patients with at least 24 months of follow-up.
Two independent investigators (K.O. and D.L.B.) screened trials and determined eligibility for inclusion. Risk of bias was also assessed using the Cochrane Collaboration tool. Trial-level data were then extracted from primary publications. Trial-level data have been previously demonstrated as the highest level of evidence for establishing the validity of surrogate end points.19,20 Extracted variables included study subject, year of the first patient enrollment, total number of randomized participants, intervention type, control type, median follow-up time, and definition of MI used for outcome adjudication. Outcomes extracted included nonfatal MI, all-cause mortality, and CV mortality. Nonfatal MI data reflected the number of adjudicated MI events based on the definition of MI used in each trial. Most trials used the World Health Organization definition or a universal definition of MI; however, trial-specific definitions were also used.21,22,23,24,25 If any outcomes data or the definition of nonfatal MI used for adjudication were unavailable in the primary publication, associated publications, or a published protocol, corresponding authors were contacted by email to request the missing data. Trials with missing data and negative response after 60 days (n = 74) were excluded. Negative responses included refusal by investigators (n = 3), no response to request (n = 55), or data declared no longer available (n = 16). The definition of CV mortality was that used in individual RCTs and was generally defined as a composite of sudden cardiac death or death from acute MI, heart failure, stroke, CV procedures, or other CV causes.
Statistical Analysis
A meta-analysis of summary statistics from each article was performed using Comprehensive Meta-Analysis, version 2.0 (Biostat Inc) software. Estimates of effect for all-cause mortality, CV mortality, and nonfatal MI were calculated from crude event rates with a random-effects model using inverse variance weighting, expressed as odds ratios (ORs) with 95% CIs and presented in forest plots. Data were analyzed according to the intention-to-treat principle. The presence of between-study heterogeneity was determined by the I2 value (ranging from 0% to 100%) and graded as follows: values less than 25% (low heterogeneity), between 25% and 50% (moderate heterogeneity), and 50% or higher (high heterogeneity).26 Funnel plots for mortality and nonfatal MI were constructed to assess for publication bias. The Egger test was used to identify asymmetry of funnel plots. The Egger test regresses the standardized effect sizes on their precisions; in the absence of publication bias, the regression intercept is expected to be 0.
To generate a graphic representation of the association between nonfatal MI and all-cause or CV mortality, the log-transformed ORs or hazard ratios (HRs) for nonfatal MI (the putative surrogate) were graphed on the x coordinate with the ORs and HRs for all-cause or CV mortality (the true end point) graphed on the y coordinate, with each trial serving as a unique data point. A horizontal line with slope = 0 indicates no association; a positive slope indicates some degree of positive association, whereas a negative slope indicates a negative association between nonfatal MI and all-cause or CV mortality. We attempted to determine the surrogate treatment effect, defined as the maximum value of the OR for nonfatal MI that needs to be observed in a trial to conclude a significant effect on all-cause or CV mortality. The surrogate treatment effect is determined by the intersection of the upper prediction limit and the horizonal line indicating log OR mortality = 0.
Because positive correlation does not necessarily meet the more stringent criteria for surrogacy, trial-level surrogacy of nonfatal MI for all-cause or CV mortality was assessed by generating a coefficient of determination, R2 (with 95% CI), between the natural logarithm of the ORs or HRs for nonfatal MI and all-cause and CV mortality using a weighted linear regression in which each study was weighted by the number of observations. The R2 values (corresponding to the explained variation) fall between 0 and 1.00, with 0 indicating the absence of surrogacy and 1.00 indicating perfect surrogacy. The CI for R2 was obtained from bootstrap resampling. The threshold for validating nonfatal MI as a surrogate for all-cause or CV mortality was set at 0.8 with the 95% CI excluding 0.6. This predefined threshold was arbitrary but has been used elsewhere.27 It was determined prospectively to limit post hoc biases. All statistical analyses other than the meta-analysis were performed in R, version 4.0 (R Foundation for Statistical Computing).
Four prespecified subgroup analyses based on study subject, era of the trial, duration of follow-up, and type of control group treatment were performed to investigate their associations with surrogacy. Study subjects included primary prevention, secondary prevention, mixed primary and secondary prevention, and revascularization trials. The time period was based on the year the first patient was enrolled and divided into 3 eras: before 2000, from 2000 to 2010, and after 2010, corresponding to the adoption of troponin in the universal definition of MI in 2000 and the widespread adoption of commercially available high-sensitive troponins in 2010.22,28 Duration of follow-up was categorized as 2.0 to 3.9 years, 4.0 to 5.9 years, and 6.0 or more years. Control groups receiving either active or placebo treatments were analyzed separately. For sensitivity analysis, we analyzed only trials that reported time-to-event data presented as HRs.
Results
Description of Trials
As illustrated in Figure 1, 1025 RCTs were identified for screening, with 298 meeting inclusion criteria. Data extraction was completed for 144 RCTs reflecting 5 726 395 patient-years of follow-up in 1 211 897 patients.25,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171 eTable 1 in the Supplement presents the trial-level characteristics of the 144 studies. eTable 2 in the Supplement provides an assessment of risk of bias, and eTable 3 in the Supplement provides the full study names. By study subject, there were 24 primary prevention, 57 mixed primary/secondary prevention, 46 secondary prevention, and 17 revascularization RCTs. By intervention, there were 117 trials of pharmacological agents, 3 trials of imaging strategies, 7 trials of lifestyle interventions, and 17 trials of interventional therapies. By time period, there were 62 trials with first patient enrollment before 2000, 44 trials that began enrollment between 2000 and 2009, and 38 trials that initiated enrollment in 2010 and after. Follow-up duration was between 2.0 and 3.9 years in 66 trials, between 4.0 and 5.9 years in 57 trials, and 6.0 or more years in 21 trials. The earliest included trial was published in May 1972, and the most recent trial was published in November 2020. Cardiovascular mortality was reported in 112 RCTs enrolling 1 023 852 patients. Hazard ratios were reported in 48 trials with 367 531 participants.
Figure 1. Preferred Reporting Items for Systematic Review and Meta-analysis Diagram.
MI indicates myocardial infarction.
In the pooled analysis of the 144 trials, randomized assignment to an investigational treatment reduced the rate of nonfatal MI from 4.2% to 3.7% (OR, 0.87; 95% CI, 0.85-0.90; overall P < .001; I2 = 58%; P < .001) and reduced all-cause mortality from 7.3% to 7.0% (OR, 0.95; 95% CI, 0.93-0.97; overall P < .001; I2 = 53%; P < .001). Funnel plots were symmetrical for both end points, indicating lack of publication bias. The Egger test did not identify asymmetry for the funnel plot for nonfatal MI (intercept, −0.16; 95% CI, −0.80 to 0.49; P = .31) or mortality (intercept, −0.44; 95% CI, −1.02 to 0.14; P = .07) (eFigure 1 in the Supplement). eFigure 2 in the Supplement demonstrates the outcomes of randomized interventions on nonfatal MI and mortality for the 144 RCTs grouped by study subject in ascending chronological order from the date of the first patient enrollment.
Correlations of Treatment Effects
Figure 2A illustrates the log-transformed graphical representation of the OR for nonfatal MI and the OR for all-cause mortality for all 144 trials. Overall, the slope of the regression line was not significantly different than the horizontal (0.09; 95% CI, −0.01 to 0.19), and the coefficient of determination confirmed that the trial-level treatment effect on nonfatal MI did not predict treatment effect on all-cause mortality (R2 = 0.02; 95% CI, 0.00-0.08) (Table). Because the upper bound of the prediction interval never crossed the log OR_mortality = 0 horizontal, the surrogate treatment effect cannot be calculated, indicating there is no OR achievable by an intervention for nonfatal MI that would predict a significant reduction in the OR for all-cause mortality. Figure 2B shows the log-transformed representation of the OR for nonfatal MI and the OR for CV mortality in 112 trials. The slope of the regression line indicates a positive correlation between nonfatal MI and CV mortality. However, at R2 = 0.11 (95% CI, 0.02-0.27), the coefficient of determination does not validate nonfatal MI as a surrogate for CV mortality (Table). Because the upper bound of the prediction interval never crosses the log OR_mortality = 0 horizontal, the surrogate treatment effect cannot be calculated, indicating there is no OR achievable by an intervention for nonfatal MI that would predict a significant reduction in the OR for CV mortality. Limiting the analysis to the 48 RCTs that reported HRs, there was no significant difference between the slope of the regression line from the horizontal (−0.05; 95% CI, −0.27 to 0.17) and no evidence of surrogacy (R2 = 0.005; 95% CI, 0.00 to 0.06) (Figure 2C) (Table).
Figure 2. Correlations of Treatment Effects on Nonfatal Myocardial Infarction (MI) and All-Cause or Cardiovascular Mortality.
