ABSTRACT
How do hierarchical gene regulation networks evolve in bacteria? Nucleoid-associated proteins (NAPs) influence the overall structure of bacterial genomes, sigma factors and global transcription factors (TFs) control thousands of genes, and many operons are regulated by highly specific TFs that in turn are controlled allosterically by cellular metabolites. These regulatory hierarchies have been shaped by millions of years of evolution to optimize fitness in response to changing environmental conditions, but it is unclear how NAPs and TFs relate and have evolved together. Cyclic AMP (cAMP) receptor protein (Crp) is the paradigmatic global TF in Escherichia coli, and here we report that mutations in the topA gene compensate for loss of cAMP, showing that the interplay between Crp and the supercoiling status of promoters is key to global stress response. Furthermore, we observed an effect of apoCrp on gene expression in the absence of its effector cAMP. This provides support for the proposed NAP-like role for Crp, suggesting that it represents an intermediate point in the evolution of a ligand-controlled TF from a NAP.
KEYWORDS: cyclic AMP receptor protein, experimental evolution, global regulatory networks, supercoiling, topoisomerases, transcription factors
INTRODUCTION
Regulation of gene expression is central to the adaptability of bacterial cells in response to changes in the environment. In the model bacterium Escherichia coli, the cyclic AMP (cAMP) receptor protein (Crp) plays an overarching role (1). Modulating the expression of hundreds of genes (2) in the E. coli genome, mostly involved in carbon metabolism (1), Crp is a global transcription factor (TF) that can act both as an activator and as a repressor upon binding its ligand cAMP. Crp is one of the most thoroughly studied TFs and provided one of the first examples that gene regulation is more complex than suggested by the Jacob-Monod operon model (3). The E. coli genome contains several thousand putative binding sites for Crp (4), of which only a fraction are known to affect promoter activity. Combined with observations of unspecific DNA binding affinity and bending of DNA by ∼90° upon binding (5) and cooperative DNA binding and condensation by apoCrp (6, 7), an additional role of Crp as a nucleoid-associated protein (NAP) involved in the global organization of the bacterial chromosome has been hypothesized (3, 8, 9).
The genome structure of E. coli is under the control of a homeostatic network comprising DNA topoisomerases, abundant NAPs, and components of the transcriptional machinery, coordinating both genes mediating stress adaptation and genes of the central metabolism. The interplay between global and local TFs is particularly important when bacteria experience stress that calls for a substantial reset of the transcriptional machinery. For example, temperature upshift from 30°C to 42°C leads to rapid induction of more than 20 heat shock proteins (HSPs), including the molecular chaperones DnaK, DnaJ/GrpE, and GroEL/GroES, as well as multiple proteases (10). HSPs are essential for survival of the cell, since they protect other proteins from thermal inactivation and reactivate aggregated proteins. Transcription of HSP genes is positively controlled by the sigma factor σ32, encoded by the rpoH gene (11).
DNA supercoiling is an important sensor for changes in the physical environment. σ32 expression is sensitive to changes in the supercoiling state of the bacterial chromosome (12) and is actively controlled by DnaKJ and GroEL/S, while σ32 levels are reduced via degradation mediated by the FtsH protease (13). The topA gene, encoding E. coli DNA topoisomerase I (TopoI), is also controlled by a σ32-dependent heat shock promoter (14). TopoI relaxes negatively supercoiled DNA, while its antagonist DNA gyrase introduces negative supercoiling into relaxed DNA (15). During heat shock, relaxation of DNA is facilitated by a tight interplay of both enzymes, which is essential to counterbalance the DNA unwinding that occurs at high temperatures in E. coli (16, 17).
The DNA topology in promoter regions directly influences gene expression. Biosynthetic genes are preferentially transcribed under conditions of high negative supercoiling, whereas transcription of catabolic genes involved in energy production are activated under conditions of DNA relaxation (18). The effective level of supercoiling is thereby determined by competition between NAPs, such as HU, H-NS, and Fis, as well as RNA polymerases, balancing the compaction and availability of DNA (19). Both Fis and Crp regulate the expression of numerous genes, needed for carbon and nucleic acid catabolism, and impact DNA topology in a growth-phase-dependent manner (1, 20). While the cAMP-Crp complex activates expression of the gyrA gene and increases the negative supercoiling level of reporter plasmids, Fis inhibits DNA gyrase and tightly regulates topA expression (20, 21). Expression of both crp and fis responds to changes in DNA topology, with fis expression being maximal at high levels of negative supercoiling and crp transcription being achieved by oscillation of multiple regulatory nucleoprotein complexes in response to the physiological state of the cell, tightly linking the global regulatory processes of Crp regulation and DNA supercoiling (22–24). However, while the specific influence of Crp on transcriptional control of genes is well documented, the extent to which unspecific or low-affinity Crp-DNA interactions directly influence global gene regulation in a NAP-like, chromatin-shaping fashion is less clear.
Several previous studies characterized the evolution of E. coli strains with deletions in the cya gene, which encodes the enzyme adenylate cyclase, responsible for the formation of cAMP from ATP (25–29). cya mutants typically experience starvation stress, as they are unable to ferment a range of Crp-dependent sugars, such as maltose and lactose. The most frequently observed compensatory mutations occur in crp and render Crp cAMP independent. Such Crp* mutations are easily identified by improved growth on, e.g., maltose. Here, in order to elucidate global effects of Crp inactivity, we sought to identify different types of cya compensatory mutations by isolating mutants that grow better at different temperatures but remain unable to efficiently ferment maltose. We sequenced the genomes of 48 mutant E. coli strains selected this way and identified new hot spot mutations that provide insights into the role and evolution of gene regulation by Crp.
RESULTS
Identification of two new mutational hot spots in an adenylate cyclase knockout strain by changing the selection regimen.
Prolonged incubation of E. coli K-12 MG1655 cya colonies on maltose MacConkey agar plates at 37°C previously led to the identification of mutations in several genomic hot spots, such as crp, cmk, rpoS, and malT (30). These mutants were selected by picking secondary colonies that spontaneously outgrew their parental colonies and turned red due to maltose fermentation and a pH indicator in the agar plates (Fig. 1) (30). Mutations within the crp gene dominated under these conditions, leading to partial cAMP independence of the TF. However, we also observed a different colony phenotype: the formation of white secondary colonies (Fig. 1) that were more pronounced and rapidly occurring when the incubation temperature was elevated to 44°C. To explore the evolutionary response to perturbations in the cAMP-Crp global stress response in greater depth, we isolated over a period of 20 days the white secondary colonies that formed on maltose MacConkey agar at 37°C and 44°C. The isolates were restreaked several times on MacConkey agar supplemented with maltose or mannitol, and strains that were unable to efficiently ferment both sugars were selected for whole-genome sequencing (WGS).
FIG 1.
Mutational hot spots and phenotypes obtained in an E. coli adenylate cyclase mutant during evolution at different temperatures. (a) Illustration of the experimental set-up for the evolution experiment. Representative pictures of red and white papillae showing the two different phenotypes when secondary colonies develop on agar plates. (b) Schematic of mutational hot spots detected within the genome of K-12 MG1655 cya after prolonged incubation on maltose MacConkey plates in the study by Sekowska et al. (30) (left) and in this study (right). Numbers of identified mutations are given in parentheses. n, total number of evolved secondary colonies sequenced in the two studies.