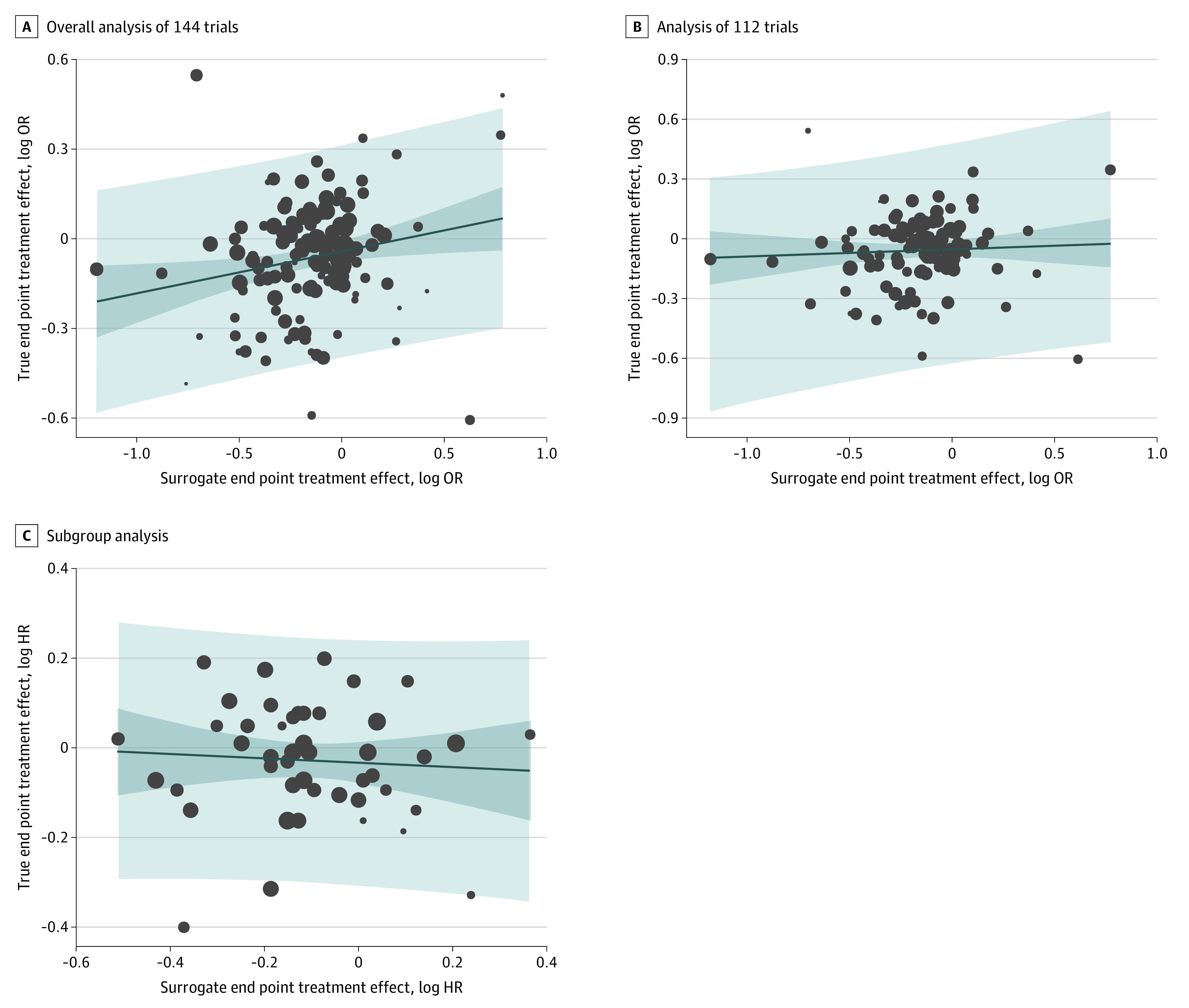
A, Overall analysis of 144 trials for the association between the logarithm of the odds ratio (log OR) for the surrogate end point of nonfatal MI and the true end point of all-cause mortality. B, Analysis of 112 trials for the association between the log OR for the surrogate end point of nonfatal MI and the true end point of cardiovascular mortality. C, Subgroup analysis of trials reporting hazard ratios (HRs) for the association between the log HR for the surrogate end point of nonfatal MI and the true end point of all-cause mortality. The dark blue area represents the 95% CI for the regression line (solid blue), light blue area represents the 95% prediction interval, and circle sizes are proportionate to the number of observations.
Table. Overall and Subgroup Analyses of the Correlation of Treatment Effects and Coefficient of Determination of MI for All-Cause and Cardiovascular Mortality.
| Analysis | Trials | Patients, No. | Regression formula | Slope (95% CI) | P value | R2 (95% CI) |
|---|---|---|---|---|---|---|
| All-cause mortality | 144 | 1 211 897 | −0.02 + 0.09 × log (OR_MI) | 0.09 (−0.01 to 0.19) | .07 | 0.02 (0.00 to 0.08) |
| Study subject | ||||||
| Primary prevention | 24 | 352 897 | 0.001 + 0.12 × log (OR_MI) | 0.12 (−0.05 to 0.29) | .14 | 0.01 (0.001 to 0.26) |
| Primary-secondary prevention | 57 | 461 329 | −0.04 + 0.02 × log (OR_MI) | 0.02 (−0.14 to 0.18) | .80 | 0.001 (0.00 to 0.08) |
| Secondary prevention | 46 | 351 823 | −0.02 + 0.14 × log (OR_MI) | 0.14 (−0.11 to 0.40) | .27 | 0.03 (0.00 to 0.20) |
| Revascularization | 17 | 45 848 | 0.04 + 0.28 × log (OR_MI) | 0.28 (−0.02 to 0.60) | .07 | 0.21 (0.002 to 0.50) |
| Era of first patient enrollment | ||||||
| <2000 | 62 | 461 103 | −0.02 + 0.27 × log (OR_MI) | 0.27 (0.14 to 0.41) | <.001 | 0.22 (0.08 to 0.36) |
| 2000-2009 | 44 | 397 766 | −0.005 + −0.08 × log (OR_MI) | −0.08 (−0.26 to 0.10) | .38 | 0.02 (0.00 to 0.17) |
| ≥2010 | 38 | 353 028 | −0.05 + −0.05 × log (OR_MI) | −0.05 (−0.27 to 0.17) | .67 | 0.01 (0.00 to 0.09) |
| Length of follow-up, y | ||||||
| 2.0-3.9 | 66 | 484 978 | −0.02 + 0.04 × log (OR_MI) | 0.04 (−0.12 to 0.20) | .61 | 0.004 (0.00 to 0.08) |
| 4.0-5.9 | 57 | 486 054 | −0.009 + 0.14 × log (OR_MI) | 0.14 (−0.01 to 0.29) | .07 | 0.06 (0.001 to 0.16) |
| ≥6.0 | 21 | 240 865 | −0.023 + 0.466 × log (OR_MI) | 0.47 (0.12 to 0.81) | .01 | 0.30 (0.01 to 0.55) |
| Control arm | ||||||
| Placebo | 86 | 827 461 | −0.02 + 0.09 × log (OR_MI) | 0.09 (−0.06 to 0.25) | .23 | 0.02 (0.00 to 0.10) |
| Active | 58 | 384 436 | −0.03 + 0.10 × log (OR_MI) | 0.10 (−0.04 to 0.23) | .16 | 0.03 (0.00 to 0.13) |
| Studies reporting time-to-event outcomes | 48 | 367 531 | −0.02 + −0.05 × log (HR_MI) | −0.05 (−0.16 to 0.26) | .63 | 0.01 (0.00 to 0.06) |
| Studies reporting cardiovascular mortality | 112 | 1 023 852 | −0.03 + 0.29 × log (OR_MI) | 0.29 (0.13 to 0.44) | .0003 | 0.11 (0.02 to 0.27) |
Abbreviations: HR, hazard ratio; MI, myocardial infarction; OR, odds ratio.
By study subject, nonfatal MI did not meet the threshold for surrogacy of all-cause mortality in primary prevention (R2 = 0.01; 95% CI, 0.001-0.26), secondary prevention (R2 = 0.03; 95% CI, 0.00-0.20), mixed primary and secondary prevention (R2 = 0.001; 95% CI, 0.00-0.08), and revascularization trials (R2 = 0.21; 95% CI, 0.002-0.50) (Figure 3) (Table). By time period of the RCT in trials with the first patient enrollment before 2000, the slope of the regression line was significantly greater than the horizontal (0.27; 95% CI, 0.14-0.41), but despite the positive association, nonfatal MI did not meet the threshold for surrogacy of all-cause mortality (R2 = 0.22; 95% CI, 0.08-0.36). There was no significant difference in the regression lines from the horizontal and even weaker evidence of surrogacy for periods 2000-2009 (R2 = 0.02; 95% CI, 0.00-0.17) and 2010 and beyond (R2 = 0.01; 95% CI, 0.00-0.09) (Figure 4) (Table).
Figure 3. Subgroup Analysis by Study Subject for the Association Between the Logarithm of the Odds Ratios (Log ORs) for the End Points.
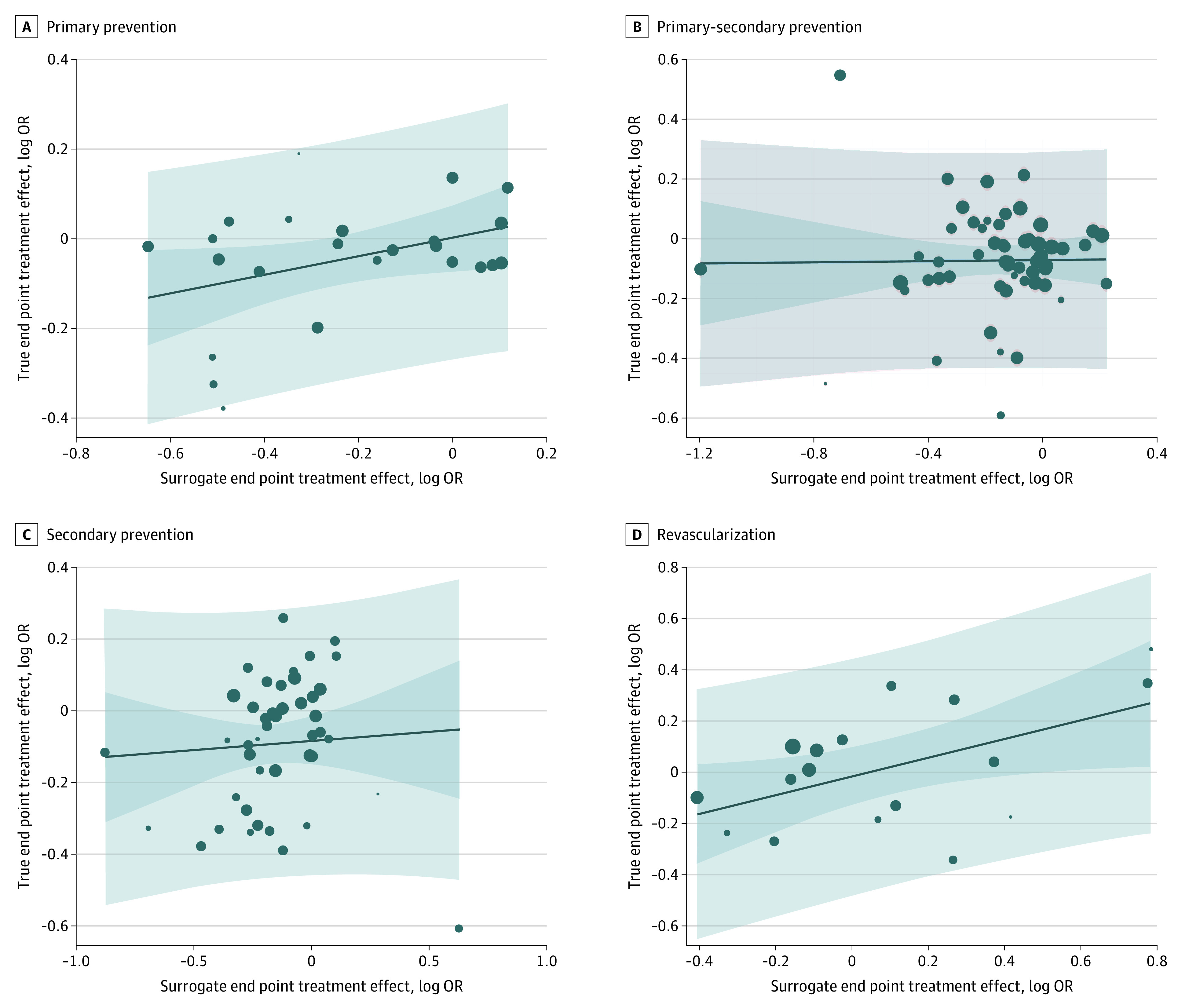
Surrogate end point of nonfatal myocardial infarction and the true end points of all-cause mortality primary prevention (A), mixed primary-secondary prevention (B), secondary prevention (C), and revascularization (D). The dark blue area represents the 95% CI for the regression line (solid blue), light blue area represents the 95% prediction interval, and circle size is proportionate to the number of observations.