In total, 48 strains were selected—15 isolated at 37°C and 33 isolated at 44°C—and a total of 58 mutations identified, presenting an average of only 1.2 mutations per genome (Table S1). Mutations in rpoS were the most frequently observed under this selection regimen, constituting 30 of the 58 mutations identified and occurring in 58% of the sequenced strains. Inactivation of RpoS happens frequently under laboratory conditions (31–33), and the majority of mutations identified in rpoS here were also frameshift mutations. Two new mutation hot spots, defined as a mutation that occurred more than once and had not been previously reported, were identified in the topA gene (5 mutations) and in an intergenic region between ygdH and sdaC (4 mutations) (Fig. 1b). The latter mutations aligned within the promoter region of the sdaC gene, encoding a serine-H+ symporter that is predominantly used to import l-serine utilized for an energy-providing role (34, 35). While these cis-acting mutations presumably enable improved utilization of an alternative carbon/energy source, the mutations identified here in the rpoS and topA gene are likely to have a more global effect on gene regulation and were therefore further investigated.
Mutations identified in 48 isolated clones by whole-genome sequencing. Download Table S1, XLSX file, 0.03 MB (28.6KB, xlsx) .
Copyright © 2021 Heyde et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
In 24 of the isolates, we identified only a single mutation; 19 of these were rpoS mutations and two were in topA, suggesting that single mutations in these loci are causative for the phenotype. Thus, this different approach toward selecting cya compensatory mutants results in the identification of fewer genomic mutations and new mutational hot spots.
trans-acting mutations that compensate for the inactivity of the global regulator Crp dominate.
In contrast to the rarely occurring cis-acting mutations, trans-acting mutations in global regulators such as Crp and the stress sigma factor RpoS are highly dominating. The so-called Crp* mutations, active in the absence of cAMP, were previously identified in 94 of 96 sequenced genomes, selected as red papillae outgrowing the initially formed colonies on maltose MacConkey agar (30). Here, by selecting only white papillae (Fig. 1), which were unable to efficiently ferment maltose and mannitol, we completely removed Crp* mutants from the observed evolutionary solution space.
A surprising new mutational hot spot found under our new selection regimen is topA. To understand the mutations obtained in the topA locus, we first mapped the alterations onto the crystal structure of the 865-amino-acid E. coli DNA topoisomerase I (TopoI) enzyme (Fig. 2a). Mutations isolated from papillae evolved at 37°C caused frameshifts close to the C-terminal end of the protein hosting the DNA binding domain. Mutations isolated at 44°C, on the other hand, were single amino acid substitutions in domains I (V73G) and IV (R168H and I187N) of the enzyme. The latter mutations were located close to a recently described R168 mutation that was shown to cause a >80-fold loss of relaxation activity and that was found to be crucial to hold the DNA substrate in proper conformation for cleavage and religation (36, 37). These findings point toward reduced TopoI function in the evolved cya topA* strains compared to the ancestral cya topA+ strain.
FIG 2.
Characterization of trans-regulatory mutations in the topA gene. (a) Structural representation of topoisomerase I (gray, cartoon) bound to DNA (yellow) (Nucleic Acid Database [NDB] no. NA3316) (59). Mutated residues are indicated together with the experimental condition they were isolated under: blue, frameshift mutations isolated in papillae evolved at 37°C; red, single nucleotide variations leading to the indicated amino acid changes evolved at 44°C. (b) Growth phenotypes of the five evolved cya topA* mutant strains (1 to 5), the parental cya strain, and wild-type K-12 MG1655 on maltose MacConkey agar at 37°C and 44°C. (c) Growth competition assay on SM agar plates comparing cya and wild-type (wt) strains and cya and cya topA* 4 strains. All strains carry the same plasmid encoding either gfp (green) or rfp (red) under the control of a constitutive promoter to discriminate between the different strains. cAMP was supplemented in the agar at the concentrations indicated. Cultures were grown individually in liquid growth medium, adjusted for cell density, and mixed (as indicated) prior to plating and incubation for 24 h at 44°C (the bottom row shows representative pictures of fluorescence on plates). (d) Relative DNA supercoiling levels in five isolated cya topA* strains (1 to 5) compared to the parental cya strain and K-12 MG1655 wild type in stationary phase at 44°C using a previously described in vivo supercoiling reporter plasmid (38). (e) Relative DNA supercoiling levels of K-12 MG1655 wild type, cya, cya crp, cya topA*, and cya topA* crp and the effect of cAMP addition in stationary phase at 44°C. (f) Relative DNA supercoiling levels of the cya crp strain when supplemented with a plasmid expressing either the wild-type Crp protein or Crp mutants carrying the amino acid substitution E72V or G162N, as indicated, an empty vector control (pSEVA-empty), and the wild type and the ancestral cya strain carrying the pSEVA-empty vector control. All measurements were performed in stationary phase at 44°C. DNA relaxation increases in the direction of the arrow on the right. RSU, relative supercoiling units. All measurements were performed in biological triplicate; error bars indicate standard errors of the means (SEM).
To explore the growth and relative fitness of the topA* mutations, we first compared the growth of topA mutants to the wild type and the parental cya strain on maltose MacConkey agar (Fig. 2b) at both 37°C and 44°C. In four of the five cases, mutations in topA resulted in increased growth relative to the topA+ cya ancestral strain. The exception was the cya topA* mutant strain 1 (Fig. 1b), which harbors an additional mutation in the gyrA gene (Table S1). To explore whether the mutations in the topA locus enable growth on maltose present in the plates, cya topA* mutant strains were restreaked on MacConkey medium without maltose and incubated at both 37°C and 44°C. At both temperatures, though most pronounced at 44°C, the evolved cya topA* mutants grew better than the parent cya strain, confirming the independence of the observed growth benefit of the sugar provided during the evolution experiment (Fig. S1). Next, competition between the wild type and the cya strain and between the cya and cya topA* strains were assayed: two strains, distinguished by expressing either gfp or rfp, were mixed at the same optical density and plated on minimal medium supplemented with maltose and different concentrations of cAMP. Following overnight incubation at 44°, cells were harvested, and relative growth estimated by quantifying green fluorescent protein (GFP) and red fluorescent protein (RFP) fluorescence in a microtiter plate reader. While the wild type, as expected, outcompeted the cya mutant, the cya topA* mutant strain 4 (R168H) strain likewise outcompeted the ancestral cya strain. This fitness benefit of cya topA* over the parental cya strain could, however, be reversed by supplementation of cAMP (Fig. 2c). These observations point toward a compensatory effect of topA mutations in a cya background in our experimental evolution setup.
Characterization of topA* mutants. Growth phenotypes of the five evolved cya topA* mutant strains (1 to 5), the parental cya strain, and wild-type K-12 MG1655 on MacConkey agar plates without (top) and with (bottom) 1% maltose supplementation at 37°C and 44°C. Download FIG S1, TIF file, 2.7 MB (2.7MB, tif) .
Copyright © 2021 Heyde et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Effect of topA and crp mutations on the supercoiling status of the cell.