Figure 4. Subgroup Analysis by Era of Trial Enrollment for the Association Between the Logarithm of the Odds Ratios (OR) for End Points.
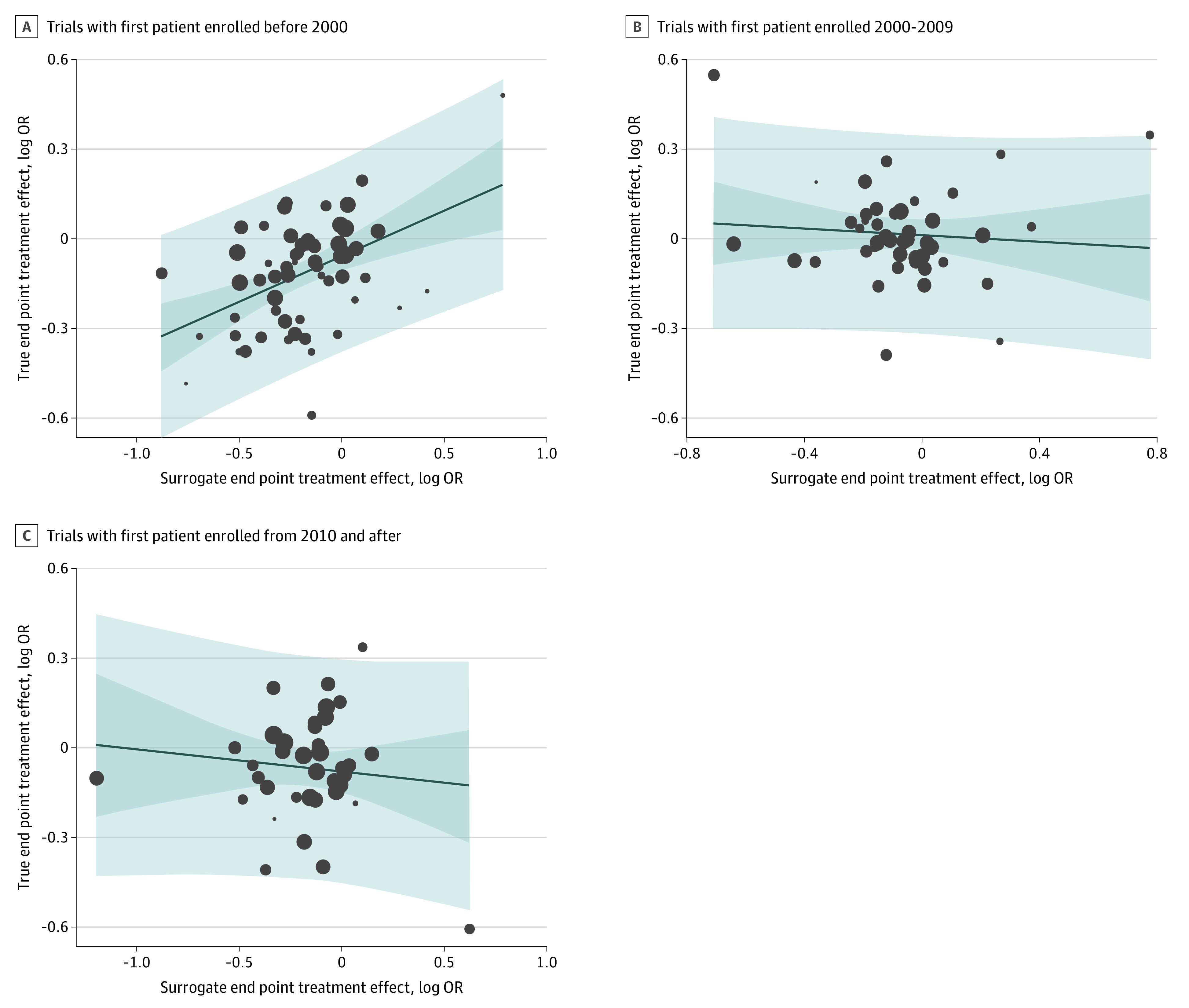
Surrogate end point of myocardial infarction (MI) and true end point of all-cause mortality in trials with first patient enrolled before 2000 (A) from 2000 to 2009 (B), and in 2010 and after (C). The dark blue area area represents the 95% CI for the regression line (solid blue). Light blue area represents the 95% prediction interval. Circle size is proportionate to trial size.
The regression lines were not significantly different from the horizonal line with no evidence of surrogacy of nonfatal MI for all-cause mortality for follow-up length of 2.0 to 3.9 years (R2 = 0.004; 95% CI, 0.00-0.08) and 4.0-5.9 years (R2 = 0.06; 95% CI, 0.001-0.16). For follow-up length of 6.0 or more years, there was a significant difference in the slope of the regression line from the horizontal (0.47; 95% CI, 0.12-0.81), but the coefficient of determination did not achieve the threshold for surrogacy (R2 = 0.30; 95% CI, 0.01-0.55) (eFigure 3 in the Supplement) (Table).
eFigure 4 in the Supplement shows the subgroup analysis based on the type of treatment in the control group (placebo or active therapy). In neither case was the slope of the regression line significantly different than the horizontal, indicating no correlation. Furthermore, R2 values were 0.02 for placebo and 0.03 for active control, indicating the absence of surrogacy of nonfatal MI for all-cause mortality (Table).
Discussion
Surrogate end points in clinical trials are biological markers or clinical events (eg, nonfatal MI) that may be observed earlier than the clinical end points (eg, death) that are of primary interest to patients and clinicians.10 Since the earliest descriptions, it has been recognized that the natural history of acute MI includes mortality.1 It has been assumed, therefore, that interventions to prevent nonfatal MI would reduce all-cause or CV mortality, supporting the notion that nonfatal MI is a surrogate for mortality. However, in this meta-analytic assessment of nonfatal MI surrogacy including 144 RCTs that randomized 1.2 million patients with 5.7 million years of follow-up to interventions to treat or prevent coronary artery disease, we found no trial-level correlation between nonfatal MI and all-cause or CV mortality. The I2 values for the 144 RCTs included in the meta-analysis revealed significant heterogeneity, which is an advantage in this setting because between-trial differences provide opportunities in subgroup analyses to find more specific settings in which surrogacy exists. However, even in subgroups divided by study subject, study era, duration of follow-up, and control arm treatment, we were unable to detect any evidence of surrogacy. We also found no evidence of surrogacy when limiting the analysis to studies that reported time-to-event data.
There are at least 3 potential explanations for the lack of trial-level surrogacy between nonfatal MI and all-cause or CV mortality. First, over the time course of this study, the extent of myocardial injury required to diagnose nonfatal MI has decreased with the introduction of progressively more sensitive biomarkers. At the same time, background primary and secondary treatments have improved. Thus, more recently detected nonfatal MIs may be so small and secondary prevention treatments so successful that nonfatal MIs are no longer on the causal pathway of mortality. However, even in the earliest era of RCTs prior to 2000 and before the widespread introduction of troponin assays to detect myocardial injury, the R2 value was only 0.27, which does not meet the threshold for surrogacy. In subsequent eras, from 2000 to 2010 before the introduction of high-sensitivity troponin assays and from 2011 to 2020 after the introduction of high-sensitivity troponin assays, there was no correlation between the treatment effects on nonfatal MI and all-cause mortality.
Second, it is plausible that the failure to demonstrate surrogacy between nonfatal MI and all-cause mortality relates to the competing risk of non-CV death that CV interventions would not be expected to reduce. In a post hoc analysis of the IMPROVE-IT trial (included in this analysis), the relative incidence of CV and non-CV death following an acute coronary syndrome was investigated.172 For patients who presented with an ST-segment-elevation MI, the incidence of CV death was higher than non-CV death for 4 years following the index event after which non-CV death predominated. For unstable angina or non–ST-segment elevation MI, the cumulative incidence of CV death remained higher than non-CV death for the entire duration of follow-up (median, 6.0 years). Although similar data for RCTs performed in different settings are lacking, our subgroup analysis showing no significant surrogacy at 2.0 to 3.9, 4.0 to 5.9, and 6.0 or more years suggests that an increasing risk of non-CV death over time is not a likely explanation for the overall lack of surrogacy. Furthermore, the lack of surrogacy of nonfatal MI for CV mortality further weakens this potential explanation.
The third potential explanation for the lack of surrogacy of nonfatal MI for all-cause or CV mortality relates to the heterogeneous nature of MI. Although the earliest descriptions attributed all acute MIs to coronary thrombosis, by 1939 it was recognized that there were both thrombotic and nonthrombotic causes of acute MI.1,173 However, it was not until 2007 that an international consensus classification of acute MI subtypes, the universal definition of MI, was published.23 By the time this classification was revised in 201224 and updated in 2018,174 5 clinical subtypes of acute MI in addition to the non-MI diagnosis of acute nonischemic myocardial injury were recognized, each with different pathophysiologic mechanisms and different mortality rates and causes.
The 2 most common MI subtypes are type 1, attributed to atherosclerotic plaque rupture or erosion and secondary thrombosis, and type 2, resulting from an acute imbalance in myocardial oxygen supply and demand without thrombosis. The reported proportion of adjudicated type 2 MI ranges from 2% to 58% of all MI presentations.175 Physician accuracy in differentiating type 1 from type 2 MI is poor.176 However, distinction of the 2 categories is important because, in addition to different pathophysiologic characteristics, type 1 and type 2 MIs require different management and have different outcomes.
For type 1 MI, treatment focuses on restoring or maintaining coronary arterial patency pharmacologically or with revascularization. Treatment of type 2 MI focuses on reversing the underlying cause of supply and demand mismatch. In most studies, both short- and long-term mortality are higher in patients with type 2 MI.176 The increased mortality in patients with nonfatal type 2 MI is associated with their greater burden of comorbidities rather than their underlying coronary disease. Thus, treatments that reduce nonfatal type 2 MI, which is a marker for comorbidities, would not be expected to reduce all-cause or CV mortality and would blunt any potential surrogacy of nonfatal type 1 MI for mortality.
Since its earliest recognition, most treatment and prevention strategies have focused on type 1 MI. Although the development of increasingly sensitive troponin assays over the past decade has increased the diagnosis of acute MI by approximately 50% and allowed the elucidation of the various MI phenotypes, it is unlikely their existence is new.177 Thus, the application of type 1 MI treatment and prevention strategies to the other MI subtypes is likely an important contributor to the lack of correlation between the effects of treatment for nonfatal MI with the treatment effect for all-cause or CV mortality.
Limitations
There are limitations to this analysis. First, by confining our search strategy to 3 journals, we did not include all potentially relevant RCTs. Second, not all trials identified by our search strategy were included because of the lack of availability of relevant data. However, unlike traditional meta-analyses, the use of nonexhaustive sets of trials are less of a concern as long as the included trials reflect a wide range of heterogeneity of treatment effects.178 Third, the specific threshold we used to grade the presence of surrogacy has not been externally validated. Others have recommended a threshold of 0.65 for coefficients of determination (R2).16 However, it is unlikely that the overall R2 of 0.02 or even the highest R2 of 0.30 identified in studies with a follow-up of 6.0 or more years would be interpreted as representing significant surrogacy by any standard.179 Fourth, the patients enrolled in RCTs may not be representative of the overall population of patients with or at risk for coronary artery disease. Fifth, it is possible that with longer follow-up, especially in primary prevention trials, the R2 values may have approached the surrogacy thresholds. Sixth, only 3 RCTs provided data on the MI subtypes, which prevented us from evaluating surrogacy as a function of MI subtype.
Conclusions
In this meta-analysis, nonfatal MI, as defined in RCTs of treatment and prevention of coronary artery disease, could not be validated as a surrogate end point for all-cause or CV mortality. Thus, interventions that reduce nonfatal MI cannot be assumed to reduce mortality. Inclusion of nonfatal MI as an end point in RCTs may still be justified based on its association with impaired quality of life and increased use of health care resources, but not based on its surrogacy for mortality.10
eFigure 1. Funnel Plots for Nonfatal Myocardial Infarction and All-cause Mortality
eFigure 2. Treatment Effect of Myocardial Infarction (MI) and All-cause Mortality by Study Type
eFigure 3. Subgroup Analysis by Years of Follow-up for the Association Between the Logarithm of the Odds Ratios (OR) for Surrogate End Point of Myocardial Infarction (MI) and True Endpoint of All-cause Mortality
eFigure 4. Subgroup Analysis by Type of Control Arm for the Association Between the Logarithm of the Odds Ratios (OR) for Surrogate End Point of Myocardial Infarction (MI) and True End Point of All-cause Mortality
eAppendix. Search Strategy
eTable 1. Trial Characteristics
eTable 2. Assessment of Risk of Bias
eTable 3. Trial Abbreviations, Names, and Date of Publication
eReferences
References
- 1.Braunwald E. Evolution of the management of acute myocardial infarction: a 20th century saga. Lancet. 1998;352(9142):1771-1774. doi: 10.1016/S0140-6736(98)03212-7 [DOI] [PubMed] [Google Scholar]
- 2.Armstrong PW, Westerhout CM. Composite end points in clinical research: a time for reappraisal. Circulation. 2017;135(23):2299-2307. doi: 10.1161/CIRCULATIONAHA.117.026229 [DOI] [PubMed] [Google Scholar]
- 3.Braunwald E, Cannon CP, McCabe CH. Use of composite endpoints in thrombolysis trials of acute myocardial infarction. Am J Cardiol. 1993;72(19):3G-12G. doi: 10.1016/0002-9149(93)90101-H [DOI] [PubMed] [Google Scholar]
- 4.Wallentin L, Ohlsson J, Swahn E, Karlsson E; Fragmin During Instability in Coronary Artery Disease (FRISC) Study Group . Low-molecular-weight heparin during instability in coronary artery disease. Lancet. Lancet. 1996;347(9001):561-568. doi: 10.1016/S0140-6736(96)91270-2 [DOI] [PubMed] [Google Scholar]
- 5.Suryapranata H, van ’t Hof AW, Hoorntje JC, de Boer MJ, Zijlstra F. Randomized comparison of coronary stenting with balloon angioplasty in selected patients with acute myocardial infarction. Circulation. 1998;97(25):2502-2505. doi: 10.1161/01.CIR.97.25.2502 [DOI] [PubMed] [Google Scholar]
- 6.Wallentin L, Swahn E, Kontny F, Husted S, Lagerqvist B, Stahle E; Fragmin and Fast Revascularisation During Instability in Coronary Artery Disease Investigators . Invasive compared with non-invasive treatment in unstable coronary-artery disease: FRISC II prospective randomised multicentre study. FRagmin and Fast Revascularisation during InStability in Coronary artery disease Investigators. Lancet. 1999;354(9180):708-715. doi: 10.1016/S0140-6736(99)07349-3 [DOI] [PubMed] [Google Scholar]
- 7.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators . Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345(7):494-502. doi: 10.1056/NEJMoa010746 [DOI] [PubMed] [Google Scholar]
- 8.Andersen HR, Nielsen TT, Rasmussen K, et al. ; DANAMI-2 Investigators . A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction. N Engl J Med. 2003;349(8):733-742. doi: 10.1056/NEJMoa025142 [DOI] [PubMed] [Google Scholar]
- 9.Wallentin L, Becker RC, Budaj A, et al. ; PLATO Investigators . Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045-1057. doi: 10.1056/NEJMoa0904327 [DOI] [PubMed] [Google Scholar]
- 10.Weintraub WS, Lüscher TF, Pocock S. The perils of surrogate endpoints. Eur Heart J. 2015;36(33):2212-2218. doi: 10.1093/eurheartj/ehv164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puymirat E, Simon T, Cayla G, et al. ; USIK, USIC 2000, and FAST-MI Investigators . Acute myocardial infarction: changes in patient characteristics, management, and 6-month outcomes over a period of 20 years in the FAST-MI Program (French Registry of Acute ST-Elevation or Non-ST-Elevation Myocardial Infarction) 1995 to 2015. Circulation. 2017;136(20):1908-1919. doi: 10.1161/CIRCULATIONAHA.117.030798 [DOI] [PubMed] [Google Scholar]
- 12.Szummer K, Wallentin L, Lindhagen L, et al. Improved outcomes in patients with ST-elevation myocardial infarction during the last 20 years are related to implementation of evidence-based treatments: experiences from the SWEDEHEART registry 1995-2014. Eur Heart J. 2017;38(41):3056-3065. doi: 10.1093/eurheartj/ehx515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeFilippis AP, Nasir K, Blaha MJ. Myocardial infarction as a clinical end point in research. Circ Res. 2019;124(12):1701-1703. doi: 10.1161/CIRCRESAHA.119.315101 [DOI] [PubMed] [Google Scholar]
- 14.Nishikawa G, Prasad V. Diagnostic expansion in clinical trials: myocardial infarction, stroke, cancer recurrence, and metastases may not be the hard endpoints you thought they were. BMJ. 2018;362:k3783. doi: 10.1136/bmj.k3783 [DOI] [PubMed] [Google Scholar]
- 15.Ciani O, Buyse M, Drummond M, Rasi G, Saad ED, Taylor RS. Time to review the role of surrogate end points in health policy: state of the art and the way forward. Value Health. 2017;20(3):487-495. doi: 10.1016/j.jval.2016.10.011 [DOI] [PubMed] [Google Scholar]
- 16.Ciani O, Buyse M, Drummond M, Rasi G, Saad ED, Taylor RS. Use of surrogate end points in healthcare policy: a proposal for adoption of a validation framework. Nat Rev Drug Discov. 2016;15(7):516. doi: 10.1038/nrd.2016.81 [DOI] [PubMed] [Google Scholar]
- 17.Bala MM, Akl EA, Sun X, et al. Randomized trials published in higher vs. lower impact journals differ in design, conduct, and analysis. J Clin Epidemiol. 2013;66(3):286-295. doi: 10.1016/j.jclinepi.2012.10.005 [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Shamseer L, Clarke M, et al. ; PRISMA-P Group . Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lassere MN. The Biomarker-Surrogacy Evaluation Schema: a review of the biomarker-surrogate literature and a proposal for a criterion-based, quantitative, multidimensional hierarchical levels of evidence schema for evaluating the status of biomarkers as surrogate endpoints. Stat Methods Med Res. 2008;17(3):303-340. doi: 10.1177/0962280207082719 [DOI] [PubMed] [Google Scholar]
- 20.Hernandez-Villafuerte K, Fischer A, Latimer N. Challenges and methodologies in using progression free survival as a surrogate for overall survival in oncology. Int J Technol Assess Health Care. 2018;34(3):300-316. doi: 10.1017/S0266462318000338 [DOI] [PubMed] [Google Scholar]