Knowing that topA is expressed under heat shock conditions (16) and observing that topA mutations compensate for the lack of cAMP, we sought to investigate the consequences of topA mutations found during the evolution experiment on the catalytic activity of the enzyme using an in vivo supercoiling genetic reporter (38). The reporter plasmid carries both a constitutive promoter driving tdtomato expression and the supercoiling-sensitive rdsA (regulator of DNA supercoiling) promoter controlling gfp. In all cases, the evolved cya topA* strains, transformed with the reporter plasmid, showed reduced TopoI activity, resulting in an increase in negative supercoiling, compared to the parental cya topA+ strain (Fig. 2d). For comparison, wild-type E. coli was included in the analysis and showed supercoiling levels similar to the cya topA* mutants. This suggests that one way in which topA* mutations compensate for the lack of cAMP is by decreasing stress-induced positive supercoiling in the cell. In line with this, addition of cAMP restored supercoiling to the wild-type level (Fig. 2e).
We additionally examined supercoiling in a crp cya topA+ background, to investigate whether the presence of Crp has an impact on the structure of DNA in the absence of cAMP. The crp deletions were created by recombineering in the cya and the evolved cya topA* strains (mutant strain 4 [R168H]). Surprisingly, plasmid relaxation was lower in the cya crp strain than in the cya crp+ topA+ background and was comparable to the supercoiling level observed in the wild type (Fig. 2e). Reintroduction of the crp wild-type gene into the cya crp background on a low-copy-number plasmid (pSEVA-crp) to complement the crp gene deletion reversed the observed decrease in positive supercoiling to the cya crp+ level, as did two Crp mutants carrying the amino acid substitutions E72V and G162N (Fig. 2f). While E72V is located within the cAMP binding domain of Crp and renders it unable to associate with its effector molecule needed for specific DNA binding in promoters, G162N is located within a surface loop of Crp interacting with the RNA polymerase and therefore interferes specifically with promoter activation but not binding (28, 29). This suggests that apoCrp plays a role in determining the structure of DNA in the cell.
Carbon starvation during heat stress triggers global transcriptional changes.
Crp and topoisomerase activities appear to be closely linked, and Crp has repeatedly been suggested to participate in the spatiotemporal organization of the bacterial genome (3, 8, 9). To explore this connection on a systems level, we analyzed the global changes in gene expression caused by the cya-compensatory topA* mutations by transcriptomics. Triplicate cultures of the evolved cya topA* strain (mutant strain 4), the parental cya strain, and the wild-type and cya crp strains were harvested in late stationary phase and sent for RNA extraction and sequencing by a commercial vendor. Principal-component analysis (PCA) of the total transcriptomics data confirmed reproducibility between the triplicate samples and illustrates general trajectories in the evolution of the different strains. For each strain, the data cluster differently depending on the temperature (Fig. 3a). Data from the evolved cya topA* strain locate it far from the unevolved cya strain at 44°C but close to cya samples isolated at 37°C, indicating systemic compensation of gene expression through the acquired topA mutation.
FIG 3.
Global gene expression analysis of topA* and crp mutants. (a) Principal-component analysis (PCA) of transcriptome sequencing (RNA-seq) data from wild-type K-12 MG1655 and the cya, cya crp, and cya topA* mutants isolated from stationary-phase cultures at 37°C or 44°C. (b) Volcano plot of all 1,806 differentially expressed genes in the cya topA* and cya topA+ data sets at 44°C. Arrows indicate changes in the differential expression pattern of the following 3 gene groups: 1, genes downregulated in the cya versus wild-type strain but upregulated in the cya topA* versus wild-type strain or vice versa; 2, genes that show a lower FC in expression in the cya topA* strain versus the wild type than in the cya strain versus the wild type; 3, genes differentially expressed only in the cya topA* versus the cya topA+ mutant but not differentially expressed in the cya topA* mutant versus the wild type. FC, fold change; n, absolute number of genes found within the group. (c) DEGs in the RpoE, RpoH, and Crp regulons (as defined by regulonDB [60]; accessed 27 October 2020), as well as all genes investigated in this study as internal control comparing the cya topA* to the cya topA+ mutant at both 37°C and 44°C. Only the fraction of DEGs in each regulon is displayed. Upregulation (pink) is shown on the left; downregulation (blue) is on the right. (d) Transcript-per-million (TPM) values for rpoE and rpoH in the RNA-seq data set at 37°C for the wild-type, cya, and cya topA* strains. (e) In vivo rpoE GFP reporter data for different strains (x axis) with or without addition of cAMP. (f) In vivo rpoH GFP reporter data for strains (x axis) with or without addition of cAMP. Data in panels e and f are from stationary-phase cultures at 44°C.
Different models can be proposed to explain the basis of the compensatory effect of the topA mutation. The global DNA supercoiling changes caused by topA* could either (i) up- or downregulate the expression of specific genes needed to reinstate/support growth in the cya background or (ii) enhance overall gene expression across the genome to compensate for the unavailability of active cAMP-Crp complexes. To explore these hypotheses, we carried out differential expression analysis. Transcript levels were determined using the CLC Genomics Workbench suite, and changes associated with a P value of ≤0.01 and changes of >1.5- and <−1.5-fold were considered to indicate significantly different expression. At 44°C, comparing the cya topA* strain with the cya topA+ strain, we identified a total of 1,809 differentially expressed genes (DEGs) (Fig. 3b)—almost half of the genes in E. coli. We next explored how expression of these DEGs compared to wild-type levels and grouped them into those that were (i) downregulated in the cya strain versus the wild type but upregulated in the cya topA* strain versus the wild type or vice versa, (ii) less differentially expressed in the cya topA* strain versus the wild type than in the cya strain versus the wild type, or (iii) differentially expressed only in the cya topA* strain versus the cya topA+ strain (Fig. 3b). This analysis showed that 896 of the DEGs represented reversions in the cya topA* strain toward more wild-type-like expression levels, suggesting that topA* mutations cause a major rewiring of global transcription in stationary phase to compensate for the lack of cAMP.
We further investigated DEGs in the cya topA* strain versus the cya strain at the two temperatures by grouping them according to their regulation by Crp or the heat stress sigma factors E and H. More Crp-regulated genes were differentially expressed in the cya topA* strain at 44°C than at 37°C (Fig. 3c), again indicating that topA* compensates for the missing cAMP at the elevated temperature. One Crp-regulated gene whose expression was consistently different between wild-type and cya strains but similar between wild-type and cya topA* strains was rmf, encoding a ribosome modulator factor (Table S2). We used this gene to validate the transcriptomics data set, by constructing an in vivo genetic reporter, fusing the rmf promoter region with gfp on a plasmid, and monitoring fluorescence under different growth conditions. Both in liquid culture and on agar plates, gfp levels from this reporter plasmid were high in the wild-type strain and in cya topA* strains but low in the cya mutant (Fig. S2). This represents an independent demonstration of a Crp-regulated gene that is reversed to wild-type expression level by a topA* mutation.
Characterization of rmf expression in different strain backgrounds. (a) Transcript-per-million (TPM) values for rmf RNA detected in the RNA-seq analysis conducted in this study. RNA was extracted from stationary-phase cultures of the wild-type, cya, and cya topA* strains grown at 37°C. (b) Relative fluorescence intensity of the cya topA* mutant strain and control strains carrying an rmf reporter plasmid (illustrated) in which gfp expression is under the control of the rmf promoter. Download FIG S2, TIF file, 2.3 MB (2.3MB, tif) .
Copyright © 2021 Heyde et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Changes in gene expression in different mutants and conditions identified by transcriptomics. Raw data were sorted in sheets as indicated in the text and the legends for Fig. 3 and 4. Download Table S2, XLSX file, 0.3 MB (340.3KB, xlsx) .