- 21.Nomenclature and criteria for diagnosis of ischemic heart disease: report of the Joint International Society and Federation of Cardiology/World Health Organization task force on standardization of clinical nomenclature. Circulation. 1979;59(3):607-609. doi: 10.1161/01.CIR.59.3.607 [DOI] [PubMed] [Google Scholar]
- 22.Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined—a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the Redefinition of Myocardial Infarction. J Am Coll Cardiol. 2000;36(3):959-969. doi: 10.1016/S0735-1097(00)00804-4 [DOI] [PubMed] [Google Scholar]
- 23.Thygesen K, Alpert JS, White HD, et al. ; Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction . Universal definition of myocardial infarction. Circulation. 2007;116(22):2634-2653. doi: 10.1161/CIRCULATIONAHA.107.187397 [DOI] [PubMed] [Google Scholar]
- 24.Thygesen K, Alpert JS, Jaffe AS, et al. ; Joint ESC/ACCF/AHA/WHF Task Force for Universal Definition of Myocardial Infarction; Authors/Task Force Members Chairpersons; Biomarker Subcommittee; ECG Subcommittee; Imaging Subcommittee; Classification Subcommittee; Intervention Subcommittee . Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60(16):1581-1598. doi: 10.1016/j.jacc.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 25.Stone GW, Kappetein AP, Sabik JF, et al. ; EXCEL Trial Investigators . Five-year outcomes after PCI or CABG for left main coronary disease. N Engl J Med. 2019;381(19):1820-1830. doi: 10.1056/NEJMoa1909406 [DOI] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paoletti X, Lewsley LA, Daniele G, et al. ; Gynecologic Cancer InterGroup (GCIG) Meta-analysis Committee . Assessment of progression-free survival as a surrogate end point of overall survival in first-line treatment of ovarian cancer: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(1):e1918939. doi: 10.1001/jamanetworkopen.2019.18939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garg P, Morris P, Fazlanie AL, et al. Cardiac biomarkers of acute coronary syndrome: from history to high-sensitivity cardiac troponin. Intern Emerg Med. 2017;12(2):147-155. doi: 10.1007/s11739-017-1612-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boden WE, O’Rourke RA, Teo KK, et al. ; COURAGE Trial Research Group . Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356(15):1503-1516. doi: 10.1056/NEJMoa070829 [DOI] [PubMed] [Google Scholar]
- 30.Hochman JS, Lamas GA, Buller CE, et al. ; Occluded Artery Trial Investigators . Coronary intervention for persistent occlusion after myocardial infarction. N Engl J Med. 2006;355(23):2395-2407. doi: 10.1056/NEJMoa066139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frye RL, August P, Brooks MM, et al. ; BARI 2D Study Group . A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360(24):2503-2515. doi: 10.1056/NEJMoa0805796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farkouh ME, Domanski M, Sleeper LA, et al. ; FREEDOM Trial Investigators . Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367(25):2375-2384. doi: 10.1056/NEJMoa1211585 [DOI] [PubMed] [Google Scholar]
- 33.Maron DJ, Hochman JS, Reynolds HR, et al. ; ISCHEMIA Research Group . Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. 2020;382(15):1395-1407. doi: 10.1056/NEJMoa1915922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The Coronary Drug Project: findings leading to further modifications of its protocol with respect to dextrothyroxine. JAMA. 1972;220(7):996-1008. doi: 10.1001/jama.1972.03200070084015 [DOI] [PubMed] [Google Scholar]
- 35.Aspirin Myocardial Infarction Study Research Group . A randomized, controlled trial of aspirin in persons recovered from myocardial infarction. JAMA. 1980;243(7):661-669. doi: 10.1001/jama.1980.03300330019023 [DOI] [PubMed] [Google Scholar]
- 36.Hjermann I, Velve Byre K, Holme I, Leren P. Effect of diet and smoking intervention on the incidence of coronary heart disease: report from the Oslo Study Group of a randomised trial in healthy men. Lancet. 1981;2(8259):1303-1310. doi: 10.1016/S0140-6736(81)91338-6 [DOI] [PubMed] [Google Scholar]
- 37.E.P.S.I.M. Research Group . A controlled comparison of aspirin and oral anticoagulants in prevention of death after myocardial infarction. N Engl J Med. 1982;307(12):701-708. doi: 10.1056/NEJM198209163071201 [DOI] [PubMed] [Google Scholar]
- 38.Taylor SH, Silke B, Ebbutt A, Sutton GC, Prout BJ, Burley DM. A long-term prevention study with oxprenolol in coronary heart disease. N Engl J Med. 1982;307(21):1293-1301. doi: 10.1056/NEJM198211183072101 [DOI] [PubMed] [Google Scholar]
- 39.Kornitzer M, De Backer G, Dramaix M, et al. Belgian Heart Disease Prevention Project: incidence and mortality results. Lancet. 1983;1(8333):1066-1070. doi: 10.1016/S0140-6736(83)91908-6 [DOI] [PubMed] [Google Scholar]
- 40.Rose G, Tunstall-Pedoe HD, Heller RF. UK Heart Disease Prevention Project: incidence and mortality results. Lancet. 1983;1(8333):1062-1066. doi: 10.1016/S0140-6736(83)91907-4 [DOI] [PubMed] [Google Scholar]
- 41.A randomized trial of propranolol in patients with acute myocardial infarction, II: morbidity results. JAMA. 1983;250(20):2814-2819. doi: 10.1001/jama.1983.03340200048027 [DOI] [PubMed] [Google Scholar]
- 42.The Lipid Research Clinics Coronary Primary Prevention Trial results. I. Reduction in incidence of coronary heart disease. JAMA. 1984;251(3):351-364. doi: 10.1001/jama.1984.03340270029025 [DOI] [PubMed] [Google Scholar]
- 43.Multicenter Diltiazem Postinfarction Trial Research Group . The effect of diltiazem on mortality and reinfarction after myocardial infarction. N Engl J Med. 1988;319(7):385-392. doi: 10.1056/NEJM198808183190701 [DOI] [PubMed] [Google Scholar]
- 44.Steering Committee of the Physicians’ Health Study Research Group . Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med. 1989;321(3):129-135. doi: 10.1056/NEJM198907203210301 [DOI] [PubMed] [Google Scholar]
- 45.Smith P, Arnesen H, Holme I. The effect of warfarin on mortality and reinfarction after myocardial infarction. N Engl J Med. 1990;323(3):147-152. doi: 10.1056/NEJM199007193230302 [DOI] [PubMed] [Google Scholar]
- 46.SHEP Cooperative Research Group . Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA. 1991;265(24):3255-3264. doi: 10.1001/jama.1991.03460240051027 [DOI] [PubMed] [Google Scholar]
- 47.The SALT Collaborative Group . Swedish Aspirin Low-Dose Trial (SALT) of 75 mg aspirin as secondary prophylaxis after cerebrovascular ischaemic events. Lancet. 1991;338(8779):1345-1349. doi: 10.1016/0140-6736(91)92233-R [DOI] [PubMed] [Google Scholar]
- 48.Dahlöf B, Lindholm LH, Hansson L, Scherstén B, Ekbom T, Wester PO. Morbidity and mortality in the Swedish Trial in Old Patients With Hypertension (STOP-Hypertension). Lancet. 1991;338(8778):1281-1285. doi: 10.1016/0140-6736(91)92589-T [DOI] [PubMed] [Google Scholar]
- 49.Pfeffer MA, Braunwald E, Moyé LA, et al. ; The SAVE Investigators . Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: results of the Survival and Ventricular Enlargement trial. N Engl J Med. 1992;327(10):669-677. doi: 10.1056/NEJM199209033271001 [DOI] [PubMed] [Google Scholar]
- 50.ETDRS Investigators . Aspirin effects on mortality and morbidity in patients with diabetes mellitus: Early Treatment Diabetic Retinopathy Study report 14. JAMA. 1992;268(10):1292-1300. doi: 10.1001/jama.1992.03490100090033 [DOI] [PubMed] [Google Scholar]
- 51.Yusuf S, Pepine CJ, Garces C, et al. Effect of enalapril on myocardial infarction and unstable angina in patients with low ejection fractions. Lancet. 1992;340(8829):1173-1178. doi: 10.1016/0140-6736(92)92889-N [DOI] [PubMed] [Google Scholar]
- 52.Juul-Möller S, Edvardsson N, Jahnmatz B, Rosén A, Sørensen S, Omblus R; The Swedish Angina Pectoris Aspirin Trial (SAPAT) Group . Double-blind trial of aspirin in primary prevention of myocardial infarction in patients with stable chronic angina pectoris. Lancet. 1992;340(8833):1421-1425. doi: 10.1016/0140-6736(92)92619-Q [DOI] [PubMed] [Google Scholar]
- 53.Anticoagulants in the Secondary Prevention of Events in Coronary Thrombosis (ASPECT) Research Group . Effect of long-term oral anticoagulant treatment on mortality and cardiovascular morbidity after myocardial infarction. Lancet. 1994;343(8896):499-503. [PubMed] [Google Scholar]
- 54.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344(8934):1383-1389. [PubMed] [Google Scholar]
- 55.Køber L, Torp-Pedersen C, Carlsen JE, et al. ; Trandolapril Cardiac Evaluation (TRACE) Study Group . A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 1995;333(25):1670-1676. doi: 10.1056/NEJM199512213332503 [DOI] [PubMed] [Google Scholar]
- 56.Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335(14):1001-1009. doi: 10.1056/NEJM199610033351401 [DOI] [PubMed] [Google Scholar]
- 57.RITA-2 Trial Participants . Coronary angioplasty versus medical therapy for angina: the second Randomised Intervention Treatment of Angina (RITA-2) trial. RITA-2 trial participants. Lancet. 1997;350(9076):461-468. doi: 10.1016/S0140-6736(97)07298-X [DOI] [PubMed] [Google Scholar]
- 58.Downs JR, Clearfield M, Weis S, et al. ; Air Force/Texas Coronary Atherosclerosis Prevention Study . Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. JAMA. 1998;279(20):1615-1622. doi: 10.1001/jama.279.20.1615 [DOI] [PubMed] [Google Scholar]
- 59.UK Prospective Diabetes Study (UKPDS) Group . Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837-853. doi: 10.1016/S0140-6736(98)07019-6 [DOI] [PubMed] [Google Scholar]
- 60.Henderson RA, Pocock SJ, Sharp SJ, et al. ; Randomised Intervention Treatment of Angina . Long-term results of RITA-1 trial: clinical and cost comparisons of coronary angioplasty and coronary-artery bypass grafting. Lancet. 1998;352(9138):1419-1425. doi: 10.1016/S0140-6736(98)03358-3 [DOI] [PubMed] [Google Scholar]