Copyright © 2021 Heyde et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
rpoH and rpoE are more highly expressed in the cya mutant, but expression is reversed to wild-type levels by topA* and crp mutations.
In contrast to Crp-regulated genes, such as rmf, the transcriptomics data showed that more RpoH- and RpoE-regulated genes were downregulated in the cya topA* strain at 37°C than at 44°C (Fig. 3c). Both RpoE and RpoH are known to be essential during heat stress and are controlled via cAMP-Crp (39, 40). To study in more detail the effect of the topA* mutation on rpoE and rpoH expression, we compared their total transcript levels (transcripts per million [TPM]) in cya topA+, cya topA*, and wild-type strains and found that they were increased in the cya background but closer to the wild-type level in the cya topA* strain (Fig. 3d). To validate this finding with more dynamic in vivo genetic reporter systems, we constructed two plasmids hosting the rpoE or rpoH promoter driving expression of gfp. Fluorescence levels were highly elevated for both promoters under heat stress conditions (44°C) in cya compared to all other strains, and this effect disappeared when cAMP was supplied in the growth medium (Fig. 3e and f). This confirms high-level expression of both rpoH and rpoE as major perturbations in the cAMP synthesis-deficient mutant, which is compensated for by mutations in topA and suggests that the supercoiling status of the cell significantly affects expression of the two sigma factors.
Inspired by the observation that apoCrp affects the supercoiling status of the cell, we additionally monitored the behavior of the rpoH and rpoE reporter plasmids in the cya crp mutant strain. In this strain, at 44°C, the fluorescence from the rpoE reporter was only slightly elevated compared to that in the wild-type strain (Fig. 3e), and for rpoH, levels were not significantly different from those in the wild type (Fig. 3f). As expected, fluorescence levels in the cya crp strains were not affected by addition of cAMP. This provides independent confirmation of a role for apoCrp in global gene regulation: deletion of the crp gene seems to reinstate wild-type-level expression of the rpoE and rpoH promoters similarly to the way higher levels of negative supercoiling facilitate this via the mutant TopoI* variants. Complementation of the crp deletion in the cya crp mutant with either pSEVA-crp or the two inactive Crp mutants increased expression of the rpoH and rpoE reporters, again indicating a regulatory role for apoCrp.
Global analysis of expression of genes affected by apoCrp.
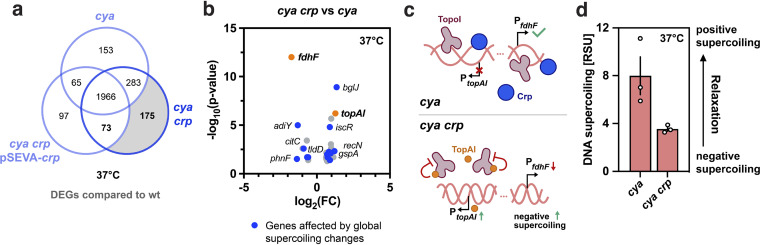
The reappearing pattern of different behavior of the cya and cya crp strains prompted us to look further into differences in gene expression in these two strains. We focused on the data obtained from stationary-phase cultures grown at 37°C, as expression changes potentially caused by apoCrp and related chromosomal rearrangements could be overshadowed by the global heat stress response at 44°C, and we wanted to depict genome-wide effects under standard conditions to understand the role of Crp as a potential nucleoid-associated protein. One hundred seventy-five genes were found to be significantly differentially expressed in the cya crp mutant compared to the wild type, but not when either the cya mutant and the wild type or the cya crp strain complemented with apoCrp (via pSEVA-crp) and the wild type were compared (Fig. 4a). We selected these genes in a comparison of the cya crp and cya data sets and plotted their distribution in a volcano plot to visualize the DEGs (Fig. 4b). Interestingly, among the 30 genes found to be significantly differentially expressed between the cya crp and crp strains, 14 genes (Fig. 4b, blue) are either directly or indirectly (Table S3) affected by the supercoiling state of the bacterial genome, pointing again toward global supercoiling-related changes in the cya crp strain. Within the DEGs identified from the data for cya crp strain versus the wild type, two supercoiling-related genes stood out: (i) topAI, encoding an inhibitor of TopoI (41), was among the highest upregulated genes, and (ii) fdhF, encoding E. coli formate dehydrogenase H previously reported to be upregulated upon gyrase inhibition (42) (i.e., downregulated by negative supercoiling), was among the most significantly downregulated genes. This suggests increased negative supercoiling upon removal of apoCrp, since upregulation of the TopoI inhibitor TopAI leads to decreased TopoI activity, which would result in more negative supercoiling and reduced expression from supercoiling sensitive genes such as fdhF (Fig. 4c). This is in line with the supercoiling reporter plasmid data, as plasmid DNA was 2-fold less negatively supercoiled at 37°C in the cya strain than the cya crp strain (Fig. 4d).
FIG 4.
Effects of apoCrp on global gene expression explored in cya and cya crp mutant strains. (a) Venn diagram of DEGs in the cya and cya crp mutants and the cya crp strain complemented with wild-type Crp via the low-copy-number plasmid vector pSEVA-crp relative to K-12 MG1655 (wild type) under standard growth conditions (stationary phase, 37°C). Genes differentially expressed only in the cya crp mutant versus the wild type but not in the cya mutant versus the wild type or the cya crp pSEVA-crp strain versus the wild type are highlighted in light gray. (b) Volcano plot of the DEGs identified in panel a (light gray) to be uniquely differentially expressed in the cya crp strain compared to the wild type. Here, the same genes are plotted comparing their expression levels in the cya crp strain versus the cya strain (n = 30). DEGs that are affected by the global supercoiling state of the cell are in blue (n = 14). The supercoiling-related genes fdhF and topAI are in orange. (c) Illustration of the connection between topAI and fdhF expression in connection with the supercoiling state of the bacterial nucleoid and the availability of apoCrp. (d) Relative DNA supercoiling levels of the cya and cya crp strains in stationary phase under standard growth conditions at 37°C.
Changes in gene expression in different mutants identified by transcriptomics. Raw data were sorted as indicated in the text and the legend for Fig. 4b. Download Table S3, XLSX file, 0.01 MB (10.6KB, xlsx) .
Copyright © 2021 Heyde et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
DISCUSSION
It is striking how mutations in global gene regulation dominate the picture in experimental evolution. RpoS and Crp* mutations have been described numerous times, but to our knowledge, the current study is the first of its kind reporting that topA mutations compensate for loss of the global stress signal cAMP. On the other hand, supercoiling has previously been suggested to have broader significance in stress-induced transcription (43, 44), supported by experimental evolution experiments that demonstrated the importance of DNA topology as a global factor in bacterial gene regulation and its sensitivity to adaptive mutations. In the long-term-evolution experiment reported by Crozat et al., parallel changes in topology were detected in 10 out of 12 initially identical populations propagated in a defined environment for 20,000 generations (45). The level of DNA supercoiling was increased due to mutations in the genes of the global regulators topA and fis. Competition assays showed that both mutations caused direct and substantial fitness benefits. Further investigations of the ancestor and evolved clones from all 12 populations showed high levels of molecular and genetic parallelism, harboring mutations in multiple genes involved in DNA supercoiling (46). Several other experiments conducted in E. coli under different selection regimens found mutations in the topA gene, further emphasizing the importance of selection of DNA topology traits (47–50).
Recently, point mutations in the topA gene were described as giving rise to mutator phenotypes influencing the mutational spectrum of E. coli to allow fast emergence of drug resistance genotypes (36). In that study, a single substitution in the conserved residue R168C in TopoI resulted in an accelerated rate of tandem duplications, known to be a source of genetic instability and to play a significant role in bacterial evolution. Here, we identified a different mutation (R168H) in the same position in TopoI, with a major effect on global gene regulation. These findings point toward a new class of fitness-enhancing mutations and confirm that the control of DNA supercoiling is a hot spot for selection in evolving bacterial populations.
Considering the frequency of observed mutations related to DNA topology in experimental evolution and the proposed role of Crp as a nucleoid-associated protein (3, 8, 9), our finding that topA mutations compensate for the lack of cAMP is not surprising. We found that almost half of the genes in E. coli change expression when the cya mutant was compared with the cya topA* mutants, which have restored growth and expression of many genes back to wild-type levels. A simple interpretation of this observation is that activator-independent activity of many of these activator-dependent promoters is increased by extra negative supercoiling. Among these genes are global factors such as the sigma factor-encoding rpoH and rpoE, and rmf encoding a ribosome modulator factor. Our suggestion that the supercoiling status of the cell influences expression of these factors is supported by earlier findings that showed supercoiling to affect the promoter activity of rpoH (12) and that expression of rmf is growth phase dependent (51), a process directly influenced by supercoiling itself (52). To our knowledge, no direct connection between rpoE expression and the supercoiling state of the cell has been reported so far, while our data strongly suggest such a connection.
It is interesting that we also isolated a strain with a single mutation in the genome in the gene encoding the FtsH protease, which is involved in controlling the stability of RpoH (13). Thus, it is possible that this ftsH mutant counteracts the perturbed expression of rpoH in the cya strain. The simultaneous occurrence of a topoisomerase and a gyrase mutation in another of the evolved strains strongly suggests a complete loss of TopoI function in this mutant. Complete loss-of-function mutations in topA are lethal for E. coli but can be compensated for by a gyrA deletion, creating a viable but growth-impaired strain (53, 54).
Crp was previously suggested to represent an example of a TF that has evolved from a NAP (3), supported by circular dichroism, sedimentation analysis, and electron microscope studies showing cooperative DNA binding and condensation by apoCrp (6, 7). In this model, Crp evolved from being a NAP with the role of shaping the genome by unspecific binding to binding more frequently in proximity to promoters and assisting in recruiting the RNA polymerase. However, this model did not discuss the role of the cAMP ligand and how this allosteric control mechanism entered the picture in evolution. The different effects of apoCrp observed here expand this model, suggesting that during evolution, apoCrp played a NAP-like role that preceded both the role of cAMP as an allosteric regulator and the binding to high-affinity sites in the DNA. This model is in good agreement with the paradoxical observation that Crp homologs exist in bacteria like Pseudomonas aeruginosa that contain little or no cAMP (55). Possibly, the sensing of cAMP and the role of Crp in carbon catabolite repression is a special case in the model bacterium E. coli and homologs more commonly play NAP-like roles in the bacterial kingdom.
MATERIALS AND METHODS
Bacterial strains.
All experiments were performed using E. coli strain K-12 MG1655 fnr cya (cya::cat Δfnr) described in Sekowska et al. (30). The strain harbors a deletion of the cya gene introduced via λRed-induced recombination, as well as a deletion of the fnr gene, a homologue of crp.
Secondary-colony-formation assay.
For growth on plates, MacConkey medium was used (Difco MacConkey agar base) supplemented with 1% maltose as carbon source and 5 mg/liter chloramphenicol. To obtain isolated colonies on the plates, early stationary-phase bacterial cultures grown in liquid lysogeny broth (LB; Thermo Fisher Inc.) medium (growth for 7 h at 37°C) were diluted in sterile water containing 9 g/liter sodium chloride to a concentration of 2.5 × 104 bacteria/ml, and 100 μl of the bacterial suspension was spread onto MacConkey plates containing the appropriate antibiotics. The plates were subsequently placed in closed plastic containers containing beakers with water to ensure constant humidity and incubated for the duration of the experiment at either 37°C or 44°C. Papillae were purified by restreaking 3 times on fresh plates supplemented with the same carbon source and antibiotic as in the secondary-colony-formation assay.
Plate reader experiments.
For growth assays of evolved and nonevolved K-12 MG1655 cya strains, in vivo supercoiling assays with the supercoiling reporter plasmid pSupR (38) and gfp promoter reporter assays cells were inoculated from single colonies and cultured for 7 h in 5 ml liquid growth medium (LB) supplemented with 2% glucose to minimize the risk of mutations arising during the cultivation time. Dilutions of 1:50 were grown subsequently for 20 h in 96-well plates and 200 rpm at 37°C or 44°C, covered with a gas-permeable adhesive seal (Thermo Fisher Scientific, Inc.) to avoid evaporation. Optical density at 600 nm (OD600) and fluorescence (GFP, excitation at 485 nm and emission at 528 nm; RFP, excitation at 488 nm and emission at 588 nm) was measured at 10-min intervals with continuous shaking using a Synergy H1 plate reader (BioTek Instruments, Inc.). All measurements were performed in biological triplicate.
Sequencing.
DNA purification for whole-genome sequencing was performed using DNeasy blood and tissue kit 50 (Qiagen, Inc.) starting with 1 ml overnight culture according to the manufacturer’s instructions. Isolated DNA was eluted twice using 200 μl 10 mM Tris (pH 8.5). The genomic libraries were generated using the TruSeq DNA HT library prep kit (Illumina Inc.). Forty-eight independent papillae were selected for whole-genome sequencing using Illumina sequencing adapters D701 (ATTACTCG) and D501 (TATAGCCT). Data analysis was performed using the breseq software developed by Deatherage and Barrick (56). Of 48 strains sequenced, 24 carried an additional mutation in an unstable IS2 element previously shown to cause a high degree of genetic variation in different E. coli strains (57), and these mutations were excluded from the total number of mutations. For transcriptomics analysis, cells were grown in LB liquid medium until stationary phase at 37°C or 44°C. After 12 h of growth, cultures were normalized to OD600, and 1 × 107 cells were harvested via centrifugation. Pellets were immediately frozen in liquid nitrogen.
RNA extraction, library preparation, and RNA sequencing were performed by Novogene, Inc., Cambridge, UK. All data analysis was performed using CLC Genomics Workbench 20. FastQ files were imported into CLC Genomics Workbench for data processing as paired reads. Reads were trimmed using a quality score limit of 0.05, ambiguous nucleotides (none allowed), and adapters (5′ adapter, AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACGCTCTTCCGATCT; 3′ adapter, GATCGGAAGAGCACACGTCTGAACTCCAGTCACATCACGATCTCGTATGCCGTCTTCTGCTTG). All sequence-based trimming followed standard alignment settings with a mismatch cost of 2, a gap cost of 3, a minimum internal score of 10, and a minimum end score of 4. Trimmed reads were then mapped to a reference sequence (GenBank no. U00096.3) using global alignment with standard alignment settings of a match score of 1, a mismatch cost of 2, a linear gap cost of 3, and length fraction and similarity fraction at 0.5 and 0.8, respectively. Following this, the reads were examined for structural variants (quality P < 0.0001 and maximum number of mismatches = 3 for end breakpoints), including deletions and insertions, and, based on this information, were realigned using local realignment (two iterations; maximum guidance variant length, 200 bp). Quality distribution followed an average PHRED score of 37, and coverage was on average 99.9% with an average nucleotide distribution of 25% and GC content of ∼50%. Read counts for each gene were then converted to TPM values by the software and exported to Excel. For subsequent comparison between conditions, samples were normalized based on a statistical model, described in the software manual to consist of TMM (trimmed mean of the M values) normalization, calculation of TMM-adjusted log counts of counts per minute, and finally, applied separately for each gene, Z-score normalization (mean = 0, standard deviation = 1). Fold change (FC) values were then calculated (applying a t test to compare groups) and reported as log2(FC) for ease of comparison along with P values and false-discovery-rate (FDR)-adjusted P values (q values) for the statistical significance.