- 61.Long-Term Intervention With Pravastatin in Ischaemic Disease (LIPID) Study Group . Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339(19):1349-1357. doi: 10.1056/NEJM199811053391902 [DOI] [PubMed] [Google Scholar]
- 62.Hansson L, Lindholm LH, Niskanen L, et al. Effect of angiotensin-converting-enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: the Captopril Prevention Project (CAPPP) randomised trial. Lancet. 1999;353(9153):611-616. doi: 10.1016/S0140-6736(98)05012-0 [DOI] [PubMed] [Google Scholar]
- 63.Rubins HB, Robins SJ, Collins D, et al. ; Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group . Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. N Engl J Med. 1999;341(6):410-418. doi: 10.1056/NEJM199908053410604 [DOI] [PubMed] [Google Scholar]
- 64.ALLHAT Collaborative Research Group . Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2000;283(15):1967-1975. doi: 10.1001/jama.283.15.1967 [DOI] [PubMed] [Google Scholar]
- 65.Hansson L, Hedner T, Lund-Johansen P, et al. Randomised trial of effects of calcium antagonists compared with diuretics and beta-blockers on cardiovascular morbidity and mortality in hypertension: the Nordic Diltiazem (NORDIL) study. Lancet. 2000;356(9227):359-365. doi: 10.1016/S0140-6736(00)02526-5 [DOI] [PubMed] [Google Scholar]
- 66.Tonkin AM, Colquhoun D, Emberson J, et al. Effects of pravastatin in 3260 patients with unstable angina: results from the LIPID study. Lancet. 2000;356(9245):1871-1875. doi: 10.1016/S0140-6736(00)03257-8 [DOI] [PubMed] [Google Scholar]
- 67.LIPID Study Group (Long-term Intervention With Pravastatin in Ischaemic Disease) . Long-term effectiveness and safety of pravastatin in 9014 patients with coronary heart disease and average cholesterol concentrations: the LIPID trial follow-up. Lancet. 2002;359(9315):1379-1387. doi: 10.1016/S0140-6736(02)08351-4 [DOI] [PubMed] [Google Scholar]
- 68.Serruys PW, de Feyter P, Macaya C, et al. ; Lescol Intervention Prevention Study (LIPS) Investigators . Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;287(24):3215-3222. doi: 10.1001/jama.287.24.3215 [DOI] [PubMed] [Google Scholar]
- 69.Grady D, Herrington D, Bittner V, et al. ; HERS Research Group . Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA. 2002;288(1):49-57. doi: 10.1001/jama.288.1.49 [DOI] [PubMed] [Google Scholar]
- 70.Heart Protection Study Collaborative Group . MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7-22. doi: 10.1016/S0140-6736(02)09327-3 [DOI] [PubMed] [Google Scholar]
- 71.Heart Protection Study Collaborative Group . MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):23-33. doi: 10.1016/S0140-6736(02)09328-5 [DOI] [PubMed] [Google Scholar]
- 72.Kjeldsen SE, Dahlöf B, Devereux RB, et al. ; LIFE (Losartan Intervention for Endpoint Reduction) Study Group . Effects of losartan on cardiovascular morbidity and mortality in patients with isolated systolic hypertension and left ventricular hypertrophy: a Losartan Intervention for Endpoint Reduction (LIFE) substudy. JAMA. 2002;288(12):1491-1498. doi: 10.1001/jama.288.12.1491 [DOI] [PubMed] [Google Scholar]
- 73.Singh RB, Dubnov G, Niaz MA, et al. Effect of an Indo-Mediterranean diet on progression of coronary artery disease in high risk patients (Indo-Mediterranean Diet Heart Study): a randomised single-blind trial. Lancet. 2002;360(9344):1455-1461. doi: 10.1016/S0140-6736(02)11472-3 [DOI] [PubMed] [Google Scholar]
- 74.Shepherd J, Blauw GJ, Murphy MB, et al. ; PROSPER Study Group . Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360(9346):1623-1630. doi: 10.1016/S0140-6736(02)11600-X [DOI] [PubMed] [Google Scholar]
- 75.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial . Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA. 2002;288(23):2998-3007. doi: 10.1001/jama.288.23.2998 [DOI] [PubMed] [Google Scholar]
- 76.Cherry N, Gilmour K, Hannaford P, et al. ; ESPRIT Team . Oestrogen therapy for prevention of reinfarction in postmenopausal women: a randomised placebo controlled trial. Lancet. 2002;360(9350):2001-2008. doi: 10.1016/S0140-6736(02)12001-0 [DOI] [PubMed] [Google Scholar]
- 77.Holdaas H, Fellström B, Jardine AG, et al. ; Assessment of LEscol in Renal Transplantation (ALERT) Study Investigators . Effect of fluvastatin on cardiac outcomes in renal transplant recipients: a multicentre, randomised, placebo-controlled trial. Lancet. 2003;361(9374):2024-2031. doi: 10.1016/S0140-6736(03)13638-0 [DOI] [PubMed] [Google Scholar]
- 78.Fox KM; EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators . Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study). Lancet. 2003;362(9386):782-788. doi: 10.1016/S0140-6736(03)14286-9 [DOI] [PubMed] [Google Scholar]
- 79.Cannon CP, Braunwald E, McCabe CH, et al. ; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators . Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350(15):1495-1504. doi: 10.1056/NEJMoa040583 [DOI] [PubMed] [Google Scholar]
- 80.Colhoun HM, Betteridge DJ, Durrington PN, et al. ; CARDS Investigators . Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364(9435):685-696. doi: 10.1016/S0140-6736(04)16895-5 [DOI] [PubMed] [Google Scholar]
- 81.Braunwald E, Domanski MJ, Fowler SE, et al. ; PEACE Trial Investigators . Angiotensin-converting-enzyme inhibition in stable coronary artery disease. N Engl J Med. 2004;351(20):2058-2068. doi: 10.1056/NEJMoa042739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352(13):1293-1304. doi: 10.1056/NEJMoa050613 [DOI] [PubMed] [Google Scholar]
- 83.LaRosa JC, Grundy SM, Waters DD, et al. ; Treating to New Targets (TNT) Investigators . Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352(14):1425-1435. doi: 10.1056/NEJMoa050461 [DOI] [PubMed] [Google Scholar]
- 84.Cannon CP, Braunwald E, McCabe CH, et al. ; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators . Antibiotic treatment of Chlamydia pneumoniae after acute coronary syndrome. N Engl J Med. 2005;352(16):1646-1654. doi: 10.1056/NEJMoa043528 [DOI] [PubMed] [Google Scholar]
- 85.Lee IM, Cook NR, Gaziano JM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294(1):56-65. doi: 10.1001/jama.294.1.56 [DOI] [PubMed] [Google Scholar]
- 86.Wanner C, Krane V, März W, et al. ; German Diabetes and Dialysis Study Investigators . Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353(3):238-248. doi: 10.1056/NEJMoa043545 [DOI] [PubMed] [Google Scholar]
- 87.Fox KA, Poole-Wilson P, Clayton TC, et al. 5-Year outcome of an interventional strategy in non-ST-elevation acute coronary syndrome: the British Heart Foundation RITA 3 randomised trial. Lancet. 2005;366(9489):914-920. doi: 10.1016/S0140-6736(05)67222-4 [DOI] [PubMed] [Google Scholar]
- 88.Dormandy JA, Charbonnel B, Eckland DJ, et al. ; PROactive Investigators . Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366(9493):1279-1289. doi: 10.1016/S0140-6736(05)67528-9 [DOI] [PubMed] [Google Scholar]
- 89.Pedersen TR, Faergeman O, Kastelein JJ, et al. ; Incremental Decrease in End Points Through Aggressive Lipid Lowering (IDEAL) Study Group . High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005;294(19):2437-2445. doi: 10.1001/jama.294.19.2437 [DOI] [PubMed] [Google Scholar]
- 90.Keech A, Simes RJ, Barter P, et al. ; FIELD Study Investigators . Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366(9500):1849-1861. doi: 10.1016/S0140-6736(05)67667-2 [DOI] [PubMed] [Google Scholar]
- 91.Bhatt DL, Fox KA, Hacke W, et al. ; CHARISMA Investigators . Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354(16):1706-1717. doi: 10.1056/NEJMoa060989 [DOI] [PubMed] [Google Scholar]
- 92.Halkes PH, van Gijn J, Kappelle LJ, Koudstaal PJ, Algra A; ESPRIT Study Group . Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet. 2006;367(9523):1665-1673. doi: 10.1016/S0140-6736(06)68734-5 [DOI] [PubMed] [Google Scholar]
- 93.Barrett-Connor E, Mosca L, Collins P, et al. ; Raloxifene Use for The Heart (RUTH) Trial Investigators . Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355(2):125-137. doi: 10.1056/NEJMoa062462 [DOI] [PubMed] [Google Scholar]
- 94.Zacharski LR, Chow BK, Howes PS, et al. Reduction of iron stores and cardiovascular outcomes in patients with peripheral arterial disease: a randomized controlled trial. JAMA. 2007;297(6):603-610. doi: 10.1001/jama.297.6.603 [DOI] [PubMed] [Google Scholar]
- 95.Anand S, Yusuf S, Xie C, et al. ; Warfarin Antiplatelet Vascular Evaluation Trial Investigators . Oral anticoagulant and antiplatelet therapy and peripheral arterial disease. N Engl J Med. 2007;357(3):217-227. doi: 10.1056/NEJMoa065959 [DOI] [PubMed] [Google Scholar]
- 96.Patel A, MacMahon S, Chalmers J, et al. ; ADVANCE Collaborative Group . Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370(9590):829-840. doi: 10.1016/S0140-6736(07)61303-8 [DOI] [PubMed] [Google Scholar]
- 97.Albert CM, Cook NR, Gaziano JM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008;299(17):2027-2036. doi: 10.1001/jama.299.17.2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tardif JC, McMurray JJ, Klug E, et al. ; Aggressive Reduction of Inflammation Stops Events (ARISE) Trial Investigators . Effects of succinobucol (AGI-1067) after an acute coronary syndrome: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371(9626):1761-1768. doi: 10.1016/S0140-6736(08)60763-1 [DOI] [PubMed] [Google Scholar]
- 99.Patel A, MacMahon S, Chalmers J, et al. ; ADVANCE Collaborative Group . Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560-2572. doi: 10.1056/NEJMoa0802987 [DOI] [PubMed] [Google Scholar]
- 100.Sacco RL, Diener HC, Yusuf S, et al. ; PRoFESS Study Group . Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359(12):1238-1251. doi: 10.1056/NEJMoa0805002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yusuf S, Teo K, Anderson C, et al. ; Telmisartan Randomised AssessmeNt Study in ACE INtolerant Subjects With CardiovascularDisease (TRANSCEND) Investigators . Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet. 2008;372(9644):1174-1183. doi: 10.1016/S0140-6736(08)61242-8 [DOI] [PubMed] [Google Scholar]
- 102.Young LH, Wackers FJ, Chyun DA, et al. ; DIAD Investigators . Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA. 2009;301(15):1547-1555. doi: 10.1001/jama.2009.476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Home PD, Pocock SJ, Beck-Nielsen H, et al. ; RECORD Study Team . Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373(9681):2125-2135. doi: 10.1016/S0140-6736(09)60953-3 [DOI] [PubMed] [Google Scholar]
- 104.Holman RR, Haffner SM, McMurray JJ, et al. ; NAVIGATOR Study Group . Effect of nateglinide on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010;362(16):1463-1476. doi: 10.1056/NEJMoa1001122 [DOI] [PubMed] [Google Scholar]
- 105.McMurray JJ, Holman RR, Haffner SM, et al. ; NAVIGATOR Study Group . Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010;362(16):1477-1490. doi: 10.1056/NEJMoa1001121 [DOI] [PubMed] [Google Scholar]
- 106.Cushman WC, Evans GW, Byington RP, et al. ; ACCORD Study Group . Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575-1585. doi: 10.1056/NEJMoa1001286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ginsberg HN, Elam MB, Lovato LC, et al. ; ACCORD Study Group . Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1563-1574. doi: 10.1056/NEJMoa1001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Armitage JM, Bowman L, Clarke RJ, et al. ; Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group . Effects of homocysteine-lowering with folic acid plus vitamin B12 vs placebo on mortality and major morbidity in myocardial infarction survivors: a randomized trial. JAMA. 2010;303(24):2486-2494. doi: 10.1001/jama.2010.840 [DOI] [PubMed] [Google Scholar]
- 109.Armitage J, Bowman L, Wallendszus K, et al. ; Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group . Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: a double-blind randomised trial. Lancet. 2010;376(9753):1658-1669. doi: 10.1016/S0140-6736(10)60310-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gerstein HC, Miller ME, Genuth S, et al. ; ACCORD Study Group . Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364(9):818-828. doi: 10.1056/NEJMoa1006524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Haller H, Ito S, Izzo JL Jr, et al. ; ROADMAP Trial Investigators . Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med. 2011;364(10):907-917. doi: 10.1056/NEJMoa1007994 [DOI] [PubMed] [Google Scholar]
- 112.Bousser MG, Amarenco P, Chamorro A, et al. ; PERFORM Study Investigators . Terutroban versus aspirin in patients with cerebral ischaemic events (PERFORM): a randomised, double-blind, parallel-group trial. Lancet. 2011;377(9782):2013-2022. doi: 10.1016/S0140-6736(11)60600-4 [DOI] [PubMed] [Google Scholar]
- 113.Boden WE, Probstfield JL, Anderson T, et al. ; AIM-HIGH Investigators . Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365(24):2255-2267. doi: 10.1056/NEJMoa1107579 [DOI] [PubMed] [Google Scholar]
- 114.Sesso HD, Christen WG, Bubes V, et al. Multivitamins in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2012;308(17):1751-1760. doi: 10.1001/jama.2012.14805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schwartz GG, Olsson AG, Abt M, et al. ; dal-OUTCOMES Investigators . Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367(22):2089-2099. doi: 10.1056/NEJMoa1206797 [DOI] [PubMed] [Google Scholar]
- 116.Wing RR, Bolin P, Brancati FL, et al. ; Look AHEAD Research Group . Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369(2):145-154. doi: 10.1056/NEJMoa1212914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Scirica BM, Bhatt DL, Braunwald E, et al. ; SAVOR-TIMI 53 Steering Committee and Investigators . Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317-1326. doi: 10.1056/NEJMoa1307684 [DOI] [PubMed] [Google Scholar]
- 118.Lincoff AM, Tardif JC, Schwartz GG, et al. ; AleCardio Investigators . Effect of aleglitazar on cardiovascular outcomes after acute coronary syndrome in patients with type 2 diabetes mellitus: the AleCardio randomized clinical trial. JAMA. 2014;311(15):1515-1525. doi: 10.1001/jama.2014.3321 [DOI] [PubMed] [Google Scholar]
- 119.White HD, Held C, Stewart R, et al. ; STABILITY Investigators . Darapladib for preventing ischemic events in stable coronary heart disease. N Engl J Med. 2014;370(18):1702-1711. doi: 10.1056/NEJMoa1315878 [DOI] [PubMed] [Google Scholar]
- 120.Maeng M, Tilsted HH, Jensen LO, et al. Differential clinical outcomes after 1 year versus 5 years in a randomised comparison of zotarolimus-eluting and sirolimus-eluting coronary stents (the SORT OUT III study): a multicentre, open-label, randomised superiority trial. Lancet. 2014;383(9934):2047-2056. doi: 10.1016/S0140-6736(14)60405-0 [DOI] [PubMed] [Google Scholar]
- 121.Landray MJ, Haynes R, Hopewell JC, et al. ; HPS2-THRIVE Collaborative Group . Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371(3):203-212. doi: 10.1056/NEJMoa1300955 [DOI] [PubMed] [Google Scholar]
- 122.Fox K, Ford I, Steg PG, Tardif JC, Tendera M, Ferrari R; SIGNIFY Investigators . Ivabradine in stable coronary artery disease without clinical heart failure. N Engl J Med. 2014;371(12):1091-1099. doi: 10.1056/NEJMoa1406430 [DOI] [PubMed] [Google Scholar]
- 123.Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA. 2014;312(23):2510-2520. doi: 10.1001/jama.2014.15690 [DOI] [PubMed] [Google Scholar]
- 124.Douglas PS, Hoffmann U, Patel MR, et al. ; PROMISE Investigators . Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372(14):1291-1300. doi: 10.1056/NEJMoa1415516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Huo Y, Li J, Qin X, et al. ; CSPPT Investigators . Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA. 2015;313(13):1325-1335. doi: 10.1001/jama.2015.2274 [DOI] [PubMed] [Google Scholar]
- 126.Hayward RA, Reaven PD, Wiitala WL, et al. ; VADT Investigators . Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372(23):2197-2206. doi: 10.1056/NEJMoa1414266 [DOI] [PubMed] [Google Scholar]
- 127.Cannon CP, Blazing MA, Giugliano RP, et al. ; IMPROVE-IT Investigators . Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387-2397. doi: 10.1056/NEJMoa1410489 [DOI] [PubMed] [Google Scholar]
- 128.Green JB, Bethel MA, Armstrong PW, et al. ; TECOS Study Group . Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232-242. doi: 10.1056/NEJMoa1501352 [DOI] [PubMed] [Google Scholar]
- 129.Zinman B, Wanner C, Lachin JM, et al. ; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi: 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 130.Wright JT Jr, Williamson JD, Whelton PK, et al. ; SPRINT Research Group . A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103-2116. doi: 10.1056/NEJMoa1511939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pfeffer MA, Claggett B, Diaz R, et al. ; ELIXA Investigators . Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247-2257. doi: 10.1056/NEJMoa1509225 [DOI] [PubMed] [Google Scholar]
- 132.Sabaté M, Brugaletta S, Cequier A, et al. Clinical outcomes in patients with ST-segment elevation myocardial infarction treated with everolimus-eluting stents versus bare-metal stents (EXAMINATION): 5-year results of a randomised trial. Lancet. 2016;387(10016):357-366. doi: 10.1016/S0140-6736(15)00548-6 [DOI] [PubMed] [Google Scholar]
- 133.Nissen SE, Wolski KE, Prcela L, et al. Effect of naltrexone-bupropion on major adverse cardiovascular events in overweight and obese patients with cardiovascular risk factors: a randomized clinical trial. JAMA. 2016;315(10):990-1004. doi: 10.1001/jama.2016.1558 [DOI] [PubMed] [Google Scholar]
- 134.Kernan WN, Viscoli CM, Furie KL, et al. ; IRIS Trial Investigators . Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374(14):1321-1331. doi: 10.1056/NEJMoa1506930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yusuf S, Bosch J, Dagenais G, et al. ; HOPE-3 Investigators . Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med. 2016;374(21):2021-2031. doi: 10.1056/NEJMoa1600176 [DOI] [PubMed] [Google Scholar]
- 136.Kelbæk H, Høfsten DE, Køber L, et al. Deferred versus conventional stent implantation in patients with ST-segment elevation myocardial infarction (DANAMI 3-DEFER): an open-label, randomised controlled trial. Lancet. 2016;387(10034):2199-2206. doi: 10.1016/S0140-6736(16)30072-1 [DOI] [PubMed] [Google Scholar]
- 137.Williamson JD, Supiano MA, Applegate WB, et al. ; SPRINT Research Group . Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315(24):2673-2682. doi: 10.1001/jama.2016.7050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Marso SP, Daniels GH, Brown-Frandsen K, et al. ; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322. doi: 10.1056/NEJMoa1603827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.McEvoy RD, Antic NA, Heeley E, et al. ; SAVE Investigators and Coordinators . CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919-931. doi: 10.1056/NEJMoa1606599 [DOI] [PubMed] [Google Scholar]
- 140.Bønaa KH, Mannsverk J, Wiseth R, et al. ; NORSTENT Investigators . Drug-eluting or bare-metal stents for coronary artery disease. N Engl J Med. 2016;375(13):1242-1252. doi: 10.1056/NEJMoa1607991 [DOI] [PubMed] [Google Scholar]
- 141.Marso SP, Bain SC, Consoli A, et al. ; SUSTAIN-6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834-1844. doi: 10.1056/NEJMoa1607141 [DOI] [PubMed] [Google Scholar]
- 142.Lamy A, Devereaux PJ, Prabhakaran D, et al. ; CORONARY Investigators . Five-year outcomes after off-pump or on-pump coronary-artery bypass grafting. N Engl J Med. 2016;375(24):2359-2368. doi: 10.1056/NEJMoa1601564 [DOI] [PubMed] [Google Scholar]