Genome engineering.
To generate the crp knockout strains MG1655 cya crp and MG1655 topA* crp (topA* 4: R168H), cells were transformed with plasmid pSIM19 (58), encoding heat-inducible λRed recombination proteins, and recombineering was performed using a tetA integration cassette flanked by 50-base homology areas for the crp locus. Colonies were screened for the desired crp knockout (crp::tetA) by Sanger sequencing.
Fitness competition assays.
To monitor fitness competition within two strains in a mixed culture, each strain was transformed with an expression vector harboring either a gfp or rfp gene under the control of a constitutive promoter. Precultures for each strain were grown individually in liquid medium for 7 h, before the number of CFU was calculated for each culture based on OD600. Equal CFU for each strain to be compared were subsequently mixed and plated onto simple MacConkey (SM) agar supplemented with maltose and up to 0.5 mM cAMP. SM medium composition for 300 ml was soybean peptone (5.1 g; 70178-100G; Sigma-Aldrich), protease peptone (0.9 g; P0431-250G; Sigma-Aldrich), NaCl (1.5 g; Sigma-Aldrich), and agar (4.05 g; Sigma-Aldrich). Colony formation was allowed overnight at 44°C. Colonies were subsequently harvested from the plates and resuspended in sterile 0.9% sodium solution, and the fluorescence signals for both GFP and RFP channels were measured. To control for plasmid-inflicted differences in growth, all experiments were repeated with reverse plasmid combinations.
Data availability.
All sequencing data connected to this study have been deposited in the European Nucleotide Archive (ENA). Whole-genome sequencing data can be accessed via accession number PRJEB43656. RNA sequencing data can be accessed via accession number PRJEB43645.
ACKNOWLEDGMENTS
We thank Eduardo A. Groisman for sharing the pSupR plasmid.
This work was supported by a grant from the Novo Nordisk Foundation, NNF17OC0027752, to M.H.H.N.
Footnotes
Citation Heyde SAH, Frendorf PO, Lauritsen I, Nørholm MHH. 2021. Restoring global gene regulation through experimental evolution uncovers a NAP (nucleoid-associated protein)-like behavior of Crp/Cap. mBio 12:e02028-21. https://doi.org/10.1128/mBio.02028-21.
Contributor Information
Morten H. H. Nørholm, Email: morno@biosustain.dtu.dk.
Sang Yup Lee, Korea Advanced Institute of Science and Technology.
REFERENCES
- 1.Gomez-Gomez JM, Baquero F, Blazquez J. 1996. Cyclic AMP receptor protein positively controls GyrA transcription and alters DNA topology after nutritional upshift in Escherichia coli. J Bacteriol 178:3331–3334. doi: 10.1128/jb.178.11.3331-3334.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martínez-Antonio A, Collado-Vides J. 2003. Identifying global regulators in transcriptional regulatory networks in bacteria. Curr Opin Microbiol 6:482–489. doi: 10.1016/j.mib.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Visweswariah SS, Busby SJW. 2015. Evolution of bacterial transcription factors: how proteins take on new tasks, but do not always stop doing the old ones. Trends Microbiol 23:463–467. doi: 10.1016/j.tim.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Shimada T, Fujita N, Yamamoto K, Ishihama A. 2011. Novel roles of cAMP receptor protein (CRP) in regulation of transport and metabolism of carbon sources. PLoS One 6:e20081. doi: 10.1371/journal.pone.0020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schultz S, Shields G, Steitz T. 1991. Crystal structure of a CAP-DNA complex: the DNA is bent by 90 degrees. Science 253:1001–1007. doi: 10.1126/science.1653449. [DOI] [PubMed] [Google Scholar]
- 6.Chang JJ, Dubochet J, Baudras A, Blazy B, Takahashi M. 1981. Electron microscope observation of a fibre structure formed by non-specific binding of cAMP receptor protein to DNA. J Mol Biol 150:435–439. doi: 10.1016/0022-2836(81)90558-1. [DOI] [PubMed] [Google Scholar]
- 7.Saxe SA, Revzin A. 1979. Cooperative binding to DNA of catabolite activator protein of Escherichia coli. Biochemistry 18:255–263. doi: 10.1021/bi00569a003. [DOI] [PubMed] [Google Scholar]
- 8.Hollands K, Busby SJW, Lloyd GS. 2007. New targets for the cyclic AMP receptor protein in the Escherichia coli K-12 genome. FEMS Microbiol Lett 274:89–94. doi: 10.1111/j.1574-6968.2007.00826.x. [DOI] [PubMed] [Google Scholar]
- 9.Dillon SC, Dorman CJ. 2010. Bacterial nucleoid-associated proteins, nucleoid structure and gene expression. Nat Rev Microbiol 8:185–195. doi: 10.1038/nrmicro2261. [DOI] [PubMed] [Google Scholar]
- 10.Arsène F, Tomoyasu T, Bukau B. 2000. The heat shock response of Escherichia coli. Int J Food Microbiol 55:3–9. doi: 10.1016/S0168-1605(00)00206-3. [DOI] [PubMed] [Google Scholar]
- 11.Nonaka G, Blankschien M, Herman C, Gross CA, Rhodius V. 2006. Regulon and promoter analysis of the of the E. coli heat-shock factor, sigma32, reveals a multifaceted cellular response to heat stress. Genes Dev 20:1776–1789. doi: 10.1101/gad.1428206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ueshima R, Fujita N, Ishihama A. 1989. DNA supercoiling and temperature shift affect the promoter activity of the Escherichia coli RpoH gene encoding the heat-shock sigma subunit of RNA polymerase. Mol Gen Genet 215:185–189. doi: 10.1007/BF00339716. [DOI] [PubMed] [Google Scholar]
- 13.Tatsuta T, Tomoyasu T, Bukau B, Kitagawa M, Mori H, Karata K, Ogura T. 1998. Heat shock regulation in the FtsH null mutant of Escherichia coli: dissection of stability and activity control mechanisms of Σ32 in vivo. Mol Microbiol 30:583–593. doi: 10.1046/j.1365-2958.1998.01091.x. [DOI] [PubMed] [Google Scholar]
- 14.Tse-Dinh YC, Qi H, Menzel R. 1997. DNA supercoiling and bacterial adaptation: thermotolerance and thermoresistance. Trends Microbiol 5:323–326. doi: 10.1016/S0966-842X(97)01080-9. [DOI] [PubMed] [Google Scholar]
- 15.Dorman CJ. 1996. Flexible response: DNA supercoiling, transcription and bacterial adaptation to environmental stress. Trends Microbiol 4:214–216. doi: 10.1016/0966-842X(96)30015-2. [DOI] [PubMed] [Google Scholar]
- 16.Qi H, Menzel R, Tse-Dinh YC. 1999. Increased thermosensitivity associated with topoisomerase I deletion and promoter mutations in Escherichia coli. FEMS Microbiol Lett 178:141–146. doi: 10.1016/S0378-1097(99)00322-5. [DOI] [PubMed] [Google Scholar]
- 17.Ogata Y, Mizushima T, Kataoka K, Miki T, Sekimizu K. 1994. Identification of DNA topoisomerases involved in immediate and transient DNA relaxation induced by heat shock in Escherichia coli. Mol Gen Genet 244:451–455. doi: 10.1007/BF00583895. [DOI] [PubMed] [Google Scholar]