- 143.Sabatine MS, Giugliano RP, Keech AC, et al. ; FOURIER Steering Committee and Investigators . Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713-1722. doi: 10.1056/NEJMoa1615664 [DOI] [PubMed] [Google Scholar]
- 144.Shroyer AL, Hattler B, Wagner TH, et al. ; Veterans Affairs ROOBY-FS Group . Five-year outcomes after on-pump and off-pump coronary-artery bypass. N Engl J Med. 2017;377(7):623-632. doi: 10.1056/NEJMoa1614341 [DOI] [PubMed] [Google Scholar]
- 145.Holman RR, Bethel MA, Mentz RJ, et al. ; EXSCEL Study Group . Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228-1239. doi: 10.1056/NEJMoa1612917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.White WB, Saag KG, Becker MA, et al. ; CARES Investigators . Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med. 2018;378(13):1200-1210. doi: 10.1056/NEJMoa1710895 [DOI] [PubMed] [Google Scholar]
- 147.Newby DE, Adamson PD, Berry C, et al. ; SCOT-HEART Investigators . Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med. 2018;379(10):924-933. doi: 10.1056/NEJMoa1805971 [DOI] [PubMed] [Google Scholar]
- 148.Vranckx P, Valgimigli M, Jüni P, et al. ; GLOBAL LEADERS Investigators . Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-label, randomised superiority trial. Lancet. 2018;392(10151):940-949. doi: 10.1016/S0140-6736(18)31858-0 [DOI] [PubMed] [Google Scholar]
- 149.Gaziano JM, Brotons C, Coppolecchia R, et al. ; ARRIVE Executive Committee . Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392(10152):1036-1046. doi: 10.1016/S0140-6736(18)31924-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bowman L, Mafham M, Wallendszus K, et al. ; ASCEND Study Collaborative Group . Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379(16):1529-1539. doi: 10.1056/NEJMoa1804988 [DOI] [PubMed] [Google Scholar]
- 151.Bowman L, Mafham M, Wallendszus K, et al. ; ASCEND Study Collaborative Group . Effects of n-3 fatty acid supplements in diabetes mellitus. N Engl J Med. 2018;379(16):1540-1550. doi: 10.1056/NEJMoa1804989 [DOI] [PubMed] [Google Scholar]
- 152.McNeil JJ, Wolfe R, Woods RL, et al. ; ASPREE Investigator Group . Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379(16):1509-1518. doi: 10.1056/NEJMoa1805819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schwartz GG, Steg PG, Szarek M, et al. ; ODYSSEY OUTCOMES Committees and Investigators . Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097-2107. doi: 10.1056/NEJMoa1801174 [DOI] [PubMed] [Google Scholar]
- 154.Rosenstock J, Perkovic V, Johansen OE, et al. ; CARMELINA Investigators . Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321(1):69-79. doi: 10.1001/jama.2018.18269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bhatt DL, Steg PG, Miller M, et al. ; REDUCE-IT Investigators . Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380(1):11-22. doi: 10.1056/NEJMoa1812792 [DOI] [PubMed] [Google Scholar]
- 156.Manson JE, Cook NR, Lee IM, et al. ; VITAL Research Group . Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2019;380(1):23-32. doi: 10.1056/NEJMoa1811403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Manson JE, Cook NR, Lee IM, et al. ; VITAL Research Group . Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380(1):33-44. doi: 10.1056/NEJMoa1809944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zenati MA, Bhatt DL, Bakaeen FG, et al. ; REGROUP Trial Investigators . Randomized trial of endoscopic or open vein-graft harvesting for coronary-artery bypass. N Engl J Med. 2019;380(2):132-141. doi: 10.1056/NEJMoa1812390 [DOI] [PubMed] [Google Scholar]
- 159.Wiviott SD, Raz I, Bonaca MP, et al. ; DECLARE–TIMI 58 Investigators . Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347-357. doi: 10.1056/NEJMoa1812389 [DOI] [PubMed] [Google Scholar]
- 160.Macdougall IC, White C, Anker SD, et al. ; PIVOTAL Investigators and Committees . Intravenous iron in patients undergoing maintenance hemodialysis. N Engl J Med. 2019;380(5):447-458. doi: 10.1056/NEJMoa1810742 [DOI] [PubMed] [Google Scholar]
- 161.Ridker PM, Everett BM, Pradhan A, et al. ; CIRT Investigators . Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med. 2019;380(8):752-762. doi: 10.1056/NEJMoa1809798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gerstein HC, Colhoun HM, Dagenais GR, et al. ; REWIND Investigators . Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121-130. doi: 10.1016/S0140-6736(19)31149-3 [DOI] [PubMed] [Google Scholar]
- 163.Roshandel G, Khoshnia M, Poustchi H, et al. Effectiveness of polypill for primary and secondary prevention of cardiovascular diseases (PolyIran): a pragmatic, cluster-randomised trial. Lancet. 2019;394(10199):672-683. doi: 10.1016/S0140-6736(19)31791-X [DOI] [PubMed] [Google Scholar]
- 164.Yasuda S, Kaikita K, Akao M, et al. ; AFIRE Investigators . Antithrombotic therapy for atrial fibrillation with stable coronary disease. N Engl J Med. 2019;381(12):1103-1113. doi: 10.1056/NEJMoa1904143 [DOI] [PubMed] [Google Scholar]
- 165.Steg PG, Bhatt DL, Simon T, et al. ; THEMIS Steering Committee and Investigators . Ticagrelor in patients with stable coronary disease and diabetes. N Engl J Med. 2019;381(14):1309-1320. doi: 10.1056/NEJMoa1908077 [DOI] [PubMed] [Google Scholar]
- 166.Mehta SR, Wood DA, Storey RF, et al. ; COMPLETE Trial Steering Committee and Investigators . Complete revascularization with multivessel PCI for myocardial infarction. N Engl J Med. 2019;381(15):1411-1421. doi: 10.1056/NEJMoa1907775 [DOI] [PubMed] [Google Scholar]
- 167.Amarenco P, Kim JS, Labreuche J, et al. ; Treat Stroke to Target Investigators . A comparison of two LDL cholesterol targets after ischemic stroke. N Engl J Med. 2020;382(1):9. doi: 10.1056/NEJMoa1910355 [DOI] [PubMed] [Google Scholar]
- 168.Ray KK, Nicholls SJ, Buhr KA, et al. ; BETonMACE Investigators and Committees . Effect of apabetalone added to standard therapy on major adverse cardiovascular events in patients with recent acute coronary syndrome and type 2 diabetes: a randomized clinical trial. JAMA. 2020;323(16):1565-1573. doi: 10.1001/jama.2020.3308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bonaca MP, Bauersachs RM, Anand SS, et al. Rivaroxaban in peripheral artery disease after revascularization. N Engl J Med. 2020;382(21):1994-2004. doi: 10.1056/NEJMoa2000052 [DOI] [PubMed] [Google Scholar]
- 170.Ferrari R, Ford I, Fox K, et al. ; ATPCI Investigators . Efficacy and safety of trimetazidine after percutaneous coronary intervention (ATPCI): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396(10254):830-838. doi: 10.1016/S0140-6736(20)31790-6 [DOI] [PubMed] [Google Scholar]
- 171.Nidorf SM, Fiolet ATL, Mosterd A, et al. ; LoDoCo2 Trial Investigators . Colchicine in patients with chronic coronary disease. N Engl J Med. 2020;383(19):1838-1847. doi: 10.1056/NEJMoa2021372 [DOI] [PubMed] [Google Scholar]
- 172.Fanaroff AC, Roe MT, Clare RM, et al. Competing risks of cardiovascular versus noncardiovascular death during long-term follow-up after acute coronary syndromes. J Am Heart Assoc. 2017;6(9):e005840. doi: 10.1161/JAHA.117.005840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Friedberg CK, Horn H. Acute myocardial infarction not due to coronary artery occlusion. JAMA. 1939;112(17):1675-1679. doi: 10.1001/jama.1939.02800170021007 [DOI] [Google Scholar]
- 174.Thygesen K, Alpert JS, Jaffe AS, et al. ; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction . Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018;72(18):2231-2264. doi: 10.1016/j.jacc.2018.08.1038 [DOI] [PubMed] [Google Scholar]
- 175.DeFilippis AP, Chapman AR, Mills NL, et al. Assessment and treatment of patients with type 2 myocardial infarction and acute nonischemic myocardial injury. Circulation. 2019;140(20):1661-1678. doi: 10.1161/CIRCULATIONAHA.119.040631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Borges FK, Sheth T, Patel A, et al. Accuracy of physicians in differentiating type 1 and type 2 myocardial infarction based on clinical information. CJC Open. 2020;2(6):577-584. doi: 10.1016/j.cjco.2020.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mills NL, Lee KK, McAllister DA, et al. Implications of lowering threshold of plasma troponin concentration in diagnosis of myocardial infarction: cohort study. BMJ. 2012;344:e1533. doi: 10.1136/bmj.e1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Xie W, Halabi S, Tierney JF, et al. A systematic review and recommendation for reporting of surrogate endpoint evaluation using meta-analyses. J Natl Cancer Inst Cancer Spectr. 2019;3(1):pkz002. doi: 10.1093/jncics/pkz002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Prasad V, Kim C, Burotto M, Vandross A. The strength of association between surrogate end points and survival in oncology: a systematic review of trial-level meta-analyses. JAMA Intern Med. 2015;175(8):1389-1398. doi: 10.1001/jamainternmed.2015.2829 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Funnel Plots for Nonfatal Myocardial Infarction and All-cause Mortality
eFigure 2. Treatment Effect of Myocardial Infarction (MI) and All-cause Mortality by Study Type
eFigure 3. Subgroup Analysis by Years of Follow-up for the Association Between the Logarithm of the Odds Ratios (OR) for Surrogate End Point of Myocardial Infarction (MI) and True Endpoint of All-cause Mortality
eFigure 4. Subgroup Analysis by Type of Control Arm for the Association Between the Logarithm of the Odds Ratios (OR) for Surrogate End Point of Myocardial Infarction (MI) and True End Point of All-cause Mortality
eAppendix. Search Strategy
eTable 1. Trial Characteristics
eTable 2. Assessment of Risk of Bias
eTable 3. Trial Abbreviations, Names, and Date of Publication
eReferences