- 18.Geertz M, Travers A, Mehandziska S, Sobetzko P, Chandra Janga S, Shimamoto N, Muskhelishvili G. 2011. Structural coupling between RNA polymerase composition and DNA supercoiling in coordinating transcription: a global role for the omega subunit? mBio 2:e00034-11. doi: 10.1128/mBio.00034-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Travers A, Muskhelishvili G. 2005. DNA supercoiling—a global transcriptional regulator for enterobacterial growth? Nat Rev Microbiol 3:157–169. doi: 10.1038/nrmicro1088. [DOI] [PubMed] [Google Scholar]
- 20.Travers A, Schneider R, Muskhelishvili G. 2001. DNA supercoiling and transcription in Escherichia coli: the FIS connection. Biochimie 83:213–217. doi: 10.1016/S0300-9084(00)01217-7. [DOI] [PubMed] [Google Scholar]
- 21.Weinstein-Fischer D, Altuvia S. 2007. Differential regulation of Escherichia coli topoisomerase I by Fis. Mol Microbiol 63:1131–1144. doi: 10.1111/j.1365-2958.2006.05569.x. [DOI] [PubMed] [Google Scholar]
- 22.González-Gil G, Kahmann R, Muskhelishvili G. 1998. Regulation of Crp transcription by oscillation between distinct nucleoprotein complexes. EMBO J 17:2877–2885. doi: 10.1093/emboj/17.10.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider R, Travers A, Muskhelishvili G. 2000. The expression of the Escherichia coli Fis gene is strongly dependent on the superhelical density of DNA. Mol Microbiol 38:167–175. doi: 10.1046/j.1365-2958.2000.02129.x. [DOI] [PubMed] [Google Scholar]
- 24.Dorman CJ, Schumacher MA, Bush MJ, Brennan RG, Buttner MJ. 2020. When is a transcription factor a NAP? Curr Opin Microbiol 55:26–33. doi: 10.1016/j.mib.2020.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daniel J, Joseph E, Ullmann A, Danchin A. 1981. CrpX mutants of Escherichia coli K-12: selection and physiological properties. FEMS Microbiol Lett 10:389–393. doi: 10.1111/j.1574-6968.1981.tb06277.x. [DOI] [Google Scholar]
- 26.Dessein A, Schwartz M, Ullmann A. 1978. Catabolite repression in Escherichia coli mutants lacking cyclic AMP. Mol Gen Genet 162:83–87. doi: 10.1007/BF00333853. [DOI] [PubMed] [Google Scholar]
- 27.Conrad TM, Joyce AR, Applebee MK, Barrett CL, Xie B, Gao Y, Palsson BT. 2009. Whole-genome resequencing of Escherichia coli K-12 MG1655 undergoing short-term laboratory evolution in lactate minimal media reveals flexible selection of adaptive mutations. Genome Biol 10:R118. doi: 10.1186/gb-2009-10-10-r118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eschenlauer AC, Reznikoff WS. 1991. Escherichia coli catabolite gene activator protein mutants defective in positive control of lac operon transcription. J Bacteriol 173:5024–5029. doi: 10.1128/jb.173.16.5024-5029.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bell A, Gaston K, Williams R, Chapman K, Kolb A, Buc H, Minchin S, Williams J, Busby S. 1990. Mutations that alter the ability of the Escherichia coli cyclic AMP receptor protein to activate transcription. Nucleic Acids Res 18:7243–7250. doi: 10.1093/nar/18.24.7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sekowska A, Wendel S, Fischer EC, Nørholm MHH, Danchin A. 2016. Generation of mutation hotspots in ageing bacterial colonies. Sci Rep 6:2–10. doi: 10.1038/s41598-016-0005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Notley-McRobb L, King T, Ferenci T. 2002. RpoS mutations and loss of general stress resistance in Escherichia coli populations as a consequence of conflict between competing stress responses. J Bacteriol 184:806–811. doi: 10.1128/JB.184.3.806-811.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farrell MJ, Finkel SE. 2003. The growth advantage in stationary-phase phenotype conferred by RpoS mutations is dependent on the PH and nutrient environment. J Bacteriol 185:7044–7052. doi: 10.1128/JB.185.24.7044-7052.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finkel SE. 2006. Long-term survival during stationary phase: evolution and the GASP phenotype. Nat Rev Microbiol 4:113–120. doi: 10.1038/nrmicro1340. [DOI] [PubMed] [Google Scholar]
- 34.Shao ZQ, Lin RT, Newman EB. 1994. Sequencing and characterization of the SdaC gene and identification of the SdaCB operon in Escherichia coli K12. Eur J Biochem 222:901–907. doi: 10.1111/j.1432-1033.1994.tb18938.x. [DOI] [PubMed] [Google Scholar]
- 35.Su H, Newman EB. 1991. A novel L-serine deaminase activity in Escherichia coli K-12. J Bacteriol 173:2473–2480. doi: 10.1128/jb.173.8.2473-2480.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bachar A, Itzhaki E, Gleizer S, Shamshoom M, Milo R, Antonovsky N. 2020. Point mutations in topoisomerase I alter the mutation spectrum in E. coli and impact the emergence of drug resistance genotypes. Nucleic Acids Res 48:761–769. doi: 10.1093/nar/gkz1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Z, Cheng B, Tse-Dinh Y-C. 2011. Crystal structure of a covalent intermediate in DNA cleavage and rejoining by Escherichia coli DNA topoisomerase I. Proc Natl Acad Sci USA 108:6939–6944. doi: 10.1073/pnas.1100300108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duprey A, Groisman EA. 2020. FEDS: a novel fluorescence-based high-throughput method for measuring DNA supercoiling in vivo. mBio 11:e01053-20. doi: 10.1128/mBio.01053-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klein G, Stupak A, Biernacka D, Wojtkiewicz P, Lindner B, Raina S. 2016. Multiple transcriptional factors regulate transcription of the RpoE gene in Escherichia coli under different growth conditions and when the lipopolysaccharide biosynthesis is defective. J Biol Chem 291:22999–23019. doi: 10.1074/jbc.M116.748954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kallipolitis BH, Valentin-Hansen P. 1998. Transcription of rpoH, encoding the Escherichia coli heat-shock regulator Σ32, is negatively controlled by the cAMP-CRP/CytR nucleoprotein complex. Mol Microbiol 29:1091–1099. doi: 10.1046/j.1365-2958.1998.00999.x. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi Y, Inouye M. 2015. An endogenous protein inhibitor, YjhX (TopAI), for topoisomerase I from Escherichia coli. Nucleic Acids Res 43:10387–10396. doi: 10.1093/nar/gkv1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Axley MJ, Stadtman TC. 1988. Anaerobic induction of Escherichia coli formate dehydrogenase (hydrogenase-linked) is enhanced by gyrase inactivation. Proc Natl Acad Sci USA 85:1023–1027. doi: 10.1073/pnas.85.4.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheung KJ, Badarinarayana V, Selinger DW, Janse D, Church GM. 2003. A microarray-based antibiotic screen identifies a regulatory role for supercoiling in the osmotic stress response of Escherichia coli. Genome Res 13:206–215. doi: 10.1101/gr.401003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martis B S, Forquet R, Reverchon S, Nasser W, Meyer S. 2019. DNA supercoiling: an ancestral regulator of gene expression in pathogenic bacteria? Comput Struct Biotechnol J 17:1047–1055. doi: 10.1016/j.csbj.2019.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crozat E, Philippe N, Lenski RE, Geiselmann J, Schneider D. 2005. Long-term experimental evolution in Escherichia coli. XII. DNA topology as a key target of selection. Genetics 169:523–532. doi: 10.1534/genetics.104.035717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crozat E, Winkworth C, Gaffé J, Hallin PF, Riley MA, Lenski RE, Schneider D. 2010. Parallel genetic and phenotypic evolution of DNA superhelicity in experimental populations of Escherichia coli. Mol Biol Evol 27:2113–2128. doi: 10.1093/molbev/msq099. [DOI] [PubMed] [Google Scholar]
- 47.Deatherage DE, Kepner JL, Bennett AF, Lenski RE, Barrick JE. 2017. Specificity of genome evolution in experimental populations of Escherichia coli evolved at different temperatures. Proc Natl Acad Sci USA 114:E1904–E1912. doi: 10.1073/pnas.1616132114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phaneuf PV, Gosting D, Palsson BO, Feist AM. 2019. Aledb 1.0: a database of mutations from adaptive laboratory evolution experimentation. Nucleic Acids Res 47:D1164–D1171. doi: 10.1093/nar/gky983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horinouchi T, Suzuki S, Kotani H, Tanabe K, Sakata N, Shimizu H, Furusawa C. 2017. High-throughput laboratory evolution of Escherichia coli under multiple stress environments. bioRxiv doi: 10.1101/143792. [DOI]
- 50.Conrad TM, Lewis NE, Palsson BO. 2011. Microbial laboratory evolution in the era of genome-scale science. Mol Syst Biol 7:509. doi: 10.1038/msb.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamagishi M, Matsushima H, Wada A, Sakagami M, Fujita N, Ishihama A. 1993. Regulation of the Escherichia coli Rmf gene encoding the ribosome modulation factor: growth phase- and growth rate-dependent control. EMBO J 12:625–630. doi: 10.1002/j.1460-2075.1993.tb05695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sobetzko P. 2016. Transcription-coupled DNA supercoiling dictates the chromosomal arrangement of bacterial genes. Nucleic Acids Res 44:1514–1524. doi: 10.1093/nar/gkw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dorman CJ, Lynch AS, Bhriain NN, Higgins CF. 1989. DNA supercoiling in Escherichia coli: TopA mutations can be suppressed by DNA amplifications involving the TolC locus. Mol Microbiol 3:531–540. doi: 10.1111/j.1365-2958.1989.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 54.Dinardo S, Voelkel KA, Sternglanz R, Reynolds AE, Wright A. 1982. Escherichia coli DNA topoisomerase I mutants have compensatory mutations in DNA gyrase genes. Cell 31:43–51. doi: 10.1016/0092-8674(82)90403-2. [DOI] [PubMed] [Google Scholar]
- 55.Green J, Stapleton MR, Smith LJ, Artymiuk PJ, Kahramanoglou C, Hunt DM, Buxton RS. 2014. Cyclic-AMP and bacterial cyclic-AMP receptor proteins revisited: adaptation for different ecological niches. Curr Opin Microbiol 18:1–7. doi: 10.1016/j.mib.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deatherage DE, Barrick JE. 2014. Identification of mutations in laboratory-evolved microbes from next-generation sequencing data using Breseq. Methods Mol Biol 1151:165–188. doi: 10.1007/978-1-4939-0554-6_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Freddolino PL, Amini S, Tavazoie S. 2012. Newly identified genetic variations in common Escherichia coli MG1655 stock cultures. J Bacteriol 194:303–306. doi: 10.1128/JB.06087-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Datta S, Costantino N, Court DL. 2006. A set of recombineering plasmids for Gram-negative bacteria. Gene 379:109–115. doi: 10.1016/j.gene.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 59.Tan K, Zhou Q, Cheng B, Zhang Z, Joachimiak A, Tse-Dinh YC. 2015. Structural basis for suppression of hypernegative DNA supercoiling by E. coli topoisomerase I. Nucleic Acids Res 43:11031–11046. doi: 10.1093/nar/gkv1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gama-Castro S, Salgado H, Santos-Zavaleta A, Ledezma-Tejeida D, Muñiz-Rascado L, García-Sotelo JS, Alquicira-Hernández K, Martínez-Flores I, Pannier L, Castro-Mondragón JA, Medina-Rivera A, Solano-Lira H, Bonavides-Martínez C, Pérez-Rueda E, Alquicira-Hernández S, Porrón-Sotelo L, López-Fuentes A, Hernández-Koutoucheva A, Del Moral-Chávez V, Rinaldi F, Collado-Vides J. 2016. RegulonDB version 9.0: high-level integration of gene regulation, coexpression, motif clustering and beyond. Nucleic Acids Res 44:D133–D143. doi: 10.1093/nar/gkv1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mutations identified in 48 isolated clones by whole-genome sequencing. Download Table S1, XLSX file, 0.03 MB (28.6KB, xlsx) .
Copyright © 2021 Heyde et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Characterization of topA* mutants. Growth phenotypes of the five evolved cya topA* mutant strains (1 to 5), the parental cya strain, and wild-type K-12 MG1655 on MacConkey agar plates without (top) and with (bottom) 1% maltose supplementation at 37°C and 44°C. Download FIG S1, TIF file, 2.7 MB (2.7MB, tif) .
Copyright © 2021 Heyde et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Characterization of rmf expression in different strain backgrounds. (a) Transcript-per-million (TPM) values for rmf RNA detected in the RNA-seq analysis conducted in this study. RNA was extracted from stationary-phase cultures of the wild-type, cya, and cya topA* strains grown at 37°C. (b) Relative fluorescence intensity of the cya topA* mutant strain and control strains carrying an rmf reporter plasmid (illustrated) in which gfp expression is under the control of the rmf promoter. Download FIG S2, TIF file, 2.3 MB (2.3MB, tif) .
Copyright © 2021 Heyde et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Changes in gene expression in different mutants and conditions identified by transcriptomics. Raw data were sorted in sheets as indicated in the text and the legends for Fig. 3 and 4. Download Table S2, XLSX file, 0.3 MB (340.3KB, xlsx) .
Copyright © 2021 Heyde et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Changes in gene expression in different mutants identified by transcriptomics. Raw data were sorted as indicated in the text and the legend for Fig. 4b. Download Table S3, XLSX file, 0.01 MB (10.6KB, xlsx) .
Copyright © 2021 Heyde et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Data Availability Statement
All sequencing data connected to this study have been deposited in the European Nucleotide Archive (ENA). Whole-genome sequencing data can be accessed via accession number PRJEB43656. RNA sequencing data can be accessed via accession number PRJEB43645.